Highlight
-
•
C11orf21 is a novel transcriptional target of RUNX1.
-
•
The expression level of C11orf21 is reduced among AML cases of t(8;21).
-
•
RUNX1-ETO dominantly down-regulates C11orf21 gene expression.
-
•
Re-analysis using public domain database for patient samples is a useful approach to reveal transcriptional targets.
Keywords: RUNX1, RUNX1-ETO, t(8:21) AML, RNA-seq, C11orf21
Abstract
The fusion protein RUNX1-ETO is an oncogenic transcription factor generated by t(8;21) chromosome translocation, which is found in FAB-M2-type acute myeloid leukemia (AML). RUNX1-ETO is known to dysregulate the normal RUNX1 transcriptional network, which should involve essential factors for the onset of AML with t(8;21). In this study, we screened for possible transcriptional targets of RUNX1 by reanalysis of public data in silico, and identified C11orf21 as a novel RUNX1 target gene because its expression was down-regulated in the presence of RUNX1-ETO. The expression level of C11orf21 was low in AML patient samples with t(8;21) and in Kasumi-1 cells, which carry RUNX1-ETO. Knockdown of RUNX1-ETO in Kasumi-1 cells restored C11orf21 expression, whereas overexpression of RUNX1 up-regulated C11orf21 expression. In addition, knockdown of RUNX1 in other human leukemia cells without RUNX-ETO, such as K562, led to a decrease in C11orf21 expression. Of note, the C11orf21 promoter sequence contains a consensus sequence for RUNX1 binding and it was activated by exogenously expressed RUNX1 based on our luciferase reporter assay. This luciferase signal was trans-dominantly suppressed by RUNX1-ETO and site-directed mutagenesis of the consensus site abrogated the reporter activity. This study demonstrated that C11orf21 is a novel transcriptional target of RUNX1 and RUNX1-ETO suppressed C11orf21 transcription in t(8;21) AML. Thus, through this in silico approach, we identified a novel transcriptional target of RUNX1, and the depletion of C11orf21, the target gene, may be associated with the onset of t(8;21) AML.
1. Introduction
The Runt-related transcription factor 1 (RUNX1) gene was initially identified from the breakpoint on chromosome 21 [1]. RUNX1 is known as an essential transcription factor for definitive hematopoiesis by regulating the expression of target genes [2,3]. The core binding factor (CBF) is a heterodimeric transcription factor composed of RUNX1 and CBFβ, which binds to the consensus sequence 5′-TGT/cGGT-3′ via the Runt domain at the N-terminus [4,5]. The CBF is a frequent target of chromosomal abnormalities in human leukemia [6,7]. De novo acute myeloid leukemia (AML) with chromosomal abnormalities affecting either of the CBF subunits is termed CBF leukemia. In addition, mutations in the RUNX1 gene are associated with many hematological diseases such as myelodysplastic syndrome (MDS) and AML [8], [9], [10], [11].
The chromosome translocation between the long arms of chromosomes 8 and 21 in AML was originally reported by Rowley et al., in 1973 [12]. This specific translocation t(8;21) involves the RUNX1 gene on chromosome 21 and ETO gene on chromosome 8, thus generating a RUNX1-ETO fusion protein [13]. The RUNX1-ETO fusion gene resulting from the t(8;21)(q22;q22) translocation is frequently found in AML with the FAB-M2 subtype [14,15]. t(8;21) AML is usually associated with a relatively good prognosis; however, no specific therapy for t(8;21) AML has been established [16,17]. Through recent studies, a number of specific molecular target therapies have been developed such as imatinib for t(9;22) chronic myeloid leukemia and all-trans retinoic acid for t(15;17) acute promyelocytic leukemia [18,19]. The RUNX1-ETO fusion gene is known as an oncogenic transcription factor that drives t(8;21) AML and RUNX1-ETO is an attractive therapeutic target [20], but no molecular target therapies against RUNX1-ETO have yet been developed because the role of RUNX1-ETO in leukemogenesis has not been fully elucidated.
RUNX1-ETO trans-dominantly inhibits the CBF complex that transcriptionally regulates many hematopoiesis-related genes [21], [22], [23]. Recent analyses of the Kasumi-1 leukemia cells that carry t(8;21) using RNA sequencing revealed that RUNX1-ETO controls the expression of important regulators of hematopoietic differentiation and self-renewal [24,25]. Furthermore, depletion of RUNX1-ETO from the Kasumi-1 cells leads to the inhibition of cellular proliferation and self-renewal, in addition to the induction of cellular differentiation [24,25]. The raw RNA sequencing data were deposited in a public domain and are open for secondary analysis.
In this study, we searched for novel RUNX1 target genes by reanalyzing data in the public domain and found the C11orf21 gene, which is localized on chromosome 11p15.5, near the Beckwith-Wiedemann syndrome region [26], as a strong candidate. Two previous reports have observed the decreased gene expression of C11orf21 in AML patients with t(8;21). In 2006, Dunne J et al. studied the effect of siRNA-mediated RUNX1-ETO depletion in the Kasumi-1 leukemia cell line and primary AML blasts using cDNA arrays, oligonucleotide arrays, and RT-PCR [27]. In 2015, Hsu CH et al. reported differentially expressed genes, including C11orf21, in pediatric AML patients with t(8;21) using RNA-seq data [28]. However, neither report clarified how transcriptional regulation of RUNX1 and RUNX1-ETO is involved in the alteration of C11orf21 expression. In addition, RNA-seq analysis was performed only for pediatric cases, and the gene expression status of this gene in adults remains to be clarified. In the current study, through the analysis of multiple public datasets, we report that the gene expression of C11orf21 was decreased in AML patients with t(8;21) regardless of their age and that RUNX1 and RUNX1-ETO function through their interaction with the C11orf21 promoter region. In addition, a combination of experiments revealed that this gene fulfills all the conditions to be a transcriptional target of RUNX1.
2. Materials and methods
2.1. Analysis of published datasets
The RNA-seq dataset (GSE60131, Ptasinska, et al., 2014) was obtained from the Gene Expression Omnibus (GEO) [24]. Analysis was performed using TopHat2 [29] and Cufflinks [30,31]. The RNA-seq reads were mapped to the hg19 human genome using TopHat2. Fragments Per Kilobase of transcript Per Million mapped reads (FPKM) values for each gene were measured and differentially expressed genes were extracted using Cufflinks [32]. Another dataset was obtained from The Cancer Genome Atlas Research Network (Acute Myeloid Leukemia, TCGA, NEJM2013) and The Beat AML program (Acute Myeloid Leukemia, OHSU, Nature 2018) using the cBioPortal for Cancer Genomics software [11,[33], [34], [35]]. ChIP-seq data (GSM722708 [25], GSM2026066 [36], GSM1595964 [37], GSM1113437 [38], GSM850824 [25], GSM2743156, GSM2743157, GSM2743158, GSM2743159 [39], GSM2026053 [36], GSM1534442 [40], GSM1082306 [41], and GSE34540 [25]) were collected from ChIP-Atlas and binding peaks were detected via Integrative Genomics Viewer (IGV) [42], [43], [44].
2.2. Cell culture
K562 cells were grown in Roswell Park Memorial Institute (RPMI) 1640 medium supplemented with 10% fetal bovine serum (FBS). Kasumi-1 cells were cultured in RPMI 1640 supplemented with 10% FBS and 2.5 μg/ml of Amphotericin B. Cells were maintained at 37 °C in humidified air with 5% CO2.
2.3. Plasmid construction
Expression plasmids carrying mouse Runx1, Runx2, and Runx3 cDNA or mouse CbfB cDNA in pRc/CMV were previously described [45]. RUNX1-ETO cDNA was subcloned into pcDNA3.1(-) at the KpnI site. C11orf21 cDNA in pcDNA3.1-C-(k)DYK (OHu07593) was purchased from GenScript (Piscataway, NJ)
The C11orf21 promoter fragment containing a RUNX1-binding site was cloned from Human male genomic DNA (Promega, Madison, WI) by PCR using the following primers (Forward: GGGGTACCGGAGGCTGCCGCCAGGTGGGGTCTGCG, Reverse: CCGGTACCGTCTTCCTGGGAAGGGCCTCAGATGTC). The amplified fragments were then inserted into the pGL3-Basic vector (Promega), which was named pGL3-C11orf21. The C11orf21 promoter fragment was constructed by insertion into the pGL3 vector through KpnI restriction enzyme digestion sites. We introduced a site-directed mutation into the RUNX1-binding sequence in pGL3-C11orf21 so as to have (TGTGGT)-to-(cGTtaT)-mutation, which was named pGL3-C11orf21-mt. To introduce a site-directed mutation, we used PCR-based methods (Takara Bio, Shiga, Japan) and primers (Forward: TCCCCGTTATTTCCAGCTGGGCAGGGGC, Reverse: TGGAAATAACGGGGAGGGGAAGGGAGGGG).
2.4. Small interfering RNA (siRNA)s and transfection
For small interfering RNA (siRNA) and plasmid DNA transfection, 1.0 × 106 Kasumi-1 or K562 cells were transfected with siRNA or DNA plasmids by electroporation using Nucleofector 2b (Lonza, Basel, Switzerland) according to the manufacturer's protocol (program T-016). RUNX1 siRNA (HSS141474) and the negative control were purchased from Invitrogen (Carlsbad, CA). RUNX1-ETO siRNA was synthesized by Eurofins (Tokyo, Japan) with the following sequences:
RUNX1-ETO siRNA No.1 (Sense: CCUCGAAAUCGUACUGAGAAG, Antisense: UCUCAGUACGAUUUCGAGGUU), RUNX1-ETO siRNA No.2 (Sense:CGAGAACCUCGAAAUCGUACU, Antisense: UACGAUUUCGAGGUUCUCGGG)
For RUNX1-ETO knock-down, RUNX1 siRNA (HSS141474) was also used as RUNX1-ETO siRNA No.3.
2.5. Cell cycle profiling analysis
C11orf21 cDNA in pcDNA3.1-C-(k)DYK was transfected into Kasumi-1 cells via electroporation using Nucleofector 2b (Lonza, Basel, Switzerland) in the program L-014. Twenty-four hours after transfection, cells were harvested and cell numbers were counted using a Bürker-Turk counting chamber. Harvested cells were suspended in PBS with 0.1% Triton X-100 and 10 µg/ml of propidium iodide (PI) and subjected to cell cycle profiling using BD FACS Canto II and BD FACS Diva software (BD Biosciences, Franklin Lakes, NJ).
2.6. RNA preparation and quantitative Real-Time PCR
RNA was extracted using Isogen (Nippon Gene, Tokyo, Japan) and reverse-transcribed into cDNA using SuperScript IV reverse transcriptase (Thermo Fisher Scientific, Waltham, MA). RT-qPCR analysis was performed on a StepOnePlus Real-Time PCR System (Thermo Fisher Scientific) with the Thunderbird SYBR qPCR mix (Toyobo, Osaka, Japan) according to the manufacturer's instructions.
2.7. Luciferase reporter assay
K562 or Kasumi-1 cells were transfected with a luciferase reporter plasmid, a control Renilla luciferase plasmid, and different combinations of transcription factor expression plasmids by electroporation (Lonza) according to the manufacturer's protocol. Luciferase assays were performed using a dual-luciferase reporter assay system (Promega) and a luminometer (Berthold Detection Systems, Pforzheim, Germany). Obtained data were normalized by Renilla luciferase activity.
2.8. Statistical analysis
The data were statistically analyzed by a two-tailed, paired Student's t-test for Figs. 1A, 2,4, 5B, 6B, and 7B, and by one-way ANOVA and Tukey HSD for Figs. 5A, 6A, and C. When p < 0.05, the difference was considered significant.
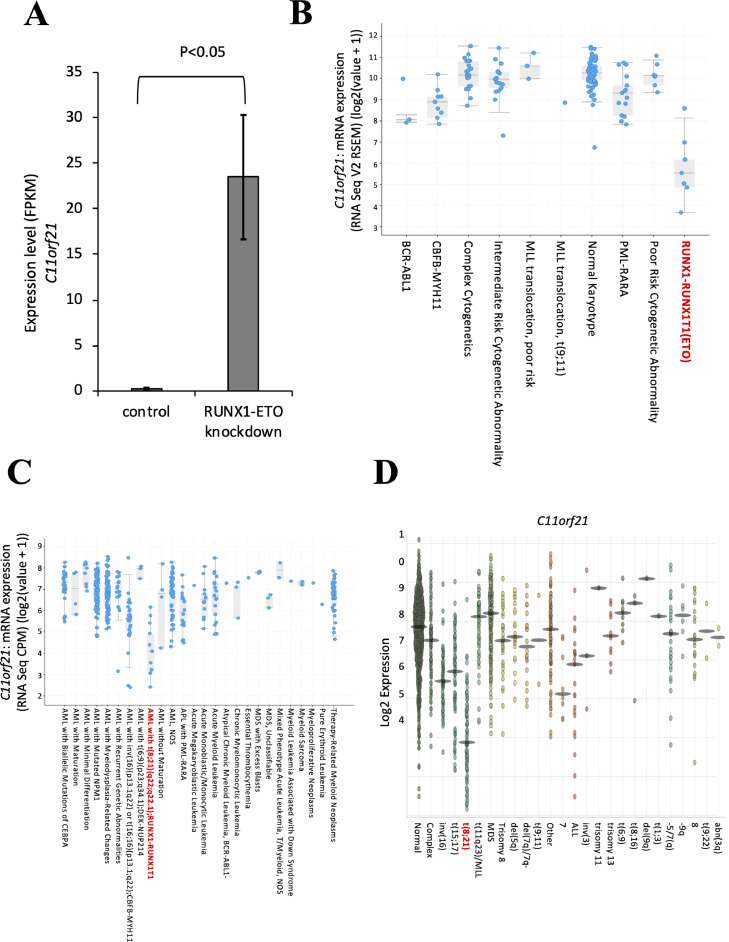
Fig. 1.
Differential gene expression analysis of published datasets revealed that C11orf21 expression is significantly suppressed in t(8;21) cells.
(A) The expression of C11orf21 was measured by RNA-seq in Kasumi-1 cells after treatment with RUNX1-ETO siRNA. We used published RNA-seq data from GSE60131.
(B, C) Reanalysis of C11orf21 expression in AML with t(8;21) and other karyotypes. Data sets were obtained from The Cancer Genome Atlas Research Network (TCGA) (B) and the Beat AML program (C). (D) Data were obtained from BloodSpot analysis using data sets GSE42519 for human normal hematopoiesis cells, and GSE13159, GSE15434, GSE61804, GSE14468, and TCGA for human AML cells.
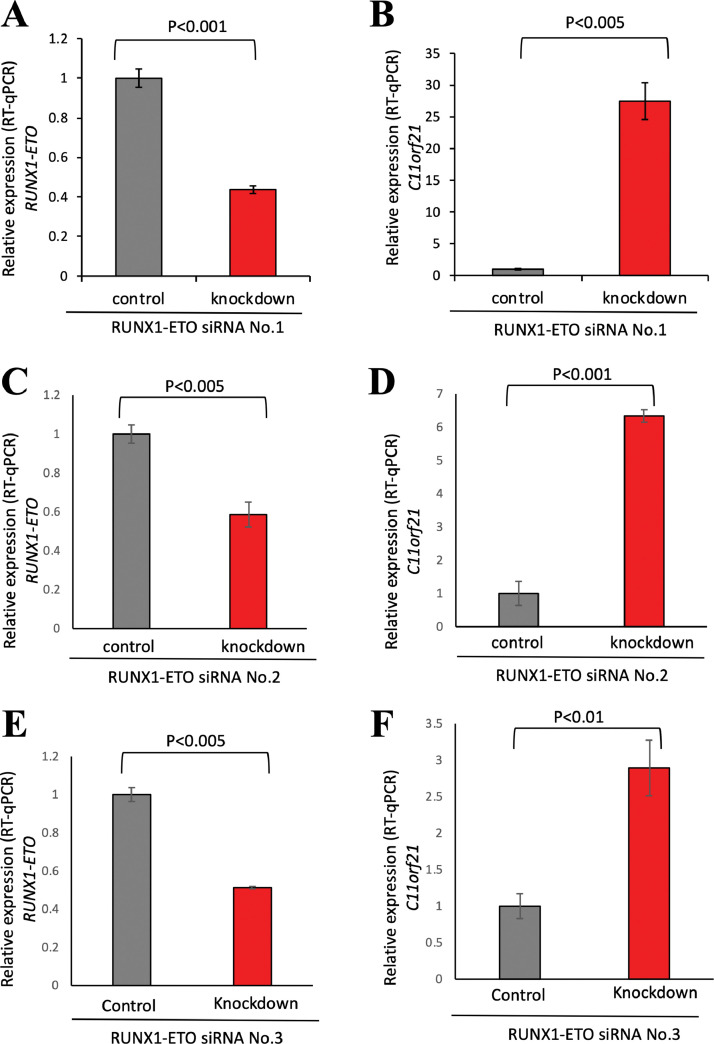
Fig. 2.
Knockdown of RUNX1-ETO in Kasumi-1 cells led to increased C11orf21 expression.
In Kasumi-1 cells, RUNX1-ETO was knocked-down by siRNA, and RT-qPCR was performed for RUNX1-ETO, C11orf21 and Actin. Relative expression of RUNX1-ETO (A) and C11orf21 (B) is shown as bar graphs. RUNX1-ETO knocked-down results performed with other siRNAs, RUNX1-ETO siRNA No.2 (C and D) and RUNX1-ETO siRNA No.3 (E and F) are indicated. Data were normalized by expression levels of Actin. Data represent the means of triplicate experiments (bars, S.D).
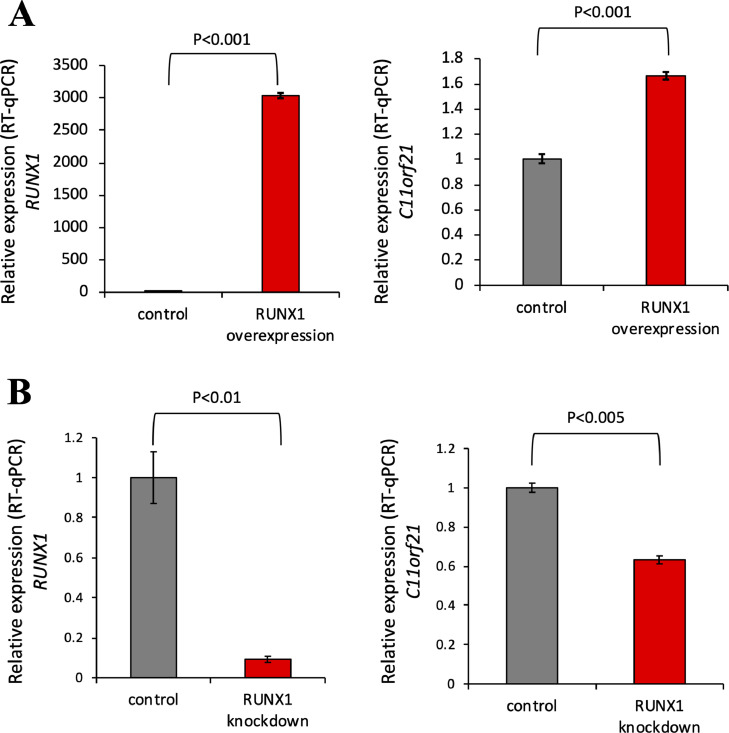
Fig. 4.
C11orf21 expression was upregulated by RUNX1 overexpression.
(A) Plasmid DNAs for RUNX1 and CBFβ were co-transfected into K562 cells, and C11orf21 (right panel) and RUNX1 (left panel) expression was measured by RT-qPCR. RUNX1 overexpression upregulated C11orf21 expression. (B) K562 cells were transfected with siRNA specific for RUNX1 by electroporation, and C11orf21 (right panel) and RUNX1 (left panel) expression was measured by RT-qPCR. The data are the means of triplicate determinations +/- S.D.
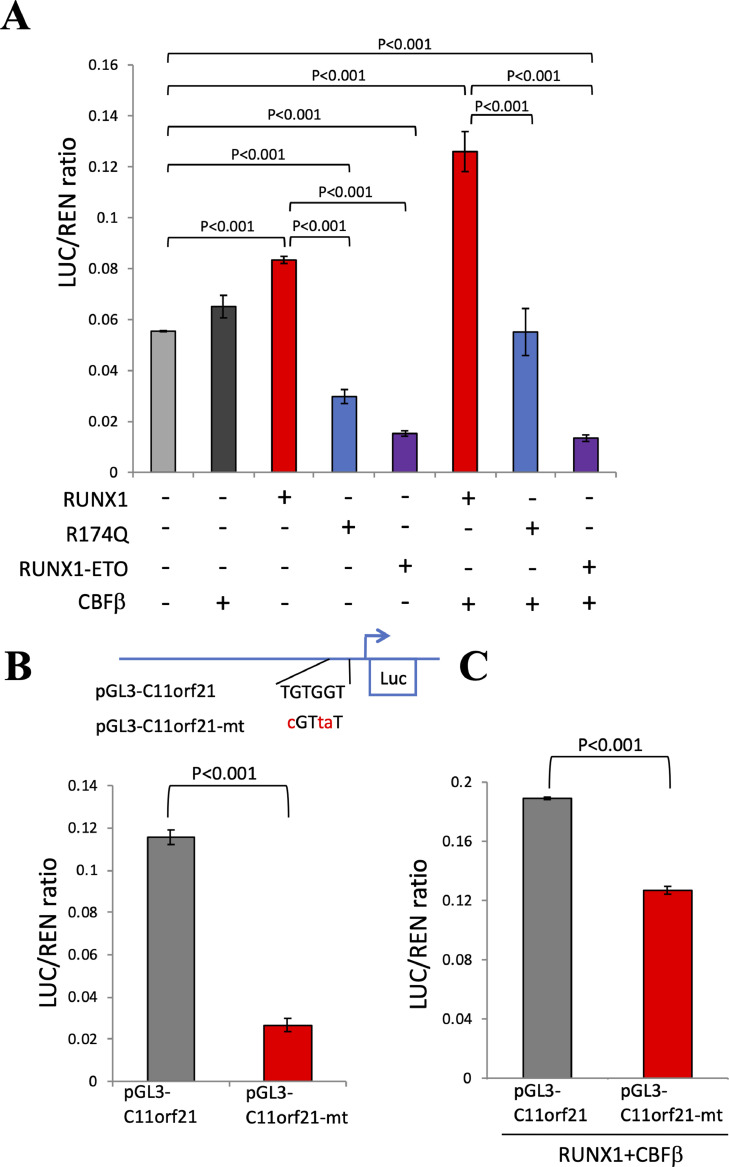
Fig. 5.
RUNX1 transactivated the C11orf21 promoter through binding to the RUNX1-binding site.
(A) pGL3-C11orf21 was transfected into K562 cells with control plasmid or RUNX1, RUNX1 R174Q-mutant, or RUNX1-ETO, with or without CBFβ expression plasmids. The result of the luciferase assay is shown as a bar graph. R174Q and RUNX1-ETO reduced C11orf21 promoter activity regardless of CBFβ status.
(B) pGL3-C11orf21 and pGL3-C11orf21-mt were transfected into K562 cells. The result of the luciferase assay is shown as a bar graph. When the RUNX1-binding site was mutated (-mt), the promoter activity of pGL3-C11orf21 was attenuated. (C) pGL3-C11orf21 and pGL3-C11orf21-mt were transfected into K562 cells with control plasmid or RUNX1 in the presence of the CBFβ expression vector. The results of the luciferase assay are shown as a bar graph. Mutation of the RUNX1-binding site reduced the response for RUNX1. The data represent the mean of triplicate determinations +/- S.D.
Fig. 6.
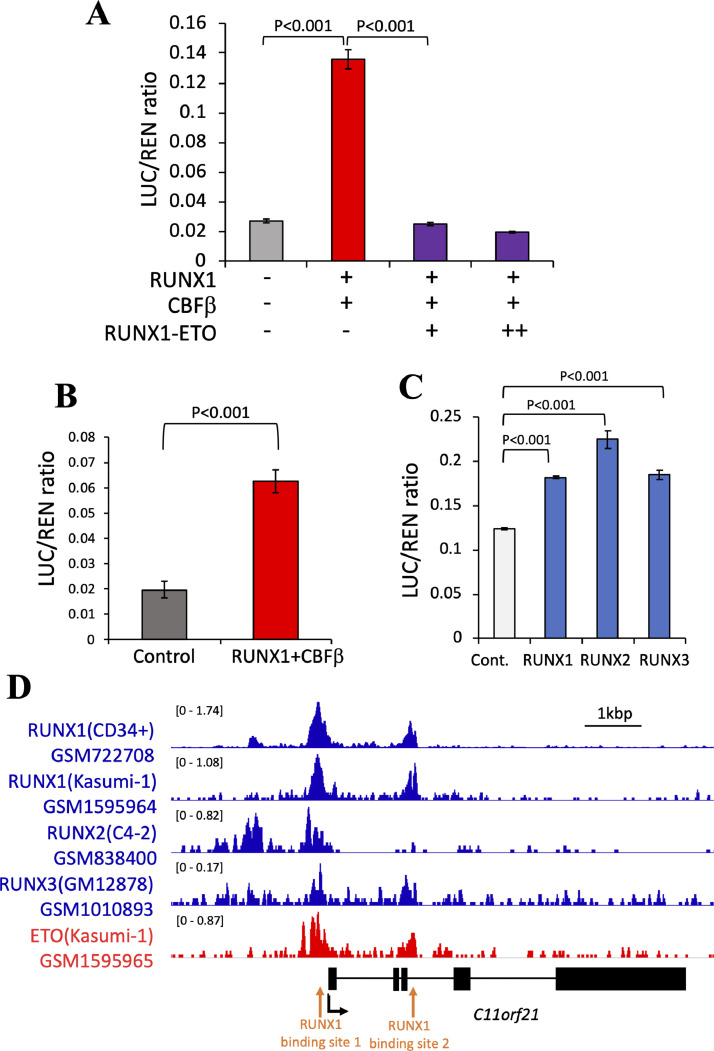
RUNX1 transactivation of C11orf21 was suppressed by RUNX1-ETO.
(A) RUNX1-ETO suppressed RUNX1 transactivation of C11orf21 in a dose-dependent manner. Fixed doses of pGL3-C11orf21, pRc/CMV-RUNX1 and pRc/CMV-CBFβ were co-transfected into K562 cells with increasing doses of RUNX1-ETO. (B) pGL3-C11orf21 was transfected into Kasumi-1 cells with or without RUNX1 and CBFβ expression plasmids. RUNX1 expression restored the transcription activity of the C11orf21 promoter. (C) RUNX2, RUNX3, and RUNX1 were competent to activate the C11orf21 promoter in the luciferase assay experiments. The data are the mean +/- S.D (n = 3). (D) Chromatin immunoprecipitation (ChIP) data. RUNX1, RUNX2, RUNX3, and ETO binding peaks detected on the C11orf21 promoter region are shown. Data were reanalyzed by ChIP-Atlas. The structure of the C11orf21 gene is shown below the histograms of ChIP data. Black boxes indicate exons. Black arrow shows the transcription start site and red arrows show RUNX1-binding sites with TGTGGT sequences.
Fig. 7.
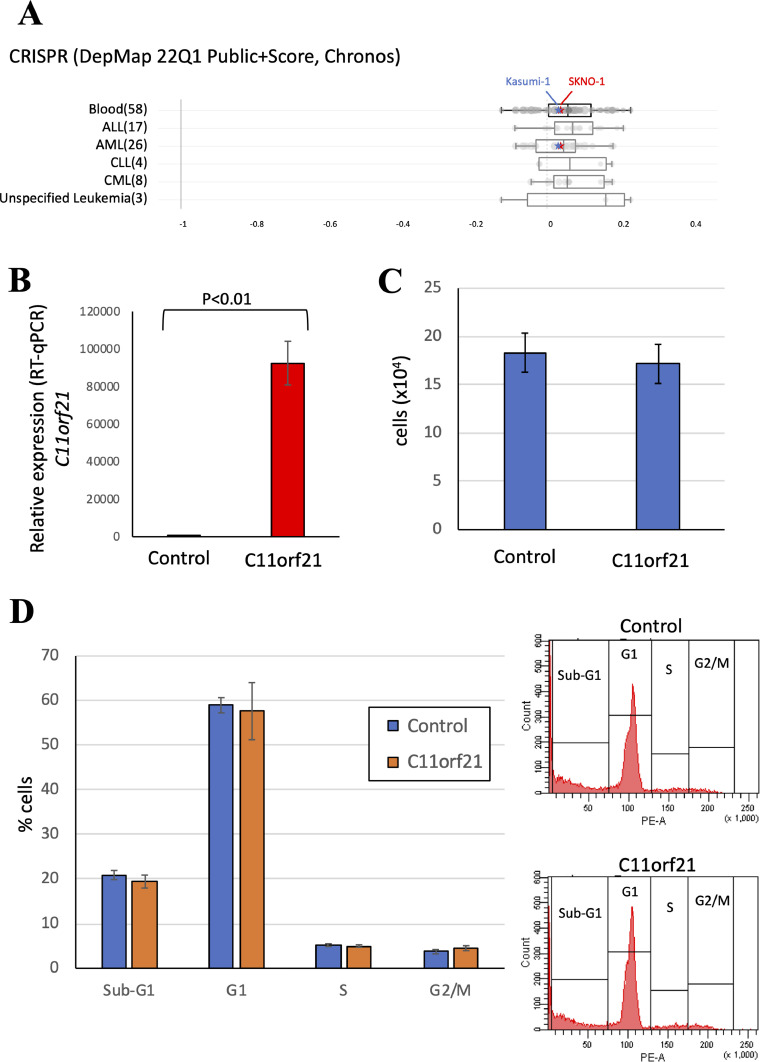
The effects of C11orf21 expression on Kasumi-1 cells.
(A) The CRISPR/Cas9-mediated gene knockout screens data were analyzed using The Cancer Dependency Map (DepMap). Knockout of C11orf21 did not affect the proliferation of t(8;21) AML cell lines such as Kasumi-1 (indicated by blue stars) or SKNO-1 (red stars). Stars were drawn by tracing the original data. (B, C and D) C11orf21 was overexpressed in Kasumi-1 cells. After 24 h of transfection, RT-qPCR for C11orf21 (B), cell counting (C) and cell cycle analysis by PI staining (D) were performed.
3. Results
3.1. C11orf21 expression levels were significantly low in t(8;21) AML cells
To identify significant changes in gene expression pattern in the presence of RUNX1-ETO, we performed differential expression analysis of RNA-seq data of the Kasumi-1 leukemia cell line with and without siRNA specific for RUNX1-ETO that were deposited in the public domain (GSE60131) using the open-source bioinformatics tools TopHat2 and Cufflinks [24,25]. Through this approach, we found that C11orf21 expression levels in the Kasumi-1 cells increased when RUNX1-ETO was knocked-down (Fig. 1A). In order to further analyze the relationship between RUNX1-ETO and C11orf21 expression levels, we next searched for samples from AML patients with t(8;21) and compared the results with those of samples of AML patients with other karyotypes by reanalyzing the data deposited in The Cancer Genome Atlas (Acute Myeloid Leukemia, TCGA, NEJM2013) (Fig. 1B) and the Beat AML program (Acute Myeloid Leukemia, OHSU, Nature 2018) (Fig. 1C) using cBioPortal for Cancer Genomics software [11,[33], [34], [35]]. Through these analyses of the open-resource data in the public domain, we noted significantly lower C11orf21 expression levels in t(8;21) AML patient samples than in samples from patients with other karyotypes (p < 0.001). In addition, BloodSpot analysis (www.bloodspot.eu/) using the data sets (GSE42519 [46], GSE13159 [47], GSE15434 [48], GSE61804 [49], GSE14468 [50], and TCGA [11]) demonstrated that C11orf21 was significantly down-regulated in t(8;21) AML patient samples compared with human normal hematopoietic cells and other human AML samples (Fig. 1D) [51]. Taken together, C11orf21 expression was suppressed in t(8;21) AML patient samples and the Kasumi-1 cell line, which was associated with the presence of RUNX1-ETO.
3.2. Knockdown of RUNX1-ETO expression by RNA interference led to an increase in C11orf21 expression in Kasumi-1 cells
In order to confirm that the low expression levels of C11orf21 in t(8;21)-positive AML cells are caused by the RUNX1-ETO fusion protein, we performed knockdown of RUNX1-ETO by transfecting small interfering RNA (siRNA) into Kasumi-1 cells (Fig. 2A). The C11orf21 expression levels in Kasumi-1 treated with and without siRNA specific for RUNX1-ETO were measured by RT-qPCR. We confirmed that the depletion of RUNX1-ETO led to increased C11orf21 expression in Kasumi-1 cells (p < 0.005) (Fig. 2B) as observed in the GSE60131 data set described above. This observation was reproducible: down-regulation of C11orf21 by use of other siRNAs against RUNX1-ETO, RUNX1-ETO siRNA No.2 (Fig. 2C and D) or RUNX1-ETO siRNA No.3 (Fig. 2E and F), also resulted in an increase of C11orf21 expression of the cell. Thus, the expression of C11orf21 was down-regulated by RUNX1-ETO and this observation was reproducible.
3.3. The RUNX1-ETO fusion protein and RUNX1 both bind the C11orf21 promoter
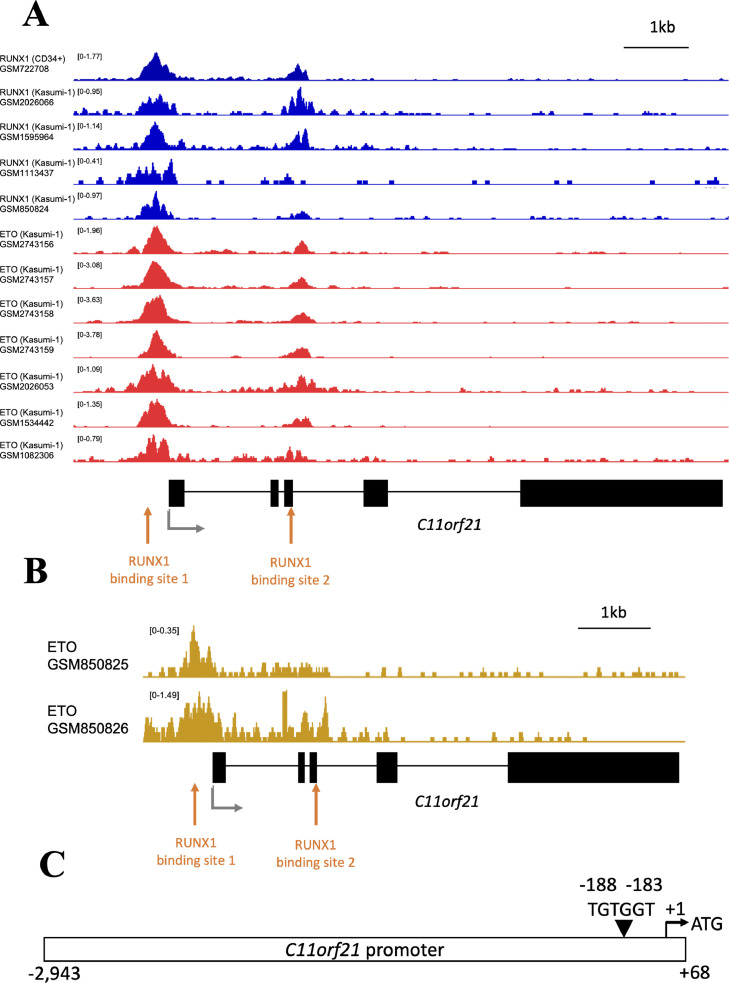
To determine the mechanism of down-regulation of C11orf21 by RUNX1-ETO, we searched for RUNX1-ETO binding on the promoter region of C11orf21 gene by examining ChIP-seq data (Fig. 3A). ChIP-seq data were obtained from ChIP-Atlas [42]. This investigation revealed that both RUNX1 and ETO binding peaks are confined to the same site on the C11orf21 promoter region where the RUNX1 binding consensus was localized in Kasumi-1 cells, suggesting that the RUNX1-ETO fusion protein bound to this site in Kasumi-1 cells (GSM2026066 [36], GSM1595964 [37], GSM1113437 [38], GSM850824 [25], GSM2743156 [39], GSM2743157 [39], GSM2743158 [39], GSM2743159 [39], GSM2026053 [36], GSM1534442 [40], and GSM1082306 [41]). In addition, a RUNX1 binding peak was detected at the same site in CD34+ hematopoietic progenitor cells (GSM722708 [25]), implying that this site serves as the RUNX1-binding site under physiological circumstances. As shown in Fig. 3B, the ChIP-seq data of RUNX1-ETO from primary t(8;21) AML cells were obtained from GSE34540 [25] containing GSM850825 and GSM850826 via ChIP-atlas. This also suggested that the RUNX1-ETO fusion protein definitely bound to the RUNX1-binding site in the C11orf21 promoter region. We cloned a human DNA fragment encompassing the upstream promoter region of the C11orf21 gene, sequenced it, and confirmed that the DNA fragment contained one RUNX1-binding consensus, TGTGGT, within the region (Fig. 3C). This suggested that RUNX1-ETO interfered with RUNX1 function by competitively binding to this RUNX1-binding site.
Fig. 3.
RUNX1-ETO directly bound the C11orf21 promoter region and down-regulated the transcription of C11orf21.
(A) Chromatin immunoprecipitation (ChIP) data. Both RUNX1 and ETO binding peaks were detected on the C11orf21 promoter region in Kasumi-1 cells, suggesting that RUNX1-ETO bound this region. RUNX1 also bound the C11orf21 promoter region in CD34+ cells. Data were obtained from ChIP-Atlas. The structure of the C11orf21 gene is shown below the histograms of ChIP data. Black boxes show exons. The transcription start site is indicated by a black arrow and RUNX1-binding sites including TGTGGT sequences are shown by red arrows. (B) Chromatin immunoprecipitation (ChIP) data of ETO for the primary t(8;21) AML cells. Data were obtained from GSE34540 containing GSM850825 and GSM850826 via ChIP-Atlas database. (C) The schematic diagram of C11orf21 promoter that we used for the experiments. The length of promoter sequence C11orf21 was 3011 bp. The triangle indicates the RUNX1 binding site (TGTGGT). “+1″ means the transcription start site and ATG was the translation start site (TSS).
3.4. C11orf21 expression was up-regulated by the overexpression of RUNX1 in human leukemia K562 cells
As mentioned above, both RUNX1-ETO and RUNX1 bound to the RUNX1-binding site on the C11orf21 promoter region according to the ChIP-seq data. We next investigated if wild-type RUNX1 functions in C11orf21 transcription. K562 cells were transfected with a RUNX1 expression vector, followed by RT-qPCR analysis. The overexpression of RUNX1 led to the increased expression of C11orf21 in K562 cells (Fig. 4A). In contrast, when we knocked-down RUNX1 by transfection with siRNA specific for RUNX1 into K562 cells, RUNX1 expression decreased and the mRNA level of C11orf21 decreased (Fig. 4B). Thus, RUNX1 is associated with C11orf21 transcription and this gene was up-regulated by RUNX1.
3.5. C11orf21 is a RUNX1 target gene whose expression is directly upregulated
As stated in the previous sections, there is one consensus site for possible RUNX1 binding, TGTGGT, in the C11orf21 promoter region. We investigated whether this site is important for the RUNX1-regulated C11orf21 expression. In order to assess this possibility, a C11orf21 promoter-reporter plasmid containing a RUNX1-binding site (pGL3-C11orf21) was constructed. Luciferase assay using this construct in K562 cells revealed that RUNX1 over-expression increases C11orf21 promoter activity, suggesting that this DNA fragment C11orf21 was regulated by RUNX1. In contrast, when K562 cells were treated with the RUNX1 R174Q-mutant (R174Q) or RUNX1-ETO expression vector, reduced C11orf21 promoter activity was observed (Fig. 5A). The R174Q mutant contains a point mutation in the runt domain, resulting in the loss of DNA binding activity and the trans-dominant suppression of wild-type RUNX1 function. RUNX1-ETO retains its DNA binding activity while interfering with RUNX1 function. Thus, the overexpression of R17Q or RUNX1-ETO reduced C11orf21 promoter activity.
The C11orf21 promoter region contains one RUNX1-binding site (TGTGGT). To analyze whether RUNX1 functions via binding to this site, we introduced mutations into the sequences: TGTGGT →cGTtaT, such that RUNX1 no longer binds to this site [52] (Fig. 5B), and performed a luciferase assay using this C11orf21 mutant promoter (pGL3-C11orf21-mt) in K562 cells. The promoter activity of the C11orf21 promoter mutant was significantly attenuated compared with that of the wild-type C11orf21 promoter construct when introduced into the K562 cells (Fig. 5B). In addition, the response to exogenous RUNX1 and CBFβ with pGL3-C11orf21-mt was much lower than that observed with wild-type pGL3-C11orf21 (Fig. 5C). This suggested that RUNX1 directly regulates C11orf21 expression through association with the binding site.
In these assays, we used the mouse RUNX1 and CBFβ expression plasmids. RUNX1 and CBFβ are highly homologous in humans and mice. Mouse RUNX1 and CBFβ can both regulate human transcription of RUNX1 target genes and are considered to be functionally interchangeable with those of humans, as ectopic human RUNX1 expression has been demonstrated to rescue the hematopoietic function lost in RUNX1-deficient mouse cells [53,54].
3.6. The RUNX1-ETO fusion protein interfered with normal RUNX1 function in C11orf21 transcription
In order to examine if the RUNX1-ETO fusion protein suppresses the transactivation of C11orf21, we carried out the following luciferase assay experiments: When wild-type RUNX1 and CBFβ were co-expressed in K562 cells, the pGL3-C11orf21 reporter construct was activated based on the high reporter signal (Red bar, in Fig. 6A). By addition of the RUNX1-ETO expression vector to the experiment system, this promoter activity was canceled in the K562 cells (Purple bars, Fig. 6A). Thus, RUNX1 increased C11orf21 promoter activity and RUNX1-ETO suppressed this transcriptional activation in a dose-dependent manner. As both RUNX1 and RUNX1-ETO bound to the same RUNX1-binding site in the C11orf21 promoter region, RUNX1-ETO was considered to have interfered with the normal transcription of RUNX1 by competing for binding to this site.
We next analyzed this using Kasumi-1 cells that contain the RUNX1-ETO fusion gene as the result of t(8;21) translocation. As previously stated, C11orf21 expression is attenuated in this cell line. As shown in Fig. 6B, C11orf21 expression increased when RUNX1 and CBFβ were expressed in Kasumi-1 cells. Lastly, the effects of other RUNX family members on C11orf21 expression were analyzed because all three RUNX members, RUNX1, RUNX2, and RUNX3, possess the conserved runt domain which can bind to the consensus, and their functions complement each other [45]. As expected, C11orf21 expression was increased not only by RUNX1, but also by either RUNX2 or RUNX3 (Fig. 6C). Reanalysis of the ChIP-seq data available in the ChIP-Atlas database demonstrated that RUNX2, RUNX3, and RUNX1 bind to the upstream promoter region of the C11orf21 gene (Fig. 6D). This indicates that the C11orf21 gene is a common downstream target of the RUNX family through binding to the consensus sequence.
3.7. Effects of C11orf21 expression on Kasumi-1 cells
According to the DepMap analysis [55], CRISPR/Cas9-mediated knockout of C11orf21 did not significantly affect the proliferation and survival of Kasumi-1 and SKNO-1 cells (Fig. 7A). In addition, we performed cell counting and cell cycle analysis of Kasumi-1 cells with forced expression of exogenous C11orf21 (Fig. 7B). C11orf21 overexpression had no definite effect on cell proliferation or cell cycle properties, suggesting that neither apoptosis nor differentiation induction was caused by C11orf21 overexpression in Kasumi-1 cells (Fig. 7C and D).
4. Discussion
In this study, we screened RUNX1 target genes in silico by reanalyzing the open-access database of RNA sequencing on leukemia cells that carry the RUNX1-ETO fusion gene, and identified C11orf21 as a strong candidate based on its expression being repressed by RUNX1-ETO. In vitro experiments support this gene being a target of RUNX1: Exogenous expression of wild-type RUNX1 upregulated C11orf21 mRNA in Kasumi-1 cells and K562 cells, and promoter sequences of C11orf21 were activated by RUNX1 depending on DNA binding. Thus, our in silico approach was successful, and C11orf21 is likely a novel RUNX1-ETO and RUNX1 target gene.
C11orf21 is located on chromosome 11p15.5, and this region is associated with Beckwith-Wiedemann syndrome [26], which has certain increased risk of tumor development [56,57]. Although it is located in the imprinted domain of 11p15.5, this gene exhibits biallelic expression (non-imprinted) when analyzed in the fetal liver [26]. C11orf21 encodes a polypeptide of 132 amino acids of no known protein motif, the role of which has not yet been established. Considering its chromosomal location, this gene may also be related to oncogenesis or growth regulation, and a low expression level of C11orf21 leads to cell-cycle dysregulation, cell growth, and leukemogenesis [26,[56], [57], [58]]. However, the data set of CRISPR/Cas9-mediated gene knockout screens, DepMap, did not show the dependence of t(8;21) AML cell lines on C11orf21, and in our initial experiments, C11orf21 overexpression alone did not affect the proliferation or apoptosis of Kasumi-1 cells. As we could not rule out the possibility that C11orf21 cooperates with other RUNX1-ETO target genes for its function, further analyses are necessary to elucidate the role of C11orf21 in t(8;21) AML.
Among adult human tissues, C11orf21 expression was detected in bone marrow, spleen, lymph node, heart, appendix, and lung (https://www.ncbi.nlm.nih.gov/gene/?term=29125%5Buid%5D Gene ID: 29,125). In fetal tissues, the C11orf21 gene was expressed in the heart, liver, brain, kidney, and muscle. This tissue distribution pattern overlaps that of RUNX family genes (https://www.ncbi.nlm.nih.gov/gene/861; https://www.ncbi.nlm.nih.gov/gene/860; https://www.ncbi.nlm.nih.gov/gene/864). Homozygous loss of RUNX1 in murine embryos resulted in a lack of fetal liver hematopoiesis and embryos died around E12.5 [2,3]. The expression of this gene in a RUNX1-deficient fetus should be examined as the next step.
No ortholog for C11orf21 has been described for the mouse (https://www.genecards.org/cgi-bin/carddisp.pl?gene=C11orf21), making it difficult to explore physiological functions of this gene using standard mouse genetics. Mouse orthologs of important human genes for hematopoietic malignancies are sometimes missing such as TEL2/ETV7 (ETS variant transcription factor 7) [59] and SAMD9 [60] (sterile α motif domain 9). Overexpression of human TEL2 that has no mouse orthologue leads to myeloproliferative disorder in mouse [59], whereas deletion of SAMD9 is often observed in -7/7q- hematopoietic disorders [60]. Although no related gene has been identified for C11orf21 based on similarity of its DNA or peptide sequences, further investigation for functionally complementing molecules should be performed.
RUNX1 is a key regulator of hematopoiesis, and is frequently involved in the pathogenesis of acute myeloid leukemia (AML) and myelodysplastic syndrome (MDS) through gene abnormalities. Among the RUNX1 gene abnormalities, t(8;21)(q22;q22), which generates the RUNX1-ETO fusion protein, is one of the most common chromosomal translocations in AML [6]. Although RUNX1-ETO is known as an oncogenic fusion protein in AML, it has been reported that RUNX1-ETO alone cannot induce the onset of AML and that additional mutations are required for the development of AML. The RUNX1-ETO fusion protein dysregulates many genes that are generally regulated by RUNX1 by trans-dominantly competing with RUNX1 for the binding sequences, leading to the ectopic recruitment of transcriptional cofactors [22,23,61,62]. Thus, RUNX1-ETO alters the normal RUNX1 transcriptional network. The RUNX1 transcriptional network dysregulated by RUNX1-ETO may involve a gene whose abnormal expression is essential for the development of AML with t(8;21). We revealed that the normal RUNX1 transcription network contains C11orf21 and its expression was dysregulated by RUNX1-ETO, suggesting that decreased C11orf21 expression in the presence of RUNX1-ETO may contribute to leukemogenesis.
Declaration of Competing Interest
The authors declare no conflict of interest.
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This study was supported by JSPS KAKENHI Grant Numbers 15K09487, 16K09857, 16K10038, 17K09936, and 19K08846. It was also partly supported by the Shimizu Foundation for Immunology and Neuroscience Grant for 2016, the Children's Cancer Association of Japan Financial Support for 2016, the Research Institute for Production Development Grant for 2017, the Sapporo Bioscience Foundation Grant for 2019, the Hokuto Bioscience Foundation for 2020 and the All Japan Coffee Association for 2021.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.bbadva.2022.100047.
Appendix. Supplementary materials
Supplemental data 1: Raw data of luciferase assay experiments for Fig. 5A and C.
References
- 1.Miyoshi H., Shimizu K., Kozu T., Maseki N., Kaneko Y., Ohki M. t(8;21) breakpoints on chromosome 21 in acute myeloid leukemia are clustered within a limited region of a single gene, AML1. Proc. Natl. Acad. Sci. USA. 1991;88:10431. doi: 10.1073/PNAS.88.23.10431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Okuda T., van Deursen J., Hiebert S.W., Grosveld G., Downing J.R. AML1, the target of multiple chromosomal translocations in human leukemia, is essential for normal fetal liver hematopoiesis. Cell. 1996;84:321–330. doi: 10.1016/S0092-8674(00)80986-1. [DOI] [PubMed] [Google Scholar]
- 3.Wang Q., Stacy T., Binder M., Marín-Padilla M., Sharpe A.H., Speck N.A. Disruption of the Cbfa2 gene causes necrosis and hemorrhaging in the central nervous system and blocks definitive hematopoiesis. Proc. Natl. Acad. Sci. USA. 1996;93:3444–3449. doi: 10.1073/pnas.93.8.3444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ogawa E., Inuzuka M., Maruyama M., Satake M., Naito-Fujimoto M., Ito Y., Shigesada K. Molecular cloning and characterization of pebp2β, the heterodimeric partner of a novel drosophila runt-related dna binding protein pebp2α. Virology. 1993;194:314–331. doi: 10.1006/viro.1993.1262. [DOI] [PubMed] [Google Scholar]
- 5.Melnikova I.N., Crute B.E., Wang S., Speck N.A. Sequence specificity of the core-binding factor. J. Virol. 1993;67:2408–2411. doi: 10.1128/jvi.67.4.2408-2411.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Look A.T. Oncogenic transcription factors in the human acute leukemias. Science. 1997;278:1059–1064. doi: 10.1126/science.278.5340.1059. [DOI] [PubMed] [Google Scholar]
- 7.Speck N.A., Gilliland D.G. Core-binding factors in haematopoiesis and leukaemia. Nat. Rev. Cancer. 2002;2:502–513. doi: 10.1038/nrc840. [DOI] [PubMed] [Google Scholar]
- 8.Osato M., Asou N., Abdalla E., Hoshino K., Yamasaki H., Okubo T., Suzushima H., Takatsuki K., Kanno T., Shigesada K., Ito Y. Biallelic and heterozygous point mutations in the runt domain of the AML1/PEBP2αB gene associated with myeloblastic leukemias. Blood. 1999;93:1817–1824. doi: 10.1182/blood.v93.6.1817.406k36_1817_1824. [DOI] [PubMed] [Google Scholar]
- 9.Song W.J., Sullivan M.G., Legare R.D., Hutchings S., Tan X., Kufrin D., Ratajczak J., Resende I.C., Haworth C., Hock R., Loh M., Felix C., Roy D.C., Busque L., Kurnit D., Willman C., Gewirtz A.M., Speck N.A., Bushweller J.H., Li F.P., Gardiner K., Poncz M., Maris J.M., Gilliland D.G. Haploinsufficiency of CBFA2 causes familial thrombocytopenia with propensity to develop acute myelogenous leukaemia. Nat. Genet. 1999;23:166–175. doi: 10.1038/13793. [DOI] [PubMed] [Google Scholar]
- 10.Nakao M., Horiike S., Fukushima-Nakase Y., Nishimura M., Fujita Y., Taniwaki M., Okuda T. Novel loss-of-function mutations of the haematopoiesis-related transcription factor, acute myeloid leukaemia 1/runt-related transcription factor 1, detected in acute myeloblastic leukaemia and myelodysplastic syndrome. Br. J. Haematol. 2004;125:709–719. doi: 10.1111/j.1365-2141.2004.04966.x. [DOI] [PubMed] [Google Scholar]
- 11.Ley T.J., Miller C., Ding L., Raphael B.J., Mungall A.J., Robertson A.G., Hoadley K., Triche T.J., Laird P.W., Baty J.D., Fulton L.L., Fulton R., Heath S.E., Eley G. Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. N. Engl. J. Med. 2013;368:2059–2074. doi: 10.1056/nejmoa1301689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rowley J.D. Identification of a translocation with quinacrine fluorescence in a patient with acute leukemia. Ann. Genet. 1973;16:109–112. https://pubmed.ncbi.nlm.nih.gov/4125056/ (accessed July 24, 2021) [PubMed] [Google Scholar]
- 13.Miyoshi H., Kozu T., Shimizu K., Enomoto K., Maseki N., Kaneko Y., Kamada N., Ohki M. The t(8;21) translocation in acute myeloid leukemia results in production of an AML1-MTG8 fusion transcript. EMBO J. 1993;12:2715–2721. doi: 10.1002/j.1460-2075.1993.tb05933.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nucifora G., Rowley J.D. AML1 and the 8;21 and 3;21 translocations in acute and chronic myeloid leukemia. Blood. 1995;86:1–14. doi: 10.1182/blood.v86.1.1.bloodjournal8611. [DOI] [PubMed] [Google Scholar]
- 15.Rowe D., Cotterill S.J., Ross F.M., Bunyan D.J., Vickers S.J., Bryon J., McMullan D.J., Griffiths M.J., Reilly J.T., Vandenberghe E.A., Wilson G., Watmore A.E., Bown N.P. Cytogenetically cryptic AML1 - ETO and CBFβ - MYH11 gene rearrangements: incidence in 412 cases of acute myeloid leukaemia. Br. J. Haematol. 2000;111:1051–1056. doi: 10.1046/j.1365-2141.2000.02474.x. [DOI] [PubMed] [Google Scholar]
- 16.Mrózek K., Bloomfield C.D. Clinical significance of the most common chromosome translocations in adult acute myeloid leukemia. J. Natl. Cancer Inst. Monogr. 2008:52–57. doi: 10.1093/JNCIMONOGRAPHS/LGN003. [DOI] [PubMed] [Google Scholar]
- 17.Byrd J.C., Dodge R.K., Carroll A., Baer M.R., Edwards C., Stamberg J., Qumsiyeh M., Moore J.O., Mayer R.J., Davey F., Schiffer C.A., Bloomfield C.D. Patients with t(8;21)(q22;q22) and acute myeloid leukemia have superior failure-free and overall survival when repetitive cycles of high-dose cytarabine are administered. J. Clin. Oncol. 1999;17:3767–3775. doi: 10.1200/JCO.1999.17.12.3767. [DOI] [PubMed] [Google Scholar]
- 18.Druker B.J., Talpaz M., Resta D.J., Peng B., Buchdunger E., Ford J.M., Lydon N.B., Kantarjian H., Capdeville R., Ohno-Jones S., Sawyers C.L. Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N. Engl. J. Med. 2001;344:1031–1037. doi: 10.1056/NEJM200104053441401. [DOI] [PubMed] [Google Scholar]
- 19.Wang Z.Y., Chen Z. Acute promyelocytic leukemia: from highly fatal to highly curable. Blood. 2008;111:2505–2515. doi: 10.1182/blood-2007-07-102798. [DOI] [PubMed] [Google Scholar]
- 20.Licht J.D. AML1 and the AML1-ETO fusion protein in the pathogenesis of t(8;21) AML. Oncogene. 2001;20:5660–5679. doi: 10.1038/sj.onc.1204593. [DOI] [PubMed] [Google Scholar]
- 21.Meyers S., Lenny N., Hiebert S.W. The t(8;21) fusion protein interferes with AML-1B-dependent transcriptional activation. Mol. Cell. Biol. 1995;15:1974–1982. doi: 10.1128/mcb.15.4.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yergeau D.A., Hetherington C.J., Wang Q., Zhang P., Sharpe A.H., Binder M., Marín-Padilla M., Tenen D.G., Speck N.A., Zhang D.E. Embryonic lethality and impairment of haematopoiesis in mice heterozygous for an AML1-ETO fusion gene. Nat. Genet. 1997;15:303–306. doi: 10.1038/ng0397-303. [DOI] [PubMed] [Google Scholar]
- 23.Okuda T., Cai Z., Yang S., Lenny N., Lyu C.J., van Deursen J.M.A., Harada H., Downing J.R. Expression of a knocked-in AML1-ETO leukemia gene inhibits the establishment of normal definitive hematopoiesis and directly generates dysplastic hematopoietic progenitors. Blood. 1998;91:3134–3143. doi: 10.1182/blood.v91.9.3134.3134_3134_3143. [DOI] [PubMed] [Google Scholar]
- 24.Ptasinska A., Assi S.A., Martinez-Soria N., Imperato M.R., Piper J., Cauchy P., Pickin A., James S.R., Hoogenkamp M., Williamson D., Wu M., Tenen D.G., Ott S., Westhead D.R., Cockerill P.N., Heidenreich O., Bonifer C. Identification of a dynamic core transcriptional network in t(8;21) AML that regulates differentiation block and self-renewal. Cell Rep. 2014;8:1974–1988. doi: 10.1016/j.celrep.2014.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ptasinska A., Assi S.A., Mannari D., James S.R., Williamson D., Dunne J., Hoogenkamp M., Wu M., Care M., McNeill H., Cauchy P., Cullen M., Tooze R.M., Tenen D.G., Young B.D., Cockerill P.N., Westhead D.R., Heidenreich O., Bonifer C. Depletion of RUNX1/ETO in t(8;21) AML cells leads to genome-wide changes in chromatin structure and transcription factor binding. Leukemia. 2012;26:1829–1841. doi: 10.1038/leu.2012.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhu X., Higashimoto K., Soejima H., Yatsuki H., Sugihara H., Mukai T., Joh K. C11orf21, a novel gene within the Beckwith-Wiedemann syndrome region in human chromosome 11p15.5. Gene. 2000;256:311–317. doi: 10.1016/S0378-1119(00)00377-2. [DOI] [PubMed] [Google Scholar]
- 27.Dunne J., Cullmann C., Ritter M., Soria N.M., Drescher B., Debernardi S., Skoulakis S., Hartmann O., Krause M., Krauter J., Neubauer A., Young B.D., Heidenreich O. siRNA-mediated AML1/MTG8 depletion affects differentiation and proliferation-associated gene expression in t(8;21)-positive cell lines and primary AML blasts. Oncogene. 2006;25:6067–6078. doi: 10.1038/sj.onc.1209638. [DOI] [PubMed] [Google Scholar]
- 28.Hsu C.H., Nguyen C., Yan C., Ries R.E., Chen Q.R., Hu Y., Ostronoff F., Stirewalt D.L., Komatsoulis G., Levy S., Meerzaman D., Meshinchi S. Transcriptome profiling of pediatric core binding factor AML. PLoS One. 2015;10:1–18. doi: 10.1371/journal.pone.0138782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim D., Pertea G., Trapnell C., Pimentel H., Kelley R., Salzberg S.L. TopHat2: accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol. 2013;14 doi: 10.1186/gb-2013-14-4-r36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Trapnell C., Williams B.A., Pertea G., Mortazavi A., Kwan G., van Baren M.J., Salzberg S.L., Wold B.J., Pachter L. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat. Biotechnol. 2010;28:511–515. doi: 10.1038/nbt.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Trapnell C., Hendrickson D.G., Sauvageau M., Goff L., Rinn J.L., Pachter L. Differential analysis of gene regulation at transcript resolution with RNA-seq. Nat. Biotechnol. 2013;31:46–53. doi: 10.1038/nbt.2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Trapnell C., Roberts A., Goff L., Pertea G., Kim D., Kelley D.R., Pimentel H., Salzberg S.L., Rinn J.L., Pachter L. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat. Protoc. 2012;7:562–578. doi: 10.1038/nprot.2012.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cerami E., Gao J., Dogrusoz U., Gross B.E., Sumer S.O., Aksoy B.A., Jacobsen A., Byrne C.J., Heuer M.L., Larsson E., Antipin Y., Reva B., Goldberg A.P., Sander C., Schultz N. The cbio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2:401–404. doi: 10.1158/2159-8290.CD-12-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gao J., Aksoy B.A., Dogrusoz U., Dresdner G., Gross B., Sumer S.O., Sun Y., Jacobsen A., Sinha R., Larsson E., Cerami E., Sander C., Schultz N. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci. Signal. 2013;6 doi: 10.1126/scisignal.2004088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tyner J.W., Tognon C.E., Bottomly D., Wilmot B., Kurtz S.E., Savage S.L., Long N., Schultz A.R., Traer E., Abel M., Agarwal A., Blucher A., Borate U., Bryant J., Burke R., Carlos A., Carpenter R., Carroll J., Chang B.H., Coblentz C., d'Almeida A., Cook R., Danilov A., Dao K.H.T., Degnin M., Devine D., Dibb J., Edwards D.K., Eide C.A., English I., Glover J., Henson R., Ho H., Jemal A., Johnson K., Johnson R., Junio B., Kaempf A., Leonard J., Lin C., Liu S.Q., Lo P., Loriaux M.M., Luty S., Macey T., MacManiman J., Martinez J., Mori M., Nelson D., Nichols C., Peters J., Ramsdill J., Rofelty A., Schuff R., Searles R., Segerdell E., Smith R.L., Spurgeon S.E., Sweeney T., Thapa A., Visser C., Wagner J., Watanabe-Smith K., Werth K., Wolf J., White L., Yates A., Zhang H., Cogle C.R., Collins R.H., Connolly D.C., Deininger M.W., Drusbosky L., Hourigan C.S., Jordan C.T., Kropf P., Lin T.L., Martinez M.E., Medeiros B.C., Pallapati R.R., Pollyea D.A., Swords R.T., Watts J.M., Weir S.J., Wiest D.L., Winters R.M., McWeeney S.K., Druker B.J. Functional genomic landscape of acute myeloid leukaemia. Nature. 2018;562:526–531. doi: 10.1038/s41586-018-0623-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mandoli A., Singh A.A., Prange K.H.M., Tijchon E., Oerlemans M., Dirks R., ter Huurne M., Wierenga A.T.J., Janssen-Megens E.M., Berentsen K., Sharifi N., Kim B., Matarese F., Nguyen L.N., Hubner N.C., Rao N.A., van den Akker E., Altucci L., Vellenga E., Stunnenberg H.G., Martens J.H.A. The hematopoietic transcription factors RUNX1 and ERG prevent AML1-ETO oncogene overexpression and onset of the apoptosis program in t(8;21) AMLs. Cell Rep. 2016;17:2087–2100. doi: 10.1016/j.celrep.2016.08.082. [DOI] [PubMed] [Google Scholar]
- 37.Li Y., Wang H., Wang X., Jin W., Tan Y., Fang H., Chen S., Chen Z., Wang K. Genome-wide studies identify a novel interplay between AML1 and AML1/ETO in t(8;21) acute myeloid leukemia. Blood. 2016;127:233–242. doi: 10.1182/blood-2015-03-626671. [DOI] [PubMed] [Google Scholar]
- 38.Ben-Ami O., Friedman D., Leshkowitz D., Goldenberg D., Orlovsky K., Pencovich N., Lotem J., Tanay A., Groner Y. Addiction of t(8;21) and inv(16) acute myeloid leukemia to native RUNX1. Cell Rep. 2013;4:1131–1143. doi: 10.1016/j.celrep.2013.08.020. [DOI] [PubMed] [Google Scholar]
- 39.Loke J., Chin P.S., Keane P., Pickin A., Assi S.A., Ptasinska A., Imperato M.R., Cockerill P.N., Bonifer C. C/EBPα overrides epigenetic reprogramming by oncogenic transcription factors in acute myeloid leukemia. Blood Adv. 2018;2:271–284. doi: 10.1182/bloodadvances.2017012781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Trombly D.J., Whitfield T.W., Padmanabhan S., Gordon J.A.R., Lian J.B., van Wijnen A.J., Zaidi S.K., Stein J.L., Stein G.S. Genome-wide co-occupancy of AML1-ETO and N-CoR defines the t(8;21) AML signature in leukemic cells. BMC Genom. 2015;16 doi: 10.1186/s12864-015-1445-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sun X.J., Wang Z., Wang L., Jiang Y., Kost N., Soong T.D., Chen W.Y., Tang Z., Nakadai T., Elemento O., Fischle W., Melnick A., Patel D.J., Nimer S.D., Roeder R.G. A stable transcription factor complex nucleated by oligomeric AML1-ETO controls leukaemogenesis. Nature. 2013;500:93–97. doi: 10.1038/NATURE12287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Oki S., Ohta T., Shioi G., Hatanaka H., Ogasawara O., Okuda Y., Kawaji H., Nakaki R., Sese J., Meno C. ChIP-Atlas: a data-mining suite powered by full integration of public ChIP-seq data. EMBO Rep. 2018;19:e46255. doi: 10.15252/EMBR.201846255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Robinson J.T., Thorvaldsdóttir H., Winckler W., Guttman M., Lander E.S., Getz G., Mesirov J.P. Integrative genomics viewer. Nat. Biotechnol. 2011;29:24–26. doi: 10.1038/nbt.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thorvaldsdóttir H., Robinson J.T., Mesirov J.P. Integrative Genomics Viewer (IGV): high-performance genomics data visualization and exploration. Brief. Bioinformatics. 2013;14:178–192. doi: 10.1093/bib/bbs017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fukushima-Nakase Y., Naoe Y., Taniuchi I., Hosoi H., Sugimoto T., Okuda T. Shared and distinct roles mediated through C-terminal subdomains of acute myeloid leukemia/Runt-related transcription factor molecules in murine development. Blood. 2005;105:4298–4307. doi: 10.1182/blood-2004-08-3372. [DOI] [PubMed] [Google Scholar]
- 46.Rapin N., Bagger F.O., Jendholm J., Mora-Jensen H., Krogh A., Kohlmann A., Thiede C., Borregaard N., Bullinger L., Winther O., Theilgaard-Mönch K., Porse B.T. Comparing cancer vs normal gene expression profiles identifies new disease entities and common transcriptional programs in AML patients. Blood. 2014;123:894–904. doi: 10.1182/blood-2013-02-485771. [DOI] [PubMed] [Google Scholar]
- 47.Kohlmann A., Kipps T.J., Rassenti L.Z., Downing J.R., Shurtleff S.A., Mills K.I., Gilkes A.F., Hofmann W.K., Basso G., Dell'Orto M.C., Foà R., Chiaretti S., de Vos J., Rauhut S., Papenhausen P.R., Hernández J.M., Lumbreras E., Yeoh A.E., Koay E.S., Li R., Liu W.M., Williams P.M., Wieczorek L., Haferlach T. An international standardization programme towards the application of gene expression profiling in routine leukaemia diagnostics: the microarray innovations in leukemia study prephase. Br. J. Haematol. 2008;142:802–807. doi: 10.1111/j.1365-2141.2008.07261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Klein H.U., Ruckert C., Kohlmann A., Bullinger L., Thiede C., Haferlach T., Dugas M. Quantitative comparison of microarray experiments with published leukemia related gene expression signatures. BMC Bioinf. 2009;10 doi: 10.1186/1471-2105-10-422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Metzelder S.K., Michel C., von Bonin M., Rehberger M., Hessmann E., Inselmann S., Solovey M., Wang Y., Sohlbach K., Brendel C., Stiewe T., Charles J., ten Haaf A., Ellenrieder V., Neubauer A., Gattenlöhner S., Bornhäuser M., Burchert A. NFATc1 as a therapeutic target in FLT3-ITD-positive AML. Leukemia. 2015;29:1470–1477. doi: 10.1038/LEU.2015.95. [DOI] [PubMed] [Google Scholar]
- 50.Wouters B.J., Löwenberg B., Erpelinck-Verschueren C.A.J., van Putten W.L.J., Valk P.J.M., Delwel R. Double CEBPA mutations, but not single CEBPA mutations, define a subgroup of acute myeloid leukemia with a distinctive gene expression profile that is uniquely associated with a favorable outcome. Blood. 2009;113:3088–3091. doi: 10.1182/BLOOD-2008-09-179895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bagger F.O., Kinalis S., Rapin N. BloodSpot: a database of healthy and malignant haematopoiesis updated with purified and single cell mRNA sequencing profiles. Nucleic Acids Res. 2019;47:D881–D885. doi: 10.1093/NAR/GKY1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Satoh Y., Matsumura I., Tanaka H., Ezoe S., Fukushima K., Tokunaga M., Yasumi M., Shibayama H., Mizuki M., Era T., Okuda T., Kanakura Y. AML1/RUNX1 works as a negative regulator of c-Mpl in hematopoietic stem cells. J. Biol. Chem. 2008;283:30045–30056. doi: 10.1074/jbc.M804768200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ito Y. Oncogenic potential of the RUNX gene family: “overview. Oncogene. 2004;23:4198–4208. doi: 10.1038/sj.onc.1207755. [DOI] [PubMed] [Google Scholar]
- 54.Goyama S., Yamaguchi Y., Imai Y., Kawazu M., Nakagawa M., Asai T., Kumano K., Mitani K., Ogawa S., Chiba S., Kurokawa M., Hirai H. The transcriptionally active form of AML1 is required for hematopoietic rescue of the AML1-deficient embryonic para-aortic splanchnopleural (P-Sp) region. Blood. 2004;104:3558–3564. doi: 10.1182/blood-2004-04-1535. [DOI] [PubMed] [Google Scholar]
- 55.Tsherniak A., Vazquez F., Montgomery P.G., Weir B.A., Kryukov G., Cowley G.S., Gill S., Harrington W.F., Pantel S., Krill-Burger J.M., Meyers R.M., Ali L., Goodale A., Lee Y., Jiang G., Hsiao J., Gerath W.F.J., Howell S., Merkel E., Ghandi M., Garraway L.A., Root D.E., Golub T.R., Boehm J.S., Hahn W.C. Defining a cancer dependency map. Cell. 2017;170:564–576. doi: 10.1016/j.cell.2017.06.010. e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Koufos A., Grundy P., Morgan K., Aleck K.A., Hadro T., Lampkin B.C., Kalbakji A., Cavenee W.K. Familial Wiedemann-Beckwith syndrome and a second Wilms tumor locus both map to 11p15.5. Am. J. Hum. Genet. 1989;44:711–719. https://pubmed.ncbi.nlm.nih.gov/2539717/ (accessed July 24, 2021) [PMC free article] [PubMed] [Google Scholar]
- 57.Scelfo R.A.M., Schwienbacher C., Veronese A., Gramantieri L., Bolondi L., Querzoli P., Nenci I., Calin G.A., Angioni A., Barbanti-Brodano G., Negrini M. Loss of methylation at chromosome 11p15.5 is common in human adult tumors. Oncogene. 2002;21:2564–2572. doi: 10.1038/sj.onc.1205336. [DOI] [PubMed] [Google Scholar]
- 58.Berndt S.I., Skibola C.F., Joseph V., Camp N.J., Nieters A., Wang Z., Cozen W., Monnereau A., Wang S.S., Kelly R.S., Lan Q., Teras L.R., Chatterjee N., Chung C.C., Yeager M., Brooks-Wilson A.R., Hartge P., Purdue M.P., Birmann B.M., Armstrong B.K., Cocco P., Zhang Y., Severi G., Zeleniuch-Jacquotte A., Lawrence C., Burdette L., Yuenger J., Hutchinson A., Jacobs K.B., Call T.G., Shanafelt T.D., Novak A.J., Kay N.E., Liebow M., Wang A.H., Smedby K.E., Adami H.O., Melbye M., Glimelius B., Chang E.T., Glenn M., Curtin K., Cannon-Albright L.A., Jones B., Diver W.R., Link B.K., Weiner G.J., Conde L., Bracci P.M., Riby J., Holly E.A., Smith M.T., Jackson R.D., Tinker L.F., Benavente Y., Becker N., Boffetta P., Brennan P., Foretova L., Maynadie M., McKay J., Staines A., Rabe K.G., Achenbach S.J., Vachon C.M., Goldin L.R., Strom S.S., Lanasa M.C., Spector L.G., Leis J.F., Cunningham J.M., Weinberg J.B., Morrison V.A., Caporaso N.E., Norman A.D., Linet M.S., de Roos A.J., Morton L.M., Severson R.K., Riboli E., Vineis P., Kaaks R., Trichopoulos D., Masala G., Weiderpass E., Chirlaque M.D., Vermeulen R.C.H., Travis R.C., Giles G.G., Albanes D., Virtamo J., Weinstein S., Clavel J., Zheng T., Holford T.R., Offit K., Zelenetz A., Klein R.J., Spinelli J.J., Bertrand K.A., Laden F., Giovannucci E., Kraft P., Kricker A., Turner J., Vajdic C.M., Ennas M.G., Ferri G.M., Miligi L., Liang L., Sampson J., Crouch S., Park J.H., North K.E., Cox A., Snowden J.A., Wright J., Carracedo A., Lopez-Otin C., Bea S., Salaverria I., Martin-Garcia D., Campo E., Fraumeni J.F., de Sanjose S., Hjalgrim H., Cerhan J.R., Chanock S.J., Rothman N., Slager S.L. Genome-wide association study identifies multiple risk loci for chronic lymphocytic leukemia. Nat. Genet. 2013;45:868–876. doi: 10.1038/NG.2652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Carella C., Potter M., Bonten J., Rehg J.E., Neale G., Grosveld G.C. The ETS factor TEL2 is a hematopoietic oncoprotein. Blood. 2006;107:1124–1132. doi: 10.1182/blood-2005-03-1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Inaba T., Honda H., Matsui H. The enigma of monosomy 7. Blood. 2018;131:2891–2898. doi: 10.1182/blood-2017-12-822262. [DOI] [PubMed] [Google Scholar]
- 61.Higuchi M., O'Brien D., Kumaravelu P., Lenny N., Yeoh E.J., Downing J.R. Expression of a conditional AML1-ETO oncogene bypasses embryonic lethality and establishes a murine model of human t(8;21) acute myeloid leukemia. Cancer Cell. 2002;1:63–74. doi: 10.1016/S1535-6108(02)00016-8. [DOI] [PubMed] [Google Scholar]
- 62.Shima T., Miyamoto T., Kikushige Y., Yuda J., Tochigi T., Yoshimoto G., Kato K., Takenaka K., Iwasaki H., Mizuno S., Goto N., Akashi K. The ordered acquisition of Class II and Class I mutations directs formation of human t(8;21) acute myelogenous leukemia stem cell. Exp. Hematol. 2014;42:955–965. doi: 10.1016/j.exphem.2014.07.267. e5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental data 1: Raw data of luciferase assay experiments for Fig. 5A and C.