Abstract
Objective
The insulin/insulin-like growth factor 1 (IGF1) pathway is emerging as a crucial component of prostate cancer progression. Therefore, we investigated the role of the novel insulin/IGF1 signaling modulator inceptor in prostate cancer.
Methods
We analyzed the expression of inceptor in human samples of benign prostate epithelium and prostate cancer. Further, we performed signaling and functional assays using prostate cancer cell lines.
Results
We found that inceptor was expressed in human benign and malignant prostate tissue and its expression positively correlated with various genes of interest, including genes involved in androgen signaling. In vitro, total levels of inceptor were increased upon androgen deprivation and correlated with high levels of androgen receptor in the nucleus. Inceptor overexpression was associated with increased cell migration, altered IGF1R trafficking and higher IGF1R activation.
Conclusions
Our in vitro results showed that inceptor expression was associated with androgen status, increased migration, and IGF1R signaling. In human samples, inceptor expression was significantly correlated with markers of prostate cancer progression. Taken together, these data provide a basis for investigation of inceptor in the context of prostate cancer.
Keywords: Insulin, IGF1R, Androgen, Signaling, Trafficking
Highlights
-
•
Inceptor levels correlate with carcinogenic markers in human prostate cancer.
-
•
Inceptor expression is associated with the androgen status of prostate cancer cells.
-
•
Overexpression of Inceptor modulates IGF1R signaling and trafficking.
-
•
Migration of a 3D spheroid model is induced by Inceptor.
Abbreviations
- 5-HT
5-hydroxytryptamine (serotonin)
- AR
Androgen Receptor
- ARE
Androgen Response Element
- BMI
Body Mass Index
- CK
Cytokeratin
- EdU
5-ethynyl-2′-deoxyuridine
- DAPI
4′,6-diamidino-2-phenylindole
- EGF
Epidermal Growth Factor
- EGFR
Epidermal Growth Factor Receptor
- ER
Estrogen Receptor
- ERE
Estrogen Response Element
- FBS
Fetal Bovine Serum
- HPA
Human Protein Atlas
- HPEC
Human Primary Prostate Epithelial Cells
- Ins
Insulin
- IGF1
Insulin-like growth factor 1
- IGF1R
Insulin-like growth factor 1 receptor
- IgG
Immunoglobulin G
- IIR/inceptor
Insulin Inhibitory Receptor
- IP
Immunoprecipitation
- IR
Insulin Receptor
- LAMP1
Lysosomal-associated membrane protein 1
- PBS
Phosphate-Buffered Saline
- PI3K
Phosphoinositide 3-kinase
- PSA
Prostate-Specific Antigen (aka KLK3)
- PSMA
Prostate-Specific Membrane Antigen (aka FOLH1)
- (q)PCR
(quantitative) Polymerase Chain Reaction
- TCGA
The Cancer Genome Atlas
- WT
Wildtype
1. Introduction
Hyperinsulinemia is known to increase the risk for many cancer types [1], thus the potential link between diabetes and cancer is of interest in exploring diagnosis and treatment options. While the risk for prostate cancer is not increased in patients with diabetes mellitus [2], the prognosis for prostate cancer patients with diabetes is worse [[3], [4], [5], [6]]. This goes along with altered carcinogenic pathways [7]. Prostate cancer is the third most frequent type of cancer among males worldwide [8]. The initial treatment for localized prostate cancer includes radical prostatectomy, radiation and chemotherapy [9]. As prostate tumor growth is highly dependent on androgens, the chemotherapeutical treatment of choice is androgen deprivation therapy via antiandrogens [10]. These substances block the androgen receptor (AR), which is a transcription factor that is dimerized after binding to androgen and translocated to the nucleus, where it binds to androgen response elements and modulates gene expression [11].
However, during the course of androgen deprivation therapy patients often become resistant to treatment and develop androgen-independent tumors. There is currently no effective treatment for this highly invasive and metastatic form of prostate cancer. Several pathways contribute to the progression towards androgen-independent prostate cancer [12]. For instance, the AR can become hypersensitive to residual androgen through gene duplication [13], or it can become promiscuous and recognize other steroids or even antiandrogens [14]. Some tumors switch to bypass pathways that activate proliferation via entirely different mechanisms [15]. Finally, AR downstream signaling can be cross-activated by other pathways, e.g. the insulin-like growth factor 1 receptor (IGF1R) pathway [16]. Immunohistochemical staining of human tissue samples has shown that IGF1R is upregulated in prostate cancer during androgen-independent progression [17,18]. As a potential mechanism, it has been suggested that IGF1R signaling induces phosphorylation of Foxo1, which subsequently leaves the nucleus and loses its ability to block AR [19].
The PI3K/Akt pathway, which is downstream of insulin receptor (IR), IGF1R and epidermal growth factor receptor (EGFR), is highly relevant in the context of cancer initiation and progression [20,21]. The combined inhibition of AR and PI3K proved effective in an animal model of androgen-independent prostate cancer [22]. Inhibitors of mTOR, which is activated by Akt, have been implicated in prostate cancer therapy in multiple studies [23,24]. Direct inhibition of IGF1R has shown promising results in androgen-independent prostate cancer as monotherapy [25] or in combination with the cytotoxic agent docetaxel [26]. IR acts via the same downstream pathways as IGF1R. Specifically the isoform IRa, which is mainly important during embryonic development and in various malignancies, has a high affinity for IGF1 and IGF2. In contrast to the more common isoform IRb, IRa predominantly activates proliferative rather than metabolic pathways [27]. It is therefore not surprising that IR activation can compensate for IGF1R inhibition in prostate cancer cells [28]. Likewise, a recent study revealed that insulin can reactivate the loss of PI3K signaling achieved by PI3K inhibitors [29]. Further, it was shown that AR signaling is elevated in prostate cancer patients with diabetes [30]. In addition to these findings, there is evidence that hyperinsulinemia can be a major driver for prostate cancer progression [31,32]. Thus, targeting the insulin/IGF1 axis might be beneficial in the treatment of metastatic prostate cancer.
We have previously found that a novel receptor, namely insulin inhibitory receptor (gene name: IIR/short form: inceptor), is a negative regulator of insulin and IGF1 signaling in pancreatic β-cells. We showed that inceptor knockout in β-cells led to increased IR and IGF1R activation in vitro and in vivo, and that inceptor desensitized IR and IGF1R by facilitating clathrin-mediated receptor endocytosis [33]. In earlier studies, inceptor was associated with various cancers. Initially, it was described as a biomarker in estrogen-related endometrial carcinoma and therefore termed estrogen-induced gene 121 (EIG121) [34]. Later it was shown that expression of this gene, also referred to as KIAA1324, was correlated to favorable prognosis in pancreatic neuroendocrine tumors and suppressed gastric cancer growth [35,36]. However, inceptor induced progression of endometrial cancer in vitro and in vivo [37] and was associated with poor prognosis in ovarian cancer [38]. The expression of inceptor in healthy tissues according to the Human Protein Atlas [39] is highest in the prostate, along with uterus, stomach and salivary glands. Further, it is expressed in the pituitary gland and other reproductive organs, i.e., testis, breast, endometrium and fallopian tube [39]. Thus, we hypothesize that due to its interaction with insulin/IGF1 signaling and its potential link to sex hormone signaling inceptor might be a drug target candidate in prostate cancer. For this reason, we investigated the expression of inceptor in benign and malignant prostate tissues and examined its potential role in prostate cancer cell lines.
2. Material and methods
2.1. Animal breeding
Mouse breeding was performed in compliance with the German Animal Protection Act and with the approved guidelines of the Society of Laboratory Animals (GV-SOLAS) and the Federation of Laboratory Animal Science Associations (FELASA). The male mice (strain: CD1xC57BL) were 4 months old and fed ad libitum.
2.2. Cell culture
LNCaP and LNCaP C4-2 cells were purchased from ATCC (CRL-1740, CRL-3314) and routinely cultured in RPMI 1640 with l-Glutamine (Gibco, 21875034), supplemented with 10% fetal bovine serum (FBS) (Pan, P40-37500). For androgen deprivation, the cells were maintained in RPMI 1640 without phenol red (Gibco, 11835030), supplemented with 10% charcoal-stripped FBS (Sigma–Aldrich, F6765). As a control, 10 nM dihydrotestosterone (DHT) (Sigma–Aldrich, D-073) was added to the charcoal-stripped medium. The medium was changed every 2 days. BPH-1 cells were purchased from DSMZ (ACC 143) and cultured in RPMI 1640 + 20% FBS + 20 ng/ml testosterone + 5 μg/ml transferrin +5 ng/ml sodium selenite + 5 μg/ml insulin. Human Prostate Epithelial cells (HPEC) (Merck Millipore, SCCE019) were cultivated using the Prostate Epithelia Complete Culture Kit (Merck Millipore, SCMP001) and PC3 cells (CLS, 300,312) were grown in DMEM F-12 (Gibco, 11,039, +5% FBS).
2.3. Cell line generation
The stable cell lines overexpressing inceptor-venus or venus only were generated by transfection of LNCaP cells using Lipofectamine 2000 (Thermo Fisher, 11668027) and selection with 1 μg/ml puromycin (Thermo Fisher, A1113803) for approx. 4 weeks. The cells were then sorted according to expression levels via fluorescence-activated cell sorting (FACS) to ensure that clones with similar expression levels were analyzed. The construct pCAG-inceptor-venus was generated as described before [33].
2.4. Immunohistochemistry
Cells were seeded in μ-slide 8 well chambers (Ibidi, 80,826) at a density of 25,000 cells per well and after 3 days were fixed in 4% PFA for 10 min at room temperature and permeabilized in 0.25% Triton-X100, 100 mM glycine for 15 min at room temperature.
Isolated mouse prostates were dehydrated in a sucrose gradient (7.5–30% sucrose in phosphate-buffered saline [PBS]) after overnight fixation in 4% PFA at 4 °C and embedded in tissue freezing medium (Leica, 14020108926) before cutting 10 μm sections. The frozen sections were rehydrated in PBS and permeabilized in 0.5% Triton-X100 for 20 min at room temperature.
Human prostate paraffin sections were deparaffinized in xylol, rehydrated in an ethanol series (100%–40%) and boiled in 100 mM sodium citrate, pH 6.0.
The cells or sections were blocked in 0.1% Tween-20, 10% FCS, 0.1% BSA and 3% donkey serum for 1 h at room temperature and incubated with the following primary antibodies overnight at 4 °C: rat anti-inceptor #16F6 or #2G6, or mouse anti-inceptor #31A11 (unpurified antibody in 1:10 dilution), rabbit anti-Cytokeratin 5 (Abcam, ab53121, 1:400), guinea pig anti-Cytokeratin 8/18 (OriGene, BP5007, 1:200), rabbit anti-AR (Abcam, ab133273, 1:200), rabbit anti-5HT/Serotonin (Neuromics, RA20080, 1:1000), rabbit anti-E-cadherin (Cell Signaling 24E10, 3195, 1:100), mouse anti-GM130 (BD, 610,822, 1:400), rabbit anti-Giantin (BioLegend, 924,302, 1:400), rat anti-LAMP1 (BD, 553,792, 1:100), rabbit anti-IR beta (SantaCruz, sc-711, 1:2000), rabbit anti-IGF1R (Cell Signaling, 3024, 1:100), rabbit anti-EGFR (Cell Signaling, 4267, 1:400). The inceptor antibodies #16F6 and #31A11 were previously validated in a murine β-cell line [33], and the validation of #2G6 is shown in Fig. S4. All inceptor antibodies were provided by Regina Feederle, Monoclonal Antibody Core Facility, Helmholtz-Zentrum München).
After washing with PBS-T (PBS + 0.1% Tween-20), the secondary antibodies were added in 1:800 dilution for 2 h at room temperature: donkey anti-rabbit immunoglobulin G (IgG) Alexa Fluor® 555 (Invitrogen, A31572); donkey anti-mouse IgG Alexa Fluor® 488 (Invitrogen, A21202); donkey anti-rabbit IgG Alexa Fluor® 488 (Invitrogen, A21206); donkey anti-guinea pig IgG Alexa Fluor® 488 (Dianova, 706-545-148); donkey anti-rat IgG Alexa Fluor® 488 (Invitrogen, A21208); donkey anti-rat IgG Alexa Fluor®647 (Dianova, 712-605-150). For subsequent nuclear counterstaining, 4′,6-diamidino-2-phenylindole (DAPI) (Sigma, 32,670) was used. Samples were stored in an aqueous embedding medium (25% Glycerol, 10% Polyvinyl alcohol, 2% 1,4-Diazabicyclo [2.2.2]octan, 100 mM Tris) and imaged on a Zeiss LSM 880 AiryScan.
The colocalization of inceptor with GM130, Giantin, LAMP1 and IGF1R/EGFR with E-cadherin was calculated using the ImageJ plugin Coloc2, using automatic threshold adjustment (Costes). The fluorescence intensity of AR and inceptor was quantified by hand-drawing regions of interest around single cells in ImageJ.
2.5. Western blot
Cells were lysed in RIPA buffer (150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS, 25 mM Tris pH 8) supplemented with protease inhibitor cocktail (Sigma, P8340, 1:100 dilution), phosphatase inhibitor cocktail 2 (Sigma, P5726, 1:100) and phosphatase inhibitor cocktail 3 (Sigma, P0044, 1:100). Protein concentrations were determined with the Pierce™ BCA protein assay kit (Thermo Fisher, 23,225). For SDS-PAGE, 20–25 μg protein were loaded in 6.5% or 7.5% acrylamide gels, and immunoblot on PVDF membrane was carried out in a Bio-Rad electrophoresis chamber and transfer system. The membranes were blocked in 5% milk powder in TBS-T for 1 h at room temperature and incubated with the following primary antibodies overnight at 4 °C: rat anti-inceptor #16F6 (1:1000 dilution), mouse anti-prostate-specific membrane antigen (PSMA) (Abcam, ab19071, 1:1000), mouse anti-AR (Santa Cruz, sc-7305, 1:500), mouse anti-IR (Cell Signaling, 3020, 1:1000), rabbit anti-IGF1R (Cell Signaling, 9750, 1:1000), rabbit anti-IGF1R phospho/IR phospho (Cell Signaling, 3024, 1:1000), rabbit anti-EGFR (Cell Signaling, 4267, 1:1000), rabbit anti-EGFR phospho (Cell Signaling, 3777, 1:1000) mouse anti-tubulin-gamma (Sigma, T5326, 1:5000), rabbit anti-prostate-specific antigen (PSA) (Abcam, ab53774, 1:5000), rabbit anti-heat shock protein 90 (Hsp90) (Cell signaling, 4874 S, 1:5000).
After washing with TBS-T, membranes were incubated for 2 h at room temperature with the secondary, horseradish peroxidase (HRP)-conjugated antibodies (1: 10,000 dilution) goat anti-mouse IgG (Dianova, 115-036-062), goat anti-rabbit IgG (Dianova, 111-036-045) or goat anti-rat IgG (Dianova, 112-035-175). The bands were detected using Clarity Western ECL Substrate (Bio-Rad, 1705061) in a ChemStudio2A (Analytik Jena). Quantification via densitometric analysis (normalized to tubulin as loading control) was performed in ImageJ.
2.6. Co-immunoprecipitation
LNCaP cells overexpressing inceptor-venus were seeded 3 days before in LNCaP medium. The confluent cultures were lysed in immunoprecipitation (IP) lysis buffer (1% Triton-X-100, 20 mM Tris–HCl pH 7.5, 150 mM NaCl, 1 mM EDTA) and centrifuged in a tabletop centrifuge at 14,000 rpm, 4 °C for 10 min; protein concentrations were determined via the Pierce™ BCA protein assay kit. SureBeads Protein G magnetic beads (Bio-Rad, 161–4021) were incubated with IR or IGF1R antibody (see Section 2.5) for 30 min at room temperature under rotation, then washed three times with PBS-T (PBS with 0.1% Tween20). Around 400 μl lysate (concentration adjusted to 1 μg/μl) were added to the antibody-conjugated SureBeads for overnight incubation at 4 °C (10 μl beads/100 μg protein). Beads were washed 3x with 500 μl lysis PBS-T and eluted with Laemmli-buffer for 10 min at 95 °C for subsequent Western blot analysis.
2.7. Proliferation assay
To assess the proliferation of LNCaP cells, they were seeded in 384-well clear bottom plates (Corning, 3770), after 2 days incubated with 10 μM 5-ethynyl-2′-deoxyuridine (EdU) for 8 h, fixated with 4% PFA and stored in PBS at 4 °C. The EdU was labeled using the Click-iT™ EdU Cell Proliferation Kit for Imaging with Alexa Fluor™ 647 (Invitrogen, C10340). Images were taken on a Zeiss Axio Observer Z1 and counted using an ImageJ Macro.
2.8. Endocytosis assay
To visualize the endocytosis of inceptor, LNCaP cells expressing inceptor-venus in μ-slide 8 well chambers (Ibidi, 80,826) were incubated with 10 μg/ml AlexaFluor555-conjugated rat anti-inceptor antibody. The cells were then observed for 5–60 min using a Zeiss LSM 880 AiryScan with incubation at 37 °C and 5% CO2 supply.
To analyze co-endocytosis of insulin/EGF and inceptor, LNCaP cells in μ-slide 8 well chambers (Ibidi, 80,826) were incubated with 100 nM AlexaFluor546-labeled insulin (provided by Oliver Plettenburg, Institute for Medicinal Chemistry, Helmholtz-Zentrum München) or 1 μg/ml AlexaFluor488-labeled EGF (Life Technologies, E13345), along with 10 μg/ml rat anti-inceptor antibody (#2G6) in normal growth medium (see Section 2.2) for 60 min at 37 °C. The cells were then fixated with 4% PFA for 10 min and permeabilized in 0.25% Triton-X100, 100 mM glycine for 15 min at room temperature. To visualize the inceptor antibody, secondary anti-rat IgG Alexa Fluor® 488 (Invitrogen, A21208) was added for 2 h at room temperature, along with SiR-Actin (Cytoskeleton, Inc., #CY-SC001, 1:1000 dilution) and DAPI for counterstaining. The samples were imaged on a Zeiss LSM 880 AiryScan and colocalization of the inceptor antibody with labeled insulin/EGF was calculated using the ImageJ Plugin JaCoP with manual thresholding.
2.9. Migration assay
To form LNCaP spheroids, the AggreWell™ 400 24 well plates (STEMCELL, Cat. No. 34411) were used. For each condition one well was rinsed using AggreWell Rinsing Solution (STEMCELL, Cat. No. 07010) and centrifuged at 1300 rcf for 5 min. Each well was washed with RPMI 1640 and filled with 1 ml prewarmed LNCaP medium (see Section 2.2) before adding the cell suspension (1.2 million cells in 1 ml) to form spheroids containing approx. 1000 cells. The plate was centrifuged at 100 rcf for 3 min and incubated for 48 h. The spheroids were then carefully collected via a 37 μm reversible cell strainer, sedimented by gravity and resuspended in 1.2 ml of a 10 μM collagen solution (Collagen type I, Sigma–Aldrich, Cat. No. C4243) buffered with 10 mM HEPES and approx. 15 mM NaOH to reach a neutral pH. The cell suspension was distributed between 4 wells of a 24-well plate (Thermo Scientific, 142,475) and incubated for 4 h. After the collagen had solidified, 1 ml of LNCaP medium (see Section 2.2) and 200 μl of mineral oil to prevent evaporation (Sigma–Aldrich, M5310) were added to each well and the plate was mounted on a Zeiss Axio Observer Z1 using incubation at 37 °C and 5% CO2 supply for live cell imaging. The area of the migrating cells was measured by hand-drawing regions of interest in ImageJ. Statistical analysis for all in vitro experiments was performed using GraphPad Prism 8 (GraphPad Software, Inc.).
2.10. Human samples
We selected 121 newly diagnosed male prostate cancer patients with a mean age of 64 years and mean body mass index (BMI) of kg/m2, who were recruited prior to radical prostatectomy. None of the patients received hormone-altering therapy prior to inclusion. Tissue sampling was performed by an experienced uropathologist. Prostate cancer (n = 50) as well as benign prostate tissues (n = 71) were immediately snap-frozen in liquid nitrogen and stored at −80 °C. For histological confirmation, hematoxylin and eosin stainings were performed on paraffinized samples and Gleason scores were determined. The tumor contents of the specimen were very variable among the patients (5%–90%). Patient characteristics are provided in Supplementary Table S2. To analyze cell types in human prostate sections, we used paraffin sections taken from prostate cancer patients aged 55–76 years with Gleason score 3–4.
Informed written consent was obtained from all participants and the Ethics Committee of the University of Tübingen approved the protocol according to the Declaration of Helsinki.
Multivariate linear regression models adjusted for age and BMI were performed using the JMP statistical software package (JMP 14.2, SAS Institute Inc.).
2.11. Real-time PCR
Total RNA from prostate cell lines and human prostate tissues was isolated using the Allprep RNA/DNA/protein kit (Qiagen) according to the manufacturer's description and cDNA was synthesized (Transcriptor First Strand cDNA synthesis kit, Roche). Real-time PCRs were performed with LightCycler 480 Probes Master (Roche) with universal probe library using LightCycler 480 (Roche) as previously described [40]. Delta–delta crossing-point (Cp) values were calculated and values were normalized to the housekeeping gene ubiquitin c (UBC) [41]. For real-time PCR analysis the following primers and probes were applied: UBC 5′-GGAAGGCATTCCTCCTGAT and 3′-CCCACCTCTGAGACGGAGTA (probe nr 11), EGFR 5′-GTGGATGGCATTGGAATCA and 3′-CAAAGGTCATCAACTCCCAAA (probe nr 50), inceptor 5′-CAGGTGCAGTCCACAGAAAA and 3′-GCCATCACAGGTCCCATC (probe nr 74) and MDM2 5′-CCATGATCTACAGGAACTTGGTAGTA and 3′-TCACTCACAGATGTACCTGAGTCC (probe nr 18). Primer sequences for AR, PSA, PSMA, IRa, IRb, IGF1R, CYP7B1 and ERb were previously published [30].
2.12. Bioinformatic analyses
Tissue expression of inceptor was extracted from the Human Protein Atlas (HPA) [39] (https://www.proteinatlas.org/ENSG00000116299-KIAA1324/tissue, last accessed on 18.09.2020). Data for the HPA dataset was collected by Illumina RNA-seq of specimen from the Uppsala Biobank. The protein sequence of inceptor was analyzed using the ENSEMBL database [42] (https://www.ensembl.org, reference ENSG00000116299, last accessed on 18.09.2020). The predicted domains were selected for the 1013aa transcript and annotated in SnapGene Viewer for visualization (Insightful Science; available at snapgene.com). The gene expression data in Figure 2 was extracted from the TCGA dataset using the GEPIA web server [43] (http://gepia.cancer-pku.cn/detail.php?gene=KIAA1324, last accessed on 18.09.2020). Mutation data in the TCGA and Neuroendocrine Prostate Cancer [44] datasets were obtained from CBioPortal [45] (https://www.cbioportal.org, last accessed on 18.09.2020). The DNA sequence of IIR was copied from the National Center for Biotechnology Information (NCBI) (available from https://www.ncbi.nlm.nih.gov/, 20.11.2020).
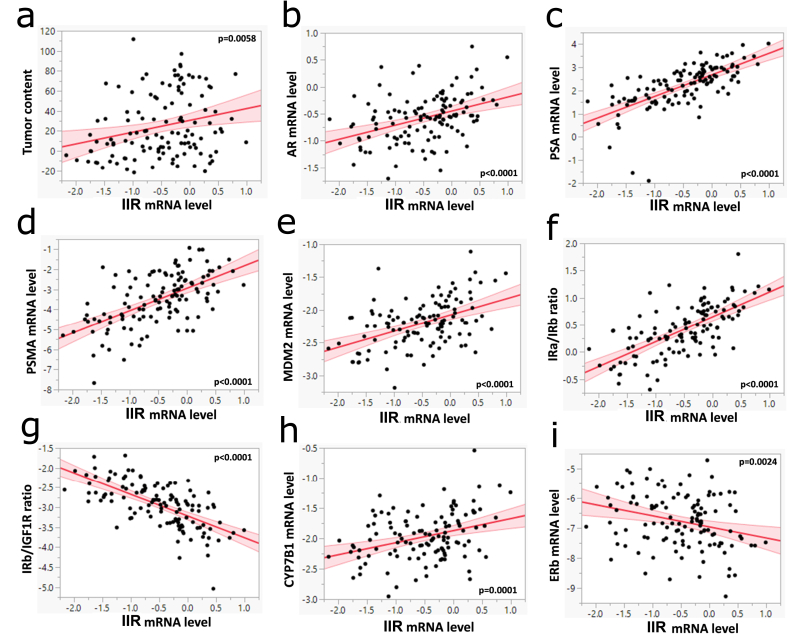
Figure 2.
Inceptor expression correlates with carcinogenic markers in human prostate samples. a. The mRNA expression of IIR in human benign prostate tissues (n = 71) and prostate cancer samples (n = 50) were determined with real-time PCR and normalized to ubiquitin c. Multivariate linear regression models were adjusted to age and BMI. Log transformed mRNA level of inceptor was correlated with tumor content (in %). b,c,d,e,h,i. Correlation of log transformed mRNA levels of inceptor with the indicated genes. f,g. Ratios of log transformed mRNA levels of key players of the Ins/IGF1 system, plotted against inceptor expression.
3. Results
3.1. Inceptor expression in healthy prostate epithelium and prostate cancer cell lines
Database searches revealed that IIR mRNA (also known as EIG121/KIAA1324) is highly expressed in human prostate (Fig. S1a). To confirm the protein expression and to identify the cell type and tissue distribution of inceptor, we used our previously generated specific monoclonal antibodies against inceptor [33] and performed immunohistochemistry on adult mouse prostate sections (Figure 1a) and on sections of human prostate biopsies (Fig. 1b). We observed inceptor in all luminal cells of the murine and human prostate epithelium, which are marked by presence of Cytokeratin 8/18 and absence of Cytokeratin 5. Moreover, the prostate epithelium contains a subset of cells that expresses both Cytokeratin 5 and Cytokeratin 8/18, usually referred to as intermediate cells [46]. We found that intermediate cells expressed inceptor, while Cytokeratin 8/18 negative basal cells did not (Figure 1a,b). There was no or only very low inceptor expression in neuroendocrine cells (Fig. 1c), which regulate growth and differentiation of the prostate epithelium by secreting peptide hormones, such as serotonin (5-HT), calcitonin and somatostatin [47].
Figure 1.
Inceptor is expressed in healthy murine prostate epithelium and human prostate cancer cell lines. a. Immunohistochemical staining of WT mouse prostate with antibodies against inceptor, Cytokeratin 8/18 marking luminal cells and Cytokeratin 5 marking basal cells. Image shows a section of dorsal prostate, representative of 14 slices from two mice, taken from the dorsal, ventral or anterior prostate. Scale bar: 100 μm (upper row), 20 μm (lower row, zoomed in). b. Immunohistochemical staining of human prostate with antibodies against inceptor (cyan), Cytokeratin 8/18 (magenta), and Cytokeratin 5 (red). Scale bar: 50 μm. c. WT mouse prostate stained for inceptor, Cytokeratin 8/18 and Serotonin (5-HT) expressed in neuroendocrine cells. Image shows a section of dorsal prostate, representative of 8 slices from teo mice, taken from the dorsal, ventral or anterior prostate. Scale bar: 100 μm (upper row), 20 μm (lower row, zoomed in). d,e. Expression levels of inceptor in different human prostate cancer cell lines, determined by qPCR and Western blot. Densitometric quantification of the Western blot is shown on the right, values are normalized on γ-tubulin as loading control (n = 3).
To analyze the expression of IIR in various androgen-dependent and independent cell lines, we performed qPCR and Western blot analyses. In qPCR, we detected IIR mRNA expression in the primary HPEC line, as well as in the androgen-dependent prostate cancer cell line LNCaP and the androgen-independent prostate cancer cell line PC3 (Fig. 1d). In Western blot we additionally analyzed the immortalized cell line BPH-1, which is a model for benign prostate hyperplasia, as well as the androgen-independent LNCaP derivative LNCaP C4-2 (Fig. 1e). For further experiments we chose LNCaP cells, which showed reliable IIR mRNA and protein expression.
3.2. Correlation of inceptor expression with carcinogenic markers in human prostate samples
To evaluate the relevance of inceptor in human prostate cancer, we searched online databases for the publicly available TCGA (The Cancer Genome Atlas) dataset. We found that, compared to healthy tissues, inceptor was downregulated in pancreatic adenocarcinoma, but upregulated in ovarian, uterine, breast and prostate carcinoma (Fig. S1b). In prostate cancer, gene expression levels of IIR correlated with AR and AR-associated genes such as PSA (prostate-specific antigen, also known as KLK3) and PSMA (also known as FOLH1) (Figs. S1c–e). Further, IIR correlated with IGF1R and IR (INSR) (Figs. S1f and g). As tumor progression often goes along with increasing mutation status, we evaluated the frequency of different types of mutations in progressively more dedifferentiated tumor stages. We found that in all stages, mutations of IIR frequently co-occurred with mutations of EGFR, IR, IGF1R, IGF2R, PI3K subunits C2a and C2b, AR, PSA and PSMA (Table S1). The mutation rate of IIR was low in prostate adenocarcinoma; however, the frequency of gene amplifications was notably enriched in castration-resistant prostate cancer and neuroendocrine carcinoma (Fig. S2a). For comparison, AR was also frequently amplified or mutated in castration-resistant prostate cancer and neuroendocrine carcinoma (Fig. S2f), presumably to facilitate androgen-dependent proliferation despite low androgen levels. A similar pattern could be observed for IGF1R, IR, EGFR and PI3K (Figs. S2b–e). Of note, deletion of IIR was very rare in all prostate cancer stages (Fig. S2a). In addition, we analyzed 121 human prostate tissue samples consisting of 71 benign and 50 cancer samples (Table S2). The IIR mRNA expression showed a significant positive correlation with the tumor content of the respective specimen (Figure 2a). Further, we plotted the IIR expression against the expression of AR and the AR-associated genes PSA and PSMA (Figure 2b–d). All three genes showed a strong positive correlation (p < 0.0001) with IIR. Moreover, there was a positive correlation between IIR and MDM2 (Fig. 2e). The expression of IIR also showed a positive correlation with IRa/IRb ratio and a negative correlation with IRb/IGF1R ratio (Figure 2f,g).
3.3. Inceptor and androgen signaling of prostate cancer cells
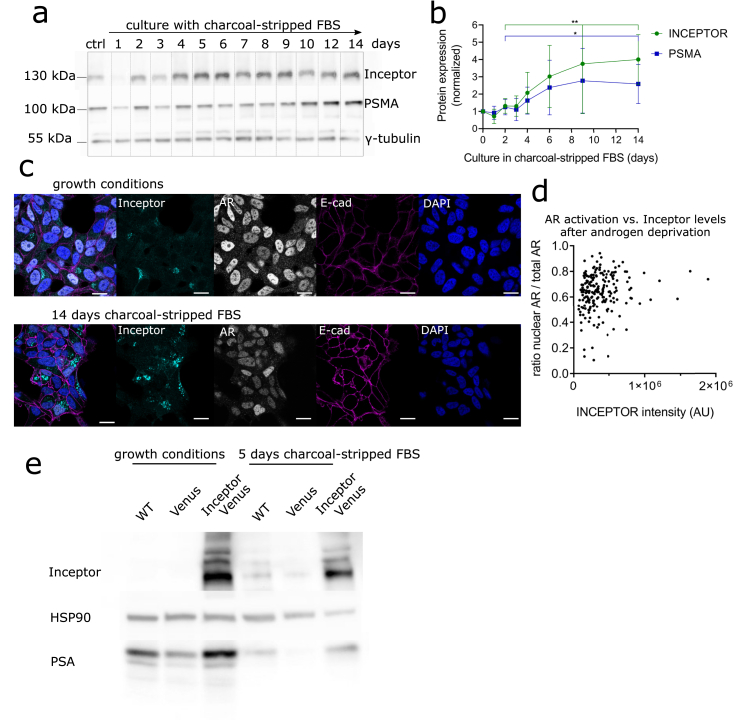
As previously reported, androgen deprivation leads to differentiation of LNCaP cells towards a more invasive, neuroendocrine cancer-like cell type [48]. To model prostate cancer progression in vitro, we cultured LNCaP cells in androgen-free medium. During deprivation, the expression levels of inceptor and PSMA increased gradually (Figure 3a,b). The increase in inceptor levels after androgen deprivation was also reflected in immunocytochemistry (Fig. 3c).
Figure 3.
Inceptor expression is dependent on the hormone status of prostate cancer cells. a. Expression of inceptor, PSMA and AR after up to 14 days of culture in RPMI 1640 + 10% charcoal-stripped FBS (androgen deprivation conditions). The control was cultured in the same medium for 14 days, with the addition of 10 nM dihydrotestosterone. b. Densitometric quantification of a, normalized to γ-tubulin as loading control (selected time points from n = 5 independent experiments). Values show fold change compared to control. P = 0.0049 for inceptor and p = 0.0441 for PSMA, determined by unpaired student's t test. c. LNCaP cells in normal growth medium (RPMI 1640 + 10% FBS) or RPMI 1640 + 10% charcoal-stripped FBS were stained for inceptor, AR and E-cadherin. Laser and detector settings were kept constant between both images (scale bar: 20 μm). d. Quantification of fluorescence intensity of nuclear AR vs. total AR, shown in dependence of inceptor intensity (Figure 3 c, left). Single cells from two independent experiments were analyzed, after 14 days in charcoal-stripped FBS (>100 cells/n), p = 0.038 (linear regression). e. LNCaP WT, LNCaP venus or LNCaP inceptor-venus cells were cultured in normal growth medium (RPMI 1640 + 10% FBS) or RPMI 1640 + 10% charcoal-stripped FBS for 5 days.
The expression of inceptor and AR, as well as the nuclear localization of AR, seemed to be heterogeneous among single cells (Fig. 3c). To investigate this heterogeneity, we measured the ratio of nuclear AR to total AR and inceptor fluorescence intensity in single cells (Fig. 3d). There was a correlation between inceptor intensity and AR nuclear localization after androgen depletion (p = 0.038). This suggests that inceptor might be associated with nuclear translocation and thus activation of AR. Therefore, we analyzed the levels of PSA, which is a direct AR target, in LNCaP cells overexpressing the fusion protein inceptor-venus compared to venus overexpressing cells and wildtype (WT) cells as controls (Fig. 3e). In normal growth conditions, as well as after 5 days of androgen deprivation, there was more PSA expression in inceptor-venus overexpressing cells.
3.4. Inceptor trafficking
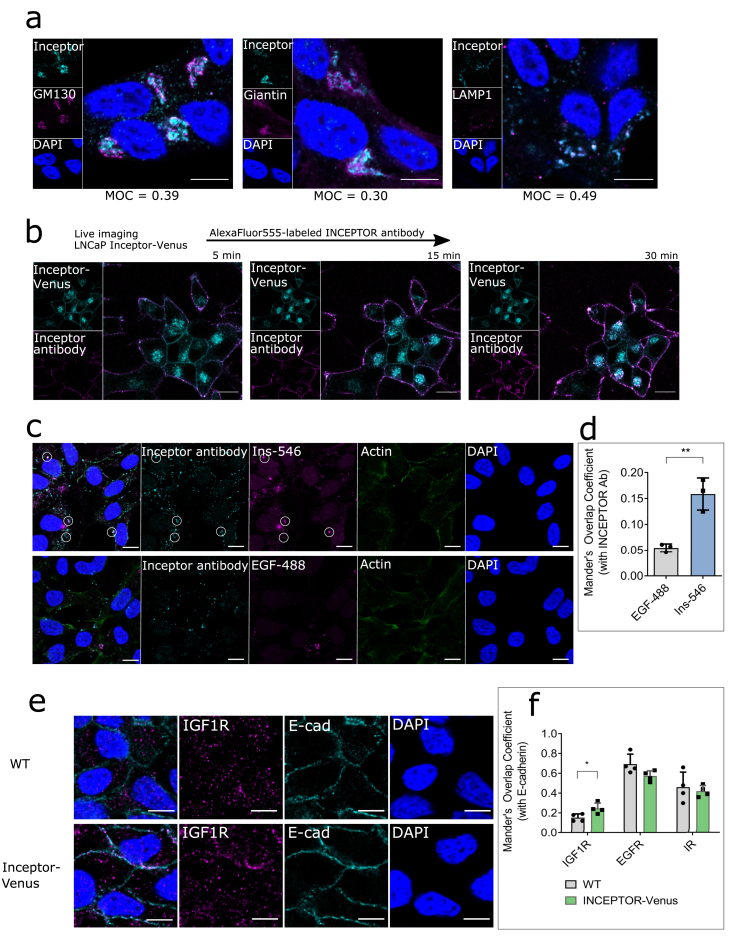
To investigate the trafficking of inceptor in prostate cancer cells, we performed co-stainings of inceptor with the Golgi markers GM130 and giantin and the lysosomal protein LAMP1 in LNCaP cells. All markers revealed a high degree of overlap with inceptor, with LAMP1 colocalization being slightly higher compared to GM130 and giantin (Figure 4a). Thus, we found the main fraction of inceptor in the endosomal-lysosomal compartment. This is consistent with bioinformatic predictions (Supplementary Table S3), particularly with the assumed mannose-6-phosphate receptor domain of inceptor (Fig. S3), as this domain is responsible for lysosomal trafficking. To track the internalization of inceptor, we used LNCaP cells overexpressing inceptor-venus. We have previously shown that inceptor-venus shows the same localization and trafficking dynamics as endogenous inceptor and is therefore a valid tool to study the function of inceptor [33]. This cell line was imaged after pulse-labeling with an AlexaFluor555-conjugated inceptor antibody (Fig. 4b). After 5 min, the antibody was visible on the plasma membrane, before it was gradually endocytosed and showed high colocalization with inceptor-venus after 30 min. These results show that inceptor is cycled between the plasma membrane, lysosomes and Golgi complex in LNCaP cells.
Figure 4.
Inceptor is involved in the trafficking of IR/IGF1R. a. Staining of LNCaP cells for inceptor and GM130 (cis-Golgi), Giantin (medial Golgi) or LAMP1 (Lysosome) to determine the subcellular localization of inceptor. The Mander's overlap coefficient was calculated from >80 cells/n (n = 3). Scale bar: 10 μm. b. LNCaP cells expressing inceptor-venus were pulse-labeled with an AlexaFluor555-conjugated inceptor antibody. Internalization of the antibody was tracked in live imaging. Images are representative for two independent experiments (>60 cells/n). Scale bar: 10 μm. c. Endocytosis assay in LNCaP C4-2 cells incubated with 10 μg/ml inceptor antibody (rat) and 100 nM AlexaFluor546-labeled insulin or 1 μg/ml AlexaFluor488-labeled EGF for 60 min, before fixation and staining with an AlexaFluor488 or 555-labeled secondary anti-rat antibody. Scale bar: 10 μm. d. Quantification of c. The Mander's Overlap Coefficient was calculated from >50 cells/n, n = 3. P = 0.0049 (unpaired student's t test). e. Staining of WT and inceptor overexpressing LNCaP cells using an IGF1R or EGFR antibody, along with an E-cadherin antibody. Scale bar: 10 μm. f. Quantification of e (including IR and EGFR, not shown in d). The Mander's Overlap Coefficient was calculated from >40 cells/n, n = 4 (p = 0.0403, unpaired student's t test).
To test if inceptor is involved in the trafficking of receptor tyrosine kinases in prostate cancer cells, we performed an endocytosis assay with labeled insulin and EGF (Figure 4c,d). We observed an overlap between the inceptor antibody and insulin, indicating that both proteins are internalized via the same endocytosis pathway. In contrast, we did not see overlap between inceptor and EGF.
To further analyze the co-trafficking of inceptor with receptor tyrosine kinases, we performed co-stainings of IR, IGF1R and EGFR with the membrane marker E-cadherin (Figure 4e,f). We found that slightly but significantly more IGF1R was localized at the plasma membrane in inceptor overexpressing cells compared to WT. There was no significant change in EGFR or IR localization (quantification in Figure 4f, images not shown).
3.5. Inceptor and insulin/IGF1 signaling
Since receptor trafficking is crucial for the initiation and termination of signaling cascades, we investigated the connection of inceptor with IR and IGF1R activation in prostate cancer cells.
To confirm IGF1R and IR as interaction partners of inceptor, we performed co-IP in LNCaP cells (Figure 5a). We successfully pulled down inceptor using an IGF1R and an IR antibody.
Figure 5.
Inceptor modulates Insulin/IGF1 signaling in prostate cancer cells. a. Co-IP using an IGF1R antibody or IR antibody or beads only (control) in LNCaP cells overexpressing inceptor-venus. To show total levels of each protein, 20 μg lysate were loaded (input), while for each IP 400 μg lysate were used. Image is representative for 4 indepenent experiments. b. Signaling assay in LNCaP cells overexpressing inceptor-venus, compared to venus only and WT cells. The cells were growth factor starved in serum-free RPMI 1640 for 4 h, before induction with 10 nM insulin, 10 nM IGF1 or 10 nM EGF for 30 min, and subsequent lysis for Western blot. c. Densitometric quantification of b (n = 4). Values were normalized to tubulin as loading control, the graph shows fold change compared to WT LNCaP. pIR/pIGF1R (+IGF1): p = 0.0017; IGF1R levels: p = 0.0636; EGFR levels: p = 0.0491 (unpaired student's t test).
Next, we analyzed the impact of inceptor on relevant signaling pathways in LNCaP cells overexpressing inceptor-venus or venus only as a control. The signaling pathways were analyzed by depletion of growth factors in the medium, followed by stimulation with insulin, IGF1 or EGF. In LNCaP cells overexpressing inceptor-venus, EGFR levels appeared to decrease slightly after androgen deprivation and after stimulation (Figure 5b,c). The phosphorylation of EGFR upon EGF induction was not changed. Upon stimulation with IGF1, the phosphorylation of IR/IGF1R was substantially increased. This was not the case for stimulation with insulin, at a concentration of 10 nM, where insulin almost exclusively activates IR [27].
3.6. Migration of LNCaP cells upon inceptor overexpression
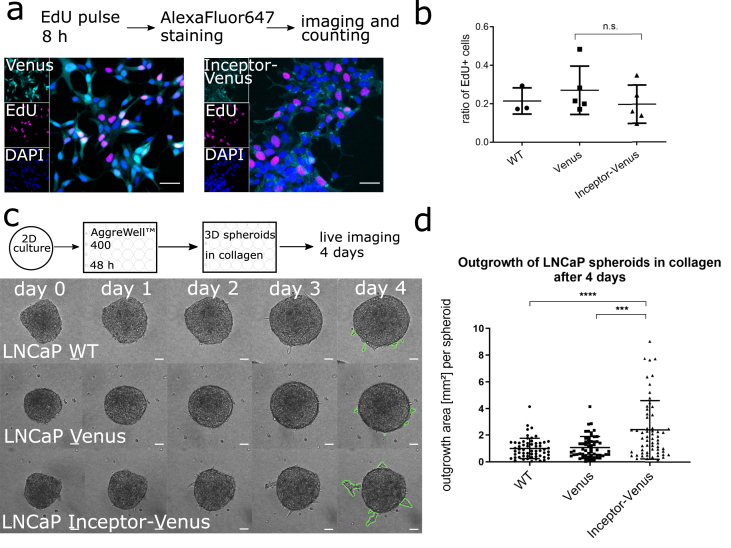
Since inceptor seems to be upregulated in more advanced prostate tumors, we investigated the functional effect of inceptor overexpression in prostate cancer cells. The proliferation rate of the inceptor-venus overexpressing cell line was assessed by incubation with EdU in normal growth medium. There was no significant difference compared to WT and Venus control (Figure 6a,b).
Figure 6.
Inceptor overexpression induces migration in prostate cancer cells. a. Proliferation assay of stable LNCaP cell lines overexpressing inceptor-venus or Venus only. b. Quantification of a, by image segmentation of EdU vs. DAPI. >5000 cells/n were counted; n = 5 for Venus and inceptor-venus overexpressing cells, n = 3 for WT cells. P = 0.3381, determined by unpaired student's t test. c. Migration of LNCaP spheroids in a collagen matrix (experimental scheme shown above). Scale bar: 50 μm. d. Quantification of c by measuring the outgrowth area (as indicated in green color in c) of 60 spheroids for each cell line from three independent experiments. P < 0.0001 as determined by unpaired student's t test; two outliers were removed, based on Grubb's outlier test.
To investigate the migration of inceptor-venus overexpressing cells, we performed a spheroid migration assay, adapted from previously published studies [49,50] (Fig. 6c). To quantify the effect, we measured the area of invading cells leaving the compact spheroid structure (marked in green color in Fig. 6c). The migration rate of the inceptor-venus overexpressing cells was significantly higher compared to WT and Venus control (Fig. 6d).
4. Discussion
According to publicly available databases, inceptor is downregulated in pancreatic adenocarcinoma compared to healthy tissue, but upregulated in ovarian, uterine, breast, and prostate carcinoma, which is in line with previous reports [[34], [35], [36]].
Both databases and our evaluation of human samples revealed that inceptor is expressed in benign and malignant prostate epithelium, with the expression in malignancies being significantly higher. The IIR gene is more frequently amplified in the more aggressive forms of castration-resistant prostate cancer and neuroendocrine carcinoma, compared to adenocarcinoma. The prostate is a secretory organ, containing mainly luminal secretory cells, basal cells and neuroendocrine cells. Inceptor is expressed in luminal and intermediate cells. This is of particular interest since luminal cells are the main origin of prostate cancer [51].
In human prostate cancer samples, the gene expression levels of IIR in this study correlated with the expression of AR, PSA and PSMA, which are well established hallmarks of disease progression [52,53]. MDM2, which also correlated with IIR expression, is an E3 ubiquitin protein ligase responsible for degradation of the tumor suppressor p53. Thus, it is considered a progression marker in many cancer types, including prostate cancer [54,55]. Notably, MDM2 has been shown to promote migration of cancer cells by ubiquitinating E-cadherin and thus tagging it for lysosomal degradation [56]. Taken together, these results show that inceptor correlates with carcinogenic markers.
Our analyses of human prostate cancer samples further showed positive correlation of inceptor with the IRa/IRb ratio and negative correlation with the IRb/IGF1R ratio. It was previously demonstrated that the ratio of insulin receptor isoforms IRa to IRb is significantly higher in prostate cancer compared to benign tissue [57]. In contrast to IRb, which only binds insulin with high affinity and is mainly responsible for mediating metabolic functions, IRa can activate proliferation via insulin, IGF1 or IGF2 binding [27]. Thus, higher IRa to IRb ratio and lower IRb/IGF1R ratios in tumors with high inceptor expression indicate an activation of mitogenic pathways rather than metabolic pathways, mediated via the insulin/IGF1 cascade.
We pulled down inceptor with IGF1R and IR, showing that inceptor physically interacts with receptor tyrosine kinases in prostate cancer cells. This is consistent with our previous results in β-cells [33]. The total levels of IGF1R were comparatively low, thus it seems that the pulldown of inceptor with IGF1R was efficient. In our endocytosis assay, insulin was internalized via the same routes as inceptor. It is important to note that insulin can be a ligand for IGF1R, especially at the high concentration used in the endocytosis experiment (100 nM) [27]. Thus, it is possible that the observed colocalization was due to co-trafficking with IGF1R rather than IR. Overexpression of inceptor led to increased IGF1R levels and more IGF1R on the membrane. Therefore, we hypothesize that inceptor has a role in the trafficking of IGF1R.
Analysis of the subcellular localization has shown that inceptor resides mainly in the Golgi complex and in the lysosome. This localization pattern is expected since inceptor contains a mannose-6-phosphate receptor domain. The cation-independent mannose-6-phosphate receptor, also known as IGF2R, is responsible for sequestering excess IGF2 from the plasma membrane and routing it to the lysosome for degradation, and for delivering lysosomal enzymes from the Golgi complex [58]. The high colocalization of inceptor with lysosomes raises the question of a potential function of inceptor in lysosomal degradation. It has previously been speculated that inceptor might be involved in EGFR degradation in breast cancer [59], although further studies are required to test this hypothesis. Taken together, our results show a correlation of inceptor levels and IGF1R signaling, which may be associated with altered IGF1R trafficking and/or degradation.
Proliferation of LNCaP cells was not affected by inceptor overexpression. This is counterintuitive, since it is known that insulin/IGF1 signaling can propagate mitogenic effects in cancer [1]. However, our data showed that the migration of LNCaP cells was increased upon inceptor overexpression. Importantly, IGF1R levels but not IR levels were elevated upon inceptor overexpression. Accordingly, IGF1 stimulation induced higher signaling activation when inceptor was overexpressed, while this effect could not be observed upon stimulation with insulin. However, we have observed increased migration in a 3D spheroid model upon inceptor overexpression. The main advantage of spheroid assays compared to conventional trans-well migration or wound-healing assays is that spheroid invasion in a 3D collagen matrix closely mimics invasion in vivo [60]. Thus, this indicates a potential involvement of inceptor in cancer cell migration. It has been shown that migration of cancer cells can be induced via IGF1 [[61], [62], [63]]. The proliferation assay was carried out in normal growth medium, in presence of androgens. As androgen signaling is the main driver for proliferation in LNCaP cells and it is known that IGF1R and IR are essential factors of androgen-independent survival mechanisms [12], the growth-promoting effect of inceptor might be masked in these conditions.
In our previous study analyzing the role of inceptor in pancreatic β-cells, we have shown that inceptor counteracts insulin signaling [33]. Based on this finding, decreased aggressiveness of cancers with high inceptor levels would be expected, which is in contrast with the results obtained in the present study. We hypothesize that inceptor has diverse effects on signaling pathways, depending on the cellular context. This hypothesis would explain the seemingly contradictory literature studies analyzing the effects of high inceptor levels in different cancers. These studies indicated favorable prognosis in pancreatic neuroendocrine tumors and gastric cancer [35,36] but poor prognosis in endometrial cancer and ovarian cancer [37,38].
We found that inceptor was upregulated upon androgen deprivation of LNCaP cells. Androgen deprivation in vitro induces differentiation towards a more aggressive cell type [48]. Cells that expressed high levels of inceptor showed more nuclear AR localization. The dynamic inceptor expression during androgen deprivation mirrored PSMA expression. Further, the PSMA gene was highly correlated to IIR in human prostate cancer. PSMA is an enzyme with peptidase and hydrolase activity whose function in prostate cancer is poorly understood. However, it is well established that PSMA is highly overexpressed in prostate cancer and increases with tumor grade [64]. It has been suggested that AR downregulates the PSMA enhancer PSME [65]. Accordingly, there are studies showing PSMA upregulation upon androgen deprivation therapy of prostate cancer patients [66,67]. Since inceptor showed similar expression dynamics as PSMA, it is possible that inceptor is downregulated by androgens in a comparable manner as PSMA. Alternatively, inceptor may be estrogen-regulated, as suggested in literature [34,38], considering that estrogen and androgen signaling are highly interconnected in prostate cancer [68]. Taken together, our results from human samples and cell culture experiments suggest that inceptor might be associated with the hormone status of prostate tumors.
In this study we demonstrate for the first time that inceptor is associated with prostate cancer, possibly connected to IGF1R signaling. Further studies are needed to evaluate the clinical significance of inceptor in prostate cancer.
Acknowledgements
Part of the results shown are based upon data generated by the TCGA Research Network: https://www.cancer.gov/tcga. We are grateful to Silvia Schirge (Helmholtz-Zentrum München) for animal breeding and support with mouse dissection, to Regina Feederle (Helmholtz-Zentrum München) for antibody generation, and to Oliver Plettenburg (Helmholtz-Zentrum München) for providing labeled insulin. Further, we want to thank Kerstin Diemer, Ines Kunze, Jessica Jaki and Lisa Appel (Helmholtz-Zentrum München) for lab organization and Alke Guirguis (University Hospital Tübingen) for technical assistance.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.molmet.2023.101706.
Conflict of interest
None declared.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- 1.Belfiore A., Malaguarnera R. Insulin receptor and cancer. Endocr Relat Cancer. 2011;18(4):R125–R147. doi: 10.1530/ERC-11-0074. [DOI] [PubMed] [Google Scholar]
- 2.Crawley D., Chamberlain F., Garmo H., Rudman S., Zethelius B., Holmberg L., et al. A systematic review of the literature exploring the interplay between prostate cancer and type two diabetes mellitus. Ecancermedicalscience. 2018;12:802. doi: 10.3332/ecancer.2018.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bensimon L., Yin H., Suissa S., Pollak M.N., Azoulay L. Type 2 diabetes and the risk of mortality among patients with prostate cancer. Cancer Causes Control. 2014;25(3):329–338. doi: 10.1007/s10552-013-0334-6. [DOI] [PubMed] [Google Scholar]
- 4.Cai H., Xu Z., Xu T., Yu B., Zou Q. Diabetes mellitus is associated with elevated risk of mortality amongst patients with prostate cancer: a meta-analysis of 11 cohort studies. Diabetes Metabol Res Rev. 2015;31(4):336–343. doi: 10.1002/dmrr.2582. [DOI] [PubMed] [Google Scholar]
- 5.Currie C.J., Poole C.D., Jenkins-Jones S., Gale E.A.M., Johnson J.A., Morgan C.L. Mortality after incident cancer in people with and without type 2 diabetes: impact of metformin on survival. Diabetes Care. 2012;35(2):299–304. doi: 10.2337/dc11-1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lutz S.Z., Todenhöfer T., Wagner R., Hennenlotter J., Ferchl J.M., Scharpf M.O, et al. Higher prevalence of lymph node metastasis in prostate cancer in patients with diabetes. Endocr Relat Cancer. 2018;25(3):L19–L22. doi: 10.1530/ERC-17-0465. [DOI] [PubMed] [Google Scholar]
- 7.Franko A., Berti L., Hennenlotter J., Rausch S., Scharpf M.O., Hrabe de Angelis M., et al. Transcript levels of aldo-keto reductase family 1 subfamily C (AKR1C) are increased in prostate tissue of patients with type 2 diabetes. J Personalized Med. 2020;10(3):124. doi: 10.3390/jpm10030124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 9.Elia I., Schmieder R., Christen S., Fendt S. In: Metabolic control. Herzig S., editor. Springer International Publishing; Cham: 2016. Organ-specific cancer metabolism and its potential for therapy; pp. 321–353. [DOI] [PubMed] [Google Scholar]
- 10.Sharifi N., Gulley J.L., Dahut W.L. Androgen deprivation therapy for prostate cancer. JAMA. 2005;294(2):238–244. doi: 10.1001/jama.294.2.238. [DOI] [PubMed] [Google Scholar]
- 11.Centenera M.M., Selth L.A., Ebrahimie E., Butler L.M., Tilley W.D. New opportunities for targeting the androgen receptor in prostate cancer. Cold Spring Harbor perspectives in medicine. 2018;8(12):a030478. doi: 10.1101/cshperspect.a030478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feldman B.J., Feldman D. The development of androgen-independent prostate cancer. Nat Rev Cancer. 2001;1(1):34–45. doi: 10.1038/35094009. [DOI] [PubMed] [Google Scholar]
- 13.Visakorpi T., Hyytinen E., Koivisto P., Tanner M., Keinänen R., Palmberg C., et al. In vivo amplification of the androgen receptor gene and progression of human prostate cancer. Nat Genet. 1995;9(4):401–406. doi: 10.1038/ng0495-401. [DOI] [PubMed] [Google Scholar]
- 14.Taplin M.-E., Bubley G.J., Ko Y.J., Small E.J., Upton M., Rajeshkumar B., et al. Selection for androgen receptor mutations in prostate cancers treated with androgen antagonist. Cancer Res. 1999;59(11):2511–2515. [PubMed] [Google Scholar]
- 15.Gioeli D., Mandell J.W., Petroni G.R., Frierson H.F., Weber M.J. Activation of mitogen-activated protein kinase associated with prostate cancer progression. Cancer Res. 1999;59(2):279–284. [PubMed] [Google Scholar]
- 16.Culig Z., Hobisch A., Cronauer M.V., Radmayr C., Trapman A., Hittmair A., et al. Androgen receptor activation in prostatic tumor cell lines by insulin-like growth factor-I, keratinocyte growth factor, and epidermal growth factor. Cancer Res. 1994;54(20):5474–5478. [PubMed] [Google Scholar]
- 17.Nickerson T., Chang F., Lrimer D., Smeekens S.P., Sawyers C.L., Pollak M. In vivo progression of LAPC-9 and LNCaP prostate cancer models to androgen independence is associated with increased expression of insulin-like growth factor I (IGF-I) and IGF-I receptor (IGF-IR) Cancer Res. 2001;61(16):6276–6280. [PubMed] [Google Scholar]
- 18.Krueckl S.L., Sikes R.A., Edlund N.M., Bell R.H., Hurtado-Coll A., Fazli L., et al. Increased insulin-like growth factor I receptor expression and signaling are components of androgen-independent progression in a lineage-derived prostate cancer progression model. Cancer Res. 2004;64(23):8620–8629. doi: 10.1158/0008-5472.CAN-04-2446. [DOI] [PubMed] [Google Scholar]
- 19.Fan W., Yanase T., Morinaga H., Okabe T., Nomura M., Daitoku H., et al. Insulin-like growth factor 1/insulin signaling activates androgen signaling through direct interactions of Foxo1 with androgen receptor. J Biol Chem. 2007;282(10):7329–7338. doi: 10.1074/jbc.M610447200. [DOI] [PubMed] [Google Scholar]
- 20.Hopkins B.D., Goncalves M.D., Cantley L.C. Insulin–PI3K signalling: an evolutionarily insulated metabolic driver of cancer. Nat Rev Endocrinol. 2020;16(5):276–283. doi: 10.1038/s41574-020-0329-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao L., Vogt P.K. Class I PI3K in oncogenic cellular transformation. Oncogene. 2008;27(41):5486–5496. doi: 10.1038/onc.2008.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carver B.S., Chapinski C., Wongvipat J., Hieronymus H., Chen Y., Chandarlapaty S., et al. Reciprocal feedback regulation of PI3K and androgen receptor signaling in PTEN-deficient prostate cancer. Cancer Cell. 2011;19(5):575–586. doi: 10.1016/j.ccr.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carracedo A., Ma L., Teruya-Feldstein J., Rojo F., Salmena L., Alimonti A., et al. Inhibition of mTORC1 leads to MAPK pathway activation through a PI3K-dependent feedback loop in human cancer. J Clin Investig. 2008;118(9):3065–3074. doi: 10.1172/JCI34739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O’Reilly K.E., Rojo F., She Q.-B., Solit D., Mills G.B., Smith D., et al. mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer Res. 2006;66(3):1500–1508. doi: 10.1158/0008-5472.CAN-05-2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen H.X., Sharon E. IGF-1R as an anti-cancer target--trials and tribulations. Chin J Cancer. 2013;32(5):242–252. doi: 10.5732/cjc.012.10263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Niu X.-B., Fu G.-B., Wang L., Ge X., Liu W.-T., Wen Y.-Y., et al. Insulin-like growth factor-I induces chemoresistence to docetaxel by inhibiting miR-143 in human prostate cancer. Oncotarget. 2017;8(63):107157–107166. doi: 10.18632/oncotarget.22362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Belfiore A., Frasca F., Pandini G., Sciacca L., Vigneri R. Insulin receptor isoforms and insulin receptor/insulin-like growth factor receptor hybrids in physiology and disease. Endocr Rev. 2009;30(6):586–623. doi: 10.1210/er.2008-0047. [DOI] [PubMed] [Google Scholar]
- 28.Weinstein D., Sarfstein R., Laron Z., Werner H. Insulin receptor compensates for IGF1R inhibition and directly induces mitogenic activity in prostate cancer cells. Nature. 2014;3(1):24. doi: 10.1530/EC-13-0086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hopkins B.D., Pauli C., Du X., Wang D.G., Li X., Wu D., et al. Suppression of insulin feedback enhances the efficacy of PI3K inhibitors. Nature. 2018;560(7719):499–503. doi: 10.1038/s41586-018-0343-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lutz S.Z., Hennenlotter J., Scharpf M.O., Sailer C., Fritsche L., Schmid V., et al. Androgen receptor overexpression in prostate cancer in type 2 diabetes. Mol Metabol. 2018;8:158–166. doi: 10.1016/j.molmet.2017.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vikram A., Jena G. Diet-induced hyperinsulinemia accelerates growth of androgen-independent PC-3 cells in vitro. Nutr Cancer. 2012;64(1):121–127. doi: 10.1080/01635581.2012.630556. [DOI] [PubMed] [Google Scholar]
- 32.Venkateswaran V., Haddad A.Q., Fleshner N.E., Fan R., Sugar L.M., Nam R., et al. Association of diet-induced hyperinsulinemia with accelerated growth of prostate cancer (LNCaP) xenografts. J Natl Cancer Inst. 2007;99(23):1793–1800. doi: 10.1093/jnci/djm231. [DOI] [PubMed] [Google Scholar]
- 33.Ansarullah Jain C., Fathi Far F., Homberg S., Wißmiller K., Gräfin von Hahn F., et al. Inceptor counteracts insulin signalling in β-cells to control glycaemia. Nature. 2021;590(7845):326–331. doi: 10.1038/s41586-021-03225-8. [DOI] [PubMed] [Google Scholar]
- 34.Deng L., Broaddus R.R., McCampbell A., Shipley G.L., Loose D.S., Stancel G.M., et al. Identification of a novel estrogen-regulated gene, EIG121, induced by hormone replacement therapy and differentially expressed in type I and type II endometrial cancer. Clin Cancer Res. 2005;11(23):8258–8264. doi: 10.1158/1078-0432.CCR-05-1189. [DOI] [PubMed] [Google Scholar]
- 35.Kang J.M., Park S., Kim S.J., Kim H., Lee B., Kim J., et al. KIAA1324 suppresses gastric cancer progression by inhibiting the oncoprotein GRP78. Cancer Res. 2015;75(15):3087–3097. doi: 10.1158/0008-5472.CAN-14-3751. [DOI] [PubMed] [Google Scholar]
- 36.Estrella J.S., Ma L.T., Milton D.R., Yao J.C., Wang H., Rashid A., et al. Expression of estrogen-induced genes and estrogen receptor beta in pancreatic neuroendocrine tumors: implications for targeted therapy. Pancreas. 2014;43(7):996–1002. doi: 10.1097/MPA.0000000000000203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ran X., Zhou P., Zhang K. Autophagy plays an important role in stemness mediation and the novel dual function of EIG121 in both autophagy and stemness regulation of endometrial carcinoma JEC cells. Int J Oncol. 2017;51(2):644–656. doi: 10.3892/ijo.2017.4047. [DOI] [PubMed] [Google Scholar]
- 38.Schlumbrecht M.P., Xie S.-S., Shipley G.L., Urbauer D.L., Broaddus R.R. Molecular clustering based on ERα and EIG121 predicts survival in high-grade serous carcinoma of the ovary/peritoneum. Mod Pathol. 2011;24(3):453–462. doi: 10.1038/modpathol.2010.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Uhlen M., Zhang C., Lee S., Sjöstedt E., Fagerberg L., Bidkhori G. A pathology atlas of the human cancer transcriptome. Science. 2017;357(6352):eaan2507. doi: 10.1126/science.aan2507. [DOI] [PubMed] [Google Scholar]
- 40.Franko A., Berti L., Guirguis A., Hennenlotter J., Wagner R., Scharpf M.O., et al. Characterization of hormone-dependent pathways in six human prostate cancer cell lines: a gene expression study. Genes. 2020;11(10):1174. doi: 10.3390/genes11101174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Franko A., Shao Y., Heni M., Hennenlotter J., Hoene M., Hu C. Human prostate cancer is characterized by an increase in urea cycle metabolites. Cancers. 2020;12(7):1814. doi: 10.3390/cancers12071814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cunningham F., Achuthan P., Akanni W., Allen J., Amode M.R., Armean I.M., et al. Ensembl 2019. Nucleic Acids Res. 2019;47(D1):D745–D751. doi: 10.1093/nar/gky1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tang Z., Li C., Kang B., Gao G., Li C., Zhang Z. GEPIA: a web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res. 2017;45(W1):W98–W102. doi: 10.1093/nar/gkx247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Beltran H., Prandi D., Mosquera J.M., Benelli M., Puca L., Carta J., et al. Divergent clonal evolution of castration-resistant neuroendocrine prostate cancer. Nat Med. 2016;22(3):298–305. doi: 10.1038/nm.4045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cerami E., Gao J., Dogrusoz U., Gross B.E., Sumer S.O., Aksoy B.A., et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2(5):401–404. doi: 10.1158/2159-8290.CD-12-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wegner K.A., Cadena M.T., Trevena R., Turco A.E., Gottschalk A., Halberg R.B., et al. An immunohistochemical identification key for cell types in adult mouse prostatic and urethral tissue sections. PLoS One. 2017;12(11) doi: 10.1371/journal.pone.0188413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Anthony Di Sant'Agnese P. Neuroendocrine differentiation in human prostatic carcinoma. Hum Pathol. 1992;23(3):287–296. doi: 10.1016/0046-8177(92)90110-o. [DOI] [PubMed] [Google Scholar]
- 48.Yuan T.-C., Veeramani S., Lin F.-F., Kondrikou D., Zelivianski S., Igawa T., et al. Androgen deprivation induces human prostate epithelial neuroendocrine differentiation of androgen-sensitive LNCaP cells. Endocrine-Related Cancer Endocr Relat Cancer. 2006;13(1):151–167. doi: 10.1677/erc.1.01043. [DOI] [PubMed] [Google Scholar]
- 49.Crosas-Molist E., Bertran E., Rodriguez-Hernandez I., Herraiz C., Cantelli G., Fabra A., et al. The NADPH oxidase NOX4 represses epithelial to amoeboid transition and efficient tumour dissemination. Oncogene. 2017;36(21):3002–3014. doi: 10.1038/onc.2016.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Valcarcel-Jimenez L., Macchia A., Crosas-Molist E., Schaub-Clerigue A., Camacho L., Martin-Martin N., et al. PGC1α suppresses prostate cancer cell invasion through ERRα transcriptional control. Cancer Res. 2019;79(24):6153–6165. doi: 10.1158/0008-5472.CAN-19-1231. [DOI] [PubMed] [Google Scholar]
- 51.Wang Z.A., Toivanen R., Bergren S.K., Chambon P., Shen M.M. Luminal cells are favored as the cell of origin for prostate cancer. Cell Rep. 2014;8(5):1339–1346. doi: 10.1016/j.celrep.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Edwards J., Krishna N.S., Grigor K.M., Bartlett J.M.S. Androgen receptor gene amplification and protein expression in hormone refractory prostate cancer. Br J Cancer. 2003;89(3):552–556. doi: 10.1038/sj.bjc.6601127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Perner S., Hofer M.D., Kim R., Shah R.B., Li H., Möller P., et al. Prostate-specific membrane antigen expression as a predictor of prostate cancer progression. Hum Pathol. 2007;38(5):696–701. doi: 10.1016/j.humpath.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 54.Leite K.R.M., Franco M.F., Srougi M., Nesrallah L.G., Bevilacqua R.G., Darini E., et al. Abnormal expression of MDM2 in prostate carcinoma. Mod Pathol. 2001;14(5):428–436. doi: 10.1038/modpathol.3880330. [DOI] [PubMed] [Google Scholar]
- 55.Wade M., Li Y.-C., Wahl G.M. MDM2, MDMX and p53 in oncogenesis and cancer therapy. Nat Rev Cancer. 2013;13(2):83–96. doi: 10.1038/nrc3430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yang J.-Y., Zong C.S., Xia W., Wei Y., Ali-Seyed M., Li Z., et al. MDM2 promotes cell motility and invasiveness by regulating E-cadherin degradation. Mol Cell Biol. 2006;26(19):7269. doi: 10.1128/MCB.00172-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Heni M., Hennenlotter J., Scharpf M., Lutz S.Z., Schwentner C., Todenhöfer T., et al. Insulin receptor isoforms A and B as well as insulin receptor substrates-1 and -2 are differentially expressed in prostate cancer. PLoS One. 2012;7(12) doi: 10.1371/journal.pone.0050953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ghosh P., Dahms N.M., Kornfeld S. Mannose 6-phosphate receptors: new twists in the tale. Nat Rev Mol Cell Biol. 2003;4(3):202–213. doi: 10.1038/nrm1050. [DOI] [PubMed] [Google Scholar]
- 59.Meseure D., Alsibai K.D., Vacher S., Hatem R., Nicolas A., Callens C., et al. Altered expression of three EGFR posttranslational regulators MDGI, MIG6, and EIG121 in invasive breast carcinomas. Anal Cell Pathol. 2020;2020:9268236. doi: 10.1155/2020/9268236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kramer N., Walzl A., Unger C., Rosner M., Krupitza G., Hengstschläger M., et al. In vitro cell migration and invasion assays. Mutat Res, Rev Mutat Res. 2013;752(1):10–24. doi: 10.1016/j.mrrev.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 61.Hellawell G.O., Turner G.D.H., Davies D.R., Poulsom R., Brewster S.F., Macaulay V.M. Expression of the type 1 insulin-like growth factor receptor is up-regulated in primary prostate cancer and commonly persists in metastatic disease. Cancer Res. 2002;62(10):2942–2950. [PubMed] [Google Scholar]
- 62.Pandini G., Mineo R., Frasca F., Roberts C.T., Marcelli M., Vigneri R., et al. Androgens up-regulate the insulin-like growth factor-I receptor in prostate cancer cells. Cancer Res. 2005;65(5):1849–1857. doi: 10.1158/0008-5472.CAN-04-1837. [DOI] [PubMed] [Google Scholar]
- 63.Burfeind P., Chernicky C.L., Rininsland F., Ilan J., Ilan J. Antisense RNA to the type I insulin-like growth factor receptor suppresses tumor growth and prevents invasion by rat prostate cancer cells in vivo. Proc Natl Acad Sci USA. 1996;93(14):7263–7268. doi: 10.1073/pnas.93.14.7263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Eder M., Eisenhut M., Babich J., Haberkorn U. PSMA as a target for radiolabelled small molecules. Eur J Nucl Med Mol Imag. 2013;40(6):819–823. doi: 10.1007/s00259-013-2374-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ghosh A., Heston W.D.W. Tumor target prostate specific membrane antigen (PSMA) and its regulation in prostate cancer. J Cell Biochem. 2004;91(3):528–539. doi: 10.1002/jcb.10661. [DOI] [PubMed] [Google Scholar]
- 66.Meller B., Bremmer F., Sahlmann C.O., Hijazi S., Bouter C., Trojan L. Alterations in androgen deprivation enhanced prostate-specific membrane antigen (PSMA) expression in prostate cancer cells as a target for diagnostics and therapy. EJNMMI Res. 2015;5(1):66. doi: 10.1186/s13550-015-0145-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Murga J.D., Moorji S.M., Han A.Q., Magargal W.W., DiPippo V.A., Olson W.C. Synergistic co-targeting of prostate-specific membrane antigen and androgen receptor in prostate cancer. Prostate. 2015;75(3):242–254. doi: 10.1002/pros.22910. [DOI] [PubMed] [Google Scholar]
- 68.Carruba G. Estrogen and prostate cancer: an eclipsed truth in an androgen-dominated scenario. J Cell Biochem. 2007;102(4):899–911. doi: 10.1002/jcb.21529. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.