Highlights
-
•
Recent findings of cryo-EM structures of mammalian F1FO-ATPase.
-
•
The membrane-embedded domain of the F1FO-ATPase and the permeability transition pore.
-
•
The Ca2+-activated 1FO-ATPase role in the mPTP is consistent with recent cryo-EM findings.
-
•
The membrane-embedded FO participates in mPTP formation in mammalian mitochondria.
-
•
Conformational changes within FO modify the inner mitochondrial membrane shape.
Keywords: F1FO-ATPase, Mitochondria, Permeability transition pore, Calcium, Structures, Membrane
The mitochondrial F-type ATPase is a reversible nano-engine responsible for ATP synthesis/hydrolysis, morphology of inner mitochondrial membrane (IMM) by shaping cristae ridges, and fulcrum of the molecular events that trigger cell death by permeability transition pore (PTP) opening [1]. Recently the cryo-EM structures of the entire mammalian complex allowed to identify the different substeps of the rotational state and improved knowledge on some up to now misinterpreted subunits of the membrane domain FO [2,3]. Moreover, the different conformational states of the Ca2+-activated F1FO-ATP(hydrol)ase activity, undetected in the presence of the natural cofactor Mg2+, may constitute subsequent steps of a gradual PTP formation [2].
The F-type (F1FO) ATPase is composed of a hydrophilic catalytic F1 domain protruding in the mitochondrial matrix, where ATP synthesis/hydrolysis takes place, and a membrane-embedded FO domain that drives H+ translocation (Fig. 1A). The two domains are functionally and structurally connected by a central stalk which rotates within F1 and by a peripheral stalk (PS) that extends laterally for the entire enzyme complex height and acts as a stator. This molecular machine transduces the proton motive force, generated by substrate oxidation during mitochondrial respiration, by torque generation to chemical energy stored in the ATP form and vice versa [4].
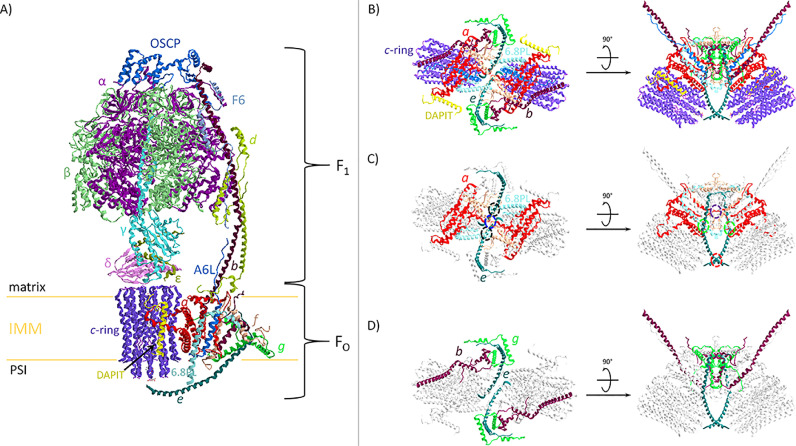
Fig. 1.
Mammalian F-type ATPase architecture. A) model of mitochondrial F1FO-ATPase monomer. Enzyme subunits are drawn as ribbon representations obtained from modified PDB ID code: 6TT7 [2]. B) Monomer-monomer interface of the membrane FO domain obtained from modified PDB ID code: 6ZA9 [2]. Top and side views of the membrane domains on the left and on the right, respectively. C) Contact sites between FO domains are shown by colored open circles with dashed lines: black (f-6.8PL) and blue (f-f) are at the matrix side; red (e-e) and green (e-6.8PL) are at the IMM positive side; violet (a-a) is in the IMM core. D) b, e, and g create the structure that bends the lipid bilayer by almost 115° The letter colors are the same as those of the subunits to which the structures belong.
The FO domain consists of c subunits that form an annular structure, the c8-ring, which in association with the a subunit, establishes the H+ translocation pathway [5]. The c-ring is linked to γ, δ, and ε subunits that extend from the IMM to the globular hexamer catalytic sector F1. Here, three catalytic β subunits alternate with three non-catalytic α subunits, sequentially changing their conformation as the rotor rotates, namely βE (empty), βDB and βTP, which differ in their affinities for nucleotides. The non-catalytic sites also undergo the three conformational states αE, αDP and αTP always occupied by adenine nucleotide [6]. The rotation direction of the rotor, transmitted to F1 by the central stalk, is anticlockwise, viewed from the matrix side, when ATP is built by ADP and Pi. As widely known, the enzyme catalysis is reversible and in the reverse mode the rotor rotates clockwise when ATP is hydrolyzed and the enzyme pumps H+ to re-energize the IMM. This reversal can occur under physiological and pathological conditions. The rotor rotation changes the properties of the three catalytic sites, namely their affinity for adenylate nucleotides, each 120° Thus, in the forward mode, each 360° rotation produces three ATP molecules. During the catalytic cycle of ATP hydrolysis the rotational states of mammalian mitochondrial F1-ATPase observed within a single 120° rotation shows 90° and 30° substeps, which differ from bacterial ones (80° and 40°, respectively) [7]. Recent advances pointed out that in mitochondria the first 90° substep is splitted into other 65° and 25° steps and then the second 30° substep completes one of the three rotational states [2]. The catalytic cycle during one 120° rotation consists of different steps, of which each corresponds to distinct rotation angles, namely 0° which corresponds to ATP binding, 65° to Pi release after ADP release, 90° to the catalytic dwell and ATP hydrolysis, which in turn generates the 30° rotation to complete the 120° rotation. All these interconverted events are based on the different subsequent conformations of the three catalytic β subunits. ADP produced by ATP hydrolysis is responsible for the absence of the steady-state F-ATPase activities by remaining entrapped in the β subunits. The adenine nucleotide bound to the non-catalytic α subunits are not essential for ATP synthesis. However, the MgADP inhibited form on the enzyme catalytic sites is only released when ATP binds to the non–catalytic sites [8]. The cryo-EM structure of F1 domain in MgADP inhibited form obtained by Sazanov's group also shows MgADP bound to all the three α subunits and in two β subunits in the βDP and βTP conformations [2]. Therefore, ATP bound to the non-catalytic sites is required during multiple ATP hydrolysis turnover, but in the α subunits adenine nucleotide exchange (ATP/ADP) may also occur, by allowing switch from active to inhibited enzyme.
The α3β3-spherical domain F1 connected to PS forms the F1FO-ATPase stator. The αTP, αDP, αE and the βDP and βE conformations of α and β subunits respectively, are joined to the OSCP (Oligomycin Sensitivity Conferring Protein) subunit, while the β subunit in the βTP conformation is only linked to F6 subunit. Moreover, α subunit in αDP conformation also interacts with F6, b, and d subunits. The PS starts from OSCP and continues with F6, d, b, and A6L in the membrane extrinsic stalk region, while the membrane portion consists of b and A6L subunits associated with the supernumerary subunits (sns) e, f, g, and 6.8 proteolipid (PL) [3]. Some sns are involved in the dimeric F1FO-ATPase structure, namely in the association of two monomers, and in the IMM bending (Fig. 1B). Accordingly, two monomers join to form a dimer and the monomer-monomer interface occupies the area matching the cristae edge. In mammals, the dimerization sites at the matrix side involve a direct f-f bond and the 6.8PL subunit of a monomer binds to the f subunit of the other monomer and vice versa. The N-terminal loops from a subunits establish a contact site within the IMM and, at the positive side of IMM (PSI), the dimer is directly linked by e-e bond plus the interaction between the 6.8PL subunit and e subunit of different monomers (Fig. 1C). Dimer association in yeast is different from mammals. Indeed, the yeast dimer is held together centrally by a and i/j subunit dimerization motifs and by interactions between k and e subunits on both sides of the supercomplex [9]. Noteworthy, the yeast i/j subunit is the ortholog of mammalian 6.8PL, while the yeast k subunit is the mammalian DAPIT subunit [2,3]. Accordingly, the a, e, f subunits, and 6.8PL lead to the mammalian F1FO-ATPase dimerization. The DAPIT subunit, considering its peripheral position in the membrane (Fig. 1B), forms rows of F1FO-ATPase dimers by side-by-side interactions of adjacent monomers with g and a subunits at the PSI and g subunits in the matrix, which corresponds to the negative side of the IMM. Moreover, DAPIT promotes the sharp bending of a subunit horizontal helix folded by a conserved proline residue to ensure a tight fit to the c-ring and to form the hydrophilic cavity of the half-channel which allows H+ exit. The formation of a larger oligomer, namely the F1FO-ATPase tetrameric structure, is due to a compact link between two g (within the IMM) and two e subunits (at the matrix side) of two monomers arranged diagonally. The inhibitory factor 1 (IF1), an endogenous protein which inhibits the mammalian F1FO-ATPase hydrolytic activity at low pHs, when is arranged in the dimeric form joins two F1 domains by targeting the βDP subunits of adjacent dimers. By such arrangement IF1 plays a structural role by stabilizing the dimer and presumably allows the physiological supramolecular arrangement of F1FO-ATPases in the mitochondrial membrane [10].
The structural architecture of the single transmembrane helix (H) of e subunit, the H3 of g subunit, and H2 and H3 of b subunit form a BAR-like domain in each monomer that is responsible for IMM bending [9] (Fig. 1D). The tight packing of helices allowed by conserved GxxxG motifs on He subunit and H3g is stabilized to the H2b by salt bridges, resulting in a compact triple transmembrane helix (TTMH) bundle [2]. Moreover, the H2b joined to H3b helix constitutes the U-turn structure which tilts the TTMH bundle. Interestingly, the e subunit is connected by a terminal lysine to a lipid plug (probably a lyso-phosphatidylserine) in the c-ring at the PSI, while the positive charges of Arg38 of c subunits coordinate the phosphatidylserine at the matrix side. The lipid regions of these two phospholipids are separated by Val16 of c subunits in the IMM core [2].
The dimeric F1FO-ATPase form, due to interactions between two identical monomers, changes during the catalysis by modifying the curvature of the mitochondrial cristae. When the enzyme is activated by Ca2+, which only supports CaATP hydrolysis [11], the Ca2+-activated F1FO-ATP(hydrol)ase causes disassembly/distortion of the entire F1FO complex with four different conformational states which are not detected when the enzyme is activated by the natural cofactor Mg2+ [2]. The structural modification of the enzyme complex is consistent with the Ca2+ signaling propagation pathway of the Ca2+-activated F1FO-ATP(hydrol)ase that, by undergoing appropriate rotational state(s), promotes the permeability transition pore [11]. The most likely hypothesis is that the channel formation is triggered when the PS accommodates conformational changes of F1 [12] during CaATP hydrolysis [11]. These conformational changes would generate a force transmitted along the b subunit to TTMH, thus pulling the lipid plug out of the c-ring by the proposed “bent-pull” model [13]. Concomitantly, water molecules fill the c-ring and push the phosphatidylserine on the matrix side (Fig S1). So, the PTP opening, arising from different conformations of the Ca2+-activated F1FO-ATP(hydrol)ase, is consistent with the enzyme monomerization and the disappearance of the cristae [2].
The recent advances on the F1FO-ATPase structures reveal quite unexpected enzyme functions at the molecular level and new biological roles of the enzyme complex in mitochondria [13].
Accordingly, the recently acquired knowledge on the enzyme structure is fully consistent with the Ca2+-activated F1FO-ATP(hydrol)ase participation in the stepwise mechanism of formation and opening of the PTP, which forms by c-ring distortion of and expulsion of the so-called lipid plug [2]. Indeed, the enzyme complex, endowed with dual catalytical role of ATP synthesis/hydrolysis, structural function in the maintenance of the cristae and functional/structural task in the formation of the lethal PTP, constitutes an amazing multitasking mechanism whose multiple functions span from health to mitochondrial diseases and death [14,15].
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.bbadva.2020.100001.
Appendix. Supplementary materials
References
- 1.Nesci S., Pagliarani A., Algieri C., Trombetti F. Mitochondrial F-type ATP synthase: multiple enzyme functions revealed by the membrane-embedded FO structure. Crit. Rev. Biochem. Mol. Biol. 2020;55:309–321. doi: 10.1080/10409238.2020.1784084. [DOI] [PubMed] [Google Scholar]
- 2.Pinke G., Zhou L., Sazanov L.A. Cryo-EM structure of the entire mammalian F-type ATP synthase. Nat. Struct. Mol. Biol. 2020 doi: 10.1038/s41594-020-0503-8. [DOI] [PubMed] [Google Scholar]
- 3.Spikes T.E., Montgomery M.G., Walker J.E. Structure of the dimeric ATP synthase from bovine mitochondria. Proc. Natl. Acad. Sci. U.S.A. 2020;117:23519–23526. doi: 10.1073/pnas.2013998117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Junge W., Sielaff H., Engelbrecht S. Torque generation and elastic power transmission in the rotary F(O)F(1)-ATPase. Nature. 2009;459:364–370. doi: 10.1038/nature08145. [DOI] [PubMed] [Google Scholar]
- 5.Klusch N., Murphy B.J., Mills D.J., Yildiz Ö., Kühlbrandt W. Structural basis of proton translocation and force generation in mitochondrial ATP synthase. Elife. 2017;6:e33274. doi: 10.7554/eLife.33274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hahn A., Parey K., Bublitz M., Mills D.J., Zickermann V., Vonck J., Kühlbrandt W., Meier T. Structure of a Complete ATP Synthase Dimer Reveals the Molecular Basis of Inner Mitochondrial Membrane Morphology. Mol. Cell. 2016;63:445–456. doi: 10.1016/j.molcel.2016.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adachi K., Oiwa K., Nishizaka T., Furuike S., Noji H., Itoh H., Yoshida M., Kinosita K. Coupling of rotation and catalysis in F(1)-ATPase revealed by single-molecule imaging and manipulation. Cell. 2007;130:309–321. doi: 10.1016/j.cell.2007.05.020. [DOI] [PubMed] [Google Scholar]
- 8.Bald D., Amano T., Muneyuki E., Pitard B., Rigaud J.L., Kruip J., Hisabori T., Yoshida M., Shibata M. ATP synthesis by F0F1-ATP synthase independent of noncatalytic nucleotide binding sites and insensitive to azide inhibition. J. Biol. Chem. 1998;273:865–870. doi: 10.1074/jbc.273.2.865. [DOI] [PubMed] [Google Scholar]
- 9.Guo H., Bueler S.A., Rubinstein J.L. Atomic model for the dimeric FO region of mitochondrial ATP synthase. Science. 2017;358:936–940. doi: 10.1126/science.aao4815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gu J., Zhang L., Zong S., Guo R., Liu T., Yi J., Wang P., Zhuo W., Yang M. Cryo-EM structure of the mammalian ATP synthase tetramer bound with inhibitory protein IF1. Science. 2019;364:1068–1075. doi: 10.1126/science.aaw4852. [DOI] [PubMed] [Google Scholar]
- 11.Algieri C., Trombetti F., Pagliarani A., Ventrella V., Bernardini C., Fabbri M., Forni M., Nesci S. Mitochondrial Ca2+ -activated F1 FO -ATPase hydrolyzes ATP and promotes the permeability transition pore. Ann. N. Y. Acad. Sci. 2019;1457:142–157. doi: 10.1111/nyas.14218. [DOI] [PubMed] [Google Scholar]
- 12.Giorgio V., Burchell V., Schiavone M., Bassot C., Minervini G., Petronilli V., Argenton F., Forte M., Tosatto S., Lippe G., Bernardi P. Ca(2+) binding to F-ATP synthase β subunit triggers the mitochondrial permeability transition. EMBO Rep. 2017;18:1065–1076. doi: 10.15252/embr.201643354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mnatsakanyan N., Jonas E.A. ATP synthase c-subunit ring as the channel of mitochondrial permeability transition: regulator of metabolism in development and degeneration. J. Mol. Cell. Cardiol. 2020;144:109–118. doi: 10.1016/j.yjmcc.2020.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nesci S. The mitochondrial permeability transition pore in cell death: a promising drug binding bioarchitecture. Med Res Rev. 2020;40:811–817. doi: 10.1002/med.21635. [DOI] [PubMed] [Google Scholar]
- 15.Carraro M., Carrer A., Urbani A., Bernardi P. Molecular nature and regulation of the mitochondrial permeability transition pore(s), drug target(s) in cardioprotection. J. Mol. Cell. Cardiol. 2020;144:76–86. doi: 10.1016/j.yjmcc.2020.05.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.