Highlights
-
•
Gaussia luciferase emits a bright blue light when oxidizing coelenterazine (CTZ).
-
•
A non-oxidizable coelenterazine (Aza-CTZ) wnabled investigation of Gluc's bioluminescence activity.
-
•
Monovalent anions are absolutely required for GLuc's bioluminescence.
-
•
NMR suggested that CTZ binds to residues in or near the hydrophobic cavity.
-
•
A large structural changes in regions remote from the hydrophobic cavity may upon the addition of CTZ.
-
•
These results point toward a unique mode of catalysis to achieve CTZ oxidative decarboxylation.
Keywords: SEP-tag, hydrophobic cavity; Intrinsically disordered region (IDR); NMR; Coelenterazine; Azacoelenterazine; Chemical shifts; Salt concentration
Abstract
Gaussia luciferase (GLuc 18.2kDa; 168 residues) is a marine copepod luciferase that emits a bright blue light when oxidizing coelenterazine (CTZ). It is a helical protein where two homologous sequential repeats form two anti-parallel bundles, each made of four helices. We previously identified a hydrophobic cavity as a prime candidate for the catalytic site, but GLuc's fast bioluminescence reaction hampered a detailed analysis. Here, we used azacoelenterazine (Aza-CTZ), a non-oxidizable coelenterazine (CTZ) analog, as a probe to investigate its binding mode to GLuc. While analysing GLuc's activity, we unexpectedly found that salt and monovalent anions are absolutely required for Gluc's bioluminescence, which retrospectively appears reasonable for a sea-dwelling organism. The NMR-based investigation, using chemical shift perturbations monitored by 15N-1H HSQC, suggested that Aza-CTZ (and thus unoxidized CTZ) binds to residues in or near the hydrophobic cavity. These NMR data are in line with a recent structural prediction of GLuc, hypothesizing that large structural changes occur in regions remote from the hydrophobic cavity upon the addition of CTZ. Interestingly, these results point toward a unique mode of catalysis to achieve CTZ oxidative decarboxylation.
Introduction
Bioluminescent organisms are found in more than half of all phyla in the animal kingdom [1] and over 100,000 species in 13 phyla and 660 genera, from bacteria to bony fishes [1,2]. Seventy-six percent of the 350,000 deep sea-dwelling organisms are endowed with bioluminescence [3]. Bioluminescence is traditionally classified as fluorescence or chemiluminescence. In the latter class, the light signal is produced by a chemical reaction resulting from a luciferin – luciferase pair. Luciferase denotes the enzyme catalyzing this reaction, and luciferin describes small chemicals acting as their substrates (calcium-dependent luciferases are also called photoproteins).
Thus, luciferase is a generic term whose members are not necessarily evolutionary related. Some luciferases require ATP or metal ions for activity, but not all. The luciferase genes (or photoprotein genes) have been cloned for more than 60 species belonging to ten different groups of organisms. Similarly, luciferins do not necessarily have a common chemical structure [1]. So far, ten types of luciferins have been characterized: Dinoflagellate luciferin [4], bacterial luciferin [5], cypridina luciferin [6], firefly luciferin [7,8], luminescent earthworm [9], shad shell latia [10], luminous mushroom [11] and coelenterazine [12].
A widely represented class of luciferin are coelenterazine (CTZ) and its derivatives. It is indeed found in at least seven species of marine luminescent organisms (radiolaria, cnidaria, combs, copepods, head-foot, decapoda, and chaerognatha) [13]. Moreover, though coelenterazine is readily oxidized by their respective luciferase, there is no strong sequence similarity or even sequence motif common to these luciferases.
Gaussia princeps is a bioluminescent copepod, which dwells in tropical or deep subtropical seas. Copepods are represented by more than 12,000 species and dominate the marine zooplankton fauna. Several bioluminescent copepods besides Gaussia have been reported: Metridia longa from the Arctic Ocean [14], Metridia pacifica from the Pacific Ocean [15], and over 20 bioluminescent copepods have been isolated around the Japanese coasts [16]. The Gaussia luciferase (GLuc) gene was cloned in 2002 [17]. Further studies pointed out common features for copepod luciferases, such as a molecular weight of about 20 kDa, which is smaller than all the non-Caecilian luciferases, as well as an N-terminal secretory signal of about 20 amino acids. In addition, copepod luciferases contain two tandem repeats of about 60 amino acids, featuring highly conserved amino acids (especially Cys, Leu, Arg, and Pro. A consensus sequence for the copepod luciferase repeat thus being: C-x(3)-C-L-x(2)-L-x(4)-C-x(8)-P-x-R-C, where x stands for any amino acid [18]. Like all copepods, GLuc uses coelenterazine as a substrate [19].
Gaussia luciferase (GLuc) is an all-alpha-helix protein made of nine helices as determined by heteronuclear multidimensional NMR [20]. GLuc surface-accessible analysis indicated that 19 residues form a hydrophobic cavity, which was hypothesized to be involved in the bioluminescence activity. However, NMR experiments could not identify interactions between GLuc and coelenterazine (CTZ) by chemical shift perturbation as monitored by HSQC, possibly because of a fast turnover of the oxidation reaction or irreversible inactivation of the enzyme as recently reported by Dijkema et al. [21].
In the present study, we analyzed the mechanism of GLuc-coelenterazine interaction using a non-oxidizable analog of coelenterazine which we named azacoelenterazine (Aza-CTZ) [22]. We found that Aza-CTZ binds to or nearby the hydrophobic cavity of GLuc. Moreover, and less anticipated, we found that salt is essential for the bioluminescence of GLuc. Retrospectively, this seems to be reasonable since luminous copepods live in halophilic environments, and thus, the natural reaction should occur in the presence of salt.
Materials and methods
Materials
Coelenterazine (CTZ) was purchased from Nanolight Technology. A stock solution of CTZ was prepared by dissolving CTZ in methanol at a concentration of 10 mM. The stock solution was diluted in the sample to the indicated final concentration for measurements. CTZ stock solutions were kept at -30 °C until use. Azacoelenterazine (Aza-CTZ) was synthesized in four steps from readily available 3-benzyl-5-(4-(benzyloxy)phenyl)-2-chloropyrazine [23] as previously described [22], and this compound was dissolved in DMSO at a concentration of 42.4 mg / mL. We expressed the recombinant Gaussia luciferase (GLuc) in E. coli using a Solubility Enhancement Peptide tag (SEP-Tag), which was essential for producing natively folded GLuc as described previously [20,[24], [25]]
Luminescence assays
Light emission spectra were measured using an FP-8000 fluorescence spectrophotometer with an emission bandwidth of 5 nm, time response of 0.2 s, and a scan speed of 20,000 nm/min at 15 °C (JASCO International Co., Ltd, Tokyo, Japan). Measurements were initiated by the addition of CTZ to the samples.
For the salt effect experiments, 40 µL of CTZ solutions, prepared with various salts (NaCl, NaBr, NaI, NaF, KCl, CaCl2, MgCl2) to final concentrations of 1, 5, 10, 50, 100, 200, 500, and 1000 mM, were added to 10 µl of GLuc solution (final concentration of 0.5 µM GLuc, 25 mM MES pH 7.0). The mixture was thoroughly mixed by pipetting. The time between the substrate addition and measurement initiation was 10 s.
Inhibition assay
A mixture of CTZ and Aza-CTZ, where CTZ concentration was kept constant at 0.5 μM and Aza-CTZ concentration was varied from 0 M to 300 μM, was prepared. 40 μL of the CTZ+Aza-CTZ mixture containing a final concentration of 0.2 M NaBr and 0.3 M ascorbic acid was added to 10 μL of GLuc solution (final concentration of GLuc was 0.2 μM in 25 mM MOPS buffer, pH 7.9). The sample was mixed by five-time pipetting, and the spectrum was measured to obtain the sample light intensity. Delta light intensity was calculated by subtracting the sample light intensity from the average maximum light intensity. The average maximum light intensity was measured using CTZ + DMSO (without Aza-CTZ). The average of three measurements was 1400, and [Delta light intensity] was thus calculated as:
NMR analysis
NMR experiments were conducted using 0.1 mM 15N labeled GLuc protein dissolved in 50 mM MES buffer pH 6.0 and 2 mM NaN3, at 293 K with 8% (v/v) D2O in a 5 mm Shigemi microtube (Shigemi co., Ltd, Tokyo, Japan). NMR spectra were acquired on a Bruker Advance-III 600 MHz spectrometer equipped with a 5 mm CPTXI cryoprobe. Two-dimensional 15N-1H heteronuclear single quantum coherence (HSQC) experiments were performed for chemical shift displacement analysis upon adding Aza-CTZ.
Results and discussion
The effects of ions type on GLuc luminescence
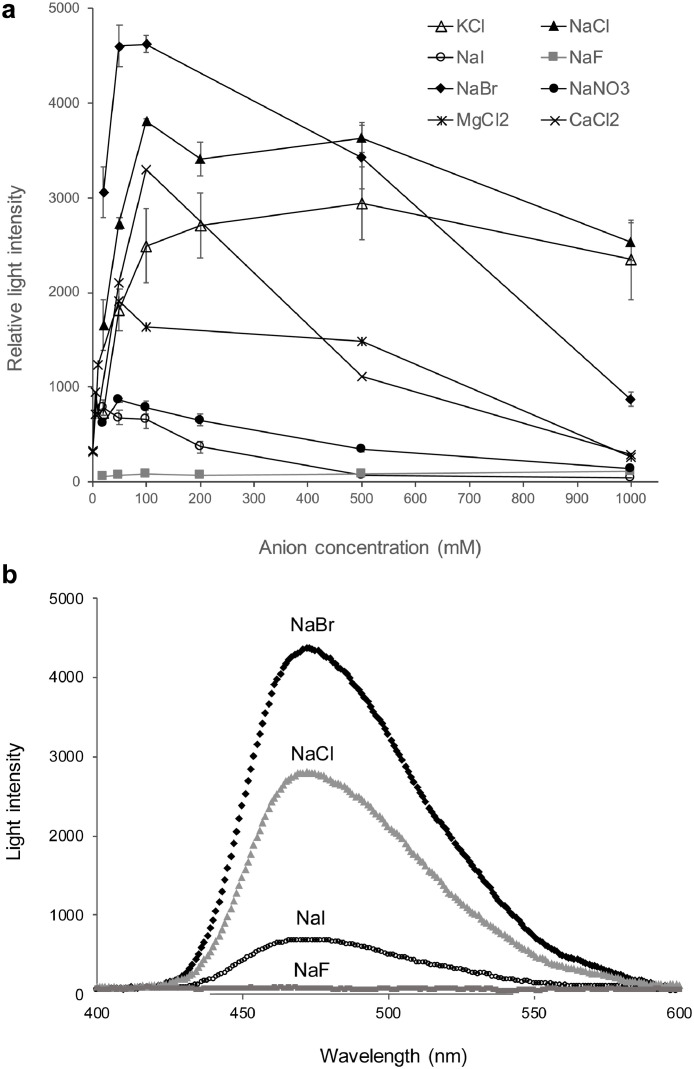
The recombinant GLuc dissolved in 50 mM Tris-HCl showed a strong bioluminescent activity upon the addition of CTZ, in line with our previous observations. However, under the same condition, but in 50 mM MES instead of Tris-HCl, GLuc was inactive. The addition of NaCl restored GLuc bioluminescence activity in MES buffer, prompting us to carefully investigate the effects of salts on GLuc's activity. We thus measured the luminescence intensity catalyzed by GLuc using five monovalent anions, four halide ions (F−, Cl−, Br−, I−), and a nitrate ion (NO3−) (Fig. 1). The Cl− and Br− ions increased GLuc's luminescence, and NO3− did so to a lesser extent while I− provided a weak emission, and F− did not induce any luminescence. These results are in line with a previous report [26]. The luciferase activity was highest between 50 and 500 mM, depending on the ion type. In nature, the luminescent reaction between GLuc and CTZ occurs in seawater. Dissolved monovalent halides content in the sea is high for chloride (0.546 mol/kg; 546 mM) but much smaller for bromine (0.844 mmol/kg; 0.844 mM) or fluorine (0.068 mmol/kg) and even less for iodine [27]. Since the reaction occurs in a pocket probably filled with seawater, it seems reasonable that our experiments showed maximum luminescence at about 500 mM for NaCl and KCl, and at a lower NaBr concentration. High concentrations of MgCl2 and CaCl2 did not enhance luminescence as much as NaCl and KCl. This might be because divalent cations affect luminescence [26].
Fig. 1.
Effect of salt on the luminescence Gaussia luciferase. (a) In the luminescence assays, 5 μM of CTZ, containing a salt to be tested, was added to the sample containing 0.5 μM of GLuc in 50 mM MOPS buffer, pH 7.9, at 15 °C. Salt concentration ranged from 20 to 1000 mM. Error bars indicate the standard deviation. The light intensity was measured at 473 nm. (b) Effect of halogen ions on the luminescence spectra. The solution conditions are the same as in (a) but with a fixed salt concentration of 50 mM.
Interestingly, only chlorine and bromine were found essential for this bioluminescence reaction. We hypothesized two reasons for the lack of activation by fluorine or the modest effect of iodine ions. First, if the anion is involved in a specific interaction, such as a salt bridge, the anion's size should be an issue. F− is the smallest monovalent halogen, whereas iodine has the biggest (anions' thermochemical radius for Cl−: 1.81 Å, Br−: 1.96 Å, and I−: 2.20 Å and F−: 1.33 Å [28]). A second hypothesis is that fluorine ions, which have the highest electronegativity, might repel each other and hamper their close contact in a cavity.
Inhibition assay
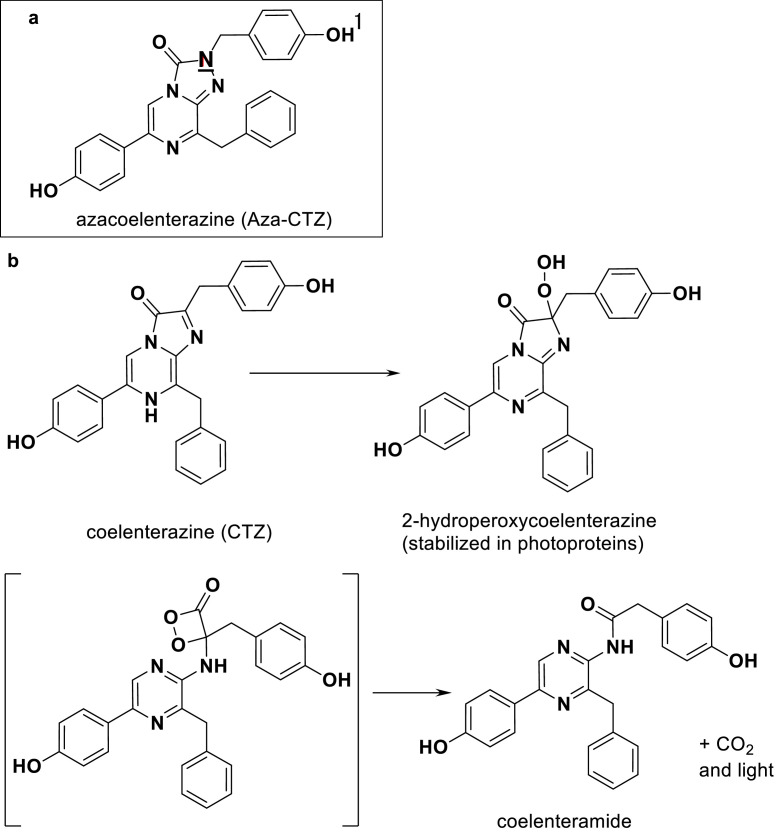
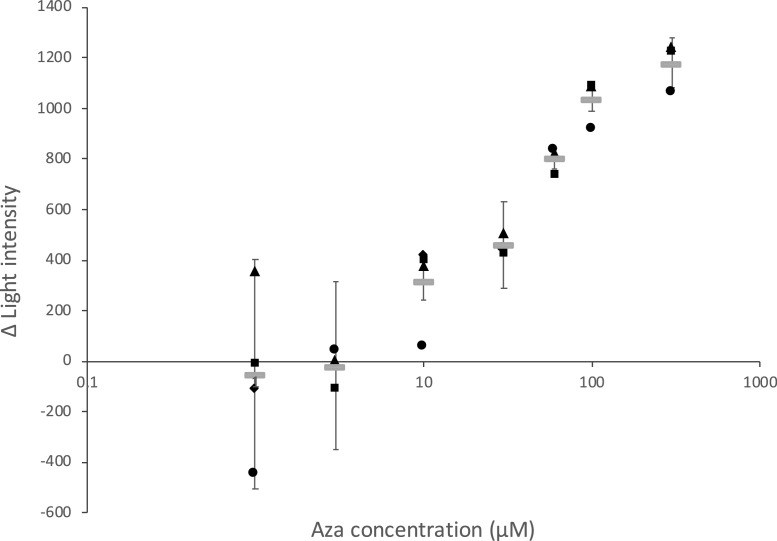
We performed inhibition assays using Aza-CTZ (Fig. 2) and CTZ. To this end, we assessed the solubility of Aza-CTZ with water, methanol, and other alcoholic solvents, as well as dimethyl sulfoxide (DMSO) to use it as a stock solution. Aza-CTZ turned out to be only suitably soluble in DMSO. DMSO is also known as a solvent used to trigger the chemiluminescence of CTZ [29], but as measured for Aza-CTZ, the baseline was low. Indeed, Aza-CTZ is stable in DMSO since the carbon oxidized in CTZ is replaced by a nitrogen. We then faced two problems with this stock solution of Aza-CTZ in DMSO. Firstly, the solution of Aza-CTZ did not completely dissolve in an aqueous solution above 150 µM. Accordingly, the final concentration of Aza-CTZ used in our experiments had to be below 150 µM. For this reason, the CTZ concentration also had to be lowered, which caused a reduction of the luminescence intensity. We then optimized the reaction pH using four buffers at different pHs: MES buffer pH 6.0, MES buffer 7.0, MOPS buffer 7.9, and Tricin buffer pH 8.8. NaOH was used to adjust the pH of the buffers. We found that the luminescence intensity was strongly pH-dependent, but the wavelength of the intensity maximum remained unchanged (Fig. S1). Eventually, MOPS buffer, at pH 7.9, was selected as it led to the strongest bioluminescence. The second issue was that the addition of DMSO suppressed the bioluminescence intensity in our experiments. We found that the final DMSO concentration had to be under 1% to avoid this. In fact, a bioluminescence reduction by DMSO has been previously observed with the CTZ-using Renilla luciferase [30]. In addition, the reaction solution had to be mixed for precisely 10 seconds to increase the measurement accuracy. Thus, we eventually used ascorbic acid (vitamin C) as a reductant to slow the oxidation reaction and improve the measurement accuracy (Fig. S2). Thus, the inhibition assays were performed in 50 mM MOPS buffer, pH 7.9, 0.2 M NaBr, containing 0.3 M vitamin C. Under these conditions, Aza-CTZ inhibited the bioluminescence intensity dose-dependently. A plot of the delta light intensity vs. Aza-CTZ concentration showed a sigmoidal curve (Fig. 3) with a 50% inhibition concentration (IC50) between 10 and 100 µM, equivalent to a CTZ/Aza-CTZ ratio of 1/20-200. Unfortunately, further refinement of this IC50 value could not be achieved because of the limitations mentioned above. A possible but unverified explanation for the surprisingly high IC50 value is that the active site needs to be saturated with Aza-CTZ since once CTZ binds to GLuc, it is oxidized and emits light. Moreover, the fact that aza-CTZ can only adopt an “oxo” conformation in contrast to the two possible tautomeric conformations of CTZ (an “oxo” and an “OH” form) is of importance (see supplementary figure S3). Such difference is the cause of the poor water solubility of Aza-CTZ and might have some effect on its luciferase binding mode and affinity. The high IC50 might be a clue on CTZ's prefered tautomeric conformation upon binding to luciferase and prior to its oxidation.
Fig. 2.
Chemical structure of Aza-CTZ and oxidative decarboxylation reaction of CTZ (a): structure of Aza-CTZ showing the carbon replaced by a nitrogen atom by an underlined N. (b) two stages of the oxidative decarboxylation process of CTZ into coelenteramide leading to the production of a photon as well as CO2.
Fig. 3.
Inhibition assay. Relationship between the concentration of Aza-CTZ and the maximum intensity of luminescence. For inhibition assay, 40 μL of CTZ mixture containing 300 μM ∼ 1 μM or 0 M of Aza-CTZ was added to 10 μL of GLuc solution and pipetted five times. Then the spectrum was measured. The final concentration of CTZ and GLuc was 0.5 μM and 0.2 μM, respectively.
NMR analysis
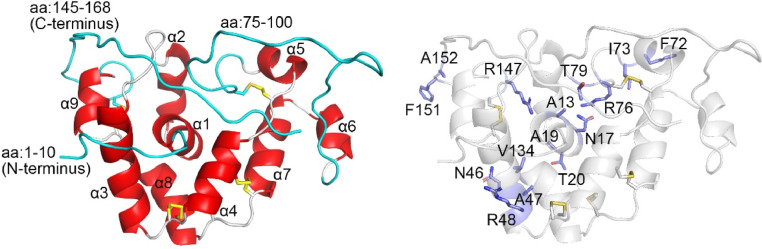
GLuc is an all-alpha-helix protein made of nine helices [20]. GLuc surface-accessible analysis indicated that 19 residues formed a hydrophobic cavity that might be involved in bioluminescence. The cavity results from the assembly of the central α1 (N10, V12, A13, V14, S16, N17, F18), α4 (L60, S61, I63, K64, C65), the functionally important but highly flexible loop (R76, C77, H78, T79), and α7 (F113, I114, V117) (Fig. 5). We did not observe chemical shift perturbation in the HSQC spectrum upon the addition of CTZ to the NMR sample, but a bright light appeared, disappearing within a few tens of seconds, which is too fast for initiating NMR measurements (unpublished observations). We speculate that the lack of chemical shift perturbation is due to the fast turnover of the reaction and that CTZ quickly dissociates from GLuc upon its decarboxylative oxidization.
Fig. 5.
Ribbon model of GLuc. (A) Ribbon model of GLuc showing the secondary structures. The helices are in red, loops are in white, the highly flexible regions (1-10; 75-100; 145-168) are in cyan, and the disulfide bonds are shown in yellow. (B) Ribbon model of GLuc showing the side-chains of residues with significantly reduced 15N-1H HSQC peak intensities upon addition of Aza-CTZ (blue).
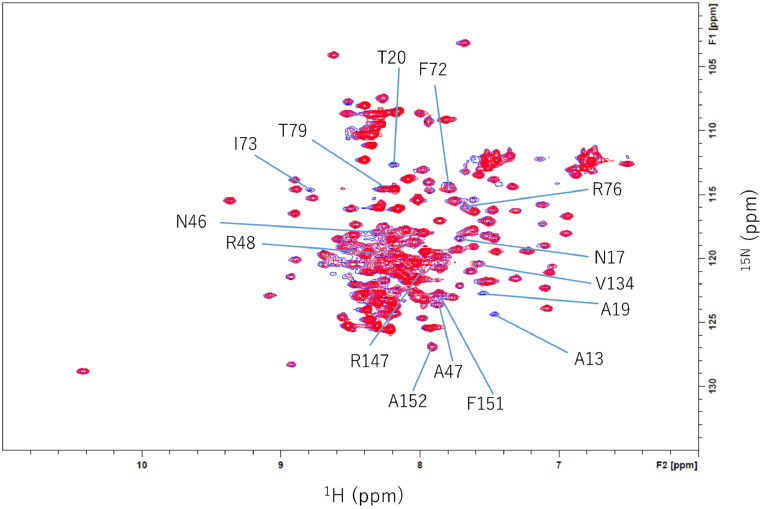
Accordingly, we expected that Aza-CTZ, which GLuc cannot oxidize, might "freeze" the reaction in the bound state, enabling us to see chemical shift perturbation and consequently deduce a putative CTZ's binding site. Chemical shift perturbation was observed for A13, N17, A19, T20, N46, A47, R48, F72, I73, R76, T79, V134, R147, F151, and A152 (Fig. 4, Fig. 5). Many of these residues are located near the cavity, in the central α1 as well as the functionally important loop. A few other residues are located in the C-terminal loop (none in α7), which is not close to the cavity observed by NMR.
Fig. 4.
2D 15N-1H HSQC of GLuc in the presence and absence of Aza-CTZ. The spectra were measured at 298 K with 0.2 mM Aza-CTZ (1% DMSO) in red and without Aza-CTZ in blue. The 1H-15N peak positions were confirmed by 3D HNCO spectrum as the sequential 1H-15N-13CO (i-1) signals tend to be less sensitive for the little difference of experimental conditions. Upon the addition of Aza-CTZ, several peaks in the 2D 15N-1H HSQC spectrum were reduced, but no significant change in the chemical shift position was observed.
GLuc contains several highly flexible or disordered regions (Residues P74 to A100 that include the functionally important loop and P145 to the C-terminus), and we thus hypothesized that the binding of CTZ to GLuc occurs according to a induced-fit model involving a large structural change. In addition, the induced-fit model would be more in line with the substrate cooperativity reported by Tzertzinis G et al. [31] than with a typical lock-and-key model where a single substrate would fit into a rigid cavity. To date, the structural change might involve an alpha-fold predicted conformation, where the C-terminal region folds into a novel helix replacing α1, which would unfold [32]. Nevertheless, further experiments are needed to assess this hypothesis.
Previous mutational analyses indicate that F72, I73, R76, H78, Y80 [33] and F113, I114, W143, L144, and F151 [34] are activity-related residues. Since F72, I73, R76 and F151 are identified by our present chemical shift perturbation NMR experiment, this strongly suggests that these four residues are directly involved in the binding of CTZ (here, we suppose that the binding mode of Aza-CTZ is identical to that of CTZ). No chemical shift perturbation was found for H78, Y80, F113, and I114, though these four residues are located in or near the hydrophobic cavity identified in the NMR structure. We speculate that these residues contribute to the formation of the hydrophobic cavity without directly participating in the binding of CTZ. On the other hand, the effect of W143 and L144 [34], which are located in the unstructured C-terminus, is difficult to rationalize from the NMR-based structure. A structural rationale for their involvement in the bioluminescence would be the aforementioned alpha-fold predicted structure [32], where W143 and L144 migrate closer to the hydrophobic cavity.
Conclusion
We have analyzed the bioluminescence activity of Gaussia luciferase (GLuc 18.2 kDa; 168 residues) using a recombinant GLuc expressed in E. coli and refolded using a SEP tag as well as Aza-CTZ, a non-oxidizable coelenterazine analog [22]. Firstly, we found that salt is required for GLuc's bioluminescence, and the optimum salts were NaCl and NaBr. The competition inhibition assay indicated that Aza-CTZ's IC50 was 10-100 µM. Secondly, we identified 15 residues whose peak intensity decreased upon adding Aza-CTZ, suggesting that CTZ binds around the cavity and the flexible functionally important loop. In addition, we speculated that Aza-CTZ binding causes a large structural change that includes the above-discussed folding of the C-terminal region into a helix [32]. Further structural analyses are needed to confirm whether such daring structural change occurs upon binding of the salt and/or CTZ.
Declaration of Competing Interest
No conflict of interest.
Acknowledgments
Funding Information
This study was supported by a JSPS Grant-in-Aid for Scientific Research (KAKENHI- 18H02385) to YK, and a visiting scholar funding by TUAT's Institute of Global Innovation Research. NW was supported by a Henan Provincial Key Scientific Research Project Plan for Colleges and Universities (Grant No. 23A180002).
Note
The SCP-tag sequences are covered by a Japanese patent 5273438 and an international (PCT) patent application PCT/JP2018/029395.
Authors' Contributions
KT, YK, TY, and YLJ designed the project. KT, YK, and TY wrote the manuscript, YLJ and NW provided the materials. KT, TY, and NK performed the experiments and analyzed and compiled the data. All authors read and approved the manuscript.
Data Sharing
All data are given in the manuscript and the supplementary data. The GLuc plasmid is deposited in ADDgene (ID: 124660).
Footnotes
Data deposition: The expression vector for GLuc-C9D (p21GLucTG) is deposited in Addgene (ID:124660).
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.bbadva.2022.100068.
Contributor Information
Toshio Yamazaki, Email: toshio.yamazaki@riken.jp.
Yutaka Kuroda, Email: ykuroda@cc.tuat.ac.jp.
Appendix. Supplementary materials
Data availability
Data will be made available on request.
References
- 1.Shimomura O., Yampolsky I. World Scientific; 2016. Bioluminescence. [Google Scholar]
- 2.Wu C., Akimoto H., Ohmiya Y. Tracer studies on dinoflagellate luciferin with [15 N]-glycine and [15 N]- l -glutamic acid in the dinoflagellate Pyrocystis lunula. Tetrahedron Lett. 2003;44:1263–1266. [Google Scholar]
- 3.Martini S., Haddock S.H. Quantification of bioluminescence from the surface to the deep sea demonstrates its predominance as an ecological trait. Sci. Rep. 2017;7:45750. doi: 10.1038/srep45750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nakamura H., Kishi Y., Shimomura O., Morse D. Structure of dinoflagellate luciferin and its enzymatic and nonenzymatic air-oxidation products. J. Am. Chem. Soc. 1989;111(19):7607–7611. [Google Scholar]
- 5.Strehler B.L., Cormier M.J. Isolation, identification, and function of long chain fatty aldehydes affecting the bacterial luciferin-luciferase reaction. J. Biol. Chem. 1954;211(1):213–225. [PubMed] [Google Scholar]
- 6.Kishi T., Goto T., Hirata Y., Shimomura O., Johnson F.H. Cypridina bioluminescence I: Structure of Cypridina luciferin. Tetrahedron Lett. 1966;7:3427–3436. [Google Scholar]
- 7.McElroy W.D. The energy source for bioluminescence in an isolated system. Proc. Natl. Acad. Sci. U. S. A. 1947;33(11):342–345. doi: 10.1073/pnas.33.11.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.White E., McCapra F., Field G., McElroy W. The structure and synthesis of firefly luciferin. J. Am. Chem. Soc. 1961 [Google Scholar]
- 9.Ohtsuka H., Rudie N.G., Wampler J.E. Structural identification and synthesis of luciferin from the bioluminescent earthworm, Diplocardia longa. Biochemistry. 1976;15(5):1001–1004. doi: 10.1021/bi00650a009. [DOI] [PubMed] [Google Scholar]
- 10.Shimomura O., Johnson F.H. The structure of Latia luciferin. Biochemistry. 1968;7(5):1734–1738. doi: 10.1021/bi00845a017. [DOI] [PubMed] [Google Scholar]
- 11.Purtov K.V., Petushkov V.N., Baranov M.S., Mineev K.S., Rodionova N.S., Kaskova Z.M., Tsarkova A.S., Petunin A.I., Bondar V.S., Rodicheva E.K., Medvedeva S.E., Oba Y., Arseniev A.S., Lukyanov S., Gitelson J.I., Yampolsky I.V. The chemical basis of fungal bioluminescence. Angew. Chem. Int. Ed. Engl. 2015;54(28):8124–8128. doi: 10.1002/anie.201501779. [DOI] [PubMed] [Google Scholar]
- 12.Inoue S., Sugiura S., Kakoi H., Hashizume K., Goto T., Iio H. Oplophorus luciferin is identical with Watasenia preluciferin. Chem. Lett. 1975:141–144. [Google Scholar]
- 13.Widder E.A. Bioluminescence in the ocean: origins of biological, chemical, and ecological diversity. Science. 2010;328(5979):704–708. doi: 10.1126/science.1174269. [DOI] [PubMed] [Google Scholar]
- 14.Markova S.V., Golz S., Frank L.A., Kalthof B., Vysotski E.S. Cloning and expression of cDNA for a luciferase from the marine copepod Metridia longa. A novel secreted bioluminescent reporter enzyme. J. Biol. Chem. 2004;279(5):3212–3217. doi: 10.1074/jbc.M309639200. [DOI] [PubMed] [Google Scholar]
- 15.Takenaka Y., Masuda H., Yamaguchi A., Nishikawa S., Shigeri Y., Yoshida Y., Mizuno H. Two forms of secreted and thermostable luciferases from the marine copepod crustacean, Metridia pacifica. Gene. 2008;425(1-2):28–35. doi: 10.1016/j.gene.2008.07.041. [DOI] [PubMed] [Google Scholar]
- 16.Takenaka Y., Yamaguchi A., Tsuruoka N., Torimura M., Gojobori T., Shigeri Y. Evolution of bioluminescence in marine planktonic copepods. Mol. Biol. Evol. 2012;29(6):1669–1681. doi: 10.1093/molbev/mss009. [DOI] [PubMed] [Google Scholar]
- 17.Verhaegen M., Christopoulos T.K. Bacterial expression of in vivo-biotinylated aequorin for direct application to bioluminometric hybridization assays. Anal. Biochem. 2002;306(2):314–322. doi: 10.1006/abio.2002.5724. [DOI] [PubMed] [Google Scholar]
- 18.Takenaka Y., Noda-Ogura A., Imanishi T., Yamaguchi A., Gojobori T., Shigeri Y. Computational analysis and functional expression of ancestral copepod luciferase. Gene. 2013;528(2):201–205. doi: 10.1016/j.gene.2013.07.011. [DOI] [PubMed] [Google Scholar]
- 19.Campbell A., Herring P. Imidazolopyrazine bioluminescence in copepods and other marine organisms. Mar. Biol. 1990;104:219–225. [Google Scholar]
- 20.Wu N., Kobayashi N., Tsuda K., Unzai S., Saotome T., Kuroda Y., Yamazaki T. Solution structure of Gaussia Luciferase with five disulfide bonds and identification of a putative coelenterazine binding cavity by heteronuclear NMR. Sci. Rep. 2020;10(1):20069. doi: 10.1038/s41598-020-76486-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dijkema F.M., Nordentoft M.K., Didriksen A.K., Corneliussen A.S., Willemoës M., Winther J.R. Flash properties of Gaussia luciferase are the result of covalent inhibition after a limited number of cycles. Protein. Sci. 2021;30(3):638–649. doi: 10.1002/pro.4023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schenkmayerova A., Toul M., Pluskal D., Baatallah R., Gagnot G., Pinto G.P., Santana V.T., Stuchla M., Neugebauer P., Chaiyen P., Damborsky J., Bednar D., Janin Y.L., Prokop Z., Marek M. A catalytic mechanism for Renilla-type bioluminescence. Nat. Catal. 2023 doi: 10.1038/s41929-022-00895-z. [DOI] [Google Scholar]
- 23.Coutant E.P., Goyard S., Hervin V.O., Gagnot G., Baatallah R., Rose T., Jacob Y., Janin Y.L. Gram-scale synthesis of luciferins derived from coelenterazine and original insights in their bioluminescence properties. Org. Biomol. Chem. 2019;17:3709–3713. doi: 10.1039/c9ob00459a. [DOI] [PubMed] [Google Scholar]
- 24.Rathnayaka T., Tawa M., Sohya S., Yohda M., Kuroda Y. Biophysical characterization of highly active recombinant Gaussia luciferase expressed in Escherichia coli. Biochim. Biophys. Acta. Proteins Proteom. 2010;1804(9):1902–1907. doi: 10.1016/j.bbapap.2010.04.014. [DOI] [PubMed] [Google Scholar]
- 25.Rathnayaka T., Tawa M., Nakamura T., Sohya S., Kuwajima K., Yohda M., Kuroda Y. Solubilization and folding of a fully active recombinant Gaussia luciferase with native disulfide bonds by using a SEP-Tag. Biochim. Biophys. Acta. Proteins Proteom. 2011;1814(12):1775–1778. doi: 10.1016/j.bbapap.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 26.Inouye S., Sahara Y. Identification of two catalytic domains in a luciferase secreted by the copepod Gaussia princeps. Biochem. Biophys. Res. Commun. 2008;365(1):96–101. doi: 10.1016/j.bbrc.2007.10.152. [DOI] [PubMed] [Google Scholar]
- 27.Culkin F. In: Chemical Oceanography. Riley J.P., Skirrow G., editors. Acad. Press; London: 1965. The major constituents of sea water; pp. 121–161. 1. [Google Scholar]
- 28.Simoes M.C., Hughes K.J., Ingham D.B., Ma L., Pourkashanian M. Estimation of the thermochemical radii and ionic volumes of complex ions. Inorg. Chem. 2017;56(13):7566–7573. doi: 10.1021/acs.inorgchem.7b01205. [DOI] [PubMed] [Google Scholar]
- 29.Teranishi K., Goto T. Synthesis and chemiluminescence of coelenterazine (Oplophorus Luciferin) analogues. Chem. Soc. Jpn. 1990;63(11) [Google Scholar]
- 30.Mishra P., Rai S., Manjithaya R. A novel dual luciferase based high throughput assay to monitor autophagy in real time in yeast. Biochem. Biophys. Rep. 2017;11:138–146. doi: 10.1016/j.bbrep.2017.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tzertzinis G, Schildkraut E, Schildkraut I. Substrate cooperativity in marine luciferases. PLoS One. 2012;7(6):e40099. doi: 10.1371/journal.pone.0040099. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang Y.J., Zhang N., Bersch B., Fidelis K., Inouye M., Ishida Y., Kryshtafovych A., Kobayashi N., Kuroda Y., Liu G., LiWang A., Swapna G.V.T., Wu N., Yamazaki T., Montelione G.T. Assessment of prediction methods for protein structures determined by NMR in CASP14: Impact of AlphaFold2. Proteins. 2021;12:1959–1976. doi: 10.1002/prot.26246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim S.B., Suzuki H., Sato M., Tao H. Superluminescent variants of marine luciferases for bioassays. Anal. Chem. 2011;83(22):8732–8740. doi: 10.1021/ac2021882. [DOI] [PubMed] [Google Scholar]
- 34.Wu N., Kamioka T., Kuroda Y. A novel screening system based on VanX-mediated autolysis-Application to Gaussia luciferase. Biotechnol. Bioeng. 2016;113(7):1413–1420. doi: 10.1002/bit.25910. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.