Highlights
-
•
Narciclasine binds to the N-terminal part of YAP
-
•
Narciclasine competes with TEAD4 for binding to YAP
-
•
Narciclasine suppresses mesothelioma cell proliferation
-
•
Narciclasine inhibits mesothelioma tumor growth when xenografted into nude mice
-
•
Narciclasine analogs may be developed for the treatment of cancers with hyperactivation and/or overexpression of YAP
Keywords: Anticancer drug, Malignant mesothelioma, Narciclasine, TEAD, YAP
Abstract
Yes-associated protein (YAP) is involved in development, cell growth, cell size, and homeostasis and plays a key role in the progression of various cancers. Among them, constitutive activation of YAP can often be observed in malignant mesothelioma, which arises in the pleura, peritoneum, and pericardium because of inactivation of the Hippo pathway. To date, however, only less-effective treatments such as chemotherapy, radiation therapy, and surgery are available for patients with malignant mesothelioma.
In this study, we identified narciclasine as a novel YAP inhibitor that prevents YAP from interacting with TEAD4 because it competes with TEAD4 for binding to YAP. Furthermore, narciclasine could perturb the cell growth and colony formation of malignant mesothelioma NCI-H290 cells in addition to inhibiting their growth in nude mice. Therefore, narciclasine might be a potential seed for a novel antitumor drug against malignant mesothelioma and other cancers in which hyperactivation and/or overexpression of YAP are observed.
1. Introduction
Malignant mesothelioma arises from mesothelial cells lining the serous membranes of the pleura, peritoneum, pericardium, and tunica vaginalis [1]. After exposure to asbestos, an incubation period of over 30 years is required for mesothelial cells in the human body to be transformed into mesothelioma [2]. When patients are diagnosed with malignant mesothelioma, the tumors cannot be completely excised through surgery and/or radiation therapy because malignant mesothelioma is a disseminated cancer. Therefore, patients with malignant mesothelioma have waited for revolutionary anti-mesothelioma drugs.
Yes-associated protein (YAP) is a key attributor to cancer cells because high levels and/or aberrant activation of YAP constitutively promote cell proliferation. In addition, YAP potentiates the ability of cancer stem cells (CSCs), including their expansion, drug resistance, and plasticity [3]. It is well known that YAP is involved in the development of distinct tumor types in humans and mice [3], [4], [5]. Thus, as an oncogene, YAP seems to be essential for tumor initiation, progression, and metastasis.
The activity of YAP is tightly regulated by the Hippo pathway, which is implicated in tissue growth, cell proliferation, differentiation, and migration in developing organs. Mechanistical stimulation such as cell-cell contact and cell polarity triggers activation of TAO kinases, followed by phosphorylation of MSTs (mammalian Ste20-like kinases). Then, MSTs catalyze the phosphorylation of Lats1/2 (large tumor suppressor 1/2). Phosphorylated Lats1/2 can facilitate their autophosphorylation to become active kinases. Subsequently, YAP and/or its homolog, TAZ (transcriptional coactivator with PDZ-binding motif) (Fig. 1A), are phosphorylated by Lats1/2 to become inactive forms. NF2 is well known as another important player that activates Hippo signaling. NF2 can directly interact with Lats1/2 or indirectly associate with them via MSTs. When the Hippo signal is therefore not transduced, YAP and/or TAZ can be localized to the nucleus, where YAP and/or TAZ bind to the TEAD (TEA domain) family (ie, TEAD1–4) to promote cell growth, survival, migration, and so forth [6]. On the other hand, phosphorylated YAP and/or TAZ are degraded via the proteasome pathway or retained in the cytosol by their interaction with 14-3-3. Consequently, interference of YAP- or TAZ-mediated transcription occurs. Besides TEADs, YAP and/or TAZ are also known to associate with other transcription factors such as AP1 [7], p73 [8], TBX5 [9], Smads [10], [11], [12], β-catenin [13], and ERG [14] to control a wide range of cellular events. Thereby, YAP and TAZ contribute to crosstalk signaling with other transcription factors [4]. Notably, the balance between the Hippo signal and YAP and/or TAZ activation is critical for YAP and/or TAZ-mediated biological responses because some of the signal components of the Hippo signal are considered to be tumor suppressor genes [3, 4]. Thus, deregulation of the Hippo pathway is thought to lead to cells becoming malignant [15, 16]. Indeed, mutations in some genes involved in the Hippo pathway are enriched in patients with malignant mesothelioma. For example, >35% of patients with mesothelioma possess a genetic mutation in the NF2 gene [15]. In addition, mutations of the BAP1 and TRAF7 genes can be frequently observed in mesothelioma [15, 17].
Figure 1.
Interaction of narciclasine with YAP. (A) Schematic structure of human YAP and TAZ. WW, WW domain; CC, coiled-coil domain. 127Serine (127S) in YAP and 89Serine (89S) in TAZ are the main phosphorylation sites by Lats1/2. (B) Structure of narciclasine. (C) Interaction of narciclasine with YAP. Interaction between narciclasine and GST-YAP was measured by SPR. GST-YAP and GST alone were captured at 8,000 and 2,900 RU, respectively. Solid line, GST-YAP; broken line, GST alone. The Kd of narciclasine to GST-YAP was 970 μM. Significant differences between GST alone and GST-YAP are indicated with asterisks.
Besides mesothelioma, high expression and/or aberrant activation of YAP can be observed in solid tumors because of gene alteration of not only Hippo-dependent but also Hippo-independent pathways [3, 4]. Recently, several inhibitors of YAP and/or TAZ have been found [16]. Verteporfin promotes dissociation of YAP and TEADs owing to its interaction with the interface between YAP and TEAD [18], [19], [20], [21], whereas CA3 inhibits YAP protein expression to perturb the YAP/TEAD-mediated transcriptional activity [22]. When mesothelioma stem cells were treated with verteporfin or CA3, these agents effectively attenuated maintenance of the mesothelioma stem cell phenotype [23]. Celastrol has also been found to be an inhibitor of the interaction between YAP and TEADs to perturb tumor cell growth [24]. A small peptide consisting of a part of VGLL4 (vestigial-like family member 4), which is known to compete with YAP for binding to TEADs, revealed a potent anticancer activity [25]. Furthermore, a natural flavonoid, luteolin, promotes degradation of YAP and TAZ to suppress epithelial-mesenchymal transition and migration of triple-negative breast cancer cells without estrogen receptor, progesterone receptor, and human epidermal growth factor receptor 2 (TNBCs) [26], and another flavonoid, apigenin, also disrupts the interaction between TEADs and YAP or TAZ to inhibit cell proliferation of TNBCs [27]. Moreover, statins, used to treat hyperlipidemia, can block nuclear translocation of YAP and TAZ [28]. However, medicines are clinically unavailable because of the requirement of high doses of inhibitors, their instability, and side effects.
In this study, we identified the alkaloid narciclasine as a novel small compound that binds to YAP. Narciclasine can decrease tumor cell growth in vitro and in vivo in addition to inhibiting YAP activity.
2. Materials and methods
2.1. Reagents, plasmids, and antibodies
Narciclasine and verteporfin were purchased from Cayman Chemical. Three biotinylated peptides (>95% purity), described in Fig. 3A were synthesized by Atlantic Peptides Inc. (PA, USA). Gal4-TEAD4 and Myc-TEAD4 were kindly gifted by Professor Guan [29]. Myc-YAP was provided by Professor Fujii [30]. 5xUAS-luc has been previously described [31]. For CTGF-luc including the fragments from -258 to +42, the promoter region of the human CTGF gene was isolated by means of PCR-based amplification using the human genome as a template and inserted into pGL3-basic (Promega). The nucleotide sequences of CTGF-luc were confirmed by sequencing. Anti-Myc9E10 mouse (sc-40) and anti-β-actin (AC-15) mouse monoclonal antibodies (mAbs) (sc-69879) were obtained from Santa Cruz. Anti-YAP rabbit polyclonal antibody (pAb) (#4912) was purchased from Cell Signaling. Anti-DYKDDDDK tag mAb (040-30953) corresponding to anti-Flag antibody was from FUJIFILM Wako. Anti-mouse IgG HRP-linked sheep (NA931-1ML) and anti-rabbit IgG HRP-linked F(ab’)2 fragment donkey pAbs (NA9340V) were purchased from GE Healthcare.
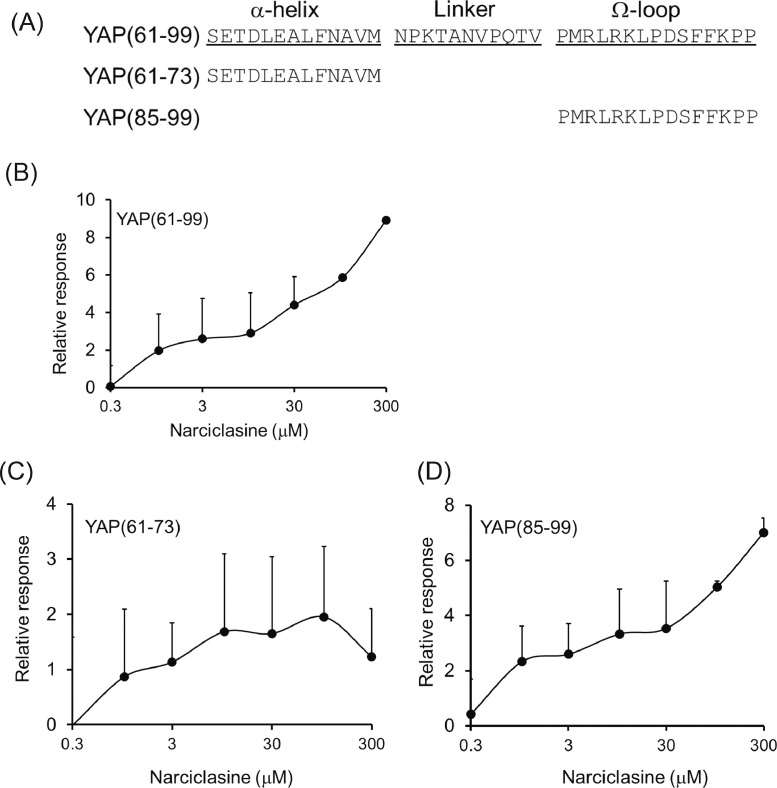
Figure 3.
Interaction of narciclasine with the Ω-loop of YAP. (A) Peptide sequences used in assays. (B–D) Binding ability of narciclasine to peptides examined. Each peptide was fixed in the surface of the chip. Then, the binding ability was measured using SPR. (B) YAP(61–99), (C) YAP(61–73), (D) YAP(85–99). YAP(61–99), YAP(61–73) and YAP(85–99) were captured at 1,900, 600 and 600 RU, respectively.
2.2. Cell culture
A549, 293A, and COS7 cells were cultured in Dulbecco's modified Eagle's medium (DMEM; Nacalai Tesque) containing 10% fetal calf serum (FCS; Invitrogen). RPMI1640 medium (Nacalai Tesque) containing 10% FCS was used for NCI-H290 cells [32].
2.3. GST fusion proteins
Human YAP was subcloned into a pGEX-4T-1 vector (GE Healthcare). YAP-N and YAP-C were amplified by means of PCR using the human YAP cDNA as a template. Each cDNA fragment was verified by means of sequencing after PCR amplification. Then, each fragment was inserted into the pGEX-4T-1 vector. GST fusion proteins from Escherichia coli, the BL21 strain, were purified according to the manufacturer's instructions (GE Healthcare).
2.4. Surface plasmon resonance (SPR)
SPR measurements were performed using a BIAcore T100 (GE Healthcare), and the obtained data were processed with BIAevaluation software (version 1.1.1). Experiments were performed in running buffer (10 mM HEPES [pH 7.4]/150 mM NaCl/3 mM EDTA/0.05% Tween20/1% DMSO). GST, GST-YAP, and its derivatives were immobilized on a carboxymethylated dextran matrix (CM5) sensor chip (GE Healthcare) in different flow cells using standard amine coupling chemistry according to the manufacturer's protocol at 25°C. The remaining activated groups were quenched using 1 M ethanolamine hydrochloride (pH 8.5). For immobilization to a streptavidin (SA) sensor chip (GE Healthcare), three biotinylated peptides, shown in Fig. 3A, were used. General procedures were performed according to the manufacturer's instructions. A reference cell containing no bound protein was used to correct for refractive index changes, nonspecific binding, and buffer subtraction. For the binding experiments, different concentrations of narciclasine were diluted in the running buffer and applied to the sensor chips for 30 sec followed by a 30 sec dissociation phase at a flow rate of 15 μL/min. Since the response unit (RU) value representing the protein-compound complexes decreased to the baseline value before the next measurement, the chip regeneration step was not performed. The experiments were triplicated at each concentration of narciclasine when the RU values were measured. To calculate a Kd value for each GST fusion protein, the value for GST alone was subtracted from that for the GST fusion protein at each concentration of narciclasine.
2.5. Screening of YAP-interacting compounds
Compound screening was supported by the Project for Development of Innovative Research on Cancer Therapeutics (P-DIRECT, Japan Agency for Medical Research and Development). In brief, 11 compound arrays were incubated with 1 μM GST-YAP. Then, each array was further mixed with an anti-GST antibody and a Cy5-cojugated antibody, followed by detection of fluorescence at 635 nm.
2.6. Luciferase assay
One day before transfection, 293A cells were seeded at 5.0 × 104 cells/well in a 12-well plate. The cells were transfected with a reporter gene, pCH110 (GE Healthcare), and the indicated plasmids, by use of polyethylenimine (PEI; Polysciences). Twenty-four hours later, different doses of narciclasine were added to the wells if necessary. Then, the cells were further cultured for 24 h. In all the experiments, β-galactosidase activity was measured to normalize for transfection efficiency. Averages and SDs were calculated for three independent wells in each transfection. The experiments were repeated three times. Representative results are shown.
2.7. RNA preparation and quantitative PCR (qPCR) analysis
Total RNAs from NCI-H290 cells were extracted using a NucleoSpin RNA kit (Macherey-Nagel). Reverse transcription was performed with a High-Capacity RNA-to-cDNA kit (Thermo Fisher Scientific). qPCR was performed using a KAPA SYBR Fast qPCR kit (KAPA). All reactions were carried out on a Thermal Cycler Dice (TAKARA). The melting curves were checked to ensure specificity. Relative quantification of mRNA expression was calculated using the standard curve method with the GAPDH level. Before qPCR, we used agarose gel electrophoresis to confirm that the DNA fragment amplified using each primer set was a single band with the correct size. The primer sets for human CTGF and GAPDH were 5’-TTTCAGACGGAGGAATGCTG-3’/3’-AAAGAAAGAGGTCGACGACG-5’ and 5’-AGGTCGGAGTCAACGGATTT-3’/3’-GGGAAGTAACTGGAGTTGAT-5’, respectively. Each sample was analyzed in triplicate at least twice for each PCR measurement. Representative results are shown.
2.8. Cell proliferation assay
NCI-H290 and A549 cells were seeded at 1000 cells/well in 96-well plates 1 day before addition of narciclasine. After the cells were cultured in DMEM/10% FCS for the indicated periods, the media were changed to DMEM/10% FCS (100 μL) including 10 μL of Cell Count Reagent SF (Nacalai Tesque). Three hours later, the absorbance of the medium was measured with 450 nm and 650 nm using Varioskan Flash (Thermo Fisher Scientific). Then, the ratio between 450 and 650 nm was calculated. The media with or without inhibitors were changed every other day. Each value was normalized with the value that was measured immediately before addition of narciclasine. Averages and SDs were calculated for three independent wells. The experiments were repeated three times. Representative results are shown.
2.9. Anchorage-independent colony formation
The anchorage-independent colony formation assay was carried out according to Fujii et al [30]. Briefly, NCI-H290 cells suspended in 0.3% low-melting agarose (dissolved in RPMI1640) were seeded on top of a layer of 0.5% low-melting agarose in a 96-well plate. Twenty-four hours later, different concentrations of inhibitors were added every other day after the media were changed. At days 8 and 16, 100 μL of Cell Count Reagent SF was added to each well. After the cells were further incubated for 4 h, the absorbance of the medium was measured as described above. Media with or without inhibitors were changed every other day. Each value was normalized with the value that was measured immediately before addition of narciclasine. Averages and SDs were calculated for three independent wells. The experiments were repeated twice. Representative results are shown.
2.10. Western blot analysis
Western blot analysis was carried out according to Nakano et al [33]. In brief, the cells were lysed in 500 μL of TNE buffer (10 mM Tris [pH 7.4], 150 mM NaCl, 1 mM ethylenediamine-N’, N’, N’, N’-tetraacetic acid [EDTA], 1% NP-40, 1 mM phenylmethylsulfonyl-l-fluoride [PMSF], 5 μg/mL leupeptin, 100 U/mL aprotinin, 2 mM sodium vanadate, 40 mM NaF, and 20 mM β-glycerophosphate). The cell lysates were boiled for 5 min in sample buffer, separated by means of SDS-polyacrylamide gel electrophoresis (SDS-PAGE), and transferred to UltraCruz Nitrocellulose Pure Transfer membranes (SantaCruz). The membranes were probed with the primary antibodies, which were detected with horseradish peroxidase-conjugated secondary antibodies and a chemiluminescent substrate (Western BLoT Quant HRP Substrate, Takara).
2.11. GST pulldown assay
A GST pulldown assay was performed as described previously [32]. Briefly, cell lysates prepared from COS7 cells transfected with Myc-TEAD4 were precleared with GST immobilized to GSH-Sepharose 4B (GE Healthcare) for 30 min at 4°C. After GST-YAP was incubated with 1 mM narciclasine for 24 h at 4°C, the above cell lysates were added together with GSH-Sepharose 4B at 4°C. Two hours later, GSH-Sepharose 4B was washed 3 times with 50 mM Tris (pH 7.4) containing 100 mM NaCl, 2 mM MgCl2, 10% glycerol, 1% NP-40, 1 mM PMSF, 5 μg/mL leupeptin, 20 U/mL aprotinin, and 5 mM benzamidine. Then, the samples were loaded for SDS-PAGE. Subsequently, the proteins were blotted on an Ultra Cruz Nitrocellulose Pure Transfer Membrane and detected with an anti-Myc9E10 antibody using a chemiluminescent substrate. To show the quantity of GST fusion proteins in each sample, Ponceau S staining was performed after the Western blot analysis. The experiments were repeated three times. Representative results are shown.
2.12. Statistical analysis
Data were expressed as means ± SDs. Significance was assessed using the t test. Probability values below 0.05, 0.01, and 0.001 were considered significant. *p<0.05, **p<0.01, and ***p<0.001.
3. Results
3.1. Identification of compounds binding to YAP
To explore a small compound(s) that binds to YAP, we performed a comprehensive screening using 11 compound arrays, on which 29,707 small compounds were totally spotted. Among them, 31 compounds were hit as YAP-binding small compounds at the first screening (Fig. S1). After we further evaluated the 31 compounds by means of SPR using GST-YAP as a binding pair, we found that four of the 31compounds (NPD5137, NPD13325, MF256 and narciclasine) could bind to GST-YAP. Among the four compounds, NPD5137 and narciclasine had relatively stronger affinity than the other two compounds (Fig. S2 and data not shown). In the following experiments, we focused only on narciclasine (Fig. 1B) because NPD5137, a natural compound, was not commercially available. As seen in Fig. 1C, narciclasine could bind to GST-YAP rather than to GST alone. Since YAP makes a family with TAZ (Fig. 1A), we could confirm that TAZ also associates with narciclasine (Fig. S3).
3.2. Determination of the binding region of YAP for narciclasine
To identify the region(s) in YAP to which narciclasine binds, we chopped YAP out into the N-terminal (1-280) and C-terminal (267-488) regions and then fused each of them to GST, calling them GST-YAP-N and GST-YAP-C (Fig. 2A), respectively. Both fusion proteins were immobilized on the surface of the sensor chip for SPR analysis. GST-YAP-N containing the N-terminal part of YAP specifically bound to narciclasine, whereas narciclasine could not interact with GST-YAP-C, including the C-terminal part of YAP (Fig. 2B and 2C).
Figure 2.
Determination of the narciclasine-interacting region in YAP. (A) Schematic structures of GST fusion protein used. (B, C) Binding ability of narciclasine to the N-terminal part of YAP (B), but not to its C-terminal part (C). Binding capacity of narciclasine to each GST fusion protein was measured using SPR. As a negative control, GST alone was used. (B) GST-YAP-N and GST alone were captured at 6,100 and 2,300 RU, respectively. The Kd of narciclasine to GST-YAP-N was 245 μM. (C) GST-YAP-C and GST alone were captured at 12,000 and 5,600 RU, respectively. Significant differences between GST alone and GST-YAP-N or GST-YAP-C are indicated with asterisks.
It has been reported that the 39 amino acid-long region from Ser61 to Pro99 in YAP is required for YAP to interact with TEADs, which are DNA-binding transcription factors as a partner of YAP. It has been demonstrated that the α-helix encoding the peptide from Ser61 to Met73 and the Ω-loop coding for Pro85 to Pro99 in YAP were involved in the interaction of YAP with TEADs, although its linker between the α-helix and Ω-loop did not directly interact with TEADs [34], [35], [36]. Since narciclasine bound to the N-terminal part of YAP, we explored the possibility that narciclasine targets the TEAD-binding region in YAP. For that purpose, three peptides termed YAP(61-99), YAP(61-73), and YAP(85-99) (Fig. 3A) were synthesized and biotinylated. Then, we examined if each biotinylated peptide trapped narciclasine on the sensor chip surface. Indeed, biotinylated YAP(85-99) including the region of the Ω-loop in YAP bound to narciclasine like biotinylated YAP(61-99). The Kds of narciclasine to YAP(61-99) and YAP(85-99) were 142 and 127 μM, respectively. On the other hand, biotinylated YAP(61-73) including the α-helix alone in YAP did not interact with narciclasine (Fig. 3B-D).
3.3. Inhibition of YAP-mediated transcriptional activity by narciclasine
Since narciclasine interacts with YAP via its TEAD-binding domain, we supposed that narciclasine might interfere in YAP-mediated transcriptional activity. Like verteporfin (VP), narciclasine could inhibit YAP-mediated transcriptional responses using a CTGF promoter whose activity is directly controlled by the YAP signal (Fig. 4A) and by Gal4-TEAD4-induced luciferase activity (Fig. 4C). Since a high dose of narciclasine decreased the expression of YAP protein (Fig. 4B and 4D), it is possible that narciclasine influences the stability of YAP protein. We will focus on this possibility in future research. Consistent with the results of both luciferase assays, narciclasine could suppress the expression of endogenous CTGF mRNA in NCI-H290 cells (Fig. 4E) under the sparse condition where YAP might be activated and localized in the nucleus. To explore the possibility that narciclasine can compete with TEAD4 for binding to YAP, we performed a GST-pull down assay. As expected, narciclasine inhibited the interaction between YAP and TEAD4, although a high dose of narciclasine was required (Fig. 4F). Since it has already been shown that the YAP-interaction domain among the TEAD family is highly conserved [36, 37], we supposed that narciclasine might also interfere in the interaction of YAP with other TEADs besides TEAD4.
Figure 4.
Inhibition of YAP-mediated transcriptional activity by narciclasine. (A) Inhibitory effect of narciclasine on the activity of CTGF-luc. 293A cells were transfected with CTGF-luc in the presence or absence of YAP. Significant differences from the control with YAP are indicated with asterisks. VP, verteporfin. (B) The expressions of YAP and β-actin in total lysates from cells transfected with CTGF-luc. Anti-Flag (upper panel) and anti-β-actin antibodies (lower panel) were used to detect proteins. VP, verteporfin. (C) Inhibitory effect of narciclasine on the activity of 5xUAS-luc. 293A cells were transfected with 5xUAS and Gal4-TEAD4 in the presence or absence of YAP. Significant differences from the control with YAP are indicated with asterisks. VP, verteporfin. (D) The expressions of YAP and β-actin in total lysates from cells transfected with 5xUAS and Gal4-TEAD4. Anti-Flag (upper panel) and anti-β-actin antibodies (lower panel) were used to detect proteins. VP, verteporfin. (E) Inhibition of endogenous CTGF mRNA expression by narciclasine. NCI-H290 cells were treated with VP or narciclasine for 24 h. CTGF mRNAs were measured by qPCR. Significant differences from the control are indicated with asterisks. VP, verteporfin. (F) Competition of narciclasine with TEAD4 for binding to YAP. Different amounts of Myc-TEAD4 were incubated with GST-YAP in the absence or presence of narciclasine. Then, a GST-pulldown assay was performed. Upper panel, Myc-TEAD4 pulled down with GST-YAP; middle panel, expression of total Myc-TEAD4; lower panel, expression of GST and GST-YAP with Ponceau S staining.
3.4. Narciclasine-mediated inhibition of cell growth and anchorage-independent colony formation for mesothelioma
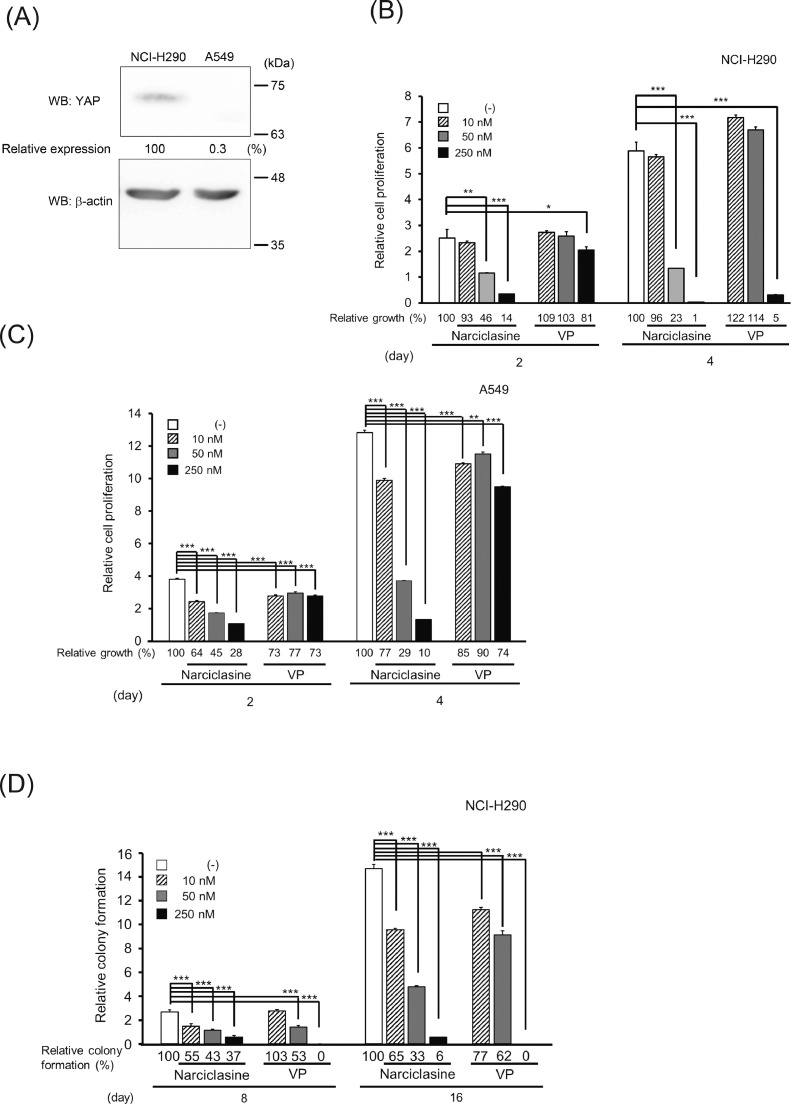
To examine the effect of narciclasine on YAP-mediated cell proliferation, we used two cell lines: the NCI-H290 cell line derived from mesothelioma and the A549 cell line from lung adenocarcinoma. It has been reported that the level of phosphorylated YAP in NCI-H290 cells is kept low because the NF2 gene is deleted in the cells [38]. As seen in Fig. 5A, NCI-H290 cells possessed a remarkable amount of YAP protein as compared with A549 cells. To explore if narciclasine counteracts the proliferation of NCI-H290 and A549 cells, we added different concentrations of narciclasine to a 96-well plate where the cells were cultured. As seen in Fig. 5B and 5C, the high concentration of narciclasine (250 nM) suppressed the cell growth of NCI-H290 cells up to 86% on day 2 and 99% on day 4, whereas this dose of narciclasine did that of A549 cells up to 72% on day 2 and 90% on day 4. Therefore, 250 nM narciclasine inhibited cell proliferation of NCI-H290 cells more efficiently than it did that of A549 cells, although both cells were sensitive to narciclasine. Since narciclasine has been known to act on other cellular proteins besides YAP [39], [40], [41], narciclasine might be able to inhibit proliferation of A549 cells as well. On the other hand, VP could specifically perturb the growth of NCI-H290 cells, although a high dose of VP slightly affected that of A549 cells. In addition, we also observed the growth inhibition of other mesothelioma NCI-H2052 cells treated with narciclasine (Fig. S4). It is known that cancer cells can grow in a semisolid culture including agarose without attachment, which is termed anchorage-independent growth. When NCI-H290 cells were cultured in a semisolid culture, their colonies could be observed with time. However, the formation of their colonies was dose-dependently inhibited by narciclasine, like VP (Fig. 5D). We also investigated whether narciclasine can affect the subcellular localization of YAP in NCI-H290 cells, but we could not observe any alteration of its subcellular localization in the presence of narciclasine (Fig. S5).
Figure 5.
Suppression of tumor cell growth by narciclasine. (A) The expression of YAP proteins in NCI-H290 and A549 cells. Upper panel, YAP; lower panel, β-actin. The intensity of the band for YAP was normalized using the intensity of the band corresponding to β-actin. Relative expression was calculated relative to the value for NCI-H290 cells. (B, C) Inhibitory effect of narciclasine on tumor cell proliferation. NCI-H290 (B) and A549 cells (C) were cultured in the presence of different doses of narciclasine or VP for 2 or 4 days. Then, the cell viability was measured. VP, verteporfin. Significant differences from the control are indicated with asterisks. Percentage of relative growth is shown below each graph. (D) Suppression of colony formation of NCI-H290 cells on semisolid agars by narciclasine. NCI-H290 cells were cultured in the presence of different doses of narciclasine or VP for 8 (left panel) and 16 (right panel) days. Significant differences from the control are indicated with asterisks. Percentage of relative colony formation is shown below each graph. VP, verteporfin.
3.5. Inhibitory effect of narciclasine on proliferation of NCI-H290 cells xenografted into the pleural cavity
After NCI-H290 cells overexpressing a luciferase gene were injected into mouse pleura we measured the tumor volumes with bioluminescence in vivo. One week after implantation of the NCI-H290 cells carrying the luciferase gene, narciclasine was intraperitoneally administered once a week 1 day before measurement of the tumor volumes. Consistent with the results of tumor cell growth in vitro, narciclasine tended to suppress tumor growth in the pleura, although we could not obtain any significant differences between the control and 0.5 mg/kg narciclasine-treated mice because of individual variability (Fig. S6). On the other hand, we could not observe weight loss in mice injected with narciclasine (Fig. S7).
4. Discussion
YAP plays an important role in the decision of organ size because active YAP is capable of controlling cell polarity, cell shape, and the cytoskeleton. Thus, dysregulation of YAP function leads cells to proliferate aberrantly [3]. Since YAP does not possess any enzymatic activity in its structure, a small compound(s) that competes with TEADs for binding to YAP might be useful to block YAP-mediated signaling because canonical YAP signaling requires TEADs to regulate the transcription of YAP target genes [16]. In this study, we used high-throughput screening and selected narciclasine as a possible small compound that binds to YAP. Indeed, narciclasine bound to the Ω-loop in YAP. It is known that its α-helix and the Ω-loop in YAP play a key role in its association with TEADs [34, 35]. Expectedly, narciclasine could dissociate the complex between YAP and TEAD4. Verteporfin, a photosensitizer for photodynamic therapy in patients with age-related macular degeneration [18, 42, 43], has been reported to inhibit YAP-mediated transcription owing to its fosterage of dissociation between YAP and TEADs [19, 44, 45]. Therefore, verteporfin can inhibit the proliferation of some tumors and established cancer cells [44, [46], [47], [48], [49]]. Like verteporfin, narciclasine might block proliferation of tumors other than mesothelioma because narciclasine possesses the same inhibitory mechanism as verteporfin.
Narciclasine is an alkaloid from the plants of the genus Narcissus L., which was widely used as a traditional medicine for anticancer treatment [[39], [40], [41], 50, 51]. Narciclasine has been reported to be selectively able to inhibit proliferation of cancer cells, but not of normal fibroblasts. In that report, narciclasine revealed a cytostatic effect via disorganization of the actin cytoskeleton because narciclasine interfered with the activities of Rho-GTP and eEF1A [41]. Although there is no homology in their amino acid sequences among YAP, Rho-GTP, and eEF1A, narciclasine might recognize a certain stochiometric structure(s) that they commonly possess in their protein structures. If a chemical compound affects a number of proteins in cells, it might have a side effect(s). Since there are many congeners of narciclasine [39], we will be able to pick up another narciclasine derivative(s) that exhibits inhibition of YAP function specifically. Furthermore, a couple of reports have recently demonstrated that narciclasine influences the activities of various intracellular proteins [51], [52], [53], [54]. Thus, it might be interesting to pursue the possibility that narciclasine inhibits the YAP/TEAD pathway through its interaction with TEADs and/or their interacting partners because the high expression of TEAD4 correlates with a poor prognosis in cancer patients [55, 56].
We could clarify that a relatively low dose of narciclasine is enough to inhibit the cell growth of mesothelioma in vitro and in vivo, although a high dose of narciclasine was required to block the interaction between YAP and TEAD4 in our GST-pulldown assay. These lines of evidence also support the notion that narciclasine targets several cellular proteins that contribute to tumor cell growth. If we can verify which groups in narciclasine play key roles in binding to YAP, it will be possible to synthesize another small compound(s) that exhibits further high affinity to interact with YAP. Furthermore, these compounds might be seeds to develop anti-tumor drugs against cancer cells whose growth is dependent on YAP activity. Recently, celastrol, a triterpenoid, has been reported to inhibit the interaction between YAP and TEADs, resulting in perturbed tumor cell growth [24]. Parts of its chemical structure seem to be more or less similar to that of narciclasine, although celastrol seems to possess the ability to dissociate the complex between YAP and TEADs at a lower dose than does narciclasine. Thus, a narciclasine derivative(s) might have much stronger activity to block the complex formation between YAP and TEADs if we can make modifications to the chemical structure of narciclasine by reference to the stoichiometric structure of celastrol.
Recently, Gao et al. designed chemically modified peptides corresponding to the α-helix of YAP because neither the α-helix nor the Ω-loop of YAP alone can efficiently bind to TEADs. Then, they showed that these peptides stably interact with TEADs [57]. Instead of targeting YAP protein, another possibility might be to find the compounds that associate with TEADs [16].
PROTAC technology using hybrid molecules between one compound for binding to a protein and another to an E3 ubiquitin (E3) ligase has been developed to degrade a targeting protein via the proteasome pathway [58], [59], [60], [61]. The group(s) in narciclasine that binds to YAP with a high affinity might become a unique and novel ligand for binding to YAP and might be useful for development of a PROTAC-mediated anti-cancer drug.
The inactivation of the Hippo signal was found in 20% to 50% of patients with malignant mesothelioma, which keeps the YAP signal constitutively active. Furthermore, Kulkarni et al. demonstrated that >35% of patients with mesothelioma possess a genetic mutation in the NF2 gene [15]. However, no specific molecularly targeted drugs have been developed against mesothelioma, although in Japan, nivolumab, which blocks recognition of PD-L1 or PD-L2 by PD-1, can be administered to patients with recurrent malignant pleural mesothelioma.
5. Conclusion
This study provides evidence for the inhibitory role of narciclasine against growth of mesothelioma expressing high levels of YAP protein. The mechanism by which narciclasine suppresses tumor growth is its competition with TEAD4 for the interaction of YAP. In future, we anticipate that narciclasine will definitely become a seed to develop molecularly targeted drugs for mesothelioma because narciclasine does not associate with YAP with a high affinity.
Author contributions
R. Kawamoto, N. Nakano, H. Ishikawa, W. Nagano, F. Kishi, and E. Tashiro carried out the in vitro and cell-based experiments. K. Sano and M. Ikuta performed the experiments using tumor-xenografted mice. T. Nakane, M. Naito, and S. Itoh designed most aspects of the research, interpreted the data, and drafted the manuscript.
Declaration of Competing Interest
The authors have no competing financial interests to declare.
Acknowledgements
This research was supported by P-DIRECT from AMED (to S. Itoh); the Vehicle Racing Commemorative Foundation (to S. Itoh); the Science Research Promotion Fund (to S. Itoh); and a GSK Japan Research Grant 2017 (to N. Nakano). We were also supported by the Joint Usage/Research Program of the Medical Research Institute, Tokyo Medical and Dental University (to S. Itoh). We thank Ms Flaminia Miyamasu for excellent English proofreading.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.bbadva.2021.100008.
Appendix. Supplementary materials
References
- 1.Yap T.A., Aerts J.G., Popat S., Fennell D.A. Novel insights into mesothelioma biology and implications for therapy. Nat. Rev. Cancer. 2017;17:475–488. doi: 10.1038/nrc.2017.42. [DOI] [PubMed] [Google Scholar]
- 2.Peto J., Hodgson J.T., Matthews F.E., Jones J.R. Continuing increase in mesothelioma mortality in Britain. Lancet. 1995;345:535–539. doi: 10.1016/s0140-6736(95)90462-x. [DOI] [PubMed] [Google Scholar]
- 3.Zanconato F., Cordenonsi M., Piccolo S. YAP/TAZ at the roots of cancer. Cancer Cell. 2016;29:783–803. doi: 10.1016/j.ccell.2016.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang K., Qi H.X., Hu Z.M., Chang Y.N., Shi Z.M., Han X.H., Han Y.W., Zhang R.X., Zhang Z., Chen T., Hong W. YAP and TAZ take center stage in cancer. Biochemistry. 2015;54:6555–6566. doi: 10.1021/acs.biochem.5b01014. [DOI] [PubMed] [Google Scholar]
- 5.Reggiani F., Gobbi G., Ciarrocchi A., Ambrosetti D.C., Sancisi V. Multiple roles and context-specific mechanisms underlying YAP and TAZ-mediated resistance to anti-cancer therapy. Biochim. Biophys. Acta Rev. Cancer. 2020;1873 doi: 10.1016/j.bbcan.2020.188341. [DOI] [PubMed] [Google Scholar]
- 6.Meng Z., Moroishi T., Guan K.L. Mechanisms of Hippo pathway regulation. Genes Dev. 2016;30:1–17. doi: 10.1101/gad.274027.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu X., Li H., Rajurkar M., Li Q., Cotton J.L., Ou J., Zhu L.J., Goel H.L., Mercurio A.M., Park J.S., Davis R.J., Mao J. Tead and AP1 coordinate transcription and motility. Cell Rep. 2016;14:1169–1180. doi: 10.1016/j.celrep.2015.12.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Strano S., Monti O., Pediconi N., Baccarini A., Fontemaggi G., Lapi E., Mantovani F., Damalas A., Citro G., Sacchi A., Del Sal G., Levrero M., Blandino G. The transcriptional coactivator Yes-associated protein drives p73 gene-target specificity in response to DNA Damage. Mol. Cell. 2005;18:447–459. doi: 10.1016/j.molcel.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 9.Murakami M., Nakagawa M., Olson E.N., Nakagawa O. A WW domain protein TAZ is a critical coactivator for TBX5, a transcription factor implicated in Holt-Oram syndrome. Proc. Natl. Acad. Sci. U. S. A. 2005;102:18034–18039. doi: 10.1073/pnas.0509109102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferrigno O., Lallemand F., Verrecchia F., L'Hoste S., Camonis J., Atfi A., Mauviel A. Yes-associated protein (YAP65) interacts with Smad7 and potentiates its inhibitory activity against TGF-β/Smad signaling. Oncogene. 2002;21:4879–4884. doi: 10.1038/sj.onc.1205623. [DOI] [PubMed] [Google Scholar]
- 11.Alarcon C., Zaromytidou A.I., Xi Q., Gao S., Yu J., Fujisawa S., Barlas A., Miller A.N., Manova-Todorova K., Macias M.J., Sapkota G., Pan D., Massagúe J. Nuclear CDKs drive Smad transcriptional activation and turnover in BMP and TGF-β pathways. Cell. 2009;139:757–769. doi: 10.1016/j.cell.2009.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Narimatsu M., Samavarchi-Tehrani P., Varelas X., Wrana J.L. Distinct polarity cues direct Taz/Yap and TGFβ; receptor localization to differentially control TGFβ;-induced Smad signaling. Dev. Cell. 2015;32:652–656. doi: 10.1016/j.devcel.2015.02.019. [DOI] [PubMed] [Google Scholar]
- 13.Rosenbluh J., Nijhawan D., Cox A.G., Li X., Neal J.T., Schafer E.J., Zack T.I., Wang X., Tsherniak A., Schinzel A.C., Shao D.D., Schumacher S.E., Weir B.A., Vazquez F., Cowley G.S., Root D.E., Mesirov J.P., Beroukhim R., Kuo C.J., Goessling W., Hahn W.C. β;-Catenin-driven cancers require a YAP1 transcriptional complex for survival and tumorigenesis. Cell. 2012;151:1457–1473. doi: 10.1016/j.cell.2012.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nguyen L.T., Tretiakova M.S., Silvis M.R., Lucas J., Klezovitch O., Coleman I., Bolouri H., Kutyavin V.I., Morrissey C., True L.D., Nelson P.S., Vasioukhin V. ERG activates the YAP1 transcriptional program and induces the development of age-related prostate tumors. Cancer Cell. 2015;27:797–808. doi: 10.1016/j.ccell.2015.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kulkarni A., Chang M.T., Vissers J.H.A., Dey A., Harvey K.F. The Hippo pathway as a driver of select human cancers. Trends Cancer. 2020;6:781–796. doi: 10.1016/j.trecan.2020.04.004. [DOI] [PubMed] [Google Scholar]
- 16.Calses P.C., Crawford J.J., Lill J.R., Dey A. Hippo pathway in cancer: aberrant regulation and therapeutic opportunities. Trends Cancer. 2019;5:297–307. doi: 10.1016/j.trecan.2019.04.001. [DOI] [PubMed] [Google Scholar]
- 17.Bueno R., Stawiski E.W., Goldstein L.D., Durinck S., De Rienzo A., Modrusan Z., Gnad F., Nguyen T.T., Jaiswal B.S., Chirieac L.R., Sciaranghella D., Dao N., Gustafson C.E., Munir K.J., Hackney J.A., Chaudhuri A., Gupta R., Guillory J., Toy K., Ha C., Chen Y.J., Stinson J., Chaudhuri S., Zhang N., Wu T.D., Sugarbaker D.J., de Sauvage F.J., Richards W.G., Seshagiri S. Comprehensive genomic analysis of malignant pleural mesothelioma identifies recurrent mutations, gene fusions and splicing alterations. Nat. Genet. 2016;48:407–416. doi: 10.1038/ng.3520. [DOI] [PubMed] [Google Scholar]
- 18.Chen W.S., Cao Z., Krishnan C., Panjwani N. Verteporfin without light stimulation inhibits YAP activation in trabecular meshwork cells: implications for glaucoma treatment. Biochem. Biophys. Res. Commun. 2015;466:221–225. doi: 10.1016/j.bbrc.2015.09.012. [DOI] [PubMed] [Google Scholar]
- 19.Liu-Chittenden Y., Huang B., Shim J.S., Chen Q., Lee S.J., Anders R.A., Liu J.O., Pan D. Genetic and pharmacological disruption of the TEAD-YAP complex suppresses the oncogenic activity of YAP. Genes Dev. 2012;26:1300–1305. doi: 10.1101/gad.192856.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brodowska K., Al-Moujahed A., Marmalidou A., Meyer Zu Horste M., Cichy J., Miller J.W., Gragoudas E., Vavvas D.G. The clinically used photosensitizer verteporfin (VP) inhibits YAP-TEAD and human retinoblastoma cell growth in vitro without light activation. Exp. Eye Res. 2014;124:67–73. doi: 10.1016/j.exer.2014.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang C., Zhu X., Feng W., Yu Y., Jeong K., Guo W., Lu Y., Mills G.B. Verteporfin inhibits YAP function through up-regulating 14-3-3σ sequestering YAP in the cytoplasm. Am. J. Cancer Res. 2016;6:27–37. [PMC free article] [PubMed] [Google Scholar]
- 22.Song S., Xie M., Scott A.W., Jin J., Ma L., Dong X., Skinner H.D., Johnson R.L., Ding S., Ajani J.A. A novel YAP1 inhibitor targets CSC-enriched radiation-resistant cells and exerts strong antitumor activity in esophageal adenocarcinoma. Mol. Cancer Ther. 2018;17:443–454. doi: 10.1158/1535-7163.MCT-17-0560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kandasamy S., Adhikary G., Rorke E.A., Friedberg J.S., Mickle M.B., Alexander H.R., Eckert R.L. The YAP1 signaling inhibitors, verteporfin and CA3, suppress the mesothelioma cancer stem cell phenotype. Mol. Cancer Res. 2020;18:343–351. doi: 10.1158/1541-7786.MCR-19-0914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nouri K., Azad T., Ling M., van Rensburg H.J.J., Pipchuk A., Shen H., Hao Y., Zhang J., Yang X. Identification of celastrol as a novel YAP-TEAD inhibitor for cancer therapy by high throughput screening with ultrasensitive YAP/TAZ-TEAD biosensors. Cancers. 2019;11:1596. doi: 10.3390/cancers11101596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jiao S., Wang H., Shi Z., Dong A., Zhang W., Song X., He F., Wang Y., Zhang Z., Wang W., Wang X., Guo T., Li P., Zhao Y., Ji H., Zhang L., Zhou Z. A peptide mimicking VGLL4 function acts as a YAP antagonist therapy against gastric cancer. Cancer Cell. 2014;25:166–180. doi: 10.1016/j.ccr.2014.01.010. [DOI] [PubMed] [Google Scholar]
- 26.Cao D., Zhu G.Y., Lu Y., Yang A., Chen D., Huang H.J., Peng S.X., Chen L.W., Li Y.W. Luteolin suppresses epithelial-mesenchymal transition and migration of triple-negative breast cancer cells by inhibiting YAP/TAZ activity. Biomed. Pharmacother. 2020;129 doi: 10.1016/j.biopha.2020.110462. [DOI] [PubMed] [Google Scholar]
- 27.Li Y.W., Xu J., Zhu G.Y., Huang Z.J., Lu Y., Li X.Q., Wang N., Zhang F.X. Apigenin suppresses the stem cell-like properties of triple-negative breast cancer cells by inhibiting YAP/TAZ activity. Cell Death Discov. 2018;4:105. doi: 10.1038/s41420-018-0124-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sorrentino G., Ruggeri N., Specchia V., Cordenonsi M., Mano M., Dupont S., Manfrin A., Ingallina E., Sommaggio R., Piazza S., Rosato A., Piccolo S., Del Sal G. Metabolic control of YAP and TAZ by the mevalonate pathway. Nat. Cell Biol. 2014;16:357–366. doi: 10.1038/ncb2936. [DOI] [PubMed] [Google Scholar]
- 29.Zhao B., Wei X., Li W., Udan R.S., Yang Q., Kim J., Xie J., Ikenoue T., Yu J., Li L., Zheng P., Ye K., Chinnaiyan A., Halder G., Lai Z.C., Guan K.L. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev. 2007;21:2747–2761. doi: 10.1101/gad.1602907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fujii M., Toyoda T., Nakanishi H., Yatabe Y., Sato A., Matsudaira Y., Ito H., Murakami H., Kondo Y., Kondo E., Hida T., Tsujimura T., Osada H., Sekido Y. TGF-β; synergizes with defects in the Hippo pathway to stimulate human malignant mesothelioma growth. J. Exp. Med. 2012;209:479–494. doi: 10.1084/jem.20111653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Itoh S., Ericsson J., Nishikawa J., Heldin C.H., ten Dijke P. The transcriptional co-activator P/CAF potentiates TGF-β;/Smad signaling. Nucleic Acids Res. 2000;28:4291–4298. doi: 10.1093/nar/28.21.4291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ikeno S., Nakano N., Sano K., Minowa T., Sato W., Akatsu R., Sakata N., Hanagata N., Fujii M., Itoh F., Itoh S. PDZK1-interacting protein 1 (PDZK1IP1) traps Smad4 protein and suppresses transforming growth factor-β; (TGF-β;) signaling. J. Biol. Chem. 2019;294:4966–4980. doi: 10.1074/jbc.RA118.004153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nakano N., Sakata N., Katsu Y., Nochise D., Sato E., Takahashi Y., Yamaguchi S., Haga Y., Ikeno S., Motizuki M., Sano K., Yamasaki K., Miyazawa K., Itoh S. Dissociation of the AhR/ARNT complex by TGF-β;/Smad signaling represses CYP1A1 gene expression and inhibits benze[a]pyrene-mediated cytotoxicity. J. Biol. Chem. 2020;295:9033–9051. doi: 10.1074/jbc.RA120.013596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hau J.C., Erdmann D., Mesrouze Y., Furet P., Fontana P., Zimmermann C., Schmelzle T., Hofmann F., Chene P. The TEAD4-YAP/TAZ protein-protein interaction: expected similarities and unexpected differences. Chembiochem. 2013;14:1218–1225. doi: 10.1002/cbic.201300163. [DOI] [PubMed] [Google Scholar]
- 35.Pobbati A.V., Chan S.W., Lee I., Song H., Hong W. Structural and functional similarity between the Vgll1-TEAD and the YAP-TEAD complexes. Structure. 2012;20:1135–1140. doi: 10.1016/j.str.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 36.Bokhovchuk F., Mesrouze Y., Meyerhofer M., Zimmermann C., Fontana P., Erdmann D., Jemth P., Chene P. An early association between the α-helix of the TEAD binding domain of YAP and TEAD drives the formation of the YAP:TEAD complex. Biochemistry. 2020;59:1804–1812. doi: 10.1021/acs.biochem.0c00217. [DOI] [PubMed] [Google Scholar]
- 37.Li Z., Zhao B., Wang P., Chen F., Dong Z., Yang H., Guan K.L., Xu Y. Structural insights into the YAP and TEAD complex. Genes Dev. 2010;24:235–240. doi: 10.1101/gad.1865810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Murakami H., Mizuno T., Taniguchi T., Fujii M., Ishiguro F., Fukui T., Akatsuka S., Horio Y., Hida T., Kondo Y., Toyokuni S., Osada H., Sekido Y. LATS2 is a tumor suppressor gene of malignant mesothelioma. Cancer Res. 2011;71:873–883. doi: 10.1158/0008-5472.CAN-10-2164. [DOI] [PubMed] [Google Scholar]
- 39.Kornienko A., Evidente A. Chemistry, biology, and medicinal potential of narciclasine and its congeners. Chem. Rev. 2008;108:1982–2014. doi: 10.1021/cr078198u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Van Goietsenoven G., Hutton J., Becker J.P., Lallemand B., Robert F., Lefranc F., Pirker C., Vandenbussche G., Van Antwerpen P., Evidente A., Berger W., Prevost M., Pelletier J., Kiss R., Kinzy T.G., Kornienko A., Mathieu V. Targeting of eEF1A with Amaryllidaceae isocarbostyrils as a strategy to combat melanomas. FASEB J. 2010;24:4575–4584. doi: 10.1096/fj.10-162263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Van Goietsenoven G., Mathieu V., Lefranc F., Kornienko A., Evidente A., Kiss R. Narciclasine as well as other Amaryllidaceae isocarbostyrils are promising GTP-ase targeting agents against brain cancers. Med. Res. Rev. 2013;33:439–455. doi: 10.1002/med.21253. [DOI] [PubMed] [Google Scholar]
- 42.Ziemssen F., Heimann H. Evaluation of verteporfin pharmakokinetics–redefining the need of photosensitizers in ophthalmology. Expert Opin. Drug Metab. Toxicol. 2012;8:1023–1041. doi: 10.1517/17425255.2012.701617. [DOI] [PubMed] [Google Scholar]
- 43.Michels S., Schmidt-Erfurth U. Photodynamic therapy with verteporfin: a new treatment in ophthalmology. Semin. Ophthalmol. 2001;16:201–206. doi: 10.1076/soph.16.4.201.10298. [DOI] [PubMed] [Google Scholar]
- 44.Gibault F., Bailly F., Corvaisier M., Coevoet M., Huet G., Melnyk P., Cotelle P. Molecular features of the YAP inhibitor verteporfin: synthesis of hexasubstituted dipyrrins as potential inhibitors of YAP/TAZ, the downstream effectors of the Hippo pathway. Chem. Med. Chem. 2017;12:954–961. doi: 10.1002/cmdc.201700063. [DOI] [PubMed] [Google Scholar]
- 45.Kandoussi I., Lakhlili W., Taoufik J., Ibrahimi A. Docking analysis of verteporfin with YAP WW domain. Bioinformation. 2017;13:237–240. doi: 10.6026/97320630013237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wei H., Wang F., Wang Y., Li T., Xiu P., Zhong J., Sun X., Li J. Verteporfin suppresses cell survival, angiogenesis and vasculogenic mimicry of pancreatic ductal adenocarcinoma via disrupting the YAP-TEAD complex. Cancer Sci. 2017;108:478–487. doi: 10.1111/cas.13138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pan W., Wang Q., Zhang Y., Zhang N., Qin J., Li W., Wang J., Wu F., Cao L., Xu G. Verteporfin can reverse the paclitaxel resistance induced by YAP over-expression in HCT-8/T cells without photoactivation through inhibiting YAP expression. Cell Physiol. Biochem. 2016;39:481–490. doi: 10.1159/000445640. [DOI] [PubMed] [Google Scholar]
- 48.Dong L., Lin F., Wu W., Liu Y., Huang W. Verteporfin inhibits YAP-induced bladder cancer cell growth and invasion via Hippo signaling pathway. Int. J. Med. Sci. 2018;15:645–652. doi: 10.7150/ijms.23460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shi G., Wang H., Han H., Gan J., Wang H. Verteporfin enhances the sensitivity of LOVO/TAX cells to taxol via YAP inhibition. Exp. Ther. Med. 2018;16:2751–2755. doi: 10.3892/etm.2018.6447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gopalakrishnan R., Matta H., Choi S., Chaudhary P.M. Narciclasine, an isocarbostyril alkaloid, has preferential activity against primary effusion lymphoma. Sci. Rep. 2020;10:5712. doi: 10.1038/s41598-020-62690-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cao C., Huang W., Zhang N., Wu F., Xu T., Pan X., Peng C., Han B. Narciclasine induces autophagy-dependent apoptosis in triple-negative breast cancer cells by regulating the AMPK-ULK1 axis. Cell Prolif. 2018;51:e12518. doi: 10.1111/cpr.12518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Julien S.G., Kim S.Y., Brunmeir R., Sinnakannu J.R., Ge X., Li H., Ma W., Yaligar J., Kn B.P., Velan S.S., Roder P.V., Zhang Q., Sim C.K., Wu J., Garcia-Miralles M., Pouladi M.A., Xie W., McFarlane C., Han W., Xu F. Narciclasine attenuates diet-induced obesity by promoting oxidative metabolism in skeletal muscle. PLoS Biol. 2017;15 doi: 10.1371/journal.pbio.1002597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stark A., Schwenk R., Wack G., Zuchtriegel G., Hatemler M.G., Brautigam J., Schmidtko A., Reichel C.A., Bischoff I., Furst R. Narciclasine exerts anti-inflammatory actions by blocking leukocyte-endothelial cell interactions and down-regulation of the endothelial TNF receptor 1. FASEB J. 2019;33:8771–8781. doi: 10.1096/fj.201802440R. [DOI] [PubMed] [Google Scholar]
- 54.Brautigam J., Bischoff I., Schurmann C., Buchmann G., Epah J., Fuchs S., Heiss E., Brandes R.P., Furst R. Narciclasine inhibits angiogenic processes by activation of Rho kinase and by downregulation of the VEGF receptor 2. J. Mol. Cell Cardiol. 2019;135:97–108. doi: 10.1016/j.yjmcc.2019.08.001. [DOI] [PubMed] [Google Scholar]
- 55.Chen M., Huang B., Zhu L., Chen K., Liu M., Zhong C. Structural and functional overview of TEAD4 in cancer biology. Onco Targets Ther. 2020;13:9865–9874. doi: 10.2147/OTT.S266649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.He L., Yuan L., Sun Y., Wang P., Zhang H., Feng X., Wang Z., Zhang W., Yang C., Zeng Y.A., Zhao Y., Chen C., Zhang L. Glucocorticoid receptor signaling activates TEAD4 to promote breast cancer progression. Cancer Res. 2019;79:4399–4411. doi: 10.1158/0008-5472.CAN-19-0012. [DOI] [PubMed] [Google Scholar]
- 57.Gao S., Wang Y., Ji L. Rational design and chemical modification of TEAD coactivator peptides to target hippo signaling pathway against gastrointestinal cancers. J. Recept. Signal Transduct. Res. 2020:1–8. doi: 10.1080/10799893.2020.1818093. [DOI] [PubMed] [Google Scholar]
- 58.Okuhira K., Demizu Y., Hattori T., Ohoka N., Shibata N., Kurihara M., Naito M. Molecular design, synthesis, and evaluation of SNIPER(ER) that induces proteasomal degradation of ERα. Methods Mol. Biol. 2016;1366:549–560. doi: 10.1007/978-1-4939-3127-9_42. [DOI] [PubMed] [Google Scholar]
- 59.Ohoka N., Ujikawa O., Shimokawa K., Sameshima T., Shibata N., Hattori T., Nara H., Cho N., Naito M. Different degradation mechanisms of inhibitor of apoptosis proteins (IAPs) by the specific and nongenetic IAP-dependent protein eraser (SNIPER) Chem. Pharm. Bull. (Tokyo) 2019;67:203–209. doi: 10.1248/cpb.c18-00567. [DOI] [PubMed] [Google Scholar]
- 60.Burslem G.M., Crews C.M. Proteolysis-targeting chimeras as therapeutics and tools for biological discovery. Cell. 2020;181:102–114. doi: 10.1016/j.cell.2019.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Khan S., He Y., Zhang X., Yuan Y., Pu S., Kong Q., Zheng G., Zhou D. PROteolysis TArgeting chimeras (PROTACs) as emerging anticancer therapeutics. Oncogene. 2020;39:4909–4924. doi: 10.1038/s41388-020-1336-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.