Introduction
Duodenal injury (DI) is infrequent. It has been reported in 0.003% to 0.5% of trauma admissions [1–3] and has been found in 3.1% to 5% trauma laparotomies [2, 4].
Most of the mortality occurs early and is related to associated lesions. Late deaths are associated with infections and multiple organ failure.
Among late morbidity, duodenal leakage (DL) and fistula have been reported in a wide range from 0 to 37.5% [5, 6], with a median of 6.1%. They are associated with higher rates of intraabdominal abscesses, prolongation of the stay in the ICU and the hospital and higher mortality [7–9].
Complex techniques, such as diverticulization [10, 11], pyloric exclusion (PE) [12], decompressive tube duodenostomy [13], were devised to prevent the exposition of the duodenal repair to saliva and gastric secretion, to reduce the pressure in the duodenal lumen or both, and as a consequence the risk and the impact of DL. They have been progressively abandoned in favor of primary repair, as in the last three decades they failed to show better outcomes.
Several authors have investigated the risk factors for DL. Still most of the evidence comes from retrospective series and lacks enough sample size, a precise definition of the studied morbidity and bivariate analyses, which precludes to know the influence of potential confounders [2, 3, 8, 9, 14–17]. Identified risk factors include shock and trauma severity. The associated pancreatic injury seems to increase the risk of DL.
Because of the mentioned limitations, the contribution of the complex techniques to reduce or increase the risk of DL has not been clarified.
A recently published multicentric study from the Panamerican Trauma Society (PTS), which had enough power, suggested that primary repair is safe in most duodenal injuries [18].
We performed a secondary analysis of the PTS database to evaluate the impact of the leakage of duodenal injuries surgically treated and to know the risk factors for DL, including the type of surgical repair.
Materials and methods
A retrospective multicenter trial was conducted, including patients from 11 PTS centers.
Recruitment methods, collection of the information, and ethical considerations were previously reported [18].
Subjects 18 years and older with duodenal injuries, surgically treated from 2006 to 2017, were included. Patients who died in the first 48 h after the trauma and subjects without classification of the duodenal lesion severity or cases in which the outcome was not registered were excluded.
Demographics, trauma mechanism, shock on admission, injury severity, associated injuries, transfusions, and type of repair were examined as potential risk factors for a leak of the duodenal repair.
The severity of the duodenal injuries was classified according to the American Association for the Surgery of Trauma (AAST) severity scale. Grade 3 wounds were categorized independently for the analysis because they exhibited a higher risk of leakage, sepsis, and death.
The duodenal repairs were classified according to their relative risk of DL as "primary repair", "suture + duodenostomy", and "complex repairs". This category included PE and ligation with reconstruction or a Whipple's procedure in a subsequent procedure.
The analysis was performed with STATA 15.1® (College Station TX). Categorical variables are presented as quantities and proportions—continuous variables as mean and standard deviation (SD) or median and interquartile range (IQR), after normality analysis.
Comparisons were made between patients who developed DL and patients who did not.
Proportions were compared with Chi2 or Fisher's exact test, as indicated. Continuous variables were compared with Student's test or Wilcoxon–Mann–Whitney test, according to normality.
Models were developed to identify predictors of duodenal leakage and sepsis. Potential predictors of DL were analyzed with simple logistic regressions. Variables with a p < 0.1, including the categorized duodenal repair, were included in a multiple logistic regression. The final models were evaluated with ROC curves and Hosmer–Lemeshow goodness-of-fit test.
Results
A total of 378 patients were registered. Ninety of them met one or more exclusion criteria, being the most frequent exclusion causes death during the first 48 h after trauma (n = 61), and age < 18 years old (n = 30). The remaining 288 were selected for the analysis.
Median age was 29 years (IQR 22–43), and 236 (81.9%) of the subjects were males. Penetrating trauma occurred in 223 (77.3%). Forty-seven patients (16.3%) were hypotensive at admission, and 126 (43.8%) received transfusions before surgery. (Table 1).
Table 1.
Descriptive statistics. Comparison by the leak of the duodenal repair
| Total (n = 288) |
No leak (n = 230) |
Leak (n = 50) |
p-value | |
|---|---|---|---|---|
| Age (years), median (IQR) | 29 (22–43) | 30 (22–43) | 26.5 (22–37) | 0.177** |
| Sex | ||||
| Males, n (%) | 236 (81.9) | 194 (81.5) | 42 (84.0) | 0.840* |
| Females, n (%) | 52 (18.1) | 44 (18.5) | 8 (16.0) | |
| Injury mechanism | ||||
| Penetrating, n (%) | 223 (77.3) | 181 (76.1) | 42 (84.0) | 0.477* |
| Blunt, n (%) | 65 (22.6) | 57 (23.9) | 8 (16.0) | |
| SBP in the ER (mm Hg), median (IQR) | 111.5 (91.5–130) | 116 (96–131) | 100 (80–120) | < 0.001** |
| Hypotension at arrival to the ER, n (%) | 47 (16.3) | 31 (13.0) | 16 (32.05) | < 0.001* |
| Transfusion before first surgery, n (%) | 126 (43.8) | 100 (42.0) | 26 (52.0) | 0.212* |
| PRBC transfused (units), median (IQR) | 2 (0–5) | 1 (0–5) | 2 (0–5) | 0.277** |
| Massive transfusion, n (%) | 64 (22.2) | 51 (21.4) | 13 (26.0) | 0.460* |
| ISS, median (IQR) | 20 (16–26) | 18 (16–25) | 25 (17–26) | 0.011** |
| Abdominal AIS, median (IQR) | 3 (3–4) | 3 (2–4) | 4 (3–4) | < 0.001** |
| Duodenal AAST grade, median (IQR) | 3 (3–3) | 3 (2–3) | 3 (3–3) | 0.248** |
| Duodenal AAST grade 3, n (%) | 180 (62.5) | 139 (58.4) | 41 (82.0) | 0.002* |
| Associated intraabdominal injuries | ||||
| None, n (%) | 28 (9.7) | 24 (10.1) | 4 (8.0) | 0.797* |
| Liver, n (%) | 119 (41.3) | 95 (39.9) | 24 (48.0) | 0.344* |
| Colon, n (%) | 102 (35.4) | 84 (35.3) | 18 (36.0) | 1.000* |
| Pancreas, n (%) | 83 (28.8) | 56 (23.5) | 27 (54.0) | < 0.001 |
| Stomach, n (%) | 67 (23.3) | 50 (21.0) | 17 (34.0) | 0.064* |
| Major vascular, n (%) | 59 (20.5) | 51 (21.4) | 8 (16.0) | 0.446* |
| Small bowel, n (%) | 47 (16.6) | 40 (16.8) | 7 (14.0) | 0.833* |
| Kidney, n (%) | 59 (20.5) | 46 (19.3) | 13 (26.0) | 0.335* |
| Spleen, n (%) | 22 (7.6) | 18 (7.6) | 4 (8.0) | 1.000* |
| Surgical treatment | ||||
| Primary repair, n (%) | 227 (78.8) | 201 (84.5) | 26 (52.0) | < 0.001* |
| Suture + duodenostomy, n (%) | 27 (9.4) | 19 (8.0) | 8 (16.0) | |
| Complex repairs, n (%)† | 34 (11.8) | 18 (7.6) | 16 (32.0) | |
SBP Systolic blood pressure, ER Emergency room, PRBC Packed red blood cells, IQR Interquartile range, AIS Abbreviated Injury Scale, AST American Association for the Surgery of Trauma, ISS Injury Severity Score
*Fisher’s exact test
**Wilcoxon–Mann–Whitney test
†Pyloric exclusion, diverticulization, others
One hundred and eight patients (38.0%) had extraabdominal injuries. This proportion was higher among blunt trauma patients (56.3% vs. 32.7%). Median (IQR) ISS was 20 (16–26) (Table 1).
The AAST duodenal injury severity grade was 3 in 180 cases (62.5%) and 4 or 5 in 40 (13.9%) (Table 2). Median (IQR) of abdominal AIS was 3 (3–4) (Table 1).
Table 2.
Trauma characteristics and outcomes according to duodenal trauma severity
| Variable | AAST Duodenal injury grade | |||
|---|---|---|---|---|
| 1 and 2 | 3 | 4 and 5 | p-value | |
| Number of patients (%) | 68 (23.6) | 180 (62.5) | 40 (13.9) | – |
| Age (years), median (IQR) | 29.5 (22–43) | 29 (22–40) | 28.5 (21–40.5) | 0.935** |
| SBP in the ER (mm Hg), median (IQR) | 112 (99–125) | 110 (90–130) | 120 (100–138) | 0.140** |
| Hypotension at arrival to the ER, n (%) | 11 (16.2) | 31 (17.2) | 5 (12.5) | 0.845* |
| Transfusion before first surgery, n (%) | 23 (33.8) | 91 (50.6) | 12 (30.0) | 0.10* |
| PRBC transfused (units), median (IQR) | 1 (0–4) | 2 (0–6) | 0 (0–3.5) | 0.231** |
| Massive transfusion, n (%) | 15 (22.1) | 41 (22.8) | 8 (20.0) | 0.978* |
| ISS, median (IQR) | 18 (15–25) | 21 (16–26) | 16 (10.5–25) | 0.005** |
| Abdominal AIS, median (IQR) | 3 (2–4) | 3 (3–4) | 3.5 (3–5) | 0.031** |
| ICU admission, n (%) | 38 (55.9) | 134 (74.4) | 29 (72.5) | 0.019* |
| Hospital LOS, n (%) | 13 (8–25) | 14.5 (9–31) | 18 (10.5–44.5) | 0.089** |
| Leak of the duodenal repair, n (%) | 5 (7.4) | 41 (22.8) | 4 (10.0) | 0.006* |
| Need for unplanned surgery, n (%) | 17 (25.0) | 69 (38.3) | 17 (42.5) | 0.094* |
| Sepsis, n (%) | 10 (14.7) | 47 (26.1) | 8 (20) | 0.165* |
| Mortality, n (%) | 0 (0) | 31 (17.2) | 5 (12.5) | < 0.001* |
AAST American Association for the Surgery of Trauma, SBP Systolic blood pressure, ER Emergency room, PRBC Packed red blood cells, IQR Interquartile range, ISS Injury Severity Score, AIS Abbreviated Injury Scale
*Fisher’s exact test
**Kruskal–Wallis test
The most frequent intraabdominal injured organ was the liver in 119 cases (41.3%), followed by the colon in 102 (35.4%), and the pancreas in 83 (28.8%). Fifty-nine (20.5%) patients had an abdominal vascular injury. In 28 cases (9.7%), there was not an abdominal associated injury (Table 1).
DL developed in 50 subjects (17.4%). Compared to those without leak, patients with leakage had significantly lower SBP at admission (100 mm Hg, IQR 80–120, vs.116 mm Hg, IQR 96–131), higher ISS (25, IQR 17–26, vs.18, IQR 16–25), higher abdominal AIS (4, IQR 3–4, vs.3, IQR 2–4), and a higher proportion of AAST grade 3 DI (82.0% vs. 58.4%). Pancreatic injury was most frequent in this group (54.0% vs. 23.5%) (Table 1).
The duodenal injury was treated most frequently by primary repair (78.8%). In 27 (9.4%) cases, a repair plus a descompressive duodenostomy was performed, in 26 (9.0%) a PE, with or without gastro-jejunostomy, and in 5, other methods of reconstruction. For the purpose of the analysis, PE and other methods were grouped as “complex repairs” due to their similar leak rate.
Compared with primary repair, patients managed with suture + duodenostomy or complex repairs leaked more frequently (Fig. 1).
Fig. 1.
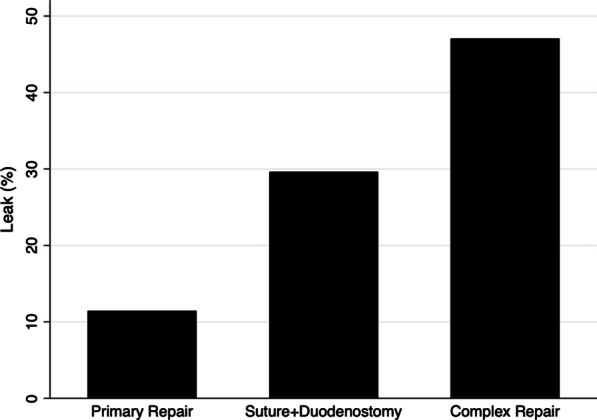
Type of repair and risk of duodenal repair leakage
Table 2 shows the comparison between grade 3 and the other grades of AAST DI. Grade 3 patients had more severe systemic trauma and associated abdominal injury, leaked, and developed sepsis more frequently. Mortality among them was higher.
Predictors of leak of the duodenal repair
Age, hypotension, ISS, abdominal AIS, duodenal AAST grade 3, associated injury of the pancreas and the liver, and the type of duodenal repair were identified as potential risk factors for DL in the univariate analysis (Table 3).
Table 3.
Analysis of risk factors for leak of duodenal repair
| Variable | Univariable analysis | Multivariable analysis | ||
|---|---|---|---|---|
| OR (95% C.I.) | p-value | OR (95% C.I.) | p-value | |
| Age, median (IQR) | 0 .963 (0.951–1.002) | 0.077 | – | 0.401 |
| Penetrating injury mechanism | 1.653 (0.734–3.726) | 0.225 | – | 0.839 |
| Hypotension at arrival | 3.142 (1.554–6.353) | 0.001 | 3.386 (1.516–7.565) | 0.003 |
| ISS | 1.034 (1.001–1.068) | 0.043 | – | 0.623 |
| Abdominal AIS | 1.908 (1.362–2.672) | < 0.001 | 1.967 (1.331–2.908) | 0.001 |
| Duodenal AAST grade | 1.262 (0.815–1.954) | 0.298 | – | |
| Duodenal AAST grade 3 | 3.245 (1.508–6.981) | 0.003 | 3.367 (1.467–7.728) | 0.004 |
| Associated intraabdominal injuries | ||||
| Liver | 1.389 (0.753—2.563) | 0.293 | – | |
| Pancreas | 3.815 (2.029–7.175) | < 0.001 | – | 0.166 |
| Major vascular | 0.698 (0.309–1.581) | 0.389 | – | |
| Stomach | 1.937 (0.998–3.759) | 0.051 | – | 0.386 |
| Surgical treatment | ||||
| Primary repair (reference) | 1 | 1 | ||
| Suture + duodenostomy | 3.255 (1.295–8.180) | 0.012 | 5.343 (1.829–15.605) | 0.002 |
| Complex repairs | 6.872 (3.126–15.105) | < 0.001 | 6.941 (2.905–16.588) | < 0.001 |
Area under ROC curve = 0.824 (0.766–0.883)
Goodness of fit p = 0.271
The MLR identified as independent predictors of leakage of the repair of the duodenal lesion hypotension on admission, O.R. (IQR) 3.386 (1.516–7.565), abdominal AIS, 1.967 (1.331–2.908) for each AIS point, duodenal AAST grade 3, 3.367 (1.467–2.908), and the duodenal repair with techniques different from primary repair, [O.R. (IQR) 5.343 (1.829–15.605) for primary suture + duodenostomy and 6.941 (2.905–16.558) for other complex repairs].
The created model had a good discriminative ability of the risk of DL (AUROC = 0.824 (0.766–0.883), and sufficient goodness to fit (p = 0.271).
Outcomes associated with the leak of the duodenal repair.
Compared with the group with no leak, the patients who leaked were admitted more frequently to the ICU (84.0% vs. 66.6%). The ICU LOS was more prolonged among the leak group [median (IQR) 21 (10–31), vs. 5.5 (3–12) days]. Additionally, patients who leaked spent more time in the hospital [median (IQR) 32 (14–52), vs. 13 (8.5–22) days].
The subjects with a leakage required more frequently unplanned surgeries, intraabdominal abscess drainage, and mechanical ventilation (Table 4).
Table 4.
Duodenal trauma. Outcomes compared by the leak of the duodenal repair
| Variable | Total (n = 288) |
No leak (n = 238) |
Leak (n = 50) |
P value |
|---|---|---|---|---|
| ICU admission, n (%) | 201 (69.8) | 159 (66.6) | 42 (84.0) | 0.017* |
| ICU LOS† days, median (IQR) | 7 (4–16) | 5.5 (3–12) | 21 (10–31) | < 0.001** |
| Hospital LOS days, median (IQR) | 15 (9–30) | 13 (8.5–22) | 32 (14–52) | < 0.001** |
| Sepsis, n (%) | 65 (22.6) | 34 (14.3) | 31 (62.0) | < 0.001* |
| Intraabdominal abscess, n (%) | 30 (10.4) | 20 (8.4) | 10 (20.0) | 0.022* |
| Need for unplanned surgery, n (%) | 103 (35.8) | 79 (33.2) | 24 (48.0) | 0.053* |
| Mechanical ventilation, n (%) | 82 (28.5) | 57 (24.0) | 25 (50.0) | < 0.001* |
| Renal replacement therapy, n (%) | 22 (7.6) | 16 (6.7) | 6 (12.0) | 0.238* |
| Hospital readmission, n (%) | 41 (14.2) | 35 (14.7) | 6 (12.0) | 0.824* |
| Mortality, n (%) | 36 (12.5) | 26 (10.9) | 10 (20.0) | 0.098* |
ICU Intensive Care Unit LOS Length of Stay
*Fisher’s Exact Test
**Wilcoxon–Mann–Whitney test
†For the patients admitted to the ICU
There were non-statistically significant increases in the need for renal replacement therapy and mortality. The readmission rate was similar in both groups (Table 4).
Multiple logistic regression identified DL as an independent risk factor for sepsis, along with hypotension, ISS, massive transfusion, and the use of complex procedures for repairing the DI (Table 5).
Table 5.
Risk factors for sepsis after duodenal trauma
| Variable | Univariable analysis | Multivariable analysis | ||
|---|---|---|---|---|
| OR (95% C.I.) | p-value | OR (95% C.I.) | p-value | |
| Age, median (IQR) | 0 .988 (0.967–1.001) | 0.280 | – | 0.466 |
| Penetrating injury mechanism | 2.445 (1.101–5.438) | 0.028 | – | 0.141 |
| Hypotension at arrival | 3.616 (1.866–7.007) | < 0.001 | 2.218 (1.003–4.905) | 0.049 |
| Massive transfusion | 2.949 (1.606–5.413) | < 0.001 | 2.553 (1.246–5.231) | 0.010 |
| ISS (every 10 points) | 1.802 (1.322–2.456) | < 0.001 | 1.651 (1.144–2.384) | 0.007 |
| Abdominal AIS | 1.505 (1.122–2.017) | 0.002 | – | 0.799 |
| Duodenal AAST grade | 1.196 (0.806–1.776) | 0.375 | – | – |
| Duodenal AAST grade 3 | 1.767 (0.994–3.238) | 0.065 | – | 0.988 |
| Associated intraabdominal injuries | ||||
| Stomach | 1.664 (0.898–3.085) | 0.106 | – | 0.582 |
| Pancreas | 3.568 (1.999–6.368) | < 0.001 | – | 0.206 |
| Kidney | 2.324 (1.242–4.346) | 0.008 | – | 0.277 |
| Leak of the duodenal repair | 7.083 (3.341–15.012) | < 0.001 | 7.083 (3.341–15.012) | < 0.001 |
| Complex repair of the duodenum* | 4.367 (2.357–8.055) | < 0.001 | 2.937 (1.425–6.051) | 0.003 |
Area under ROC curve = 0.819 (0.758–0.879)
Goodness of fit p = 0.546
IQR Interquartile range, ISS Injury Severity Score, AAST The American Association for the Surgery of Trauma
*Duodenal suture + duodenostomy or pyloric exclusion or diverticulizaction or other complex repairs
Discussion
Leakage of the repair of a duodenal lesion with or without fistula formation is one of the most feared complications in the surgical treatment of duodenal trauma, with a median of 6.3% in the published series [1, 6, 8, 9, 12–15, 17–33]. It has been associated with a higher risk of intraabdominal infection [8, 15], the need for support [8, 15, 29], prolonged stay [8, 15, 29], and a higher death risk [1, 8, 9, 13, 15, 20, 21, 24, 25, 27, 29].
In the PTS cohort, we identified leakages in 17.4% of the cases, which showed association with a higher risk of intraabdominal abscess, sepsis, ICU admission, and ventilatory support. ICU and hospital stay were longer.
The multivariate analysis of the sepsis risk factors revealed that DL contributes independently of trauma severity, shock, massive transfusions, and the technique used to repair the duodenal injury.
The probability of death was 1.8 times higher in the subjects with leakage. This difference did not reach statistical significance. Except for Levison’s study [23], which reported a slightly lower mortality rate in the group of the patients who leaked, the authors who analyzed this association found a higher risk of death in the leak subjects, with a median of 2.8 [1, 8, 9, 13, 15, 20, 21, 24, 25, 27, 29]. The intriguing Levinson's finding may be the consequence of survival bias. The author did not exclude the early deaths. Eight of the 17 patients who died did it intraoperatively by exsanguination. They did not have a chance to leak despite the severity of their trauma, modifying the result falsely.
The risk factors for DL have not been appropriately studied. Previous publications examined all duodenal complications, performed univariate analyses, or had low statistical power. In 1999, Timaran and coworkers studied 152 patients, 27 of them with duodenal complications. In a multivariate analysis, they found shock, ATI > 25, and the coexistence of colonic, pancreatic, or superior mesenteric vessels injury as independent risk factors [15]. In 2008, Fraga et al., in univariate analysis of duodenal and non-duodenal complications, occurring in 47 of 77 patients, identified association with altered RTS, ATI > 25, ISS > 25, and procedures different to primary repair [17]. In 2016, Schroeppel et al. compared subjects who leaked with individuals who did not. They did not identify significant differences in the compared variables [8]. In 2019, Weale published a similar comparison reporting a lower arterial PH, a higher lactic acid, and more frequent damage control surgeries in the patients who developed a duodenal leak [9].
Our study collected patients from 11 trauma centers from North, Central, and South America. It included an adequate number of subjects and outcomes to perform the statistical analysis required to identify the variables associated with the leak of the duodenal repair. We confirm the role of shock and trauma severity as risk factors for DL and evidence the risk associated with the more complex repairs, independently of the presence or the magnitude of the other factors.
Complex procedures were devised, to decompress the duodenum or to deviate the intestinal content from the repair, to prevent the fistula formation or to ameliorate its impact. Some of them, such as diverticulization, proved to be excessively aggressive or morbid. The merits of others, such as pyloric exclusion or duodenal decompression, are still debated.
Pyloric exclusion with gastro-jejunostomy, as described by Vaughan [12], or without it as proposed by Ginzburg [34] and Ferrada [35], has been the preferred method to treat duodenal injuries judged as severe.
One of the main difficulties in selecting candidates for a PE is the definition of severe duodenal trauma. Ben Taub Hospital [12, 22] and Denver Hospital [36] surgeons reported using PE in severe duodenal or pancreatoduodenal injuries without clearly defining severe trauma. Both groups reported PE in 41% of their cases. Nassoura et al., on the other hand, proposed ATI > 40 or duodenal injury score ≥ 4 as severity criteria. They performed PE in 3 out of 66 patients [14]. Additionally, the reports describing the surgical treatment according to trauma severity showed PE was used among severity grades 2 to 5, giving evidence of inconsistencies in the indication [18, 27, 36, 37].
The technique was created to reduce the risk of complications, which has not been proven. The publications from Houston containing the technique's description showed leakages only in the group treated by PE [12, 22].
Some studies have evaluated the impact of PE. Seamon and coworkers studied patients with penetrating DI OIS ≥ 2, who survived > 48 h. They compared 14 subjects with PE with 15 managed with PR. PE patients had a higher proportion of grade 4 injuries (21% vs. 0), suffered complications more frequently (71% vs. 33%), and had a more extended hospital stay (24.3 ± 19.7 vs. 13.5 ± 7.7 days). None of the differences reached statistical significance [6].
Velmahos et al. included 50 patients with OIS > 2 DI, 16 with PE. The proportion of cases with injuries in D1 and D2 and subjects with pancreatic trauma were higher in the PE group (79% vs. 42%, p = 0.02) and (63% vs. 24%, p = 0.02), respectively. DL, intraabdominal infections, and systemic complications occurred with similar frequencies [31].
Dubose and coworkers analyzed patients from the National Trauma Data Bank with DI grades 2 to 5 who survived more than 24 h. They compared 119 subjects primarily repaired with 28 patients treated with PE. The proportions of patients with ISS > 20, abdominal AIS > 3, and DI > 3 were higher in the PE group, without statistical significance. Adjusted morbidity, mortality, ICU stay, hospital stay, and hospital charges were similar [30].
Our data showed a fourfold increase in the risk of leakage when a PE was used. It cannot be attributed to the trauma severity. The association persisted after adjustment by the other identified risk factors.
Duodenal decompression with tubes comprises a heterogeneous set of intraluminal lines, including gastrostomy, duodenostomy, and proximal and distal jejunostomy. It was proposed by Stone et al. as an adjunct to reduce the pressure within the duodenal lumen without opening or resecting the stomach [38]. Original Stone's publication reported zero duodenal complications in 18 patients treated with this method [38]. Corley and coworkers informed 15% of duodenal complications in decompressed patients, compared with 26% in not decompressed subjects [1]. Stone and Fabian reported 302 cases of DI. Decompression was used in 78%. Duodenal complications occurred in 0.4% of the patients treated with decompression and in 19% of the cases treated without it [13].
Other authors reported a high frequency of use of decompression, without similar results. Snyder et al. complemented the duodenal repair with decompression techniques in 53% of their cases. Duodenal morbidity was more frequent among decompressed patients, 12% versus 8% [21]. Schroeppel and coworkers informed using decompression in 50% of their cases. Duodenal leakage happened in 10% when decompression was used and 2% when it was not [8].
In our report, DL was three times more frequent in the repair + duodenostomy. The association persisted and its strength increased after the multivariate analysis. It confirms the independent contribution and suggest a role in increasing the risk of DL.
Nassoura et al. proposed primary repair as the management technique for most penetrating DI. Duodenal fistula developed in 4% of the PR patients [14]. Some authors have documented an increase in PR use without a parallel increase in the complications [39, 40]. In most contemporary reports, Talving and Weale informed PR in 87% and 97% of their cases, respectively, with a low leakage rate [9, 29].
The available literature and our results identify trauma severity (systemic and local) as the main determinant of leakage after the repair of a duodenal injury [9, 15, 17, 28]. Complex procedures including diverticulization, pyloric exclusion, and tube duodenostomy have failed to reduce the risk of duodenal complications. In fact, as our analysis shows, they can contribute to increase the risk. Resecting, practicing incisions, and anastomoses or inserting tubes for decompression sum to the traumatic burden and the operation's length, which can increase the risk of infectious complications. There is enough evidence of the biological and clinical impact of the trauma from the injury and the surgery [41–44] and the additional risk derived from unnecessary procedures [45–48]. Our findings can be considered part of this evidence.
Our study has several limitations. First, the retrospective nature introduces the risk of information bias. It was mitigated by using clear and simple definitions. Second, the collected information covers 10 years, with possible changes in the diagnostic strategies, surgical procedures, and resuscitation principles. The available information did not let us analyze the influence of the trends over time on the risk factors or the outcomes. Third, duodenal trauma is infrequent. The exposition of each surgeon is limited, and as a consequence, the practices may be inconsistent. Despite this, the associations between the complex procedures and the duodenal complication were robust.
On the other hand, the investigation has some strengths which must be mentioned. First, patients from 11 high-volume trauma centers from North America, Central America, and South America were included. It makes our conclusions more generalizable. Second, the explored information and used definitions permitted us to analyze the most critical technical aspects. Third, the assembled cohort's sample size and the number of outcomes observed allowed the analyses we performed.
Conclusion
This retrospective multicentric analysis included 288 patients from 11 North and Latin America trauma centers. Hypotension at arrival, abdominal AIS, duodenal OIS = 3, and complex surgical procedures were identified as independent risk factors for the leakage of the repair of the duodenal injuries. Our findings permit us to recommend abandoning complex surgical procedures, including duodenal tube decompression, in favor of primary repair.
Author contributions
Study design and conception: AFG, AIS, PF, TS. Data Analysis: AFG, AIS. Manuscript elaboration: AFG, AIS, PF, LW, JD, GF, EB, AC, CM, BP, MR, MQ, GP, JCS, VK, RI, TS. Figures: AFG. All authors reviewed the manuscript.
Funding
None of the authors of this manuscript received any funding during the development of the study and the writing of the manuscript.
Availability of data and materials
Yes.
Declarations
Ethical approval and consent to participate
The Institutional Review Board of each of the participating centers approved the conduction of the study.
Consent for publication
The institutional Review Board of each institution approved the conduction and publication of the study results.
Competing interests
None of the authors declare any conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Alberto García, Email: alberto.garcia@correounivalle.edu.co, Email: alberto.garcia@fvl.org.co.
Alvaro I. Sanchez, Email: alvaro.sanchez@fvl.org.co
Paula Ferrada, Email: paula.ferrada@inova.org.
Luke Wolfe, Email: luke.wolfe@vcuhealth.org.
Juan Duchesne, Email: jduchesn@tulane.edu.
Gustavo P. Fraga, Email: fragagp2008@gmail.com
Elizabeth Benjamin, Email: Elizabeth.benjamin@med.usc.edu.
Andre Campbell, Email: andre.campbell@ucsf.edu.
Carlos Morales, Email: Hernando.morales@udea.edu.co.
Bruno M. Pereira, Email: dr.bruno@gruposurgical.com.br
Marcelo Ribeiro, Email: mribeiro@cwaynet.com.br.
Martha Quiodettis, Email: traumahst@gmail.com.
Gregory Peck, Email: peckgr@rwjms.rutgers.edu.
Juan C. Salamea, Email: jsalamea@gmail.com
Rao Ivatury, Email: raoivatury@gmail.com.
Thomas Scalea, Email: tscalea@umm.edu.
References
- 1.Corley RD, Norcross WJ, Shoemaker WC. Traumatic injuries to the duodenum: a report of 98 patients. Ann Surg. 1975;181(1):92–98. doi: 10.1097/00000658-197501000-00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huerta S, Bui T, Porral D, Lush S, Cinat M. Predictors of morbidity and mortality in patients with traumatic duodenal injuries. Am Surg. 2005;71(9):763–767. doi: 10.1177/000313480507100914. [DOI] [PubMed] [Google Scholar]
- 3.Phillips B, Turco L, McDonald D, Mause A, Walters RW. Penetrating injuries to the duodenum: an analysis of 879 patients from the National Trauma Data Bank, 2010 to 2014. J Trauma Acute Care Surg. 2017;83(5):810–817. doi: 10.1097/TA.0000000000001604. [DOI] [PubMed] [Google Scholar]
- 4.Asensio JA, Feliciano DV, Britt LD, Kerstein MD. Management of duodenal injuries. Curr Probl Surg. 1993;30(11):1023–1093. doi: 10.1016/0011-3840(93)90063-M. [DOI] [PubMed] [Google Scholar]
- 5.Fraga GP, Biazotto G, Bortoto JB, Andreollo NA, Mantovani M. The use of pyloric exclusion for treating duodenal trauma: case series. Sao Paulo Med J. 2008;126(6):337–341. doi: 10.1590/S1516-31802008000600009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seamon MJ, Pieri PG, Fisher CA, Gaughan J, Santora TA, Pathak AS, et al. A ten-year retrospective review: does pyloric exclusion improve clinical outcome after penetrating duodenal and combined pancreaticoduodenal injuries? J Trauma. 2007;62(4):829–833. doi: 10.1097/TA.0b013e318033a790. [DOI] [PubMed] [Google Scholar]
- 7.Teixeira PG, Inaba K, Dubose J, Salim A, Brown C, Rhee P, et al. Enterocutaneous fistula complicating trauma laparotomy: a major resource burden. Am Surg. 2009;75(1):30–32. doi: 10.1177/000313480907500106. [DOI] [PubMed] [Google Scholar]
- 8.Schroeppel TJ, Saleem K, Sharpe JP, Magnotti LJ, Weinberg JA, Fischer PE, et al. Penetrating duodenal trauma: a 19-year experience. J Trauma Acute Care Surg. 2016;80(3):461–465. doi: 10.1097/TA.0000000000000934. [DOI] [PubMed] [Google Scholar]
- 9.Weale RD, Kong VY, Bekker W, Bruce JL, Oosthuizen GV, Laing GL, et al. Primary repair of duodenal injuries: a retrospective cohort study from a major trauma centre in South Africa. Scand J Surg. 2019 doi: 10.1177/1457496918822620. [DOI] [PubMed] [Google Scholar]
- 10.Berne CJ, Donovan AJ, Hagen WE. Combined duodenal pancreatc trauma. The role of end-to-side gastrojejunostomy. Arch Surg. 1968;96(5):712–722. doi: 10.1001/archsurg.1968.01330230020004. [DOI] [PubMed] [Google Scholar]
- 11.Berne CJ, Donovan AJ, White EJ, Yellin AE. Duodenal "diverticulization" for duodenal and pancreatic injury. Am J Surg. 1974;127(5):503–507. doi: 10.1016/0002-9610(74)90305-5. [DOI] [PubMed] [Google Scholar]
- 12.Vaughan GD, 3rd, Frazier OH, Graham DY, Mattox KL, Petmecky FF, Jordan GL., Jr The use of pyloric exclusion in the management of severe duodenal injuries. Am J Surg. 1977;134(6):785–790. doi: 10.1016/0002-9610(77)90325-7. [DOI] [PubMed] [Google Scholar]
- 13.Stone HH, Fabian TC. Management of duodenal wounds. J Trauma. 1979;19(5):334–338. doi: 10.1097/00005373-197905000-00006. [DOI] [PubMed] [Google Scholar]
- 14.Nassoura ZE, Ivatury RR, Simon RJ, Kihtir T, Stahl WM. A prospective reappraisal of primary repair of penetrating duodenal injuries. Am Surg. 1994;60(1):35–39. [PubMed] [Google Scholar]
- 15.Timaran CH, Martinez O, Ospina JA. Prognostic factors and management of civilian penetrating duodenal trauma. J Trauma. 1999;47(2):330–335. doi: 10.1097/00005373-199908000-00019. [DOI] [PubMed] [Google Scholar]
- 16.Tyburski JG, Dente CJ, Wilson RF, Shanti C, Steffes CP, Carlin A. Infectious complications following duodenal and/or pancreatic trauma. Am Surg. 2001;67(3):227–230. doi: 10.1177/000313480106700305. [DOI] [PubMed] [Google Scholar]
- 17.Fraga GP, Biazotto G, Villaca MP, Andreollo NA, Mantovani M. Duodenal trauma: factors related to morbimortality. Rev Col Bras Cir. 2008;35(2):94–102. doi: 10.1590/S0100-69912008000200006. [DOI] [Google Scholar]
- 18.Ferrada P, Wolfe L, Duchesne J, Fraga GP, Benjamin E, Alvarez A, et al. Management of duodenal trauma: a retrospective review from the Panamerican Trauma Society. J Trauma Acute Care Surg. 2019;86(3):392–396. doi: 10.1097/TA.0000000000002157. [DOI] [PubMed] [Google Scholar]
- 19.Lucas CE, Ledgerwood AM. Factors influencing outcome after blunt duodenal injury. J Trauma. 1975;15(10):839–846. doi: 10.1097/00005373-197510000-00001. [DOI] [PubMed] [Google Scholar]
- 20.Flint LM, Jr, McCoy M, Richardson JD, Polk HC., Jr Duodenal injury. Analysis of common misconceptions in diagnosis and treatment. Ann Surg. 1980;191(6):697–702. doi: 10.1097/00000658-198006000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Snyder WH, 3rd, Weigelt JA, Watkins WL, Bietz DS. The surgical management of duodenal trauma. Precepts based on a review of 247 cases. Arch Surg. 1980;115(4):422–429. doi: 10.1001/archsurg.1980.01380040050009. [DOI] [PubMed] [Google Scholar]
- 22.Martin TD, Feliciano DV, Mattox KL, Jordan GL., Jr Severe duodenal injuries. Treatment with pyloric exclusion and gastrojejunostomy. Arch Surg. 1983;118(5):631–635. doi: 10.1001/archsurg.1983.01390050097019. [DOI] [PubMed] [Google Scholar]
- 23.Levison MA, Petersen SR, Sheldon GF, Trunkey DD. Duodenal trauma: experience of a trauma center. J Trauma. 1984;24(6):475–480. doi: 10.1097/00005373-198406000-00003. [DOI] [PubMed] [Google Scholar]
- 24.Moore JB, Moore EE. Changing trends in the management of combined pancreatoduodenal injuries. World J Surg. 1984;8(5):791–797. doi: 10.1007/BF01655784. [DOI] [PubMed] [Google Scholar]
- 25.Ivatury RR, Gaudino J, Ascer E, Nallathambi M, Ramirez-Schon G, Stahl WM. Treatment of penetrating duodenal injuries: primary repair vs. repair with decompressive enterostomy/serosal patch. J Trauma. 1985;25(4):337–341. doi: 10.1097/00005373-198504000-00011. [DOI] [PubMed] [Google Scholar]
- 26.Ivatury RR, Nallathambi M, Gaudino J, Rohman M, Stahl WM. Penetrating duodenal injuries. Analysis of 100 consecutive cases. Ann Surg. 1985;202(2):153–158. doi: 10.1097/00000658-198508000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cogbill TH, Moore EE, Feliciano DV, Hoyt DB, Jurkovich GJ, Morris JA, et al. Conservative management of duodenal trauma: a multicenter perspective. J Trauma. 1990;30(12):1469–1475. doi: 10.1097/00005373-199012000-00005. [DOI] [PubMed] [Google Scholar]
- 28.Blocksom JM, Tyburski JG, Sohn RL, Williams M, Harvey E, Steffes CP, et al. Prognostic determinants in duodenal injuries. Am Surg. 2004;70(3):248–255. doi: 10.1177/000313480407000311. [DOI] [PubMed] [Google Scholar]
- 29.Talving P, Nicol AJ, Navsaria PH. Civilian duodenal gunshot wounds: surgical management made simpler. World J Surg. 2006;30(4):488–494. doi: 10.1007/s00268-005-0245-0. [DOI] [PubMed] [Google Scholar]
- 30.DuBose JJ, Inaba K, Teixeira PG, Shiflett A, Putty B, Green DJ, et al. Pyloric exclusion in the treatment of severe duodenal injuries: results from the National Trauma Data Bank. Am Surg. 2008;74(10):925–929. doi: 10.1177/000313480807401009. [DOI] [PubMed] [Google Scholar]
- 31.Velmahos GC, Constantinou C, Kasotakis G. Safety of repair for severe duodenal injuries. World J Surg. 2008;32(1):7–12. doi: 10.1007/s00268-007-9255-4. [DOI] [PubMed] [Google Scholar]
- 32.Ordonez C, Garcia A, Parra MW, Scavo D, Pino LF, Millan M, et al. Complex penetrating duodenal injuries: less is better. J Trauma Acute Care Surg. 2014;76(5):1177–1183. doi: 10.1097/TA.0000000000000214. [DOI] [PubMed] [Google Scholar]
- 33.Turan U, Kilavuz H. Surgical management of penetrating duodenal injury: role of primary repair. J Coll Phys Surg Pak. 2020;30(10):1078–1081. doi: 10.29271/jcpsp.2020.10.1078. [DOI] [PubMed] [Google Scholar]
- 34.Ginzburg E, Carrillo EH, Sosa JL, Hertz J, Nir I, Martin LC. Pyloric exclusion in the management of duodenal trauma: is concomitant gastrojejunostomy necessary? Am Surg. 1997;63(11):964–966. [PubMed] [Google Scholar]
- 35.Ferrada R. Pancreas and Duodenum. Comment. In: Mattox KL, Moore EE, Feliciano DV, editors. TRAUMA. 4th ed. USA: TRAUMA; 2001; p. 759–2.
- 36.Mansour MA, Moore JB, Moore EE, Moore FA. Conservative management of combined pancreatoduodenal injuries. Am J Surg. 1989;158(6):531–535. doi: 10.1016/0002-9610(89)90185-2. [DOI] [PubMed] [Google Scholar]
- 37.Velmahos GC, Kamel E, Chan LS, Hanpeter D, Asensio JA, Murray JA, et al. Complex repair for the management of duodenal injuries. Am Surg. 1999;65(10):972–975. doi: 10.1177/000313489906501016. [DOI] [PubMed] [Google Scholar]
- 38.Stone HH, Garoni WJ. Experiences in the Management of Duodenal Wounds. South Med J. 1966;59(7):864–867. doi: 10.1097/00007611-196607000-00023. [DOI] [Google Scholar]
- 39.Mayberry J, Fabricant L, Anton A, Ham B, Schreiber M, Mullins R. Management of full-thickness duodenal laceration in the damage control era: evolution to primary repair without diversion or decompression. Am Surg. 2011;77(6):681–685. doi: 10.1177/000313481107700619. [DOI] [PubMed] [Google Scholar]
- 40.Aiolfi A, Matsushima K, Chang G, Bardes J, Strumwasser A, Lam L, et al. Surgical trends in the management of duodenal injury. J Gastrointest Surg. 2019;23(2):264–269. doi: 10.1007/s11605-018-3964-x. [DOI] [PubMed] [Google Scholar]
- 41.Borlase BC, Moore EE, Moore FA. The abdominal trauma index–a critical reassessment and validation. J Trauma. 1990;30(11):1340–1344. doi: 10.1097/00005373-199011000-00006. [DOI] [PubMed] [Google Scholar]
- 42.Vanzant EL, Lopez CM, Ozrazgat-Baslanti T, Ungaro R, Davis R, Cuenca AG, et al. Persistent inflammation, immunosuppression, and catabolism syndrome after severe blunt trauma. J Trauma Acute Care Surg. 2014;76(1):21–29. doi: 10.1097/TA.0b013e3182ab1ab5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brakenridge SC, Wang Z, Cox M, Raymond S, Hawkins R, Darden D, et al. Distinct immunologic endotypes are associated with clinical trajectory after severe blunt trauma and hemorrhagic shock. J Trauma Acute Care Surg. 2021;90(2):257–267. doi: 10.1097/TA.0000000000003029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dobson GP, Morris JL, Biros E, Davenport LM, Letson HL. Major surgery leads to a proinflammatory phenotype: Differential gene expression following a laparotomy. Ann Med Surg (Lond) 2021;71:102970. doi: 10.1016/j.amsu.2021.102970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nelson R, Singer M. Primary repair for penetrating colon injuries. Cochrane Database Syst Rev. 2003;3:CD002247. doi: 10.1002/14651858.CD002247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Talving P, Mohseni S, Inaba K, Plurad D, Branco BC, Lam L, et al. Closed suction drain after isolated hollow viscus injury: a friend or foe? J Trauma. 2011;70(6):1424–1428. doi: 10.1097/TA.0b013e31821c6337. [DOI] [PubMed] [Google Scholar]
- 47.Martin MJ, Hatch Q, Cotton B, Holcomb J. The use of temporary abdominal closure in low-risk trauma patients: helpful or harmful? J Trauma Acute Care Surg. 2012;72(3):601–606. doi: 10.1097/TA.0b013e31824483b7. [DOI] [PubMed] [Google Scholar]
- 48.George MJ, Adams SD, McNutt MK, Love JD, Albarado R, Moore LJ, et al. The effect of damage control laparotomy on major abdominal complications: a matched analysis. Am J Surg. 2018;216(1):56–59. doi: 10.1016/j.amjsurg.2017.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Yes.


