Abstract
Liver transplant (LT) outcomes have markedly improved in the recent decades, even if long-term morbidity and mortality are still considerable. Most of late deaths are independent from graft function and different comorbidities, including complications of metabolic syndrome and de novo neoplasms, seem to play a key role in determining long-term outcomes in LT recipients. This review discusses the main factors associated with late mortality and suggests possible strategies to improve long-term management and follow-up after liver transplantation. In particular, the reduction of drug toxicity, the use of tools to identify high-risk patients, and setting up a multidisciplinary team also for long-term management of LT recipients may further improve survival after liver transplantation.
Keywords: Alcohol, Liver transplantation, Long term survival, Metabolic syndrome, Renal dysfunction, Therapy adherence
Core Tip: Survival after liver transplantation has increased in the last decades due to an improvement in early post-transplantation outcomes, underlining the need to shift the focus towards long-term outcomes. We herein discuss the main factors related to long-term morbidity and mortality in liver transplant recipients and outline the main management suggestions and recommendations to improve long-term outcomes.
INTRODUCTION
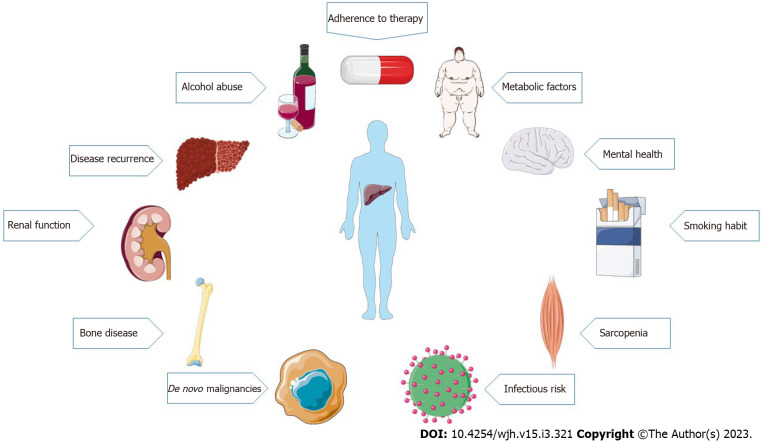
Liver transplantation (LT) is the only lifesaving treatment option for patients with end-stage liver disease and acute liver failure, and for selected patients with hepatocellular carcinoma in whom other curative treatment options have failed or are not suitable[1]. Over the past decades, early post-transplantation outcomes have significantly improved[2], while the 20-year survival rate still remains only approximately 50%[3]. In fact, long-term survivors have an increased morbidity risk, not only related to “classical” transplant-related complications, such as graft dysfunction, rejection, or liver disease recurrence, but also to factors that are not strictly related to the graft[4]. Metabolic complications, cardiovascular disease, renal dysfunction, and extrahepatic malignancies play a major role in long-term morbidity and mortality of LT patients[1]. Long-term post-transplant management is complex and requires a close follow-up to recognize, manage, and prevent medical complications and comorbidities[1] (Figure 1). The purpose of this review is to discuss the factors associated with long-term morbidity and mortality after LT, describing current recommendations and suggesting possible strategies to improve the management and the follow-up of these patients.
Figure 1.
Main factors affecting long-term morbidity and mortality after liver transplantation.
BIBLIOGRAPHIC SEARCH
A bibliographic search was conducted by two Authors (Fuochi E and Anastasio L) using PubMed and EMBASE databases, with the following terms: “liver transplant", "liver transplantation", and “orthotopic liver transplantation”. Searches of the databases were run on September 13th, 2022. Only papers written in English language were considered. After exclusion of duplicates, the search results were double-blind screened by two reviewers (TI and ENL), and abstracts assessed for eligibility. Reviews, conference abstracts and book chapters were excluded. Articles were declared not relevant by consensus.
METABOLIC FACTORS
Metabolic syndrome (MetS) is generally defined as the presence of three of five risk factors among elevated fasting glucose, reduced high-density lipoprotein cholesterol, elevated triglycerides, obesity, and hypertension[5]. It has been estimated that approximately a quarter of the world population is affected by this condition and its prevalence is still increasing[6].
There is a two-way correlation between metabolic syndrome and LT: On one hand, non-alcoholic steatohepatitis (NASH) is currently the second leading cause for LT waitlist registration/LT in general population and the first leading cause in females, at least in Western countries[7]; on the other hand, the majority of patients who have undergone transplantation develop diabetes mellitus, hyperlipidemia, and arterial hypertension[8]. It has been established that 50%–60% of these patients fulfill the criteria of metabolic syndrome[2]. In the United States, obesity is observed in 30%-40% of LT recipients within the first 5 years after transplantation[9]. Moreover, about 30% of patients suffer from diabetes after transplantation, and pretransplant diabetes is a predisposing factor[10]. Post-transplant development of MetS is due to multiple factors, such as reversal of cirrhosis, increased appetite, use of steroids, and may partly be due to the dysmetabolism of fats and sugars deriving from the use of immunosuppressants[11].
It is widely recognized that there is a strong association between metabolic syndrome and cardiovascular events. Since cardiovascular diseases are listed as the third cause of late mortality in patients who underwent LT[12], estimating cardiovascular risk and managing cardiovascular risk factors are central elements in the management of these patients. In all transplant candidates a cardiac evaluation is mandatory, although there is no ideal way to assess it. Several efforts have been made to find a more complete scoring system capable of accurately evaluating cardiovascular risk in these patients. For example, VanWagner has recently proposed the CAR-OLT score, based on age, sex, race, working status, education, liver pathology, and comorbidities to evaluate the coronary risk at one year after transplantation[13]. In 2021, Rachwan et al[14] elaborated another score, The coronary artery disease (CAD)-LT score and algorithm, that stratified significant CAD risk as low (≤ 2%), intermediate (3% to 9%), and high ≥ 10%). The score seemed to identify 97% of all significant CAD and potentially avoided unnecessary testing such as cardiac catheterization in low-risk patients[14]. However, none of these scores has been validated yet and it is uncertain whether they are able to predict long-term cardiovascular outcomes after LT.
In the post-transplant setting, European Association for the Study of the Liver guidelines recommend a continuous cardiovascular risk stratification and an aggressive management of metabolic syndrome, with a prompt detection and treatment of modifiable risk factors by means of lifestyle changes, pharmacological therapies, and modifications of the immunosuppression in order to prevent serious cardiovascular complications[1]. American Association for the Study of Liver Diseases (AASLD) Guidelines also recommend dietary counseling for all LT patients to avoid obesity. For patients who fail behavioral weight-loss programs, bariatric surgery may be considered[4]. There is still a debate about the ideal timing of bariatric surgery with respect to transplantation. A recent metanalysis compared the outcomes of bariatric surgery performed before, during, and after LT in a large cohort of obese patients. In all the analyzed groups, the 30-d mortality after surgery was 0%, although patients who underwent bariatric surgery after LT had a higher mortality rate beyond 30 d (7%). The graft survival rate after 1 year was 70% in patients operated before LT, while it rose to 100% for patients who underwent bariatric surgery during LT. Thirty-day minor and major complication rates were 4% and 1%, respectively, if bariatric surgery was performed before transplantation[15]. Further studies are required to define the optimal bariatric procedure and its timing with respect to transplantation[4].
SARCOPENIA
Sarcopenia is defined as the loss of skeletal muscle mass, quality, and function[16].
The overall prevalence of sarcopenia among patients with cirrhosis is 37.5% with an estimated higher prevalence in males, alcohol-related liver disease, and greater severity of cirrhosis[17,18]. Moreover, sarcopenia not only can be present before transplant but may also develop after surgery. This condition can be related to multiple factors such as infections, renal dysfunction, lack of specific nutritional diets, and specific medications[19].
To investigate the correlation between post-transplant sarcopenia and long-term outcome, a study on a population of 382 adult LT recipients has been recently performed in the Netherlands. Stam et al[20] measured post-transplant urinary creatinine 24 h excretion rate (24-h CER, a noninvasive marker of total body muscle mass) one year post-transplantation and found that low CER was associated with increased 10-year mortality and graft failure risk, independently of age, sex, and body surface area. Similarly, patients within the lowest tertile of CER values had worst outcomes in terms of mortality and graft failure, compared to transplant recipients in the highest tertile[20]. It must be noted that, although the 24-h CER index is an established method for assessing skeletal muscle mass, computed tomography (CT) or magnet resonance studies are currently considered the gold standard to assess sarcopenia[21]. However, urinary CER might be an inexpensive and accessible sarcopenia marker, without the need for costly exams or exposure to radiation[22].
In a recent Chinese study, sarcopenia was assessed by measuring psoas muscle index from tomography images obtained within 1 mo after transplantation in 70 male patients. Sarcopenia was identified as being significantly associated with worse post-transplant overall survival (OS) for an average of 63.3 mo of follow-up. Interestingly, sarcopenic patients seem to suffer from higher rates of hepatocarcinoma recurrence, although this difference did not reach statistical significance[23].
Another Asian study also found that sarcopenia, assessed as height-normalized psoas muscle thickness on computed tomography within 2 mo before surgery, was associated with a higher risk of tumor recurrence after transplantation for hepatocellular carcinoma. Authors hypothesized that sarcopenia may promote tumor progression by decreasing levels of certain cytokines (myokines and adipokines) and increasing others, such as tumor necrosis factor (TNF)-α[24]. Further studies are required to confirm these results.
It is important to underline that sarcopenia can also be found in obese people. In a German meta-analysis on 1515 patients, pre-transplant sarcopenic obesity (SO), assessed with different methods, was found to increase overall mortality compared to non-SO at 1, 3 and 5-years follow-up[25]. Unfortunately, using sarcopenia as a predictor of post-transplant survival is still limited by the significant heterogeneity among studies[26]. Many questions remain, including the best modality for assessing muscle mass, the optimal cut-off values for sarcopenia, the ideal timing and frequency of muscle mass assessment, and how to best incorporate the concept of sarcopenia into clinical decision making[27]. In our opinion, sarcopenia should be evaluated before transplantation, for example using CT scan, which is generally easily available as it is required for the global evaluation of the patient before transplantation. Then, the evaluation should be repeated one year after transplantation, using the same method and possibly the same CT machine, in order to compare results.
Treatment of sarcopenia is based on lifestyle modifications. Even if there are no standardized exercise programs, Tandon et al[28] recommend 150 min of mild aerobic activity divided in 3-5 d per week and more than two days per week of resistance training in cirrhotic patients. Nutritional intervention prior to transplantation may also play an important role although, to date, studies have been unable to identify strategies that offers convincing benefits. Furthermore, given that sarcopenia can also develop after transplantation, dietary advice by a nutritionist may help to improve patient prognosis. Nutritional supplementations may also play a role in this condition. For example, a recent Italian randomized pilot study reported that a 12-wk supplementation after LT with β-hydroxy-β-methyl-butyrate, an active metabolite of leucine with anabolic effect that inhibits muscle proteolysis, seems to significantly improve muscle mass values in sarcopenic LT patients[29]. However, these supplementations are usually expensive and further studies with larger cohort of patients are needed to confirm these results.
BONE DISEASES
Osteoporosis was defined by the World Health Organization in 1994 as a bone mineral density of less than 2.5 standard deviations below the sex-specific young adult mean[30]. Reduced bone density leads to decreased mechanical strength, thus making the skeleton more prone to fractures[31]. Many studies have reported how fragility fractures cause a significant morbidity and mortality burden in the general population[32], with hip fractures being the most serious, with a 33% cumulative mortality rate in the 12 mo after fracture[33].
One-third of LT recipients have a bone mineral density below the fracture threshold[34] and the fracture rate in these patients has been reported to be as high as 24%–65%[35]. Up to 55% of waitlisted patients might already have osteoporosis, especially women. In this setting osteopenia can be related to different factors such as malnutrition, physical inactivity, malabsorption of vitamin D in cholestatic liver disease, steroid use in patients with autoimmune hepatitis, and direct toxicity in alcohol-related liver damage[36]. Older age, female sex, and low body mass index (BMI) are also risk factors for osteoporosis in the general population. Furthermore, patients with end-stage liver disease present with decreased bone density compared with the age-matched control population[1].
It has been established that low bone mineral density before LT is a risk factor for developing osteoporosis after transplantation[37]. Bone loss often peaks at 6 mo after transplantation, resulting in a high fracture risk[38], even if this trend tends to reverse in the following period, with no deterioration afterward[34]. Multiple factors contribute to increased bone loss after transplantation, including use of corticosteroids, poor nutritional status, vitamin D deficiency, immobility, sarcopenia, hypogonadism, smoking, and alcohol abuse[39].
Current guidelines recommend regular measurement of bone mineral density pre- and post- LT. If osteopenic bone disease is confirmed or if atraumatic fractures are present, patients should be assessed for risk factors for bone loss; in particular, this should include an assessment of calcium intake and 25-hydroxy-vitamin D levels, an evaluation of gonadal and thyroid function, a full medication history, and thoracolumbar radiography[4]. The management of osteopenia and osteoporosis in transplant recipients correlates with recommendations for the general population and involves calcium and vitamin D replacement (if deficient) and weight-bearing exercise (whenever possible). Bisphosphonate therapy must be considered for patients with osteoporosis and/or recurrent fractures[1]. In particular, a recent multicenter randomized double-blind controlled trial evaluated the efficacy of neridronate (an amino-bisphosphonate) in patients with reduced bone mass after transplantation of the heart, liver, or lung. Neridronate, at the dose of 25 mg i.m./mo for 12 mo, significantly increased lumbar bone mineral density in these patients, with a good safety profile, even in case of minor renal impairment[40].
PSYCHOLOGICAL ASPECTS AND QUALITY OF LIFE
The World Health Organization defines quality of life (QoL) as “the individual’s perception of their position in life in the context of the culture and value systems in which they live and in relation to their goals, expectations, standards and concerns”[41]. Several studies have highlighted the importance of considering as determinants of successful transplantation not only mere survival rates, but also functional recovery and health-related QoL[42].
Few data are available on long-term QoL perception in transplant recipients. In a single-center cross-sectional study performed in England, QoL perception 10 and 30 years post-transplantation was found to be generally good, being reduced only in older individuals[43]. An American multicenter longitudinal study of 381 patients also reported that the general health perception declined over time in LT patients. There was also a general and progressive worsening of the distress or emotional burden related to physical symptoms such as fatigue, muscle weakness, headaches, and backaches[44]. These results could be explained by the normal age-related general health perception decline. Immunosuppression-related side effects could be at least partly responsible for the worsening of long-term QoL perception[45]. There is no clear association between gender or etiology of liver disease and QoL perception[46,47], although female sex and hepatitis C virus (HCV) related cirrhosis could be associated with worse QoL and higher levels of anxiety[48,49].
Moreover, in the post-transplant setting, patients tend to experience more clinically relevant symptoms of anxiety, depression, and post-traumatic stress compared to the general population[50].
Depressive symptoms appear to determine worse outcomes[51]. DiMartini et al[52] divided a cohort of 167 patients transplanted for alcoholic cirrhosis in three groups, according to the evolution of depressive symptoms within the first post-surgery year (consistently low depression levels at all time points; depression levels that rose over time; consistently high depression levels). The Authors found that recipients with increasing depression or persisting depression were more than twice as likely to die for all-cause mortality within the subsequent years. At 10 years follow-up, post-survival rate were significantly lower for the increasing-depression and high-depression groups compared with the low-depression one[52]. In another prospective cohort study, 134 LT patients were assessed for depressive symptoms using a validated questionnaire administrated 3 mo after surgery. Depressive symptoms were significantly associated with a higher 5-year mortality rate. Moreover, the questionnaire score correlated with the mortality rate in this population[53].
Interestingly, some Authors reported how patients receiving appropriate pharmacotherapy for early post-transplant depression had similar long-term survival rates to non-depressed liver-transplant recipients[54]. In addition, a recent Italian study reported how a personalized aerobic and strength training program not only improved metabolic aspects, but also the QoL perception in LT patients, so lifestyle modifications should probably be considered part of mental health management in these patients[55].
European Guidelines suggest that clinical physicians should identify depressive symptoms in the early post-transplantation period and treat them accordingly when present[1]. Unfortunately, the assessment of QoL in LT recipients has not yet been studied thoroughly and is not standardized[56]. More studies are needed to find effective strategies to manage psychological problems in this specific population. In our opinion, studies on larger cohorts of LT patients should be performed to compare different QoL and depressive symptoms questionnaires (for example, questionnaires that are already been validated in other populations such as elderly people or oncologic patients) in order to select the ones that better correlate with long term outcomes after LT.
RENAL DYSFUNCTION
Most of liver-transplanted patients develop impaired kidney function with a variable degree of severity. Within the first 10 years post-surgery, 30%-80% of patients develop chronic kidney disease stages 3-4[57] and 25–5% of patients require dialysis[58].
Renal function impairment may already be present before LT or develop or worsen after surgery. In LT candidates, renal dysfunction can be related to cirrhosis itself but also to other coexisting conditions such as diabetes, glomerulosclerosis, or IgA nephropathy[59]. Hepatorenal syndrome (HRS)-related kidney injury deriving from intense renal vasoconstriction secondary to complex circulatory changes in cirrhotic patients may not be fully reversible after transplantation[60]. Liver transplantation is considered the definitive treatment for HRS because renal failure is functional and liver disease is the actual cause of the renal impairment[61]. Patients with hepato-renal syndrome seem to have worse survival expectancy than other patients with cirrhosis for any given value of model for end-stage liver disease (MELD) score, which suggests that HRS may be considered a poor-prognosis factor after LT[62]. However, a recent meta-analysis demonstrated that about 83% of HRS patients achieved HRS reversal after LT[63] and HRS-non acute kidney injury seem to have worse outcomes compared to HRS-acute kidney injury[64]. Living-donor LT results in identical long-term outcome when compared with deceased-donor LT in patients with HRS[65]. Many factors may contribute to the development or worsening of kidney failure after transplantation, including perioperative acute kidney injury, hypertension, diabetes mellitus, atherosclerosis and, most importantly, exposure to calcineurin inhibitors (CNI)-based immunosuppressive regimens, especially when it comes to long-term therapies[1]. CNIs might be responsible for more than 70% of chronic kidney injury in post-transplant setting[57].
Many studies have reported an increased risk of death, myocardial infarction, stroke, and major bleeding in chronic kidney disease, especially in its most severe stages[66]; thus, preventing renal deterioration and preserving its function may be a key element in the management of transplanted patients.
Continuous monitoring of renal function is recommended to detect and treat kidney disease at an early stage. Not only serum creatinine, but also an estimating equation to evaluate the glomerular filtration rate should routinary be used. Urinary protein quantification using the concentration ratio of protein to creatinine in a spot urine specimen should be evaluated at least once yearly[4]. Sufficient treatment of potential risk factors such as diabetes and hypertension and avoiding nephrotoxic drugs is recommended and should be started immediately after transplantation[1]. Adjustment of the immunosuppression (IS) (usually on an individual level, especially in patients with impaired kidney function), is mandatory. In particular, reduction or withdrawal of CNI associated-immunosuppression or alternative CNI-free protocols should be considered as soon as possible in patients with impaired renal function[1]. For example, a recent meta-analysis on 769 patients has detected higher estimated glomerular filtration rates (eGFR) at one, 3, and 5 years post transplantation in patients on everolimus therapy (EVR) compared to those receiving CNI standard therapy[67]. In 2019, the observational CERTITUDE study, following patients who had completed the SIMCER trial, found that patients starting EVR therapy at month 1 after transplant with stepwise tacrolimus (TAC) withdrawal had a mean eGFR which was significantly higher compared to patients that were on standard tacrolimus-based regimen at 24 mo after transplant[68]. A phase 2, multicenter, randomized, open-label trial has evaluated the safety and efficacy of EVR initiation even earlier than 1 mo after LT[69]. In this study, patients treated with corticosteroids, TAC, and basiliximab were randomized to receive EVR (1.5 mg twice daily) from the eighth day post-surgery and to gradually minimize or withdraw TAC when EVR was stable at > 5 ng/mL or to continue TAC at 6-12 ng/mL (control group). eGFR was significantly higher in the EVR group, as early as 2 wk after randomization, with similar efficacy rates in the two groups at 3 mo follow up. These studies suggest that EVR based IS, started early after transplantation, might be a valid alternative to CNI-based therapies in patients with renal dysfunction. Despite its positive effect on renal function, early switch to EVR has been associated with higher biopsy-proven acute rejection at 6 mo follow up[70], so that the choice of IS treatment should always be personalized and made weighing up the risks and benefits of the different therapeutic strategies.
Kidney transplantation from deceased or living donors is beneficial in improving survival and should be considered the optimal therapy for LT recipients who develop end-stage renal disease[4].
INFECTION RISK AND VACCINATION
Solid organ transplant recipients are at an increased risk of infection because of the IS required to prevent graft rejection[71]. Vaccination is considered an important strategy to prevent infectious risk not only in the general population but also in transplanted patients[1]. As immunodepression can reduce immune response to vaccines[72] and live attenuated vaccines are not recommended in immunocompromised patient[73], guidelines suggest to perform HAV, HBV, Varicella, Pneumococcus, influenza, and tetanus vaccinations prior to transplantation, if possible[1]. Many national guidelines recommend annual influenza vaccination of immunocompromised patients, although the decision to vaccinate is usually at clinical discretion[74]. A meta-analysis conducted on 209 studies has found that transplanted patients, together with HIV and cancer patients, are those who benefit most from the annual boost as it significantly decreases the rate of laboratory-confirmed influenza cases in these patients[75].
Influenza is a considerable public health issue due to its dissemination and contagiousness, causing annually about 4 million severe infection cases and about half of million deaths each year[76]. The precise epidemiology of influenza in the transplant population is not well known because little data are available describing the incidence of influenza in multi-season and multicenter prospective cohorts, in particular for recipients of allografts other than lung[77]. However, influenza seems to be more common among solid organ transplant recipients compared to the general population, as showed in a 10-year longitudinal study with an incidence of 4.3 cases per 1000 person years[78]. In patients with impaired immunity, influenza is more likely to lead to a lower respiratory infection and can also have unusual manifestations such as rhabdomyolysis and myocarditis[78,79].
A recent multicenter prospective study including 606 transplanted patients from twenty centers in the U.S., Canada, and Spain showed that receiving vaccination for influenza is associated with a decrease in disease severity as determined by the presence of pneumonia and Intensive Care Unit admission[80]. Similar results have been found in another recent Italian study, in which vaccination was associated with fewer hospital admissions for infectious respiratory diseases compared to unvaccinated patients (9.7% vs 23.5%). The main reason for vaccination refusal was fear of adverse reaction, impaired health status, or low vaccine efficacy. Interestingly, receiving advice of Reference Center physicians was positively associated with influenza vaccination, highlighting the important role of the transplant hepatologist with regard to vaccine communication and recommendation for high-risk patients[81].
In addition, there is no consistent evidence suggesting an association between influenza vaccine and graft rejection, worsening of allograft function, or other serious adverse events in immunocompromised patients[75].
European and American Guidelines recommend influenza vaccination in transplanted candidates and annual influenza vaccination in liver transplanted patients[1,4]. The Infectious Disease Society of America guidelines suggest administering inactivated influenza vaccine starting from one month after transplantation during community influenza outbreaks[82].
Adherence to seasonal influenza vaccination is still low in immunocompromised patients, reaching a maximum of 50%-60% of patients[83,84]. Accurate counseling by the hepatologist may increase the percentage of vaccinated patients and therefore improve the long-term outcome of these patients[81].
A recent meta-analysis of randomized controlled trial has compared the clinical benefit of high dose trivalent influenza vaccine (TIV) vs standard dose in adult patients. One of the 10 analyzed studies also included immunocompromised patients. The meta-analysis found that laboratory-confirmed influenza A (H3N2) was significantly reduced with high-dose TIV, especially in older adults, even if no difference in mortality or hospitalizations was demonstrated[85]. Further studies are needed to compare the efficacy of high-dose vs standard-dose TIV in LT patients.
Another strategy to prevent influenza disease is the prophylaxis with neuraminidase inhibitor (Oseltamivir) during periods of local influenza circulation. A randomized controlled trial on 477 immunocompromised subjects, mostly solid organ transplant adult recipients, has found that Oseltamivir, given orally at the dosage of 75 mg daily, significantly reduced laboratory-confirmed influenza incidence in these patients and it was also well tolerated[86]. Also, Oseltamivir does not affect the steady-state pharmacokinetic characteristics of cyclosporine, mycophenolate, or tacrolimus, at least in adult renal transplant patients[87]. In our opinion, Oseltamivir prophylaxis may be considered as a strategy to prevent influenza disease in LT recipients along with vaccination. Oseltamivir is also indicated to prevent serious complications when influenza is established. A recent randomized, placebo-controlled, phase 3 trial found that a single dose of Baloxavir marboxil, a selective inhibitor of influenza cap-dependent endonuclease, has similar efficacy to Oseltamivir in improving influenza symptoms in high-risk healthy individuals[88]. To our knowledge, specific studies on LT population comparing these medications are lacking and further studies are needed to support the use of this drug in transplant patients.
DE NOVO NEOPLASMS
Besides cardiovascular diseases, de novo malignancies are the leading cause of mortality after the first post-LT year[1]. LT patients have an 11-fold higher risk of developing cancer compared to the general population[89]. The overall incidence of de novo malignancies is considered between 3.1 and 14.4%, with a cumulative risk gradually increasing with posttransplant graft survival, rising to 55% at 15 years[90]. The overall estimated survival rates for all types of neoplasms are reportedly 70, 48, and 39% after 1, 5, and 10 years, respectively[90]. Notably, the probability of survival is generally worse than for a non-transplanted patient with the same tumor at the same stage and location[91].
Recent data suggest that solid organ tumors are becoming the most frequent malignancy in these patients, followed by skin cancers and lymphoproliferative disorders. In particular, Rademacher et al[92] have analyzed 1616 LT patients and have found that solid organ tumors were responsible for more than 50% of all the novo malignancies after a mean follow-up of 28 years.
The major causes of de novo malignancies in the post-LT course are related not only to the loss of immunovigilance induced by immunosuppressive agents but also to other carcinogenesis risk factors that are shared with the general population[1].
For example, Epstein Barr virus seropositivity before transplantation and aggressive immunosuppressive regimens are considered risk factors for developing lymphoproliferative disorders after transplantation[93]. On the contrary, major risk factors for developing non-melanoma skin cancers in these patients are older age, chronic sun exposure and sunburn, fair skin, and a history of previous skin cancers[94]. Considering solid organ tumors, significantly higher rates of colorectal cancer have been reported in patients with primary sclerosing cholangitis (PSC) and inflammatory bowel disease (IBD), even after LT[95]. Metabolic syndrome, that is common in transplanted patients, as previously discussed[7,8], is associated with a higher risk of endometrial, pancreatic, breast, and colorectal cancer[96]. Human papilloma virus (HPV) infection is associated with various cancers and, especially, with cervical cancer in women[97]. Patients with alcoholic cirrhosis are of particularly have a higher risk of developing upper gastrointestinal, oropharyngeal-laryngeal, and lung cancers, especially if there is also a positive present or past smoking history[98].
Treating modifiable risk factors and thus preventing cancer onset must be part of the clinical management of LT recipients. Smoking cessation and alcohol withdrawal should be promoted to reduce lung and head-neck cancers incidence[99]. Metabolic syndrome, obesity, and diabetes should be managed in order not only to prevent cardiovascular disease, but also to reduce cancer burden in these patients[100]. Sunbed use and sun exposure without adequate protection should be avoided to prevent skin cancers[101]. HPV vaccination is safe in immunosuppressed patients and is indicated to prevent cervical cancer[102]. A recent study has compared the immunological response and tolerability of HPV vaccination in pediatric kidney transplant (KT) recipients vs KT non immunosuppressed candidates. The study established that antibody concentration against HPV and seroconversion rates were significantly lower in patients vaccinated after KT compared to those who had been vaccinated before KT. The vaccination was well tolerated in both groups. This study suggests the importance of advocating for HPV vaccination prior to transplantation and acknowledges its safety after transplantation[103]. To our knowledge, there are no specific studies comparing HPV vaccination prior and after LT. Also, future studies are needed to investigate the effect of a supplemental dose of HPV vaccine in transplant recipients who do not seroconvert and to evaluate the long-term persistence of antibodies post-transplantation.
European and American guidelines highlight the importance of cancer screening protocols after LT, especially in high-risk populations, in order to detect de novo tumors at an early and potentially curative stage[1,4]. Patients transplanted for alcoholic liver disease should undergo a more intensive surveillance protocol for the detection of upper gastrointestinal, oropharyngeal, laryngeal, and lung cancers[1]. Patients transplanted for PSC with associated IBD should undergo annual colonoscopy to allow early detection of colorectal cancers[1]. American guidelines suggest that all LT recipients should see a dermatologist after transplantation to assess cutaneous lesions. Then, annual evaluation should be performed at least 5 years after transplantation for skin cancer prevention[4]. More data, however, are needed to define the optimal surveillance protocol after LT with individualized emphasis laid on patients’ particular risk profiles[1]. A proposal for a possible screening protocol is shown in Table 1.
Table 1.
Screening protocols proposal for the surveillance of de novo neoplasms in liver transplantation population
| Malignancy | Screening proposal |
| Skin cancer | Annual dermatological visit[4], shorter follow up interval for high risk patients (i.e. every six months) |
| Lung cancer | Annual thoracic X-Ray; CT-scan in active or past smokers[282] |
| Colorectal cancer | Perform baseline colonoscopy on patients > 50 years old; annual fecal occult blood test in younger patients or if colonoscopy is negative; annual colonoscopy if patient affected by PSC + inflammatory bowel disease[1] |
| Ear, nose, and throat cancers | Annual otolaryngological visit in patient with active or past alcohol and/or smoking habit[124] |
| Renal cancer | Annual abdominal ultrasound |
| Cervical cancer | Annual papanicolau-test; annual gynecological visit |
| Breast cancer | Annual mammography, ultrasound evaluation if needed |
| Prostate cancer | Annual PSA and PSA ratio evaluation |
CT: Computed tomography; PSC: Primary sclerosing cholangitis; PSA: Prostate specific antigen.
Immunosuppressants also play a key role in promoting cancer development and progression, not only by inhibiting the body’s immune surveillance, but also by several other mechanisms, including the induction of insulin resistance and direct carcinogenic effects[104,105]. A lower incidence of neoplastic disease has been reported in patients treated with mammalian target of rapamycin inhibitors (mTORi) with a gradual tapering of CNI, if compared with patients on standard-dose CNI[106,107]. For this reason many transplant centers frequently add mTORi to CNI or convert to an mTOR inhibitor IS regimen when there are risk factors for malignancy after transplantation, or when a tumor has been diagnosed[1]. Nevertheless, all immunosuppressant regimens could increase de novo neoplasms risk, including those based on mTORi[89], so it is advisable to keep IS levels as low as possible, when feasible[108].
SMOKING
The prevalence of patients with a lifetime history of smoking before LT varies between 47% and 60%, while that of active smokers at the time of LT ranges between 10%-12%, with a relapse rate of 7%-12%[109-111]. As expected, patients who quit smoking for a shorter time before LT are those with higher rates of relapse[109]. These figures are subject to variation depending on the considered population, as there are higher rates of smokers among patients who underwent LT for alcohol-associated liver disease (ALD)[112,113]. Cigarette smoke is in fact clearly linked to alcohol consumption[114], which is why smoking habits should be especially investigated in individuals with previous ALD or current known alcohol use.
Other risk factors associated with being an active smoker until and after LT are younger age, higher MELD score, comorbid substance use disorder, and six months or less of alcohol abstinence before LT; alcohol dependence awareness is a protective factor from smoking after LT, underlining the close relationship between the two habits[115].
The majority of studies investigating smoking habits of LT recipients uses self-reported instruments (i.e. questionnaires) which appear to be sufficiently reliable in LT candidates, with about 10% not disclosing their smoking habit. In this setting, the use of a biomarker such as serum cotinine can be helpful in detecting deceptive reporting[116].
Tobacco smoking hampers long-term survival[112,117], with a worse prognosis in ALD-transplanted recipients who are active smokers at the time of LT compared to former smokers[115]. Smokers have a 79% higher risk of dying compared to nonsmokers[115]. A history of smoking is not only a well-known risk factor leading to the major causes of death in the long-term post-LT, such as development of cardiovascular complications and de novo neoplasms, as showed by a recent meta-analysis[118], but it has also been associated with alcohol relapse in ALD-transplanted patients[119], recurrent viral-hepatitis[120], an augmented risk of IBD flare in PSC-transplanted patients[121], and with an increase in biliary complications[122]. On the other hand, an increased time from smoking cessation to transplantation seems to be a protective factor against developing biliary complications[123].
Undoubtedly, special attention during follow-up is warranted for patients with a history of smoking, by means of screening (annual chest CT and ear-nose-throat evaluation) for early detection of de novo malignancies[124] and by actively assessing their smoking status at each visit, focusing on those with particular risk of relapse, implementing tobacco cessation treatments, and, if needed, providing a referral to start behavioral and/or pharmacological treatment[115,116,125,126].
MAINTENANCE IMMUNOSUPPRESSION AND ADHERENCE TO THERAPY
The transplanted liver becomes partially tolerant to immune-mediated injury, so the need for IS declines after the first 90 d[4]. Since the liver is considered a privileged organ in terms of immunological interaction, the clinician’s aim has switched from trying to achieve complete suppression of acute rejection to obtaining a reduction of IS-related side effects, as long-term direct and indirect side effects of immunosuppressive therapy are a major cause of morbidity and mortality[1].
Maintenance IS therapy after LT is mainly based on CNI, with TAC being favored over Cyclosporine, with a variable use of other two classes: Antimetabolites like Azathioprine and mycophenolate mofetil, and mTORi, such as sirolimus (SRL) and everolimus. Management of immunosuppressants should take into consideration recipient characteristics, etiology of primary liver disease, and magnitude of alloimmune activation[108].
Each of these drugs has adverse effects (Table 2); for this reason, given the tolerogenic aspect of LT, an immunosuppressant minimization strategy should be considered for each patient (except for those with a history of graft rejection or those transplanted for immune-mediated diseases), while waiting for further development of personalized therapies[108].
Table 2.
Maintenance immunosuppressants main adverse effects
| Drug class | Adverse effects |
| CNI | Nephrotoxicity[283], recurrence of HCC[188,284], risk of de novo neoplasia[285-287], new onset diabetes mellitus (TAC more than CyA)[288,289], hypertension (CyA more than TAC)[290], dyslipidemia[291] (CyA more than TAC)[292], neurotoxicty[293], weight gain[294,295] |
| Antimetabolites | Leukopenia, thrombocytopenia, gastrointestinal disturbances (MMF and AZA) diarrhea, CMV reactivation (MMF)[296], pancreatitis, hepatotoxicity, risk of de novo neoplasia (AZA)[296,297] |
| mTORi | Leukopenia, dyslipidemia[298,299], cutaneous and mucosal alterations[300], wound complications, lymphocele[301], hypertension[302] |
CNI: Calcineurin inhibitors; HCC: Hepatocellular carcinoma; TAC: Tacrolimus; CyA: Cyclosporine; MMF: Mycophenolate mofetil; AZA: Azathioprine; mTORi: Mammalian target of Rapamicin inhibitors.
Even though IS complete withdrawal should only be limited to clinical trials[108] it always remains an interesting perspective. Recently, Levitsky et al[127] conducted a pilot clinical trial of SRL monotherapy withdrawal in 15 selected recipients, who were followed-up for 12 mo after complete IS withdrawal with serial peripheral blood and graft biomarker assessments: 8 (53%) patients were successfully withdrawn from SRL at a median of 18 wk. Interestingly the authors found higher percentages of tolerogenic dendritic cells (HLA-DR +CD11c+ILT3+ILT4+ DC) prior to and after successful SRL withdrawal, compared to those who failed withdrawal. Furthermore, the authors previously identified a real-time PCR based biopsy signature relating to iron metabolism that predicted tolerance and found that this same signature on pre-weaning biopsy accurately predicted tolerance to withdrawal, with 88% sensitivity, 83% specificity, 88% positive predictive value and 83% negative predictive value.
On the other hand, it must be noted that poor adherence to therapy and/or low blood levels of immunosuppressant are associated with a higher number of acute rejection episodes[128,129], which has been linked to chronic rejection that may lead to re-transplantation or death[130].
Poor-adherence to therapy has been reported in up to 50% of LT recipients[131], even though there is a substantial heterogeneity in the definition of non-compliance[132], often causing difficult comparisons among study results.
Assessing adherence to therapy is also an issue. Electronic monitoring (e.g. using pill bottles with a special cap that contains microelectronics to register the time and date of every bottle opening) yields detailed and reliable data but it is time- and cost-consuming[133]. There is also debate for its use as a gold standard, since it may not be feasible in clinical practice[134,135]. Trough levels can be affected by a variety of conditions, such as graft function and the concomitant use of other drugs[136]. Self-reporting could be a reliable method[137], but it lacks of objectivity[138].
A study conducted in kidney transplant recipients showed how a composite score using self-reported non-adherence and/or collateral-reported non-adherence and/or non-therapeutic blood assay variability had the highest sensitivity in assessing non-adherence to therapy[139].
Factors associated with poor compliance to therapy are: Young age[140], divorce, history of substance or alcohol use, mental health disorders, missing clinic appointments[131], belief in alternative medications, high regimen complexity, poor knowledge about medications, and cost issues[137].
As showed by a recent meta-analysis of randomized controlled trials conducted on solid organ recipients (mainly kidney)[134], adherence-enhancing interventions can result in significant increases in total adherence, medication dosing, and timing adherence rates, and even if there is insufficient evidence to assess which type of intervention (mobile health, cognitive, or behavioral) may be maximally effective, probably a combination of multiple interventions led by a multidisciplinary team may improve the immunosuppressive therapy adherence rate for solid organ recipients.
In a review by Burra et al[136], the Authors underlined the need to adopt a multidisciplinary approach for LT patient management, where multidisciplinary measures are developed by professional educators, supported by psychologists, and coordinated by physicians.
DISEASE RECURRENCE
ALD
ALD is the main indication for LT in Europe[141] and the United States[142].
There is no standardized definition for relapse thus reported relapse rates vary greatly: from nearly 50% if relapse is intended as alcohol use of any measure[143], to 12% if relapse is intended as harmful alcohol consumption, starting as soon as 1 mo after LT[144]. Interestingly, the study from Faure et al[145] reports excessive alcohol consumption post-LT in about 10% of the patients who were not transplanted for ALD as a primary indication, but who reported excessive alcohol consumption before LT, and about 3% of patients who did not report excessive alcohol consumption before LT. For this reason, a thorough history and ongoing monitoring of alcohol consumption in all patients is of great importance during follow-up of LT patients.
Factors associated with alcohol relapse are psychiatric comorbidities, pre-transplant abstinence of less than 6 mo[146], smoking[147], alcohol consumption from an early age[148], noncompliance with appointments or medication[149], and the lack of social support, in particular the absence of a companion in life[150]. Satapathy et al[151] have proposed a “Harmful Alcohol Relapse after Liver Transplant” score, that included 4 variables (i.e. age at LT, alcohol abstinence measured in months, daily alcohol use, and history of non-alcohol-related criminal history) to help identify ALD patients at high-risk for harmful alcohol relapse, with an Area Under the Curve (AUC) of 0.79 for predicting relapse after LT.
Lee et al[152] developed an artificial intelligence model to predict post-LT harmful alcohol consumption in patients who underwent early liver transplant for alcohol associated hepatitis, using variables generated through content analysis: These variables included the identification of a primary support person, the presence of young children or grandchildren living with the patient, being a home caregiver for children or elderly relatives, opioid abuse, and being religious; this model could predict harmful alcohol consumption in the external validation set with a positive predictive value of 0.82 (95%CI: 0.625–1.000) and a negative predictive value of 0.81 (95%CI: 0.803–0.819), with and AUC of 0.69, indicating potential for AI to assist in the discovery of novel predictors of post-LT hazardous alcohol use, which may be used as a tool to tailor therapies for alcohol use disorder based on a projected likelihood of relapse.
While transplantation for ALD has a favorable outcome even when compared to other etiologies, de novo malignancies and cardiovascular events are still more frequent in this category of patients[153]. Excessive alcohol consumption post-LT is associated with a further reduction of long-term survival, with cancer and cardiovascular events as the main causes of death[154,155]. It seems that an average of 5 years follow-up post-LT is needed to observe an increase in liver-related mortality in the excessive alcohol consumption group[156]. It is important to keep in mind that excessive alcohol consumption-related impact on long-term survival can be an issue for every LT recipient, no matter what the primary indication for LT was[145].
Early diagnosis and prevention of relapse is important, given the clinical influence of excessive alcohol consumption post LT. Self-reported alcohol use can lead to deceptive reporting, so that biomarkers can be a supportive tool, in particular liver function tests and metabolites of alcohol, such as urinary ethyl glucuronide[157,158].
A structured management of patients at risk of relapse by a multidisciplinary team, including transplant hepatologist, clinical psychologists, psychiatrists with expertise in alcoholism and social workers is an effective strategy to prevent relapse post-LT[159,160].
In the study by Addolorato et al[161] , follow up of LT-recipients by an alcohol addiction unit, formed by internists, physicians in training, and psychologists with expertise in alcoholism, hepatology, and neuroscience, providing multimodal treatment (clinical and medical management, including counseling and pharmacological treatment), proved to be effective in reducing alcohol recidivism and mortality.
To our knowledge, there is still no published RCT evaluating the best intervention to prevent alcohol relapse in LT recipients. However, there is evidence suggesting that a multidisciplinary team approach is an effective way to prevent relapse[159-161]. We still do not have an ideal tool to predict who will relapse after LT, but some risk factors have been identified[147-151], allowing the clinician to focus on specific psychosocial features. Ongoing monitoring for alcohol-relapse is necessary for ALD patients[162], but a thorough history of alcohol consumption and assessment use during follow-up is also important for non-ALD recipients, since excessive alcohol consumption cannot be excluded in this category, also many years after LT[163]. Referral to psychiatric treatment or counseling is recommended in case of relapse, and every patient who underwent LT for ALD should also be encouraged to undertake smoking cessation[1,4].
Hepatocellular carcinoma
Hepatocellular carcinoma (HCC) is an indication for LT[1,164] in the first stages of the neoplastic disease, or after downstaging[165]. Recurrence of the disease develops in 8-18% of the recipients after a median time of 12 mo[166,167]. It significantly affects survival, especially if it appears in the first two years after LT, with a median survival after LT of 4 years vs 12 years in those without recurrence[168]. Among the many pre- and post-operative risk factors associated with recurrence[169], we can find tumor stage[170,171], vascular invasion[172], high Alpha-fetoprotein (AFP) levels[171] and differentiation grade[173-175], size, and number of nodules[175].
Several prognostic models have been developed using some of these risk factors; these models can be divided in pre- and post-transplant. Among the pre-transplant prognostic models the Milan criteria (solitary HCC with diameter < 5 cm or up to 3 nodules with diameter ≤ 3 cm) represent the benchmark for the selection of HCC patients for LT and the basis for comparison with other proposed criteria[1]. Attempts to expand the Milan criteria usually occur at the expense of HCC recurrence[176]. Other pre-transplant criteria which have demonstrated comparable survival results are those relying on size/number of nodules alone, such as the University of California San Francisco (UCSF) criteria (single nodule ≤ 6.5 cm or 2–3 nodules ≤ 4.5 cm and total tumor diameter ≤ 8 cm)[177] and the Up-to-7 criteria (sum of the largest tumor size and number of lesions < 7)[178] and those including AFP beside the size/number of nodules, such as Metroticket 2.0 (AFP levels + tumor number and size of the largest tumor) which showed good results compared to the above-mentioned models[179]. Every nodule with an intermediate-to-high probability of harboring HCC according to LI-RADS protocol seems to contribute to tumor burden and should be entered in the Metroticket 2.0 calculator in order to grant appropriate performance[180]. It has also been demonstrated how incorporating the modified RECIST criteria in response to neoadjuvant therapies into the Metroticket 2.0 framework can improve its predictive ability[181]. In any case, it is mandatory to evaluate not only preoperative but also postoperative predictors of recurrence as there may be a mismatch between radiological findings before surgery and postoperative pathological assessments[182].
Among the post-transplant criteria, Parfitt et al[183] developed a risk score to predict HCC recurrence, based on microvascular invasion, tumor size, satellitosis and giant/bizarre cells visible at low power which was subsequently externally validated[184], showing a sensitivity of 80%, specificity of 79%, and an area under the ROC curve (AUROC) of 0.80.
Mehta et al[185] developed a simple prognostic score (RETREAT score) involving patients transplanted within the Milan Criteria, using 3 variables: AFP levels, the presence of microvascular invasion, and the sum of the diameter of the largest viable tumor plus the number of viable tumors. The score was able to stratify 5-year HCC recurrence risks ranging from less than 3% in those with a risk score of 0 to higher than 75% with a risk score of 5 or higher.
In this setting, the choice of the IS regimen is extremely important since mTORi, primary SRL, seem to have a protective effect against HCC recurrence[186,187] while CNI therapy is associated with an increased risk of tumor recurrence[188], even though the current recommendation is to minimize IS[108], with no mention to a specific IS regimen. AASLD guidelines also suggest considering an IS regimen including SRL, started several weeks after transplantation, for patients undergoing transplantation for HCC[4].
Strategies for preventing HCC recurrence mainly rely on an adequate pre-transplant selection of candidates[176] and on optimizing IS regimen[108] since there currently is no approved adjuvant therapy that has demonstrated prolonged disease-free survival[189-191]. On this basis, early diagnosis of HCC recurrence gains a central role in the post-LT care but there is no consensus about screening for recurrence, translating in a significant variability in center practices. In fact, Aggarwal et al[192] recently conducted a survey among 48 American adult liver transplant centers: There was considerable variation in the duration of surveillance, with 48% of the reporting centers maintaining surveillance for 5 years, while 18% discontinued surveillance after 2 years; 38 out of 48 centers used a risk stratification method for disease recurrence post-LT, categorizing patients into high and low risk groups, mostly based on the presence of microvascular invasion, tumor differentiation grade, discrepancy between pretransplant radiologic tumor size or number and explant pathology, and serum AFP measured before LT or at the time of LT. As expected, AFP was the most commonly used biomarker for detecting recurrence and 13 centers used specific cutoff values for serum AFP (between 100 and 500 ng/mL). On the other hand, 21% of the reporting centers employed solely abdominal/pelvic imaging and only 5% including bone imaging. The most frequently used imaging monitoring routine was every 3–4 mo in the first year, followed by every 6 mo in the second year, and every 6–12 mo at 3 years or beyond. For patients who were thought to be at a higher risk for HCC recurrence, 21 of the 38 facilities that stratified HCC recurrence risk had a more stringent "high risk" surveillance protocol, with a significant variability among the centers: In the first five years after liver transplantation, imaging was most frequently reported every 3 to 6 mo. Only in a few centers surveillance was interrupted after two years of follow-up (14%).
A reasonable surveillance strategy should therefore include chest and abdomen imaging and serum AFP monitoring, and should be more rigorous for those patients with high risk features for recurrence, especially during the first year after LT[193]. Most authors report monitoring for HCC recurrence post-LT with thoracic CT, abdominal CT or MRI, and AFP levels with 3- to 6-mo intervals in the first 2 or 3 years, increasing the interval between exams after the 2 or 3-year timepoint[194].
Increased surveillance may improve post-recurrence survival, though optimal surveillance strategies have yet to be proven; currently, no surveillance guidelines exist in this setting[195,196].
In fact, it is not clear whether screening for recurrence is worthwhile at all, due to poor results of systemic treatment for recurrence after LT[197]. Thus, surveillance should be customized according to a known recurrence pattern (i.e. frequent time and space frame) in order to be more cost-effective[198], considering that recurrence beyond 5 year is less common and associated with better prognosis[199]. Recurrence of HCC can be intrahepatic and/or extrahepatic, with the lung and bones as the most common extrahepatic sites of recurrence[199,200].
The study from Ladabaum et al[201] suggests that the relatively small gains in life-expectancy that may be achieved by screening for recurrence after LT are likely to be associated with relatively high incremental costs per life-year gained, and that the greatest benefit of screening is more likely to be derived by screening patients whose explant pathology exceeded the Milan criteria and by limiting screening to the first two years after LT.
Lee et al[195] showed how increased surveillance, measured by cumulative exposure to surveillance (CETS-i.e. the cumulative sum of all the protected intervals that each surveillance test provides) is associated with improved post-recurrence survival and a higher probability of aggressive treatment: In particular 252 d of CETS in the first 24 mo after LT would yield the best sensitivity and specificity for identifying disease which can be treated with either resection or ablation.
The above mentioned RETREAT score underwent a subsequent validation by Mehta et al[202] who found that a higher score is associated with a shorter time to HCC recurrence; the authors proposed a cost-saving surveillance strategy in which no surveillance is needed for patients with a score of 0, surveillance every 6 mo for 2 years is warranted for those with a score of 1-3 and for 5 years for those with a score of 4, while for those with a score > 5 surveillance is warranted every 3– 4 mo for 2 years then every 6 mo for 2-5 years.
Further studies addressing the optimal strategy, the survival benefit, and the cost-effectiveness of surveillance for HCC recurrence should be undertaken, given that not being amenable to a curative-intent treatment has been found to be a poor prognosis factor in recurrent HCC post-LT[203].
Regarding treatment options, European guidelines suggest that treatment of HCC recurrence should probably follow the same algorithms used for immunocompetent patients, and also considering re-transplantation in selected cases[1], while American guidelines state that resection or ablation is usually the treatment of choice for a solitary extrahepatic metastasis or intrahepatic recurrence of HCC and that ablation with radiofrequency (RFA) is the best treatment for small solitary recurrences[4]. It seems that patients undergoing surgical treatment have a better post-recurrence survival, for both in intra-[204] and extrahepatic[205] recurrence, compared to those not undergoing resection, with a reported OS of 20-27 mo after HCC recurrence, in those who underwent surgery, significantly superior to patients who received only nonsurgical therapy (9-10 mo) or best supportive care (2-4 mo)[204,206]. There is still a dearth of information regarding locoregional treatments for the management of HCC recurrence following LT, so further data is needed. Among the few studies evaluating locoregional treatment we can find a study by Huang et al[207], which was conducted on 78 patients who had recurrence of HCC post-LT and found no significant difference in terms of OS or recurrence-free survival between the group undergoing surgical resection and the group undergoing RFA.
In a retrospective study conducted on 28 patients with HCC recurrence, Zhou et al[208] compared the outcomes of 14 patients receiving chemoembolization to 14 matched control subjects not receiving chemoembolization: Patients who underwent chemoembolization had significantly longer OS after LT and after the diagnosis of HCC recurrence (median OS after LT 865 d, median OS after HCC recurrence 286 d) compared to those who did not (median OS after LT 228 days, median OS after HCC recurrence 85 d), respectively, with no severe complications, and 57% in the treatment group showing partial response. The development of new recurrence, both intra- and extrahepatic, was still high in both groups (86% in those receiving chemoembolization vs 93% in those who did not), implying that the improved survival in patients receiving chemoembolization is likely attributed to the control of established tumors instead of the prevention of new lesions.
Regarding systemic therapies, Mancuso et al[209] conducted a meta-analysis of studies on survival and safety of sorafenib for HCC recurrence after LT with the aim of estimating the 1-year rates of survival: Overall the median survival was 10.5 mo (range 5 to 21.3). The pooled estimate of the 1-year survival rate was 63% (range, 18%–90%) with a significant heterogeneity among studies (P < 0.0001). Studies on sorafenib have shown that systemic therapy improves survival when compared to optimal supportive care alone. Patients experienced considerable medication toxicity, along with poorly tolerated side effects[210,211]. Close monitoring is necessary and should be even closer if IS regimen includes mTOR inhibitors as well, as the association between Sorafenib and mTORi showed an increased frequency of dose reduction and discontinuation due to adverse events[212,213]. Regorafenib could be an option in patients progressing while on treatment with sorafenib[214].
Recently Iavarone et al[215] conducted an observational multicenter retrospective study on 81 LT patients with HCC recurrence who discontinued first-line sorafenib (36 treated subsequently with regorafenib and 45 undergoing BSC at sorafenib discontinuation): The median OS was significantly longer in the group treated with regorafenib than in the group undergoing BSC (13.1 mo vs 5.5 mo, P < 0.01); treatment with regorafenib was an independent predictor of reduced mortality (hazard ratio, 0.37, P = 0.02).
Scarce data is currently available for other tyrosine kinase inhibitors (lenvatinib and cabozantinib), which would allow to propose new treatment sequences for patients with HCC recurrence after LT[198].
Another promising option for post-transplant HCC recurrence includes immunotherapy: The combination of atezolizumab (an anti-PD-L1) and bevacizumab (anti-vascular endothelial growth factor monoclonal antibody) proved to be superior to sorafenib in non-LT setting[216].
It must be noted that there is evidence that immunotherapy can interfere with post-transplant immunological tolerance and lead to allograft rejection that is resistant to treatment[217].
Luo et al[218] recently conducted a pooled analysis of the published cases of post-LT immunotherapy-treated HCC, including 29 patients: The overall response rate (complete response and partial response) to immunotherapy was 31.3% , including 18.8% with complete response and 12.5% with partial response
In the immune checkpoint inhibitors subgroups, including 19 patients, rejection was experienced by 6 out of 19 patients (32%), including 5 receiving nivolumab and 1 receiving pembrolizumab; allograft rejection exhibited a tendency to occur shortly after immunotherapy initiation, at a median time of 12 d and patients who started immunotherapy shortly after LT seemed to be at a higher risk of rejection than those starting after a longer interval of time.
After a median follow-up of 3 mo 68% of patients died (13/19), but only 23% (3/13) of those deaths were due to early rejection; the authors concluded that allograft rejection can be lethal, but the possibility of rejection-related death justifies considering immunotherapy as a backup plan because disease progression invariably results in death.
Post-transplant HCC recurrence currently represents a diagnostic and therapeutic challenge, and as such, patients transplanted for HCC need a close surveillance and an individualized management discussed in a multidisciplinary team in case of recurrence.
Autoimmune diseases
Reported recurrence rates vary among the different autoimmune diseases: The prevalence of recurrent primary biliary cholangitis (PBC) ranges between 9% and 35% with mean time to recurrence between 1.6 and 6.5 years[219]. Autoimmune hepatitis (AIH) recurs in 8%-12% of patients within the first year after LT and 36%-68% after 5 years[220].
PSC recurs in about 20% of patients after a median time of 4.6 years[221] with a cumulative incidence up to 45% at 6 years[222].
Diagnosing recurrence of autoimmune disease can be challenging, leading to a substantial variation of the data reported in the literature[219], since there are many conditions in the transplanted liver that can mimic autoimmune diseases (e.g. ischemia related biliary insults, hepatic artery thrombosis and/or chronic ductopenic rejection, infectious cholangitis can mimic PSC; acute cellular rejection can mimic PBC and AIH)[223].
Recurrence of PBC has little impact on patient and graft survival, with a reported patient survival at 5 and 10 year of 96% and 83% in those with disease recurrence[224], with a proportion of graft lost to disease recurrence of about 5%[225].
Reported patient survival for AIH is approximately 79% and 70% at 5 and 10 years, while graft survival is 73% and 63% after 5 and 10 years of follow up. Compared to recurrence of PBC and PSC, AIH recurrence leads to an increased risk of death due to infection or graft rejection[226].
Patient survival for PSC recurrence is approximately 86% and 70% at 5 and 10 years whereas reported graft survival at 5 and 10 years is 79% and 60%, respectively[227]. There is some controversy about whether recurrence of the disease affects survival[219,221,227,228]. A recent study conducted analyzing the European Liver Transplant Registry[229] shows that PSC recurrence has a negative impact on both graft and patient survival, leading to higher number of re-transplantations and a 33% decrease in 10-year graft survival.
As showed by a recent meta-analysis, the only identified risk factor for PBC recurrence[230] is the use of tacrolimus while the use of preventive UDCA was a protective factor. UDCA is also an effective treatment for disease recurrence[231,232], while there is a lack of data on the use of Obeticholic Acid and Fibrates for the treatment of recurrent PBC.
Risk factors associated with recurrent AIH are younger age at LT, use of mycophenolate mofetil post-LT, sex mismatch and high IgG pre-LT[233], suboptimal IS, disease type and severity[234], histological findings of severe disease in native liver[235]; of note, long term use of low-dose corticosteroid after LT seems to reduce the incidence of recurrent disease with a good safety profile[233,236].
The choice of the best treatment for recurrent AIH depends on the severity of presentation: For mild recurrence, such as asymptomatic disease with minimal changes in liver biochemistry and histology, an adjustment of the IS regimen may be sufficient, while severe disease recurrence may require re-introducing or increasing the dose of corticosteroids, or adding another immunosuppressive drugs[237]. Although this strategy remains controversial[233]. Re-transplantation may be required for patients with recurrent AIH who present with liver failure and graft loss[237].
Another meta-analysis showed that identified risk factors for PSC recurrence[238] are intact colon before LT and IBD presence, cholangiocarcinoma, advanced donor age, higher MELD score, acute cellular rejection (ACR) and multiple episodes of ACR.
Autoimmune etiology is a risk factor for Late T-Cell mediated rejection, which is associated with reduced graft survival, that is why particular attention is warranted in the IS protocol, even though the optimal IS regimen has not been defined[108].
In this scenario, early diagnosis of recurrence gains particular importance, even though diagnosis could be challenging[223] and liver function tests alteration should be considered highly suspicious for disease recurrence[239], while also considering other risk factors[240].
Montano-Loza et al[240] proposed strategies to reduce the risk of autoimmune liver disease recurrence after LT, acting on the main risk factors for recurrence: Treatment of active cirrhosis and normalization of transaminases and IgG for AIH in the pre-transplantation period plus long term corticosteroid use after LT; use of preventive Ursodeoxycholic Acid for PBC; control of IBD and considering pre- or post-transplant colectomy in patients with PSC.
In summary, prevention of recurrence in the future will probably rely on identification of risk profiles starting from the pre-transplantation phase and on tailored IS protocols.
Non-alcoholic fatty liver disease /NASH
The proportion of transplants performed for NASH has increased significantly over time[241], outpacing HCV[142], and metabolic liver disease has become a top indication for LT worldwide[242]. Post-transplant outcomes of NASH patients are generally good, even though there is some controversy about the overall survival rates for patients transplanted for NASH cirrhosis or HCC compared to other etiologies; graft survival rates are comparable[241,243,244].
Pre-LT screening for MetS is mandatory given the high prevalence in this population[245].
The distinction between recurrent and de novo non-alcoholic fatty liver disease (NAFLD) after LT is made clinically by accurately identifying the preexisting liver disease. There are few data on NAFLD after LT and a broad range in the recurrence or de novo rate depending on which diagnostic criteria is used[246]. There are no histologic characteristics to distinguish recurrent from de novo NAFLD after LT and histological findings in recurrent or de novo NAFLD in the allograft are considered to be the same as in immune-competent native livers[246].
Recurrence of NAFLD is up to 100% in patients who were transplanted for NASH after 1 to 5 years of follow-up, while reported NASH recurrence rates over a comparable follow-up period are between 4%–57%, with 2%-5% demonstrating compensated cirrhosis. Over a similar time period, the incidence of de novo NAFLD ranged from 18%- 78%, whereas de novo NASH ranged from 13% to 17%, showing lower rates of de novo disease compared to recurrence[247-249]. A meta-analysis on the incidence and risk of NAFLD/NASH post-LT reported the rate of cirrhosis in recurrent NAFLD recipients to range from 1 to 11%, with one study reporting the rate as high as 29%, whereas the rate of cirrhosis in de novo NAFLD LT recipients was 14% at five years after LT[250]. Despite the lack of data, NAFLD/NASH cirrhosis post-LT is probably a rare cause of death or graft loss in the first years post-LT given the good 5-year graft survival rate[246], but further data is needed on the long term. A recent study has pointed at recent pre-LT cardiovascular history and a combined donor-recipient age of 135 as major prognostic factors[251].
Management recommendations for LT recipients are the same as those for other NAFLD/NASH patients[252].
Diet and lifestyle changes have in fact a main role in the treatment of fatty liver disease[253]. NASH can be resolved with a weight loss of at least 7% of total body weight, and fibrosis can be stabilized or can regress with a weight loss of at least 10% of total body weight; a lower target of weight loss of 3%-5% is advised for patients with lean NAFLD[254]. Patients with fatty liver disease should follow the Mediterranean diet, mainly constituted by fresh fruit, vegetables, legumes, whole grains, fish, olive oil, nuts, and seeds while limiting the consumption of red and processed meat as well as commercially produced fructose[254]. This type of diet has been showed to reduce liver steatosis[255] and improve liver stiffness[256]. Patients with NAFLD should also consider engaging in regular physical activity, aiming for 150–300 min of moderate–intensity aerobic exercise per week[254], since it has been found that exercise alone, even without dietary intervention, can significantly decrease liver fat. Both European[257] and American[252] guidelines recommend that pharmacological treatments, aimed primarily at improving liver disease, should be limited to those with biopsy-proven NASH and fibrosis. It is also recommended to consider pharmacological treatment for patients with less severe disease who are at high risk of disease progression (i.e. with diabetes, MetS, persistently increased ALT, high necroinflammation). As of today, no NASH drug has been approved by Food and Drugs Administration, European Medicines Agency, or any other leading regulatory agencies[258]. Bariatric surgery should be proposed in case of non-response to lifestyle changes and pharmacotherapy[257].
Regarding modification to the IS regimen, ILTS advises corticosteroids minimization where possible, since they carry a significant risk for all components of MetS, and CNI minimization to mitigate post-transplant weight gain and hypertension[108].
There are no societal or professional guidelines for post-transplant surveillance in NASH LT patients at the moment, nor a frequency of post-LT monitoring for recurrent or de novo NASH has been defined, given the low likelihood of clinically significant recurrence of NASH. Conversely, high-risk individuals identified during the pretransplant work-up, such as those with PNPLA3 polymorphism or hypopituitarism, definitely require closer surveillance[246].
Suggested strategies for NAFLD/NASH screening in LT patients include annual ultrasound and liver enzymes monitoring: If fatty liver disease is identified or suspected then the patient should undergo noninvasive tests for fibrosis assessment[259]. A metanalysis by Bhat et al[260] showed how transient elastography (TE) performed better that APRI and FIB-4 at diagnosing recurrent fibrosis in LT recipients, but none of the studies included in the analysis was specific for NAFLD/NASH. A recent study from Siddiqui et al[261] conducted on 99 patients who underwent LT, showed how TE can detect advanced fibrosis with an AUROC of 0.94 and exclude advanced fibrosis with a negative predictive value of 0.99 when a liver stiffness cutoff value of 10.5 kPa is used; furthermore a controlled attenuation parameter cutoff value of 270 dB/m can identify any hepatic steatosis with an AUROC of 0.88. Along with TE, also Magnetic Resonance Elastography is an accurate method for assessing liver fibrosis in LT recipients[262]. Singh et al[263] conducted a pooled analysis including 6 cohorts where the mean AUROC values for diagnosis of advanced fibrosis and cirrhosis were respectively 0.83 (0.61-0.88) and 0.96 (0-93-0.98), with a good diagnostic performance even after stratification based on sex, BMI and degree of inflammation.
Beyond being a way to perform a differential diagnosis with other potential causes for elevated liver enzymes, liver biopsy remains the gold standard for the diagnosis of post-LT NAFLD/NASH; patients who have an established diagnosis of recurrent or de novo NAFLD can probably be followed with serial noninvasive testing to diagnose advanced fibrotic disease, but given the lack of data regarding fibrosis monitoring and the presence of factors that can influence these tests, suspected fibrotic disease at noninvasive testing still needs confirmation with biopsy[259].
While de novo or recurrent NASH appears to have little effect on prognosis, infections and cardiovascular diseases are among the leading causes of mortality[241,264].
A multidisciplinary approach in the management of NAFLD/NASH transplanted patients, promoting increased physical activity, diet modifications, behavioral therapy, and pharmacological treatment, when necessary, should be explored[265,266].
Unfortunately, in many cases a full multidisciplinary team is not available for the patient due to limited resources; either way the active assistance of the physicians is essential since their advice to lose weight has favorable impacts on the likelihood that patients will adhere to the suggested lifestyle changes[253].
Viral hepatitis
HCV recurrence post-LT was an issue with a major impact on the prognosis in the pre-direct acting antiviral (DAA) era, given its shortened natural history in the LT setting (development of cirrhosis in 10-30% of patients after a median of 5 years)[267-269].
The advent of DAA therapy was a “game changer”, with HCV recurrence as cause of death or re-transplantation decreasing from 5.89% in the Interferon era to 0.60% in DAA era over a three-year period[270].
LT may not remove HBV from a persistently infected host because HBV may reside in extra-hepatic sites and serve as a source of reactivation. As a result, in a chronically infected patient, after LT, prophylaxis is used to avoid reactivation rather than re-infection or recurrence of HBV. Because of this, lifetime antiviral prophylaxis is required[271]. Treatment with hepatitis B immunoglobulin (HBIG) and Nucleos(t)ide Analogues is an effective strategy to prevent HBV recurrence in most HBV-infected patients undergoing LT, showing very low recurrence rates[272]; monotherapy with entecavir or tenofovir is probably not sufficient to prevent graft reinfection but is considered sufficient to prevent disease recurrence[1].
Risk factors for HBV recurrence include a high HBV-DNA level at LT, presence of HBeAg positivity, HCC, anti-viral drug resistance, and HBIG monoprophylaxis[1,273,274].
Recurrence is defined by the presence of HBsAg in the serum and detectable quantities of DNA, and it is typically linked to clinical evidence of recurrent disease. The goal of treatment is to keep HBV replication under control throughout time to prevent graft loss, even though there is no standard follow-up protocol for early diagnosis[1,275]. Therapy with ETV and/or TDF seems to be efficient and safe when used for treatment of HBV recurrence after LT[276]. Treated recurrence is associated with good prognosis[277-279] but care should be taken in patients transplanted for HCC since HBV recurrence could be a signal of HCC recurrence[277,278].
CONCLUSION
With the dramatic improvement in short-term survival of LT recipients that occurred in recent years, the focus of the physician is shifting to the improvement of long-term outcomes[280]. The main causes of late mortality in this category are not liver related[2]. In this review, we described the main comorbidities and risk factors affecting LT recipients, which in most cases are preventable, can be treated, or are amenable of screening measures, even though there is a lack of consensus to define the best strategy for the follow-up and management of part of these factors, for which we reported some of the suggested approaches (Table 3).
Table 3.
Comorbidity/risk factors impairing long-term survival with suggested follow-up and management
|
Risk Factor/Comorbidity
|
Follow-up
|
Management
|
| Metabolic syndrome | (1) Electrocardiogram and transthoracic echocardiography before transplantation. If patient older than 50 and has multiple cardiovascular risk factors, perform a cardiopulmonary exercise test. If coronary disease is suspected, coronary angiography should be executed[1]; and (2) Repeatedly perform a cardiovascular risk stratification after transplantation (for example, every 6 mo)[1]. | (1) Aggressive and rapid management of metabolic risk factors in the form of lifestyle changes, pharmacological therapies and modifications of the IS[1]; (2) Dietary counseling for all LT patients[4]; and (3) Consider bariatric surgery in patients who failed behavioral weight-loss programs[4]. |
| Sarcopenia | (1) Sarcopenia evaluation before LT (for example, measuring skeletal mass from CT scan, even required for other reasons)[23]; and (2) New assessment at 1 year after transplantation (possibly using the same method and the same CT machine, in order to compare results. Consider also 24-CER evaluation[20]). | (1) Manage sarcopenia with lifestyle modification and nutritional interventions both pre and post transplantation[1,28]; (2) Consider nutritional counseling after transplantation; and (3) Consider nutritional supplementations (e.g. with HMB)[29]. |
| Osteoporosis | (1) Regular measurement of bone mineral density pre- and post- LT, with a follow up timing dependent on the severity of the disease; and (2) If osteopenic bone disease is confirmed or if atraumatic fractures are present, assess calcium intake, vitamin D levels, gonadal and thyroid function, a full medication history, and thoracolumbar radiography[4]. | (1) Manage osteoporosis with calcium and vitamin D replacement (if deficient), consider a weight-bearing exercise pre-operative program[1]; and (2) Consider bisphosphonate therapy (for example, Neridronate, at the dose of 25 mg i.m./mo for 12 mo)[40]. |
| Psychological health and QoL | Actively look for depressive symptoms since the early post-transplantation period[1]. | (1) Treat promptly depressive symptoms with adequate pharmacotherapy[54]; (2) Consider psychological support by a specialist if needed; and (3) Propose lifestyle modifications (in particular, personalized aerobic and strength training programs)[55]. |
| Renal dysfunction | (1) Continuous monitoring of renal function with serum creatinine and glomerular filtration rate measurements[1]; and (2) Urinary protein quantification at least once yearly[4]. | (1) Treat potential risk factors (diabetes, hypertension); (2) Avoid nephrotoxic drugs[1]; (3) Adjustment of the IS; reduction or withdrawal of CNI or use alternative CNI-free protocols as soon as possible in impaired renal function (for example, EVR combination regimens starting 1 mo after transplantation[67,68]; and (4) Consider kidney transplantation in end-stage renal disease[4]. |
| Infectious risk | Hepatologist accurate counseling to increase the percentage of vaccinated patients[81]. | (1) Perform HAV, HBV, varicella, Pneumococcus, influenza and tetanus vaccinations prior to transplantation; (2) Administer inactivated influenza vaccine starting one month after transplantation during community influenza outbreak; (3) Annual influenza vaccination in liver transplanted patients[4]; and (4) Consider Oseltamivir prophylaxis during periods of local influenza circulation[86]. |
| De novo malignancies | Define and follow a surveillance protocol with individualized emphasis laid on patients’ particular risk profiles[1]. | (1) Treating modifiable risk factors: stop smoking, alcohol withdrawal[99], metabolic syndrome management[100], avoid sunbed use and sun exposure[101], promote HPV vaccination[102]; and (2) Use mTOR- based therapy if possible[1] or a CNI-mTOR combined therapy always at the lowest effective dose. |
| Smoking | (1) Assess smoking status at each visit, focusing on those with particular risk of relapse (ALD); and (2) Use of biomarkers (serum Cotinine)[116]. | (1) Encourage to undertake smoking cessation[4]; and (2) Referral for behavioral/pharmacological therapy[125,126]. |
| Adherence to therapy | Self- and collateral-reported non-adherence, trough levels (considering graft-function and concomitant use of other drugs)[136,139] with particular attention to younger patients and those missing clinic appointments, with a history of substance or alcohol use, mental health needs, divorced or high regimen complexity[131,137,140]. | Multidisciplinary measures developed by professional educators, supported by psychologists, and coordinated by physicians[1,136]. |
| Alcohol abuse relapse | (1) Assessing alcohol use at each clinic visit, with particular attention to those transplanted for ALD with pre-transplant abstinence of less than 6 mo, with psychiatric comorbidities, smoking, noncompliant with clinic appointment or medication and lacking social support[147,149,150]; and (2) Assessment by self-reported alcohol use, liver function tests and metabolites of alcohol, such as urinary ethyl glucuronide[157,158]. | (1) Preventive structured management by a multidisciplinary team including transplant hepatologist, clinical psychologists and psychiatrists with expertise in alcoholism and social workers[159,160]; (2) Encouraging smoking cessation; and (3) Referral to psychiatric treatment or counselling in case of relapse[1,4]. |
| HCC recurrence | (1) Thoracic CT – abdominal CT or MRI and AFP levels with 3- to 6-mo intervals in the first 2 or 3 years, increasing the interval between exams from that date[194]; (2) Selection of patients who needs a stricter follow-up using prognostic criteria such as: AFP levels, the presence of microvascular invasion, the diameter of the largest viable tumor and the number of viable tumors[202]; and (3) Particular attention to patients transplanted outside of Milan Criteria[176]. | (1) Minimizing overall IS; consider adding mTORi[108]; (2) Individualized management of HCC discussed in a multidisciplinary team[303]; and (3) Surgical treatment when feasible[204,205]. |
| Autoimmune disease recurrence | (1) Monitoring liver function tests[223,229] and performing liver biopsy and/or cholangiography when deemed necessary[1]; and (2) Exclude mimicking conditions (ischemia related biliary insults, hepatic artery thrombosis and/or chronic ductopenic rejection, infectious cholangitis for PSC, rejection histological mimicking for PBC and AIH)[223]. | (1) AIH: Treatment of active cirrhosis and normalization of transaminases and IgG in the pre-transplantation period plus long-term corticosteroid use after LT; (2) PBC: Use of preventive Ursodeoxycholic Acid; and (3) PSC: Control of IBD and considering pre- or post-transplant colectomy in patients with difficult to control PSC[240]. |
| NASH recurrence | (1) Early identification of Metabolic Syndrome components pre- and post-transplant[304]; (2) Annual screening with US and liver function tests; (3) Noninvasive testing and liver stiffness measurement in case of alterations at the annual screening; and (4) Biopsy in case of suspected fibrotic disease[259]. | (1) Management as for other NAFLD/NASH patients (multidisciplinary approach with diet and lifestyle modification, pharmacological treatment and bariatric surgery when necessary[252,257]; (2) Treating metabolic syndrome components)[265,266]; and (3) Corticosteroids and CNI minimization when possible[108]. |
| Viral hepatitis recurrence | (1) Liver function test monitoring; (2) HCV-RNA titres[111]; (3) HBV DNA and HBsAg monitoring[1,275]; (4) Regular assessment of graft damage[1]; and (5) Particular attention to patients transplanted for HCC with HBV recurrence/reactivation[277,278]. | (1) Treating HCV before LT when possible; use of DAA[1,164]; and (2) HBIG and NUCs to prevent HBV recurrence/reactivation[1,272]. |
LT: Liver transplantation; CT: Computed tomography; CNI: Calcineurin inhibitors; EVR: Everolimus; HAV: Hepatitis A virus; HBV: Hepatitis B virus; HPV: Human papilloma virus; mTORi: Mammalian target of Rapamicin inhibitors; ALD: Alcohol-related liver disease; HCC: Hepatocellular carcinoma; AFP: Alpha fetoprotein; PSC: Primary sclerosing cholangitis; PBC: Primary biliary cholangitis; AIH: Autoimmune hepatitis; NASH: Non-alcoholic steatohepatitis; HCV: Hepatitis C virus; HBIG: Hepatitis B immunoglobulin; NUCs: Nucleos(t)ide analogues.
The hepatologist’s role in long-term management of LT recipients is becoming more complex with the increase of comorbidities/risk factors that can affect long-term outcomes. A multidisciplinary approach could help overcome this complexity. The importance of a multidisciplinary team is underlined by current guidelines[1,164] with regard to pre-LT evaluation, and its value is recognized in some particular settings such as the prevention and management of alcohol relapse or to improve adherence to therapy[1,161].
The availability of a “long-term management multidisciplinary team” dedicated to LT recipients, could handle or prevent the onset of the aforementioned comorbidities/risk factors and should be composed by psychiatrists, psychologists, cardiologists, general practitioners, nutritionists, dieticians, social workers, and should be coordinated by the transplant hepatologist. We believe this approach could improve the long-term outcomes of the LT recipients.
A body of the evidence is already available for the identification of high-risk patients[14,151,185] and such prognostic ability could allow healthcare providers to focus on those who could benefit the most from preventive measures in a cost-effective manner.
Furthermore, a special mention should go to maintenance immunosuppression, which has a strong impact on patient long-term survival: Although further studies are needed to propose IS withdrawal[108], which should therefore be limited to clinical trials, early minimization of IS (when feasible) seems a rational strategy to limit adverse events and improve long-term outcomes. A deeper understanding of the immunological pathways of rejection would allow to design more specific and safer drugs, in order to tailor therapy[281].
The question “How can we improve long-term outcomes after liver transplantation?” has no clear and simple answer. The combination of reduction of drugs toxicity, the use of precise instruments that allow to detect high-risk patients and the presence of a multidisciplinary team coordinated by an hepatologist could probably be the key for the improvement of long-term outcomes after LT.
ACKNOWLEDGEMENTS
The figures were drawn by using pictures from Servier Medical Art. Servier Medical Art by Servier is licensed under a Creative Commons Attribution 3.0 Unported License.
Footnotes
Conflict-of-interest statement: Authors declare no conflict of interests for this article.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: United European Gastroenterology; Società Italiana Di Gastroenterologia Ed Endoscopia Digestiva.
Peer-review started: October 18, 2022
First decision: November 14, 2022
Article in press: February 22, 2023
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chang A, Thailand; Spataru A, Canada S-Editor: Wang JL L-Editor: A P-Editor: Wang JL
Contributor Information
Elisa Fuochi, Gastroenterology Research Unit, Department of Experimental and Clinical Biomedical Sciences “Mario Serio”, University of Florence, Florence 50134, Italy.
Lorenzo Anastasio, Gastroenterology Research Unit, Department of Experimental and Clinical Biomedical Sciences “Mario Serio”, University of Florence, Florence 50134, Italy.
Erica Nicola Lynch, Gastroenterology Research Unit, Department of Experimental and Clinical Biomedical Sciences “Mario Serio”, University of Florence, Florence 50134, Italy.
Claudia Campani, Department of Experimental and Clinical Medicine, University of Florence, Florence 50134, Italy.
Gabriele Dragoni, Gastroenterology Research Unit, Department of Experimental and Clinical Biomedical Sciences “Mario Serio”, University of Florence, Florence 50134, Italy; Department of Medical Biotechnologies, University of Siena, Siena 53100, Italy.
Stefano Milani, Gastroenterology Research Unit, Department of Experimental and Clinical Biomedical Sciences “Mario Serio”, University of Florence, Florence 50134, Italy.
Andrea Galli, Gastroenterology Research Unit, Department of Experimental and Clinical Biomedical Sciences “Mario Serio”, University of Florence, Florence 50134, Italy.
Tommaso Innocenti, Gastroenterology Research Unit, Department of Experimental and Clinical Biomedical Sciences “Mario Serio”, University of Florence, Florence 50134, Italy.
References
- 1.European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Liver transplantation. J Hepatol. 2016;64:433–485. doi: 10.1016/j.jhep.2015.10.006. [DOI] [PubMed] [Google Scholar]
- 2.Watt KD, Pedersen RA, Kremers WK, Heimbach JK, Charlton MR. Evolution of causes and risk factors for mortality post-liver transplant: results of the NIDDK long-term follow-up study. Am J Transplant. 2010;10:1420–1427. doi: 10.1111/j.1600-6143.2010.03126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schoening WN, Buescher N, Rademacher S, Andreou A, Kuehn S, Neuhaus R, Guckelberger O, Puhl G, Seehofer D, Neuhaus P. Twenty-year longitudinal follow-up after orthotopic liver transplantation: a single-center experience of 313 consecutive cases. Am J Transplant. 2013;13:2384–2394. doi: 10.1111/ajt.12384. [DOI] [PubMed] [Google Scholar]
- 4.Lucey MR, Terrault N, Ojo L, Hay JE, Neuberger J, Blumberg E, Teperman LW. Long-term management of the successful adult liver transplant: 2012 practice guideline by the American Association for the Study of Liver Diseases and the American Society of Transplantation. Liver Transpl. 2013;19:3–26. doi: 10.1002/lt.23566. [DOI] [PubMed] [Google Scholar]
- 5.Expert Panel on Detection, Evaluation , and Treatment of High Blood Cholesterol in Adults. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 6.Rochlani Y, Pothineni NV, Kovelamudi S, Mehta JL. Metabolic syndrome: pathophysiology, management, and modulation by natural compounds. Ther Adv Cardiovasc Dis. 2017;11:215–225. doi: 10.1177/1753944717711379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Noureddin M, Vipani A, Bresee C, Todo T, Kim IK, Alkhouri N, Setiawan VW, Tran T, Ayoub WS, Lu SC, Klein AS, Sundaram V, Nissen NN. NASH Leading Cause of Liver Transplant in Women: Updated Analysis of Indications For Liver Transplant and Ethnic and Gender Variances. Am J Gastroenterol. 2018;113:1649–1659. doi: 10.1038/s41395-018-0088-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cigrovski Berkovic M, Virovic-Jukic L, Bilic-Curcic I, Mrzljak A. Post-transplant diabetes mellitus and preexisting liver disease - a bidirectional relationship affecting treatment and management. World J Gastroenterol. 2020;26:2740–2757. doi: 10.3748/wjg.v26.i21.2740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Watt KD. Extrahepatic implications of metabolic syndrome. Liver Transpl. 2013;19 Suppl 2:S56–S61. doi: 10.1002/lt.23726. [DOI] [PubMed] [Google Scholar]
- 10.García-Pajares F, Peñas-Herrero I, Sánchez-Ocaña R, Torrres-Yuste R, Cimavilla-Román M, Carbajo-López A, Almohalla-Alvarez C, Pérez-Saborido B, Muñoz-Conejero E, Gonzalez-Sagrado M, Caro-Patón A, Sánchez-Antolín G. Metabolic Syndrome After Liver Transplantation: Five-Year Prevalence and Risk Factors. Transplant Proc. 2016;48:3010–3012. doi: 10.1016/j.transproceed.2016.07.038. [DOI] [PubMed] [Google Scholar]
- 11.de Carvalho L, Parise ER, Samuel D. Factors associated with nutritional status in liver transplant patients who survived the first year after transplantation. J Gastroenterol Hepatol. 2010;25:391–396. doi: 10.1111/j.1440-1746.2009.06033.x. [DOI] [PubMed] [Google Scholar]
- 12.Kim NG, Sharma A, Saab S. Cardiovascular and metabolic disease in the liver transplant recipient. Best Pract Res Clin Gastroenterol. 2020;46-47:101683. doi: 10.1016/j.bpg.2020.101683. [DOI] [PubMed] [Google Scholar]
- 13.VanWagner LB, Ning H, Whitsett M, Levitsky J, Uttal S, Wilkins JT, Abecassis MM, Ladner DP, Skaro AI, Lloyd-Jones DM. A point-based prediction model for cardiovascular risk in orthotopic liver transplantation: The CAR-OLT score. Hepatology. 2017;66:1968–1979. doi: 10.1002/hep.29329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rachwan RJ, Kutkut I, Timsina LR, Bou Chaaya RG, El-Am EA, Sabra M, Mshelbwala FS, Rahal MA, Lacerda MA, Kubal CA, Fridell JA, Ghabril MS, Bourdillon PD, Mangus RS. CAD-LT score effectively predicts risk of significant coronary artery disease in liver transplant candidates. J Hepatol. 2021;75:142–149. doi: 10.1016/j.jhep.2021.01.008. [DOI] [PubMed] [Google Scholar]
- 15.Lee Y, Tian C, Lovrics O, Soon MS, Doumouras AG, Anvari M, Hong D. Bariatric surgery before, during, and after liver transplantation: a systematic review and meta-analysis. Surg Obes Relat Dis. 2020;16:1336–1347. doi: 10.1016/j.soard.2020.05.012. [DOI] [PubMed] [Google Scholar]
- 16.Hamaguchi Y, Kaido T, Okumura S, Fujimoto Y, Ogawa K, Mori A, Hammad A, Tamai Y, Inagaki N, Uemoto S. Impact of quality as well as quantity of skeletal muscle on outcomes after liver transplantation. Liver Transpl. 2014;20:1413–1419. doi: 10.1002/lt.23970. [DOI] [PubMed] [Google Scholar]
- 17.Dasarathy S, Merli M. Sarcopenia from mechanism to diagnosis and treatment in liver disease. J Hepatol. 2016;65:1232–1244. doi: 10.1016/j.jhep.2016.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tantai X, Liu Y, Yeo YH, Praktiknjo M, Mauro E, Hamaguchi Y, Engelmann C, Zhang P, Jeong JY, van Vugt JLA, Xiao H, Deng H, Gao X, Ye Q, Zhang J, Yang L, Cai Y, Liu N, Li Z, Han T, Kaido T, Sohn JH, Strassburg C, Berg T, Trebicka J, Hsu YC, IJzermans JNM, Wang J, Su GL, Ji F, Nguyen MH. Effect of sarcopenia on survival in patients with cirrhosis: A meta-analysis. J Hepatol. 2022;76:588–599. doi: 10.1016/j.jhep.2021.11.006. [DOI] [PubMed] [Google Scholar]
- 19.Marasco G, Serenari M, Renzulli M, Alemanni LV, Rossini B, Pettinari I, Dajti E, Ravaioli F, Golfieri R, Cescon M, Festi D, Colecchia A. Clinical impact of sarcopenia assessment in patients with hepatocellular carcinoma undergoing treatments. J Gastroenterol. 2020;55:927–943. doi: 10.1007/s00535-020-01711-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stam SP, Osté MCJ, Eisenga MF, Blokzijl H, van den Berg AP, Bakker SJL, de Meijer VE. Posttransplant muscle mass measured by urinary creatinine excretion rate predicts long-term outcomes after liver transplantation. Am J Transplant. 2019;19:540–550. doi: 10.1111/ajt.14926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cooper C, Fielding R, Visser M, van Loon LJ, Rolland Y, Orwoll E, Reid K, Boonen S, Dere W, Epstein S, Mitlak B, Tsouderos Y, Sayer AA, Rizzoli R, Reginster JY, Kanis JA. Tools in the assessment of sarcopenia. Calcif Tissue Int. 2013;93:201–210. doi: 10.1007/s00223-013-9757-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Proctor DN, O'Brien PC, Atkinson EJ, Nair KS. Comparison of techniques to estimate total body skeletal muscle mass in people of different age groups. Am J Physiol. 1999;277:E489–E495. doi: 10.1152/ajpendo.1999.277.3.E489. [DOI] [PubMed] [Google Scholar]
- 23.Tan Y, Duan T, Li B, Zhang B, Zhu Y, Yan K, Song J, Lv T, Yang J, Jiang L, Wen T, Yan L. Sarcopenia defined by psoas muscle index independently predicts long-term survival after living donor liver transplantation in male recipients. Quant Imaging Med Surg. 2022;12:215–228. doi: 10.21037/qims-21-314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim YR, Park S, Han S, Ahn JH, Kim S, Sinn DH, Jeong WK, Ko JS, Gwak MS, Kim GS. Sarcopenia as a predictor of post-transplant tumor recurrence after living donor liver transplantation for hepatocellular carcinoma beyond the Milan criteria. Sci Rep. 2018;8:7157. doi: 10.1038/s41598-018-25628-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hegyi PJ, Soós A, Hegyi P, Szakács Z, Hanák L, Váncsa S, Ocskay K, Pétervári E, Balaskó M, Eröss B, Pár G. Pre-transplant Sarcopenic Obesity Worsens the Survival After Liver Transplantation: A Meta-Analysis and a Systematic Review. Front Med (Lausanne) 2020;7:599434. doi: 10.3389/fmed.2020.599434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aby ES, Lee E, Saggi SS, Viramontes MR, Grotts JF, Agopian VG, Busuttil RW, Saab S. Pretransplant Sarcopenia in Patients With NASH Cirrhosis Does Not Impact Rehospitalization or Mortality. J Clin Gastroenterol. 2019;53:680–685. doi: 10.1097/MCG.0000000000001109. [DOI] [PubMed] [Google Scholar]
- 27.Carey EJ, Lai JC, Sonnenday C, Tapper EB, Tandon P, Duarte-Rojo A, Dunn MA, Tsien C, Kallwitz ER, Ng V, Dasarathy S, Kappus M, Bashir MR, Montano-Loza AJ. A North American Expert Opinion Statement on Sarcopenia in Liver Transplantation. Hepatology. 2019;70:1816–1829. doi: 10.1002/hep.30828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tandon P, Ismond KP, Riess K, Duarte-Rojo A, Al-Judaibi B, Dunn MA, Holman J, Howes N, Haykowsky MJF, Josbeno DA, McNeely M. Exercise in cirrhosis: Translating evidence and experience to practice. J Hepatol. 2018;69:1164–1177. doi: 10.1016/j.jhep.2018.06.017. [DOI] [PubMed] [Google Scholar]
- 29.Lattanzi B, Giusto M, Albanese C, Mennini G, D'Ambrosio D, Farcomeni A, Ginanni Corradini S, Rossi M, Merli M. The Effect of 12 Weeks of β-Hydroxy-β-Methyl-Butyrate Supplementation after Liver Transplantation: A Pilot Randomized Controlled Study. Nutrients. 2019;11 doi: 10.3390/nu11092259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kanis JA. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis: synopsis of a WHO report. WHO Study Group. Osteoporos Int. 1994;4:368–381. doi: 10.1007/BF01622200. [DOI] [PubMed] [Google Scholar]
- 31.Glaser DL, Kaplan FS. Osteoporosis. Definition and clinical presentation. Spine (Phila Pa 1976) 1997;22:12S–16S. doi: 10.1097/00007632-199712151-00003. [DOI] [PubMed] [Google Scholar]
- 32.Compston JE, McClung MR, Leslie WD. Osteoporosis. Lancet. 2019;393:364–376. doi: 10.1016/S0140-6736(18)32112-3. [DOI] [PubMed] [Google Scholar]
- 33.Guzon-Illescas O, Perez Fernandez E, Crespí Villarias N, Quirós Donate FJ, Peña M, Alonso-Blas C, García-Vadillo A, Mazzucchelli R. Mortality after osteoporotic hip fracture: incidence, trends, and associated factors. J Orthop Surg Res. 2019;14:203. doi: 10.1186/s13018-019-1226-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hamburg SM, Piers DA, van den Berg AP, Slooff MJ, Haagsma EB. Bone mineral density in the long term after liver transplantation. Osteoporos Int. 2000;11:600–606. doi: 10.1007/s001980070081. [DOI] [PubMed] [Google Scholar]
- 35.Bush H, Golabi P, Younossi ZM. Pediatric Non-Alcoholic Fatty Liver Disease. Children (Basel) 2017;4 doi: 10.3390/children4060048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sethi A, Stravitz RT. Review article: medical management of the liver transplant recipient - a primer for non-transplant doctors. Aliment Pharmacol Ther. 2007;25:229–245. doi: 10.1111/j.1365-2036.2006.03166.x. [DOI] [PubMed] [Google Scholar]
- 37.Leidig-Bruckner G, Hosch S, Dodidou P, Ritschel D, Conradt C, Klose C, Otto G, Lange R, Theilmann L, Zimmerman R, Pritsch M, Ziegler R. Frequency and predictors of osteoporotic fractures after cardiac or liver transplantation: a follow-up study. Lancet. 2001;357:342–347. doi: 10.1016/S0140-6736(00)03641-2. [DOI] [PubMed] [Google Scholar]
- 38.Pennisi P, Trombetti A, Giostra E, Mentha G, Rizzoli R, Fiore CE. Pamidronate and osteoporosis prevention in liver transplant recipients. Rheumatol Int. 2007;27:251–256. doi: 10.1007/s00296-006-0196-2. [DOI] [PubMed] [Google Scholar]
- 39.Maalouf NM, Shane E. Osteoporosis after solid organ transplantation. J Clin Endocrinol Metab. 2005;90:2456–2465. doi: 10.1210/jc.2004-1978. [DOI] [PubMed] [Google Scholar]
- 40.Giannini S, Poci C, Fusaro M, Egan CG, Marcocci C, Vignali E, Cetani F, Nannipieri F, Loy M, Gambino A, Adami G, Braga V, Rossini M, Arcidiacono G, Baffa V, Sella S. Effect of neridronate in osteopenic patients after heart, liver or lung transplant: a multicenter, randomized, double-blind, placebo-controlled study. Panminerva Med. 2021;63:214–223. doi: 10.23736/S0031-0808.21.04401-3. [DOI] [PubMed] [Google Scholar]
- 41.. The World Health Organization Quality of Life assessment (WHOQOL): position paper from the World Health Organization. Soc Sci Med. 1995;41:1403–1409. doi: 10.1016/0277-9536(95)00112-k. [DOI] [PubMed] [Google Scholar]
- 42.Crespo G. Long-term outcomes after liver transplantation for ACLF - Don't forget quality of life! Liver Int. 2021;41:430–431. doi: 10.1111/liv.14789. [DOI] [PubMed] [Google Scholar]
- 43.Desai R, Jamieson NV, Gimson AE, Watson CJ, Gibbs P, Bradley JA, Praseedom RK. Quality of life up to 30 years following liver transplantation. Liver Transpl. 2008;14:1473–1479. doi: 10.1002/lt.21561. [DOI] [PubMed] [Google Scholar]
- 44.Ruppert K, Kuo S, DiMartini A, Balan V. In a 12-year study, sustainability of quality of life benefits after liver transplantation varies with pretransplantation diagnosis. Gastroenterology. 2010;139:1619–1629, 1629.e1. doi: 10.1053/j.gastro.2010.06.043. [DOI] [PubMed] [Google Scholar]
- 45.Hathaway D, Winsett R, Prendergast M, Subaiya I. The first report from the patient outcomes registry for transplant effects on life (PORTEL): differences in side-effects and quality of life by organ type, time since transplant and immunosuppressive regimens. Clin Transplant. 2003;17:183–194. doi: 10.1034/j.1399-0012.2003.00024.x. [DOI] [PubMed] [Google Scholar]
- 46.Tome S, Wells JT, Said A, Lucey MR. Quality of life after liver transplantation. A systematic review. J Hepatol. 2008;48:567–577. doi: 10.1016/j.jhep.2007.12.013. [DOI] [PubMed] [Google Scholar]
- 47.Aberg F, Höckerstedt K, Roine RP, Sintonen H, Isoniemi H. Influence of liver-disease etiology on long-term quality of life and employment after liver transplantation. Clin Transplant. 2012;26:729–735. doi: 10.1111/j.1399-0012.2012.01597.x. [DOI] [PubMed] [Google Scholar]
- 48.De Bona M, Ponton P, Ermani M, Iemmolo RM, Feltrin A, Boccagni P, Gerunda G, Naccarato R, Rupolo G, Burra P. The impact of liver disease and medical complications on quality of life and psychological distress before and after liver transplantation. J Hepatol. 2000;33:609–615. doi: 10.1034/j.1600-0641.2000.033004609.x. [DOI] [PubMed] [Google Scholar]
- 49.Cowling T, Jennings LW, Goldstein RM, Sanchez EQ, Chinnakotla S, Klintmalm GB, Levy MF. Liver transplantation and health-related quality of life: scoring differences between men and women. Liver Transpl. 2004;10:88–96. doi: 10.1002/lt.20013. [DOI] [PubMed] [Google Scholar]
- 50.Annema C, Roodbol PF, Stewart RE, Porte RJ, Ranchor AV. Prevalence of psychological problems and associated transplant-related variables at different time periods after liver transplantation. Liver Transpl. 2015;21:524–538. doi: 10.1002/lt.24075. [DOI] [PubMed] [Google Scholar]
- 51.Dew MA, Rosenberger EM, Myaskovsky L, DiMartini AF, DeVito Dabbs AJ, Posluszny DM, Steel J, Switzer GE, Shellmer DA, Greenhouse JB. Depression and Anxiety as Risk Factors for Morbidity and Mortality After Organ Transplantation: A Systematic Review and Meta-Analysis. Transplantation. 2015;100:988–1003. doi: 10.1097/TP.0000000000000901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.DiMartini A, Dew MA, Chaiffetz D, Fitzgerald MG, Devera ME, Fontes P. Early trajectories of depressive symptoms after liver transplantation for alcoholic liver disease predicts long-term survival. Am J Transplant. 2011;11:1287–1295. doi: 10.1111/j.1600-6143.2011.03496.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Corruble E, Barry C, Varescon I, Falissard B, Castaing D, Samuel D. Depressive symptoms predict long-term mortality after liver transplantation. J Psychosom Res. 2011;71:32–37. doi: 10.1016/j.jpsychores.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 54.Rogal SS, Dew MA, Fontes P, DiMartini AF. Early treatment of depressive symptoms and long-term survival after liver transplantation. Am J Transplant. 2013;13:928–935. doi: 10.1111/ajt.12164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Totti V, Tamè M, Burra P, Mosconi G, Roi GS, Sella G, Ermolao A, Ferrarese A, Sgarzi S, Savino G, Parodi G, Poggioli G, Ricchiuti A, Di Michele R, Trerotola M, Nanni Costa A. Physical Condition, Glycemia, Liver Function, and Quality of Life in Liver Transplant Recipients After a 12-Month Supervised Exercise Program. Transplant Proc. 2019;51:2952–2957. doi: 10.1016/j.transproceed.2019.03.087. [DOI] [PubMed] [Google Scholar]
- 56.Jay CL, Butt Z, Ladner DP, Skaro AI, Abecassis MM. A review of quality of life instruments used in liver transplantation. J Hepatol. 2009;51:949–959. doi: 10.1016/j.jhep.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gonwa TA, Mai ML, Melton LB, Hays SR, Goldstein RM, Levy MF, Klintmalm GB. End-stage renal disease (ESRD) after orthotopic liver transplantation (OLTX) using calcineurin-based immunotherapy: risk of development and treatment. Transplantation. 2001;72:1934–1939. doi: 10.1097/00007890-200112270-00012. [DOI] [PubMed] [Google Scholar]
- 58.Allen AM, Kim WR, Therneau TM, Larson JJ, Heimbach JK, Rule AD. Chronic kidney disease and associated mortality after liver transplantation--a time-dependent analysis using measured glomerular filtration rate. J Hepatol. 2014;61:286–292. doi: 10.1016/j.jhep.2014.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Trawalé JM, Paradis V, Rautou PE, Francoz C, Escolano S, Sallée M, Durand F, Valla D, Lebrec D, Moreau R. The spectrum of renal lesions in patients with cirrhosis: a clinicopathological study. Liver Int. 2010;30:725–732. doi: 10.1111/j.1478-3231.2009.02182.x. [DOI] [PubMed] [Google Scholar]
- 60.Durand F, Graupera I, Ginès P, Olson JC, Nadim MK. Pathogenesis of Hepatorenal Syndrome: Implications for Therapy. Am J Kidney Dis. 2016;67:318–328. doi: 10.1053/j.ajkd.2015.09.013. [DOI] [PubMed] [Google Scholar]
- 61.Salerno F, Gerbes A, Ginès P, Wong F, Arroyo V. Diagnosis, prevention and treatment of hepatorenal syndrome in cirrhosis. Gut. 2007;56:1310–1318. doi: 10.1136/gut.2006.107789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Alessandria C, Ozdogan O, Guevara M, Restuccia T, Jiménez W, Arroyo V, Rodés J, Ginès P. MELD score and clinical type predict prognosis in hepatorenal syndrome: relevance to liver transplantation. Hepatology. 2005;41:1282–1289. doi: 10.1002/hep.20687. [DOI] [PubMed] [Google Scholar]
- 63.Utako P, Emyoo T, Anothaisintawee T, Yamashiki N, Thakkinstian A, Sobhonslidsuk A. Clinical Outcomes after Liver Transplantation for Hepatorenal Syndrome: A Systematic Review and Meta-Analysis. Biomed Res Int. 2018;2018:5362810. doi: 10.1155/2018/5362810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tan HK, Marquez M, Wong F, Renner EL. Pretransplant Type 2 Hepatorenal Syndrome Is Associated With Persistently Impaired Renal Function After Liver Transplantation. Transplantation. 2015;99:1441–1446. doi: 10.1097/TP.0000000000000557. [DOI] [PubMed] [Google Scholar]
- 65.Goldaracena N, Marquez M, Selzner N, Spetzler VN, Cattral MS, Greig PD, Lilly L, McGilvray ID, Levy GA, Ghanekar A, Renner EL, Grant DR, Selzner M. Living vs. deceased donor liver transplantation provides comparable recovery of renal function in patients with hepatorenal syndrome: a matched case-control study. Am J Transplant. 2014;14:2788–2795. doi: 10.1111/ajt.12975. [DOI] [PubMed] [Google Scholar]
- 66.Pilmore HL, Xiong F, Choi Y, Poppe K, Lee M, Legget M, Kerr A. Impact of chronic kidney disease on mortality and cardiovascular outcomes after acute coronary syndrome: A nationwide data linkage study (ANZACS-QI 44) Nephrology (Carlton) 2020;25:535–543. doi: 10.1111/nep.13703. [DOI] [PubMed] [Google Scholar]
- 67.Bzeizi KI, Smith R, Albenmousa A, Dama M, Aba-Alkhail F, Jalan R, Broering D. Long-Term Outcomes of Everolimus Therapy in De Novo Liver Transplantation: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Transplant Proc. 2021;53:148–158. doi: 10.1016/j.transproceed.2020.09.021. [DOI] [PubMed] [Google Scholar]
- 68.Saliba F, Duvoux C, Dharancy S, Dumortier J, Calmus Y, Gugenheim J, Kamar N, Salamé E, Neau-Cransac M, Vanlemmens C, Durand F, Pageaux G, Leroy V, Hardwigsen J, Gharbi H, Masson C, Tindel M, Conti F. Early Switch From Tacrolimus to Everolimus After Liver Transplantation: Outcomes at 2 Years. Liver Transpl. 2019;25:1822–1832. doi: 10.1002/lt.25664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cillo U, Saracino L, Vitale A, Bertacco A, Salizzoni M, Lupo F, Colledan M, Corno V, Rossi G, Reggiani P, Baccarani U, Bresàdola V, De Carlis L, Mangoni I, Ramirez Morales R, Agnes S, Nure E. Very Early Introduction of Everolimus in De Novo Liver Transplantation: Results of a Multicenter, Prospective, Randomized Trial. Liver Transpl. 2019;25:242–251. doi: 10.1002/lt.25400. [DOI] [PubMed] [Google Scholar]
- 70.Saliba F, Duvoux C, Gugenheim J, Kamar N, Dharancy S, Salamé E, Neau-Cransac M, Durand F, Houssel-Debry P, Vanlemmens C, Pageaux G, Hardwigsen J, Eyraud D, Calmus Y, Di Giambattista F, Dumortier J, Conti F. Efficacy and Safety of Everolimus and Mycophenolic Acid With Early Tacrolimus Withdrawal After Liver Transplantation: A Multicenter Randomized Trial. Am J Transplant. 2017;17:1843–1852. doi: 10.1111/ajt.14212. [DOI] [PubMed] [Google Scholar]
- 71.Stucchi RSB, Lopes MH, Kumar D, Manuel O. Vaccine Recommendations for Solid-Organ Transplant Recipients and Donors. Transplantation. 2018;102:S72–S80. doi: 10.1097/TP.0000000000002012. [DOI] [PubMed] [Google Scholar]
- 72.Karbasi-Afshar R, Izadi M, Fazel M, Khedmat H. Response of transplant recipients to influenza vaccination based on type of immunosuppression: A meta-analysis. Saudi J Kidney Dis Transpl. 2015;26:877–883. doi: 10.4103/1319-2442.164556. [DOI] [PubMed] [Google Scholar]
- 73.Croce E, Hatz C, Jonker EF, Visser LG, Jaeger VK, Bühler S. Safety of live vaccinations on immunosuppressive therapy in patients with immune-mediated inflammatory diseases, solid organ transplantation or after bone-marrow transplantation - A systematic review of randomized trials, observational studies and case reports. Vaccine. 2017;35:1216–1226. doi: 10.1016/j.vaccine.2017.01.048. [DOI] [PubMed] [Google Scholar]
- 74.Grohskopf LA, Alyanak E, Ferdinands JM, Broder KR, Blanton LH, Talbot HK, Fry AM. Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices, United States, 2021-22 Influenza Season. MMWR Recomm Rep. 2021;70:1–28. doi: 10.15585/mmwr.rr7005a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Beck CR, McKenzie BC, Hashim AB, Harris RC University of Nottingham Influenza and the ImmunoCompromised (UNIIC) Study Group,, Nguyen-Van-Tam JS. Influenza vaccination for immunocompromised patients: systematic review and meta-analysis by etiology. J Infect Dis. 2012;206:1250–1259. doi: 10.1093/infdis/jis487. [DOI] [PubMed] [Google Scholar]
- 76.Clayville LR. Influenza update: a review of currently available vaccines. P T. 2011;36:659–684. [PMC free article] [PubMed] [Google Scholar]
- 77.Mombelli M, Kampouri E, Manuel O. Influenza in solid organ transplant recipients: epidemiology, management, and outcomes. Expert Rev Anti Infect Ther. 2020;18:103–112. doi: 10.1080/14787210.2020.1713098. [DOI] [PubMed] [Google Scholar]
- 78.Vilchez RA, McCurry K, Dauber J, Lacono A, Griffith B, Fung J, Kusne S. Influenza virus infection in adult solid organ transplant recipients. Am J Transplant. 2002;2:287–291. doi: 10.1034/j.1600-6143.2002.20315.x. [DOI] [PubMed] [Google Scholar]
- 79.Sellers SA, Hagan RS, Hayden FG, Fischer WA 2nd. The hidden burden of influenza: A review of the extra-pulmonary complications of influenza infection. Influenza Other Respir Viruses. 2017;11:372–393. doi: 10.1111/irv.12470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kumar D, Ferreira VH, Blumberg E, Silveira F, Cordero E, Perez-Romero P, Aydillo T, Danziger-Isakov L, Limaye AP, Carratala J, Munoz P, Montejo M, Lopez-Medrano F, Farinas MC, Gavalda J, Moreno A, Levi M, Fortun J, Torre-Cisneros J, Englund JA, Natori Y, Husain S, Reid G, Sharma TS, Humar A. A 5-Year Prospective Multicenter Evaluation of Influenza Infection in Transplant Recipients. Clin Infect Dis. 2018;67:1322–1329. doi: 10.1093/cid/ciy294. [DOI] [PubMed] [Google Scholar]
- 81.Restivo V, Vizzini G, Mularoni A, Di Benedetto C, Gioè SM, Vitale F. Determinants of influenza vaccination among solid organ transplant recipients attending Sicilian reference center. Hum Vaccin Immunother. 2017;13:346–350. doi: 10.1080/21645515.2017.1264792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rubin LG, Levin MJ, Ljungman P, Davies EG, Avery R, Tomblyn M, Bousvaros A, Dhanireddy S, Sung L, Keyserling H, Kang I Infectious Diseases Society of America. 2013 IDSA clinical practice guideline for vaccination of the immunocompromised host. Clin Infect Dis. 2014;58:e44–100. doi: 10.1093/cid/cit684. [DOI] [PubMed] [Google Scholar]
- 83.Loubet P, Kernéis S, Groh M, Loulergue P, Blanche P, Verger P, Launay O. Attitude, knowledge and factors associated with influenza and pneumococcal vaccine uptake in a large cohort of patients with secondary immune deficiency. Vaccine. 2015;33:3703–3708. doi: 10.1016/j.vaccine.2015.06.012. [DOI] [PubMed] [Google Scholar]
- 84.Weltermann B, Herwig A, Dehnen D, Herzer K. Vaccination Status of Pneumococcal and Other Vaccines in 444 Liver Transplant Patients Compared to a Representative Population Sample. Ann Transplant. 2016;21:200–207. doi: 10.12659/aot.896436. [DOI] [PubMed] [Google Scholar]
- 85.Leibovici Weissman Y, Cooper L, Sternbach N, Ashkenazi-Hoffnung L, Yahav D. Clinical efficacy and safety of high dose trivalent influenza vaccine in adults and immunosuppressed populations - A systematic review and meta-analysis. J Infect. 2021;83:444–451. doi: 10.1016/j.jinf.2021.08.028. [DOI] [PubMed] [Google Scholar]
- 86.Ison MG, Szakaly P, Shapira MY, Kriván G, Nist A, Dutkowski R. Efficacy and safety of oral oseltamivir for influenza prophylaxis in transplant recipients. Antivir Ther. 2012;17:955–964. doi: 10.3851/IMP2192. [DOI] [PubMed] [Google Scholar]
- 87.Lam H, Jeffery J, Sitar DS, Aoki FY. Oseltamivir, an influenza neuraminidase inhibitor drug, does not affect the steady-state pharmacokinetic characteristics of cyclosporine, mycophenolate, or tacrolimus in adult renal transplant patients. Ther Drug Monit. 2011;33:699–704. doi: 10.1097/FTD.0b013e3182399448. [DOI] [PubMed] [Google Scholar]
- 88.Ison MG, Portsmouth S, Yoshida Y, Shishido T, Mitchener M, Tsuchiya K, Uehara T, Hayden FG. Early treatment with baloxavir marboxil in high-risk adolescent and adult outpatients with uncomplicated influenza (CAPSTONE-2): a randomised, placebo-controlled, phase 3 trial. Lancet Infect Dis. 2020;20:1204–1214. doi: 10.1016/S1473-3099(20)30004-9. [DOI] [PubMed] [Google Scholar]
- 89.Zhou J, Hu Z, Zhang Q, Li Z, Xiang J, Yan S, Wu J, Zhang M, Zheng S. Spectrum of De Novo Cancers and Predictors in Liver Transplantation: Analysis of the Scientific Registry of Transplant Recipients Database. PLoS One. 2016;11:e0155179. doi: 10.1371/journal.pone.0155179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Burra P, Rodriguez-Castro KI. Neoplastic disease after liver transplantation: Focus on de novo neoplasms. World J Gastroenterol. 2015;21:8753–8768. doi: 10.3748/wjg.v21.i29.8753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Finkenstedt A, Graziadei IW, Oberaigner W, Hilbe W, Nachbaur K, Mark W, Margreiter R, Vogel W. Extensive surveillance promotes early diagnosis and improved survival of de novo malignancies in liver transplant recipients. Am J Transplant. 2009;9:2355–2361. doi: 10.1111/j.1600-6143.2009.02766.x. [DOI] [PubMed] [Google Scholar]
- 92.Rademacher S, Seehofer D, Eurich D, Schoening W, Neuhaus R, Oellinger R, Denecke T, Pascher A, Schott E, Sinn M, Neuhaus P, Pratschke J. The 28-year incidence of de novo malignancies after liver transplantation: A single-center analysis of risk factors and mortality in 1616 patients. Liver Transpl. 2017;23:1404–1414. doi: 10.1002/lt.24795. [DOI] [PubMed] [Google Scholar]
- 93.Yanik EL, Smith JM, Shiels MS, Clarke CA, Lynch CF, Kahn AR, Koch L, Pawlish KS, Engels EA. Cancer Risk After Pediatric Solid Organ Transplantation. Pediatrics. 2017;139 doi: 10.1542/peds.2016-3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Herrero JI, España A, Quiroga J, Sangro B, Pardo F, Alvárez-Cienfuegos J, Prieto J. Nonmelanoma skin cancer after liver transplantation. Study of risk factors. Liver Transpl. 2005;11:1100–1106. doi: 10.1002/lt.20525. [DOI] [PubMed] [Google Scholar]
- 95.Watt KD, Pedersen RA, Kremers WK, Heimbach JK, Sanchez W, Gores GJ. Long-term probability of and mortality from de novo malignancy after liver transplantation. Gastroenterology. 2009;137:2010–2017. doi: 10.1053/j.gastro.2009.08.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Esposito K, Chiodini P, Colao A, Lenzi A, Giugliano D. Metabolic syndrome and risk of cancer: a systematic review and meta-analysis. Diabetes Care. 2012;35:2402–2411. doi: 10.2337/dc12-0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yuan Y, Cai X, Shen F, Ma F. HPV post-infection microenvironment and cervical cancer. Cancer Lett. 2021;497:243–254. doi: 10.1016/j.canlet.2020.10.034. [DOI] [PubMed] [Google Scholar]
- 98.Herrero JI, Pardo F, D'Avola D, Alegre F, Rotellar F, Iñarrairaegui M, Martí P, Sangro B, Quiroga J. Risk factors of lung, head and neck, esophageal, and kidney and urinary tract carcinomas after liver transplantation: the effect of smoking withdrawal. Liver Transpl. 2011;17:402–408. doi: 10.1002/lt.22247. [DOI] [PubMed] [Google Scholar]
- 99.Zhang YB, Pan XF, Chen J, Cao A, Zhang YG, Xia L, Wang J, Li H, Liu G, Pan A. Combined lifestyle factors, incident cancer, and cancer mortality: a systematic review and meta-analysis of prospective cohort studies. Br J Cancer. 2020;122:1085–1093. doi: 10.1038/s41416-020-0741-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lee J, Lee KS, Kim H, Jeong H, Choi MJ, Yoo HW, Han TH, Lee H. The relationship between metabolic syndrome and the incidence of colorectal cancer. Environ Health Prev Med. 2020;25:6. doi: 10.1186/s12199-020-00845-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Raimondi S, Suppa M, Gandini S. Melanoma Epidemiology and Sun Exposure. Acta Derm Venereol. 2020;100:adv00136. doi: 10.2340/00015555-3491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Garland SM, Brotherton JML, Moscicki AB, Kaufmann AM, Stanley M, Bhatla N, Sankaranarayanan R, de Sanjosé S, Palefsky JM IPVS. HPV vaccination of immunocompromised hosts. Papillomavirus Res. 2017;4:35–38. doi: 10.1016/j.pvr.2017.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Nailescu C, Nelson RD, Verghese PS, Twombley KE, Chishti AS, Mills M, Mahan JD, Slaven JE, Shew ML. Human Papillomavirus Vaccination in Male and Female Adolescents Before and After Kidney Transplantation: A Pediatric Nephrology Research Consortium Study. Front Pediatr. 2020;8:46. doi: 10.3389/fped.2020.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chakkera HA, Kudva Y, Kaplan B. Calcineurin Inhibitors: Pharmacologic Mechanisms Impacting Both Insulin Resistance and Insulin Secretion Leading to Glucose Dysregulation and Diabetes Mellitus. Clin Pharmacol Ther. 2017;101:114–120. doi: 10.1002/cpt.546. [DOI] [PubMed] [Google Scholar]
- 105.Gutierrez-Dalmau A, Campistol JM. Immunosuppressive therapy and malignancy in organ transplant recipients: a systematic review. Drugs. 2007;67:1167–1198. doi: 10.2165/00003495-200767080-00006. [DOI] [PubMed] [Google Scholar]
- 106.Aguiar D, Martínez-Urbistondo D, D'Avola D, Iñarrairaegui M, Pardo F, Rotellar F, Sangro B, Quiroga J, Herrero JI. Conversion from Calcineurin Inhibitor-Based Immunosuppression to Mycophenolate Mofetil in Monotherapy Reduces Risk of De Novo Malignancies After Liver Transplantation. Ann Transplant. 2017;22:141–147. doi: 10.12659/aot.901556. [DOI] [PubMed] [Google Scholar]
- 107.Cholongitas E, Mamou C, Rodríguez-Castro KI, Burra P. Mammalian target of rapamycin inhibitors are associated with lower rates of hepatocellular carcinoma recurrence after liver transplantation: a systematic review. Transpl Int. 2014;27:1039–1049. doi: 10.1111/tri.12372. [DOI] [PubMed] [Google Scholar]
- 108.Charlton M, Levitsky J, Aqel B, OʼGrady J, Hemibach J, Rinella M, Fung J, Ghabril M, Thomason R, Burra P, Little EC, Berenguer M, Shaked A, Trotter J, Roberts J, Rodriguez-Davalos M, Rela M, Pomfret E, Heyrend C, Gallegos-Orozco J, Saliba F. International Liver Transplantation Society Consensus Statement on Immunosuppression in Liver Transplant Recipients. Transplantation. 2018;102:727–743. doi: 10.1097/TP.0000000000002147. [DOI] [PubMed] [Google Scholar]
- 109.Van Der Heide F, Dijkstra G, Porte RJ, Kleibeuker JH, Haagsma EB. Smoking behavior in liver transplant recipients. Liver Transpl. 2009;15:648–655. doi: 10.1002/lt.21722. [DOI] [PubMed] [Google Scholar]
- 110.Ehlers SL, Rodrigue JR, Widows MR, Reed AI, Nelson DR. Tobacco use before and after liver transplantation: a single center survey and implications for clinical practice and research. Liver Transpl. 2004;10:412–417. doi: 10.1002/lt.20087. [DOI] [PubMed] [Google Scholar]
- 111.Rammohan A. Current management & future directions in post-liver transplant recurrence of viral hepatitis. J Liver Transpl . 2021;3:100027. [Google Scholar]
- 112.Carenco C, Faure S, Herrero A, Assenat E, Duny Y, Danan G, Bismuth M, Chanques G, Ursic-Bedoya J, Jaber S, Larrey D, Navarro F, Pageaux GP. Incidence of solid organ cancers after liver transplantation: comparison with regional cancer incidence rates and risk factors. Liver Int. 2015;35:1748–1755. doi: 10.1111/liv.12758. [DOI] [PubMed] [Google Scholar]
- 113.DiMartini A, Javed L, Russell S, Dew MA, Fitzgerald MG, Jain A, Fung J. Tobacco use following liver transplantation for alcoholic liver disease: an underestimated problem. Liver Transpl. 2005;11:679–683. doi: 10.1002/lt.20385. [DOI] [PubMed] [Google Scholar]
- 114.De Leon J, Rendon DM, Baca-Garcia E, Aizpuru F, Gonzalez-Pinto A, Anitua C, Diaz FJ. Association between smoking and alcohol use in the general population: stable and unstable odds ratios across two years in two different countries. Alcohol Alcohol. 2007;42:252–257. doi: 10.1093/alcalc/agm029. [DOI] [PubMed] [Google Scholar]
- 115.López-Lazcano AI, Gual A, Colmenero J, Caballería E, Lligoña A, Navasa M, Crespo G, López E, López-Pelayo H. Active Smoking Before Liver Transplantation in Patients with Alcohol Use Disorder: Risk Factors and Outcomes. J Clin Med. 2020;9 doi: 10.3390/jcm9092710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bright RP, Civalier KM, Krahn L. Reliability of self-reported nicotine use as determined by serum cotinine levels in patients referred for liver transplantation. Psychosomatics. 2010;51:395–400. doi: 10.1176/appi.psy.51.5.395. [DOI] [PubMed] [Google Scholar]
- 117.Mangus RS, Fridell JA, Kubal CA, Loeffler AL, Krause AA, Bell JA, Tiwari S, Tector J. Worse Long-term Patient Survival and Higher Cancer Rates in Liver Transplant Recipients With a History of Smoking. Transplantation. 2015;99:1862–1868. doi: 10.1097/TP.0000000000000671. [DOI] [PubMed] [Google Scholar]
- 118.Li Q, Wang Y, Ma T, Liu X, Wang B, Wu Z, Lv Y, Wu R. Impact of cigarette smoking on early complications after liver transplantation: A single-center experience and a meta-analysis. PLoS One. 2017;12:e0178570. doi: 10.1371/journal.pone.0178570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Skladany L, Adamcova Selcanova S, Koller T. Alcohol Use Relapse Following Liver Transplantation for Alcoholic Liver Disease. Ann Transplant. 2019;24:359–366. doi: 10.12659/AOT.914690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bhat M, Deschenes M, Tan X, Martel M, Bhat V, Wong P, Metrakos P, Ghali P. Smoking increases recurrent viral hepatitis after liver transplantation. Liver Transpl. 2012;18:828–833. doi: 10.1002/lt.23444. [DOI] [PubMed] [Google Scholar]
- 121.Joshi D, Bjarnason I, Belgaumkar A, O'Grady J, Suddle A, Heneghan MA, Aluvihare V, Rela M, Heaton N, Agarwal K. The impact of inflammatory bowel disease post-liver transplantation for primary sclerosing cholangitis. Liver Int. 2013;33:53–61. doi: 10.1111/j.1478-3231.2011.02677.x. [DOI] [PubMed] [Google Scholar]
- 122.Mathur AK, Ranney DN, Patel SP, Lee DS, Bednar F, Lynch RJ, Welling TH, Englesbe MJ. The effect of smoking on biliary complications following liver transplantation. Transpl Int. 2011;24:58–66. doi: 10.1111/j.1432-2277.2010.01146.x. [DOI] [PubMed] [Google Scholar]
- 123.Dulaney DT, Dokus KM, McIntosh S, Al-Judaibi B, Ramaraju GA, Tomiyama K, Levstik M, Hernandez-Alejandro R, Orloff MS, Kashyap R. Tobacco Use is a Modifiable Risk Factor for Post-Transplant Biliary Complications. J Gastrointest Surg. 2017;21:1643–1649. doi: 10.1007/s11605-017-3519-6. [DOI] [PubMed] [Google Scholar]
- 124.Mouchli MA, Singh S, Loftus EV Jr, Boardman L, Talwalkar J, Rosen CB, Heimbach JK, Wiesner RH, Hasan B, Poterucha JJ, Kymberly WD. Risk Factors and Outcomes of De Novo Cancers (Excluding Nonmelanoma Skin Cancer) After Liver Transplantation for Primary Sclerosing Cholangitis. Transplantation. 2017;101:1859–1866. doi: 10.1097/TP.0000000000001725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Harrington C, Kosirog M, Campbell P, Gregory D, Daud A, Levitsky J, Holl JL, Lloyd-Jones DM, VanWagner LB. Poor Practitioner Adherence to Clinical Tobacco Use Guidelines in Liver Transplant Recipients. Transplant Direct. 2022;8:e1288. doi: 10.1097/TXD.0000000000001288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zwar NA, Mendelsohn CP, Richmond RL. Supporting smoking cessation. BMJ. 2014;348:f7535. doi: 10.1136/bmj.f7535. [DOI] [PubMed] [Google Scholar]
- 127.Levitsky J, Burrell BE, Kanaparthi S, Turka LA, Kurian S, Sanchez-Fueyo A, Lozano JJ, Demetris A, Lesniak A, Kirk AD, Stempora L, Yang GY, Mathew JM. Immunosuppression Withdrawal in Liver Transplant Recipients on Sirolimus. Hepatology. 2020;72:569–583. doi: 10.1002/hep.31036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Mor E, Gonwa TA, Husberg BS, Goldstein RM, Klintmalm GB. Late-onset acute rejection in orthotopic liver transplantation--associated risk factors and outcome. Transplantation. 1992;54:821–824. doi: 10.1097/00007890-199211000-00010. [DOI] [PubMed] [Google Scholar]
- 129.Berlakovich GA, Langer F, Freundorfer E, Windhager T, Rockenschaub S, Sporn E, Soliman T, Pokorny H, Steininger R, Mühlbacher F. General compliance after liver transplantation for alcoholic cirrhosis. Transpl Int. 2000;13:129–135. doi: 10.1007/s001470050298. [DOI] [PubMed] [Google Scholar]
- 130.Jain A, Demetris AJ, Kashyap R, Blakomer K, Ruppert K, Khan A, Rohal S, Starzl TE, Fung JJ. Does tacrolimus offer virtual freedom from chronic rejection after primary liver transplantation? Liver Transpl. 2001;7:623–630. doi: 10.1053/jlts.2001.25364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Lamba S, Nagurka R, Desai KK, Chun SJ, Holland B, Koneru B. Self-reported non-adherence to immune-suppressant therapy in liver transplant recipients: demographic, interpersonal, and intrapersonal factors. Clin Transplant. 2012;26:328–335. doi: 10.1111/j.1399-0012.2011.01489.x. [DOI] [PubMed] [Google Scholar]
- 132.Drent G, Haagsma EB, Geest SD, van den Berg AP, Ten Vergert EM, van den Bosch HJ, Slooff MJ, Kleibeuker JH. Prevalence of prednisolone (non)compliance in adult liver transplant recipients. Transpl Int. 2005;18:960–966. doi: 10.1111/j.1432-2277.2005.00170.x. [DOI] [PubMed] [Google Scholar]
- 133.Klein A, Otto G, Krämer I. Impact of a pharmaceutical care program on liver transplant patients' compliance with immunosuppressive medication: a prospective, randomized, controlled trial using electronic monitoring. Transplantation. 2009;87:839–847. doi: 10.1097/TP.0b013e318199d122. [DOI] [PubMed] [Google Scholar]
- 134.Shi YX, Liu CX, Liu F, Zhang HM, Yu MM, Jin YH, Shang SM, Fu YX. Efficacy of Adherence-Enhancing Interventions for Immunosuppressive Therapy in Solid Organ Transplant Recipients: A Systematic Review and Meta-Analysis Based on Randomized Controlled Trials. Front Pharmacol. 2020;11:578887. doi: 10.3389/fphar.2020.578887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Whitsett M, Levitsky J. Medication nonadherence in liver transplantation. Clin Liver Dis (Hoboken) 2017;10:157–160. doi: 10.1002/cld.680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Burra P, Germani G, Gnoato F, Lazzaro S, Russo FP, Cillo U, Senzolo M. Adherence in liver transplant recipients. Liver Transpl. 2011;17:760–770. doi: 10.1002/lt.22294. [DOI] [PubMed] [Google Scholar]
- 137.Jain M, Venkataraman J, Reddy MS, Rela M. Determinants of Medication Adherence in Liver Transplant Recipients. J Clin Exp Hepatol. 2019;9:676–683. doi: 10.1016/j.jceh.2019.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Shemesh E, Shneider BL, Savitzky JK, Arnott L, Gondolesi GE, Krieger NR, Kerkar N, Magid MS, Stuber ML, Schmeidler J, Yehuda R, Emre S. Medication adherence in pediatric and adolescent liver transplant recipients. Pediatrics. 2004;113:825–832. doi: 10.1542/peds.113.4.825. [DOI] [PubMed] [Google Scholar]
- 139.Schäfer-Keller P, Steiger J, Bock A, Denhaerynck K, De Geest S. Diagnostic accuracy of measurement methods to assess non-adherence to immunosuppressive drugs in kidney transplant recipients. Am J Transplant. 2008;8:616–626. doi: 10.1111/j.1600-6143.2007.02127.x. [DOI] [PubMed] [Google Scholar]
- 140.O'Carroll RE, McGregor LM, Swanson V, Masterton G, Hayes PC. Adherence to medication after liver transplantation in Scotland: a pilot study. Liver Transpl. 2006;12:1862–1868. doi: 10.1002/lt.20828. [DOI] [PubMed] [Google Scholar]
- 141.Adam R, Karam V, Delvart V, O'Grady J, Mirza D, Klempnauer J, Castaing D, Neuhaus P, Jamieson N, Salizzoni M, Pollard S, Lerut J, Paul A, Garcia-Valdecasas JC, Rodríguez FS, Burroughs A All contributing centers (www. eltr.org); European Liver and Intestine Transplant Association (ELITA). Evolution of indications and results of liver transplantation in Europe. A report from the European Liver Transplant Registry (ELTR) J Hepatol. 2012;57:675–688. doi: 10.1016/j.jhep.2012.04.015. [DOI] [PubMed] [Google Scholar]
- 142.Cholankeril G, Ahmed A. Alcoholic Liver Disease Replaces Hepatitis C Virus Infection as the Leading Indication for Liver Transplantation in the United States. Clin Gastroenterol Hepatol. 2018;16:1356–1358. doi: 10.1016/j.cgh.2017.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.DiMartini A, Dew MA, Day N, Fitzgerald MG, Jones BL, deVera ME, Fontes P. Trajectories of alcohol consumption following liver transplantation. Am J Transplant. 2010;10:2305–2312. doi: 10.1111/j.1600-6143.2010.03232.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.De Gottardi A, Spahr L, Gelez P, Morard I, Mentha G, Guillaud O, Majno P, Morel P, Hadengue A, Paliard P, Scoazec JY, Boillot O, Giostra E, Dumortier J. A simple score for predicting alcohol relapse after liver transplantation: results from 387 patients over 15 years. Arch Intern Med. 2007;167:1183–1188. doi: 10.1001/archinte.167.11.1183. [DOI] [PubMed] [Google Scholar]
- 145.Faure S, Herrero A, Jung B, Duny Y, Daures JP, Mura T, Assenat E, Bismuth M, Bouyabrine H, Donnadieu-Rigole H, Navarro F, Jaber S, Larrey D, Pageaux GP. Excessive alcohol consumption after liver transplantation impacts on long-term survival, whatever the primary indication. J Hepatol. 2012;57:306–312. doi: 10.1016/j.jhep.2012.03.014. [DOI] [PubMed] [Google Scholar]
- 146.Louvet A, Labreuche J, Moreno C, Vanlemmens C, Moirand R, Féray C, Dumortier J, Pageaux GP, Bureau C, Chermak F, Duvoux C, Thabut D, Leroy V, Carbonell N, Rolland B, Salamé E, Anty R, Gournay J, Delwaide J, Silvain C, Lucidi V, Lassailly G, Dharancy S, Nguyen-Khac E, Samuel D, Duhamel A, Mathurin P QuickTrans trial study group. Early liver transplantation for severe alcohol-related hepatitis not responding to medical treatment: a prospective controlled study. Lancet Gastroenterol Hepatol. 2022;7:416–425. doi: 10.1016/S2468-1253(21)00430-1. [DOI] [PubMed] [Google Scholar]
- 147.Chuncharunee L, Yamashiki N, Thakkinstian A, Sobhonslidsuk A. Alcohol relapse and its predictors after liver transplantation for alcoholic liver disease: a systematic review and meta-analysis. BMC Gastroenterol. 2019;19:150. doi: 10.1186/s12876-019-1050-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Yu TW, Chen YM, Wang CC, Lin CC, Huang KT, Liu YW, Hsu LW, Li WF, Chan YC, Chen CL, Chen CC. Incidence and Risk Factors of Alcohol Relapse after Liver Transplantation: Analysis of Pre-Transplant Abstinence and Psychosocial Features. J Clin Med. 2020;9 doi: 10.3390/jcm9113716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Gish RG, Lee A, Brooks L, Leung J, Lau JY, Moore DH 2nd. Long-term follow-up of patients diagnosed with alcohol dependence or alcohol abuse who were evaluated for liver transplantation. Liver Transpl. 2001;7:581–587. doi: 10.1053/jlts.2001.25455. [DOI] [PubMed] [Google Scholar]
- 150.Pfitzmann R, Schwenzer J, Rayes N, Seehofer D, Neuhaus R, Nüssler NC. Long-term survival and predictors of relapse after orthotopic liver transplantation for alcoholic liver disease. Liver Transpl. 2007;13:197–205. doi: 10.1002/lt.20934. [DOI] [PubMed] [Google Scholar]
- 151.Satapathy SK, Thornburgh C, Heda R, Jiang Y, Kedia SK, Nair SP, Eason JD, Maluf D. Predicting harmful alcohol relapse after liver transplant: The HALT score. Clin Transplant. 2020;34:e14003. doi: 10.1111/ctr.14003. [DOI] [PubMed] [Google Scholar]
- 152.Lee BP, Roth N, Rao P, Im GY, Vogel AS, Hasbun J, Roth Y, Shenoy A, Arvelakis A, Ford L, Dawe I, Schiano TD, Davis JP, Rice JP, Eswaran S, Weinberg E, Han H, Hsu C, Fix OK, Maddur H, Ghobrial RM, Therapondos G, Dilkina B, Terrault NA. Artificial intelligence to identify harmful alcohol use after early liver transplant for alcohol-associated hepatitis. Am J Transplant. 2022;22:1834–1841. doi: 10.1111/ajt.17059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Burra P, Senzolo M, Adam R, Delvart V, Karam V, Germani G, Neuberger J ELITA; ELTR Liver Transplant Centers. Liver transplantation for alcoholic liver disease in Europe: a study from the ELTR (European Liver Transplant Registry) Am J Transplant. 2010;10:138–148. doi: 10.1111/j.1600-6143.2009.02869.x. [DOI] [PubMed] [Google Scholar]
- 154.Cuadrado A, Fábrega E, Casafont F, Pons-Romero F. Alcohol recidivism impairs long-term patient survival after orthotopic liver transplantation for alcoholic liver disease. Liver Transpl. 2005;11:420–426. doi: 10.1002/lt.20386. [DOI] [PubMed] [Google Scholar]
- 155.Dumortier J, Guillaud O, Adham M, Boucaud C, Delafosse B, Bouffard Y, Paliard P, Scoazec JY, Boillot O. Negative impact of de novo malignancies rather than alcohol relapse on survival after liver transplantation for alcoholic cirrhosis: a retrospective analysis of 305 patients in a single center. Am J Gastroenterol. 2007;102:1032–1041. doi: 10.1111/j.1572-0241.2007.01079.x. [DOI] [PubMed] [Google Scholar]
- 156.Dumortier J, Dharancy S, Cannesson A, Lassailly G, Rolland B, Pruvot FR, Boillot O, Faure S, Guillaud O, Rigole-Donnadieu H, Herrero A, Scoazec JY, Mathurin P, Pageaux GP. Recurrent alcoholic cirrhosis in severe alcoholic relapse after liver transplantation: a frequent and serious complication. Am J Gastroenterol. 2015;110:1160–6; quiz 1167. doi: 10.1038/ajg.2015.204. [DOI] [PubMed] [Google Scholar]
- 157.European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of alcohol-related liver disease. J Hepatol. 2018;69:154–181. doi: 10.1016/j.jhep.2018.03.018. [DOI] [PubMed] [Google Scholar]
- 158.Litten RZ, Bradley AM, Moss HB. Alcohol biomarkers in applied settings: recent advances and future research opportunities. Alcohol Clin Exp Res. 2010;34:955–967. doi: 10.1111/j.1530-0277.2010.01170.x. [DOI] [PubMed] [Google Scholar]
- 159.Björnsson E, Olsson J, Rydell A, Fredriksson K, Eriksson C, Sjöberg C, Olausson M, Bäckman L, Castedal M, Friman S. Long-term follow-up of patients with alcoholic liver disease after liver transplantation in Sweden: impact of structured management on recidivism. Scand J Gastroenterol. 2005;40:206–216. doi: 10.1080/00365520410009591. [DOI] [PubMed] [Google Scholar]
- 160.Attilia ML, Lattanzi B, Ledda R, Galli AM, Farcomeni A, Rotondo C, Di Gregorio V, Mennini G, Poli E, Attilia F, Ginanni Corradini S, Rossi M, Merli M. The multidisciplinary support in preventing alcohol relapse after liver transplantation: A single-center experience. Clin Transplant. 2018;32:e13243. doi: 10.1111/ctr.13243. [DOI] [PubMed] [Google Scholar]
- 161.Addolorato G, Mirijello A, Leggio L, Ferrulli A, D'Angelo C, Vassallo G, Cossari A, Gasbarrini G, Landolfi R, Agnes S, Gasbarrini A Gemelli OLT Group. Liver transplantation in alcoholic patients: impact of an alcohol addiction unit within a liver transplant center. Alcohol Clin Exp Res. 2013;37:1601–1608. doi: 10.1111/acer.12117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Addolorato G, Bataller R, Burra P, DiMartini A, Graziadei I, Lucey MR, Mathurin P, OʼGrady J, Pageaux G, Berenguer M. Liver Transplantation for Alcoholic Liver Disease. Transplantation. 2016;100:981–987. doi: 10.1097/TP.0000000000001156. [DOI] [PubMed] [Google Scholar]
- 163.Bravata DM, Olkin I, Barnato AE, Keeffe EB, Owens DK. Employment and alcohol use after liver transplantation for alcoholic and nonalcoholic liver disease: a systematic review. Liver Transpl. 2001;7:191–203. doi: 10.1053/jlts.2001.22326. [DOI] [PubMed] [Google Scholar]
- 164.Martin P, DiMartini A, Feng S, Brown R Jr, Fallon M. Evaluation for liver transplantation in adults: 2013 practice guideline by the American Association for the Study of Liver Diseases and the American Society of Transplantation. Hepatology. 2014;59:1144–1165. doi: 10.1002/hep.26972. [DOI] [PubMed] [Google Scholar]
- 165.Reig M, Forner A, Rimola J, Ferrer-Fàbrega J, Burrel M, Garcia-Criado Á, Kelley RK, Galle PR, Mazzaferro V, Salem R, Sangro B, Singal AG, Vogel A, Fuster J, Ayuso C, Bruix J. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J Hepatol. 2022;76:681–693. doi: 10.1016/j.jhep.2021.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Chagas AL, Felga GEG, Diniz MA, Silva RF, Mattos AA, Silva RCMA, Boin IFSF, Garcia JHP, Lima AS, Coelho JCU, Bittencourt PL, Alves VAF, D'Albuquerque LAC, Carrilho FJ Brazilian HCC Study Group. Hepatocellular carcinoma recurrence after liver transplantation in a Brazilian multicenter study: clinical profile and prognostic factors of survival. Eur J Gastroenterol Hepatol. 2019;31:1148–1156. doi: 10.1097/MEG.0000000000001448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Roayaie S, Schwartz JD, Sung MW, Emre SH, Miller CM, Gondolesi GE, Krieger NR, Schwartz ME. Recurrence of hepatocellular carcinoma after liver transplant: patterns and prognosis. Liver Transpl. 2004;10:534–540. doi: 10.1002/lt.20128. [DOI] [PubMed] [Google Scholar]
- 168.Foerster F, Hoppe-Lotichius M, Vollmar J, Marquardt JU, Weinmann A, Wörns MA, Otto G, Zimmermann T, Galle PR. Long-term observation of hepatocellular carcinoma recurrence after liver transplantation at a European transplantation centre. United European Gastroenterol J. 2019;7:838–849. doi: 10.1177/2050640619840221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Toniutto P, Fornasiere E, Fumolo E, Bitetto D. Risk factors for hepatocellular carcinoma recurrence after liver transplantation. Hepatoma Res . :2020. [Google Scholar]
- 170.Mazzaferro V, Bhoori S, Sposito C, Bongini M, Langer M, Miceli R, Mariani L. Milan criteria in liver transplantation for hepatocellular carcinoma: an evidence-based analysis of 15 years of experience. Liver Transpl. 2011;17 Suppl 2:S44–S57. doi: 10.1002/lt.22365. [DOI] [PubMed] [Google Scholar]
- 171.Takayasu K, Arii S, Ikai I, Omata M, Okita K, Ichida T, Matsuyama Y, Nakanuma Y, Kojiro M, Makuuchi M, Yamaoka Y Liver Cancer Study Group of Japan. Prospective cohort study of transarterial chemoembolization for unresectable hepatocellular carcinoma in 8510 patients. Gastroenterology. 2006;131:461–469. doi: 10.1053/j.gastro.2006.05.021. [DOI] [PubMed] [Google Scholar]
- 172.Shah SA, Tan JC, McGilvray ID, Cattral MS, Levy GA, Greig PD, Grant DR. Does microvascular invasion affect outcomes after liver transplantation for HCC? J Gastrointest Surg. 2007;11:464–471. doi: 10.1007/s11605-006-0033-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Guerrini GP, Pinelli D, Di Benedetto F, Marini E, Corno V, Guizzetti M, Aluffi A, Zambelli M, Fagiuoli S, Lucà MG, Lucianetti A, Colledan M. Predictive value of nodule size and differentiation in HCC recurrence after liver transplantation. Surg Oncol. 2016;25:419–428. doi: 10.1016/j.suronc.2015.09.003. [DOI] [PubMed] [Google Scholar]
- 174.Tamura S, Kato T, Berho M, Misiakos EP, O'Brien C, Reddy KR, Nery JR, Burke GW, Schiff ER, Miller J, Tzakis AG. Impact of histological grade of hepatocellular carcinoma on the outcome of liver transplantation. Arch Surg. 2001;136:25–30; discussion 31. [PubMed] [Google Scholar]
- 175.Jonas S, Bechstein WO, Steinmüller T, Herrmann M, Radke C, Berg T, Settmacher U, Neuhaus P. Vascular invasion and histopathologic grading determine outcome after liver transplantation for hepatocellular carcinoma in cirrhosis. Hepatology. 2001;33:1080–1086. doi: 10.1053/jhep.2001.23561. [DOI] [PubMed] [Google Scholar]
- 176.Al-Ameri AAM, Wei X, Wen X, Wei Q, Guo H, Zheng S, Xu X. Systematic review: risk prediction models for recurrence of hepatocellular carcinoma after liver transplantation. Transpl Int. 2020;33:697–712. doi: 10.1111/tri.13585. [DOI] [PubMed] [Google Scholar]
- 177.Yao FY, Xiao L, Bass NM, Kerlan R, Ascher NL, Roberts JP. Liver transplantation for hepatocellular carcinoma: validation of the UCSF-expanded criteria based on preoperative imaging. Am J Transplant. 2007;7:2587–2596. doi: 10.1111/j.1600-6143.2007.01965.x. [DOI] [PubMed] [Google Scholar]
- 178.Mazzaferro V, Llovet JM, Miceli R, Bhoori S, Schiavo M, Mariani L, Camerini T, Roayaie S, Schwartz ME, Grazi GL, Adam R, Neuhaus P, Salizzoni M, Bruix J, Forner A, De Carlis L, Cillo U, Burroughs AK, Troisi R, Rossi M, Gerunda GE, Lerut J, Belghiti J, Boin I, Gugenheim J, Rochling F, Van Hoek B, Majno P Metroticket Investigator Study Group. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: a retrospective, exploratory analysis. Lancet Oncol. 2009;10:35–43. doi: 10.1016/S1470-2045(08)70284-5. [DOI] [PubMed] [Google Scholar]
- 179.Mazzaferro V, Sposito C, Zhou J, Pinna AD, De Carlis L, Fan J, Cescon M, Di Sandro S, Yi-Feng H, Lauterio A, Bongini M, Cucchetti A. Metroticket 2.0 Model for Analysis of Competing Risks of Death After Liver Transplantation for Hepatocellular Carcinoma. Gastroenterology. 2018;154:128–139. doi: 10.1053/j.gastro.2017.09.025. [DOI] [PubMed] [Google Scholar]
- 180.Centonze L, Di Sandro S, Lauterio A, De Carlis R, Sgrazzutti C, Ciulli C, Vella I, Vicentin I, Incarbone N, Bagnardi V, Vanzulli A, De Carlis L. A retrospective single-centre analysis of the oncological impact of LI-RADS classification applied to Metroticket 2.0 calculator in liver transplantation: every nodule matters. Transpl Int. 2021;34:1712–1721. doi: 10.1111/tri.13983. [DOI] [PubMed] [Google Scholar]
- 181.Cucchetti A, Serenari M, Sposito C, Di Sandro S, Mosconi C, Vicentin I, Garanzini E, Mazzaferro V, De Carlis L, Golfieri R, Spreafico C, Vanzulli A, Buscemi V, Ravaioli M, Ercolani G, Pinna AD, Cescon M. Including mRECIST in the Metroticket 2.0 criteria improves prediction of hepatocellular carcinoma-related death after liver transplant. J Hepatol. 2020;73:342–348. doi: 10.1016/j.jhep.2020.03.018. [DOI] [PubMed] [Google Scholar]
- 182.Silva MF, Sherman M. Criteria for liver transplantation for HCC: what should the limits be? J Hepatol. 2011;55:1137–1147. doi: 10.1016/j.jhep.2011.05.012. [DOI] [PubMed] [Google Scholar]
- 183.Parfitt JR, Marotta P, Alghamdi M, Wall W, Khakhar A, Suskin NG, Quan D, McAllister V, Ghent C, Levstik M, McLean C, Chakrabarti S, Garcia B, Driman DK. Recurrent hepatocellular carcinoma after transplantation: use of a pathological score on explanted livers to predict recurrence. Liver Transpl. 2007;13:543–551. doi: 10.1002/lt.21078. [DOI] [PubMed] [Google Scholar]
- 184.Aziz S, Sey M, Marotta P, Driman D, Parfitt J, Teriaky A, Brahmania M, Skaro A, Qumosani K. Recurrent Hepatocellular Carcinoma After Liver Transplantation: Validation of a Pathologic Risk Score on Explanted Livers to Predict Recurrence. Transplant Proc. 2021;53:1975–1979. doi: 10.1016/j.transproceed.2021.05.007. [DOI] [PubMed] [Google Scholar]
- 185.Mehta N, Heimbach J, Harnois DM, Sapisochin G, Dodge JL, Lee D, Burns JM, Sanchez W, Greig PD, Grant DR, Roberts JP, Yao FY. Validation of a Risk Estimation of Tumor Recurrence After Transplant (RETREAT) Score for Hepatocellular Carcinoma Recurrence After Liver Transplant. JAMA Oncol. 2017;3:493–500. doi: 10.1001/jamaoncol.2016.5116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Schnitzbauer AA, Zuelke C, Graeb C, Rochon J, Bilbao I, Burra P, de Jong KP, Duvoux C, Kneteman NM, Adam R, Bechstein WO, Becker T, Beckebaum S, Chazouillères O, Cillo U, Colledan M, Fändrich F, Gugenheim J, Hauss JP, Heise M, Hidalgo E, Jamieson N, Königsrainer A, Lamby PE, Lerut JP, Mäkisalo H, Margreiter R, Mazzaferro V, Mutzbauer I, Otto G, Pageaux GP, Pinna AD, Pirenne J, Rizell M, Rossi G, Rostaing L, Roy A, Turrion VS, Schmidt J, Troisi RI, van Hoek B, Valente U, Wolf P, Wolters H, Mirza DF, Scholz T, Steininger R, Soderdahl G, Strasser SI, Jauch KW, Neuhaus P, Schlitt HJ, Geissler EK. A prospective randomised, open-labeled, trial comparing sirolimus-containing versus mTOR-inhibitor-free immunosuppression in patients undergoing liver transplantation for hepatocellular carcinoma. BMC Cancer. 2010;10:190. doi: 10.1186/1471-2407-10-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Thorat A, Jeng LB, Yang HR, Yeh CC, Hsu SC, Chen TH, Poon KS. Assessing the role of everolimus in reducing hepatocellular carcinoma recurrence after living donor liver transplantation for patients within the UCSF criteria: re-inventing the role of mammalian target of rapamycin inhibitors. Ann Hepatobiliary Pancreat Surg. 2017;21:205–211. doi: 10.14701/ahbps.2017.21.4.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Rodríguez-Perálvarez M, Tsochatzis E, Naveas MC, Pieri G, García-Caparrós C, O'Beirne J, Poyato-González A, Ferrín-Sánchez G, Montero-Álvarez JL, Patch D, Thorburn D, Briceño J, De la Mata M, Burroughs AK. Reduced exposure to calcineurin inhibitors early after liver transplantation prevents recurrence of hepatocellular carcinoma. J Hepatol. 2013;59:1193–1199. doi: 10.1016/j.jhep.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 189.European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69:182–236. doi: 10.1016/j.jhep.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 190.Bruix J, Chan SL, Galle PR, Rimassa L, Sangro B. Systemic treatment of hepatocellular carcinoma: An EASL position paper. J Hepatol. 2021;75:960–974. doi: 10.1016/j.jhep.2021.07.004. [DOI] [PubMed] [Google Scholar]
- 191.Wang ZY, Geng L, Zheng SS. Current strategies for preventing the recurrence of hepatocellular carcinoma after liver transplantation. Hepatobiliary Pancreat Dis Int. 2015;14:145–149. doi: 10.1016/s1499-3872(15)60345-9. [DOI] [PubMed] [Google Scholar]
- 192.Aggarwal A, Te HS, Verna EC, Desai AP. A National Survey of Hepatocellular Carcinoma Surveillance Practices Following Liver Transplantation. Transplant Direct. 2021;7:e638. doi: 10.1097/TXD.0000000000001086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Uchida K, Levi DM, Nishida S, Selvaggi G, Tekin A, Fan J, Hibi T, Dohi T, El Hinnawi A, Ruiz P, Tzakis AG. An Evidence-Based Strategy for HCC Surveillance after Liver Transplantation. Transplantation. 2012;94:636. [Google Scholar]
- 194.Filgueira NA. Hepatocellular carcinoma recurrence after liver transplantation: Risk factors, screening and clinical presentation. World J Hepatol. 2019;11:261–272. doi: 10.4254/wjh.v11.i3.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Lee DD, Sapisochin G, Mehta N, Gorgen A, Musto KR, Hajda H, Yao FY, Hodge DO, Carter RE, Harnois DM. Surveillance for HCC After Liver Transplantation: Increased Monitoring May Yield Aggressive Treatment Options and Improved Postrecurrence Survival. Transplantation. 2020;104:2105–2112. doi: 10.1097/TP.0000000000003117. [DOI] [PubMed] [Google Scholar]
- 196.Hoffman D, Mehta N. Recurrence of hepatocellular carcinoma following liver transplantation. Expert Rev Gastroenterol Hepatol. 2021;15:91–102. doi: 10.1080/17474124.2021.1823213. [DOI] [PubMed] [Google Scholar]
- 197.Roberts JP. Tumor surveillance-what can and should be done? Liver Transpl. 2005:S45–S46. doi: 10.1002/lt.20605. [DOI] [PubMed] [Google Scholar]
- 198.Pelizzaro F, Gambato M, Gringeri E, Vitale A, Cillo U, Farinati F, Burra P, Russo FP. Management of Hepatocellular Carcinoma Recurrence after Liver Transplantation. Cancers (Basel) 2021;13 doi: 10.3390/cancers13194882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Alshahrani AA, Ha SM, Hwang S, Ahn CS, Kim KH, Moon DB, Ha TY, Song GW, Jung DH, Park GC, Cho HD, Kwon JH, Kang SH, Lee SG. Clinical Features and Surveillance of Very Late Hepatocellular Carcinoma Recurrence After Liver Transplantation. Ann Transplant. 2018;23:659–665. doi: 10.12659/AOT.910598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Shin WY, Suh KS, Lee HW, Kim J, Kim T, Yi NJ, Lee KU. Prognostic factors affecting survival after recurrence in adult living donor liver transplantation for hepatocellular carcinoma. Liver Transpl. 2010;16:678–684. doi: 10.1002/lt.22047. [DOI] [PubMed] [Google Scholar]
- 201.Ladabaum U, Cheng SL, Yao FY, Roberts JP. Cost-effectiveness of screening for recurrent hepatocellular carcinoma after liver transplantation. Clin Transplant. 2011;25:283–291. doi: 10.1111/j.1399-0012.2010.01212.x. [DOI] [PubMed] [Google Scholar]
- 202.Mehta N, Dodge JL, Roberts JP, Yao FY. Validation of the prognostic power of the RETREAT score for hepatocellular carcinoma recurrence using the UNOS database. Am J Transplant. 2018;18:1206–1213. doi: 10.1111/ajt.14549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Sapisochin G, Goldaracena N, Astete S, Laurence JM, Davidson D, Rafael E, Castells L, Sandroussi C, Bilbao I, Dopazo C, Grant DR, Lázaro JL, Caralt M, Ghanekar A, McGilvray ID, Lilly L, Cattral MS, Selzner M, Charco R, Greig PD. Benefit of Treating Hepatocellular Carcinoma Recurrence after Liver Transplantation and Analysis of Prognostic Factors for Survival in a Large Euro-American Series. Ann Surg Oncol. 2015;22:2286–2294. doi: 10.1245/s10434-014-4273-6. [DOI] [PubMed] [Google Scholar]
- 204.Bodzin AS, Lunsford KE, Markovic D, Harlander-Locke MP, Busuttil RW, Agopian VG. Predicting Mortality in Patients Developing Recurrent Hepatocellular Carcinoma After Liver Transplantation: Impact of Treatment Modality and Recurrence Characteristics. Ann Surg. 2017;266:118–125. doi: 10.1097/SLA.0000000000001894. [DOI] [PubMed] [Google Scholar]
- 205.Fernandez-Sevilla E, Allard MA, Selten J, Golse N, Vibert E, Sa Cunha A, Cherqui D, Castaing D, Adam R. Recurrence of hepatocellular carcinoma after liver transplantation: Is there a place for resection? Liver Transpl. 2017;23:440–447. doi: 10.1002/lt.24742. [DOI] [PubMed] [Google Scholar]
- 206.Yang Z, Wang S, Tian XY, Xie QF, Zhuang L, Li QY, Chen CZ, Zheng SS. Impact of treatment modalities on patients with recurrent hepatocellular carcinoma after liver transplantation: Preliminary experience. Hepatobiliary Pancreat Dis Int. 2020;19:365–370. doi: 10.1016/j.hbpd.2020.06.002. [DOI] [PubMed] [Google Scholar]
- 207.Huang J, Yan L, Wu H, Yang J, Liao M, Zeng Y. Is radiofrequency ablation applicable for recurrent hepatocellular carcinoma after liver transplantation? J Surg Res. 2016;200:122–130. doi: 10.1016/j.jss.2015.07.033. [DOI] [PubMed] [Google Scholar]
- 208.Zhou B, Shan H, Zhu KS, Jiang ZB, Guan SH, Meng XC, Zeng XC. Chemoembolization with lobaplatin mixed with iodized oil for unresectable recurrent hepatocellular carcinoma after orthotopic liver transplantation. J Vasc Interv Radiol. 2010;21:333–338. doi: 10.1016/j.jvir.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 209.Mancuso A, Mazzola A, Cabibbo G, Perricone G, Enea M, Galvano A, Zavaglia C, Belli L, Cammà C. Survival of patients treated with sorafenib for hepatocellular carcinoma recurrence after liver transplantation: a systematic review and meta-analysis. Dig Liver Dis. 2015;47:324–330. doi: 10.1016/j.dld.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 210.de'Angelis N, Landi F, Nencioni M, Palen A, Lahat E, Salloum C, Compagnon P, Lim C, Costentin C, Calderaro J, Luciani A, Feray C, Azoulay D. Role of Sorafenib in Patients With Recurrent Hepatocellular Carcinoma After Liver Transplantation. Prog Transplant. 2016;26:348–355. doi: 10.1177/1526924816664083. [DOI] [PubMed] [Google Scholar]
- 211.Sposito C, Mariani L, Germini A, Flores Reyes M, Bongini M, Grossi G, Bhoori S, Mazzaferro V. Comparative efficacy of sorafenib versus best supportive care in recurrent hepatocellular carcinoma after liver transplantation: a case-control study. J Hepatol. 2013;59:59–66. doi: 10.1016/j.jhep.2013.02.026. [DOI] [PubMed] [Google Scholar]
- 212.Invernizzi F, Iavarone M, Zavaglia C, Mazza S, Maggi U, Cesarini L, Antonelli B, Airoldi A, Manini MA, Sangiovanni A, Rossi G, Donato MF, Saverio Belli L, Lampertico P. Experience With Early Sorafenib Treatment With mTOR Inhibitors in Hepatocellular Carcinoma Recurring After Liver Transplantation. Transplantation. 2020;104:568–574. doi: 10.1097/TP.0000000000002955. [DOI] [PubMed] [Google Scholar]
- 213.De Simone P, Crocetti L, Pezzati D, Bargellini I, Ghinolfi D, Carrai P, Leonardi G, Della Pina C, Cioni D, Pollina L, Campani D, Bartolozzi C, Lencioni R, Filipponi F. Efficacy and safety of combination therapy with everolimus and sorafenib for recurrence of hepatocellular carcinoma after liver transplantation. Transplant Proc. 2014;46:241–244. doi: 10.1016/j.transproceed.2013.10.035. [DOI] [PubMed] [Google Scholar]
- 214.Bruix J, Qin S, Merle P, Granito A, Huang YH, Bodoky G, Pracht M, Yokosuka O, Rosmorduc O, Breder V, Gerolami R, Masi G, Ross PJ, Song T, Bronowicki JP, Ollivier-Hourmand I, Kudo M, Cheng AL, Llovet JM, Finn RS, LeBerre MA, Baumhauer A, Meinhardt G, Han G RESORCE Investigators. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389:56–66. doi: 10.1016/S0140-6736(16)32453-9. [DOI] [PubMed] [Google Scholar]
- 215.Iavarone M, Invernizzi F, Ivanics T, Mazza S, Zavaglia C, Sanduzzi-Zamparelli M, Fraile-López M, Czauderna C, Di Costanzo G, Bhoori S, Pinter M, Manini MA, Amaddeo G, Yunquera AF, Piñero F, Blanco Rodríguez MJ, Anders M, Aballay Soteras G, Villadsen GE, Yoon PD, Cesarini L, Díaz-González Á, González-Diéguez ML, Tortora R, Weinmann A, Mazzaferro V, Romero Cristóbal M, Crespo G, Regnault H, De Giorgio M, Varela M, Prince R, Scudeller L, Donato MF, Wörns MA, Bruix J, Sapisochin G, Lampertico P, Reig M. Regorafenib Efficacy After Sorafenib in Patients With Recurrent Hepatocellular Carcinoma After Liver Transplantation: A Retrospective Study. Liver Transpl. 2021;27:1767–1778. doi: 10.1002/lt.26264. [DOI] [PubMed] [Google Scholar]
- 216.Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, Kudo M, Breder V, Merle P, Kaseb AO, Li D, Verret W, Xu DZ, Hernandez S, Liu J, Huang C, Mulla S, Wang Y, Lim HY, Zhu AX, Cheng AL IMbrave150 Investigators. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N Engl J Med. 2020;382:1894–1905. doi: 10.1056/NEJMoa1915745. [DOI] [PubMed] [Google Scholar]
- 217.Rajendran L, Ivanics T, Claasen MP, Muaddi H, Sapisochin G. The management of post-transplantation recurrence of hepatocellular carcinoma. Clin Mol Hepatol. 2022;28:1–16. doi: 10.3350/cmh.2021.0217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Luo Y, Teng F, Fu H, Ding GS. Immunotherapy in liver transplantation for hepatocellular carcinoma: Pros and cons. World J Gastrointest Oncol. 2022;14:163–180. doi: 10.4251/wjgo.v14.i1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Mendes F, Couto CA, Levy C. Recurrent and de novo autoimmune liver diseases. Clin Liver Dis. 2011;15:859–878. doi: 10.1016/j.cld.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 220.Mack CL, Adams D, Assis DN, Kerkar N, Manns MP, Mayo MJ, Vierling JM, Alsawas M, Murad MH, Czaja AJ. Diagnosis and Management of Autoimmune Hepatitis in Adults and Children: 2019 Practice Guidance and Guidelines From the American Association for the Study of Liver Diseases. Hepatology. 2020;72:671–722. doi: 10.1002/hep.31065. [DOI] [PubMed] [Google Scholar]
- 221.Fosby B, Karlsen TH, Melum E. Recurrence and rejection in liver transplantation for primary sclerosing cholangitis. World J Gastroenterol. 2012;18:1–15. doi: 10.3748/wjg.v18.i1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Ueda Y, Kaido T, Okajima H, Hata K, Anazawa T, Yoshizawa A, Yagi S, Taura K, Masui T, Yamashiki N, Haga H, Nagao M, Marusawa H, Seno H, Uemoto S. Long-term Prognosis and Recurrence of Primary Sclerosing Cholangitis After Liver Transplantation: A Single-Center Experience. Transplant Direct. 2017;3:e334. doi: 10.1097/TXD.0000000000000751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Faisal N, Renner EL. Recurrence of autoimmune liver diseases after liver transplantation. World J Hepatol. 2015;7:2896–2905. doi: 10.4254/wjh.v7.i29.2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Montano-Loza AJ, Wasilenko S, Bintner J, Mason AL. Cyclosporine A protects against primary biliary cirrhosis recurrence after liver transplantation. Am J Transplant. 2010;10:852–858. doi: 10.1111/j.1600-6143.2009.03006.x. [DOI] [PubMed] [Google Scholar]
- 225.Rowe IA, Webb K, Gunson BK, Mehta N, Haque S, Neuberger J. The impact of disease recurrence on graft survival following liver transplantation: a single centre experience. Transpl Int. 2008;21:459–465. doi: 10.1111/j.1432-2277.2007.00628.x. [DOI] [PubMed] [Google Scholar]
- 226.Heinemann M, Adam R, Berenguer M, Mirza D, Malek-Hosseini SA, O'Grady JG, Lodge P, Pratschke J, Boudjema K, Paul A, Zieniewicz K, Fronek J, Weiss KH, Karam V, Duvoux C, Lohse A, Schramm C all the other contributing centers (www. eltr.org) and the European Liver and Intestine Transplant Association (ELITA). Longterm Survival After Liver Transplantation for Autoimmune Hepatitis: Results From the European Liver Transplant Registry. Liver Transpl. 2020;26:866–877. doi: 10.1002/lt.25739. [DOI] [PubMed] [Google Scholar]
- 227.Graziadei IW, Wiesner RH, Marotta PJ, Porayko MK, Hay JE, Charlton MR, Poterucha JJ, Rosen CB, Gores GJ, LaRusso NF, Krom RA. Long-term results of patients undergoing liver transplantation for primary sclerosing cholangitis. Hepatology. 1999;30:1121–1127. doi: 10.1002/hep.510300501. [DOI] [PubMed] [Google Scholar]
- 228.Alabraba E, Nightingale P, Gunson B, Hubscher S, Olliff S, Mirza D, Neuberger J. A re-evaluation of the risk factors for the recurrence of primary sclerosing cholangitis in liver allografts. Liver Transpl. 2009;15:330–340. doi: 10.1002/lt.21679. [DOI] [PubMed] [Google Scholar]
- 229.Visseren T, Erler NS, Polak WG, Adam R, Karam V, Vondran FWR, Ericzon BG, Thorburn D, IJzermans JNM, Paul A, van der Heide F, Taimr P, Nemec P, Pirenne J, Romagnoli R, Metselaar HJ, Darwish Murad S European Liver and Intestine Transplantation Association (ELITA) Recurrence of primary sclerosing cholangitis after liver transplantation - analysing the European Liver Transplant Registry and beyond. Transpl Int. 2021;34:1455–1467. doi: 10.1111/tri.13925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Li X, Peng J, Ouyang R, Yang Y, Yu C, Lin H. Risk factors for recurrent primary biliary cirrhosis after liver transplantation: A systematic review and meta-analysis. Dig Liver Dis. 2021;53:309–317. doi: 10.1016/j.dld.2020.12.005. [DOI] [PubMed] [Google Scholar]
- 231.Lindor KD, Bowlus CL, Boyer J, Levy C, Mayo M. Primary Biliary Cholangitis: 2018 Practice Guidance from the American Association for the Study of Liver Diseases. Hepatology. 2019;69:394–419. doi: 10.1002/hep.30145. [DOI] [PubMed] [Google Scholar]
- 232.European Association for the Study of the Liver. EASL Clinical Practice Guidelines: The diagnosis and management of patients with primary biliary cholangitis. J Hepatol. 2017;67:145–172. doi: 10.1016/j.jhep.2017.03.022. [DOI] [PubMed] [Google Scholar]
- 233.Montano-Loza AJ, Ronca V, Ebadi M, Hansen BE, Hirschfield G, Elwir S, Alsaed M, Milkiewicz P, Janik MK, Marschall HU, Burza MA, Efe C, Calışkan AR, Harputluoglu M, Kabaçam G, Terrabuio D, de Quadros Onofrio F, Selzner N, Bonder A, Parés A, Llovet L, Akyıldız M, Arikan C, Manns MP, Taubert R, Weber AL, Schiano TD, Haydel B, Czubkowski P, Socha P, Ołdak N, Akamatsu N, Tanaka A, Levy C, Martin EF, Goel A, Sedki M, Jankowska I, Ikegami T, Rodriguez M, Sterneck M, Weiler-Normann C, Schramm C, Donato MF, Lohse A, Andrade RJ, Patwardhan VR, van Hoek B, Biewenga M, Kremer AE, Ueda Y, Deneau M, Pedersen M, Mayo MJ, Floreani A, Burra P, Secchi MF, Beretta-Piccoli BT, Sciveres M, Maggiore G, Jafri SM, Debray D, Girard M, Lacaille F, Lytvyak E, Mason AL, Heneghan M, Oo YH International Autoimmune Hepatitis Group (IAIHG) Risk factors and outcomes associated with recurrent autoimmune hepatitis following liver transplantation. J Hepatol. 2022;77:84–97. doi: 10.1016/j.jhep.2022.01.022. [DOI] [PubMed] [Google Scholar]
- 234.Hübscher SG. Recurrent autoimmune hepatitis after liver transplantation: diagnostic criteria, risk factors, and outcome. Liver Transpl. 2001;7:285–291. doi: 10.1053/jlts.2001.23085. [DOI] [PubMed] [Google Scholar]
- 235.Ayata G, Gordon FD, Lewis WD, Pomfret E, Pomposelli JJ, Jenkins RL, Khettry U. Liver transplantation for autoimmune hepatitis: a long-term pathologic study. Hepatology. 2000;32:185–192. doi: 10.1053/jhep.2000.9077. [DOI] [PubMed] [Google Scholar]
- 236.Krishnamoorthy TL, Miezynska-Kurtycz J, Hodson J, Gunson BK, Neuberger J, Milkiewicz P, Oo YH. Longterm corticosteroid use after liver transplantation for autoimmune hepatitis is safe and associated with a lower incidence of recurrent disease. Liver Transpl. 2016;22:34–41. doi: 10.1002/lt.24323. [DOI] [PubMed] [Google Scholar]
- 237.Stirnimann G, Ebadi M, Czaja AJ, Montano-Loza AJ. Recurrent and De Novo Autoimmune Hepatitis. Liver Transpl. 2019;25:152–166. doi: 10.1002/lt.25375. [DOI] [PubMed] [Google Scholar]
- 238.Steenstraten IC, Sebib Korkmaz K, Trivedi PJ, Inderson A, van Hoek B, Rodriguez Girondo MDM, Maljaars PWJ. Systematic review with meta-analysis: risk factors for recurrent primary sclerosing cholangitis after liver transplantation. Aliment Pharmacol Ther. 2019;49:636–643. doi: 10.1111/apt.15148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Cholongitas E, Burroughs AK. Recurrence of autoimmune liver diseases after liver transplantation: clinical aspects. Auto Immun Highlights. 2012;3:113–118. doi: 10.1007/s13317-012-0040-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Montano-Loza AJ, Bhanji RA, Wasilenko S, Mason AL. Systematic review: recurrent autoimmune liver diseases after liver transplantation. Aliment Pharmacol Ther. 2017;45:485–500. doi: 10.1111/apt.13894. [DOI] [PubMed] [Google Scholar]
- 241.Haldar D, Kern B, Hodson J, Armstrong MJ, Adam R, Berlakovich G, Fritz J, Feurstein B, Popp W, Karam V, Muiesan P, O'Grady J, Jamieson N, Wigmore SJ, Pirenne J, Malek-Hosseini SA, Hidalgo E, Tokat Y, Paul A, Pratschke J, Bartels M, Trunecka P, Settmacher U, Pinzani M, Duvoux C, Newsome PN, Schneeberger S European Liver and Intestine Transplant Association (ELITA) Outcomes of liver transplantation for non-alcoholic steatohepatitis: A European Liver Transplant Registry study. J Hepatol. 2019;71:313–322. doi: 10.1016/j.jhep.2019.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Sayiner M, Younossi ZM. Nonalcoholic Steatohepatitis Is Becoming a Top Indication for Liver Transplantation Worldwide. Liver Transpl. 2019;25:10–11. doi: 10.1002/lt.25387. [DOI] [PubMed] [Google Scholar]
- 243.Weinmann A, Alt Y, Koch S, Nelles C, Düber C, Lang H, Otto G, Zimmermann T, Marquardt JU, Galle PR, Wörns MA, Schattenberg JM. Treatment and survival of non-alcoholic steatohepatitis associated hepatocellular carcinoma. BMC Cancer. 2015;15:210. doi: 10.1186/s12885-015-1197-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Nagai S, Collins K, Chau LC, Safwan M, Rizzari M, Yoshida A, Abouljoud MS, Moonka D. Increased Risk of Death in First Year After Liver Transplantation Among Patients With Nonalcoholic Steatohepatitis vs Liver Disease of Other Etiologies. Clin Gastroenterol Hepatol. 2019;17:2759–2768.e5. doi: 10.1016/j.cgh.2019.04.033. [DOI] [PubMed] [Google Scholar]
- 245.Yang KC, Hung HF, Lu CW, Chang HH, Lee LT, Huang KC. Association of Non-alcoholic Fatty Liver Disease with Metabolic Syndrome Independently of Central Obesity and Insulin Resistance. Sci Rep. 2016;6:27034. doi: 10.1038/srep27034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Cotter TG, Charlton M. Nonalcoholic Steatohepatitis After Liver Transplantation. Liver Transpl. 2020;26:141–159. doi: 10.1002/lt.25657. [DOI] [PubMed] [Google Scholar]
- 247.Bhati C, Idowu MO, Sanyal AJ, Rivera M, Driscoll C, Stravitz RT, Kohli DR, Matherly S, Puri P, Gilles H, Cotterell A, Levy M, Sterling RK, Luketic VA, Lee H, Sharma A, Siddiqui MS. Long-term Outcomes in Patients Undergoing Liver Transplantation for Nonalcoholic Steatohepatitis-Related Cirrhosis. Transplantation. 2017;101:1867–1874. doi: 10.1097/TP.0000000000001709. [DOI] [PubMed] [Google Scholar]
- 248.Contos MJ, Cales W, Sterling RK, Luketic VA, Shiffman ML, Mills AS, Fisher RA, Ham J, Sanyal AJ. Development of nonalcoholic fatty liver disease after orthotopic liver transplantation for cryptogenic cirrhosis. Liver Transpl. 2001;7:363–373. doi: 10.1053/jlts.2001.23011. [DOI] [PubMed] [Google Scholar]
- 249.Dumortier J, Giostra E, Belbouab S, Morard I, Guillaud O, Spahr L, Boillot O, Rubbia-Brandt L, Scoazec JY, Hadengue A. Non-alcoholic fatty liver disease in liver transplant recipients: another story of "seed and soil". Am J Gastroenterol. 2010;105:613–620. doi: 10.1038/ajg.2009.717. [DOI] [PubMed] [Google Scholar]
- 250.Saeed N, Glass L, Sharma P, Shannon C, Sonnenday CJ, Tincopa MA. Incidence and Risks for Nonalcoholic Fatty Liver Disease and Steatohepatitis Post-liver Transplant: Systematic Review and Meta-analysis. Transplantation. 2019;103:e345–e354. doi: 10.1097/TP.0000000000002916. [DOI] [PubMed] [Google Scholar]
- 251.Villeret F, Dharancy S, Erard D, Abergel A, Barbier L, Besch C, Boillot O, Boudjema K, Coilly A, Conti F, Corpechot C, Duvoux C, Faitot F, Faure S, Francoz C, Giostra E, Gugenheim J, Hardwigsen J, Hilleret MN, Hiriart JB, Houssel-Debry P, Kamar N, Lassailly G, Latournerie M, Pageaux GP, Samuel D, Vanlemmens C, Saliba F, Dumortier J. Liver transplantation for NAFLD cirrhosis: Age and recent coronary angioplasty are major determinants of survival. Liver Int. 2022;42:2428–2441. doi: 10.1111/liv.15385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Clin Liver Dis (Hoboken) 2018;11:81. doi: 10.1002/cld.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Romero-Gómez M, Zelber-Sagi S, Trenell M. Treatment of NAFLD with diet, physical activity and exercise. J Hepatol. 2017;67:829–846. doi: 10.1016/j.jhep.2017.05.016. [DOI] [PubMed] [Google Scholar]
- 254.Younossi ZM, Corey KE, Lim JK. AGA Clinical Practice Update on Lifestyle Modification Using Diet and Exercise to Achieve Weight Loss in the Management of Nonalcoholic Fatty Liver Disease: Expert Review. Gastroenterology. 2021;160:912–918. doi: 10.1053/j.gastro.2020.11.051. [DOI] [PubMed] [Google Scholar]
- 255.Ryan MC, Itsiopoulos C, Thodis T, Ward G, Trost N, Hofferberth S, O'Dea K, Desmond PV, Johnson NA, Wilson AM. The Mediterranean diet improves hepatic steatosis and insulin sensitivity in individuals with non-alcoholic fatty liver disease. J Hepatol. 2013;59:138–143. doi: 10.1016/j.jhep.2013.02.012. [DOI] [PubMed] [Google Scholar]
- 256.Katsagoni CN, Papatheodoridis GV, Ioannidou P, Deutsch M, Alexopoulou A, Papadopoulos N, Papageorgiou MV, Fragopoulou E, Kontogianni MD. Improvements in clinical characteristics of patients with non-alcoholic fatty liver disease, after an intervention based on the Mediterranean lifestyle: a randomised controlled clinical trial. Br J Nutr. 2018;120:164–175. doi: 10.1017/S000711451800137X. [DOI] [PubMed] [Google Scholar]
- 257.European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO) EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64:1388–1402. doi: 10.1016/j.jhep.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 258.Xu HE, Guo JS. All about NASH: disease biology, targets, and opportunities on the road to NASH drugs. Acta Pharmacol Sin. 2022;43:1101–1102. doi: 10.1038/s41401-022-00900-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Shetty A, Giron F, Divatia MK, Ahmad MI, Kodali S, Victor D. Nonalcoholic Fatty Liver Disease after Liver Transplant. J Clin Transl Hepatol. 2021;9:428–435. doi: 10.14218/JCTH.2020.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Bhat M, Tazari M, Sebastiani G. Performance of transient elastography and serum fibrosis biomarkers for non-invasive evaluation of recurrent fibrosis after liver transplantation: A meta-analysis. PLoS One. 2017;12:e0185192. doi: 10.1371/journal.pone.0185192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Siddiqui MS, Idowu MO, Stromberg K, Sima A, Lee E, Patel S, Ghaus S, Driscoll C, Sterling RK, John B, Bhati CS. Diagnostic Performance of Vibration-Controlled Transient Elastography in Liver Transplant Recipients. Clin Gastroenterol Hepatol. 2021;19:367–374. doi: 10.1016/j.cgh.2020.03.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Melekoglu Ellik Z, Idilman IS, Kartal A, Balaban Y, Elhan AH, Karcaaltincaba M, Ozkan H, Idilman R. Evaluation of Magnetic Resonance Elastography and Transient Elastography for Liver Fibrosis and Steatosis Assessments in the Liver Transplant Setting. Turk J Gastroenterol. 2022;33:153–160. doi: 10.5152/tjg.2022.21705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Singh S, Venkatesh SK, Keaveny A, Adam S, Miller FH, Asbach P, Godfrey EM, Silva AC, Wang Z, Murad MH, Asrani SK, Lomas DJ, Ehman RL. Diagnostic accuracy of magnetic resonance elastography in liver transplant recipients: A pooled analysis. Ann Hepatol. 2016;15:363–376. doi: 10.5604/16652681.1198808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Yalamanchili K, Saadeh S, Klintmalm GB, Jennings LW, Davis GL. Nonalcoholic fatty liver disease after liver transplantation for cryptogenic cirrhosis or nonalcoholic fatty liver disease. Liver Transpl. 2010;16:431–439. doi: 10.1002/lt.22004. [DOI] [PubMed] [Google Scholar]
- 265.Kallwitz ER. Metabolic syndrome after liver transplantation: preventable illness or common consequence? World J Gastroenterol. 2012;18:3627–3634. doi: 10.3748/wjg.v18.i28.3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Fatourou EM, Tsochatzis EA. Management of metabolic syndrome and cardiovascular risk after liver transplantation. Lancet Gastroenterol Hepatol. 2019;4:731–741. doi: 10.1016/S2468-1253(19)30181-5. [DOI] [PubMed] [Google Scholar]
- 267.Féray C, Gigou M, Samuel D, Paradis V, Wilber J, David MF, Urdea M, Reynes M, Bréchot C, Bismuth H. The course of hepatitis C virus infection after liver transplantation. Hepatology. 1994;20:1137–1143. doi: 10.1002/hep.1840200506. [DOI] [PubMed] [Google Scholar]
- 268.Berenguer M. Natural history of recurrent hepatitis C. Liver Transpl. 2002;8:S14–S18. doi: 10.1053/jlts.2002.35781. [DOI] [PubMed] [Google Scholar]
- 269.Coilly A, Roche B, Samuel D. Current management and perspectives for HCV recurrence after liver transplantation. Liver Int. 2013;33 Suppl 1:56–62. doi: 10.1111/liv.12062. [DOI] [PubMed] [Google Scholar]
- 270.Belli LS, Perricone G, Adam R, Cortesi PA, Strazzabosco M, Facchetti R, Karam V, Salizzoni M, Andujar RL, Fondevila C, De Simone P, Morelli C, Fabregat-Prous J, Samuel D, Agarwaal K, Moreno Gonzales E, Charco R, Zieniewicz K, De Carlis L, Duvoux C all the contributing centers (www. eltr.org) and the European Liver and Intestine Transplant Association (ELITA). Impact of DAAs on liver transplantation: Major effects on the evolution of indications and results. An ELITA study based on the ELTR registry. J Hepatol. 2018;69:810–817. doi: 10.1016/j.jhep.2018.06.010. [DOI] [PubMed] [Google Scholar]
- 271.Fung J. Prevention of hepatitis B virus recurrence. Hepatoma Res . 2021 [Google Scholar]
- 272.Cholongitas E, Goulis J, Akriviadis E, Papatheodoridis GV. Hepatitis B immunoglobulin and/or nucleos(t)ide analogues for prophylaxis against hepatitis b virus recurrence after liver transplantation: a systematic review. Liver Transpl. 2011;17:1176–1190. doi: 10.1002/lt.22354. [DOI] [PubMed] [Google Scholar]
- 273.Xu X, Tu Z, Wang B, Ling Q, Zhang L, Zhou L, Jiang G, Wu J, Zheng S. A novel model for evaluating the risk of hepatitis B recurrence after liver transplantation. Liver Int. 2011;31:1477–1484. doi: 10.1111/j.1478-3231.2011.02500.x. [DOI] [PubMed] [Google Scholar]
- 274.Lenci I, Milana M, Grassi G, Manzia TM, Gazia C, Tisone G, Angelico R, Baiocchi L. Hepatitis B virus recurrence after liver transplantation: An old tale or a clear and present danger? World J Gastroenterol. 2020;26:2166–2176. doi: 10.3748/wjg.v26.i18.2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Verna EC. Updated Hepatitis B Guidance: Implications for liver transplant patients. Liver Transpl. 2018;24:465–469. doi: 10.1002/lt.25037. [DOI] [PubMed] [Google Scholar]
- 276.Jiménez-Pérez M, Sáez-Gómez AB, Mongil Poce L, Lozano-Rey JM, de la Cruz-Lombardo J, Rodrigo-López JM. Efficacy and safety of entecavir and/or tenofovir for prophylaxis and treatment of hepatitis B recurrence post-liver transplant. Transplant Proc. 2010;42:3167–3168. doi: 10.1016/j.transproceed.2010.05.127. [DOI] [PubMed] [Google Scholar]
- 277.Li H, Fan MQ, Men TY, Wang YP, Xing TH, Fan JW, Peng ZH, Zhong L. Long-Term Outcomes of Simultaneous Liver-Kidney Transplant Patients with Hepatitis B Compared to with Liver Transplant Alone. Med Sci Monit. 2016;22:332–340. doi: 10.12659/MSM.895757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Chou HS, Cheng CH, Hung HC, Lee JC, Wang YC, Wu TH, Lee CF, Wu TJ, Chan KM, Lee WC. Significance of Hepatitis B Recurrence in Liver Transplantation Recipients. Biomed Res Int. 2020;2020:2489526. doi: 10.1155/2020/2489526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Cotter TG, Paul S, Sandıkçı B, Couri T, Bodzin AS, Little EC, Sundaram V, Charlton M. Improved Graft Survival After Liver Transplantation for Recipients With Hepatitis C Virus in the Direct-Acting Antiviral Era. Liver Transpl. 2019;25:598–609. doi: 10.1002/lt.25424. [DOI] [PubMed] [Google Scholar]
- 280.Rana A, Ackah RL, Webb GJ, Halazun KJ, Vierling JM, Liu H, Wu MF, Yoeli D, Kueht M, Mindikoglu AL, Sussman NL, Galván NT, Cotton RT, O'Mahony CA, Goss JA. No Gains in Long-term Survival After Liver Transplantation Over the Past Three Decades. Ann Surg. 2019;269:20–27. doi: 10.1097/SLA.0000000000002650. [DOI] [PubMed] [Google Scholar]
- 281.Rodríguez-Perálvarez M, De la Mata M, Burroughs AK. Liver transplantation: immunosuppression and oncology. Curr Opin Organ Transplant. 2014;19:253–260. doi: 10.1097/MOT.0000000000000069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.de Koning HJ, van der Aalst CM, de Jong PA, Scholten ET, Nackaerts K, Heuvelmans MA, Lammers JJ, Weenink C, Yousaf-Khan U, Horeweg N, van 't Westeinde S, Prokop M, Mali WP, Mohamed Hoesein FAA, van Ooijen PMA, Aerts JGJV, den Bakker MA, Thunnissen E, Verschakelen J, Vliegenthart R, Walter JE, Ten Haaf K, Groen HJM, Oudkerk M. Reduced Lung-Cancer Mortality with Volume CT Screening in a Randomized Trial. N Engl J Med. 2020;382:503–513. doi: 10.1056/NEJMoa1911793. [DOI] [PubMed] [Google Scholar]
- 283.Karie-Guigues S, Janus N, Saliba F, Dumortier J, Duvoux C, Calmus Y, Lorho R, Deray G, Launay-Vacher V, Pageaux GP. Long-term renal function in liver transplant recipients and impact of immunosuppressive regimens (calcineurin inhibitors alone or in combination with mycophenolate mofetil): the TRY study. Liver Transpl. 2009;15:1083–1091. doi: 10.1002/lt.21803. [DOI] [PubMed] [Google Scholar]
- 284.Vivarelli M, Cucchetti A, La Barba G, Ravaioli M, Del Gaudio M, Lauro A, Grazi GL, Pinna AD. Liver transplantation for hepatocellular carcinoma under calcineurin inhibitors: reassessment of risk factors for tumor recurrence. Ann Surg. 2008;248:857–862. doi: 10.1097/SLA.0b013e3181896278. [DOI] [PubMed] [Google Scholar]
- 285.Jain AB, Yee LD, Nalesnik MA, Youk A, Marsh G, Reyes J, Zak M, Rakela J, Irish W, Fung JJ. Comparative incidence of de novo nonlymphoid malignancies after liver transplantation under tacrolimus using surveillance epidemiologic end result data. Transplantation. 1998;66:1193–1200. doi: 10.1097/00007890-199811150-00014. [DOI] [PubMed] [Google Scholar]
- 286.Tjon AS, Sint Nicolaas J, Kwekkeboom J, de Man RA, Kazemier G, Tilanus HW, Hansen BE, van der Laan LJ, Tha-In T, Metselaar HJ. Increased incidence of early de novo cancer in liver graft recipients treated with cyclosporine: an association with C2 monitoring and recipient age. Liver Transpl. 2010;16:837–846. doi: 10.1002/lt.22064. [DOI] [PubMed] [Google Scholar]
- 287.Wimmer CD, Angele MK, Schwarz B, Pratschke S, Rentsch M, Khandoga A, Guba M, Jauch KW, Bruns C, Graeb C. Impact of cyclosporine versus tacrolimus on the incidence of de novo malignancy following liver transplantation: a single center experience with 609 patients. Transpl Int. 2013;26:999–1006. doi: 10.1111/tri.12165. [DOI] [PubMed] [Google Scholar]
- 288.Heisel O, Heisel R, Balshaw R, Keown P. New onset diabetes mellitus in patients receiving calcineurin inhibitors: a systematic review and meta-analysis. Am J Transplant. 2004;4:583–595. doi: 10.1046/j.1600-6143.2003.00372.x. [DOI] [PubMed] [Google Scholar]
- 289.Haddad EM, McAlister VC, Renouf E, Malthaner R, Kjaer MS, Gluud LL. Cyclosporin versus tacrolimus for liver transplanted patients. Cochrane Database Syst Rev. 2006;2006:CD005161. doi: 10.1002/14651858.CD005161.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Canzanello VJ, Textor SC, Taler SJ, Schwartz LL, Porayko MK, Wiesner RH, Krom RA. Late hypertension after liver transplantation: a comparison of cyclosporine and tacrolimus (FK 506) Liver Transpl Surg. 1998;4:328–334. doi: 10.1002/lt.500040404. [DOI] [PubMed] [Google Scholar]
- 291.Canzanello VJ, Schwartz L, Taler SJ, Textor SC, Wiesner RH, Porayko MK, Krom RA. Evolution of cardiovascular risk after liver transplantation: a comparison of cyclosporine A and tacrolimus (FK506) Liver Transpl Surg. 1997;3:1–9. doi: 10.1002/lt.500030101. [DOI] [PubMed] [Google Scholar]
- 292.Manzarbeitia C, Reich DJ, Rothstein KD, Braitman LE, Levin S, Munoz SJ. Tacrolimus conversion improves hyperlipidemic states in stable liver transplant recipients. Liver Transpl. 2001;7:93–99. doi: 10.1053/jlts.2001.21289. [DOI] [PubMed] [Google Scholar]
- 293.Bechstein WO. Neurotoxicity of calcineurin inhibitors: impact and clinical management. Transpl Int. 2000;13:313–326. doi: 10.1007/s001470050708. [DOI] [PubMed] [Google Scholar]
- 294.Charlton M, Rinella M, Patel D, McCague K, Heimbach J, Watt K. Everolimus Is Associated With Less Weight Gain Than Tacrolimus 2 Years After Liver Transplantation: Results of a Randomized Multicenter Study. Transplantation. 2017;101:2873–2882. doi: 10.1097/TP.0000000000001913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Richards J, Gunson B, Johnson J, Neuberger J. Weight gain and obesity after liver transplantation. Transpl Int. 2005;18:461–466. doi: 10.1111/j.1432-2277.2004.00067.x. [DOI] [PubMed] [Google Scholar]
- 296.Hussaini T, Erb S, Yoshida EM. Immunosuppressive pharmacotherapy in liver transplantation. AME Med J. 2018;3:18–18. [Google Scholar]
- 297.Benlloch S, Berenguer M, Prieto M, Moreno R, San Juan F, Rayón M, Mir J, Segura A, Berenguer J. De novo internal neoplasms after liver transplantation: increased risk and aggressive behavior in recent years? Am J Transplant. 2004;4:596–604. doi: 10.1111/j.1600-6143.2004.00380.x. [DOI] [PubMed] [Google Scholar]
- 298.Fischer L, Klempnauer J, Beckebaum S, Metselaar HJ, Neuhaus P, Schemmer P, Settmacher U, Heyne N, Clavien PA, Muehlbacher F, Morard I, Wolters H, Vogel W, Becker T, Sterneck M, Lehner F, Klein C, Kazemier G, Pascher A, Schmidt J, Rauchfuss F, Schnitzbauer A, Nadalin S, Hack M, Ladenburger S, Schlitt HJ. A randomized, controlled study to assess the conversion from calcineurin-inhibitors to everolimus after liver transplantation--PROTECT. Am J Transplant. 2012;12:1855–1865. doi: 10.1111/j.1600-6143.2012.04049.x. [DOI] [PubMed] [Google Scholar]
- 299.Abdelmalek MF, Humar A, Stickel F, Andreone P, Pascher A, Barroso E, Neff GW, Ranjan D, Toselli LT, Gane EJ, Scarola J, Alberts RG, Maller ES, Lo CM Sirolimus Liver Conversion Trial Study Group. Sirolimus conversion regimen versus continued calcineurin inhibitors in liver allograft recipients: a randomized trial. Am J Transplant. 2012;12:694–705. doi: 10.1111/j.1600-6143.2011.03919.x. [DOI] [PubMed] [Google Scholar]
- 300.Campistol JM, de Fijter JW, Flechner SM, Langone A, Morelon E, Stockfleth E. mTOR inhibitor-associated dermatologic and mucosal problems. Clin Transplant. 2010;24:149–156. doi: 10.1111/j.1399-0012.2010.01232.x. [DOI] [PubMed] [Google Scholar]
- 301.Pengel LH, Liu LQ, Morris PJ. Do wound complications or lymphoceles occur more often in solid organ transplant recipients on mTOR inhibitors? Transpl Int. 2011;24:1216–1230. doi: 10.1111/j.1432-2277.2011.01357.x. [DOI] [PubMed] [Google Scholar]
- 302.Di Stefano C, Vanni E, Mirabella S, Younes R, Boano V, Mosso E, Nada E, Milazzo V, Maule S, Romagnoli R, Salizzoni M, Veglio F, Milan A. Risk factors for arterial hypertension after liver transplantation. J Am Soc Hypertens. 2018;12:220–229. doi: 10.1016/j.jash.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 303.Agarwal PD, Lucey MR. Management of hepatocellular carcinoma recurrence after liver transplantation. Ann Hepatol. 2022;27:100654. doi: 10.1016/j.aohep.2021.100654. [DOI] [PubMed] [Google Scholar]
- 304.Spiritos Z, Abdelmalek MF. Metabolic syndrome following liver transplantation in nonalcoholic steatohepatitis. Transl Gastroenterol Hepatol. 2021;6:13. doi: 10.21037/tgh.2020.02.07. [DOI] [PMC free article] [PubMed] [Google Scholar]