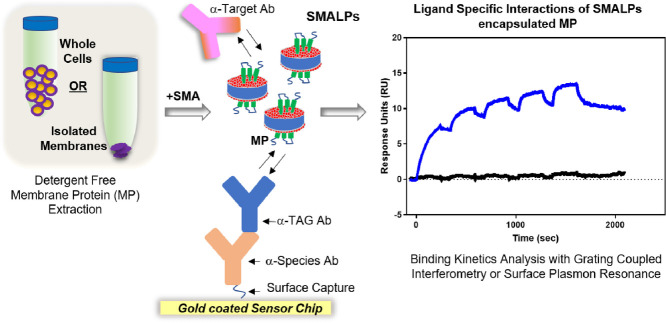
Highlights
-
•
Encapsulation of target membrane protein (MP) complexes with SMALP (Styrene maleic acid lipid Particles) was established as detergent free approach for binding characterization.
-
•
Binding kinetics of interactions of therapeutic antibodies or tool reagents associated with target MP were determined by Grating-coupled interferometry (GCI) and surface plasmon resonance based biosensor platforms creoptix WAVEsystem and biacore respectively.
-
•
SMALPs based purification were observed to be capable of both qualitative and quantitative analysis of membrane protein interactions with a variety of antibodies in specific manner.
-
•
SPR data demonstrated that SMA based stabilization of membrane proteins is a precise and efficient procedure for label-free kinetic analysis of diverse antibody binding interactions as both purified and complex mixture environment.
-
•
Integrated SMALP-Biosensor platform developed for multiple membrane proteins of interest demonstrated the strength of the two techniques and provides a robust label-free approach to quantify membrane target binding directly with diverse ligands.
Keywords: Grating-coupled Interferometry (GCI), Surface Plasmon Resonance (SPR), Antibodies and SMALPs (Styrene Maleic Acid Lipid Particles), Membrane Proteins (MP), Binding Kinetics, Label Free, Detergent Free
Abstract
The fundamental importance of membrane protein (MP) targets in central biological and cellular events has driven a marked increase in the use of membrane mimetics for exploring these proteins as therapeutic targets. The main challenge associated with biophysical analysis of membrane protein is the need for detergent extraction from the bilayer environment, which in many cases causes the proteins to become insoluble, unstable or display altered structure or activity. Recent technological advances have tried to limit the exposure of purified membrane protein to detergents. One such method involves the amphipathic co-polymer of styrene and maleic acid (SMA), which can release lipids and integral membrane proteins into water soluble native particles (or vesicles) termed SMALPs (Styrene Maleic Acid Lipid Particles). In this study, assay conditions that leverage SMA for membrane protein stabilization were developed to perform kinetic analysis of antibody binding to integral membrane protein and complexes in SMALPs in both purified and complex mixture settings using multiple biosensor platforms. To develop a robust and flexible platform using SMALPs technology, we optimized various SPR assay formats to analyze SMALPs produced with cell membrane pellets as well as whole cell lysates from the cell lines overexpressing membrane protein of interest. Here we emphasize the extraction of model membrane proteins of diverse architecture and function from native environments to encapsulate with SMALPs. Given the importance of selected membrane targets in central biological events and therapeutic relevance, MP-specific or tag-specific antibodies were used as a proof-of-principal to validate the SMALPs platform for ligand binding studies to support drug discovery or tool generation processes. MP-SMALPs that retain specific binding capability in multiple assay formats and biosensors, such as waveguide interferometry and surface plasmon resonance, would be a versatile platform for a wide range of downstream applications.
Graphical abstract
Introduction
Membrane proteins (MP) comprise a significant percentage of the expressed cellular proteome and their interactions control many important cellular functions such as transport and signal transduction [1]. Consequently, membrane proteins represent a major portion of current therapeutic targets [2]. Despite their significance in cellular function and potential for therapeutic intervention, biophysical analysis of membrane proteins remains understudied because of inherent issues related to their isolation. Membrane protein are not only difficult to express in quantities necessary for study, but their purification and analysis are challenging due to hydrophobic patches that associate within the bilayer environment. Typically, in vitro studies rely on detergents to extract membrane proteins from the bilayer, followed by solubilization and stabilization of membrane proteins [3]. Although there are several successful reports to solubilize membrane protein into detergent micelles for various applications, [4], [5], [6], [7], [8], detergent treatment can often be quite harsh, resulting in the disruption and unfolding of the native protein structure, and in turn affecting protein function. This alteration of protein structure and function often is a direct result of the membrane protein being removed from its native lipid environment. To confound the issues with detergents, expensive and labor-intensive screening campaigns to find a detergent that works well for membrane protein solubilization are often needed [9,10]. These challenges associated with solubilizing membrane proteins in a detergent environment while preserving native protein structure and enabling biophysical study have led to the development of alternative membrane mimetics.
Typically, to avoid aggregation of transmembrane surfaces, detergents are used at 10–20 times above their critical micellar concentration (CMC), which leads to formation of free micelles [6,8]. In the presence of free micelles, membrane protein-associated lipids, co-factors and subunits can dissociate from the complex into free micelles and may often cause the inactivation of the membrane protein [11]. The development of reagents that have a high affinity for the transmembrane domains have limited dissociation and protein inactivation. An example of a high affinity transmembrane domain-binding molecule are the amphipathic polymers termed amphipols (AmPol) [12]. The polymeric nature of an amphipol produces many more transmembrane domain association points compared to a traditional detergent, which leads to higher affinity and avoids dissociation of the membrane protein. Taken together, amphipols can complex with membrane proteins at their transmembrane domains and render them water soluble and stable. Amphipols, however cannot efficiently solubilize proteins from membranes, even though they are amphipathic and can partition into the membrane [13], [14], [15], [16]. Therefore, membrane proteins need to first be extracted and purified before they can be transferred to amphipols.
The lipid environment found in the bilayer can affect proper folding, assembly and function of membrane proteins. Thus, providing the proper lipid environment during membrane protein purification can have a direct impact on successful biophysical analysis of membrane proteins [17]. Since detergent micelles take the membrane protein out of its native environment methods such as nanodiscs were developed to maintain membrane protein/native lipid interaction. Nanodiscs are discoidal lipoprotein bilayers which stabilize membrane protein and bilayer-lipid interactions with two encircling amphipathic protein belts termed membrane scaffold proteins (MSPs). MSPs can be engineered to produce particles ranging in size from 6 to 16 nm [18,19]. Nanodiscs undergo a self-assembly process after combining all detergent-solubilized components (lipid, membrane protein and MSP), which is followed by incubation with hydrophobic beads for detergent removal. The stabilized lipoprotein particle renders the membrane protein complexes stable in an aqueous solution and allows their study with several biophysical techniques, such as SPR and NMR [20]. However, like AmPol, solubilization of membrane is required prior to nanodiscs assembly.
Given the diversity of the membrane proteome, there is not a one-size-fits-all solution for detergent extraction and stabilization of membrane proteins. Although advances have been made, newer techniques (e.g., AmPol and Nanodiscs) developed to study membrane proteins still suffer from the need for detergent extraction prior to stabilization with these novel mimetic platforms [21], [22], [23]. Recently, the use of the polymer SMA [poly (styrene-co-maleic acid)] has been investigated for membrane protein purification with the aim to avoid the necessity for detergent extraction [22]. The co-polymer SMA is comprised of alternating groups of maleic acid (hydrophilic) and styrene (hydrophobic) that by nature make it amphipathic and able to insert into biological membranes [24]. Studies have shown that SMA intercalates into membranes by styrene groups inserting between the acyl chains of the lipid bilayer, while maleic acid groups face the solvent environment [22]. Through this association within the bilayer, SMA assembles into disk-like structures that contain and stabilize portions of the lipid bilayer that are surrounded by an SMA belt-like structure [21]. The disk-like structures of SMALPs (Styrene Maleic Acid Lipid Particles) are similar in structure to the nanodiscs previously described, with a diameter ranging from 9 to 11 nm [22]. After treatment of membranes with SMA, the SMALPs can contain an integral membrane protein. Many studies have shown that recombinantly expressed membrane protein can be purified and studied using conventional methods once encapsulated into a SMALP [21,[24], [25], [26], [27], [28], [29], [30]].
Biosensor instruments employing various technologies such as Surface Plasmon Resonance (SPR – Biacore), Grating-coupled Interferometry (GCI – Creoptix) and Biolayer Interferometry (BLI – Octet) are used for real time and label-free investigation of molecular interactions [3,31]. Real time measurement is advantageous because it allows kinetic analysis for determination of association and dissociation rates and binding affinities. The versatility of these instruments allows for a wide range of interactions to be measured, such as protein-protein, small molecule-protein and antibody-antigen interactions, in studies ranging from a single molecule to a large library of molecules. Biosensors-based binding methods have become well adapted for kinetic analysis of soluble proteins, [32], [33], [34], [35], [36], [37], [38], [39], [40] but implementation of the technology on membrane protein targets are challenging due to a variety of reasons, such as purification of the conformationally active state [3]. With binding interaction studies as well as with other types of membrane protein analysis, the main hurdle is to purify, and surface capture the membrane protein under conditions that maintain its native structure and activity.
The assay we report here demonstrates the application for kinetic analysis of antibodies binding to membrane proteins that have been stabilized in SMALPs in both a purified and crude cell lysate setting. The optimal conditions were developed to be seamlessly transferable to various biosensor instruments (SPR and GCI) platforms as well as potentially wide range of membrane proteins.
In this current report, we selected MPR1 (Membrane Protein Receptor 1), MPA1 (Membrane Protein Adaptor 1) and MPR2 (Membrane Protein Receptor 2) as model transmembrane protein targets for development of SMALPs technique based purification and binding assays. Selected sets of membrane proteins with different architectures and oligomeric states can be routinely purified in SMALPs, and the approaches presented here could be applicable on diverse membrane protein of research and therapeutic interest.
MPA1 belongs to a family of type I adaptor proteins [41] which associates with a variety of immune signaling receptors in the transmembrane domain such as MPR1. MPA1 is a prototype transmembrane protein that consists of nearly 30 amino acid (aa) residues with a molecular weight (MW) range of 10–12 kDa and is widely expressed in diverse immune cells. MPR1 has average MW ∼ 30 kDa depending on post-translational modifications. Association with MPA1 is critical for signal transfer activity of MPR1 to recognize a wide range of ligands in mammalian tissues [42].
A type-II transmembrane receptor MPR2 of estimated MW ∼ 33 kDa is another model membrane protein target explored in this study [43]. MPR2 is generally expressed on cell surface and recognize a diverse range of ligands for downstream signal processing to mediate inflammation and immune responses. All three membrane proteins MPA1, MPR1 and MPR2 may have biological and therapeutic relevance in critical disorders, and interaction with various ligands could play an important role in the drug discovery process [42].
Here we emphasize the detergent free extraction of membrane proteins MPR1, MPA1 and MPR2 using SMALPs technology. Given the importance of selected membrane proteins in biological events, target-specific or tag-specific antibodies were used as a proof-of-principle to validate SMALPs with future potential for binding studies to support drug discovery or reagent generation processes. The integrated SMALPs-Biosensor platform for membrane protein targets of interest we present demonstrates the strength of the two techniques and provides a reliable label-free platform to quantify membrane proteins direct binding with diverse affinity and kinetics ligands.
Materials and methods
Reagents
All general biochemical reagents were obtained from ThermoFisher (Waltham, MA) and Sigma Aldrich (St. Louis, MO). Biacore reagents were obtained from GE Healthcare (Piscataway, NJ). Creoptix Waveguide reagents were from Creoptix (Wadenswil, Switzerland). Tag-specific (α-TAG) antibodies, anti-FLAG and anti-His, Anti-Human (hu) Fc (Fragment crystallizable region), anti-Murine (mu) Fc were obtained from Jackson Laboratories (West Grove, PA) or Sigma Aldrich (Munich, Germany). Target-specific (α-target) antibodies anti-MPR1 and anti-MPR2 were developed with our in-house research tool development programs (method generation of antibodies is beyond the scope of this paper). SMA-2000 was from Cray-Valley (Exton, PA). 96-well microtiter plates used for sample injections were obtained from Costar/Corning Inc. (Corning, NY).
Expression of membrane proteins
HEK293–6E cells were used for transient co-expression of MPR1/MPA1. HEK293–6E cells transfection was done with polyethylenimine (PEI) from Polysciences. Non-linearized DNA and PEI were diluted and mixed in Freestyle F-17 media (Life Technologies #13,835) for 10 min at room temperature. HEK293–6E cells were washed with PBS and resuspended in F-17 media. The resuspended cells were then mixed with the DNA/PEI complex with final cell density at 1e6 /ml (milliliter) and incubated for 5–6 h at 37 °C + 5% CO2 with shaking. After the incubation, yeastolate (BD Biosciences #292,805) was added and incubated at 37 °C + 5% CO2 for 7 days for protein expression. Finally, cells were frozen as whole-cell pellets with confirmed co-expression of MPA1 and MPR1 with N-term FLAG and C-term His-tags respectively by Western blot analysis with tag or target-specific antibodies.
CHO-S cells transfection and protein expression for MPR2
CHO-S cells transfection was done by Lipofectamine LTX reagent from ThermoFisher Scientific (Cat. No.15338500) in a 24-well format. Non-linearized DNA and Lipofectamine LTX were diluted in 0.5 ml Opti-MEM media respectively. Then, the diluted DNA and Lipofectamine LTX were mixed together thoroughly and incubated at room temperature for 15–20 min. 1e6 viable CHO-S cells were washed with PBS and resuspended in 1 ml of Opti-MEM. The resuspended CHO-S cells were then mixed with the DNA/Lipofectamine complex and incubated for 5–6 h at 37 °C + 5% (percentage) CO2 with shaking. After the incubation, an additional 2 ml of MIX-6 media (50% CD-CHO; 50% imMEDIAte Advantage (SAFC #66,714–1000ML Formerly known as Sigma CHO Medium 5 #C0363–1 L) was added. After 2 days, the media was changed to selection media (MIX-6 media with 10ug/ml puromycin (Gibco #A11138–03). Cell viability was measured, and the selection media was changed every 2 days until viability reached 90%. The recovered cells were expanded and seeded at 1.5e6 /ml density in MIX-6 media for protein expression at 37 °C + 5% CO2 for 7 days. Harvested cells were frozen as whole-cell pellets with confirmed expression of MPR2 with FLAG tags and His-tags by Western blot analysis.
Preparation of cell membranes
Cell pellets previously harvested and stored at −80 °C were thawed and resuspended in Tris Buffered Saline at pH 8.0 (TBS) (Sigma Aldrich) with Roche protease inhibitor (4,693,132,001) (1 tablet/25 ml suspension) and disrupted using a Parr cell disruption bomb at 70 Barr for 30 min. After disruption, cell lysates were Dounce homogenized on ice with 30 strokes. Cell homogenates were centrifuged at 3000 x g for 7 min to pellet remaining cells and nuclei, followed by ultracentrifugation at 100,000 x g for 1 hour to pellet membranes. Membrane pellets were resuspended in 1X TBS and Dounce homogenized with 30 strokes. Protein concentration was determined using a Pierce BCA protein assay (Thermo Fisher) and membranes were adjusted to 30 mg/ml total protein, snap frozen in liquid nitrogen and stored at −80 °C in 1.5 ml aliquots.
Hydrolysis of styrene maleic anhydride copolymer
The SMA copolymer (2:1 styrene to maleic anhydride ratio) (Cray Valley) used was hydrolyzed as previously described by Dafforn et al. [23].
SMALPs generation from cell membranes
Frozen cell membranes were thawed on ice and resuspended in an equal volume of 4% SMA in 1X TBS with Roche protease inhibitor (1 tablet/25 ml suspension). The membrane suspension was then rotated at 4 °C overnight, followed by ultracentrifugation at 100,000 x g for 60 min to pellet any insoluble material. Alternatively, for small volumes the solubilized material was filtered using 0.45 µm (micrometer) centrifugal filters (Thermo Fisher). After clarification by centrifugation or filtration, the material was now ready for purification of SMALPs containing the membrane protein of interest or binding studies using total SMALPs.
SMALPs generation from frozen cell pellets
Frozen cell pellets were thawed on ice and resuspended in 2% SMA in 1X TBS with Roche protease inhibitor (4,693,132,001) (1 tablet/25 ml suspension) and 100 U of Benzonase at a concentration of 50 mg/ml based on wet weight of the cell pellet (e.g. 2 liters of spun down cell culture pellets in 50 ml 1X TBS with additives adjusted). The membrane suspension was then pipetted to ensure proper mixing followed by rotated incubation at 4 °C overnight to solubilize the membrane. The solubilized material was then centrifuged at 100,000 x g for 60 min to pellet any insoluble material. Alternatively, for small volumes the solubilized material was filtered using 0.45 µm centrifugal filters (Thermo Fisher). The supernatant was now ready for purification of SMALPs containing the membrane protein of interest by affinity chromatography or binding studies using total SMALPs.
Purification of histidine-tagged membrane proteins in SMALPs
Clarified total SMALPs were combined with TBS pre-equilibrated Ni-NTA resin (100 µl bed volume (bv) per ml of solubilized protein,) (Qiagen) and allowed to rotate overnight at 4°°C. The material was placed in an empty column and allowed to settle. After collection of the column flow through, the following sequential steps were performed; (1) 30 column volumes (CV) of TBS, (2) 20 CV of TBS + 20 mM (millimolar) imidazole (Thermo Fisher) and (3) elution of bound proteins with 10 CV of TBS + 200 mM imidazole. Eluted samples were buffer exchanged and concentrated into TBS using microcentrifuge spin concentrators (Sigma Aldrich) with the appropriate molecular weight cutoff (MWCO).
Purification of FLAG-Tagged membrane proteins in SMALPs
Clarified total SMALPs were combined with TBS pre-equilibrated anti-FLAG resin (150 µl /1 ml purified protein) (Sigma Aldrich) and allowed to rotate overnight at 4 °C. The material was placed in an empty column and allowed to settle. After collection of the column flow through, the following sequential steps were performed; (1) 30 column volumes (CV) of TBS plus 350 mM NaCl and (2) elution of bound proteins with 5 CV of TBS + FLAG peptide (Sigma Aldrich). Eluted samples were buffer exchanged and concentrated into TBS using microcentrifuge spin concentrators (Sigma Aldrich) with the appropriate molecular weight cutoff (MWCO).
Minimal biotinylation of capture antibodies
Anti-Human Fc, anti-Murine Fc (Jackson ImmunoResearch) and anti-FLAG M2 (Sigma Aldrich) antibodies were diluted to 1.0 mg/ml (6.7 µM) in phosphate buffered saline (PBS) (Gibco). EZ-Link Biotin Reagent (Thermo Fisher) stocks were prepared at 10 mM in 100% DMSO (Sigma Aldrich) and frozen in single-use aliquots at −80 °C. Working stocks were prepared by dilution to 4.7 µM in PBS. To mitigate over-biotinylation issues, minimal biotinylation of antibodies was performed at random lysine residues, a protein (6.7 µM) to biotin reagent (4.7 µM) ratio of 0.7 was used as reported previously [44,45]. Biotinylation reactions were run for 1 hour at room temperature before desalting into PBS using Zeba 7000 MWCO spin filters (Thermo Fisher). Single-use aliquots of the various antibodies were made and frozen at −80 °C.
Grating-coupled interferometry experiments
Grating-coupled interferometry experiments were conducted on a Creoptix Waveguide system (Creoptix WAVEdelta) [46], [47], [48]. A PCH (Polycarboxylate Hydrogel) chip was used to create an anti-Human Fc (Jackson ImmunoResearch) surface for capture of specific anti-target antibodies (MPR1 and MPR2). Specifically, in 0.2X PBS (Gibco) a PCH chip was conditioned for 3 min at 10 µl/min with 0.1 M borate/1 M NaCl at pH 9.0 (Creoptix) followed by activation with 1-ethyl-3-(3-dimethylaminopropylcarbodi-imide (EDC)/N-hydroxysuccinimide for 7 min at 10 µl/min. The anti-species antibody was then introduced to the surface at 10 µg/ml in 10 mM Sodium Acetate (pH 4.5) for 10 min at 10 µl/min. Finally, the remaining activated free carboxyl groups on the chip surface were inactivated by injection of 1 M ethanolamine for 7 min at 10 µl/min. Target-specific antibodies were then captured to the chip surface through their Fc (fragment crystallizable region) by injection of a 200 nM antibody solution for 5 min at 10 µl/min. Capture level or Binding level are presented with RU (response units). Multi cycle binding experiments were performed with a running buffer composed of 50 mM Tris pH 8.0, 250 mM NaCl and 0.002% SMA. Various concentrations of SMALPs were injected over the chip surface at flow rate of 30 µl/min with an association time of 240 s followed by a dissociation time of 600 s.
Surface plasmon resonance experiments
Surface plasmon resonance (SPR) experiments were conducted on a Biacore T100 system that has been upgraded to T200 sensitivity (GE Healthcare) [49], with a running buffer of 10 mM HEPES pH 7.4, 150 mM NaCl, 0.1 mg/ml BSA, 1 mM CaCl2, 0.013% Tween-20. A Biacore CAP chip was used for all experiments. The CAP chip was functionalized by (1) Regeneration for 3 × 60 s at 10 µl/min with 6 M Guanidine/0.25 M NaOH, (2) Hybridization of oligonucleotide Biotin capture reagent (GE Healthcare) for 6 min at 2 µl/min and (3) capture of biotinylated antibody at a concentration of 100 nM for 6 min at 10 µl/min. Total SMALPs produced from either isolated membranes or frozen cell pellets were diluted to ∼0.5 mg/ml total protein and captured to various capture antibodies for 10 min at 5 µl/min. Single cycle binding experiments were performed with 5 concentrations of antibodies injected over the chip surface at a flow rate of 50 µl/min with an association time of 240 s followed by a dissociation time of 480 s.
Gel electrophoresis of purified SMALPs
Purified SMALPs were analyzed by the electrophoresis methods of denaturing SDS-PAGE and native SMA-PAGE as described previously by Pollock et al. [50]. Briefly, for native SMA-PAGE, 4–20% precast gradient gels (Mini-Protean TGX and Criterion TGX, Bio-Rad, U.K.) were used. SMA-PAGE was run using Tris/glycine running buffer (25 mM Tris pH 8.8, 192 mM glycine) at 4 °C, 150 V for 60 min or until the dye migrated to the lowest visible area of the gel. 1 mg/ml bromophenol blue in 20 mM Tris pH 8.0 and 50% (v/v) glycerol was used as native loading dye. SDS-PAGE was run using NuPAGE™ Novex™ 4–12% Bis-Tris pre-cast polyacrylamide gels and NuPAGE™ MOPS buffer with 0.1% (w/v) SDS as running buffer to obtain different separation ranges. The denaturing gel sample buffer was 4X LDS (Invitrogen, U.K.) and gels were run at 180 V for 45 min at room temperature. To detect protein, gels were stained with InstantBlue (Expedeon, U.K.) silver stain or Ponceau S stain according to standard protocols and manufacturers' instructions. Invitrogen Novex SeeBlue Plus2 Pre-Stained MW Standard (198–3 kDa) was loaded with each native and denaturing gel. In-gel digestion on collected bands was performed with trypsin/Lys-C from Promega and then run on Thermo FUSION with previously published methods [51], [52], [53].
Data processing and management
Analysis of binding interactions was performed using instrument supplied software (Biacore T200 Evaluation v3.2 or Scrubber software v2.0c (Biologic Software). Sensorgrams from various experiments were plotted using Prism v5.01 (GraphPad).
Results
We selected co-expressed membrane proteins MPR1/MPA1 and a different type of receptor protein MPR2 as model membrane protein to develop SMALPs-based extraction and label-free SPR and GCI binding assays. MPR1/MPA1 function as a dimer that require co-expression of the two genes, and MPR2 was expressed separately from MPR1/MPA1. For MPR1/MPA1 co-expression, incorporation of an N-terminal FLAG tag allowed unambiguous immunodetection by exploiting the high selectivity and specificity of anti-FLAG M2 antibodies. On the other hand, the C-terminal 10xHis tag was introduced to ensure a more stable interaction with the Ni–NTA resin used for the next step in purification of SMALPs [24]. C-terminal His-tags were preceded by the TEV site for subsequent cleavage in planned future experiments. Overexpressed proteins (MPA1::TEV::FLAG + MPR1::TEV::10xHis), MPR2::TEV::10xHis and MPR2::TEV:: FLAG were incorporated into SMALPs and purified from cells using either anti-FLAG or Ni-NTA resin. Total SMALPs generated from the membrane isolation step and whole-cell pellets were examined for potential target-specific SMALPs capture on a biosensor chip followed by antibody binding and comparison of the results. Membrane protein extraction directly from whole cells was carried out to determine the feasibility of excluding the additional step of membrane preparation (or isolation) prior to purification of the target of interest.
Preparation and characterization of MPR1/MPA1 and MPR2 SMALPs
MPR1-MPA1 complex SMALPs and MPR2 SMALPs produced from isolated membranes or whole cell pellets were analyzed by both denaturing (SDS) and non-denaturing (SMA) polyacrylamide gel electrophoresis (PAGE) to check the quality of purified membrane proteins.
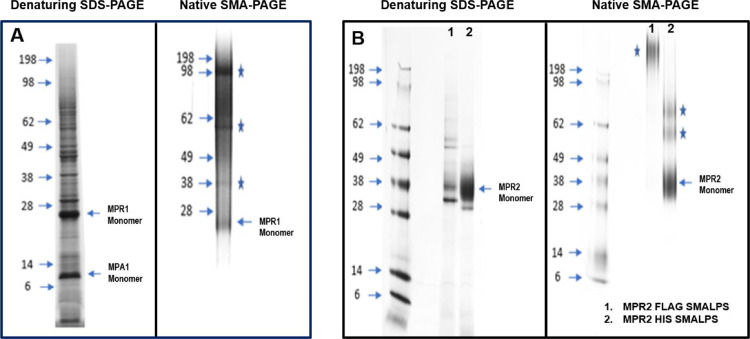
Molecular mass of the proteins MPR1/MPA1 was first estimated by gel electrophoresis with denaturing SDS-PAGE (Fig. 1A). The size of MPR1 and MPA1 monomer was observed as expected from sequencing around 28 kDa and 12 kDa respectively. MPR2 monomer was observed as smeared bands between 28 kDa and 38 kDa within range of the expected MW of 33 kDa (Fig. 1B). These MW estimations were approximately matched with target bands or smears observed in native gel. SDS-PAGE observed bands were more defined for monomeric proteins, on the other hand SMA-PAGE revealed more smeared bands for MPR1/MPA1 as well as MPR2, indicating large sized complexes as expected for SMALPs captured membrane proteins. Consistent with recent reports, [23,50] we observed that SMA migrates separately from the protein (Fig. 1) and is sensitive to standard protein stains. SMA bands were observed only in denaturing gel at a MW of around 5–10 kDa, (Fig. 1) however this staining was not observed in the native gel. This observation suggested that membrane protein remains associated in SMA-PAGE and membrane lipid can be extracted from protein bands after SMA-PAGE [24,26,50]. Both electrophoresis analyses were further combined with in-gel digestion and mass spectrometric analysis [52] to evaluate the proteins’ integrity, MW, and sequences (data not shown). Co-expressed proteins MPR1 and MPA1 can exist as hetero-dimers or higher order oligomers, whereas MPR2 can exist both as monomer and dimer. SDS-PAGE and SMA-PAGE analysis also identified SMALPs containing membrane proteins of various quaternary structures or protein complex stoichiometries. The SMA-PAGE analysis allowed us to understand whether the MPR1-MPA1 complex and MPR2 migrated as intact SMALPs and to find if they retained associated lipids. Interestingly, from in-gel digestion analysis we observed membrane lipids such as apolipoprotein A in protein bands from SMA-PAGE, but not from SDS-PAGE of the same samples. Moreover, with SMA-PAGE for SMALPs containing MPR1/MPA1 complex, we observed a dimer and higher order oligomer around 98 and 198 (as marked with stars in left panels of Fig. 1A and 1B) which is consistent with previous reports for this receptor-adaptor pair. Additionally, due to its extremely hydrophobic aa sequence as well as complex formation, the MPA1 band was very weak in SMA-PAGE compared to SDS-PAGE.
Fig. 1.
Purification and Characterization of SMALPs produced from whole cell pellets and isolated membranes. SMALPs produced from isolated membranes or whole-cell pellets were analyzed by both denaturing and non-denaturing polyacrylamide gel electrophoresis (PAGE). (A) MPR1/MPA1 SMALPs purified from whole cells using either anti-FLAG or Ni-NTA resin. The SMALPs were analyzed by SDS-PAGE and SMA-PAGE. (B) MPR2 SMALPs purified from isolated membranes using either anti-FLAG or Ni-NTA resin. The SMALPs were analyzed by SDS-PAGE and SMA-PAGE. Blue stars identify SMALPs containing membrane proteins of various quaternary structures or protein complex stoichiometries.
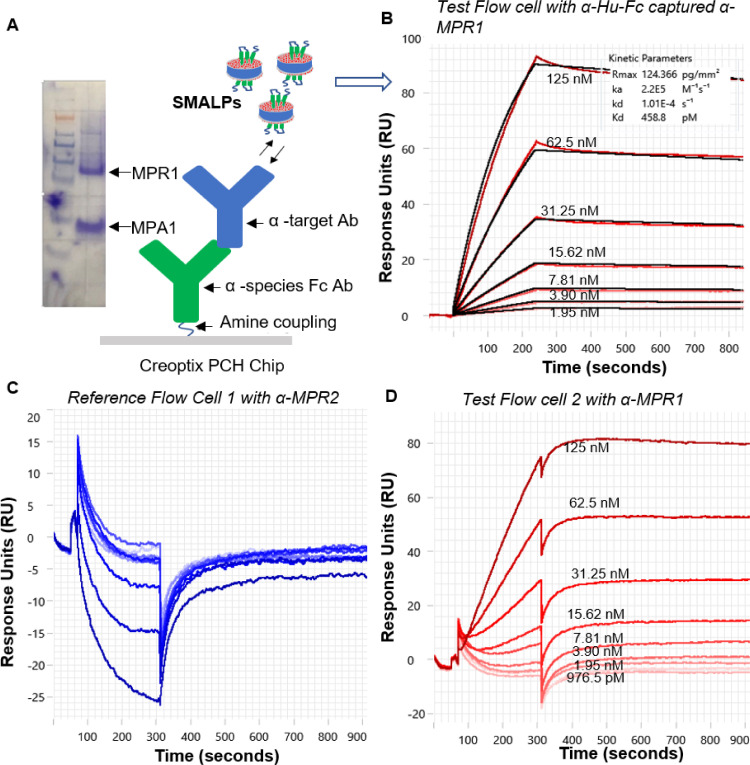
Demonstration of specific antibody binding interactions with purified SMALPs using grating-coupled interferometry
The functionality and specific antibody binding interactions of the purified membrane protein into SMALPs were analyzed through SPR binding assays on a Creoptix Waveguide GCI system. The surface was prepared by amine coupling of anti-human Fc on a PCH chip followed by capture of specific α-target antibodies (MPR2 and MPR1) (Fig. 2A). Increasing concentrations of purified MPR1/MPA1 SMALPs in solution were interacted with surface-bound α-target antibodies. These target-specific antibodies were developed into other discovery campaigns and were found to be highly active in modulating MPR1 and MPR2 functions in various cell-based studies (unpublished data). Binding was confirmed by kinetic analysis of purified MPR1/MPA1 SMALPs and α-MPR1 antibody. A 1:1 kinetic model fit of referenced binding response resulted in a KD of 459 pM (picomolar) with all dose-response sensorgrams under the calculated maximum response (Rmax). Very slow association and dissociation rates were observed for MPR1/MPA1 and α-MPR1 antibody interactions (Fig. 2B). Binding kinetics or association and dissociation rates could be influenced by avidity, very high (pM range) affinity, specific conformation of ligands, or diffusion coefficient of the SMALPs into SPR chip environment. Therefore, not all sites may saturate with binding [54]. Curvature in the dissociation phase suggests it's a true binding event, and that controlling ligand and analyte concentrations, as well as parameters such as flowrate and injection time, can potentially modulate the equilibrium phase to a more saturable binding.
Fig. 2.
Purified SMALPs bind specifically to surface captured α-Target antibodies. Increasing concentrations of purified MPR1/MPA1 SMALPs in solution were interacted with surface bound α-target antibodies on a Creoptix Waveguide GCI system. (A) SDS-PAGE analysis of MPR1/MPA1 SMALPs and the assay configuration for binding analysis shown in panel B. (B) Referenced dose-dependent binding response sensorgrams of purified MPR1/MPA1 SMALPs and α-MPR1 antibody captured to surface coupled α-Hu Fc antibody (C) No binding detection from sensorgram responses of increasing concentrations of purified MPR1/MPA1 SMALPs and a directly surface coupled α-MPR2 antibody. (D) Sensorgram binding responses of increasing concentrations of purified MPR1/MPA1 SMALPs and a directly surface coupled α-MPR1 antibody.
In a subsequent experiment, we tested a slightly different assay configuration where α-MPR1 and α-MPR2 antibodies were directly immobilized via amine coupling on different flow cells. Sensorgrams of increasing concentrations of purified MPR1/MPA1 SMALPs and a surface captured unrelated antibody (α-MPR2) showed no positive binding responses, indicating specificity of observed binding for α-MPR1 and adequately purified target extraction with SMALPs (Fig. 2C). In the case of directly surface coupled α-MPR1, binding levels of 80–100 RU were observed with MPR1/MPA1 SMALPs and with anti-human Fc bound α-MPR1 (Fig. 2D). This observation indicated that specific antibody interactions for SMALPs-encapsulated targets could be observed in both assay formats tested here.
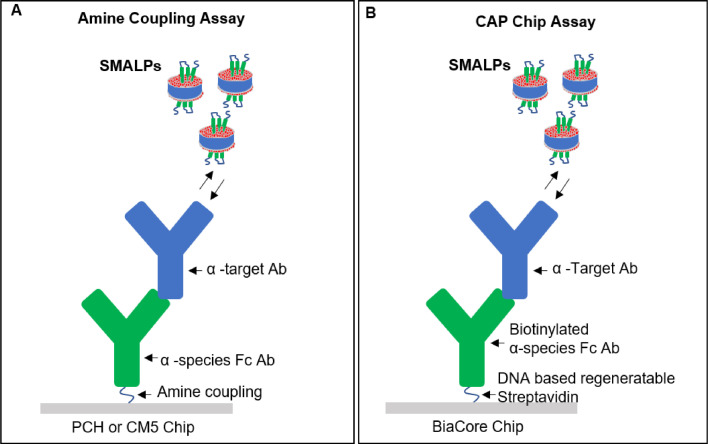
Specific SMALP capture from crude total SMALP using SPR surface captured anti-TAG antibodies
Multiple SPR assays were designed to confirm functionality of stabilized membrane proteins in SMALPs by measuring specific antibody interactions. Designs of SPR binding assays are depicted in Fig. 3 and were configured onto PCH or CM5 chips by amine coupling and Biacore CAP chip by DNA-based Streptavidin (SA) capture (Fig. 3). SPR binding experiments carried out using the above assay strategies are explained in follow up results and binding data shown with Fig. 4, Fig. 5, Fig. 6. These experiments were performed and compared for crude total SMALPs with MPR1/MPA1 or MPR2 using surface captured tag or target-specific antibodies. Figs. 4,5, and 6 represent results from SMALPs capture on a unique and new biosensor chip used each time for the designed SPR binding assay format. The response levels (RU) of captured reagent on flow cells 1–4 are depicted with vertical bars instead of sensorgrams for clarity of comparison. The results shown here are representative data of several experiments during optimization of purified MP-SMALPs and were found to be highly reproducible in nature.
Fig. 3.
Comparison of assay setups for binding analysis of antibodies and membrane proteins stabilized in SMALPs. Assay configurations for SMALPs vs antibody binding studies using amine coupling or complementary DNA strands are visualized. (A) Assay design for amine coupling of captured antibodies are shown using PCH Creoptix chips. (B) Assay design for streptavidin immobilization of biotinylated capture antibodies are shown using the Biacore CAP chips.
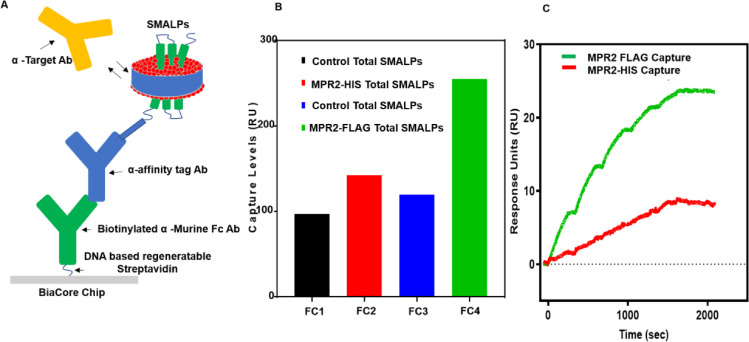
Fig. 4.
Antibody binding responses after SMALPs capture from total cellular SMALPs. Total cellular SMALPs produced with cell membrane pellets from cell lines expressing MPR2-His-and MPR2-FLAG were used to capture MPR2 SMALPs with surface captured α-TAG antibodies. Subsequent captured SMALPs were then used for antibody binding studies. (A) Assay configuration for SMALPs capture and target-specific antibody binding. (B) Capture levels for all flow cells using total SMALPs from overexpressing cell lines and parental cell lines as a background control. (C) Dose-dependent binding response levels of α-MPR2 antibody with surface captured α-His-or α -FLAG MPR2 SMALPs.
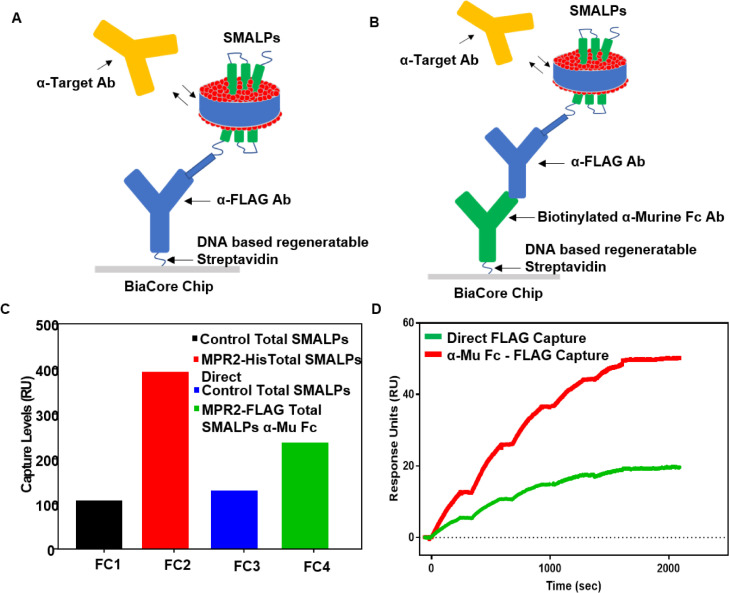
Fig. 5.
Comparison of assay formats for antibody binding to FLAG captured MPR2 SMALP. Total cellular SMALPs produced with cell membrane pellets from a cell line expressing MPR2 -FLAG was used to capture MPR2 SMALPs with surface captured α-FLAG antibody in two separate formats. Subsequent captured SMALPs were then used for antibody binding studies. (A) Assay configuration for SMALPs capture and target- specific antibody binding using a direct surface capture of a biotinylated α-FLAG antibody. (B) Assay configuration for SMALPs capture and target-specific antibody binding using a stacked surface capture of biotinylated α-Mu Fc antibody with α-FLAG antibody. (C) Capture levels for all flow cells using total SMALPs from an overexpressing MPR2-FLAG cell line and the parental cell lines as a background control. (D) Dose-dependent binding response levels of α-MPR2 antibody with surface captured α-FLAG MPR2 SMALPs using the two different capture formats.
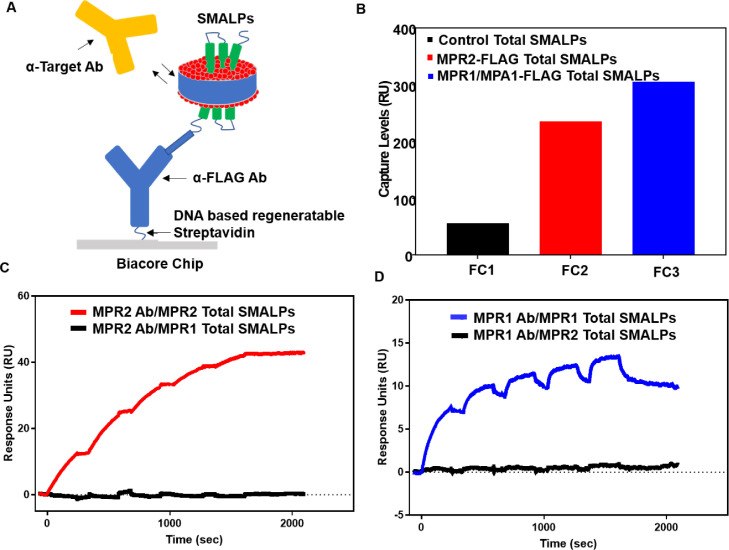
Fig. 6.
Demonstration of antibody binding specificity with MPR2 and MPR1/MPA1 SMALPs using target-specific antibodies and SMALPs α-FLAG capture. Total cellular SMALPs produced with cell membrane pellets from a cell line expressing MPR2-FLAG and total cell pellets from a cell line overexpressing MPR1/MPA1 were used to demonstrate the specificity of target-specific antibodies and the various SMALPs species. SMALPs were captured on the chip surface using a cell surface captured biotinylated α-FLAG antibody and the Biacore CAP chip. (A) Assay configuration for SMALPs capture and target-specific antibody binding using a direct surface capture of a biotinylated α-FLAG antibody using the Biacore CAP chip. (B) Capture levels for all flow cells using total SMALPs from an overexpressing MPR2 -FLAG cell line and the parental cell lines as a background control. (C) Dose-dependent binding response levels of α-MPR2 antibody to α-FLAG captured MPR2 and MPR1/MPA1 SMALPs. (D) Dose-dependent binding response levels of α-MPR1 antibody to α-FLAG captured MPR2 and MPR1/MPA1 SMALPs.
Comparison of direct streptavidin or α-Fc capture of α-FLAG antibodies for binding studies with total crude SMALPs
To explore and compare total cellular SMALPs produced with cell membrane pellets from cell lines expressing MPR2-His-and MPR2-FLAG, corresponding α-TAG antibodies were surface captured (Fig. 4A). Biotinylated α-murine (mu) Fc antibody was first captured using a DNA-based regeneratable SA surface. α-TAG antibodies were then captured, followed by binding measurement of crude SMALPs with MPR2-TAG. In this format, anti-target antibodies’ SPR binding levels to crude SMALPs were also explored.
Capture levels were compared for all flow cells using total SMALPs from overexpressing cell lines and parental cell lines as a background control (Fig. 4B). Dose-dependent binding response levels of α-MPR2 antibody with surface captured α-His-or α-FLAG MPR2 SMALPs were overlapped for comparison. In this analysis for total SMALPs captured on all flow cells (Fc1–4), MPR2-FLAG displayed the highest binding levels followed by MPR2-His-compared to control flow cells 1 or 3 (Fig. 4C).
Demonstration of specific antibody binding to surface captured SMALPs from crude total MPR2 and MPR1/MPA1 SMALPs
Subsequently, we optimized two separate formats of SPR assay to explore the robustness and flexibility of cellular SMALPs produced with cell membrane pellets from a cell line expressing MPR2-FLAG. First, we verified SMALPs capture and target-specific antibody binding using a direct surface capture of a biotinylated α-FLAG antibody (Fig. 5A). Next, we established SMALPs capture and target-specific antibody binding using a stacked surface capture of biotinylated α-muFc antibody with α-FLAG antibody (Fig. 5B). Single-cycle kinetics sensorgrams from interactions of α-MPR2 antibody with surface captured α-FLAG MPR2 SMALPs confirmed incorporation of conformationally active levels of MPR2-FLAG in both above assay configurations (Fig. 5D).
SPR binding outcomes were compared from both assays and these were found to correlate well for binding kinetics by the shape of the sensorgrams as well as the calculated kinetics values. This observation verified that specific antibody binding to surface captured SMALPs from crude total MPR2-FLAG SMALPs was attainable with the methods described in this report.
A comparison of the binding levels observed with the two approaches to capture total SMALPs revealed that direct capture of biotinylated α-FLAG antibody on a chip surface is more efficient compared to stacked surface capture of biotinylated α-muFc antibody with α-FLAG antibody (Fig. 5C). Total SMALPs capture levels or RU were elevated up to 1.6-fold in the direct capture method, and overall binding response levels were also translated to α-MPR2 antibody interactions with surface captured α-FLAG MPR2 SMALPs, as observed in the comparative binding levels of two the methods (Fig. 5D). Nevertheless, similar kinetics and affinity parameters of measured interactions further indicated membrane protein orientations in SMALPs-encapsulation remains the same during both SPR strategies explored to capture total SMALPs. These assay configurations were also established for MPR2-His-total SMALPs (Fig. 6) capture levels, where a similar ratio of SMALPs capture levels were observed as described above.
Capture levels were compared for all flow cells using total SMALPs from an overexpressing MPR2-FLAG cell line and the parental cell lines as background control. An almost 4-fold increase in capture levels (RU) of MPR2-FLAG SMALPs was observed in the direct capture α-FLAG antibody assay format compared to the total SMALPs control. However, only a 1.8-fold increase was observed in RU of MPR2-SMALPs in the second stacked binding format with α-muFc antibody. This observation indicates that there is higher binding surface density from the direct binding method for α-FLAG antibody and MPR2-FLAG SMALPs interactions.
In the next step we investigated whether SMALPs-encapsulated membrane proteins MPR1/MPA1 and MPR2 display specific binding only to corresponding α-TAG antibodies. This was determined by cross testing total SMALPs samples in a SPR binding experiment (Fig. 6C and D). Biotinylated α-FLAG antibody was surface captured using the Biacore CAP chip and dose-dependent binding response levels of α-MPR2 antibody and α-MPR1 antibody were compared to corresponding as well as switched targets with SMALPs. No binding was detected for α-MPR2 antibody and MPR1/MPA1 SMALPs as well as for α-MPR1 antibody and MPR2-FLAG SMALPs. Additionally, expected binding response and kinetics were observed for α-MPR1 and MPR1/MPA1 SMALPs as well as α-MPR2 and MPR2-FLAG SMALPs. These results demonstrated that total cellular SMALPs produced with cell membrane pellets expressing MPR2-FLAG or MPR1/MPA1 are specific to membrane protein targets.
Although SMALPs are reported as a highly stable formulation for membrane protein solubilization [55], SMA is susceptible to divalent cations such as magnesium (Mg2+) due to affinity and after cation binding the SMA polymer becomes insoluble [22,50]. SMALPs encapsulated lipids and proteins precipitate in the presence of divalent cations due to SMA disassociation from the SMALPs. To ensure that membrane protein under study remain associated with SMALPs during binding assays, we compared the buffer conditions used in current binding assays with conditions found to disintegrate SMALPs. Presence of 5–10 mM MgCl2 is known to disintegrate SMALPs with various experimental evidence. To observe similar effects on MP-SMALPs generated samples for current SPR studies, we also investigated the sensitivity of SMALPs made with SMA polymer by addition of Mg2+ at a concentration of approximately 5 mM at ambient temperature, with 30 min of incubation [56], [57], [58]. SMA precipitation was observed and pelleted by centrifuging for 1–2 min at 21,000 x g and supernatant was analyzed for the protein's activity or concentration. SMALPs disintegration was confirmed by absence of SPR binding data or loss of protein in a concentration measurement assay in the presence of 5 mM MgCl2. As a requirement for biosensor instrument fluidics, we used SPR buffer in addition to a commonly used a non-ionic surfactant, Tween-20 (CMC 0.007%). This surfactant was used mainly to prevent non-specific binding at a recommended concentration of 0.005%−0.013% which is almost 10–20 times lower than concentrations required to form stable Tween-20 micelles sometimes used for membrane extraction [11,59,60]. Presence of 5 mM MgCl2 completely mitigated SMALPs-based binding data in both assay formats described above. This observation further validated that all presented binding data for membrane protein of interest is based on the SMALPs-encapsulated form of intact membrane proteins or complexes. Protein concentration analysis as well as early SPR tests on such samples indicated complete loss of solubilized membrane protein as well as further validated integrity of SMALPs-solubilized MP's after purification.
Discussion
The importance of membrane protein extraction in a native-like environment to validate and characterize target integrity and interactions as well as associated challenges with detergent use are well recognized [[21], [22], [23], 58]. This prompted us to develop a platform with systematic strategies to extract diverse types of membrane protein with SMALPs for validation of membrane target engagement with various antibodies or analytes on biosensor systems. SMALPs encapsulation for membrane protein characterization is a fast-growing area [55] and recently several structural studies also reported application of SMALPs to obtain high resolution structures [61,62]. Combining biosensors-based binding assays and SMALPs solubilization for studying membrane proteins will be a versatile platform to enable robust analysis of biomolecular interactions for decision making [31]. The biophysical assays with SPR and GCI techniques developed in the current study validated the application of SMALPs-based extraction from both isolated membranes and whole-cell settings to enable kinetic analyses of antibodies binding to membrane protein targets. To the best of our knowledge, studies presented here are one of the early reports to implement SMALPs purification for membrane protein and antibody binding characterization on multiple biosensor platforms. Furthermore, for the biologically relevant prototype targets (MPR1/MPA1 pair in immune signaling and MPR2 in inflammation) selected for current studies, this is the first report on SMALP-biosensor platform methodologies.
SMALPs deliver several benefits compared to traditional membrane protein preparations due to direct extraction of target protein from the membranes that avoids membrane protein exposure to detergents [22,50,55]. For current studies, although the purity of the SMALPs-encapsulated membrane proteins might be limited, the SMALPs-solubilized membrane proteins were quite stable as well as active for highly specific binding assays. SMALP production from isolated membrane as well as whole-cell allowed characterization of the highly specific and robust binding kinetic analysis of antibodies with membrane protein that was free from detergent's influence on its conformation [63]. Various SPR assay formats optimized here represent the flexibility of SMALPs applications for diverse membrane protein systems. SMALPs were reported to retain endogenously bound lipids which may be crucial for the correct folding and membrane protein function [26]. Therefore, SMALPs represent a promising technology that keeps extracted membrane protein in environment as native as possible. The presented methods not only replace the detergent requirement for extraction from cell membrane preparations, but also suggest a whole-cell method likely be adequate for analytical purposes like ligand binding assays.
The electrophoresis study of three different membrane proteins by SMA-PAGE demonstrated that proteins in SMALPs remained intact and migrated consistently in a native gel electrophoresis.
The observed migration of MPR1/MPA1 complex as well as MPR2 aligned well not only with their expected molecular weight (MW), but also retained their oligomeric states, which was likely due to the high negative charge density of the maleic acid groups in the SMA copolymer during electrophoresis [50]. Denaturing SDS-PAGE and native SMA-PAGE analyses verified SMALPs containing membrane protein of various quaternary structures or protein complex with correct stoichiometries.
Co-expressed transmembrane receptor-adaptor complex of MPR1/MPA1 and another receptor MPR2 were solubilized with SMALPs and purified on anti-FLAG and Ni-NTA resin. Full-length membrane protein with correctly folded secondary and ternary structures are expected to achieve allocated biological functions and interactions of target or tag-specific antibodies were therefore explored. SPR binding assays in various formats were developed to experimentally demonstrate the direct interactions, specificity, and binding kinetics of purified SMALPs containing target proteins.
Figs. 4,5, and 6 represent results from SMALPs captured on the unique and new biosensor chip used each time for the designed SPR binding assay format. We used vertical bars to represent binding level in response units (RU) of captured reagents and showed a comparison on flow cells 1 to 4 for each assay. Due to the configuration of independent channels in Biacore T200 and Creoptix WAVEdelta instruments, there are only 4 flow cells available on each chip. To demonstrate specificity of binding events, we have utilized each flow cell for a unique binding interaction instead of identical replicate. The results shown here are representative data of multiple experiments during optimization of reagents such as purified SMALPs, and data are highly reproducible in nature. Robustness or reproducibility of data could be observed by comparing the bar graphs in Figs. 4,5, and 6 with total percent SMALPs capture levels ∼100 RU each time, with MPR1-His-and MPR2-His-or MPR2-FLAG levels remaining in range of 200–300 RU if captured with similar experimental parameters.
A combination of various antibodies was utilized to demonstrate the potential of SMALPs for membrane protein solubilization and interaction analyses. These optimal SPR strategies were found impeccably transferable to multiple biosensor instruments (SPR and GCI) platforms. Membrane protein binding kinetics are traditionally difficult to get, and the results are often questionable, depending on the methods used. Here, we show newly developed methods using SPR and GCI-based biosensor assays which can be used for label-free measurements of biosensors to study SMALPs-membrane protein binding kinetics with various antibodies. The robustness and flexibility of membrane protein reagent generation methods can be evidenced by the consistent value of determined kinetic parameters and similar outcomes from various assay formats. Together, the integration of SMALPs membrane protein and biosensors enable accurate cross-validation of binding kinetics, providing reliable results for molecular interaction analysis. The preservation of native lipid encapsulated in SMALPs- membrane protein combined with the advantages of biosensor assays described above establishes a versatile platform for investigating antibody or ligand and membrane protein interactions in an environment very close to that of native membranes.
Conclusion
The systematic studies reported here show that encapsulation of membrane protein complexes with SMALPs provide a detergent-free and robust approach to study therapeutic candidates with target membrane proteins. Given the importance of membrane proteins in therapeutic intervention, these studies validate SMALPs-based nano-encapsulation of membrane protein can be successfully applied for binding studies in the drug discovery process. Multiple biosensor-based label-free assay strategies developed here, along with solubilization and purification of membrane proteins with SMALPs are capable of both qualitative and quantitative analysis of membrane protein interactions with a variety of antibodies. Specifically, with diverse membrane protein targets and two affinity tags, this study demonstrated that SMA-based stabilization of membrane proteins is an efficient way for kinetic analysis of diverse antibody interactions specifically to integral membrane proteins in both purified and complex mixture environments. We anticipate SMALPs-enabled kinetic binding analysis will emerge as a widely applicable platform to aid therapeutic developments of membrane protein targets.
Credit author statement
Pooja Sharma: Writing- Original draft preparation, Writing - Review & Editing Investigation, Methodology; Validation, Investigation;
Matthew Plant: Conceptualization, Methodology; Investigation, Writing- Original draft preparation; Reviewing
Sheung Kwan Lam: Resources (cell pellets), Reviewing;
Qing Chen: Supervision, Reviewing
Declaration of Competing Interest
Pooja Sharma, Sheung Kwan Lam and Qing Chen are current employees and own stock of Amgen, Inc. Matthew Plant is previous employee of Amgen, Inc at the time when the experiments were performed. No other potential conflicts of interest were disclosed.
Acknowledgements
We would like to recognize Karl Bedke for procurement and hydrolysis of SMA-2000 polymer from Cray Valley. We would like to thank Hao Chen and AssayWorks Inc for supplying cell mass material for membrane preparations and frozen cell pellets, Nikolai Sharkov for in-gel digestion assays and Chris Fotsch for useful discussions on SMALPs applications for various systems.
Contributor Information
Pooja Sharma, Email: psharm03@amgen.com.
Matthew Plant, Email: mplant@momatx.com.
Sheung Kwan Lam, Email: shlam@amgen.com.
Qing Chen, Email: qchen@amgen.com.
References
- 1.Wallin E., von Heijne G. Genome-wide analysis of integral membrane proteins from eubacterial, archaean, and eukaryotic organisms. Protein Sci. 1998;7:1029–1038. doi: 10.1002/pro.5560070420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wishart D.S., Knox C., Guo A.C., Shrivastava S., Hassanali M., Stothard P., Chang Z., Woolsey J. DrugBank: a comprehensive resource for in silico drug discovery and exploration. Nucleic. Acids. Res. 2006;34:D668–D672. doi: 10.1093/nar/gkj067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Patching S.G. Surface plasmon resonance spectroscopy for characterisation of membrane protein-ligand interactions and its potential for drug discovery. Biochim. Biophys. Acta. 2014;1838:43–55. doi: 10.1016/j.bbamem.2013.04.028. [DOI] [PubMed] [Google Scholar]
- 4.Tamm L.K., Abildgaard F., Arora A., Blad H., Bushweller J.H. Structure, dynamics and function of the outer membrane protein A (OmpA) and influenza hemagglutinin fusion domain in detergent micelles by solution NMR. FEBS Lett. 2003;555:139–143. doi: 10.1016/s0014-5793(03)01127-x. [DOI] [PubMed] [Google Scholar]
- 5.Susac L., Horst R., Wuthrich K. Solution-NMR characterization of outer-membrane protein A from E. coli in lipid bilayer nanodiscs and detergent micelles. ChemBioChem. 2014;15:995–1000. doi: 10.1002/cbic.201300729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Renault M., Saurel O., Demange P., Reat V., Milon A. Solution-state NMR spectroscopy of membrane proteins in detergent micelles: structure of the Klebsiella pneumoniae outer membrane protein A, KpOmpA. Methods Mol. Biol. 2010;654:321–339. doi: 10.1007/978-1-60761-762-4_17. [DOI] [PubMed] [Google Scholar]
- 7.Ng E.Y., Loh Y.R., Li Y., Li Q., Kang C. Expression, purification of Zika virus membrane protein-NS2B in detergent micelles for NMR studies. Protein Expr. Purif. 2019;154:1–6. doi: 10.1016/j.pep.2018.09.013. [DOI] [PubMed] [Google Scholar]
- 8.Hiller S., Garces R.G., Malia T.J., Orekhov V.Y., Colombini M., Wagner G. Solution structure of the integral human membrane protein VDAC-1 in detergent micelles. Science. 2008;321:1206–1210. doi: 10.1126/science.1161302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McDevitt C.A., Collins R.F., Conway M., Modok S., Storm J., Kerr I.D., Ford R.C., Callaghan R. Purification and 3D structural analysis of oligomeric human multidrug transporter ABCG2. Structure. 2006;14:1623–1632. doi: 10.1016/j.str.2006.08.014. [DOI] [PubMed] [Google Scholar]
- 10.Telbisz A., Ozvegy-Laczka C., Hegedus T., Varadi A., Sarkadi B. Effects of the lipid environment, cholesterol and bile acids on the function of the purified and reconstituted human ABCG2 protein. Biochem. J. 2013;450:387–395. doi: 10.1042/BJ20121485. [DOI] [PubMed] [Google Scholar]
- 11.Arachea B.T., Sun Z., Potente N., Malik R., Isailovic D., Viola R.E. Detergent selection for enhanced extraction of membrane proteins. Protein Expr. Purif. 2012;86:12–20. doi: 10.1016/j.pep.2012.08.016. [DOI] [PubMed] [Google Scholar]
- 12.Autzen H.E., Julius D., Cheng Y. Membrane mimetic systems in CryoEM: keeping membrane proteins in their native environment. Curr. Opin. Struct. Biol. 2019;58:259–268. doi: 10.1016/j.sbi.2019.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Popot J.L., Althoff T., Bagnard D., Baneres J.L., Bazzacco P., Billon-Denis E., Catoire L.J., Champeil P., Charvolin D., Cocco M.J., Cremel G., Dahmane T., de la Maza L.M., Ebel C., Gabel F., Giusti F., Gohon Y., Goormaghtigh E., Guittet E., Kleinschmidt J.H., Kuhlbrandt W., Bon C.Le, Martinez K.L., Picard M., Pucci B., Sachs J.N., Tribet C., van Heijenoort C., Wien F., Zito F., Zoonens M. Amphipols from A to Z. Annu. Rev. Biophys. 2011;40:379–408. doi: 10.1146/annurev-biophys-042910-155219. [DOI] [PubMed] [Google Scholar]
- 14.Gohon Y., Vindigni J.D., Pallier A., Wien F., Celia H., Giuliani A., Tribet C., Chardot T., Briozzo P. High water solubility and fold in amphipols of proteins with large hydrophobic regions: oleosins and caleosin from seed lipid bodies. Biochim. Biophys. Acta. 2011;1808:706–716. doi: 10.1016/j.bbamem.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 15.Popot J.L. Amphipols, nanodiscs, and fluorinated surfactants: three nonconventional approaches to studying membrane proteins in aqueous solutions. Annu. Rev. Biochem. 2010;79:737–775. doi: 10.1146/annurev.biochem.052208.114057. [DOI] [PubMed] [Google Scholar]
- 16.Tribet C., Audebert R., Popot J.L. Amphipols: polymers that keep membrane proteins soluble in aqueous solutions. Proc. Natl. Acad. Sci. U. S. A. 1996;93:15047–15050. doi: 10.1073/pnas.93.26.15047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Navratilova I.H., Aristotelous T., Bird L.E., Hopkins A.L. Surveying GPCR solubilisation conditions using surface plasmon resonance. Anal. Biochem. 2018;556:23–34. doi: 10.1016/j.ab.2018.06.012. [DOI] [PubMed] [Google Scholar]
- 18.Hagn F., Nasr M.L., Wagner G. Assembly of phospholipid nanodiscs of controlled size for structural studies of membrane proteins by NMR. Nat. Protoc. 2018;13:79–98. doi: 10.1038/nprot.2017.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Denisov I.G., Grinkova Y.V., Lazarides A.A., Sligar S.G. Directed self-assembly of monodisperse phospholipid bilayer Nanodiscs with controlled size. J. Am. Chem. Soc. 2004;126:3477–3487. doi: 10.1021/ja0393574. [DOI] [PubMed] [Google Scholar]
- 20.Rouck J.E., Krapf J.E., Roy J., Huff H.C., Das A. Recent advances in nanodisc technology for membrane protein studies (2012-2017) FEBS Lett. 2017;591:2057–2088. doi: 10.1002/1873-3468.12706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gulati S., Jamshad M., Knowles T.J., Morrison K.A., Downing R., Cant N., Collins R., Koenderink J.B., Ford R.C., Overduin M., Kerr I.D., Dafforn T.R., Rothnie A.J. Detergent-free purification of ABC (ATP-binding-cassette) transporters. Biochem. J. 2014;461:269–278. doi: 10.1042/BJ20131477. [DOI] [PubMed] [Google Scholar]
- 22.Knowles T.J., Finka R., Smith C., Lin Y.P., Dafforn T., Overduin M. Membrane proteins solubilized intact in lipid containing nanoparticles bounded by styrene maleic acid copolymer. J. Am. Chem. Soc. 2009;131:7484–7485. doi: 10.1021/ja810046q. [DOI] [PubMed] [Google Scholar]
- 23.Lee S.C., Knowles T.J., Postis V.L., Jamshad M., Parslow R.A., Lin Y.P., Goldman A., Sridhar P., Overduin M., Muench S.P., Dafforn T.R. A method for detergent-free isolation of membrane proteins in their local lipid environment. Nat. Protoc. 2016;11:1149–1162. doi: 10.1038/nprot.2016.070. [DOI] [PubMed] [Google Scholar]
- 24.Hesketh S.J., Klebl D.P., Higgins A.J., Thomsen M., Pickles I.B., Sobott F., Sivaprasadarao A., Postis V.L.G., Muench S.P. Styrene maleic-acid lipid particles (SMALPs) into detergent or amphipols: an exchange protocol for membrane protein characterisation. Biochim. Biophys. Acta Biomembr. 2020;1862 doi: 10.1016/j.bbamem.2020.183192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Radoicic J., Park S.H., Opella S.J. Macrodiscs Comprising SMALPs for Oriented Sample Solid-State NMR Spectroscopy of Membrane Proteins. Biophys. J. 2018;115:22–25. doi: 10.1016/j.bpj.2018.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Orekhov P.S., Bozdaganyan M.E., Voskoboynikova N., Mulkidjanian A.Y., Steinhoff H.J., Shaitan K.V. Styrene/Maleic Acid Copolymers Form SMALPs by Pulling Lipid Patches out of the Lipid Bilayer. Langmuir. 2019;35:3748–3758. doi: 10.1021/acs.langmuir.8b03978. [DOI] [PubMed] [Google Scholar]
- 27.Hellwig N., Peetz O., Ahdash Z., Tascon I., Booth P.J., Mikusevic V., Diskowski M., Politis A., Hellmich Y., Hanelt I., Reading E., Morgner N. Native mass spectrometry goes more native: investigation of membrane protein complexes directly from SMALPs. Chem. Commun. (Camb.) 2018;54:13702–13705. doi: 10.1039/c8cc06284f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harding B.D., Dixit G., Burridge K.M., Sahu I.D., Dabney-Smith C., Edelmann R.E., Konkolewicz D., Lorigan G.A. Characterizing the structure of styrene-maleic acid copolymer-lipid nanoparticles (SMALPs) using RAFT polymerization for membrane protein spectroscopic studies. Chem. Phys. Lipids. 2019;218:65–72. doi: 10.1016/j.chemphyslip.2018.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Craig A.F., Clark E.E., Sahu I.D., Zhang R., Frantz N.D., Al-Abdul-Wahid M.S., Dabney-Smith C., Konkolewicz D., Lorigan G.A. Tuning the size of styrene-maleic acid copolymer-lipid nanoparticles (SMALPs) using RAFT polymerization for biophysical studies. Biochim. Biophys. Acta. 2016;1858:2931–2939. doi: 10.1016/j.bbamem.2016.08.004. [DOI] [PubMed] [Google Scholar]
- 30.Bali A.P., Sahu I.D., Craig A.F., Clark E.E., Burridge K.M., Dolan M.T., Dabney-Smith C., Konkolewicz D., Lorigan G.A. Structural characterization of styrene-maleic acid copolymer-lipid nanoparticles (SMALPs) using EPR spectroscopy. Chem. Phys. Lipids. 2019;220:6–13. doi: 10.1016/j.chemphyslip.2019.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Olaru A., Bala C., Jaffrezic-Renault N., Aboul-Enein H.Y. Surface plasmon resonance (SPR) biosensors in pharmaceutical analysis. Crit. Rev. Anal. Chem. 2015;45:97–105. doi: 10.1080/10408347.2014.881250. [DOI] [PubMed] [Google Scholar]
- 32.Wang X., McKay P., Yee L.T., Dutina G., Hass P.E., Nijem I., Allison D., Cowan K.J., Lin K., Quarmby V., Yang J. Impact of SPR biosensor assay configuration on antibody: neonatal Fc receptor binding data. MAbs. 2017;9:319–332. doi: 10.1080/19420862.2016.1261774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Coppari E., Santini S., Bizzarri A.R., Cannistraro S. Kinetics and binding geometries of the complex between beta2-microglobulin and its antibody: an AFM and SPR study. Biophys. Chem. 2016;211:19–27. doi: 10.1016/j.bpc.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 34.Khurana S., King L.R., Manischewitz J., Coyle E.M., Golding H. Novel antibody-independent receptor-binding SPR-based assay for rapid measurement of influenza vaccine potency. Vaccine. 2014;32:2188–2197. doi: 10.1016/j.vaccine.2014.02.049. [DOI] [PubMed] [Google Scholar]
- 35.Lee S.K., Kim H.C., Cho S.J., Jeong S.W., Jeon W.B. Binding behavior of CRP and anti-CRP antibody analyzed with SPR and AFM measurement. Ultramicroscopy. 2008;108:1374–1378. doi: 10.1016/j.ultramic.2008.04.064. [DOI] [PubMed] [Google Scholar]
- 36.Li R.S., Ni M.C., Zhu H.J., Ma Q.Q., Fu M., Lu P. [Establishment of Quantitative SPR Assay for Antibodies Against Human Platelet Antigen-1a] Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2021;29:239–242. doi: 10.19746/j.cnki.issn.1009-2137.2021.01.039. [DOI] [PubMed] [Google Scholar]
- 37.Rispens T., Te Velthuis H., Hemker P., Speijer H., Hermens W., Aarden L. Label-free assessment of high-affinity antibody-antigen binding constants. Comparison of bioassay, SPR, and PEIA-ellipsometry. J. Immunol. Methods. 2011;365:50–57. doi: 10.1016/j.jim.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 38.Singh P. Surface plasmon resonance (SPR) based binding studies of refolded single chain antibody fragments. Biochem. Biophys. Rep. 2018;14:83–88. doi: 10.1016/j.bbrep.2018.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Townsend S., Finlay W.J., Hearty S., O'Kennedy R. Optimizing recombinant antibody function in SPR immunosensing. The influence of antibody structural format and chip surface chemistry on assay sensitivity. Biosens. Bioelectron. 2006;22:268–274. doi: 10.1016/j.bios.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 40.Zhao H., Gorshkova, Fu G.L., Schuck P. A comparison of binding surfaces for SPR biosensing using an antibody-antigen system and affinity distribution analysis. Methods. 2013;59:328–335. doi: 10.1016/j.ymeth.2012.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peterson E.J., Clements J.L., Fang N., Koretzky G.A. Adaptor proteins in lymphocyte antigen-receptor signaling. Curr. Opin. Immunol. 1998;10:337–344. doi: 10.1016/s0952-7915(98)80173-8. [DOI] [PubMed] [Google Scholar]
- 42.Kirkbride K.C., Ray B.N., Blobe G.C. Cell-surface co-receptors: emerging roles in signaling and human disease. Trends Biochem. Sci. 2005;30:611–621. doi: 10.1016/j.tibs.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 43.Goder V., Spiess M. Topogenesis of membrane proteins: determinants and dynamics. FEBS Lett. 2001;504:87–93. doi: 10.1016/s0014-5793(01)02712-0. [DOI] [PubMed] [Google Scholar]
- 44.Geuijen K.P., Egging D.F., Bartels S., Schouten J., Schasfoort R.B., Eppink M.H. Characterization of low affinity Fcgamma receptor biotinylation under controlled reaction conditions by mass spectrometry and ligand binding analysis. Protein Sci. 2016;25:1841–1852. doi: 10.1002/pro.2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Papalia G., Myszka D. Exploring minimal biotinylation conditions for biosensor analysis using capture chips. Anal. Biochem. 2010;403:30–35. doi: 10.1016/j.ab.2010.03.044. [DOI] [PubMed] [Google Scholar]
- 46.Patko D., Cottier K., Hamori A., Horvath R. Single beam grating coupled interferometry: high resolution miniaturized label-free sensor for plate based parallel screening. Opt. Express. 2012;20:23162–23173. doi: 10.1364/OE.20.023162. [DOI] [PubMed] [Google Scholar]
- 47.Kozma P., Kehl F., Ehrentreich-Forster E., Stamm C., Bier F.F. Integrated planar optical waveguide interferometer biosensors: a comparative review. Biosens. Bioelectron. 2014;58:287–307. doi: 10.1016/j.bios.2014.02.049. [DOI] [PubMed] [Google Scholar]
- 48.Hopkins F.K., Jackson H.E., Boyd J.T. In-plane scattering measurements in a planar optical waveguide by an integrated technique. Appl. Opt. 1981;20 doi: 10.1364/AO.20.2761_1. [DOI] [PubMed] [Google Scholar]
- 49.Sambrook J., Russell D.W. Analysis of Interacting Proteins with SPR Spectroscopy Using BIAcore * Stage 1: preparation of the Capture Surface and Test Binding. CSH Protoc. 2006;2006 doi: 10.1101/pdb.prot3894. [DOI] [PubMed] [Google Scholar]
- 50.Pollock N.L., Rai M., Simon K.S., Hesketh S.J., Teo A.C.K., Parmar M., Sridhar P., Collins R., Lee S.C., Stroud Z.N., Bakker S.E., Muench S.P., Barton C.H., Hurlbut G., Roper D.I., Smith C.J.I., Knowles T.J., Spickett C.M., East J.M., Postis V.L.G., Dafforn T.R. SMA-PAGE: a new method to examine complexes of membrane proteins using SMALP nano-encapsulation and native gel electrophoresis. Biochim Biophys Acta Biomembr. 2019;1861:1437–1445. doi: 10.1016/j.bbamem.2019.05.011. [DOI] [PubMed] [Google Scholar]
- 51.Quach T.T., Li N., Richards D.P., Zheng J., Keller B.O., Li L. Development and applications of in-gel CNBr/tryptic digestion combined with mass spectrometry for the analysis of membrane proteins. J. Proteome Res. 2003;2:543–552. doi: 10.1021/pr0340126. [DOI] [PubMed] [Google Scholar]
- 52.Shevchenko A., Tomas H., Havlis J., Olsen J.V., Mann M. In-gel digestion for mass spectrometric characterization of proteins and proteomes. Nat. Protoc. 2006;1:2856–2860. doi: 10.1038/nprot.2006.468. [DOI] [PubMed] [Google Scholar]
- 53.Huynh M.L., Russell P., Walsh B. Tryptic digestion of in-gel proteins for mass spectrometry analysis. Methods Mol. Biol. 2009;519:507–513. doi: 10.1007/978-1-59745-281-6_34. [DOI] [PubMed] [Google Scholar]
- 54.Vauquelin G., Charlton S.J. Exploring avidity: understanding the potential gains in functional affinity and target residence time of bivalent and heterobivalent ligands. Br. J. Pharmacol. 2013;168:1771–1785. doi: 10.1111/bph.12106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Simon K.S., Pollock N.L., Lee S.C. Membrane protein nanoparticles: the shape of things to come. Biochem. Soc. Trans. 2018;46:1495–1504. doi: 10.1042/BST20180139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hall S.C.L., Tognoloni C., Charlton J., Bragginton E.C., Rothnie A.J., Sridhar P., Wheatley M., Knowles T.J., Arnold T., Edler K.J., Dafforn T.R. An acid-compatible co-polymer for the solubilization of membranes and proteins into lipid bilayer-containing nanoparticles. Nanoscale. 2018;10:10609–10619. doi: 10.1039/c8nr01322e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.de Jonge P.A., Smit Sibinga D.J.C., Boright O.A., Costa A.R., Nobrega F.L., Brouns S.J.J., Dutilh B.E. Development of Styrene Maleic Acid Lipid Particles as a Tool for Studies of Phage-Host Interactions. J. Virol. 2020;94 doi: 10.1128/JVI.01559-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Teo A.C.K., Lee S.C., Pollock N.L., Stroud Z., Hall S., Thakker A., Pitt A.R., Dafforn T.R., Spickett C.M., Roper D.I. Analysis of SMALP co-extracted phospholipids shows distinct membrane environments for three classes of bacterial membrane protein. Sci. Rep. 2019;9:1813. doi: 10.1038/s41598-018-37962-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li X., Song Y., Sanders C.R., Buxbaum J.N. Transthyretin Suppresses Amyloid-beta Secretion by Interfering with Processing of the Amyloid-beta Protein Precursor. J. Alzheimers Dis. 2016;52:1263–1275. doi: 10.3233/JAD-160033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Huelgas-Morales G., Sanders M., Mekonnen G., Tsukamoto T., Greenstein D. Decreased mechanotransduction prevents nuclear collapse in a Caenorhabditis elegans laminopathy. Proc. Natl. Acad. Sci. U S A, 2020;117:31301–31308. doi: 10.1073/pnas.2015050117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sun C., Benlekbir S., Venkatakrishnan P., Wang Y., Hong S., Hosler J., Tajkhorshid E., Rubinstein J.L., Gennis R.B. Structure of the alternative complex III in a supercomplex with cytochrome oxidase. Nature. 2018;557:123–126. doi: 10.1038/s41586-018-0061-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Broecker J., Eger B.T., Ernst O.P. Crystallogenesis of Membrane Proteins Mediated by Polymer-Bounded Lipid Nanodiscs. Structure. 2017;25:384–392. doi: 10.1016/j.str.2016.12.004. [DOI] [PubMed] [Google Scholar]
- 63.Dominik P.K., Borowska M.T., Dalmas O., Kim S.S., Perozo E., Keenan R.J., Kossiakoff A.A. Conformational Chaperones for Structural Studies of Membrane Proteins Using Antibody Phage Display with Nanodiscs. Structure. 2016;24:300–309. doi: 10.1016/j.str.2015.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]