Highlights
-
•
Probucol is unable to completely inhibit cholesterol efflux in foam cells.
-
•
Functional nHDL is released from foam cells in the presence of probucol.
-
•
ABCA1 expression is the same in non-foam and foam cells, but with different efflux.
-
•
In probucol pretreated foam cells a new mechanism exists that is ABCA1-independent.
Keywords: Probucol, ABCA1, Apolipoprotein A-I, High-density lipoprotein, Cholesterol efflux, Cholesterol pool
Abstract
Objective
Probucol is a cholesterol-lowering agent whose ability to prevent atherosclerosis is currently under study. Herein, we investigate the putative mechanism of probucol by observation of changes in cellular cholesterol efflux and lipid droplet morphology in macrophages.
Results
The inhibitory activity of probucol was assessed in non-foam or foam cell macrophages expressing ABCA1 generated by treatment with fetal calf serum (FCS) alone or in combination with acetylated LDL, respectively. Probucol inhibited cholesterol efflux to apolipoprotein A-I (apoA-I) by 31.5±0.1% in THP-1 non-foam cells and by 18.5±0.2% in foam cells. In probucol-treated non-foam THP-1 cells, nascent high density lipoprotein (nHDL) particles with a diameter < 7 nm were generated, while in probucol-treated THP-1 foam cells nHDL particles of > 7 nm in diameter containing cholesterol were produced. Foam cells also displayed a significant accumulation of free cholesterol at the plasma membrane, as measured by percent cholestenone formed. Intracellularly, there was a significant decrease in lipid droplet number and an increase in size in probucol-treated THP-1 foam cells when compared to non-treated cells.
Conclusions
We report for the first time that probucol is unable to completely inhibit cholesterol efflux in foam cells to the same extent as in non-foam cells. Indeed, functional nHDL is released from foam cells in the presence of probucol. This difference in inhibitory effect could potentially be explained by changes in the plasma membrane pool as well as intracellular cholesterol storage independently of ABCA1.
1. Introduction
The current intensive low-density lipoprotein (LDL)-lowering therapy is designed to significantly attenuate the rate of progression of atherosclerotic vascular disease. Treatment typically involves statins and proprotein convertase subtilisin/kexin type 9 (PCSK9) antibodies such as evolocumab [1] and alirocumab [2] to improve liver clearance of LDL, as well as ezetimibe to inhibit cholesterol absorption in the intestine [3]. Despite these therapies, not all cardiovascular events are completely prevented [4]. Hence, additional effective pharmacological intervention to mitigate the observed residual risk of coronary events may be necessary in the future. Probucol is a unique diphenolic antilipidemic compound originally designed for the treatment of hypercholesterolemia [5]. This drug is a powerful nonpolar antioxidant which is believed to stabilize high-risk plaques [4,6]. Additionally, probucol appears to inhibit both the activity and degradation of ATP-binding cassette A1 (ABCA1) [7,8]. Strikingly, in vivo oxidation products of probucol (spiroquinone and diphenoquinone), stabilize ABCA1 by protecting it from calpain-mediated degradation [7]. Although many papers have described the effect of probucol, its mechanism of action has not yet been elucidated in detail [5,[9], [10], [11]]. Several well-accepted mechanisms of probucol-mediated lipid lowering are thought to occur via increasing LDL catabolism independent of the LDL receptor, exerting anti-inflammatory activities, and causing scavenger receptor suppression [4,12]. Although clinical trials were stopped [13], probucol is still being investigated for its effect on the inhibition of atherosclerosis initiation in vitro and in animal models [14], [15], [16], [17]. A paradox has surrounded the lipid lowering effect of probucol. Probucol both reduces high density lipoprotein cholesterol (HDL-C) as well as xanthomas and atheromatous vascular lesions [14,18]. Available evidence suggests that it may alleviate atherosclerosis and improve HDL function in vivo [16]. Notably, the pharmacological inhibition of ABCA1-mediated cholesterol efflux by probucol seems to be in contrast to its antilipidemic antiatherosclerotic activities [19]. Although the true effect of this drug and the mechanisms by which it influences efflux remain unclear [4,5,8,20,21], there is no doubt that treatment of cells with probucol inhibits cellular cholesterol efflux [8]. To date, the effect of probucol on cholesterol removal from foam cells is not well documented. We hypothesize that in foam cells probucol only partially inhibits efflux of cholesterol enhancing a mechanism that is independent of ABCA1 activity. In this study, we aim to clarify the mechanism of action of probucol in macrophage foam cells in mediating cellular cholesterol efflux and to explore regulation of the cellular cholesterol pool following probucol treatment. We demonstrate that probucol is unable to completely inhibit cholesterol efflux in foam cells to the same extent as in non-foam cells. We find that despite probucol treatment, foam cells generated partially lipidated small nascent HDL (nHDL) particles with a size greater than 7 nm in diameter, which were functional in transferring cholesterol to apolipoprotein B (apoB)-containing lipoproteins. We demonstrate that probucol treatment resulted in less accessible plasma membrane (PM) cholesterol as measured by their accessibility to oxidation by cholesterol oxidase, but that despite treatment with probucol, foam cells retained more accessible PM cholesterol compared to non-foam cells. This decrease in accessible PM cholesterol coincided with a significant reduction in lipid droplets (LD) number and an increase in their size. Taken together, these results demonstrate that cholesterol efflux persists in probucol treated foam cells versus non-foam by an ABCA1-independent mechanism.
2. Experimental procedures
2.1. Materials
Tamm-Horsfall protein 1 (THP-1) monocytes were purchased from American Type Tissue Collection (ATCC, Camden, NJ). Mouse-derived peritoneal macrophages J774 cells were purchased from (ATCC TIB-67, Cedarlane, Burlington, Ontario, Canada). Baby hamster kidney (BHK) cells were the generous gift from Drs. Oram and Vaughan (University of Washington, Seattle, WA). These cells were stably transfected with an ABCA1 cDNA gene insert that is inducible by treating the cells with mifepristone [22]. CHO-K1 cells (ATCC) stably transfected with hABCG1 were a gift from Dr. J Wendy Jessup (Centre for Vascular Research, School of Medical Sciences, University of New South Wales, Kensington, Australia) and conducted as described previously [23]. Cell culture media and phosphate-buffered saline (PBS) for washing were purchased from Wisent (Walkersville, MD); Fetal calf serum (FCS), bovine serum albumin (BSA), 22- hydroxycholesterol (22-OH), cis-9-retinoic acid (9cRA), 8-(4-Chlorophenylthio) adenosine 3’, 5’-cyclic monophosphate (cpt-cAMP), glutamine, gentamicin, sodium pyruvate, Cholesterol oxidase (streptomyces enzyme) and probucol were obtained from Sigma-Aldrich (St. Louis, MO, USA). Tissue culture flasks and plates were obtained from Corning (Corning, NY, USA). Purified human apolipoprotein A-I (apoA-I) was obtained from Biodesign (Memphis, TN, USA). Mifepristone was from Invitrogen (Canada) and anti-apoA-I polyclonal antibody was purchased from Novus Biologicals Co. (USA). Acetylated LDL (AcLDL) were prepared as previously described [12] and verified by native agarose gel electrophoresis stained with Sudan black.
2.1.1. Cells
The J774 murine macrophage and THP-1 human monocyte cell lines were cultured in Roswell Park Memorial Institute (RPMI) medium 1640 supplemented with 10% FCS. Baby hamster kidney (BHK) cells expressing human ABCA1 were grown in Dulbecco's Modified Eagle's Medium (DMEM) with 5% FCS. All the culture media were supplemented with 50 µg/ml gentamicin.
2.1.2. Assay of cellular cholesterol efflux
Cholesterol efflux was determined as previously described [24] with minor modifications. Briefly, cells were seeded in 24-well plates. BHK (150,000 cells/well) were cultured in DMEM, J774 (150,000 cells/well), and THP-1 (0.5 × 105 cells/ml) cells were respectively cultured in RPMI 1640 supplemented with 10% FCS in a humidified 37°C, 5% CO2 incubator under experimental treatments. J774 cells were labeled with 0.5ml/well of 2 µCi/ml [3H]cholesterol (Perkin Elmer, Milan, Italy) for 48 h and subsequently incubated overnight in medium containing 0.2% BSA with or without 0.3 mM of (cpt-cAMP) for 18–20 h to induce the expression of ABCA1 [25]. Cells were then treated without or with probucol (10 µM) for 2h. The washed cells were incubated in the presence of 10 μg/ml apoA-I for 24 h and the cholesterol efflux rate is calculated [24]. In the case of BHK cells, medium consisted of DMEM with 0.1% BSA in the presence or absence of 10 nM mifepristone, for 18–20 h [22]. Cells were then treated without or with probucol (10 µM) for 2h. The washed cells were incubated in the presence of 10 μg/ml apoA-I for 24 h and the cholesterol efflux rate is calculated [24]. In THP-1 cells the efflux process was evaluated as previously described [26]. Briefly, THP-1 monocytes were cultured in RPMI 1640 medium supplemented with 10% FCS at 37°C in 5% CO2. To perform the experiments, cells were seeded in 24-well plates at the density of 5 × 105 cells/well in the presence of 50 µg/ml phorbol myristate acetate (PMA) for 72 h to allow differentiation into macrophages [26]. In all experiments, cells were labeled with 2 µCi [3H]cholesterol for 48 h. THP-1 cells were then exposed for 16 h to 0.2% BSA with or without 10 µM 9-cis-retinoic acid (9cRA) plus 5 µg/ml 22-hydroxycholesterol (22-OH) for activation of retinoid X receptor (RXR) and liver X receptor (LXR) respectively. 22-OH and 9cRA were dissolved in dimethyl sulfoxide (DMSO) at 2 mg/ml and 4 mM respectively and stored at -20 ˚C. Cells were then washed and incubated for 2 h with or without 10 μM probucol before efflux time. The efflux (4 h or as indicated) was promoted by 25 µg/ml of apoA-I. CHO-K1 Cells were initially labeled for 24 hours with 1 µCi/ml [3H]cholesterol (PerkinElmer, Milan, Italy). The cells were then equilibrated for 2h in the presence of absence of probucol. Efflux was promoted for 6 h to 12.5 µg/ml of HDL [27]. Cholesterol efflux capacity values were expressed as percentage ratio between the radioactivity released in the medium and the total radioactivity incorporated by the cells. The difference between cholesterol efflux capacity of transfected cells and the non-transfected cells allowed to evaluate the contribution of ABCG1 [28].
2.1.3. THP-1 foam cells
In a separate experiment, adherent macrophages J774 or THP-1 cells were incubated with 50 µg/ml AcLDL and 2 µCi/ml [3H]cholesterol in serum-free RPMI 1640 medium containing 1% FCS for 48 h. 0.3 mM cAMP in RPMI (0.2% BSA) was used to upregulate ABCA1 for 18-20 h in J774 cells. A condition of non-stimulated J774 foam cells was also used. J774 and THP-1 foam cells were then washed and incubated for 2 h with or without 10 μM probucol before the efflux period. The medium was collected and centrifuged for 10 min at 1.5 xg to pellet cellular debris. An aliquot of the medium was counted to quantitate the effluxed cholesterol label. Meanwhile, the cells were incubated overnight with 0.1 N NaOH at room temperature, whereupon the radioactivity remaining within the cells was determined by liquid scintillation counting (Packard 1600CA Tri-Carb, Packard, Meriden, CT). Cholesterol efflux was expressed as a percentage of the radioactivity released to the medium over the total radioactivity incorporated by cells [8]. Following AcLDL loading, THP-1 foam cells were either used for the visualization of intracellular neutral LD by fluorescent microscopy or the quantification of changes of the LD morphology.
2.1.4. In vitro lipid transfer assay
THP-1 radiolabelled [3H]cholesterol foam cells (1% FCS, 2 µCi/ml, 48 h) were incubated with or without 10 μM probucol for 2 h, before apoA-I (25 µg/ml) exposure, 24 h. Media cell culture containing 3 [H]-nHDL-particles were concentrated with a cutoff filter of (10 kDa) and incubated in human normolipidemic plasma (10 μg:100 μg/ml, relative to plasma apoA-I) at 37 ˚C for 1 h [24]. After incubation, the plasma apoB fraction was precipitated with an equal volume of 13% polyethylene glycol (PEG) 6000. Transfer of total [3H]cholesterol to plasma apoB was examined by counting radioactivity in (PEG) precipitated plasma fraction (apoB) and supernatant (HDL).
2.1.5. ABCA1 inhibition
Efflux was promoted from cell lines expressing ABCA1 after the incubation of cells with or without 10 μM probucol [8,26]. Probucol was prepared as follows. A stock solution of probucol dissolved at 10 mM in ethanol was prepared and stored at -20°C. For experiments, an initial solution containing 200 µM probucol, 2% ethanol (v/v) and 4% BSA in FCS-free culture medium was prepared and then diluted to final concentration of 10 µM probucol, 0.1% ethanol (v/v) and 0.2% BSA. Control medium was prepared without probucol.
2.1.6. Analysis of HDL species by 2D-PAGGE
Two-dimensional-nondenaturing gradient gel electrophoresis (2D-PAGGE) 5% to 35% was performed as previously described [24,26]. Briefly, media cell cultures (100 μl) were separated in the first dimension (according to their charge) by 0.75% agarose gel electrophoresis (100 V, 3 h, 4°C) and in the second dimension (according to their size) by 5–35% polyacrylamide concave gradient gel electrophoresis (125 V, 24 h, 4°C) along with a molecular weight protein standard mixture (GE Healthcare, UK). Electrophoretically separated samples were electro transferred (30 V, 24 h, 4°C) onto nitrocellulose membranes (Hybond ECL; Amersham). Molecular weight markers were revealed by Ponceau S sodium salt. ApoA-I containing particles were detected with an anti-human-apoA-I antibody. Quantification of lipid content of the particles formed in the presence of probucol was performed from 2D-PAGGE. We use a film detection method for tritium-labelled proteins in 2D-PAGGE according to Bonner et al. with minor modifications [29]. Briefly, directly after electrophoresis, gels were incubated two times in DMSO separately for 20 min, afterward gels were immersed in 4 volumes of 20% scintillation liquid in DMSO for 3h. After 1h wash in water, gels were dried under vacuum (Bio-Rad) for 2h. Area of dried gels corresponding to each condition were cut in slices. Slices were dissolved in scintillation liquid for overnight at 37°C and counted for radioactivity by beta counter.
2.1.7. Assays of total accessible plasma membrane cholesterol by cholesterol oxidase
Cholesterol oxidase treatment was performed as previously described [8,22]. Briefly, cells were labeled with 3 μCi/ml [3H]cholesterol for 48 h. Washed cells were then incubated with or without 10 μM probucol. After 2 h of probucol treatment, cells were washed and then incubated with RPMI medium containing 10 μg/ml lipid-free apoA-I for 24 h. The enzyme cholesterol oxidase (1 U/ml) in Dulbecco's PBS (DPBS) was added, and cells were incubated for 4 h at 37 ˚C. Lipid was extracted with isopropanol, and radioactive cholesterol and cholestenone were separated using thin-layer chromatography (mobile phase: 96:15:8 ratio of hexane:methanol:ethyl ether). Quantification was accomplished using liquid scintillation counting.
2.1.8. Visualization of and quantification of intracellular neutral lipids
Cells were stained using the neutral LD specific (BODIPY TM 493/503, Thermo Fisher Scientific) as described previously [30]. Cover slips were incubated with 4μM BODIPY staining solution for 45 minutes at room temperature. Coverslips were washed with fish skin gelatin (FSG) then mounted on microscope slides with MOWIOL (Calbiochem, San Diego, CA, USA) mounting media. BODIPY fluorescence was visualized using the Olympus IX81 inverted microscope (Olympus Corporation, Tokyo, Japan) equipped with a 60x oil immersion PlanApo N 1.42 objective and a QuantEM:512SC electron-multiplying CCD (EMCCD) camera operated by the MetaMorph software. BODIPY emission signals were detected using FITC filter, and images were saved as 16-bit TIF images. Images were then processed by the free software ImageJ/Fiji (https://imagej.nih.gov/ij/), National Institutes of Health and the Laboratory for Optical and Computational Instrumentation (LOCI, University of Wisconsin, US).
2.1.9. Western blot analysis by SDS PAGE
Cells were lysed at 4°C in a lysis buffer (20 mM Tris-HCl, pH 7.4, containing 5 mM NaCl, and 5 mM EGTA, pH 7.5 and protease inhibitor mixture (1 tablet/50 ml, Roche)) after being washed twice with PBS 1x. Cell lysates were centrifuged at 1.5 × g for 5 min, and the resulting supernatant was subjected to protein assay (Bio-Rad, California, US). Proteins were resolved by SDS-PAGE (8-28%) and transferred to a nitrocellulose membrane (Amersham, Darmstadt, Germany). The ABCA1 and ABCG1 proteins were detected by the affinity-purified human anti-ABCA1 and anti-ABCG1 antibodies (Novus Biological, Ontario, Canada) respectively using an enhanced chemiluminescence (ZmTech Scientifique, QC, Canada) according to the manufacturer's instructions. Standard molecular weight (Bio-Rad, California, US) is shown on the left of the gel. Membranes were stripped and re-probed with rabbit anti-β-actin (abcam, Ontario, Canada) as a loading-control. Band densities were evaluated with Alpha Imager HP Imaging Densitometer with the Multi-Analyst software (Alpha Innotech Corporation, California, USA).
2.1.10. Quantification of lipid droplet number and diameter
A total of 166 images were acquired for all experimental conditions. ImageJ/Fiji software was used to manually produce regions of interest (ROIs) corresponding to each LD in a given cell using thresholding tools, allowing for quantification of changes in LD number, diameter, and volume. For assessment of the average number of LD per cell for a given condition (NLD), cells from each condition were randomly selected and LD were counted using the multi-point function in ImageJ/Fiji and saved as a ROI. In total, 5740 LD from the 166 images were counted. For assessment of the average LD diameter per cell (µm) for a given condition (Davg), cells were measured using the Oval selection tool in ImageJ/Fiji.
2.1.11. Statistical analysis
Results were presented as means and standard deviations (SD) of triplicate determinations. Efflux efficiency (Km) was calculated using the Michaelis-Menten equation (Graph-Pad Prism). Student's t-tests were used to examine the difference between continuous variables; a P value of 0.05 (2-tailed) was considered statistically significant for all studies. One-way analysis of variance ANOVA was used when comparing LD quantification values. Statistical analyses were performed using GraphPad Prism software, version 6.0 (GraphPad Software Inc, La Jolla, CA).
3. Results
Many previous researchers have demonstrated the effect of probucol on ABCA1-mediated cholesterol efflux. We have attempted to dissect this mechanism by loading macrophages with [3H]cholesterol labeled FCS vs [3H]cholesterol labeled FCS and AcLDL. These conditions are designed to generate non-foam cells vs foam cells respectively. By doing this, we have provided radioactive cholesterol to two separates, but partially overlapping pools of intracellular cholesterol that allow us to discern which pool ABCA1 draws upon to promote cholesterol efflux.
3.1. Probucol influences the kinetics of cholesterol transfer to lipid free apoA-I
We evaluated the inactivation of cholesterol efflux by probucol in non-foam vs foam THP-1 macrophages, Fig. 1A, B respectively. Fig. 1A shows the effect of probucol treatment on the cholesterol efflux activation curve after incubation for 4 h in non-foam cells. The efflux efficiency (Km) was 5.46±0.93% µg/ml for apoA-I alone and 6.31±1.27 µg/ml for apoA-I in the presence of probucol. Probucol shifted the activation curve of cholesterol efflux to apoA-I. The inhibitory effect of probucol was associated with a lower Vmax 5.65±0.28% efflux/4 h than apoA-I alone 8.1±0.33% efflux/4 h. Thus, in non-foam cells, apoA-I promoted the efflux of cholesterol more efficiently (higher Km rate) but less cholesterol (lower Vmax) in the presence of probucol. The kinetics of efflux efficiency in foam cells Fig 1B (Km) was 3.08±0.08% µg/ml for apoA-I and 2.60±0.08 µg/ml for apoA-I in the presence of probucol. Efficient cholesterol efflux removal to apoA-I persisted in foam cells treated with probucol. The inhibitory effect of probucol was associated with a higher Vmax (4.19±0.42% efflux/4 h) than apoA-I alone (3.24±0.59% efflux/4 h). Thus, in foam cells, apoA-I promoted the efflux of cholesterol less efficiently (lower Km rate) but more cholesterol (higher Vmax) in the presence of probucol. We further examined the effect of probucol concentration on apoA-I cholesterol efflux from J774 cells and THP-1 macrophages (Fig. 1C). J774 cells pre-treated with cAMP were exposed to probucol for 2 h. Increasing doses of probucol were able to decrease apoA-I efflux more dramatically in J774 cells than in THP-1 macrophages, p=0.015. We found that 1 μM of probucol inhibited efflux by 50% in these cells and maximum inhibition was achieved at 5 μM. However, in THP-1 macrophages under the same experimental conditions, 1 µM probucol inhibited efflux by 30% and was able to reach maximum inhibition at 2 µM. Accordingly, probucol affects apoA-I/ABCA1 efflux kinetics and has a different effect on J774 cells when compared to THP-1 macrophages. This may relate to the amount of ABCA1 present in each cell type. It was previously shown that probucol is effective against cholesterol efflux after 15 minutes and reaches a maximum activity at 2 h that remains until 4h in J774 cells [8]. We specifically quantify this effect in BHK cells expressing human ABCA1 under mifepristone induction and observed probucol significantly inhibited apoA-I cholesterol efflux by 93±0.01% when compared to apoA-I alone, p=0.018 (black and blue bars, Fig. 1D). This result indicated that ABCA1 concentration, but not apoA-I concentration, is rate limiting for cholesterol efflux. We obtained a similar level of cholesterol efflux inhibition in THP-1 non-foam macrophages, regardless of apoA-I concentration (> 25 µg/ml) (Fig. 1A).
Fig. 1.
Probucol inhibits cholesterol efflux differently in NF cells vs F cells. The effect of a fixed probucol dose on apoA-I cholesterol efflux capacity in THP-1 non-foam (A) and foam cells (B). THP-1 cells were radiolabeled with [3H]cholesterol (48 h) or [3H]cholesterol, AcLDL (50 µg/ml) and 1% FBS. Cells were incubated for 48 h and treated with 10 µM 9cRA and 5 µg/ml 22-OH for 18 h as indicated in “Methods.” Cholesterol efflux was then initiated by the addition of apoA-I at the indicated doses for 4 h. The fractional cholesterol efflux is plotted as a function of acceptor concentration. In all experiments, efflux of [3H]cholesterol is expressed as mean±SD of triplicate measurements and represents three experiments. C. Concentration-dependent effect of probucol on apoA-I-mediated cholesterol efflux in THP-1 and J774 non foam cells respectively. Monolayers were radio labeled for 48 h as described in “Methods.” THP-1 and J774 cells were then equilibrated with 0.2% BSA and incubated with 10 µM 9cRA and 5 µg/ml 22-OH or cAMP (0.3 mM) for 18 h respectively. Monolayers were then incubated in the presence of increasing concentrations of probucol for 2h. After probucol treatment, cells were washed and incubated with RPMI containing 25 µg/ml lipid-free apoA-I for 4 h. Data are from a representative experiment with triplicate wells (n=3). Values are expressed as means + S.D. D. Efflux to apoA-I in the presence of probucol is almost abolished, in the BHK expressing ABCA1 cell type. BHK cells were grown as described in “Methods.” ABCA1-expressing BHK cells were radio labeled with [3H]cholesterol for 48 h in 1% FCS. To induce expression of ABCA1, BHK cells were incubated with 10 nM mifepristone containing 0.1% BSA for 18–20 h. Cell were treated with probucol for 2 h, washed and incubated with apoA-I for 24 h. Insert represents specific cholesterol efflux after subtracting efflux to BSA (0.2%) including diffusion. Results shown are representative of three independent experiments.
3.2. Probucol inhibits efflux to a lesser degree in foam cells than in non-foam cells
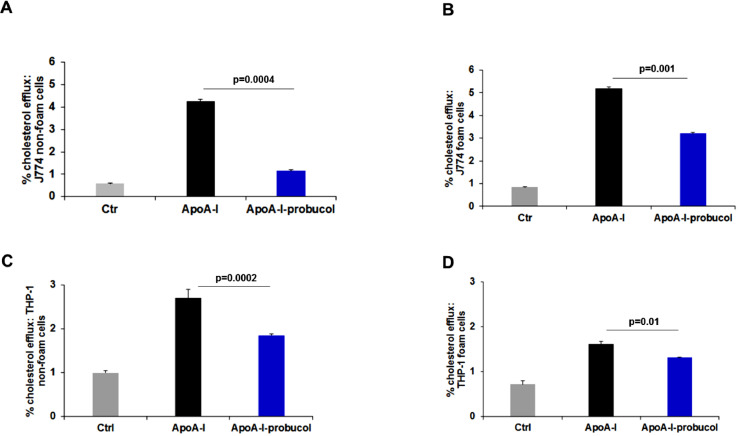
The observed difference of probucol inhibition across cell lines may in part account for the effect of probucol on foam cells. Thus, we assessed the drug's effect on cholesterol efflux in foam cells. ApoA-I–mediated cholesterol efflux from J774 non-foam cells (treated with cAMP agonists) after treatment with probucol was maximally inhibited by 68.7±0.8% (Fig. 2A, black and blue bars). In J774 foam cells and under similar loading conditions, probucol inhibited efflux by only 42±0.60% (Fig. 2B, black and blue bars). In THP-1 non-foam macrophages, probucol treatment inhibited this efflux by 31.48±0.10% (Fig. 2C, black and blue bars). However, we report only an 18.51±0.31% inhibition of efflux in THP-1 foam cells (Fig. 2D, black and blue bars). Therefore, probucol has less effect on foam cells than non-foam cells (Table 1).
Fig. 2.
Probucol results in less efflux inhibition in foam cells than non-foam cells. (A, B, C and D) Monolayers of J774 cells (A, B) and THP-1 cells (C, D) were labeled with 2 μCi/mL [3H]cholesterol (non-foam, A, C) or [3H]cholesterol and AcLDL (foam, B, D; 50 µg/ml), for 48 h in RPMI medium 1640 with 1% FCS. Cells were then incubated for 18 h with 0.2% BSA or not in the presence (solid bars) or absence (hatched bars) of 0.3 mM cpt-cAMP followed by incubation with or without 10 μM probucol as described in “Methods.” After 2 h of probucol treatment, cells were washed and then incubated with RPMI medium 1640 containing 25 μg/ml lipid-free apoA-I for 4 h. Data are from a representative experiment with triplicate wells (n=3). Values are expressed as mean ± SD. *P < 0.05 by Student's t-test.
Table 1.
Macrophage foam cells are less sensitive to probucol-mediated inhibition of cholesterol efflux than non-foam cells.
| Cell type | Experimental condition | % efflux± SD | Total cpm count (media and cells) | % efflux inhibition± SD | p-value |
|---|---|---|---|---|---|
| J774 Non-foam |
ApoA-I | 4.25±0.05 |
879035 |
68.70±0.82 | 0.0004 |
| ApoA-I + Pb | 1.33±0.05 |
994868 |
|||
| J774 Foam |
ApoA-I | 5.12±0.07 |
488575 |
42.00±0.60 | 0.0002 |
| ApoA-I + Pb | 2.96±0.03 |
547263 |
|||
| THP-1 Non-foam |
ApoA-I | 2.7±0.2 |
347488 |
31.48±0.10 | 0.0002 |
| ApoA-I + Pb | 1.85±0.04 |
325383 |
|||
| THP-1 Foam |
ApoA-I | 1.62±0.05 |
174866 |
18.51±0.31 | 0.01 |
| ApoA-I + Pb | 1.32±0.01 |
174285 |
3.3. Probucol's inhibition is independent of ABCA1 expression levels
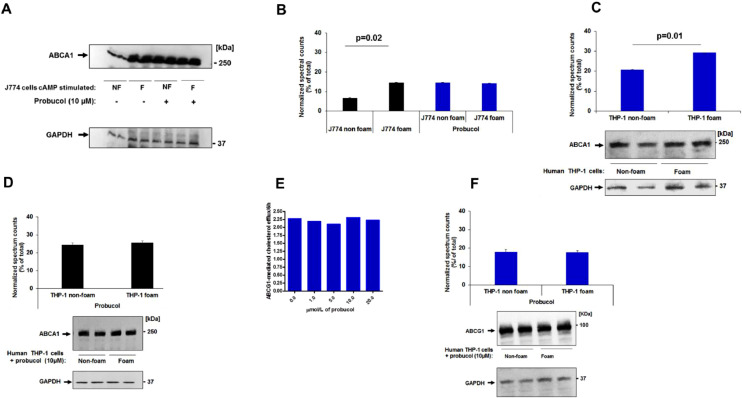
To further define the role of ABCA1 in the observed efflux and test our hypothesis, we used J774 cells non-treated with cAMP agonist. In this case, we would expect ABCA1 protein levels to be very low. In J774 non-foam cells, probucol inhibited efflux by 34±0.01% (Fig. 3A, black and blue bars) (Table 2). In J774 foam cells non treated with cAMP agonists, probucol inhibited efflux by 20.63±0.88% (Fig. 3B, black and blue bars) (Table 2). The putative mechanism of probucol was also observed in non-induced BHK cells with 33±0.1 % inhibition of total efflux (Fig. 3C) and 70±0.2% in specific efflux (Fig. 3C, inset), defined as cholesterol efflux after subtracting efflux to BSA (0.2%) and diffusion. These cell lines do not express detectable ABCA1 (Fig. 3D) [31]. The effect of probucol on ABCA1 expression was further examined in J774 cells stimulated with cAMP and in THP-1 macrophages. Probucol treatment did not significantly alter ABCA1 protein levels in non-foam cells and foam J774 cells when stimulated with cAMP (Fig. 4C). J774 cells not treated with probucol were used as a control (Figs. 4A, B), consistent with previous studies [8,32]. Probucol treatment did not significantly affect ABCA1 protein expression in cholesterol efflux to apoA-I in J774 foam cells (Fig. 4B). This result was consistent with THP-1 macrophages (Figs. 4C, D). Since this data shows that efflux is different but ABCA1 expression is not, this suggests that cholesterol efflux removal from foam cells is partially independent of ABCA1 expression.
Fig. 3.
The effect of probucol on cholesterol efflux in foam cells without ABCA1 expression. Probucol produces a lesser degree of efflux inhibition in non-cAMP stimulated J774 foam cells vs non-foam cells. Monolayers were labeled with 2 μCi/ml [3H]cholesterol (A) or [3H]cholesterol and AcLDL (B, 50 µg/ml), for 48 h in RPMI medium 1640 with 1% FCS. Cells were then incubated for 18 h or not with 0.2% BSA in the absence of 0.3 mM cpt-cAMP followed by incubation with 10 μM probucol for 2 h. Cells were washed and then incubated with medium containing 25 μg/ml lipid-free apoA-I for 24 h. C. Probucol completely inhibits cholesterol efflux in the absence of ABCA1 expression in non-mifepristone induced BHK cells. BHK cells were labelled with [3H]cholesterol 48 h, 1% FCS. Afterwards, cells were incubated in DMEM containing 0.2% BSA for 18–20 h. This was followed by an incubation with 10 μM probucol for 2 h. Non-induced BHK cells were washed and incubated with medium containing 10 μg/ml lipid-free apoA-I for 24 h. BHK cells incubated alone were used as control. Inset represents specific cholesterol efflux inhibited by probucol obtained after subtracted cholesterol efflux from background.D. Non-stimulated J774 and BHK cells do not express ABCA1 transporter protein. Cells were grown as described in Materials and methods, cells were lysed at 4°C with 20 mM Tris, 5 mM EDTA, and 5 mM EGTA; pH 7.5 containing 0.5% n-dodecylmaltoside. Protein concentration was determined by standard assay (Bio-Rad). Cells were separated by SDS-PAGE (4–22.5%) and immunoblotted using antibodies against human ABCA1 and the loading control glyceraldehyde 3-phosphate dehydrogenase (GAPDH). The molecular weight (Bio-Rad) is shown on the right of the gel. Data are from a representative experiment with triplicate wells (n=3). Values are expressed as mean±SD. *P < 0.05 by Student's t-test. NF; non foam, F; foam
Table 2.
Probucol inhibits cholesterol efflux in J774 macrophages non-expressing ABCA1.
| Cell type | Experimental condition | % efflux ± SD | % efflux inhibition ± SD | p-value |
|---|---|---|---|---|
| J774 -cAMP Non-foam |
ApoA-I | 1.04±0.06 | 34±0.01 | 0.001 |
| ApoA-I + Pb | 0.69±0.01 | |||
| J774 -cAMP Foam |
ApoA-I | 1.89±0.09 | 20.63±0.88 | 0.01 |
| ApoA-I + Pb | 1.50±0.05 |
Fig. 4.
Probucol does not alter ABCA1 nor ABCG1 protein expression in foam cells and non-foam cells. Cells were treated as described above, and after probucol incubation they were washed with PBS and solubilized and separated by (4-22.5%) SDS-PAGE. (A, D) ABCA1 from J774 and THP-1 cells lysis was detected by anti-ABCA1 antibody respectively. (B, C, and D) Changes in ABCA1 protein expression were determined by normalizing against the densitometric intensity of GAPDH. (E) Human ABCG1-overexpressing CHO-K1 cells were labeled with 1 µCi/ml [3H]cholesterol for 24 h, washed, and then equilibrated for 2 h in the presence of absence of different concentration of probucol. Efflux was promoted for 6 h to 12.5 µg/ml of HDL. (F) Probucol treatment did not significantly affect ABCG1 protein expression in THP-1 foam cells. ABCG1 from THP-1 cells lysis was detected by anti-ABCG1 antibody. Changes in ABCA1 protein expression were determined by normalizing against the densitometric intensity of GAPDH. Each sample was run in triplicate. Values are expressed as means ± SD. GAPDH was used as a loading control. *P < 0.05 by Student's t-test.
We further address ABCG1 activity in the presence of probucol in foam cells, given that LXR upregulation will also increase ABCG1 expression in foam cells to a much greater extent than ABCA1 [33]. Our data indicate that increased doses of probucol did not affect ABCG1 cholesterol efflux activity to HDL particles in CHO-K1 cells (Fig. 4E). Importantly, probucol treatment did not significantly affect ABCG1 protein expression in THP-1 cells (Fig. 4F) as previously reported [34]. These results suggest that ABCG1 activity is unaffected by probucol in our model.
3.4. The formation of small lipidated HDL particles > 7 nm from foam cells in the presence of probucol activate cholesterol transfer to plasma apoB particles
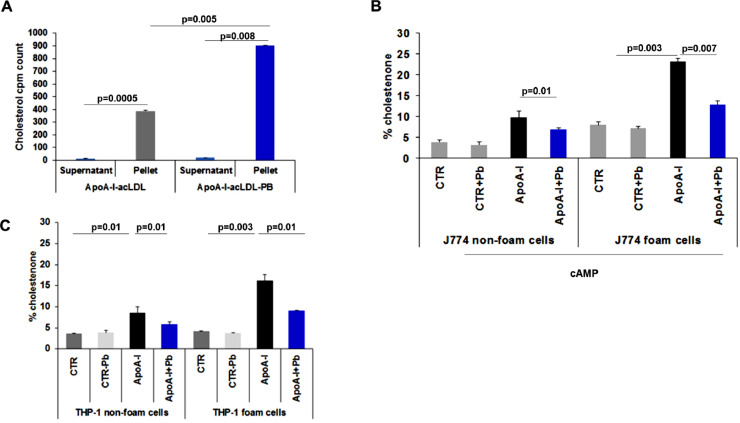
The effect of probucol on HDL formation was evaluated in J774 foam vs. non foam cells not expressing ABCA1 by 2D-PAGGE. Our data confirmed an aggregate of lipid free apoA-I with molecular size ≥ 4 nm (Figs. 5A, B) respectively. In THP-1 macrophages non foam cells (with LXR and RXR agonists), probucol effect was confirmed by a lack of formation of spherical or α-migrating HDL (Figs. 5C, D). In non foam THP-1, nHDL had a diameter less than 7 nm (Fig. 5C left panel). THP-1 foam cells generated discrete apoA-I particles with a diameter greater than 7 nm (Fig. 5D left panel). To characterize the lipids in the particles released from foam cells in the presence or absence of probucol we assessed lipid composition by determining [3H]cholesterol radioactivity by 2D gel electrophoresis. We found that probucol decreased lipid association by 50±3.73%, p=0.02, in foam cells (Fig. 5E). The decrease in lipid association by probucol is 88±0.59%, p=0.004, in non-foam cells (Fig. 5F). This means that probucol inhibits the transfer of [3H]cholesterol into nHDL particles less in foam cells. Moreover, the question was raised whether these particles were functional. We assayed the ability of these particles to activate the transfer of radiolabeled cholesterol to plasma lipoproteins (apoB particles) ex vivo, isolated by PEG precipitation. These particles significantly activate the transfer of cholesterol to plasma apoB, p=0.008 when compared to particles created in foam cells in the absence of probucol, p=0.0005 (Fig. 6A). Therefore, fully functional nHDL particles are produced in probucol treated foam cells.
Fig. 5.
Characterization of lipidated apoA-I-containing particles generated from pretreated THP-1 cells with probucol. Monolayers were labeled with 2 μCi/ml [3H]cholesterol for 48 h in RPMI medium 1640 with 1% FCS. After 2 h of probucol treatment, cells were washed and then incubated with RPMI medium 1640 containing 10 μg/ml lipid-free apoA-I for 24 h. Afterwards, the media were collected and prepared as described in “Experimental Procedures.” Samples were separated by 2D-PAGGE and apoA-I was detected by anti-apoA-I antibody as described in “Methods.” (A, B): J774 non-foam and foam cells whole medium. (C, D): Human THP-1 macrophage non foam and foam cells whole medium. Molecular size markers (diameter in nm) are indicated. The HDL particle diameters are derived from the relative mobility values of the centers of the various bands. Molecular weight markers were revealed by Ponceau S. (E, F). Nascent HDL (nHDL) particles formed from foam cells pretreated with probucol contain more lipids than those from non foam cells. radioactivity applied to gel electrophoresis. Radioactivity appearing in gels corresponding to apoA-I and apoA-I + probucol was counted as described in “Methods.” [3H]cholesterol (E) from foam cells versus (F) non foam cells in the presence of apoA-I or apoA-I + probucol was expressed as a percentage of control (100%, in the absence of apoA-I). Results shown are representative of three independent experiments. *P < 0.05 by Student's t-test.
Fig. 6.
Dynamics of nascent HDL generated from foam cells in the presence of probucol. Radiolabelled [3H]cholesterol loaded nascent HDL (nHDL)-like lipoprotein apoA-I derived from THP-1 foam cells pretreated with or without probucol were incubated with normolipidemic human plasma for 6 h at 37°C. After incubation, apoB was precipitated with 50/50 vol/vol 20% PEG 6000. Apo B containing particle fractions were dialyzed and [3H]cholesterol transfer was calculated between nHDL and apo B fractions as indicated (A). Counts were made in triplicates. B. Probucol effect on free cholesterol oxidation by cholesterol oxidase in J774 mouse macrophages (C) and in THP-1 non-foam and foam macrophages. Monolayers were labeled with 3 μCi/ml [3H]cholesterol for 48 h with 1% FCS or with 3 μCi/ml [3H]cholesterol, AcLDL (50 µg/ml) and 1% FCS for 48 h with 1% FCS. Cells were treated with probucol as described above, but the incubation with cAMP was done with 10 μM 9cRA and 5 μg/ml 22-OH. Data are from a representative experiment with triplicate wells (n=3). Values are expressed as mean±SD. *P < 0.05 by Student's t-test. Control cells (CTR) shows the results from non-treated cells, while CTR-probucol (PB) shows the results from cells treated with probucol 10 mM for 2h.
3.5. Probucol significantly reduced total accessible plasma membrane cholesterol in foam cells when compared to non-foam cells
We report that probucol causes less inhibition of cholesterol efflux in foam cells in comparison to non-foam cells. To address this issue, we sought to determine which cholesterol pool is accessible to probucol activity in cholesterol efflux from foam cells. To discern between cholesterol that can be effluxed through ABCA1 and the cholesterol that cannot, we used a cholesterol oxidase assay to measure total accessible PM cholesterol content. In this assay, cholesterol oxidase interacts with cholesterol within the outer leaflet of the PM (and the cholesterol that can flop from the inner leaflet to the outer leaflet) and converts it to cholestenone. All cholestenone formed is now designated as the total accessible PM cholesterol content. In J774 foam cells in the presence of probucol, percent cholestenone shifts from 8.00±0.78% in the absence of apoA-I (control) to 23.24±0.62 in the presence of apoA-I, p=0.00036 (Table 3). This suggests that incubation with apoA-I promotes recruitment of accessible free cholesterol to the PM. When cells treated with apoA-I are subjected to a 2 h incubation with probucol, this significantly reduces this value to 12.82±0.92, p=0.007 (inhibition of cholestenone formation by almost 45%). In J774 non-foam cells, percent cholestenone was 9.8±1.55 in the presence of apoA-I, and incubation with probucol reduced this value significantly to 6.99±0.31, p=0.01 (Fig. 6B) an inhibition of 29%. In THP-1 foam cells, percent cholestenone was 4.15±0.09 in non-treated cells (control) and 16.19±1.46 in the presence of apoA-I, p=0.034. A 2 h incubation with probucol reduced this value significantly to 9.06±0.09, p=0.01, suggesting an inhibition of cholestenone formation of 44%. Under similar conditions and in THP-1 non foam cells, percent cholestenone was 3.6±0.07 in non-treated cells (control) and 8.50±1.55 in the presence of apoA-I, p=0.01. A 2 h incubation with probucol reduces this value significantly to 5.8±0.59, p=0.01, resulting in an inhibition of cholestenone formation by almost 32% (Fig. 6C) (Table 3). Taken together, probucol treatment resulted in less accessible PM cholesterol for all cell types and treatments (Table 3). However, most importantly, more accessible PM cholesterol remains in foam cells compared to non-foam cells. As reported previously, differences in the cholesterol oxidase sensitive pool could be explained by a diminished ability of ABCA1 to redistribute membrane cholesterol to cell-surface domains accessible to treatment with the enzyme cholesterol oxidase [22]. Of note, in foam and in non-foam cells, probucol alone did not influence the total accessible plasma cholesterol level (Fig. 6C, CTR + probucol bars).
Table 3.
Macrophage foam cells are more sensitive to probucol-mediated decrease of the total accessible plasma membrane pool of cholesterol than non-foam cells.
| Cell type | Experimental condition | % cholestenone± SD | % cholestenone inhibition± SD | p-value |
|---|---|---|---|---|
| J774 Non-foam |
ApoA-I | 9.8±1.55 | 29± 0.5 | 0.01 |
| ApoA-I + Pb | 6.99±0.31 | |||
| J774 Foam |
ApoA-I | 23.24±0.62 | 45±1.41 | 0.007 |
| ApoA-I + Pb | 12.82±0.92 | |||
| THP-1 Non-foam |
ApoA-I | 8.50±1.55 | 32±0.1 | 0.01 |
| ApoA-I + Pb | 5.8±0.59 | |||
| THP-1 Foam |
ApoA-I | 16.19±1.46 | 44.03±0.1 | 0.01 |
| ApoA-I + Pb | 9.06±0.09 |
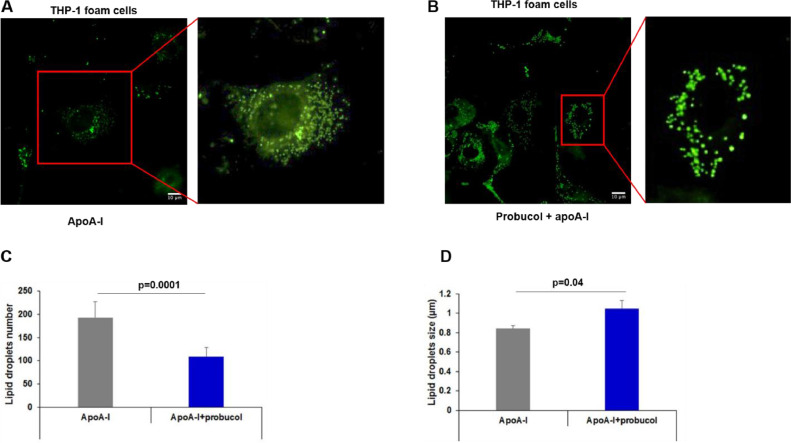
3.6. Probucol alters lipid droplet number and size in foam cells
We next sought to provide further insight into the intracellular regulation of the cholesterol pool following probucol treatment of foam cells. Alterations of lipid metabolism are often apparent through changes of the LD morphology (number and diameter of LD per cell) [30]. Because macrophage foam cells mainly contain cholesteryl esters (CE) harbored within LD [35], we tested whether probucol interfered with LD metabolism in these cells. Intracellular LD of THP-1 foam cells treated with apoA-I in the presence or absence of probucol are visualized by fluorescent microscopy and shown in Figs. 7A, B. In comparison to the apoA-I alone condition (Fig. 7A), addition of probucol decreases the total number of LD per cell (Fig. 7B). Quantitative data shows that probucol treatment caused a significant decrease in the number of foam cell LD (Fig. 7C), p=0.0001. Interestingly, this reduction was also associated with a significant increase in LD size, p=0.04 (Fig. 7D). Therefore, probucol treatment changes the size and number of LD, such that the THP-1 foam cells have fewer but larger LD.
Fig. 7.
Probucol effect on lipid droplet distribution in THP-1 foam cells treated with apoA-I (A), and a combination of apoA-I and probucol (B). THP-1 foam cells were prepared as described in “Methods” and incubated with apoA-I or ApoA-I and probucol. Cells were stained with BODIPY and LD were visualized with an Olympus IX81 inverted microscope. Images were then processed in ImageJ/FIJI. C, D. Probucol effect on LD number and size in THP-1 foam cells. FIJI was used to manually count the number of LD (C) and the size of each LD (D) in THP-1 foam cells. Values are displayed as mean±SD. E. Probucol effect on apparent LD volume per cell. Total LD volume was estimated per cell based on mean LD number and diameter as shown in “Methods.” Values are displayed as mean±SD. *P < 0.05.
4. Discussion
It remains undisputed that the treatment of macrophages with probucol can influence cellular cholesterol efflux. However, the mechanisms by which it produces changes in efflux remain paradoxical [5,20]. It has been proven that probucol binds to and directly inhibits ABCA1 [8]; however, cholesterol efflux is differentially affected by probucol in foam and in non-foam cells at both the kinetic and physiologic level.
We distinguish differences in cholesterol efflux kinetics across cell lines when exposed to probucol. A fixed probucol dose tested against various doses of apoA-I was found to lower apoA-I cholesterol efflux efficiency, as judged by a higher Km and lower Vmax values. The kinetics of cholesterol transfer to apoA-I increases in the presence of probucol, while the kinetics of increased doses of probucol differs across cell lines. In J774 cells, 5 μM probucol was able to reach maximum inhibition of cholesterol efflux. However, in THP-1 macrophages a 2 µM dose of probucol was efficient to reach maximum inhibition. This difference likely has to do with the different expression level of ABCA1 in these cells. Nevertheless, long-term treatment with probucol (48 h) with doses higher than 10 µM (30, 50, and 100 μM) may cause ubiquitin-mediated protein degradation as observed in human embryonic kidney cells (HEK293) expressing ABCA1 [36]. This evidence may point to the importance of appropriate drug dosage [14].
Based on our data from two cell lines (J774 and THP-1 macrophages), we propose that probucol inhibits ABCA1, thereby inhibiting the recruitment of free cholesterol to the ABCA1 microdomain in the PM (as shown in Fig. 6), which in turn blocks the shuttling of cholesterol from intracellular sources (such as LD) to the PM, and ultimately leads to the reorganization of LD morphology (Fig. 7). We predict that probucol inhibits ABCA1 from effluxing the "ABCA1-accessible" pool. Therefore, the cholesterol that is loaded by AcLDL is not entirely in an "ABCA1-accessible" pool. We attempted to use the cholesterol oxidase assay as a second method to measure the ABCA1-accessible cholesterol pool (besides the efflux measurements in non-foam and foam cells). We concluded that probucol treatment resulted in more accessible PM cholesterol in foam cells than non-foam cells (Table 3). Because treatment of apoA-I in foam cells resulted in a greater recruitment of accessible PM cholesterol than in non-foam cells, probucol elicited a greater inhibitory effect on accessible PM cholesterol. This evidence is in line with the effect of probucol in reducing the total accessible PM cholesterol pool in J774 foam cells [37] and diminishing cholesterol microdomains in differentiated wildtype mouse macrophages incubated with AcLDL [20].
We further identified the formation of functional lipidated-small nHDL (preβ-1 like HDL particles) with diameter size ~7-8 nm, in foam cells in the presence of probucol. In non-foam cells treated with probucol, nHDL particles were ~4 nm and poorly lipidated. Transient elevation of lipidated nHDL particles is thought to be beneficial in removing cholesterol from the artery wall [38]. Several lines of evidence suggest that preβ1-HDL concentration associates with the amount of CETP protein [39]. This evidence, in part, supports our finding that small nHDL particles collected from foam cells are active in promoting transfer of unesterified cholesterol content to apoB-containing particles in plasma ex vivo. In support of this concept, plasma HDL reduction in a probucol-treated hypercholesterolemic patient may accelerate cholesterol transport through the HDL system out of cholesterol-enriched foam cells [11,40]. It is possible that probucol-treated cells may generate such lipoprotein particles that are more active in promoting net cholesterol efflux from foam cells [41]. Moreover, this is consistent with the observation that probucol attenuates the accumulation of lipid-laden macrophages in xanthoma lesions of familial hypercholesterolemia patients [7]. Inactivation of ABCA1-dependent cholesterol efflux by probucol in mouse primary hepatocytes was shown to be responsible for increasing the fecal excretion of HDL-derived cholesterol in vivo [18]. Although expected to lead to a decrease in HDL-C levels, probucol may lead to increased reverse cholesterol transport. This may provide an explanation for the beneficial effects of probucol on reducing xanthomas. Moreover, an earlier published report demonstrated an ABCA1-independent mechanism in primary hepatocytes in vitro [42]. However, the relevance of this pathway in regard to our finding in macrophages is yet to be determined. We believe that the use of probucol has effectively revealed an ABCA1-independent mechanism of HDL generation in our model. While the total HDL production is much less, this novel mechanism does produce nHDL with cholesterol lipidation.
The amount of cholesterol accumulated in LD, as well as ABCA1 activity, are potentially rate limiting steps in cholesterol efflux [35]. However, it has been shown that probucol inhibits the translocation of ABCA1 to the PM [8,43]. We thus propose that the rate limiting step of the observed efflux in the probucol-treated foam cell condition could be the hydrolysis of CE from LD independently of ABCA1. The enhanced efficiency of probucol-mediated inhibition of cholesterol efflux in non-foam cells when compared to foam cells can not be explained by the mechanism of action of probucol alone. Indeed, the differences in cholesterol loading also likely play a role in the observed effect. In this study we propose that loading macrophages with cholesterol derived from FCS (non-foam) differs from cholesterol from AcLDL (foam). The data would argue that cholesterol that enters macrophages through different lipoproteins and different receptors is handled differently by the macrophage. We can then use probucol as a precise tool to probe intracellular cholesterol trafficking stimulated by apoA-I, creation of a cholesterol enriched PM ABCA1 microdomain, and ABCA1 mediated cholesterol efflux (Table 4). Altogether these observations provide substantial evidence for a revised model of cholesterol trafficking in macrophages (Fig. 8).
Table 4.
Summary of main findings.
| Main finding: a non-ABCA1 pathway of cholesterol efflux in foam cells is unmasked by probucol |
|
|
|
|
|
|
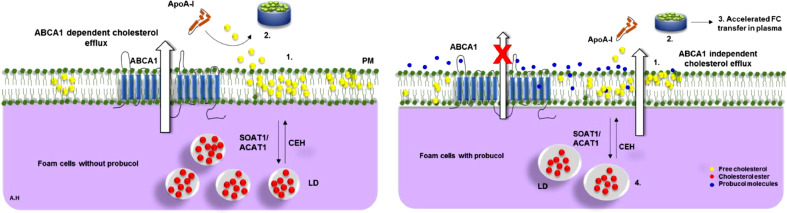
Fig. 8.
A proposed model for probucol interaction in foam cells. A) ABCA1 dependent cholesterol efflux without probucol. 1, 2) ApoA-I interacts with ABCA1 to produce preβ-HDL. B) ABCA1 independent cholesterol efflux. Probucol inhibits the ABCA1 activity with apoA-I and decreases the ABCA1-accessible cholesterol pool in PM. This process would modulate: 1) PM ABCA1-accessible and inaccessible cholesterol where probucol treatment resulted in less PM cholesterol labeling/recruitment. 2) Generation of small nHDL particles (> 7 nm) partially lipidated, 3) which were functional in transferring cholesterol to apoB-containing lipoproteins. 4) Intracellularly, this was associated with increased storage of CE, a decrease in LD number and increase in size. Free cholesterol, FC; cholesteryl ester, CE; Acyl-coenzyme A:cholesterol acyltransferase 1, ACAT1; Cholesteryl ester hydrolase, CEH; Apolipoprotein A-I, ApoA-I; nascent high density lipoprotein, nHDL; Sterol O-acyltransferase (acyl-Coenzyme A: cholesterol acyltransferase) 1, SOAT1; lipid droplets, LD.
5. Conclusion
In conclusion, we are unmasking an ABCA1-independent efflux mechanism by treating macrophages with probucol (to inhibit the ABCA1-dependent efflux) and by loading the cells with different lipoproteins such as AcLDL. This is the first report of an ABCA1-independent mechanism of HDL generation in macrophages. While the total HDL (7 to 9 nm in size) production is significantly reduced, this novel mechanism produces nHDL with partial cholesterol lipidation. We demonstrate that probucol influences cholesterol trafficking through intracellular (LD), PM, and extracellular (efflux) means.
Funding
This work was supported by grant FVRLDR_RICERCA_IST from University of Parma, Italy (E.F.).
Data availability
All data reported in this study are located within the article.
CRediT authorship contribution statement
Anouar Hafiane: Conceptualization, Investigation, Methodology, Formal analysis, Writing – original draft, Writing – review & editing, Data curation, Formal analysis, Visualization. Alessandro Pisaturo: Writing – review & editing. Annalisa Ronca: Methodology, Formal analysis, Writing – original draft. Matteo Incerti: Methodology, Formal analysis, Writing – original draft. Robert S. Kiss: Conceptualization, Investigation, Methodology, Formal analysis, Writing – original draft, Writing – review & editing. Elda Favari: Conceptualization, Investigation, Methodology, Formal analysis, Writing – original draft, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors would like to thank Prof. George H. Rothblat for his long-standing scientific mentorship.
Contributor Information
Anouar Hafiane, Email: anouar.hafiane@mail.mcgill.ca.
Alessandro Pisaturo, Email: alessandro.pisaturo@mail.mcgill.ca.
Annalisa Ronca, Email: annalisa.ronca@unipr.it.
Matteo Incerti, Email: matteo.incerti@unipr.it.
Robert S. Kiss, Email: robert.kiss@mcgill.ca.
Elda Favari, Email: elda.favari@unipr.it.
References
- 1.Nicholls SJ, Puri R, Anderson T, Ballantyne CM, Cho L, Kastelein JJ, et al. Effect of evolocumab on progression of coronary disease in statin-treated patients: the GLAGOV randomized clinical trial. JAMA. 2016;316(22):2373–2384. doi: 10.1001/jama.2016.16951. [DOI] [PubMed] [Google Scholar]
- 2.Cannon CP, Cariou B, Blom D, McKenney JM, Lorenzato C, Pordy R, et al. Efficacy and safety of alirocumab in high cardiovascular risk patients with inadequately controlled hypercholesterolaemia on maximally tolerated doses of statins: the ODYSSEY COMBO II randomized controlled trial. Eur. Heart J. 2015;36(19):1186–1194. doi: 10.1093/eurheartj/ehv028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Takase S, Matoba T, Nakashiro S, Mukai Y, Inoue S, Oi K, et al. Ezetimibe in combination with statins ameliorates endothelial dysfunction in coronary arteries after stenting: the CuVIC trial (effect of cholesterol absorption inhibitor usage on target vessel dysfunction after coronary stenting), a multicenter randomized controlled trial. Arterioscler. Thromb. Vasc. Biol. 2017;37(2):350–358. doi: 10.1161/ATVBAHA.116.308388. [DOI] [PubMed] [Google Scholar]
- 4.Hafiane A. Vulnerable plaque, characteristics, detection, and potential therapies. J. Cardiovasc. Dev. Dis. 2019;6(3) doi: 10.3390/jcdd6030026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yamamoto A. A uniqe antilipidemic drug–probucol. J. Atheroscler. Thromb. 2008;15(6):304–305. doi: 10.5551/jat.e621. [DOI] [PubMed] [Google Scholar]
- 6.Sirtori CR, Yamashita S, Greco MF, Corsini A, Watts GF, Ruscica M. Recent advances in synthetic pharmacotherapies for dyslipidaemias. Eur. J. Preventive Cardiol. 2020;27(15):1576–1596. doi: 10.1177/2047487319845314. [DOI] [PubMed] [Google Scholar]
- 7.Arakawa R, Tsujita M, Iwamoto N, Ito-Ohsumi C, Lu R, Wu CA, et al. Pharmacological inhibition of ABCA1 degradation increases HDL biogenesis and exhibits antiatherogenesis. J. Lipid Res. 2009;50(11):2299–2305. doi: 10.1194/jlr.M900122-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Favari E, Zanotti I, Zimetti F, Ronda N, Bernini F, Rothblat GH. Probucol inhibits ABCA1-mediated cellular lipid efflux. Arterioscler. Thromb. Vasc. Biol. 2004;24(12):2345–2350. doi: 10.1161/01.ATV.0000148706.15947.8a. [DOI] [PubMed] [Google Scholar]
- 9.Lu R, Tsuboi T, Okumura-Noji K, Iwamoto N, Yokoyama S. Caveolin-1 facilitates internalization and degradation of ABCA1 and probucol oxidative products interfere with this reaction to increase HDL biogenesis. Atherosclerosis. 2016;253:54–60. doi: 10.1016/j.atherosclerosis.2016.08.025. [DOI] [PubMed] [Google Scholar]
- 10.Tsujita M, Yokoyama S. Selective inhibition of free apolipoprotein-mediated cellular lipid efflux by probucol. Biochemistry. 1996;35(40):13011–13020. doi: 10.1021/bi960734h. [DOI] [PubMed] [Google Scholar]
- 11.Adlouni A, El Messal M, Saile R, Parra H, Fruchart J, Ghalim N. Probucol promotes reverse cholesterol transport in heterozygous familial hypercholesterolemia. Effects on apolipoprotein AI-containing lipoprotein particles. Atherosclerosis. 2000;152(2):433–440. doi: 10.1016/s0021-9150(99)00493-1. [DOI] [PubMed] [Google Scholar]
- 12.Basu SK, Goldstein JL, Anderson GW, Brown MS. Degradation of cationized low density lipoprotein and regulation of cholesterol metabolism in homozygous familial hypercholesterolemia fibroblasts. PNAS. 1976;73(9):3178–3182. doi: 10.1073/pnas.73.9.3178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miida T, Seino U, Miyazaki O, Hanyu O, Hirayama S, Saito T, et al. Probucol markedly reduces HDL phospholipids and elevated prebeta1-HDL without delayed conversion into alpha-migrating HDL: putative role of angiopoietin-like protein 3 in probucol-induced HDL remodeling. Atherosclerosis. 2008;200(2):329–335. doi: 10.1016/j.atherosclerosis.2007.12.031. [DOI] [PubMed] [Google Scholar]
- 14.Yamamoto A, Hara H, Takaichi S, Wakasugi J, Tomikawa M. Effect of probucol on macrophages, leading to regression of xanthomas and atheromatous vascular lesions. Am. J. Cardiol. 1988;62(3) doi: 10.1016/s0002-9149(88)80048-1. 31b-6b. [DOI] [PubMed] [Google Scholar]
- 15.Li T, Chen W, An F, Tian H, Zhang J, Peng J, et al. Probucol attenuates inflammation and increases stability of vulnerable atherosclerotic plaques in rabbits. Tohoku J. Exp. Med. 2011;225(1):23–34. doi: 10.1620/tjem.225.23. [DOI] [PubMed] [Google Scholar]
- 16.Zhong JK, Guo ZG, Li C, Wang ZK, Lai WY, Tu Y. Probucol alleviates atherosclerosis and improves high density lipoprotein function. Lipids Health Dis. 2011;10:210. doi: 10.1186/1476-511X-10-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guo X, Wang L, Xia X, Wang P, Li X. Effects of atorvastatin and/or probucol on recovery of atherosclerosis in high-fat-diet-fed apolipoprotein E-deficient mice. Biomed. Pharmacother. 2019;109:1445–1453. doi: 10.1016/j.biopha.2018.10.184. [DOI] [PubMed] [Google Scholar]
- 18.Yamamoto S, Tanigawa H, Li X, Komaru Y, Billheimer JT, Rader DJ. Pharmacologic suppression of hepatic ATP-binding cassette transporter 1 activity in mice reduces high-density lipoprotein cholesterol levels but promotes reverse cholesterol transport. Circulation. 2011;124(12):1382–1390. doi: 10.1161/CIRCULATIONAHA.110.009704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamashita S, Masuda D, Matsuzawa Y. Did we abandon probucol too soon? Curr. Opin. Lipidol. 2015;26(4):304–316. doi: 10.1097/MOL.0000000000000199. [DOI] [PubMed] [Google Scholar]
- 20.Freeman SR, Jin X, Anzinger JJ, Xu Q, Purushothaman S, Fessler MB, et al. ABCG1-mediated generation of extracellular cholesterol microdomains. J. Lipid Res. 2014;55(1):115–127. doi: 10.1194/jlr.M044552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang SH, Reddick RL, Avdievich E, Surles LK, Jones RG, Reynolds JB, et al. Paradoxical enhancement of atherosclerosis by probucol treatment in apolipoprotein E-deficient mice. J. Clin. Invest. 1997;99(12):2858–2866. doi: 10.1172/JCI119479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vaughan AM, Oram JF. ABCA1 redistributes membrane cholesterol independent of apolipoprotein interactions. J. Lipid Res. 2003;44(7):1373–1380. doi: 10.1194/jlr.M300078-JLR200. [DOI] [PubMed] [Google Scholar]
- 23.Gelissen IC, Harris M, Rye K-A, Quinn C, Brown AJ, Kockx M, et al. ABCA1 and ABCG1 synergize to mediate cholesterol export to ApoA-I. Arterioscler. Thromb. Vasc. Biol. 2006;26(3):534–540. doi: 10.1161/01.ATV.0000200082.58536.e1. [DOI] [PubMed] [Google Scholar]
- 24.Hafiane A, Bielicki JK, Johansson JO, Genest J. Novel Apo E-derived ABCA1 agonist peptide (CS-6253) promotes reverse cholesterol transport and induces formation of prebeta-1 HDL in vitro. PLoS One. 2015;10(7) doi: 10.1371/journal.pone.0131997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Favari E, Lee M, Calabresi L, Franceschini G, Zimetti F, Bernini F, et al. Depletion of pre-beta-high density lipoprotein by human chymase impairs ATP-binding cassette transporter A1- but not scavenger receptor class B type I-mediated lipid efflux to high density lipoprotein. J. Biol. Chem. 2004;279(11):9930–9936. doi: 10.1074/jbc.M312476200. [DOI] [PubMed] [Google Scholar]
- 26.Hafiane A, Johansson JO, Genest J. ABCA1 agonist mimetic peptide CS-6253 induces microparticles release from different cell types by ABCA1-efflux-dependent mechanism. Can. J. Cardiol. 2019;35(6):770–781. doi: 10.1016/j.cjca.2019.02.018. [DOI] [PubMed] [Google Scholar]
- 27.Favari E, Calabresi L, Adorni MP, Jessup W, Simonelli S, Franceschini G, et al. Small discoidal pre-beta1 HDL particles are efficient acceptors of cell cholesterol via ABCA1 and ABCG1. Biochemistry. 2009;48(46):11067–11074. doi: 10.1021/bi901564g. [DOI] [PubMed] [Google Scholar]
- 28.Zimetti F, Favari E, Cagliero P, Adorni MP, Ronda N, Bonardi R, et al. Cholesterol trafficking-related serum lipoprotein functions in children with cholesteryl ester storage disease. Atherosclerosis. 2015;242(2):443–449. doi: 10.1016/j.atherosclerosis.2015.08.007. [DOI] [PubMed] [Google Scholar]
- 29.Bonner WM, Laskey RA. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur. J. Biochem. 1974;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- 30.Exner T, Beretta CA, Gao Q, Afting C, Romero-Brey I, Bartenschlager R, et al. Lipid droplet quantification based on iterative image processing. J. Lipid Res. 2019;60(7):1333–1344. doi: 10.1194/jlr.D092841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Favari E, Zimetti F, Bortnick AE, Adorni MP, Zanotti I, Canavesi M, et al. Impaired ATP-binding cassette transporter A1-mediated sterol efflux from oxidized LDL-loaded macrophages. FEBS Lett. 2005;579(29):6537–6542. doi: 10.1016/j.febslet.2005.10.042. [DOI] [PubMed] [Google Scholar]
- 32.Hafiane A, Genest J. ATP binding cassette A1 (ABCA1) mediates microparticle formation during high-density lipoprotein (HDL) biogenesis. Atherosclerosis. 2017;257:90–99. doi: 10.1016/j.atherosclerosis.2017.01.013. [DOI] [PubMed] [Google Scholar]
- 33.Larrede S, Quinn CM, Jessup W, Frisdal E, Olivier M, Hsieh V, et al. Stimulation of cholesterol efflux by LXR agonists in cholesterol-loaded human macrophages is ABCA1-dependent but ABCG1-independent. Arterioscler. Thromb. Vasc. Biol. 2009;29(11):1930–1936. doi: 10.1161/ATVBAHA.109.194548. [DOI] [PubMed] [Google Scholar]
- 34.Hafiane A, Daskalopoulou SS. Adiponectin’s mechanisms in high-density lipoprotein biogenesis and cholesterol efflux. Metabolism. 2020;113 doi: 10.1016/j.metabol.2020.154393. [DOI] [PubMed] [Google Scholar]
- 35.Ouimet M, Franklin V, Mak E, Liao X, Tabas I, Marcel YL. Autophagy regulates cholesterol efflux from macrophage foam cells via lysosomal acid lipase. Cell Metab. 2011;13(6):655–667. doi: 10.1016/j.cmet.2011.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shi YQ, Yan CC, Zhang X, Yan M, Liu LR, Geng HZ, et al. Mechanisms underlying probucol-induced hERG-channel deficiency. Drug Des. Dev. Ther. 2015;9:3695–3704. doi: 10.2147/DDDT.S86724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kellner-Weibel G, Luke SJ, Rothblat GH. Cytotoxic cellular cholesterol is selectively removed by apoA-I via ABCA1. Atherosclerosis. 2003;171(2):235–243. doi: 10.1016/j.atherosclerosis.2003.08.024. [DOI] [PubMed] [Google Scholar]
- 38.Waksman R, Torguson R, Kent KM, Pichard AD, Suddath WO, Satler LF, et al. A first-in-man, randomized, placebo-controlled study to evaluate the safety and feasibility of autologous delipidated high-density lipoprotein plasma infusions in patients with acute coronary syndrome. J. Am. Coll. Cardiol. 2010;55(24):2727–2735. doi: 10.1016/j.jacc.2009.12.067. [DOI] [PubMed] [Google Scholar]
- 39.Miida T, Ozaki K, Murakami T, Kashiwa T, Yamadera T, Tsuda T, et al. Prebeta1-high-density lipoprotein (prebeta1-HDL) concentration can change with low-density lipoprotein-cholesterol (LDL-C) concentration independent of cholesteryl ester transfer protein (CETP) Clin. Chim. Acta. 2000;292(1–2):69–80. doi: 10.1016/s0009-8981(99)00259-4. [DOI] [PubMed] [Google Scholar]
- 40.de La Llera-Moya M, Connelly MA, Drazul D, Klein SM, Favari E, Yancey PG, et al. Scavenger receptor class B type I affects cholesterol homeostasis by magnifying cholesterol flux between cells and HDL. J. Lipid Res. 2001;42(12):1969–1978. [PubMed] [Google Scholar]
- 41.Rinninger F, Wang N, Ramakrishnan R, Jiang XC, Tall AR. Probucol enhances selective uptake of HDL-associated cholesteryl esters in vitro by a scavenger receptor B-I-dependent mechanism. Arterioscler. Thromb. Vasc. Biol. 1999;19(5):1325–1332. doi: 10.1161/01.atv.19.5.1325. [DOI] [PubMed] [Google Scholar]
- 42.Kiss RS, McManus DC, Franklin V, Tan WL, McKenzie A, Chimini G, et al. The lipidation by hepatocytes of human apolipoprotein A-I occurs by both ABCA1-dependent and -independent pathways. J. Biol. Chem. 2003;278(12):10119–10127. doi: 10.1074/jbc.M300137200. [DOI] [PubMed] [Google Scholar]
- 43.Zambon S, Brazg R, Aviram M, Oram JF, Bierman EL. The effect of probucol on HDL-mediated sterol translocation and efflux from cells. Atherosclerosis. 1992;94(1):51–60. doi: 10.1016/0021-9150(92)90187-l. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data reported in this study are located within the article.