Abstract
A paper by Sun et al. identified the Ca2+-activated Cl− channel anoctamin 1 or ANO1 (TMEM16A) as an important regulator of osteoclast function by interacting with RANKL activating signaling pathways involved in bone resorption. Although Cl− transporters (e.g. ClC7, CLIC5) have been known to be involved in the active process of bone resorption, ANO1 appears to control osteoclast differentiation and function to levels beyond those of other Cl−transporters. Regulating ANO1 function might be a useful target for therapeutics in osteoporosis.
Normal bone remodeling is a balance of bone formation by the osteoblasts and bone breakdown or resorption by the osteoclasts. The latter cells have unique characteristics and abilities to resorb the mineralized and organic phases of bone [1]. They are multinucleated cells formed by fusion that seal to the mineral surface of bone creating an extracellular “lysosome” space. Into this sealed zone, the cells export H+ and Cl− ions across the ruffled border, acidifying this space to dissolve the hydroxyapatite of the mineralized bone matrix, releasing Ca2+ and Pi that are then transported across the cell to the extracellular fluid.
Osteoclasts differentiate from monocytic cells in the bone marrow. This requires M-CSF (macrophage-colony stimulating factor) for proliferation and maintenance of the monocytic cells, and RANKL (receptor activator of nuclear factor kappa-B ligand) to differentiate the monocytic cells to osteoclasts, for their fusion and terminal state of differentiation. Both M-CSF and RANKL are produced by the osteoblast, demonstrating the tight coupling that occurs between osteoblasts and osteoclasts. RANKL binds and acts through its receptor, RANK, triggering a cascade of intracellular events culminating in the activation of NFATC1 (nuclear factor of activated T cells).
Osteoporosis is a bone disease that primarily affects women after menopause due to loss of estrogen [2]. In female mice, this can be mimicked by ovariectomy causing bone loss. Loss of estrogen leads to an imbalance in bone remodeling, with greater numbers of osteoclasts causing a decrease in bone mass, thinning of trabeculae and, in some cases, leading to fractures. There are a number of treatments available for osteoporosis, and several target the osteoclast, inhibiting bone resorption. These include the bisphosphonates, the RANKL antibody, denosumab, and the cathepsin K inhibitor (although the latter is not approved in the U.S. due to cerebo-cardiovascular issues).
Until recently, the acidification of resorption lacunae by Cl− transporters was attributed mainly to ClC-7. Sun et al. report a novel role of Anoctamin 1 (ANO1, TMEM16A) in bone resorption and suggest that it may be a possible target for therapeutic intervention in osteoporosis [3]. ANO1 is a Ca2+-activated Cl− channel activated by modest elevations in intracellular Ca2+ that does not involve calmodulin [4]. ANO1 has been associated with several functions including Ca2+ dependent fluid transport in epithelia including salivary and sweat glands [5, 6], mucin secretion [7] and cell proliferation and metastasis [8]. The authors first identified several Cl− channels highly expressed in osteoclasts (ANO1, CFTR, ClC7 and ClCN4), with ANO1 and ClC7 being highly expressed during osteoclast differentiation. ClC7 is a secondary active Cl−/H+ antiporter expressed in the ruffled border of the osteoclasts providing the necessary acidification for the digestion of bone matrix within the resorption lacunae. Human mutations in the CLC-7 gene result in a full spectrum of osteopetrosis, a disorder causing bones to grow abnormally and become very dense. Sun et al. [3] noted that siRNA knockdown of Ano1 in murine osteoclasts elicited a more robust decrease of TRAP+ multinucleated cells (markers of osteoclasts) than the knockdown of Clc-7. These findings were followed by whole cell patch clamp Cl− current measurements in osteoclasts that could not be suppressed by blocking Clc-7 but could be nearly abolished by pharmacological inhibition of Ano1. The generation of osteoclast-specific conditional KO of Ano1 mice (Ctsk-Cre;Ano1fl/fl showed a decrease in the expression of several osteoclast markers such as Nfatc1, Acp5 and Mmp9 in bone cells, and further analysis by micro-computerized tomography of the femurs and vertebrae showed higher bone volume, consistent with abnormal osteoclastic bone resorption, which was also supported by a decrease in staining of TRAP+ multinucleated osteoclasts. Conversely, overexpressing Ano1 in transgenic mice using the Acp5 promoter revealed decreased bone volume in mice concomitant with elevated expression of the same markers.
As noted above, RANKL signaling is important for osteoclast differentiation and the authors found, through microarray analysis of cultured osteoclasts from the Ctsk-Cre;Ano1fl/fl mice, that several pathways detected in the bioinformatic analysis coalesced as being downstream from RANKL-RANK signaling, prompting them to consider Ano1 as an upstream effector of RANKL [3]. In fact, they identified that RANK and ANO1 associated, using co-immunoprecipitation, but only in cells cultured with RANKL for 5 d The authors then extended their work to include the analysis of samples from patients with osteoporosis. They found that ANO1 protein levels were significantly higher in bone tissue of patients with osteoporosis than in non-osteoporotic samples. Interestingly, ANO1 levels were also higher in bone tissue of ovariectomized mice prompting the authors to address the role of ANO1 in the treatment of osteoporosis. Ovariectomy of Ctsk-Cre;Ano1fl/fl mice revealed higher bone mass than in Ano1fl/fl mice suggesting that reduction of ANO1 levels might be a protective mechanism against OVX-induced bone loss placing ANO1 in a position to be considered a possible therapeutic target for osteoporosis treatment.
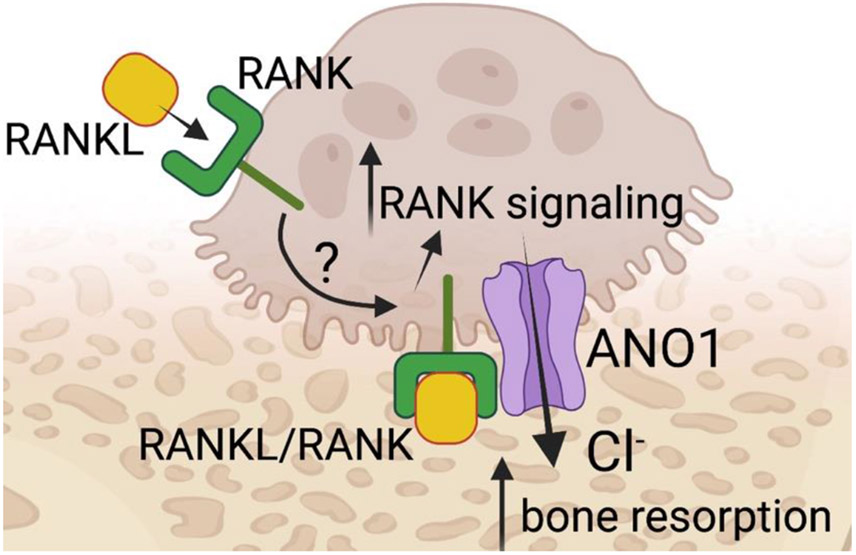
This comprehensive study suggests that ANO1 has a dual function in bone biology; it is important in osteoclast differentiation and function; and interacts with RANK when stimulated by RANKL thereby forming an important feedback loop promoting signaling pathways evoked by RANK/RANKL interactions. The latter, however, is a point that seemingly requires additional clarification given that RANKL stimulation is likely on the basolateral surface of the osteoclast whereas ANO1 is likely positioned in the ruffled border facing the bone matrix (Fig. 1). The study raises questions about the potential use of ANO1 in osteoporosis. Attenuating ANO1 function would decrease bone resorption and may promote bone formation. Yet such treatments may also contend with off-target effects due to the function of ANO1 in other tissues than bone. Regardless, ANO1 now has a new defined role in bone biology that should be seriously considered.
Fig. 1.
ANO1 function has dual relevance in bone biology. RANKL stimulation leads its receptor, RANK, to associate with ANO1 promoting RANK signaling. ANO1 also provides Cl− to enhance bone resorption.
Acknowledgements
This work was partially funded by NIH grant R01DK047420 to N.C.P. and NIDCR grants R01DE027679, R01DE027981 to R.S.L.
References
- [1].Teitelbaum SL, Ross FP, Genetic regulation of osteoclast development and function, Nat. Rev. Genet 4 (8) (2003) 638–649. [DOI] [PubMed] [Google Scholar]
- [2].Almeida M, et al. , Estrogens and Androgens in Skeletal Physiology and Pathophysiology, Physiol. Rev 97 (1) (2017) 135–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Sun W, et al. , Anoctamin 1 controls bone resorption by coupling Cl(−) channel activation with RANKL-RANK signaling transduction, Nat. Commun 13 (1) (2022) 2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Yu K, et al. , Activation of the Ano1 (TMEM16A) chloride channel by calcium is not mediated by calmodulin, J. Gen. Physiol 143 (2) (2014) 253–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Romanenko VG, et al. , Tmem16A encodes the Ca2+-activated Cl− channel in mouse submandibular salivary gland acinar cells, J. Biol. Chem 285 (17) (2010) 12990–13001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Concepcion AR, et al. , Store-operated Ca2+ entry regulates Ca2+-activated chloride channels and eccrine sweat gland function, J. Clin. Invest 126 (11) (2016) 4303–4318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Huang F, et al. , Calcium-activated chloride channel TMEM16A modulates mucin secretion and airway smooth muscle contraction, Proc. Natl. Acad. Sci. USA 109 (40) (2012) 16354–16359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Britschgi A, et al. , Calcium-activated chloride channel ANO1 promotes breast cancer progression by activating EGFR and CAMK signaling, Proc. Natl. Acad. Sci. USA 110 (11) (2013) E1026–E1034. [DOI] [PMC free article] [PubMed] [Google Scholar]