HIGHLIGHTS
-
•
Lipid fingerprints are comprised of similar lipid classes
-
•
Sites of specific lipid contacts, including CHOL, varies between transporters
-
•
Changes in lipid annulus result in variable local membrane biophysical properties
-
•
Membrane composition, including that of complex membranes, affects lipid annulus
KEYWORDS: nNeuronal membrane, lLipid-protein interactions, SLC6 transport proteins, mMembrane transporters, mMolecular dynamics, mMembrane analysis
Abstract
The local lipid annulus, or “fingerprint”, of four SLC6 transporters (dDAT, hDAT, hSERT, and GlyT2) embedded in a complex neuronal membrane were compared and characterised using molecular dynamics. Our analysis included the development of new tools to improve membrane leaflet detection and the analysis of leaflet-dependent properties. Overall, the lipid fingerprints of the four transporters are comprised of similar lipids when grouped by headgroup or tail saturation. The enrichment and depletion of specific lipids, including sites of cholesterol contacts, varies between transporters. The subtle differences in lipid fingerprints results in varying membrane biophysical properties near the transporter. Our results highlight that the lipid-fingerprint of SLC6 transporters in complex membranes is highly dependent on membrane composition. Our results further characterize how the presence and identity of membrane proteins affects the complex interplay of lipid-protein interactions, influencing the local lipid environment and membrane biophysical properties.
Graphical abstract
Introduction
The composition of cell membranes encompasses tremendous chemical diversity. A typical bilayer contains hundreds of different lipid species, distributed asymmetrically between the two leaflets [1]. Membrane environments are also constantly changing, as lipids mix laterally to form distinct domains and translocate between leaflets in a variety of biological processes [2]. The complex and dynamic lipid environment gives rise to correspondingly complex interactions with embedded membrane proteins. Lipids play roles in protein trafficking, localization, and activity [3]. They modulate membrane protein behaviour both specifically, by binding to the protein at distinct binding sites [4], and non-specifically, through changes in the biophysical properties of the surrounding membrane environment that in turn affects the protein function [5]. Several properties are known to affect protein behaviour, including the thickness and the lateral pressure field of the membrane, as well as the organization of charges at the protein-lipid interface [6].
A typical membrane lipid is comprised of a polar headgroup and one or more acyl chain tail groups, and the combinatorial possibilities result in membranes that incorporate extensive chemical diversity [1]. Headgroups common to mammalian membranes include phosphatidylcholines (PC), phosphatidylethanolamines (PE), phosphatidylserines (PS), phosphatidylinositols (PI), and phosphatidic acid (PA). In addition, PI lipids may be phosphorylated to form phosphorylated PIs (PIPs), while PC and PE headgroups can be combined with a sphingosine backbone to form sphingomyelin (SM). Alternatively, the headgroup of lipids with a sphingosine backbone can be a hydroxyl group, creating ceramides (CER), or a carbohydrate, creating glycosphingolipids (GS). GS lipids are typically found only in the extracellular leaflet, and PI, PA and PS lipids in the intracellular leaflet. Acyl chains range in length from 16–22 carbons in synaptic membrane phospholipids [7], and vary in saturation from 0 to 6 cis double bonds, with further variation in the position of these double bonds. The tails join phosphoglycerides at two positions, denoted sn-1 and sn-2. In mammals, the sn-1 tail is commonly unsaturated, whereas the sn-2 tail is often mono- or polyunsaturated [8]. Sterols are also present in membranes, most commonly in eukaryotes as cholesterol (CHOL) [1].
The combinatorial complexity of membranes can be difficult to study at a molecular level using experimental techniques, which typically yield ensemble-averaged data. Molecular dynamics (MD) simulations probe membrane and transmembrane protein interactions at a molecular resolution, enabling us to study time-dependent behaviour and quantify subtle changes to membrane properties. Coarse-graining, the practice of representing multiple atoms with one “bead”, allows the simulation of larger systems for longer timescales than atomistic simulations. The balance between fine spatiotemporal resolution and breadth of accessible size and timescales has made coarse-grain (CG) MD a popular technique for quantitative studies of membrane behaviour [9].
Recent studies have used CG MD to model an average plasma membrane [10] and a neuronal membrane [11] from experimentally determined lipid compositions. These are the first computational models to approach the complexity characteristic of realistic membranes. Until the publication of these models, MD simulations of transmembrane proteins incorporated at most four or five different lipid species [9]; the neuronal and plasma membrane models each contain over fifty lipid species. The average properties of the neuronal membrane model are comparable with those of the plasma membrane in properties such as bilayer thickness, tail order, diffusion, and flip-flop, although diffusion and flip-flop rates are slightly slower in the neuronal model. A previous study examining ten different classes of membrane proteins embedded in the average plasma membrane showed that each protein induced changes in the local membrane environment to form a lipid annulus unique to that protein class. The distinct, non-uniform distributions of different lipid types around each protein perturb local membrane properties to form a protein-specific “lipid fingerprint” [12]. These local membrane properties are highly dependent on lipid composition. Another study compared the complex neuronal membrane to a series of model membranes of decreasing chemical complexity; the results suggest that a complex model incorporating diverse lipid species is important for the accurate representation of a number of properties that can modulate transmembrane protein behaviour, such as membrane fluidity, cholesterol localization, lipid clustering, and membrane curvature [13]. Therefore, the lipid fingerprints observed around each protein in an averaged membrane may not be representative of the true environment that may emerge in a tissue-specific membrane.
One of the proteins examined in the previous average plasma membrane study was the Drosophila melanogaster dopamine transporter (dDAT) [12], a neurotransmitter transporter from the solute carrier 6 (SLC6) family. SLC6 neurotransmitter transporters are responsible for neurotransmitter reuptake from the synaptic cleft. Dysregulation of SLC6 neurotransmitter transporters leads to disruption of neurotransmission and has been implicated in a range of disorders including Parkinson's disease, addiction, depression, chronic pain, and epilepsy [14]. Here we examine the lipid environment obtained when dDAT and three other SLC6 neurotransmitter transporters, hDAT (52.42% sequence identity to dDAT), hSERT (48.61% sequence identity to dDAT) and GlyT2 (45.76% sequence identity to dDAT), are embedded in a complex neuronal membrane. We investigate whether lipid fingerprints are unique to members within the same protein family; whether the local lipid environment is substantially different in the neuronal membrane compared to the plasma membrane; and the effect of the presence of a protein in the neuronal membrane on membrane properties. We then embed the same proteins in a simplified model membrane (POPC/CHOL) comprised of 80% 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC) and 20% cholesterol, to explore whether the presence of membrane protein mitigates or modifies the known changes in bilayer properties between simple and complex membranes.
METHODS
All simulations were prepared and performed using the 2019.1 version of the Groningen Machine for Chemical Simulation (GROMACS) [15] and version 2.2 of the MARTINI forcefield [16]. The coordinates of the Drosophila melanogaster dopamine transporter, dDAT (PDB ID: 4XP1) [17], and human serotonin transporter, hSERT (PDB ID: 5I6X) [18], in the outward-occluded conformation were obtained from the Protein Data Bank. The homology model of hDAT in the outward-occluded conformation was generated using the Phyre2 server [19]. The homology model of GlyT2 in the outward-occluded conformation was obtained from Subramanian et al. [20]. Mutations to the wild-type sequence that were introduced to help with crystallography were reversed (specifically, V50A and L350A in dDAT; and I218A, T366S, C418A and C507A in hSERT). GlyT2, dDAT, hDAT and hSERT were coarse-grained using the martinize protocol [21]. In order to maintain protein secondary structure, Go̅ -like potentials were applied to each protein using the GoMARTINI protocol [22]. Each coarse-grained protein was then inserted into a lipid bilayer generated using a custom version of the insane script [23]. The position and relative distribution of individual lipid species within the membrane was assigned randomly in each of the three replicate simulations of each system. The model two-component bilayer comprised 80% phosphatidylcholine (POPC) and 20% cholesterol, while the neuronal membrane was set up using previously reported lipid compositions (Table S1 and Figure S1) [11]. Parameters for the lipids were obtained from the same paper [11]. Due to the known limitations with the standard MARTINI coarse-grain water [21,24], the system was solvated using polarizable coarse-grained water. Each system contained ~1230 molecules in a box size of 20×20×14 nm with the membrane oriented in x-y plane. NaCl was added to a physiological concentration of 0.15 M. Additional ions were added to give overall charge neutrality for each system.
All 6 systems were energy minimized using a steepest descent algorithm. The systems were then equilibrated for 200 ns: we performed a series of five sequential 10 ns simulations with a 10 fs timestep with stepwise 1000, 500, 100, 50 or 10 kJ mol−1 nm−2 position restraints on the protein. Position restraints were removed, and a further equilibration of 150 ns was performed, using a 20 fs timestep. Each system was simulated in triplicate for 10 μs, again using a 20 fs timestep, in the recommended configuration for MARTINI [25]. Random velocities were assigned at the beginning of each simulation to initiate the simulation. The lengths of the covalent solute bonds were constrained using the LINCS algorithm. Simulations were performed in the NPT ensemble, with the temperature maintained at 310 K using the Bussi-Donadio-Parrinello velocity-rescaling thermostat [26] with a coupling constant of τt = 1 ps. The pressure was maintained at 1 bar through coupling to a Parrinello-Rahman barostat [27] with a coupling constant of τp = 12 ps and an isothermal compressibility of 3×10−4 bar. The non-covalent interactions were calculated with a 1.1 nm cut-off.
Analysis was performed on frames at 1 ns intervals unless otherwise specified. The membrane thickness was calculated with g_thickness [28], using the PO4 and GM1 beads of lipids. Lipid diffusion in the xy plane was calculated using gmx msd on the final 5 μs of the simulation. The 200 to 500 ns lag-time portion of the MSD was used to avoid ballistic trajectories obtained at short lag-times and poor averaging obtained at long lag-times [29]. To characterize how lipids within the complex neuronal membrane bind to the neurotransmitter transporters, residues within 6 Å of a lipid were calculated using the MDAnalysis package [30,31]. Contacts between lipid groups and protein residues were defined such that a lipid group is considered to be a contact of the protein if any part of it lies within 6 Å of the protein for ≥ 40% a single trajectory. A “long-lasting” lipid contact was defined as being within 6 Å of the protein for ≥ 70% a single trajectory. The regions and amino acids of the transporters with which these contacts formed were compared to the composition of the regions at the protein-membrane interface. The protein-membrane interface was defined as any residues within 6 Å of a lipid for at least 50% of a trajectory, across all 3 replicates. Lipid-lipid contact fractions were calculated as described in Wilson et al., 2020 [13]. All other membrane analyses (leaflet detection, flip-flop rates, area per lipid, and depletion-enrichment index calculation) were carried out using Python code built upon MDAnalysis [30,31]. The flip-flop analysis was carried out on the trajectory at 10 ns intervals in order to remain consistent and obtain comparable results to earlier studies [13]. Further details about the code are provided in the SI. Simulation parameter files, the initial and final coordinates of the simulations, and custom Python code are available at https://github.com/OMaraLab/SLC6_lipid_fingerprints.
Results
The local lipid environment is similar around different SLC6 membrane proteins
In order to monitor changes in the protein structure over the simulations, we calculated the protein RMSD and RMSF for each trajectory with respect to the initial protein conformation. No significant changes to the protein structure were observed in the 10 μs simulation time (RMSD < 3.5 Å and RMSF < 3.0 Å, Figures S2–S4). We characterized the lipid annulus around each SLC6 transporter with lipid depletion-enrichment and contact analyses. Lipid depletion-enrichment indices (DEI) summarize the enrichment or depletion of a certain lipid group within the lipid annulus around the protein, compared to the composition of the bulk membrane, over the simulation. A lipid annulus of 6 Å from the edge of the SLC6 transporter, with a 2 Å buffer around this annulus was used to quantify the immediate lipid environment. To identify specific lipid-protein interactions, we examined which lipid groups form contacts. In general, the local lipid environment around each SLC6 transporter is similar across major lipid headgroup types, although differences arise when inspecting the interactions between the transporter and individual lipid species. The behaviour of lipids was investigated both by headgroup and tail saturation.
The lipid annulus around dDAT, hDAT, GlyT2, and hSERT is enriched in polyunsaturated lipids
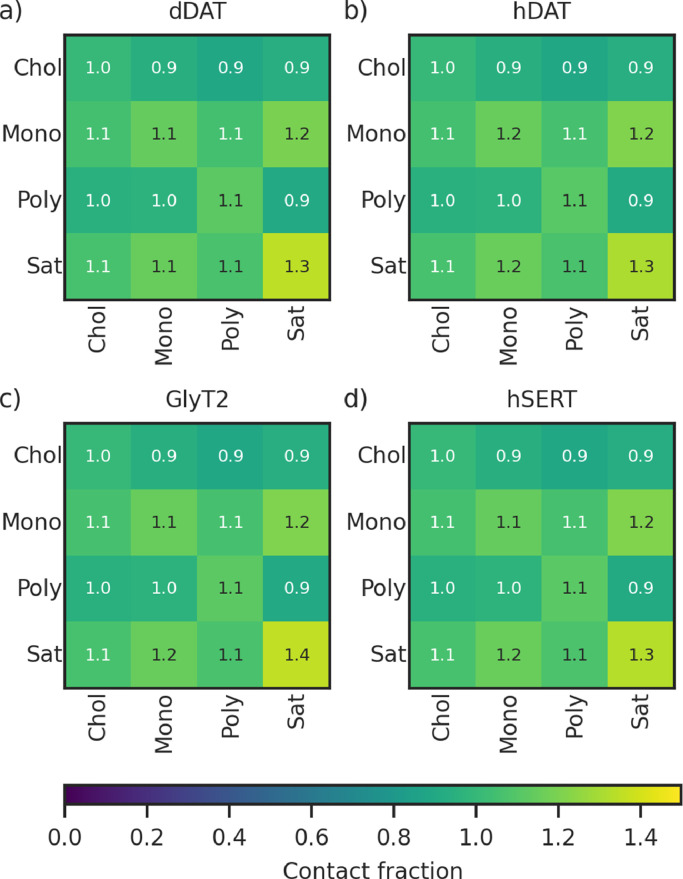
The neuronal membrane contains saturated, monounsaturated, and polyunsaturated species (Figures 1 and S1, Table S1). These are distributed asymmetrically across the intracellular and extracellular leaflet. On average, lipids in the extracellular leaflet contain 2.26 unsaturated bonds, while the intracellular leaflet contains an average of 3.62 unsaturations per lipid. Previous studies have shown that endogenous polyunsaturated fatty acids and their synthetic derivatives regulate the activity of SLC6 transporters, in particular GlyT2 [32] and the dopamine transporter [33,34]. Polyunsaturated lipids are enriched around all four transporters in both leaflets (DEIextracellular = 1.35 ± 0.16, DEIintracellular = 1.29 ± 0.08, Figure 2). For each embedded SLC6 transporter, saturated lipids are depleted around the protein (DEIextracellular = 0.44 ± 0.12; DEIintracellular = 0.33 ± 0.11). Regardless of the presence of the transporter, saturated lipids are depleted in the vicinity of polyunsaturated lipids and cholesterol, as shown by the lipid contact fractions in Figure 3. Monounsaturated lipids are neither significantly enriched nor depleted in the extracellular leaflet (DEIextracellular = 0.89 ± 0.15) and are depleted in the intracellular leaflet (DEIintracellular = 0.69 ± 0.12).
Figure 1.
Membrane composition by headgroup (top: a–d) and starting orientation of protein-bilayer systems (bottom: d–g) for neuronal system (left: a, b, d, e) and the model POPC-CHOL system (right: c, d, f, g). Proteins are shown in grey. Full composition and definition of acronyms are given in Table S1.
Figure 2.
The mean depletion-enrichment index (DEI) of lipids in the protein annulus grouped by tail saturation, in the a) extracellular leaflet; and b) the intracellular leaflet. The standard error of the mean is represented by the error bars. DEI values of each lipid species are shown in Table S2. Blue: dDAT, green: hDAT, yellow: GlyT2, pink: hSERT.
Figure 3.
The average lipid contact fractions of the neuronal membrane, by tail saturation, for membranes with embedded: a) dDAT, b) hDAT, c) GlyT2, and d) hSERT.
Phosphatidylcholine lipids form few and non-specific sustained contacts with SLC6 transporters
Phosphatidylcholines (PC) are the most abundant class of glycerophospholipid in the neuronal membrane (24% of the extracellular and 13% of the intracellular leaflets), and the second most abundant lipid class in the membrane after cholesterol. Six polyunsaturated species (PAPC, DOPC, PUPC, PFPC, OIPC, OUPC), one monounsaturated species (POPC) and one saturated species (DPPC) are present in the neuronal membrane. Despite their abundance in the membrane, as a class, PC lipids are largely depleted in the lipid annulus around the SLC6 transporters in both the intracellular and extracellular leaflets of the membrane (DEIextracellular = 0.76 ± 0.11; DEIintracellular = 0.56 ± 0.06, Figure 4). The population of PC lipids within the membrane appears to influence which particular lipid species form contacts with each transporter. Contacts (defined as persisting for >40% of each trajectory) are only formed with lipids that constitute at least 1% of the membrane: PUPC, PAPC, DOPC, and POPC. These contacts are formed with different regions of each transporter, indicating no overall pattern or specificity in the contacts formed. For example, PAPC and PUPC form long-lasting contacts with every transporter, but the number and lengths of the contacts vary between the transporters. Overall, no specific contacts with PC lipids are consistently formed with any one region of a transporter across all three replicates of a single transporter.
Figure 4.
The mean depletion-enrichment index (DEI) of lipids in the protein annulus grouped by head group, in the a) extracellular leaflet; and b) the intracellular leaflet. The error bars give the standard error of the mean. DEI values of each lipid species are shown in Table S2. Blue: dDAT, green: hDAT, yellow: GlyT2, pink: hSERT.
Polyunsaturated phosphatidylserine lipids are enriched around dDAT, hDAT, GlyT2 and hSERT
Phosphatidylserine (PS) lipids are only found in the intracellular leaflet and comprise 10% lipids in the intracellular leaflet. As a class, PS lipids are neither enriched nor depleted around each transporter (DEIintracellular = 1.07 ± 0.21). However, this analysis averages the enrichment behaviour of each PS lipid (Figure S5). Two highly unsaturated lipid species are enriched around each transporter: OUPS (DEI = 1.92 ± 0.99), which contains 7 unsaturations (C18:1/22:6 tails), and PUPS (DEI = 1.45 ± 0.32), containing 6 unsaturations (C16:0/22:6). Despite its enrichment, OUPS lipids only contact hSERT for 42–44% a single replicate and do not form contacts with dDAT, hDAT or GlyT2. In contrast, PUPS lipids form contacts with each transporter. PUPS interacts with residues in TM10 in two replicates of dDAT and hSERT and one replicate of GlyT2 and hDAT for up to 53% of a single trajectory. PUPS lipids also form contacts with residues in TM11 in one replicate each of dDAT, hDAT, and hSERT; and finally, PUPS interacts with one residue in TM7 of hDAT. PAPS, a polyunsaturated lipid with 4 unsaturations (C16:0/20:4) is enriched around dDAT (DEI = 1.21 ± 0.13) and hDAT (DEI = 1.18 ± 0.43), but depleted around hSERT (DEI = 0.82 ± 0.12) and GlyT2 (DEI = 0.77 ± 0.02). Contacts are formed between PAPS and residues in TM10 and TM11 of hDAT, but not with any other transporter. In contrast to OUPS and PUPS, the saturated DPPS (DEI = 0.43 ± 0.48) and monounsaturated POPS (DEI = 0.57 ± 0.15) are generally depleted around each transporter.
Phosphatidylethanolamines interact differently with dDAT, hDAT, GlyT2, and hSERT
Phosphatidylethanolamine (PE) lipids are the second most abundant glycerophospholipid in the neuronal plasma membrane. The neuronal membrane contains five polyunsaturated species (PUPE, PAPE, OAPE, OUPE and OIPE) and a single monounsaturated (POPE) species. PE lipids are the only lipid family to have different depletion-enrichment profiles over the intracellular and extracellular leaflets. PE lipids comprise 22% of the intracellular leaflet. Despite their relative abundance in the intracellular leaflet, PE lipids are depleted around all four transporters in this leaflet (DEIintracellular = 0.86 ± 0.08). The enrichment varies by protein in the extracellular leaflet, where PE lipids comprise 11 mol% of the total lipids; the relative depletion-enrichment index shows that they are enriched around dDAT (DEIextracellular = 1.29 ± 0.19), hDAT (DEIextracellular = 1.42 ± 0.40), and GlyT2 (DEIextracellular = 1.58 ± 0.31), but not hSERT (DEIextracellular = 1.09 ± 0.22). No leaflet- or protein-dependent trend is apparent in the lipid enrichment profiles of individual PE lipid (Figure S5). For example, lipids in the extracellular leaflet such as OAPE and OUPE are the most enriched around GlyT2, following the trend of the overall PE class. Inversely, lipids in the intracellular leaflet such as OUPE and PUPE are the most depleted around GlyT2.
The contact profiles of lipid-protein interactions correlate with the DEI trends in the extracellular leaflet, but highlight differences in lipid interaction behaviour between the SLC6 transporters (Figure S6). hDAT forms the greatest number (124) of contacts with PE lipids, followed by hSERT (108) and GlyT2 (106). dDAT forms the fewest contacts (88). Furthermore, the contacts are maintained for longer in each 10 µs replicate for hDAT (maximum 84% and median of 48% of each 10 µs simulation), than for hSERT (max 81% and median 48%) or GlyT2 (max 70% and median 46%). Although dDAT formed fewer contacts with PE lipids than GlyT2, these contacts were maintained for longer (max 75% and median 47%). Most of the interactions with PE lipids take place in helical regions of each transporter (dDAT: 82%, hDAT: 77%, GlyT2: 69%, hSERT: 77%) and do not occur as frequently in loop regions. The most frequently occurring contact sites of PE lipids for dDAT are in TM5 (18 ± 5%) and TM12 (19 ± 8%) (Figure 5a and S6). Residues in TM12 of hDAT also form frequent contacts with PE lipids, accounting for 25 ± 3% of contacts with PE over the replicates. However, contacts with TM5 of hDAT are relatively few, comprising only 6 ± 2% of the interactions with PE lipids. Likewise, contacts between hSERT and PE lipids occur mostly with residues in TM12 (14 ± 3%) and TM11 (14 ± 1%) but not with TM5 (3 ± 3%). In contrast, contacts with TM5 in GlyT2 account for 14 ± 2% of PE contacts and interactions with TM11 comprise a further 18 ± 5% of contacts. The PE contacts with the transporter are very dynamic and PE lipids exchange rapidly in each binding site. While PUPE binds to each transporter for >40% of the 30 µs of total simulation time, the binding sites of these PE lipids vary between replicates. The PE contacts are driven almost entirely by tail group interactions; only 2% (GlyT2) – 6% (hDAT) of the contacts over all replicates occur between the protein and a PE headgroup.
Figure 5.
The fraction of contacts formed by each class of lipids with the protein, classified by protein region. a) PE lipids; b) GS lipids; c) PI lipids; d) cholesterol. The error bars (black) give the standard error of the mean. Blue: dDAT, green: hDAT, yellow: GlyT2, pink: hSERT.
PE lipids also interact preferentially with certain amino acids, as shown in Figure 6a. Across all four SLC6 transporters, protein-PE lipid contacts preferentially form with phenylalanine (29 ± 2%, 27 ± 3%, 32 ± 3%, and 27 ± 6% of contacts over three replicates for dDAT, hDAT, GlyT2 and hSERT, respectively). Phe comprises between 9.3% (hSERT) and 12.7% (dDAT) of the membrane-facing amino acids in each of the four transporters (Figure S7). PE lipids also form frequent contacts with tryptophan across all four transporters (13 ± 2%, 15 ± 2%, 13 ± 2%, 19 ± 2% of contacts for dDAT, hDAT, GlyT2 and hSERT, respectively) despite only comprising 4.1–4.9% of the membrane-facing amino acids. In addition, PE lipids interact with glycine and alanine to a lesser extent than may be expected from their relative abundance within the transmembrane interface of each transporter. The prevalence of tail group contacts with aromatic groups suggests that the double bond beads in the highly unsaturated lipid tails may be interacting preferentially with aromatic amino acids in a manner that may map to CH-π or π-π interactions in atomistic structures. These non-covalent interactions have been noted to provide stability of up to ~30 kJ/mol to protein-protein [35], nucleic acid-protein [36,37] and carbohydrate-protein complexes [38,39] but have not been previously identified in lipid-protein interactions.
Figure 6.
The fraction of contacts formed by each class of lipids with the protein, classified by amino acid. a) PE lipids; b) GS lipids; c) PI lipids; d) cholesterol. The error bars (black) give the standard error of the mean. Blue: dDAT, green: hDAT, yellow: GlyT2, pink: hSERT.
Glycosphingolipids form long-lived headgroup interactions with dDAT, hDAT, GlyT2, and hSERT loop regions
Glycosphingolipids (GS) make up 10% the extracellular leaflet of the model neuronal membrane and include glucosylceramides and glycosylated gangliosides. Glycosphingolipid species in the neuronal membrane comprise the monounsaturated (DPGS and DBGS) and polyunsaturated glucosylceramides (PNGS and POGS), monounsaturated monosialotetrahexosylgangliosides (DPG1) and the monounsaturated monosialodihexosylgangliosides (DPG3). As a group, glycosphingolipids are enriched around each transporter. GS lipids are less enriched around GlyT2 (DEI = 1.60 ± 0.35; 88 contacts) than dDAT (DEI = 1.96 ± 0.46; 101 contacts), hDAT (DEI = 2.10 ± 0.44; 120 contacts), and hSERT (DEI = 2.11 ± 0.76; 154 contacts). Individually, the DEI and lipid contact profiles vary between transporters, as extreme DEI values can be driven by the low population of the glycosphingolipids. The most common glycosphingolipid, DPGS, comprising 5% the extracellular leaflet, is consistently enriched around every transporter (DEI = 1.70 ± 0.51). The second most populous GS lipid, PNGS (comprising 1% the extracellular leaflet), is enriched around dDAT (DEI = 2.55 ± 0.90), hDAT (DEI = 2.43 ± 0.59), and hSERT (2.86 ± 0.76), but depleted around GlyT2 (DEI = 0.58 ± 0.26). Lower population glycosphingolipids vary much more; most are not systematically enriched or depleted around any of the SLC6 transporters but are both enriched and depleted in different replicates of at least one transporter. Five molecules each of DPG1, DPG3, and DBGS are present in the membrane, constituting 0.8% the extracellular leaflet, while one molecule each of DBG1, DBG3, PNG1, and PNG3 are present in the extracellular leaflet. Accordingly, these low population lipids are either highly enriched or depleted around each transporter in different replicates. The variation in individual lipid profile underscores that the lipid annulus is a dynamic environment and that differences in the average lipid fingerprint at the individual lipid species level (particularly for low abundancy species) may arise from the stochastic nature of lipid diffusion.
The contacts formed by lipids to each transporter suggest that the nature of interactions differs between each of the GS lipids (Figure S8). Long-lasting interactions (maintained for ≥ 70% any single trajectory) comprise 30%, 47%, 40%, and 49% of the total GS contacts (maintained for ≥ 40% any single trajectory) with dDAT, hDAT, GlyT2, and hSERT, respectively. Individual glycosphingolipids containing polysaccharide headgroups (G1 and G3) form more long-lived contacts than individual lipids with monosaccharide headgroups. These interactions also vary between transporters, particularly between hSERT and the other three SLC6 transporters. DBG3 forms long-lived interactions with two replicates of hSERT, but no other transporter, while DPG3 forms long-lived contacts with one replicate each of dDAT, hDAT, and GlyT2, but all three replicates of hSERT. No other species with polysaccharide headgroups forms long-lived contacts with hSERT, however DPG1 forms long-lasting contacts with one replicate each of hDAT and GlyT2 and two replicates of dDAT. PNG1 has long-lasting interactions with dDAT, hDAT and GlyT2 in one replicate each. Overall, these low population glycosphingolipids form few contacts with each protein, but the contacts formed are long-lasting. For example, one replicate of GlyT2 interacts with PNG3 ≥70% of the trajectory and one replicate of hDAT interacts with DBG1 ≥70% of the trajectory.
The lipids with a glucosylceramide headgroup form fewer sustained contacts with each transporter than is suggested by their high enrichment. Although enriched around all transporters in all replicates, DPGS forms long-lived contacts with two replicates each of dDAT, hDAT, and hSERT, and one replicate of GlyT2. Similarly, despite being enriched around all replicates of dDAT, hDAT, and hSERT, PNGS only forms long-lasting contacts with one replicate of hDAT and hSERT. DBGS forms long-lasting contacts with one replicate each of GlyT2 and hSERT, while POGS forms long-lasting contacts with one replicate of GlyT2.
SLC6 transporter interactions with GS lipids are localized to particular structural regions, but not to specific amino acid residues (Figure 7). GS lipids most commonly interact with the extracellular loop regions in all four transporters. Contacts with EL2 account for 17 ± 9%, 29 ± 4%, 31 ± 9%, and 29 ± 8% of GS contacts over the three replicates with dDAT, hDAT, GlyT2, and hSERT, respectively. Interactions with EL3 comprise a further 36 ± 2%, 21 ± 5%, and 32 ± 13% of GS contacts with dDAT, hDAT, and hSERT, respectively. Unlike the other SLC6 transporters, only 7 ± 7% of GS contacts with GlyT2 occur with residues in EL3 (2% of the total contacts). Instead, EL6 is the second most frequently occurring loop interaction for GlyT2 (9 ± 7% of contacts over the replicates). The frequencies of lipid interaction with particular amino acid residues largely fall within 6% of their abundance at the membrane interface (Figure 6b). Exceptions include threonine contacts in hDAT, which form 14 ± 2% of all GS contacts, but only comprises 6% the membrane-facing residues; and isoleucine contacts in hSERT, which form 4 ± 2% of the GS contacts but comprise 14% membrane-facing residues.
Figure 7.
Top-down view of density of lipid groups around a) dDAT, b) hDAT, c) GlyT2, and d) hSERT. Surfaces represent spaces with at least 0.005 A−3 density. Proteins are aligned. High-density sites are represented in by pink: cholesterol; yellow: GS; and orange: PE. Lower density sites are represented by grey: DAG (PADG); green: PC; blue: PI; and purple: PS. SM lipids are too low-density to be visible.
Phosphatidylinositols interact specifically with dDAT, hDAT, GlyT2, and hSERT, but interactions vary in number and location between the transporters
Phosphoinisotol (PI) lipids comprise 6 mol% of the intracellular leaflet of the neuronal plasma membrane and occur as monounsaturated phosphatidylinositol (POPI), polyunsaturated phosphatidylinositol (PUPI, PAPI and PIPI), and polyunsaturated phosphatidylinositol mono-, bi- or triphosphate (PAP1, PAP2 and PAP3). As a group, PI lipids are enriched around all four SLC6 transporters investigated (DEI = 3.10 ± 0.32). However, the quantity, length, and location of these interactions varies between each SLC6 transporter. PI lipids form more contacts with dDAT (82 contacts) and hDAT (72 contacts) than GlyT2 (66 contacts) and hSERT (51 contacts); however, more of the interactions are long-lasting (persisting for ≥ 70% of single 10 µs replicate simulation) in hDAT (15 contacts) and hSERT (13 contacts) than dDAT (5 contacts) and GlyT2 (9 contacts). Despite the lower abundance of PI lipids in the membrane compared to PE lipids (5 mol% vs 22 mol% of the intracellular leaflet), dDAT forms a comparable number of contacts with PI lipids as with PE lipids (88 contacts). This is not the case for hDAT, GlyT2, and hSERT, where interactions with PI lipids occur less frequently than those with PE or GS lipids.
PUPI, the most abundant PI lipid (comprising 2 mol% of the intracellular leaflet), is enriched around each transporter (DEI = 4.22 ± 0.98) and forms contacts with all four transporters across all three 10 µs replicates. As the lipid population decreases for the different non-phosphorylated PI lipids, more sporadic interactions are observed. The next most abundant species, POPI and PAPI, each comprise 1.3% the intracellular leaflet. Although they are both enriched around the transporter across all replicates (DEIPOPI = 1.66 ± 0.45, DEIPAPI = 2.69 ± 0.77), POPI lipids only form contacts >40% a single trajectory with one replicate each of hDAT and hSERT, while PAPI lipids form contacts with two replicates of hDAT and hSERT. No contacts between these lipid species and GlyT2 or dDAT residues were observed. Similarly, PIPI lipids comprise 0.5% the intracellular leaflet and are enriched around each transporter (DEI = 2.72 ± 1.30), but only comes into contact with one residue of hDAT for 41% of a single replicate. Only one molecule each of the phosphorylated lipids PAP1, PAP2, PAP3, POP1, POP2, and POP3 exist in the membrane. Two replicates of hDAT, GlyT2, and hSERT each interact with a phosphorylated lipid, although the species differs between replicates – hDAT forms contacts with POP1 and POP2, GlyT2 forms contacts with POP1 and PAP1, and hSERT forms contacts with PAP1 and PAP3. Phosphorylated lipids do not form any contacts with dDAT in any of the simulations.
PI lipids interact most commonly with helical regions in each transporter. Contacts with the TM5 helix are the most common, comprising 14 ± 7% of the interactions with PI lipids for dDAT, 20 ± 10% for hDAT, 10 ± 5% for GlyT2, and 10 ± 7% for hSERT (Figure 5c and Figure S9). Each transporter also has unique interaction sites. PI lipids form contacts with dDAT in the region of TM11 (12 ± 5% of contacts) and TM12 (11 ± 6% of contacts). In hDAT, PI lipid contacts occur with TM1 (22 ± 1% of contacts) and TM7 (20 ± 5% of contacts). For GlyT2, PI lipid contacts occur primarily in the IL4 region (31 ± 22% of contacts), followed by TM12 (12 ± 11% of contacts). In hSERT, 10 ± 7% of contacts are formed with TM5, 9 ± 5% with IL3 and 12 ± 6% with TM12. Regardless of the transporter, both the lipid tail (57–64%) and PI lipid headgroup (36–43%) are involved in these interactions.
Figure 6c shows that the specific amino acids in contact with PI lipids also varies between transporters. In hSERT, 30 ± 11% of all amino acid/PI interactions are with lysine, while in hDAT and GlyT2 lysine forms 20 ± 0% and 17 ± 3% of the interactions with PI lipids across replicates, respectively. This is lower in dDAT, where lysine is only involved in 12 ± 4% of the PI interactions and interactions are also spread across phenylalanine (21 ± 1%). As lysine only comprises between 3.6 and 4.5% of residues in the membrane-facing regions of each protein, these contacts occur far more frequently than may be expected from random interactions. The enrichment of interactions with cationic amino acids is consistent with the formation of electrostatic interactions with the PI lipid headgroup. This has been previously reported to be important for stabilizing PI interactions with proteins [40].
The binding of phosphatidylinositol (4,5)-bisphosphate to the N-terminus (Lys3 and Lys5) of hDAT has been shown to support dopamine release in response to amphetamine [40]. PI lipids form contacts with the N-terminus of all four transporters. However, these interactions do not exclusively occur with phosphatidylinositol bisphosphate lipids. Rather, most interactions with the N-terminus occur with non-phosphorylated PI lipids. Additionally, while previous contacts were reported with the Lys3 and Lys5 of hDAT, the homology model of hDAT has 57 amino acids truncated from the N-terminus, while the dDAT, GlyT2 and hSERT structures have 24, 196, and 73 amino acids truncated from the N-terminus, respectively. Thus, different amino acids form the basis of the interactions. In dDAT, PUPI lipids form contacts with Ser31; in hDAT, PUPI and POP1 interact with Lys65. In GlyT2, PUPI and POP1 lipids form contacts with Trp194, Ser195, and Lys197. In hSERT, Lys84 forms a contact with PAPI. The interaction pattern of the N-terminus with PI lipids as a class is similar, with Ser195 in GlyT2 being homologous to Ser31 in dDAT, and Lys65 in dDAT being homologous to Lys84 in hSERT and Lys196 in GlyT2 (adjacent to the PI interaction site Lys197).
Phosphatidic acids, sphingomyelins, diacylglycerols and ceramides form few sustained contacts with dDAT, hDAT, GlyT2, or hSERT
The interactions of several lipid classes with dDAT, hDAT, GlyT2 and hSERT are distinctly depleted (Figure 4) or transient. Sphingomyelin (SM) lipids comprise 9% of the extracellular leaflet and 2% of the intracellular leaflet but form few contacts with each transporter. SM lipids are depleted around all proteins in both leaflets (DEIextracellular = 0.44 ± 0.09; DEIintracellular = 0.33 ± 0.10) and do not form direct contacts with any of the SLC6 transporters for >50% of each 10 µs replicate simulation. The low concentration lipids, CER (ceramide, 1 DPCE in each leaflet) and PA (1 PAPA in the membrane), do not form contacts with any transporter, while DAG (1 PADG in each leaflet) has transient contacts with dDAT, hDAT, and hSERT. None of these lipids formed systematic contacts with any transporter over multiple replicate simulations. However, due to the low number of these lipids within each leaflet, any localization around the transporter was magnified in the DEI analysis. Despite the presence of only two molecules of PADG in the neuronal membrane, PADG is significantly enriched around each of the three transporters in both leaflets (DEIextracellular = 4.93 ± 3.23; DEIintracellular = 3.06 ± 1.97). Nonetheless, it forms contacts only in one replicate of dDAT, one replicate of hDAT, two replicates of hSERT and no contacts are observed with GlyT2. PADG flips between leaflets, and the glycerol groups of PADG interact directly with the transporters near the interleaflet interface. Specifically, a single PADG lipid interacts for 41% of a single 10 µs dDAT replicate simulation in the region between TM2 and TM7, and for 44% of a single simulation with TM10 in hDAT. PADG also forms contacts with hSERT for up to 64% of a trajectory, in the region of TM12 in one replicate and in the region of TM7 in another. This indicates that PADG selectively aggregates around each transporter but does not form sustained contacts with any single region. Overall, sphingomyelin, phosphatidic acid, and ceramide lipids do not form contacts with any of the four neurotransmitter transporters examined.
Cholesterol binds to SLC6 transporters at specific sites on each protein
Cholesterol makes up 46% of the neuronal plasma membrane model and is known to play a key role in neurotransmission, and the activity of DAT [41, 42] and SERT [43,44]. GlyT2 is modulated by different concentrations of cholesterol in the membrane [45]. Bound cholesterol has been found at the interface of TM1a, TM5, and TM7 (which form the CHOL1 binding site) in all structures of dDAT [17,46,47], while the cholesterol analogue, cholesteryl hemisuccinate, is bound at the interface of TM2 and TM7 (at the CHOL2 binding site) in a subset of these structures [17,47]. Previous molecular dynamics studies found that cholesterol occupied the corresponding CHOL1 and CHOL2 binding sites of hSERT. The CHOL1 site was occupied to a higher extent [48,49] and experiments mutating the CHOL1 site in hSERT to improve or weaken cholesterol binding found that the binding of cholesterol to CHOL1 modulates the protein conformation between an outward-facing conformation (when cholesterol is bound) and an inward-facing conformation (when cholesterol is depleted) [49]. A potential third cholesterol binding site (CHOL3) has also been identified on TM12 of the hSERT crystal structure [18]. In addition, previous coarse-grained simulations of dDAT, hDAT, and hSERT in POPC/CHOL membranes located another three binding sites around TM4, TM5, and TM8 (CHOL4); TM9 and EL2 (CHOL5); and TM10, TM11, and TM12 (CHOL6) [48]. There are also numerous occurrences of cholesterol-binding motifs (CRAC and CARC) in each protein. Specifically, 4 CRAC motifs and 10 CARC motifs are present in dDAT, 6 CRAC motifs and 9 CARC motifs are on hDAT, 5 of each CRAC and CARC motifs reside on GlyT2, and 6 CRAC and 5 CARC motifs are present on hSERT. While these motifs can be predictive of cholesterol binding [50], previous simulations have found that most of these motifs are not near major binding sites [48]. Similarly, we observe that cholesterol aggregates preferentially around two particular cholesterol-binding motifs, instead of binding equally to every motif present.
Across our simulations, cholesterol is slightly depleted around all four SLC6 transporters in the neuronal membranes (DEIextracellular = 0.95 ± 0.02, DEIintracellular = 0.91 ± 0.06, Figure 4). However, the remaining interactions with the protein are highly localised to certain regions (Figure S7). Cholesterol forms 432 contacts (lasting >40% of each 10 µs replicate simulation) with dDAT, 473 contacts with hDAT, 496 contacts with GlyT2, and 513 contacts with hSERT. Of these, 115 contacts in dDAT are sustained for >90% of the simulation time for each 10 µs replicate, compared to 169 in hDAT, 155 in GlyT2, and 168 in hSERT. Due to the high proportion of cholesterol in the membrane, only these “high occupancy” contacts (lasting for >90% of each trajectory) will be discussed further. Moreover, the proportion of contacts formed with particular regions of the protein is discussed with respect to the total number of contacts over all replicates, rather than the mean and standard error as above. As expected, the high occupancy contacts are formed almost exclusively in helical regions (96% in dDAT and hDAT, 99% in GlyT2 and 94% in hSERT).
A substantial number of these high occupancy contacts (Figure S10) are formed with residues within 6 Å of previously identified binding sites, particularly those present in the crystal structures. In the case of dDAT, 21% of these cholesterol contacts are formed with residues within 6 Å of the crystallographic cholesterol at binding site CHOL1, 6% are within 6 Å of crystallographic cholesterol at binding site CHOL2, and another 3% are with residues around CHOL3. Cholesterol contacts are distributed differently in hDAT; with 21% of the high occupancy contacts formed with residues at CHOL2, while only 12% are formed with CHOL1 and another 4% with CHOL3. In GlyT2, 26% of the high occupancy contacts are also formed at regions corresponding to the crystallographic binding sites: cholesterol is approximately equally distributed between CHOL1 (8% high occupancy contacts), CHOL2 (10%), and CHOL3 (8%). In the case of hSERT, 27% of the of high occupancy contacts are at the crystallographic binding sites; while cholesterol is found at CHOL1 and CHOL2 and with TM12. In general, cholesterol rarely interacts at CHOL3 (3 high occupancy contacts).
Cholesterol is also found to occupy locations corresponding to the binding sites previous identified in simulation by Zeppelin et al. [48]. Many high occupancy cholesterol contacts are formed with residues at binding site CHOL6 across each transporter: 27% of high occupancy contacts with dDAT, 20% contacts with hDAT, 17% with GlyT2 and hSERT. In dDAT and hDAT, cholesterol also accumulates around CHOL4. Here, 12% of high occupancy contacts are formed with CHOL4 in dDAT, and 17% in hDAT, compared to 7% in GlyT2 and 11% in hSERT. Contacts with CHOL5 are more common in GlyT2, where 13% of the high occupancy contacts are formed with cholesterol at CHOL5, compared to 9% in dDAT, 7% in hDAT, and 11% in hSERT.
While site-specific binding of cholesterol is observed in all four SLC6 transporters, CRAC and CARC motifs comprise a high percentage of the protein sequence (~25%), and cholesterol does not preferentially bind to all these cholesterol-binding motifs in dDAT, hDAT, hSERT or GlyT2. Instead, the majority of cholesterol binding at cholesterol-binding motifs is targeted to two specific cholesterol-binding motifs (CRAC motif on TM4 and IL2 and CARC motif on TM11). Specifically, cholesterol binds to CRAC motif on TM4 and IL2 on all four transporters and to residues of the CARC motif on TM11 for dDAT and GlyT2. This latter motif is not present on hSERT. In total, another 7% of high occupancy contacts are formed with the specified CRAC and CARC motifs on dDAT, compared to 17% in hDAT, 7% in GlyT2, and 4% in hSERT. The lack of the CARC motif on TM11 on hSERT, but substantial binding to a binding site (CHOL6) that includes TM11, is further indication that cholesterol-binding motifs are not a prerequisite for cholesterol-binding. This is supported by the binding of cholesterol to specific motifs rather than all CARC and CRAC motifs.
Cholesterol forms high occupancy contacts with isoleucine 12% more frequently than the abundance of Ile in the membrane-facing region of dDAT and GlyT2. It also forms high occupancy contacts with leucine for 7% (GlyT2) – 10% (hDAT) more than the abundance of Leu in the relevant region of dDAT, hDAT, and GlyT2. Finally, high occupancy contacts with Val are formed 9% (hDAT) – 11% (dDAT) more with dDAT, hDAT, and hSERT than their abundance within the membrane-facing region would suggest. This is consistent with branched amino acids (e.g., Ile, Leu and Val) associating with the β-face of cholesterol through van der Waals interactions [51]. All other amino acids of the transporters interact with CHOL at a rate within 5% of their occurrence. Residues on TM5 account for a large number of high occupancy contacts with cholesterol for all four transporters. In dDAT, contacts with TM12 (21%) and TM5 (19%) comprise the greatest proportion of high occupancy contacts (21%) (Figure 5d). A number of high occupancy contacts are formed with residues in TM5 of hDAT (18%) and hSERT (18%). However, in GlyT2, cholesterol contacts with TM5 only comprise 14% of the high occupancy contacts. Instead, most cholesterol interactions for GlyT2 occur with TM12 (24%) and TM9 (15%). Overall, while similar binding sites are observed for each SLC6 transporter, cholesterol binds preferentially at different sites for each protein: while CHOL6 is highly occupied in each transporter, cholesterol forms many contacts with CHOL1 in dDAT, CHOL4 in hDAT, CHOL5 in GlyT2, and CHOL2 in hSERT.
The presence of embedded SLC6 transporters alter some membrane properties
To evaluate the effects of each SLC6 transporter on the neuronal membrane biophysical properties, we calculated the membrane thickness, area per lipid, lipid lateral self-diffusivity and distribution of lipid species for each neurotransmitter transporter system. Due to the asymmetric composition of the neuronal membrane, results are determined for each leaflet individually.
The average membrane thickness does not vary significantly when the four SLC6 transporters are embedded in the membrane, with all four systems having an average membrane thickness of 41.8 – 41.9 ± 0.6 Å (Table 1). These values are consistent with the membrane thickness derived from previously published simulations of neuronal membrane (41.5 ± 0.2 Å) [13] in the absence of membrane proteins, suggesting that the presence of the transporter does not significantly affect this property. The thickness is not a uniform property across the membrane (Figure 8) but varies by proximity to the embedded transporter. In all cases, the membrane is thinner closer to the protein-lipid interface. In the neuronal systems, the maximum membrane thickness is 46.0, 46.1, 46.6, and 48.0 Å for hSERT, hDAT, GlyT2, and dDAT, respectively (Table 1). At its thinnest point, the membrane thickness is 34.2, 35.5, 37.0 and 37.0 Å for hSERT, GlyT2, dDAT and hDAT, respectively.
Table 1.
Neuronal membrane thickness and area per lipid of each leaflet with and without SLC6 transporters
| SLC6 transporter | Average thickness (Å) | Minimum thickness (Å) | Maximum thickness (Å) | Area per lipid (APL) | Lateral self-diffusivity | ||
| Intracellular leaflet (Å2) | Extracellular leaflet (Å2) | Intracellular leaflet (10–7 cm2/s) | Extracellular leaflet (10–7 cm2/s) | ||||
| dDAT | 41.8 ± 0.7 | 36.9 | 48.0 | 49.2 ± 0.4 | 46.4 ± 0.3 | 1.75 ± 0.10 | 0.88 ± 0.07 |
| hDAT | 41.8 ± 0.5 | 37.0 | 46.1 | 49.4 ± 0.4 | 46.3 ± 0.3 | 1.65 ± 0.08 | 0.81 ± 0.03 |
| GlyT2 | 41.9 ± 0.6 | 35.6 | 46.7 | 49.2 ± 0.4 | 46.2 ± 0.3 | 1.67 ± 0.06 | 0.91 ± 0.04 |
| hSERT | 41.8 ± 0.6 | 34.2 | 46.1 | 49.3 ± 0.4 | 46.4 ± 0.3 | 1.63 ± 0.07 | 0.85 ± 0.04 |
| No proteina | 42.2 ± 0.2 | 41.6 | 42.9 | 47.8 ± 0.3 | 45.1 ± 0.3 | 1.34 ± 0.06 | 0.63 ± 0.03 |
| No proteinbww | 40.6 ± 0.02 | -c | -c | 48.5 ± 0.1 | 46.0 ± 0.1 | 2.8 ± 0.2 | 1.6 ± 0.2 |
Figure 8.
Membrane thickness across the x-y plane of the neuronal membrane embedded with a) dDAT; b) hDAT, c) GlyT2; and d) hSERT. Proteins are not aligned to each other. The colour bar is truncated and does not represent the full range of values. Average and standard deviation of the thickness is shown below each plot, followed by the minimum and maximum values.
The area per lipid (APL) is related to the packing, phase, and fluidity of the membrane [52,53]. The average APL is not sensitive to which protein is embedded but differs between leaflets. Table 1 shows that the average APL of the extracellular leaflet (46.2 – 46.4 ± 0.3 Å2) is lower than that of the intracellular leaflet (49.2 – 49.4 ± 0.4 Å2). The APL is constant throughout the time-course of the simulation (Figure S11). Notably, these values lie within the range previously reported for simulations of neuronal membranes in the absence of protein (Table 1) [11,13], suggesting that the presence of the embedded SLC6 transporter does not significantly affect the APL. One factor that affects the APL is the proportion of polyunsaturated lipids in the membrane, as polyunsaturated lipids have larger areas per lipid (Figure S12) [54]. In the neuronal membrane, 36% the intracellular leaflet is comprised of polyunsaturated lipids, compared to only 23% the extracellular. In addition, the relative enrichment of low APL lipids in the extracellular leaflet suggests a possible basis for the difference in areas per lipid between the leaflets. Of the lipid species present, glycosphingolipids (46.2–46.8 Å2) and sphingomyelins (53.0–53.7 Å2) have the smallest areas per lipid (Figure 9) and are predominantly found in the extracellular leaflet (Table S1), explaining the lower APL of the extracellular leaflet.
Figure 9.
Area per lipid for each lipid class in the neuronal membrane, for a) the extracellular leaflet; and b) the intracellular leaflet. The standard deviation is represented by the error bars. Blue: dDAT, Green: hDAT, yellow: GlyT2, pink: hSERT.
Regardless of which SLC6 transporter is present, the lateral self-diffusivity of the lipids in the extracellular leaflet (0.81 – 0.91×10–7 cm2/s) is slower than in the intracellular leaflet (1.63 – 1.75×10–7 cm2/s; Table 1) This trend is consistent with the lateral self-diffusivities derived from previously published simulations of neuronal membrane (1.34×10–7 cm2/s and 0.63×10–7 cm2/s, respectively) [13]. Notably, the absolute magnitude of the lateral self-diffusivity is higher than our previous work conducted on the protein-free neuronal membrane under the same simulation conditions and box size [13], but is lower than the value obtained by Ingolfsson et al. [11] using a significantly larger system. Despite this, changes in lateral self-diffusivity across the four SLC6 systems do not appear to be correlated to the propensity of the lipid to form interactions with the transporters (Figure 10 and Table 1).
Figure 10.
Self- diffusivity of lipid classes in the neuronal membrane, for a) the extracellular leaflet; and b) the intracellular leaflet. The error bars give the standard error of the mean. Blue: dDAT, green: hDAT, yellow: GlyT2, pink: hSERT.
A consistent pattern of domain formation occurs across the neuronal membranes regardless of which SLC6 transporter is present (Figure 11). Specifically, CHOL is equally distributed throughout the membrane and PC lipids are slightly enriched around SM lipids (1.2), but otherwise equally distributed. PE lipids are enriched around PE (1.2–1.3), PS (1.4), and PI (1.4), but depleted around GS lipids (0.7). PS lipids are highly enriched in the vicinity of PS (2.0–2.1) and PI lipids (2.1) and are depleted around SM (0.4). SM and GS are enriched around themselves and each other and significant rafting of GS is observed (3.3–3.4), while SM-SM and GS-SM clustering is enriched to 1.5–1.6. PI lipids also raft (2.1–2.2) and are depleted around SM (0.4). In general, the domain formation is similar to that observed in the neuronal membrane in the absence of the embedded transporters [13]. The exceptions are that PI domain formation occurs less in the presence of the transporter and GS domain formation occurs more in the presence of the transporters. Since both PI and GS lipids form contacts with the SLC6 transporters, this indicates that these interactions are changing how the lipids are positioned in the membrane. Indeed, transporters have previously been shown to interact with GS rafts within membranes [55].
Figure 11.
The average lipid contact fractions of the neuronal membrane, by head group, for membranes with embedded: a) dDAT, b) hDAT, c) GlyT2, and d) hSERT.
Cholesterol flip-flop between leaflets occurs at a faster rate (3.5–3.6×106 s−1) in the neuronal membrane with the transporter embedded than the rates reported for corresponding membranes without embedded proteins (2.3×106 s−1), but slower than the rate of cholesterol flip-flop (4.820×106 s−1) in the much larger neuronal membrane reported by Ingolfsson et al. [11]. Absolute flip-flop rates are notoriously hard to compare as they change depending on the time interval of the frames included for analysis (flip-flop events can be missed within large time intervals). The reduction in flip-flop in the neuronal membrane reflects previous research showing that cholesterol flip-flop decreases as cholesterol content is increased, and increases with bilayer polyunsaturation [56]. The flip-flop rate does not change significantly when transporters are embedded in the membrane.
In general, the properties of the neuronal membrane appear to vary within the same range as those of the neuronal membrane without a transporter embedded, indicating that the presence of a protein does not significantly alter properties. The exceptions are the lipid lateral self-diffusivity (increased for all lipid groups) and PI and GS domain formation (which is altered by interactions with the SLC6 transporters). A comparison of membrane properties containing different embedded transporters showed the thickness profile of the membrane as a function of distance from the transporter varied slightly between the four SLC6 transporters. The area-per-lipid, cholesterol flip-flop rate, and domain formation, however, remained constant between different transporters.
Effect of complex membrane composition
We compared membrane properties of the neuronal membrane and the model membrane to investigate the effect of the SLC6 transporters on properties that are affected by membrane composition (Table 2). There is less variation in the thickness of the POPC/CHOL model membrane systems compared to the neuronal membrane, with an average membrane thickness of 40.4 Å for all systems. The presence of protein does not significantly influence the average membrane thickness. The average membrane thickness is consistent with the values derived from previously published simulations of POPC/CHOL (41.0 ± 0.2 Å) membranes without SLC6 transporters embedded [13]. As noted previously, the thickness of the membrane varies with proximity to the protein in the neuronal membrane. As with the neuronal membrane, the maximum thickness of the membrane varied for each transporter in the model POPC/CHOL systems, from 41.2 to 42.6 Å. The minimum membrane thickness occurs closest to the protein and is 33.7 to 34.6 Å. Overall, the neuronal membrane systems are 1–2 Å thicker than the model membrane systems (Figure S13).
Table 2.
POPC/CHOL model average membrane thickness and area per lipid with and without SLC6 transporters
| SLC6 transporter | Average thickness (Å) | Minimum thickness (Å) | Maximum thickness (Å) | Area per lipid (APL) | |
| Intracellular leaflet (Å2) | Extracellular leaflet (Å2) | ||||
| dDAT | 40.4 ± 0.6 | 34.6 | 42.7 | 56.5 ± 0.3 | 56.4 ± 0.3 |
| hDAT | 41.4 ± 0.7 | 34.5 | 41.2 | 56.8 ± 0.3 | 56.3 ± 0.3 |
| GlyT2 | 40.4 ± 0.7 | 33.8 | 42.6 | 56.6 ± 0.3 | 56.3 ± 0.3 |
| hSERT | 40.4 ± 0.6 | 33.7 | 41.2 | 56.6 ± 0.3 | 56.4 ± 0.3 |
| No proteina | 40.8 ± 0.1 | 40.5 | 41.0 | 55.4 ± 0.3 | 55.4 ± 0.3 |
Data taken from Wilson et al., 2020 [13] and recalculated with g_thickness to be consistent with the present work.
POPC has a greater average area per lipid in the POPC/CHOL simulations (59.4 – 59.5 ± 0.2 Å2) than the neuronal membrane systems (48.6 – 48.8 ± 0.8 Å2), regardless of which SLC6 transporter is embedded (Table 2). Consistent with the results obtained for the complex neuronal membrane, the area per lipid is constant throughout the time-course of the simulation (Figure S14). The higher concentration of cholesterol in the neuronal membrane is likely to play a role in the difference in properties between the neuronal and POPC/CHOL membranes. Cholesterol has been shown to induce the ordering of lipid fatty acid tails, which would increase membrane thickness and correspondingly reduce the area per lipid [57]. Experimentally determined APLs of simplified membranes show decreasing average areas as the concentration of cholesterol increases [13]. The average APL for a 50% CHOL/50% POPC membrane has been determined to be 45.1 ± 0.9 Å2 [58], and the average APL for a 40% CHOL/60% POPC membrane to be 46 Å2 [59]. In addition, we find that polyunsaturated lipids have a significantly larger APL (53.6 ± 17.2 Å2) compared to monounsaturated (45.6 ± 13.5 Å2) and saturated (47.0 ± 12.5 Å2) lipids in the neuronal membrane, supporting the supposition that the increased ordering is one reason for the difference in APL.
Cholesterol flip-flop between leaflets occurs at a slower rate (3.5–3.6×106 s−1) in the neuronal membrane than the POPC/CHOL membranes (4.6×106 s−1). As with the neuronal membrane, flip-flop in the simple membrane does not vary significantly between different proteins and occurs substantially faster than a corresponding model membrane without the transporter embedded (POPC/CHOL, 3.7×106 s−1) [13]. The reduction in flip-flop in the neuronal membrane reflects previous research showing that cholesterol flip-flop decreases as cholesterol content is increased, and increases with bilayer polyunsaturation [56].
The properties of the simple POPC/CHOL model do not vary significantly between the different SLC6 transporters. It is likely that a diversity of lipids is necessary for individual lipid-protein interactions to have a meaningful effect on average membrane properties. Furthermore, as previously discussed many contacts are formed between PE, PI and GS lipids in the neuronal membrane that will not be accounted for in a simplified POPC/CHOL membrane system.
DISCUSSION
Overall, the lipid annuli of dDAT, hDAT, GlyT2 and hSERT are broadly similar
The lipid annuli around the SLC6 transporters are similar in composition and dynamics when interactions are grouped by lipid class, although differences are apparent in the number and sites of interactions, especially when examined at the lipid species level. Polyunsaturated lipids are enriched around each transporter, and saturated lipids are depleted. Lipids interacted differently with the transporter depending on their headgroup. In general, PC and SM lipids were depleted around all four SLC6 transporters and formed few sustained contacts with each transporter. Similarly, PS, PA, DAG and CER lipids were neither enriched nor depleted around the four SLC6 transporters and did not form sustained contacts. Nevertheless, several classes of lipids were notably enriched around each SLC6 transporter, and each class interacted in different ways.
PE lipids were enriched around all four SLC6 transporters and formed non-specific contacts. While there is some overlap in the most prevalent sites of PE contacts for each of the investigated SLC6 transporters, there are also significant differences between these sites of interaction. These interactions occurred mainly between the lipid tail and aromatic amino acids (i.e., Phe and Trp). The preference of these protein-tail lipid interactions towards PE lipids is striking as the tail groups are present with other headgroups (e.g., PUPC, PAPC, OUPC, PUPS and OUPS) but these lipids do not form contacts with the transporters. The high occupancy of PE lipids around the SLC6 transporters may occur due to the negative curvature induced by the small lipid headgroup and polyunsaturated tails, which can curve the membrane to suit the hydrophobic depth of the transporter.
GS lipids are enriched around each transporter. However, the nature of the interactions differs between the different GS lipids. Individual glycosphingolipids containing polysaccharide headgroups (G1 and G3) form more long-lived contacts than individual lipids with monosaccharide headgroups. While lipids with a glucosylceramide headgroups formed more mobile and transient interactions with each transporter. The majority of the long-lived GS contacts occur between the glycosphingolipid headgroup and a loop region of the protein (particularly EL2). GS interactions do not show a preference for interacting with any specific amino acid.
Generally, phosphorylated PI lipids show greater enrichment around the SLC6 transporters than the non-phosphorylated PI lipids. Additionally, throughout the simulations there is more exchange with the non-phosphorylated PI lipids than the phosphorylated PI lipids. While PI lipids commonly interact with TM5 in all four transporters, additional transporter-specific interactions are seen with TM1, IL3, IL4, TM7, TM11, and TM12. PI interactions occur equally with the lipid tail and head group and commonly involve Lys and Phe. Consistent with previous literature indicating the importance of PI interactions with the N-terminus of hDAT [40], interactions are observed in a homologous region in the N-terminus of all four SLC6 transporters.
Previous literature has shown that CHOL plays a key role in the activity of DAT [17,41,42,46,47], SERT [43,44,48,49], and GlyT2 [45]. In the context of dDAT specifically, we note that Drosophila melanogaster neurons contain a number of sterols, of which cholesterol is a relatively minor species [60]. It should be noted that cholesterol was the only sterol present in the current simulations, and the concentration was appropriate for a human neuronal membrane. Nevertheless, across our simulations cholesterol is slightly depleted around all four SLC6 transporters, and we observe site-specific binding of CHOL to each transporter. These CHOL contacts did not preferentially involve CARC or CRAC motifs but are often within proximity to the previously identified CHOL binding sites. Specifically, while all four transporters show significant CHOL binding to CHOL6, differences in CHOL binding are also observed. Indeed, binding to CHOL1, CHOL2 or CHOL5 is preferred for dDAT, hDAT, or GlyT2, respectively. For hSERT, equal binding is observed to CHOL1 and CHOL2. Unsurprisingly, based on the preference of CHOL interactions to occur with branched amino acids [51], a significant number of contacts are formed with Ile, Leu and Val. These results further highlight the site-specific binding of CHOL to SLC6 transporters.
Overall, the general protein structure and membrane biophysical properties remained consistent between the four SLC6 transporters over the course of the simulations, despite differences in the lipid annulus of each transporter. This suggests that although lipid fingerprints of proteins within the same family may be broadly similar: unique differences may emerge at the level of interaction with individual lipid species.
The local lipid environment of SLC6 transporters in the neuronal membrane differs from the plasma membrane
The composition of the neuronal membrane differs from the average plasma membrane in several ways. The neuronal membrane contains significantly more cholesterol by proportion than the average membrane; cholesterol constitutes 46.1% of the neuronal membrane [13,61], compared to 30.1% of the average plasma membrane model [10]. By tail saturation, the neuronal membrane contains three saturated lipid species (PPC, DPPC, and DPPS) that together form 4.6% of the neuronal membrane; the average plasma membrane, on the other hand, contains only a single saturated species (PPC) that comprises 0.3% of the average membrane [10]. Monounsaturated lipids are less abundant in the neuronal membrane, especially the intracellular leaflet. They comprise 13.5% of the intracellular leaflet of the neuronal membrane, compared to 23.1% of the intracellular leaflet of the average [10]. The proportion of polyunsaturated lipids is also substantially lower in the neuronal membrane (29.5%) than the average plasma membrane (44.5%) [10]. By headgroup, the biggest differences in membrane composition are with the PC, PE, and SM lipids. PC and SM lipids are less abundant in the neuronal membrane than the average plasma membrane, particularly in the extracellular leaflet. PC lipids comprise 24.1%, and SM lipids only 9.0% of the extracellular leaflet of the neuronal membrane, compared to 35.6% and 19.0% of the extracellular leaflet of the average plasma membrane, respectively [10]. PE lipids are somewhat more abundant in the extracellular leaflet of the neuronal membrane (11.0% compared to 5.6% of the average plasma membrane) but less abundant in the intracellular leaflet (21.9% compared to 25.7% of the average plasma membrane). Membrane behaviour is highly dependent on membrane composition, which varies between tissue types and the organism that a particular tissue is derived from. In the absence of high-resolution tissue or organism specific lipidomic data, it is worthwhile to investigate to what extent a protein “lipid fingerprint” varies between the average plasma and neuronal membranes. As dDAT has been simulated in complex models of both the average plasma [12] and neuronal membrane, this provides an ideal test case to investigate the effects of composition on the “lipid fingerprint”.
We observe that although the general composition of the lipid annulus around dDAT is similar between the membranes, the proportions differ significantly. For example, polyunsaturated lipids are enriched around dDAT in both membranes. However, a leaflet-averaged DEI value of 4.16 ± 1.34 was calculated for polyunsaturated lipids within a 7 Å radius around dDAT in the average membrane [12]. This indicates markedly higher enrichment of polyunsaturated lipids around dDAT in the average membrane compared to the neuronal membrane (DEIextracellular = 1.35 ± 0.16, DEIintracellular = 1.29 ± 0.08). Polyunsaturated lipids comprise a significantly lower portion of the neuronal membrane (29.5%) than the average plasma membrane (44.5%) [10], which means that similar numbers of polyunsaturated lipids aggregating around the transporter would result in much higher DEI values for the neuronal membrane than the average. This suggests that the difference in membrane composition has resulted in substantially lower numbers of polyunsaturated lipids around dDAT. The behaviour of saturated lipids is also significantly affected, although this may be due in part to their low numbers. Fully saturated lipids are slightly enriched around dDAT (DEI = 1.26 ± 0.22) in the average membrane [12]; they are significantly depleted around dDAT in both leaflets of the neuronal membrane (DEI = 0.42 ± 0.10). Monounsaturated lipids are depleted in the intracellular leaflet (DEI = 0.71 ± 0.04) but neither enriched nor depleted in the extracellular leaflet (DEI = 0.91 ± 0.11). The enrichment or depletion of monounsaturated lipids as a class is not reported by Ingólfsson et al. for the average membrane [12].
Differences also occur based on the enrichment of lipids when classified by headgroups as a function of membrane composition. There are significant differences in the enrichment profiles of DAG, CER, and PA lipids, but these are likely due to their low population in the neuronal membrane (0.2%, 0.2%, and 0.1%, respectively; 1–2 lipid molecules). While these groups are also a small part of the average plasma membrane, they are both more numerous in absolute number (40 – 46) and comprise proportionally more of the membrane (0.6 –0.7%). Glycosphingolipids are far more enriched around dDAT in the average membrane (DEI = 6.37 ± 0.29) [12] than the neuronal membrane (DEI = 1.96 ± 0.46), although the proportion of glycosphingolipids in the neuronal membrane (5%) is nearly double the proportion in the average membrane (3%). PC lipids are less depleted around dDAT in the neuronal membrane (DEI = 0.67 ± 0.13) than the average membrane (DEI = 0.46 ± 0.05) [12], possibly due to lower abundance in the former. SM lipids are similarly less depleted in the neuronal membrane (DEI = 0.44 ± 0.12) than the average plasma membrane (DEI = 0.18 ± 0.06) [12]. As with PC lipids, SM lipids comprise significantly less of the neuronal membrane than the average plasma membrane. PE lipids are enriched around dDAT in the average membrane (DEI = 1.34 ± 0.15). While PE lipids are enriched to a similar extent in the extracellular leaflet of the neuronal membrane (DEI = 1.29 ± 0.19), they are depleted in the intracellular leaflet (DEI = 0.89 ± 0.09), despite PE lipids comprising a similar proportion of the intracellular leaflet in both membranes (~22% in the neuronal membrane and~26% in the average plasma membrane). Given that PE contacts are primarily driven by lipid tail interactions, this difference may reflect differences in tail composition between the average plasma membrane and the neuronal membrane. For example, PUPE and PAPE comprise ~16% the intracellular leaflet of the neuronal membrane, but only combine to ~8% the average plasma membrane. Several species present in the average plasma membrane (DOPE, PQPE, PIPE, DAPE, DUPE) are absent from the neuronal membrane, and vice versa (OAPE, OIPE, OUPE). PI lipids comprise ~6% of the intracellular leaflet in both the average plasma and neuronal membranes and are highly enriched around dDAT in both membranes. Overall, differences between the lipid annulus of dDAT in the neuronal and average plasma membrane highlight the importance of studying a transporter in a realistic tissue-specific membrane environment and the need for further organism-specific lipidomic data.
CONCLUSION
In this study we investigate the differences between the lipid annulus of four SLC6 transporters, both within members of the same class of proteins and in membranes of different composition. We found that the overall lipid fingerprints were similar between each SLC6 transporter when lipids were grouped by saturation and headgroup. Polyunsaturated lipids, which comprise the vast majority of the membrane, are enriched around each transporter. PC, SM and PS lipids form few, non-specific and non-sustained contacts with each transporter. PE, PI and GS lipids are enriched around each transporter, but different interactions were formed with each SLC6 transporter. The tails of PE lipids interacted non-specifically with each transporter, while PI headgroups and tails interact non-specifically with each transporter in similar ratios. In contrast, GS headgroups formed contacts with protein loop regions. However, there were unique differences at the chemical species level in each lipid class with respect to enrichment around the transporter, or the number and length of contacts formed. In addition, cholesterol clusters around the structurally identified binding sites conserved across SLC6 transporters but binds preferentially at different binding sites for each transporter. We find that the composition of the lipid annulus is heavily dependent on the composition of the membrane. The local lipid environment of dDAT embedded in an average plasma membrane compared to the neuronal membrane, highlighted that the enrichment of chemically distinct lipid species around the transporter, and specific lipid-protein interactions, were likely to be affected by the precise membrane composition.
The presence of embedded SLC6 transporters did not perturb the biophysical properties of simple and complex membranes for metrics such as the area per lipid and the average thickness. While properties such as the local membrane thickness and lipid lateral self-diffusivity varied between SLC6 transporters in the neuronal membrane, no such variation was observed in the simple POPC/CHOL model. Overall, this work highlights the importance of studying membrane-protein interactions in a membrane model that accurately represents both the complexity and composition of the biological environment.
FUNDING
This work was supported by a grant from the National Health and Medical Research Council (APP1144429). Y.L. is the recipient of a Replacing Animals in Medical Research Honours Scholarship from the Medical Advances Without Animals Trust.
Data Availability
Code and input files are avaliable on GitHub.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
Megan L. O'Mara and Katie A. Wilson reports equipment, drugs, or supplies was provided by National Computational Infrastructure. Yie Chang Lin reports financial support was provided by Medical Advances Without Animals (MAWA) Trust. Megan L. O'Mara reports a relationship with National Health and Medical Research Council that includes: funding grants. This author has no patents to disclose. The corresponding author reports no additional activities to disclose.
ACKNOWLEDGMENTS
The research was undertaken with the assistance of resources and services from the National Computational Infrastructure (NCI), which is supported by the Australian Government.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.bbadva.2021.100010.
Appendix. Supplementary materials
References
- 1.Wilson K.A., Wang L., MacDermott-Opeskin H., O'Mara M.L. The Fats of Life: Using Computational Chemistry to Characterise the Eukaryotic Cell Membrane. Aust. J. Chem. 2020;73:85–95. doi: 10.1071/CH19353. [DOI] [Google Scholar]
- 2.Allhusen J.S., Conboy J.C. The Ins and Outs of Lipid Flip-Flop. Acc. Chem. Res. 2017;50:58–65. doi: 10.1021/acs.accounts.6b00435. [DOI] [PubMed] [Google Scholar]
- 3.van Meer G., Voelker D.R., Feigenson G.W. Membrane lipids: where they are and how they behave. Nat. Rev. Mol. Cell Biol. 2008;9:112–124. doi: 10.1038/nrm2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Laganowsky A., Reading E., Allison T.M., Ulmschneider M.B., Degiacomi M.T., Baldwin A.J., Robinson C.V. Membrane proteins bind lipids selectively to modulate their structure and function. Nature. 2014;510:172–175. doi: 10.1038/nature13419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Corradi V., Sejdiu B.I., Mesa-Galloso H., Abdizadeh H., Noskov S.Yu., Marrink S.J., Tieleman D.P. Emerging Diversity in Lipid–Protein Interactions. Chem. Rev. 2019;119:5775–5848. doi: 10.1021/acs.chemrev.8b00451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.F.-X. Contreras, A.M. Ernst, F. Wieland, B. Brügger, Specificity of Intramembrane Protein–Lipid Interactions, Cold Spring Harb. Perspect. Biol. 3 (2011) a004705. https://doi.org/10.1101/cshperspect.a004705. [DOI] [PMC free article] [PubMed]
- 7.Cotman C.W., Blank M.L., Moehl A., Snyder F. Lipid composition of synaptic plasma membranes isolated from rat brain by zonal centrifugation. Biochemistry. 1969;8:4606–4612. doi: 10.1021/bi00839a056. [DOI] [PubMed] [Google Scholar]
- 8.Yamashita A., Hayashi Y., Nemoto-Sasaki Y., Ito M., Oka S., Tanikawa T., Waku K., Sugiura T. Acyltransferases and transacylases that determine the fatty acid composition of glycerolipids and the metabolism of bioactive lipid mediators in mammalian cells and model organisms. Prog. Lipid Res. 2014;53:18–81. doi: 10.1016/j.plipres.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 9.Marrink S.J., Corradi V., Souza P.C.T., Ingólfsson H.I., Tieleman D.P., Sansom M.S.P. Computational Modeling of Realistic Cell Membranes. Chem. Rev. 2019;119:6184–6226. doi: 10.1021/acs.chemrev.8b00460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ingólfsson H.I., Melo M.N., van Eerden F.J., Arnarez C., Lopez C.A., Wassenaar T.A., Periole X., de Vries A.H., Tieleman D.P., Marrink S.J. Lipid Organization of the Plasma Membrane. J. Am. Chem. Soc. 2014;136:14554–14559. doi: 10.1021/ja507832e. [DOI] [PubMed] [Google Scholar]
- 11.H.I. Ingólfsson, T.S. Carpenter, H. Bhatia, P.-T. Bremer, S.J. Marrink, F.C. Lightstone, Computational Lipidomics of the Neuronal Plasma Membrane, Biophys. J. 113 (2017) 2271–2280. https://doi.org/10.1016/j.bpj.2017.10.017. [DOI] [PMC free article] [PubMed]
- 12.Corradi V., Mendez-Villuendas E., Ingólfsson H.I., Gu R.-X., Siuda I., Melo M.N., Moussatova A., DeGagné L.J., Sejdiu B.I., Singh G., Wassenaar T.A., Delgado Magnero K., Marrink S.J., Tieleman D.P. Lipid–Protein Interactions Are Unique Fingerprints for Membrane Proteins. ACS Cent. Sci. 2018;4:709–717. doi: 10.1021/acscentsci.8b00143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.K.A. Wilson, H.I. MacDermott-Opeskin, E. Riley, Y. Lin, M.L. O'Mara, Understanding the Link between Lipid Diversity and the Biophysical Properties of the Neuronal Plasma Membrane, Biochemistry. 59 (2020) 3010–3018. https://doi.org/10.1021/acs.biochem.0c00524. [DOI] [PubMed]
- 14.P.A. Bala, J. Foster, L. Carvelli, L.K. Henry, SLC6 Transporters: Structure, Function, Regulation, Disease Association and Therapeutics, Mol. Aspects Med. 34 (2013) 197–219. https://doi.org/10.1016/j.mam.2012.07.002. [DOI] [PMC free article] [PubMed]
- 15.M.J. Abraham, T. Murtola, R. Schulz, S. Páll, J.C. Smith, B. Hess, E. Lindahl, GROMACS: High performance molecular simulations through multi-level parallelism from laptops to supercomputers, SoftwareX. 1–2 (2015) 19–25. https://doi.org/10.1016/j.softx.2015.06.001.
- 16.de Jong D.H., Singh G., Bennett W.F.D., Arnarez C., Wassenaar T.A., Schäfer L.V., Periole X., Tieleman D.P., Marrink S.J. Improved Parameters for the Martini Coarse-Grained Protein Force Field. J. Chem. Theory Comput. 2013;9:687–697. doi: 10.1021/ct300646g. [DOI] [PubMed] [Google Scholar]
- 17.Wang K.H., Penmatsa A., Gouaux E. Neurotransmitter and psychostimulant recognition by the dopamine transporter. Nature. 2015;521:322–327. doi: 10.1038/nature14431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coleman J.A., Green E.M., Gouaux E. X-ray structures and mechanism of the human serotonin transporter. Nature. 2016;532:334–339. doi: 10.1038/nature17629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kelley L.A., Mezulis S., Yates C.M., Wass M.N., Sternberg M.J.E. The Phyre2 web portal for protein modeling, prediction and analysis. Nat. Protoc. 2015;10:845–858. doi: 10.1038/nprot.2015.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Subramanian N., Scopelitti A.J., Carland J.E., Ryan R.M., O'Mara M.L., Vandenberg R.J. Identification of a 3rd Na+ Binding Site of the Glycine Transporter, GlyT2. PLOS ONE. 2016;11 doi: 10.1371/journal.pone.0157583. e0157583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marrink S.J., Risselada H.J., Yefimov S., Tieleman D.P., de Vries A.H. The MARTINI Force Field: Coarse Grained Model for Biomolecular Simulations. J. Phys. Chem. B. 2007;111:7812–7824. doi: 10.1021/jp071097f. [DOI] [PubMed] [Google Scholar]
- 22.Poma A.B., Cieplak M., Theodorakis P.E. Combining the MARTINI and Structure-Based Coarse-Grained Approaches for the Molecular Dynamics Studies of Conformational Transitions in Proteins. J. Chem. Theory Comput. 2017;13:1366–1374. doi: 10.1021/acs.jctc.6b00986. [DOI] [PubMed] [Google Scholar]
- 23.Wassenaar T.A., Ingólfsson H.I., Böckmann R.A., Tieleman D.P., Marrink S.J. Computational Lipidomics with insane: A Versatile Tool for Generating Custom Membranes for Molecular Simulations. J. Chem. Theory Comput. 2015;11:2144–2155. doi: 10.1021/acs.jctc.5b00209. [DOI] [PubMed] [Google Scholar]
- 24.Marrink S.J., de Vries A.H., Mark A.E. Coarse Grained Model for Semiquantitative Lipid Simulations. J. Phys. Chem. B. 2004;108:750–760. doi: 10.1021/jp036508g. [DOI] [Google Scholar]
- 25.de Jong D.H., Baoukina S., Ingólfsson H.I., Marrink S.J. Martini straight: Boosting performance using a shorter cutoff and GPUs. Comput. Phys. Commun. 2016;199:1–7. doi: 10.1016/j.cpc.2015.09.014. [DOI] [Google Scholar]
- 26.Bussi G., Donadio D., Parrinello M. Canonical sampling through velocity-rescaling. J. Chem. Phys. 2007;126 doi: 10.1063/1.2408420. [DOI] [PubMed] [Google Scholar]
- 27.Parrinello M., Rahman A. Polymorphic transitions in single crystals: A new molecular dynamics method. J. Appl. Phys. 1981;52:7182–7190. doi: 10.1063/1.328693. [DOI] [Google Scholar]
- 28.Castillo N., Monticelli L., Barnoud J., Tieleman D.P. Free energy of WALP23 dimer association in DMPC, DPPC, and DOPC bilayers. Chem. Phys. Lipids. 2013;169:95–105. doi: 10.1016/j.chemphyslip.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 29.Maginn E.J., Messerly R.A., Carlson D.J., Roe D.R., Elliott J.R. Best Practices for Computing Transport Properties 1. Self-Diffusivity and Viscosity from Equilibrium Molecular Dynamics [Article v1.0] Living J. Comput. Mol. Sci. 2018;1:6324. doi: 10.33011/livecoms.1.1.6324. [DOI] [Google Scholar]
- 30.Michaud-Agrawal N., Denning E.J., Woolf T.B., Beckstein O. MDAnalysis: A toolkit for the analysis of molecular dynamics simulations. J. Comput. Chem. 2011;32:2319–2327. doi: 10.1002/jcc.21787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gowers R.J., Linke M., Barnoud J., Reddy T.J.E., Melo M.N., Seyler S.L., Domański J., Dotson D.L., Buchoux S., Kenney I.M., Beckstein O. Proc. 15th Python Sci. Conf. 2016. MDAnalysis: A Python Package for the Rapid Analysis of Molecular Dynamics Simulations; pp. 98–105. [DOI] [Google Scholar]
- 32.Mostyn S.N., Carland J.E., Shimmon S., Ryan R.M., Rawling T., Vandenberg R.J. Synthesis and Characterization of Novel Acyl-Glycine Inhibitors of GlyT2. ACS Chem. Neurosci. 2017;8:1949–1959. doi: 10.1021/acschemneuro.7b00105. [DOI] [PubMed] [Google Scholar]
- 33.L'hirondel M., Chéramy A., Godeheu G., Glowinski J. Effects of Arachidonic Acid on Dopamine Synthesis, Spontaneous Release, and Uptake in Striatal Synaptosomes from the Rat. J. Neurochem. 1995;64:1406–1409. doi: 10.1046/j.1471-4159.1995.64031406.x. [DOI] [PubMed] [Google Scholar]
- 34.Regulation of the functional activity of the human dopamine transporter by the arachidonic acid pathway. Eur. J. Pharmacol. 1996;315:345–354. doi: 10.1016/S0014-2999(96)00646-2. [DOI] [PubMed] [Google Scholar]
- 35.Vernon R.M., Chong P.A., Tsang B., Kim T.H., Bah A., Farber P., Lin H., Forman-Kay J.D. Pi-Pi contacts are an overlooked protein feature relevant to phase separation. ELife. 2018;7:e31486. doi: 10.7554/eLife.31486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wilson K.A., Kellie J.L., Wetmore S.D. DNA–protein π-interactions in nature: abundance, structure, composition and strength of contacts between aromatic amino acids and DNA nucleobases or deoxyribose sugar. Nucleic Acids Res. 2014;42:6726–6741. doi: 10.1093/nar/gku269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wilson K.A., Wetmore S.D. Combining crystallographic and quantum chemical data to understand DNA-protein π-interactions in nature. Struct. Chem. 2017;28:1487–1500. doi: 10.1007/s11224-017-0954-7. [DOI] [Google Scholar]
- 38.Hudson K.L., Bartlett G.J., Diehl R.C., Agirre J., Gallagher T., Kiessling L.L., Woolfson D.N. Carbohydrate–Aromatic Interactions in Proteins. J. Am. Chem. Soc. 2015;137:15152–15160. doi: 10.1021/jacs.5b08424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Montalvillo-Jiménez L., Santana A.G., Corzana F., Jiménez-Osés G., Jiménez-Barbero J., Gómez A.M., Asensio J.L. Impact of Aromatic Stacking on Glycoside Reactivity: Balancing CH/π and Cation/π Interactions for the Stabilization of Glycosyl-Oxocarbenium Ions. J. Am. Chem. Soc. 2019;141:13372–13384. doi: 10.1021/jacs.9b03285. [DOI] [PubMed] [Google Scholar]
- 40.Sanchez K.M., Kang G., Wu B., Kim J.E. Tryptophan-Lipid Interactions in Membrane Protein Folding Probed by Ultraviolet Resonance Raman and Fluorescence Spectroscopy. Biophys. J. 2011;100:2121–2130. doi: 10.1016/j.bpj.2011.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Belovich A.N., Aguilar J.I., Mabry S.J., Cheng M.H., Zanella D., Hamilton P.J., Stanislowski D.J., Shekar A., Foster J.D., Bahar I., Matthies H.J.G., Galli A. A network of phosphatidylinositol (4,5)-bisphosphate (PIP 2) binding sites on the dopamine transporter regulates amphetamine behavior in Drosophila Melanogaster. Mol. Psychiatry. 2019:1–14. doi: 10.1038/s41380-019-0620-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hong W.C., Amara S.G. Membrane cholesterol modulates the outward facing conformation of the dopamine transporter and alters cocaine binding. J. Biol. Chem. 2010;285:32616–32626. doi: 10.1074/jbc.M110.150565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jones K.T., Zhen J., Reith M.E.A. Importance of cholesterol in dopamine transporter function. J. Neurochem. 2012;123:700–715. doi: 10.1111/jnc.12007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bjerregaard H., Severinsen K., Said S., Wiborg O., Sinning S. A Dualistic Conformational Response to Substrate Binding in the Human Serotonin Transporter Reveals a High Affinity State for Serotonin. J. Biol. Chem. 2015;290:7747–7755. doi: 10.1074/jbc.M114.573477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Scanlon S.M., Williams D.C., Schloss P. Membrane Cholesterol Modulates Serotonin Transporter Activity. Biochemistry. 2001;40:10507–10513. doi: 10.1021/bi010730z. [DOI] [PubMed] [Google Scholar]
- 46.Divito C.B., Amara S.G. Close Encounters of the Oily Kind: Regulation of Transporters by Lipids. Mol. Interv. 2009;9:252–262. doi: 10.1124/mi.9.5.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Penmatsa A., Wang K.H., Gouaux E. X-ray structure of dopamine transporter elucidates antidepressant mechanism. Nature. 2013;503:85–90. doi: 10.1038/nature12533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Penmatsa A., Wang K.H., Gouaux E. X-ray structures of Drosophila dopamine transporter in complex with nisoxetine and reboxetine. Nat. Struct. Mol. Biol. 2015;22:506–508. doi: 10.1038/nsmb.3029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zeppelin T., Ladefoged L.K., Sinning S., Periole X., Schiøtt B. A direct interaction of cholesterol with the dopamine transporter prevents its out-to-inward transition. PLOS Comput. Biol. 2018;14 doi: 10.1371/journal.pcbi.1005907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Laursen L., Severinsen K., Kristensen K.B., Periole X., Overby M., Müller H.K., Schiøtt B., Sinning S. Cholesterol binding to a conserved site modulates the conformation, pharmacology, and transport kinetics of the human serotonin transporter. J. Biol. Chem. 2018;293:3510–3523. doi: 10.1074/jbc.M117.809046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fantini J., Barrantes F.J. How cholesterol interacts with membrane proteins: an exploration of cholesterol-binding sites including CRAC, CARC, and tilted domains. Front. Physiol. 2013:4. doi: 10.3389/fphys.2013.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fantini J., Carlus D., Yahi N. The fusogenic tilted peptide (67–78) of α-synuclein is a cholesterol binding domain. Biochim. Biophys. Acta BBA - Biomembr. 2011;1808:2343–2351. doi: 10.1016/j.bbamem.2011.06.017. [DOI] [PubMed] [Google Scholar]
- 53.Park S., Beaven A.H., Klauda J.B., Im W. How Tolerant are Membrane Simulations with Mismatch in Area per Lipid between Leaflets? J. Chem. Theory Comput. 2015;11:3466–3477. doi: 10.1021/acs.jctc.5b00232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mathai J.C., Tristram-Nagle S., Nagle J.F., Zeidel M.L. Structural Determinants of Water Permeability through the Lipid Membrane. J. Gen. Physiol. 2008;131:69–76. doi: 10.1085/jgp.200709848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ermilova I., Lyubartsev A.P. Extension of the Slipids Force Field to Polyunsaturated Lipids. J. Phys. Chem. B. 2016;120:12826–12842. doi: 10.1021/acs.jpcb.6b05422. [DOI] [PubMed] [Google Scholar]
- 56.Prinetti A., Loberto N., Chigorno V., Sonnino S. Glycosphingolipid behaviour in complex membranes. Biochim. Biophys. Acta BBA - Biomembr. 2009;1788:184–193. doi: 10.1016/j.bbamem.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 57.Bennett W.F.D., MacCallum J.L., Hinner M.J., Marrink S.J., Tieleman D.P. Molecular View of Cholesterol Flip-Flop and Chemical Potential in Different Membrane Environments. J. Am. Chem. Soc. 2009;131:12714–12720. doi: 10.1021/ja903529f. [DOI] [PubMed] [Google Scholar]
- 58.de Meyer F., Smit B. Effect of cholesterol on the structure of a phospholipid bilayer. Proc. Natl. Acad. Sci. U. S. A. 2009;106:3654–3658. doi: 10.1073/pnas.0809959106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.A. Leftin, T.R. Molugu, C. Job, K. Beyer, M.F. Brown, Area per Lipid and Cholesterol Interactions in Membranes from Separated Local-Field 13C NMR Spectroscopy, Biophys. J. 107 (2014) 2274–2286. https://doi.org/10.1016/j.bpj.2014.07.044. [DOI] [PMC free article] [PubMed]
- 60.Smaby J.M., Momsen M.M., Brockman H.L., Brown R.E. Phosphatidylcholine acyl unsaturation modulates the decrease in interfacial elasticity induced by cholesterol. Biophys. J. 1997;73:1492–1505. doi: 10.1016/S0006-3495(97)78181-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Carvalho M., Sampaio J.L., Palm W., Brankatschk M., Eaton S., Shevchenko A. Effects of diet and development on the Drosophila lipidome. Mol. Syst. Biol. 2012;8:600. doi: 10.1038/msb.2012.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Code and input files are avaliable on GitHub.