Highlights
-
•
Molecular dissections of Connexin39.4 and connexin 41.8.
-
•
CT domain of Cx39.4 is not essential for skin pattern formation.
-
•
CT domain of Cx41.8 is required, depending on the expression of Cx39.4.
-
•
Cx39.4 supports Cx41.8 localization to the cell membrane.
Keywords: C-terminal domain, Connexin, Gap junction, Pigment cell, Skin-pattern formation, Zebrafish
Abstract
Background
Zebrafish display a striped skin pattern on their body; two types of connexins, namely, Connexin39.4 (Cx39.4) and Connexin41.8 (Cx41.8), are involved in stripe pattern formation. Herein, we investigated the role of the C-terminal (CT) domains of Cx39.4 and Cx41.8 in vivo and in vitro.
Methods
To investigate the role of CT domains in vivo, we established transgenic zebrafish lines expressing the CT-domain-modified connexin series in pigmented cells and observed skin patterns in fish. To investigate the role of the CT domains in vitro, we expressed the CT-domain modified connexin series in Neuro-2a (N2a) cells and calculated the plaque formation frequency.
Results
The overexpression of Cx39.4 lacking a CT domain produced skin patterns similar to that produced by full-length Cx39.4 in the cx39.4−/− mutant and in cx39.4 and cx41.8 double-knockout mutant zebrafish. Fluorescence-protein-fused CT-domain-modified Cx39.4 formed gap junction plaques between N2a cells. The overexpression of CT-truncated Cx41.8 rescued the mutant phenotype in the cx41.8−/− mutant but did not function in the double knockout zebrafish. Fluorescence-protein-fused CT-truncated Cx41.8 hardly formed plaques between N2a cells without Cx39.4 but formed gap junction plaques when co-expressed with Cx39.4.
Conclusions
The CT domain of Cx39.4 is not required for protein function, at least in the pigment cells of zebrafish. However, the need for the CT domain of Cx41.8 depends on Cx39.4 expression.
General significance
These results provide evidence for the interactions between Cx39.4 and Cx41.8 in pigment cells of zebrafish and suggest that at least one connexin must have a CT domain.
1. Introduction
Cell–cell interaction is a key factor in morphogenesis and organogenesis during the development of living organisms. To understand the molecular basis of cell–cell interactions, molecular factors, including membrane proteins and release factors, have been actively analyzed [1], [2], [3], [4], [5]. Gap junctions (GJs) are intercellular channels that mediate the direct transfer of small molecules, ions, metabolites, and second messengers with molecular weights <1000 between connected cells [6]. In chordate species, the GJ is composed of connexin protein subunits. Six connexin proteins oligomerize into hexameric structures called hemichannels, and a hemichannel docks with the corresponding hemichannel of the neighboring cell to form a GJ; thus, a GJ consists of 12 connexin protein subunits [3,7,8]. Approximately 20 connexin isotypes have been cloned in mammals, whereas approximately 40 have been predicted in teleosts [9,10]. The connexin protein is a 4-pass transmembrane (TM) protein; it includes several domains. The N-terminal (NT), intracellular loop (IL), and C-terminal (CT) domains are localized in the cytoplasm, and two extracellular loops (EL1 and EL2) are located outside the cell [11]. Several studies have demonstrated that CT domains play multiple roles in GJ function, including anchoring the connexin to the cell membrane through interaction with other membrane proteins [12], [13], [14], [15], [16], [17], [18], [19], [20], localization of connexins to the cell membrane [21,22], and function in channel gating [23], [24], [25], [26]. Mutations in CT domains have been reported to be associated with several human diseases [27], [28], [29], [30], [31], [32], [33]. The possibility of the formation of a heteromeric hemichannel/GJ consisting of different types of connexins has been demonstrated by the combinations of cell imaging and electrophysiological analysis [34], [35], [36]. Heteromeric channels provide diversity with regard to the exchanged molecules, protein trafficking, and electrophysiological properties of hemichannels/GJs [35,[37], [38], [39]]. However, the necessity of the formation of such a heteromer in vivo remains largely obscure [35].
The zebrafish (Danio rerio) is a small tropical fish that has attracted attention owing to its applications in molecular biology; the transparency of zebrafish embryos for easy analysis, the ease of breeding this species, and the ease of gene transfer using transposon make the zebrafish an excellent model organism for research [[40], [41], [42]]. This fish exhibits black-yellow stripes on its body surface, composed of three types of pigment cells: melanophores (black pigment cells), xanthophores (yellow pigment cells), and iridophores (silver cells spread over the fish trunk) (Fig. 1A) [43], [44], [45], [46], [47], [48]. These characteristics make zebrafish an excellent model for both experimental and theoretical studies in biology for studying cell-autonomous skin pattern formation [46], [47], [48], [49], [50], [51], [52], [53]. In the stripe pattern formation of zebrafish, two types of connexin proteins, Connexin39.4 (Cx39.4) and Connexin41.8 (Cx41.8), are involved (Fig. 1) [54], [55], [56]. Cx39.4 is a teleost lineage-specific connexin and Cx41.8 is an ortholog of mammalian Cx40 (Fig. S1) [10,57]. Oocyte clamp experiments have shown that Cx39.4 exhibits loose closing, whereas Cx41.8 exhibits a channel-gating property similar to that of Rattus Cx40 [55,58]. Cx41.8 was identified from the zebrafish skin pattern mutant leopard (leo), which exhibits a spot pattern on its skin (Fig 1A) [56]. Several leopard alleles were examined and mutations were identified; for example, leot1 (cx41.8−/−; Cx41.8_R68STOP) had a nonsense mutation, whereas leotq270 (Cx41.8_I203F) and leotw28 (Cx41.8_I31F) had amino acid substitutions [56]. As the leotq270 mutant exhibited a smaller spot phenotype than the leot1 null mutant, the involvement of one or more connexin genes in skin patterning and the formation of heteromeric/heterotypic GJs with Cx41.8 were predicted [56]. A decade later, Cx39.4 was identified from a zebrafish skin pattern mutant, luchs (luc), which exhibits a labyrinth/wavy stripe pattern (Fig. 1A) [54,55]. The double knockout of cx39.4 and cx41.8 [WKO; (cx39.4−/−;cx41.8−/−)] was generated by crossing between the mutant lines cx39.4−/− and cx41.8−/−; the obtained WKO fish showed a pattern-less phenotype (Fig. 1A) [54,55]. The expression of connexin genes in melanophores and xanthophores was investigated using reverse transcriptase-polymerase chain reaction (RT-PCR), and it was concluded that cx39.4 and cx41.8 were expressed in melanophores and xanthophores, whereas the expression of other connexin genes was not detected [55,59]. In addition, the requirement of connexin expression in pigment cells was examined using transgenic experiments, and it was concluded that the expression of cx39.4 in melanophores and cx41.8 in xanthophores are minimal requirements for stripe patterning, and the expression of cx41.8 in melanophores has a supportive function for the stable stripe pattern formation [59,60]. In theoretical studies, xanthophores were predicted to have a function whereby they support the survival of melanophores [52,61]. In the experimental studies that used the laser ablation of pigment cells on the live fish skin, the requirement of xanthophores for the survival of melanophores was demonstrated [52,62]. GJs formed by Cx39.4 and Cx41.8 were hypothesized to be involved in the survival signal transfer from xanthophores to melanophores; however, the mechanism underlying this process is unclear [54,55,59,60,63]. The possibility of the formation of heteromeric GJs by Cx39.4 and Cx41.8 was further investigated using transgenic and electrophysiological experiments [55]. The N-terminus-truncated Cx41.8 mutant named Cx41.8M7, in which 6 amino acid residues from the 2nd to 7th positions of Cx41.8 were removed and the initial methionine was placed at the 7th position, showed a strong dominant negative effect on GJ formation. Cx41.8M7 overexpression in the melanophores of leopard fish caused the pattern-less phenotype as shown by the WKO mutant, indicating that Cx41.8M7 inactivated Cx39.4 in pigment cells. In addition, inactivation of WT-Cx39.4 and WT-Cx41.8 by Cx41.8M7 were detected by performing electrophysiological analysis using Xenopus oocytes. These results showed that Cx41.8 and Cx39.4 possibly formed heteromeric hemichannels/GJs that play a role in skin pattern formation [55].
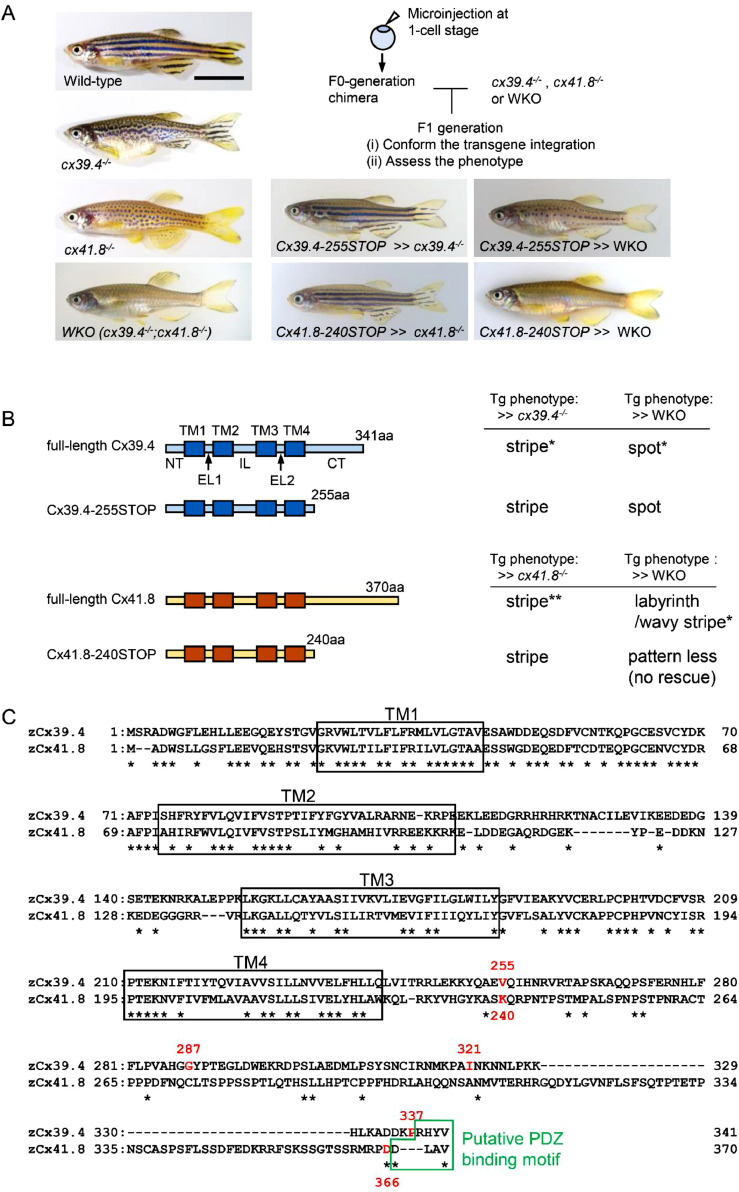
Fig. 1.
Transgenic experiments using CT-truncated connexins. (A) Images of the wild-type, connexin mutants [luchs (cx39.4−/−), leopard (cx41.8−/−)], double knockout [WKO; (cx39.4−/−;cx41.8−/−)], and transgenic lines of zebrafish. “>>” indicates transgene integration. The scheme to generate transgenic zebrafish lines is shown in the upper right. Scale bar; 10 mm. (B) Description of the Cx39.4 and Cx41.8 fragments used for the transgenic experiments. The left column indicates the construct names and connexin structures; the light-blue boxes indicate the Cx39.4 peptides, the light-orange boxes indicate the Cx41.8 peptides, and the dark-blue boxes and brown boxes indicate the transmembrane domains (TM1–4). NT, EL1, EL2, IL, and CT indicate the N-terminal domain, extracellular loop-1, extracellular loop-2, intracellular loop, and C-terminal domain, respectively. The right column indicates the results of the transgenic experiments using the fish with the cx39.4−/− or cx41.8−/− and WKO backgrounds. “Tg” means transgenic. * and **; see references (*Usui et al., Development, 2019 [59], and **Watanabe & Kondo, Pigment Cell Melanoma Res, 2012 [60]). (C) Alignment of the amino-acid sequences of zebrafish Cx39.4 and Cx41.8. The asterisks indicate amino-acid residues conserved between Cx39.4 and Cx41.8. The 255th, 287th, 321st, and 337th positions in Cx39.4, and the 240th and 366th positions in Cx41.8 are shown in red. The boxes with the black line indicate the putative transmembrane regions (TM1–4), and the box with the green line indicates the putative PDZ-binding motif. Two sequences were aligned using the ClustalW software [65]
In this study, we focused on the CT domains of Cx39.4 and Cx41.8 and investigated their roles in skin pattern formation in zebrafish. CT-truncated Cx39.4 and Cx41.8 were introduced in mutant fish genome, and their functionalities were examined. To compensate for the disadvantage of the lack of commercially available antibodies in zebrafish and for the further investigation of protein localization in live fish, enhanced green fluorescent protein (EGFP)/red fluorescent protein (RFP)-fused connexins were designed and used in this study. The functionalities of EGFP-tagged connexins in pigment cells were confirmed to understand whether they rescue the mutant phenotypes [59].
2. Material and methods
2.1. Zebrafish
All experiments in this study were performed in accordance with the guidelines and approved protocols for animal care and use (approval numbers: FBS-14–002–1 for animal use and 04294 for transgenic experiment) of the Osaka University. Zebrafish (Danio rerio) were maintained under standard conditions at 28.5 °C on a 14/10-hour light/dark cycle. We used previously reported strains and lines: wild-type (Tübingen), cx39.4−/− (cx39.4ou2025/ou2025), and cx41.8−/− (cx41.8t1/t1) [55,56]. Our results were obtained by randomly selecting male and female fishes. The new lines were established as follows: transgenic (Tg) lines were generated using the Tol2 transposon vector system [41,42]. Briefly, each plasmid (30 ng/μL) and transposase mRNA (25 ng/μL) synthesized in vitro were co-injected into the fertilized eggs of cx39.4−/− or cx41.8−/− zebrafish at a single-cell stage (Fig. 1A). F0 generation chimera fish were crossed with cx39.4−/−, cx41.8−/−, or WKO (cx39.4−/−; cx41.8−/−) zebrafish to establish transgenic zebrafish lines. WKO background transgenic lines were obtained by crossing the F0 chimera of single-knockout background fish with WKO fish twice. In other words, F1 fish obtained from the cross between F0 and WKO fish were crossed with WKO fish again to obtain F2 fish with a WKO background. The integration of the transgenes was detected by performing PCR, and genetic background was confirmed by cx39.4 and cx41.8 sequencing. Hence, the effects of modified connexin protein in cx39.4−/− or cx41.8−/−fish were assessed in the F1 generation fish, and the effects in WKO fish were assessed in the F2 generation fish.
2.2. Plasmid construction
To generate transgenic zebrafish lines, the fragments of CT domain-modified Cx39.4 series and Cx41.8 series were amplified using the primer sets listed in Supplementary table and cloned into Tol2 plasmids containing a 1.5-kb mitfa promoter for the Cx39.4 series [64] and a 4.5-kb cx41.8 promoter for the Cx41.8 series [60]. To express connexin proteins in Neuro-2a (N2a) cells, CT-modified Cx39.4, CT-modified Cx41.8, and Cx41.8IMM [58] were cloned into the multi-cloning site of the pIRES2-DsRed or pEGFP-N1 vector plasmid (Clontech). The details of plasmid construction are described in the Supplementary Information.
2.3. Cell culture
N2a cells were cultivated using Dulbecco's Modified Eagle's Medium (Sigma, USA) supplemented with 10% (v/v) fetal bovine serum (Sigma) and penicillin and streptomycin (100 U/mL and 10 mg/mL, respectively; Sigma). The cells were maintained at 37 °C in a humidified atmosphere containing 5% CO2. The N2a cells were transfected with the plasmids using the Lipofectamine 3000 reagent (Thermo Fisher Scientific, USA) according to the manufacturer's instructions. GJ plaques were observed at 24 h post-transfection. To observe the formation of homomeric–heterotypic GJs consisting of Cx39.4ins287EGFP and Cx41.8del241–370RFP, the N2a cells transfected with each plasmid were mixed and re-seeded at 24 h post-transfection. The homomeric–heterotypic GJs were observed at 24 h after re-seeding. The expression and localization of connexin proteins in N2a cells were detected using the Keyence BZ-X700 and Carl Zeiss LSM780 microscopes.
2.4. Immunostaining
The plaque-formation efficiency of Cx41.8IMM was detected by performing the immunofluorescence assay. “IMM” indicates a 2x Myc tag sequence inserted into the IL domain of Cx41.8, resulting in the formation of Cx41.8IMM [58]. Cx41.8IMM was previously confirmed to be functional in vivo; it rescued the cx41.8−/− mutant phenotype. After 24 h of transfection, the N2a cells were fixed with 4% paraformaldehyde in PBS for 15 min, permeabilized with 0.02% Triton X-100 in PBS for 1 h, and blocked with 5% bovine serum albumin (Sigma) in PBS for 1 h. The cells were then incubated with anti-c-Myc rabbit polyclonal antibodies (QED Bioscience, Cat# 18826) overnight at 4 °C and subsequently stained with Goat anti-Rabbit IgG (H + L) Highly Cross-Adsorbed Secondary Antibody, Alexa Fluor 488 (Invitrogen, Cat# 11034) and Hoechst 33324 (Invitrogen) for 1 h at 25 °C. Cx41.8IMM localization in the N2a cells was detected using the Keyence BZ-X700 microscope.
3. Results
3.1. Requirement of the CT domains of Cx39.4 and Cx41.8 for the stripe pattern formation in zebrafish
We investigated whether the CT domains of Cx39.4 and Cx41.8 are essential for the stripe pigment pattern formation of zebrafish (Fig. 1A). We designed the following CT-truncated connexins: Cx39.4–255STOP, in which 86 amino-acid residues from the 256th to 341st positions were removed, and Cx41.8–240STOP, in which 130 amino-acid residues from the 241st to 370th positions were removed (Fig. 1B). The 240th position in Cx41.8 corresponded to the 255th position in Cx39.4 (Fig. 1C). The respective mutant connexin proteins were expressed in each mutant zebrafish line: Cx39.4–255STOP in the cx39.4−/− mutant fish and Cx41.8–240STOP in the cx41.8−/− mutant fish. We used the mitfa-promoter to express Cx39.4 in melanophores and the cx41.8-promoter to express Cx41.8 in melanophores and xanthophores, as these promoters within each connexin gene are sufficient to rescue the mutant phenotypes [60,64].
We microinjected Tol2 transposase [41,42] and each plasmid construct into the corresponding mutant line, cx39.4−/− or cx41.8−/−, and obtained F0 generation chimera fish (Fig. 1A upper right). Each F0 chimera fish was then crossed with cx39.4−/− or cx41.8−/− fish, resulting in the formation of Tg lines at the F1 generation (Fig. 1A upper right). “Cx39.4–255STOP >> cx39.4−/−” indicates that Cx39.4–255STOP is expressed in fish with the cx39.4−/− background under the control of the mitfa-promoter, and “Cx41.8–240STOP >> cx41.8−/−” indicates that Cx41.8–240-STOP is expressed in fish with the cx41.8−/− background under the control of the cx41.8-promoter. We assessed whether the CT-truncated connexins are functional in skin pattern formation at the F1 generation. Unexpectedly, we observed stripe skin patterns in both these Tg lines at the F1 generation (Fig. 1A, pictures of the fish in the center column). In other words, both Cx39.4–255STOP and Cx41.8–240STOP rescued the cx39.4−/− or cx41.8−/− mutant phenotypes, like the full-length connexins did (stripe* and stripe** in Fig. 1B right column) [59,60]. The individual Tg fish in each experiment showed almost the same phenotype. The variations in the Tg phenotypes are shown in Fig. S2.
.
3.2. Role of the CT domains of Cx39.4 and Cx41.8 in double-knockout fish
We examined whether these CT-truncated connexins function in the WKO mutant (Fig. 1). The WKO lines harboring Cx39.4–255STOP or Cx41.8–240STOP were generated by crossing the F0 chimera fish with the WKO fish twice. The chimera fish (F0) (Cx39.4–255STOP-injected cx39.4−/− mutant fish) were crossed with WKO fish, generating fish with a cx39.4−/−;cx41.8+/− genetic background (F1). Then, these Tg fish (F1) were crossed with WKO fish, generating WKO fish with the cx39.4−/−;cx41.8−/− genetic background (F2). The integration of the transgene was determined by performing PCR at each step, and the genetic background was confirmed by cx39.4 and cx41.8 sequencing. Via the same scheme, F0 chimera fish (Cx41.8–240STOP injected cx41.8−/− mutant fish) were crossed with WKO fish, generating cx39.4+/−;cx41.8−/- fish (F1), which were then crossed with WKO fish. As a result, we obtained the “Cx41.8–240STOP >> WKO” Tg line (F2). In the case of Cx41.8, the Cx41.8–240STOP transgene did not change the WKO mutant phenotype at the F2 generation (Fig. 1A; “Cx41.8–240STOP >> WKO”), which raised questions regarding whether the Cx41.8–240STOP protein was expressed but nonfunctional or not expressed because of experimental problems such as the inactivation of the insertion site of the transgene. Based on the results obtained, the former possibility seems to be correct. The Tg fish “Cx41.8–240STOP >> WKO” (F2) were generated as explained above by crossing “Cx41.8–240 >> cx39.4+/−;cx41.8−/−” (F1) with WKO fish. As these “Cx41.8–240 >> cx39.4+/−;cx41.8−/−” (F1) fish showed a stripe pattern because of Cx41.8–240STOP insertion, the inserted transgene was expressed in this Tg line, whereas zebrafish with the cx39.4+/−; cx41.8−/− genetic background without the transgene showed a spot pattern (data not shown). Via this experiment, we concluded that Cx39.4–255STOP was functional; the WKO phenotype was changed to the spot pattern due to the effects of Cx39.4–255STOP, but Cx41.8–240STOP was nonfunctional in fish with the WKO background (Fig 1A, B).
By considering the results from experiments using the single- and double-knockout mutant lines, we found that in zebrafish skin pattern formation, the Cx39.4 CT domain is not indispensable; however, the Cx41.8 CT domain is required, depending on the existence of Cx39.4. The CT-truncated Cx41.8 requires Cx39.4 for its function, membrane localization, and/or GJ function.
3.3. Visualization of Cx39.4 and Cx41.8
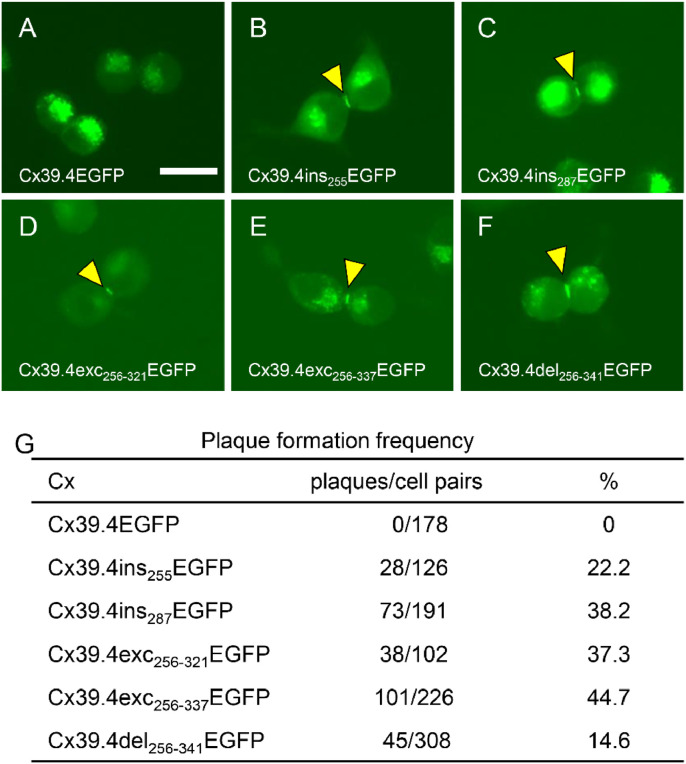
We designed fluorescent protein-tagged Cx39.4 and Cx41.8 that were applicable for the further investigation of protein localization in cultured cells and live fish, to compensate for the disadvantage of the lack of commercially available antibodies in zebrafish. Results from previous studies have shown that EGFP-fused full-length Cx39.4 (Cx39.4EGFP) and Cx41.8 (Cx41.8EGFP) are nonfunctional in the cx39.4−/− or cx41.8−/− mutant (Figs. S3A, S4A) [58,59]. Recently, we successfully obtained functional EGFP-tagged Cx39.4, Cx39.4exc256–321EGFP, in which the EGFP sequence replaced the amino-acid residues from the 256th to 321st positions in the Cx39.4 CT domain (Fig. S3A) [59]. This EGFP-tagged Cx39.4 rescued the cx39.4−/− mutant phenotype and formed GJ plaques between melanophores on the fish skin and between the N2a cells [59]. In this study, we designed several EGFP-tagged Cx39.4 constructs (Fig. S3A, B) and used them to examine the function of the CT domain in GJ plaque formation between the N2a cells (Fig. 2). In addition, the GJ plaque formation between melanophores was observed (Fig. S3C; yellow arrowhead). Cx39.4exc256–321EGFP, Cx39.4exc256–337EGFP, and Cx39.4del256–341EGFP (Fig. S3A) were designed to examine the requirement of the PDZ binding motif located at the CT-end of Cx39.4 (Figs. 1C, S3A, S3B,). These three connexins rescued cx39.4−/− mutant phenotype, but Cx39.4del256–341EGFP, which did not have the PDZ binding motif, had a smaller effect on WKO (Figs. S3A; S3B).
Fig. 3.
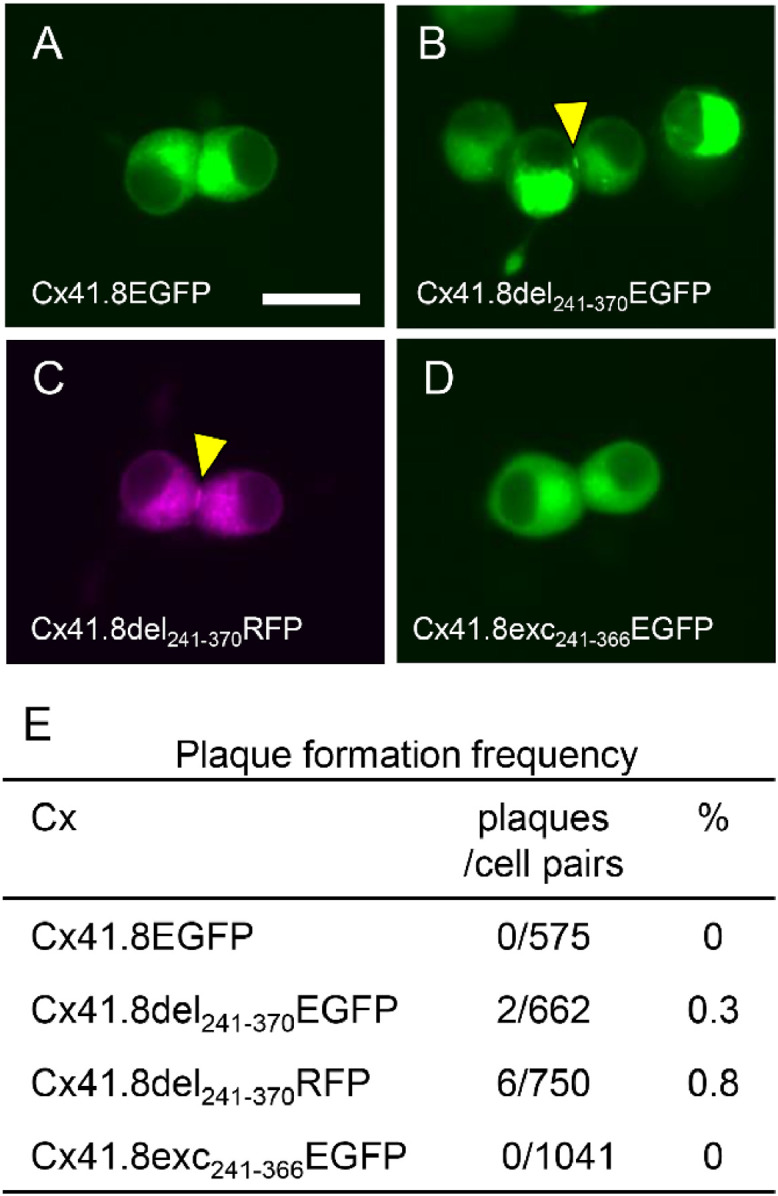
Cx41.8-gap junction reconstruction between N2a cells. (A–D) Representative fluorescence images of gap-junction plaques in N2a cells expressing fluorescent protein-fused connexin. (A) Cx41.8EGFP, (B) Cx41.8del241–370EGFP, (C) Cx41.8del241–370RFP, and (D) Cx41.8exc241–366EGFP. The yellow arrowheads indicate gap junction plaques. (E) The frequency of gap-junction plaque formation between N2a cells. Scale bar: 20 µm. Connexin structures are shown in Fig. S4.
Fig. 2.
Cx39.4-gap junction reconstruction between N2a cells. (A–F) Representative fluorescence images of gap-junction plaques between N2a cells expressing enhanced green fluorescent protein (EGFP)-fused connexins. (A) Cx39.4EGFP, (B) Cx39.4ins255EGFP, (C) Cx39.4ins287EGFP, (D) Cx39.4exc256–321EGFP, (E) Cx39.4exc256–337EGFP, and (F) Cx39.4del256–341EGFP. The yellow arrowheads indicate gap junction plaques. (G) The frequency of gap-junction plaque formation between N2a cells. Scale bar: 20 µm. Connexin structures are shown in Fig. S3A.
In contrast, to visualize Cx41.8 GJs, we designed EGFP-tagged CT-truncated Cx41.8 (Fig. S4A) because CT-truncated Cx41.8 rescues the cx41.8−/− mutant phenotype (Fig. 1). Interestingly, Cx41.8del241–370EGFP rescued the cx41.8−/− mutant phenotype but did not function in the WKO mutant (Fig. S4B) as Cx41.8–240STOP (Fig. 1). Using this construct, we attempted to detect EGFP signals on the fish skin, but this was unsuccessful. One reason for this might be weak promoter activity, due to which the expression of cx41.8 was weakly induced. Another reason for this might be the autofluorescence from xanthophores, in which the Cx41.8 protein is primarily expressed. To compensate for the difficulty in using EGFP in xanthophores, RFP was used (Fig. S4A). The RFP signal derived from Cx41.8del241–370RFP was slightly detected by increasing the exposure of the camera to the upper limit of fluorescence intensity; however, GJ plaques that should be present on the adhesion surface between the cells were not detected in this experiment (Fig. S4C).
3.4. Formation of GJs between cultured cells
Using these plasmid constructs, EGFP-tagged Cx39.4 s and Cx41.8 s were transiently expressed in N2a cells; N2a cells are known to have low levels of endogenous connexins and are commonly used in GJ-related studies [66], [67], [68]. The plaque formation frequency was calculated among the cell pairs showing the EGFP signal (Figs. 2,3). As mentioned above, EGFP-fused full-length Cx39.4 and Cx41.8 did not form GJ plaques (Fig. 2A, 3A) [58,59]. Cx39.4 s having the EGFP tag inside the CT domain showed a GJ plaque formation frequency of approximately 20%–40% (Fig. 2B–G). With regard to the requirement of the PDZ-binding motif, the Cx39.4del256–341EGFP (Fig. 2F) showed a GJ plaque formation frequency that was approximately half or less than half of that of the other Cx39.4 constructs (Fig. 2G: 14.6%; vs. Fig. 2B–E, G: 22.2–44.7%). This suggests that the PDZ-binding motif has some role in Cx39.4 function.
In contrast, Cx41.8del241–370EGFP and Cx41.8del241–370RFP rarely formed GJ plaques between N2a cells (Fig 3B, C. E; 0.3% and 0.8%), although the EGFP-fused CT-truncated Cx41.8 Cx41.8del241–370EGFP was functional in the cx41.8−/− mutant (Fig. S4). Cx41.8exc241–366EGFP, made by attaching a PDZ domain binding motif to the C-terminus of Cx41.8del241–370EGFP, did not improve the plaque formation efficiency in EGFP-tagged Cx41.8 (Fig. 3D, E). To eliminate the possibility that the EGFP or RFP sequence hampered the formation of GJs between N2a cells, we used “Cx41.8IMM”, which had a 2x Myc tag sequence at the IL domain of Cx41.8 (Fig. S5A). Cx41.8IMM was previously confirmed to be functional in vivo; it rescued the cx41.8−/− mutant phenotype [58]; however, we failed to detect the Cx41.8IMM-GJ plaques between N2a cells (Fig. S5B). These results showed that Cx41.8 shows a low efficiency of membrane localization in N2a cells.
3.5. Heteromeric/heterotypic GJ formation
We examined GJ formation in heteromeric and heterotypic conditions. CT-truncated Cx41.8 (Cx41.8–240STOP) rescued the mutant phenotype cx41.8−/−; however, it did not function in the WKO mutant (Fig. 1A, B). Based on this, we hypothesized that Cx39.4 might assist Cx41.8 in performing its function. To test this hypothesis, we examined the ability of Cx39.4 to form plaques in heteromeric/homomeric-heterotypic GJs with Cx41.8 between N2a cells. We transfected Cx39.4ins287EGFP and Cx41.8del241–370RFP and calculated the frequency of the formation of heteromeric GJ plaques between the N2a cells, such that both green and red fluorescent signals were observed in both the cells (Fig. 4A–A"). The plaque-forming frequency of heteromeric GJs was almost the same as that observed in case of Cx39.4 alone (compare Fig. 2C, G; 38.2% and Fig 4A–A", C; 37.6%). This result indicates that Cx39.4 resulted in the uptake of Cx41.8 to the cell membrane efficiently; this is in good agreement with the in vivo transgenic results showing that the CT-truncated Cx41.8, Cx41.8–240STOP required Cx39.4 expression to perform its function in vivo (Fig. 1A, B). In contrast, the plaque-forming frequency in case of homomeric–heterotypic GJs was higher than that in case of Cx41.8 alone (compare Fig. 4B–B", C; 3.8% to Fig. 3C, E; 0.8%); however, the efficiency was lower than that for heteromeric GJs.
Fig. 4.
Heteromeric/heterotypic gap-junction formation by Cx39.4 and Cx41.8. (A–A”) The heteromeric gap-junction plaque formed by Cx39.4ins287EGFP and Cx41.8del241–370RFP. (B–B”) The homomeric-heterotypic gap-junction plaque formed by Cx39.4ins287EGFP and Cx41.8del241–370RFP hemichannels. (A, B) Green fluorescence by Cx39.4ins287EGFP, (A', B') red fluorescence by Cx41.8del241–370RFP, and (A”, B”) Merged images. The yellow arrowheads indicate the gap junction plaques. (C) The frequency of heteromeric/heterotypic gap junction plaque formation. Scale bar; 20 μm.
4. Discussion
We investigated the role of the CT domains of Cx39.4 and Cx41.8 in skin-pattern formation in vivo and plaque formation in vitro. Both CT-truncated Cx39.4 and CT-truncated Cx41.8 rescued their respective mutant phenotypes (Fig. 1). Therefore, it was unlikely that the CT domains would be required for zebrafish pattern formation. CT-truncated Cx39.4 functioned in the WKO mutant, but CT-truncated Cx41.8 did not (Fig. 1). Although these results appear to be contradictory, they lead to the following hypotheses: (i) the functions of CT-truncated Cx41.8 depend on Cx39.4 expression and (ii) Cx39.4 plays a supportive role for Cx41.8 function in vivo. A similar conclusion was obtained from in vitro experiments with cultured cells (Figs 2,3, and 4). Cx41.8, which was unlikely to reach the cell membrane in cultured cells alone, was able to form heteromeric GJs with Cx39.4, reaching the cell membrane with high efficiency (Fig. 4).
Our previous study identified the minimal GJ network among pigment cells required for the formation of stripe patterns in zebrafish [59]. We observed that the combination of Cx41.8 expression in xanthophores and Cx39.4 expression in melanophores is sufficient to form stripes; however, the mRNA expression of both cx41.8 and cx39.4 was detected in melanophores and xanthophores [55,59]. It is assumed that Cx41.8 in melanophores might perform a supportive function to form clear stripe patterns, as the shape of dark stripes appeared clearer when the melanophores expressed both cx41.8 and cx39.4 than when they expressed only cx39.4. This result might be related to the electrophysiological characteristics of GJs. Both Cx41.8 and Cx39.4 exhibit a polyamine-binding motif at their NT domain, and this might contribute to the spermidine-dependent rectification property of GJs [58,59,69]. We showed that Cx39.4 exhibits a spermidine dependency; nonetheless, the effect of this property was not sufficient to provide directionality to the GJ channel [59]. Further studies are needed to determine the effects of the co-existence of Cx39.4 and Cx41.8 on the rectification property of GJs.
The effects of the co-existence of connexins were previously reported in case of Cx43 and Cx40.8, which represent a paralogous pair in zebrafish. Interestingly, they showed complicated behaviors with regard to membrane localization in vivo and in vitro [70,71]. In cultured cells, Cx43 supported the membrane localization of Cx40.8; without Cx43, it was unlikely for Cx40.8 to be localized in the cell membrane. However, they exhibited different behaviors between developing cells and regenerating cells. Cx43 and Cx40.8 were co-localized in the plasma membrane of mesenchymal cells at the tip of the developing fin. In contrast, in regenerating fins, Cx43 was localized in the plasma membrane but Cx40.8 was retained in the Golgi apparatus in the proliferating cell population of the blastema. These observations hypothesized that the membrane localization of Cx40.8 suppresses the Cx43-dependent rapid growth of blastema cells, indicating that Cx40.8 controls the speed of cell growth in the development and regeneration of fish fins [71]. Cx39.4 is a teleost lineage-specific connexin that is ubiquitously expressed in zebrafish tissues (Usui et al., unpublished data). As cx39.4 is widely distributed in fish genomes and the amino-acid sequence of the protein encoded by this gene is highly conserved, it is predicted to perform a fundamental function in addition to skin-pigment pattern formation; however, no phenotype other than skin patterning was detected in the cx39.4 mutant fish.
The CT domains of connexins play important roles in channel function, and mutations in this domain are occasionally responsible for genetic diseases [27,29,30]. In the case of Cx41.8 in pigment cells, precise control of the channel properties might not be required to form specific skin patterns; however, the mechanisms underlying these processes remain unclear. In addition, little is known regarding the function of the CT domain in Cx39.4. Only one study has reported the interaction of proteins with the CT domains of Cx39.4 and Cx41.8; yeast two-hybrid experiments using the CT motifs of Cx39.4 and Cx41.8 as probes concluded that the tight-junction protein ZO1a (encoded by tjp1a) possibly binds to the CT domains of both these connexins [72]. Originally, ZO1a was isolated as the causative gene for the zebrafish pattern mutant schachbrett, which shows a different type of spot pattern compared with that in case of the cx41.8−/− mutant. In mammals, the ZO1 protein binds to the PDZ-binding motif at the CT of Cx43 [19,20], and this binding was also expected in case of Cx39.4 and Cx41.8 in zebrafish [72]. However, ZO1a functions neither in melanophores nor in xanthophores, but in iridophores, for stripe pattern formation [72]. In addition, as described above, we identified the minimal requirement for Cx39.4 and Cx41.8 expression and concluded that Cx39.4 expression in melanophores and Cx41.8 expression in xanthophores are sufficient to form stripes [59]. Altogether, the binding of ZO1a to the CT end of Cx39.4 or Cx41.8 is not efficient for the formation of the striped skin pattern. In contrast, this study demonstrated that the 4 amino-acid sequence “RHYV” at the CT end of Cx39.4 plays a role in GJ formation in vitro (Fig. 2D–G). “YV” sequences at the CT end of claudin-1–claudin-8 are predicted as the PDZ-binding motifs for ZO1, ZO2, and ZO3 [73]. Further studies are required to identify the binding of proteins to Cx39.4 and clarify their interactions.
Credit author statement
Y. U. and M. W. conceived the project, performed the experiments, analyzed the data, and wrote the text.
Declaration of interest
None.
Acknowledgments
Funding
This work was supported by the Japan Society for the Promotion of Science, JSPS Fellows Grant [19J12372 (to Y. U.)], and KAKENHI Grant [15K0079 and 17H03683 (to M.W.)].
Acknowledgements
The authors thank N. Tanimoto, M. Kadota, and N. Takada for breeding and maintaining the zebrafish.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.bbadva.2021.100006.
Appendix. Supplementary materials
References
- 1.Artavanis-Tsakonas S., Rand M.D., Lake R.J. Notch signaling: cell fate control and signal integration in development. Science. 1999;284:770–776. doi: 10.1126/science.284.5415.770. [DOI] [PubMed] [Google Scholar]
- 2.Stark G.R., Kerr I.M., Williams B.R.G., Silverman R.H., Schreiber R.D. How cells respond to interferons. Annu. Rev. Biochem. 1998;67:227–264. doi: 10.1146/annurev.biochem.67.1.227. [DOI] [PubMed] [Google Scholar]
- 3.Saez J.C., Berthoud V.M., Branes M.C., Martinez A.D., Beyer E.C. Plasma membrane channels formed by connexins: their regulation and functions. Physiol. Rev. 2003;83:1359–1400. doi: 10.1152/physrev.00007.2003. [DOI] [PubMed] [Google Scholar]
- 4.Danial N.N., Korsmeyer S.J. Cell death: critical control points. Cell. 2004;116:205–219. doi: 10.1016/s0092-8674(04)00046-7. [DOI] [PubMed] [Google Scholar]
- 5.Camussi G., Deregibus M.C., Bruno S., Cantaluppi V., Biancone L. Exosomes/microvesicles as a mechanism of cell-to-cell communication. Kidney Int. 2010;78:838–848. doi: 10.1038/ki.2010.278. [DOI] [PubMed] [Google Scholar]
- 6.Simpson I., Rose B., Loewenstein W.R. Size limit of molecules permeating the junctional membrane channels. Science. 1977;195:294–296. doi: 10.1126/science.831276. [DOI] [PubMed] [Google Scholar]
- 7.Bruzzone R., White T.W., Paul D.L. Connections with connexins: the molecular basis of direct intercellular signaling. Eur. J. Biochem. /FEBS. 1996;238:1–27. doi: 10.1111/j.1432-1033.1996.0001q.x. https://www.ncbi.nlm.nih.gov/pubmed/8665925 [DOI] [PubMed] [Google Scholar]
- 8.Kumar N.M., Gilula N.B. The gap junction communication channel. Cell. 1996;84:381–388. doi: 10.1016/s0092-8674(00)81282-9. [DOI] [PubMed] [Google Scholar]
- 9.Kosakovsky Pond S.L., Mannino F.V., Gravenor M.B., Muse S.V., Frost S.D. Evolutionary model selection with a genetic algorithm: a case study using stem RNA. Mol. Biol. Evol. 2007;24:159–170. doi: 10.1093/molbev/msl144. [DOI] [PubMed] [Google Scholar]
- 10.Eastman S.D., Chen T.H., Falk M.M., Mendelson T.C., Iovine M.K. Phylogenetic analysis of three complete gap junction gene families reveals lineage-specific duplications and highly supported gene classes. Genomics. 2006;87:265–274. doi: 10.1016/j.ygeno.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 11.Unger V.M., Kumar N.M., Gilula N.B., Yeager M. Three-dimensional structure of a recombinant gap junction membrane channel. Science. 1999;283:1176–1180. doi: 10.1126/science.283.5405.1176. https://www.ncbi.nlm.nih.gov/pubmed/10024245 [DOI] [PubMed] [Google Scholar]
- 12.Ambrosi C., Ren C., Spagnol G., Cavin G., Cone A., Grintsevich E.E., Sosinsky G.E., Sorgen P.L. Connexin43 forms supramolecular complexes through non-overlapping binding sites for drebrin, tubulin, and ZO-1. PLoS ONE. 2016;11 doi: 10.1371/journal.pone.0157073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Batra N., Riquelme M.A., Burra S., Kar R., Gu S., Jiang J.X. Direct regulation of osteocytic connexin 43 hemichannels through AKT kinase activated by mechanical stimulation. J. Biol. Chem. 2014;289:10582–10591. doi: 10.1074/jbc.M114.550608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bouvier D., Kieken F., Kellezi A., Sorgen P.L. Structural changes in the carboxyl terminus of the gap junction protein connexin 40 caused by the interaction with c-Src and zonula occludens-1. Cell Commun Adhes. 2008;15:107–118. doi: 10.1080/15419060802014347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen V.C., Kristensen A.R., Foster L.J., Naus C.C. Association of connexin43 with E3 ubiquitin ligase TRIM21 reveals a mechanism for gap junction phosphodegron control. J. Proteome Res. 2012;11:6134–6146. doi: 10.1021/pr300790h. [DOI] [PubMed] [Google Scholar]
- 16.Fanning A.S., Anderson J.M. Zonula occludens-1 and -2 are cytosolic scaffolds that regulate the assembly of cellular junctions. Ann. N Y Acad. Sci. 2009;1165:113–120. doi: 10.1111/j.1749-6632.2009.04440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moorer M.C., Hebert C., Tomlinson R.E., Iyer S.R., Chason M., Stains J.P. Defective signaling, osteoblastogenesis and bone remodeling in a mouse model of connexin 43 C-terminal truncation. J. Cell. Sci. 2017;130:531–540. doi: 10.1242/jcs.197285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sun Y., Zhao X., Yao Y., Qi X., Yuan Y., Hu Y. Connexin 43 interacts with Bax to regulate apoptosis of pancreatic cancer through a gap junction-independent pathway. Int. J. Oncol. 2012;41:941–948. doi: 10.3892/ijo.2012.1524. [DOI] [PubMed] [Google Scholar]
- 19.Giepmans B.N., Moolenaar W.H. The gap junction protein connexin43 interacts with the second PDZ domain of the zona occludens-1 protein. Curr. Biol. 1998;8:931–934. doi: 10.1016/s0960-9822(07)00375-2. [DOI] [PubMed] [Google Scholar]
- 20.Toyofuku T., Yabuki M., Otsu K., Kuzuya T., Hori M., Tada M. Direct association of the gap junction protein connexin-43 with ZO-1 in cardiac myocytes. J. Biol. Chem. 1998;273:12725–12731. doi: 10.1074/jbc.273.21.12725. [DOI] [PubMed] [Google Scholar]
- 21.Langlois S., Cowan K.N., Shao Q., Cowan B.J., Laird D.W. Caveolin-1 and -2 interact with connexin43 and regulate gap junctional intercellular communication in keratinocytes. Mol. Biol. Cell. 2008;19:912–928. doi: 10.1091/mbc.e07-06-0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park D.J., Wallick C.J., Martyn K.D., Lau A.F., Jin C., Warn-Cramer B.J. Akt phosphorylates Connexin43 on Ser373, a "mode-1" binding site for 14-3-3. Cell Commun. Adhes. 2007;14:211–226. doi: 10.1080/15419060701755958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gu H., Ek-Vitorin J.F., Taffet S.M., Delmar M. Coexpression of connexins 40 and 43 enhances the pH sensitivity of gap junctions: a model for synergistic interactions among connexins. Circ.. Res.. 2000;86:E98–E103. http://www.ncbi.nlm.nih.gov/pubmed/10827142 [PubMed] [Google Scholar]
- 24.Homma N., Alvarado J.L., Coombs W., Stergiopoulos K., Taffet S.M., Lau A.F., Delmar M. A particle-receptor model for the insulin-induced closure of connexin43 channels. Circ. Res. 1998;83:27–32. doi: 10.1161/01.res.83.1.27. http://www.ncbi.nlm.nih.gov/pubmed/9670915 [DOI] [PubMed] [Google Scholar]
- 25.Zhou L., Kasperek E.M., Nicholson B.J. Dissection of the molecular basis of pp60(v-src) induced gating of connexin 43 gap junction channels. J. Cell Biol. 1999;144:1033–1045. doi: 10.1083/jcb.144.5.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moreno A.P., Chanson M., Elenes S., Anumonwo J., Scerri I., Gu H., Taffet S.M., Delmar M. Role of the carboxyl terminal of connexin43 in transjunctional fast voltage gating. Circ. Res. 2002;90:450–457. doi: 10.1161/hh0402.105667. http://www.ncbi.nlm.nih.gov/pubmed/11884375 [DOI] [PubMed] [Google Scholar]
- 27.van Steensel M.A., Spruijt L., van der Burgt I., Bladergroen R.S., Vermeer M., Steijlen P.M., van Geel M. A 2-bp deletion in the GJA1 gene is associated with oculo-dento-digital dysplasia with palmoplantar keratoderma. Am. J. Med. Genet A. 2005;132A:171–174. doi: 10.1002/ajmg.a.30412. [DOI] [PubMed] [Google Scholar]
- 28.Vreeburg M., de Zwart-Storm E.A., Schouten M.I., Nellen R.G., Marcus-Soekarman D., Devies M., van Geel M., van Steensel M.A. Skin changes in oculo-dento-digital dysplasia are correlated with C-terminal truncations of connexin 43. Am. J. Med. Genet A. 2007;143:360–363. doi: 10.1002/ajmg.a.31558. [DOI] [PubMed] [Google Scholar]
- 29.Gong X.Q., Shao Q., Lounsbury C.S., Bai D., Laird D.W. Functional characterization of a GJA1 frameshift mutation causing oculodentodigital dysplasia and palmoplantar keratoderma. J. Biol. Chem. 2006;281:31801–31811. doi: 10.1074/jbc.M605961200. [DOI] [PubMed] [Google Scholar]
- 30.Churko J.M., Langlois S., Pan X., Shao Q., Laird D.W. The potency of the fs260 connexin43 mutant to impair keratinocyte differentiation is distinct from other disease-linked connexin43 mutants. Biochem. J. 2010;429:473–483. doi: 10.1042/BJ20100155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guo S.X., Yang Z.Y., Wang R.X., Yang Y., Cao H.M., Zhang T. Association between C1019T polymorphism of the connexin37 gene and coronary heart disease in patients with in-stent restenosis. Exp. Ther. Med. 2013;5:539–544. doi: 10.3892/etm.2012.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wong C.W., Christen T., Pfenniger A., James R.W., Kwak B.R. Do allelic variants of the connexin37 1019 gene polymorphism differentially predict for coronary artery disease and myocardial infarction? Atherosclerosis. 2007;191:355–361. doi: 10.1016/j.atherosclerosis.2006.03.031. [DOI] [PubMed] [Google Scholar]
- 33.Wong C.W., Christen T., Roth I., Chadjichristos C.E., Derouette J.P., Foglia B.F., Chanson M., Goodenough D.A., Kwak B.R. Connexin37 protects against atherosclerosis by regulating monocyte adhesion. Nat. Med. 2006;12:950–954. doi: 10.1038/nm1441. [DOI] [PubMed] [Google Scholar]
- 34.Diez J.A., Ahmad S., Evans W.H. Assembly of heteromeric connexons in guinea-pig liver en route to the Golgi apparatus, plasma membrane and gap junctions. Eur. J. Biochem. 1999;262:142–148. doi: 10.1046/j.1432-1327.1999.00343.x. [DOI] [PubMed] [Google Scholar]
- 35.Cottrell G.T., Burt J.M. Functional consequences of heterogeneous gap junction channel formation and its influence in health and disease. Biochim. Biophys. Acta. 2005;1711:126–141. doi: 10.1016/j.bbamem.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 36.Vaney D.I., Weiler R. Gap junctions in the eye: evidence for heteromeric, heterotypic and mixed-homotypic interactions. Brain Res. Brain Res. Rev. 2000;32:115–120. doi: 10.1016/s0165-0173(99)00070-3. [DOI] [PubMed] [Google Scholar]
- 37.Goldberg G.S., Valiunas V., Brink P.R. Selective permeability of gap junction channels. Biochim. Biophys. Acta. 2004;1662:96–101. doi: 10.1016/j.bbamem.2003.11.022. [DOI] [PubMed] [Google Scholar]
- 38.Koval M., Harley J.E., Hick E., Steinberg T.H. Connexin46 is retained as monomers in a trans-Golgi compartment of osteoblastic cells. J. Cell Biol. 1997;137:847–857. doi: 10.1083/jcb.137.4.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Suchyna T.M., Nitsche J.M., Chilton M., Harris A.L., Veenstra R.D., Nicholson B.J. Different ionic selectivities for connexins 26 and 32 produce rectifying gap junction channels. Biophys. J. 1999;77:2968–2987. doi: 10.1016/S0006-3495(99)77129-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kimmel C.B. Genetics and early development of zebrafish. Trends Genet. 1989;5:283–288. doi: 10.1016/0168-9525(89)90103-0. [DOI] [PubMed] [Google Scholar]
- 41.Kawakami K., Koga A., Hori H., Shima A. Excision of the tol2 transposable element of the medaka fish, oryzias latipes, in zebrafish, danio rerio. Gene. 1998;225:17–22. doi: 10.1016/s0378-1119(98)00537-x. [DOI] [PubMed] [Google Scholar]
- 42.Kawakami K., Shima A., Kawakami N. Identification of a functional transposase of the Tol2 element, an Ac-like element from the Japanese medaka fish, and its transposition in the zebrafish germ lineage. Proc. Natl. Acad. Sci. U S A. 2000;97:11403–11408. doi: 10.1073/pnas.97.21.11403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Milos N., Dingle A.D., Milos J.P. Dynamics of pigment pattern formation in the zebrafish, Brachydanio rerio. III. Effect of anteroposterior location of three-day lateral line melanophores on colonization by the second wave of melanophores. J. Exp. Zool. 1983;227:81–92. doi: 10.1002/jez.1402270112. [DOI] [PubMed] [Google Scholar]
- 44.Odenthal J., Rossnagel K., Haffter P., Kelsh R.N., Vogelsang E., Brand M., van Eeden F.J., Furutani-Seiki M., Granato M., Hammerschmidt M., Heisenberg C.P., Jiang Y.J., Kane D.A., Mullins M.C., Nusslein-Volhard C. Mutations affecting xanthophore pigmentation in the zebrafish. Danio rerio, Dev. 1996;123:391–398. doi: 10.1242/dev.123.1.391. https://dev.biologists.org/content/123/1/391.long [DOI] [PubMed] [Google Scholar]
- 45.Hirata M., Nakamura K., Kanemaru T., Shibata Y., Kondo S. Pigment cell organization in the hypodermis of zebrafish. Dev. Dyn. 2003;227:497–503. doi: 10.1002/dvdy.10334. [DOI] [PubMed] [Google Scholar]
- 46.Patterson L.B., Parichy D.M. Interactions with iridophores and the tissue environment required for patterning melanophores and xanthophores during zebrafish adult pigment stripe formation. PLoS. Genet. 2013;9 doi: 10.1371/journal.pgen.1003561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Frohnhofer H.G., Krauss J., Maischein H.M., Nusslein-Volhard C. Iridophores and their interactions with other chromatophores are required for stripe formation in zebrafish. Development. 2013;140:2997–3007. doi: 10.1242/dev.096719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gur D., Bain E.J., Johnson K.R., Aman A.J., Pasoili H.A., Flynn J.D., Allen M.C., Deheyn D.D., Lee J.C., Lippincott-Schwartz J., Parichy D.M. situ differentiation of iridophore crystallotypes underlies zebrafish stripe patterning. Nat. Commun. 2020;11:6391. doi: 10.1038/s41467-020-20088-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Eom D.S., Bain E.J., Patterson L.B., Grout M.E., Parichy D.M. Long-distance communication by specialized cellular projections during pigment pattern development and evolution. Elife. 2015:4. doi: 10.7554/eLife.12401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mahalwar P., Walderich B., Singh A.P., Nusslein-Volhard C. Local reorganization of xanthophores fine-tunes and colors the striped pattern of zebrafish. Science. 2014;345:1362–1364. doi: 10.1126/science.1254837. [DOI] [PubMed] [Google Scholar]
- 51.Yamanaka H., Kondo S. In vitro analysis suggests that difference in cell movement during direct interaction can generate various pigment patterns in vivo. Proc. Natl. Acad. Sci. U S A. 2014;111:1867–1872. doi: 10.1073/pnas.1315416111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nakamasu A., Takahashi G., Kanbe A., Kondo S. Interactions between zebrafish pigment cells responsible for the generation of Turing patterns. Proc. Natl. Acad. Sci. U S A. 2009;106:8429–8434. doi: 10.1073/pnas.0808622106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Volkening A., Sandstede B. Iridophores as a source of robustness in zebrafish stripes and variability in Danio patterns. Nat. Commun. 2018;9:3231. doi: 10.1038/s41467-018-05629-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Irion U., Frohnhofer H.G., Krauss J., Colak Champollion T., Maischein H.M., Geiger-Rudolph S., Weiler C., Nusslein-Volhard C. Gap junctions composed of connexins 41.8 and 39.4 are essential for colour pattern formation in zebrafish. Elife. 2014;3:e05125. doi: 10.7554/eLife.05125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Watanabe M., Sawada R., Aramaki T., Skerrett I.M., Kondo S. The physiological characterization of connexin41.8 and connexin39.4, which are involved in the striped pattern formation of zebrafish. J. Biol. Chem. 2016;291:1053–1063. doi: 10.1074/jbc.M115.673129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Watanabe M., Iwashita M., Ishii M., Kurachi Y., Kawakami A., Kondo S., Okada N. Spot pattern of leopard Danio is caused by mutation in the zebrafish connexin41.8 gene. EMBO Rep. 2006;7:893–897. doi: 10.1038/sj.embor.7400757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Watanabe M. Gap Junction in the Teleost Fish Lineage: duplicated Connexins May Contribute to Skin Pattern Formation and Body Shape Determination. Front Cell Dev Biol. 2017;5:13. doi: 10.3389/fcell.2017.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Watanabe M., Watanabe D., Kondo S. Polyamine sensitivity of gap junctions is required for skin pattern formation in zebrafish. Sci. Rep. 2012;2:473. doi: 10.1038/srep00473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Usui Y., Aramaki T., Kondo S., Watanabe M. The minimal gap-junction network among melanophores and xanthophores required for stripe pattern formation in zebrafish. Development. 2019;146 doi: 10.1242/dev.181065. [DOI] [PubMed] [Google Scholar]
- 60.Watanabe M., Kondo S. Changing clothes easily: connexin41.8 regulates skin pattern variation. Pigment Cell Melanoma Res. 2012;25:326–330. doi: 10.1111/j.1755-148X.2012.00984.x. [DOI] [PubMed] [Google Scholar]
- 61.Watanabe M., Kondo S. Is pigment patterning in fish skin determined by the Turing mechanism? Trends Genet. 2015;31:88–96. doi: 10.1016/j.tig.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 62.Yamaguchi M., Yoshimoto E., Kondo S. Pattern regulation in the stripe of zebrafish suggests an underlying dynamic and autonomous mechanism. Proc. Natl. Acad. Sci. U S A. 2007;104:4790–4793. doi: 10.1073/pnas.0607790104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mahalwar P., Singh A.P., Fadeev A., Nusslein-Volhard C., Irion U. Heterotypic interactions regulate cell shape and density during color pattern formation in zebrafish. Biol. Open. 2016;5:1680–1690. doi: 10.1242/bio.022251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lister J.A., Robertson C.P., Lepage T., Johnson S.L., Raible D.W. nacre encodes a zebrafish microphthalmia-related protein that regulates neural-crest-derived pigment cell fate. Development. 1999;126:3757–3767. doi: 10.1242/dev.126.17.3757. https://dev.biologists.org/content/126/17/3757.long [DOI] [PubMed] [Google Scholar]
- 65.Larkin M.A., Blackshields G., Brown N.P., Chenna R., McGettigan P.A., McWilliam H., Valentin F., Wallace I.M., Wilm A., Lopez R., Thompson J.D., Gibson T.J., Higgins D.G. Clustal W and Clustal X version 2.0. Bioinformatics. 2007;23:2947–2948. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- 66.Connexin methods and protocols, humana press inc., springer protocols, 2001. doi:https://doi.org/10.1385/1592590438.
- 67.Mao A.J., Bechberger J., Lidington D., Galipeau J., Laird D.W., Naus C.C. Neuronal differentiation and growth control of neuro-2a cells after retroviral gene delivery of connexin43. J. Biol. Chem. 2000;275:34407–34414. doi: 10.1074/jbc.M003917200. [DOI] [PubMed] [Google Scholar]
- 68.Veenstra R.D., Wang H.Z., Westphale E.M., Beyer E.C. Multiple connexins confer distinct regulatory and conductance properties of gap junctions in developing heart. Circ. Res. 1992;71:1277–1283. doi: 10.1161/01.res.71.5.1277. [DOI] [PubMed] [Google Scholar]
- 69.Frohnhofer H.G., Geiger-Rudolph S., Pattky M., Meixner M., Huhn C., Maischein H.M., Geisler R., Gehring I., Maderspacher F., Nusslein-Volhard C., Irion U. Spermidine, but not spermine, is essential for pigment pattern formation in zebrafish. Biol. Open. 2016;5:736–744. doi: 10.1242/bio.018721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gerhart S.V., Eble D.M., Burger R.M., Oline S.N., Vacaru A., Sadler K.C., Jefferis R., Iovine M.K. The Cx43-like connexin protein Cx40.8 is differentially localized during fin ontogeny and fin regeneration. PLoS ONE. 2012;7:e31364. doi: 10.1371/journal.pone.0031364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gerhart S.V., Jefferis R., Iovine M.K. Cx40.8, a Cx43-like protein, forms gap junction channels inefficiently and may require Cx43 for its association at the plasma membrane. FEBS. Lett. 2009;583:3419–3424. doi: 10.1016/j.febslet.2009.09.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fadeev A., Krauss J., Frohnhofer H.G., Irion U., Nusslein-Volhard C. Tight Junction Protein 1a regulates pigment cell organisation during zebrafish colour patterning. Elife. 2015;4:e06545. doi: 10.7554/eLife.06545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Itoh M., Furuse M., Morita K., Kubota K., Saitou M., Tsukita S. Direct binding of three tight junction-associated MAGUKs, ZO-1, ZO-2, and ZO-3, with the COOH termini of claudins. J. Cell. Biol. 1999;147:1351–1363. doi: 10.1083/jcb.147.6.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.