Abstract
Moenomycins, such as moenomycin A, are phosphoglycolipid specialized metabolites produced by a number of actinobacterial species. They are among the most potent antibacterial compounds known to date, which drew numerous studies directed at various aspects of the chemistry and biology of moenomycins. In this review, we outline the advances in moenomycin research over the last decade. We focus on biological aspects, highlighting the contribution of the novel methods of genomics and molecular biology to the deciphering of the biosynthesis and activity of moenomycins. Specifically, we describe the structural diversity of moenomycins as well as the underlying genomic variations in moenomycin biosynthetic gene clusters. We also describe the most recent data on the mechanism of action and assembly of complicated phosphoglycolipid scaffold. We conclude with the description of the genetic control of moenomycin production by Streptomyces bacteria and a brief outlook on future developments.
Keywords: Phosphoglycolipid antibiotics, Bacterial cell wall, Streptomyces, Peptidoglycan glycosyltransferases, Biosynthetic gene clusters
Introduction
Phosphoglycolipid antibiotics, first described in the 1960s, constitute a rather compact, in terms of chemical diversity, family of natural products produced by Gram-positive bacteria of the Actinobacteria phylum [1]. Moenomycin A (MmA, Fig. 1), an archetypal member of this family, proved to be a formidable challenge for medicinal chemistry efforts to generate analogs to probe MmA mode of action and improve its pharmacological properties [2,3]. This, along with the availability of more promising drug leads, has put moenomycins (as well as many other classes of natural products [4]) into the drawers of the antibiotic development industry of the 20th century. The relentless rise of multidrug-resistant nosocomial infections in the last two decades, recently exacerbated by the coronavirus pandemic [5], has renewed the need for antibiotics that would possess novel mechanisms of action and, thus, slow down the development of antimicrobial resistance. MmA in this regard represents a very attractive drug candidate. First, it is a subnanomolar inhibitor of peptidoglycan glycosyltransferases (PGTs), essential peptidoglycan (PG) assembly enzymes not currently exploited by any other drug [6]. Second, many of the issues that plagued the rational design of MmA-based PGT inhibitors are now solved. We have robust chemical and biological tools to construct complex phosphoglycolipid scaffolds and very detailed insight into MmA-PGT interactions. Third, the available evidence suggests that by targeting the active sites of numerous distinct PGTs in the cell moenomycins become a very tough target for drug resistance evolution. Part of the aforementioned breakthroughs has been achieved in the first decade of the 21st century, and these have been exhaustively reviewed [1]. Here we mainly focus on studies of cell and molecular biology of phosphoglycolipid antibiotics that were carried out since 2010. An update of our understanding of the chemical diversity of moenomycins will be first given. Then important developments in the area of MmA mode of action and antibiotic activity will be reviewed, as well as genomics of moenomycin biosynthetic gene clusters. A description of the complete phosphoglycolipid biosynthetic pathway, recent regulatory and structural insights into the former, as well as the outlook of future research directions in the field, will conclude the review.
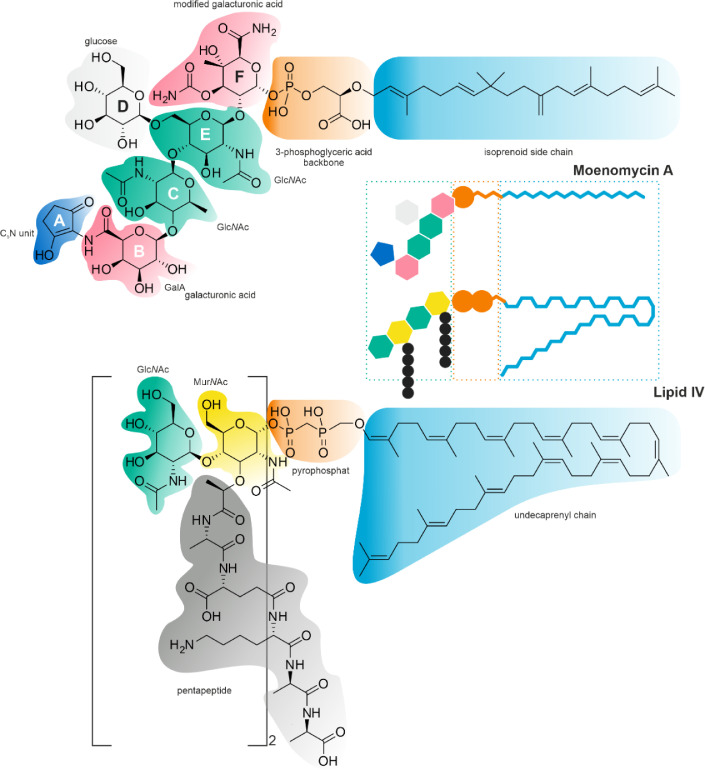
Fig. 1.
Structural diversity of naturally occurring moenomycins. MmA is shown in top left corner of the figure.
Diversity of naturally occurring phosphoglycolipid antibiotics: chemical and genetic points of view
3-phosphoglycerate is a structural unit (G; Fig. 1) that distinguishes moenomycins from the other classes of carbohydrate- and lipid-containing natural products. Unit G bridges a lipid moiety and the complex carbohydrate scaffold bristling with functional groups. As can be seen from Fig. 1, chemical diversity around the phosphoglycolipid scaffold arises mainly from the presence/absence of substituents on carbohydrate units B, C, and D. A suite of unit B modifications is especially striking as it includes the presence of carboxamide and amino acid-decorated compounds. Glycine-bearing moenomycins have been reported previously under typical submerged fermentation conditions, whereas transfer of alanine, serine, and cysteine residues onto unit B has been observed only upon overexpression of amidotransferase gene moeH5 in moenomycin-producing streptomycetes [7]. Moenocinol is usually found as a lipid chain of moenomycins; the only exception is AC326-α which features diumycinol, a moenocinol isomer. The presence of diumycinol is also suggested (but not proven) for teichomycins on the basis of analysis of a gene cluster for teichomycin biosynthesis in Actinoplanes (A.) teichomyceticus; this will be detailed further in this review. Streptomyces (S.) sp. K04–0144, a nosokomycin producer, was reported to accumulate nosokophic acid, an early phosphoglycolipid intermediate consisting of units F, G, and H [8]. There are several in vitro assays to monitor the activity of MmA target enzymes, PGTs, which, theoretically, can be used for screening the bacterial extracts to turn up novel phosphoglycolipid antibiotics. At the moment these assays were validated on libraries of known compounds and no screening of natural sources was reported yet [9], [10], [11], [12]. Сollision-induced dissociation patterns of phosphoglycolipid antibiotics in multistage mass spectrometry (MS) experiments provide another means for their identification in complex mixtures [13,14]. Facile loss of lipid moiety (428 Da, anion) in MS2 is a primary signal for the presence of moenomycins in the sample.
Recent mining of a sizable collection of Streptomyces genomes led to the identification of tens of thousands of biosynthetic gene clusters (BGCs) for specialized metabolites, where phosphoglycolipid BGCs were among the least abundant ones [15]. As of the time of writing of this review (July 2022), we were able to reveal 20 actinobacterial and three enterobacterial (Photorhabdus) species carrying moenomycin BGCs (Fig. 2). Many of these 20 actinobacterial species carry identical moenomycin BGCs, and so the latter can be classified into 7 closely related types. The BGCs differ mainly in the presence/absence of genes for chromophore C5N moiety (unit A) biosynthesis (moeA4, moeC4) and conversion of glucosamine into quinovosamine (unit C; moeR5, moeS5). Moenomycin BGCs from Actinoplanes species also harbor genes putatively involved in lipid chain biosynthesis, such as the supply of isoprene building blocks and conversion of moenocinol to diumycinol. Gene clusters for phosphoglycolipid biosynthesis in Photorhabdus bear a little resemblance to actinobacterial moenomycin BGCs, and so might encode molecules significantly different from those depicted in Fig. 1. Except for S. ghanaensis (=viridisporus), S. prasinus, S. umbrinus and Actinoplanes teichomyceticus, no phosphoglycolipid production was described in the strains mentioned in Fig. 2. Particularly extensive metabolomic analysis has been undertaken recently for S. clavuligerus (carries type 3 moenomycin BGC, see Fig. 2) and Photorhabdus species (heterogeneous type 8) [16], [17], [18]. Failure to identify phosphoglycolipid metabolites in these strains likely implies that their production is strictly regulated and may require very specific induction signal(s).
Fig. 2.
Moenomycin biosynthesis gene clusters (BGCs) identified in bacterial genomes. Gene function in the BGCs are color-coded, and the BGCs are binned into eight types, see bottom of the figure for the graphical legend.
Mode of action, resistance mechanisms, and the spectrum of activity against various bacteria
MmA interferes with the cell wall biosynthesis in Gram-positive and some Gram-negative bacteria. It does so via inhibiting PGT domains of penicillin-binding proteins (PBPs) and monofunctional transglycosylases (TGases) [19]. The available data agree with the idea that MmA is a structural analog of natural substrates of aforementioned enzymes, such as Lipids II and IV. The 25-carbon moenocinol chain corresponds to Lipid II/IV undecaprenyl chain (Fig. 3), 3-phosphoglycerate resembles pyrophosphate, and the oligosaccharide portion of MmA mimics GlcNAc-MurNAc region of Lipid IV [1]. Biochemical assays show that MmA competes with Lipid IV for binding to the donor site of PGT, and thus affects the initiation of synthesis of PG strands [20], although some debate over this issue remains [9]. Moenocinol is crucial for the biological activity of MmA, assuring its anchoring in the lipid membrane. Indeed, the introduction of the terminal hydroxyl group into the lipid chain of MmA renders the antibiotic inactive [1]. 3-phosphoglycerate and the oligosaccharide portion of MmA interact with the PGT domain.
Fig. 3.
Alignment of MmA and Lipid IV, analogous structural parts are highlighted with the same color: isoprenoid side chain and undecaprenyl chain; 3-phosphoglyceric acid backbone and pyrophosphate moiety; oligosaccharide portions. A sketch representation of MmA and Lipid IV will aid understanding of the Fig. 4 scheme.
The interaction of MmA with PGT domains was elucidated with the help of X-ray analysis and/or cryoelectron microscopy (cryo-EM). Structural data are available for MmA co-complexed with Mtg (monofunctional TGase) and PBP2 of Staphylococcus (St.) aureus [21], [22], [23], [24], PBP1b of Escherichia (E.) coli [25], [26], [27], [28], [29], and PBP1a of Aquifex aeolicus [30,31]. All PGTs mentioned above share transmembrane (TM) N-terminal α-helix and PGT domain, while PBPs carry also the transpeptidase (TP) domain (Fig. 4A). E. coli PBP1b harbors, in addition, a UB2H domain (UvrB domain 2 homolog). The role of UB2H in PGT and TP reactions is unclear, but it might be important for binding co-factor proteins [32,33]. The PGTs are a challenging target for structural biologists as these enzymes adopt various conformations in solution and the growth of well-diffracting crystals required a ligand. MmA serves well as the latter, while attempts to co-crystalize PGT with Lipid II/IV have not yet met with success. It was, however, possible to infer the 3D structure of PBP1a from E. coli in apo form using cryoEM [25]. Being rather diverged at the level of primary structure, the PGT domains possess similar folds with two subdomains, the “head” and “jaw” ones, delineated with a catalytic groove [34] (see Fig. 4A). Donor (for Lipid II/IV/nascent PG/MmA) and acceptor (for Lipid II) sites are located within the catalytic groove. The “flap” region is the most dynamic part of the “jaw”. PGT domain undergoes a series of conformational changes to achieve one cycle of PG extension [21], and the “flap” region assists in shuttling the Lipid II and growing PG between donor and acceptor sites [24,28]. Certain segments of the “jaw” and “flap” subdomains (likely involved in Lipid II recognition) are partially embedded into the membrane [21].
Fig. 4.
Simplified schematic representation of (A) domain architectures of three PGTs with crystal structure solved experimentally and (B) moenomycin binding to the PGT domain of PBP blocking PG biosynthesis.
Although we assume that MmA mimics Lipid II/IV binding to PGT (Fig. 4B), this mimicry is not complete. Rather, MmA appears to lock the PGT domain in a certain conformation which impedes proper functioning, but this conformation is not identical to the one that PGT adopts in presence of natural substrates [25]. EFCB units of MmA bind within the catalytic groove where EF disaccharide forms a network of hydrogen bonds to the active site. CB disaccharide corresponds to the GlcNAc-MurNAc backbone of the growing PG and contacts the amino acids of the donor binding site [28]. A sketch of MmA-PBP interaction is given in Fig. 4B. Finally, MmA might also have an additional non-canonical binding site at the “flap” region [21], although it is unknown whether such binding occurs in vivo. Studies of MmA-PGT interactions inspired the creation of new PGT inhibitors, either MmA derivatives or its structural analogs [1,29,[35], [36], [37], [38], [39], [40]].
A rather unexpected feature of MmA is the ability to inhibit conjugal transfer of plasmids in E. coli and Enterococcus (Ent.) feacalis [41,42] and “cure” bacterial cultures of plasmids (i.e. block normal plasmid segregation in course of cell division) [43]. These effects were observed for different types of plasmids utilizing sub-inhibitory concentrations of MmA. Since some of the investigated plasmids encode lytic TGases (required for PG remodeling prior to conjugal transfer) – it was speculated that MmA might inhibit these enzymes [41]. This, however, does not explain how MmA affects the transfer of plasmids not encoding lytic TGases. Possessing detergent-like properties [44], MmA could as well destabilize cell membranes, interrupting the protein complexes required for conjugal transfer and segregation of plasmids. Whatever the real explanation is, such a phenomenon leads to one peculiar outcome justifying MmA application in animal husbandry. It is known that MmA is not metabolized and adsorbed in the animal digestive system, thus it accumulates in feces [45], “curing” fecal plasmid mobilome potentially mediating the spread of antibiotic resistance determinants.
Actinomycetes producing moenomycins [1,[46], [47], [48]] require certain auto-resistance mechanisms to avoid suicide caused by antibiotic accumulation. Genes for ABC transporters found in each moenomycin BGC (see Fig. 2) appeared to impact MmA titers but not resistance to it, as studies on S. ghanaensis have shown [49]. High-level resistance to MmA is widespread among moenomycin non-producing actinomycetes, with S. albidoflavus (=albus) J1074 being one notable exception. This species is 10,000-fold more sensitive to MmA when compared to S. ghanaensis and S. coelicolor, although its genetic basis remains enigmatic [50,51]. It is uncertain whether MmA resistance in actinomycetes is determined by some specific mechanisms or simply caused by a thick cell wall, physically hindering the delivery of MmA to the target. There is no direct evidence that actinomycete PGTs are inhibited by MmA in vitro, and no attempts to co-crystallize actinobacterial PGTs with MmA were made. Treatment of S. coelicolor with MmA changes the expression of hundreds of genes (including those for PGTs), suggesting the involvement of general stress response [52].
Gram-positives such as St. aureus, St. epidermidis, St. haemolyticus, Streptococcus pyogenes, Listeria monocytogenes, some strains of Ent. faecalis, and Ent. faecium are sensitive to ng/mL MmA concentrations [1,[53], [54], [55], [56]]. Since only a couple of hundred PBPs are found in typical Gram-positive cell [57], extremely low amounts of MmA are needed to exhaustively bind all of them. On the contrary, other Gram-positive bacteria, such as Bacillus (B.) subtilis, are intrinsically resistant to MmA. B. subtilis possesses four MmA-susceptible class A PBPs. Deletion of all four corresponding genes does not terminate PG biosynthesis [58], implying the presence of a bypass route. Exposure to MmA significantly induces the expression of SigM (σM)-regulon in B. subtilis [59], where SigM is an extracytoplasmic function (ECF) σ-factor sensing the environmental stressors of the cell envelope [60]. Knockout of sigM rendered B. subtilis hypersensitive to MmA [59]. A recent investigation of SigM regulon revealed the rodA gene for SEDS (shape, elongation, division, and sporulation) family protein exhibiting PGT activity that is not subject to inhibition by MmA [61,62]. SEDS proteins are widely distributed across Terrabacteria, including actinobacteria, and might explain the high level of MmA resistance in the latter. Finally, one environmental B. subtilis isolate was reported to degrade MmA enzymatically [63].
It is possible to raise MmA-resistant (Mmr) mutants of Gram-positive bacteria, such as St. aureus. Thickened PG was the reason for Mmr phenotype of St. aureus mutants in the first known report on this topic [64]. In the other case, Mmr St. aureus strains were found carrying two point amino acid substitutions (Y196D and P234Q) in the donor binding site of PBP2 (see above) [65]. The same substitutions hindering MmA binding were found in more than 30 screened Mmr clones. The Y196D and P234Q substitutions seemed to prevent non-specific interactions of MmA and PBP2. The other mutations, e.g. in sites responsible for more specific interactions, are probably impossible. MmA and Lipid IV specifically interact with the same amino acids. Hence, mutations disrupting specific binding will interfere with the normal docking of Lipid IV to PBP2, leading to a lethal effect. The aforementioned Mmr mutations came at a cost: mutant PBP2 appeared to generate shorter PG chains than the wild type enzyme, causing cell morphology and division abnormalities. Notably, such changes were observed only if mutants were grown in presence of MmA since TGases SgtA and SgtB complement mutated PBP2 in the absence of MmA [65]. Type III [66] ABC transporter AbcA was also shown to be involved in MmA resistance in St. aureus, where knockout of the corresponding gene led to the increment in MmA susceptibility, while its overexpression increased MmA resistance [67]. AbcA is a typical transmembrane efflux pump and its contribution to MmA resistance is not clear, given that MmA acts on the cell surface. However, AbcA might be involved in maintaining the fitness of the PG. Reduced levels of phosphatidyl glycerol in a daptomycin-resistant B. subtilis mutant also led to the increase in MmA resistance [68].
Gram-negative bacteria are usually several-fold more resistant to MmA as compared to Gram-positives [69]. At the same time, PBPs from Gram-negative and Gram-positive bacteria are inhibited by MmA in vitro to the same extent [70,71]. An obvious explanation for this is the outer membrane of the Gram-negative cell envelope shielding PBPs. Indeed, E. coli mutants with impaired outer membrane are more sensitive to MmA [55]. At the same time, some Gram-negatives, such as Neisseria, Brucella, Pasteurella, and Pseudomonas, are relatively sensitive to MmA. Recent findings also suggest that MmA is effective against notable Gram-negative pathogens. For example, the MIC of MmA for Helicobacter (H.) pylori is 2 µg/mL; moreover, MmA remains efficient against a variety of clinical H. pylori isolates, including multidrug-resistant ones [71]. MmA shows excellent activity against H. suis [72]. A few studies depict MmA as an extremely active antibiotic against Neisseria (N.) gonorrhoeae, with MICs within the 0.008–0.06 µg/mL range [48,73]. Unlike in E. coli, MmA was found to penetrate easily the outer membrane of N. gonorrhoeae. The presence of lipooligosacharides instead of lipopolysacharides in N. gonorrhoeae outer membrane might be a key reason for this difference [48].
Biosynthesis of moenomycins
Our current understanding of biosynthetic logic behind moenomycins is summarized in Fig. 5. Pathway from phosphoglycerate to nosokomycin A has been first described in 2009 [74] and reviewed in 2010; we therefore will not discuss this point further. The decoration of terminal glucuronic acid (unit B, see Fig. 1) with chromophore C5N (unit A), amine, or glycine has been deciphered in 2013 for S. ghanaensis (=viridosporus) ATCC14672 [7]. MoeH5, an amidotransferase of glutamine amidotransferase (GAT) superfamily was shown to control all of these modifications of NoA. MoeH5 resembles MoeF5, another GAT type amidotransferase involved in the carboxyamidation of unit F of moenomycins. MoeF5 is a canonical GAT enzyme that harbors an intact N terminal hydrolase domain (absolutely necessary for the hydrolysis of glutamine to produce free amine) and catalyzes a single reaction. MoeH5 appears to lose the hydrolase domain and instead directly transfers various amine-bearing moieties (as diverse as C5N and ammonium) onto the carboxyl group of unit B (see Fig. 5). Interestingly, MoeH5 ortholog encoded within A. teichomyceticus teichomycin BGC, retains hydrolase domain in its sequence and is able to produce only carboxyamidated moenomycins [46]. Future studies of the promiscuity of MoeH5 orthologous group enzymes towards their donor and acceptor substrates are of interest given that such modifications improve the antibacterial properties of moenomycins [7].
Fig. 5.
Biosynthetic pathway from 3-phosphoglycerate (3-PG) to MmA. Steps from 3-PG to nosokomycin A are shown in a condensed manner, for more details readers are referred to extensive 2010 review of this stage of MmA production [1].
Prenyltransferases MoeO5 and MoeN5 involved in MmA lipid chain assembly have been purified and characterized in vitro. MoeO5 was shown to catalyze the transfer of the farnesyl group onto oxygen of 3-phosphoglycerate. The mechanism of this reaction includes the isomerization of prenyl substrate farnesyl pyrophosphate into either nerolidyl or (Z,E)-farnesyl pyrophosphate, giving rise to cis-allylic double bond observed in the lipid chain of MmA [75]. MoeO5 was crystallized as a dimer which features a small catalytic pocket capable of accommodating a single molecule of the product, 2-(Z,E)-farnesyl-3-phosphoglycerate (FPG), in a bent (around C8=C9 double bond) conformation. This appears to be essential for isomerization to occur [76]. These studies underscore that MoeO5 employs a mechanism distinct from that reported for the other homologous TIM barrel prenyltransferases. Crystal structures of MoeN5 in complex with various substrate analogs were also reported, they revealed the dimeric structure of the protein and two aspartate-rich motifs likely involved in substrate binding [77,78]. Although structural data for MoeN5 do not challenge the mechanism of moenocinol formation proposed by Arigoni two decades ago [79], new insight into this reaction also has not yet emerged due to the low resolution of the X-ray data and the use of substrate analogs quite different from the native trisaccharide intermediate of MmA.
Recently there has been reported crystal structure of protein TchmY from teichomycin BGC of A. teichomyceticus [80]. TchmY crystallized as a monomer and possessed (α/α)6-barrel fold typical for many prenylcyclases. This observation is in agreement with the suggestion that TchmY is involved in the production of diumycinol, a terminally cyclized version of moenocinol.
Regulation of moenomycin biosynthesis
Unlike the majority of known BGCs in actinobacteria [81], moenomycin BGCs do not harbor pathway-specific (cluster-situated) regulatory genes (see Fig. 2). Hence, MmA production must be governed by pleiotropic (global) regulators, e.g. those acting on more than one biosynthetic pathway. Current knowledge of regulatory mechanisms governing MmA production (mostly studied in ATCC 14672) is summarized in Fig. 6 and described below.
Fig. 6.
Graphical summary of current knowledge of regulatory mechanisms involved in regulation of MmA production by S. ghanaensis (=viridosporus) ATCC 14672. See graphical legend and main text for more details.
The peculiar codon-based regulatory mechanism was the first global control circuit reported to limit MmA production to the late stages of the life cycle. This circuit consists of a gene bldA for leucyl tRNAUAA and its cognate codons TTA scattered in certain genes. The TTA codon is the rarest one in streptomycete genomes and can be decoded only by the bldA-encoded tRNA; the latter accumulates in significant quantities in the stationary phase of growth [82]. In ATCC 14672 TTA codons are present in key moe genes (moeE5, moeO5) which effectively confines the translation of moe mRNAs to the late stage of growth. On the other hand, the TTA codon is also present in a gene for transcriptional factor AdpA, a master regulator of morphogenesis and specialized metabolism of Streptomyces. AdpA was shown to directly interact with key moe gene promoters and upregulate their transcription. In sum, bldA-based regulation exerts control over MmA biosynthesis in ATCC 14672 by limiting the AdpA-dependent transcription of the moe BGC and translation of TTA-harboring moe mRNAs [83]. The effect of bldA on MmA production was also reported under heterologous expression conditions [84]. It is obvious that any factor that impairs the accumulation of mature bldA tRNA will affect MmA production. Indeed, mutations within genes miaA and miaB for hypermodification of adenosine in the 37th (A37) position of tRNAXXA in S. ghanaensis and S. albus J1074 severely decreased the levels of moenomycin production [85,86]. AdpA also upregulates bldA expression, creating a positive feedback loop between these regulators [82]. Likewise, factors that impact AdpA abundance will also leave a footprint on MmA biosynthesis. For example, the absB gene for double-stranded RNA-specific endoribonuclease is known to perform cleavage of adpA mRNA in various streptomycetes. Manipulations of absB expression were shown to influence MmA biosynthesis, pointing to the existence of the regulatory triad BldA-AdpA-AbsB in this metabolic pathway [83]. The other factors influencing the function of this triad will be described in a paragraph devoted to BldD.
Deletion of a gene wblA for Fe-S cluster-containing transcriptional regulator has been shown to enhance MmA production [87]. WblA falls into the WhiB-like (Wbl) family of proteins whose members are distributed exclusively within the phylum Actinobacteria, where they are typically involved in the late steps of morphogenesis, yet, due to their pleiotropicity, also often influence specialized metabolism [88]. Overexpression of SSFG_01620 for the Streptomyces subtilisin inhibitor (SSI) is thought to be responsible for MmA overproduction by the wblA knockout strain, although the exact mechanism remains unknown [87].
Expression of adpA, bldA, and wblA in ATCC 14672 was shown to be under the control of the master regulator BldD [89]. BldD is one of the most conserved regulatory proteins in Actinobacteria. It sits at the top of the regulatory cascade controlling morphological progression in Streptomyces by inhibiting the expression of sporulation genes during vegetative growth. In S. coelicolor, BldD exerts its control by regulating the expression of at least 167 genes, where a large portion includes the well-known regulators required for the maturation of spores, cell division, chromosome segregation, and secondary metabolite production [90]. Deletion of bldD severely impaired the morphogenesis in ATCC 14672 and nearly completely abolished MmA biosynthesis. Transcription of adpA and the key structural moe genes required for MmA assembly was strongly reduced in the bldD mutant [89]. In contrast to S. coelicolor, where transcription of adpA is repressed by BldD [90], adpA in ATCC 14672 is activated by BldD, similarly to what is observed in daptomycin producer S. roseosporus [91]. BldD also controls the expression of wblA in ATCC 14672 via repression of its transcription, which stems from the binding of BldD to the wblA promoter [89]. Deregulated expression of dozens of genes including adpA, wblA, and bldA seems to be one of the main contributors to the observed phenotype of S. ghanaenis ΔbldD strain.
The regulatory activity of BldD is mediated by the second messenger, cyclic dimeric 3′−5′ guanosine monophosphate (c-di-GMP) [92]. In the presence of c-di-GMP, two monomers of BldD form a complex bound to four c-di-GMP molecules, which then proceeds to bind to target promoter sites [92,93]. The intracellular pool of c-di-GMP is replenished by diguanylate cyclases (DGCs) and degraded by phosphodiesterases (PDEs). DGCs make c-di-GMP from two molecules of GTP, whereas PDEs break c-di-GMP either to linear dinucleotide 5′-phosphoguanylyl-(3′→5′)-guanosine (pGpG) or directly to two molecules of GMP, depending on a class of PDE [94]. ATCC 14672 genome encodes nine proteins for c-di-GMP metabolism [89]. Deletion of cdgB for a highly and constitutively expressed DGC reduced both c-di-GMP and MmA accumulation. In contrast, MmA production was boosted in ATCC 14672 strain deficient in rmdB for active PDE [89]. Deletion of the gene for active DGC SSFG_02181 (CdgC) negatively influenced MmA accumulation, leading to precocious sporulation, while the overexpression of ssfg_02181 blocked sporulation and remarkably improved the antibiotic titer [95]. Furthermore, individual deletion of rmdA, cdgA, and cdgD encoding a bifunctional DGC/PDE, an active PDE, and a predicted DGC, respectively, positively influenced MmA accumulation, whereas deletion of cdgE for a DGC had no impact on MmA titers [96]. The transcription of most DGC-encoding genes is repressed by BldD, forming a reciprocal regulatory loop, where DGCs are proposed to synthesize c-di-GMP to stimulate the activity of BldD [89,[94], [95], [96]]. When the c-di-GMP pool reaches a certain threshold level, BldD represses the transcription of DGC genes. BldD controls the expression of TTA-containing rmdB indirectly at the translational level by regulating the abundance of BldA to avoid premature PDE activity [89]. Additionally, to adjust c-di-GMP levels in response to fluctuating environmental or intracellular signals, most DGCs and PDEs are accompanied by auxiliary regions required either for the recognition of specific triggers or spatial allocation [94]. Altogether, the c-di-GMP-mediated regulatory network in ATCC 14672 seems to be immensely intricate and includes numerous layers to ensure proper coordination of morphological progression and antibiotic production.
Prokaryotes, including streptomycetes, have evolved an efficient system of intercellular communications mediated by low molecular weight signaling compounds (LMWCs) [97]. For instance, the archetypal gamma-butyrolactone A-factor triggers morphogenesis and production of streptomycin in S. griseus by binding to the TetR family transcriptional repressor ArpA. This releases ArpA from the promoter of adpA that activates the expression of genes related to morphological progression and secondary metabolite biosynthesis [81]. In silico analysis of the ATCC 14672 genome revealed the presence of genes for at least two classes of LMWCs, γ-butyrolactones, and avenolides [98]. Deletion of SSFG_07725 encoding a putative γ-butyrolactone synthase in ATCC 14672 abrogated the production of diffusible LMWCs leading to morphogenetic deficiency and somewhat decreasing MmA production [99]. Introduction of extra copies of SSFG_07725 into the wild-type strain affected neither morphogenesis nor MmA production [98]. No moenomycin production was observed during the heterologous expression of MmA BGC in S. lividans M707 carrying a deletion of the γ-butyrolactone synthase gene scbA [98]. Thus, the LMWC-based regulatory pathway seems to impact MmA biosynthesis in a species-dependent manner. Likely, the expression of adpA could be either γ-butyrolactone-dependent (S. griseus) or independent (S. coelicolor and ATCC 14672).
Several genes influencing MmA production were recently revealed in course of Tn5 and mariner transposon mutagenesis of ATCC 14672 [85,100]. No further elucidation of these genes beyond initial annotation has been carried out yet. While for some of the identified genes (such as those for kinases and RNA polymerase subunit) a putative role can be easily put forward, an association of the majority of these genes with MmA remains vague.
Both native and heterologous producers of MmA were engineered to reveal the factors limiting MmA production [101], [102], [103]. The production of moenomycins varies greatly in different heterologous hosts, indicating their different metabolic and regulatory backgrounds influenced moenomycin biosynthesis. None of the tested strains was superior over ATCC 14672 in terms of moenomycin productivity. Nonetheless, the obtained data about the regulatory pathways governing moenomycin biosynthesis in heterologous hosts laid a useful background for the exploration of its regulation in the natural MmA producer. For instance, overexpression of the S. coelicolor ppGpp synthetase gene relA has been shown to positively correlate with moenomycin accumulation in both native and heterologous hosts [101]. Similarly, duplication of moe genes via the introduction of an additional copy of the moe cluster offers a beneficial way to significantly improve MmA titers [101,104,105]. Several genome-engineering approaches were employed to boost MmA production. Intriguingly, not only moenomycin accumulation but also the growth dynamic was greatly improved after in vivo elimination of binding sites for the pleiotropic regulator AdpAgh in the oriC region of the S. ghanaensis chromosome [106]. Rational combination of moe gene dosage along with the overexpression of the pleiotropic regulator bldA led to a notable increase in MmA synthesis [104].
Strong improvement in moenomycin accumulation was achieved during the expression of a hybrid BGC derived from ATCC 14672 and S. lincolnensis NRRL2936 in S. albus J1074 [103]. Promoter refactoring along with the overexpression of the gene salb-PBP2 for peptidoglycan biosynthetic protein PBP2 and media optimization experiments further elevated the antibiotic titers. This study illustrates the power of synthetic biotechnology in tackling some of the toughest problems in the development of industrial antibiotic producers.
Outlook
The unique structure and mode of action of moenomycins fueled decades of investigations, which culminated in total synthesis of MmA, atomic-level view of its interaction with PGTs, and delineation of its biosynthetic logic. MmA proved to be an invaluable chemical probe to understand cell wall biosynthesis. It helped overproduce Lipid II [107], validate high-throughput screens of PGT inhibitors, and reveal an entirely new SEDS family of peptidoglycan synthases. Nevertheless, a much sought-after application, the one as a drug to treat human diseases, still eludes this highly promising class of compounds. The harsh reality of the economics of development and marketing of any new antibacterial is indeed a roadblock [4,108], yet one that fades gradually as the antimicrobial resistance crisis becomes urgent [109]. Below we suggest how biology-oriented studies of moenomycins can help transform this class of natural products into a drug.
MmA is not orally bioavailable and exhibits an extremely long half-life in the bloodstream [1]. These are the main drawbacks (from a point of view of pharmacology) of MmA as well as all studied to date members of this family. The diversity of naturally occurring moenomycins is low, and so are the chances of finding new congeners with different pharmacological profiles. Nevertheless, identification of very distinct moenomycin-like BGCs in Photorhabdus and Actinomyces spp. (see Fig. 2) suggests that chemical space around the phosphoglycolipid scaffold is larger than we know at the moment. In this regard, it is difficult to underestimate the value of fundamental research into regulatory mechanisms that are involved in sensing and handling the MmA-induced stress response, and silencing of moenomycin BGCs. The former will find their use to develop inexpensive cell-based tools for screening of moenomycin producers in large strain collections (akin LiaRS system for Lipid II binders [110]), the latter can be used to reveal the compounds encoded by cryptic BGCs. Characterization of several moenomycin biosynthetic enzymes, such as MoeH5, MoeN5, and MoeO5 paves the road to the chemoenzymatic production of moenomycin analogs inaccessible naturally. Here it is crucial to continue the studies of all enzymes involved in the assembly of moenocinol, as the latter is the main cause of the poor pharmacokinetics of MmA. More effort should be focused on enzymes for moenuronamide, the most densely modified carbohydrate unit (F, see Fig. 1) of MmA and part of its pharmacophore. Radical SAM cobalamin-dependent methyltransferase MoeK5 is of particular interest, as it carries out crucial biotransformation on unit F via a mechanism that remains largely speculative [111]. Finally, the elucidation of antibacterial effects and mechanisms of MmA action on a wider set of pathogenic bacteria is as important as finding more novel moenomycins; recent works on Helicobacter and Neisseria clearly demonstrate this point.
CRediT authorship contribution statement
Bohdan Ostash: Conceptualization, Funding acquisition, Visualization, Writing – original draft, Writing – review & editing. Roman Makitrynskyy: Visualization, Writing – original draft, Writing – review & editing. Oleksandr Yushchuk: Visualization, Writing – original draft, Writing – review & editing. Victor Fedorenko: Conceptualization, Funding acquisition, Supervision, Writing – review & editing.
Declaration of Competing Interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
V.F. and B.O. thank the Ministry of Education and Science of Ukraine for the continuous support of the moenomycin-related research at Lviv University over the last 15 years (grants BG-01F, -98F, -09F, -41Nr, -21F). B.O. was supported by DAAD (A/12/04489) and VRU5517-VI fellowships. Authors thank all coworkers at Lviv and Freiburg groups who contributed to the current understanding of moenomycin biology.
Data availability
No data was used for the research described in the article.
References
- 1.Ostash B., Walker S. Moenomycin family antibiotics: chemical synthesis, biosynthesis, and biological activity. Nat. Prod. Rep. 2010;27:1594–1617. doi: 10.1039/c001461n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Welzel P. Syntheses around the transglycosylation step in peptidoglycan biosynthesis. Chem. Rev. 2005;105:4610–4660. doi: 10.1021/cr040634e. [DOI] [PubMed] [Google Scholar]
- 3.Taylor J., Li X., Oberthür M., Zhu W., Kahne D. The total synthesis of moenomycin A. J. Am. Chem. Soc. 2006;128:15084–15085. doi: 10.1021/ja065907x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bisacchi G., Manchester J. A new-class antibacterial-almost. Lessons in drug discovery and development: a critical analysis of more than 50 years of effort toward ATPase inhibitors of DNA gyrase and topoisomerase IV. ACS Infect. Dis. 2015;1:4–41. doi: 10.1021/id500013t. [DOI] [PubMed] [Google Scholar]
- 5.Bengoechea J., Bamford C. SARS-CoV-2, bacterial co-infections, and AMR: the deadly trio in COVID-19? EMBO Mol. Med. 2020;12:e12560. doi: 10.15252/emmm.202012560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Galley N., O'Reilly A., Roper D. Prospects for novel inhibitors of peptidoglycan transglycosylases. Bioorg. Chem. 2014;55:16–26. doi: 10.1016/j.bioorg.2014.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ostash B., Campbell J., Luzhetskyy A., Walker S. MoeH5: a natural glycorandomizer from the moenomycin biosynthetic pathway. Mol. Microbiol. 2013;90:1324–1338. doi: 10.1111/mmi.12437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koyama N., Tokura Y., Takahashi Y., Tomoda H. Discovery of nosokophic acid, a predicted intermediate of moenomycins, from nosokomycin-producing Streptomyces sp. K04-0144. Bioorg. Med. Chem. Lett. 2013;23:860–863. doi: 10.1016/j.bmcl.2012.11.044. [DOI] [PubMed] [Google Scholar]
- 9.Huang S., Wu W., Huang L., Huang W., Fu W., Chen P., Fang J., Cheng W., Cheng T., Wong C.H. New continuous fluorometric assay for bacterial transglycosylase using Förster resonance energy transfer. J. Am. Chem. Soc. 2013;135:17078–17089. doi: 10.1021/ja407985m. [DOI] [PubMed] [Google Scholar]
- 10.Dahmane C.M., Matagne A., Dumbre S., Herdewijn P., Terrak M. Peptidoglycan glycosyltransferase-ligand binding assay based on tryptophan fluorescence quenching. Biochimie. 2018;152:1–5. doi: 10.1016/j.biochi.2018.06.010. [DOI] [PubMed] [Google Scholar]
- 11.Boes S.O., Mohammadi T., Breukink E., Terrak M. Fluorescence anisotropy assays for high throughput screening of compounds binding to lipid II, PBP1b, FtsW and MurJ. Sci Rep. 2020;10:6280. doi: 10.1038/s41598-020-63380-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hernández-Rocamora V., Baranova N., Peters K., Breukink E., Loose M., Vollmer W. Real-time monitoring of peptidoglycan synthesis by membrane-reconstituted penicillin-binding proteins. Elife. 2021;10:e61525. doi: 10.7554/eLife.61525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zehl M., Pittenauer E., Rizzi A., Allmaier G. Characterization of moenomycin antibiotic complex by multistage MALDI-IT/RTOF-MS and ESI-IT-MS. J. Am. Soc. Mass Spectrom. 2006;17:1081–1090. doi: 10.1016/j.jasms.2006.04.019. [DOI] [PubMed] [Google Scholar]
- 14.Gallo P., Fabbrocino S., Serpe L., Fiori M., Civitareale C., Stacchini P. Determination of the banned growth promoter moenomycin A in feed stuffs by liquid chromatography coupled to electrospray ion trap mass spectrometry. Rapid Commun. Mass Spectrom. 2010;24:1017–1024. doi: 10.1002/rcm.4478. [DOI] [PubMed] [Google Scholar]
- 15.Belknap K., Park C., Barth B., Andam C. Genome mining of biosynthetic and chemotherapeutic gene clusters in Streptomyces bacteria. Sci. Rep. 2020;10:2003. doi: 10.1038/s41598-020-58904-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.AbuSara N., Piercey B., Moore M., Shaikh A., Nothias L., Srivastava S., Cruz-Morales P., Dorrestein P., Barona-Gómez F., Tahlan K. Comparative genomics and metabolomics analyses of clavulanic acid-producing Streptomyces species provides insight into specialized metabolism. Front. Microbiol. 2019;10:2550. doi: 10.3389/fmicb.2019.02550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shaikh A., Nothias L., Srivastava S., Dorrestein P., Tahlan K. Specialized metabolites from ribosome engineered strains of Streptomyces clavuligerus. Metabolites. 2021;11:239. doi: 10.3390/metabo11040239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shi Y., Hirschmann M., Shi Y., Ahmed S., Abebew D., Tobias N., Grün P., Crames J., Pöschel L., Kuttenlochner W., Richter C., Herrmann J., Müller R., Thanwisai A., Pidot S., Stinear T., Groll M., Kim Y., Bode H. Global analysis of biosynthetic gene clusters reveals conserved and unique natural products in entomopathogenic nematode-symbiotic bacteria. Nat. Chem. 2022;14:701–712. doi: 10.1038/s41557-022-00923-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sauvage E., Kerff F., Terrak M., Ayala J., Charlier P. The penicillin-binding proteins: structure and role in peptidoglycan biosynthesis. FEMS Microbiol. Rev. 2008;32:234–258. doi: 10.1111/j.1574-6976.2008.00105.x. [DOI] [PubMed] [Google Scholar]
- 20.Gampe C., Tsukamoto H., Wang T., Walker S., Kahne D. Modular synthesis of diphospholipid oligosaccharide fragments of the bacterial cell wall and their use to study the mechanism of moenomycin and other antibiotics. Tetrahedron. 2011;67:9771–9778. doi: 10.1016/j.tet.2011.09.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Punekar A., Samsudin F., Lloyd A., Dowson C., Scott D., Khalid S., Roper D. The role of the jaw subdomain of peptidoglycan glycosyltransferases for lipid II polymerization. Cell Surf. 2018;2:54–66. doi: 10.1016/j.tcsw.2018.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang C., Shih H., Lin L., Tien Y., Cheng T., Cheng W., Wong C., Ma C. Crystal structure of Staphylococcus aureus transglycosylase in complex with a lipid II analog and elucidation of peptidoglycan synthesis mechanism. Proc. Natl. Acad. Sci. USA. 2012;109:6496–6501. doi: 10.1073/pnas.1203900109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heaslet H., Shaw B., Mistry A., Miller A. Characterization of the active site of S. aureus monofunctional glycosyltransferase (Mtg) by site-directed mutation and structural analysis of the protein complexed with moenomycin. J. Struct. Biol. 2009;167:129–135. doi: 10.1016/j.jsb.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 24.Lovering A., de Castro L., Lim D., Strynadka N. Structural insight into the transglycosylation step of bacterial cell-wall biosynthesis. Science. 2007;315:1402–1405. doi: 10.1126/science.1136611. [DOI] [PubMed] [Google Scholar]
- 25.Caveney N., Workman S., Yan R., Atkinson C., Yu Z., Strynadka N. CryoEM structure of the antibacterial target PBP1b at 3.3Å resolution. Nat. Commun. 2021;12:1–7. doi: 10.1038/s41467-021-23063-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boes A., Kerff F., Herman R., Touze T., Breukink E., Terrak M. The bacterial cell division protein fragment EFtsN binds to and activates the major peptidoglycan synthase PBP1b. J. Biol. Chem. 2020;295:18256–18265. doi: 10.1074/jbc.RA120.015951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sung M., Lai Y., Huang C., Chou L., Shih H., Cheng W., Wong C., Ma C. Crystal structure of the membrane-bound bifunctional transglycosylase PBP1b from Escherichia coli. Proc. Natl. Acad. Sci. USA. 2009;106:8824–8829. doi: 10.1073/pnas.0904030106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.King D., Wasney G., Nosella M., Fong A., Strynadka N., Guengerich F.P. Structural insights into inhibition of Escherichia coli penicillin-binding protein 1B. J. Biol. Chem. 2017;292:979–993. doi: 10.1074/jbc.M116.718403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.King A., King D., French S., Brouillette E., Asli A., Alexander J., Vuckovic M., Maiti S., Parr T., Brown E., et al. Structural and kinetic characterization of diazabicyclooctanes as dual inhibitors of both serine-β-lactamases and penicillin-binding proteins. ACS Chem. Biol. 2016;11:864–868. doi: 10.1021/acschembio.5b00944. [DOI] [PubMed] [Google Scholar]
- 30.Yuan Y., Fuse S., Ostash B., Sliz P., Kahne D., Walker S. Structural analysis of the contacts anchoring moenomycin to peptidoglycan glycosyltransferases and implications for antibiotic design. ACS Chem. Biol. 2008;3:429–436. doi: 10.1021/cb800078a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fuse S., Tsukamoto H., Yuan Y., Wang T., Zhang Y., Bolla M., Walker S., Sliz P., Kahne D. Functional and structural analysis of a key region of the cell wall inhibitor moenomycin. ACS Chem. Biol. 2010;5:701–711. doi: 10.1021/cb100048q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Markovski M., Bohrhunter J., Lupoli T., Uehara T., Walker S., Kahne D., Bernhardt T. Cofactor bypass variants reveal a conformational control mechanism governing cell wall polymerase activity. Proc. Natl. Acad. Sci. USA. 2016;113:4788–4793. doi: 10.1073/pnas.1524538113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.King D., Lameignere E., Strynadka N. Structural insights into the lipoprotein outer membrane regulator of penicillin-binding protein 1B. J. Biol. Chem. 2014;289:19245–19253. doi: 10.1074/jbc.M114.565879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen X., Wong C., Ma C. Targeting the bacterial transglycosylase: antibiotic development from a structural perspective. ACS Infect. Dis. 2019;5:1493–1504. doi: 10.1021/acsinfecdis.9b00118. [DOI] [PubMed] [Google Scholar]
- 35.Boes A., Brunel J., Derouaux A., Kerff F., Bouhss A., Touze T., Breukink E., Terrak M. Squalamine and aminosterol mimics inhibit the peptidoglycan glycosyltransferase activity of PBP1b. Antibiotics. 2020;9:1–11. doi: 10.3390/antibiotics9070373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang Y., Cheong W., Liang Z., So L., Chan K., So P., Chen Y., Wong W., Wong K. Hydrophobic substituents on isatin derivatives enhance their inhibition against bacterial peptidoglycan glycosyltransferase activity. Bioorg. Chem. 2020;97 doi: 10.1016/j.bioorg.2020.103710. [DOI] [PubMed] [Google Scholar]
- 37.Mesleh M., Rajaratnam P., Conrad M., Chandrasekaran V., Liu C., Pandya B., Hwang Y., Rye P., Muldoon C., Becker B., et al. Targeting bacterial cell wall peptidoglycan synthesis by inhibition of glycosyltransferase activity. Chem. Biol. Drug Des. 2016;87:190–199. doi: 10.1111/cbdd.12662. [DOI] [PubMed] [Google Scholar]
- 38.Zuegg J., Muldoon C., Adamson G., McKeveney D., Thanh G.Le, Premraj R., Becker B., Cheng M., Elliott A., Huang J., et al. Carbohydrate scaffolds as glycosyltransferase inhibitors with in vivo antibacterial activity. Nat. Commun. 2015;6:1–11. doi: 10.1038/ncomms8719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang Y., Chan F., Sun N., et al. Structure-based design, synthesis, and biological evaluation of isatin derivatives as potential glycosyltransferase inhibitors. Chem. Biol. Drug Des. 2014;84:685–696. doi: 10.1111/cbdd.12361. [DOI] [PubMed] [Google Scholar]
- 40.Dumbre S., Derouaux A., Lescrinier E., Piette A., Joris B., Terrak M., Herdewijn P. Synthesis of modified peptidoglycan precursor analogues for the inhibition of glycosyltransferase. J. Am. Chem. Soc. 2012;134:9343–9351. doi: 10.1021/ja302099u. [DOI] [PubMed] [Google Scholar]
- 41.Riedl S., Ohlsen K., Werner G., Witte W., Hacker J. Impact of flavophospholipol and vancomycin on conjugational transfer of vancomycin resistance plasmids. Antimicrob. Agents Chemother. 2000;44:3189–3192. doi: 10.1128/AAC.44.11.3189-3192.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Poole T., McReynolds J., Edrington T., Byrd J., Callaway T., Nisbet D. Effect of flavophospholipol on conjugation frequency between Escherichia coli donor and recipient pairs in vitro and in the chicken gastrointestinal tract. J. Antimicrob. Chemother. 2006;58:359–366. doi: 10.1093/jac/dkl249. [DOI] [PubMed] [Google Scholar]
- 43.Kudo H., Usui M., Nagafuji W., Oka K., Takahashi M., Yamaguchi H., Tamura Y. Inhibition effect of flavophospholipol on conjugative transfer of the extended-spectrum β-lactamase and vanA genes. J. Antibiot. 2019;72:79–85. doi: 10.1038/s41429-018-0113-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Volke F., Waschipky R., Pampel A., Donnerstag A., Lantzsch G., Pfeiffer H., Richter W., Klose G., Welzel P. Characterization of antibiotic moenomycin A interaction with phospholipid model membranes. Chem. Phys. Lipids. 1997;85:115–123. doi: 10.1016/S0009-3084(96)02649-7. [DOI] [PubMed] [Google Scholar]
- 45.Bauer F., Dost G. Moenomycin in animal nutrition. Antimicrob. Agents Chemother. 1965;5:749–752. [PubMed] [Google Scholar]
- 46.Horbal L., Ostash B., Luzhetskyy A., Walker S., Kalinowski J., Fedorenko V. A gene cluster for the biosynthesis of moenomycin family antibiotics in the genome of teicoplanin producer Actinoplanes teichomyceticus. Appl. Microbiol. Biotechnol. 2016;100:7629–7638. doi: 10.1007/s00253-016-7685-3. [DOI] [PubMed] [Google Scholar]
- 47.Yushchuk O., Homoniuk V., Datsiuk Y., Ostash B., Marinelli F., Fedorenko V. Development of a gene expression system for the uncommon actinomycete Actinoplanes rectilineatus NRRL B-16090. J. Appl. Genet. 2020;61:141–149. doi: 10.1007/s13353-019-00534-7. [DOI] [PubMed] [Google Scholar]
- 48.Yarlagadda V., Rao V., Kaur M., Guitor A., Wright G. A screen of natural product extracts identifies moenomycin as a potent antigonococcal agent. ACS Infect. Dis. 2021;7:1569–1577. doi: 10.1021/acsinfecdis.1c00040. [DOI] [PubMed] [Google Scholar]
- 49.Ostash B., Doud E., Walker S. ABC transporter genes from Streptomyces ghanaensis moenomycin biosynthetic gene cluster: roles in antibiotic production and export. Arch. Microbiol. 2012;194:915–922. doi: 10.1007/s00203-012-0827-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ostash B., Yushchuk O., Koshla O., Rebets Y., Ostash I., Sehin Y., Busche T., Kalinowski J., Muth G., Fedorenko V. Elucidation of the genetic mechanisms contributing to moenomycin resistance in actinobacteria. Factors Exp. Evol. Org. 2018;22:203–209. https://europub.co.uk/articles/-A-618757 [Google Scholar]
- 51.Shashkov A., Streshinskaya G., Tul'skaya E., Senchenkova S., Baryshnikova L., Dmitrenok A., Ostash B., Fedorenko V. Cell wall glycopolymers of Streptomyces albus, Streptomyces albidoflavus and Streptomyces pathocidini. Antonie Van Leeuwenhoek. 2016;109:923–936. doi: 10.1007/s10482-016-0691-8. [DOI] [PubMed] [Google Scholar]
- 52.Hesketh A., Hill C., Mokhtar J., Novotna G., Tran N., Bibb M., Hong H. Genome-wide dynamics of a bacterial response to antibiotics that target the cell envelope. BMC Genomics. 2011;12:226. doi: 10.1186/1471-2164-12-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Baizman E., Branstrom A., Longley C., Allanson N., Sofia M., Gange D., Goldman R. Antibacterial activity of synthetic analogues based on the disaccharide structure of moenomycin, an inhibitor of bacterial transglycosylase. Microbiology. 2000;146:3129–3140. doi: 10.1099/00221287-146-12-3129. [DOI] [PubMed] [Google Scholar]
- 54.Goldman R., Baizman E., Branstrom A., Longley C. Differential antibacterial activity of moenomycin analogues on gram-positive bacteria. Bioorganic Med. Chem. Lett. 2000;10:2251–2254. doi: 10.1016/S0960-894X(00)00443-1. [DOI] [PubMed] [Google Scholar]
- 55.He H., Shen B., Korshalla J., Siegel M., Carter G. Isolation and structural elucidation of AC326α, a new member of the moenomycin group. J. Antibiot. 2000;53:191–195. doi: 10.7164/antibiotics.53.191. [DOI] [PubMed] [Google Scholar]
- 56.Uchida R., Iwatsuki M., Kim Y., Ohte S., Mura S., Tomoda H. Nosokomycins, new antibiotics discovered in an in vivo-mimic infection model using silkworm larvae. I: fermentation, isolation and biological properties. J. Antibiot. 2010;63:151–155. doi: 10.1038/ja.2010.9. [DOI] [PubMed] [Google Scholar]
- 57.Pucci M., Dougherty T. Direct quantitation of the numbers of individual penicillin-binding proteins per cell in Staphylococcus aureus. J. Bacteriol. 2002;184:588–591. doi: 10.1128/JB.184.2.588-591.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.McPherson D., Popham D. Peptidoglycan synthesis in the absence of class A penicillin-binding proteins in Bacillus subtilis. J. Bacteriol. 2003;185:1423–1431. doi: 10.1128/JB.185.4.1423-1431.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Salzberg L., Luo Y., Hachmann A., Mascher T., Helmann J. The Bacillus subtilis GntR family repressor YtrA responds to cell wall antibiotics. J. Bacteriol. 2011;193:5793–5801. doi: 10.1128/JB.05862-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Helmann J. Bacillus subtilis extracytoplasmic function (ECF) sigma factors and defense of the cell envelope. Curr. Opin. Microbiol. 2016;30:122–132. doi: 10.1016/j.mib.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Meeske A., Riley E., Robins W., Uehara T., Mekalanos J., Kahne D., Walker S., Kruse A., Bernhardt T., Rudner D. SEDS proteins are a widespread family of bacterial cell wall polymerases. Nature. 2016;537:634–638. doi: 10.1038/nature19331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Emami K., Guyet A., Kawai Y., Devi J., Wu L., Allenby N., Daniel R., Errington J. RodA as the missing glycosyltransferase in Bacillus subtilis and antibiotic discovery for the peptidoglycan polymerase pathway. Nat. Microbiol. 2017;2:1–8. doi: 10.1038/nmicrobiol.2016.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Metten K., Hobert K., Marzian S., Hackler U., Heinz U., Welzel P., Aretz W., Böttger D., Hedtmann U., Seibert G., et al. The first enzymatic degradation products of the antibiotic moenomycin A. Tetrahedron. 1992;48:8401–8418. doi: 10.1016/S0040-4020(01)86589-3. [DOI] [Google Scholar]
- 64.Nishi H., Komatsuzawa H., Yamada S., Fujiwara T., Ohara M., Ohta K., Sugiyama M., Ishikawa T., Sugai M. Moenomycin-resistance is associated with vancomycin-intermediate susceptibility in Staphylococcus aureus. Microbiol. Immunol. 2003;47:927–935. doi: 10.1111/j.1348-0421.2003.tb03466.x. [DOI] [PubMed] [Google Scholar]
- 65.Rebets Y., Lupoli T., Qiao Y., Schirner K., Villet R., Hooper D., Kahne D., Walker S. Moenomycin resistance mutations in Staphylococcus aureus reduce peptidoglycan chain length and cause aberrant cell division. ACS Chem. Biol. 2014;9:459–467. doi: 10.1021/cb4006744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Méndez C., Salas J. ABC transporters in antibiotic-producing actinomycetes. FEMS Microbiol. Lett. 1998;158:1–8. doi: 10.1016/S0378-1097(97)00434-5. [DOI] [PubMed] [Google Scholar]
- 67.Villet R., Truong-Bolduc Q., Wang Y., Estabrooks Z., Medeiros H., Hooper D. Regulation of expression of abcA and its response to environmental conditions. J. Bacteriol. 2014;196:1532–1539. doi: 10.1128/JB.01406-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hachmann A., Sevim E., Gaballa A., Popham D., Antelmann H., Helmann J. Reduction in membrane phosphatidylglycerol content leads to daptomycin resistance in Bacillus subtilis. Antimicrob. Agents Chemother. 2011;55:4326–4337. doi: 10.1128/AAC.01819-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Van Heijenoort Y., Leduc M., Singer H., Van Heijenoort J. Effects of moenomycin on Escherichia coli. J. Gen. Microbiol. 1987;133:667–674. doi: 10.1099/00221287-133-3-667. [DOI] [PubMed] [Google Scholar]
- 70.Yuan Y., Barrett D., Zhang Y., Kahne D., Sliz P., Walker S. Crystal structure of a peptidoglycan glycosyltransferase suggests a model for processive glycan chain synthesis. Proc. Natl. Acad. Sci. USA. 2007;104:5348–5353. doi: 10.1073/pnas.0701160104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tseng Y., Liou J., Hsu T., Cheng W., Wu M., Wong C. Development of bacterial transglycosylase inhibitors as new antibiotics: moenomycin A treatment for drug-resistant Helicobacter pylori. Bioorganic Med. Chem. Lett. 2014;24:2412–2414. doi: 10.1016/j.bmcl.2014.04.041. [DOI] [PubMed] [Google Scholar]
- 72.De Witte C., Taminiau B., Flahou B., Hautekiet V., Daube G., Ducatelle R., Haesebrouck F. In-feed bambermycin medication induces anti-inflammatory effects and prevents parietal cell loss without influencing Helicobacter suis colonization in the stomach of mice. Vet. Res. 2018;49:1–16. doi: 10.1186/s13567-018-0530-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yang F., Gao S., Yan J., Lin X., van der Veen S. Moenomycin is broadly active against multidrug-resistant Neisseria gonorrhoeae and clears an infection from a murine vaginal tract infection model. J. Antimicrob. Chemother. 2022;77:2461–2469. doi: 10.1093/jac/dkac202. [DOI] [PubMed] [Google Scholar]
- 74.Ostash B., Doud E., Lin C., Ostash I., Perlstein D., Fuse S., Wolpert M., Kahne D., Walker S. Complete characterization of the seventeen step moenomycin biosynthetic pathway. Biochemistry. 2009;48:8830–8841. doi: 10.1021/bi901018q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Doud E., Perlstein D., Wolpert M., Cane D., Walker S. Two distinct mechanisms for TIM barrel prenyltransferases in bacteria. J. Am. Chem. Soc. 2011;133:1270–1273. doi: 10.1021/ja109578b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ren F., Ko T., Feng X., Huang C., Chan H., Hu Y., Wang K., Ma Y., Liang P., Wang A., Oldfield E., Guo R. Insights into the mechanism of the antibiotic-synthesizing enzyme MoeO5 from crystal structures of different complexes. Angew. Chem. Int. Ed. Engl. 2012;51:4157–4160. doi: 10.1002/anie.201108002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhang L., Ko T., Malwal S., Liu W., Zhou S., Yu X., Oldfield E., Guo R., Chen C. Complex structures of MoeN5 with substrate analogues suggest sequential catalytic mechanism. Biochem. Biophys. Res. Commun. 2019;511:800–805. doi: 10.1016/j.bbrc.2019.02.131. [DOI] [PubMed] [Google Scholar]
- 78.Zhang L., Chen C., Ko T., Huang J., Zheng Y., Liu W., Wang I., Malwal S., Feng X., Wang K., Huang C., Hsu S., Wang A., Oldfield E., Guo R. Moenomycin biosynthesis: structure and mechanism of action of the prenyltransferase MoeN5. Angew. Chem. Int. Ed. Engl. 2016;55:4716–4720. doi: 10.1002/anie.201511388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Schuricht U., Hennig L., Findeisen M., Endler K., Welzel P., Arigoni D. The biosynthesis of moenocinol, the lipid part of the moenomycin antibiotics. Tetrahedron Lett. 2001;42:3835–3837. [Google Scholar]
- 80.Yang Z., Zhang L., Yu X., Wu S., Yang Y., Hu Y., Li Q., Shang N., Guo R., Chen C., Dai L., Liu W. Crystal structure of TchmY from Actinoplanes teichomyceticus. Acta Crystallogr. F. 2019;75:570–575. doi: 10.1107/S2053230X19010914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ostash B. Pleiotropic regulatory genes as a tool for Streptomyces strains bioprospecting and improvement. Curr. Biotech. 2021;10:18–31. doi: 10.2174/2211550110666210217105112. [DOI] [Google Scholar]
- 82.Liu G., Chater K.F., Chandra G., Niu G., Tan H. Molecular regulation of antibiotic biosynthesis in streptomyces, Microbiol. Mol. Biol. Rev. 2013;77:112–143. doi: 10.1128/MMBR.00054-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Makitrynskyy R., Ostash B., Tsypik O., Rebets Y., Doud E., Meredith T., Luzhetskyy A., Bechthold A., Walker S., Fedorenko V. Pleiotropic regulatory genes bldA, adpA and absB are implicated in production of phosphoglycolipid antibiotic moenomycin. Open Biol. 2013;3 doi: 10.1098/rsob.130121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Koshla O., Lopatniuk M., Rokytskyy I., Yushchuk O., Dacyuk Y., Fedorenko V., Luzhetskyy A., Ostash B. Properties of Streptomyces albus J1074 mutant deficient in tRNALeuUAA gene bldA. Arch. Microbiol. 2017;199:1175–1183. doi: 10.1007/s00203-017-1389-7. [DOI] [PubMed] [Google Scholar]
- 85.Sehin Y., Koshla O., Dacyuk Y., Zhao R., Ross R., Myronovskyi M., Limbach P.A., Luzhetskyy A., Walker S., Fedorenko V., Ostash B. Gene ssfg_01967 (miaB) for tRNA modification influences morphogenesis and moenomycin biosynthesis in Streptomyces ghanaensis ATCC14672. Microbiology. 2019;165:233–245. doi: 10.1099/mic.0.000747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Koshla O., Yushchuk O., Ostash I., Dacyuk Y., Myronovskyi M., Jäger G., Süssmuth R.D., Luzhetskyy A., Byström A., Kirsebom L.A., Ostash B. Gene miaA for post-transcriptional modification of tRNAXXA is important for morphological and metabolic differentiation in Streptomyces. Mol. Microbiol. 2019;112:249–265. doi: 10.1111/mmi.14266. [DOI] [PubMed] [Google Scholar]
- 87.Rabyk M., Ostash B., Rebets Y., Walker S., Fedorenko V. Streptomyces ghanaensis pleiotropic regulatory gene wblA(gh) influences morphogenesis and moenomycin production. Biotechnol. Lett. 2011;33:2481–2486. doi: 10.1007/s10529-011-0728-z. [DOI] [PubMed] [Google Scholar]
- 88.Nah H.-.J., Park J., Choi S., Kim E.-.S. WblA, a global regulator of antibiotic biosynthesis in Streptomyces. J. Ind. Microbiol. Biotechnol. 2021;48 doi: 10.1093/jimb/kuab007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Makitrynskyy R., Tsypik O., Nuzzo D., Paululat T., Zechel D.L., Bechthold A. Secondary nucleotide messenger c-di-GMP exerts a global control on natural product biosynthesis in streptomycetes. Nucleic Acids Res. 2020;48:1583–1598. doi: 10.1093/nar/gkz1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.den Hengst C.D., Tran N.T., Bibb M.J., Chandra G., Leskiw B.K., Buttner M.J. Genes essential for morphological development and antibiotic production in Streptomyces coelicolor are targets of BldD during vegetative growth. Mol. Microbiol. 2010;78:361–379. doi: 10.1111/j.1365-2958.2010.07338.x. [DOI] [PubMed] [Google Scholar]
- 91.Yan H., Lu X., Sun Di, Zhuang S., Chen Q., Chen Z., Li J., Wen Y. BldD, a master developmental repressor, activates antibiotic production in two Streptomyces species. Mol. Microbiol. 2020;113:123–142. doi: 10.1111/mmi.14405. [DOI] [PubMed] [Google Scholar]
- 92.Tschowri N., Schumacher M.A., Schlimpert S., Chinnam N.B., Findlay K.C., Brennan R.G., Buttner M.J. Tetrameric c-di-GMP mediates effective transcription factor dimerization to control Streptomyces development. Cell. 2014;158:1136–1147. doi: 10.1016/j.cell.2014.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Römling U., Galperin M.Y., Gomelsky M. Cyclic di-GMP: the first 25 years of a universal bacterial second messenger. Microbiol. Mol. Biol. Rev. 2013;77:1–52. doi: 10.1128/MMBR.00043-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Al-Bassam M.M., Haist J., Neumann S.A., Lindenberg S., Tschowri N. Expression patterns, genomic conservation and input into developmental regulation of the GGDEF/EAL/HD-GYP domain proteins in Streptomyces. Front. Microbiol. 2018;9:2524. doi: 10.3389/fmicb.2018.02524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Nuzzo D., Makitrynskyy R., Tsypik O., Bechthold A. Cyclic di-GMP cyclase SSFG_02181 from Streptomyces ghanaensis ATCC14672 regulates antibiotic biosynthesis and morphological differentiation in streptomycetes. Sci. Rep. 2020;10:12021. doi: 10.1038/s41598-020-68856-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nuzzo D., Makitrynskyy R., Tsypik O., Bechthold A. Identification and characterization of four c-di-GMP-metabolizing enzymes from Streptomyces ghanaensis ATCC14672 involved in the regulation of morphogenesis and moenomycin A biosynthesis. Microorganisms. 2021;9 doi: 10.3390/microorganisms9020284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Atkinson S., Williams P. Quorum sensing and social networking in the microbial world. J. R. Soc. Interface. 2009;6:959–978. doi: 10.1098/rsif.2009.0203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mutenko H., Makitrinskyy R., Tsypik O., Walker S., Ostash B., Fedorenko V. Genes for biosynthesis of butenolide-like signalling molecules in Streptomyces ghanaensis, their role in moenomycin production. Rus. J. Genet. 2014;50:563–568. doi: 10.1134/S1022795414060076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kuzhyk Y., Mutenko H., Fedorenko V., Ostash B. Analysis of Streptomyces ghanaensis ATCC14672 gene SSFG_07725 for putative γ-butyrolactone synthase. Folia Microbiol. 2018;63:701–706. doi: 10.1007/s12223-018-0614-3. [DOI] [PubMed] [Google Scholar]
- 100.Kuzhyk Y., Rebets Y., Popko I., Ostash I., Walker S., Fedorenko V., Ostash B. Tn5-based transposon mutagenesis of Streptomyces ghanaensis ATCC14672: searching for novel regulators of moenomycin production. Visn. Lviv Univ. Ser. Biol. 2019;81:50–56. doi: 10.30970/vlubs.2019.81.06. [DOI] [Google Scholar]
- 101.Makitrynskyy R., Rebets Y., Ostash B., Zaburannyi N., Rabyk M., Walker S., Fedorenko V. Genetic factors that influence moenomycin production in streptomycetes. J. Ind. Microbiol. Biotechnol. 2010;37:559–566. doi: 10.1007/s10295-010-0701-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lopatniuk M., Ostash B., Luzhetskyy A., Walker S., Fedorenko V. Generation and study of the strains of streptomycetes –heterologous hosts for production of moenomycin. Russ. J. Genet. 2014;50:360–365. doi: 10.1134/S1022795414040085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Li X., Hu X., Sheng Y., Wang H., Tao M., Ou Y., Deng Z., Bai L., Kang Q. Adaptive optimization boosted the production of moenomycin A in the microbial chassis Streptomyces albus J1074. ACS Synth. Biol. 2021;10:2210–2221. doi: 10.1021/acssynbio.1c00094. [DOI] [PubMed] [Google Scholar]
- 104.Kuzhyk Y., Lopatniuk M., Luzhetskyy A., Fedorenko V., Ostash B. Genome engineering approaches to improve nosokomycin A production by Streptomyces ghanaensis B38.3. Indian J. Microbiol. 2019;59:109–111. doi: 10.1007/s12088-018-0761-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ostash B., Makitrinskyy R., Walker S., Fedorenko V. Identification and characterization of Streptomyces ghanaensis ATCC14672 integration sites for three actinophage-based plasmids. Plasmid. 2009;61:171–175. doi: 10.1016/j.plasmid.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Makitrynskyy R., Tsypik O., Bechthold A. Genetic engineering of Streptomyces ghanaensis ATCC14672 for improved production of moenomycins. Microorganisms. 2021;10 doi: 10.3390/microorganisms10010030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Qiao Y., Srisuknimit V., Rubino F., Schaefer K., Ruiz N., Walker S., Kahne D. Lipid II overproduction allows direct assay of transpeptidase inhibition by β-lactams. Nat. Chem. Biol. 2017;13:793–798. doi: 10.1038/nchembio.2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Morel C., Lindahl O., Harbarth S., de Kraker M., Edwards S., Hollis A. Industry incentives and antibiotic resistance: an introduction to the antibiotic susceptibility bonus. J. Antibiot. 2020;73(7):421–428. doi: 10.1038/s41429-020-0300-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.de Kraker M., Stewardson A., Harbarth S. Will 10 million people die a year due to antimicrobial resistance by 2050? PLoS Med. 2016;13 doi: 10.1371/journal.pmed.1002184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.de la Cruz M., González I., Parish C., Onishi R., Tormo J., Martín J., Peláez F., Zink D., El Aouad N., Reyes F., Genilloud O., Vicente F. Production of ramoplanin and ramoplanin analogs by actinomycetes. Front. Microbiol. 2017;8:343. doi: 10.3389/fmicb.2017.00343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lichstrahl M., Townsend C., Sinner E. Stereochemical course of cobalamin-dependent radical SAM methylation by TokK and ThnK. RSC Chem. Biol. 2022;3:1028–1034. doi: 10.1039/d2cb00113f. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.