Abstract
The Förster resonance energy transfer (FRET) is a well-established and versatile spectroscopic technique extensively used for exploring a variety of biomolecular interactions and processes. The present review is intended to cover the main results of our FRET studies focused on amyloid fibrils, a particular type of disease-associated protein aggregates. Based on the examples of several fibril-forming proteins including insulin, lysozyme and amyloidogenic variants of N-terminal fragment of apolipoprotein A-I, it was demonstrated that: (i) the two- and three-step FRET with the classical amyloid marker Thioflavin T as an input donor has a high amyloid-sensing potential and can be used to refine the amyloid detection assays; (ii) the intermolecular time-resolved and single-molecule pulse interleaved excitation FRET can give quantitative information on the nucleation of amyloid fibrils; (iii) FRET between the membrane fluorescent probes and protein-associated intrinsic or extrinsic fluorophores is suitable for monitoring the membrane binding of fibrillar proteins, exploring their location relative to lipid-water interface and restructuring on a lipid matrix; (iv) the FRET-based distance estimation between fibril-bound donor and acceptor fluorophores can serve as one of the verification criteria upon structural modeling of amyloid fibrils.
Keywords: Förster resonance energy transfer; Amyloid fibrils, Lysozyme; N-terminal fragment of apolipoprotein A-I; Insulin; Amyloid detection and structural analysis
1. Introduction
The last decades have seen considerable progress in the development of the ratiometric fluorescence sensor systems based on the Fӧrster resonance energy transfer (FRET), a process in which the energy absorbed by a donor molecule is passed to an acceptor molecule by a long-range dipole–dipole mechanism. The dependence of energy transfer efficiency on the donor-acceptor distance, photophysical characteristics of the chromophores, their spatial arrangement and orientational behavior, renders FRET a powerful tool with a broad range of applications among which are signal transduction, optical data storage, cryptography, light harvesting, plasmonics, theranostics and nanoscale structural analysis of biological systems, from single molecules to membranes and cells [1], [2], [3], [4], [5], [6], [7]. The recent advances in FRET studies highlight the following main tendencies: (i) the arsenal of photostable FRET donors and acceptors, including fluorescent proteins, organic dyes, semiconductor nanoparticles, etc. is steadily expanding [8,9]; (ii) FRET is increasingly combined with confocal, two-photon, time-resolved and super resolution fluorescence microscopy [10,11]; (iii) a growing attention is paid to devising the FRET-based DNA probes [12] and biosensors [13]; (iv) significant research efforts are focused on the development of multiplexed FRET platforms for simultaneous monitoring of different biological processes in vitro and in vivo [14,15]; (v) the ensembles of light-harvesting chromophores implicated in the cascade FRET (cFRET) attract considerable interest in nanotechnological and biomedical contexts [16]; (vi) FRET is becoming more and more important in exploring the biomolecular interactions of the drug nanocarriers and their distribution in the organism [17]. Another important research area in which FRET can provide unique and versatile information concerns structural transition of misfolded proteins into the amyloid fibril state that is associated with a range of human diseases, including neurodegenerative disorders, type II diabetes, systemic amyloidoses, etc. [18]. Amyloid fibrils represent a special class of the ordered protein aggregates with characteristic cross-β spine in which the intermolecular β-sheets extend parallel to the long fibril axis [19]. These protein assemblies nowadays attract increasing attention not only from a biomedical point of view, but also from a nanotechnological perspective [20]. Along with other powerful physical techniques currently used for detection and structural characterization of amyloid fibrils, among which are cryo- AFM, TEM, cryoTEM [14], [15], [16], [17], CD, FTIR, NMR, fluorescence spectroscopy [21], [22], [23], [24], [25], [26], [27], [28], FRET appeared to be a valuable tool suitable for elucidating multiple aspects of amyloid growth and reactivity. For instance, FRET between the classical amyloid marker Thioflavin T (ThT) and carbocyanine dye JC-1 has been employed for identification of the amyloid fibrils of α-synuclein [29]. The sensitivity of FRET to various polymorphs of Aβ(1-42) fibrils has been revealed using the donor-acceptor pair ThT-Nile red [30]. The energy transfer between bis-ANS and a styrylquinoxalin derivative has been measured to monitor the formation of oligomers and fibrils of amyloidogenic peptides in situ [31]. The donor-acceptor pair Alexa Fluor 488 - Alexa Fluor 647 has been used to examine the oligomeric populations of α-synuclein [32] and tau protein [33,34]. A fluorescence lifetime FRET sensor based on the energy transfer between a yellow fluorescent protein and the growing protein oligomers has been developed and applied to monitor the aggregation of α-synuclein in solution, cells in culture and in vivo [35]. Novel methodology based on FRET between quinolimide derivative and Nile Blue A, has been evolved for quantitative imaging of apoferritin amyloid formation [36,37]. FRET-based assay as a prototype of high throughput platform for non-invasive characterization of α-synuclein fibrillar species [38]. Using cyan and yellow fluorescent probes as a donor-acceptor pair, Takahashi et al. constructed FRET sensor for detection of the oligomeric assemblies of amyloid β-peptide and its mutants [39]. Single-molecule FRET between 5-carboxytetramethylrhodamine and HiLyte Fluor 647 has been utilized for assessing the stability of Aβ-peptide aggregates in physiological solutions [40]. The effect of Zn(II) on the neurotoxicity of Aβ(1-21)-peptide amyloid fibrils was assessed by steady-state FRET between newly synthesized dye A-1 and 1-naphthylethylenediamine [41]. FRET as an analytical tool has been employed for the analysis of different surfaces as scaffolds for amyloid formation by Aβ(1-40)-peptide [42]. The list of the above examples is far from being exhausted and represents only the most prominent cases.
The present paper outlines the previous and ongoing research activities of our laboratory in probing the amyloid structural state of protein molecules using the FRET technique. The main issues addressed here concern: (i) the FRET approaches to differentiating between the native and amyloid conformations of protein molecules; (ii) structural modeling of fibrillized proteins based on the results FRET measurements; (iii) association of amyloid fibrils with lipid bilayers monitored by FRET; (iv) FRET evidence for fibril nucleation on a membrane template; (v) membrane-mediated topological rearrangement of fibril structure.
2. FRET in amyloid detection
Our starting point for application of FRET in amyloid identification emerged from the idea to combine two the most prominent amyloid markers, the benzothiazole dye Thioflavin T (ThT) and the azo dye Congo red (CR) [43], [44], [45], [46] in the one detection assay. A distinctive feature of amyloid assemblies is a core β-sheet structure with the solvent-exposed grooves lined with amino acid side chains and running along the fibril axis [19,47]. These grooves provide a unique binding motif for amyloid-specific chromo- and fluorophores that is thought to be critical in determining their characteristic spectral responses [48], [49], [50], [51]. The binding of ThT to fibrillar aggregates is accompanied by considerable long-wavelength shifts of its excitation and emission bands, coupled with a dramatic fluorescence upsurge that can be of several orders of magnitude depending on the fibril type [52,53]. This effect is interpreted as arising from a significant restriction of the torsional oscillations of the benzothiazole and aminobenzoyl rings of the dye accommodating within the fibril groove [54], [55], [56]. This mode of the dye-protein complexation prevents the formation of a weakly-fluorescent twisted internal charge-transfer state and renders the ThT molecule nearly planar [55]. Another dye that has traditionally been used as amyloid marker is Congo Red, whose association with amyloid assemblies produces a characteristic green-yellow birefringence under polarized light and a bathochromic shift of absorption maximum from ∼ 490 to ∼ 540 nm [57,58].
Allowing for the remarkable amyloid-sensing properties of Thioflavin T and Congo Red, along with the fact that the spectra of ThT emission and CR absorption are significantly overlapped we came to the idea of designing the combined fluorescence ThT-CR amyloid test. To verify this idea, the donor-acceptor pair ThT-CR has been employed to distinguish between the amyloid and native forms of three proteins, viz. lysozyme, insulin and N-terminal fragment of apolipoprotein A-I (apoA-I) [59]. It appeared that the energy transfer between ThT and CR is much more effective in the fibrillar state of these proteins than in the non-fibrillized state (Fig. 1).
Fig. 1.
The efficiency of energy transfer measured as a function of acceptor concentration for the donor-acceptor pair Thioflavin T-Congo Red in fibrillar (F) and non-fibrillar (NF) states of N-terminal fragment of apoA-I, insulin and lysozyme. The concentration of Thioflavin T was 1 μM.
Similar effects were further observed while measuring the FRET between ThT as a donor and one of a series of the novel trimethine cyanine dyes (Fig. 2, A) as an acceptor [60]. The energy was transferred from ThT to cyanines only in the presence of fibrillar, but not native insulin, depending on the cyanine chemical structure and decreasing in the order: AK3-3 > AK3-7 > AK3-1 > AK3-5 > AK3-8 > AK3-11 (Fig. 2, B). It was assumed that the observed very high FRET efficiencies for the ThT - cyanine donor-acceptor pairs arise from accumulation of the dyes in the cavities within suprafibrillar structures and/or energy transfer from ThT to the cyanine H-aggregates assembled on a fibril template [60].
Fig. 2.
Chemical structures of the cyanine dyes under study (A). The efficiency of energy transfer measured as a function of acceptor concentration for the donor-acceptor pairs ThT-AK3-dye. The protein and ThT concentrations were 1.44 µM and 0.2 µM, respectively
The revealed significant enhancement of FRET between fibril-bound fluorophores may reflect the contributions of various factors among which are: (i) high-affinity dye binding to specific structural elements of amyloid fold such as the surface-exposed grooves, resulting in considerable increase of the donor quantum yield and spectral overlap between the donor and acceptor; (ii) a particular fluorophore arrangement within fibril structure; (iii) a specific mutual orientation of the fibril-bound donors and acceptors.
As a next logical step towards extending the FRET applicability in amyloid research, we explored the possibility of amyloid-sensing with the cascade or multi-step FRET (msFRET). The multi-step FRET has found numerous applications in a wide range of areas such as biosensing [61], theranostics [62,63], light harvesting [64], optical computing [65], fabrication of photonic nanowires [64,66] and light-emitting diodes [67], etc. To host the donor and acceptor fluorophores for msFRET, a variety of matrices have been employed including DNA [64,68], micelles [69], dendrimers [70] and quantum dots [71], but amyloid nanostructures have not been investigated in this aspect. To fill this gap, we started from a simplest format, the two-step energy transfer cascade involving the three fluorophores, a classical amyloid marker ThT, a phosphonium dye TDV and a squaraine dye SQ1 (Fig. 3, A).
Fig. 3.
Chemical structures of the dyes involved in the two-step FRET chain (A). Thioflavin T fluorescence intensity at 480 nm measured during 14 days of fibrillization reaction for WT and mutated variants of the N-terminal (1-83) fragment of apolipoprotein A-I.
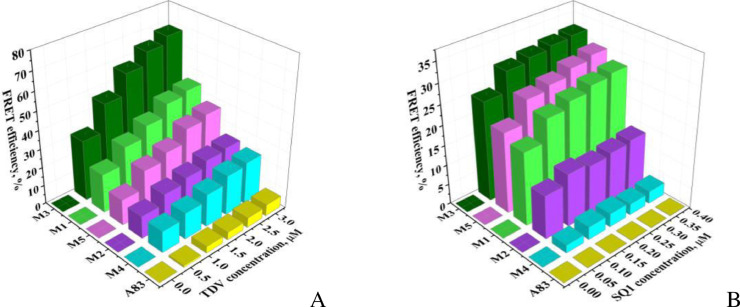
The apolipoprotein A-I variants differing in their amyloid-forming ability were used to produce the fibrillar scaffolds for co-localization of the input donor (ThT), relay dye (TDV) and the output acceptor (SQ1) [72]. ApoA-I is a main protein component of the high-density lipoproteins participating in the efflux of phospholipids and cholesterol from the cell plasma membranes [73]. The substitution mutant of the human apoA-I, G26R, is associated with the hereditary systemic amyloidosis, with the N-terminal fragment being a major constituent of fibrillar deposits [74,75]. A panel of the examined apoA-I variants was comprised of the WT N-terminal (1-83) fragment of the protein and its five amyloidogenic mutants with the mutation G26R and the substitutions of tryptophan by phenylalanine (W8F, W50F, W72F) and aspartic acid by alanine (D48A). This set of polypeptides (denoted here as A83 (WT), M1 (A83/G26R/W@72), M2 (A83/G26R/W@50), M3 (A83/G26R/W@8), M4 (A83/G26R/W@8/D48A), M5 (A83/G26R)) appeared to be a suitable model system for evaluating the applicability of two-step FRET to amyloid detection, since the examined apoA-I mutants differed in the extent of their fibrillization quantitated by measuring the ThT fluorescence intensity (Fig. 3, B). The FRET efficiencies measured for the pairs ThT-TDV and TDV-SQ1 were found to correlate with the amount of fibrillized protein. As seen in Fig. 4, , the FRET efficiencies for the pairs ThT-TDV and TDV-SQ1 follow the order M3>M5≥M1>M2>M4>A83, in accord with the ThT spectral responses.
Fig. 4.
The efficiency of energy transfer measured as a function of acceptor concentration for the donor-acceptor pairs ThT-TDV (A) and TDV-SQ1 (B). The donor concentrations were 13.6 μM (A) and 3.0 μM (B).
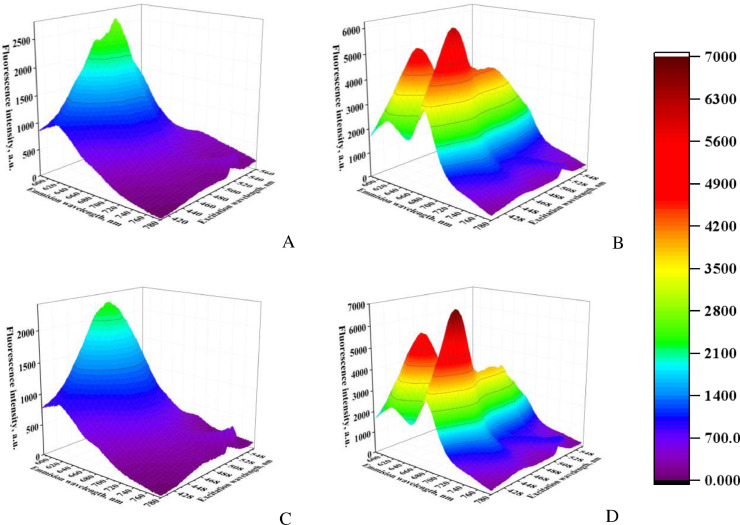
It should be noted that the existence of a correlation between the FRET efficiencies and the extent of protein fibrillization could not be expected a priori because of the intrinsic complexity of energy tansfer process. All factors governing the rate of FRET (the donor-acceptor distance, spatial and orientational distribution of the donors and acceptors, the donor quantum yield, the overlap between the donor emission and acceptor absorption spectra, the refractive index of the medium) seem to be substantially different for the dyes associated with the monomeric and fibrillar forms of a protein molecule. Remarkably, these differences become more apparent while using an ensemble of fluorophores organized in the FRET chain instead of the one donor-acceptor pair. This is clearly seen in the 3D fluorescence spectra of the aforementioned apoA-I mutants measured at the excitation wavelengths from the region of ThT and TDV absorption (410-560 nm), and emission wavelengths from the range of TDV and SQ1 fluorescence (585-780 nm). As illustrated in Fig. 5, A, a nearly elliptical low-intensity 3D pattern centered at the TDV emission ( ∼ 620 nm) and excitation maxima (∼ 495 nm) with no signs of FRET is observed for the non-fibrillized apoA-I variant A83. In contrast, the 3D contour map of M5 and M3 (Fig. 5, B, D), the apoA-I variants with the greatest extent of fibril formation, contains the high-intensity fluorescence peaks / ∼ 450 /∼ 620 nm, ∼ 495 /∼ 620 nm (TDV emission excited through ThT or directly, respectively) and ∼ 450 /∼ 680 nm, ∼ 495 /∼ 680 nm reflecting the energy transfer to SQ1 (Fig. 5, B). Analogously, 3D spectra of M3 at the first (Fig. 5, C) and last (Fig. 5, D) of fibrillization are clearly different.
Fig. 5.
3D fluorescence spectra recorded at the 1-st (C) and14th days (A, B, D) of the fibrillization reaction for the apoA-I variants: A83 (A), M5 (B), M3 (C, D). The excitation and emission wavelengths were varied within the ranges 410–560 nm and 585-780 nm, respectively. The concentrations of ThT, TDV and SQ1 were 15.4, 3.1 μM and 0.3 μM, respectively. The protein concentration was 0.16 μM.
To extend this line of research, in the following experiments we explored the amyloid sensitivity of the three-step FRET and evaluated the ability of a range of dyes to serve as mediators (a series of benzanthrones [76], styryl pyridinium dyes DSM, DSP6, DSP12, squaraine dye SQ4 [77]) and terminal acceptors (squaraine dyes SQ1, SQ2, SQ3 [77] chain with ThT as a principal donor. The insulin amyloid fibrils were used to host these four-chromophore systems. Insulin, a proteinaceous hormone that plays a dominant role in glucose metabolism, readily undergoes fibrillization both in vivo (at the sites of its multiple injection in diabetic patients) and in vitro (during the storage of therapeutic insulin and under amyloidogenic conditions). Here we employed insulin as a model protein to uncover the peculiarities of the three-step energy transfer on the amyloid scaffold. The FRET experiments were conducted in the two formats: (i) measuring the decrease of the donor fluorescence upon sequential titration of the ThT+insulin mixture by the other three donor/acceptor fluorophores of the FRET cascade, followed by deconvolution of the overall emission spectra into separate components; (ii) monitoring the enhancement of the acceptor fluorescence in the presence of a donor. It appeared that for all donor-acceptor pairs of the examined FRET chains the energy transfer was more efficient for the fibrillar insulin compared to its non-fibrillized counterpart, with the most significant difference being observed for the pair ThT-first mediator. Accordingly, the collective spectral response of the ensemble of four fluorophores accounts for a characteristic 3D fluorescence fingerprint of amyloid protein conformation. To exemplify, the 3D fluorescence spectra of the system ThT-DSP12-SQ4-SQ1 + fibrillar insulin (Fig. 6, A) contain the peaks reflecting the energy transfer from ThT to DSP12, SQ4, SQ1 (the peaks / 450 nm / 598 nm, 450 nm / 647 nm, 450 nm / 689 nm), and from DSP12 to SQ4 and SQ1 (the peaks / 512 nm / 647 nm, 512 nm / 689 nm), while 3D pattern of non-aggregated insulin is quite different (Fig. 6, B).
Fig. 6.
3D fluorescence spectra recorded in the presence of fibrillar (A) and non-fibrillized (B) insulin for the FRET chain ThT-DSP12-SQ4-SQ1. The dye concentrations were 3.4 μM (ThT), 0.61 μM (DSP12), 0.15 μM (SQ4) and 0.42 μM (SQ1). The protein concentration was 13.2 μM.
Taken together, our findings clearly demonstrate that multistep FRET represents a promising tool for identifying the amyloid state of protein molecules. The main advantages of msFRET over the traditional one-step format can be outlined as follows: (i) a large effective Stokes shift (more than 200 nm in the examined systems with ThT as an input donor and squaraine dye as an output acceptor) allowing to perform the FRET measurements in the first optical window of biological tissues (650–950 nm) and achieve a higher sensitivity of amyloid detection both in vitro and in vivo; (ii) minimizing the risk of false positive or negative results due to synergistic combination of multiple amyloid-specific fluorophores in one assay; (iii) convenience and handiness of obtaining the amyloid signatures, particularly, the unique 3D fluorescence patterns.
3. FRET monitoring of membrane-mediated nucleation of amyloid fibrils
It is becoming generally accepted that amyloid formation proceeds through aggregation of misfolded protein conformers into intermediate oligomeric species that are currently regarded as a key determinant of amyloid cytotoxicity [11]. Amyloid intermediates are complex in nature, and represent heterogeneous in size and conformation continuum of states, ranging from dimers to higher-order oligomers. In mapping such states FRET represents one of the best alternatives to other physical techniques. In the present section we reflect on the utilization of advanced FRET modifications, viz. intermolecular time-resolved FRET (tr-FRET) and single-molecule pulse interleaved excitation FRET (PIE-FRET) to quantitatively characterize the oligomerization of polycationic protein lysozyme (Lz) in the presence of lipid vesicles composed of phosphatidylcholine (PC) and its mixture with 5, 10, 20 or 40 mol% of phosphatidylglycerol (PG) (PG5, PG10, PG20 or PG40, respectively). The donor-acceptor pairs were represented by fluorescein 5’-isothiocyanate (Fl) and DyLight 549 (DyL549) (tr-FRET studies) or Fl and SeTau-647-di-NHS (SeTau647) (PIE-FRET experiments), covalently attached to Lz. More specifically, our experimental and theoretical efforts were concentrated at: (i) monitoring the formation of lipid-mediated Lz aggregates; (ii) estimating the degree of oligomerization; the distance between the monomers in protein assembly and the fraction of donors present in oligomers; (iii) deciphering the impact of membrane charge and surface coverage with the adsorbed protein on the above parameters. While analyzing the results outlined in this section, we utilized the theoretical formalism, based on the model of FRET in protein oligomers [78]. The intermolecular FRET from Fl to DyL549 covalently bound to Lz (Lz-Fl and Lz-DyL549, respectively) was detected upon protein binding to PG10, PG20 or PG40 membranes. In contrast, no FRET was observed in buffer solution and in the presence of PC or PG5 liposomes. Notably, the energy transfer may occur between donor and acceptor attached to either aggregated protein species or adjacent non-aggregated freely diffusing molecules. These cases were distinguished using the approach proposed by John and Jähnig [79]. It appeared that in the presence of weakly charged lipid bilayers (PG10) Lz retains its monomeric conformation, while higher concentrations of anionic lipid (20 and 40 mol%) give rise to the protein aggregation (Fig. 7). The FRET data obtained for PG20 and PG40 membranes were analyzed in terms of the model of energy transfer in protein oligomers [78] by minimizing the error function through varying the set of the above oligomer parameters. Next, to gain further insight into the membrane-induced self-association of lysozyme, a sophisticated PIE-FRET technique based on the excitation with two separate but synchronized laser pulses with different wavelengths has been applied. The laser pulses are delayed with respect to each other in order to produce the sequence of independent decays for donor and acceptor excited by each pulse separately. For short wavelength excitation pulse both donor and acceptor are excited, while exclusive excitation of acceptor molecules occurs at long wavelength pulse, so that only acceptor decay can be observed.
Fig. 7.

Schematic representation of the processes occurred in the model membrane systems containing lysozyme and negatively charged lipid bilayers.
In this way, the interfering artifacts in standard FRET procedure are eliminated and more reliable quantitative information can be derived from the experimental data [80,81]. Overall, both tr-FRET and PIE-FRET provided evidence for the enhancement of lysozyme aggregation potential upon increasing the molar content of anionic lipids, indicating that electrostatic protein-lipid interactions play a key role in the protein conversion into oligomeric state.
4. Biomolecular interactions of amyloid fibrils probed by FRET
The traditional way of applying FRET lies in monitoring various types of binding events occurring in biomolecular systems [82,83]. In the amyloid context, we have employed FRET to gain insight into lipid bilayer interactions of amyloidogenic proteins in the native and fibrillar states. The FRET between tryptophan as a donor and the membrane-incorporated fluorescent probe 4-dimethylaminochalcone as an acceptor was used to investigate the binding of the single tryptophan variants of apoA-I (1-83, 1-83/G26R, 1-83/G26R/W@8 and 1-83/G26R/W@50) to lipid bilayers from phosphatidylcholine and its mixture with 30 mol% of cholesterol (Chol) [84]. To quantitatively interpret the results of FRET measurements the model of Fung & Stryer [85] was modified to allow for the distance dependence of orientation factor in two-dimensional systems [86], donor distribution in the plane located at a distance from the membrane center and acceptor distribution over two planes separated by a distance . The FRET efficiency was calculated as:
| (1) |
| (2) |
| (3) |
| (4) |
where and are the quenching contributions describing the energy transfer to the outer and inner acceptor planes, respectively; , assuming that the donor emission and acceptor absorption transition moments are symmetrically distributed within the cones about certain axes Dx and Ax, and are the axial depolarization factors related to the steady-state () and fundamental () anisotropies of a donor and an acceptor as [87]; , where are the angles made by Dx and Ax with the bilayer normal. The analysis of the experimental FRET profiles in terms of this model allowed us to evaluate the donor separation of the protein tryptophan residues from the membrane midplane (). It appeared that: (i) the amyloidogenic mutation G26R results in a more exposed location of the Trp residues in PC bilayer, with W8 and W50 being located at the distances 1.5-2 nm from the bilayer center; (ii) cholesterol favors a much shallower location of the proteins relative to the membrane surface, with W8 and W50 residing in the aqueous phase, at the distances 3.2-3.9 nm from the bilayer midplane.
Another example of FRET application to detection of protein-lipid association comes from our study of the interactions between amyloid fibrils from apoA-I variants 1-83/G26R and 1-83/G26R/W@8 with PC and PC/Chol (30 mol%) lipid bilayers [88]. In this case the membrane fluorescent probe Laurdan was recruited as a donor while amyloid-specific dye Thioflavin T served as an acceptor. The binding of amyloid fibrils to the model membranes was found to produce the amyloid-induced enhancement of energy transfer correlating with the degree of protein fibrillization, increasing in the row 1-83, 1-83/G26R, 1-83/G26R/W@8. The efficiency of energy transfer in membrane systems is determined by the number of acceptors per unit area and the randomness of their distribution in a lipid bilayer. In the case of non-fibrillizing polypeptide 1-83, in which specific binding sites for ThT are lacking, the amount of membrane-bound acceptors and their arrangement relative to the donor plane turned out to be similar to those observed for the neat PC membranes, resulting in the indistinguishable FRET profiles for PC and PC+1-83 systems. On the contrary, association of amyloid fibrils from 1-83/G26R and 1-83/G26R/W@8 with PC bilayer was followed by significant increase of the FRET efficiency due to the rise in the amount of ThT molecules within the distance of energy transfer, their particular orientation and ordered distribution within fibril structure. To determine the distance between Laurdan and ThT, the FRET efficiency was calculated for different spatial arrangements of the donors and acceptors. In the acceptor configuration consistent with the experimental data the β-strands of 1-83/G26R/W@8 fibrils were found to reside in the interfacial region of PC bilayer, with ThT-binding grooves facing the aqueous phase. Another noteworthy finding of this FRET study is the ability of cholesterol to reduce the extent of fibril-membrane binding and prevent the penetration of β-strands in the lipid bilayer interior.
5. Amyloid fibril modeling based on FRET data
This section is intended to illustrate how FRET can be used for refinement and/or verification of the structural models of amyloid fibrils. The possibility of FRET application to structural modeling of amyloids is analyzed on the example of the apoA-I amyloidogenic mutant 1-83/G26R/W@8. The N-terminal (1-83) fragment of apoA-I contains three tryptophan residues, Trp8, Trp50 and Trp72, one of which, Trp8, served as an energy donor for ThT in the FRET experiments, while two other Trp residues were substituted by Phe [89]. Based on several lines of evidence, including the theoretical prediction of aggregation-prone regions, EPR and proteolytic accessibility data [90], together with X-ray crystallographic [74] and AFM results, the β-strand–loop–β-strand structural model of the 1-83/G26R/W@8 fibrils has been proposed, in which the polypeptides in the U-shape are registered in parallel, and the β-strands composed of 14-31 and 41-58 residues tend to form a self-complementary steric zipper [89]. The superficial grooves that could accommodate ThT are formed by the residues L14_T16_Y18_D20_L22_D24_R26_D28_V30 in the N-terminal β-sheet layer and by the residues Q41_N43_K45_L47_N49_D51, S52_T54_T56_S58 in the C-terminal β-sheet layer. Since ThT prefers to associate with the grooves lined with aromatic residues, particularly, Tyr and Phe [91], possesses high affinity for hydrophobic amino acid residues [92] and may be targeted to fibril surface by the negatively charged residues we considered the five grooves T16_Y18 (G1), Y18_D20 (G2), D20_L22 (G3), L22_D24 (G4), D28_V30 (G5) as the most probable sites for ThT binding. Taking into account that the propagation of an extended polypeptide chain is ∼ 0.35 nm per residue and Trp radius is ∼ 0.3 nm the maximum distance between Trp8 and the above grooves was found to lie between 3.5 nm and 7.6 nm. Allowing for rigidity of fibril structure the grooves were regarded as being separated by a fixed distance and the analysis of the FRET data was intended to ascertain what calculated distances from Trp8 to G1-G5 agree with those derived from the FRET data fitting. To verify the above structural model, the Trp-ThT distance constraints were recovered from the results of FRET measurements through the use of the classical expression for distance dependence of energy transfer efficiency and the calculation of value from the coordinates of donors and acceptors generated in a virtual elongated cell modeling the fluorophore distribution within the fibril structure:
| (5) |
where , are the numbers of donors and acceptors in a cell, respectively. This simulation-based approach yielded the sets of possible distances between Trp8 and ThT linear arrays consistent with the experimental values of the energy transfer efficiency. The comparison of the distance ranges obtained by varying the orientation factor in the widest limits showed that the distance constraints recovered from the Trp8-ThT FRET analysis are consistent with the proposed β-strand–loop–β-strand structural model of apoA-I 1-83/G26R/W@8 fibrils and allowed us to propose that the grooves T16_Y18 and D20_L22 are the most likely candidates for the ThT binding centers [89].
6. FRET in structural analysis of fibrillized proteins
Another important aspect of amyloid research that can be addressed by FRET concerns the structural characterization of amyloid fold. In our early study we demonstrated a principal possibility of elucidating the structural peculiarities of fibrillar aggregates, in particular, determining the fractal-like dimension of amyloid fibrils [93]. The idea was to combine the FRET data from several donor-acceptor pairs and analyze them in terms of the stretched exponential model that considers the energy transfer efficiency () as a function of the dimensionality of fluorophore distribution [94]:
| (6) |
where , is the donor fluorescence lifetime in the absence of acceptor; is the volume of d-dimensional sphere of radius , is the dimensionality of fluorophore distribution (fractal-like dimension); , is the total protein concentration; is the volume of a protein molecule in a fibrillar state; is the molar concentration of bound acceptor. The examined protein-dye systems were composed of the lysozyme amyloid fibrils, the benzanthrone dye ABM, squaraine dye SQ1 and polymethine dye V2 which serve as the components of the two donor-acceptor pairs, ABM – SQ-1 and SQ-1 – V2. The approximation of the experimental FRET profiles by Eq. (6) with the orientation factor being varied in the widest possible limits (0-4) allowed us to derive the most probable sets and to recover the lower (2.2) and upper (2.9) bounds for the fractal-like dimension of fibrillar lysozyme which are indicative of the fibril heterogeneity [93].
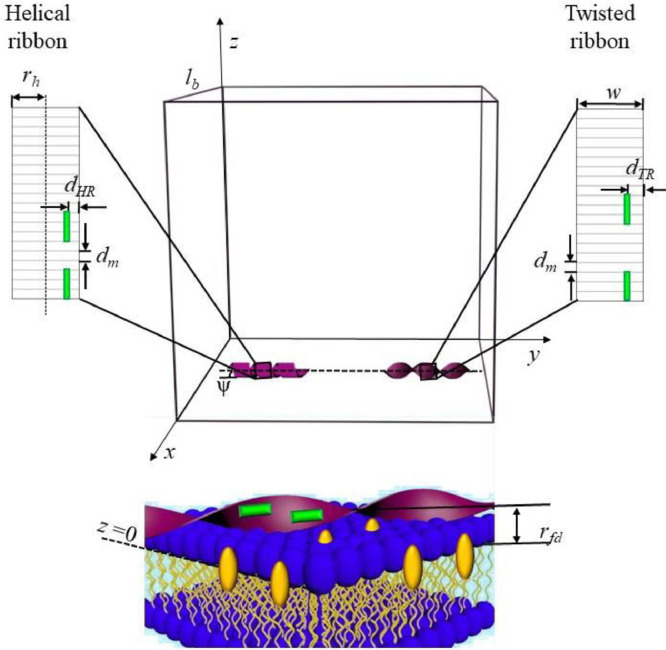
Another example of the FRET application to structural characterization of amyloid assemblies comes from our investigation of the lipid bilayer interactions of amyloid fibrils from N-terminal fragment of apoA-I 1-83/G26R/W@8 [88]. The importance of such kind of studies arises from the fact that the toxic action of amyloids is primarily targeted at cell membranes [95,96] and correlate with fibril morphology [97]. At the level of mature fibrils amyloid polymorphs may vary in their topology, forming the structures such as twisted ribbons, helical ribbons and nanotubes, as has been reported, for instance, for lysozyme [98], β-lactoglobulin [99], Aβ-peptide [100] and bovine serum albumin [101]. Likewise, the structures with helical (HR) and twisted ribbon (TR) morphologies have been revealed for the apoA-I variant 1-83/G26R/W@8 [89]. On the one hand, supramolecular structure of fibrillar assemblies control their membrane binding and toxicity, but, on the other hand, the morphological features of fibrillar fold can be altered in a lipid bilayer environment. To uncover the effects of fibril-lipid interactions on the structural features of 1-83/G26R/W@8, we measured the FRET efficiency between the membrane fluorescent probe Laurdan as a donor and ThT as an acceptor. Since ThT molecules align in the solvent-exposed grooves spanning parallel to fibril axis, the spatial distribution of this dye and, as a consequence, the FRET efficiency may be expected to depend on the topography of fibril surface. While analyzing the experimental FRET results we employed the simulation-based approach in which the donors were considered to reside in a plane parallel to the membrane surface and separated by a certain distance from the fibril axis (Fig. 8). The lengths of HR () and TR () fibrils relative to the edge length of the square simulation box () were defined by the following parameters , . In the case when fibril axis is parallel to Y-axis, the coordinates of ThT molecules distributed over the twisted ribbon (,,) or helical ribbon (,,) structures are given by:
| (7) |
| (8) |
where is the fibril width; , are the pitches of TR and HR; , are the distances between the protein monomers (along Y-axis) in TR and HR, respectively); , are the numbers of protein monomers per one acceptor molecule in TR and HR; , are the distances from ThT to the protofilament edge in the TR and HR structures; is helix radius; is the pitch angle; is a random number chosen from a uniform distribution between 0 and 1.
Fig. 8.
Schematic representations of the parameters used in the simulation-based analysis of the FRET data. ThT molecules are depicted as green rectangles, while Laurdan molecules are drawn as yellow ovals.
The eqns (7)-(8) were used to approximate the FRET profiles of the donor-acceptor pair Laudan-ThT obtained for the TR and HR polymorphs whose structural parameters were found from the AFM images of the 1-83/G26R/W@8 fibrils nm; nm; nm; ). The value of was taken as 0.47 nm, the interstrand distance in β-sheet, whereas and were derived from the ThT-fibril binding data, and . The estimates for and were obtained from the aforementioned structural model of the 1-83/G26R/W@8 fibrils [89]. The energy transfer efficiency was calculated from the donor and acceptor coordinates as:
| (9) |
where is the distance between j-th donor and i-th acceptor; , are the numbers of donors and acceptors in the simulation box given by:
| (10) |
here is the box square; is the concentration of accessible lipids related to the total lipid concentration (L) as ; is the mean area per lipid molecule taken as 0.65 nm2 for PC bilayer and 0.47 nm2 for PC/Chol bilayer; , are the molar concentrations of the membrane-bound Laurdan and ThT, respectively; is the part of Förster radius independent of the orientation factor, . This way of data analysis yielded the set of optimizing parameters providing the best agreement between the experimental and calculated values. It appeared that the experimental FRET profiles can be described by the meaningful combination of the above parameters only on the assumption that the fibril-bound acceptors are arranged along the lines parallel to the lipid bilayer surface. This implies that the fibrillar 1-83/G26R/W@8 undergoes the lipid-induced structural alterations involving the untwisting of the helical and twisted ribbons. To conclude, this example illustrates that analysis of FRET between the membrane-located donors and acceptors associated with fibril grooves can provide information not only on the lipid binding of amyloid fibrils, but also on the changes in their morphology on the membrane surface.
7. Concluding remarks
The Förster resonance energy transfer is one of the most powerful spectroscopic tools providing unique information on the structure and dynamics of a wide variety of biomolecular systems. Here we overview the results of FRET studies conducted by our laboratory over the past decade in the rapidly expanding field of amyloid research. The main outcomes of these studies can be outlined as follows:
-
(i)
It has been demonstrated that sensitivity and reliability of amyloid detection assay can be increased by using the ensembles of donor and acceptor fluorophores organized in the FRET chains, offering the advantages of large effective Stokes shift, amyloid-specific 3D fluorescence patterns and significant enhancement of the output acceptor fluorescence;
-
(ii)
An approach based on the intermolecular time-resolved and single-molecule pulse interleaved excitation FRET has been proposed and applied to quantitatively characterize the formation of oligomeric amyloid intermediates of cationic protein lysozyme on the negatively charged lipid matrix;
-
(iii)
The energy transfer involving the membrane fluorescent probes and protein-associated fluorophores has been examined to gain evidence for membrane binding of amyloid fibrils from apoA-I variants and to determine their location relative to lipid-water interface;
-
(iv)
On the example of amyloidogenic mutant of apoA-I it has been illustrated how FRET can be used to confirm the validity of the structural model of amyloid fibril;
-
(v)
It has been evidenced that FRET can be used for structural analysis of amyloid folds and monitoring of fibril restructuring on a membrane surface.
The future perspectives of the above lines of research seem to lie in: (i) applying the msFRET to detection not only of mature fibrils but also oligomeric amyloid intermediates; (ii) searching for the most effective donor and acceptor fluorophores of the FRET chain in terms of quantum yield in a fibril-bound state, spectral overlap, amyloid affinity and specificity, NIR emission of a terminal acceptor, etc.; (iii) maximizing the efficiency of msFRET by adjusting a molar ratio of the employed dyes; (iv) exploring the performance of multichromophoric ensembles under conditions of amyloid complexation with membranes, functional proteins, DNA, etc.; (v) evaluating the possibility of integrating amyloid fibrils functionalized with msFRET fluorophores into nanophotonic devices; (vi) development of simulation-based approaches allowing to extract structural information from the FRET data.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Data availability
No data was used for the research described in the article.
References
- 1.Okamoto K., Sako Y. Recent advances in FRET for the study of protein interactions and dynamics. Curr. Opin. Struct. Biol. 2017;46:16–23. doi: 10.1016/j.sbi.2017.03.010. [DOI] [PubMed] [Google Scholar]
- 2.Klein W.P., Rolczynski B.S., Oliver S.M., Zadegan R., Buckhout-White S., Ancona M.G., Cunningham P.D., Melinger J.S., Vora P.M., Kuang W., Medintz I.L., Díaz S.A. DNA origami chromophore scaffold exploiting homoFRET energy transport to create molecular photonic wires. ACS Appl. Nano Mater. 2020;3:3323–3336. doi: 10.1021/acsanm.0c00038. [DOI] [Google Scholar]
- 3.Imani M., Mohajeri N., Rastegar M., Zarghami N. Recent advances in FRET-Based biosensors for biomedical applications. Anal. Biochem. 2021;630 doi: 10.1016/j.ab.2021.114323. [DOI] [PubMed] [Google Scholar]
- 4.Pettersson K., Kyrychenko A., Ronnow E., Ljungdahl T., Martensson J., Albinsson B. Singlet energy transfer in porphyrin-based donor-bridge-acceptor systems: interaction between bridge length and bridge energy. J. Phys. Chem. A. 2006;110:310–318. doi: 10.1021/jp053819d. [DOI] [PubMed] [Google Scholar]
- 5.Andreasson J., Kyrychenko A., Martensson J., Albinsson B. Temperature and viscosity dependence of the triplet energy transfer process in porphyrin dimers. Photochem. Photobiol. Sci. 2002;1:111–119. doi: 10.1039/b108200k. [DOI] [PubMed] [Google Scholar]
- 6.Posokhov Ye., Merzlyakov M., Hristova K., Ladokhin A. A simple "proximity" correction for Förster resonance energy transfer efficiency determination in membranes using lifetime measurements. Anal. Biochem. 2008;380:134–136. doi: 10.1016/j.ab.2008.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lakowicz J.R. Springer; New York: 2006. Principles of fluorescence spectroscopy. [Google Scholar]
- 8.Algar W., Hilderbrandt N., Vogel S., Medintz I. FRET as a biomolecular research tool – understanding its potential while avoiding pitfalls. Nat. Methods. 2019;16:815–829. doi: 10.1038/s41592-019-0530-8. [DOI] [PubMed] [Google Scholar]
- 9.Kyrychenko A., Rodnin M., Ghatak C., Ladokhin A. Joint refinement of FRET measurements using spectroscopic and computational tools. Anal. Biochem. 2017;522:1–9. doi: 10.1016/j.ab.2017.01.011. [DOI] [PubMed] [Google Scholar]
- 10.Posokhov Ye., Kyrychenko A., Ladokhin A. Steady-state and time-resolved fluorescence quenching with transition metal ions as short-distance probes for protein conformation. Anal. Biochem. 2010;407:284–286. doi: 10.1016/j.ab.2010.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Szalai A.M., Siarry B., Lukin J., Giusti S., Unsain N., Cáceres A., Steiner F., Tinnefeld P., Refojo D., Jovin T.M., Stefani F.D. Super-resolution imaging of energy transfer by intensity-based STED-FRET. Nano Lett. 2021;21:2296–2303. doi: 10.1021/acs.nanolett.1c00158. [DOI] [PubMed] [Google Scholar]
- 12.Quan K., Yi C., Yang X., He X., Huang J., Wang K. FRET-based nucleic acid probes: basic designs and applications in bioimaging. Trends Anal. Chem. 2020;124 doi: 10.1016/j.trac.2019.115784. [DOI] [Google Scholar]
- 13.Pehlivan Z., Torabfam M., Kurt H., Ow-Yang C., Hildebrandt N., Yüce M. Aptamer and nanomaterial based FRET biosensors: a review on recent advances (2014-2019) Microchim. Acta. 2019;186:563. doi: 10.1007/s00604-019-3659-3. [DOI] [PubMed] [Google Scholar]
- 14.Kaur A., Dhakal S. Recent applications of FRET-based multiplexed techniques. Trends Anal. Chem. 2020;123 doi: 10.1016/j.trac.2019.115777. [DOI] [Google Scholar]
- 15.Posokhov Ye., Kyrychenko A. Location of fluorescent probes (2′-hydroxy derivatives of 2,5-diaryl-1,3-oxazole) in lipid membrane studied by fluorescence spectroscopy and molecular dynamics simulation. Biophys. Chem. 2018;235:9–18. doi: 10.1016/j.bpc.2018.01.005. [DOI] [PubMed] [Google Scholar]
- 16.Brown C.W., III, Samanta A., Díaz S.A., Buckhout-White S., Walper S.A., Goldman E.R., Medintz I.L. Dendrimeric DNA nanostructures as scaffolds for efficient bidirectional BRET–FRET cascades. Adv. Opt. Mater. 2017;5 doi: 10.1002/adom.201700181. [DOI] [Google Scholar]
- 17.Chen T., He B., Tao J., He Y., Deng H., Wang X., Zheng Y. Application of Förster resonance energy transfer (FRET) technique to elucidate intracellular and in vivo biofate of nanomedicines. Adv. Drug Deliv. Rev. 2019;143:177–205. doi: 10.1016/j.addr.2019.04.009. [DOI] [PubMed] [Google Scholar]
- 18.Ke P., Zhou R., Serpell L., Riek R., Knowles T., Lashuel H., Gazit E., Hamley I., Davis T., Fändrich M., Otzen D., Chapman M., Dobson C., Eisenberg D., Mezzenga R. Half a century of amyloids: past, present and future. Chem. Soc. Rev. 2020;49:5473–5509. doi: 10.1039/C9CS00199A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taylor A., Staniforth R. General principles underpinning amyloid structure. Front. Neurosci. 2022;16 doi: 10.3389/fnins.2022.878869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Knowles T.P., Mezzenga R. Amyloid fibrils as building blocks for natural and artificial functional materials. Adv. Mater. 2016;28:6546–6561. doi: 10.1002/adma.201505961. [DOI] [PubMed] [Google Scholar]
- 21.Serpell L.C., Sunde M., Fraser P.E., Luther P.K., Morris E.P., Sangren O., Lundgren E., Blake C.C. Examination of the structure of the transthyretin amyloid fibril by image reconstruction from electron mcrographs. J. Mol. Biol. 1995;254:113–118. doi: 10.1006/jmbi.1995.0604. [DOI] [PubMed] [Google Scholar]
- 22.Serpell L., Sunde M., Benson M., Tennent G., Pepys M., Fraser P. The protofilament substructure of amyloid fibrils. J. Mol. Biol. 2000;300:1033–1039. doi: 10.1006/jmbi.2000.3908. [DOI] [PubMed] [Google Scholar]
- 23.Goldsbury C., Goldie K., Pellaud J., Seelig J., Frey P., Muller S.A., Kistler J., Cooper G.J., Aebi U. Amyloid fibril formation from full-length and fragments of amylin. J. Struct. Biol. 2000;130:352–362. doi: 10.1006/jsbi.2000.4268. [DOI] [PubMed] [Google Scholar]
- 24.Jimenez J.L., Guijarro J.I., Orlova E., Zurdo J., Dobson C.M., Sunde M., Saibil H.R. Cryo-electron microscopy structure of an SH3 amyloid fibril and model of the molecular packing. EMBO J. 1999;18:815–821. doi: 10.1093/emboj/18.4.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shobo A., Röntgen A., Hancock M., Multhaup G. Biophysical characterization as a tool to predict amyloidogenic and toxic properties of amyloid-β42 peptides. FEBS Lett. 2022;596:1401–1411. doi: 10.1002/1873-3468.14358. [DOI] [PubMed] [Google Scholar]
- 26.Moran S., Zanni M. How to get insight into amyloid structure and formation from infrared spectroscopy. J. Phys. Chem. Lett. 2014;5:1984–1993. doi: 10.1021/jz500794d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Karamanos T.K., Kalverda A.P., Thompson G.S., Radford S.E. Mechanisms of amyloid formation revealed by solution NMR. Prog. Nucl. Magn. Reson. Spectrosc. 2015;88-89:86–104. doi: 10.1016/j.pnmrs.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hammarström P., Lindgren M., Nilsson K.P.R., Otzen D.E. Amyloid Fibrils and Prefibrillar Aggregates. 2013. Fluorescence spectroscopy as a tool to characterize amyloid oligomers and fibrils. (Ed.) [DOI] [Google Scholar]
- 29.Lee J.H., Lee I.H., Choe Y.J., Kang S., Kim H.Y., Gai W.P., Hahn J.S., Paik S.R. Real-time analysis of amyloid fibril formation of α-synuclein using a fibrillation-state-specific fluorescent probe of JC-1. Biochem. J. 2009;418:311–323. doi: 10.1042/BJ20081572. [DOI] [PubMed] [Google Scholar]
- 30.Mishra R., Sjölander D., Hammarström P. Spectroscopic characterization of diverse amyloid fibrils in vitro by the fluorescent dye Nile red. Mol. BioSyst. 2011;7:1232–1240. doi: 10.1039/c0mb00236d. [DOI] [PubMed] [Google Scholar]
- 31.Alies B., Eury H., Essassi E.M., Pratviel G., Hureau C., Faller P. Concept for simultaneous and specific in situ monitoring of amyloid oligomers and fibrils via Förster resonance energy transfer. Anal. Chem. 2014;86:11877–11882. doi: 10.1021/ac503509g. [DOI] [PubMed] [Google Scholar]
- 32.Cremades N., Cohen S.I.A., Deas E., Abramov A.Y., Chen A.Y., Orte A., Sandal M., Clarke R.W., Dunne P., Aprile F.A., Bertoncini C.W., Wood N.W., Knowles T.P.J., Dobson C.M., Klenerman D. Direct observation of the interconversion of normal and toxic forms of alpha-synuclein. Cell. 2012;149:1048–1059. doi: 10.1016/j.cell.2012.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shammas S.L., Garcia G.A., Kumar S., Kjaergaard M., Horrocks M.H., Shivji N., Mandelkow E., Knowles T.P.J., Mandelkow E., Klenerman D. A mechanistic mode of tau amyloid aggregation based on direct observation of oligomers. Nat. Commun. 2015;6:7025. doi: 10.1038/ncomms8025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kjaergaard M., Dear A.J., Kundel F., Qamar S., Meisl G., Knowles T.P.J., Klenerman D. Oligomer diversity during the aggregation of the repeat region of tau. ACS Chem. Neurosci. 2018;9:3060–3071. doi: 10.1021/acschemneuro.8b00250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kaminski Schierle G.S., Bertoncini C.W., Chan F.T.S., van der Goot A.T., Schwedler S., Skepper J., Schlachter S., van Ham T., Esposito A., Kumita J.R., Nollen E.A.A., Dobson C.M., Kaminski C.F. A FRET sensor for non-invasive imaging of amyloid formation in vivo. ChemPhysChem. 2011;12:673–680. doi: 10.1002/cphc.201000996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ruiz-Arias A., Jurado R., Fueyo-Gonzalez F., Herranz R., Galvez N., Gonzalez-Vera J.A., Orte A. A FRET pair for quantitative and superresolution imaging of amyloid fibril formation. Sens. Actuators. 2022;350:130882–130890. doi: 10.1016/j.snb.2021.130882. [DOI] [Google Scholar]
- 37.Ruiz-Arias A., Jurado R., Fueyo-Gonzalez F., Herranz R., Galvez N., Gonzalez-Vera J.A., Orte A. Selecting FRET pairs for visualizing amyloid aggregation. Results Chem. 2022;4:100275–100279. doi: 10.1016/j.rechem.2021.100275. [DOI] [Google Scholar]
- 38.Schierle G., Bertoncini C., Chan F.T.S., van der Goot A., Schwedler S., Skepper J., Schlachter S., van Ham T., Esposito A., Kumita J., Nollen E., Dobson C., Kaminski C. A FRET sensor for non-invasive imaging of amyloid formation in vivo. ChemPhysChem. 2011;12:673–680. doi: 10.1002/cphc.201000996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Takahashi T., Mihara H. FRET detection of amyloid b-peptide oligomerization using a fluorescent protein probe presenting a pseudo-amyloid structure. Chem. Commun. 2012;48:1568–1570. doi: 10.1039/C1CC14552E. [DOI] [PubMed] [Google Scholar]
- 40.Han J., Mei E., Kung M.-P., Kung H., Yuan J.-M., Dai H.-L. Single-molecule fluorescence resonance energy transfer studies of β-amyloid clusters in physiological solutions. Biochem. Biophys. Prot. Aggreg. 2017:297–311. doi: 10.1142/9789813202382_0008. [DOI] [Google Scholar]
- 41.Lee H., Lee Y., Kang J., Yang S., Kim J., Ghisaidoobe A., Kang H., Lee S.-R., Lim M., Chung S. Monitoring metal-amyloid-β complexation by a FRET-based probe: design, detection, and inhibitor screening. Chem. Sci. 2019;10:1000–1009. doi: 10.1039/C8SC04943B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Katina N., Mikhaylina A., Ilina N., Eliseeva I., Balobanov V. Near-wall aggregation of amyloidogenic Aβ 1-40 peptide: direct observation by the FRET. Molecules. 2021;26:7590–7597. doi: 10.3390/molecules26247590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sulatskaya A.I., Rychkov G.N., Sulatsky M.I., Mikhailova E.V., Melnikova N.M., Andozhskaya V.S., Kuznetsova I.M., Turoverov K.K. New evidence on a distinction between Aβ40 and Aβ42 amyloids: Thioflavin T binding modes, clustering tendency, degradation resistance, and cross-seeding. Int. J. Mol. Sci. 2022;23:5513. doi: 10.3390/ijms23105513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Groenning M. Binding mode of Thioflavin T and other molecular probes in the context of amyloid fibrils-current status. J. Chem. Biol. 2010;3:1–18. doi: 10.1007/s12154-009-0027-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yakupova E., Bobyleva L., Vikhlyantsev I., Bobylev A. Congo Red and amyloids: history and relationship. Biosci. Rep. 2019;39 doi: 10.1042/BSR20181415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schutz A.K., Soragni A., Hornemann S., Aguzzi A., Ernst M., Bockmann A., Meier B. The amyloid-Congo Red interface at atomic resolution. Angew. Chem. Int. Ed. 2011;50:5956–5960. doi: 10.1002/anie.201008276. [DOI] [PubMed] [Google Scholar]
- 47.Sinnige T. Molecular mechanisms of amyloid formation in living systems. Chem. Sci. 2022;13:7070–7097. doi: 10.1039/D2SC01278B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hammarstrom P., Lindgren M., Nilsson P. Amyloid Fibrils and Prefibrillar Aggregates: Molecular and Biological Properties. 2013. Fluorescence spectroscopy as a tool to characterize amyloid oligomers and fibrils; pp. 211–240. [DOI] [Google Scholar]
- 49.Zhang Y., Ding C., Li C., Wang X. Advances in fluorescent probes for detection and imaging of amyloid-β peptides in Alzheimer's disease. Adv. Clin. Chem. 2021;103:135–190. doi: 10.1016/bs.acc.2020.08.008. [DOI] [PubMed] [Google Scholar]
- 50.Lee D., Kim S., Kim H., Kim Y. Fluorescence chemicals to detect insoluble and soluble amyloid-β aggregates. ACS Chem. Neurosci. 2019;10:2647–2657. doi: 10.1021/acschemneuro.9b00199. [DOI] [PubMed] [Google Scholar]
- 51.Jun Y.W., Cho S.W., Jung J., Huh Y., Kim Y., Kim D., Ahn K.H. Frontiers in probing Alzheimer's disease biomarkers with fluorescent small molecules. ACS Cent. Sci. 2019;5:209–217. doi: 10.1021/acscentsci.8b00951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Naiki H., Higuchi K., Hosokawa M., Takeda T. Fluorometric determination of amyloid fibrils in vitro using the fluorescent dye, Thioflavin T. Anal. Biochem. 1989;177:244–249. doi: 10.1016/0003-2697(89)90046-8. [DOI] [PubMed] [Google Scholar]
- 53.LeVine H. Thioflavin T Interaction with synthetic Alzheimer's disease Aβ - amyloid peptides: detection of amyloid aggregation in solution. Protein Sci. 1993;2:404–410. doi: 10.1002/pro.5560020312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sulatskaya A.I., Maskevich A.A., Kuznetsova I.M., Uversky V.N., Turoverov K.K. Fluorescence quantum yield of Thioflavin T in rigid isotropic solution and incorporated into the amyloid fibrils. PLOS One. 2010;5:e15385. doi: 10.1371/journal.pone.0015385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stsiapura V.I., Maskevich A.A., Kuznetsova I.M. Computational study of thioflavin T torsional relaxation in the excited state. J. Phys. Chem. A. 2007;111:4829–4835. doi: 10.1021/jp070590o. [DOI] [PubMed] [Google Scholar]
- 56.Hawe A., Sutter M., Jiskoot W. Extrinsic fluorescent dyes as tools for protein characterization. Pharm. Res. 2007;25:1487–1499. doi: 10.1007/s11095-007-9516-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Klunk W.E., Pettegrew J.W., Abraham D.J. Quantitative evaluation of Congo Red binding to amyloid-like proteins with a beta-pleated sheet conformation. J. Histochem. Cytochem. 1989;37:1273–1281. doi: 10.1177/37.8.2666510. [DOI] [PubMed] [Google Scholar]
- 58.Howie A.J., Brewer D.B. Optical properties of amyloid stained by Congo red: history and mechanisms. Micron. 2009;40:285–301. doi: 10.1016/j.micron.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 59.Girych M., Gorbenko G., Maliyov I., Trusova V., Mizuguchi C., Saito H., Kinnunen P. Combined thioflavin T – Congo red fluorescence assay for amyloid fibril detection. Methods Appl. Fluor. 2016;4 doi: 10.1088/2050-6120/4/3/034010. [DOI] [PubMed] [Google Scholar]
- 60.Zhytniakivska O., Kurutos A., Shchuka M., Vus K., Tarabara U., Trusova V., Gorbenko G. Fӧrster resonance energy transfer between Thioflavin T and unsymmetrical trimethine cyanine dyes on amyloid fibril scaffold. Chem. Phys. Lett. 2021;785 doi: 10.1016/j.cplett.2021.139127. [DOI] [Google Scholar]
- 61.Modi S., Nizak C., Surana S., Halder S., Krishnan Y. Two DNA nanomachines map pH changes along intersecting endocytic pathways inside the same cell. Nat. Nanotechnol. 2013;8:459–467. doi: 10.1038/nnano.2013.92. [DOI] [PubMed] [Google Scholar]
- 62.Zhao Y-X., Shaw A., Zeng X., Benson E., Nyström A.M., Högberg B. DNA origami delivery system for cancer therapy with tunable release properties. ACS Nano. 2012;6:8684–8691. doi: 10.1021/nn3022662. [DOI] [PubMed] [Google Scholar]
- 63.Chen T., Wu C.S., Jimenez E., Zhu Z., Dajac J.G., You M.X., Han D., Zhang X.B., Tan W.H. DNA micelle flares for intracellular mRNA imaging and gene therapy. Angew. Chem. Int. Ed. 2013;52:2012–2016. doi: 10.1002/anie.201209440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Buckhout-White S., Spillmann C.M., Algar W.R., Khachatrian A., Melinger J.S., Goldman E.R., Ancona M.G., Medintz I.L. Assembling programmable FRET-based photonic networks using designer DNA scaffolds. Nat. Commun. 2014;5:5615. doi: 10.1038/ncomms6615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang F., Lu C.H., Willner I. From cascaded catalytic nucleic acids to enzyme-DNA nanostructures: controlling reactivity, sensing, logic operations, and assembly of complex structures. Chem. Rev. 2014;114:2881–2941. doi: 10.1021/cr400354z. [DOI] [PubMed] [Google Scholar]
- 66.Spillmann C.M., Buckhout-White S., Oh E., Goldman E.R., Anconac M.G., Medintz I.L. Extending FRET cascades on linear DNA photonic wires. Chem. Commun. 2014;50:7246–7249. doi: 10.1039/C4CC01072H. [DOI] [PubMed] [Google Scholar]
- 67.Baldo M.A., Thompson M.E., Forrest S.R. High-efficiency fluorescent organic light-emitting devices using a phosphorescent sensitizer. Nature. 2000;403:750–753. doi: 10.1038/35001541. [DOI] [PubMed] [Google Scholar]
- 68.Boeneman K., Prasuhn D.E., Blanco-Canosa J.B., Dawson P.E., Melinger J.S., Ancona M., Stewart M.H., Susumu K., Huston A., Medintz I.L. Self-assembled quantum dot-sensitized multivalent DNA photonic wires. J. Am. Chem. Soc. 2010;132:18177–18190. doi: 10.1021/ja106465x. [DOI] [PubMed] [Google Scholar]
- 69.Afzal S., Lone M.S., Bhat P.A., Dar A.A. Multi-step fluorescence resonance energy transfer between the fluorophores via cosolubilization in cationic, anionic and non-ionic micelles. J. Photochem. Photobiol. 2018;365:220–231. doi: 10.1016/j.jphotochem.2018.08.002. [DOI] [Google Scholar]
- 70.Brown C.W., III, Samanta A., Díaz S.A., Buckhout-White S., Walper S.A., Goldman E.R., Medintz I.L. Dendrimeric DNA Nanostructures as scaffolds for efficient bidirectional BRET–FRET cascades. Adv. Opt. Mater. 2017;5 doi: 10.1002/adom.201700181. [DOI] [Google Scholar]
- 71.Algar W.R., Wegner D., Huston A.L., Blanco-Canosa J.B., Stewart M.H., Armstrong A., Dawson P.E., Hildebrandt N., Medintz I.L. Quantum dots as simultaneous acceptors and donors in time-gated Förster resonance energy transfer relays: characterization and biosensing. J. Am. Chem. Soc. 2012;134:1876–1891. doi: 10.1021/ja210162f. [DOI] [PubMed] [Google Scholar]
- 72.Gorbenko G., Trusova V., Deligeorgiev T., Gadjev N., Mizuguchi C., Saito H. Two-step FRET as a tool for probing the amyloid state of proteins. J. Mol. Liq. 2019;294 doi: 10.1016/j.molliq.2019.111675. [DOI] [Google Scholar]
- 73.Rosenson R.S., Brewer Jr H.B., Davidson W.S., Fayad Z.A., Fuster V., Goldstein J., Hellerstein M., Jiang X., Phillips M.C., Rader D.J., Remaley A.T., Rothblat G.H., Tall A.R., Yvan-Charvet L. Cholesterol efflux and atheroprotection: advancing the concept of reverse cholesterol transport. Circulation. 2012;125:1905–1919. doi: 10.1161/CIRCULATIONAHA.111.066589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gursky O., Mei X., Atkinson D. The crystal structure of the C-terminal truncated apolipoprotein A-I sheds new light on amyloid formation by the N-terminal fragment. Biochemistry. 2012;51:10–18. doi: 10.1021/bi2017014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rowczenio D., Dogan A., Theis J.D., Vrana J.A., Lachmann Ashutosh H.J., Wechalekar D., Gilbertson J.A., Hunt T., Gibbs S.D., Sattianayagam P.T., Pinney J.H., Hawkins P.N., Gillmore J.D. Amyloidogenicity and clinical phenotype associated with five novel mutations in apolipoprotein A-I. Am. J. Pathol. 2011;179:1978–1987. doi: 10.1016/j.ajpath.2011.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tarabara U., Kirilova E., Kirilov G., Vus K., Zhytniakivska O., Trusova. G. Gorbenko V. Benzanthrone dyes as mediators of cascade energy transfer in insulin amyloid fibrils. J. Mol. Liq. 2021;324 doi: 10.1016/j.molliq.2020.115102. [DOI] [Google Scholar]
- 77.Gorbenko G., Zhytniakivska O., Vus K., Tarabara U., Trusova V. Three-step Förster resonance energy transfer on an amyloid fibril scaffold. Phys. Chem. Chem. Phys. 2021;23:14746–14754. doi: 10.1039/D1CP01359A. [DOI] [PubMed] [Google Scholar]
- 78.Li M., Reddy L.G., Bennett R., Silva N., Larry J., Jones R., Thomas D. A fluorescence energy transfer method for analyzing protein oligomeric structure: application to phospholamban. Biophys. J. 1999;76:2587–2599. doi: 10.1016/S0006-3495(99)77411-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.John E., Jähnig F. Aggregation state of melittin in lipid vesicle membranes. Biophys. J. 1991;60:319–328. doi: 10.1016/S0006-3495(91)82056-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kapanidis A., Lee N., Laurence T., Doose S., Margeat E., Weiss A. Fluorescence-aided molecule sorting: analysis of structure and interactions by alternating-laser excitation of single molecule. Proc. Natl. Acad. Sci. USA. 2004;101:8936–8941. doi: 10.1073/pnas.0401690101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lee N., Kapanidis A., Wang Y., Michalet Y., Mukhopadhyay J., Ebright R., Weiss S. Accurate FRET measurements within single diffusing biomolecules using alternating-laser excitation. Biophys. J. 2005;88:2939–2953. doi: 10.1529/biophysj.104.054114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhang X., Hu Y., Yang X., Tang Y., Han S., Kang A., Deng H., Chi Y., Zhu D., Lu Y. Fӧrster resonance energy transfer (FRET)-based biosensors for biological applications. Biosens. Bioelectron. 2019;138 doi: 10.1016/j.bios.2019.05.019. [DOI] [PubMed] [Google Scholar]
- 83.Szabó Á., Szendi-Szatmári T., Szöllősi J., Nagy P. Quo vadis FRET? Förster's method in the era of superresolution. Methods Appl. Fluoresc. 2020;8 doi: 10.1088/2050-6120/ab9b72. [DOI] [PubMed] [Google Scholar]
- 84.Gorbenko G., Trusova V., Molotkovsky J. Förster resonance energy transfer study of cytochrome c-lipid interactions. J. Fluoresc. 2018;28:79–88. doi: 10.1007/s10895-017-2176-1. [DOI] [PubMed] [Google Scholar]
- 85.Fung B., Stryer L. Surface density determination in membranes by fluorescence energy transfer. Biochemistry. 1978;17:5241–5248. doi: 10.1021/bi00617a025. [DOI] [PubMed] [Google Scholar]
- 86.Gorbenko G., Handa T., Saito H., Molotkovsky J., Tanaka M., Egashira M., Nakano M. Effect of cholesterol on bilayer location of the class A peptide Ac-18A-NH2 as revealed by fluorescence resonance energy transfer. Eur. Biophys. J. 2003;32:703–709. doi: 10.1007/s00249-003-0333-8. [DOI] [PubMed] [Google Scholar]
- 87.Dale R, Eisinger J, Blumberg W. The orientational freedom of molecular probes. The orientation factor in intramolecular energy transfer. Biophys. J. 1979;26:161–194. doi: 10.1016/S0006-3495(79)85243-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gorbenko G., Trusova V., Girych M., Adachi E., Mizuguchi C., Akaji K., Saito H. FRET evidence for untwisting of amyloid fibrils on the surface of model membranes. Soft Matter. 2015;11:6223–6234. doi: 10.1039/C5SM00183H. [DOI] [PubMed] [Google Scholar]
- 89.Girych M., Gorbenko G., Trusova V., Adachi E., Mizuguchi C., Nagao K., Kawashima H., Akaji K., Phillips M., Saito H. Interaction of Thioflavin T with amyloid fibrils of apolipoprotein A-I N-terminal fragment: resonance energy transfer study. J. Struct. Biol. 2014;185:116–124. doi: 10.1016/j.jsb.2013.10.017. [DOI] [PubMed] [Google Scholar]
- 90.Lagerstedt J., Cavigiolio G., Roberts L., Hong H., Jin L., Fitzgerald P., Oda M., Voss J. Mapping the structural transition in an amyloidogenic apolipoprotein A-I. Biochemistry. 2007;46:9693–9699. doi: 10.1021/bi7005493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Biancalana M., Makabe K., Koide A., Koide S. Molecular mechanism of Thioflavin-T binding to the surface of beta-rich peptide self-assemblies. J. Mol. Biol. 2009;385:1052–1063. doi: 10.1016/j.jmb.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wu C., Bowers M., Shea J. On the origin of the stronger binding of PIB over Thioflavin T to protofibrils of the Alzheimer amyloid-β peptide: a molecular dynamics study. Biophys. J. 2011;100:1316–1324. doi: 10.1016/j.bpj.2011.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gorbenko G., Trusova V., Kirilova E., Kirilov G., Kalnina I., Vasilev A., Kaloyanova S., Deligeorgiev T. New fluorescent probes for detection and characterization of amyloid fibrils. Chem. Phys. Lett. 2010;495:275–279. doi: 10.1016/j.cplett.2010.07.005. [DOI] [Google Scholar]
- 94.Drake J., Klafter J., Levitz P. S Chemical and biological microstructures as probed by dynamic processes. Science. 1991;251:1574–1579. doi: 10.1126/science.201173. [DOI] [PubMed] [Google Scholar]
- 95.Bucciantini M., Rigacci S., Stefani M. Amyloid aggregation: role of biological membranes and the aggregate–membrane system. J. Phys. Chem. Lett. 2014;5:517–527. doi: 10.1021/jz4024354. [DOI] [PubMed] [Google Scholar]
- 96.Butterfield S., Lashuel H. Amyloidogenic protein–membrane interactions: mechanistic insight from model systems. Angew. Chem. Int. Ed. 2010;49:5628–5654. doi: 10.1002/anie.200906670. [DOI] [PubMed] [Google Scholar]
- 97.Petkova A., Leapman R., Guo Z., Yau W., Mattson M., Tycko R. Self-propagating, molecular-level polymorphism in Alzheimer's ß-amyloid fibrils. Science. 2005;307:262–265. doi: 10.1126/science.1105850. [DOI] [PubMed] [Google Scholar]
- 98.Lara C., Adamcik J., Jordens S., Mezzenga R. General Self-assembly mechanism converting hydrolyzed globular proteins into giant multistranded amyloid ribbons. Biomacromolecules. 2011;12:1868–1875. doi: 10.1021/bm200216u. [DOI] [PubMed] [Google Scholar]
- 99.Adamcik J., Jung J., Flakowski J., De Los Rios P., Dietler G., Mezzenga R. Understanding amyloid aggregation by statistical analysis of atomic force microscopy images. Nat. Nanotechnol. 2010;5:423–428. doi: 10.1038/nnano.2010.59. [DOI] [PubMed] [Google Scholar]
- 100.Adamcik J., Castelletto V., Bolisetty S., Hamley I.W., Mezzenga R. Direct observation of time-resolved polymorphic states in the self-assembly of end-capped heptapeptides. Angew. Chem. Int. Ed. 2011;50:5495–5498. doi: 10.1002/ange.201100807. [DOI] [PubMed] [Google Scholar]
- 101.Usov I., Adamcik J., Mezzenga R. Polymorphism complexity and handedness inversion in serum albumin amyloid fibrils. ACS Nano. 2013;7:10465–10474. doi: 10.1021/nn404886k. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.