Highlights
-
•
PrPc /APP imbalance affects cancer cell survival in a 3D tissue like environment.
-
•
APP gene silencing enhances cytoplasmic PrPcprotein accumulation.
-
•
Impaired APP expression induces HTRA2 and destabilizes lipid raft-associated protein partners (e.g. CD24).
-
•
HTRA2 up- or down-expression dysregulates PRNP.
-
•
HTRA2 is a new modulator for PrPcproteostasis in human cancer cells.
Keywords: PRNP, PrPc, APP, HTRA2, CD24, cancer cells, proteostasis, pEBVsiRNA, RNA interference, CRISPR-Cas9
Abstract
Cellular protein homeostasis (proteostasis) requires an accurate balance between protein biosynthesis, folding, and degradation, and its instability is causally related to human diseases and cancers. Here, we created numerous engineered cancer cell lines targeting APP (amyloid ß precursor protein) and/or PRNP (cellular prion) genes and we showed that APP knocking-down impaired PRNP mRNA level and vice versa, suggesting a link between their gene regulation. PRNPKD, APPKD and PRNPKD/APPKD HeLa cells encountered major difficulties to grow in a 3D tissue-like environment. Unexpectedly, we found a cytoplasmic accumulation of the PrPc protein without PRNP gene up regulation, in both APPKD and APPKO HeLa cells. Interestingly, APP and/or PRNP gene ablation enhanced the chaperone/serine protease HTRA2 gene expression, which is a protein processing quality factor involved in Alzheimer's disease. Importantly, HTRA2 gene silencing decreased PRNP mRNA level and lowered PrPc protein amounts, and conversely, HTRA2 overexpression increased PRNP gene regulation and enhanced membrane-anchored and cytoplasmic PrPc fractions. PrPc, APP and HTRA2 destabilized membrane-associated CD24 protein, suggesting changes in the lipid raft structure. Our data show for the first time that APP and the dual chaperone/serine protease HTRA2 protein could modulate PrPc proteostasis hampering cancer cell behavior.
Introduction
The prion protein (PRNP gene) and the amyloid ß precursor protein (APP gene) have been extensively studied in neural functions and pathologies. While the APP and the normal cellular PrP (PrPc) proteins are distributed all over the organism, independently of their roles in neurodegeneration, their relationships remain still elusive in cancer cells. APP has been shown to be upregulated in some cancers such as breast cancers by promoting cell proliferation and motility [1,2]. Besides, an overexpression of PrPc have been reported in numerous tumors, related to cancer cell migration, invasion and metastasis, and also associated with poor prognosis [3], [4], [5]. A putative role of APP and PrPc protein in cell mobility and cancer spreading could arise from their localization on lipid rafts (lipid raft-like microdomains) [6], which allows numerous interactions with components of the extracellular matrix [6,7]. As pivotal scaffold proteins, they also allow the transduction of intracellular signals. For this reason, we sought to bring insights into the implication of both endogenous PrPc and APP proteins on growth and progression of cancer cells.
Originally, the PrPc protein got considerable attention for its crucial involvement in neurodegenerative pathogenesis (e.g. Creutzfeldt-Jakob disease) since it became the center of the protein conformational hypothesis in neurodegeneration through its conversion from a normal alpha helix structure to an abnormal β–sheet enriched form, called the scrapie PrPSc form [8]. However, while this conversion occurs in brain, PrPc is ubiquitously expressed in all known mammals, where it is mostly located at the cell surface tightly tethered to the plasma membrane through a glycosyl-phosphatidylinositol (GPI) anchor [9]. Indeed, at the cell membrane, the localization of PrPc on lipid rafts allows its interaction with numerous neighbouring proteins including receptors, signaling molecules and components of the extracellular matrix, such as glycosaminoglycans (GAGs) [10,11]. The cellular functions of PrPc, while still debated, include cell adhesion and mobility, signaling, and differentiation [4,12]. Interestingly, some of these functions have also been conferred to APP, another ubiquitous protein expressed in the central nervous system and in peripheric organs and tissues. The APP protein is a transmembrane protein with a large extracellular ectodomain, an hydrophobic transmembrane domain and an intracellular cytoplasmic tail that contains the regulatory AICD domain (amyloid precursor protein intracellular domain) [13,14], reported to act as a transcriptional enhancer [15]. APP alternative splicing generate ten isoforms from which APP675, APP751 and APP770 are the most abundant ones. APP675 is predominantly expressed in neurons while APP751 and APP770 are found in non-neuronal cells. .APP is involved in the pathogenesis of Alzheimer disease (AD) through an abnormal accumulation of the amyloid-β peptide (Aβ) in several brain regions, including the hippocampus and cortex.
Synthesis and maturation of PrPc and APP seems to be fine-tuned and common components in their interactomes could participate to both processes. Several reports have brought evidences of functional links between PrPc and APP in AD [11,[16], [17], [18]]. For instance, PrPc is a key neuronal receptor for β-amyloid oligomers [19] in that the N-terminal PrPc fragment (amino acids 95 to 113) can trap oligomeric Aβ [20]. PrPc could also lowered the amyloidogenic processing of APP by inhibiting its β secretase cleavage, reducing Aβ formation [11]. Interestingly, during this process PrPc has to be localized in the cholesterol and glycosphingolipid-rich lipid rafts, where it interacts with BACE1 (β-site APP cleaving enzyme) via GAG side chains in the proteoglycans coreceptors. As APP, PrPc is proteolytically cleaved from the cell surface by the disintegrin and metalloprotease 10 (ADAM10), which reduces cellular binding and toxicity of amyloid-β oligomers [21]. Therefore, both physical and functional relationships between PrPc and APP can be proposed to contribute not only to the regulation of each of their own normal biological functions but also to the fine-tuned regulation of cell growth in a tissue environment. Because PrPc and APP (whole protein vs Aβ fragment) are aggregation-prone molecules, it is conceivable that their cytoplasmic accumulation could affect various stress events such as the endoplasmic reticulum unfolded protein response (UPRER) [22,23].
Cellular proteostasis requires an accurate balance between protein synthesis, folding, conformational maintenance, and degradation. During evolution, highly sophisticated pathways have been conserved to finely monitor the fate of unfolded or aggregated proteins through the proteasome or autophagy pathways, always in cooperation with an adaptive network of molecular chaperones that recognize misfolded proteins [24,25]. In addition to UPRER-associated quality control (QC) factors, mitochondria possess an array of endogenous chaperones and proteases that maintain mitochondrial protein homeostasis. Among the mitochondrial UPR (UPRmt) players, there is HTRA2, a member of the HTRA family (High-Temperature Requirement serine protease A). HTRA proteins are homo-oligomeric and ATP-independent serine proteases, highly conserved during evolution in different organisms, from bacteria to human [26,27]. In human, heat shock HTRA proteins encompass four paralogs (HTRA1 - 4) displaying serine protease and molecular chaperone activities [28]. They participate to regulation of apoptosis, autophagy, cell signaling, and aging and they are involved in neurodegenerative disorders and mitochondrial dysfunctions, as observed in the Cockayne's syndrome or Alzheimer's disease [27,[29], [30], [31], [32]]. After various stresses, such as heat shock, hypoxia, ischemia/reperfusion injury or heart failure, these proteins can switch their function from chaperones (for refolding proteins) to proteases (for hygrolizing proteins) to efficiently reduce the amount of damaged or unfolded proteins in order to eliminate or refold them, or to trigger apotosis [33], [34], [35].
HTRA2, previously named as OMI, is the member of the HTRA family which is located in the mitochondrial intermembrane space [36]. HTRA2 is released from the mitochondria to the cytoplasm during stress or apoptosis [26]. A strong positive association between HTRA2 and AD has been highlighted in that AD patient brain extracts, which displayed a higher cytosolic distribution of HTRA2 protein and a significant enhanced activity [30]. Furthermore, APP appeared to be directly cleaved by HTRA2 in mitochondria, and APP interacted with HTRA2 through its Aβ region [37]. A fraction of HTRA2 localizes in the cytosolic side of ER membranes tethered with the immature form of APP prompting its degradation [38]. Moreover, aberrant HTRA2 function have been shown to lead to neurodegeneration and parkinsonian phenotypes. Hence, HTRA2 appears to be a regulatory APP-interacting chaperone protease. To our knowledge, nothing is known about the relationship between HTRA2 and PrPc in cancer cells.
Here, based on evidence reported by observational studies with a battery of cancer cell lines and engineered cells, we described for the first time a link between APP, HTRA2 and PrPc. We show that silencing APP in HeLa cells results in enhanced HTRA2 protein expression and cytoplasmic PrPc accumulation and that HTRA2 up- and down-regulation affects PRNP gene expression. Hence, impaired APP and HTRA2 can disrupt the PrPc proteostasis, contributing to hamper cancer cell growth in a tissue-like environment, probably through change in the lipid rafts composition.
Results
Great diversity of PrPcprotein expression profiles
As APP and PrPc are both expressed in numerous cells and tissues, we sought to determine whether these two proteins could coordinately work to influence the complex regulation of tumor cell growth. To first investigate this possibility, we cultivated in parallel 33 human tumor cell lines from diverse tissue origins (Table S1). By using three different anti-PrPc antibodies (SAF32, SHA31 and BAR233), we showed that the protein was expressed in most cell lines, although at different levels and sub-cellular locations (Fig. 1a, S1a and S1d). It was noticeable that PrPc levels and localizations were dependent on the cell proliferation state (Figure S2). In epithelial cells, PrPc was mainly localized on the cytoplasmic membrane, in particular in colon carcinoma cells (e.g. HCT-116, HT-29, SW480, RKO) but also in HeLa cells. In other cell lines, PrPc staining was diffuse and localized into intense foci, closely to the nuclear compartment (e.g. MIA PaCa-2, H1299, SK-N-AS, and T98G cells). These dense fluorescent foci might correspond to the rough endoplasmic reticulum and the Golgi apparatus, where processed and misprocessed PrPc protein could accumulate [39]. We also observed significant variations in the location of the PrPc protein depending on tissue origin (e.g. neuroblastoma vs glioblastoma) (Fig. 1a and S1a). For instance, neuroblastoma L-A-N-1 and SH-SY5Y cells exhibited a nearly undetectable PrPc protein level. On the other side, APP protein was difficult to visualize at the cell membrane possibly because only 10% of nascent APP reaches the cytoplasmic membrane by immunocytochemical (ICC) staining [14]. Accordingly, APP was mostly observed inside the cytoplasmic compartment, likely in the trans-Golgi network, where APP is processed, as observed in T98G, MDA-MB-231 and MIA Paca-2 cells (Fig. 1d and S1e).
Fig. 1.
Quantitative and qualitative diversity for PrPc and APP protein content. Cells were plated (9.105 cells per 6 cm diameter dishes) and cultivated at the same time. Cells were recovered 2 days later for protein analyses. (a) ICC stainings were performed with a pool of 2 antibodies against PrPc, SAF32 (epitope amino acids 79–92) and SHA31 (aa 148–159), this allows detection of all putative PrPc fragments. Antibodies epitope location is depicted in Fig. S1c. Cells were counterstained with DAPI to visualize nuclei (scale bar 25 µm). (b) Western blot analysis of PrPc was performed with 5 µg of protein per slot or (c) 105 cell equivalents. Proteins were loaded and separated on a 12% SDS-polyacrylamide gel. Western blots b) and c) represented two independent culture experiments. Western blot were quantified in using ImageJ. Color code corresponds to the TP53 genotype of each cell lines: green = wild-type, blue = null, red = mutated, black = wild-type with viral protein or transcription-inactive p53β variant. (d) ICC staining against APP using the monoclonal 22C11 antibody and counterstained with DAPI (scale bar 25 µm). Relationship between endogenous PRNP and APP gene expressions in parental cell lines. (e) In parallel to protein analyses, mRNA was quantified by RT qPCR, and ordered in function of their relative quantification. Experiments were reproduced twice.
Western blot analyses confirmed the wide range of PrPc protein expression with both quantitative and qualitative differences, suggesting complex cell dependent PrPc production and post-translational maturation, as evidenced by three independent experiments (Fig. 1b, 1c and S1b). T98G, H1299, MDA-MB-231, MIA PaCa-2, and SK-MEL-37 cells were among cells that constitutively overexpressed PrPc.
Together, these results suggest that depending on the cell in which it is expressed, PrPc follows different cellular paths with different post-translational maturation and levels of protein expression.
High diversity and relationship between of PRNP and APP gene expressions
To confirm the variability of PrPc expression at the transcript level in the different cells and to assess a possible correlation with APP expression, PRNP and APP transcripts were analysed by means of RT qPCR in using RPLPO as an internal control. Figure S3a and b show the PRNP/RPLPO and APP/RPLPO ratios. We observed a wide range of endogenous PRNP gene expression unrelated to tissue of origin (Figure S3a), with elevated PRNP gene expressions in most mutated TP53 cell lines as compared to cells harboring a wild-type TP53 genotype. As expected, among a same tissue origin, different cell lines displayed a wide diversity of PRNP expression, as evidenced in glioblastoma cells (T98G 308%, Malme-3 M 158% and U87-MG 84%). It was noteworthy that neuroblastoma cells expressed nearly undetectable PRNP mRNA contents. A correlation between PRNP mRNA and PrPc protein contents was observed in most cases (Fig. 1 vs S3a). In parallel, we also detected a large range of APP gene expression levels albeit lower than that observed for PRNP (Figure S3b). Only lymphoma cells failed to express detectable APP mRNA. Interestingly, PRNP and APP genes expression was not restricted to brain-derived cells, since three melanoma cell lines were among cells that constitutively overexpressed PRNP, and the pancreatic MIA PaCa-2 cells were found among APP expressing cells we found.
Thus, we calculated the RQ factor to compare the relative expression of PRNP and APP in each cell lines using HeLa cells as calibrator. Hence, we could classify our cell lines in three categories (Fig. 1e): (i) cells with high APP gene expression but low PRNP mRNA level (↑APP, ↓ PRNP), (ii) cells displaying an equilibrium between both genes, and (iii) cells exhibiting high PRNP gene expression but reduced APP mRNA content (PRNP ↑, ↓ APP). These data support the existence of a link between PRNP and APP gene expressions.
Relation between PRNP and APP gene expressions
To study the link between PRNP and APP gene expression we silenced APP, PRNP, and for the first time, PRNP and APP simultaneously in different cell lines by using replicative pEBVsiRNA vectors. Each cell line was transfected with two pEBVsiRNA plasmids per gene in at least three independent experiments. We observed a dramatic decrease in cell survival for most of the PRNPKD and APPKD cell lines (Figure S4a, b & c). Only HeLaKD and RKOKD cells exhibited moderate effects on their growth potential in 2D culture. It was noteworthy that simultaneous PRNP and APP gene silencing failed to trigger synthetic lethality in middle term experiments. However, with longer times in 2D culture, double PRNPKD/APPKD cells displayed a decreased growth potential (Figure S4d). Together, these results show that efficient disruption of PrPc and/or APP expression strongly affects growth in most PRNPKD and APPKD cancer cells grown in 2D cultures.
Thereafter, we focused on interest on MDA-MB-231KD, HCT-116KD and HeLaKD cells because they exhibited different TP53 genotypes. RT qPCR analyses showed that, several weeks after transfection, silencing of the PRNP gene in MDA-MB-231 cells (3% of remaining PRNP mRNA as compared to control) induced a lowered APP transcription (57%; Fig. 2a). In opposition, HCT-116 and HeLa PRNPKD cells displayed an increased APP mRNA content (167% and 140% respectively as compared to control) (Fig. 2b and c). Interestingly, APP shutting down always decreased PRNP transcription at 64%, 49% and 74% in MDA-MB-231, HCT-116 and HeLa cells respectively (Fig. 2a, b and d). These results were consistent with a link between APP and PRNP gene expressions, which was cell dependent. It was noticeable that TP53 deficiency triggered a reduced amount of APP mRNA in MDA-MB-231 cells (65% as compared to control) and an enhanced amount in HCT-116 cells (185%) (Fig. 2a and b). Because HCT-116 cells harbor a wild type TP53 genotype, our result supported the idea that activation of wild type TP53 could repress APP gene expression, as previously reported [40].
Fig. 2.
Relationship between PRNP and APP gene expression. RT qPCR analysis of PRNP and APP in stable (a) MDA-MB-231 or (b) HCT-116 cells. Reversion experiment in HeLaKD cells. Stable HeLaKD clones were maintained in culture for 20 and 40 additional days with (c) or without (d) hygromycin B to revert gene silencing. At indicated time, PRNP and APP mRNA were analysed by RT qPCR. REVP means reversed PRNPKD, REVA means reversed APPKD, REVP/A means reversed PRNPKD/APPKD. Primers used are indicated in Table S4.
To confirm these findings, reversion experiments were performed in HeLaKD cells. The use of replicative pEBVsiRNA vectors allowed us to reverse the imposed gene silencing after selection pressure withdrawal, which allows a slow disappearance of pEBV plasmids with phenotypic reversion [29,41]. Reversed PRNPKD (REVP) and APPKD (REVA) HeLa cells recovered a normal PRNP and APP mRNA content as compared to control (Fig. 2d). It was noteworthy that reversed PRNPKD/APPKD (REVP/A) cells maintained very high expression levels of both PRNP and APP genes even 40 days post-reversion but not latter. This observation outlines the existence of an adaptive response that might take place during the gene silencing period possibly to bypass the loss of PrPc and APP proteins without success, as previously described [29].
PRNP and APP gene silencing impaired cell growth in 3D culture
To explore the cellular phenotypes induced by PRNP and/or APP silencing we focused on HeLaKD cells because they endured the loss of PRNP and/or APP expression very well in 2D culture albeit exhibiting stable gene silencing, as evidenced by RT PCR (Fig. 3a). Firstly, we observed that PRNPKD HeLa cells acquired a fibroblastic-like appearance during the transient period following transfection (14 days) as evidenced by quantitative ImageJ analysis using circularity and roudness shape indicators (Fig. 3b and S5c). This suggested a regulation of cell adhesion cytoskeleton dynamics, as previously reported [4]. As control, we established CD24 and CD44 deficient HeLa cells (respectively CD24KD and CD44KD cells). The GPI-anchored CD24 protein is a heavily glycosylated protein with diverse physiological functions, including cell adhesion, spreading, and cell-cell and cell-matrix interactions [42]. CD44 is a hyaluronic acid receptor and a stem cell marker, also associated to lipid rafts and tightly related to CD24 during tumor development [43]. Change in cell morphology was also observed in CD24KD cells. After about 20 days, PRNPKD and CD24KD HeLa cells always recovered their epithelial shape, as tumor cell plasticity was transient and reversible [44]. In opposition, CD44KD, APPKD and PRNPKD/APPKD HeLa cells showed no change in cell morphology. Hence, PrPc and CD24 are likely necessary to maintain a stable morphology
Fig. 3.
PRNP and/or APP gene silencing impairs 3D cell growth. (a) Characterization of stable HeLaKD cells by RT PCR; primers used are indicated in Table S4. (b) PRNPKD and CD24KD cells acquired a fibroblastic-like appearance. Illustrative bright field observations of HeLaKD cells 14 days after transfection are shown (scale bar: 50 µm). At least 3 fields corresponding to 300 cells per cell line were quantified with ImageJ for shape indicators (circularity and roundness, mean +/- SEM), and statistical analysis was performed using GraphPad Prism v5 right), n = 2. (c) Culture in soft agar. HeLaKD cells were cultivated in presence or not of hygromycin B for 2 weeks and thereafter 5000 HeLa cells were seeded in soft agar (+/- hygromycin B) for 18 additional days. Each point represents the mean of 3 wells +/- SD. (d) Culture in a tissue-like environment. 3000 stable HeLaKD clones were embedded into collagen matrices (three wells per point) and cultivated for 17 days. Collagen gels were analysed with a binocular microscope or an indirect microscope, as indicated in M&M. Growing colonies were counted. Indicated values correspond to mean +/-SD. Experiments have been reproduced four times.
Because the PrPc protein and a fraction of APP are facing the outside of the cell, we carried out three dimensional cell cultures, in either soft agar or collagen type I. Soft agar is a stringent assay for studying malignant potential in anchorage independent condition [45], and collagen matrices mimic a tissue-like environment [46,47]. In soft agar, PRNPKD and APPKD HeLa cells exhibited a tremendous decrease in colony formation as compared to control cells, which was resumed in reverted clones (Fig. 3c), suggesting a direct link between growth without anchorage and expression of both PrPc and APP. Importantly, PrPc and/or APP deficient cells were unable to colonize collagen type I matrices (Fig. 3d). This was confirmed with different HeLaKD clones through independent experiments (Figure S5a). As a positive control, we used TP53KD HeLa cells, which displayed enhanced colony formation capabilities. Together, these results light up that loss of PrPc and/or APP in HeLa cells strongly disrupt cell growth in 3D culture, especially in collagen type I matrices in which cell proliferation, invasion and survival are dependent of cell-microenvironment interactions.
Stable PRNPKDand/or APPKD altered membrane-anchored CD24 content
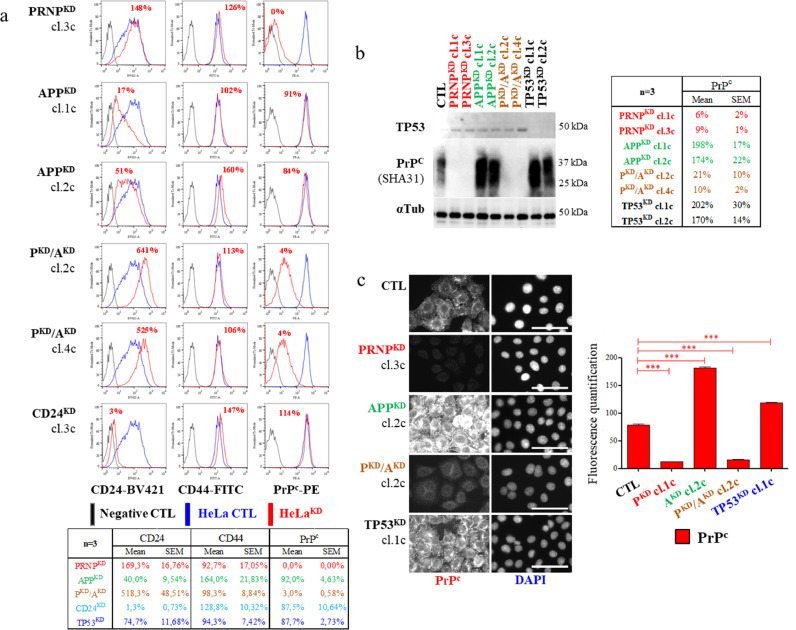
Because PrPc and APP co-localized on lipid raft microdomains on the plasma membrane [48], we assumed that their impaired expression could affected lipid raft stability, hampering 3D cell growth. It has been suggested that GPI anchored proteins, such as CD24, act as gate-keepers for lipid rafts and thus they represent selective markers of raft stability [49,50]. Thus; we quantified the cell membrane-anchored CD24 in living HeLaKD cells by means of flow cytometry. On one hand it was noteworthy to observe that APPKD cells displayed a tremendous decrease in the membrane-anchored CD24, as evidenced by median fluorescence intensities, in either nearly confluent (21% and 53% respectively for clones 1c and 2c, compared to the control) or proliferating cells (clone 1c: 21%, clone 2c: 33%) (Fig. 4a and S5d). These APPKD cells also showed a moderate increase in the content of membrane-anchored CD44. Interestingly, these cells failed to change their membrane-associated PrPc content (Fig. 4a and S5d); as confirmed by ICC with short integration times (Figure S6a). On the other hand, PRNPKD and PRNPKD/APPKD cells showed nearly undetectable membrane-anchored PrPc (between 0% to 4%), with no significant CD24 change in PRNPKD cells, but with an enhanced membrane-associated CD24 in either confluent (clone 1c: 615%, clone 2c: 505%) and proliferating (clone 1c: 190%, clone 2c: 180%) PRNPKD/APPKD cells (Fig. 4a and S5d). Thereafter, we analyzed the PrPc protein content in the KD clones by Western blot analyses (Fig. 4b) and ICC stainings (Fig. 4c). Strikingly, we observed a cytoplasmic PrPc accumulation in APPKD HeLa cells without significant enhanced PRNP transcription, as aforementioned. These data lent to support that APP and PRNP/APP deficiencies disrupted lipid rafts in an opposite way.
Fig. 4.
Altered membrane-anchored CD24 content in PRNP and APP deficient stable HeLa cells. (a) Membrane-associated CD24, CD44, and PrPc proteins were analysed by flow cytometry in living stable HeLaKD clones 57 days after transfection. 200,000 cells were plated in 6 cm diameter dishes and analyzed 6 days later at about 95% of confluence. Percentages correspond to the mean fluorescence intensity as compared to control HeLa cells carrying the pBD650 plasmid. The Table underneath the panel (a) represents the mean +/-SEM of three independent flow cytometry experiments performed with different clones and/or different culture conditions. Cytoplasmic accumulation of PrPc in APPKD HeLa clones (b) Representative Western blot were quantified with ImageJ (mean +/- SEM, n = 3). (c) Illustrative PrPc stainings in HeLaKD cells (scale bar = 25 µm). 1237 individual cells in 4 to 7 fields per sample were analysed with ImageJ and GraphPad Prism (mean+/- SEM, n = 3).
HTRA2 monitored PRNP gene expression
Lipid rafts are master regulators of signal transduction, and changes in their composition could lead to misregulated processes triggering various stresses [7]. Because APPKD HeLa cells accumulated intracellular PrPc protein without upregulation of the PRNP gene, we thought that a defect of the QC proteins could be involved. We thus focused our interest on HTRA2, known to directly interact with APP to cleave it. We first investigate the correlation between HTRA2 and PrPc proteins in different cancer cell lines. It appeared that cells which overexpressed PrPc also exhibited enhanced HTRA2 levels, such as MIA Paca2 or SKMEL-37 cells (Fig. 5). We then silenced HTRA2 gene in HeLa cells with two pEBVsiHTRA2 plasmids displaying either high (HTRA2KD1) or low (HTRA2KD2) inhibition efficiencies. We found that silencing the HTRA2 gene with the highly efficient plasmid (HTRA2KD1 cells) lowered the PRNP gene expression at a greater extent than with its less efficient counterpart (Fig. 6b). Moreover, both HTRA2KD1 cells and HTRA2 knock out cells (HTRA2KO) displayed a lowered PrPc protein content as well as a decreased amount of CD44 (Fig. 6c and S7). Accordingly, flow cytometry analyses revealed that the membrane-associated PrPc mean fluorescence intensity fell down to 33% (day 9 after transfection) and 50% (day 11) in HTRA2KD1 cells as compared to control (Fig. 6d). We also found a tremendous decrease of the GPI-anchored CD24 protein amounts, reaching 17% and 33% respectively at day 9 and 11 after HTRA2 knocking down (Fig. 6d). These results highlighted that HTRA2 deficiency could induce major changes in the lipid raft composition and directly affect PrPc protein content.
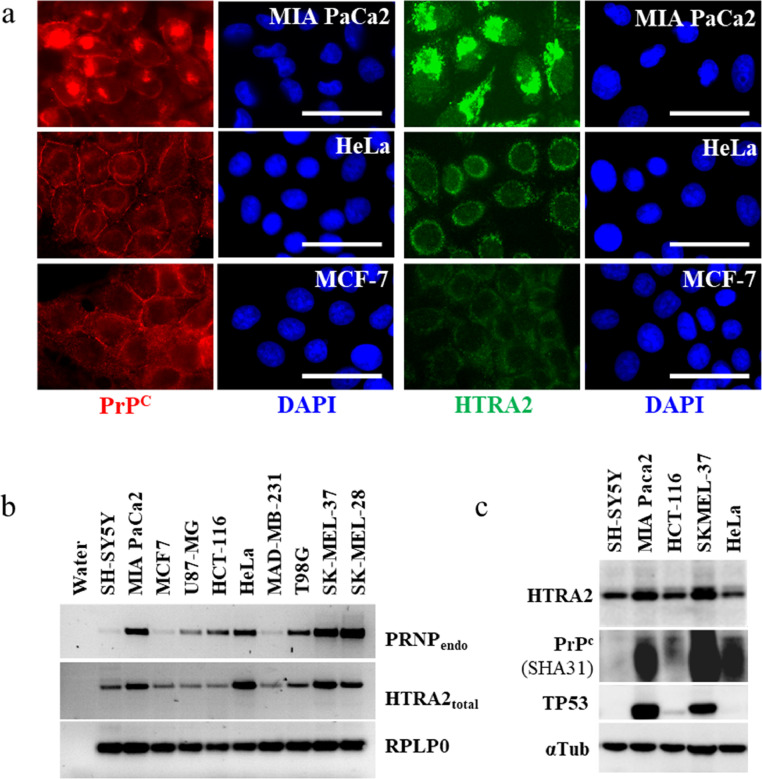
Fig. 5.
HTRA2 and PrPc co-expression. (a) ICC detection of HTRA2 in three tumor-derived cells cultivated at the same time. Cells were cultivated as indicated in legend of Fig. 1, and stained for PrPc (SHA31 + SAF32 antibodies) or HTRA2. They were counterstained with DAPI to visualize nuclei (scale bar 25 µm). (b) RT PCR analysis of PRNP and HTRA2 expression in 10 cell lines cultivated in parallel (n = 2). Primer used are described in Table S4. (c) Western blot analysis of HTRA2 in 5 cell lines.
Fig. 6.
HTRA2 down regulation lowers PRNP gene expression. (a) Representative PrPc (SHA31 + SAF32 antibodies), APP or HTRA2 stainings 14 days after transfection of HeLa cells. Cells were counterstained with DAPI to visualize nuclei (scale bar 25 µm). 1637 individual cells in 4 to 5 fields per sample were analysed with ImageJ and GraphPad Prism (mean+/- SEM, n = 3). (b) RT qPCR analysis of HTRA2 and PRNP gene expression in 26 days old HTRA2KD HeLa cell populations. Reactions were carried out in triplicate and values were normalized with control BD650 HeLa cells (mean +/- SEM). Cells were transfected with pEBVsiHTRA2 plasmids displaying a high (HTRA2KD1, pBD3115 vector) or moderate (HTRAKD2, pBD3116 vector) gene silencing efficiency. For HTRA2 mRNA quantification, 2 sets of primers were used: primers 1 (pr1) detecting either total HTRA2 (short and long HTRA2 mRNA chains) and primers 2 (pr2) detecting only long chain. Primers are described in Table S4. (c) Western blot analysis of the HTRA2 protein content in stable knock down (HTRA2KD1) and knock out (HTRA2KO) HeLa clones. Protein equivalent to 105 cells were loaded onto a 10% PAGE. PrPc was revealed with both SHA31 and SAF32 antibodies. (d) Flow cytometry analysis of membrane-associated CD24, CD44, and PrPc in living HeLaKD cells 9 or 11 days after transfection in 2 independent culture experiments. Percentages correspond to the mean fluorescence intensity as compared to control. On the right of the panel d the loss of membrane-anchored PrPc was revealed after PrPc stainings in HTRA2KD1 cells as compared to CTL (scale bar 25 µm).
In parallel, we found that both APPKD and PRNPKD cells displayed an enhanced HTRA2 protein level few days after transfection (day 14; Fig. 6a) or in stable clones (Fig. 7a and 7b). Moreover, in two independent experiments, we detected moderate up-regulation of the HTRA2 gene transcription in PRNPKD, APPKD and PRNPKD/APPKD HeLa clones (Fig. 7c and S5e). To confirm results obtained with stable KD HeLa cells, we established knock out HeLa (HeLaKO) cells. As expected, APPKO cells also showed a higher level of the intracellular PrPc protein compared to control (Fig. 7d). Besides, PRNPKO, APPKO and PRNPKO/APPKO cells displayed an enhanced HTRA2 protein content, as found in their KD counterparts. These results further confirm the crosstalk between APP, HTRA2 and PrPc.
Fig. 7.
Intracellular accumulation of PrPc in APPKD and APPKO HeLa cells. Characterization of different HeLaKD clones by: (a) Representative ICC stainings of HeLaKD clones stained for PrPc (SHA31 + SAF32 antibodies), or HTRA2, and counterstained with DAPI to visualize nuclei (scale bar 25 µm). 1632 individual cells in 4 to 7 fields per sample were analysed with ImageJ and GraphPad Prism (mean+/- SEM, n = 3). (b) Western blot was performed with protein extracted from 105 cells and were loaded onto a 10% PAGE. (c) For RT PCR, the used primers are indicated in Table S4. (d) CRISPR-Cas9-engineered HeLaKO cells were analysed by ICC stainings. Cells were stained and analysed as mentioned in panel a (scale bar 25 µm).
Because silenced HTRA2 gene expression lowered the PrPc content, overexpression experiments were undertaken. Very high expression of ectopic PrPc in HeLa cells did not affect cell viability (data not shown), nor HTRA2 protein and mRNA levels (Fig. 8a and 8b). Consequently, HTRA2 up regulation in APPKD cells was not due to PrPc protein accumulation. Instead, HTRA2 overexpression triggered intracellular PrPc accumulation (Fig. 7a and 7c) and enhanced PRNP transcription (Fig. 8b). Besides, 10 and 14 days after transfection, HTRA2+ cells exhibited elevated membrane-associated PrPc amount with 150% (day 11) or 225% (day 14) as compared to control cells carrying an empty vector (Fig. 8d and 8e). In PRNP+ cells, we also detected an enhanced intracellular CD44 accumulation without change at the membrane level (Fig. 8a and 8d). It was also noticeable that PRNP+, HTRA2+ and HTRA3+ cells displayed an enhanced APP transcription (Fig. 8b). Furthermore, HTRA2+ cells showed an increased amount of cytoplasmic (Fig. 8a) and membrane-bound CD44 (213% at day 11 and 338% at day 14) (Fig. 8d and e) and a lowered membrane-anchored CD24 (22% at day 11 and 8% at day 14). Again, this suggested that altered HTRA2 expression induces major changes in lipid raft composition. Besides, it was noteworthy that in contrast to PrPc in PRNP+ cells, most of ectopic CD24 protein in CD24+ cells localized at the plasma membrane (4674%), lowering membrane-anchored PrPc (59%) and CD44 (65%) protein levels (Fig. 8d). It can then be assumed that overexpressed PrPc exhibited both preferential intracellular accumulation and extracellular export in HeLa cells. These data reinforced the idea of a tight relationship between HTRA2, PRNP and APP.
Fig. 8.
Overexpression of HTRA2 enhances PrPc protein content. (a) Representative stainings of PrPc (SHA31 + SAF32 antibodies), HTRA2 and CD44 performed 10 days after transfection of PRNP or HTRA2 CDS in HeLa cells. Cells were counterstained with DAPI to visualize nuclei (scale bar 25 µm). 1399 individual cells in 5 to 8 fields per sample were analysed with ImageJ and GraphPad Prism (mean+/- SEM). (b) RT PCR analysis of HeLa cells, 52 days after transfection with either PRNP, APP695, HTRA2 or HTRA3 CDS. Primers used are indicated in Table S4. (c) Western blot analysis of HTRA2 and PrPc (SHA31 antibody) in HeLa cells silenced for HTRA2 (KD1 and KO) and overexpressing HTRA2 (HTRA2+). Cells were transfected with two different pEBVCAG-HTRA2 plasmids (1 means pBD3357; 2 means pBD3696). Proteins extracted from 105 cells were loaded onto a 10% PAGE. *: unspecific band. Plasmids were described in Table S3. Altered expression of membrane-associated CD24, CD44 and PrPc in living HTRA2+ HeLa cells. (d) 11 and (e) 14 days after transfection, living HeLa cells were stained for CD24, CD44 and PrPc and analysed by flow cytometry as indicated in M&M. PRNP+ and CD24+ cells are used as controls. Panels d and e represent two independent transfection experiments. Percentages correspond to the ΔΔMFI as compared to control.
Discussion
For a long time, PrPc and APP proteins have been studied for their involvement in neurodegenerative diseases through a common molecular mechanism characterized by the aggregation of specific host proteins, which became abnormal and toxic. This is the prion-like concept, which underlies the propagation of toxic protein aggregates from cell to cell [51,52]. This concept is currently considered in the propagation of several protein aggregates including the prion protein (PrP) in prion diseases, α-synuclein, β-amyloid and/or MAPT (microtubule-associated protein tau) in other neurodegenerative diseases such as Parkinson's and Alzheimer's diseases [53]. However, a general consensus on the exact biological role of PrPc and APP outside the nervous system remains debated, in particular in cancer cells. Herein, using various tumor cell lines and engineered MBA-MB-231, HCT-116 and HeLa cells, we highlighted a link between endogenous PRNP and APP gene expressions.
Under physiological conditions, constitutive PrPc protein is subjected to sequential and complex proteolytic processing generating several polypeptides and harboring either protective or deleterious roles [54,55]. Accordingly, our results on PrPc immunoblotting show profiles in agreement with the complexity of newly synthesized PrPc fragments, as previously described [56]. The PrPc expression and protein localization variability in each cell line strongly suggests differences in the protein maturation process, which can greatly influence the biological functions of PrPc, especially when these polypeptides can move to different subcellular locations. PrPc and APP proteins are mainly localized in outer plasma membrane lipid rafts (lipid raft-like microdomains), which play an essential role in tumor cell adhesion and migration [7]. However, the GPI-anchored PrPc protein is a mobile element, shifting from different membrane domains to internal organelles. Hence, PrPc constantly undergoes endocytic cycles between the plasma membrane, the trans-Golgi compartment, and ultimately the ER to be recycled back to the plasma membrane [57,58]. Thus, the internalized PrPc can reach membrane structures close to the nucleus [59]. This was observed in various cell lines, such as MIA PaCa-2, H1299, SK-N-AS, and T98G, where the endogenous PrPc was mainly localized closely to the nuclear compartment, presumably in the rough ER and the Golgi apparatus, where processed and misprocessed PrPc protein could accumulate [39]. This retrograde transport may lead to an accumulation of PrPc in the ER. Indeed, a member of the Ras superfamily of small GTPase (e.g. Rab6a) stimulates this PrP delocalization and increases PrPRes production in prion-infected neuroblastoma N2a cells [60]. Hence, we assume that the loss of membrane-associated APP in the APPKD and APPKO cells might enhance this process. Because the cellular PrPc is handled by the ER-associated degradation (ERAD)-proteasome pathway [61], it is conceivable to expect UPRER and UPRmt protein recruitment. For the first time, it was here shown that HTRA2, a UPRmt factor, could participate to the global UPR response leading to intracellular PrPc protein accumulation, highlighting a role of HTRA2 in PrPc proteostasis.
The involvement of HTRA2 in the PrPc proteostasis was observed in cells exhibited an impaired APP expression, such as APPKD and APPKO HeLa cells, leading to the intracellular accumulation of PrPc in its trafficking, probably through a retrograde mechanism [60,61]. The interplay between HTRA2 and PrPc was confirmed in HTRA2KD and HTRA2KO HeLa cells, where HTRA2 deficiency reduced both PRNP transcription and PrPc protein contents. In opposition, HTRA2 overexpression enhanced PRNP mRNA levels and PrPc protein amounts. We also showed that, among our set of tumor cells, MIA Paca2 and SKMEL-37 cells exhibited a very high endogenous PrPc protein content as well as an elevated HTRA2 protein level.
The member of the HTRA family are stress sensors and regulators of UPR to manage the fate of misfolded, aggregated, and mislocated proteins. Especially, HTRA1 can cleave proteins associated with AD, such as tau (MAPT), APP, and APOE4 [62], [63], [64]. In this way, the mitochondrial HTRA2 member participates to the QC pathway as an adaptive dual chaperone protease [35]. Herein, we had postulated that HTRA2 acted in coordination with UPRER factors to manage PrPc, leading to the accumulation of aggregation-prone PrP molecules in the cytosol, as observed after proteasome dysfunction and ER stress [22,23,61]. Indeed, different studies have already shown that some UPRER factors, such as ATF6A, spliced XBP1 (sXBP1) or CREB3/Luman, were able to enhance PRNP gene expression, suggesting a protective role of PrPc in the global response to misfolded proteins [65]. For instance, ATF6A and sXBP1 transactivated PRNP gene expression through ER stress response elements (ERSE) motifs stretch on the human PRNP promoter [66]. Similarly, GRP78/BIP (also termed HSPA5), another master regulator of ER homeostasis and a key component for sensing ER stress, is also an ER chaperone of the cellular PrPc [23,67]. Accordingly, GRP78/BIP was shown to modulate the propagation of the pathogenic and infectious form of the prion protein PrPSc, reducing prion pathogenesis [68]. Moreover, in murine model of scrapie, [69]GRP58 (also termed PDIA3/ERP57), another ER molecular chaperone, showed to interact with the lectin chaperone calnexin and to modulate the folding of mutant PrP protein through its disulfide isomerase activity [69]. These compelling data highlight the tight regulation of PrPc by key building blocks of UPRER. In HeLa cells, the PrPc protein has also been associated with a UPRmt-related chaperone, namely HSP60 [70]. Interestingly, both HSP60 and HTRA2 expressions were upregulated in Aβ25–35-treated human neuroblastoma SH-SY5Y cells and in APPsw/PS1dE9 trangenic mice [71], indicating that these two UPRmt chaperones participate to the PrPc proteostasis, particularly during an abnormal state for APP metabolism.
On another hand, in APPKD and PRNPKD/APPKD HeLa cells we observed tremendous changes in membrane-associated CD24, which are usually concentrated on plasma membrane lipid rafts. As the detection of lipid rafts is very tricky [72,73], the fate of GPI-anchored CD24 in living cells can reveal their stability [6,74]. Our results suggested that impaired APP and/or PRNP gene expression disrupt lipid rafts composition. Better results were obtained in HeLa cells displaying impaired HTRA2 gene expression. As lipid rafts articulate a set of synergistic signaling factors and their composition plays a key role in normal PrPc functions [75], disruption of these lipid rafts should alter cellular PrPc signaling. On another hand, CD24 expression in intact lipid rafts has been tightly correlated with tumor invasion and metastasis [42,76]. Hence, we observed a hampered growth of APPKD and/or PRNPKD HeLa cells in 3D-culture, reinforcing the idea that intact lipid rafts are essential for cell growth in collagen matrices [47].
To conclude, our observational data revealed that HTRA2 modify the PRNP gene expression and alters the subcellular localization of PrPc, particularly in APP deficient cells. The HTRA family encompasses mitochondrial, cytoplasmic and extracellular members. It is noteworthy that HTRA1 and HTRA3 are secreted into the intracellular space where they degrade various substrates of the ECM, playing a major role in extracellular matrix homeostasis. For instance, HTRA1 degrade fibronectin, type II collagen, biglycan, clusterin, vitronectin, aggrecan, decorin or fibromodulin [62,[77], [78], [79]]. Interestingly, Kaur et al. [80] showed that staurosporine-treated HUVEC cells released HTRA2 in the culture medium. An emerging question is now to known whether these three members of the HTRA family may play a role in the behavior of extracellular PrPc and its spreading.
Materials and methods
Cell culture and survival assays
Cell origins: MCF7, MDA-MB-231, MDA-MB-436, MIA PaCa2, PANC-1, HeLa, T47D, ZR-75-1, BE(2)-C (ATCC); SK-MEL-37 (Dr R. Chammas, Sao Paulo University, Brazil); SK-MEL-5, SK-MEL-28, Malme-3 M (Dr C Gazin, CNRS—CEA, Evry, France), RKO (Dr M.F. Poupon, CNRS, Paris, France); HCT-116 (Dr B. Bugler, CNRS Toulouse, France); SK-N-AS, SH-SY5Y, LA-N-1; LA-N-5, KELLY, T98G (Theranexus, CEA Fontenay aux Roses, France); U-87 MG, U-251 MG (Dr L. Gauthier, CEA, Fontenay aux Roses, France); K562, RAMOS, BL2 (Dr I. Délic, CEA Fontenay aux Roses, France); HOS, H1299 (Dr P. May, CNRS Villejuif, France), HEK293 (Dr A. Delaunay, CEA, Saclay) Main features of the cells were indicated in the Table S1. Parental cell lines were always cultivated in parallel.
Cells were maintained in Dulbecco's modified Eagle's medium (DMEM; Sigma) supplemented with 10% foetal calf serum (FCS), 100 U/mL penicillin / 100 µg/mL streptomycin / 2 mM glutamin, 10 mM HEPES (Sigma products), under 5% CO2. Transfection experiments were performed with either JetPrime (Ozyme) or FugeneHD (Promega) reagents following manufacturer's recommendations. As control of transfection we used a pEBV vector over-expressing the YFP protein. 24 h after transfection, cells were cultured in the presence of hygromycin B at relevant concentrations (Table S1). Transfected cells were plated in 6 cm diameter dishes to follow cell growth. Depending on the cell type, cells were counted 2 to 4 weeks later. Transfection experiments were repeated at least three times, and more than ten times for HeLa cells. Average numbers of survival cells from each experiment were plotted (+/- SD). For long term survival, cells were trypsinized, counted and plated at 5000 cells per dish in 6-well dishes for an additional period in culture. Three wells per condition were counted and experiments were reproduced twice in different independent transfection experiments. For very long-term cultures (more than one month) numerous knock down (KD) clones were established in independent transfection experiments (e.g. for HeLa cells). Clones were picked up randomly, propagated and analyzed. Gene silencing efficiency was constantly assessed.
pEBVsiRNA and overexpressing pEBV vectors
pEBVsiRNA plasmids and establishment of stable knock down cells have been extensively described [41,81,82]. Cloning was performed using our replicative pEBV2siRNA LacZ’ vectors for either single or double gene silencing. For dual expression of two shRNAs, transcription cartridges were fitted side by side in the same orientation (in tandem). These vectors carried the H1 RNA polymerase III promoter (RNA pol III) to drive the transcription of short hairpin RNA (shRNA), which gives rise to siRNA like molecules in vivo. Because the crucial step in the quality of RNA interference is the unfailing recognition of the cognate mRNA by the siRNA sequence, we used the DSIR program which includes an exact similarity search algorithm for potential off-target detection [83]. For each gene, 3 to 5 vectors were built and only 2 of them were selected for efficient gene silencing without side effects [82]. Control cells (termed as CTL) were obtained by transfection with the vector pBD650, which carries an inefficient shRNA [41]. All plasmids, target sequences, and predicted efficiencies are presented in Table S2. CRISPR-Cas9 cell lines were established using new homemade plasmids carrying one or two H1-sgRNA transcription cassette and allowing a very high level of gene silencing (manuscript in preparation). These cells were analysed at the DNA genomic level and thereafter by RT PCR and ICC staining. For expression of ectopic proteins, we used our well-validated pEBV plasmids [84]. Transcription was driven by either a CMV or a CAG promoter. Name and main features of these plasmids are mentioned in Table S3. All plasmids have been checked by sequencing (Eurofins Genomics).
Soft agar assay for colony formation
Soft agar assay was performed in six-well plate using a 2 ml basal layer of 0.63% bacto-agar (Becton Dickinson) in complemented DMEM. Cells were trypsinized, counted and plated at 6000 cells per well in 0.42% bacto-agar in complemented DMEM medium. Depending on the cell lines, after 3 to 4 weeks, the colonies were stained with 0.4% crystal violet overnight at 37 °C and counted. Images were captured using Argimed software (Microvision instruments, France) and quantified using NHI ImageJ software. Only particles ranging from 30 to 1000 000 pixels were counted. Each experiment was repeated at least twice with two replicates each. Average numbers of colonies from each experiment were plotted.
Culture in a tissue-like environment (collagen matrices)
Cell growth in a tissue-like environment has been adapted from study on in vitro reconstructed skin [85]. Briefly, ice-cold collagen type I isolated from rat tail tendons (4 mg/ml) was mixed with Hank's balanced saline solution and neutralised with NaOH. Complemented DMEM containing 3000 cells per gel was added and cell-populated collagens were poured in independent wells of 24-well plates. For each cell lines, three wells were seeded. Collagen gels were allowed to polymerize for 1–2 h in a humid incubator and complemented DMEM was added onto each well. Culture proceeded for two weeks. Collagen specimens were fixed with 4% buffered formol (VWR) for 10 min and stained with Crystal Violet (0.004%, Sigma) overnight. analyze was performed with a binocular microscope and an indirect microscope. Experiments were reproduced 4 times with different pools of cells.
Western blot and immunostaining staining
Procedures were described elsewhere [41]. For PrPc, we used the following monoclonal antibodies: SAF32, BAR233, and SHA31 (CEA). We also used antibodies against APP (clone 22C11, Affymetrix), α-tubulin (clone B-5-1-2, Sigma), APE-1/Ref-1 (H-6 sc-55,498, Santa Cruz), TP53 (DO-7, Tébu), HTRA2 (GTX54154, GeneTex), SOD2 (ABIN204760, Antibodies-online), and CD44 (clone EPR1013Y, Abcam). ICC stainings were analysed with a Leica DM6000 microscope. For ICC stainings and for each experiments, more than 1500 individual cells were circled in different fields and analysed. To evaluate change in cell shape, 300 cells were quantified in at least three fields per sample using ImageJ shape indicators (circularity and roundness).
RNA extraction and quantitative RT qPCR
RNA was extracted with NucleoSpinRNA II kit (Macherey-Nagel, France) and quantified using Nanodrop (ND-1000 spectrophotometer). For RT experiments, 1 µg of each RNA sample was reverse-transcripted into cDNA using iScriptcDNA Synthesis Kit (Bio-Rad, USA) with 20 µL of random hexamer primers, oligo-dT mixes and incubated at 25 °C for 5 min, then at 42 °C for 30 min and at 85 °C for 5 min. Relative gene expression level of each target gene in cells was determined by real-time quantitative PCR (RT-qPCR). Ribosomal protein RPLPO was used as a reference gene. Primer sequences were mentioned in the Table S4. Two microliters of cDNA (1:5 diluted) and 0.3 µM of primers were mixed with components from the IQ™ SYBR® Green Supermix kit (Biorad, France) in a final volume of 10 µL. Reactions in triplicate were carried out in the MiniOpticon real-time PCR machine (Biorad, France) under the following conditions: initial denaturation at 95 °C for 3 min and then 45 cycles of denaturation at 95 °C for 10 s, annealing/extension at 60 °C for 30 s. Melting curves were obtained to examine the purity of amplified products. Absolute quantitative data and Cq values were obtained by analysis with BioRad MFX Software 2.0 by second derivative method. Normalization of data was done with RPLP0 as reference gene and HeLa cells as calibrator. We used the following equation: [copy numbers of target gene in a sample/copy numbers of RPLPO in a sample)]/[copy numbers of target gene in HeLa cells/copy numbers of RPLPO in HeLa cells]. To reveal a correlation between PRNP and APP mRNA expression (Fig. 1e), we calculated the RQ factor using the following equation: RQ= 2(puissance (Cq RPLPO – Cq target)), Cq target being Cq APP or Cq PRNP.
Alternatively, total RNA was isolated from cells using a TRIzoL® Reagent according to the manufacturer's recommendations (ThermoFisher). Extracted RNAs were treated with recombinant rDNase (New England Biolabs) for 15 min at 37 °C and deactivated at 75 °C for 10 min. RT was performed in 1 µg of RNA that was reverse transcribed with the GoScript™ Reverse Transcription Mix kit (Promega). PCR reactions were performed with the OneTaq® Quick-load 2X Master Mix. Primers sequences were indicated in the Table S4 and the level of ribosomal protein RPLPO mRNA was used as internal control. PCR reactions were carried out as followed: initial denaturation (98 °C 30 s), 26 cycles of denaturation (98 °C 10 s) - annealing (64–70 °C depending of the primer's TM, 30 s) - extension (72 °C 30 s). cDNAs were loaded onto 2% agarose gels and analyzed with a U:Genius device (Syngene).
Flow cytometry analysis
Living cells were recovered and stained with the antibodies against CD24 (clone ML5), CD44 (clone C26), and CD230 (PrP, clone 4D5) from eBioscience. Isotypic controls were from BD Biosciences (San Jose, CA, USA). Cell immunolabelling was performed in PBS 1% BSA according to manufacturer's recommendations. Cells were analyzed on a SORP LSR-II analyzer (Configuration: 488 nm, 561 nm, 405 nm, 355 nm, and 635 nm). For each experiment, 10,000 to 20,000 cells were analyzed. Gating strategy was performed following exclusion of debris and cellular aggregates and live/dead discrimination. The positive populations were identified using an isotypic control to determine the negative populations. Data were treated with FlowJo v7.6.1 software (Tree Star). ΔΔMFI (median fluorescence intensity) and percentage expression as compared to control were calculated.
Statistical analysis
ICC stainings and Western blots were quantified using ImageJ v1.49o (NIH, USAStatistical analysis was carried out with GraphPad Prism v5 (GraphPad Software, La Jolla, CA, USA).). The same software was used to design graphs. Values were subjected to a Shapiro-Wilk normality test. When data did not show a normal distribution, they were compared by a Kruskal-Wallis test with a Dunns (compare all pairs of columns) post test. Differences were considered statistically significant for p values < 0.05.
Authors contributions
B.D.S.F. conceived and designed experiments. B.D.S.F. created and validated KD and KO cells, performed 2D and 3D-culture in collagen type I experiments, ICC stainings and qualitative RT PCR. R.J. realized RT qPCR experiments & analysis, and soft agar assays. N.R. performed Western blots. F.L. carried out flow cytometry analyses. B.D.S.F. drafted the manuscript, which was revised by D.P-G., N.R., F.L. and R.J. All authors have read and approved the final version of the manuscript before publication.
Funding
Internal sources were used to found the experiments.
Declaration of Competing Interest
The authors declare no competing interests.
Acknowledgements
Denis Biard dedicates this work to his wife, daughter, son and son-in-law, as well as to all health professionals in hospitals during the COVID-19 pandemic. D. Biard thanks Prof. A. Sarasin for its evaluation of our manuscript. D. Biard is indebted to Caroline Jan (CEA, MIRCEN) for access to the MIRCEN's microscopy facility. D. Biard is also grateful for Dr R. Chammas (Sao Paulo University, Brazil), Dr P. May (CNRS Villejuif, France), Dr C Gazin (CNRS-CEA, Evry, France), Dr M.F. Poupon (CNRS, Paris, France), Dr B. Bugler (CNRS, Toulouse, France), Theranexus and Drs L. Gauthier and I. Délic (CEA, Fontenay aux Roses, France) for providing validated cell lines. All experiments have been carried out in accordance with the quality management program developed by the CEA and otherwise described in details in an UPEC teaching program (Master STA2E parcours Biologie Intégrative).
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.bbadva.2021.100035.
Appendix. Supplementary materials
References
- 1.Lim S., Yoo B.K., Kim H.-.S., Gilmore H.L., Lee Y., Lee H., Kim S.-.J., Letterio J., Lee H. Amyloid-β precursor protein promotes cell proliferation and motility of advanced breast cancer. BMC Cancer. 2014;14:928. doi: 10.1186/1471-2407-14-928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu X., Chen S., Lu C. Amyloid precursor protein promotes the migration and invasion of breast cancer cells by regulating the MAPK signaling pathway. Int. J. Mol. Med. 2020;45:162–174. doi: 10.3892/ijmm.2019.4404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mehrpour M., Codogno P. Prion protein: from physiology to cancer biology. Cancer Lett. 2010;290:1–23. doi: 10.1016/j.canlet.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 4.Prado M.B., Melo Escobar M.I., Alves R.N., Coelho B.P., de L. Fernandes C.F., Boccacino J.M., Iglesia R.P., Lopes M.H. Prion Protein at the Leading Edge: its Role in Cell Motility. Int. J. Mol. Sci. 2020;21 doi: 10.3390/ijms21186677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ryskalin L., Biagioni F., Busceti C.L., Giambelluca M.A., Morelli L., Frati A., Fornai F. The Role of Cellular Prion Protein in Promoting Stemness and Differentiation in Cancer. Cancers (Basel) 2021;13 doi: 10.3390/cancers13020170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Simons K., Gerl M.J. Revitalizing membrane rafts: new tools and insights. Nat. Rev. Mol. Cell Biol. 2010;11:688–699. doi: 10.1038/nrm2977. [DOI] [PubMed] [Google Scholar]
- 7.Greenlee J.D., Subramanian T., Liu K., King M.R. Rafting Down the Metastatic Cascade: the Role of Lipid Rafts in Cancer Metastasis, Cell Death, and Clinical Outcomes. Cancer Res. 2021;81:5–17. doi: 10.1158/0008-5472.CAN-20-2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lasmézas C.I., Deslys J.P., Robain O., Jaegly A., Beringue V., Peyrin J.M., Fournier J.G., Hauw J.J., Rossier J., Dormont D. Transmission of the BSE agent to mice in the absence of detectable abnormal prion protein. Science. 1997;275:402–405. doi: 10.1126/science.275.5298.402. [DOI] [PubMed] [Google Scholar]
- 9.Linden R. The Biological Function of the Prion Protein: a Cell Surface Scaffold of Signaling Modules. Front. Mol. Neurosci. 2017;10 doi: 10.3389/fnmol.2017.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Warner R.G., Hundt C., Weiss S., Turnbull J.E. Identification of the heparan sulfate binding sites in the cellular prion protein. J. Biol. Chem. 2002;277:18421–18430. doi: 10.1074/jbc.M110406200. [DOI] [PubMed] [Google Scholar]
- 11.Parkin E.T., Watt N.T., Hussain I., Eckman E.A., Eckman C.B., Manson J.C., Baybutt H.N., Turner A.J., Hooper N.M. Cellular prion protein regulates beta-secretase cleavage of the Alzheimer's amyloid precursor protein. Proc. Natl. Acad. Sci. U.S.A. 2007;104:11062–11067. doi: 10.1073/pnas.0609621104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gasperini L., Legname G. Prion protein and aging, Front. Cell Dev. Biol. 2014;2 doi: 10.3389/fcell.2014.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.U.C. Müller, H. Zheng, Physiological functions of APP family proteins, Cold Spring Harb. Perspect. Med. 2 (2012) a006288. 10.1101/cshperspect.a006288. [DOI] [PMC free article] [PubMed]
- 14.Wang X., Zhou X., Li G., Zhang Y., Wu Y., Song W. Modifications and Trafficking of APP in the Pathogenesis of Alzheimer’s Disease. Front. Mol. Neurosci. 2017;10:294. doi: 10.3389/fnmol.2017.00294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang H., Ma Q., Zhang Y., Xu H. Proteolytic processing of Alzheimer's β-amyloid precursor protein. J. Neurochem. 2012;120(Suppl 1):9–21. doi: 10.1111/j.1471-4159.2011.07519.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gimbel D.A., Nygaard H.B., Coffey E.E., Gunther E.C., Laurén J., Gimbel Z.A., Strittmatter S.M. Memory impairment in transgenic Alzheimer mice requires cellular prion protein. J. Neurosci. 2010;30:6367–6374. doi: 10.1523/JNEUROSCI.0395-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Griffiths H.H., Whitehouse I.J., Baybutt H., Brown D., Kellett K.A.B., Jackson C.D., Turner A.J., Piccardo P., Manson J.C., Hooper N.M. Prion protein interacts with BACE1 protein and differentially regulates its activity toward wild type and Swedish mutant amyloid precursor protein. J. Biol. Chem. 2011;286:33489–33500. doi: 10.1074/jbc.M111.278556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laurén J., Gimbel D.A., Nygaard H.B., Gilbert J.W., Strittmatter S.M. Cellular prion protein mediates impairment of synaptic plasticity by amyloid-beta oligomers. Nature. 2009;457:1128–1132. doi: 10.1038/nature07761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Salazar S.V., Strittmatter S.M. Cellular prion protein as a receptor for amyloid-β oligomers in Alzheimer's disease. Biochem. Biophys. Res. Commun. 2017;483:1143–1147. doi: 10.1016/j.bbrc.2016.09.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meslin F., Hamaï A., Gao P., Jalil A., Cahuzac N., Chouaib S., Mehrpour M. Silencing of prion protein sensitizes breast adriamycin-resistant carcinoma cells to TRAIL-mediated cell death. Cancer Res. 2007;67:10910–10919. doi: 10.1158/0008-5472.CAN-07-0512. [DOI] [PubMed] [Google Scholar]
- 21.Jarosz-Griffiths H.H., Corbett N.J., Rowland H.A., Fisher K., Jones A.C., Baron J., Howell G.J., Cowley S.A., Chintawar S., Cader M.Z., Kellett K.A.B., Hooper N.M. Proteolytic shedding of the prion protein via activation of metallopeptidase ADAM10 reduces cellular binding and toxicity of amyloid-β oligomers. J. Biol. Chem. 2019;294:7085–7097. doi: 10.1074/jbc.RA118.005364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Godsave S.F., Peters P.J., Wille H. Subcellular distribution of the prion protein in sickness and in health. Virus Res. 2015;207:136–145. doi: 10.1016/j.virusres.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 23.Nunziante M., Ackermann K., Dietrich K., Wolf H., Gädtke L., Gilch S., Vorberg I., Groschup M., Schätzl H.M. Proteasomal dysfunction and endoplasmic reticulum stress enhance trafficking of prion protein aggregates through the secretory pathway and increase accumulation of pathologic prion protein. J. Biol. Chem. 2011;286:33942–33953. doi: 10.1074/jbc.M111.272617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen B., Retzlaff M., Roos T., Frydman J. Cellular Strategies of Protein Quality Control. Cold Spring Harb. Perspect. Biol. 2011;3 doi: 10.1101/cshperspect.a004374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klaips C.L., Jayaraj G.G., Hartl F.U. Pathways of cellular proteostasis in aging and disease. J. Cell Biol. 2018;217:51–63. doi: 10.1083/jcb.201709072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Clausen T., Southan C., Ehrmann M. The HtrA family of proteases: implications for protein composition and cell fate. Mol. Cell. 2002;10:443–455. doi: 10.1016/s1097-2765(02)00658-5. [DOI] [PubMed] [Google Scholar]
- 27.Skorko-Glonek J., Zurawa-Janicka D., Koper T., Jarzab M., Figaj D., Glaza P., Lipinska B. HtrA protease family as therapeutic targets. Curr. Pharm. Des. 2013;19:977–1009. doi: 10.2174/1381612811319060003. [DOI] [PubMed] [Google Scholar]
- 28.Clausen T., Kaiser M., Huber R., Ehrmann M. HTRA proteases: regulated proteolysis in protein quality control. Nat. Rev. Mol. Cell Biol. 2011;12:152–162. doi: 10.1038/nrm3065. [DOI] [PubMed] [Google Scholar]
- 29.Chatre L., Biard D.S.F., Sarasin A., Ricchetti M. Reversal of mitochondrial defects with CSB-dependent serine protease inhibitors in patient cells of the progeroid Cockayne syndrome. Proc. Natl. Acad. Sci. U.S.A. 2015;112:E2910–E2919. doi: 10.1073/pnas.1422264112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Darreh-Shori T., Rezaeianyazdi S., Lana E., Mitra S., Gellerbring A., Karami A., Bogdanovic N., Lithner C.U., Winblad B., Behbahani H. Increased Active OMI/HTRA2 Serine Protease Displays a Positive Correlation with Cholinergic Alterations in the Alzheimer's Disease Brain. Mol. Neurobiol. 2019;56:4601–4619. doi: 10.1007/s12035-018-1383-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kang S., Fernandes-Alnemri T., Alnemri E.S. A novel role for the mitochondrial HTRA2/OMI protease in aging. Autophagy. 2013;9:420–421. doi: 10.4161/auto.22920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moisoi N., Klupsch K., Fedele V., East P., Sharma S., Renton A., Plun-Favreau H., Edwards R.E., Teismann P., Esposti M.D., Morrison A.D., Wood N.W., Downward J., Martins L.M. Mitochondrial dysfunction triggered by loss of HtrA2 results in the activation of a brain-specific transcriptional stress response. Cell Death Differ. 2009;16:449–464. doi: 10.1038/cdd.2008.166. [DOI] [PubMed] [Google Scholar]
- 33.Chen Z., Huang L., Tso A., Wang S., Fang X., Ouyang K., Han Z. Mitochondrial Chaperones and Proteases in Cardiomyocytes and Heart Failure. Front. Mol. Biosci. 2021;8 doi: 10.3389/fmolb.2021.630332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Spiess C., Beil A., Ehrmann M. A temperature-dependent switch from chaperone to protease in a widely conserved heat shock protein. Cell. 1999;97:339–347. doi: 10.1016/s0092-8674(00)80743-6. [DOI] [PubMed] [Google Scholar]
- 35.Toyama Y., Harkness R.W., Lee T.Y.T., Maynes J.T., Kay L.E. Oligomeric assembly regulating mitochondrial HtrA2 function as examined by methyl-TROSY NMR. Proc. Natl. Acad. Sci. U. S. A. 2021;118 doi: 10.1073/pnas.2025022118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goo H.-.G., Jung M.K., Han S.S., Rhim H., Kang S. HtrA2/Omi deficiency causes damage and mutation of mitochondrial DNA. Biochim. Biophys. Acta. 2013;1833:1866–1875. doi: 10.1016/j.bbamcr.2013.03.016. [DOI] [PubMed] [Google Scholar]
- 37.Park H.-.J., Kim S.-.S., Seong Y.-.M., Kim K.-.H., Goo H.G., Yoon E.J., Min D.S., Kang S., Rhim H. Beta-amyloid precursor protein is a direct cleavage target of HtrA2 serine protease. Implications for the physiological function of HtrA2 in the mitochondria. J. Biol. Chem. 2006;281:34277–34287. doi: 10.1074/jbc.M603443200. [DOI] [PubMed] [Google Scholar]
- 38.Huttunen H.J., Guénette S.Y., Peach C., Greco C., Xia W., Kim D.Y., Barren C., Tanzi R.E., Kovacs D.M. HtrA2 regulates beta-amyloid precursor protein (APP) metabolism through endoplasmic reticulum-associated degradation. J. Biol. Chem. 2007;282:28285–28295. doi: 10.1074/jbc.M702951200. [DOI] [PubMed] [Google Scholar]
- 39.Harris D.A. Cellular Biology of Prion Diseases. Clin. Microbiol. Rev. 1999;12:429–444. doi: 10.1128/cmr.12.3.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cuesta A., Zambrano A., Royo M., Pascual A. The tumour suppressor p53 regulates the expression of amyloid precursor protein (APP) Biochem. J. 2009;418:643–650. doi: 10.1042/BJ20081793. [DOI] [PubMed] [Google Scholar]
- 41.Biard D.S.F., Despras E., Sarasin A., Angulo J.F. Development of new EBV-based vectors for stable expression of small interfering RNA to mimick human syndromes: application to NER gene silencing. Mol. Cancer Res. 2005;3:519–529. doi: 10.1158/1541-7786.MCR-05-0044. [DOI] [PubMed] [Google Scholar]
- 42.Baumann P., Thiele W., Cremers N., Muppala S., Krachulec J., Diefenbacher M., Kassel O., Mudduluru G., Allgayer H., Frame M., Sleeman J.P. CD24 interacts with and promotes the activity of c-src within lipid rafts in breast cancer cells, thereby increasing integrin-dependent adhesion. Cell Mol. Life Sci. 2012;69:435–448. doi: 10.1007/s00018-011-0756-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jaggupilli A., Elkord E. Significance of CD44 and CD24 as cancer stem cell markers: an enduring ambiguity. Clin. Dev. Immunol. 2012;708036 doi: 10.1155/2012/708036. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chao Y., Wu Q., Acquafondata M., Dhir R., Wells A. Partial mesenchymal to epithelial reverting transition in breast and prostate cancer metastases. Cancer Microenviron. 2012;5:19–28. doi: 10.1007/s12307-011-0085-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dodson M.G., Slota J., Lange C., Major E. Distinction of the phenotypes of in vitro anchorage-independent soft-agar growth and in vivo tumorigenicity in the nude mouse. Cancer Res. 1981;41:1441–1446. [PubMed] [Google Scholar]
- 46.Sapudom J., Pompe T. Biomimetic tumor microenvironments based on collagen matrices. Biomater. Sci. 2018;6:2009–2024. doi: 10.1039/c8bm00303c. [DOI] [PubMed] [Google Scholar]
- 47.Yamada K.M., Cukierman E. Modeling Tissue Morphogenesis and Cancer in 3D. Cell. 2007;130:601–610. doi: 10.1016/j.cell.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 48.Taylor D.R., Hooper N.M. The prion protein and lipid rafts. Mol. Membr. Biol. 2006;23:89–99. doi: 10.1080/09687860500449994. [DOI] [PubMed] [Google Scholar]
- 49.Patschan S., Li H., Brodsky S., Sullivan D., De Angelis D.A., Patschan D., Goligorsky M.S. Probing lipid rafts with proximity imaging: actions of proatherogenic stimuli. Am. J. Physiol. Heart Circ. Physiol. 2006;290:H2210–H2219. doi: 10.1152/ajpheart.01112.2005. [DOI] [PubMed] [Google Scholar]
- 50.Runz S., Mierke C.T., Joumaa S., Behrens J., Fabry B., Altevogt P. CD24 induces localization of beta1 integrin to lipid raft domains. Biochem. Biophys. Res. Commun. 2008;365:35–41. doi: 10.1016/j.bbrc.2007.10.139. [DOI] [PubMed] [Google Scholar]
- 51.Scheckel C., Aguzzi A. Prions, prionoids and protein misfolding disorders. Nat. Rev. Genet. 2018;19:405–418. doi: 10.1038/s41576-018-0011-4. [DOI] [PubMed] [Google Scholar]
- 52.Stopschinski B.E., Diamond M.I. The prion model for progression and diversity of neurodegenerative diseases. The Lancet Neurology. 2017;16:323–332. doi: 10.1016/S1474-4422(17)30037-6. [DOI] [PubMed] [Google Scholar]
- 53.Goedert M. NEURODEGENERATION. Alzheimer’s and Parkinson’s diseases: the prion concept in relation to assembled Aβ, tau, and α-synuclein. Science. 2015;349 doi: 10.1126/science.1255555. [DOI] [PubMed] [Google Scholar]
- 54.Wulf M.-.A., Senatore A., Aguzzi A. The biological function of the cellular prion protein: an update. BMC Biol. 2017;15:34. doi: 10.1186/s12915-017-0375-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Béland M., Motard J., Barbarin A., Roucou X. PrP(C) homodimerization stimulates the production of PrPC cleaved fragments PrPN1 and PrPC1. J. Neurosci. 2012;32:13255–13263. doi: 10.1523/JNEUROSCI.2236-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Orsi A., Fioriti L., Chiesa R., Sitia R. Conditions of endoplasmic reticulum stress favor the accumulation of cytosolic prion protein. J. Biol. Chem. 2006;281:30431–30438. doi: 10.1074/jbc.M605320200. [DOI] [PubMed] [Google Scholar]
- 57.Mattei V., Matarrese P., Garofalo T., Tinari A., Gambardella L., Ciarlo L., Manganelli V., Tasciotti V., Misasi R., Malorni W., Sorice M. Recruitment of cellular prion protein to mitochondrial raft-like microdomains contributes to apoptosis execution. Mol. Biol. Cell. 2011;22:4842–4853. doi: 10.1091/mbc.E11-04-0348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Alves R.N., Iglesia R.P., Prado M.B., Melo Escobar M.I., Boccacino J.M., de L. Fernandes C.F., Coelho B.P., Fortes A.C., Lopes M.H. A New Take on Prion Protein Dynamics in Cellular Trafficking. Int. J. Mol. Sci. 2020;21:E7763. doi: 10.3390/ijms21207763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lee K.S., Magalhães A.C., Zanata S.M., Brentani R.R., Martins V.R., Prado M.A. Internalization of mammalian fluorescent cellular prion protein and N-terminal deletion mutants in living cells. J. Neurochem. 2001;79:79–87. doi: 10.1046/j.1471-4159.2001.00529.x. [DOI] [PubMed] [Google Scholar]
- 60.Béranger F., Mangé A., Goud B., Lehmann S. Stimulation of PrP(C) retrograde transport toward the endoplasmic reticulum increases accumulation of PrP(Sc) in prion-infected cells. J. Biol. Chem. 2002;277:38972–38977. doi: 10.1074/jbc.M205110200. [DOI] [PubMed] [Google Scholar]
- 61.Ma J., Lindquist S. Wild-type PrP and a mutant associated with prion disease are subject to retrograde transport and proteasome degradation. Proc. Natl. Acad. Sci. U. S. A. 2001;98:14955–14960. doi: 10.1073/pnas.011578098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tennstaedt A., Pöpsel S., Truebestein L., Hauske P., Brockmann A., Schmidt N., Irle I., Sacca B., Niemeyer C.M., Brandt R., Ksiezak-Reding H., Tirniceriu A.L., Egensperger R., Baldi A., Dehmelt L., Kaiser M., Huber R., Clausen T., Ehrmann M. Human high temperature requirement serine protease A1 (HTRA1) degrades tau protein aggregates. J. Biol. Chem. 2012;287:20931–20941. doi: 10.1074/jbc.M111.316232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chu Q., Diedrich J.K., Vaughan J.M., Donaldson C.J., Nunn M.F., Lee K.-.F., Saghatelian A. HtrA1 Proteolysis of ApoE In Vitro Is Allele Selective. J. Am. Chem. Soc. 2016;138:9473–9478. doi: 10.1021/jacs.6b03463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Grau S., Baldi A., Bussani R., Tian X., Stefanescu R., Przybylski M., Richards P., Jones S.A., Shridhar V., Clausen T., Ehrmann M. Implications of the serine protease HtrA1 in amyloid precursor protein processing. Proc. Natl. Acad. Sci. U. S. A. 2005;102:6021–6026. doi: 10.1073/pnas.0501823102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Déry M.-.A., LeBlanc A.C. Luman contributes to brefeldin A-induced prion protein gene expression by interacting with the ERSE26 element. Sci. Rep. 2017;7:42285. doi: 10.1038/srep42285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Déry M.-.A., Jodoin J., Ursini-Siegel J., Aleynikova O., Ferrario C., Hassan S., Basik M., LeBlanc A.C. Endoplasmic reticulum stress induces PRNP prion protein gene expression in breast cancer. Breast Cancer Res. 2013;15:R22. doi: 10.1186/bcr3398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jin T., Gu Y., Zanusso G., Sy M., Kumar A., Cohen M., Gambetti P., Singh N. The chaperone protein BiP binds to a mutant prion protein and mediates its degradation by the proteasome. J. Biol. Chem. 2000;275:38699–38704. doi: 10.1074/jbc.M005543200. [DOI] [PubMed] [Google Scholar]
- 68.Park K.-.W., Kim G.Eun, Morales R., Moda F., Moreno-Gonzalez I., Concha-Marambio L., Lee A.S., Hetz C., Soto C. The Endoplasmic Reticulum Chaperone GRP78/BiP Modulates Prion Propagation in vitro and in vivo. Sci. Rep. 2017;7:44723. doi: 10.1038/srep44723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hetz C., Russelakis-Carneiro M., Wälchli S., Carboni S., Vial-Knecht E., Maundrell K., Castilla J., Soto C. The disulfide isomerase Grp58 is a protective factor against prion neurotoxicity. J. Neurosci. 2005;25:2793–2802. doi: 10.1523/JNEUROSCI.4090-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Edenhofer F., Rieger R., Famulok M., Wendler W., Weiss S., Winnacker E.L. Prion protein PrPc interacts with molecular chaperones of the Hsp60 family. J. Virol. 1996;70:4724–4728. doi: 10.1128/JVI.70.7.4724-4728.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shen Y., Ding M., Xie Z., Liu X., Yang H., Jin S., Xu S., Zhu Z., Wang Y., Wang D., Xu L., Zhou X., Wang P., Bi J. Activation of Mitochondrial Unfolded Protein Response in SHSY5Y Expressing APP Cells and APP/PS1 Mice. Front. Cell Neurosci. 2019;13:568. doi: 10.3389/fncel.2019.00568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Klymchenko A.S., Kreder R. Fluorescent probes for lipid rafts: from model membranes to living cells. Chem. Biol. 2014;21:97–113. doi: 10.1016/j.chembiol.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 73.Levental I., Levental K.R., Heberle F.A. Lipid Rafts: controversies Resolved, Mysteries Remain. Trends Cell Biol. 2020;30:341–353. doi: 10.1016/j.tcb.2020.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sharma P., Varma R., Sarasij R.C., Ira null, Gousset K., Krishnamoorthy G., Rao M., Mayor S. Nanoscale organization of multiple GPI-anchored proteins in living cell membranes. Cell. 2004;116:577–589. doi: 10.1016/s0092-8674(04)00167-9. [DOI] [PubMed] [Google Scholar]
- 75.Lewis V., Hooper N.M. The role of lipid rafts in prion protein biology. Front. Biosci. (Landmark Ed) 2011;16:151–168. doi: 10.2741/3681. [DOI] [PubMed] [Google Scholar]
- 76.Mierke C.T., Bretz N., Altevogt P. Contractile forces contribute to increased glycosylphosphatidylinositol-anchored receptor CD24-facilitated cancer cell invasion. J. Biol. Chem. 2011;286:34858–34871. doi: 10.1074/jbc.M111.245183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.An E., Sen S., Park S.K., Gordish-Dressman H., Hathout Y. Identification of novel substrates for the serine protease HTRA1 in the human RPE secretome. Invest. Ophthalmol. Vis. Sci. 2010;51:3379–3386. doi: 10.1167/iovs.09-4853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Grau S., Richards P.J., Kerr B., Hughes C., Caterson B., Williams A.S., Junker U., Jones S.A., Clausen T., Ehrmann M. The role of human HtrA1 in arthritic disease. J. Biol. Chem. 2006;281:6124–6129. doi: 10.1074/jbc.M500361200. [DOI] [PubMed] [Google Scholar]
- 79.Tsuchiya A., Yano M., Tocharus J., Kojima H., Fukumoto M., Kawaichi M., Oka C. Expression of mouse HtrA1 serine protease in normal bone and cartilage and its upregulation in joint cartilage damaged by experimental arthritis. Bone. 2005;37:323–336. doi: 10.1016/j.bone.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 80.Kaur G., Stallmann D., Schanze N., Laumann R., Heger L.A., Steinfurt J., Stachon P., Peter K., Bode C., Moser M., Ahrens I., Duerschmied D., Hortmann M. Extracellular HtrA2 Induces Apoptosis in Human Umbilical Vein Endothelial Cells. Int. J. Mol. Sci. 2019;20:E5446. doi: 10.3390/ijms20215446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.D.S. Biard, New Insight on Entangled DNA Repair Pathways: stable Silenced Human Cells for Unraveling the DDR Jigsaw, DNA Repair - On the Pathways to Fixing DNA Damage and Errors. (2011). 10.5772/23681. [DOI]
- 82.Biard D.S.F. Untangling the relationships between DNA repair pathways by silencing more than 20 DNA repair genes in human stable clones. Nucleic Acids Res. 2007;35:3535–3550. doi: 10.1093/nar/gkm195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Vert J.-.P., Foveau N., Lajaunie C., Vandenbrouck Y. An accurate and interpretable model for siRNA efficacy prediction. BMC Bioinformatics. 2006;7:520. doi: 10.1186/1471-2105-7-520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Biard D.S., Kannouche P., Lannuzel-Drogou C., Mauffrey P., Apiou F., Angulo J.F. Ectopic expression of (Mm)Kin17 protein inhibits cell proliferation of human tumor-derived cells. Exp. Cell Res. 1999;250:499–509. doi: 10.1006/excr.1999.4515. [DOI] [PubMed] [Google Scholar]
- 85.Biard D.S., Saintigny Y., Maratrat M., Vozenin M.C., Martin M., Daburon F., Angulo J.F. Differential expression of the HsKin17 protein during differentiation of in vitro reconstructed human skin. Arch. Dermatol. Res. 1997;289:448–456. doi: 10.1007/s004030050220. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.