Highlights
-
•
PK2β intraperitoneal administration does not reduce food intake.
-
•
PK2, but not PK2β, activates STAT3 and ERK in hypothalamus explants.
-
•
PKR1-KO mice adipocytes show impaired levels of STAT3 and ERK activation.
-
•
PKR1-KO mice adipocytes present variation of pk2 splicing variants, PK2 and PK2L.
Keywords: Food intake regulation, Adipocytes, Prokineticin 2, Prokineticin 2β, Prokineticin receptor 1
Abstract
The secreted bioactive peptide prokineticin 2 (PK2) is a potent adipokine and its central and peripheral administration reduces food intake in rodents. The pk2 gene has two splice variants, PK2 and PK2L (PK2 long form), which is cleaved into an active peptide, PK2β, that preferentially binds prokineticin receptor 1 (PKR1). We investigated the role of PK2β in the regulation of food intake. We demonstrated that intraperitoneal injection of PK2β, in contrast to PK2, did not reduce food intake in mice. Exposure of hypotalamic explants to PK2, but not PK2β, induced phosphorylation of STAT3 and ERK. We also evidenced that in adipocytes from PKR1 knock-out mice, a model of obesity, there were higher PK2β levels than PK2 inducing a decreased activation of STAT3 and ERK. Our results suggest that variations in PK2 and PK2β levels, due to modulation of pk2 gene splicing processes, affect food intake in mice.
Graphical abstract
Introduction
Regulation of food intake, involving reciprocal signals between the central nervous system and the periphery, allows the storage of an adequate amount of triglycerides in adipose tissue and allows survival during periods of food deprivation. Dysregulation of the hypothalamic mechanisms that control food intake and energy expenditure, and thus energy homeostasis, is currently known to play an important role in weight gain and the development of obesity [1]. The hypothalamic neuropeptide-related effects on feeding behavior can be divided into anorexigenic neuropeptides that reduce food intake, such as α-melanocyte-stimulating hormone (α-MSH) and oxytocin, and orexigenic neuropeptides that stimulate food intake, such as neuropeptide Y (NPY), galanin, agouti-related protein (AgRP), and orexin [2].
Specific adipokines, cytokines secreted by adipocytes, direct the hypothalamus in maintaining energy homeostasis and food intake. In particular, leptin binding to its hypothalamic receptors, activates STAT3 transcription and modulates the expression of α-MSH, NPY and AgRP [3,4].
Prokineticin 2 (PK2) and prokineticin 2β (PK2β) are signaling molecules generated by alternative splicing of the pk2 gene. They bind two G-coupled receptors, PKR1 and PKR2, with different selectivity: while PK2 binds both receptors with the same affinity [5], PK2β binds preferentially to PKR1 and, unlike PK2 [6,7], does not induce STAT3 phosphorylation [5].
The prokineticin system is expressed in various tissues and is involved in numerous essential physiological processes such as: neurogenesis, angiogenesis, tissue development, regulation of circadian rhythm, inflammation, and nociception [8].
Recently, has been demonstrated that PK2, acting as an adipokine, binds PKR1 and, via the hypothalamic ARC melanocortin system, reduces food intake and adipose tissue proliferation [9,10]. Global ablation of PK2 or PKR1 in mice leads to obesity [11,12].
The aim of this study was to investigate whether PK2, acting as an adipokine, reduces STAT3 activation triggered by food intake. We also investigated the role of PK2β in the regulation of feeding and how the concentration ratios between PK2 and PK2β can modulate the activation of the transcription factor STAT3, which affects food intake.
Materials and methods
Expression of PK2 and PK2β in Pichia pastoris
The expression of PK2 and PK2β in yeast Pichia pastoris strain GS115 was performed as described in our previous article [5]. In brief, induction of PK2L synthesis was performed in BMMY for 120 h, with daily addition of 1% methanol. The recombinant proteins were purified from the crude culture supernatants by CM-Sephadex, followed by reverse-phase HPLC on a Vydac C-18 column, as described [13]. Protein concentration was measured by integrating the area of two peaks 33–35 min from the C-18 reverse column and comparing with a natural Bv8 standard.
Animals
Experiments were carried out in male wild-type (WT) and PKR1 knock-out (PKR1-KO) C57BL/6 J mice (generated by Lexicon Genetics the Woodlands, TX) weighing 25–30 g. Mice were housed in individual plastic cages, with light/dark cycle of 12 h, in a temperature-controlled environment (22 ± 2 °C), humidity of 55 ± 10%, with food and water ad libitum. All procedures involving the care or treatment of animals were performed in accordance with EU Directive 2010/63/EU and approved by the Animal Care and Use Committee of the Italian Ministry of Health (approval number 116/2015-PR). Every effort was made to minimize animal suffering and to reduce the number of animals use.
Food intake evaluation
The effects of PK2β and PK2 on food intake were studied in mice at the beginning of the dark phase. Prior to drug administration mice were singly housed in individually ventilated cages and fasted for 24 h. One hour after the light went out, saline, PK2 60 nmol kg−1 and PK2β 60 nmol kg−1 were intraperitoneally (i.p.) administrated in mice (n = 7). After drug administration, a known amount of food returned to the cages, and the food was reweighed 1, 2, 4 and 12 h after injection. The PK2 dose of 60 nmol kg−1 was chosen because it had previously been shown to significantly reduce acute food intake [9].
Adipose tissue and hypothalamus explants
Epididymal adipose tissue was collected from 10 month old WT (n = 4) and PKR1-KO (n = 4) mice, rapidly frozen on dry ice and stored at −80 °C until RNA or protein was extracted. Mouse hypothalami were collected from male 4 month old WT mice (n = 12) immediately following decapitation and placed in oxygenated ice-cold Krebs–Ringer bicarbonate buffer (pH 7.4; 4 °C). Hypothalami were successively cut into 300 μm thick slices using a vibratome and each hypothalamic slice was incubated separately at 37 °C, 5% CO2 and 95% O2 in Dulbecco's Modified Eagle Medium (DMEM, Merck KGaA, Darmstadt, Germany) containing 1% of Fetal Bovine Serum (FBS, Merck KGaA, Darmstadt, Germany) for 2 h [14,15]. Slices were then serum-starved and treated with PK2 (100 nM), PK2β (100 nM) or PK2 plus STAT-3 inhibitor WP1066 (Merck KGaA, Darmstadt, Germany) for 1 h. WP1066 (5 μM) was added 1 h before the addition of PK2 [6]. At the end of the incubation period, the explants were quickly frozen on dry ice and stored at −80 °C until RNA or protein was extracted.
RNA extraction and real time-PCR
Total RNA was isolated from adipose tissue and hypothalamus after homogenization with Trizol reagent (Thermo Fisher Scientific) as shown in the manufacturer's instructions. The RNA concentration was determined spectrophotometrically, and purity was evaluated with 260/280 and 260/230 ratios. The RNA was reverse transcribed using SensiFAST cDNA Synthesis Kit (Meridian Bioscience, Cincinnati, USA) and the cDNA was used to perform Real-Time PCR (iCycler; Bio-Rad, Hercules, California, USA). All reactions were performed with 25 ng cDNA and blank in triplicate using iQ SYBR Green Supermix (Meridian Bioscience, Cincinnati, USA), under the same conditions: polymerase activation at 95 °C for 10 min followed by 40 cycles at 95 °C for 30 s for the denaturation phase, 56–60 °C (depending on the melting temperature of the primer used) for 30 s for the annealing phase and the extension phase at 72 °C for 30 s.
Comparative threshold method was used to quantify the result. The Ct value of the specific gene of interest (Table 1) was normalized to the Ct value of the endogenous control, glyceraldehydes-3-phosphate dehydrogenase (GAPDH), then the comparative Ct method (2−ΔΔCt) was applied using WT mice as the reference.
Table 1.
Primers used for quantitative real-time PCR.
| Gene | Forward | Reverse |
| PK2 | 5′-CTC GGA AAG TTC CAT TTT GG-3′ | 5′-TTC CGG GCC AAG CAA ATA AAC C-3′ |
| PK2L | 5′-CAA ATG GAA GGC AGG AAA GAA G-3′ | 5′-TTC CGG GCC AAG CAA ATA AAC C-3′ |
| SOCS-3 | 5′-ACC TTC AGC TCC AAA AGC GAG TAC-3′ | 5′-CGC TCC AGT AGA ATC CGC TCT C-3′ |
| GAPDH | 5′-GCC AAG GCT GTG GGC AAG GT-3′ | 5′-TCT CCA GGC GGC ACG TCA GA-3′. |
Western blotting analysis
Tissue was lysed, proteins were extracted and quantified by the Bradford method. 20 μg of total proteins were mixed with standard Laemmli buffer, heated at 95 °C for 3 min and loaded onto a 10% SDS-PAGE gel which was analyzed by Western Blotting as described above. Experiments were performed using rabbit anti-ERK (Santa Cruz, sc-153) and mouse anti-p-ERK (Cell Signaling Technology, #9106S), mouse anti-STAT3 (MA1–13,042) and rabbit anti-p-STAT3(Tyr705) (# 44–380 G) (Invitrogen-Thermo Fisher Scientific). Quantification of the signal intensity of the western blotting bands was performed using ImageJ software and relative protein expression was calculated after normalization to total protein of interest. Data were obtained from three separate experiments.
Data analysis
All data are represented as mean ± SEM. One-way ANOVA followed by Tukey's post test and Student's t-test were performed when appropriate, using GraphPad Prism 6. Differences were considered significant at p <0.05.
Results
PK2β peripheral administration does not reduce food intake
As previously demonstrated, intraperitoneal administration of 60 nmol kg−1 PK2 significantly reduced 0–1 h food intake in WT mice [10]. The same dose of PK2β was ineffective at 0–1 h and showed a non-significant trend to increase at 2–4 h compared to saline controls (Fig. 1).
Fig. 1.
Evaluation of food intake in WT mice following i.p. injection of saline, PK2 (60 nmol kg−1) or PK2β (60 nmol kg−1). Data are expressed as mean ± SEM. Statistical analyses were performed using One-way ANOVA followed by Tukey's post test. **p < 0.01 vs saline, n = 7 mice per group.
PK2 but not PK2β activates STAT3 in hypothalamus explants
We evaluated STAT3 phosphorylation by Western Blot analysis in hypothalamic ex vivo explants incubated with PK2, PK2 plus WP1066 (STAT3 inhibitor) or PK2β. PK2 exposure significantly increased STAT3 phosphorylation and this activation was abolished by pretreatment with the STAT3 inhibitor WP1066 (Fig. 2). Conversely, p-STAT3 levels after incubation with PK2β were comparable to those observed in control explants (Fig. 2).
Fig. 2.
STAT3 activation in hypothalamic explants treated with PK2 (100 nM), PK2 (100 nM) plus STAT3 inhibitor WP1066 (5 μM) and PK2β (100 nM). Representative Western Blot analysis shows phospho-STAT3 (p-STAT3) and STAT3 protein. Bar graphs show densitometric analysis of p-STAT3/STAT3 protein ratio expressed as percentage of controls. Data are expressed as mean ± SEM of three separate experiments (n = 3 per group). Statistical analyses were performed using One-way ANOVA followed by Tukey's post test where ***p < 0.001 vs CTRL; °°p < 0.01 vs PK2.
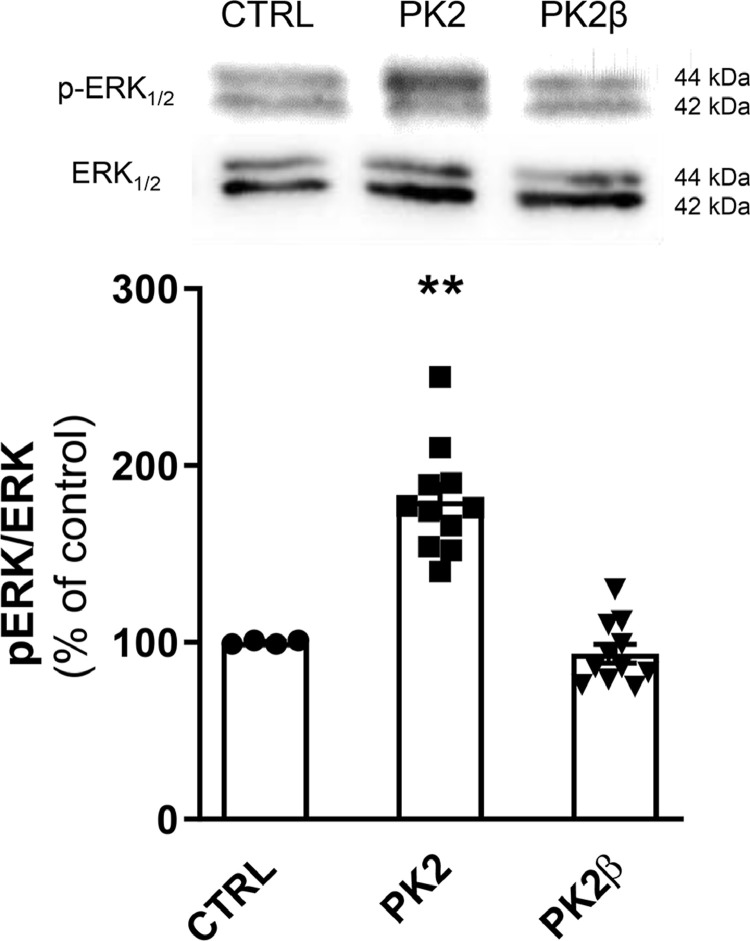
PK2 but not PK2β activates ERK in hypothalamus explants
We analyzed, by Western Blot, ERK activation in hypothalamic ex vivo explants treated with PK2 or PK2β. Exposure to PK2 significantly increased ERK phosphorylation compared to control explants. Conversely, p-ERK levels after incubation with PK2β were comparable to those observed in control explants (Fig. 3).
Fig. 3.
ERK activation in hypothalamic explants treated with PK2 (100 nM) and PK2β (100 nM). Representative Western Blot analysis shows p-ERK and ERK protein. Bar graphs show densitometric analysis of p-ERK/ERK protein ratio expressed as a percentage of controls. Data are expressed as mean ± SEM of three separate experiments (n = 3 per group). Statistical analyses were performed using One-way ANOVA followed by Tukey's post test where **p < 0.01 vs CTRL.
Adipocytes from PKR1-KO mice show impaired levels of STAT3 and ERK activation
In adipose tissue from WT and PKR1-KO mice, we examined the phosphorylation of STAT3 and ERK. We performed western blotting experiments on total proteins using anti-STAT3/p-STAT3 and anti-ERK/p-ERK antibodies. As shown in Fig. 4, we demonstrated that there was activation of STAT3 and ERK proteins in adipocytes of WT mice (Fig. 4A, B). Conversely, in PKR1-KO mice the levels of p-STAT3 and p-ERK were significantly reduced. Experiments were performed in animals at 40 weeks of age, as this is the known age at which the obese phenotype is achieved [16].
Fig. 4.
STAT3 and ERK activation in adipocytes from WT and PKR1-KO mice. Representative western blot analysis showing the ratio of protein levels of p-STAT3/STAT3 (A) and p-ERK/ERK (B), performed on adipose tissue collected from 40 week old mice. Bar graphs show densitometric analysis of the ratio of p-STAT3/STAT3 and p-ERK/ERK proteins expressed as percentage of controls. Real Time RT-PCR analysis of SOCS-3 mRNA levels in adipose tissue from 15- and 40 week old WT and PKR1-KO mice (C). Data are expressed as mean ± SEM of three separate experiments (n = 4 per group). Statistical analyses were performed using Student's t-test where *p< 0.05; **p < 0.01 vs WT.
STAT3 regulates the transcription of the Socs-3 gene [17]. A decrease in SOCS-3 mRNA levels was observed in the adipose tissue 40 week old PKR1-KO mice compared to WT mice. Conversely, SOCS-3 mRNA levels in adipose tissue of 15 week old PKR1-KO mice are significantly higher compared to WT mice (Fig. 4C).
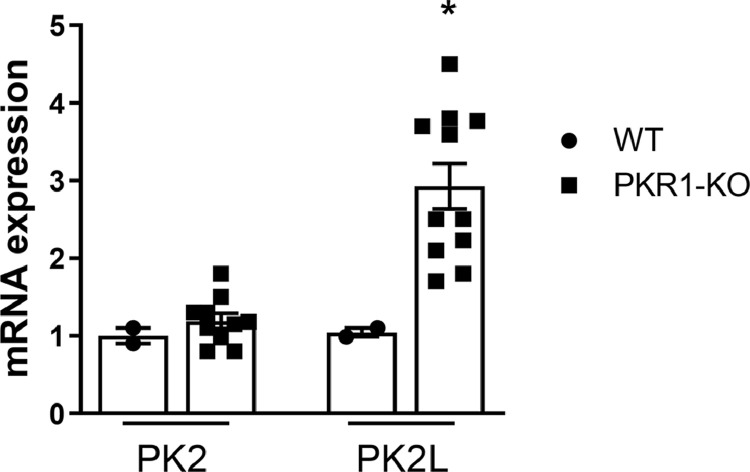
PKR1-KO mice adipocytes present variation of pk2 splicing gene
The expression levels of PK2 are comparable in the adipose tissue of WT and PKR1-KO mice whereas the levels of PK2L mRNA increase significantly in the adipocytes of PKR1-KO mice (Fig. 5). PK2L mRNA is the longer transcript of the pk2 gene. The PK2L protein produced undergoes rapid to proteolytic cleavage generating PK2β [18]. Thus, it is possible to quantify PK2β transcriptional expression by evaluating PK2L mRNA levels.
Fig. 5.
Real Time RT-PCR analysis showing PK2 and PK2L mRNA levels in adipocytes from WT and PKR1-KO mice. Data are expressed as mean ± SEM of three separate experiments (n = 4 per group). Statistical analyses were performed using Student's t-test where *p < 0.05 vs WT.
The increase in PK2β levels was found only in 40 week old PKR1-KO mice, in agreement with published data [16].
Discussion
Alternative splicing is an important mechanism for the expansion of proteome diversity, and incorrect splicing is associated with a large array of human diseases; in particular, significant changes in exon skipping and in splicing factor expression are observed in pathological conditions of obesity [19].
Alternative splicing of the pk2 gene generates two splice variants with different specificity and activity: PK2 consists of 81 amino acids and binds with the same affinity to both prokineticin receptors PKR1 and PKR2, while the PK2β peptide of 57 amino acids, derived from proteolytic cleavage of the long-form PK2L, binds explicitly to PKR1 [5,18]. PK2β is a biased agonist compared to PK2 as it is unable to couple Gαi and to activate STAT3 [5], [6], [7].
The aim of this work was to establish the role of PK2β in the regulation of food intake. It has been previously shown that intraperitoneal and intracerebroventricular injection of PK2 strongly inhibits food intake in rodents [9,10]. In contrast, our results show that in mice, intraperitoneal injection of PK2β does not reduce food intake.
Leptin regulation of food intake in the hypothalamus is modulated by STAT3, which induces the expression of anorectic peptides, such as α-MSH, and downregulates orexigenic peptides [20]. PK2 has been shown to increase the release of α-MSH from ex vivo hypothalamic explants [9,10].
Our results from ex vivo hypothalamic explants suggest that PK2 triggers STAT3 activation. Conversely, treatment of hypothalamic explants with PK2β does not induce STAT3 phosphorylation. These results suggest that STAT3 regulates the PK2-induced anorectic action and explain the inability of PK2β to induce a comparable effect. Indeed, a reduction in STAT3 activation has been shown to lead to dysregulation of energy metabolic pathways that promote obesity [21,22].
Since in some pathological conditions, such as obesity, the splicing process is dysregulated, as mentioned above, we investigated the modulation of pk2 gene splicing. As a model for obese adipocytes, we used adipocytes from PKR1-KO mice because they exhibit a hypoxic state with a high concentration of HIF-1α [16]: the increased size of adipocytes reduces oxygen diffusion from the vasculature.
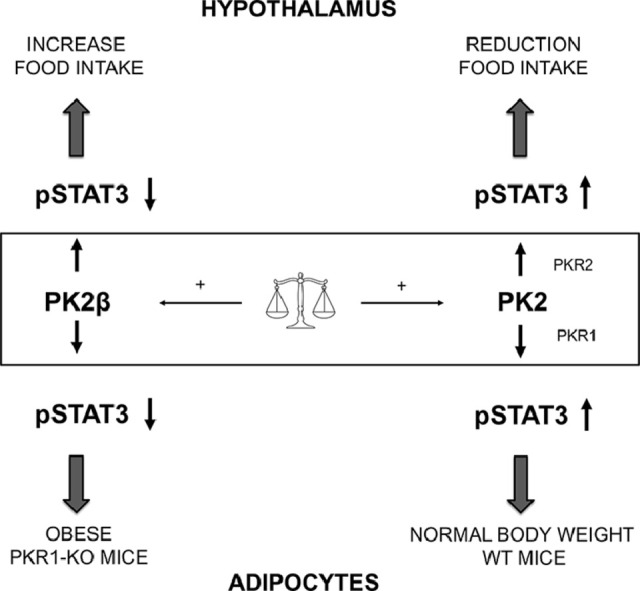
PKR1-KO mice show a diabetic phenotype: in particular, reduced glucose tolerance in the 15-week-old mice [16], low expression of insulin receptors and low levels of insulin-stimulated Akt phosphorylation [23]. Based on our results, we can hypothesize that the low insulin response may also be due to an increase in SOCS3 levels in adipocytes from 15-week-old PKR1-KO mice. SOCS-3 inhibits IR-dependent IRS-1 tyrosine phosphorylation, thereby suppressing insulin-stimulated glucose uptake [24], leading to local insulin resistance in adipose tissue [25]. The induction of SOCS-3 transcription is likely due to the increased TNFa levels that determine NFκB activation in PKR1-KO mice [16]. We have shown that in adipocytes from PKR1-KO mice, PK2β concentration is increased compared to PK2. This increase in PK2β levels may explain the decreased activation of STAT3 and ERK in PKR1-KO mice. The decreased activation of STAT3, a key factor in the regulation of metabolic pathways in the cell [23], which is also affected by a decrease in leptin levels [23], may explain why PKR1-KO mice show dysregulation in lipid and carbohydrate metabolism, as reflected by high FFA and glucose levels in the blood [16,23].
The regulation of fuel stores and energy balance is a complex physiological system, coordinated by the action of numerous transcription factors acting at different levels. Under normal physiological conditions, PK2 and PK2β levels are balanced and PK2 expression is cell-specific and regulated by STAT3 and HIF-1α [6,7,26], but in obesity, alterations in pk2 gene expression and splicing processes could increase the levels concentration of either splice variant, affecting food intake.
Previously, an increase in PK2/PK2β levels has been demonstrated in adipocytes of human obese individuals [16], without distinguishing the prevalence of either form.
In this study, we analyzed for the first time, the expression levels ratio between PK2 and PK2β, demonstrating a strong increase of PK2β levels in the adipocytes of obese mice.
Conclusion
In this study, we brought evidence that peripheral administration of PK2β shows a tendency to increase food intake, likely due to its impaired ability to induce STAT3 phosphorylation in the hypothalamus. In adipocytes from PKR1-KO mice, we detected a high level of PK2β compared with PK2, which correlates with impaired STAT3 and ERK activation. In summary, our data show that changes in pk2 gene splicing processes with variations in PK2 and PK2β expression levels in the hypothalamus and adipocytes play a role in appetite regulation.
Declaration of Competing Interest
The authors claim no conflict of interest.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- 1.Hill J.O., Wyatt H.R., Peters J.C. Energy balance and obesity. Circulation. 2012;126(1):126–132. doi: 10.1161/CIRCULATIONAHA.111.087213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coll A.P., Farooqi I.S., O’Rahilly S. The hormonal control of food intake. Cell. 2007;129(2):251–262. doi: 10.1016/j.cell.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gao Q., Wolfgang M.J., Neschen S., Morino K., Horvath T.L., Shulman G.I., Fu X.Y. Disruption of neural signal transducer and activator of transcription 3 causes obesity, diabetes, infertility, and thermal dysregulation. PNAS. 2004;101(13):4661–4666. doi: 10.1073/pnas.0303992101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hu W., Lv J., Han M., Yang Z., Li T., Jiang S., Yang Y. STAT3: the art of multi-tasking of metabolic and immune functions in obesity. Prog. Lipid. Res. 2018;70:17–28. doi: 10.1016/j.plipres.2018.04.002. [DOI] [PubMed] [Google Scholar]
- 5.Lattanzi R., Maftei D., Negri L., Fusco I., Miele R. PK2β ligand, a splice variant of prokineticin 2, is able to modulate and drive signaling through PKR1 receptor. Neuropeptides. 2018;71:32–42. doi: 10.1016/j.npep.2018.06.005. [DOI] [PubMed] [Google Scholar]
- 6.Qu X., Zhuang G., Yu L., Meng G., Ferrara N. Induction of Bv8 expression by granulocyte colony-stimulating factor in CD11b+Gr1+ cells: key role of Stat3 signaling. J. Biol. Chem. 2012;287(23):19574–19584. doi: 10.1074/jbc.M111.326801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xin H., Lu R., Lee H., Zhang W., Zhang C., Deng J., Liu Y., Shen S., Wagner K.U., Forman S., Jove R., Yu H. G-protein-coupled receptor agonist Bv8/prokineticin-2 and STAT3 protein form a feed-forward loop in both normal and malignant myeloid cells. J. Biol. Chem. 2013;288(19):13842–13849. doi: 10.1074/jbc.M113.450049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Negri L., Ferrara N. The Prokineticins: neuromodulators and mediators of inflammation and myeloid cell-dependent angiogenesis. Physiol. Rev. 2018;98(2):1055–1082. doi: 10.1152/physrev.00012.2017. [DOI] [PubMed] [Google Scholar]
- 9.Gardiner J.V., Bataveljic A., Patel N.A., Bewick G.A., Roy D., Campbell D., Greenwood H.C., Murphy K.G., Hameed S., Jethwa P.H., Ebling F.J.P., Vickers S.P., Cheetham S., Ghatei M.A., Bloom S.R., Dhillo W.S. Prokineticin 2 Is a Hypothalamic Neuropeptide That Potently Inhibits Food Intake. Diabetes. 2010;59(2):397–406. doi: 10.2337/db09-119819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beale K., Gardiner J.V., Bewick G.A., Hostomska K., Patel N.A., Hussain S.S., Jayasena C.N., Ebling F.J.P., Jethwa P.H., Prosser H.M., Lattanzi R., Negri L., Ghatei M.A., Bloom S.R., Dhillo W.S. Peripheral administration of prokineticin 2 potently reduces food intake and body weight in mice via the brainstem. Br. J. Pharmacol. 2012;168(2):403–410. doi: 10.1111/j.1476-5381.2012.02191.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Von Hunolstein J.J., Nebigil C.G. Can prokineticin prevent obesity and insulin resistance? Curr. Opin. Endocrinol. Diabetes. Obes. 2015;22(5):367–373. doi: 10.1097/MED.0000000000000185. [DOI] [PubMed] [Google Scholar]
- 12.Nebigil C.G. Prokineticin Is a New Linker between Obesity and Cardiovascular Diseases. Front. Cardiovasc. Med. 2017;4:20. doi: 10.3389/fcvm.2017.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miele R., Lattanzi R., Bonaccorsi Di Patti M.C., Paiardini A., Negri L., Barra D. Expression of Bv8 in Pichia Pastoris to identify structural features for receptor binding. Protein. Expr. Purif. 2010;73(1):10–14. doi: 10.1016/j.pep.2010.04.012. [DOI] [PubMed] [Google Scholar]
- 14.Rucinski M., Ziolkowska A., Szyszka M., Hochol A., Malendowicz L.K. Evidence suggesting that ghrelin O-acyl transferase inhibitor acts at the hypothalamus to inhibit hypothalamo-pituitary-adrenocortical axis function in the rat. Peptides. 2012;35(2):149–159. doi: 10.1016/j.peptides.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 15.Kruse K.S., Suarez L.G., Coirini H. Regulation of the expression of LXR in rat hypothalamic and hippocampal explants. Neurosci. Lett. 2017;639:53–58. doi: 10.1016/j.neulet.2016.12.065. [DOI] [PubMed] [Google Scholar]
- 16.Szatkowski C., Vallet J., Dormishian M., Messaddeq N., Valet P., Boulberdaa M., Metzger D., Chambon P., Nebigil C.G. Prokineticin receptor 1 as a novel suppressor of preadipocyte proliferation and differentiation to control obesity. PLoS ONE. 2013;8(12):81175. doi: 10.1371/journal.pone.0081175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kershaw N.J., Murphy J.M., Lucet I.S., Nicola N.A., Babon J.J. Regulation of Janus Kinases by SOCS proteins. Biochem. Soc. Trans. 2013;41(4):1042–1047. doi: 10.1042/BST20130077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen J., Kuei C., Sutton S., Wilson S., Yu J., Kamme F., Mazur C., Lovenberg T., Liu C. Identification and pharmacological characterization of prokineticin 2 beta as a selective ligand for prokineticin receptor 1. Mol. Pharmacol. 2005;67(6):2070–2076. doi: 10.1124/mol.105.011619. [DOI] [PubMed] [Google Scholar]
- 19.Wong C.M., Xu L., Yau M.Y.C. Alternative mRNA Splicing in the Pathogenesis of Obesity. Int. J. Mol. Sci. 2018;19(2):632. doi: 10.3390/ijms19020632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aaronson D.S., Horvath C.M. A road map for those who don’t know JAK–STAT. Science. 2002;296(5573):1653–1655. doi: 10.1126/science.1071545. [DOI] [PubMed] [Google Scholar]
- 21.Jiang C., Kim J.H., Li F., Qu A., Gavrilova O., Shah Y.M., Gonzalez F.J. Hypoxia-inducible factor 1α regulates a SOCS3-STAT3-adiponectin signal transduction pathway in adipocytes. J. Biol. Chem. 2013;288(6):3844–3857. doi: 10.1074/jbc.M112.426338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lu H., Gao Z., Zhao Z., Weng J., Ye J. Transient hypoxia reprograms differentiating adipocytes for enhanced insulin sensitivity and triglyceride accumulation. Int. J. Obes. 2016;40(1):121–128. doi: 10.1038/eye.2015.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dormishian M., Turkeri G., Urayama K., Nguyen T.L., Boulberdaa M., Messaddeq N., Renault G., Henrion D., Nebigil C.G. Prokineticin Receptor-1 Is a New Regulator of Endothelial Insulin Uptake and Capillary Formation to Control Insulin Sensitivity and Cardiovascular and Kidney Functions. J. Am. Heart. Assoc. 2013;2(5) doi: 10.1161/JAHA.113.000411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cawthorn W.P., Sethi J.K. TNF-α and adipocyte biology. FEBS Lett. 2008;582(1):117–131. doi: 10.1016/j.febslet.2007.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shi H., Cave B., Inouye K., Bjorbaek C., Flier J.S. Overexpression of suppressor of cytokine signaling 3 in adipose tissue causes local but not systemic insulin resistance. Diabetes. 2006;55:699–707. doi: 10.2337/diabetes.55.03.06.db05-0841. [DOI] [PubMed] [Google Scholar]
- 26.LeCouter J., Zlot C., Tejada M., Peale F., Ferrara N. Bv8 and endocrine gland-derived vascular endothelial growth factor stimulate hematopoiesis and hematopoietic cell mobilization. PNAS. 2004;101(48):16813–16818. doi: 10.1073/pnas.0407697101. [DOI] [PMC free article] [PubMed] [Google Scholar]