Abstract
It is now well established that transition metals, such as Iron (Fe), Copper (Cu), and Zinc (Zn) are necessary for healthy brain function. Although Fe, Cu, and Zn are essential to the brain, imbalances in the amount, distribution, or chemical form (“metallome”) of these metals is linked to the pathology of numerous brain diseases or disorders. Despite the known importance of metal ions for both brain health and disease, the metallome that exists within specific types of brain cells is yet to be fully characterised. The aim of this mini-review is to present an overview of the current knowledge of the metallome found within specific brain cells (oligodendrocytes, astrocytes, microglia, and neurons), as revealed by direct elemental mapping techniques. It is hoped this review will foster continued research using direct elemental mapping techniques to fully characterise the brain cell metallome.
Keywords: Microscopy, Metals, Neuroscience, Hippocampus, Neurodegeneration
Introduction
In general terms, brain cells can be classified into two types, neurons and glia. The defining anatomical features of neurons are dendrites and axons, and their defining physiological characteristic is the ability to generate an action potential. Neurons can be further divided into a range of sub-classes, such as pyramidal neurons, motor neurons, Purkinje neurons, and interneurons to name a few. Even within these classes, further sub-division is possible based on the nature of synaptic connections and the neurotransmitters used [1,2]. In contrast to neurons, glial cells do not generate an action potential. Although glia contain many fibre like “processes”, they do not contain dendrites or axons. As with neurons, the glia lineage can be further broken down into cell types, such as astrocytes, microglia, and oligodendrocytes. In general, astrocytes provide critical metabolic support for brain neurons, microglia are the resident immune cells of the brain, and oligodendrocytes produce the lipid rich myelin sheath that insulates axons [3], [4], [5]. Indeed, as is the case with neurons and interneurons, further sub-division and classifications of astrocytes, microglia, and oligodendrocytes is possible, but is beyond the scope of this review.
In recent years there have been major advances in our understanding of the metabolome, genome, and proteome of different types of brain cells, and how this relates to healthy brain function, or brain malfunction (e.g., during disease or after injury). Despite the large advances in metabolomics, proteomics, and genomics, our understanding of brain metallomics remains largely incomplete. The cell metallome refers to the complement of different chemical forms of metal ions that exist within a cell. It is critical for the field of neuroscience to continue to further identify, characterise, and understand the metallome within different brain cells, as there is substantive evidence linking balanced metal homoeostasis to healthy brain function [6], [7], [8], [9], [10], [11]. Further, metal dyshomeostasis is strongly implicated in neurodegeneration and cognitive decline in multiple diseases (e.g., Alzheimer's disease, [6,[12], [13], [14], [15], [16], [17], [18], [19]] Parkinson's disease [20], [21], [22], [23], amyotrophic lateral sclerosis [24,25], multiple sclerosis [26], [27], [28]), neurological disorders (e.g., epilepsy [29], [30], [31]), or following brain injuries (e.g., traumatic injury [32], [33], [34] and stroke [35], [36], [37], [38], [39], [40], [41]).
As a consequence of coordination chemistry, the transition metal ions (Fe, Cu, and Zn) are able to deliver a diverse range of chemical function to support cell biology. A detailed description of the wide range of cell processes supported by metal ions is beyond the scope of this review, but many excellent reviews already exist on the topic [6,[8], [9], [10], [11],[42], [43], [44], [45]]. Detailed knowledge of the differences in the metallome between cell types and cell sub-types can shed vital information on the inherent functioning of brain cells, or identify potential pathways or conditions that may render certain cells vulnerable to damage or impaired function [12,13,46,47]. As an example, the classical histochemical method for detecting brain Fe, the Perl's stain, has repeatedly shown oligodendrocytes are enriched in Fe [48,49]. Knowledge of the abundance of histochemically detectable Fe within oligodendrocytes has been linked to the high metabolic turnover required for myelin synthesis [4,50]. This knowledge of Fe metallomics within oligodendrocytes has directed research to investigate the possible susceptibility of oligodendrocytes to oxidative stress during disease or injury, along an axis of Fe-mediated free-radical production [51]. Another prominent example of where knowledge of brain metallomics has provided valuable insight into disease pathology, was the discovery of metal enrichment within amyloid-β plaques during Alzheimer's disease [52], [53], [54], [55], [56], [57], [58], [59]. Fe, Cu, and Zn enrichment has been observed in amyloid-β plaques, in human and pre-clinical animal tissues [52], [53], [54], [55], [56], [57], [58], [59], which subsequently drove studies to reveal that metal ions can catalyse fibril formation, possibly driving or accelerating plaque formation and associated pathologies (e.g. oxidative stress) [16,[60], [61], [62], [63], [64], [65]].
The above examples are just two cases where knowledge of the brain metallome provides vital insight into pathways of disease. Despite the clear importance of studying the metallome of brain cells, progress is not always easy, largely due to the difficulty in imaging and quantifying the different chemical forms of metal ions found in different brain cells. Although a range of cells can be grown in cell culture, it is well established that brain cells in vitro can be metabolically and physiologically different to brain cells in vivo [66]. It is therefore, imperative to study the metallome of different brain cell types and sub-types in conditions as close as possible to that found in vivo. There is now a suite of direct elemental mapping techniques available to characterise the metallome of individual brain cells, in situ within ex vivo brain tissue sections. Such techniques include X-ray fluorescence (XRF) microscopy, [67], [68], [69] laser-ablation inductively coupled plasma mass spectrometry (LA-ICP-MS) [70], [71], [72], [73], [74], [75], [76], secondary ion mass spectrometry (SIMS), [77], [78], [79] and proton induced X-ray emission spectrometry (PIXE) [80], [81], [82], [83]. The application of these elemental mapping techniques, which are direct and label free, has become an attractive analytical option for the field of neuroscience, as they can minimise or prevent distortions in metal ion homoeostasis that are known to occur during sample preparations associated with histochemical methods [84], [85], [86], [87], [88], [89].
A detailed description of the techniques themselves is beyond the scope of this review, however the interested reader will find detailed descriptions of XRF, LA-ICP-MS, SIMS, and PIXE, in the literature cited herein. As a general overview: XRF offers the capability for in situ analysis of dehydrated (freeze-dried or air-dried) tissue sections at ambient temperature and pressure, or analysis of hydrated tissue sections (frozen) under cryogenic conditions. The method typically provides detection limits approximately at the PPM level, with spatial resolution ranging from 1–30 µm (microprobe) or 100–500 nm (nanoprobe) [67], [68], [69]. PIXE has similar capabilities to XRF, but generally the spatial resolution and detection limits are slightly poorer, and analysis under vacuum conditions is required, which prevents analysis of hydrated specimens [80], [81], [82], [83]. LA-ICP-MS offers superior detection limits compared to XRF (PPB levels), however spatial resolution is typically on the order of 10′s of µm (1 µm is possible with state-of-the-art instrumentation, but comes at the expense of sensitivity) [70], [71], [72], [73], [74], [75], [76]. LA-ICP-MS is very well suited for scanning large sample regions with high sensitivity. SIMS offers excellent detection limits (PPM to PPB) and outstanding spatial resolution (e.g., ∼ 50 nm), however the measurement is highly surface sensitive (which can be an advantage or disadvantage, depending on the application) and vacuum conditions are required [77], [78], [79].
A detailed discussion of the sample preparation considerations is not presented in this review, however there exists a range of studies that have examined in detail the effects of sample preparation on metal ion content and distribution in brain tissues [84], [85], [86], [87], [88], [89]. In addition to the effects of sample preparation, careful consideration must be given when interpreting the results of indirect analyzes (e.g., detection of metal ions using histochemistry). As is the case for metal ion redistributions that can occur during chemical fixation and sample preparation, there is substantial opportunity for metal ion redistribution to occur during staining steps in histochemical protocols. Furthermore, histochemical methods (or metal-sensitive fluorescence sensors) may display heightened sensitivity to specific chemical forms of a metal ion, and insensitivity towards other chemical forms (often by design). The variability in sensitivity of detection that exists between different chemical forms of metal ions should always be thoroughly considered when interpreting the results from analyses using indirect analytical methods. The direct elemental mapping techniques discussed in this review (e.g., XRF, LA-ICP-MS, PIXE, SIMS) are advantageous as they do not show preferential sensitivity to a specific chemical form of a metal ion. However, it should also be stated that in some applications increased sensitivity towards a specific chemical form of a metal ion may offer more in-depth mechanistic insight.
Herein, this review examines current knowledge regarding the metallome of Fe, Cu, and Zn within different types of brain cells, neurons and glia, which has been determined using direct elemental mapping techniques. Specific focus is given to studies of cellular metal content determined from tissue sections, as opposed to in vitro cell culture.
Discussion
Zinc (Zn)
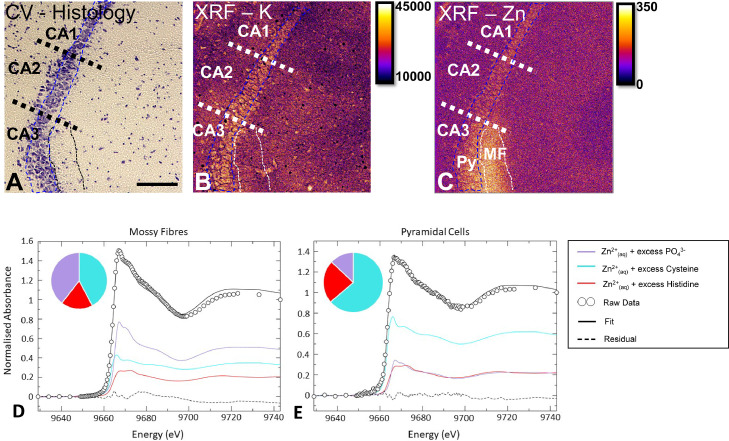
The brain is particularly enriched in Zn (∼150 µM), [90] of which a substantial portion is mobile and labile (i.e., the chelatable Zn2+ pool) [9,[90], [91], [92], [93]]. The chelatable Zn2+ pool has long been detected with classical histochemical stains, such as the methods developed by Timm's and Danscher [91,[94], [95], [96]]. In addition to histochemical detection, fluorescent Zn chelators, such as TSQ and Newport Green (to name a few) have been developed to detect labile Zn2+ [92,97]. The chelatable Zn2+ pool is released during neurotransmission from the synapses of a subset of excitatory glutamatergic neurons (“zincergic” neurons) [6,9,98]. The zincergic neurons are enriched in specific brain regions, including the CA3 sector of the hippocampus, layer II/III and V of the cortex, and olfactory bulb [90,91]. The exact chemical pathways modulated by chelatable Zn within zincergic neurons remains unknown, as does the specific chemical form of labile Zn. However, depletion of chelatable brain Zn, either by dietary induced Zn deficiency, or via disease pathologies, is associated with cognitive impairment [98]. Likewise genetic manipulation (Zn-transporter 3 knockout, ZnT3-KO) depletes chelatable Zn in the hippocampus and induces memory deficits that resemble facets of dementia [15,93]. Interestingly, while much research attention in the field of Alzheimer's disease has focussed on the accumulation of Zn in amyloid-β plaques, a recent study used elemental mapping to demonstrate that the neuropil in the CA3 mossy fibre sub-region of the hippocampus becomes depleted in Zn, adjacent to Zn enriched plaques [52]. This finding provides additional evidence through which amyloid plaques may effect cognitive function during Alzheimer's disease (i.e. through depletion of the local labile Zn pool).
Direct elemental mapping has made valuable contributions to our understanding of the chelatable Zn pool, including a seminal study in which synchrotron radiation XRF was used to quantify the magnitude of hippocampal Zn depletion that occurred in ZnT3-KO mice [93]. More recently, synchrotron radiation XRF has been used to identify depleted Zn within the tissue region that contains the synaptic terminals of the CA3 zincergic neurons (the “mossy fibres”), in an animal model of accelerated ageing [99]. Although synchrotron radiation XRF probes used in that study did not have sufficient spatial resolution to resolve individual synapses, the fact that the mossy fibre region contains almost exclusively synapses is strong evidence for synaptic Zn depletion. Possibly of little surprise, the cell body (soma) of CA3 pyramidal neurons has also been confirmed to be particularly enriched in Zn, relative to adjacent CA2 and CA1 pyramidal neurons (Fig. 1) [100]. In addition, recent work using X-ray absorption near-edge structure (XANES) spectroscopy has revealed distinctive differences in the chemical form of Zn found in the neuron cell body (soma) compared to tissue regions containing synapses [101]. Given the important role of chelatable Zn in memory function, and the fact that depletion in chelatable Zn is associated with cognitive decline, there has been much interest in modulating or restoring Zn pools in the brain [46]. Studies using direct elemental mapping with XRF or LA-ICP-MS have made several important contributions by revealing that various therapeutic compounds restore Zn across brain regions [46,47]. There is much potential for future studies to employ elemental mapping techniques (XRF, LA-ICP-MS, SIMS, PIXE) in combination with techniques that reveal Zn speciation (e.g., XANES) [101], [102], [103], to greatly improve our understanding of the chemical mechanisms through which Zn modulates brain function (as highlighted in Fig. 1).
Fig. 1.
Imaging the Zn metallome in brain neurons. (A) Cresyl violet histology showing the hippocampal pyramidal neuron cell layer (blue dashed region), which can be divided into 3 sub-regions, containing phenotypically different pyramidal cells (CA1, CA2, CA3). The dendrites of the CA3 pyramidal cells are enriched in Zn, and this anatomical location is known as the “mossy fibres” (black or white dash region). (B) K elemental map, collected using synchrotron radiation XRF, allows visualisation of individual cells. (C) Zn elemental map indicates the abundance of Zn within the mossy fibre region. Panels A-C are on the same scale, scale bar in A = 100 µm. Py = pyramidal cell, MF = mossy fibres. Areal density units for panel B, C are ng cm−2. (D) X-ray absorption near edge structure spectroscopy (XANES) can be used in combination with XRF, to determine the chemical form of Zn in specific brain regions, such as the mossy fibres, or E) the pyramidal neurons.
Panels A-C adapted with permission from reference 100. Panels D, E adapted with permission from reference 101.
Copper (Cu)
Cu in Astrocytes– Cu is an essential trace element for a multitude of biochemical pathways in the brain, where it is incorporated into enzymes or serves as a critical cofactor to support: metabolism (Cytochrome c oxidase), anti-oxidant defence (super-oxide dismutase) and neurotransmitter synthesis (Dopamin-b-monoxygenase) [11,104]. The import, storage, and subsequent export or transport of Cu ions is vital to healthy brain function, and astrocytes (from the glial cell lineage) are central to these roles [104]. The “end-feet” of astrocyte processes form a key component of the blood-brain barrier (BBB), which enables astrocytes direct control of Cu entry into the brain across the BBB [105]. Astrocytes and another specialised class of glial cells, ependymal cells, line the ventricle walls of the brain, and form a key component of the brain – ventricle barrier, which is also responsible for Cu import / export in the brain [105]. Consistent with in vivo roles for Cu import and storage, astrocytes have remarkable capacity to safely sequester Cu ions in vitro [104].
Direct elemental mapping techniques have provided valuable information on the Cu levels within astrocytes, highlighting how astrocyte Cu homoeostasis changes during ageing [106,107]. One would expect that with a prominent role in Cu import and storage, astrocytes would be enriched in Cu, and indeed this has been directly confirmed using elemental mapping [100,[106], [107], [108], [109], [110]]. Multiple research groups have used synchrotron radiation XRF to demonstrate Cu enrichment in astrocytes and ependymal cells of the brain lateral ventricles, and also Cu enrichment within the corpus callosum white matter, in mice, rats, and human tissues (as shown in Fig. 2) [100,[106], [107], [108], [109], [110], [111]]. Studies by Pushkar et al., used an especially elegant approach of multi-modal XRF and immuno-histochemistry, to convincingly show Cu enrichment in a subset of astrocytes [106,107,109]. Further, the works by Pushie et al., [111] highlight a role for Prion Protein in brain Cu homoeostasis, with elevated Prion Protein expression resulting in increased Cu levels along the ependymal cell lining of the lateral ventricle wall. Interestingly, but perhaps not surprisingly, Cu transport and storage in the brain is not static, and Cu accumulation is observed in glial cells during ageing, concomitant with decreased Cu transport into the brain [104,105]. LA-ICP-MS in combination with 67Cu auto-radiography played a key role in identifying the association between total brain Cu accumulation during natural ageing and decreased Cu import into the brain [74].
Fig. 2.
Localising the Cu metallome within astrocytes. (A) A representative example of Cu enrichment within cells of the corpus callosum, which are most likely astrocytes, as proven by other studies.106–109 (B-E) Cu enrichment is also observed in the ependymal cell layer of the lateral ventricles, which can be seen with a comparison of B) Cl distribution, C) K distribution, D) Cu distribution, and E) overlay. Scale bar in A = 500 µm. Scale bar in E = 100 µm. Areal density units for panel A is ng cm−2.
Panel A is adapted with permission from reference 100. Panels B-E are adapted with permission from reference 110.
The physiological consequences of Cu accumulation within astrocytes during ageing, on a background of decreased brain-Cu import remain unknown however, impacts on SOD-1 function, brain metabolism, and neurotransmitter synthesis seem likely. Further studies are now needed, and indeed are currently underway in many groups, to elucidate the specific biochemical pathways perturbed by age-related alterations to astrocyte Cu homoeostasis.
Cu in Neuron Synapses and Dendrites – The most prevalent transition metal ion found in synapses and involved in neurotransmission is Zn2+, as already discussed, however there is also mounting evidence for important roles of Cu ions in neurotransmission [7,112]. A number of authors have provided indirect evidence of a labile Cu+ pool (detected with fluorescence metal sensors), which increases in concentration following neuro-stimulation [7]. Direct elemental mapping with synchrotron radiation XRF has revealed distinct Cu enrichment within the dentate gyrus molecular layer of the hippocampus, a tissue region rich in the dendrites and synapses of dentate gyrus granule neurons (as shown in Fig. 2) [52,113]. At this stage, elemental mapping of this tissue region has only been performed at the micron level, and sub-micron resolution will be needed to resolve the Cu to individual dendrites in tissues. The elemental maps do however, help support that Cu may have important roles in synaptic function. In addition, although Cu distributions in dendrites are not yet known in brain tissue, elemental mapping of cultured neuronal cells has revealed Cu enrichment in dendritic processes [114], providing further evidence that supports involvement of Cu in regulating neurotransmission.
Iron (Fe)
Fe in Oligodendrocytes and Astrocytes – Very few studies have used elemental mapping techniques to characterise the Fe content of glial cells, such as oligodendrocytes and astrocytes. However, there is one detailed investigation that has characterised the Fe content of oligodendrocytes, astrocytes, microglia, and neurons, using PIXE [83]. The results revealed oligodendrocytes are the most Fe enriched class of brain cell, with approximately 30% more Fe than the other cell types [83]. This finding is in excellent agreement with the result of Perl's histochemical Fe staining, and Ferritin immuno-histochemistry, which have shown oligodendrocytes are especially Fe rich [48,49]. The abundance of Fe within oligodendrocytes has been linked to the high metabolic rates required for myelin synthesis [52]. Astrocytes play an important role in Fe import and transport in the brain (as they do for Cu, as already described), which most likely originates from the intimate contact between astrocyte foot processes and brain blood-capillaries. The study by Reinert et al., also suggests Fe enrichment in astrocytes [83], but not to the same extent as oligodendrocytes, which is consistent with the known fact that astrocytes do not have the same rate of oxidative metabolism as oligodendrocytes, nor do astrocytes synthesise myelin [115]. The results of Reinert are in agreement with a nano-SIMS investigation of Fe distribution in brain tissue [77]. Although the nano-SIMS study did not differentiate between astrocytes and oligodendrocytes, Fe enrichment was observed within glial cells [77]. One important consideration when interpreting the results of Reinert et al. is the fact that formalin-fixed tissues were used. It is well established that chemical fixation can result in metal ion contamination of the sample [87], in addition to redistribution or leaching of labile or mobile metal ions [85,87,89]. Therefore, the contribution of the labile metal ion pool to total metal content will likely not have been accurately captured in the studies by Reinert et al.
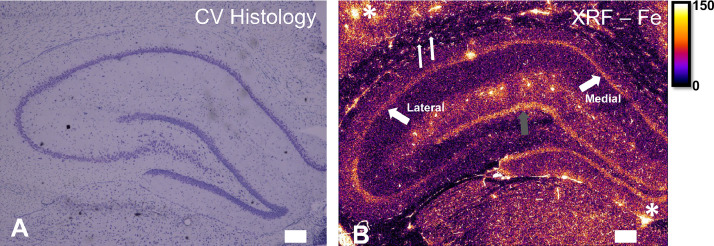
Fe within Pyramidal Neurons – Pyramidal neurons are a major neuron type found throughout the brain cortical layers, and within key brain structures such as the hippocampus. Interestingly, pyramidal neurons are typically thought to contain relatively low levels of Fe, as they often do not show strong Perl's staining [49,116]. However, it has now been shown that there is a substantial portion of intracellular neuronal Fe, at least within hippocampal pyramidal neurons, which can be detected with elemental mapping (as shown in Fig. 3), or modified histochemical methods using minimum exposure of tissues to fixatives, acids, and solvents [116]. These results support that neurons do contain a substantive intracellular Fe pool. This is not particularly surprising due to the fairly high metabolic requirements of neurons associated with neurotransmission, and the requirement of Fe as a co-factor for synthesis of several neuro-transmitters. There is evidence of systematic variation in neuronal Fe levels as a consequence of anatomical location within the hippocampus [100]. Specifically, CA1 pyramidal neurons located in the medial portions of the rodent (mouse and rat) hippocampus, which are more excitatory, contain greater Fe levels than neurons located in lateral regions of the hippocampus [100]. Interestingly, multiple sub-cellular PIXE elemental mapping studies have revealed the presence of elevated Fe in neuron nucleoli [82,83]. The studies analyzed chemically fixed tissues, so it remains to be demonstrated that the nucleoli are Fe-enriched in vivo, but further investigation into the role of Fe in neuronal nucleoli function is certainly warranted and intriguing.
Fig. 3.
An example of imaging the Fe metallome in the rat hippocampus. (A) Cresyl violet histology reveals the anatomical structure of the hippocampus. (B) An XRF elemental map of Fe distribution in the hippocampus and surrounding structures. XRF reveals an abundant pool of Fe in hippocampal pyramidal neurons, with medial neurons containing more Fe than lateral neurons (thick white arrows). The dentate gyrus granule cells (grey arrow) also appear Fe enriched relative to surrounding tissue. Small, highly Fe enriched cells can be seen in the corpus callosum white matter, which are most likely oligodendrocytes (thin white arrows). White asterisks in top left and bottom right of the image indicate blood vessels. Scale bar = 100 µm. Areal density units for panel B are ng cm−2. Panels A, B adapted with permission from reference 100.
Fe within Dopaminergic Neurons – The substantia nigra is a unique brain region, responsible for regulating motor movement. Neurons within the substantia nigra are typically characterised by being highly pigmented (high melanin content) and dopaminergic (synthesis and release of dopamine). A host of analytical methods have now demonstrated the importance of Fe within dopaminergic neurons of the substantia nigra – specifically, elemental mapping studies have made important contributions localising Fe to dopamine rich vesicles [22,23,114,117,118]. Dysregulated Fe homoeostasis has been implicated with pathology of dopaminergic neurons within the substantia nigra, particularly in Parkinson's disease [22,23,114,117,118].
Fe within Inter-Neurons There has been little research attention given to date on the metallome within interneurons, such as the granule cells of the hippocampal dentate gyrus, or the cerebellar granule cells. Interestingly, in published elemental maps from rats but not mice hippocampal tissues, one can often observe the location of the dentate gyrus as a region of elevated Fe content [46,52,100,108]. This suggests possible species differences in the metallome of interneurons, which requires further study.
Fe in Microglia – Perl's histochemistry frequently shows Fe deposits within microglia, the brains resident immune cells [48,49]. Subsequent immuno-histochemistry confirms that microglia can store large reserves of Ferritin, [119], [120], [121] which likely accounts for the Perl's positive staining. Not unexpectedly, substantial Fe content is observed within microglia in elemental mapping of fixed tissue sections [83]. Following tissue injury or neurodegeneration there is often a strong inflammatory response and recruitment of microglia and other Fe rich inflammatory cells (e.g., macrophages) to the site of tissue damage. On the basis that inflammatory cells are enriched in Fe, one would therefore expect elevated Fe content at or surrounding the site of tissue damage, and indeed this has been demonstrated in conditions such as ischaemic stroke, [37] haemorrhagic stroke, [39] multiple sclerosis, [26] and traumatic brain injury [34].
Conclusions and future perspectives
There is now substantial evidence that metal ions are essential for healthy brain function, and disturbed brain metal homoeostasis is implicated in neurodegeneration and altered cognitive function. Unfortunately, our understanding of the brain metallome lags behind that of the genome, proteome, or metabolome. Nonetheless, with increased technique development and greater access to elemental mapping techniques such as XRF, LA-ICP-MS, PIXE, and nano-SIMs, the field is now well positioned for in-depth study and characterisation of the brain metallome. In particular, greater understanding of the sub-cellular localisation of metal ions within specific cells in situ within tissue sections, is urgently needed. It is anticipated that the increasing availability of nano-probes will provide the tools to address this knowledge gap in the near future. This review has aimed to summarise the current knowledge of differences in the metallome (Fe, Cu, Zn) of different types of brain cells (astrocytes, oligodendrocytes, microglia, and neurons), and it should be clear from this literature that there are distinct differences in the intracellular metallome between different brain cells. There is now need for detailed fundamental studies to examine exactly how natural brain physiology influences the brain metallome, in addition to elucidating how the metallome within specific brain cells helps maintain brain health. Provision of such information may then help identify precisely how metal ions are involved in the development and progression of neurodegenerative diseases.
Declarations of Competing Interest
The authors declare no conflicts of interest.
Acknowledgments
MJH gratefully acknowledges current support from the Australian Research Council (ARC Future Fellowship FT190100017). MJH acknowledges past support from the Dementia Australia Research Foundation, Mamutil New Investigator Project Grant (11646), and past support from the Canadian Institute of Health Research (CIHR). ALH acknowledges the support from the Australian Government through an Australian Government Research Training Program Scholarship and an Australian Institute of Nuclear Science and Engineering Post Graduate Research Award (AINSE-PGRA). GE acknowledges support from the Australian Government through an Australian Institute of Nuclear Science and Engineering Early Career Researcher Grant (AINSE-ECRG).
References
- 1.Yuste R., et al. A community-based transcriptomics classification and nomenclature of neocortical cell types. Nat. Neurosci. 2020;23:1456–1468. doi: 10.1038/s41593-020-0685-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Poulin J.F., Gaertner Z., Moreno-Ramos O.A., Awatramani R. Classification of Midbrain Dopamine Neurons Using Single-Cell Gene Expression ProfilingApproaches. Trends Neurosci. 2020;43:155–169. doi: 10.1016/j.tins.2020.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Watson C., Kirkcaldie M., Paxinos G. Academic Press; 2010. The brain: An Introduction to Functional Neuroanatomy. [Google Scholar]
- 4.Simons M., Trotter J. Wrapping it up: the cell biology of myelination. Curr. Opin. Neurobiol. 2007;17:533–540. doi: 10.1016/j.conb.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 5.Cameron R.S., Rakic P. Glial cell lineage in the cerebral cortex: a review and synthesis. Glia. 1991;4:124–137. doi: 10.1002/glia.440040204. [DOI] [PubMed] [Google Scholar]
- 6.Frederickson C.J., Koh J.Y., Bush A.I. The neurobiology of zinc in health and disease. Nat. Rev. Neurosci. 2005;6:449–462. doi: 10.1038/nrn1671. [DOI] [PubMed] [Google Scholar]
- 7.Opazo C.M., Greenough M.A., Bush A.I. Copper: from neurotransmission to neuroproteostasis. Front. Aging Neurosci. 2014;6:143. doi: 10.3389/fnagi.2014.00143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Metal-Based Neurodegeneration. John Wiley and Sons Ltd; 2013. Role of metal ions in brain function, metal transport, storage and homoeostasis; pp. 23–50. [Google Scholar]
- 9.Frederickson C.J., Suh S.W., Silva D., Frederickson C.J., Thompson R.B. Importance of Zn in the central nervous system: the Zinc-containing neuron. J. Nutr. 2000;130:1471–1483. doi: 10.1093/jn/130.5.1471S. [DOI] [PubMed] [Google Scholar]
- 10.Takeda A. Movement of zinc and its functional significance in the brain. Brain Res. Rev. 2000;34:137–148. doi: 10.1016/s0165-0173(00)00044-8. [DOI] [PubMed] [Google Scholar]
- 11.Bush A.I. Metals and neuroscience. Curr. Opin. Chem. Biol. 2000;4:184–191. doi: 10.1016/s1367-5931(99)00073-3. [DOI] [PubMed] [Google Scholar]
- 12.Barnham K.J., Masters C.L., Bush A.I. Neurodegenerative diseases and oxidative stress. Nat. Rev. Drug Discov. 2004;3:205. doi: 10.1038/nrd1330. [DOI] [PubMed] [Google Scholar]
- 13.Kaur D., et al. Genetic or pharmacological iron chelation prevents MPTP-induced neurotoxicity in vivo: a novel therapy for Parkinson's disease. Neuron. 2003;37:899–909. doi: 10.1016/s0896-6273(03)00126-0. [DOI] [PubMed] [Google Scholar]
- 14.Adlard P.A., Bush A.I. Metals and Alzheimer';s disease. J. Alzheimer's Dis. 2006;10:145–163. doi: 10.3233/jad-2006-102-303. [DOI] [PubMed] [Google Scholar]
- 15.Adlard P.A., Parncutt J.M., Finkelstein D.I., Bush A.I. Cognitive loss in zinc transporter-3 knock-out mice: a phenocopy for the synaptic and memory deficits of Alzheimer's disease? J. Neurosci. 2010;30:1631–1636. doi: 10.1523/JNEUROSCI.5255-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bush A.I. The metallobiology of Alzheimer's disease. Trends Neurosci. 2003;26:207–214. doi: 10.1016/S0166-2236(03)00067-5. [DOI] [PubMed] [Google Scholar]
- 17.Greenough M.A., Camakaris J., Bush A.I. Metal dyshomeostasis and oxidative stress in Alzheimer's disease. Neurochem. Int. 2013;62:540–555. doi: 10.1016/j.neuint.2012.08.014. [DOI] [PubMed] [Google Scholar]
- 18.Huang X., Moir R.D., Tanzi R.E., Bush A.I., Rogers J.T. Redox-Active Metals, Oxidative Stress, and Alzheimer's Disease. Ann. N. Y. Acad. Sci. 2004;1012:153–163. doi: 10.1196/annals.1306.012. [DOI] [PubMed] [Google Scholar]
- 19.James S.A., Roberts B.R., Hare D.J., de Jonge M.D., Birchall I.E., Jenkins N.L., Cherny R.A., Bush A.I., McColl G. Direct in vivo imaging of ferrous iron dyshomeostasis in ageing Caenorhabditis elegans. Chem. Sci. 2015;6:2952–2962. doi: 10.1039/c5sc00233h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hare D.J., George J.L., Grimm R., Wilkins S., Adlard P.A., Cherny R.A., Bush A.I., Finkelstein D.I., Doble P. Three-dimensional elemental bio-imaging of Fe, Zn, Cu, Mn and P in a 6-hydroxydopamine lesioned mouse brain. Metallomics. 2010;2:745–753. doi: 10.1039/c0mt00039f. [DOI] [PubMed] [Google Scholar]
- 21.Popescu B.F.G., George M.J., Bergmann U., Garachtchenko A.V., Kelly M.E., McCrea R.P.E., Lüning K., Devon R.M., George G.N., Hanson A.D. Mapping metals in Parkinson's and normal brain using rapid-scanning x-ray fluorescence. Phys. Med. Biol. 2009;54:651. doi: 10.1088/0031-9155/54/3/012. [DOI] [PubMed] [Google Scholar]
- 22.Chwiej J., Adamek D., Szczerbowska-Boruchowska M., Krygowska-Wajs A., Wojcik S., Falkenberg G., Manka A., Lankosz M. Investigations of differences in iron oxidation state inside single neurons from substantia nigra of Parkinson's disease and control patients using the micro-XANES technique. J. Bio. Inorg. Chem. 2007;12:204–211. doi: 10.1007/s00775-006-0179-5. [DOI] [PubMed] [Google Scholar]
- 23.Carmona A., Roudeau S., Perrin L., Carcenac C., Vantelon D., Savasta M., Ortega R. Mapping Chemical Elements and Iron Oxidation States in the Substantia Nigra of 6-Hydroxydopamine Lesioned Rats Using Correlative Immunohistochemistry With Proton and Synchrotron Micro-Analysis. Front. Neurosci. 2019;13:1014. doi: 10.3389/fnins.2019.01014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lelie H.L., Liba A., Bourassa M.W., Chattopadhyay M., Chan P.K., Gralla E.B., Miller L.M., Borchelt D.R., Valentine J.S., Whitelegge J.P. Copper and Zinc Metallation Status of Copper-Zinc Superoxide Dismutase from Amyotrophic Lateral Sclerosis Transgenic Mice. J. Biol. Chem. 2011;286:2795–2806. doi: 10.1074/jbc.M110.186999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tomik B., Chwiej J., Szczerbowska-Boruchowska M., Lankosz M., Wójcik S., Adamek D., Falkenberg G., Bohic S., Simionovici A., Stegowski Z., Szczudlik A. Implementation of X-ray Fluorescence Microscopy for Investigation of Elemental Abnormalities in Amyotrophic Lateral Sclerosis. Neurochem. Res. 2006;31:321–331. doi: 10.1007/s11064-005-9030-6. [DOI] [PubMed] [Google Scholar]
- 26.Popescu B.F., Frischer J.M., Webb S.M., Tham M., Adiele R.C., Robinson C.A., Fitz-Gibbon P.D., Weigand S.D., Metz I., Nehzati S., George G.N., Pickering I.J., Brück W., Hametner S., Lassmann H., Parisi J.E., Yong G., Lucchinetti C.F. Pathogenic implications of distinct patterns of iron and zinc in chronic MS lesions. Acta Neuropathol. 2017;134:45–64. doi: 10.1007/s00401-017-1696-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Popescu B.F.G., Robinson C.A., Chapman L.D., Nichol H. Synchrotron X-ray fluorescence reveals abnormal metal distributions in brain and spinal cord in spinocerebellar ataxia: A case report. Cerebellum. 2009;8:74–79. doi: 10.1007/s12311-009-0102-z. [DOI] [PubMed] [Google Scholar]
- 28.Popescu B.F.G., Robinson C.A., Rajput A., Rajput A.H., Harder S.L., Nichol H. Iron, Copper and Zn distribution of the cerebellum. Cerebellum. 2009;8:74–79. doi: 10.1007/s12311-008-0091-3. [DOI] [PubMed] [Google Scholar]
- 29.Chwiej J., Janeczko K., Marciszko M., Czyzycki M., Rickers K., Setkowicz Z. Neuroprotective action of FK-506 (tacrolimus) after seizures induced with pilocarpine: quantitative and topographic elemental analysis of brain tissue. J. Bio. Inorg. Chem. 2010;15:283–289. doi: 10.1007/s00775-009-0597-2. [DOI] [PubMed] [Google Scholar]
- 30.Chwiej J., Kutorasinska J., Janeczko K., Gzielo-Jurek K., Uram L., Appel K., Simon R., Setkowicz Z. Progress of elemental anomalies of hippocampal formation in the pilocarpine model of temporal lobe epilepsy: an X-ray fluorescence microscopy study. Anal. Bioanal. Chem. 2012;404:3071–3080. doi: 10.1007/s00216-012-6425-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chwiej J., Winiarski W., Ciarach M., Janeczko K., Lankosz M., Rickers K., Setkowicz Z. The role of trace elements in the pathogenesis and progress of pilocarpine-induced epileptic seizures. J. Bio. Inorg. Chem. 2008;13:1267–1274. doi: 10.1007/s00775-008-0411-6. [DOI] [PubMed] [Google Scholar]
- 32.Hartnell D., Gillespie-Jones K., Ciornei C., Hollings A., Thomas A., Harrild E., Reinhardt J., Paterson D.J., Alwis D., Rajan R., Hackett M.J. Characterization of Ionic and Lipid Gradients within Corpus Callosum White Matter after Diffuse Traumatic Brain Injury in the Rat. ACS Chem. Neurosci. 2019;11:248–257. doi: 10.1021/acschemneuro.9b00257. [DOI] [PubMed] [Google Scholar]
- 33.Chwiej J., Sarapata A., Janeczko K., Stegowski Z., Appel K., Setkowicz Z. X-ray fluorescence analysis of long-term changes in the levels and distributions of trace elements in the rat brain following mechanical injury. J. Bio. Inorg. Chem. 2011;16:275–283. doi: 10.1007/s00775-010-0724-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Portbury S.D., Hare D.J., Sgambelloni C., Finkelstein D.I., Adlard P.A. A time-course analysis of changes in cerebral metal levels following a controlled cortical impact. Metallomics. 2016;8:193–200. doi: 10.1039/c5mt00234f. [DOI] [PubMed] [Google Scholar]
- 35.Caine S., Hackett M.J., Hou H., Kumar S., Maley J., Ivanishvili Z., Suen B., Szmigielski A., Jiang Z., Sylvain N.J., Nichol H., Kelly M.E. A novel multi-modal platform to image molecular and elemental alterations in ischemic stroke. Neurobiol. Dis. 2016;91:132–142. doi: 10.1016/j.nbd.2016.03.006. [DOI] [PubMed] [Google Scholar]
- 36.Hackett M.J., DeSouza M., Caine S., Bewer B., Nichol H., Paterson P.G., Colbourne F. A New Method To Image Heme-Fe, Total Fe, and Aggregated Protein Levels after Intracerebral Hemorrhage. ACS Chem. Neurosci. 2015;6:761–770. doi: 10.1021/acschemneuro.5b00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pushie M., Sylvain N., Hou H., Caine S., Hackett M., Kelly M. Tracking elemental changes in an ischemic stroke model with X-ray fluorescence imaging. Sci. Rep. 2020;10:1–14. doi: 10.1038/s41598-020-74698-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pushie M.J., Crawford A.M., Sylvain N.J., Hou H., Hackett M.J., George G.N., Kelly M.E. Revealing the Penumbra through Imaging Elemental Markers of Cellular Metabolism in an Ischemic Stroke Model. ACS Chem. Neurosci. 2018;9:886–893. doi: 10.1021/acschemneuro.7b00382. [DOI] [PubMed] [Google Scholar]
- 39.Williamson M.R., Dietrich K., Hackett M.J., Caine S., Nadeau C.A., Aziz J.R., Nichol H., Paterson P.G., Colbourne F. Rehabilitation Augments Hematoma Clearance and Attenuates Oxidative Injury and Ion Dyshomeostasis After Brain Hemorrhage. Stroke. 2016;48:195–203. doi: 10.1161/STROKEAHA.116.015404. [DOI] [PubMed] [Google Scholar]
- 40.Ali M.H., Rakib F., Abdelalim E.M., Limbeck A., Mall R., Ullah E., Mesaeli N., McNaughton D., Ahmed T., Al-Saad K. Fourier-transform infrared imaging spectroscopy and laser ablation-ICPMS new vistas for biochemical analyses of ischemic stroke in rat brain. Front. Neurosci. 2018;12:647. doi: 10.3389/fnins.2018.00647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Balbekova A., Lohninger H., van Tilborg G.A., Dijkhuizen R.M., Bonta M., Limbeck A., Lendl B., Al-Saad K.A., Ali M., Celikic M. Fourier transform infrared (FT-IR) and laser ablation inductively coupled plasma–mass spectrometry (LA-ICP-MS) imaging of cerebral ischemia: combined analysis of rat brain thin cuts toward improved tissue classification. Appl. Spectrosc. 2018;72:241–250. doi: 10.1177/0003702817734618. [DOI] [PubMed] [Google Scholar]
- 42.Andreini C., Bertini I., Cavallaro G., Holliday G.L., Thornton J.M. Metal ions in biological catalysis: from enzyme databases to general principles. J. Bio. Inorg. Chem. 2008;13:1205–1218. doi: 10.1007/s00775-008-0404-5. [DOI] [PubMed] [Google Scholar]
- 43.Huang E.P. Metal ions and synaptic transmission: Think zinc. Proc. Natl. Acad. Sci. U. S. A. 1997;94:13386–13387. doi: 10.1073/pnas.94.25.13386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chang C.J. Searching for harmony in transition-metal signalling. Nat. Chem. Biol. 2015;11:744–747. doi: 10.1038/nchembio.1913. [DOI] [PubMed] [Google Scholar]
- 45.Que E.L., Domaille D.W., Chang C.J. Metals in neurobiology: probing their chemistry and biology with molecular imaging. Chem. Rev. 2008;108:1517–1549. doi: 10.1021/cr078203u. [DOI] [PubMed] [Google Scholar]
- 46.Adlard P.A., Parncutt J., Lal V., James S., Hare D., Doble P., Finkelstein D.I., Bush A.I. Metal chaperones prevent zinc-mediated cognitive decline. Neurobiol. Dis. 2015;81:196–202. doi: 10.1016/j.nbd.2014.12.012. [DOI] [PubMed] [Google Scholar]
- 47.Adlard P.A., Sedjahtera A., Gunawan L., Bray L., Hare D., Lear J., Doble P., Bush A.I., Finkelstein D.I., Cherny R.A. A novel approach to rapidly prevent age-related cognitive decline. Aging Cell. 2014;13:351–359. doi: 10.1111/acel.12178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hill J.M., Switzer R.C. The regional distribution and cellular localization of iron in the rat brain. Neuroscience. 1984;11:595–603. doi: 10.1016/0306-4522(84)90046-0. [DOI] [PubMed] [Google Scholar]
- 49.Meguro R., Asano Y., Odagiri S., Li C., Shoumura K. Cellular and subcellular localizations of nonheme ferric and ferrous iron in the rat brain: a light and electron microscopic study by the perfusion-Perls and -Turnbull methods. Arch. Histol. Cytol. 2008;71:205–222. doi: 10.1679/aohc.71.205. [DOI] [PubMed] [Google Scholar]
- 50.Todorich B., Pasquini J.M., Garcia C.I., Paez P.M., Connor J.R. Oligodendrocytes and myelination: the role of iron. Glia. 2009;57:467–478. doi: 10.1002/glia.20784. [DOI] [PubMed] [Google Scholar]
- 51.Thorburne S.K., Juurlink B.H. Low glutathione and high iron govern the susceptibility of oligodendroglial precursors to oxidative stress. J. Neurochem. 1996;67:1014–1022. doi: 10.1046/j.1471-4159.1996.67031014.x. [DOI] [PubMed] [Google Scholar]
- 52.James S.A., Churches Q.I., de Jonge M.D., Birchall I.E., Streltsov V., McColl G., Adlard P.A., Hare D.J. Iron, Copper, and Zinc Concentration in Aß Plaques in the APP/PS1 Mouse Model of Alzheimer's Disease Correlates with Metal Levels in the Surrounding Neuropil. ACS Chem. Neurosci. 2017;8:629–637. doi: 10.1021/acschemneuro.6b00362. [DOI] [PubMed] [Google Scholar]
- 53.Bourassa M.W., Leskovjan A.C., Tappero R.V., Farquhar E.R., Colton C.A., Van Nostrand W.E., Miller L.M. Elevated copper in the amyloid plaques and iron in the cortex are observed in mouse models of Alzheimer's disease that exhibit neurodegeneration. Biomed. Spectrosc. imaging. 2013;2:129–139. doi: 10.3233/BSI-130041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Leskovjan A.C., Kretlow A., Lanzirotti A., Barrea R., Vogt S., Miller L.M. Increased brain iron coincides with early plaque formation in a mouse model of Alzheimer's disease. Neuroimage. 2011;55:32–38. doi: 10.1016/j.neuroimage.2010.11.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Telling N.D., Everett J., Collingwood J.F., Dobson J., van der Laan G., Gallagher J.J., Wang J., Hitchcock A.P. Iron Biochemistry is Correlated with Amyloid Plaque Morphology in an Established Mouse Model of Alzheimer's Disease. Cell Chem. Biol. 2017;24 doi: 10.1016/j.chembiol.2017.07.014. 1205–15.e3. [DOI] [PubMed] [Google Scholar]
- 56.Wang H., Wang M., Wang B., Li M., Chen H., Yu X., Zhao Y., Feng W., Chai Z. The distribution profile and oxidation states of biometals in APP transgenic mouse brain: dyshomeostasis with age and as a function of the development of Alzheimer's disease. Metallomics. 2012;4:289–296. doi: 10.1039/c2mt00104g. [DOI] [PubMed] [Google Scholar]
- 57.Wang H.J., Wang M., Wang B., Meng X.Y., Wang Y., Li M., Feng W.Y., Zhao Y.L., Chai Z.F. Quantitative imaging of element spatial distribution in the brain section of a mouse model of Alzheimer's disease using synchrotron radiation X-ray fluorescence analysis. J. Anal. At. Spectrom. 2010;25:328–333. [Google Scholar]
- 58.Summers K.L., Fimognari N., Hollings A., Kiernan M., Lam V., Tidy R.J., Paterson D., Tobin M.J., Takechi R., George G.N., Pickering I.J., Mamo J.C., Harris H.H., Hackett M.J. A Multimodal Spectroscopic Imaging Method To Characterize the Metal and Macromolecular Content of Proteinaceous Aggregates (‘Amyloid Plaques’) Biochemistry. 2017:4107–4116. doi: 10.1021/acs.biochem.7b00262. [DOI] [PubMed] [Google Scholar]
- 59.Miller L.M., Wang Q., Telivala T.P., Smith R.J., Lanzirotti A., Miklossy J. Synchrotron-based infrared and X-ray imaging shows focalized accumulation of Cu and Zn colocalized with beta-amyloid deposits in Alzheimer's disease. J. Struct. Biol. 2006;155:30–37. doi: 10.1016/j.jsb.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 60.Adlard P.A., Bush A.I. Metals and Alzheimer's Disease: How Far Have We Come in the Clinic? J. Alzheimer's Dis. 2018;62:1369–1379. doi: 10.3233/JAD-170662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bush A., Pettingell W., Multhaup G., d Paradis M., Vonsattel J., Gusella J., Beyreuther K., Masters C., Tanzi R. Rapid induction of Alzheimer A beta amyloid formation by zinc. Science. 1994;265:1464–1467. doi: 10.1126/science.8073293. [DOI] [PubMed] [Google Scholar]
- 62.Crouch P.J., White A.R., Bush A.I. The modulation of metal bio-availability as a therapeutic strategy for the treatment of Alzheimer's disease. FEBS J. 2007;274:3775–3783. doi: 10.1111/j.1742-4658.2007.05918.x. [DOI] [PubMed] [Google Scholar]
- 63.Duce J.A., Bush A.I. Biological metals and Alzheimer's disease: implications for therapeutics and diagnostics. Prog. Neurobiol. 2010;92:1–18. doi: 10.1016/j.pneurobio.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 64.Huang X., Atwood C.S., Hartshorn M.A., Multhaup G., Goldstein L.E., Scarpa R.C., Cuajungco M.P., Gray D.N., Lim J., Moir R.D., Tanzi R.E., Bush A.I. The Aß Peptide of Alzheimer's Disease Directly Produces Hydrogen Peroxide through Metal Ion Reduction. Biochemistry. 1999;38:7609–7616. doi: 10.1021/bi990438f. [DOI] [PubMed] [Google Scholar]
- 65.Bush A.I., Tanzi R.E. Therapeutics for Alzheimer's Disease Based on the Metal Hypothesis. Neurotherapeutics. 2008;5:421–432. doi: 10.1016/j.nurt.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zilberter Y., Zilberter T., Bregestovski P. Neuronal activity in vitro and the in vivo reality: the role of energy homeostasis. Trends Pharmacol. Sci. 2010;31:394–401. doi: 10.1016/j.tips.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 67.Pushie M.J., Pickering I.J., Korbas M., Hackett M.J., George G.N. Elemental and Chemically Specific X-ray Fluorescence Imaging of Biological Systems. Chem. Rev. 2014;114:8499–8541. doi: 10.1021/cr4007297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hartnell D., Andrews W., Smith N., Jiang H., McAllum E., Rajan R., Colbourne F., Fitzgerald M., Lam V., Takechi R., Pushie M.J., Kelly M.E., Hackett M.J. A Review of ex vivo Elemental Mapping Methods to Directly Image Changes in the Homeostasis of Diffusible Ions (Na+, K+, Mg2 +, Ca2 +, Cl–) Within Brain Tissue. Front. Neurosci. 2020;13:1415. doi: 10.3389/fnins.2019.01415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pushie M., Kelly M., Hackett M. Direct label-free imaging of brain tissue using synchrotron light: a review of new spectroscopic tools for the modern neuroscientist. Analyst. 2018;143:3761–3774. doi: 10.1039/c7an01904a. [DOI] [PubMed] [Google Scholar]
- 70.Becker J.S., Matusch A., Becker J.S., Wu B., Palm C., Becker A.J., Salber D. Mass spectrometric imaging (MSI) of metals using advanced BrainMet techniques for biomedical research. Int. J. Mass Spectrom. 2011;307:3–15. [Google Scholar]
- 71.Becker J.S., Matusch A., Wu B. Bioimaging mass spectrometry of trace elements – recent advance and applications of LA-ICPMS: A review. Anal. Chim. Acta. 2014;835:1–18. doi: 10.1016/j.aca.2014.04.048. [DOI] [PubMed] [Google Scholar]
- 72.Pozebon D., Dressler V.L., Mesko M.F., Matusch A., Becker J.S. Bioimaging of metals in thin mouse brain section by laser ablation inductively coupled plasma mass spectrometry: novel online quantification strategy using aqueous standards. J. Anal. At. Spectrom. 2010;25:1739–1744. [Google Scholar]
- 73.Becker J.Sabine, Matusch A., Palm C., Salber D., Morton K.A., Becker J.Susanne. Bioimaging of metals in brain tissue by laser ablation inductively coupled plasma mass spectrometry (LA-ICP-MS) and metallomics. Metallomics. 2010;2:104–111. doi: 10.1039/b916722f. [DOI] [PubMed] [Google Scholar]
- 74.Wang L.M., Becker J.S., Wu Q., Oliveira M.F., Bozza F.A., Schwager A.L., Hoffman J.M., Morton K.A. Bioimaging of copper alterations in the aging mouse brain by autoradiography, laser ablation inductively coupled plasma mass spectrometry and immunohistochemistry. Metallomics. 2010;2:348–353. doi: 10.1039/c003875j. [DOI] [PubMed] [Google Scholar]
- 75.Hare D.J., Lee J.K., Beavis A.D., van Gramberg A., George J., Adlard P.A., Finkelstein D.I., Doble P.A. Three-dimensional atlas of iron, copper, and zinc in the mouse cerebrum and brainstem. Anal. Chem. 2012;84:3990–3997. doi: 10.1021/ac300374x. [DOI] [PubMed] [Google Scholar]
- 76.Paul B., Hare D.J., Bishop D.P., Paton C., Cole N., Niedwiecki M.M., Andreozzi E., Vais A., Billings J.L., Bray L. Visualising mouse neuroanatomy and function by metal distribution using laser ablationinductively coupled plasma-mass spectrometry imaging. Chem. Sci. 2015;6:5383–5393. doi: 10.1039/c5sc02231b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Quintana C., Wu T.D., Delatour B., Dhenain M., Guerquin-Kern J.l., Croisy A. Morphological and chemical studies of pathological human and mice brain at the subcellular level: Correlation between light, electron, and nanosims microscopies. Microsc. Res. Tech. 2007;70:281–295. doi: 10.1002/jemt.20403. [DOI] [PubMed] [Google Scholar]
- 78.Lozić I., Bartlett C.A., Shaw J.A., Iyer K.S., Dunlop S.A., Kilburn M.R., Fitzgerald M. Changes in subtypes of Ca microdomains following partial injury to the central nervous system. Metallomics. 2014;6:455–464. doi: 10.1039/c3mt00336a. [DOI] [PubMed] [Google Scholar]
- 79.Payne S.C., Bartlett C.A., Harvey A.R., Dunlop S.A., Fitzgerald M. Chronic swelling and abnormal myelination during secondary degeneration after partial injury to a central nervous system tract. J. Neurotrauma. 2011;28:1077–1088. doi: 10.1089/neu.2010.1665. [DOI] [PubMed] [Google Scholar]
- 80.Hackett M.J., Siegele R., El-Assaad F., McQuillan J.A., Aitken J.B., Carter E.A., Grau G.E., Hunt N.H., Cohen D., Lay P.A. Investigation of the mouse cerebellum using STIM and u-PIXE spectrometric and FTIR spectroscopic mapping and imaging. Nucl. Instrum. Methods B. 2011;269:2260–2263. [Google Scholar]
- 81.Lee J., Siegele R., Pastuovic Z., Hackett M.J., Hunt N.H., Grau G.E., Cohen D.D., Lay P.A. Light and heavy ion beam analysis of thin biological sections. Nucl. Instrum. Methods B. 2013;306:129–133. [Google Scholar]
- 82.Fiedler A., Reinert T., Morawski M., Brückner G., Arendt T., Butz T. Intracellular iron concentration of neurons with and without perineuronal nets. Nucl. Instrum. Methods B. 2007;260:153–158. [Google Scholar]
- 83.Reinert A., Morawski M., Seeger J., Arendt T., Reinert T. Iron concentrations in neurons and glial cells with estimates on ferritin concentrations. BMC Neurosci. 2019;20:1–14. doi: 10.1186/s12868-019-0507-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Perrin L., Carmona A., Roudeau S., Ortega R. Evaluation of sample preparation methods for single cell quantitative elemental imaging using proton or synchrotron radiation focused beams. J. Anal. At. Spectrom. 2015;30:2525–2532. [Google Scholar]
- 85.Chwiej J., Szczerbowska-Boruchowska M., Lankosz M., Wojcik S., Falkenberg G., Stegowski Z., Setkowicz Z. Preparation of tissue samples for X-ray fluorescence microscopy. Spectrochim. Acta. B At. Spectrosc. 2005;60:1531–1537. [Google Scholar]
- 86.Hackett M.J., Britz C.J., Nichol H., Paterson P.G., Pickering I.J., George G.N. In situ Bio-Spectroscopic Investigation of Rapid Ischemic and Post-mortem Induced Biochemical Alterations in the Rat Brain. ACS Chem. Neurosci. 2015;6:226–238. doi: 10.1021/cn500157j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hackett M.J., McQuillan J.A., El-Assaad F., Aitken J.B., Levina A., Cohen D.D., Siegele R., Carter E.A., Grau G.E., Hunt N.H., Lay P.A. Chemical alterations to murine brain tissue induced by formalin fixation: implications for biospectroscopic imaging and mapping studies of disease pathogenesis. Analyst. 2011;136:2941–2952. doi: 10.1039/c0an00269k. [DOI] [PubMed] [Google Scholar]
- 88.Hackett M.J., Smith S.E., Paterson P.G., Nichol H., Pickering I.J., George G.N. X-ray Absorption Spectroscopy at the Sulfur K-Edge: A New Tool to Investigate the Biochemical Mechanisms of Neurodegeneration. ACS Chem. Neurosci. 2012;3:178–185. doi: 10.1021/cn200097s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pushie M.J.J., Hollings A., Reinhardt J., Webb S.M., Lam V., Takechi R., Mamo J., Paterson P., Kelly M.E., George G.N., Hackett M.J. Sample preparation with sucrose cryoprotection dramatically alters Zn distribution in the rodent hippocampus, as revealed by elemental mapping. J. Anal. At. Spectrom. 2020;35:2498–2508. doi: 10.1039/d0ja00323a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Frederickson C.J., Klitenick M.A., Manton W.I., Kirkpatrick J.B. Cytoarchitectonic distribution of zinc in the hippocampus of man and the rat. Brain Res. 1983;273:335–339. doi: 10.1016/0006-8993(83)90858-2. [DOI] [PubMed] [Google Scholar]
- 91.Frederickson C., Rampy B., Reamy-Rampy S., Howell G. Distribution of histochemically reactive zinc in the forebrain of the rat. J. Chem. Neuroanat. 1992;5:521–530. doi: 10.1016/0891-0618(92)90007-d. [DOI] [PubMed] [Google Scholar]
- 92.Frederickson C.J., Kasarskis E., Ringo D., Frederickson R. A quinoline fluorescence method for visualizing and assaying the histochemically reactive zinc (bouton zinc) in the brain. J. Neurosci. Methods. 1987;20:91–103. doi: 10.1016/0165-0270(87)90042-2. [DOI] [PubMed] [Google Scholar]
- 93.Linkous D.H., Flinn J.M., Koh J.Y., Lanzirotti A., Bertsch P.M., Jones B.F., Giblin L.J., Frederickson C.J. Evidence that the ZNT3 protein controls the total amount of elemental zinc in synaptic vesicles. J. Histochem. Cytochem. 2008;56:3–6. doi: 10.1369/jhc.6A7035.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Danscher G. Histochemical demonstration of heavy metals. Histochemistry. 1981;71:1–16. doi: 10.1007/BF00592566. [DOI] [PubMed] [Google Scholar]
- 95.Danscher G., Zimmer J. An improved Timm sulphide silver method for light and electron microscopic localization of heavy metals in biological tissues. Histochem. Cell Biol. 1978;55:27–40. doi: 10.1007/BF00496691. [DOI] [PubMed] [Google Scholar]
- 96.Pe´rez-Clausell J., Danscher G. Intravesicular localization of zinc in rat telencephalic boutons. A histochemical study. Brain Res. 1985;337:91–98. doi: 10.1016/0006-8993(85)91612-9. [DOI] [PubMed] [Google Scholar]
- 97.Thompson R., Peterson D., Mahoney W., Cramer M., Maliwal B.P., Suh S.W., Frederickson C., Fierke C., Herman P. Fluorescent zinc indicators for neurobiology. J. Neurosci. Methods. 2002;118:63–75. doi: 10.1016/s0165-0270(02)00144-9. [DOI] [PubMed] [Google Scholar]
- 98.Frederickson R.E., Frederickson C.J., Danscher G. In situ binding of bouton zinc reversibly disrupts performance on a spatial memory task. Behav. Brain Res. 1990;38:25–33. doi: 10.1016/0166-4328(90)90021-6. [DOI] [PubMed] [Google Scholar]
- 99.Fimognari N., Hollings A., Lam V., Tidy R.J., Kewish C.M., Albrecht M.A., Takechi R., Mamo J.C.L., Hackett M.J. Biospectroscopic Imaging Provides Evidence of Hippocampal Zn Deficiency and Decreased Lipid Unsaturation in an Accelerated Aging Mouse Model. ACS Chem. Neurosci. 2018;9:2774–2785. doi: 10.1021/acschemneuro.8b00193. [DOI] [PubMed] [Google Scholar]
- 100.Hackett M.J., Hollings A., Caine S., Bewer B.E., Alaverdashvili M., Takechi R., Mamo J.C.L., Jones M.W.M., de Jonge M.D., Paterson P.G., Pickering I.J., George G.N. Elemental characterisation of the pyramidal neuron layer within the rat and mouse hippocampus. Metallomics. 2019;11:151–165. doi: 10.1039/c8mt00230d. [DOI] [PubMed] [Google Scholar]
- 101.Hollings A.L., Lam V., Takechi R., Mamo J.C., Reinhardt J., de Jonge M.D., Kappen P., Hackett M.J. Revealing differences in the chemical form of zinc in brain tissue using K-edge X-ray absorption near-edge structure spectroscopy. Metallomics. 2020;12:2134–2144. doi: 10.1039/d0mt00198h. [DOI] [PubMed] [Google Scholar]
- 102.McCubbin Stepanic O., Ward J., Penner-Hahn J.E., Deb A., Bergmann U., DeBeer S. Probing a Silent Metal: A Combined X-ray Absorption and Emission Spectroscopic Study of Biologically Relevant Zinc Complexes. Inorg. Chem. 2020;59:13551–13560. doi: 10.1021/acs.inorgchem.0c01931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Thomas S.A., Mishra B., Myneni S.C.B. High Energy Resolution-X-ray Absorption Near Edge Structure Spectroscopy Reveals Zn Ligation in Whole Cell Bacteria. J. Phys. Chem. Lett. 2019;10:2585–2592. doi: 10.1021/acs.jpclett.9b01186. [DOI] [PubMed] [Google Scholar]
- 104.Scheiber I.F., Dringen R. Astrocyte functions in the copper homeostasis of the brain. Neurochem. Int. 2013;62:556–565. doi: 10.1016/j.neuint.2012.08.017. [DOI] [PubMed] [Google Scholar]
- 105.Choi B.S., Zheng W. Copper transport to the brain by the blood-brain barrier and blood-CSF barrier. Brain Res. 2009;1248:14–21. doi: 10.1016/j.brainres.2008.10.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Pushkar Y., Robison G., Sullivan B., Fu S.X., Kohne M., Jiang W., Rohr S., Lai B., Marcus M.A., Zakharova T. Aging results in copper accumulations in glial fibrillary acidic protein-positive cells in the subventricular zone. Aging Cell. 2013;12:823–832. doi: 10.1111/acel.12112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sullivan B., Robison G., Pushkar Y., Young J.K., Manaye K.F. Copper accumulation in rodent brain astrocytes: A species difference. J. Trace Elem. Med. Biol. 2017;39:6–13. doi: 10.1016/j.jtemb.2016.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Robison G., Zakharova T., Fu S., Jiang W., Fulper R., Barrea R., Zheng W., Pushkar Y. X-ray fluorescence imaging of the hippocampal formation after manganese exposure. Metallomics. 2013;(5):1554–1565. doi: 10.1039/c3mt00133d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sullivan B., Robison G., Osborn J., Kay M., Thompson P., Davis K., Zakharova T., Antipova O., Pushkar Y. On the nature of the Cu-rich aggregates in brain astrocytes. Redox Biol. 2017;11:231–239. doi: 10.1016/j.redox.2016.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lins B.R., Pushie J.M., Jones M., Howard D.L., Howland J.G., Hackett M.J. Mapping Alterations to the Endogenous Elemental Distribution within the Lateral Ventricles and Choroid Plexus in Brain Disorders Using X-Ray Fluorescence Imaging. PLoS One. 2016;11 doi: 10.1371/journal.pone.0158152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Pushie M.J., Pickering I.J., Martin G.R., Tsutsui S., Jirik F.R., George G.N. Prion protein expression level alters regional copper, iron and zinc content in the mouse brain. Metallomics. 2011;3:206–214. doi: 10.1039/c0mt00037j. [DOI] [PubMed] [Google Scholar]
- 112.Peters C., Muñoz B., Sepúlveda F.J., Urrutia J., Quiroz M., Luza S., De Ferrari G.V., Aguayo L.G., Opazo C. Biphasic effects of copper on neurotransmission in rat hippocampal neurons. J. Neurochem. 2011;119:78–88. doi: 10.1111/j.1471-4159.2011.07417.x. [DOI] [PubMed] [Google Scholar]
- 113.Hackett M.J., Hollings A., Majimbi M., Brook E., Cochran B., Giles C., Lam V., Nesbit M., Rye K.A., Mamo J.C. Multimodal Imaging Analyses of Brain Hippocampal Formation Reveal Reduced Cu and Lipid Content and Increased Lactate Content in Non-Insulin-Dependent Diabetic Mice. ACS Chem. Neurosci. 2019;10:2533–2540. doi: 10.1021/acschemneuro.9b00039. [DOI] [PubMed] [Google Scholar]
- 114.Carmona A., Cloetens P., Devès G., Bohic S., Ortega R. Nano-imaging of trace metals by synchrotron X-ray fluorescence into dopaminergic single cells and neurite-like processes. J. Anal. At. Spectrom. 2008;23:1083–1088. [Google Scholar]
- 115.Edmond J., Robbins R.A., Bergstrom J.D., Cole R.A., de Vellis J. Capacity for substrate utilization in oxidative metabolism by neurons, astrocytes, and oligodendrocytes from developing brain in primary culture. J. Neurosci. Res. 1987;18:551–561. doi: 10.1002/jnr.490180407. [DOI] [PubMed] [Google Scholar]
- 116.Sands S.A., Leung-Toung R., Wang Y., Connelly J., LeVine S.M. Enhanced Histochemical Detection of Iron in Paraffin Sections of Mouse Central Nervous System Tissue: Application in the APP/PS1 Mouse Model of Alzheimer's Disease. ASN Neuro. 2016;8 doi: 10.1177/1759091416670978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.J. Chwiej. The use of cluster and discriminant analysis in the investigations of the role of trace metals in the pathogenesis of Parkinson's disease. J. Trace Elem. Med. Biol. 2010, 24, 78–88. [DOI] [PubMed]
- 118.Yoshida S., Ide-Ektessabi A., Fujisawa S. Application of Synchrotron Radiation in Neuromicrobiology: Role of Iron in Parkinson's Disease. Struct. Chem. 2003;14:85–95. [Google Scholar]
- 119.Benkovic S.A., Connor J.R. Ferritin, transferrin, and iron in selected regions of the adult and aged rat brain. J. Comp. Neurol. 1993;338:97–113. doi: 10.1002/cne.903380108. [DOI] [PubMed] [Google Scholar]
- 120.Mehlhase J., Gieche J., Widmer R., Grune T. Ferritin levels in microglia depend upon activation: modulation by reactive oxygen species. Biochim. Biophys. Acta Mol. Cell Res. 2006;1763:854–859. doi: 10.1016/j.bbamcr.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 121.Roskams A.J.I., Connor J.R. Iron, Transferrin, and Ferritin in the Rat Brain During Development and Aging. J. Neurochem. 1994;63:709–716. doi: 10.1046/j.1471-4159.1994.63020709.x. [DOI] [PubMed] [Google Scholar]