Highlights
-
•
Zinc oxide (ZnO) is a metal oxide nanostructure which has unique photocatalytic activity.
-
•
ZnO nanoflower is one of the shapes which has huge surface area to volume ratio due to the extended petal structures.
-
•
The surface modification and functionalization is also possible to be used for various biomedical applications, catalysis and bioremediation etc.
-
•
The ZnO nanoflower can also act as an antimicrobial agent for water remediation.
-
•
The ZnO nanoflower has been used as therapeutic agents for many diseases and designing of biosensors.
Keywords: Metal oxide nanoparticles, Zinc oxide nanoflower; Biosensors; Photocatalysis; Bionanotechnology; Biomedical application
Abstract
Zinc oxide (ZnO) nanostructures can be synthesized in nanoforms of spheres, rods, flowers, disks, walls, etc., among which nanoflowers have gained special attention due to their versatile biomedical and pollutant remedial applications in waste water and air. ZnO nanoflowers have an ultrasmall size with a huge surface area to volume ratio due to their hexagonal petal structures which render them superior compared to the nanoparticles of other shapes. The ZnO nanoflowers have bandgap energy equivalent to a semiconductor that makes them have unique photophysical properties. We have used the appropriate keywords in Google Scholar and PubMed to obtain the recent publications related to our topic. We have selected the relevant papers and utilized them to write this review. The different methods of synthesis of ZnO nanoflowers are chemical vapor deposition, facile hydrothermal, thermal evaporation, chemical reduction, bio route of synthesis, and solvothermal method, etc. which are mentioned in this review. ZnO nanoparticles are used in paints, cosmetics, and other products due to their high photocatalytic activity. The different applications of ZnO nanoflowers in the diagnosis of disease biomarkers, biosensors, catalysts, and the therapeutic process along with wastewater remediation and gas sensing applications will be discussed in this review.
Graphical abstract
1. Introduction
Nanotechnology is one of the major developing fields which has versatile applications in various fields of biomedical sciences, chemical catalysis, nano-enabled drug delivery, nanoformulations of drugs, contrast agents in biomedical imaging, biosensors for different disease biomarkers detection, antimicrobial agents, and therapeutics such as bone regeneration [43], etc. Nanostructures can be synthesized using various physical and chemical methods, and the nanoparticles can be altered or modified using simple methods to yield enhanced physical and chemical properties [45]. Among various types of metal oxide based nanoparticles, zinc oxide nanoparticle (ZnO NPs) is mostly used because of their applications in various fields such as beauty products, biosensors, antibacterial cream, and biomedical applications. ZnO NPs are naturally white and thermally stable, have a typical bandgap which gives them the semiconductor properties and because of these kinds of properties, it has a wide range of applications. ZnO NPs is a promising metal oxide nanoparticle, as zinc (Zn) can act as both an active element as well as a strong reducing agent, which is very much helpful in the synthesis of ZnO NPs. ZnO has unique optical properties, chemical sensing ability, semiconducting nature, piezoelectric, and electrical conducting properties. The ZnO NPs have a direct wide bandgap of nearly 3.3 eV which falls in near UV wavelength and high excitation binding energy at 60 meV at room temperature.
ZnO NPs were coated with different types of polymers, e.g., natural polymers like chitosan and synthetic polymer like polyethylene glycol (PEG), and has been exploited for their UV light scavenging activities. It was found that chitosan coating was superior in UV light scavenging as well it had high biocompatibility [24]. The biosynthesis of nano-structure-based biomaterials is eco-friendly, mostly biocompatible and non-toxic, and is also cost-effective. Green synthesis of ZnO NPs have been carried out using a wide range of metabolites from natural sources, which can act as both reducing agent as well as stabilizing agent [56]. Researchers have shown that ZnO nanoparticles can also be used as nanofertilizers [60]. The biogenic synthesis of ZnO and its effect on maize seed germination was also studied earlier and at 100 mg/L concentration, the ZnO primed seeds showed maximum germination [32]. Anti- bacterial wound healing agents were also developed by entrapping ZnO NPs in sodium alginate to make a nanocomposite [7].
ZnO NPs can be synthesized in various morphologies like spherical, nanocombs, nanorings, nanohelices, nanobelts, nanowires, nanocages, and nanoflowers. The ZnO NPs and ZnO nanoflowers (ZnO NFs) can be synthesized using various physical, chemical, and biological processes such as chemical vapor deposition, facile hydrothermal, thermal evaporation, chemical reduction, bio route of synthesis, and solvothermal method [37]. The various applications of the nanoflower structure of ZnO have been elaborately discussed which is schematically represented through a diagram shown in Fig. 1.
Fig. 1.
Application of ZnO Nanoflowers in biomedical sciences.
2. Zinc oxide nanoflowers synthesized using different methods and their applications
ZnO NFs have many petals which provide more surface area to volume ratio that renders more active sites for the analyte molecule adsorption. This property of the ZnO NFs makes it more sensitive than any other ZnO NPs and thin films [4]. The thermal evaporation method was utilized for the synthesis of ZnO NFs, using pure Zn powder in the presence of a catalyst, for ZnO NFs growth on a silicon surface. At high-temperature heating (850 °C) with a continuous flow of Argon gas, the ZnO NFs started to grow on the silicon wafer yielding the size of 60–200 nm wide nanoflowers [1]. The synthesized ZnO NFs were characterized using different photophysical tools like SEM, XRD, spectrophotometry, etc. A research group has reported the synthesis of ZnO NFs at low temperature using zinc acetate dehydrate as a precursor and sodium hydroxide as reducing agent, by solution process at 90 °C. The nanoflowers had hexagonal-shaped petals with sharp tips as visualized under SEM. They had a good crystalline structure with minimum structural defects as wurtzite hexagonal phase, exhibiting unique optical properties [69]. Another group of researchers has synthesized rose like ZnO NFs by using the chemical vapor deposition method. The nanoflowers were grown over SiC substrate and characterized using SEM, TEM, and XRD. At different parts of the substrate, some other types of structures were also observed like micro-plates, self-assembled nanosheets, and micro-palmerworms due to differences in Zn gas concentrations during synthesis. The PL spectrum of the ZnO NFs showed an emission peak at nearly 491 nm representing a crystal defect-related emission [80]. Single-step synthesis of one-dimensional ZnO NFs by the hydrothermal method was executed at different time of reactions. At 15 min, the petals were rod-like obelisk-shaped, but for a longer time of reactions like 30 min, 2 h, and 4 h the tips of the petals were tapered, although the base of the petals was hexagonal rod in shape. At 6 h and 48 h, the tips of the flowers were also hexagonal in shape but again at 72 h reaction time, the petals became rod-like obelisk shaped. The length of the rod was maximum at 30 h of reaction and the ZnO NFs kept for a lower time like 5 min or 10 min produced perfect flower structure but contained impurities [15]. A layer of polystyrene beads was deposited by colloidal lithography over a Si substrate which was then coated with a ZnO nanoparticle layer. These nanoparticles acted as a seed to grow the ZnO nanowires, which gave an overall appearance of a nanoflower resembling a sunflower. The petal rods were perfectly hexagonal in shape, making these structures useful for designing different optoelectronic devices [73]. Facile succinate and PEG-supported hydrothermal process was used to synthesize ZnO NFs and were characterized using XRD, SEM, UV–visible spectroscopy, and PL spectroscopy. Keeping the concentration of PEG constant, the precursor, succinate, and sodium hydroxide concentrations were varied for tuning the shape and size of the synthesized nanostructures. Apart from ZnO NFs, the other structures formed were sheet-like, vesicular and some undefined shapes (a mixture of rod and walls). The shape of the flower also varied between wall-like petals and hexagonal rod-shaped petals. The synthesized nanoflowers were also shown to reduce Rhodamine B through photocatalysis [2]. ZnO NFs were synthesized using an ultrasonic-assisted hydrothermal method at a temperature of 95 °C to yield ZnO NFs with hexagonal rod-shaped petals. The synthesized NFs exhibited UV light emission and three visible light emissions in the range of violet, blue-green and yellow [35]. Gamma radiation was used to synthesize ZnO and zinc sulfide nanoparticles with the help of polyvinyl pyrrolidone and their antifungal activity was observed pre and post-harvesting of the orange and pomegranate seeds. The combination of nano ZnO and zinc sulfide showed antifungal activities against Aspergillus niger (A.niger) and Penicillin digitatum (P.digitatum) [29]. Studies exist where ZnO NFs were grown atop nanosilver coated glass substrate by using spherical ZnO nanoparticles as a seed for the flower growth. The complete characterization showed perfect crystal structure and hexagonal shaped petals. It was used to design a biosensor for the detection of insulin amyloids [3]. Another application of ZnO NFs was its use for the dissociation of insulin amyloids. The degradation was monitored with an increase in its zeta potential values in magnitude. The electrokinetic potential was used as a measure of amyloid degradation [21]. Thus, it was shown in the above-mentioned studies that ZnO NFs could be synthesized using different routes with different flower morphology and its size can be tuned by controlling the experimental conditions.
3. Photocatalytic activity of zinc oxide nanoflowers-uses in wastewater remediation
Photocatalysis is the reaction in which light is used to accelerate the speed of the reaction. ZnO NFs and zinc oxide nanorods (ZnO NRs) were used in the photocatalytic degradation of 4-chlorophenol. The reaction of ZnO NFs and ZnO NRs with the 4-chlorophenol was compared and the results showed that the ZnO NFs exhibited higher photocatalytic properties than ZnO NRs. The Raman and photoluminescence spectrum of ZnO revealed that ZnO NFs have more oxygen valances than ZnO NRs. This showed that the change in the morphology of the ZnO NPs can possess superior photocatalytic activity due to the presence of higher oxygen valancies [72]. The doping of elements with ZnO NFs can also provide enhanced photocatalytic activity. A small amount of cadmium (Cd2+) doped with ZnO nanostructures could capture photo-generated electrons as well as holes on the surface. This could restrain the carrier recombination process, which improved the photocatalytic activity of the ZnO NFs doped with Cd2+. It was evidenced that there was an expansion in the absorption range and the spectral range of response of the ZnO NFs to the visible spectrum and the solar energy utilization was enhanced by the addition of Cd2+ to ZnO NFs [77]. The photocatalytic activity of ZnO NFs synthesized via green route using Calliandra haematocephala leave extract for the photodegradation of methylene blue (MB) under visible light was studied. The sample was stirred for 30 min under dark conditions to reach adsorption-desorption equilibrium and the degradation of the dye with and without catalyst was compared. The dye with ZnO NFs as catalyst showed 88% of dye degradation within 270 min, whereas no degradation was observed without the catalyst [76]. Lanthanum is a heavy metal and it was used to dope ZnO NFs at different doping amounts. The resultant La-doped ZnO NFs were tested for the degradation of the dye MB under UV light irradiation. The dye degradation capacity of the La-doped ZnO NFs was compared with pure ZnO NPs, and it was found that the La-doped ZnO NFs were more efficient in the MB dye degradation. The optimum concentration of La dopant was 3 mol% which showed maximum dye degradation within 60 min irradiation to UV light [30]. Doping of Copper (Cu) in ZnO NFs was synthesized using a facile hydrothermal route taking PEG and sodium citrate. A detailed study was done on the effect of OH− ions, metal ion concentration, and synthesis conditions of the surface morphology of the Cu doped ZnO NFs. MB dye degradation potential was monitored for the Cu doped ZnO after sunlight exposure and it was found that 100 mL of 100 pm dye was completely degraded within 30 min of treatment with the synthesized nanostructure (sample CZ-01) [40]. Dye-sensitized solar cell (DSSC) was fabricated using ZnO NFs as photoanode because of the unique structure of the flower which increased the amount of light absorption increasing the short-circuit current density. This made the ZnO NFs a good candidate for high-efficiency ZnO-based DSSCs [13].
Reduction of textile dyes using ZnO NFs has been shown by many researchers using the green synthesis methods for the preparation of ZnO NFs and directing the research towards wastewater remediation. The panos plant extract was taken from a group of four plants namely- Kalopanax septemlobus, Panax ginseng, Dendropanax morbifera, and Acanthopanax senticosus, and was utilized for the synthesis of ZnO NFs which appeared in shape like a quaker ladies flower and was named as ZnO/QNF. The ZnO/QNF was further used for the degradation of dyes like malachite green, Eosin Y, MB, after exposure to UV light. The synthesized ZnO/QNF was efficient in the degradation of all the above-mentioned dyes and was also reusable for another five cycles without losing its dye degradation capacity significantly [34]. The same group also investigated the dye degradation capacity of ZnO NFs synthesized through bio route using sea buckthorn fruit extract. The dyes used for the degradation study were-eosin Y, Congo red, malachite green, and MB at a concentration used in the previous study (15 mg/L). The synthesized ZnO NFs were added to the respective dyes followed by UV light exposure resulting in dye degradation up to 99% for all the dyes used. The ZnO NFs were also recycled and reused for dye degradation and could show equal efficiency up to 5 cycles of use. The study yielded an effective nanocatalyst for wastewater treatment [54]. Another study has shown the green synthesis of ZnO NFs using the aqueous extract of the leaves of the plant Calliandra haematocephala. The synthesized ZnO NFs were investigated for the degradation of the dye MB (20 mg/L) in the presence of sunlight. Within 270 min, 88% of the MB dye was degraded after exposure to sunlight and catalytic action of the synthesized ZnO NFs [67]. Researchers have shown that ZnO NFs synthesized through bio-route using Thlaspi arvense I plant extract as a reducing agent could be used to degrade harmful dyes like MB and 4‐nitrophenol (4- NP). The synthesized ZnO NFs possessed a size of 70–90 nm, whereas the zeta potential was +31 mV, showing high stability. A complete degradation was achieved within 49 min for MB. The conversion of harmful 4-NP to benign 4-aminophenol (4-AP) was also seen when treated with the synthesized ZnO NFs [65]. Tripathi et al., synthesized ZnO NFs using bio route by utilizing the extracts from the bacteria Bacillus licheniformis MTCC 9555. The size of the ZnO NFs was in the range of 250 nm to 1 µm and it exhibited MB dye degradation with high efficiency compared to the earlier reports. The reason for enhanced photocatalytic activity was proposed to be due to the higher content of oxygen vacancy. The photocatalyst was reusable for 3 cycles of MB dye degradation [63]. Green synthesis of ZnO NFs were conducted using the pod extract of Peltophorum pterocarpum with high specific surface area 19.61 m2/g as revealed by BET analysis. The synthesized NFs also showed its dye degradation properties on methylene blue [68]. Thus, it could be concluded that ZnO NFs can be utilized for the efficient degradation of harmful dyes and can be a possible wastewater remediation agent.
4. Applications of zinc oxide nanoflowers as a sensor
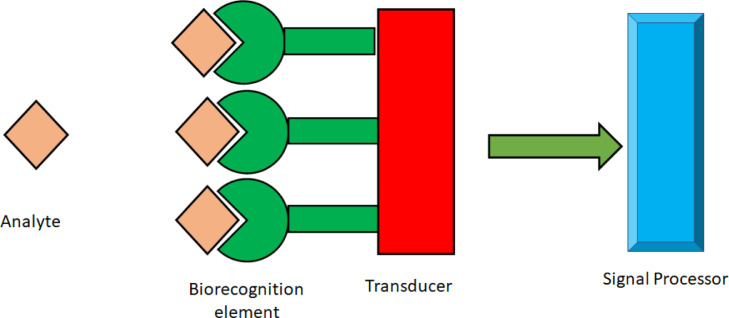
The detection of different toxic chemicals, gases, and metabolites related to different diseases can be effectively executed by sensors or biosensors. Biosensors are miniaturized devices that uses the target analyte reaction for detecting different bioanalytes with high specificity and sensitivity. Biosensors contain a basic structure as shown in Fig. 2, with a biorecognition element that can specifically bind to the analyte of interest and convert the binding event into a signal which is converted using a transducer to a measurable signal. The analytes to be detected can be enzymes, antibody, cells, microbes, gases, membrane, cofactors, DNA, miRNAs, protein, toxin, peptide, vitamin, sugar, metal ions etc. The energy transducer is attached to the biorecognition element which is a chemical or physical transducer that converts the output of the reaction between bioanalyte and biorecognition element into a form of energy that can be measurable like conductance, impedance, potential, current, heat, change in absorbance, change in fluorescence mass change etc.
Fig. 2.
The basic structure of a biosensor. The analyte is detected by the recognition element which converts the event to a measurable signal through the transducer.
There are different types of biosensors classified based on the principle of transduction, such as, electrochemical (amperometric, conductometric and potentiometric), optical (Surface plasmon resonance biosensors, Localized surface plasmon resonance, Evanescent wave fluorescence biosensors, Optical waveguide interferometric biosensors, Surface-enhanced Raman scattering biosensors), piezoelectric, calorimetric and magnetic biosensors [22, 23, 41, 62]. The principle of these biosensors is described briefly below:
-
(i)
Electrochemical Biosensors: The electrochemical biosensors utilize predominantly the enzyme substrate reaction to detect the analyte because this biocatalysis reaction is highly specific. When the recognition element (enzyme) detects the analyte (substrate), the reaction results in generation of a measurable current (amperometric), or can cause accumulation of charge or potential (potentiometric) that can be measured, or can influence the conduction of the medium (conductometric) that is measurable. These changes in current, conductance and potential are measured in reference to standard electrodes available. The more the amount of analyte present in the sample, the more signal is detected as current, potential or conductance respectively for the amperometric, potentiometric and conductimetric biosensors. In field- effect biosensors, transistor technology is used to assess the current due to the potentiometric effect in gate electrode.
-
(ii)
Optical Biosensors: In optical biosensors, the recognition element reacts with the analyte to produce an optical signal with change in absorbance or change in fluorescence signal which is recorded using spectrophotometer, fluorimeter or plate readers. The fluorescence biosensor works on the principle of switch-on or switch-off based on the signal enhancement or decrease respectively, of the fluorochrome molecule attached to the analyte. In case of DNA based biosensors, one of the strands is immobilized on a solid surface and the sample containing the target strand is made to hybridize with the immobilized DNA. After hybridization the fluorochrome and the quencher which were tagged at the two ends of the single strand, gets separated and the fluorescence signal enhances.
-
(iii)
Piezoelectric Biosensors: Piezoelectricity is a physical phenomenon where a piezoelectric material can produce voltage if there is application of a mechanical stress. This process is applicable in the reverse situation also, e.g., if we apply voltage to the surface of a piezoelectric material, it will cause mechanical stress leading to oscillation in the crystal. Piezoelectric biosensors are based on this principle of detection of signals, i.e., after the binding of the analyte with the recognition element immobilized on a piezoelectric crystal, there will be a mass change which will be proportional to the number of binding events. As the mass will change the potential generated across the crystal will also change and the change will be proportional to the concentration of the analyte present in the sample.
-
(iv)
Calorimetric: Calorimetric or thermometric biosensors are based on the principle that in many biological reactions, heat is either evolved or absorbed and the amount of such heat is proportional to the total number of molecules present in the product. Calorimetric biosensors can measure the heat change and convert it to electrical signal.
-
(v)
Magnetic biosensors: This type of biosensors utilizes the paramagnetic or super-paramagnetic particles, also known as magnetic nanoparticles. The particles detect the biological interactions leading to change in the magnetic properties such as, coil resistance, inductance or magneto-optical parameters. There is involvement of many technologies for particle detection in magnetic biosensors that includes GMR devices, Hall Effect devices, coils and different optical and imaging techniques.
There are many types of nanomaterials such as, carbon tubes, semiconductor metal oxides (SMO) and polymers, which are used for sensing different gases because these materials offer changes in electrical properties when the gas molecules get adsorbed over them. Apart from electrical properties, these materials also show differences in acoustic, optic, calorimetric and gas chromatographic properties when certain gas molecules are adsorbed atop them. As per the method of signal measurement gas sensors can be classified as (i) Field Effect Transistors (FET) based gas sensors, (ii) Photoluminescence based gas sensors (iii) DC conductometric gas sensors [14].
ZnO NFs based sensors have shown to be highly efficient and have been used to design many types of sensors. The ZnO NFs were doped with Gallium to form Gallium zinc oxide (GZO) nanostructures. This preparation of GZO could be done using various known methods and this was used in various research areas like sensors, photocatalysis, and dye-sensitized solar cells. The main advantage of these GZO and undoped ZnO nanoparticles is they are non-toxic semiconducting nanoparticles. In this study, the fluorescent spectroscopy confirmed that the Green Fluorescent Protein was immobilized on the GZO nanodisks and nanoflowers. The prepared nanomaterial showed definite activity as a UV light sensor and exhibited green color fluorescence with high sensitivity [52]. The sensing property of the ZnO NFs is high compared to other zinc oxide structures. The sensors with ZnO NFs coating showed great sensitivity of 5.75 pm/ (μg • L − 1) and selectivity for ammonia gas detection at room temperature. This study showed that ammonia gas can be sensed using sensors coated with ZnO NFs and high sensitivity levels were obtained due to the high surface area to volume ratio [81].
The other types of sensors using ZnO NFs are given in Table 1.
Table 1.
The different types of sensors based on the ZnO NF structure.
| S No. | Type of nanostructure used | Finding of the study | Method of detection | References |
|---|---|---|---|---|
| 1. | ZnO nanoworms, nanoflowers, nanowalls, and nanorods | Nanoworms, nanorods, and nanowalls were prepared by the pulsed laser ablation (PLD) method. During nanoworm and nanowall synthesis secondary intermittent product which was ZnO NF was formed. The PLD-grown ZnO nanostructures were elaborately explained according to the different growth planes and experimental conditions. ZnO nanowall due to its very high surface area to volume ratio was immobilized on Al2O3 and has shown its high-efficiency gas sensor capacity for CO and ethanol gases detection. The detection limit was 50 ppm at 350 °C for CO and 400 °C for ethanol gas. | Electrochemical (conductometric) | [38] |
| 2. | ZnO nanodisk, nanoflower, and nanospindle | Aqueous chemical route was used to synthesize ZnO nanostructures like nanodisk, nanorod, nanoflower and nanospindle by varying the pH from 5.0 to 10.0. The acetone gas sensor was designed using the above nanostructures and the results showed that compared to nanodisks the nanoflower gave higher limit of detection for acetone gas. The lower response of the nanodisks was attributed to the sporadic distribution of the nanodisk over the total substrate which reduced the active surface area required for the adsorption of oxygen resulting low gas response. On the other hand, for the ZnO NFs, the effective surface area was higher due to the extended flower petals which facilitated elevated interaction of acetone gas the molecules of oxygen that were adsorbed. | Electrochemical (conductometric) | [50] |
| 3. | Micro-patternable double-faced (DF) zinc oxide nanoflowers (ZnO NFs) | On a polyimidie substrate, ZnO nanoshells were laid at regular intervals over which the nanorods were grown inside and outside the shells to give a maximum surface area. The three-dimensional structure contained many gas diffusion channels and was used efficiently as a gas sensor for NO2 detection with Single-walled carbon nanotubes (SWCNTs) as electrodes. The high selectivity for NO2 gas was observed showing a stable response of 218.1, with rapid rising and decay timings as 25.0 s and 14.1 s respectively. The recovery percentage of the gas sensor was 98% after exposure to NO2. | semiconductor metal oxides (SMO) | [36] |
| 4. | ZnO NFs | An amperometric biosensor based on ZnO NFs was designed to detect hydrogen peroxide (H2O2). A modification was made in the glassy carbon electrode by electrodeposition of ZnO NFs over a thin film of multi-walled carbon tubes (MWCNTs). H2O2 was detected with high sensitivity at −0.11 V in a liner range from 9.9 × 10 − 7 to 2.9 × 10 − 3 mol/L with a very short response time of less than 5 s. The rapid response time, excellent stability, and a linear range of response were observed in this H2O2 sensor. |
Amperometric | [6] |
| 5. | ZnO nanowires, nanorods and nanoflowers | One dimensional nanostructure of ZnO was employed to design an oxygen sensor. The nanostructures used were nanowires, nanorods, and nanoflowers which were grown on substrates of silicon and alumina to assess the gas sensor activity under different conditions. The results revealed that alumina substrate was superior compared to silicon substrate for gas sensing abilities. Different temperatures and gas flow of oxygen were used to determine the gas sensing sensitivity and it was found that the maximum sensitivity was near ambient temperature using a nanowire structure. | Conductometric | [42] |
| 6. | ZnO NFs | A simple co-precipitation method was used for the synthesis of ZnO NFs for designing an electrochemical sensor for detection of Sunset Yellow (SY) dye in which the carbon paste electrode was modified by coating it with the synthesized ZnO NFs. The modified electrode had a higher surface area which yielded an enhancement of electrochemical oxidation of SY and a linear detection of SY was executed in the range of 0.50–10 μg/L and 10–70 μg/L. | Electrochemical (Amperometric) | [74] |
| 7. | ZnO NFs | ZnO NFs composed of ZnO nanorods were synthesized by facile one-step hydrothermal method and its application was studied for sensing flammable gases as well as corrosive vapors. The sensor was highly sensitive, had fast response-recovery characteristics, and was stable. | Conductometric | [39] |
| 8. | ZnO NFs | The ZnO NF was immobilized on an indium tin oxide (ITO) electrode using hydrothermal decomposition method and utilized for detection of heavy metal lead (Pb+2) by Pb(2+)-dependent DNAzyme based biosensor. The detection was done using fluorescence emitted after DNA hybridization and the sensor was linear in the range of 0.5–20 nM and had a limit of detection of 0.1 nM. | Photoelectrochemical sensor | [78] |
| 9. | Ag-doped ZnO NFs | Low temperature hydrothermal synthesis of Ag-doped ZnO NFs was achieved with high density and they possessed a well-crystalline structure. The fabricated nanostructure was used as a chemical sensor for the detection of phenylhydrazine by employing a current-voltage (I-V) technique. The detection was achieved with high sensitivity ≈ 557.108 ± 0.012 mAcm(−2)(mol L(−1))(−1) and detection limit of ≈ 5 × 10(−9) mol L(−1). | Current-voltage (I-V) technique | [31] |
| 10. | ZnO-Ag hybrids | A surface-enhanced Raman scattering (SERS) spectroscopy-based sensors designed by 4-aminothiophenol (4-ATP) functionalized ZnO–Ag hybrid NFs was used for the detection of explosives (Trinitrotoluene, TNT). The sensor provided excellent sensitivity for TNT detection with concentrations as low as 5 × 10−9 M. The sensor was also sensitive for other derivatives of TNT detection compared to the available sensors. | Surface-enhanced Raman scattering (SERS) spectroscopy |
[28] |
| 11. | Ag-doped ZnO NFs | A low-cost hydrothermal synthesis of an ultraviolet-enhanced (UV-enhanced) nitric oxide (NO) sensor engineered by silver-doped zinc oxide (ZnO) nanoflowers was established. Ag ions improved the sensitivity of NO detection and the limit increased from 73.91 to 89.04% post-exposure to UV light-emitting diode (UV-LED). The UV-enhanced Ag-doped ZnO NFs sensor exhibited lower response time which was reduced to 60 s from 120 s without Ag. The temperature for detection of NO was also reduced to 150 °C from 200 °C with an increase in detection amount to 15.13%. The gas sensor property was also possible to be calibrated by using the self-photoelectric effect under the illumination of UV light. | Field Effect Transistor (FET) | [64] |
The huge surface area of the ZnO NFs petals imparts it with superior gas sensing ability and also for its use as other sensors.
5. Zinc oxide nanoflowers as diagnostic biosensor
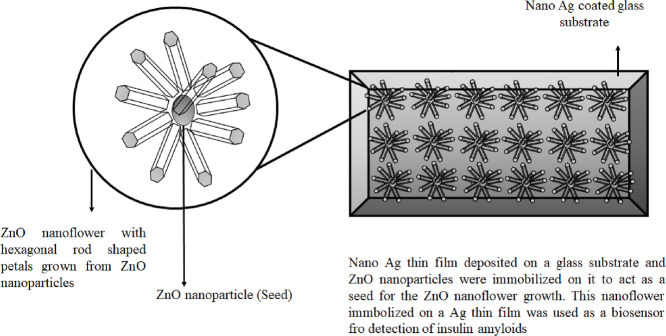
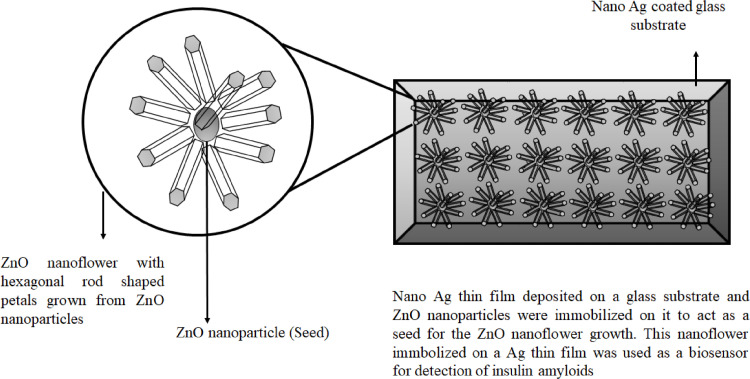
The understanding of amyloid protein was fascinating and made many researchers to work on it because of the protein folding dynamics, self-assembly, and the pathogenic mechanism of some human diseases. Amyloidosis defines the spectrum of diseases which involve the accumulation of amyloidogenic protein aggregates in the parts of the brain and different organs of the body like Alzheimer's disease, Parkinson's disease, Creutzfledt Jakob syndrome, type II diabetes, etc. Alzheimer disease is one of the neurodegenerative diseases caused by misfolding of amyloid protein and its aggregation in the neuropil of the brain and forming neurofibrillary tangles [53]. Alzheimer's disease is considered a worldwide health problem and also as late-life dementia. Due to strong neurotoxicity, amyloid-β protein (Aβ 1–42) was proved that it was responsible for the pathogenesis of Alzheimer's disease compared to Aβ 1–40. The ceria doped ZnO NFs (Ce:ZONFs) were used for designing a luminol-based electrochemiluminescence (ECL) immunosensor for the detection of amyloid-β protein (Aβ). The Ce:ZONFs were synthesized by the green method using lysine as a reducing agent and this ECL immunosensor has shown high sensitivity for detection of Aβ having a broad linear range from 80 fg/mL to 100 ng/mL. The lowest limit of detection was 52 fg/mL which made this sensor useful for effective detection of Aβ [71]. The biosensor for the detection of insulin amyloid was also developed using ZnO NFs grown on different substrates and the efficiency was compared between them. Insulin amyloid, being structurally similar to other amyloids related to neurodegenerative disorders, was used as model amyloid for the study. The ThT (Thioflavin T) dye which can bind to amyloids specifically to emit fluorescence was adsorbed over the ZnO NFs and the detection sensitivity enhanced nearly 10 folds compared to fluorescence emitted from the bare glass substrate. Thus, an enhanced sensitivity was observed in this engineered biosensor for insulin amyloid detection [3]. In Fig. 3 we can see that on an Ag coated glass substrate, the ZnO NPs are seeded and ZnO NFs were grown taking the NPs as seeds to design a biosensor. The nano Ag film coating helps in fluorescence enhancement by metal enhanced fluorescence (MEF) [11].
Fig. 3.
An illustration showing the growth of ZnO nanoflower from ZnO nanoparticles as a seed which is immobilized on an Ag thin film coated glass substrate. This nanostructure was used to design biosensor for detection of insulin amyloids [3].
In another study, the ZnO NFs were used for the detection of diabetes. The Na element acting as a p-element was incorporated in ZnO lattice. The Na:ZnO nanoflower was a p-type semiconductor and was utilized as a gas sensor and its sensing ability was activated by the illumination of UV light. The Na:ZnO nanoflower sensor showed a high response to acetone gas. In cancer and type-1 diabetes patients, it was found that the acetone gas was exhaled by them exceeded the range between 1.0 ppm and 1.8 ppm while in normal person the acetone gas present in the exhaled air was found in the range from 0.3 to 0.9 ppm. The solid-gas reaction was prompted by the metal oxide activation through UV illumination and consequent ionosorption of the oxygen species over the surface of metal oxide was found. The prepared gas biosensor exhibited hole transport of electrons and showed a high level of sensitivity to acetone gas [33].
Facile solution-based synthesis of ZnO NFs was done and its efficiency as a highly sensitive non-enzymatic sensor for glucose measurement was assessed. The ZnO NFs were found to be an efficient electron mediator and the biosensor had a sensitivity of ∼411 μA M–1 cm–2. The limit of detection of the sensor was ∼1.25 mM and it had a quick response time of nearly 10.0 s [66]. Oxygen plasma treated ZnO NFs were used as a coating of electrode designed to detect dopamine (DA), and diclofenac sodium (DS) in an electrochemical biosensor. The different photophysical tools were used for the characterization of ZnO NFs and the large surface area of the petals demonstrated good electrochemical performance. The cyclic voltammetry in the potential range of 0.3–1 V showed a linear response in the range of 0.1–300 µM for DA and 0.01–40 µM for DS with sensitivities of 0.26 µA•μM−1cm−2 and 0.37 µM•μM−1cm−2 for DA and DS respectively with the limits of detection 0.28, and 0.11 μM. The sensor could detect both DA and DS simultaneously in urine samples with good sensitivity [79]. The sonochemical technique was used to synthesize graphene oxide covered ZnO NFs (ZnO NFs@GOS) to be utilized as a modified electrode for the detection of 8‑hydroxy-2′-deoxyguanosine (8-HDG) in the urine sample. 8-HDG is a biomarker related to oxidative stress and cancer. The range of detection of the electrochemical biosensor was 0.05–536.5 µM with a detection limit of 8.67 nM [25]. In another research work, gold nanoparticles were spotted on ZnO NFs, to design a nanohybrid that was used to detect pathogenic Leptospirosis-causing strain's DNA through hybridization technique and mismatch analyses. Using impedance spectroscopy, the limit of detection was as low as 100 fM [51]. Nicotine is a potent carcinogen and its detection was achieved by a SPR based fiber optic sensor. The sensor was composed of coatings of Ag and graphene doped four types of ZnO nanostructure over the unclad core of the optical fiber. The four types of ZnO nanostructures used were nanocomposites, nanotubes, nanoflowers, and nanofibers. The ZnO nanostructure-based core produced high sensitivity for the detection of nicotine compared to other available methods [61]. A three-dimensional (3D) cloves bud resembling gadolinium doped ZnONFs speckled with reduced graphene oxide (GZO@rGO) was used to modify the glassy carbon electrode for the detection of l-Dopa. The electrochemical sensor showed l-Dopa oxidation current response to be directly proportional to the l-Dopa concentration giving a linear range of response from 10 to 100 nM. The designed sensor was sensitive at 0.1 μA nM−1 cm−2 and had a limit of detection of 0.82 nM [16]. The other electrochemical sensors based on different ZnO nanostructures have been reviewed earlier [44]. The development of biosensors for the detection of various biomarkers related to different diseases was thus shown to be designed using the unique properties of ZnO NFs. ZnO NFs are also useful in therapeutics. Some of the therapeutic applications of ZnO NFs are discussed.
7. Therapeutic applications of zinc oxide nanoflowers
7.1. Formation of blood vessels by zinc oxide nanoflowers
Angiogenesis is the process of new blood vessel formation from the pre-existing blood vessels. It is a very complex process and it involves many major steps like the stimulation of endothelial cells induced by the growth factors, the proliferation of endothelial cells, migration of endothelial cells followed by the formation of intravenous blood vessels. The cell proliferation assay was conducted for endothelial cells with ZnO NFs. The endothelial cells were stimulated by the ZnO NFs, exhibiting the proangiogenic nature of the ZnO NFs. The chick embryo angiogenesis assay showed new intravenous blood vessel formation from the pre-existing blood vessels when exposed to ZnO NFs. The results showed that H2O2, the signaling molecule may be one of the reasonable mechanisms for the induced formation of blood vessels by the ZnO NFs. The same group reported that the angiogenesis executed by the ZnO NFs was mediated through the nitric oxide formation through MAPK/Akt/eNOS pathway where the nitric oxide acted in a cGMP dependent manner [9, 49].
7.2. Zinc oxide nanoflowers in cerebral ischemia
Neuritogenesis is a major process in which the differentiation of neurons, exons and several dendrites takes place. After seeing the angiogenetic property of the ZnO NFs researchers conjectured that these nanoflowers can also be used as a therapeutic agent for improving the neuritogenic activity by increasing the mRNA expressions of different neurotrophins. Cerebral ischemia can lead to decreased brain function and sometimes brain death. There are few therapies available to restore the function and structure of the damaged neuronal tissue related to cerebral ischemia. Some of the therapies include thrombolytic tissue plasminogen activator, pro-angiogenic growth factors as well as nerve growth factors. Cerebral ischemia was induced in the Fischer rat model and the ZnO NFs possessing pro-angiogenic properties were injected into the cerebral region. The ZnO NFs showed neuroprotective ability as evidenced by the neuronal studies and staining of the Golgi-Cox in sections of brain tissue. The results showed improved synaptic connections with improved dendritic spine density, establishing the important role of neurabin-2 and NT3 protein. This is a regenerative approach directing possible cerebral ischemia treatment through in vivo process. This study showed that ZnO NFs not only had angiogenetic properties but also demonstrated neuroprotective and neuritogenic properties [8].
7.3. Zinc oxide nanoflowers on osteoblast growth and osseointegration
Osteoblasts are the cells that are responsible for the formation of bones. The fabrication of nanomaterials with biomaterials could make the material more biocompatible and more effective. In a study, the surface effect of MC3T3-E3 osteoblast growth and osseointegration for applications in bone tissue engineering were studied. The ZnO NFs were prepared on a silicon substrate in the presence of a layer of photoresist to manipulate the inter-distancing in the nanoflower structure. The Si substrate was coated with the ZnO NFs and the electron holes were made by photolithography. The substrate was aligned and oxygen plasma treatment was done to remove the organic residues present on the patterned holes and the substrate was dipped in the nutrient solution for the ZnO NFs growth. Then the photo resist layer was removed by treating with acetone. The MC3T3-E1 osteoblast cells were cultivated on the top of TCPS, ZnO film, and ZnO NFs respectively. After 16 days it showed that the ZnO NFs had an increased amount of DNA content and ALP activity. After centrifugation, it was concluded that ZnO NFs showed enhanced surface effects on differentiation, adhesion, and growth of the osteoblast cells compared to other substrates. The ZnO NFs on the substrate were used for implantation to the calvarial bone defect in the SD rat model and covered with HA-PLGA films. After an incubation period of 4 weeks, the X-ray tomography showed that the bones have started to regenerate on the surface of the ZnO NFs substrate. This showed that ZnO NFs could promote cell proliferation, adhesion, growth, as well as osseointegration in a superior way compared to ZnO film. This novel concept can be used in dental implants and other medical applications [47]. This unique method for the growth of ZnO NFs structure on biomaterials has raised interest in various tissue engineering applications.
7.4. Enhanced toxicity of zinc oxide nanoflowers for killing cancer cells
The ZnO NFs were synthesized at low temperature and its cell killing capacity was monitored for human cervical carcinoma (HeLa) cells as well as towards a noncancerous murine fibroblast cell line (L929). The nanoflowers were synthesized using different reaction times like 0.5 h, 2 h, and 4 h and the hydrodynamic diameter in cell culture medium with serum was minimum (7.53 ± 0.53 nm) for the ZnO NFs grown for 4 h. The cellular uptake of the different ZnO NFs was monitored using flow cytometry and the uptake was higher for the HeLa cells compared to L929 cells for all the different ZnO NFs. The cell killing through apoptosis and necrosis was also monitored for HeLa and L929 cells after exposure to the different concentrations of the different types of ZnO NFs by flow cytometry post staining with Annexin V, FITC/propidium iodide. Ten times more apoptosis and necrosis were induced in HeLa cells compared to L929 cells. A similar result was obtained when studied using fluorescence microscopy. Although the production of ROS was significantly higher than negative control in both HeLa and L929 cells for all the doses (0.1, 1.0, and 10.0 μM) of the three types pf ZnO NFs (named as ZnO NP 4 h, ZnO NP 2 h, and ZnO NP 30 min), but the response was more pronounced in case of HeLa cells compared to L929 cells. Thus, from the above study, it was concluded that the ZnO NFs could induce higher cell killing in cancer cells compared to normal cells [46]. Cai et al., [10] have shown that the different morphologies of the petals in ZnO NFs, like fusiform flowers, rod flowers, and petal flowers have shown toxicity towards HeLa cells. ZnO NFs along with other self-styled ZnO nanostructures like nanoplates (NPls), nanosheets (NSs), and nanorods (NRs) were exploited for their effect on cancer cells in a study by [70]. Human T98G gliomas, kidney HEK293, and MRC5 fibroblast cells were used to study the induction of apoptosis at a fixed pH. The results demonstrated that all the nanostructures were capable of inducing cell death in the malignant T98 G glioma cells, although the nanorods exhibited higher killing by inducing caspase dependent apoptosis in these cells. Chronic low dose exposure to oxidative stress plays a pivotal role in the etiology of cancer [12]. Inhibition of caspase dependent apoptosis was also seen in Chinese hamster fibroblasts cells, conditioned with chronic low dose exposure to H2O2, when exposed to gamma radiation [19] or cisplatin, a chemotherapeutic drug [18].
8. Antimicrobial activity of zinc oxide nanoflowers
The ZnO nanoparticles showed growth inhibition properties in many of the Gram- positive and gram-negative bacteria. The facile solution based synthesis of ZnO NFs was tested for the antimicrobial activity against E.coli bacteria. The minimum inhibitory concentration for killing E. coli was 25 µg/mL of ZnO NFs. The interaction of the ZnO NFs with the bacteria is responsible for the disruption of the cell membrane as a result of ROS production from water and oxygen leading to cell killing [66]. Graphene oxide was coated with metal and metal oxide nanoparticles like Ag, ZnO NFs, and TiO2 to give GO–Ag, GO–TiO2@ZnO, and GO–Ag–TiO2@ZnO nanocomposites. The synthesized nanocomposites were tested for the antimicrobial activities of Gram-positive bacteria (B. anthracoides and S. aureus) as well as strains of Gram-negative bacteria (P. multocida and E. coli) by using the disc diffusion method. The study showed both the Gram-positive and Gram-negative bacteria were strongly inhibited by GO–Ag and GO–TiO2@ZnO nanocomposites and higher killing activity was performed for the Gram-negative bacteria. Although, GO–Ag, GO–TiO2@ZnO, and GO–Ag–TiO2@ZnO nanocomposites have shown antimicrobial activity towards both Gram-positive and negative bacteria, but only Ag NPs exhibited minimum inhibitory effect against all the bacteria tested [17]. In wastewater treatment green synthesized ZnO NFs using Carica papaya milk were used. These ZnO NFs showed more antibacterial activity against Pseudomonas aeruginosa and Staphylococcus aureus compared to Klebsiella aerogenes and Pseudomonas desmolyticum. The culture was cultivated with the bacteria and the disk loaded with ZnO NFs was placed on the culture and incubated. After the incubation period, the Gram- negative bacteria showed a maximum zone of inhibition compared to Gram- positive bacteria. This is because the outer membrane of the Gram- positive bacteria is made of a thick peptidoglycan layer which consists of linear polysaccharide chains with short peptide cross- links making the membrane rigid but in Gram- negative bacteria the cell wall possesses only layers of peptide-glycan. The ZnO NFs could not penetrate the rigid cell membrane of the Gram-positive bacteria but entered the Gram-negative bacteria ruptured them using reacting species like O2•−, OH•−, H2O2 generated by the surface of ZnO NFs. This experiment was conducted in the dark because white light may enhance the generation of electron-holes by the activation of ZnO NFs. The holes were generated which split the H2O molecules present in the suspension of ZnO into hydroxyl radicals (OH−) and hydrogen ions (H+). Further, the oxygen molecules that are dissolved in the solution are converted to superoxide radicals (O2•−) which react with H+ ions to form hydrogen peroxide radicals (H2O2•). The hydroxyl radicals collide with electrons to yield hydrogen peroxide anions (H2O2−) which further react with hydrogen ions for the production of H2O2 molecules. The H2O2 can penetrate into the cell membrane and disrupt the bacteria while the OH− and O2•− cannot penetrate into the bacteria as they possess a negative charge. The small-sized nanoparticle could easily penetrate into the cell wall of the bacteria. The ZnO NFs were synthesized and characterized by XRD and the size of 11 nm was obtained which was optimal to investigate the antibacterial activity. The UV visible spectroscopy results showed the band-gap energy of the ZnO NFs has significantly increased. This study showed that the small crystallite size, shape, and concentration were the essential features responsible for the improved antibacterial activity of the ZnO NFs [58].
Yittrium doped 3D ZnO NFs with ZnO nanorod shaped petals were synthesized using the hydrothermal method with Yittrium doping at 2% and 4% to give YZO. The antibacterial activity against E.coli and S. aureus was evaluated for the ZnO and YZO. There was a complete elimination of both the bacteria, Gram- negative and Gram- positive after 6 h of incubation with YZO. Thus, an inhibitory effect was established for Yittrium doped ZnO NFs [[58], [59]]. Surface functionalized eggshell membranes (ESM) were used as a template to grow ZnO NFs decorated with Ag nanoparticles through sonochemical synthesis to prepare a nanobiocomposite. The bactericidal properties of the synthesized nanobiocomposite were monitored for Gram-negative, E. coli, or P. aeruginosa along with Gram-positive, S. aureus, or B. subtilis. Excellent bactericidal activity was observed with cell killing up to 97% for both Gram- positive and Gram- negative bacteria. The biocompatibility was tested by using NIH 3T3 fibroblast cells which showed lower cell killing after exposure to the nanocomposite. The reason was justified as the Ag got agglomerated when it was exposed to the cells because the culture medium containing the nanobiocomposite was not changed for three days. It is known that if the Ag nanoparticles have a higher size, which happened due to agglomeration, it can exert higher killing of the mammalian cells. Under such a situation it can be considered that the synthesized ZnO NF decorated with Ag nanoparticles grown over ESM, can be used as a bactericidal agent [26]. In a recent study, bioinspired synthesis of ZnO NFs was done using Withania coagulans extract as a reducing agent, and its antibacterial ability and high efficiency algae harvesting was assessed. The ZnO NFs have typical triangular shaped petals with asteroideal projections as visualized under TEM. The presence of phenolic and amino group on the surface of the synthesized nanoflowers was observed which acted as a stabilizing agent and the antibacterial activity was more pronounced for the Gram-positive bacteria Staphylococcus aureus in comparison to Gram-negative bacteria Pseudomonas aeruginosa. The ZnO NFs were porous in nature which could entangle with the algae for its harvest. The algae harvesting efficiency was nearly 96% which was very useful for the production of bioethanol and has shown a promising industrial application [27]. In another study, green synthesis of ZnO NFs was done by facile method using extracts from the plant Thlaspi arvense. Complete characterization of the as-fabricated ZnO NFs was done using UV- visible spectrometry, FTIR, XRD, SEM, HRTEM, and EDS. The bactericidal activity of the synthesized ZnO NFs was done for MDR bacterial strain Escherichia coli (E. coli, BL21 DE3) and was compared with that of ZnO nanoparticles which were commercially available. The killing was found to be higher for the ZnO NFs which was demonstrated due to excess generation of ROS and higher membrane damage induced by the nanoflowers. The SEM images have shown that the E. coli cells were completely ruptured after exposure to biosynthesized ZnO NFs. Moreover, the photocatalytic activity of the ZnO NFs was also demonstrated which has been discussed in earlier sections [65]. Three types of flower petal structures namely fusiform, petal, and rod-shaped petal was synthesized controlling the experimental conditions for ZnO NFs and their antibacterial activity was found to be promising for all the petal structures [10]. An in silico study exploring the molecular electrostatic potential study of ZnO NPs and bacteria has shown that the ZnO NPs have a high surface area along with structural defects that allows the bacterial protein to bind electrostatically weakly or in an adsorbed state with ZnO [55]. A recent study reported the synthesis of Phyto-reflexive surface decorated ZnO NFs which exhibited antimicrobial activity against Gram-negative Pseudomonas aeruginosa and Gram-positive Staphylococcus aureus, and also anti- fungal activity against Aspergillus niger and Candida Albicans [57]. Among a wide range of research studies discussed above, it was observed that ZnO NFs could act as a potent antibacterial agent with their effect extended for both Gram-positive and Gram-negative bacteria.
9. Molecular and mechanistic insights into zinc oxide nanoflower–protein interactions
To study the interaction of ZnO nanoflowers with the proteins BSA and human insulin (HI), researchers have synthesized ZnO nanoflowers and used wet lab as well as in silico tools for the analysis. Solvothermal route was used to synthesize ZnO nanoflowers and microwave assisted route was employed for polyol-coated ZnO NPs (ZnO@PEG NPs) synthesis which was smaller in size (40 nm) compared to the nanoflowers. The fibrillation and antifibrillation of the proteins BSA and HI after the addition of these nanostructures were monitored by Thioflavin T (ThT) fluorescence measurement. Both the structures exhibited their role in amyloid formation and dissociation, but the ZnO NFs could degrade both the proteins with high efficiency. Acetylsalicylic acid (ASA) was used as a positive control to induce the fibrillation. The insulin fibrillation was inhibited to 47% by the ZnO NFs and 44% by the ZnO@PEG NPs. Molecular docking studies were done to find the structural interactions between the nanostructures and the two proteins. The global binding energies of the ZnO NPs when docked with BSA and the HI targets were calculated and was found to be −5.44 kcal.mol−1 for BSA and −1.56 kcal.mol−1 for HI. The data showed that ZnO NPs had a higher capacity to bind with BSA and they can act as catalysts during the process of fibrillation of the albumin monomers [20]. ZnO NPs are incorporated in foodstuffs as a dietary source of zinc and have the possibility of interacting with the biological molecules which may impart some toxic effects. In a study, the interactions between ZnO NPs and skim milk, a representative protein food matrix, were monitored with emphasis on the different components of the skim milk such as the protein, minerals, and saccharides. Cellular uptake, cytotoxicity, structural deformation of the proteins, intestinal transport, and the efficiency of digestion was assessed. Fluorescence quenching of tryptophan was used to study the interaction of the protein casein, a component of skim milk, with the ZnO NPs. HPLC was employed to find out the amount of saccharide, lactose, adsorbed above the ZnO NPs. This was done to study the interaction between the saccharides and the ZnO NPs. The adsorption of the mineral calcium over the ZnO NPs was observed for skim milk containing calcium and only calcium solution. The calcium adsorption was only 10–29% for skim milk, whereas it was 60% for only calcium solution. All the above data for the interaction between ZnO NPs with saccharides, proteins, and minerals present in skim milk did not show any significant difference with different times of ZnO NP incubation. Along with the data of molecular as well as cellular interactions, the ZnO NPs were found to be safe for human consumption [5]. The interaction of ZnO NPs with biomatrices was studied in another study by the same group of researchers to reveal its effect on oral absorption, distribution, and toxic effect of ZnO NPs. The effect of ZnO size was assessed on the different physicochemical properties such as quenching of protein fluorescence, solubility, particle-protein corona, and transport inside simulated gastrointestinal (GI) and plasma fluids. The outcome of the study showed that the protein fluorescence quenching was more pronounced for ZnO nanoparticles compared to the bulk particles. On the other hand, the solubility of ZnO and the mechanism of intestinal transport were not affected by the particle size. Proteomics studies were done to establish that fibrinogen, albumin, and fibronectin play an important role in the particle-plasma protein corona, independent of the size of the particle. The ZnO nanoparticles had a more strong interaction with fibrinogen and fibronectin, the proteins responsible for the innate immune system. Thus, ZnO nanoparticles may influence the immune system [75]. On the other hand, another group of researchers has reported the deleterious effect of ZnO nanostructures (nanoneedles and nanoflowers) on different blood components such as erythrocytes, human serum albumin (HSA), and human isolated primary neutrophils. There was no significant difference found in the intrinsic and extrinsic fluorescence and circular dichroism of HSA after incubation with ZnO nanostructures showing that these nanostructures did not cause any denaturation of this protein. The drug carrying capacity of the HSA was also not altered after treatment with the ZnO NFs and ZnO nanoneedles. Neutrophil activation was also not elicited by any of these ZnO nanostructures as documented by the lucigenin- and luminol-dependent chemiluminescence as well as by the release of hydrogen peroxide in the extracellular medium. However, it was found that the ZnO—NFs could induce hemolysis of the erythrocytes which was not observed for the ZnO nanoneedles. This finding of ZnO NF made an exception for the capacity of ZnO nanostructures to be used in biomaterials [48]. Thus, there was a differential effect of ZnO nanostructures on the proteins in biological fluids.
10. Conclusion
Nanomaterials of metals and their oxides have been found to have immense potential application in healthcare. Of particular importance is the ZnO nanoparticles, where it has been observed that the varied shapes and sizes have distinctive advantages. Important among them are the NFs because of the enhanced surface area due to the multifaceted petal like appearance, which enables them to be functionalized with versatile applications. The various routes of synthesis for obtaining differently shaped NFs have been discussed. Small defects in the crystal structure have given the ZnO NFs high oxygen availability on the surface and could act as a photocatalyst with high efficiency. In many cases, the ZnO NFs were doped with Ceria or decorated over a GO layer to enhance their efficiency. The gas sensor application has been discussed elaborately for the nanostructures and composite that contained ZnO NFs. For different harmful dye remediation, ZnO NFs have shown their promising photocatalytic activity and have been used for wastewater remediation. Its use in designing biosensors is significant for their high selectivity. It has been possible to detect Aβ, insulin amyloids, acetone gas which is usually evolved for patients with diabetes and cancer, etc., and the efficiency was higher with high sensitivity. The induction of angiogenesis, osteoblast growth, and osseointegration, and probable killing of cancer cells have also been observed to be induced by ZnO NFs. A wide range of Gram-positive, as well as Gram-negative bacteria are killed by the ZnO NFs or doped ZnO NFs, showing its role in killing bacteria. The interaction of ZnO NFs and ZnO NPs was also briefly discussed with different biological macromolecules. The review aims to provide the researchers an insight into ZnO NF technology. The future perspective of ZnO NFs directs the researchers to develop different therapeutic strategies, biosensors with more accuracy and sensitivity, environmental remediation applications using bare or surface functionalized ZnO NFs.
Declaration of Competing Interest
The authors declare no conflict of interest.
Acknowledgment
The authors (VJR, AG & KG) are grateful to Chettinad Academy of Research and Education for providing the infrastructural support and the other author (RG) acknowledges University of Kalyani, West Bengal, DST-PURSE and UGC-SAP, GoI for supporting this work. The authors acknowledge funding from Council of Scientific and Industrial Research (CSIR), INDIA, Scheme no. 01(2868)/17/EMR-II.
References
- 1.Abdulgafour H.I., Hassan Z., Al-Hardan N., Yam F.K. Growth of zinc oxide nanoflowers by thermal evaporation method. Phys. B: Condens. Matter. 2010;405(11):2570–2572. [Google Scholar]
- 2.Adhyapak P.V., Meshram S.P., Mulla I.S., Pardeshi S.K., Amalnerkar D.P. Controlled synthesis of zinc oxide nanoflowers by succinate-assisted hydrothermal route and their morphology -dependent photocatalytic performance. Mater. Sci. Semicond. Process. 2014;27:197–206. [Google Scholar]
- 3.Akhtar N., Metkar S.K., Girigoswami A., Girigoswami K. ZnO nanoflower based sensitive nano-biosensor for amyloid detection. Mater. Sci. Eng. C Mater. Biol. Appl. 2017;78:960–968. doi: 10.1016/j.msec.2017.04.118. [DOI] [PubMed] [Google Scholar]
- 4.Amna T. Shape-controlled synthesis of three-dimensional zinc oxide nanoflowers for disinfection of food pathogens. Z Naturforsch. C J. Biosci. 2018;73(7–8):297–301. doi: 10.1515/znc-2017-0195. [DOI] [PubMed] [Google Scholar]
- 5.Bae S.H., Yu J., Lee T.G., Choi S.J. Protein food matrix–ZnO nanoparticle interactions affect protein conformation, but may not be biological responses. Int. J.Mol. Sci. 2018;19(12):3926. doi: 10.3390/ijms19123926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bai H.P., Lu X.X., Yang G.M., Yang Y.H. Hydrogen peroxide biosensor based on electrodeposition of zinc oxide nanoflowers onto carbon nanotubes film electrode. Chin. Chem. Lett. 2008;19(3):314–318. [Google Scholar]
- 7.Bakil S.N.A., Kamal H., Abdullah H.Z., Idris M.I. Sodium alginate-zinc oxide nanocomposite film for antibacterial wound healing applications. Biointerface Res. Appl. Chem. 2020;10(2):6245–6252. [Google Scholar]
- 8.Barui A.K., Jhelum P., Nethi S.K., Das T., Bhattacharya D., Vinothkumar B., Karri S., Chakravarty S., Patra C.S. Potential therapeutic application of zinc oxide nanoflowers in the cerebral ischemia rat model through neuritogenic and neuroprotective properties. Bioconjug. Chem. 2020;31(3):895–906. doi: 10.1021/acs.bioconjchem.0c00030. [DOI] [PubMed] [Google Scholar]
- 9.Barui A.K., Nethi S.K., Patra C.R. Investigation of the role of nitric oxide driven angiogenesis by zinc oxide nanoflowers. J. Mater. Chem. B. 2017;5(18):3391–3403. doi: 10.1039/c6tb03323g. [DOI] [PubMed] [Google Scholar]
- 10.Cai Q., Gao Y., Gao T., Lan S., Simalou O., Zhou X., Zhang Y., Harnoode C., Gao G., Dong A. Insight into biological effects of zinc oxide nanoflowers on bacteria: why morphology matters. ACS Appl. Mater. Interfaces. 2016;8(16):10109–10120. doi: 10.1021/acsami.5b11573. [DOI] [PubMed] [Google Scholar]
- 11.Centeno A., Xie F. Towards optimizing metal enhanced fluorescence (MEF) for improved detection of disease biomarkers. Biointerface Res. Appl. Chem. 2014;4(3):731–735. [Google Scholar]
- 12.Cerutti P., Ghosh R., Oya Y., Amstad P. The role of the cellular antioxidant defense in oxidant carcinogenesis. Environ. Health Perspect. 1994;102(10):123–129. doi: 10.1289/ehp.94102s10123. suppl. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen X., Tang Y., Liu W. Efficient dye-sensitized solar cells based on nanoflower-like ZnO photoelectrode. Molecules. 2017;22(8):1284. doi: 10.3390/molecules22081284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Comini E. Metal oxide nano-crystals for gas sensing. Anal. Chim. Acta. 2006;568(1–2):28–40. doi: 10.1016/j.aca.2005.10.069. [DOI] [PubMed] [Google Scholar]
- 15.Cunha D.M., Souza F.L. Facile synthetic route for producing one-dimensional zinc oxide nanoflowers and characterization of their optical properties. J. Alloys Compd. 2013;577:158–164. [Google Scholar]
- 16.Dhanalakshmi N., Priya T., Karthikeyan V., Thinakaran N. 3D cloves bud like Gd doped ZnO strewn rGO hybrid for highly selective determination of L-dopa in the presence of carbidopa and ascorbic acid. J. Pharm. Biomed. Anal. 2019;174:182–190. doi: 10.1016/j.jpba.2019.05.047. [DOI] [PubMed] [Google Scholar]
- 17.El-Shafai N., El-Khouly M.E., El-Kemary M., Ramadan M., Eldesoukey I., Masoud M. Graphene oxide decorated with zinc oxide nanoflower, silver and titanium dioxide nanoparticles: fabrication, characterization, DNA interaction, and antibacterial activity. RSC Adv. 2019;9(7):3704–3714. doi: 10.1039/c8ra09788g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ghosh R., Girigoswami K., Guha D. Suppression of apoptosis leads to cisplatin resistance in V79 cells subjected to chronic oxidative stress. Ind. J. Biochem. Biophys. 2012;49:363–370. [PubMed] [Google Scholar]
- 19.Ghosh R., Girigoswami K., Guha D. Caspase dependent apoptosis is only inhibited on γ irradiation of cells conditioned by repetitive oxidative stress. Int. J. Sci. Res. 2013;2:12–18. [Google Scholar]
- 20.Giannousi K., Geromichalos G., Kakolyri D., Mourdikoudis S., Dendrinou-Samara C. Interaction of ZnO nanostructures with proteins: in vitro fibrillation/antifibrillation studies and in silico molecular docking simulations. ACS Chem. Neurosci. 2020;11(3):436–444. doi: 10.1021/acschemneuro.9b00642. [DOI] [PubMed] [Google Scholar]
- 21.Girigoswami A., Ramalakshmi M., Akhtar N., Metkar S.K., Girigoswami K. ZnO nanoflower petals mediated amyloid degradation - an in vitro electrokinetic potential approach. Mater. Sci. Eng. C Mater. Biol. Appl. 2019;101:169–178. doi: 10.1016/j.msec.2019.03.086. [DOI] [PubMed] [Google Scholar]
- 22.Girigoswami K., Akhtar N. Nanobiosensors and fluorescence based biosensors: an overview. Int. J. Nano Dimens. 2019;10(1):1–17. http://www.ijnd.ir/article_660965.html [Google Scholar]
- 23.Girigoswami K., Girigoswami A. A Review on role of nanosensors in detecting cellular miRNA expression in colorectal cancer. Endocr. Metab. Immune Disord. Drug Targets. 2021;21(1):12–26. doi: 10.2174/1871530320666200515115723. [DOI] [PubMed] [Google Scholar]
- 24.Girigoswami K., Viswanathan M., Murugesan R., Girigoswami A. Studies on polymer-coated zinc oxide nanoparticles: UV-blocking efficacy and in vivo toxicity. Mater. Sci. Eng. C Mater. Biol. Appl. 2015;56:501–510. doi: 10.1016/j.msec.2015.07.017. [DOI] [PubMed] [Google Scholar]
- 25.Govindasamy M., Wang S.F., Subramanian B., Ramalingam R.J., Al-Lohedan H., Sathiyan A. A novel electrochemical sensor for determination of DNA damage biomarker (8-hydroxy-2′-deoxyguanosine) in urine using sonochemically derived graphene oxide sheets covered zinc oxide flower modified electrode. Ultrason. Sonochem. 2019;58 doi: 10.1016/j.ultsonch.2019.104622. [DOI] [PubMed] [Google Scholar]
- 26.Guha Ray P., Biswas S., Roy T., Ghosh S., Majumder D., Basak P., Roy S., Dhara S. Sonication assisted hierarchical decoration of Ag-NP on Zinc Oxide nanoflower impregnated eggshell membrane: evaluation of antibacterial activity and in vitro cytocompatibility. ACS Sustain. Chem. Eng. 2019;7(16):13717–13733. [Google Scholar]
- 27.Hasan M., Altaf M., Zafar A., Gul Hassan S., Ali Z., Mustafa G., Munawar T., Saqib Saif M., Tariq T., Iqbal F., Khan M.W., Mahmood A., Mahmood N., Shu X. Bioinspired Bioinspired synthesis of zinc oxide Nano-flowers: a surface enhanced antibacterial and harvesting efficiency. Mater. Sci. Eng. C Mater. Biol. Appl. 2021;119 doi: 10.1016/j.msec.2020.111280. [DOI] [PubMed] [Google Scholar]
- 28.He X., Wang H., Li Z., Chen D., Zhang Q. ZnO-Ag hybrids for ultrasensitive detection of trinitrotoluene by surface-enhanced Raman spectroscopy. Phys. Chem. Chem. Phys. 2014;16(28):14706–14712. doi: 10.1039/c4cp01723d. [DOI] [PubMed] [Google Scholar]
- 29.Helmy K.G., Partila A.M., Salah M. Gamma radiation and polyvinyl pyrrolidone mediated synthesis of Zinc oxide /Zinc sulfide nanoparticles and evaluation of their antifungal effect on pre and post harvested orange and pomegranate fruits. Biocat. Agric. Biotech. 2020;29 doi: 10.1016/j.bcab.2020.101728. [DOI] [Google Scholar]
- 30.Hemalatha P., Karthick S.N., Hemalatha K.V., Yi M., Kim H.-.J., Alagar M. La-doped ZnO nanoflower as photocatalyst for methylene blue dye degradation under UV irradiation. J. Mater. Sci: Mater. Electron. 2016;27:2367–2378. [Google Scholar]
- 31.Ibrahim A.A., Dar G.N., Zaidi S.A., Umar A., Abaker M., Bouzid H., Baskoutas S. Growth and properties of Ag-doped ZnO nanoflowers for highly sensitive phenyl hydrazine chemical sensor application. Talanta. 2012;93:257–263. doi: 10.1016/j.talanta.2012.02.030. [DOI] [PubMed] [Google Scholar]
- 32.Itroutwar P.D., Kasivelu G., Raguraman V., Malaichamy K., Sevathapandian S.K. Effects of biogenic zinc oxide nanoparticles on seed germination and seedling vigor of maize (Zea mays) Biocat. Agric. Biotech. 2020;29 doi: 10.1016/j.bcab.2020.101778. [DOI] [Google Scholar]
- 33.Jaisutti R., Lee M., Kim J., Choi S., Ha T.J., Kim J., Kim H., Park S.K., Kim Y.H. Ultrasensitive room-temperature operable gas sensors using p-type Na:ZnO nanoflowers for diabetes detection. ACS Appl. Mater. Interfaces. 2017;9(10):8796–8804. doi: 10.1021/acsami.7b00673. [DOI] [PubMed] [Google Scholar]
- 34.Kaliraj L., Ahn J.C., Rupa E.J., Abid S., Lu J., Yang D.C. Synthesis of panos extract mediated ZnO nano-flowers as photocatalyst for industrial dye degradation by UV illumination. J. Photochem. Photobiol. B. 2019;199 doi: 10.1016/j.jphotobiol.2019.111588. [DOI] [PubMed] [Google Scholar]
- 35.Katiyar A., Kumar N., Shukla R.K., Srivastava A. Substrate free ultrasonic-assisted hydrothermal growth of ZnO nanoflowers at low temperature. SN Appl. Sci. 2020;2(8):1386. doi: 10.1007/s42452-020-3186-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim J.W., Porte Y., Ko K.Y., Kim H., Myoung J.M. Micropatternable double-faced ZnO nanoflowers for flexible gas sensor. ACS Appl. Mater. Interfaces. 2017;9(38):32876–32886. doi: 10.1021/acsami.7b09251. [DOI] [PubMed] [Google Scholar]
- 37.Król A., Pomastowski P., Rafińska K., Railean-Plugaru V., Buszewski B. Zinc oxide nanoparticles: synthesis, antiseptic activity and toxicity mechanism. Adv. Colloid Interface Sci. 2017;249:37–52. doi: 10.1016/j.cis.2017.07.033. [DOI] [PubMed] [Google Scholar]
- 38.Labis J.P., Al-Anazi A.Q., Al-Brithen H.A., Hezam M., Alduraibi M.A., Algarni A., Alharbi A.A., Al-Awadi A.S., Khan A., El-Toni A.M. Designing zinc oxide nanostructures (nanoworms, nanoflowers, nanowalls, and nanorods) by pulsed laser ablation technique for gas-sensing application. J. Am. Ceram. Soc. 2019;102:4367–4375. doi: 10.1111/jace.16270. [DOI] [Google Scholar]
- 39.Ma S., Li R., Lv C., Xu W., Gou X. Facile synthesis of ZnO nanorod arrays and hierarchical nanostructures for photocatalysis and gas sensor applications. J. Hazard Mater. 2011;192(2):730–740. doi: 10.1016/j.jhazmat.2011.05.082. [DOI] [PubMed] [Google Scholar]
- 40.Mardikar S.P., Kulkarni S., Adhyapak P.V. Sunlight driven highly efficient degradation of methylene blue by CuO-ZnO nanoflowers. J. Environ. Chem. Eng. 2020;8(2) [Google Scholar]
- 41.Metkar S.K., Girigoswami K. Diagnostic biosensors in medicine- a review. Biocatal. Agric. Biotech. 2019;17:271–283. doi: 10.1016/j.bcab.2018.11.029. [DOI] [Google Scholar]
- 42.Minaee H., Mousavi S.H., Haratizadeh H., de Oliveira P.W. Oxygen sensing properties of zinc oxide nanowires, nanorods, and nanoflowers: the effect of morphology and temperature. Thin Solid Films. 2013;545:8–12. [Google Scholar]
- 43.Mitri F.F., Ingle A.P., Rai M. Nanotechnology in the management of bone diseases and as regenerative medicine. Curr. Nanosci. 2018;14:95. doi: 10.2174/1573413713666171123164027. [DOI] [Google Scholar]
- 44.Napi M.L.M., Sultan S.M., Ismail R., How K.W., Ahmad M. K. electrochemical-based biosensors on different zinc oxide nanostructures: a review. Materials (Basel) 2019;12(18):2985. doi: 10.3390/ma12182985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ojo O.A., Olayide I.I., Akalabu M.C., Ajiboye B.O., Ojo A.B., Oyinloye B.E., Ramalingam M. Nanoparticles and their biomedical applications. Biointerface Res. Appl. Chem. 2021;11(1):8431–8445. [Google Scholar]
- 46.Paino I.M.J., Gonçalves F., Souza F.L., Zucolotto V. Zinc oxide flower-like nanostructures that exhibit enhanced toxicology effects in cancer cells. ACS Appl. Mater. Interfaces. 2016;8(48):32699–32705. doi: 10.1021/acsami.6b11950. [DOI] [PubMed] [Google Scholar]
- 47.Park J.K., Kim Y.J., Yeom J., Jeon J.H., Yi G.C., Je J.H., Hahn S.K. The topographic effect of zinc oxide nanoflowers on osteoblast growth and osseointegration. Adv. Mater. 2010;22(43):4857–4861. doi: 10.1002/adma.201002255. [DOI] [PubMed] [Google Scholar]
- 48.Pastrello B., Paracatu L.C., de Carvalho Bertozo L., et al. Synthesis and evaluation of the potential deleterious effects of ZnO nanomaterials (nanoneedles and nanoflowers) on blood components, including albumin, erythrocytes and human isolated primary neutrophils. J. Nanopart. Res. 2016;18:216. [Google Scholar]
- 49.Patra C.R., Barui A.K. Nanoflowers: a future therapy for cardiac and ischemic disease? Nanomedicine (Lond) 2013;8(11):1735–1738. doi: 10.2217/nnm.13.161. [DOI] [PubMed] [Google Scholar]
- 50.Pawar R.C., Shaikh J.S., Suryavanshi S.S., Patil P.S. Growth of ZnO nanodisk, nanospindles and nanoflowers for gas sensor: pH dependency. Curr. Appl. Phys. 2012;12(3):778–783. [Google Scholar]
- 51.Perumal V., Hashim U., Gopinath S.C., Haarindraprasad R., Foo K.L., Balakrishnan S.R., Poopalan P. Spotted nanoflowers’: gold-seeded zinc oxide nanohybrid for selective bio-capture. Sci. Rep. 2015;5:12231. doi: 10.1038/srep12231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ramalingam R.J., Radika T., Al-Lohedan H.A. Preparation and surface characterization of nanodisk/nanoflower-structured gallium-doped zinc oxide as a catalyst for sensor applications. Chinese J. Catal. 2016;37(8):1235–1241. [Google Scholar]
- 53.Roychaudhuri R., Yang M., Hoshi M.M., Teplow D.B. Amyloid beta-protein assembly and Alzheimer disease. J. Biol. Chem. 2009;284(8):4749–4753. doi: 10.1074/jbc.R800036200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rupa E.J., Kaliraj L., Abid S., Yang D.C., Jung S.K. Synthesis of a zinc oxide nanoflower photocatalyst from sea buckthorn fruit for degradation of industrial dyes in wastewater treatment. Nanomaterials (Basel) 2019;9(12):1692. doi: 10.3390/nano9121692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sabry N.M., Tolba S.T.M., Abdel-Gawad F.K., Bassem S.M., Nassar H., El-Taweel G.E., Ibrahim M.A. On the molecular modeling analyses of the interaction between nano zinc oxide and bacteria. Biointerface Res. Appl. Chem. 2018;8(3):3294–3297. [Google Scholar]
- 56.Saha R., Subramani K., Sikdar S., Fatma K., Rangaraj S. Effects of processing parameters on green synthesised ZnO nanoparticles using stem extract of Swertia chirayita. Biocat. Agric. Biotech. 2021;101968 doi: 10.1016/j.bcab.2021.101968. [DOI] [Google Scholar]
- 57.Saif M.S., Zafar A., Waqas M., Hassan S.G., ul Haq A., Tariq T., Batool S., Irshad M., Hasan M., Shu X. Phyto-reflexive zinc oxide nano-flowers synthesis: an advanced photocatalytic degradation and infectious therapy. J. Mater. Res. Technol. 2021 doi: 10.1016/j.jmrt.2021.05.107. [DOI] [Google Scholar]
- 58.Sharma S.C. ZnO nano-flowers from Carica papaya milk: degradation of Alizarin Red-S dye and antibacterial activity against Pseudomonas aeruginosa and Staphylococcus aureus. Optik – Int. J. Light Electron Optics. 2016;127(16):6498–6512. [Google Scholar]
- 59.Sharma S.K., Pamidimarri D.V.N.S., Kim D.Y., Na J.-.G. Y-doped zinc oxide (YZO) nanoflowers, microstructural analysis and test their antibacterial activity. Mater. Sci. Eng. C Mater. Biol. Appl. 2015;53:104–110. doi: 10.1016/j.msec.2015.04.007. [DOI] [PubMed] [Google Scholar]
- 60.Sheoran P., Grewal S., Kumari S., Goel S. Enhancement of growth and yield, leaching reduction in Triticum aestivum using biogenic synthesized zinc oxide nanofertilizer. Biocat. Agric. Biotech. 2021;32 doi: 10.1016/j.bcab.2021.101938. [DOI] [Google Scholar]
- 61.Tabassum R., Gupta B.D. Simultaneous tuning of electric field intensity and structural properties of ZnO: graphene nanostructures for FOSPR based nicotine sensor. Biosens. Bioelectron. 2017;91:762–769. doi: 10.1016/j.bios.2017.01.050. [DOI] [PubMed] [Google Scholar]
- 62.Thendral V., Dharshni T., Ramalakshmi M., Girigoswami A., Girigoswami K. Cerium oxide nanocluster based nanobiosensor for ROS detection. Biocatal. Agric. Biotech. 2019;19 doi: 10.1016/j.bcab.2019.101124. [DOI] [Google Scholar]
- 63.Tripathi R.M., Bhadwal A.S., Gupta R.K., Singh P., Shrivastav A., Shrivastav B.R. ZnO nanoflowers: novel biogenic synthesis and enhanced photocatalytic activity. J. Photochem. Photobiol. B: Biology. 2014;141:288–295. doi: 10.1016/j.jphotobiol.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 64.Tsai Y.T., Chang S.J., Ji L.W., Hsiao Y.J., Tang I.T., Lu H.Y., Chu Y.L. High sensitivity of NO gas sensors based on novel Ag-doped ZnO nanoflowers enhanced with a UV light-emitting diode. ACS Omega. 2018;3(10):13798–13807. doi: 10.1021/acsomega.8b01882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ullah S., Ahmad A., Ri H.I., Khan A.U., Khan U.A., Yuan Q. Green synthesis of catalytic zinc oxide nano-flowers and their bacterial infection therapy. Appl. Organometal Chem. 2020;34:e5298. [Google Scholar]
- 66.Umar A., Chauhan M.S., Chauhan S., Kumar R., Sharma P., Tomar K.J., Wahab R., Al-Hajry A., Singh D. Applications of ZnO nanoflowers as antimicrobial agents for Escherichia coli and enzyme-free glucose sensor. J. Biomed. Nanotechnol. 2013;9(10):1794–1802. doi: 10.1166/jbn.2013.1751. [DOI] [PubMed] [Google Scholar]
- 67.Vinayagam R., Selvaraj R., Arivalagan P., Varadavenkatesan T. Synthesis, characterization and photocatalytic dye degradation capability of Calliandra haematocephala-mediated zinc oxide nanoflowers. J. Photochem. Photobiol. B. 2020;203 doi: 10.1016/j.jphotobiol.2019.111760. [DOI] [PubMed] [Google Scholar]
- 68.Vinayagam R., Pai S., Murugesan G., et al. Synthesis of photocatalytic zinc oxide nanoflowers using Peltophorum pterocarpum pod extract and their characterization. Appl. Nanosci. 2021 doi: 10.1007/s13204-021-01919-z. (2021) [DOI] [Google Scholar]
- 69.Wahab R., Ansari S.G., Kim Y.S., Seo H.K., Kim G.S., Khang G., Shin H.-.S. Low temperature solution synthesis and characterization of ZnO nano-flowers. Mater. Res. Bull. 2007;42(9):1640–1648. [Google Scholar]
- 70.Wahab R., Kaushik N., Khan F., Kaushik N.K., Choi E.H., Musarrat J., Al-Khedhairy A.A. Self-styled ZnO nanostructures promotes the cancer cell damage and supresses the epithelial phenotype of glioblastoma. Sci. Rep. 2016;6:19950. doi: 10.1038/srep19950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang J.X., Zhuo Y., Zhou Y., Wang H.J., Yuan R., Chai Y.Q. Ceria doped zinc oxide nanoflowers enhanced luminol-based electrochemiluminescence immunosensor for amyloid-β detection. ACS Appl. Mater. Interface. 2016;8(20):12968–12975. doi: 10.1021/acsami.6b00021. [DOI] [PubMed] [Google Scholar]
- 72.Wang Y., Li X., Wang N., Quan X., Chen Y. Controllable synthesis of ZnO nanoflowers and their morphology-dependent photocatalytic activities. Sep. Purif. Technol. 2008;62(3):727–732. [Google Scholar]
- 73.Xie F., Centeno A., Zou B., Ryan M.P., Riley D.J., Alford N.M. Tunable synthesis of ordered zinc oxide nanoflower-like arrays. J. Colloid Interface Sci. 2013;395:85–90. doi: 10.1016/j.jcis.2012.12.028. [DOI] [PubMed] [Google Scholar]
- 74.Ya Y., Jiang C., Li T., Liao J., Fan Y., Wei Y., Yan F., Xie L. A zinc oxide nanoflower-based electrochemical sensor for trace detection of sunset yellow. Sensors (Basel) 2017;17(3):545. doi: 10.3390/s17030545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yu J., Kim H.J., Go M.R., Bae S.H., Choi S.J. ZnO interactions with biomatrices: effect of particle size on ZnO-protein corona. Nanomaterials (Basel). 2017;7(11):377. doi: 10.3390/nano7110377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yuan J., Choo E.S., Tang X., Sheng Y., Ding J., Xue J. Synthesis of ZnO-Pt nanoflowers and their photocatalytic applications. Nanotechnology. 2010;21(18) doi: 10.1088/0957-4484/21/18/185606. [DOI] [PubMed] [Google Scholar]
- 77.Zhai Y.J., Li J.H., Fang X., Chen X.Y., Fang F., Chu X.Y., Wei Z.P., Wang X.H. Preparation of cadmium-doped zinc oxide nanoflowers with enhanced photocatalytic activity. Mater. Sci. Semicond. Process. 2014;26:225–230. [Google Scholar]
- 78.Zhang B., Lu L., Hu Q., Huang F., Lin Z. ZnO nanoflower-based photoelectrochemical DNAzyme sensor for the detection of Pb2+ Biosens. Bioelectron. 2014;56:243–249. doi: 10.1016/j.bios.2014.01.026. [DOI] [PubMed] [Google Scholar]
- 79.Zhang C., Cao Z., Zhang G., Yan Y., Yang X., Chang J., Song Y., Jia Y., Pan P., Mi W., Yang Z., Zhao J., Wei J. An electrochemical sensor based on plasma-treated zinc oxide nanoflowers for the simultaneous detection of dopamine and diclofenac sodium. Microchem. J. 2020;158 doi: 10.1016/j.microc.2020.105237. [DOI] [Google Scholar]
- 80.Zhang N., Yi R., Shi R., Gao G., Chen G., Liu X. Novel rose-like ZnO nanoflowers synthesized by chemical vapor deposition. Materials Lett. 2009;63(3–4):496–499. [Google Scholar]
- 81.Zhu Y., Fu H., Ding J., Li H., Zhang M., Zhang J., Liu Y. Fabrication of three-dimensional zinc oxide nanoflowers for high-sensitivity fiber-optic ammonia gas sensors. Appl. Opt. 2018;57(27):7924–7930. doi: 10.1364/AO.57.007924. [DOI] [PubMed] [Google Scholar]