Highlights
-
•
mTOR and AKG are involved in the regulation of energy homeostasis.
-
•
An imbalance of mTOR signaling is correlated with neurological disorders.
-
•
AKG metabolism could overlap with mTOR signaling via ATP synthase inhibition.
-
•
AKG may activate mTOR by replenishing the TCA-intermediate pool.
-
•
Modulation of mTOR activity with AKG may increase the effectiveness of therapeutic strategies in brain disorders treatment.
Keywords: mTOR, α-ketoglutarate (AKG), Metabolism, Autophagy, Aging, Neurodegeneration, Neuroprotection
Abstract
Cerebral disorders are largely associated with impaired cellular metabolism, despite the regulatory mechanisms designed to ensure cell viability and adequate brain function. Mechanistic target of rapamycin (mTOR) signaling is one of the most crucial factors in the regulation of energy homeostasis and its imbalance is linked with a variety of neurodegenerative diseases. Recent advances in the metabolic pathways’ modulation indicate the role of α-ketoglutarate (AKG) as a major signaling hub, additionally highlighting its anti-aging and neuroprotective properties, but the mechanisms of its action are not entirely clear. In this review, we analyzed the physiological and pathophysiological aspects of mTOR in the brain. We also discussed AKG’s multifunctional properties, as well as mTOR/AKG-mediated functional communications in cellular metabolism. Thus, this article provides a broad overview of the mTOR/AKG-mediated signaling pathways, in the context of neurodegeneration and endogenous neuroprotection, with the aim to find novel therapeutic strategies.
1. Introduction
Understanding the neurodegenerative mechanisms of various genesis and the search for effective neuroprotection, remain relevant to the present day. Most brain diseases are accompanied by metabolic changes caused by circulatory failure, defects of protein synthesis/degradation machinery and/or dysregulation of signaling pathways. Depending on the degree, this can lead to both cognitive impairment and critical manifestations of diseases (stroke, Alzheimer’s disease (AD), Parkinson’s disease (PD), etc.). The pathogenesis involves such mechanisms as ion imbalance, glutamate excitotoxicity, oxidative stress, disruption of the blood-brain barrier, inflammation, etc. [1,2].
As a central regulator of metabolic homeostasis and protein synthesis/degradation mTOR (mammalian/mechanistic target of rapamycin) signaling is vital for maintaining cell viability and adequate brain function, hence its imbalance is associated with a broad spectrum of diseases (stroke, cancer, epilepsy, aging, etc.) [3,4]. In the context of neurodegenerative/neuroprotective mechanisms, the role of mTOR-modulated autophagy has become the subject of several recent studies [5,6]. The modulation of autophagy is considered an auspicious direction for the possible treatment of neurodegeneration.
One of the promising protective agents for confronting brain disorders is a multifunctional molecule involved in numerous metabolic and cellular pathways, α-ketoglutarate (AKG). Due to its diverse protective functions, AKG is already applied in therapy, including its usage in brain function improvement [7]. It has been shown to play a key role in maintaining the energy balance of the cell, utilization of reactive oxygen species (ROS), amino acid metabolism and ammonia homeostasis, as well as in cell survival during hypoxia [8]. By converting glutamate into AKG, glutamate dehydrogenase involves an additional amount of AKG into the Krebs cycle, thereby increasing the functional activity of mitochondria and promoting cell survival [9]. Moreover, AKG functions as a signaling molecule, as well as a regulator of epigenetic processes and cellular signaling via protein binding [10].
In this review, we summarized the advances in the mTOR and AKG research fields and their impact on cellular metabolism. Then, we discussed the overlap between AKG metabolism and mTOR signaling to promote the understanding of their metabolic pathways in the brain in order to improve the treatment efficacy for a wide range of neurodegenerative diseases.
2. mTOR and its signaling network in the brain
2.1. mTOR organization and function under physiological conditions in the brain
mTOR is a 289 kDa serine/threonine protein kinase, which belongs to the phosphatidylinositol-3-kinase-related kinase (PIKK) family. It is evolutionarily highly conserved and ubiquitously expressed throughout the body, including neural cells. It assembles two different multiprotein complexes: mTOR complex 1 (mTORC1) or mTOR complex 2 (mTORC2). As the name of mTOR implies, it is a target of a molecule named rapamycin, a macrolide antibiotic compound produced by the bacterium Streptomyces hygroscopicus, which was isolated in a soil sample from Easter Island (Rapa Nui in Polynesian) [3].
In the brain, mTOR not only controls basic cellular functions such as protein synthesis, energy metabolism, proliferation, migration, lipid metabolism, autophagy, mitochondria and lysosome biogenesis as it does in peripheral tissues, but it is also involved in more specific processes such as dendritic spine growth, axonal sprouting, axonal regeneration and myelination, ionic and receptor channel expression. mTOR-controlled signaling pathways in neurons and glial cells regulate higher physiological functions of the nervous system including neuronal excitability, neuronal survival, synaptic plasticity, memory storage, cognition, feeding, and control of circadian rhythm. Thus, any mutations in genes encoding mTOR regulators, or deregulated expression of proteins implicated in the mTOR pathway, are potentially involved in brain diseases [11,12].
Structurally, mTOR contains several functional domains, including C-carboxyterminal FAT domain (FATC), C-terminal kinase domain (KD), FKBP12 rapamycin-binding domain (FRB), the FAT domain (FKBP-associated protein/ataxia-telangiectasia mutated/transactivation-transformation domain-associated protein), and an N-terminal domain with at least 20 HEAT (Huntingtin elongation factor 3 A subunit of PP2A TOR1) repeats. The last provides sites for the protein interaction between mTOR complex with Raptor (regulatory-associated protein with mTOR) or Rictor (rapamycin-insensitive companion of mTOR). It is the association with either Raptor or Rictor that determines whether mTOR is a component of mTORC1 or mTORC2. mTOR complexes share four components that are identical: (1) a catalytic subunit (mTOR itself), (2) a small protein known as mLSt8 (mammalian lethal with SEC13 protein 8), (3) the Tti1/Tel2 (Tel two-interacting protein 1/ telomere maintenance 2) associated regulatory proteins, which create a scaffold for recruitment of substrates; and (4) the negative regulator Deptor (DEP domain-containing mTOR-interacting protein), which inhibits the substrate binding. mTORC1 contains the scaffold protein Raptor and the inhibitory subunit PRAS40 (proline-rich Akt substrate 40 kDa) as key components, and mTORC2 specifically associates with the regulatory subunit Protor 1/2, scaffold proteins Rictor, and mSIN1, which help the complex assembly [13]. Noteworthy, mTORC2 was initially described to be insensitive to rapamycin inhibition in contrast to mTORC1 [14], but later it was uncovered that prolonged exposure to this macrolide resulted in the disruption of the mTORC2 assembly and integrity, thereby causing the functional inhibition of the complex [15]. mTORCs are considered to be cellular energy sensors, and as such, they act as signal convergence centers from extra- and intra-cellular “energetic factors”. In nutrient-rich conditions, mTOR is activated through phosphorylation of its specific residues (e.g., phosphorylation of Raptor through several pathways that involve the protein Ras homolog enriched in brain (Rheb)) stimulating anabolic processes such as translation, transcription, lipid synthesis and inhibiting catabolic processes. In contrast, nutrient-deficient conditions can down-regulate mTOR activity inducing protein degradation and autophagy in order to maintain minimal biological processes for survival. Moreover, mTORC1 activity is modulated by multiple pathways based on the presence of trophic and growth factors, nutrients (especially amino acids and glucose), and oxygen, while mTORC2 is regulated mainly by growth factors, hormones, and neurotransmitters via the PI3K (phosphatidylinositol-3-kinase) activity upregulation [16], [17], [18]. Although, knowledge about the regulation of mTORC2 in the central neural system is still limited, in several studies mTORC2 was shown to play an important role in maintaining the actin cytoskeleton and morphological regulation of actin-rich dendritic spines. Neuronal mTORC2 is activated by neurotrophins, glutamate, N-methyl-D-aspartate (NMDA), inducers of long-term potentiation (LTP), and is involved in synaptic plasticity and memory modulation [12,19,20].
2.1.1. Upstream regulation of mTOR pathway
Extracellular (e.g., brain-derived neurotrophic factor (BDNF), netrin 1, reelin, insulin, insulin-like growth factor 1 (IGF1), vascular endothelial growth factor (VEGF) and ciliary neurotrophic factor (CNTF), glutamate, dopamine, serotonin, acetylcholine, orexin) and intracellular stimuli are usually conveyed through mTOR via PI3K-protein kinase B (Akt)-tuberous sclerosis complex (TSC)-Rheb signaling (Fig. 1). The binding of the effector molecules to the tyrosine kinase receptors (RTKs), or to G-protein-coupled receptors (GPCRs) leads to the subsequent activation of the kinases PI3K and Akt with involvement of the second messenger – phosphatidylinositol-3,4,5-phosphate (PIP3). PI3K-Akt signaling phosphorylates and inhibits the TSC, a trimeric complex formed by TSC1 (hamartin), TSC2 (tuberin), and the scaffold protein TSC1D7. TSC is the GTPase-activating protein (GAP) for the small GTPases Rheb and Rhes (a form of Rheb specifically expressed in the striatum). Rheb is present in an inactive or active state by binding to GDP or GTP, respectively. Rheb-GTP activates mTORC1 by directly interacting with mTOR [11,12,21].
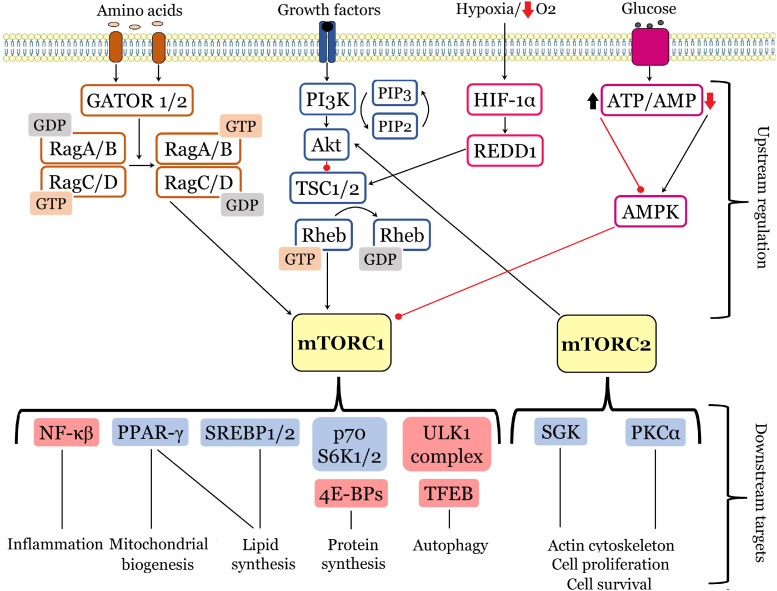
Fig. 1.
mTOR signaling network. At the cellular level, mTOPC1/mTOPC2 integrate signals from multiple stimuli. The top diagram illustrates the upstream regulation of mTOR by basic stimuli: amino acids (signaling pathway indicated by orange boxes), growth factors (blue boxes), oxygen levels (pink boxes), and energy status (purple boxes). Downstream targets upregulated by mTOR activity are marked with a blue field, whereas those downregulated – are with a red field. Black arrows and red lines respectively represent positive and negative regulation.
The levels of amino acids are sensed with the recruitment of heterodimers of Rag small GTPases (RagA/B and RagC/D). These GTPases are retained inactive by a lysosome-bound complex “Ragulator” and controlled by two multiprotein complexes GATOR1 and GATOR2. Amino acid accessibility allows the active conformation of Rag to bind directly to Raptor and cause the recruitment of mTORC1 to the lysosomal membrane [22].
Nutrient deprivation and hypoxia inhibit mTORC1 signaling, thus cells adapt to limit energy consumption. A decrease in glucose supply causes a rapid reduction in intracellular ATP levels, leading to 5′-AMP-activated protein kinase (AMPK) activation through its phosphorylation by LKB1 tumor suppression kinase. AMPK serves as a cellular sensor of ADP/ATP and AMP/ATP ratios, consequently when these ratios are high AMPK inhibits mTORC1 activity through phosphorylation on two targets, namely TSC2 and Raptor. AMPK phosphorylates TSC2 increasing its GAP activity toward Rheb, promoting the inactive state of Rheb-GDP, and inducing the inhibition of mTORC1 activity. Raptor phosphorylation leads to its association with 14-3-3 protein and subsequent allosteric inhibition of mTORC1 [11,18].
Additionally, hypoxia induces an increase in the expression of regulated DNA damage and development 1 protein (REDD1; also known as RTP801/DDIT4), which is controlled by the transcription factor hypoxia-inducible factor 1 (HIF-1). REDD1 can activate the TSC1/TSC2 complex by competing with TSC2 for 14-3-3 protein binding, stabilizing the interaction between TSC1 and TSC2 and inducing mTORC1 inhibition [18]. Furthermore, it was shown that activation of REDD1 and mTORC1 could be induced by metformin (antidiabetic type 2 drug), which in addition to its antitumoral effects, exerts neuroprotective effects in neurodegenerative diseases such as Alzheimer’s and Huntington’s diseases [18,23].
2.1.2. Downstream outputs of mTORCs
The main downstream cellular functions of mTORC1 and mTORC2 include protein synthesis and degradation, lipid synthesis, autophagy, lysosome biogenesis, energy metabolism, cell survival and cytoskeleton rearrangements (Fig. 1). In response to growth signals, mTOR phosphorylates and activates/inhibits several translation regulators, including eukaryotic translation initiation factor 4E (eIF4E)-binding proteins (4E-BPs) and the p70 ribosomal S6 kinase 1 and 2 (p70 S6K1/2), which in turn promote mRNA biogenesis and activates the 5′-cap-dependent protein translation. S6K1 phosphorylates the ribosomal protein S6, eukaryotic elongation factor-2 kinase (eEF2K), eIF4B, S6K1 Aly/REF (SKAR)-like substrate, a cell growth regulator, and CBP80 (cap-binding protein 80), thus regulating translation initiation or elongation [11,22,24]. mTOR also regulates local protein synthesis, which is essential for neuronal development. mTOR and p70 S6K are necessary for the local translation of Tau protein and collapsin response mediator protein 2 (CRMP2), which contribute to neuronal polarization. During the navigation of axonal growth cones mTOR-dependent local translation is required for the proper response to chemoattractants and chemorepellents [20]. mTORC1 controls lipid metabolism, a key aspect during axonal growth, myelination and dendritic arborization as well as in nutrient-deficient conditions, when glucose is lacking. mTORC1 stimulates de novo lipogenesis via phosphorylation by S6K1 of sterol regulatory element-binding proteins 1 and 2 (SREBP1/2), which serve as transcription factors for numerous genes involved in fatty acid and cholesterol synthesis. Additionally, mTORC1 activates proliferator-activated receptor-γ (PPAR-γ) involved in adipogenesis and modulates lipin 1 localization to suppress its inhibitory effects on SREBP1/2 [22,25].
Nucleotide synthesis as well could be enhanced by mTOR signaling pathway through increasing ATF-dependent expression of MTHFD2, the key enzyme in mitochondrial tetrahydrofolate (mTHF) cycle. mTORC1 also stimulates glycolysis and glucose uptake through modulating the transcription factor hypoxia-inducible factor (HIF-1α) [25,26].
A key process of damaged cellular components and protein degradation – autophagy – is regulated by a crosstalk between mTOR and AMPK signaling pathways. Thus, mTOR activation leads to macroautophagy and mitophagy inhibition, whereas mTOR inhibition and AMPK activation trigger protein degradation. Due to its homeostatic role in cellular physiology, autophagy has been associated with a wide spectrum of metabolic and neurodegenerative disorders [17,27].
In nutrient-rich conditions, active mTORC1 controls autophagy through inhibition of the unc-51-like kinase 1 (ULK1) complex, which consists of several autophagy-related proteins (ULK1, Atg13, FIP200), by its phosphorylation. Therefore, inhibition of mTORC1 by rapamycin leads to dephosphorylation of ULK1, which initiates autophagy. In contrast, in nutrient-deficient conditions, reduced mTORC1 and enhanced AMPK activity stimulates autophagy, which leads to the elimination of proteins and organelles to compensate for nutrient starvation [27]. Active ULK1 phosphorylated by AMPK, in its turn, phosphorylates and activates Beclin-1, which is also a part of a protein complex PI3KC3 (also called the VPS34 complex). Beclin-1 promotes the lipidation of cytosolic LC3-I to generate LC3-II. LC3-II localizes to the autophagosome membrane, enabling elongation of the limiting membrane of the phagophore, which seals to form LC3-II (+) autophagosomes. Autophagosomes degrade cargo adaptors such as p62, and subsequently deliver proteins and organelles to lysosomes for degradation [28]. mTORC2 is reported to participate in neuronal morphology rearrangements, specifically in the actin cytoskeleton remodeling, by controlling the activity of Akt, serine/threonine-protein kinase 1 (SGK1), and protein kinase Cα (PKCα). Moreover, mTORC2 controls the integrity of mitochondrion-associated membranes (MAMs), which facilitate the transfer of calcium and lipids between the endoplasmic reticulum and mitochondria [17,20,29].
2.2. mTOR-related pathologies of the nervous system
Due to mTOR diverse functions in cellular metabolism and homeostasis, dysregulation of mTOR signaling pathway has been implicated in various diseases. The list of diseases includes both mental illnesses (depression, mental retardation, schizophrenia, Down syndrome, autistic disorders, etc.) and neurological disorders (such as stroke, epilepsy, PD, AD, injuries and tumors of the brain) [11,17].
In majority of cases, it is an increase in mTOR activity or mTOR-dysregulated autophagy that are responsible for the progression of pathology. Thus, to reverse the symptoms of pathologies in animal models and some preclinical trials mTOR inhibitor – rapamycin was applied. A systematic review and meta-analysis of animal model studies indicated that low-dose rapamycin treatment may be an effective therapeutic option for stroke [30]. Rapamycin and rapalogs were also shown to protect against toxicity created by several misfolded proteins including alpha-synuclein, TDP43, and hyperphosphorylated tau. In tuberous sclerosis clinical studies, it was pointed out that rapamycin treatment reduced subependymal giant cell astrocytomas (SEGAs), specific brain tumors associated with this disease, and white matter abnormalities. However, direct or indirect stimulation of mTORC1 can be beneficial for other pathologies such as Rett syndrome, some forms of PD, central nerve injury and depression [11,31].
There are available studies concerning the role of mTOR signaling pathway in epileptogenesis. Even in the absence of any other associated pathology seizures themselves increase mTORC1 activity. Therefore, it was investigated in organotypic hippocampal cultures that posttraumatic epileptogenesis can be blocked by a transient inhibition of mTORC1 [32].
Oxygen-poor and nutrient-deficient conditions caused by ischemia modulate mTOR activities via all upstream pathways resulting in dysregulation of mTORCs. However, it is worth noting that the effects of cerebral ischemia on mTOR signaling are not homogeneous. In ischemic core region, it causes a decrease in mTORC1 activity, whereas in penumbra area it triggers mTORC1 activation [17,28,33]. Administration of the mTOR inhibitor rapamycin after middle cerebral artery occlusion (MCAO) reduced mTORC1 activity, consequently increasing autophagy – a crucial mechanism in neuron protection against ischemic damage, thereby diminishing neuronal apoptosis. It signifies the inverse causal relation between mTOR, autophagy, and neuronal death. In addition, ischemia was shown to induce the expression of hamartin (TSC1), an upstream inhibitor of mTOR, in CA3 hippocampal neurons, which could explain CA3 neurons resistance to ischemia-induced cell death [34]. In CA1 hippocampal neurons destined to die ischemia suppresses mTOR and stimulates autophagy. Astrocytes and microglia are also involved in mTOR modulation under these conditions. Hence, oxygen-glucose deprivation (OGD) causes a decrease in astrocytic mTORC1 activity via AMPK or tuberin (TSC2), afterwards increasing autophagic flow and decreasing the release of pro-inflammatory cytokines, as a result improving neuronal viability [35]. mTORC1 inhibition after ischemia leads to an increase in the M2 phenotype microglia (in contrast to deleterious M1 phenotype) and a reduction in pro-inflammatory cytokine expression [36].
The contribution of mTOR to the control of both translation and autophagy implies its role in the development of neurodegenerative diseases, which later was assessed and confirmed in various cellular and animal models. In post-mortem brains of AD patients an upregulation of mTORC1 was detected, which correlated with toxic oligomeric Aβ accumulation. However, available studies indicate heterogeneous effects of mTORC1 in AD: (1) upregulation of mTORC1 is detrimental via the blockade of autophagy; or (2) activation of mTOR is essential for long-term potentiation and memory and protects against Aβ toxicity. Life-long prophylactic rapamycin administration in the triple transgenic AD mouse model (APP, presenilin 1 - PS1, tau genes mutations) was shown to induce autophagy, decrease the number of plaques and tangles, and reduce cognitive deficits, but if the rapamycin treatment was implemented after plaques and tangles were formed, there was no positive effect observed [11,[37], [38], [39]]. mTOR signaling pathway is reported to participate in PD pathogenesis in several ways and depending on the pathology’s origin both positive and negative mTORC1 modulation is considered advantageous for pathology treatment [38]. On the one hand, it is revealed that mTORC1 inactivation and autophagy stimulation might preserve dopaminergic neurons by protecting them against the accumulation of ubiquitinated α-synuclein. On the other hand, some PD models showed that activation of mTORC1 and overexpression of constitutively activated Akt have a neuroprotective effect on both neuronal cell bodies and axons of the nigrostriatal projections for dopaminergic neurons [11,40,41].
Owing to its role in nutrients sensing and energy balance control mTOR was identified as a pivotal element in lifespan and aging regulation. It was shown that mTORC1 pathway inhibition through genetic depletion of mTOR or rapamycin treatment extended lifespan in diverse organisms such as yeast, nematodes, fruit flies, rats, mice, and primates [38,42]. Nutrient-sensing pathways, such as the insulin/insulin-like growth factor 1 (IGF-I) signaling network, are believed to determine longevity. Nutrients/mTOR axis suppression is one of the supporting mechanisms of the beneficiary effects of dietary restriction, which not only prolongs lifespan, but also delays the onset of age-related pathologies [43]. Moreover, some studies found rapamycin to improve spatial learning, memory and explorative activity if chronically administered during aging, but it had analogous effects in young mice. Chronic rapamycin treatment has been shown to elevate monoamines (dopamine, norepinephrine, and serotonin) levels, which could explain the improvements in learning and memory, as well as the stimulatory effect on exploratory activity. This study also showed that chronic rapamycin administration tended to increase dentate gyrus DCX (doublecortin) immunoreactivity (neurogenesis marker) in aged animals, but this effect was far from reaching statistical significance [44]. Given that dietary restriction and rapamycin treatment appear to extend lifespan via overlapping mechanisms, it raises the demand for further research about the effects of rapamycin on age-related phenotypes especially pertaining to the mammalian brain.
2.3. mTOR-related autophagy impairment in brain disorders
Autophagy is vital lysosome-mediated self-degradative process regulated by mTOR which is essential for balancing energy sources in response to nutrient stress. Studies suggest that autophagy impairment is linked with brain aging and neurodegenerative disorders, such as stroke, PD, and AD, where a defect along the autophagy pathway occurs at different stages. It has been proposed that decreased autophagy in AD brains results in the accumulation of protein aggregates via the hyperactivation of the PI3K/Akt/mTOR axis [31].
Autophagy is involved in the control of axon homeostasis, which is supported by the axonal pathology identified in autophagy-deficient mouse models, as well as in humans with congenital disorders of autophagy. Autophagosomes are constructed at the distal end of axons and transported retrogradely to the cell soma for degradation, which is why any defect disrupting this crucial mechanism is likely to cause neuronal pathology [45].
Moreover, autophagy impacts mitochondrial turn-over and ensures a pool of healthy mitochondria. It was shown that decreased mTOR signaling and dietary restriction might improve mitochondrial function both by enhanced mitochondrial quality control via mitochondrial autophagy (mitophagy) and by reducing ROS due to an increase in mitochondrial localization of TERT (telomerase reverse transcriptase) protein [6,46].
It was discovered that cerebral ischemia/reperfusion injury could be attenuated by autophagy stimulation with eugenol (active compound isolated from traditional Chinese medicine Acorus gramineus) via AMPK/mTOR/P70 S6K signaling pathway. Rapamycin treatment promoted inhibitory effects of eugenol against ischemia-induced apoptosis, as well as decreased infarct volume and neurological score in both in vitro and in vivo models [47].
The research about the effects of ischemic stress on autophagy revealed a vital role of mTOR-dependent autophagy-lysosomal machinery in the maintenance of synaptic structures. With the usage of OGD model in hippocampal neurons as well as a mouse model of MCAO it was shown that initial transient upregulation of autophagy leads to an accumulation of undegraded cargoes in neurites, and the following mTOR-dependent lysosomal biogenesis is not sufficient to clear these undegraded materials resulting in the impaired dynamic turnover of synaptic proteins [6]. Therefore, autophagy and lysosomal degradation modulation should be considered in searching for new therapeutic strategies against synaptic dysfunction.
3. α-ketoglutarate (AKG) metabolic pathways
3.1. Biochemical characteristics and physiological functions of AKG
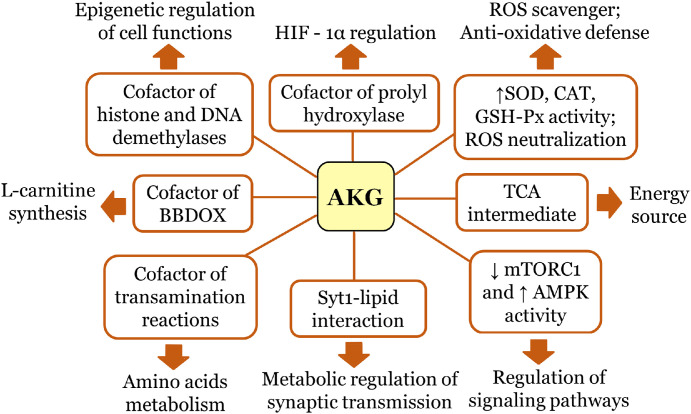
Alpha-ketoglutarate (AKG), also known as 2-ketoglutaric acid, 2-oxoglutaric acid, 2-oxoglutamate (IUPAC name: 2-oxopentanedioic acid), plays a crucial role in cellular energy metabolism. It is an important source of glutamine and glutamate that stimulates protein synthesis and an alternative energy source for use in the tricarboxylic acid (TCA) cycle under energy depleted conditions [48]. AKG is at the intersection of important physiological pathways due to its functions as a TCA intermediate, a cofactor for numerous transamination reactions, a cofactor for the prolyl hydroxylase-dependent hydroxylations of the transcription factor HIF-1 and a cofactor for epigenetic enzymes, histone, and DNA demethylases (Fig. 2) [10]. Thus, this crucial for energy-generating metabolic network keto-acid contributes to a multitude of cellular processes, such as anti-oxidative defence, energy production, amino acid metabolism, modulation of signaling systems and genetic modification [9,[48], [49], [50], [51], [52], [53]].
Fig. 2.
AKG multifaceted functions in cellular metabolism.
Endogenous AKG is mainly generated from oxidative decarboxylation of isocitrate catalysed by NAD-dependent isocitrate dehydrogenase (IDH) and from oxidative deamination of glutamate conducted by glutamate dehydrogenase (GDH) [8,9]. In the TCA cycle, AKG is utilized by AKG dehydrogenase through decarboxylation to succinyl-CoA and CO2, which subsequently contributes to ATP production via mitochondrial oxidative phosphorylation [50]. Noteworthy, IDH was shown to regulate the migration of primary glioblastoma cells by altering AKG levels with involvement of PI3K/Akt/mTOR pathway regulation [54]. GDH activators were reported to increase AKG production and improve overall bioenergetic in the ischemia/reperfusion injury model, thus suggesting GDH/AKG mediation of glutamate oxidation as a new therapeutic intervention for neurodegenerative disorders, including stroke [48]. Moreover, GDH is involved in glutamate metabolization by brain endothelial cells, thus replenishing TCA-intermediates and producing ATP under hypoglycaemic conditions [55].
AKG plays a significant role in the biosynthesis of amino acids. It can be transaminated with glutamine to form the excitatory neurotransmitter glutamate, which may be decarboxylated to the inhibitory neurotransmitter gamma-aminobutyric acid (GABA), thus protecting from excitotoxicity [50]. AKG supplementation also resulted in the inhibition of seizures and subsequent mitochondrial DNA damage induced by the excitotoxic/neurotoxic agent, kainic acid [56]. Transamination reaction with AKG is essential in utilizing ammonia, which accumulation can lead to neurological symptoms such as memory related disorders. Therefore, AKG is considered to have neuroprotective traits as it scavenges ammonia and contributes to glutamate/glutamine homeostasis [50,[56], [57], [58].
The effects of exogenous AKG administered as a dietary supplement in the form of various salts (ornithine, sodium, calcium) were investigated by many in vivo studies [10,49,59]. It was revealed that AKG is absorbed by the organism and can be metabolised to glutamine, glutamate, proline, and arginine. This keto-acid is mainly present inside cells (in mitochondria and cytoplasm), but it can also be found in small quantities (mg/ml) in the bloodstream [60]. Even more, one research uncovered that AKG level in blood is progressively decreased with age in people to the quantity of ng/ml, which is why the reduction of endogenous levels of AKG was proposed to serve as a marker of aging. In human studies after physical exercise, AKG blood level was shown to increase. It was assumed that AKG in blood might derive from the bacterial flora residing in the intestine, as different bacteria produce this metabolite [58,59,[61], [62], [63]].
Several studies indicate that AKG can cross the blood–brain barrier by a carrier-mediated process and by simple diffusion. AKG easily diffuses through channels, such as voltage-dependent anion channels in the outer mitochondrial membrane, and it is transported across the inner mitochondrial membrane through the oxoglutarate carrier (oxoglutarate/malate antiporter). Experiment with the sodium salt of 14C-labelled AKG discovered the presence of AKG carbon in various tissues (liver, brain, skin, muscle, bone tissue) already after 3 h of administration of the compound, which could signify the AKG ability to enter the bloodstream as well as to penetrate through blood-brain barrier [57,59,[64], [65], [66]].
One of the mechanisms of AKG protective action could be explained by its anti-oxidative properties. The keto-acid contributes to ROS scavenging, as it can directly and instantly neutralize hydrogen peroxide (H2O2), superoxide, and other reactive species, as well as increase the enzymatic activity of superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GSH-Px) [67]. AKG treatment prevented the lipid peroxidation in rat brain in vitro when exposed to Fe2+ ions as well as it exerted a good H2O2-scavenging activity in in vitro and in vivo studies [68], [69], [70]. Supplementation with AKG exhibited a protective effect against oxidative damage in the brain in experimental ischemia and damage to mitochondrial DNA induced by free radicals in mouse neural cells [69]. It is also acknowledged that AKG protects the brain and liver of rats from injury caused by cyanide (a rapidly acting neurotoxin that induces the formation of ROS) [71], [72], [73], [74].
As a substrate of hydroxylases AKG influences prolyl/aspartyl/lysyl hydroxylations, which in turn controls the stability of the HIF-1. AKG increases succinate accumulation in cytoplasm resulting in the inhibition of prolyl hydroxylase 2 (PHD2) and subsequent stabilization and activation of the alpha subunit of HIF-1 (HIF-1α) [75]. HIF-1α can further regulate the expression of downstream proteins involved in glucose metabolism, angiogenesis, and hypoxic response. Thus, it denotes the role of AKG in oxidative tension sensing and regulation of hypoxia [8,52].
Moreover, it was shown that AKG plays a regulatory role in synaptic transmission at the metabolite level. In in vitro and in vivo models, AKG treatment improved the Ca2+ sensitivity of mammalian synaptotagmin 1 and promoted its C2 cytoplasmic domains interaction with membrane’s acidic phospholipids, triggering membrane fusion and neurotransmitter release. It was detected that fusion of vesicles induced by AKG is conducted in the presence of mouse SNAREs and mouse synaptotagmin 1 at low Ca2+ concentration, which cannot be promoted by glutamate, thus emphasizing the specificity of AKG [76].
3.2. Role of AKG in metabolism during aging
Metabolism and aging are highly connected. Due to AKG pleiotropic functions in cell metabolism, including amino acid biosynthesis, protein degradation, and antioxidant system, recent studies have focused attention on AKG potential anti-aging properties. It was identified that AKG dietary supplementation can slow aging, as well as prevent the progress of age-related pathologies [70]. AKG treatment supports the self-renewal of naive primed human pluripotent stem cells, potentially by promoting the demethylation of histones and DNA [77]. It also extends lifespan, compresses morbidity, and promotes healthier life associated with decreased levels of inflammatory cytokines in mice [78]. Moreover, recent study highlighted the associations between AKG and reproductive aging in mice, swine, and humans. In fruit fly model AKG supplementation in addition to lifespan-prolonging effects increased locomotor activity and stress resistance [79,80].
Molecular mechanisms underlying the lifespan-extending effects of AKG became clear in studies with Caenorhabditis elegans and Drosophila melanogaster [79,81]. The authors identified mitochondrial ATP synthase as a direct target of AKG, which mediated the inhibition of mTOR signaling pathway and promoted AMPK activity accompanied by increased autophagy level. Experiments in C. elegans model with usage of small-molecule target identification strategy DARTS (drug affinity responsive target stability) revealed ATP synthase subunit β to be a binding protein for AKG [81]. Thus, AKG inhibits ATP synthase, consequently reducing ATP level and oxygen consumption, which leads to indirect mTOR inhibition and autophagy activation. Regarding mTOR inhibition, AKG acts as a mimetic of caloric restriction. The geroprotective effect of AKG is linked with metabolic pathways modulation which is similar to the state of caloric restriction [70].
Noteworthy, supporting the role of the mTOR pathway in the effects of AKG-induced longevity, it was uncovered that AKG-indirect AMPK activation stimulates the phosphorylation of transcription factor Fork head box Other (FoxO) leading to increased expression of genes involved in oxidative stress response, apoptosis, glucose, and lipid metabolism. AMPK activation also inhibits NF-κB signaling pathway, in turn suppressing inflammatory processes which are known to be activated during aging. Thus, AKG’s geroprotective properties might be due to the involvement of AMPK and NF-κB-mediated signaling pathways [70,82,83].
Another mechanism that could explain AKG’s control of lifespan is genome-wide demethylation. It is acknowledged that aging is accompanied by significant changes in genome-wide methylation levels. AKG controls the activity of DNA demethylases TET1-3, thus triggering the demethylation of GpC loci in DNA [82,84].
Many age-related pathologies are accompanied by oxidative stress. Moreover, the free radical theory of aging created by Denham Harman states that living organisms age due to the accumulation of ROS and irreversibly oxidized biomolecules [85]. In this regard, AKG’s antioxidative properties could diminish manifestations of age-associated oxidative stress and age-related pathologies. AKG supplementation prevented an age-related increase in oxidative damage to biomolecules and modulated antioxidant defence in the aged mice [72].
In summary, the effect of AKG on lifespan is associated with the inhibition of ATP synthase and mTOR, activation of AMPK, suppression of inflammatory responses, prevention of age-related epigenetic changes in gene expression, and antioxidative defence system. Overall, the anti-aging effect of AKG confirmed in various models implies the possibility of age-related brain pathologies treatment with AKG, however, further experiments are required.
3.3. AKG and neuroprotection
The brain is one of the most energy-consuming organs in the human body and any malfunction of energy metabolism is intricately connected with the manifestation of disease. In addition to dysfunctional energy metabolism, the pathogenesis of many brain disorders includes neuroinflammation, oxidative stress, mitochondria dysfunction and autophagy impairment [86].
Due to its pleiotropic metabolic properties, AKG has emerged as a possible neuroprotective agent against numerous brain disorders. It was indicated that a composition comprising AKG, and one or more enzymes (lipase, protease, amylase) improves brain function and has potential medical uses in the treatment of neurological and/or neurodegenerative diseases, including AD, PD, mild cognitive impairment, Huntington’s disease, amyotrophic lateral sclerosis, ischemic stroke, traumatic brain injury, depression and disorders of cognitive performance or memory. AKG supplementation in a gerbil model induced an increase in adult neurogenesis, along with an improved cognitive performance and synaptic vesicle spatial rearrangements, resulting in enhanced efficacy of synaptic transmission. The number of synaptic vesicles strongly correlated with the neurotransmitter amount. Therefore, an AKG-induced increase in synaptic vesicles can be explained by AKG involvement in neurotransmitter synthesis [7].
The neuroprotective effect of AKG was also delineated in ischemic injury models, indicating its role in the cellular response to glutamate excitotoxicity and mitochondrial dysfunction [71]. Treatment with AKG was shown to attenuate neuronal death and reactive astrogliosis preventing the damage of neural cells that usually takes place during ischemic pathology. In cerebellar granule neuron culture preincubation with AKG synthetic analog protected cells from the irreversible impairment of mitochondrial function and delayed calcium deregulation [87,88].
Neurological disorders are often accompanied by the generation of elevated levels of ROS as well as oxidized lipids, proteins, and nucleic acids. Hence, AKG’s antioxidative activity coupled with the ability to fuel the mitochondria can provide neuroprotection during oxidative stress. AKG promotes the synthesis of L-carnitine – an amino acid crucial for the effective metabolism of fatty acids into ATP. In particular, AKG serves as a cofactor of butyrobetaine dioxygenase (BBDOX), which is found to be downregulated in AD and ROS stress. Because the dyslipidemia observed in astrocytes was described to be attenuated after keto-acids administration, it is suggested that AKG could alleviate the symptoms associated with AD [89,90].
Noteworthy, ornithine-AKG treatment of mongrel dogs increased brain oxygen and glucose utilization while diminishing metabolic disturbances caused by hypoxia. Similarly, intravenous infusion of ornithine-AKG to the patients within 96 h of stroke significantly improved consciousness and neurological impairment scores in comparison to the placebo group. The positive effect of AKG on brain oxygenation is associated with the post-ischemic decrease in glutamate concentration and protein degradation, thus changing functional activity in the brain [53].
Additionally, it was discovered that AKG exhibits neuroprotective properties against MPTP-induced neurotoxicity and dopaminergic neuron damage in mice. MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) is a neurotoxin associated with drug abuse and causes permanent symptoms of PD. In the same study AKG treatment was found to reduce the loss of motor coordination, oxidative stress, and diminished activity of complex I (NADH: ubiquinone oxidoreductase), as well as minimize the accumulation of alpha-synuclein in the midbrain region. Thus, it is proposed that AKG could be of potential therapeutic value in the treatment of PD [57].
AKG/mTOR interplay modulation was reported to be a promising direction in the treatment of Leigh syndrome, a mitochondrial disorder (defects observed in a subunit NDUFS4 of the mitochondrial electron transport chain complex I) characterized by progressive focal neurodegenerative lesions in specific brain regions. The research revealed a direct link between NDUFS4 deficiency and decreased glutamine/glutamate/AKG levels. Preservation of these metabolites by mTOR inhibition with rapamycin treatment attenuated disease by providing AKG as a functional mitochondrial complex I substrate to support oxidative phosphorylation or/and through the rescue of neuron-specific metabolic pathways related to neurotransmitter metabolism [91].
4. The crosstalk between AKG and mTOR signaling pathways
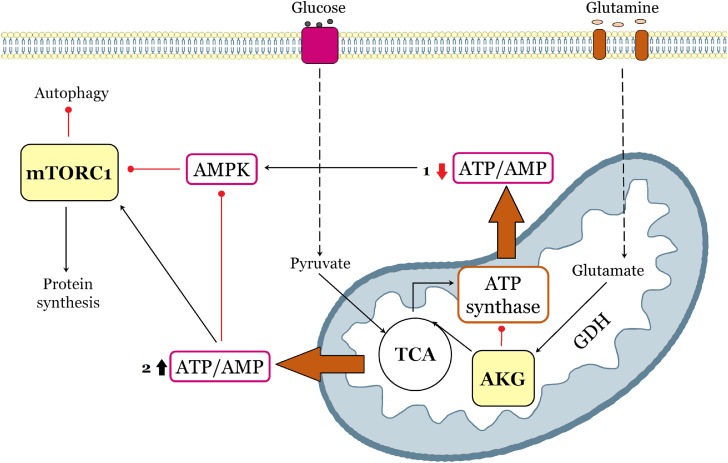
mTOR signaling pathway plays a crucial role in nutrient sensing and is highly involved in cellular metabolism. As a key molecule in many important physiological pathways, AKG as well is tightly associated with metabolism regulation. Attention is drawn to the cooperation between AKG and mTOR signaling in the context of metabolic regulation. Recent studies point out the involvement of the mTOR signaling pathway in aging, as well as the ability of AKG to indirectly inhibit mTOR and act as an anti-aging agent [70,81]. Lifespan-prolonging effects of AKG supplementation were reported in mice, yeast, nematode C. elegans and fruit fly D. melanogaster models [78,79,81]. It was shown that AKG directly inhibits ATP synthase and indirectly mTOR, thereby promoting autophagy and catabolic reactions similar to those observed under caloric restriction (Fig. 3).
Fig. 3.
Model of mTOR/α-ketoglutarate-mediated signaling communications. The scheme indicates two AKG-mediated event variants (marked with numbers and highlighted with orange arrows): (1) AKG directly inhibits ATP synthase, followed by AMPK activation and mTOR suppression, thereby promoting autophagy and catabolic reactions, (2) AKG replenishes the pool of TCA intermediates, promoting additional ATP synthesis, thus triggering activation of mTOR signaling pathway and anabolic reactions. Black arrows and red lines respectively represent positive and negative regulation.
In AKG-treated flies’ study after AKG supplementation the expression of transcription factors in the mTOR pathway (TFEB, PGC, SREBP, HIF-1α), and the upstream gene PI3K, as well as TORC were significantly downregulated, while FKBP12, PRAS40 and AMPK were upregulated. The mRNA levels of autophagy-related genes and the number of phagosomes confirmed by phagosome staining were also significantly increased in flies fed with AKG, indicating enhanced autophagy [79]. Hence, it has been suggested that AKG metabolic pathway could overlap with mTOR signaling pathway with the involvement of ATP synthase inhibition, reductions in both ATP levels and the ATP/ADP ratio and AMPK activation.
However, another group of studies indicated that AKG may activate mTOR by replenishing TCA-intermediate pool and by amino acid metabolism (Fig. 3). AKG is a precursor for the biosynthesis of such amino acids as glutamate, glutamine, leucine, and proline. It was found that exogenous AKG treatment enhanced protein biosynthesis and triggered activation of mTOR signaling pathway in animal cell cultures and young pigs [92,93]. AKG treatment increased the phosphorylated (active state) levels of mTOR, 4E-BP1, and P70 S6K1, thereby promoting the initiation of protein synthesis. Moreover, glutamine-derived AKG was reported to activate mTORC1 by stimulation of GTPases ADP ribosylation factor 1 (Arf1) -Rheb-phospholipase D signaling apparatus [94] and by stimulating GTP loading of RagB that induces the Rag-mediated translocation of mTORC1 to the lysosome surface [95]. Therefore, these studies also verify the hypothesis of AKG and mTOR pathways interaction, highlighting the link via amino acid metabolism [92,93].
Cooperation between AKG and mTOR is hypothesized to be attributed not only to AKG metabolic functions in energy and amino acid/protein metabolism, but also to its antioxidant properties and its role as a signaling molecule, which participates in the control of DNA methylation and chromatin modifications, regulation of nutrient-sensitive pathways, response to hypoxia and inflammation [70]. It should be noted that the multidirectional effect of AKG on mTOR signaling may depend on the type of tissue or experimental model, therefore, it is assumed that in different cells various mechanisms of AKG and mTOR signaling could be involved and both catabolic and anabolic reactions can be triggered [50]. The interplay between AKG and mTOR pathways has been observed in different tissues and several model organisms. However, there are no descriptions of clearly specific mechanisms of such crosstalk in the nervous system. It could be speculated that the involvement of AKG in mTOR inhibition and subsequent autophagy activation or amino acid metabolism stimulation by AKG/mTOR explain the neuroprotective effect of AKG in neurological disorders. We also hypothesize that the phenomenon of AKG-mediated regulation of neurotransmission and mTOR-controlled synaptic plasticity may be indirect evidence for the interaction of AKG/mTOR signaling pathways. However, further studies are required to determine the mechanistic basis of AKG/mTOR-mediated cooperation in the brain. The study of molecular communications between a universal metabolite and an important cellular regulator may reveal new prospects for the prevention and treatment of diseases associated with metabolism, age, etc.
5. Conclusions
As summarized above, mTOR signaling pathway is a principal regulator of cellular physiological processes, including energy homeostasis, cell growth, protein synthesis, autophagy, etc. An imbalance of mTOR signaling is correlated with brain abnormalities and neurological disorders.
AKG’s multifaceted functions in energy production, amino acid metabolism, anti-oxidative defence, modulation of signaling systems, and genetic modification indicate its crucial role in cellular metabolism. AKG is reported to have anti-aging and neuroprotective properties. mTOR and AKG essential functions in metabolic control imply the possible crosstalk between the signaling pathways of these molecules. Suppression of mTOR function by AKG metabolism delineated by age-related studies confirms the overlap between mTOR and AKG-signaling pathways, thus indicating a possibility of mTOR-related neurological pathologies treatment with AKG.
Specified mechanisms of AKG/mTOR interplay in the brain remain to be elucidated. To what extent/mode inhibition or activation of mTOR determines the neuroprotective effects of AKG on brain cells in neuropathology is something that needs to be studied. It would be important to investigate the mechanisms of AKG influence on mTOR-regulated autophagy, amino acid metabolism, and protection against oxidative stress in neuronal tissue. Analyzed findings suggest a novel role of AKG metabolism in mTOR signaling pathway regulation, and modulation of mTOR activity could be considered as a therapeutic strategy in brain disorders treatment.
CRediT authorship contribution statement
Olha Kostiuchenko: Conceptualization, Writing – original draft, Visualization. Iryna Lushnikova: Conceptualization, Writing – review & editing, Supervision. Magdalena Kowalczyk: Writing – review & editing. Galyna Skibo: Writing – review & editing, Supervision.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
All authors approved the final version of the submitted paper.
Acknowledgments
This article was funded by National Academy of Science of Ukraine. State registration number of work is 0118U007350. This review study was also supported by NAWA scholarship program “Solidarni z Ukrainą” and “IBB START” scholarship of the Institute of Biochemistry and Biophysics PAS.
Contributor Information
Olha Kostiuchenko, Email: kostiuchenko.olha@biph.kiev.ua.
Iryna Lushnikova, Email: ivlook@ukr.net.
Magdalena Kowalczyk, Email: mk@ibb.waw.pl.
Galyna Skibo, Email: skibo@biph.kiev.ua.
Data availability
No data was used for the research described in the article.
References
- 1.Datta A., Sarmah D., Mounica L., Kaur H., Kesharwani R., Verma G., Veeresh P., Kotian V., Kalia K., Borah A., Wang X., Dave K.R., Yavagal D.R., Bhattacharya P. Cell death pathways in ischemic stroke and targeted pharmacotherapy. Transl. Stroke Res. 2020;11:1185–1202. doi: 10.1007/s12975-020-00806-z. [DOI] [PubMed] [Google Scholar]
- 2.Fakih W., Zeitoun R., AlZaim I., Eid A.H., Kobeissy F., Abd-Elrahman K.S., El-Yazbi A.F. Early metabolic impairment as a contributor to neurodegenerative disease: Mechanisms and potential pharmacological intervention. Obes. Silver Spring Md. 2022;30:982–993. doi: 10.1002/oby.23400. [DOI] [PubMed] [Google Scholar]
- 3.Saxton R.A., Sabatini D.M. mTOR signaling in growth, metabolism, and disease. Cell. 2017;168:960–976. doi: 10.1016/j.cell.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wong M. Mammalian target of rapamycin (mTOR) pathways in neurological diseases. Biomed. J. 2013;36 doi: 10.4103/2319-4170.110365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hou K., Xu D., Li F., Chen S., Li Y. The progress of neuronal autophagy in cerebral ischemia stroke: mechanisms, roles and research methods. J. Neurol. Sci. 2019;400:72–82. doi: 10.1016/j.jns.2019.03.015. [DOI] [PubMed] [Google Scholar]
- 6.Zhang X., Wei M., Fan J., Yan W., Zha X., Song H., Wan R., Yin Y., Wang W. Ischemia-induced upregulation of autophagy preludes dysfunctional lysosomal storage and associated synaptic impairments in neurons. Autophagy. 2021;17:1519–1542. doi: 10.1080/15548627.2020.1840796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.S. Pierzynowski, Compositions for improvement of brain function, US9592211B2, 2017. https://patents.google.com/patent/US9592211B2/no (Accessed 26 May 2022).
- 8.Legendre F., MacLean A., Appanna V.P., Appanna V.D. Biochemical pathways to α-ketoglutarate, a multi-faceted metabolite. World J. Microbiol. Biotechnol. 2020;36:123. doi: 10.1007/s11274-020-02900-8. [DOI] [PubMed] [Google Scholar]
- 9.Kim A.Y., Jeong K.-H., Lee J.H., Kang Y., Lee S.H., Baik E.J. Glutamate dehydrogenase as a neuroprotective target against brain ischemia and reperfusion. Neuroscience. 2017;340:487–500. doi: 10.1016/j.neuroscience.2016.11.007. [DOI] [PubMed] [Google Scholar]
- 10.Zdzisińska B., Żurek A., Kandefer-Szerszeń M. Alpha-ketoglutarate as a molecule with pleiotropic activity: well-known and novel possibilities of therapeutic use. Arch. Immunol. Ther. Exp. (Warsz.). 2017;65:21–36. doi: 10.1007/s00005-016-0406-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bockaert J., Marin P. mTOR in brain physiology and pathologies. Physiol. Rev. 2015;95:1157–1187. doi: 10.1152/physrev.00038.2014. [DOI] [PubMed] [Google Scholar]
- 12.Karalis V., Bateup H.S. Current approaches and future directions for the treatment of mTORopathies. Dev. Neurosci. 2021;43:143–158. doi: 10.1159/000515672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang H., Rudge D.G., Koos J.D., Vaidialingam B., Yang H.J., Pavletich N.P. mTOR kinase structure, mechanism and regulation. Nature. 2013;497:217–223. doi: 10.1038/nature12122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jacinto E., Loewith R., Schmidt A., Lin S., Rüegg M.A., Hall A., Hall M.N. Mammalian TOR complex 2 controls the actin cytoskeleton and is rapamycin insensitive. Nat. Cell Biol. 2004;6:1122–1128. doi: 10.1038/ncb1183. [DOI] [PubMed] [Google Scholar]
- 15.Sarbassov D.D., Ali S.M., Sengupta S., Sheen J.-H., Hsu P.P., Bagley A.F., Markhard A.L., Sabatini D.M. Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol. Cell. 2006;22:159–168. doi: 10.1016/j.molcel.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 16.Kim S.G., Buel G.R., Blenis J. Nutrient regulation of the mTOR complex 1 signaling pathway. Mol. Cells. 2013;35:463–473. doi: 10.1007/s10059-013-0138-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Villa-González M., Martín-López G., Pérez-Álvarez M.J. Dysregulation of mTOR signaling after brain ischemia. Int. J. Mol. Sci. 2022;23:2814. doi: 10.3390/ijms23052814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Castellanos M., Gubern C., Kadar E. In: Mol. Med. MTOR. Maiese K., editor. Academic Press; Boston: 2016. Chapter 7 - mTOR: exploring a new potential therapeutic target for stroke; pp. 105–122. Ed. [DOI] [Google Scholar]
- 19.Huang W., Zhu P.J., Zhang S., Zhou H., Stoica L., Galiano M., Krnjević K., Roman G., Costa-Mattioli M. mTORC2 controls actin polymerization required for consolidation of long-term memory. Nat. Neurosci. 2013;16:441–448. doi: 10.1038/nn.3351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Switon K., Kotulska K., Janusz-Kaminska A., Zmorzynska J., Jaworski J. Molecular neurobiology of mTOR. Neuroscience. 2017;341:112–153. doi: 10.1016/j.neuroscience.2016.11.017. [DOI] [PubMed] [Google Scholar]
- 21.van Dam T.J.P., Zwartkruis F.J.T., Bos J.L., Snel B. Evolution of the TOR pathway. J. Mol. Evol. 2011;73:209–220. doi: 10.1007/s00239-011-9469-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Laplante M., Sabatini D.M. mTOR signaling in growth control and disease. Cell. 2012;149:274–293. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ben Sahra I., Regazzetti C., Robert G., Laurent K., Marchand-Brustel Y.Le, Auberger P., Tanti J.-F., Giorgetti-Peraldi S., Bost F. Metformin, independent of AMPK, induces mTOR inhibition and cell-cycle arrest through REDD1. Cancer Res. 2011;71:4366–4372. doi: 10.1158/0008-5472.CAN-10-1769. [DOI] [PubMed] [Google Scholar]
- 24.Zoncu R., Efeyan A., Sabatini D.M. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat. Rev. Mol. Cell Biol. 2011;12:21–35. doi: 10.1038/nrm3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mao Z., Zhang W. Role of mTOR in glucose and lipid metabolism. Int. J. Mol. Sci. 2018;19:2043. doi: 10.3390/ijms19072043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ben-Sahra I., Hoxhaj G., Ricoult S.J.H., Asara J.M., Manning B.D. mTORC1 induces purine synthesis through control of the mitochondrial tetrahydrofolate cycle. Science. 2016;351:728–733. doi: 10.1126/science.aad0489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dunlop E.A., Tee A.R. mTOR and autophagy: a dynamic relationship governed by nutrients and energy. Semin. Cell Dev. Biol. 2014;36:121–129. doi: 10.1016/j.semcdb.2014.08.006. [DOI] [PubMed] [Google Scholar]
- 28.Hwang J.-Y., Gertner M., Pontarelli F., Court-Vazquez B., Bennett M.V.L., Ofengeim D., Zukin R.S. Global ischemia induces lysosomal-mediated degradation of mTOR and activation of autophagy in hippocampal neurons destined to die. Cell Death Differ. 2017;24:317–329. doi: 10.1038/cdd.2016.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Betz C., Stracka D., Prescianotto-Baschong C., Frieden M., Demaurex N., Hall M.N. MTOR complex 2-Akt signaling at mitochondria-associated endoplasmic reticulum membranes (MAM) regulates mitochondrial physiology. Proc. Natl. Acad. Sci. U. S. A. 2013;110:12526–12534. doi: 10.1073/pnas.1302455110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Beard D.J., Hadley G., Thurley N., Howells D.W., Sutherland B.A., Buchan A.M. The effect of rapamycin treatment on cerebral ischemia: a systematic review and meta-analysis of animal model studies. Int. J. Stroke. 2019;14:137–145. doi: 10.1177/1747493018816503. [DOI] [PubMed] [Google Scholar]
- 31.Ryskalin L., Limanaqi F., Frati A., Busceti C.L., Fornai F. mTOR-related brain dysfunctions in neuropsychiatric disorders. Int. J. Mol. Sci. 2018;19:2226. doi: 10.3390/ijms19082226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Berdichevsky Y., Dryer A.M., Saponjian Y., Mahoney M.M., Pimentel C.A., Lucini C.A., Usenovic M., Staley K.J. PI3K-Akt signaling activates mTOR-mediated epileptogenesis in organotypic hippocampal culture model of post-traumatic epilepsy. J. Neurosci. Off. J. Soc. Neurosci. 2013;33:9056–9067. doi: 10.1523/JNEUROSCI.3870-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu M., Zhang H., Kai J., Zhu F., Dong J., Xu Z., Wong M., Zeng L. Rapamycin prevents cerebral stroke by modulating apoptosis and autophagy in penumbra in rats. Ann. Clin. Transl. Neurol. 2017;5:138–146. doi: 10.1002/acn3.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Papadakis M., Hadley G., Xilouri M., Hoyte L.C., Nagel S., McMenamin M.M., Tsaknakis G., Watt S.M., Drakesmith C.W., Chen R., Wood M.J.A., Zhao Z., Kessler B., Vekrellis K., Buchan A.M. Tsc1 (hamartin) confers neuroprotection against ischemia by inducing autophagy. Nat. Med. 2013;19:351–357. doi: 10.1038/nm.3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zha H., Fan Y., Yang L., Yin M., Miao W., He J., Wang Y. Autophagy protects against cerebral ischemic reperfusion injury by inhibiting neuroinflammation. Am. J. Transl. Res. 2021;13:4726–4737. [PMC free article] [PubMed] [Google Scholar]
- 36.Li D., Wang C., Yao Y., Chen L., Liu G., Zhang R., Liu Q., Shi F.-D., Hao J. mTORC1 pathway disruption ameliorates brain inflammation following stroke via a shift in microglia phenotype from M1 type to M2 type. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2016;30:3388–3399. doi: 10.1096/fj.201600495R. [DOI] [PubMed] [Google Scholar]
- 37.Swiech L., Perycz M., Malik A., Jaworski J. Role of mTOR in physiology and pathology of the nervous system. Biochim. Biophys. Acta. 2008;1784:116–132. doi: 10.1016/j.bbapap.2007.08.015. [DOI] [PubMed] [Google Scholar]
- 38.Lipton J.O., Sahin M. The Neurology of mTOR. Neuron. 2014;84:275–291. doi: 10.1016/j.neuron.2014.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Caccamo A., Majumder S., Richardson A., Strong R., Oddo S. Molecular interplay between mammalian target of rapamycin (mTOR), amyloid-beta, and Tau: effects on cognitive impairments. J. Biol. Chem. 2010;285:13107–13120. doi: 10.1074/jbc.M110.100420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Crews L., Spencer B., Desplats P., Patrick C., Paulino A., Rockenstein E., Hansen L., Adame A., Galasko D., Masliah E. Selective molecular alterations in the autophagy pathway in patients with Lewy body disease and in models of α-synucleinopathy. PLOS ONE. 2010;5:e9313. doi: 10.1371/journal.pone.0009313. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 41.Cheng H.C., Kim S.R., Oo T.F., Kareva T., Yarygina O., Rzhetskaya M., Wang C., During M., Talloczy Z., Tanaka K., Komatsu M., Kobayashi K., Okano H., Kholodilov N., Burke R.E. Akt suppresses retrograde degeneration of dopaminergic axons by inhibition of macroautophagy. J. Neurosci. 2011;31:2125–2135. doi: 10.1523/JNEUROSCI.5519-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Papadopoli D., Boulay K., Kazak L., Pollak M., Mallette F.A., Topisirovic I., Hulea L. mTOR as a central regulator of lifespan and aging. F1000Research. 2019;8:F1000. doi: 10.12688/f1000research.17196.1. Faculty Rev-998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu G.Y., Sabatini D.M. mTOR at the nexus of nutrition, growth, ageing and disease. Nat. Rev. Mol. Cell Biol. 2020;21:183–203. doi: 10.1038/s41580-019-0199-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Neff F., Flores-Dominguez D., Ryan D.P., Horsch M., Schröder S., Adler T., Afonso L.C., Aguilar-Pimentel J.A., Becker L., Garrett L., Hans W., Hettich M.M., Holtmeier R., Hölter S.M., Moreth K., Prehn C., Puk O., Rácz I., Rathkolb B., Rozman J., Naton B., Ordemann R., Adamski J., Beckers J., Bekeredjian R., Busch D.H., Ehninger G., Graw J., Höfler H., Klingenspor M., Klopstock T., Ollert M., Stypmann J., Wolf E., Wurst W., Zimmer A., Fuchs H., Gailus-Durner V., Hrabe de Angelis M., Ehninger D. Rapamycin extends murine lifespan but has limited effects on aging. J. Clin. Invest. 2013;123:3272–3291. doi: 10.1172/JCI67674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maday S., Wallace K.E., Holzbaur E.L.F. Autophagosomes initiate distally and mature during transport toward the cell soma in primary neurons. J. Cell Biol. 2012;196:407–417. doi: 10.1083/jcb.201106120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Miwa S., Saretzki G. Telomerase and mTOR in the brain: the mitochondria connection. Neural Regen. Res. 2017;12:358–361. doi: 10.4103/1673-5374.202922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sun X., Wang D., Zhang T., Lu X., Duan F., Ju L., Zhuang X., Jiang X. Eugenol attenuates cerebral ischemia-reperfusion injury by enhancing autophagy via AMPK-mTOR-P70S6K pathway. Front. Pharmacol. 2020;11:84. doi: 10.3389/fphar.2020.00084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Meng X., Liu H., Peng L., He W., Li S. Potential clinical applications of alpha‑ketoglutaric acid in diseases (Review) Mol. Med. Rep. 2022;25:1–8. doi: 10.3892/mmr.2022.12667. [DOI] [PubMed] [Google Scholar]
- 49.Harrison A.P., Pierzynowski S.G. Biological effects of 2-oxoglutarate with particular emphasis on the regulation of protein, mineral and lipid absorption/metabolism, muscle performance, kidney function, bone formation and cancerogenesis, all viewed from a healthy ageing perspective state of the art–review article. J. Physiol. Pharmacol. Off. J. Pol. Physiol. Soc. 2008;59(Suppl 1):91–106. [PubMed] [Google Scholar]
- 50.Wu N., Yang M., Gaur U., Xu H., Yao Y., Li D. Alpha-ketoglutarate: physiological functions and applications. Biomol. Ther. 2016;24:1–8. doi: 10.4062/biomolther.2015.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bruegge K., Jelkmann W., Metzen E. Hydroxylation of hypoxia-inducible transcription factors and chemical compounds targeting the HIF-alpha hydroxylases. Curr. Med. Chem. 2007;14:1853–1862. doi: 10.2174/092986707781058850. [DOI] [PubMed] [Google Scholar]
- 52.Kuo C.-Y., Cheng C.-T., Hou P., Lin Y.-P., Ma H., Chung Y., Chi K., Chen Y., Li W., Kung H.-J., Ann D.K. HIF-1-alpha links mitochondrial perturbation to the dynamic acquisition of breast cancer tumorigenicity. Oncotarget. 2016;7:34052–34069. doi: 10.18632/oncotarget.8570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gyanwali B., Lim Z.X., Soh J., Lim C., Guan S.P., Goh J., Maier A.B., Kennedy B.K. Alpha-Ketoglutarate dietary supplementation to improve health in humans. Trends Endocrinol. Metab. 2022;33:136–146. doi: 10.1016/j.tem.2021.11.003. [DOI] [PubMed] [Google Scholar]
- 54.Shen X., Wu S., Zhang J., Li M., Xu F., Wang A., Lei Y., Zhu G. Wild‑type IDH1 affects cell migration by modulating the PI3K/AKT/mTOR pathway in primary glioblastoma cells. Mol. Med. Rep. 2020;22:1949–1957. doi: 10.3892/mmr.2020.11250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hinca S.B., Salcedo C., Wagner A., Goldeman C., Sadat E., Aibar M.M.D., Maechler P., Brodin B., Aldana B.I., Helms H.C.C. Brain endothelial cells metabolize glutamate via glutamate dehydrogenase to replenish TCA-intermediates and produce ATP under hypoglycemic conditions. J. Neurochem. 2021;157:1861–1875. doi: 10.1111/jnc.15207. [DOI] [PubMed] [Google Scholar]
- 56.Yamamoto H., Mohanan P.V. Effect of alpha-ketoglutarate and oxaloacetate on brain mitochondrial DNA damage and seizures induced by kainic acid in mice. Toxicol. Lett. 2003;143:115–122. doi: 10.1016/s0378-4274(03)00114-0. [DOI] [PubMed] [Google Scholar]
- 57.Satpute R., Lomash V., Kaushal M., Bhattacharya R. Neuroprotective effects of alpha-ketoglutarate and ethyl pyruvate against motor dysfunction and oxidative changes caused by repeated 1-methyl-4-phenyl-1,2,3,6 tetrahydropyridine exposure in mice. Hum. Exp. Toxicol. 2013;32:747–758. doi: 10.1177/0960327112468172. [DOI] [PubMed] [Google Scholar]
- 58.Pierzynowski S., Pierzynowska K. Alpha-ketoglutarate, a key molecule involved in nitrogen circulation in both animals and plants, in the context of human gut microbiota and protein metabolism. Adv. Med. Sci. 2022;67:142–147. doi: 10.1016/j.advms.2022.02.004. [DOI] [PubMed] [Google Scholar]
- 59.Filip R., Pierzynowski S.G. The absorption, tissue distribution and excretion of entirely administered alpha-ketoglutarate in rats. J. Anim. Physiol. Anim. Nutr. 2008;92:182–189. doi: 10.1111/j.1439-0396.2007.00725.x. [DOI] [PubMed] [Google Scholar]
- 60.Wagner B.M., Donnarumma F., Wintersteiger R., Windischhofer W., Leis H.J. Simultaneous quantitative determination of alpha-ketoglutaric acid and 5-hydroxymethylfurfural in human plasma by gas chromatography-mass spectrometry. Anal. Bioanal. Chem. 2010;396:2629–2637. doi: 10.1007/s00216-010-3479-0. [DOI] [PubMed] [Google Scholar]
- 61.Loï C., Nakib S., Neveux N., Arnaud-Battandier F., Cynober L. Ornithine alpha-ketoglutarate metabolism in the healthy rat in the postabsorptive state. Metabolism. 2005;54:1108–1114. doi: 10.1016/j.metabol.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 62.Junghans P., Derno M., Pierzynowski S., Hennig U., Rudolph P.Eberhard, Souffrant W.B. Intraduodenal infusion of alpha-ketoglutarate decreases whole body energy expenditure in growing pigs. Clin. Nutr. Edinb. Scotl. 2006;25:489–496. doi: 10.1016/j.clnu.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 63.Otto C., Yovkova V., Barth G. Overproduction and secretion of α-ketoglutaric acid by microorganisms. Appl. Microbiol. Biotechnol. 2011;92:689–695. doi: 10.1007/s00253-011-3597-4. [DOI] [PubMed] [Google Scholar]
- 64.Oldendorf W.H. Carrier-mediated blood-brain barrier transport of short-chain monocarboxylic organic acids. Am. J. Physiol. 1973;224:1450–1453. doi: 10.1152/ajplegacy.1973.224.6.1450. [DOI] [PubMed] [Google Scholar]
- 65.Conn A.R., Steele R.D. Transport of alpha-keto analogues of amino acids across blood-brain barrier in rats. Am. J. Physiol. 1982;243:E272–E277. doi: 10.1152/ajpendo.1982.243.4.E272. [DOI] [PubMed] [Google Scholar]
- 66.Monné M., Miniero D.V., Iacobazzi V., Bisaccia F., Fiermonte G. The mitochondrial oxoglutarate carrier: from identification to mechanism. J. Bioenerg. Biomembr. 2013;45:1–13. doi: 10.1007/s10863-012-9475-7. [DOI] [PubMed] [Google Scholar]
- 67.Liu S., He L., Yao K. The antioxidative function of alpha-ketoglutarate and its applications. BioMed Res. Int. 2018;2018 doi: 10.1155/2018/3408467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Puntel R.L., Nogueira C.W., Rocha J.B.T. Krebs cycle intermediates modulate thiobarbituric acid reactive species (TBARS) production in rat brain in vitro. Neurochem. Res. 2005;30:225–235. doi: 10.1007/s11064-004-2445-7. [DOI] [PubMed] [Google Scholar]
- 69.Bayliak M.M., Lylyk M.P., Vytvytska O.M., Lushchak V.I. Assessment of antioxidant properties of alpha-keto acids in vitro and in vivo. Eur. Food Res. Technol. 2016;2:179–188. doi: 10.1007/s00217-015-2529-4. [DOI] [Google Scholar]
- 70.Bayliak M.M., Lushchak V.I. Pleiotropic effects of alpha-ketoglutarate as a potential anti-ageing agent. Ageing Res. Rev. 2021;66 doi: 10.1016/j.arr.2020.101237. [DOI] [PubMed] [Google Scholar]
- 71.T.N. Kovalenko, G.A. Ushakova, I. Osadchenko, G.G. Skibo, S.G. Pierzynowski, The neuroprotective effect of 2-oxoglutarate in the experimental ischemia of hippocampus, (n.d.) 8. [PubMed]
- 72.Niemiec T., Sikorska J., Harrison A., Szmidt M., Sawosz E., Wirth-Dzieciolowska E., Wilczak J., Pierzynowski S. Alpha-ketoglutarate stabilizes redox homeostasis and improves arterial elasticity in aged mice. J. Physiol. Pharmacol. Off. J. Pol. Physiol. Soc. 2011;62:37–43. [PubMed] [Google Scholar]
- 73.Faulkner A., Tamiato A., Cathery W., Rampin A., Caravaggi C.M., Jover E., Allen S., Mellor H., Hauton D., Heather L.C., Spinetti G., Madeddu P. Dimethyl-2-oxoglutarate improves redox balance and mitochondrial function in muscle pericytes of individuals with diabetes mellitus. Diabetologia. 2020;63:2205–2217. doi: 10.1007/s00125-020-05230-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bhattacharya R., Rao P.V.L., Vijayaraghavan R. In vitro and in vivo attenuation of experimental cyanide poisoning by alpha-ketoglutarate. Toxicol. Lett. 2002;128:185–195. doi: 10.1016/s0378-4274(02)00012-7. [DOI] [PubMed] [Google Scholar]
- 75.Hansen G.E., Gibson G.E. The α-ketoglutarate dehydrogenase complex as a hub of plasticity in neurodegeneration and regeneration. Int. J. Mol. Sci. 2022;23:12403. doi: 10.3390/ijms232012403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ugur B., Bao H., Stawarski M., Duraine L.R., Zuo Z., Lin Y.Q., Neely G.G., Macleod G.T., Chapman E.R., Bellen H.J. The krebs cycle enzyme isocitrate dehydrogenase 3A couples mitochondrial metabolism to synaptic transmission. Cell Rep. 2017;21:3794–3806. doi: 10.1016/j.celrep.2017.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.TeSlaa T., Chaikovsky A.C., Lipchina I., Escobar S.L., Hochedlinger K., Huang J., Graeber T.G., Braas D., Teitell M.A. α-Ketoglutarate accelerates the initial differentiation of primed human pluripotent stem cells. Cell Metab. 2016;24:485–493. doi: 10.1016/j.cmet.2016.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Asadi Shahmirzadi A., Edgar D., Liao C.-Y., Hsu Y.-M., Lucanic M., Asadi Shahmirzadi A., Wiley C.D., Gan G., Kim D.E., Kasler H.G., Kuehnemann C., Kaplowitz B., Bhaumik D., Riley R.R., Kennedy B.K., Lithgow G.J. Alpha-Ketoglutarate, an endogenous metabolite, extends lifespan and compresses morbidity in aging mice. Cell Metab. 2020;32:447–456. doi: 10.1016/j.cmet.2020.08.004. e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Su Y., Wang T., Wu N., Li D., Fan X., Xu Z., Mishra S.K., Yang M. Alpha-ketoglutarate extends Drosophila lifespan by inhibiting mTOR and activating AMPK. Aging. 2019;11:4183–4197. doi: 10.18632/aging.102045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lylyk M.P., Bayliak M.M., Shmihel H.V., Storey J.M., Storey K.B., Lushchak V.I. Effects of alpha-ketoglutarate on lifespan and functional aging of drosophila melanogaster flies, Ukr. Biochem. J. 2018;90:49–61. doi: 10.15407/ubj90.06.049. [DOI] [Google Scholar]
- 81.Chin R.M., Fu X., Pai M.Y., Vergnes L., Hwang H., Deng G., Diep S., Lomenick B., Meli V.S., Monsalve G.C., Hu E., Whelan S.A., Wang J.X., Jung G., Solis G.M., Fazlollahi F., Kaweeteerawat C., Quach A., Nili M., Krall A.S., Godwin H.A., Chang H.R., Faull K.F., Guo F., Jiang M., Trauger S.A., Saghatelian A., Braas D., Christofk H.R., Clarke C.F., Teitell M.A., Petrascheck M., Reue K., Jung M.E., Frand A.R., Huang J. The metabolite α-ketoglutarate extends lifespan by inhibiting ATP synthase and TOR. Nature. 2014;510:397–401. doi: 10.1038/nature13264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sharma R., Ramanathan A. The aging metabolome—biomarkers to hub metabolites. Proteomics. 2020;20 doi: 10.1002/pmic.201800407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Salminen A., Kaarniranta K. AMP-activated protein kinase (AMPK) controls the aging process via an integrated signaling network. Ageing Res. Rev. 2012;11:230–241. doi: 10.1016/j.arr.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 84.An J., Rao A., Ko M. TET family dioxygenases and DNA demethylation in stem cells and cancers. Exp. Mol. Med. 2017;49:e323. doi: 10.1038/emm.2017.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Harman D. Free radical theory of aging: an update - Increasing the functional life span. Ann. N. Y. Acad. Sci. 2006;1067:10–21. doi: 10.1196/annals.1354.003. [DOI] [PubMed] [Google Scholar]
- 86.Kapogiannis D. Energy metabolism and the brain: a bidirectional relationship. Ageing Res. Rev. 2015;20:35–36. doi: 10.1016/j.arr.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dakshayani K.B., Subramanian P. Alpha-ketoglutarate modulates the circadian patterns of lipid peroxidation and antioxidant status during N-nitrosodiethylamine-induced hepatocarcinogenesis in rats. J. Med. Food. 2006;9:90–97. doi: 10.1089/jmf.2006.9.90. [DOI] [PubMed] [Google Scholar]
- 88.Kabysheva M.S., Storozhevykh T.P., Pinelis V.G., Bunik V.I. Synthetic regulators of the 2-oxoglutarate oxidative decarboxylation alleviate the glutamate excitotoxicity in cerebellar granule neurons. Biochem. Pharmacol. 2009;77:1531–1540. doi: 10.1016/j.bcp.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 89.Thomas S.C., Alhasawi A., Appanna V.P., Auger C., Appanna V.D. Brain metabolism and Alzheimer's disease: the prospect of a metabolite-based therapy. J. Nutr. Health Aging. 2015;19:58–63. doi: 10.1007/s12603-014-0511-7. [DOI] [PubMed] [Google Scholar]
- 90.Gasior M., Rogawski M.A., Hartman A.L. Neuroprotective and disease-modifying effects of the ketogenic diet. Behav. Pharmacol. 2006;17:431–439. doi: 10.1097/00008877-200609000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Johnson S.C., Kayser E.-B., Bornstein R., Stokes J., Bitto A., Park K.Y., Pan A., Sun G., Raftery D., Kaeberlein M., Sedensky M.M., Morgan P.G. Regional metabolic signatures in the Ndufs4(KO) mouse brain implicate defective glutamate/α-ketoglutarate metabolism in mitochondrial disease. Mol. Genet. Metab. 2020;130:118–132. doi: 10.1016/j.ymgme.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wang L., Yi D., Hou Y., Ding B., Li K., Li B., Zhu H., Liu Y., Wu G. Dietary supplementation with α-ketoglutarate activates mTOR signaling and enhances energy status in skeletal muscle of lipopolysaccharide-challenged piglets. J. Nutr. 2016;146:1514–1520. doi: 10.3945/jn.116.236000. [DOI] [PubMed] [Google Scholar]
- 93.Yao K., Yin Y., Li X., Xi P., Wang J., Lei J., Hou Y., Wu G. Alpha-ketoglutarate inhibits glutamine degradation and enhances protein synthesis in intestinal porcine epithelial cells. Amino Acids. 2012;42:2491–2500. doi: 10.1007/s00726-011-1060-6. [DOI] [PubMed] [Google Scholar]
- 94.Bernfeld E., Menon D., Vaghela V., Zerin I., Faruque P., Frias M.A., Foster D.A. Phospholipase D-dependent mTOR complex 1 (mTORC1) activation by glutamine. J. Biol. Chem. 2018;293:16390–16401. doi: 10.1074/jbc.RA118.004972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bodineau C., Tomé M., Courtois S., Costa A.S.H., Sciacovelli M., Rousseau B., Richard E., Vacher P., Parejo-Pérez C., Bessede E., Varon C., Soubeyran P., Frezza C., del S. Murdoch P., Villar V.H., Durán R.V. Two parallel pathways connect glutamine metabolism and mTORC1 activity to regulate glutamoptosis. Nat. Commun. 2021;12:4814. doi: 10.1038/s41467-021-25079-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.