Abstract
Background
Neonates might be exposed to numerous painful procedures due to diagnostic reasons, therapeutic interventions, or surgical procedures.
Options for pain management include opioids, non‐pharmacological interventions, and other drugs. Morphine, fentanyl, and remifentanil are the opioids most often used in neonates. However, negative impact of opioids on the structure and function of the developing brain has been reported.
Objectives
To evaluate the benefits and harms of opioids in term or preterm neonates exposed to procedural pain, compared to placebo or no drug, non‐pharmacological intervention, other analgesics or sedatives, other opioids, or the same opioid administered by a different route.
Search methods
We used standard, extensive Cochrane search methods. The latest search date was December 2021.
Selection criteria
We included randomized controlled trials conducted in preterm and term infants of a postmenstrual age (PMA) up to 46 weeks and 0 days exposed to procedural pain where opioids were compared to 1) placebo or no drug; 2) non‐pharmacological intervention; 3) other analgesics or sedatives; 4) other opioids; or 5) the same opioid administered by a different route.
Data collection and analysis
We used standard Cochrane methods. Our primary outcomes were pain assessed with validated methods and any harms. We used a fixed‐effect model with risk ratio (RR) for dichotomous data and mean difference (MD) for continuous data, and their confidence intervals (CI). We used GRADE to assess the certainty of the evidence for each outcome.
Main results
We included 13 independent studies (enrolling 823 newborn infants): seven studies compared opioids to no treatment or placebo (the main comparison in this review), two studies to oral sweet solution or non‐pharmacological intervention, and five studies (of which two were part of the same study) to other analgesics and sedatives. All studies were performed in a hospital setting.
Opioids compared to placebo or no drug
Compared to placebo, opioids probably reduce pain score assessed with the Premature Infant Pain Profile (PIPP)/PIPP‐Revised (PIPP‐R) scale during the procedure (MD −2.58, 95% CI −3.12 to −2.03; 199 participants, 3 studies; moderate‐certainty evidence); may reduce Neonatal Infant Pain Scale (NIPS) during the procedure (MD −1.97, 95% CI −2.46 to −1.48; 102 participants, 2 studies; low‐certainty evidence); and may result in little to no difference in pain score assessed with the Douleur Aiguë du Nouveau‐né (DAN) scale one to two hours after the procedure (MD −0.20, 95% CI −2.21 to 1.81; 42 participants, 1 study; low‐certainty evidence). The evidence is very uncertain about the effect of opioids on pain score assessed with the PIPP/PIPP‐R scale up to 30 minutes after the procedure (MD 0.14, 95% CI −0.17 to 0.45; 123 participants, 2 studies; very low‐certainty evidence) or one to two hours after the procedure (MD −0.83, 95% CI −2.42 to 0.75; 54 participants, 2 studies; very low‐certainty evidence). No studies reported any harms. The evidence is very uncertain about the effect of opioids on episodes of bradycardia (RR 3.19, 95% CI 0.14 to 72.69; 172 participants, 3 studies; very low‐certainty evidence). Opioids may result in an increase in episodes of apnea compared to placebo (RR 3.15, 95% CI 1.08 to 9.16; 199 participants, 3 studies; low‐certainty evidence). The evidence is very uncertain about the effect of opioids on episodes of hypotension (RR not estimable, risk difference 0.00, 95% CI −0.06 to 0.06; 88 participants, 2 studies; very low‐certainty evidence). No studies reported parent satisfaction with care provided in the neonatal intensive care unit (NICU).
Opioids compared to non‐pharmacological intervention
The evidence is very uncertain about the effect of opioids on pain score assessed with the Crying Requires oxygen Increased vital signs Expression Sleep (CRIES) scale during the procedure when compared to facilitated tucking (MD −4.62, 95% CI −6.38 to −2.86; 100 participants, 1 study; very low‐certainty evidence) or sensorial stimulation (MD 0.32, 95% CI −1.13 to 1.77; 100 participants, 1 study; very low‐certainty evidence). The other main outcomes were not reported.
Opioids compared to other analgesics or sedatives
The evidence is very uncertain about the effect of opioids on pain score assessed with the PIPP/PIPP‐R during the procedure (MD −0.29, 95% CI −1.58 to 1.01; 124 participants, 2 studies; very low‐certainty evidence); up to 30 minutes after the procedure (MD −1.10, 95% CI −2.82 to 0.62; 12 participants, 1 study; very low‐certainty evidence); and one to two hours after the procedure (MD −0.17, 95% CI −2.22 to 1.88; 12 participants, 1 study; very low‐certainty evidence). No studies reported any harms. The evidence is very uncertain about the effect of opioids on episodes of apnea during (RR 3.27, 95% CI 0.85 to 12.58; 124 participants, 2 studies; very low‐certainty evidence) and after the procedure (RR 2.71, 95% CI 0.11 to 64.96; 124 participants, 2 studies; very low‐certainty evidence) and on hypotension (RR 1.34, 95% CI 0.32 to 5.59; 204 participants, 3 studies; very low‐certainty evidence). The other main outcomes were not reported.
We identified no studies comparing different opioids (e.g. morphine versus fentanyl) or different routes for administration of the same opioid (e.g. morphine enterally versus morphine intravenously).
Authors' conclusions
Compared to placebo, opioids probably reduce pain score assessed with PIPP/PIPP‐R scale during the procedure; may reduce NIPS during the procedure; and may result in little to no difference in DAN one to two hours after the procedure. The evidence is very uncertain about the effect of opioids on pain assessed with other pain scores or at different time points. No studies reported if any harms occurred. The evidence is very uncertain about the effect of opioids on episodes of bradycardia or hypotension. Opioids may result in an increase in episodes of apnea. No studies reported parent satisfaction with care provided in the NICU. The evidence is very uncertain about the effect of opioids on any outcome when compared to non‐pharmacological interventions or to other analgesics. We identified no studies comparing opioids to other opioids or comparing different routes of administration of the same opioid.
Plain language summary
Opioids for managing pain in babies exposed to painful procedures
Key messages
• Due to a lack of strong evidence, the benefits and risks of opioids for managing pain in babies exposed to painful procedures are unclear.
• Compared to placebo (a 'dummy' treatment, or sham treatment, that does not contain any medicine but looks identical to the medicine being tested), opioids may reduce pain assessed with certain scales during the procedure, but may not make a difference with other scales one to two hours after the procedure.
• The evidence is very uncertain about the effect of opioids on: pain assessed with other pain scores or at different time points, episodes of bradycardia (slow heart rate), or hypotension (low blood pressure). Opioids may increase episodes of breathing stops.
Why are opioids given to manage pain during procedures in babies?
Babies (particularly in the first four weeks after birth) are frequently exposed to painful procedures during hospitalization. Similar to adults, they require uninterrupted pain management and control during these procedures. Opioids, a broad group of pain‐relieving medications that work by interacting with opioid receptors in the body's cells, are commonly used in babies.
What did we want to find out?
We wanted to find out the effect of opioids in babies exposed to painful procedures, compared to:
• no treatment or placebo;
• non‐drug treatments (such as sweet solutions);
• other drugs;
• different types of opioids;
• or same opioid administered by a different route, for example by mouth compared to by injection.
What did we do?
We searched for studies looking at the five comparisons described above. We compared and summarized study results and rated our confidence in the evidence based on factors such as study methods and sizes.
What did we find?
We included 13 studies involving a total of 823 babies. The largest study was in 150 babies, and the smallest in 12 babies. All studies were performed in a hospital. Four studies were conducted in India, two each in Italy and the UK, one each in Canada, Finland, Iran, and the USA, and one was an international study conducted in France and the USA.
Seven studies compared opioids to placebo; two studies compared opioids to oral sweet solution or other treatments such as touching the baby's body; and five studies compared opioids to another drug.
Compared to placebo, opioids probably result in a reduction in pain score assessed with certain scales during the procedure, but in little or no difference between groups with other scales one to two hours after the procedure. The evidence is very uncertain about the effect of opioids on pain assessed with other pain scores or at different time points. Harms were not reported. The evidence is very uncertain about the effect of opioids on episodes of bradycardia or hypotension. Opioids may increase episodes of breathing stops. No studies reported parent satisfaction with medical care.
The evidence is very uncertain about the effect of opioids on any outcome when compared to other treatments, such as touching the baby's body or giving other drugs.
What are the limitations of the evidence?
We are not confident in the evidence because there are not enough studies to be certain about the results of our outcomes. Moreover, it is possible that people in the studies were aware of what treatment they were giving to the babies. Few studies provided data about everything that we were interested in.
How up‐to‐date is this review?
We searched for studies up to December 2021.
Summary of findings
Summary of findings 1. Opioids compared to no treatment/placebo for procedural pain in neonates.
| Opioids compared to no treatment/placebo for procedural pain in neonates | ||||||
| Patient or population: neonates exposed to procedural pain Setting: neonatal units Intervention: opioids Comparison: no treatment/placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with no treatment/placebo | Risk with opioids | |||||
| Pain, assessed using the PIPP/PIPP‐R pain scale, ranging from 0 to 21 and 0 to 18 in preterm infants < 28 weeks' gestational age and full‐term infants, respectively, during procedure | Mean PIPP/PIPP‐R during procedure ranged 8 to 11. | MD 2.58 lower (3.12 lower to 2.03 lower) | ‐ | 199 (3 RCTs) | ⊕⊕⊕⊝ Moderate 1 | Opioids probably reduce pain score assessed with the PIPP/PIPP‐R scale during the procedure compared to placebo. |
| Pain, assessed using the PIPP/PIPP‐R pain scale up to 30 min after procedure | Mean PIPP/PIPP‐R up to 30 min after procedure ranged 3 to 6. | MD 0.14 higher (0.17 lower to 0.45 higher) | ‐ | 123 (2 RCTs) | ⊕⊝⊝⊝ Very low 2 | The evidence is very uncertain about the effect of opioids on pain score assessed with the PIPP/PIPP‐R scale up to 30 min after the procedure compared to placebo. |
| Pain, assessed using the PIPP/PIPP‐R pain scale 1 to 2 hours after procedure | Mean PIPP/PIPP‐R 1 to 2 hours after procedure ranged 4 to 11. | MD −0.83 (2.42 lower to 0.75 higher) | ‐ | 54 (2 RCTs) | ⊕⊝⊝⊝ Very low 3 | The evidence is very uncertain about the effect of opioids on pain score assessed with the PIPP/PIPP‐R scale 1 to 2 hours after the procedure compared to placebo. |
| Pain, assessed using the DAN pain scale, ranging from 0 to 21, 1 to 2 hours after procedure | Mean DAN 1 to 2 hours after procedure was 5. | MD 0.2 lower (2.21 lower to 1.81 higher) | ‐ | 42 (1 RCT) | ⊕⊕⊝⊝ Low 4 | Opioids may result in little to no difference in pain score assessed with the DAN scale 1 to 2 hours after the procedure compared to placebo. |
| Pain, assessed using the NIPS, ranging from 0 to 7, during procedure | Mean NIPS during procedure ranged 5 to 6. | MD 1.97 lower (2.46 lower to 1.48 lower) | ‐ | 102 (2 RCTs) | ⊕⊕⊝⊝ Low 5 | Opioids may reduce NIPS during the procedure compared to placebo. |
| Any harms | ‐ | ‐ | ‐ | ‐ | ‐ | This outcome was not reported. |
| Episodes of bradycardia | Study population | RR 3.19
(0.14 to 72.69) RD 0.01, (−0.03 to 0.06) |
172 (3 RCTs) | ⊕⊝⊝⊝ Very low 6 | The evidence is very uncertain about the effect of opioids on episodes of bradycardia compared to placebo. | |
| 0 per 1000 (No events in the 3 RCTs) |
0 per 1000 (0 to 0) | |||||
| Episodes of apnea | Study population | RR 3.15
(1.08 to 9.16) RD 0.07 (0.01 to 0.14) NNTH = 14 |
199 (3 RCTs) | ⊕⊕⊝⊝ Low 7 | Opioids may result in an increase in episodes of apnea compared to placebo. | |
| 30 per 1000 | 95 per 1000 (33 to 278) | |||||
| Hypotension | Study population | ‐ | 88 (2 RCTs) | ⊕⊝⊝⊝ Very low 8 | The evidence is very uncertain about the effect of opioids on hypotension compared to placebo. | |
| See comment | See comment | |||||
| Parent satisfaction with care provided in the NICU (as measured by a validated instrument/tool)—not reported | ‐ | ‐ | ‐ | ‐ | This outcome was not reported. | This outcome was not reported. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; DAN: Douleur Aiguë du Nouveau‐né; MD: mean difference; NICU: neonatal intensive care unit; NIPS: Neonatal Infant Pain Scale; NNTH: number needed to treat for an additional harmful outcome; OR: odds ratio; PIPP: Premature Infant Pain Profile; PIPP‐R: PIPP‐Revised; RCT: randomized controlled trial; RD: risk difference; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1Downgraded one level for inconsistency (moderate heterogeneity, I² = 62%); not downgraded for imprecision (narrow CIs). 2Downgraded one level for inconsistency (substantial heterogeneity, I² = 85%) and two levels for imprecision (two small trials with low sample size; CIs overlapping no effect). 3Downgraded one level for study limitations (unclear risk of selection, performance, detection, and reporting bias) and two levels for imprecision (two small trials with low sample size; CIs overlapping no effect). 4Downgraded two levels for imprecision (one small trial with low sample size; CIs overlapping no effect). 5Downgraded one level for inconsistency (moderate heterogeneity, I² = 60%) and one level for imprecision (two small trials with low sample size; CIs not overlapping no effect). 6Downgraded one level for study limitations (unclear risk of selection bias in the informative study) and two levels for imprecision (three small trials with low sample size; CI overlapping no effect). 7Downgraded one level for study limitations (unclear risk of selection and reporting bias) and one level for imprecision (wide CIs). 8Downgraded one level for study limitations (unclear risk of selection and reporting bias) and two levels for imprecision (two small trials with no events).
Summary of findings 2. Opioids compared to oral sweet solution or non‐pharmacological intervention for procedural pain in neonates.
| Opioids compared to oral sweet solution or non‐pharmacological intervention for procedural pain in neonates | ||||||
| Patient or population: neonates exposed to procedural pain Setting: neonatal units Intervention: opioids Comparison: oral sweet solution or non‐pharmacological intervention | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with oral sweet solution or non‐pharmacological intervention | Risk with opioids | |||||
| Pain, assessed using the CRIES pain scale, ranging from 0 to 10, during the procedure— opioids versus facilitated tucking | Mean CRIES—opioids versus facilitated tucking was 9. | MD 4.62 lower (6.38 lower to 2.86 lower) | ‐ | 100 (1 RCT) | ⊕⊝⊝⊝ Very low 1 | The evidence is very uncertain about the effect of opioids on pain score assessed with the CRIES scale during the procedure compared to facilitated tucking. |
| Pain, assessed using the CRIES pain scale, ranging from 0 to 10, during the procedure— opioids versus sensorial stimulation | Mean CRIES—opioids versus sensorial stimulation was 4. | MD 0.32 higher (1.13 lower to 1.77 higher) | ‐ | 100 (1 RCT) | ⊕⊝⊝⊝ Very low 1 | The evidence is very uncertain about the effect of opioids on pain score assessed with the CRIES scale during the procedure compared to sensorial stimulation. |
| Any harms | ‐ | ‐ | ‐ | ‐ | ‐ | This outcome was not reported. |
| Episodes of bradycardia—not reported | ‐ | ‐ | ‐ | ‐ | ‐ | This outcome was not reported. |
| Episodes of apnea—not reported | ‐ | ‐ | ‐ | ‐ | ‐ | This outcome was not reported. |
| Hypotension—not reported | ‐ | ‐ | ‐ | ‐ | ‐ | This outcome was not reported. |
| Parent satisfaction with care provided in the NICU—not reported | ‐ | ‐ | ‐ | ‐ | ‐ | This outcome was not reported. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; CRIES: Crying Requires oxygen Increased vital signs Expression Sleep; MD: mean difference; NICU: neonatal intensive care unit; RCT: randomized controlled trial | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1Downgraded two levels for study limitations (high risk of performance bias; unclear risk of bias for the other domains) and one level for imprecision (one small trial with low sample size; CIs overlapping no effect).
Summary of findings 3. Opioids compared to other analgesics for procedural pain in neonates.
| Opioids compared to other analgesics for procedural pain in neonates | ||||||
| Patient or population: procedural pain in neonates Setting: neonatal units Intervention: opioids Comparison: other analgesics | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with other analgesics | Risk with opioids | |||||
| Pain, assessed using the PIPP/PIPP‐R pain scale, ranging from 0 to 21 and 0 to 18 in preterm infants < 28 weeks gestational age and full‐term infants, respectively, during procedure | Mean PIPP/PIPP‐R during procedure ranged 5 to 7. | MD 0.29 lower (1.58 lower to 1.01 higher) | ‐ | 124 (2 RCTs) | ⊕⊝⊝⊝ Very low 1 | The evidence is very uncertain about the effect of opioids on pain score assessed with the PIPP/PIPP‐R during the procedure compared to other analgesics. |
| Pain, assessed using the PIPP/PIPP‐R pain scale up to 30 min after procedure | Mean PIPP/PIPP‐R up to 30 min after procedure was 5. | MD 1.1 lower (2.82 lower to 0.62 higher) | ‐ | 12 (1 RCT) | ⊕⊝⊝⊝ Very low 2 | The evidence is very uncertain about the effect of opioids on pain score assessed with the PIPP/PIPP‐R up to 30 min after the procedure compared to other analgesics. |
| Pain, assessed using the PIPP/PIPP‐R pain scale 1 to 2 hours after procedure | Mean PIPP/PIPP‐R 1 to 2 hours after procedure was 4. | MD 0.17 lower (2.22 lower to 1.88 higher) | ‐ | 12 (1 RCT) | ⊕⊝⊝⊝ Very low 2 | The evidence is very uncertain about the effect of opioids on pain score assessed with the PIPP/PIPP‐R 1 to 2 hours after the procedure compared to other analgesics. |
| Any harms | ‐ | ‐ | ‐ | ‐ | ‐ | This outcome was not reported. |
| Episodes of apnea—during the procedure | Study population | RR 3.27 (0.85 to 12.58) | 124 (2 RCTs) | ⊕⊝⊝⊝ Very low 1 | The evidence is very uncertain about the effect of opioids on episodes of apnea during the procedure compared to other analgesics. | |
| 33 per 1000 | 109 per 1000 (28 to 419) | |||||
| Episodes of apnea—after the procedure | Study population | RR 2.71 (0.11 to 64.96) | 124 (2 RCTs) | ⊕⊝⊝⊝ Very low 1 | The evidence is very uncertain about the effect of opioids on episodes of apnea after the procedure compared to other analgesics. | |
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Episodes of bradycardia—not reported | ‐ | ‐ | ‐ | ‐ | ‐ | This outcome was not reported. |
| Hypotension | Study population | RR 1.34 (0.32 to 5.59) | 204 (3 RCTs) | ⊕⊝⊝⊝ Very low 3 | The evidence is very uncertain about the effect of opioids on hypotension compared to other analgesics. | |
| 20 per 1000 | 26 per 1000 (6 to 110) | |||||
| Parent satisfaction with care provided in the NICU—not reported | ‐ | ‐ | ‐ | ‐ | ‐ | This outcome was not reported. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; NICU: neonatal intensive care unit; PIPP: Premature Infant Pain Profile; PIPP‐R: PIPP‐Revised; RCT: randomized controlled trial; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1Downgraded two levels for study limitations (high risk of performance bias; unclear risk of selection and other biases) and one level for imprecision (two small trials with low sample size; CI overlapping no effect). 2Downgraded one level for study limitations (unclear risk of selection, performance, and detection bias) and two levels for imprecision (one small trial with low sample size; CIs overlapping no effect). 3Downgraded two levels for study limitations (high risk of performance bias; unclear risk of selection and other biases) and one level for imprecision (three small trials with low sample size; CIs overlapping no effect).
Background
Description of the condition
A painful procedure can be defined as a procedure causing skin or mucosal damage by inserting or removing foreign bodies and disturbing the body integrity through therapeutic or diagnostic methods (Kassab 2019). Most newborn infants undergo painful procedures early in life, including routine therapeutic interventions (such as intramuscular vitamin K injection) and diagnostic testing (such as metabolic screening). Infants that require neonatal intensive care are subjected to numerous invasive and painful procedures, with as many as 14 to 16 painful procedures a day being reported (Courtois 2016; Johnston 2013). The most unwell and the most preterm infants are exposed to a higher number of painful procedures compared to older and healthier infants (Cruz 2016). This is unfortunate as preterm infants are especially vulnerable to the negative effects of pain due to their immature and still‐developing nervous system (Maxwell 2019).
Painful procedures have immediate negative physiological effects as well as long‐term negative effects such as altered pain processing, pain sensitivity, and response to pain (Ranger 2014); reduction in cortical thickness (Ranger 2013); and lower cognitive and motor function (Ranger 2014). Painful procedures can be diagnostic (such as venipuncture and eye screening for retinopathy of prematurity (ROP)), therapeutic (such as tracheal suctioning or bladder catheterization), or surgical (such as circumcision) (Williams 2020). The most common painful procedures that neonates undergo are nasal/tracheal aspiration and heel lance (Carbajal 2008; Cruz 2016), while the procedures that are considered the most painful by nurses and physicians are endotracheal intubation, lumbar puncture, and insertion of a chest tube (Andersen 2010). The likelihood of developing hypersensitivity or persistent pain, or both, later in life increases with the number of painful procedures experienced as a neonate (Williams 2020).
Description of the intervention
Interventions for pain management can be classified as non‐pharmacological or pharmacological. In the policy statement regarding the prevention and management of procedural pain in the neonate, the American Academy of Pediatrics (AAP) recommends the consistent use of non‐pharmacological strategies, coupled with pharmacological strategies when necessary (AAP 2016). Non‐pharmacological interventions like swaddling, positioning, skin‐to‐skin care (SSC), breastfeeding, oral sweet solutions, non‐nutritive sucking, multi‐sensory stimulation, and facilitated tucking have the advantage of not being associated with the short‐ and long‐term side effects caused by analgesic drugs, and have increasingly gained importance in the pain management of this most vulnerable population (Mehler 2013; van den 2016). These interventions to keep infants comfortable could be as effective as analgesics administered for painful procedures (Gomes Neto 2020; Shah 2012). They may be used alone or in adjunct with other interventions to address mild to moderate pain secondary to various procedures in the neonatal intensive care unit (NICU) (Squillaro 2019).
Pharmacological interventions signify the administration of analgesic drugs, of which opioids play a major role. Opioids are well‐known to provide both analgesia and sedation across all age groups, and administration of opioids has traditionally been the first choice for pain management in the NICU, where critically ill infants were often under moderate to severe pain as well as being exposed to numerous painful procedures (McKechnie 2008). However, the optimal regimen for the use of the many opioids available for different procedures is not completely understood. Recent studies have reported conflicting results toward the ongoing use of opioids to control pain: Hartley and colleagues reported that 0.1 mg/kg of body weight of oral morphine, given to non‐ventilated preterm infants one hour prior to an eye examination or heel lance, was not effective and may even be harmful (Hartley 2018), and Gitto and colleagues reported that 1 μg/kg of body weight to 2 μg/kg intravenous fentanyl or sensorial saturation given before every heel lance provided effective analgesia (Gitto 2012). Moreover, past studies have reported adverse effects of opioids in the smallest patients, which raises concerns for safety and emphasizes the need for establishing an effective dosing schedule with minimum side effects (Hartley 2018; Orsini 1996).
Of the opioids, morphine and fentanyl are the most commonly used, and therefore the most studied, in neonates. Other fentanyl derivatives (e.g. remifentanil, alfentanil, sufentanil) and other opioids (e.g. codeine, oxycodone, hydrocodone) are used more sporadically (Hall 2014; Thigpen 2019). Codeine is a prodrug (i.e. a substance that, after administration, is metabolized into a pharmacologically active drug) that is converted to morphine, with one‐tenth the potency of morphine; oxycodone and hydrocodone are structurally similar to codeine but do not need to be metabolized for action. Fentanyl is a purely synthetic opioid that is 50 to 100 times more potent than morphine, and sufentanil is more potent while alfentanil and remifentanil are shorter‐acting. Several administration routes are physiologically possible, but morphine is usually administered by intravenous and oral routes in the NICU, and fentanyl and its derivatives are usually administered intravenously (Thigpen 2019). Though classified into the same drug group, each opioid should be administered in an individualized manner based on the condition of the patient and the pharmacokinetic and pharmacodynamic profiles of the opioid. In addition, neonates ranging from preterm to term have differing liver metabolism and renal clearance, owing to the maturation of enzymes and physiologic processes over time (Tibboel 2005). Due to the historical fact that most drugs used in the NICU started off as 'off‐label' use of those drugs given to adults and the older pediatric population (Balan 2018; Krzyżaniak 2016), pharmacological data in small and preterm infants are still lacking and are continuously being updated (Norman 2019). Morphine has the longest time to onset and the longest half‐life and elimination time compared to fentanyl and remifentanil, while remifentanil exhibits rapid action and elimination with twice the potency of fentanyl (Thigpen 2019). Short‐term adverse effects of opioids include hypotension, bradycardia, respiratory depression, chest‐wall rigidity, gastrointestinal dysmotility, and urinary retention, while studies suggesting negative impact on the developing brain have raised concerns about the possible long‐term adverse effects of opioids (McPherson 2015; Zwicker 2016). Some studies indicate an association between the use of opioids and the development of intraventricular hemorrhage in premature infants (Khanafer‐Larocque 2019; Riskin 2015), while others show no evidence of this (Jiang 2012; McPherson 2015). There might also be a correlation between the use of opioids and necrotizing enterocolitis (Hällström 2003; Riskin 2015; Zvizdic 2019).
Neonatal pain management is challenging because physicians must find the balance between achieving sufficient pain control and minimizing oversedation and negative consequences of opioid use, therefore a multifactorial approach is called for. Non‐opioid alternatives (i.e. non‐pharmacological interventions and non‐opioid pharmacological agents) have emerged over the years as understanding of neonatal pain has improved and practice has evolved. Non‐opioid agents include paracetamol (acetaminophen), non‐steroidal anti‐inflammatory drugs (NSAIDs) (e.g. ketorolac, ibuprofen), and alpha 2‐agonists (e.g. clonidine, dexmedetomidine). Paracetamol has been used for its opioid‐sparing effects in treating mild to moderate pain in neonates, in which the pharmacokinetics and metabolism of this drug have been summarized, but recent reviews found that paracetamol failed to provide effective procedural pain management (Allegaert 2020; Ohlsson 2020). Although a Cochrane Review recently concluded that there is still insufficient evidence to recommend the use of clonidine for pain management—including prevention or treatment of procedural pain (Romantsik 2020)—alpha 2‐agonists such as clonidine are administered in combination with opioids to decrease their required doses for sedation and analgesia in critically ill children (Duffett 2012).
Results from the EUROpean Pain Audit In Neonates (EUROPAIN) prospective cohort study showed wide variations in the clinical practice of neonatal sedation and analgesia across institutions and countries (Carbajal 2015). Not all NICUs implement routine analgesia before daily heel pricks and venipunctures, and pain prevention and treatment among neonates is far from optimal (Bellieni 2018). Considering the ethical and practical challenges of accumulating evidence in the youngest patients, as well as the significant impact of using the evidence for their care, the current situation emphasizes the urgent need to organize the evidence at hand, investigate unanswered questions by well‐designed studies (Dotta 2011), and develop common guidelines, in order to ultimately manage neonatal pain in the safest and most effective evidence‐based way possible.
How the intervention might work
Opioids are used for the treatment of moderate to severe, acute, perioperative, and chronic pain in patients of all ages and provide both analgesia and sedation (Nafziger 2018). Moreover, they also attenuate physiological stress responses and have a wide therapeutic window (Nafziger 2018). The positive and adverse effects of opioids depend on their binding affinity to the different opioid G protein‐coupled receptors (mu, delta and kappa), which are present at virtually all neural loci related to pain in both the peripheral and central nervous systems. When a neonate requiring intensive care is exposed to repeated invasive procedures such as blood sampling and tracheal suctioning, continuous administration of low‐dose opioids or intermittent administration of boluses prior to each procedure may be beneficial (Anand 2005). However, caution has been called for on the neonatal use of opioids, with recommendations to only use them in cases when non‐pharmacological interventions are considered insufficient (Anand 2007).
Several findings have supported the positive impact of opioid use in managing procedural pain, even in the smallest patients. For example, it was shown that endogenous opioids, such as endorphins and mu‐receptor agonists, tend to reduce the stress response and produce stress adaptation by preventing overactivation of the hypothalamic–pituitary–adrenal axis (Bali 2015). A retrospective study found that intranasal midazolam and fentanyl were well‐tolerated in preterm and term infants requiring intensive care (Ku 2019). The effectiveness of opioids in relieving procedural pain has been well‐established, primarily starting from older patients, like any major drug. Sedoanalgesia with oral fentanyl citrate and midazolam has been shown to be highly effective in reducing pain during bone marrow aspiration and biopsy in adult patients with hematological malignancies (Cerchione 2020). Similarly, remifentanil, alfentanil, and midazolam were effective in reducing pain during bone marrow aspiration (Antmen 2005). The effectiveness of these drugs in the youngest patients has been studied vigorously over the years, accumulating evidence related especially to the main opioids, morphine and fentanyl. It has been reported that a single dose of fentanyl to ventilated preterm neonates decreased changes in heart rate and increased growth hormone levels, as well as decreasing behavioral measures of pain and stress (Guinsburg 1998). A number of studies have indicated that continuous morphine infusion to neonates during heel lances and endotracheal tube suctioning decreased pain responses compared to control patients (Anand 1999; Scott 1999). However, some recent studies, including the NEOPAIN (Neurologic Outcomes and Pre‐emptive Analgesia In Neonates) trial, have reported conflicting results regarding the effectiveness of morphine analgesia for acute procedural pain, which raises questions warranting further research (Carbajal 2005; Simons 2003). Results from the Poppi (PrOcedural Pain in Premature Infants) randomized controlled trial (a study investigating the effectiveness of oral morphine to infants before procedures) have provided more reason to be cautious about the potential adverse effects of opioids (Monk 2019).
Why it is important to do this review
Safe and effective management of procedural pain is important for humanitarian and ethical reasons but also to minimize the detrimental effects of repeated pain. Pain should be assessed with validated methods for the specific type of pain and newborn infant undergoing the procedural pain (Giordano 2019; Olsson 2021). A Cochrane Review on the validity and reliability of neonatal pain scales is in preparation (Bruschettini 2022).
Cochrane Reviews on opioid administration in newborn infants for postoperative pain (Kinoshita 2021; Kinoshita 2023), sedation during mechanical ventilation (Bellù 2021), pain or sedation management during therapeutic hypothermia (Bäcke 2022), elective endotracheal intubation (Ayed 2017), and the prevention of pain during endotracheal suctioning (Pirlotte 2019), are available or currently underway. However, no systematic reviews have been conducted on opioids for procedural pain. Of note, painful procedures and inadequate pain management in early life may lead to long‐term negative effects (Walker 2019). It is therefore important to synthesize and appraise the available evidence on opioids for procedural pain in neonates.
Objectives
To evaluate the benefits and harms of opioids in term or preterm neonates exposed to procedural pain, compared to placebo or no drug, non‐pharmacological intervention, other analgesics or sedatives, other opioids, or the same opioid administered by a different route.
Methods
Criteria for considering studies for this review
Types of studies
We included prospective randomized controlled trials (RCTs), quasi‐RCTs, cluster‐RCTs, and cross‐over RCTs.
Types of participants
We included preterm and term infants of a postmenstrual age (PMA) up to 46 weeks and 0 days—irrespective of their gestational age at birth—receiving opioids for procedural pain such as during dialysis, extracorporeal membrane oxygenation (ECMO) treatment, before screening for ROP, placement of Broviac catheter, air leak drainage, insertion of a central line, heel lance, lumbar puncture, venipuncture, arterial line placement, and any other painful procedures.
We excluded infants:
receiving opioids during mechanical ventilation for respiratory morbidity (assessed in a separate Cochrane Review, Bellù 2021);
receiving opioids pre‐intubation (assessed in a separate Cochrane Review, Ayed 2017);
undergoing endotracheal suctioning (assessed in a separate Cochrane Review, Pirlotte 2019);
receiving opioids for postoperative pain (assessed in separate Cochrane Reviews, Kinoshita 2021; Kinoshita 2023);
treated for neonatal abstinence syndrome (assessed in a separate Cochrane Review, Osborn 2021);
undergoing therapeutic hypothermia (assessed in a separate Cochrane Review, Bäcke 2022);
undergoing invasive procedures during the postoperative period and in other excluded conditions.
Types of interventions
We included studies on any opioids (e.g. morphine, diamorphine, fentanyl, alfentanil, sufentanil, pethidine, meperidine, codeine) for procedural pain. We included any systemic route of administration. We included the following comparisons.
Comparison 1: opioids versus no treatment or placebo.
Comparison 2: opioids versus oral sweet solution or non‐pharmacological intervention (skin‐to‐skin contact, music exposure, non‐nutritive sucking, swaddling, etc.).
Comparison 3: opioids versus other analgesics (e.g. paracetamol) and sedatives (e.g. midazolam and other benzodiazepines).
Comparison 4: head‐to‐head comparison of different opioids.
Comparison 5: different routes for administration of the same opioid.
Types of outcome measures
The following outcome measures did not form part of the eligibility criteria.
Primary outcomes
Pain assessed with the following scales: ABC scale (Bellieni 2005); Bernese Pain Scale for Neonates (Cignacco 2004); Behavioral Indicators of Infant Pain (BIIP) (Holsti 2008); Douleur Aiguë du Nouveau‐né (DAN) (Acute Pain in Newborn infants, APN, English version) (Carbajal 1997); Neonatal Infant Pain Scale (NIPS) (Lawrence 1993); Neonatal Pain, Agitation, and Sedation Scale (N‐PASS) (Hummel 2008); Premature Infant Pain Profile (PIPP)/PIPP‐Revised (PIPP‐R) (Gibbins 2014; Stevens 1996). We planned to report the median and mean values of each pain scale assessed during the procedure; up to 30 minutes after the procedure; and at one to two hours after the procedure.
Any harms.
Secondary outcomes
All‐cause neonatal mortality (death until postnatal day 28).
All‐cause mortality during initial hospitalization.
Use of additional pharmacological intervention for the relief of procedural pain.
Episodes of bradycardia, defined as a fall in heart rate of more than 30% below the baseline or less than 100 beats per minute for 10 seconds or longer.
Episodes of desaturation, defined as a decrease of arterial oxygen saturation (SpO²) to less than 80%, with no minimum duration specified.
Episodes of apnea (mean rates of apnea).
Hypotension requiring medical therapy (vasopressors or fluid boluses).
Parent satisfaction with care provided in the NICU (as measured by a validated instrument/tool) (Butt 2013).
Intraventricular hemorrhage (IVH; all (grade 1 or 2) or severe (grade 3 or greater) on cranial ultrasound, according to Papile classification (Papile 1978)).
Necrotizing enterocolitis (NEC) (modified Bell stage 2/3; Walsh 1986).
Constipation during the course of treatment, defined as a delay in defecation sufficient to cause significant distress to the infant.
Major neurodevelopmental disability: cerebral palsy, developmental delay (Bayley Scales of Infant Development ‐ Mental Development Index Edition II (BSID‐MDI‐II; Bayley 1993), Bayley Scales of Infant and Toddler Development ‐ Edition III Cognitive Scale (BSITD‐III) (Bayley 2005)), or Griffiths Mental Development Scale ‐ General Cognitive Index (GCI) (Griffiths 1954; Griffiths 1970), assessment greater than two standard deviations (SDs) below the mean, intellectual impairment (intelligence quotient (IQ) greater than two SDs below the mean), blindness (vision less than 6/60 in both eyes), or sensorineural deafness requiring amplification (Jacobs 2013). We planned to separately assess data on children aged 18 to 24 months and those aged three to five years.
Cognitive and educational outcomes in children aged more than five years old.
Search methods for identification of studies
We conducted the searches in December 2021. The Cochrane Sweden and Cochrane Neonatal Information Specialists developed a draft search strategy for Ovid MEDLINE in consultation with the review authors (Appendix 1). This strategy was peer reviewed by an Information Specialist using the Peer Review of Electronic Search Strategies (PRESS) checklist (McGowan 2016a; McGowan 2016b). The MEDLINE strategy was translated, using appropriate syntax, for other databases. Methodological filters based on those developed by Cochrane, Lefebvre 2021; RCT‐Filter EMBASE, and the Canadian Agency for Drugs and Technologies in Health (CADTH), CADTH 2016, were used to limit retrieval to RCTs and quasi‐RCTs, and systematic reviews. We conducted the searches without restriction on language, publication year, publication type, or publication status.
The timeline for this publication was disrupted by the COVID‐19 pandemic and staffing issues at the Cochrane Neonatal editorial base. As a result, publication of this review has been delayed, and the literature search is more than one year old. We will endeavor to undertake an updated search within the next calendar year.
Electronic searches
We searched the following databases.
Cochrane Central Register of Controlled Trials (CENTRAL), via Wiley (2021, Issue 12) (16 December 2021)
MEDLINE via PubMed (1966 to 16 December 2021)
Embase, via Elsevier (1974 to 16 December 2021)
CINAHL Complete, via EBSCOhost (1982 to 16 December 2021)
Searching other resources
We identified trial registration records using CENTRAL and by independent searches of the US National Library of Medicine ClinicalTrials.gov (clinicaltrials.gov/) and the World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) on 17 December 2021. We identified conference abstracts using CENTRAL, Embase, and the Eastern Society for Pediatric Research (ESPR) (2019; 2018).
We screened the reference lists of included studies for studies not identified by the database searches. We searched for errata or retractions for included studies published on PubMed (ncbi.nlm.nih.gov/pubmed). In addition to searching for related systematic reviews via databases, we searched the Epistemonikos registry of systematic reviews (epistemonikos.org).
We conducted a grey literature search to identify reports of trials conducted by or referenced in research by agencies or organizations. We identified sources by consulting the Technical Supplement of the searching chapter in the Cochrane Handbook for Systematic Reviews of Interventions (Lefebvre 2021).
Data collection and analysis
We collected information regarding the method of randomization, blinding, intervention, stratification, and whether the trial was single‐ or multicenter for each included study. We noted information regarding trial participants, including birthweight, gestational age, number of participants, type of procedural pain, modality of administration, and dose of opioids. We analyzed the clinical outcomes noted above in Types of outcome measures.
Selection of studies
We used Cochrane’s Screen4Me workflow to help assess the search results. Screen4Me comprises three components: known assessments—a service that matches records in the search results to records that have already been screened in Cochrane Crowd and been labeled as an RCT or as Not an RCT; the RCT classifier—a machine learning model that distinguishes RCTs from non‐RCTs; and, if appropriate, Cochrane Crowd—Cochrane’s citizen science platform where the Crowd help to identify and describe health evidence.
For more information about Screen4Me and the evaluations that have been done, please visit the Screen4Me webpage on the Cochrane Information Specialist’s portal. In addition, more detailed information regarding evaluations of the Screen4Me components can be found in the following publications: Marshall 2018; Noel‐Storr 2020; Noel‐Storr 2021; Thomas 2020.
We included all RCTs, quasi‐RCTs, and cluster‐RCTs fulfilling our inclusion criteria. Two review authors (EO, FB) reviewed the results of the search and separately selected studies for inclusion. Any disagreements were resolved by discussion or by involving a third review author when necessary. We recorded the selection process in sufficient detail to complete a PRISMA flow diagram and Characteristics of excluded studies table (Moher 2009).
Data extraction and management
Two review authors (EO, FB) independently extracted data using a data extraction form integrated with a modified version of the Cochrane Effective Practice and Organisation of Care Group data collection checklist (Cochrane EPOC Group 2017). We piloted the form within the review team, using a sample of included studies. We extracted the following characteristics from each included study.
Administrative details: study author(s); published or unpublished; year of publication; year in which study was conducted; presence of vested interest; details of other relevant papers cited.
Study: study design; type, duration, and completeness of follow‐up (e.g. greater than 80%); country and location of study; informed consent; ethics approval.
Participants: sex, birthweight, gestational age, number of participants.
Interventions: initiation, dose, and duration of opioids administration.
Outcomes as mentioned above in Types of outcome measures.
Any disagreements were resolved by discussion. We described any ongoing studies identified by our search, detailing the primary author, research question(s), methods, and outcome measures, together with an estimate of the reporting date, in the Characteristics of ongoing studies table.
In the case of queries or where additional data were required, we contacted study investigators/authors for clarification. Two review authors (MB, MK) used Cochrane statistical software Review Manager 5 for data entry (Review Manager 2020). We replaced any standard error of the mean (SEM) by the corresponding SD.
Assessment of risk of bias in included studies
Two review authors (EO, FB) independently assessed the risk of bias (low, high, or unclear) of the included trials using the Cochrane risk of bias tool for the following domains (Higgins 2011).
Random sequence generation (selection bias)
Allocation concealment (selection bias)
Blinding of participants and personnel (performance bias)
Blinding of outcome assessment (detection bias)
Incomplete outcome data (attrition bias)
Selective reporting (reporting bias)
Any other bias
Any disagreements were resolved by discussion or by consulting a third review author (MK). See Appendix 2 for a more detailed description of risk of bias for each domain. We assessed overall risk of bias according to three categories, as follows.
Low risk of bias: we classified the outcome result of a trial as being at low risk of bias overall only if all domains were classified as being at low risk of bias.
Unclear risk of bias: we classified the outcome result of a trial as being at unclear risk of bias overall if one or more domains were classified as being at unclear risk of bias, and no domain was at high risk of bias.
High risk of bias: we classified the outcome result of a trial as being at high risk of bias overall if at least one domain was classified as being at high risk of bias.
Measures of treatment effect
We performed the statistical analyses using Review Manager 5 (Review Manager 2020). We summarized the data in a meta‐analysis if they were sufficiently homogeneous, both clinically and statistically.
Dichotomous data
For dichotomous data, we presented results using risk ratios (RRs) and risk differences (RDs) with 95% confidence intervals (CIs). We calculated the number needed to treat for an additional beneficial outcome (NNTB), or number needed to treat for an additional harmful outcome (NNTH) with 95% CIs if there was a statistically significant reduction (or increase) in RD.
Continuous data
For continuous data, we used the mean difference (MD) when outcomes were measured in the same way between trials. We used the standardized mean difference (SMD) to combine trials that measured the same outcome but used different methods. However, we did not pool in the same analysis pain scores assessed with different scales. Where trials reported continuous data as median and interquartile range (IQR) and data passed the test of skewness, we converted median to mean and estimated the SD as IQR/1.35.
Unit of analysis issues
The unit of analysis was the participating infant in individually randomized trials, and an infant was considered only once in the analysis. The participating neonatal unit or section of a neonatal unit or hospital was to be the unit of analysis in cluster‐randomized trials. We planned to analyze these using an estimate of the intracluster correlation coefficient (ICC) derived from the trial (if possible), or from a similar trial or from a study with a similar population as described in Section 16 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2020). If we were to use ICCs from a similar trial or from a study with a similar population, we would report this and conduct a sensitivity analysis to investigate the effect of variation in the ICC. In the end, no cluster‐randomized trial was included.
Had we identified both cluster‐randomized trials and individually randomized trials, we would only combine the results from both if there was little heterogeneity between the study designs, and if the interaction between the effect of the intervention and the choice of randomization unit was considered to be unlikely; however, in the end, no cluster‐randomized trial was included. In the event that we identified cross‐over trials, in which the reporting of continuous outcome data precluded paired analysis, we would not include these data in a meta‐analysis, in order to avoid unit of analysis error. Where carry‐over effects were thought to exist, and where sufficient data existed, we would only include data from the first period in the analysis (Higgins 2021). We planned to acknowledge any possible heterogeneity in the randomization unit and perform a sensitivity analysis to investigate possible effects of the randomization unit. However, no cross‐over trials were included.
Dealing with missing data
Where feasible, we carried out analysis on an intention‐to‐treat basis for all outcomes. Whenever possible, we analyzed all participants in the treatment group to which they had been randomized, regardless of the actual treatment received. If we identified important missing data (in the outcomes), or unclear data, we contacted the original investigators to request the missing data. If a trial contained a mixed population (i.e. postoperative and non‐operative infants were combined in the report), we first assessed whether subgroup results for non‐operative infants were reported. If not, we contacted the trial authors. If results were not available, we included all the trial data if non‐operative infants made up 50% or more of the total trial population. We planned to carry out a sensitivity analysis to assess the impact of including studies with mixed populations, if we were unable to get the subgroup data from trialists; in the end, we were able to get the trial results upon contact with the authors.
We made explicit the assumptions of any methods used to deal with missing data. We planned to perform sensitivity analyses to assess how sensitive results were to reasonable changes in the undertaken assumptions. We also planned to address the potential impact of missing data on the findings of the review in the Discussion section. Ultimately, there were no missing data.
Assessment of heterogeneity
We estimated the treatment effects of individual trials and examined heterogeneity among trials by inspecting the forest plots and quantifying the impact of heterogeneity using the I² statistic (Deeks 2020). We graded the degree of heterogeneity using the following parameters:
0% to 40%: might not represent important heterogeneity;
30% to 60%: may represent moderate heterogeneity;
50% to 90%: may represent substantial heterogeneity;
more than 75%: may represent considerable heterogeneity.
If we noted statistical heterogeneity (indicated by an I² value greater than 50%), we explored the possible causes (e.g. differences in study quality, participants, intervention regimens, or outcome assessments) and considered conducting sensitivity analysis (see Sensitivity analysis).
Assessment of reporting biases
We created and examined a funnel plot to explore possible small‐study biases. When interpreting funnel plots, we examined the different possible reasons for funnel plot asymmetry, as outlined in Section 10 of the Cochrane Handbook for Systematic Reviews of Interventions (Sterne 2017), and related this to the results of the review. We planned that if we were able to pool more than 10 trials, we would undertake formal statistical tests to investigate funnel plot asymmetry (Sterne 2017); however, this was precluded by the number of included studies.
To assess outcome reporting bias, we checked trial protocols against published reports. For studies published after 1 July 2005, we screened the WHO ICTRP for the a priori trial protocol. We evaluated whether selective reporting of outcomes were present.
Data synthesis
We used a fixed‐effect model to combine data where it was reasonable to assume that studies were estimating the same underlying treatment effect. If we judged meta‐analysis to be inappropriate, we analyzed and interpreted individual trials separately. If there was evidence of clinical heterogeneity, we attempted to explain this based on the different study characteristics and subgroup analyses.
Subgroup analysis and investigation of heterogeneity
Tests for subgroup differences in effects need to be interpreted with caution given the potential for confounding with other study characteristics and the observational nature of the comparisons (Deeks 2022). In particular, subgroup analyses with fewer than five studies per category are unlikely to be adequate to ascertain valid difference in effects and would not be highlighted in our results. We conducted stratified meta‐analysis and a formal statistical test for interaction to examine subgroup differences that could account for effect heterogeneity (e.g. Cochran’s Q test, meta‐regression) (Borenstein 2013; Higgins 2020).
Given the potential differences in the intervention effectiveness related to gestational age (extremely preterm infants are more vulnerable), type and route of opioids administration (which might affect the outcomes), presence of co‐interventions (which might interact with opioids), we planned to conduct subgroup comparisons to see if the intervention is more effective for the following groups for subgroup analysis where data were available. We restricted these analyses to the primary outcomes.
Gestational age: term infants (37 weeks' gestation or greater); preterm infants (less than 37 weeks' gestation); extreme preterm (less than 28 weeks' gestation).
Type of administration: with or without loading dose; bolus or continuous infusion.
Route of administration: enteral or intravenous; between other different routes.
With or without other pharmacological sedation/analgesia as co‐interventions.
Within studies that included co‐interventions: studies in which the protocol allowed co‐interventions for sedation/analgesia for one or both of the intervention groups; studies in which the protocol mandated sedation/analgesia with co‐interventions.
Participants with specific clinical conditions, e.g. infants undergoing dialysis or extracorporeal membrane oxygenation.
Sensitivity analysis
Where we identified substantial heterogeneity, we would conduct sensitivity analysis to determine if the findings were affected by inclusion of only those trials considered to have used adequate methodology with a low risk of bias selection and performance bias. We reported results of sensitivity analyses for primary outcomes only.
Summary of findings and assessment of the certainty of the evidence
We used the GRADE approach, as outlined in the GRADE Handbook (Schünemann 2013), to assess the certainty of evidence for the following (clinically relevant) outcomes.
-
Pain assessed with the following scales: ABC scale (Bellieni 2005); Bernese Pain Scale for Neonates (Cignacco 2004); BIIP (Holsti 2008); DAN (Carbajal 1997); NIPS (Lawrence 1993); N‐PASS (Hummel 2008); PIPP/PIPP‐R (Gibbins 2014; Stevens 1996):
during the procedure;
up to 30 minutes after the procedure;
at one to two hours after the procedure.
Any harms.
Episodes of bradycardia, defined as a fall in heart rate of more than 30% below the baseline or less than 100 beats per minute for 10 seconds or longer.
Episodes of apnea (mean rates of apnea).
Hypotension requiring medical therapy (vasopressors or fluid boluses).
Parent satisfaction with care provided in the NICU (as measured by a validated instrument/tool) (Butt 2013).
Two review authors (MK, MB) independently assessed the certainty of the evidence for each of the outcomes above. We planned to include a summary of findings table for each of the five comparisons specified in Types of interventions; however, we could include only three (Table 1; Table 2; Table 3) because no studies were included for the other two comparisons. We considered evidence from RCTs as high certainty, and downgraded the evidence by one level for serious (or two levels for very serious) limitations based upon the following: design (risk of bias), consistency across studies, directness of the evidence, precision of estimates, and presence of publication bias. We used GRADEpro GDT software to create a summary of findings table to report the certainty of the evidence (GRADEpro GDT).
The GRADE approach resulted in an assessment of the certainty of a body of evidence in one of the following four grades.
High: we are very confident that the true effect lies close to that of the estimate of the effect.
Moderate: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
Low: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect.
Very low: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect.
Results
Description of studies
See Characteristics of included studies; Characteristics of excluded studies; Characteristics of studies awaiting classification; Characteristics of ongoing studies.
Results of the search
The search identified a total of 6974 references (6919 via databases; 55 via other methods). After removal of 322 duplicates, 6652 records remained. These 6652 records were evaluated using Cochrane’s Screen4Me workflow The results of the Screen4Me assessment process are shown in Figure 1. Screen4Me eliminated 3367 records; the remaining 3285 records were screened by the review authors. We excluded 3250 records based on title/abstract and reviewed 35 full texts. We included 13 studies (Characteristics of included studies); excluded 12 studies (Characteristics of excluded studies); classified 3 studies as awaiting classification (Characteristics of studies awaiting classification); and identified 7 ongoing studies (Characteristics of ongoing studies). Details of study selection are presented in Figure 2.
1.
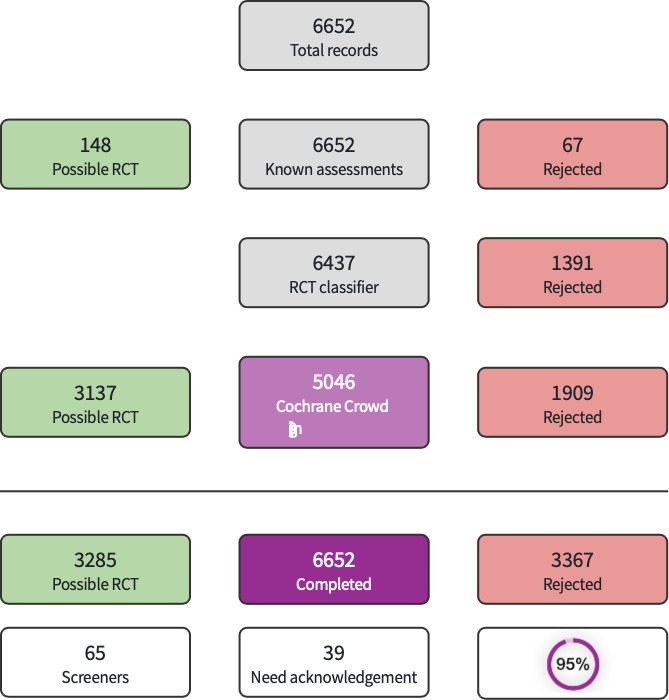
Screen4Me summary diagram.
2.
Study flow diagram.
Included studies
We included 13 independent studies (enrolling 823 newborn infants), considering the study by Madathil and colleagues as two separate studies (Madathil 2021a; Madathil 2021b), because the doses of fentanyl and ketamine were increased after the first 97 newborn infants were enrolled. For detailed information, see Characteristics of included studies and Table 4.
1. Overview of included studies, listed by type of comparison.
| Author | Country | No. of infants in intervention and control group, respectively | GA in intervention and control group, respectively | Procedure | Intervention | Comparison |
| Comparison 1: Opioids versus no treatment or placebo | ||||||
| Carbajal 2005 | France, USA | 21/21 | 27.3 (1.8)/27.2 (1.7)a | Heel lances | Morphine 100 μg/kg loading dose and 10 to 30 μg/kg/h continuous infusion (intravenously) | Placebo 5% dextrose (intravenously) |
| Hartley 2018 | UK | 15/16 | 28.1 (26.3 to 30.1)/28.6 (27.9 to 29.7)b | Heel lances and ROP screening examination | Morphine 100 μg/kg single dose (orally) | Placebo (orally) |
| Manjunatha 2009 | UK | 6/6 | NR | ROP screening examination | Morphine 200 μg/kg single dose (orally) | Placebo (orally) |
| Fallah 2016 | Iran | 23/22 | 37.4 (0.8)/37.6 (0.6)a | Lumbar puncture | Fentanyl 2 μg/kg single dose (intravenously) | Placebo normal saline (intravenously) |
| Sindhur 2020 | India | 56/55 | 30.7 (1.7)/31.0 (1.7)a | ROP screening | Fentanyl 2 μg/kg single dose (intranasal) | Placebo normal saline (intranasally) |
| Lago 2008 | Italy | 27/27 | 28 (2)/29 (2)a | PICC insertion | Remifentanil 0.03 μg/kg/min continuous infusion (intravenously) | Placebo 5% dextrose continuous infusion (intravenously) |
| Pokela 1994 | Finland | 42/42 | 31.6 (25 to 40)/32.9 (24 to 41)c | Daily routine care proceduresd and tracheal suction | Meperidine 1 mg/kg single dose (intravenously) | 0.9% saline single dose (intravenously) |
| Comparison 2: Opioids versus oral sweet solution or non‐pharmacological intervention (skin‐to‐skin contact, music exposure, non‐nutritive sucking, swaddling, etc.) | ||||||
| Gitto 2012 | Italy | 50/50/50 | NR | Heel lances | Fentanyl 1 to 2 μg/kg bolus injection (intravenously) | Facilitated tucking/sensorial saturation |
| Sethi 2020 | India | 29/29 | 30.3 (2.2)/30.3 (2.4)a | Laser for ROP | Fentanyl 1 μg/kg/h continuous infusion (intravenously) | 24% oral sucrose single dose |
| Comparison 3: Opioids versus other analgesics (e.g. paracetamol) and sedatives (e.g. midazolam and other benzodiazepines) | ||||||
| Manjunatha 2009 | UK | 6/6 | NR | ROP screening examination | Morphine 200 μg/kg single dose (orally) | Paracetamol 20 mg/kg single dose (orally) |
| Cordero 1991 | USA | 15/14 | 28 (2)/27 (2)a | Broviac catheter placement | Fentanyl 2 μg/kg single dose (intravenously) | Secobarbital 1 mg/kg single dose (intravenously) |
| Madathil 2021a | India | 51/46 | 29.7 (1.9)/29.8 (1.5)a | Laser for ROP | Fentanyl 2 μg/kg followed by a continuous infusion of 1 μg/kg/h increased to a maximum of 3 µg/kg/h (intravenously) | Ketamine 0.5 mg/kg, followed by further intermittent intravenous bolus doses of 0.5 mg/kg to a maximum of 2 mg/kg (intravenously) |
| Madathil 2021b | India | 13/14 | 30.3 (1.3)/30.5 (2.4)a | Laser for ROP | Fentanyl 2 μg/kg followed by infusion of 2 μg/kg/h to a maximum of 5 μg/kg/h (intravenously) | Ketamine 1 mg/kg followed by intermittent bolus doses of 0.5 mg/kg to a maximum of 4 mg/kg (intravenously) |
| Taddio 2006 | Canada | 38/42 | 29.6 (4.9)/30 (5.1)a | PICC placement | Morphine 100 μg/kg single dose (intravenously) | Tetracaine 0.5 g 4% gel applied to the insertion site |
| We included no studies for the following comparisons: head‐to‐head comparison of different opioids; different routes of administration of the same opioid. | ||||||
GA: gestational age; NR: not reported; PICC: peripherally inserted central catheter; ROP: retinopathy of prematurity aMean (standard deviation). bMedian (interquartile range). cMean (range). dWeighing, washing, temperature measurement, chest roentgenogram.
All studies were performed in a hospital setting, most at a NICU (Carbajal 2005; Fallah 2016; Gitto 2012; Lago 2008; Manjunatha 2009; Pokela 1994; Sindhur 2020; Taddio 2006), two at a neonatal unit (Hartley 2018; Sethi 2020), one in a regional perinatal center (Cordero 1991), and one at a pediatric high‐dependency unit (Madathil 2021a; Madathil 2021b).
We pooled the included studies in three separate comparisons: seven studies in the comparison opioids versus no treatment or placebo (Carbajal 2005; Fallah 2016; Hartley 2018; Lago 2008; Manjunatha 2009; Pokela 1994; Sindhur 2020); two studies in the comparison opioids versus oral sweet solution or non‐pharmacological intervention (Gitto 2012; Sethi 2020); and five studies in the comparison opioids versus other analgesics (e.g. paracetamol) and sedatives (Cordero 1991; Madathil 2021a; Madathil 2021b; Manjunatha 2009; Taddio 2006). Manjunatha 2009 had three groups (morphine, placebo, paracetamol) and is included in both the first comparison (morphine versus placebo) and third comparison (morphine versus paracetamol). Overall, fentanyl and morphine were used in seven studies (Cordero 1991; Fallah 2016; Gitto 2012; Madathil 2021a; Madathil 2021b; Sethi 2020; Sindhur 2020), and four studies (Carbajal 2005; Hartley 2018; Manjunatha 2009; Taddio 2006), respectively; remifentanil, Lago 2008, and meperidine, Pokela 1994, were used in the remaining two studies. Among the two studies in the comparison opioids versus oral sweet solution or non‐pharmacological intervention, the comparator was facilitated tucking/sensorial saturation in Gitto 2012 and 24% oral sucrose in Sethi 2020.
The sample size of the studies ranged from 12 infants, Manjunatha 2009, to 150 infants, Gitto 2012. One study done was stopped after 31 infants were recruited, because predefined stopping boundary was crossed due to occurrence of adverse events in intervention group (Hartley 2018). Most studies enrolled mainly preterm infants; Table 4 reports the values for each study, which were reported as either mean with SD, mean with range, or median with IQR. Four studies were conducted in India (Madathil 2021a; Madathil 2021b; Sethi 2020; Sindhur 2020), two in Italy (Gitto 2012; Lago 2008), two in the UK (Hartley 2018; Manjunatha 2009), and one each in Canada (Taddio 2006), Finland (Pokela 1994), Iran (Fallah 2016), and the USA (Cordero 1991). Carbajal 2005 was an international multicenter study conducted in France and the USA.
Opioids were administered for different indications: laser for ROP or screening for ROP in five studies (Madathil 2021a; Madathil 2021b; Manjunatha 2009; Sethi 2020; Sindhur 2020), central catheter placement in three studies (Cordero 1991; Lago 2008; Taddio 2006), heel stick or heel lance in two studies (Carbajal 2005; Gitto 2012), routine care procedures and tracheal suction in one study (Pokela 1994), and lumbar puncture in one study (Fallah 2016), and in one study, opioids were administered for heel lance and ROP screening examination (Hartley 2018).
No funding was reported for the studies by Cordero 1991, Gitto 2012, Lago 2008, Madathil 2021a, Madathil 2021b, Manjunatha 2009, and Sindhur 2020. Carbajal 2005 received funds from the Foundation CNP and National Institute for Child Health and Human Development grants HD36484 and HD36270. Hartley 2018 received funds from the Wellcome Trust and National Institute for Health Research. Fallah 2016 received a grant from Deputy for Research of Shahid Sadoughi University of Medical Sciences, Yazd, Iran. Pokela 1994 received funding from Foundation for Paediatric Research in Finland, Helsinki and the Alma and K.A. Snellman Foundation, Oulu, Finland. In the study by Sethi 2020, Prof Velpandian, Department of Ocular Pharmacy, AIIMS, New Delhi and Mr Ujjwal provided the 24% oral glucose. Taddio 2006 received funding from the Canadian Society of Hospital Pharmacists, the Canadian Institutes of Health Research New Investigator Award, and the Ontario Student Opportunity Trust Fund ‐ Hospital for Sick Children Foundation Student Scholarship Program.
Ongoing studies
We identified seven ongoing studies (see Characteristics of ongoing studies): five on fentanyl (CTRI/2017/12/011035; CTRI/2020/08/027144; NCT02125201; NCT03718507; NCT03735563), one on morphine (CTRI/2018/04/012926), and one on remifentanil (NCT04073173).
Indications for opioids administration were less invasive surfactant administration (LISA) in four studies, therapy or screening for ROP in two studies, and overall pain prevention in one study.
Studies awaiting classification
We assessed three studies as awaiting classification (ACTRN12612000385842; Gadzinowski 2000; Li 1997); see Characteristics of studies awaiting classification.
Abstract and full text were not available for two of these studies (Gadzinowski 2000; Li 1997). ACTRN12612000385842 is an RCT comparing intravenous infusion of remifentanil with placebo in term and preterm neonates requiring insertion of a central venous catheter. The protocol was registered in 2012; results are not available.
Excluded studies
We excluded 12 studies at full‐text stage; see Characteristics of excluded studies.
Reasons for exclusion were either study design, patient population, or comparator not matching the inclusion criteria of this review, that is:
study design in six studies: Bell 2019 and NCT03897452 are phase II trials; Campbell‐Yeo 2018 and Soffer 2019 are commentaries; Moustogiannis 1996 is not an RCT; and in Axelin 2009 opioids was administered at the very end, without randomization;
patient population in four studies: Chambers 2002 is conducted on surgical pain; NCT00571636 and Valkenburg 2015 on sedation during mechanical ventilation; and Rosen 2000 in infants older than one month;
comparator in two studies that compared high versus low dose of the same opioid (Shin 2013; Shin 2014).
Risk of bias in included studies
The overall risk of bias assessment for each study, including all domain evaluations and justifications for judgement, is displayed in Figure 3 and Figure 4, as well as in the risk of bias section (Characteristics of included studies), and on the right side of all forest plots. The overall quality of studies was good (Figure 3), as nearly two‐thirds of the studies had low risk of bias for each domain in the Cochrane risk of bias tool. The only exception was blinding of participants and personnel, for which about a third of the studies were assessed as high risk of bias.
3.

Risk of bias graph.
4.

Risk of bias summary.
Allocation
The majority of included studies provided details on random sequence generation and allocation concealment. Madathil 2021a; Madathil 2021b; Manjunatha 2009 described that infants were randomized and envelopes were used to conceal allocation, but without further details regarding the specific method of randomization. In contrast, Hartley 2018 stated that a "web‐based facility hosted by the NPEU CTU (National Perinatal Epidemiology Unit Clinical Trials Unit)" was used to randomize infants, but failed to explain how the treatment allocation was masked. Cordero 1991 and Gitto 2012 did not provide sufficient details about study allocation.
Blinding
Blinding of caregivers and assessors to the intervention was established in more than half (7 out of 13) of the included studies; blinding of outcome assessors was described in all but two studies (Gitto 2012; Manjunatha 2009). An exception was Cordero 1991, where blinding was not described but was judged as at low risk of bias for blinding of outcome assessors, given that all study outcomes were objective measurements. The seven double‐blinded trials usually had a pharmacist or a nurse not involved in clinical care prepare the drugs, thereby ensuring the blinding of the clinicians and nurses assisting the infants.
While two studies did not sufficiently explain how the study participants were blinded and were thus judged as unclear risk of bias (Cordero 1991; Manjunatha 2009), four studies could not blind participants due to the different types of intervention (e.g. continuous infusion versus intermittent bolus) and were thus judged as high risk of bias (Gitto 2012; Madathil 2021a; Madathil 2021b; Sethi 2020).
Incomplete outcome data
Follow‐up and outcome data were complete for all studies except Gitto 2012. In Gitto 2012, 150 infants were randomized to receive either intravenous fentanyl, facilitated tucking, or sensorial stimulation, and it was unclear whether secondary outcomes (i.e. levels of cytokines during painful procedures) were reported for all 150 infants.
Selective reporting
Two‐thirds of the included studies reported the trial registration number, and there were no relevant differences between outcomes in the study protocol and those reported in the published article. For five studies a protocol was not available; these studies were judged to be at unclear risk of bias (Carbajal 2005; Cordero 1991; Gitto 2012; Lago 2008; Pokela 1994).
Other potential sources of bias
In Madathil 2021a and Madathil 2021b, the drug regimens were revised upon recommendation from the study steering committee with regard to inadequate analgesia, thus the study was divided into two separate phases, each with smaller numbers of infants than initially planned.
In Pokela 1994, the pain score was modified in the study to be used with intubated neonates, and needs to be further improved and validated.
Effects of interventions
See: Table 1; Table 2; Table 3
Comparison 1: Opioids versus no treatment or placebo
We included seven studies in this comparison (Carbajal 2005; Fallah 2016; Hartley 2018; Lago 2008; Manjunatha 2009; Pokela 1994; Sindhur 2020). Certainty of the evidence is reported for the seven outcomes specified for the summary of findings table (Table 1).
Primary outcomes
Pain assessed with the following scales
PIPP/PIPP‐R during procedure
Three trials reported this outcome (Hartley 2018; Lago 2008; Sindhur 2020). Opioids probably reduce pain score assessed with the PIPP/PIPP‐R scale during the procedure compared to placebo (mean difference (MD) −2.58, 95% confidence interval (CI) −3.12 to −2.03; 199 participants, 3 studies; I² = 62%; moderate‐certainty evidence; Analysis 1.1). As Hartley 2018 reported pain scores following two procedures (ROP screening and heel stick), we halved the number of randomized infants reported to avoid double counting in the meta‐analysis.
1.1. Analysis.

Comparison 1: Opioids versus no treatment/placebo, Outcome 1: PIPP/PIPP‐R during procedure
PIPP/PIPP‐R up to 30 minutes after procedure
Two trials reported this outcome (Manjunatha 2009; Sindhur 2020). The evidence is very uncertain about the effect of opioids on pain score assessed with the PIPP/PIPP‐R scale up to 30 minutes after the procedure compared to placebo (MD 0.14, 95% CI −0.17 to 0.45; 123 participants, 2 studies; I² = 85%; very low‐certainty evidence; Analysis 1.2).
1.2. Analysis.

Comparison 1: Opioids versus no treatment/placebo, Outcome 2: PIPP/PIPP‐R up to 30 min after procedure
PIPP/PIPP‐R one to two hours after procedure
Two trials reported this outcome (Carbajal 2005; Manjunatha 2009). The evidence is very uncertain about the effect of opioids on pain score assessed with the PIPP/PIPP‐R scale one to two hours after the procedure compared to placebo (MD −0.83, 95% CI −2.42 to 0.75; 54 participants, 2 studies; I² = 47%; very low‐certainty evidence; Analysis 1.3).
1.3. Analysis.

Comparison 1: Opioids versus no treatment/placebo, Outcome 3: PIPP/PIPP‐R 1 to 2 hours after procedure
DAN one to two hours after procedure
One trial reported this outcome (Carbajal 2005). Opioids may result in little to no difference in pain score assessed with the DAN scale one to two hours after the procedure compared to placebo (MD −0.20, 95% CI −2.21 to 1.81; 42 participants, 1 study; I² not applicable; low‐certainty evidence; Analysis 1.4).
1.4. Analysis.

Comparison 1: Opioids versus no treatment/placebo, Outcome 4: DAN 1 to 2 hours after procedure
NIPS during procedure
Two trials reported this outcome (Fallah 2016; Lago 2008). Opioids may reduce NIPS during the procedure compared to placebo (MD −1.97, 95% CI −2.46 to −1.48; 102 participants, 2 studies; I² = 60%; low‐certainty evidence; Analysis 1.5).
1.5. Analysis.

Comparison 1: Opioids versus no treatment/placebo, Outcome 5: NIPS during procedure
Any harms
No trials reported this outcome.
Secondary outcomes
Mortality
Two trials reported this outcome, with no events (Fallah 2016; Lago 2008). Opioids may result in little to no difference in mortality compared to placebo (risk ratio (RR) not estimable, risk difference (RD) 0.00, 95% CI −0.05 to 0.05; 102 participants, 2 studies; I² for RR: not applicable, I² for RD = 0%; low‐certainty evidence; Analysis 1.6).
1.6. Analysis.

Comparison 1: Opioids versus no treatment/placebo, Outcome 6: Mortality
Episodes of bradycardia
Three trials reported this outcome (Hartley 2018; Lago 2008; Pokela 1994). The evidence is very uncertain about the effect of opioids on episodes of bradycardia compared to placebo (RR 3.19, 95% CI 0.14 to 72.69; RD 0.01, 95% CI −0.03 to 0.06; 172 participants, 3 studies; I² for RR: not applicable, I² for RD = 0%; very low‐certainty evidence; Analysis 1.7).
1.7. Analysis.

Comparison 1: Opioids versus no treatment/placebo, Outcome 7: Episodes of bradycardia
Episodes of desaturation
Three trials reported this outcome (Hartley 2018; Lago 2008; Sindhur 2020). The evidence is very uncertain about the effect of opioids on episodes of desaturation compared to placebo (RR 1.82, 95% CI 0.72 to 4.58; RD 0.05, 95% CI −0.02 to 0.12; 199 participants, 3 studies; I² for RR = 0%, I² for RD = 63%; very low‐certainty evidence; Analysis 1.8).
1.8. Analysis.

Comparison 1: Opioids versus no treatment/placebo, Outcome 8: Episodes of desaturation
Episodes of apnea
Three trials reported this outcome (Hartley 2018; Lago 2008; Sindhur 2020). Opioids may result in an increase in episodes of apnea compared to placebo (RR 3.15, 95% CI 1.08 to 9.16; RD 0.07, 95% CI 0.01 to 0.14; number needed to treat for an additional harmful outcome (NNTH) = 14; 199 participants, 3 studies; I² for RR = 0%, I² for RD = 78%; low‐certainty evidence; Analysis 1.9). We obtained outcome data from Sindhur 2020 via personal communication with study authors.
1.9. Analysis.

Comparison 1: Opioids versus no treatment/placebo, Outcome 9: Episodes of apnea
Hypotension
Two trials reported this outcome, with no events (Hartley 2018; Lago 2008). The evidence is very uncertain about the effect of opioids on episodes of hypotension compared to placebo (RR not estimable, RD 0.00, 95% CI −0.06 to 0.06; 88 participants, 2 studies; I² for RR: not applicable, I² for RD = 0%; very low‐certainty evidence; Analysis 1.10).
1.10. Analysis.

Comparison 1: Opioids versus no treatment/placebo, Outcome 10: Hypotension
Comparison 2: Opioids versus oral sweet solution or non‐pharmacological intervention
We included two studies in this comparison (Gitto 2012; Sethi 2020); Gitto 2012 and Sethi 2020 reported one and none of the outcomes specified in this review, respectively. Certainty of the evidence is reported for the seven outcomes specified for the summary of findings table (Table 2).
Primary outcomes
Pain assessed with the following scales
CRIES during procedure
One trial reported this outcome (Gitto 2012). The evidence is very uncertain about the effect of opioids on pain score assessed with the CRIES scale during the procedure compared to facilitated tucking (MD −4.62, 95% CI −6.38 to −2.86; 100 participants, 1 study; I² not applicable; Analysis 2.1 (first subgroup)) or sensorial stimulation (MD 0.32, 95% CI −1.13 to 1.77; 100 participants, 1 study; I² not applicable; Analysis 2.1 (second subgroup)).
2.1. Analysis.

Comparison 2: Opioids versus oral sweet solution or non‐pharmacological intervention, Outcome 1: CRIES
Any harms
No trials reported this outcome.
Secondary outcomes
Comparison 3: Opioids versus other analgesics and sedatives
We included five studies in this comparison (Cordero 1991; Madathil 2021a; Madathil 2021b; Manjunatha 2009; Taddio 2006). Certainty of the evidence is reported for the seven outcomes specified for the summary of findings table (Table 3).
Primary outcomes
Pain assessed with the following scales
PIPP/PIPP‐R during procedure
Two trials reported this outcome (Madathil 2021a; Madathil 2021b). The evidence is very uncertain about the effect of opioids on pain score assessed with the PIPP/PIPP‐R during the procedure compared to other analgesics (MD −0.29, 95% CI −1.58 to 1.01; 124 participants, 2 studies; I² = 0%; very low‐certainty evidence; Analysis 3.1).
3.1. Analysis.

Comparison 3: Opioids versus other analgesics, Outcome 1: PIPP/PIPP‐R during procedure
PIPP/PIPP‐R up to 30 minutes after procedure
One trial reported this outcome (Manjunatha 2009). The evidence is very uncertain about the effect of opioids on pain score assessed with the PIPP/PIPP‐R up to 30 minutes after the procedure compared to other analgesics (MD −1.10, 95% CI −2.82 to 0.62; 12 participants, 1 study; I² not applicable; very low‐certainty evidence; Analysis 3.2).
3.2. Analysis.

Comparison 3: Opioids versus other analgesics, Outcome 2: PIPP/PIPP‐R up to 30 min after procedure
PIPP/PIPP‐R one to two hours after procedure
One trial reported this outcome (Manjunatha 2009). The evidence is very uncertain about the effect of opioids on pain score assessed with the PIPP/PIPP‐R one to two hours after the procedure compared to other analgesics (MD −0.17, 95% CI −2.22 to 1.88; 12 participants, 1 study; I² not applicable; very low‐certainty evidence; Analysis 3.3).
3.3. Analysis.

Comparison 3: Opioids versus other analgesics, Outcome 3: PIPP/PIPP‐R 1 to 2 hours after procedure
Any harms
No trials reported this outcome.
Secondary outcomes
Episodes of apnea
Two trials reported this outcome (Madathil 2021a; Madathil 2021b). The evidence is very uncertain about the effect of opioids on episodes of apnea during the procedure compared to other analgesics (RR 3.27, 95% CI 0.85 to 12.58; RD 0.09, 95% CI 0.00 to 0.19; 124 participants, 2 studies; I² for RR = 6%, I² for RD = 76%; very low‐certainty evidence; Analysis 3.4 (first subgroup)) or after the procedure (RR 2.71, 95% CI 0.11 to 64.96; RD 0.02, 95% CI −0.04 to 0.07; 124 participants, 2 studies; I² for RR and RD = 0%; very low‐certainty evidence; Analysis 3.4 (second subgroup)).
3.4. Analysis.

Comparison 3: Opioids versus other analgesics, Outcome 4: Episodes of apnea
Hypotension
Three trials reported this outcome (Madathil 2021a; Madathil 2021b; Taddio 2006). The evidence is very uncertain about the effect of opioids on hypotension compared to other analgesics (RR 1.34, 95% CI 0.32 to 5.59; RD 0.01, 95% CI −0.04 to 0.06; 204 participants, 3 studies; I² for RR = 67%, I² for RD = 54%; very low‐certainty evidence; Analysis 3.5).
3.5. Analysis.

Comparison 3: Opioids versus other analgesics, Outcome 5: Hypotension
Comparison 4: Head‐to‐head comparison of different opioids
No studies were included in this comparison.
Comparison 5: Different routes for administration of the same opioid
No studies were included in this comparison.
Discussion
Summary of main results
We evaluated the benefits and harms of opioids for procedural pain in newborn infants. Seven studies compared opioids to no treatment or placebo (Carbajal 2005; Fallah 2016; Hartley 2018; Lago 2008; Manjunatha 2009; Pokela 1994; Sindhur 2020), two studies to oral sweet solution or non‐pharmacological intervention (Gitto 2012; Sethi 2020), and five studies to other analgesics and sedatives (Cordero 1991; Madathil 2021a; Madathil 2021b; Manjunatha 2009; Taddio 2006). Manjunatha 2009 has three groups (morphine, placebo, paracetamol) and is included in both the first comparison (morphine versus placebo) and third comparison (morphine versus paracetamol).
Compared to placebo, opioids probably reduce pain score assessed with the PIPP/PIPP‐R scale during the procedure; however, the evidence is very uncertain when pain score is assessed with the PIPP/PIPP‐R scale up to 30 minutes after the procedure or one to two hours after the procedure. Opioids may reduce NIPS during the procedure and may result in little to no difference in DAN scale one to two hours after the procedure. No studies reported harms. The evidence is very uncertain about the effect of opioids on episodes of bradycardia or hypotension. Opioids may result in an increase in episodes of apnea. No studies reported parent satisfaction with care provided in the NICU.
The evidence is very uncertain about the effect of opioids on pain score assessed with the CRIES scale during the procedure compared to facilitated tucking or sensorial stimulation. No studies in this comparison reported other pain scales; any harms, episodes of bradycardia or apnea, or hypotension; or parent satisfaction with care provided in the NICU.
The evidence is very uncertain about the effect of opioids on pain scores assessed with the PIPP/PIPP‐R scale during or after the procedure compared to other analgesics. No studies in this comparison reported any harms. The evidence is very uncertain about the effect of opioids on episodes of apnea or hypotension. No studies reported bradycardia or parent satisfaction with care provided in the NICU.
We identified no studies comparing different opioids (e.g. morphine versus fentanyl) or different routes for administration of the same opioid (e.g. morphine enterally versus morphine intravenously).
Overall completeness and applicability of evidence
To date, 13 trials comparing opioids versus placebo or other interventions for procedural pain have enrolled 823 infants. Study authors report limited data about the potential adverse effects of opioids and did not report relevant, long‐term outcomes such as major neurodevelopmental disability and cognitive and educational outcomes. We identified seven ongoing trials. High versus low dose of the same opioid was assessed in a study done during mechanical ventilation, which is outside the scope of this review (Shin 2013; Shin 2014). Phase II trials investigate the influence of opioid use on neonates brain activity (Bell 2019; NCT03897452). We could not perform an appropriate a priori subgroup analysis to detect differential effects because of the paucity of included trials.
Quality of the evidence
According to the GRADE approach, the overall certainty of evidence for critical outcomes for opioids administration for procedural pain ranged from moderate to very low (see Table 1; Table 2; Table 3). We downgraded all outcomes except one for imprecision, in most cases by two levels, because of the paucity and small sample size of the included studies. We downgraded most outcomes for limitations in study design (one level in most cases) (i.e. unclear or high risk of bias in different domains, mainly selection, detection, and reporting bias). We downgraded a couple of outcomes for inconsistency because of moderate or substantial heterogeneity. We explored publication bias by means of a funnel plot (Figure 5); however, its value is limited due to the few studies reporting the outcome.
5.

Funnel plot for PIPP/PIPP‐R during procedure, opioids versus placebo.
Potential biases in the review process
We used the standard methods of Cochrane Neonatal in conducting this systematic review. It is unlikely that the literature search missed relevant trials. We are confident that this systematic review summarizes all the currently available evidence from randomized trials on opioids for procedural pain in neonates. We applied no language restrictions. We succeeded in obtaining additional information from some study authors. Following full‐text screening, studies were excluded because of study design (e.g. phase II trial), characteristics of patient population (e.g. surgical pain or sedation during mechanical ventilation), or type of comparator (e.g. comparing high versus low dose of the same opioid). As prespecified in the protocols (Types of participants), multiple indications for opioids administration to neonates were excluded (i.e. during mechanical ventilation for respiratory morbidity, pre‐intubation or endotracheal suctioning, for postoperative pain, neonatal abstinence syndrome, and therapeutic hypothermia). One study done was stopped prematurely due to safety concerns in the opioid group, thus potentially affecting the point estimate (Hartley 2018). We reported pain scores at three time points: during the procedure; up to 30 minutes after the procedure; and at one to two hours after the procedure. The last might be too late for accurate detection of pain; however, the sickest infants can have a delayed reaction to pain and be affected by the pain long after the procedure. The authors of this Cochrane Review were not involved in any of the included trials.
Agreements and disagreements with other studies or reviews
This is the first systematic review on opioids for procedural pain in neonates. A systematic review with considerably broader inclusion criteria, including both opioids and alpha‐2‐agonists, and assessing any type of analgesia and sedation in newborn infants is ongoing (Kinoshita 2020). One Cochrane Review on propofol for procedural sedation/anesthesia in neonates included one study with 63 neonates (Prakeshkumar 2011). The authors' conclusions did not seek to directly affect current practice, but time to complete procedure and for recovery to previous clinical status was shorter in the propofol group compared to morphine‐atropine‐suxamethonium; no difference was detected in clinically significant side effects; however, the number of events was small.
Authors' conclusions
Implications for practice.
Compared to placebo, opioids probably reduce pain score assessed with Premature Infant Pain Profile (PIPP)/PIPP‐Revised (PIPP‐R) scale during the procedure; may reduce Neonatal Infant Pain Scale (NIPS) during the procedure; and may result in little to no difference in Douleur Aiguë du Nouveau‐né (DAN) one to two hours after the procedure. The evidence is very uncertain about the effect of opioids on pain assessed with other pain scores or at different time points. No studies reported if any harms had occurred. The evidence is very uncertain about the effect of opioids on episodes of bradycardia or hypotension. Opioids may result in an increase in episodes of apnea. None of the studies reported on parent satisfaction with care provided in the neonatal intensive care unit (NICU). The evidence is very uncertain about the effect of opioids on any outcome when compared to non‐pharmacological interventions or to other analgesics. We identified no studies comparing opioids to other opioids or to the same opioid by different route of administration.
Implications for research.
This systematic review highlights the need for large randomized controlled trials to evaluate the effectiveness of opioid analgesics compared to placebo or no drug, non‐pharmacological intervention, other opioids or analgesics, or other opioid by different route of administration in neonates undergoing painful procedures in the NICU. Past studies have focused on morphine and fentanyl, the most commonly used opioids in neonates (Hall 2014), and they may reduce pain during procedure and increase apneic episodes. Since neonatal pain management is about the effective combination of pharmacological and non‐pharmacological interventions (AAP 2016), future trials should focus on specific comparisons that include non‐pharmacological interventions as well as different routes of administration to allow assessment of the optimal intervention across various painful procedures in the NICU. If opioids are indeed effective in reducing procedural pain and beneficial for critical outcomes without significant harm, further comparisons of opioids with placebo or no drug would be unnecessary as we continue our search for optimal pain management. Neurodevelopmental consequences of neonatal management would require time to develop, thus recruited infants would need to be efficiently followed to obtain valuable data.
History
Protocol first published: Issue 12, 2021
Acknowledgements
The Methods section of this review is based on a standard template used by Cochrane Neonatal.
Maria Björklund (Library and ICT services, Lund University) designed and ran the literature searches.
We would like to thank study authors Dr Shamnad Madathil, Madathil 2021a; Madathil 2021b, and Dr Haribalakrishna Balasubramanian, Sindhur 2020, for providing additional information on the included studies.
We would like to acknowledge and thank the following people for their help in assessing the search results for this review via Cochrane’s Screen4Me workflow: Rahul Gujarathi, Nikolaos Sideris, Rubyath Binte Hasan, Dave Lock, Theodoros Aslanidis, Galia Maderi, Ciara Gleeson, Maria Sol Fritz, Bernardo Costa, Luis Coloma, Katja Matthias, Stefanie Rosumeck, Sunu Alice Cherian, Denise Schulz, Jens Thorsen, Paul Whittaker, Susanna Wisniewski, Vighnesh Devulapalli, Akhilanand Chaurasia, Gianluca Gazzaniga, Jehath Syed, Nicole Askin, Ahmad Ozair, Matias Mena, Igor Svintsitskyi, Shammas Mohammed, Mary MacCara, Riccardo Guarise, Cathy Wellan, Angela Gunn, Max Sarmet, Prabina Bhattarai, Lai Ogunsola, Julia Robertson, Phoebe Spink, Iván Javier Ramos Cortés, Amin Sharifan, Ilkin Mengusoglu, Ana‐Marija Ljubenković.
We would like to thank Cochrane Neonatal: Michelle Fiander and Jane Cracknell (Managing Editors), Roger Soll (Co‐coordinating Editor), and Bill McGuire (Co‐coordinating Editor), who provided editorial and administrative support.
A Cochrane Neonatal Associate Editor, Nai Ming Lai, and Senior Editor, Gautham Suresh, peer reviewed and offered feedback for the protocol (Kinoshita 2021).
We would like to thank Tim Disher, PhD, Senior Director of Biostatistics ‐ EVERSANA, Nova Scotia, Canada for peer reviewing the review.
Appendices
Appendix 1. Search strategies
No language restrictions or publication date limitations were applied.
PubMed
Date of search: 2021‐10‐05, revised 2021‐10‐15, main search 2021‐12‐16
#1 general surgery[Mesh:noexp] 40061
“2 (Surgical procedures, Operative[MeSH Terms]) 3359331
#3 ("Perioperative Medicine"[Mesh]) OR "Perioperative Care"[Mesh] OR "Perioperative Period"[Mesh] OR "Perioperative Nursing"[Mesh] 255451
#4 ((((((("Catheterization"[Mesh]) OR "Catheters"[Mesh:NoExp]) OR "Cannula"[Mesh]) OR "Catheter Obstruction"[Mesh]) OR "Catheters, Indwelling"[Mesh]) OR "Urinary Catheters"[Mesh]) OR "Vascular Access Devices"[Mesh]) OR ("Cardiac Catheters"[Mesh]) OR "Central Venous Catheters"[Mesh] OR "Chest Tubes"[Mesh] 225319
#5 "Retinopathy of Prematurity"[Mesh] 6474
#6 "Spinal Puncture"[Mesh] 6531
#7 catheter*[Title/Abstract] OR cannula*[Title/Abstract] OR surgery[Title/Abstract] OR surgical*[Title/Abstract] OR retinopathy[Title/Abstract] OR puncture[Title/Abstract] OR needle[Title/Abstract] OR needles[Title/Abstract] OR "heel lanc*"[Title/Abstract] OR heellanc*[Title/Abstract] OR "chest tube"[Title/Abstract] OR "chest tubes"[Title/Abstract] 2426192
#8 operativ*[Title/Abstract] OR postoperat*[Title/Abstract] OR post‐operat*[Title/Abstract] OR perioperativ*[Title/Abstract] OR peri‐operativ*[Title/Abstract] OR preopera*[Title/Abstract] OR pre‐opera*[Title/Abstract] 1038896
#9 presurgi*[Title/Abstract] OR pre‐surgi*[Title/Abstract] 12781
#10 "invasive procedure*"[Title/Abstract] 19469
#11 (injection*[Title/Abstract] OR intravenous*[Title/Abstract] OR "Needles"[Mesh]) OR ((((((("Injections, Intravenous"[Mesh]) OR "Anesthesia, Intravenous"[Mesh]) OR "Immunoglobulins, Intravenous"[Mesh]) OR "Administration, Intravenous"[Mesh]) OR "Fat Emulsions, Intravenous"[Mesh]) OR "Anesthetics, Intravenous"[Mesh]) OR "Infusions, Intravenous"[Mesh])
993858
#12 drainage[Title/Abstract] OR suction*[Title/Abstract] OR ("Drainage"[Mesh:NoExp]) OR "Suction"[Mesh] 143054
#13 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 [SURGERY, PROCEDURES] 5595888
#14 "Pain, Postoperative"[Mesh] 45627
#15 "procedural pain"[Title/Abstract] OR "pain* procedure*"[Title/Abstract] 1622
#16 ((pain[mh] OR "pain management"[mh] OR pain measurement[mh] OR pain threshold[mh]) OR (Anxiety[mh] or Behavior[mh] or Crying[mh] or Facial expressions[mh] or Fear[mh] or Gestures[mh] or Heart Rate[mh] or Infant Behavior[mh] or Oxygen Consumption[mh] or Panic[mh] or Wakefulness[mh])) OR (anxiet*[Title/Abstract] OR anxious[Title/Abstract] OR behavior*[Title/Abstract] OR behaviour*[Title/Abstract] OR crying[Title/Abstract] OR discomfort*[Title/Abstract] OR distress*[Title/Abstract] OR Douleur Aigue du Nouveau ne[Title/Abstract] OR DAN[Title/Abstract] OR facial expression*[Title/Abstract] OR fear*[Title/Abstract] OR fright*[Title/Abstract] OR gesture*[Title/Abstract] OR grimac*[Title/Abstract] OR heart rate*[Title/Abstract] OR Median Premature Infant Pain Profile score*[Title/Abstract] OR Neonatal Facial Action*[Title/Abstract] OR Neonatal Facial Activity Coding System[Title/Abstract] OR Neonatal Facial Coding Score*[Title/Abstract] OR NFCS[Title/Abstract] OR neonatal facial coding system[Title/Abstract] OR nociceptive reaction*[Title/Abstract] OR orosensorial antinociceptive effect*[Title/Abstract] OR oxygen consumption[Title/Abstract] OR oxygen saturation*[Title/Abstract] OR pain*[Title/Abstract] OR panic*[Title/Abstract] OR sleep wake state*[Title/Abstract] OR wakefulness[Title/Abstract]) 4365981
#17 #14 OR #15 OR #16 [BROADER PAIN TERMS]
4365981
#18 "Analgesics, Opioid"[Mesh] OR "Morphine Derivatives"[Mesh] 90273
#19 opioid*[Title/Abstract] OR opiat*[Title/Abstract] 121782
#20 alfentanil[Title/Abstract] OR sufentanil[Title/Abstract] OR morphine[Title/Abstract] OR meperidine[Title/Abstract] OR codeine[Title/Abstract] OR remifentanil[Title/Abstract] OR piperidines[Title/Abstract] OR opioid*[Title/Abstract] OR analgesi*[Title/Abstract] OR fentanyl[Title/Abstract] OR alfentanil[Title/Abstract] OR sufentanil[Title/Abstract] OR diamorphine[Title/Abstract] OR meperidine[Title/Abstract] OR pethidine[Title/Abstract] OR codeine[Title/Abstract] OR remifentanil[Title/Abstract] 257784
#21 (((((("fentanyl"[MeSH Terms]) OR ("alfentanil"[MeSH Terms])) OR ("sufentanil"[MeSH Terms])) OR ("meperidine"[MeSH Terms])) OR ("codeine"[MeSH Terms])) OR ("remifentanil"[MeSH Terms])) OR ("piperidines"[MeSH Terms]) 141793
#22 #18 OR #19 OR #20 OR #21 394001 [OPIATES]
#23 ((("infant, newborn"[MeSH Terms]) ) OR (intensive care, neonatal[MeSH Terms])) OR (intensive care unit, neonatal[MeSH Terms]) 643230
#24 baby*[TIAB] OR babies[TIAB] OR infant[TIAB] OR infants[TIAB] OR infant s[TIAB] OR infant's[TIAB] OR infantile[TIAB] OR infancy[TIAB] OR low birth weight[TIAB] OR low birthweight[TIAB] OR neonat*[TIAB] OR newborn*[TIAB] OR new born[TIAB] OR new borns[TIAB] OR newly born[TIAB] OR premature[TIAB] OR prematures[TIAB] OR prematurity[TIAB] OR preterm[TIAB] OR preterms[TIAB] OR pre term[TIAB] OR preemie[TIAB] OR preemies[TIAB] OR premies[TIAB] OR premie[TIAB] OR VLBW[TIAB] OR LBW[TIAB] OR ELBW[TIAB] OR NICU[TIAB] 953275
#25 #23 OR #24 [NEONATES] 1232711
#26 (((randomized controlled trial[Publication Type]) OR (controlled clinical trial[Publication Type])) OR (randomi*[TIAB] OR placebo[TIAB] OR randomly[TIAB] OR trial[TIAB] OR groups[TIAB] ) OR ("drug therapy"[MeSH Subheading])) OR (quasirandom*[Title/Abstract] OR quasi‐random*[Title/Abstract])
5305918
#27 ("Animals"[Mesh]) NOT "Humans"[Mesh]
4930434
#28 #26 NOT #27 [RCTs] 4623615
#29 #13 OR #17 9191208
#30 #29 AND #22 AND #25 AND #28 4139
Embase (Elsevier)
Date of search: 20211005, revised 2021‐10‐15, main search 2021‐12‐16
#1 'general surgery'/exp OR ‘general surgery’ 158463
#2 'surgery'/exp 5543382
#3 'perioperative medicine'/exp OR 'perioperative period'/exp OR 'perioperative nursing'/exp 65304
#4 'catheterization'/exp OR 'catheter'/exp OR 'cannula'/exp OR 'cannulation'/exp OR 'catheter occlusion'/exp OR 'indwelling catheter'/exp OR 'urinary catheter'/exp OR 'vascular access device'/exp OR 'heart catheter'/exp OR 'central venous catheter'/exp OR 'chest tube'/exp 451043
#5 'retrolental fibroplasia'/exp 11617
#6 'lumbar puncture'/exp 26912
#7 catheter*:ab,ti OR cannula*:ab,ti OR surgery:ab,ti OR surgical*:ab,ti OR retinopathy:ab,ti OR puncture:ab,ti OR needle:ab,ti OR needles:ab,ti OR 'heel lanc*':ab,ti OR heellanc*:ab,ti OR 'chest tube':ab,ti OR 'chest tubes':ab,ti 3267152
#8 operativ*:ab,ti OR postoperat*:ab,ti OR 'post operat*':ab,ti OR perioperativ*:ab,ti OR 'peri operativ*':ab,ti OR preopera*:ab,ti OR 'pre opera*':ab,ti 1403037
#9 'pre surgi*':ab,ti OR presurgi*:ab,ti 19399
#10 'invasive procedure*':ab,ti 30550
#11 injection*:ab,ti OR intravenous*:ab,ti OR 'intravenous fat emulsion':ab,ti OR 'needle'/exp OR 'intravenous anesthesia'/exp OR 'immunoglobulin'/exp OR 'intravenous drug administration'/exp 2,018971
#12 'suction'/exp OR 'drainage'/de OR drainage:ab,ti OR suction*:ab,ti 167535
#13 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12
[Procedures]
8448310
#14 'postoperative pain'/exp 78007
#15 'procedural pain':ab,ti OR 'pain* procedure*':ab,ti 4777
#16 'pain'/exp OR 'analgesia'/exp OR 'pain measurement'/exp OR 'pain threshold'/exp OR 'anxiety'/exp OR 'behavior'/exp OR 'crying'/exp OR 'facial expression'/exp OR 'fear'/exp OR 'gesture'/exp OR 'heart rate'/exp OR 'child behavior'/exp OR 'oxygen consumption'/exp OR 'panic'/exp OR 'wakefulness'/exp OR anxiet*:ab,ti OR anxious:ab,ti OR behavior*:ab,ti OR behaviour*:ab,ti OR crying:ab,ti OR discomfort*:ab,ti OR distress*:ab,ti OR 'douleur aigue du nouveau ne':ab,ti OR dan:ab,ti OR 'facial expression*':ab,ti OR fear*:ab,ti OR fright*:ab,ti OR gesture*:ab,ti OR grimac*:ab,ti OR 'heart rate*':ab,ti OR 'median premature infant pain profile score*':ab,ti OR 'neonatal facial action*':ab,ti OR 'neonatal facial activity coding system':ab,ti OR 'neonatal facial coding score*':ab,ti OR nfcs:ab,ti OR 'neonatal facial coding system':ab,ti OR 'nociceptive reaction*':ab,ti OR 'orosensorial antinociceptive effect*':ab,ti OR 'oxygen consumption':ab,ti OR 'oxygen saturation*':ab,ti OR pain*:ab,ti OR panic*:ab,ti OR 'sleep wake state*':ab,ti OR wakefulness:ab,ti 7940246
#17 #14 OR #15 OR #16 [Broader pain terms] 7940246
#18 'narcotic analgesic agent'/exp OR 'morphine derivative'/exp 400596
#19 opioid*:ab,ti OR opiat*:ab,ti 164723
#20 'fentanyl'/exp OR 'alfentanil'/exp OR 'sufentanil'/exp OR 'pethidine'/exp OR 'codeine'/exp OR 'remifentanil'/exp OR 'piperidine'/exp 125016
#21 'sulfentanil':ab,ti OR 'morphine':ab,ti OR 'remifentanil':ab,ti OR 'piperidines':ab,ti OR 'opioid*':ab,ti OR 'analgesi*':ab,ti OR 'fentanyl':ab,ti OR 'alfentanil':ab,ti OR 'sufentanil':ab,ti OR 'diamorphine':ab,ti OR 'meperidine':ab,ti OR 'pethidine':ab,ti OR 'codeine':ab,ti OR remifentanil:ab,ti 345819
#22 #18 OR #19 OR #20 OR #21 [Opioids]
551529
#23 'newborn'/de OR 'prematurity'/de 697816
#24 infant:ti,ab,kw OR infants:ti,ab,kw OR infant$:ti,ab,kw OR infantile:ti,ab,kw OR infancy:ti,ab,kw OR newborn*:ti,ab,kw OR 'new born':ti,ab,kw OR 'new borns':ti,ab,kw OR 'newly born':ti,ab,kw OR neonat*:ti,ab,kw OR baby*:ti,ab,kw OR babies:ti,ab,kw OR premature:ti,ab,kw OR prematures:ti,ab,kw OR prematurity:ti,ab,kw OR preterm:ti,ab,kw OR preterms:ti,ab,kw OR 'pre term':ti,ab,kw OR preemie:ti,ab,kw OR preemies:ti,ab,kw OR premies:ti,ab,kw OR 'low birth weight':ti,ab,kw OR 'low birthweight':ti,ab,kw OR vlbw:ti,ab,kw OR lbw:ti,ab,kw OR elbw:ti,ab,kw OR nicu:ti,ab,kw 1184493
#25 #23 OR #24 [Neonates] 1443525
#26 'randomized controlled trial'/de OR 'controlled clinical trial'/de 864345
#27 random*:ti,ab,kw 1737121
#28 'randomization'/de 92395
#29 placebo:ti,ab,kw 334732
#30 ((double OR single OR doubly OR singly) NEAR/2 (blind OR blinded OR blindly)):ti,ab,kw 254710
#31 'double blind procedure'/de 190961
#32 (controlled NEAR/7 (study OR design OR trial)):ti,ab,kw 403354
#33 'parallel group$':ti,ab 28482
#34 crossover:ti,ab OR 'cross over':ti,ab 114093
#35 ((assign* OR match OR matched OR allocation) NEAR/5 (alternate OR group$ OR intervention$ OR patient$ OR subject$ OR participant$)):ti,ab 367630
#36 (open NEAR/2 label):ti,ab 92731
#37 #26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34 OR #35 OR #36 2487408
#38 ('animal'/exp OR 'invertebrate'/exp OR 'animal experiment'/de OR 'animal model'/de OR 'animal tissue'/de OR 'animal cell'/de OR 'nonhuman'/de) AND ('human'/de OR 'normal human'/de OR 'human cell'/de) 224177751
#39 'animal'/exp OR 'invertebrate'/exp OR 'animal experiment'/de OR 'animal model'/de OR 'animal tissue'/de OR 'animal cell'/de OR 'nonhuman'/de 31562444
#40 #39 NOT #38 7384693
#41 #37 NOT #40 2218822
#42 #13 OR #17 14657360
#43 (Procedures OR Pain), broader terms AND opiates AND neonates AND RCTs
##42 AND #22 AND #25 AND #40
2917
#44 #43 AND [embase]/lim NOT ([embase]/lim AND [medline]/lim)
895 records
CINAHLComplete (EbscoHost)
Date of search: 2021‐10‐06, revised 2021‐10‐15, main search 2021‐12‐16
#1 (MH "Surgery, Operative+") 717556
#2 (MH "Perioperative Care+") OR (MH "Perioperative Medicine+") OR (MH "Perioperative Nursing+") 73977
#3 (MH "Catheterization+") 66025
#4 (MH "Catheters") 5479
#5 (MH "Catheter Occlusion") 633
#6 (MH "Catheters, Urinary+") 2226
#7 (MH "Vascular Access Devices+") 8853
#8 (MH "Central Venous Catheters+") 4340
#9 (MH "Chest Tubes") 1391
#10 (MH "Retinopathy of Prematurity") 187
#11 (MH "Spinal Puncture") 2211
#12 TI ( catheter* OR cannula* OR surgery OR surgical* OR retinopathy OR puncture OR needle OR needles OR "heel lanc*" OR heellanc* OR "chest tube" OR "chest tubes” ) OR AB ( catheter* OR cannula* OR surgery OR surgical* OR retinopathy OR puncture OR needle OR needles OR "heel lanc*" OR heellanc* OR "chest tube" OR "chest tubes” ) 499044
#13 TI ( operativ* OR postoperat* OR post‐operat* OR perioperativ* OR peri‐operativ* OR preopera* OR pre‐opera* ) OR AB ( operativ* OR postoperat* OR post‐operat* OR perioperativ* OR peri‐operativ* OR preopera* OR pre‐opera* ) 208080
#14 TI ( presurgi* OR pre‐surgi* ) OR AB ( presurgi* OR pre‐surgi* ) 2714
#15 TI invasive procedure* OR AB invasive procedure* 15387
#16 (MH "Needles") 4945
#17 (MH "Injections, Intravenous") 5638
#18 (MH "Anesthesia, Intravenous") 2207
#19 (MH "Immunoglobulins, Intravenous") 3059
#20 (MH "Administration, Central Venous") 37
#21 (MH "Fat Emulsions, Intravenous") 1323
#22 (MH "Infusions, Intravenous") 11441
#23 TI ( injection* OR intravenous* ) OR AB ( injection* OR intravenous* ) 118093
#24 (MH "Drainage+") 11035
#25 (MH "Suction+") 3557
#26 TI ( suction* OR drainage ) OR AB ( suction* OR drainage ) 17603
#27 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR#17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 [Procedures/surgery] 1127027
#28 (MH "Postoperative Pain") 19303
#29 TI ( "procedural pain" OR pain* procedure* ) OR AB ( "procedural pain" OR pain* procedure* ) 24640
#30 ( (MH "Pain Management") OR (MH "Pain Measurement") OR (MH "Pain Threshold") ) OR MH “Pain+” 243413
#31 (MH "Anxiety+") 53414
#32 (MH "Behavior+") 1078963
#33 (MH "Crying") 1646
#34 (MH "Facial Expression") 4594
#35 (MH "Fear+") 16632
#36 (MH "Heart Rate+") 34830
#37 (MH "Infant Behavior") 3518
#38 (MH "Oxygen Consumption+") 20064
#39 (MH "Wakefulness") 2272
#40 TI ( (anxiet* OR anxious OR behavior* OR behaviour* OR crying OR discomfort* OR distress* OR Douleur Aigue du Nouveau ne OR DAN OR facial expression* OR fear* OR fright* OR gesture* OR grimac* OR heart rate* OR Median Premature Infant Pain Profile score* OR Neonatal Facial Action* OR Neonatal Facial Activity Coding System OR Neonatal Facial Coding Score* OR NFCS OR neonatal facial coding system OR nociceptive reaction* OR orosensorial antinociceptive effect* OR oxygen consumption OR oxygen saturation* OR pain* OR panic* OR sleep wake state* OR wakefulness) ) OR AB ( (anxiet* OR anxious OR behavior* OR behaviour* OR crying OR discomfort* OR distress* OR Douleur Aigue du Nouveau ne OR DAN OR facial expression* OR fear* OR fright* OR gesture* OR grimac* OR heart rate* OR Median Premature Infant Pain Profile score* OR Neonatal Facial Action* OR Neonatal Facial Activity Coding System OR Neonatal Facial Coding Score* OR NFCS OR neonatal facial coding system OR nociceptive reaction* OR orosensorial antinociceptive effect* OR oxygen consumption OR oxygen saturation* OR pain* OR panic* OR sleep wake state* OR wakefulness) ) 828797
#41 #28 OR #29 OR #30 OR #1 OR #32 OR #33 OR #34 OR #35 OR #36 OR #37 OR #38 OR #39 OR #40 [Pain broader terms] 1761993
#42 (MH "Analgesics, Opioid+") 41711
#43 (MH "Morphine+") 18039
#44 TI ( opioid* OR opiate* ) OR AB ( opioid* OR opiate* ) 42595
#45 (MH "Alfentanil") 488
#46 (MH "Sufentanil") 569
#47 (MH "Meperidine") 1033
#48 (MH "Codeine+") 2843
#49 (MH "Remifentanil") 68
#50 (MH "Piperidines+") 4741
#51 TI ( alfentanil OR sufentanil OR morphine OR meperidine OR codeine OR remifentanil OR piperidines OR opioid* OR analgesi* OR fentanyl OR alfentanil OR sufentanil OR diamorphine OR meperidine OR pethidine OR codeine OR remifentanil ) OR AB ( alfentanil OR sufentanil OR morphine OR meperidine OR codeine OR remifentanil OR piperidines OR opioid* OR analgesi* OR fentanyl OR alfentanil OR sufentanil OR diamorphine OR meperidine OR pethidine OR codeine OR remifentanil ) 75473
#52 #42 OR #43 OR #44 OR #45 OR #46 OR #47 OR #48 OR #49 OR #50 [Opioids] 96120
#53 (MH "Infant, Newborn+") 151396
#54 (MH "Intensive Care, Neonatal+") 6260
#55 (MH "Intensive Care Units, Neonatal") 14683
#56 TI ( baby* OR babies OR infant OR infants OR infant s OR infant's OR infantile OR infancy OR low birth weight OR low birthweight OR neonat*OR newborn* OR new born OR new borns OR newly born OR premature OR prematures OR prematurity OR preterm OR preterms OR pre term OR preemie OR preemies OR premies OR premie OR VLBW OR LBW OR ELBW OR NICU ) OR AB ( baby* OR babies OR infant OR infants OR infant s OR infant's OR infantile OR infancy OR low birth weight OR low birthweight OR neonat*OR newborn* OR new born OR new borns OR newly born OR premature OR prematures OR prematurity OR preterm OR preterms OR pre term OR preemie OR preemies OR premies OR premie OR VLBW OR LBW OR ELBW OR NICU ) 209226
#57 #53 OR #54 OR #55 OR #56 [Neonatal terms] 292539
#58 PT randomized controlled trial OR controlled clinical trial 138126
#59 MH drug therapy 16713
#60 TI ( randomi* OR placebo OR randomly OR trial OR groups OR quasirandom* OR quasi‐random* ) OR AB ( randomi* OR placebo OR randomly OR trial OR groups OR quasirandom* OR quasi‐random* ) 1176503
#61 MH animals NOT humans 93609
#62 #57 OR #58 OR #59 1206224
#63 #62 NOT #61 [Randomized studies] 1191318
#64 #27 OR #41 2660650
#65 #64 AND #52 AND #57 AND #63 1189
CENTRAL (via Cochrane Library)
Date of search: 2021‐10‐06, main search 2021‐12‐16
#1 MeSH descriptor: [General Surgery] explode all trees 362
#2 MeSH descriptor: [Surgical Procedures, Operative] explode all trees 125601
#3 MeSH descriptor: [Perioperative Medicine] explode all trees 0
#4 MeSH descriptor: [Perioperative Care] explode all trees 12678
#5 MeSH descriptor: [Perioperative Period] explode all trees 9114
#6 MeSH descriptor: [Perioperative Nursing] explode all trees 129
#7 MeSH descriptor: [Catheterization] explode all trees 9743
#8 MeSH descriptor: [Catheters] this term only 317
#9 MeSH descriptor: [Cannula] explode all trees 145
#10 MeSH descriptor: [Catheter Obstruction] explode all trees 27
#11 MeSH descriptor: [Catheters, Indwelling] explode all trees 1052
#12 MeSH descriptor: [Urinary Catheters] explode all trees 102
#13 MeSH descriptor: [Vascular Access Devices] explode all trees 403 records
#14 MeSH descriptor: [Cardiac Catheters] explode all trees 92
#15 MeSH descriptor: [Central Venous Catheters] explode all trees 178
#16 MeSH descriptor: [Chest Tubes] explode all trees 275
#17 MeSH descriptor: [Retinopathy of Prematurity] explode all trees 406
#18 MeSH descriptor: [Spinal Puncture] explode all trees 302
#19 (catheter* OR cannula* OR surgery OR surgical* OR retinopathy OR puncture OR needle OR needles OR heel lanc* OR heellanc* OR chest tube OR chest tubes):ti,ab 250964
#20 (operativ* OR postoperat* OR post‐operat* OR perioperativ* OR peri‐operativ* OR preopera* OR pre‐opera*):ti,ab 149331
#21 (presurgi* OR pre‐surgi*):ti,ab 1194
#22 invasive procedure*:ti,ab 8539
#23 (injection* OR intravenous*):ti,ab 152121
#24 MeSH descriptor: [Needles] explode all trees 1268
#25 MeSH descriptor: [Injections, Intravenous] explode all trees 7730
#26 MeSH descriptor: [Immunoglobulins, Intravenous] explode all trees
885
#27 MeSH descriptor: [Administration, Intravenous] explode all trees 19060
#28 MeSH descriptor: [Fat emulsions, Intravenous] explode all trees 482
#29 MeSH descriptor: [Anesthetics, Intravenous] explode all trees 3710
#30 MeSH descriptor: [Infusions, Intravenous] explode all trees
10495
#31 MeSH descriptor: [Drainage] explode all trees 2967
#32 MeSH descriptor: [Suction] explode all trees 942
#33 (drainage OR suction*):ti,ab 12210
#34 #1 OR #2 OR # OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33. [Procedures/surgery]
458669
#35 MeSH descriptor: [Pain, Postoperative] explode all trees 16592
#36 (procedural pain OR pain* procedure*):ti,ab 27343
#37 MeSH descriptor: [Pain] explode all trees 52513
#38 MeSH descriptor: [Pain Management] explode all trees 4186
#39 MeSH descriptor: [Pain Measurement] explode all trees 22446
#40 MeSH descriptor: [Pain Threshold] explode all trees 1770
#41 MeSH descriptor: [Anxiety] explode all trees 8729
#42 MeSH descriptor: [Crying] explode all trees 329
#43 MeSH descriptor: [Facial Expression] explode all trees 689
#44 MeSH descriptor: [Fear] explode all trees 1633
#45 MeSH descriptor: [Gestures] explode all trees 70
#45 MeSH descriptor: [Heart Rate] explode all trees 19818
#47 MeSH descriptor: [Infant Behavior] explode all trees 341
#48 MeSH descriptor: [Oxygen Consumption] explode all trees 6864
#49 MeSH descriptor: [Panic] explode all trees 265
#50 MeSH descriptor: [Wakefulness] explode all trees 1037
#51 (anxiet* OR anxious OR behavior* OR behaviour* OR crying OR discomfort* OR distress* OR Douleur Aigue du Nouveau ne OR DAN OR facial expression* OR fear* OR fright* OR gesture* OR grimac* OR heart rate* OR Median Premature Infant Pain Profile score* OR Neonatal Facial Action* OR Neonatal Facial Activity Coding System OR Neonatal Facial Coding Score* OR NFCS OR neonatal facial coding system OR nociceptive reaction* OR orosensorial antinociceptive effect* OR oxygen consumption OR oxygen saturation* OR pain* OR panic* OR sleep wake state* OR wakefulness):ti,ab 397438
#52 #35 OR #36 OR #37 OR #38 OR #39 OR #40 OR #41 OR #42 OR #43 OR #44 OR #45 OR #46 OR #47 OR #48 OR #49 OR #50 OR #51 [Pain broader terms]
407054
#53 MeSH descriptor: [Analgesics, Opioid] explode all trees 8127
#54 MeSH descriptor: [Morphine Derivatives] explode all trees 7350
#55 (opioid* OR opiate*):ti,ab 23883
#56 MeSH descriptor: [Fentanyl] explode all trees 5691
#57 MeSH descriptor: [Alfentanil] explode all trees 702
#58 MeSH descriptor: [Sufentanil] explode all trees 971
#59 MeSH descriptor: [Meperidine] explode all trees 1170
#60 MeSH descriptor: [Codeine] explode all trees 1787
#61 MeSH descriptor: [Remifentanil] explode all trees 1821
#62 MeSH descriptor: [Piperidines] explode all trees 18783
#63 (alfentanil OR sulfentanil OR morphine OR meperidine OR codeine OR remifentanil OR piperidines OR opioid* OR analgesi* OR fentanyl OR alfentanil OR sufentanil OR diamorphine OR meperidine OR pethidine OR codeine OR remifentanil):ti,ab 80253
#64 #53 OR #54 OR #55 OR #56 OR#57 OR #58 OR #59 OR #60 OR #61 OR #62 OR #63 [Opioids] 93185
#65 MeSH descriptor: [Infant, Newborn] explode all trees 16999
#66 MeSH descriptor: [Intensive Care, Neonatal] explode all trees 347
#67 MeSH descriptor: [Intensive Care Units, Neonatal] explode all trees 821
#68 (baby* OR babies OR infant OR infants OR infant s OR infant's OR infantile OR infancy OR low birth weight OR low birthweight OR neonat*OR newborn* OR new born OR new borns OR newly born OR premature OR prematures OR prematurity OR preterm OR preterms OR pre term OR preemie OR preemies OR premies OR premie OR VLBW OR LBW OR ELBW OR NICU):ti,ab 74934
#69 #65 OR #66 OR #67 OR #68 [Neonatal terms]
79862
#71 (#34 OR #52) AND #64 AND #69 [Procedures AND Pain AND Opiates AND Neonates] 2720
2548 Trials
In total 8771 records in EndNote before deduplication, after deduplication 6919.
**********************************************************************************
Ongoing studies/conference abstracts
Clinicaltrials.gov
Date of search: 2021‐12‐17
Advanced search
Condition or disease: procedural pain
Filter: 0 years
22 records
ICTRP
(procedural pain) AND (neonate* OR newborn* OR infant*)
Recruitment status: All
34 records for 33 trials
Eastern Society for Pediatric Research ESPR 2018
easternspr.org/wp‐content/uploads/2018/09/2018ESPR_Programv6.pdf
Pdf file with conference presentations
Search Cmd+F “pain” 5 hits, no relevant records, OR “procedural pain”‐ no records.
Eastern Society for Pediatric Research ESPR 2019
https://plan.core‐apps.com/espr2019/search?query=pain&type=abstracts
Search for “pain”
10 results, one about procedural pain but not opioids
Pediatric Academic Societies PAS
https://virtual2021.pas-meeting.org/searchGlobal.asp
Search procedural pain‐ 6 results, some relevant, see screenshot below
Records from 2017 available as csv/txt file, but not 2016, 2018 or 2019.
Two records mention procedural pain, but these are not opioid studies
Record number 2694429 and 2703014
Appendix 2. Risk of bias tool
We will use the standard methods of Cochrane and Cochrane Neonatal to assess the methodological quality of the trials. For each trial, we will seek information regarding the method of randomization, blinding, and reporting of all outcomes of all the infants enrolled in the trial. We will assess each criterion as being at low, high, or unclear risk of bias. Two review authors will separately assess each study. Any disagreements will be resolved by discussion. We will add this information to the 'Characteristics of included studies' table. We will evaluate the following issues and enter the findings into the risk of bias table.
Sequence generation (checking for possible selection bias). Was the allocation sequence adequately generated?
For each included study, we will categorize the method used to generate the allocation sequence as being at:
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number); or
unclear risk of bias.
Allocation concealment (checking for possible selection bias). Was allocation adequately concealed?
For each included study, we will categorize the method used to conceal the allocation sequence as being at:
low risk of bias (e.g. telephone or central randomization; consecutively numbered, sealed, opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth); or
unclear risk of bias.
Blinding of participants and personnel (checking for possible performance bias). Was knowledge of the allocated intervention adequately prevented during the study?
For each included study, we will categorize the methods used to blind study participants and personnel from the knowledge of which intervention a participant had received. We will assess blinding separately for different outcomes or classes of outcomes. We will categorize the methods as being at:
low, high, or unclear risk of bias for participants; and
low, high, or unclear risk of bias for personnel.
Blinding of outcome assessment (checking for possible detection bias). Was knowledge of the allocated intervention adequately prevented at the time of outcome assessment?
For each included study, we will categorize the methods used to blind outcome assessment. We will assess blinding separately for different outcomes or classes of outcomes. We will categorize the methods as being at:
low risk of bias for outcome assessors;
high risk of bias for outcome assessors; or
unclear risk of bias for outcome assessors.
Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations). Were incomplete outcome data adequately addressed?
For each included study and for each outcome, we will describe the completeness of data, including attrition and exclusions from the analysis. We will note whether attrition and exclusions were reported, the numbers included in the analysis at each stage (compared with the total randomized participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information is reported or supplied by the trial authors, we will re‐include missing data in the analyses. We will categorize the methods as being at:
low risk of bias (less than 20% missing data);
high risk of bias (20% or more missing data); or
unclear risk of bias.
Selective reporting bias. Are reports of the study free of the suggestion of selective outcome reporting?
For each included study, we will describe how we investigated the possibility of selective outcome reporting bias and what we found. For studies in which study protocols were published in advance, we will compare the prespecified outcomes versus outcomes reported in the published results. If the study protocol was not published in advance, we will contact study authors to gain access to the study protocol. We will assess the methods as being at:
low risk of bias (where it is clear that all of the study's prespecified outcomes and all expected outcomes of interest to the review have been reported);
high risk of bias (where not all of the study's prespecified outcomes have been reported; one or more reported primary outcomes were not prespecified outcomes of interest and are reported incompletely and so cannot be used; the study fails to include results of a key outcome that would be expected to have been reported); or
unclear risk of bias.
Other sources of bias. Did the study appear to be free of other problems that could put it at high risk of bias?
For each included study, we will describe any important concerns we had about other possible sources of bias (e.g. whether there was a potential source of bias related to the specific study design or whether the trial was stopped early due to some data‐dependent process). We will assess whether each study was at:
low risk of other bias;
high risk of other bias;
unclear risk of other bias.
If needed, we plan to explore the impact of the level of bias by undertaking sensitivity analyses.
Data and analyses
Comparison 1. Opioids versus no treatment/placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 PIPP/PIPP‐R during procedure | 3 | 199 | Mean Difference (IV, Fixed, 95% CI) | ‐2.58 [‐3.12, ‐2.03] |
| 1.2 PIPP/PIPP‐R up to 30 min after procedure | 2 | 123 | Mean Difference (IV, Fixed, 95% CI) | 0.14 [‐0.17, 0.45] |
| 1.3 PIPP/PIPP‐R 1 to 2 hours after procedure | 2 | 54 | Mean Difference (IV, Fixed, 95% CI) | ‐0.83 [‐2.42, 0.75] |
| 1.4 DAN 1 to 2 hours after procedure | 1 | 42 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐2.21, 1.81] |
| 1.5 NIPS during procedure | 2 | 102 | Mean Difference (IV, Fixed, 95% CI) | ‐1.97 [‐2.46, ‐1.48] |
| 1.6 Mortality | 2 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 1.7 Episodes of bradycardia | 3 | 172 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.19 [0.14, 72.69] |
| 1.8 Episodes of desaturation | 3 | 199 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.82 [0.72, 4.58] |
| 1.9 Episodes of apnea | 3 | 199 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.15 [1.08, 9.16] |
| 1.10 Hypotension | 2 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
Comparison 2. Opioids versus oral sweet solution or non‐pharmacological intervention.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 CRIES | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1.1 Opioids vs facilitated tucking | 1 | 100 | Mean Difference (IV, Fixed, 95% CI) | ‐4.62 [‐6.38, ‐2.86] |
| 2.1.2 Opioids vs sensorial stimulation | 1 | 100 | Mean Difference (IV, Fixed, 95% CI) | 0.32 [‐1.13, 1.77] |
Comparison 3. Opioids versus other analgesics.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 PIPP/PIPP‐R during procedure | 2 | 124 | Mean Difference (IV, Fixed, 95% CI) | ‐0.29 [‐1.58, 1.01] |
| 3.2 PIPP/PIPP‐R up to 30 min after procedure | 1 | 12 | Mean Difference (IV, Fixed, 95% CI) | ‐1.10 [‐2.82, 0.62] |
| 3.3 PIPP/PIPP‐R 1 to 2 hours after procedure | 1 | 12 | Mean Difference (IV, Fixed, 95% CI) | ‐0.17 [‐2.22, 1.88] |
| 3.4 Episodes of apnea | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.4.1 During the procedure | 2 | 124 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.27 [0.85, 12.58] |
| 3.4.2 After the procedure | 2 | 124 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.71 [0.11, 64.96] |
| 3.5 Hypotension | 3 | 204 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.34 [0.32, 5.59] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Carbajal 2005.
| Study characteristics | ||
| Methods | Randomized, double‐blind, multicenter (16 participating NICUs), placebo‐controlled trial | |
| Participants | 42 neonates born at 23 to 32 weeks of gestation, intubated before 72 hours of age and ventilated for < 8 hours at inclusion. Exclusion criteria: congenital anomalies, birth asphyxia, intrauterine growth retardation, maternal opioid addiction and participation in other clinical trials. Morphine group (n = 21; mean GA 27.2 SD 1.7 weeks; mean BW 947 SD 269 g) 5% dextrose (placebo) group (n = 21; mean GA 27.3 SD 1.8 weeks; mean BW 972 SD 270 g) | |
| Interventions | Morphine group: 100 μg/kg loading dose, infused intravenously in 1 hour and 10 to 30 μg/kg/h continuous infusion (intravenously) 5% dextrose (placebo) group (n = 21; mean GA 27.3 SD 1.8 weeks; mean BW 972 SD 270 g) | |
| Outcomes | Pain assessment by DAN and PIPP‐R scale during heel stick before the loading dose, the heel stick 2 to 3 hours after the loading dose, and the heel stick after 20 to 28 hours of morphine infusion | |
| Funding sources | Grant funds from the Fondation CNP (Paris, France) and National Institute for Child Health and Human Development grants HD36484 and HD36270 | |
| Declaration of interest among the primary researchers | No conflict of interest declared. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization to the morphine and placebo groups occurred with an automated telephone response system located in the United States, followed by faxed confirmation of the coded treatment assignment to the NICU and the hospital pharmacy. Neonates were randomized to 8 study drug codes, with 4 codes each for the morphine and placebo groups." |
| Allocation concealment (selection bias) | Low risk | Quote: "parents of each enrolled infant. Assignment Randomization to the morphine and placebo groups occurred with an automated telephone response system located in the United States, followed by faxed confirmation of the coded treatment assignment to the NICU and the hospital pharmacy. Neonates were randomized to 8." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Physicians and nurses in charge of neonates were blinded to the treatments received by the patients. Study drug syringes were dispensed by a research pharmacist who did not participate in the routine care of neonates." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Pain assessments were conducted by an independent observer, who did not participate in the procedure" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes are reported for all randomized infants—see Fig 1. Trial profile and participant flow. |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available. |
| Other bias | Low risk | None |
Cordero 1991.
| Study characteristics | ||
| Methods | Randomized controlled trial | |
| Participants | 29 infants whose birthweight ranged from 600 to 1350 g No inclusion and exclusion criteria defined. Fentanyl group (n = 15; mean GA 28 SD 2 weeks; mean BW 953 SD 205 g) Secobarbital group (n = 14; mean GA 27 SD 2 weeks; mean BW 931 SD 209 g) | |
| Interventions | Fentanyl group: 2 μg/kg single dose (intravenously) Secobarbital group: 1 mg/kg single dose (intravenously) | |
| Outcomes | Heart rate, oxygen saturation, blood pressure, blood glucose, plasma catecholamines, duration of procedure | |
| Funding sources | Not reported | |
| Declaration of interest among the primary researchers | Not reported | |
| Notes | Quote: "Local anesthesia was accomplished by infiltrating 5 mg/kg of lidocaine 1% subcutaneously in three to four divided doses" | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "After informed consent was obtained from the parents, infants were randomly assigned to receive either fentanyl 2 µg/kg or secobarbital 1 mg/kg intravenously." |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not reported, but outcomes were all lab or monitor values (objective) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes are reported for all randomized infants—see Table 1. |
| Selective reporting (reporting bias) | Unclear risk | No protocol available. |
| Other bias | Low risk | None |
Fallah 2016.
| Study characteristics | ||
| Methods | Randomized controlled trial | |
| Participants | 45 neonates with gestational age of more than 34 weeks, birthweight of more than 1800 g, and those who underwent lumbar puncture based on clinical judgement of the neonatologist of research. Exclusion criteria: major congenital anomalies, severe hypoxic ischemic encephalopathy, neuromuscular disorders, hemodynamic or respiratory instability, aged more than 2 months, decrease in the level of consciousness, used sedative or analgesic drugs 12 h before lumbar puncture. Fentanyl group (n = 23; mean GA 37.4 SD 0.8 weeks; BW not reported) Saline (placebo) group (n = 22; mean GA 37.6 SD 0.6 weeks; BW not reported) | |
| Interventions | Fentanyl group: 2 μg/kg single dose (intravenously) Normal saline (placebo) group: 0.2 mL | |
| Outcomes | NIPS during needle insertion to skin, clinical side effects, serious adverse events | |
| Funding sources | This study was funded by a grant from the Deputy for Research of Shahid Sadoughi University of Medical Sciences, Yazd, Iran. | |
| Declaration of interest among the primary researchers | The researchers received no financial support from the drug company. The authors declare that there are no conflicts of interest. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Simple randomization of the study was computer generated by random numbers and allocation ratio was 1:1 for the three groups." |
| Allocation concealment (selection bias) | Low risk | Quote: "A trained NICU nurse was in charge of allocating each neonate in the randomized treatment group, and she guaranteed that the two preparations would not differentiate." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "A trained NICU nurse was in charge of allocating each neonate in the randomized treatment group, and she guaranteed that the two preparations would not differentiate." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Data gatherers, outcome assessors and data analysts were all allocation blinded." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes are reported for all randomized infants—see Fig 1. CONSORT 2010 Flow Diagram. |
| Selective reporting (reporting bias) | Low risk | Registration number 2014022616761N150 |
| Other bias | Low risk | None |
Gitto 2012.
| Study characteristics | ||
| Methods | Randomized controlled trial | |
| Participants | 150 newborns with gestational age from 27 to 32 weeks. Exclusion criteria: the presence of an infectious disease, congenital malformations of the brain, or inborn errors of metabolism. Fentanyl group (n = 50; mean GA not reported; BW not reported) Facilitated tucking group (n = 50; mean GA not reported; BW not reported) Sensorial stimulation group (n = 50; mean GA not reported; BW not reported) | |
| Interventions | Fentanyl group: 1 to 2 μg/kg bolus injection 2 min prior to every painful heel lance, single dose (intravenously) Facilitated tucking group: holding the infant with extremities flexed and close to trunk Sensorial stimulation group: the use of various non‐painful stimuli, is based on competition of various gentle stimuli, given during the painful event | |
| Outcomes | CRIES score during painful procedure, level of cytokines (IL6, IL8, and TNF‐α) | |
| Funding sources | Not reported | |
| Declaration of interest among the primary researchers | The authors declare that they have no conflicts of interest. Authors have no financial relationship with the organization that sponsored the research. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "The primary outcome of the study was to evaluate the utility of three different pharmacological or non‐pharmacological treatments to alleviate the procedural pain due to heel‐lances performed within 2 days of birth." Not possible to blind due to the nature of the interventions |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported |
| Selective reporting (reporting bias) | Unclear risk | No protocol available. |
| Other bias | Low risk | None |
Hartley 2018.
| Study characteristics | ||
| Methods | Blinded randomized placebo‐controlled trial | |
| Participants | 31 neonates born prematurely at less than 32 weeks’ gestation or with a birthweight less than 1501 g. Inclusion criteria: infants who were inpatients at the time of the study and aged 34 to 42 weeks’ gestation, and required a heel lance and ROP screening on the same test occasion. Exclusion criteria: grade 3 or 4 intraventricular hemorrhages, short bowel syndrome, infants were nil by mouth, congenital malformations or genetic conditions known to affect neurodevelopment, neonates who received analgesics or sedatives in the previous 24 hours or opiates in the previous 72 hours, had a documented opiate sensitivity, or were born to mothers who regularly used opiates during pregnancy or while breastfeeding or expressing breast milk. No infants were mechanically ventilated at the time of study. Morphine group (n = 15; median GA 28.1 IQR 26.3 to 30.1 weeks; BW 1107 SD 329 g) Placebo group (n = 15; median GA 28.1 IQR 26.3 to 30.1 weeks; BW 1107 SD 329 g) | |
| Interventions | Morphine group: 100 μg/kg single dose (orally) Placebo group: single dose (orally) | |
| Outcomes | PIPP‐R 30 s after ROP examination, EEG (noxious evoked brain activity), withdrawal, PIPP‐R after heel lance, episodes of oxygen desaturation, bradycardia, tachycardia, apnea, requirements for increase in respiratory support | |
| Funding sources | Wellcome Trust and National Institute for Health Research—the funder had no role in study design, data collection, data analysis, data interpretation, or writing of the report | |
| Declaration of interest among the primary researchers | Authors declare no competing interests. | |
| Notes | Quote: "Trial recruitment prematurely stopped due to adverse effects. Topical local anaesthetic (proxymetacaine 0·5%) eye drops were instilled before starting the ROP screening" | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "We randomised infants to receive either morphine or placebo, using a webbased facility hosted by the NPEU CTU" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Morphine sulphate (at a concentration of 200 µg/mL) and placebo solutions were indistinguishable by colour, odour, and flow, and were dispensed in 10 mL glass amber bottles with tamperevident caps and a pack identification label (appendix). Researchers, clinicians, outcome assessors, and parents were masked to treatment allocation" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Morphine sulphate (at a concentration of 200 µg/mL) and placebo solutions were indistinguishable by colour, odour, and flow, and were dispensed in 10 mL glass amber bottles with tamperevident caps and a pack identification label (appendix). Researchers, clinicians, outcome assessors, and parents were masked to treatment allocation" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes are reported for all randomized infants—see Fig 1: Trial profile. |
| Selective reporting (reporting bias) | Low risk | Quote: "This trial is registered with the European Clinical Trials Database (number 2014‐003237‐25)" |
| Other bias | Low risk | None |
Lago 2008.
| Study characteristics | ||
| Methods | Randomized, double‐blind, controlled trial | |
| Participants | 54 preterm infants ≤ 32 gestational age needing PICC during their first 2 weeks of life were eligible for the study. Exclusion criteria: major congenital anomalies, perinatal asphyxia, severe intracerebral hemorrhage, neurological disorders, sepsis, or concomitant infusion of other opioids, sedatives, or neuromuscular blockers. Remifentanil group (n = 27; mean GA 28 SD 2 weeks; BW 1108 SD 371 g) 5% dextrose (placebo) group (n = 27; mean GA 29 SD 2 weeks; BW 1144 SD 307 g) | |
| Interventions | Remifentanil group: 0.03 μg/kg/min continuous infusion (intravenously) 5% dextrose (placebo) continuous infusion (intravenously) | |
| Outcomes | NIPS and PIPP (scored before procedure, during skin preparation, needle insertion, and recovery phase lasting 15 min after completing the maneuver), changes in oxygen demand, RR, HR, SaO², blood pressure, body movements | |
| Funding sources | Not reported | |
| Declaration of interest among the primary researchers | Not reported | |
| Notes | Quote: "All enrolled infants also received 0.3 ml of a 12% sucrose solution (0.036 g) per os and non‐nutritive sucking 2 min before the procedure" | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The infants were randomized sequentially, using a random numbers table" |
| Allocation concealment (selection bias) | Low risk | Quote: "A single pharmacist responsible for allocating each neonate to the randomized treatment group also ensured that the two preparations were indistinguishable" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "A single pharmacist responsible for allocating each neonate to the randomized treatment group also ensured that the two preparations were indistinguishable" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "a digital camera and the PIPP and NIPS scores were awarded independently and blindly by two researchers unaware of which infants received the treatment" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes are reported for all randomized infants—see Trial profile. |
| Selective reporting (reporting bias) | Unclear risk | No protocol available. |
| Other bias | Low risk | None |
Madathil 2021a.
| Study characteristics | ||
| Methods | Open‐label randomized trial. In the second part of this study, higher doses were used; see Madathil 2021b. | |
| Participants | 97 neonates Inclusion criteria: hemodynamically stable infants with type 1 ROP requiring laser photocoagulation. Exclusion criteria: anemia, grade III to IV intraventricular hemorrhage, congenital malformations, patent ductus arteriosus or necrotizing enterocolitis and the anticipated duration of procedure was more than 30 min; infants receiving respiratory support and/or admitted to NICU. Fentanyl group (n = 51; mean GA 29.7 SD 1.9 weeks; mean BW 1227.8 280 g) Ketamine group (n = 46; mean GA 29.8 SD 1.5 weeks; mean BW 1202.9 SD 254 g) | |
| Interventions | Fentanyl group: 2 μg/kg bolus over 5 min, 15 min prior to the procedure, followed by a continuous infusion of 1 μg/kg/h increased to a maximum of 3 µg/kg/h (intravenously) Ketamine group: 0.5 mg/kg bolus 1 min prior to the procedure, followed by further intermittent bolus doses of 0.5 mg/kg given every 10 min to a maximum of 2 mg/kg | |
| Outcomes | PIPP‐R scores measured every 15 min less than 7, proportion of the procedure time the infant spent crying less than 5% apnea during and postprocedure, need for supplemental oxygen during and postprocedure, change in mean cardiorespiratory stability scores requiring up‐gradation of respiratory support, hemodynamic instability requiring fluid boluses or vasoactive support, feed intolerance, urinary retention, need for NICU admission for 24 hours or longer | |
| Funding sources | The authors have not declared a specific grant for this research from any funding agency in the public, commercial, or not‐for‐profit sectors. | |
| Declaration of interest among the primary researchers | None declared. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Low risk | Quote: "We used serially numbered, sealed and opaque envelopes for allocation concealment. The unit nurse opened the envelope and assigned the infant to a group" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unblinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "the outcome assessors were blinded to the groups" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes are reported for all randomized infants—see Fig 1. |
| Selective reporting (reporting bias) | Low risk | Quote: "Trial registration number CTRI/2018/03/012878" |
| Other bias | Unclear risk | Quote: "Small number of infants enrolled in the second phase of the study due to logistical constraints as we had to stop the trial after achieving the target sample size." "Accordingly, the study steering committee recommended revision of the regimens: higher dose of intravenous fentanyl (intravenous bolus dose of 2 µg/ kg followed by an intravenous infusion of 2 µg/kg/ hour to maximum of 5 µg/kg/hour) and intravenous ketamine (bolus dose of 1 mg/kg followed by intermit‐ tent bolus doses of 0.5 mg/kg to a maximum of 4 mg/kg) were recommended. Subsequently, we enrolled 27 more infants (13 in fentanyl group and 14 in ketamine group). The results are described separately as initial phase and revised regimen phase" |
Madathil 2021b.
| Study characteristics | ||
| Methods | Open‐label randomized trial. In the first part of this study, lower doses were used; see Madathil 2021a. | |
| Participants | 27 neonates Inclusion criteria: hemodynamically stable infants with type 1 ROP requiring laser photocoagulation. Exclusion criteria: anemia, grade III to IV intraventricular hemorrhage, congenital malformations, patent ductus arteriosus or necrotizing enterocolitis and the anticipated duration of procedure was more than 30 min; infants receiving respiratory support and/or admitted to NICU. Fentanyl group (n = 13; mean GA 30.3 SD 1.3 weeks; mean BW 1281.6 SD 267 g) Ketamine group (n = 14; mean GA 30.5 SD 2.4 weeks; mean BW 1301.0 SD 338 g) | |
| Interventions | Fentanyl group: 2 μg/kg bolus, followed by a continuous infusion of 2 μg/kg/h increased to a maximum of 5 µg/kg/h (intravenously) Ketamine group: 1 mg/kg bolus, followed by further intermittent bolus doses of 0.5 mg/kg given to a maximum of 4 mg/kg (intravenously) | |
| Outcomes | PIPP‐R scores measured every 15 min less than 7, proportion of the procedure time the infant spent crying less than 5% apnea during and postprocedure, need for supplemental oxygen during and postprocedure, change in mean cardiorespiratory stability scores requiring up‐gradation of respiratory support, hemodynamic instability requiring fluid boluses or vasoactive support, feed intolerance, urinary retention, need for NICU admission for 24 hours or longer | |
| Funding sources | The authors have not declared a specific grant for this research from any funding agency in the public, commercial, or not‐for‐profit sectors. | |
| Declaration of interest among the primary researchers | None declared. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Low risk | Quote: "We used serially numbered, sealed and opaque envelopes for allocation concealment. The unit nurse opened the envelope and assigned the infant to a group" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unblinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "the outcome assessors were blinded to the groups" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes are reported for all randomized infants—see Fig 1. |
| Selective reporting (reporting bias) | Low risk | Quote: "Trial registration number CTRI/2018/03/012878" |
| Other bias | Unclear risk | Quote: "Small number of infants enrolled in the second phase of the study due to logistical constraints as we had to stop the trial after achieving the target sample size." "Accordingly, the study steering committee recommended revision of the regimens: higher dose of intravenous fentanyl (intravenous bolus dose of 2 µg/ kg followed by an intravenous infusion of 2 µg/kg/ hour to maximum of 5 µg/kg/hour) and intravenous ketamine (bolus dose of 1 mg/kg followed by intermit‐ tent bolus doses of 0.5 mg/kg to a maximum of 4 mg/kg) were recommended. Subsequently, we enrolled 27 more infants (13 in fentanyl group and 14 in ketamine group). The results are described separately as initial phase and revised regimen phase" |
Manjunatha 2009.
| Study characteristics | ||
| Methods | Double‐blind randomized controlled trial | |
| Participants | 18 neonates undergoing ROP screening Only information was inclusion criteria: GA of 31 weeks or younger. Exclusion criteria: not reported. Morphine group (n = 6; mean GA not reported; mean BW not reported) Paracetamol group (n = 6; mean GA not reported; mean BW not reported) Placebo group (n = 6; mean GA not reported; mean BW not reported) | |
| Interventions | Morphine group: 200 μg/kg single dose, orally Paracetamol group: 20 mg/kg single dose, orally Placebo group: not reported | |
| Outcomes | PIPP (5 minutes prior, 5, 30, 60, and 120 minutes postprocedure), apnea, gastrointestinal side effects, oxygen requirements for 24 hours after the screening | |
| Funding sources | Not reported | |
| Declaration of interest among the primary researchers | Not reported | |
| Notes | Quote: "The trial was stopped prematurely not reaching the number of infants needed for power." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Low risk | Quote: "Blinded randomisation was done by picking up consecutive envelopes, providing a random allocation of patients to these groups" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "The infant’s face, saturation monitor and time frame cards were recorded on videotape over a 1‐2 minute period, at five minutes before, then at five minutes, 30 minutes, one hour, two hour and three hours after the procedure. Two separate individuals subsequently scored the information independently. Babies’ details were recorded on a pro‐forma. As the recording time was between 1‐2 minutes, the observer chose the first 30‐second period per time frame for analysis" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes are reported for all randomized infants—see Table 2. |
| Selective reporting (reporting bias) | Low risk | Protocol not available. |
| Other bias | Low risk | None |
Pokela 1994.
| Study characteristics | ||
| Methods | Randomized and placebo‐controlled study | |
| Participants | 84 mechanically ventilated distressed neonates Inclusion criteria: need for sedation or analgesia, respiratory distress, age < 1 week, newborns hypoxemia during previous nursing care. Exclusion criteria: newborns with fatal anomalies. Meperidine group (n = 42; mean GA 31.6 range 25 to 40 weeks; mean BW 1700 range 810 to 4120 g) 0.9% saline group (n = 42; mean GA 32.9 range 24 to 41 weeks; mean BW 2180 range 670 to 4260 g) | |
| Interventions | Meperidine group: 1 mg/kg single dose (intravenously) administered intravenously 15 minutes before tracheal suction or routine nursing care (weighing, washing, temperature measurement, chest X‐ray) 0.9% saline group: single dose (intravenously) administered intravenously 15 minutes before tracheal suction or routine nursing care (weighing, washing, temperature measurement, chest X‐ray) | |
| Outcomes | Duration of hypoxemia, arterial blood pressure, heart rate, or plasma beta‐endorphin, cortisol, and glucose concentration during treatment procedures, Behavioral Pain Score assessed during 2‐hour study period | |
| Funding sources | This work was supported by the Foundation for Paediatric Research in Finland, Helsinki and the Alma och K. A. Sneilman Foundation, Oulu, Finland. | |
| Declaration of interest among the primary researchers | Not reported | |
| Notes | Quote: "Outcome data were mostly not separated for tracheal suction and routine nursing care" | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "from identically numbered ampules. The randomization was performed in advance using numbers taken from a randomization table by the independent person. Mependine or saline was administered" |
| Allocation concealment (selection bias) | Low risk | Quote: "The randomization was performed in advance using numbers taken from a randomization table by the independent person" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Eighty‐four patients were randomized into groups blindly receiving either I mg/kg mependine (meperidine group, n = 42) or 0.9% saline (saline group, n = 42) from identically numbered ampules. The randomization was performed in advance" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "together with measured cardiorespiratory changes. Pain during the 2‐hour study period was scored by the researcher in terms of the behavioral pain score (Table 2), without knowing which of the patients had been given meperidine. Pain reactions TABLE 2. Behavioral Pain" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes are reported for all randomized infants—see Fig 1. |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available. |
| Other bias | Unclear risk | Quote: "The pain score used here is modified from the previously presented pain scales24’ such that it could be useful with intubated neonates, but it has many limitations and needs to be improved further and validated" |
Sethi 2020.
| Study characteristics | ||
| Methods | Open‐label parallel randomized clinical trial | |
| Participants | 58 spontaneously breathing preterm infants undergoing laser for ROP Exclusion criteria: neonates with gross congenital malformations, on anticonvulsants/sedatives prior to the procedure, and cholestasis. Fentanyl group (n = 29; mean GA 30.3 SD 2.2 weeks; mean BW 1347 SD 291 g) Sucrose group (n = 29; mean GA 30.3 SD 2.4 weeks; mean BW 1321 SD 275 g) | |
| Interventions | Fentanyl infusion: 1 μg/kg/hour, infusion started 15 min prior to the procedure, and the infusion was continued till the procedure was over Sucrose group 24% single dose, infusion started 15 min prior to the procedure, and the infusion was continued till the procedure was over | |
| Outcomes | Proportion of time spent crying, salivary cortisol levels before, immediately after, and 12 to 24 hours after procedure, PIPP‐R score every 10 min during the procedure, proportion of infants requiring mechanical ventilation in the first 24 hours postprocedure, proportion of infants having feeding intolerance and urinary retention in the first 24 hours postprocedure | |
| Funding sources | Not reported | |
| Declaration of interest among the primary researchers | The authors declare that they have no conflicts of interest. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "We used computer generated random numbers with variable block size (2 to 8) to allocate the neonates into the two groups" |
| Allocation concealment (selection bias) | Low risk | Quote: "An investigator, who did not participate in collecting baseline data, applying the intervention or measurement of outcomes, prepared the randomization sequence. To ensure allocation concealment, random treatment assignment was placed in serially numbered opaque and sealed envelopes" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unblinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "However, the outcome assessor was blinded to the group assignment" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes are reported for all randomized infants—see Fig 2 Trial flow. |
| Selective reporting (reporting bias) | Low risk | Quote: "This trial was registered with Central Trial Registry‐India with registration no. CTRI/2017/07/008977" |
| Other bias | Low risk | None |
Sindhur 2020.
| Study characteristics | ||
| Methods | Double‐blind randomized controlled trial | |
| Participants | 111 neonates with postmenstrual age 30 to 34 weeks, undergoing their first ROP screening Exclusion criteria: neonates who were mechanically ventilated, hemodynamically unstable, and/or sedated, and those with congenital malformations or neurological dysfunction. Fentanyl group (n = 56; mean GA 30.7 SD 1.7 weeks; BW 1409 SD 410 g) Saline (placebo) group (n = 55; mean GA 31.0 SD 1.7 weeks; BW 1537 SD 432 g) | |
| Interventions | Fentanyl group: 2 μg/kg single dose, intranasally Saline (placebo) group: normal saline, intranasally | |
| Outcomes | PIPP‐R during procedure, PIPP‐R at 1 and 5 minutes postprocedure; heart rate, oxygen saturation during the procedure and 5 minutes postprocedure; average duration of procedure, total crying time, and adverse effects of the intervention | |
| Funding sources | None of the authors received any grant for producing the manuscript. | |
| Declaration of interest among the primary researchers | The authors declare that they have no conflicts of interest. | |
| Notes | Quote: "Both groups received oral sucrose 24% at a dose of 0.5 ml and topical ophthalmic analgesic ‐ 0.5% proparacaine." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The random number sequence was generated in variable block sizes of two or four using Stata (version 13.1) software" |
| Allocation concealment (selection bias) | Low risk | Quote: "Allocation concealment was performed using sequentially numbered opaque‐sealed envelopes" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The intervention group received intranasal fentanyl (Verfen, Verve Health care ltd, 50 mcg/ml) at a dose of 2 mcg/kg, diluted with normal saline to a volume of 0.3 ml. The control group received 0.3 ml normal saline intranasally. Both injections were prepared by a senior nurse, who was not involved in the clinical care of the infant. A trained senior resident administered the study preparations by rapid insufflation method into both nostrils using a mucosal atomization device (Teleflex Medical, USA). The study preparations were colorless, odorless, and of the same volume. Clinical neonatologists, ophthalmologists, nurses, and parents were therefore blinded to the intervention" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Two pediatricians who were blinded to the study assignment independently reviewed the videos of the procedure. The PIPP‐R score at baseline (before speculum insertion) and the score during the 30 s time frame following speculum insertion in the right eye was recorded. The PIPP‐R scores at 1 and 5 minutes were assessed in real time by the senior resident" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes are reported for all randomized infants—see Fig 1 Flow diagram of study enrollment process. |
| Selective reporting (reporting bias) | Low risk | Quote: "Clinical Trial Registration CTRI/2017/12/011016" |
| Other bias | Low risk | None |
Taddio 2006.
| Study characteristics | ||
| Methods | Double‐blind, randomized controlled trial | |
| Participants | 132 newborns: the study participants were medically stable neonates who required insertion of a PICC and were receiving ventilatory support in the form of high‐frequency oscillation, conventional ventilation, or continuous positive airway pressure. Exclusion criteria: clinical seizures, concomitant muscle relaxant or inotrope therapy, or skin disorders with visually apparent skin lesions or disruptions in skin integrity. Intravenous morphine group (n = 38; mean GA 29.6 SD 4.9 weeks; BW 1380 SD 900 g) Tetracaine group (n = 42; mean GA 30 SD 5.1 weeks; BW 1450 SD 900 g) Tetracaine and morphine group (n = 31; mean GA 28.9 SD 4.6 weeks; BW 1300 SD 830 g) No analgesia group: not randomized (n = 21; mean GA 27.5 SD 3.4 weeks; BW 900 SD 400 g) | |
| Interventions | Morphine group: 0.1 mg/kg delivered over 20 minutes, single dose, intravenously Tetracaine group: 0.5 g of 4% single dose, gel applied to the insertion site Tetracaine and morphine group: tetracaine: 0.5 g of 4% single dose, gel applied to the insertion site; morphine: 0.1 mg/kg delivered over 20 minutes, single dose, intravenously No analgesia group: not randomized | |
| Outcomes | Facial grimacing (brow bulge) scored in 2‐second intervals for the first 20 seconds of each phase of the procedure, safety assessment (blood pressure and ventilatory support (at 15, 30, and 60 minutes after the beginning of the infusion), local skin reactions) | |
| Funding sources | Funding for this study was provided by the Canadian Society of Hospital Pharmacists. Dr Taddio is supported by the Canadian Institutes of Health Research New Investigator Award. Ms Lee was supported through a studentship by the Ontario Student Opportunity Trust Fund–Hospital for Sick Children Foundation Student Scholarship Program. | |
| Declaration of interest among the primary researchers | The authors did not report any financial disclosures. | |
| Notes | Quote: "Identical‐appearing placebos were available for both tetracaine and morphine (ie, double dummy), so that all neonates received either tetracaine or placebo applied to the insertion site and all neonates received either morphine or placebo infusions prior to the procedure." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "using a computerized random‐number generator" "Concealment of treatment allocation was achieved by having the research pharmacist prepare the randomization assignment before enrollment of neonates using a computerized random‐number generator, stratified by corrected gestational age (30 weeks, 30‐36 weeks, or 36 weeks) and in random block sizes of 6 or 9, with an equal probability of being allocated to each active treatment" |
| Allocation concealment (selection bias) | Low risk | Quote: "The randomization assignment was stored in a secure location that could not be accessed by study personnel" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Identical‐appearing placebos were available for both tetracaine and morphine (ie, double dummy), so that all neonates received either tetracaine or placebo applied to the insertion site and all neonates received either morphine or placebo infusions prior to the procedure" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The facial grimacing activity of the neonates, expressed as the proportion of time that the neonate had bulging of the brow, was assessed by a trained research assistant who was unaware of treatment assignment" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Outcomes are reported for all randomized infants—see Figure Flow of Study Participants" |
| Selective reporting (reporting bias) | Low risk | Quote: "ClinicalTrials.gov Identifier NCT00213200" |
| Other bias | Low risk | None |
BW: birthweight; CRIES: Crying Requires oxygen Increased vital signs Expression Sleep; DAN: Douleur Aiguë Nouveau‐né; EEG: electroencephalography; GA: gestational age; HR: heart rate; IL6: interleukin 6; IL8: interleukin 8; IQR: interquartile range; NICU: neonatal intensive care unit; NIPS: Neonatal Infant Pain Scale; PICC: peripherally inserted central catheter; PIPP: Premature Infant Pain Profile; PIPP‐R: Premature Infant Pain Profile ‐ Revised; ROP: retinopathy of prematurity; RR: respiratory rate; SaO²: saturation of oxygen; SD: standard deviation; TNF‐α: tumor necrosis factor alpha
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Axelin 2009 | Study design ("Opioid was administered always before the last nursing care episode because of a potential carry‐over effect") |
| Bell 2019 | Study design: phase II trial |
| Campbell‐Yeo 2018 | Study design: commentary |
| Chambers 2002 | Patient population: surgical pain |
| Moustogiannis 1996 | Study design: not a randomized trial |
| NCT00571636 | Patient population: sedation during mechanical ventilation |
| NCT03897452 | Study design: phase II trial |
| Rosen 2000 | Patient population: infants older than 1 month |
| Shin 2013 | Comparator: comparing high versus low dose of the same opioid |
| Shin 2014 | Comparator: comparing high versus low dose of the same opioid |
| Soffer 2019 | Study design: commentary |
| Valkenburg 2015 | Patient population: sedation during mechanical ventilation |
Characteristics of studies awaiting classification [ordered by study ID]
ACTRN12612000385842.
| Methods | Randomized, double‐blind, placebo‐controlled trial |
| Participants | Medically stable neonates requiring insertion of a central venous catheter at a NICU Level 3 Nursery, who are 24 to 44 weeks corrected gestational age at the time of procedure |
| Interventions | Intravenous infusion of 0.1 μg/kg/min remifentanil until central catheter has been threaded to its final position and secured or awaiting confirmation of catheter tip position by imaging |
| Outcomes | Primary: PIPPS and facial grimacing derived from clinical monitoring data and facial coding data; secondary: steady‐state pharmacokinetic data for analgesic infusions of remifentanil |
| Notes | Status: currently recruiting Main Co‐ordinating Center: John Hunter Children's Hospital, NSW, Australia Contact: Ian.Wright@hnehealth.nsw.gov.au |
Gadzinowski 2000.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Abstract not available |
Li 1997.
| Methods | Randomized to 4 groups of diode or argon laser photocoagulation |
| Participants | 8 infants with threshold ROP |
| Interventions | Midazolam or fentanyl |
| Outcomes | Continuous 16‐channel EEGs |
| Notes |
EEG: electroencephalogram; NICU: neonatal intensive care unit; PIPPS: Premature Infant Pain Profile; ROP: retinopathy of prematurity
Characteristics of ongoing studies [ordered by study ID]
CTRI/2017/12/011035.
| Study name | Single dose intranasal fentanyl for the reduction of pain associated with laser therapy for retinopathy of prematurity: a randomized controlled trial |
| Methods | Randomized controlled trial |
| Participants | 46 |
| Interventions | Fentanyl or placebo |
| Outcomes | Primary: PIPP during laser photocoagulation time point (every 10 min during procedure). Secondary: PIPP, HR, RR at 1, 5, 10 minutes |
| Starting date | Not yet recruiting |
| Contact information | Jagdish Kathwate, no email provided |
| Notes | Registered 2017 and not yet started |
CTRI/2018/04/012926.
| Study name | Oral morphine for analgesia in neonates undergoing ROP screening |
| Methods | Randomized controlled trial |
| Participants | 100 |
| Interventions | Oral morphine or oral glucose |
| Outcomes | Primary: mean pain score 1 min postprocedure. Secondary: mean pain score at 5 and 15 min, adverse events (tachycardia, apnea requiring intervention, abdominal distension, change in requirement of respiratory support) |
| Starting date | Not started |
| Contact information | Prakash Vinayagam; dr_praky76@yahoo.com |
| Notes |
CTRI/2020/08/027144.
| Study name | Analgesia during less invasive surfactant administration (LISA): a randomized controlled trial |
| Methods | Randomized controlled trial |
| Participants | 34 |
| Interventions | Fentanyl or no fentanyl |
| Outcomes | Primary: percentage of infants with PIPP‐R < 10 within 10 min of procedure |
| Starting date | August 2020 |
| Contact information | Bijan Saha; bijansaha18@gmail.com |
| Notes |
NCT02125201.
| Study name | Effectiveness of intranasal versus intravenous fentanyl in preterm and term newborns for pain prevention |
| Methods | Randomized controlled trial |
| Participants | 21 |
| Interventions | Fentanyl low dose or fentanyl high dose |
| Outcomes | Primary: N‐PASS |
| Starting date | June 2014 |
| Contact information | Marina Peniakov, no email found |
| Notes | Study completion date 2015 |
NCT03718507.
| Study name | Study on the effects of different premedication for LISA on stress and cerebral tissue oxygenation in preterm infants (SAFE LISA) |
| Methods | Randomized controlled trial |
| Participants | 20 |
| Interventions | Fentanyl or oral sucrose |
| Outcomes | Primary: PIPP |
| Starting date | Estimated 2019 |
| Contact information | Ilia Bresesti, no email address |
| Notes | Says "withdrawn (no recruitment started)" |
NCT03735563.
| Study name | Premedication for less invasive surfactant administration |
| Methods | Randomized controlled trial |
| Participants | 40 |
| Interventions | Ketamine or fentanyl |
| Outcomes | Primary: adverse events. Secondary: duration of procedure, number of attempts to get the catheter intratracheally, pain core (NIAPAS), the need for additional dosing of study drug or midazolam, edi‐signals |
| Starting date | 11 February 2019 |
| Contact information | Eveliina Ronkainen; eveliina.ronkainen@oulu.fi |
| Notes |
NCT04073173.
| Study name | Stress assessment with and without analgesia during surfactant therapy in preterm infants |
| Methods | Randomized controlled trial |
| Participants | 80 (but probably 40 undergoing LISA) |
| Interventions | Remifentanil or no remifentanil for LISA or INSURE |
| Outcomes | Primary: cortisol concentration. Secondary: galvanic skin response, heart rate, brain oxygenation, oxygen saturation, markers of oxidative stress |
| Starting date | 1 November 2020 |
| Contact information | Virgilio Carnielli; v.carnielli@staff.univpm.it |
| Notes |
HR: heart rate; INSURE: intubation‐surfactant‐extubation; LISA: less invasive surfactant administration; NIAPAS: Neonatal Infant Acute Pain Assessment Scale; N‐PASS: Neonatal Pain, Agitation and Sedation Scale; PIPP: Premature Infant Pain Profile; PIPP‐R: PIPP‐Revised; ROP: retinopathy of prematurity; RR: respiratory rate
Differences between protocol and review
We made the following change to the protocol (Kinoshita 2021): we added the outcome 'any harms' to the summary of findings tables.
Contributions of authors
Conceiving the protocol: MK, EO, MB
Designing the review: MK, MB
Co‐ordinating the review: MB
Data collection for the review: FB, MK, EO
Screening search results: EO, FB
Organizing retrieval of papers: MK, FB
Screening retrieved papers against eligibility criteria: EO, FB
Appraising quality of papers: EO, FB
Extracting data from papers: MK, EO, FB
Writing to authors of papers for additional information: EO
Data management for the review: MK, MB
Entering data into Review Manager 5: MK, MB, EO, FB
Analysis of data: MK, MB
Interpretation of data: MK, MB
Providing a methodological and a clinical perspective: MB
Sources of support
Internal sources
-
Institute for Clinical Sciences, Lund University, Lund, Sweden, Sweden
MB is employed by this organization.
External sources
-
Vermont Oxford Network, USA
Cochrane Neonatal Reviews are produced with support from Vermont Oxford Network, a worldwide collaboration of health professionals dedicated to providing evidence‐based care of the highest quality for newborn infants and their families.
-
Region Skåne, Skåne University Hospital, Lund University and Region Västra Götaland, Sweden
Cochrane Sweden is supported by Region Skåne, Skåne University Hospital Lund University and Region Västra Götaland.
Declarations of interest
MK works as a physician in the Department of Pediatrics, Yokohama Municipal Citizens' Hospital.
EO works clinically (part‐time) as a registered nurse at Örebro University Hospital, and therefore cares for newborns exposed to procedural pain.
FB has started a neonatology residency in II Department of Neonatology in GPSK clinical hospital, Poznan University of Medical Sciences, Poznan, Poland.
MB is an Associate Editor for the Cochrane Neonatal Group. However, his participation in the editorial group has not impacted this review.
New
References
References to studies included in this review
Carbajal 2005 {published data only}
- Carbajal R, Lenclen R, Jugie M, Paupe A, Barton B, Anand KS. Morphine does not alleviate acute pain in preterm neonates. Pediatric Research 2004;55:38. [DOI] [PubMed] [Google Scholar]
- Carbajal R, Lenclen R, Jugie M, Paupe A, Barton BA, Anand KJ. Morphine does not provide adequate analgesia for acute procedural pain among preterm neonates. Pediatrics 2005;115(6):1494-500. [DOI: 10.1542/peds.2004-1425] [PMID: ] [DOI] [PubMed] [Google Scholar]
Cordero 1991 {published data only}
- Cordero L, Gardner D K, O'Shaughnessy R. Analgesia versus sedation during broviac catheter placement. American Journal of Perinatology 1991;8(4):284-7. [DOI: 10.1055/s-2007-999398] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Cordero L, Gardner D, O'Shaughnessy R. Low dose analgesia for broviac catheter placement (BCP). Pediatric Research 1990;27:203A. [Google Scholar]
Fallah 2016 {published data only}
- Fallah R, Habibian S, Noori-Shadkam M. Efficacy and safety of single low dose intravenous fentanyl in pain reduction of lumbar puncture in near term neonates by a randomized clinical trial. Iranian Journal of Child Neurology 2016;10(2):60-6. [PMID: ] [PMC free article] [PubMed] [Google Scholar]
- IRCT2014022616761N1. Effect of fentanyl on pain reduction. https://trialsearch.who.int/Trial2.aspx?TrialID=IRCT2014022616761N1 (first received 16 April 2014). [CENTRAL: CN-01863513]
Gitto 2012 {published data only}
- Aversa S, Marseglia L, Manfrida M, Reiter RJ, Gitto E. Stress response and procedural pain in the preterm newborn: the role of pharmacological and non-pharmacological treatments. Journal of Maternal-Fetal & Neonatal Medicine 2012;25:134. [DOI] [PubMed] [Google Scholar]
- Gitto E, Pellegrino S, Manfrida M, Aversa S, Trimarchi G, Barberi I, et al. Stress response and procedural pain in the preterm newborn: the role of pharmacological and non-pharmacological treatments. European Journal of Pediatrics 2012;171(6):927-33. [DOI: 10.1007/s00431-011-1655-7] [PMID: ] [DOI] [PubMed] [Google Scholar]
Hartley 2018 {published data only}
- EUCTR2014-003237-25-GB. Does morphine provide effective pain relief during painful procedures performed in newborn babies as part of their essential medical care? trialsearch.who.int/Trial2.aspx?TrialID=EUCTR2014-003237-25-GB (first received 20 July 2015). [CENTRAL: CN-01844452]
- Hartley C, Moultrie F, Hoskin A, Green G, Monk V, Bell JL, et al. Analgesic efficacy and safety of morphine in the Procedural Pain in Premature Infants (Poppi) study: randomised placebo-controlled trial. Lancet 2018;392(10164):2595-605. [DOI: 10.1016/S0140-6736(18)31813-0] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISRCTN82342359. Is morphine an effective analgesic for procedural pain in infants? trialsearch.who.int/Trial2.aspx?TrialID=ISRCTN82342359 (first received 29 July 2015). [CENTRAL: CN-01863765]
- Slater R, Hartley C, Moultrie F, Adams E, Juszczak E, Rogers R, et al, Poppi Trial Team. A blinded randomised placebo-controlled trial investigating the efficacy of morphine analgesia for procedural pain in infants: trial protocol. Wellcome Open Research 2016;1:7. [DOI: 10.12688/wellcomeopenres.10005.2] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lago 2008 {published data only}
- Lago P, Tiozzo C, Boccuzzo G, Allegro A, Zacchello F. Remifentanil for percutaneous intravenous central catheter placement in preterm infant: a randomized controlled trial. Paediatric Anaesthesia 2008;18(8):736-44. [DOI: 10.1111/j.1460-9592.2008.02636.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
Madathil 2021a {published data only}
- Madathil S, Thomas D, Chandra P, Agarwal R, Sankar MJ, Thukral A, et al. 'NOPAIN-ROP' trial: intravenous fentanyl and intravenous ketamine for pain relief during laser photocoagulation for retinopathy of prematurity (ROP) in preterm infants: a randomised trial. BMJ Open 2021;11(9):e046235. [DOI: 10.1136/bmjopen-2020-046235] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Madathil 2021b {published data only}
- Madathil S, Thomas D, Chandra P, Agarwal R, Sankar MJ, Thukral A, et al. 'NOPAIN-ROP' trial: intravenous fentanyl and intravenous ketamine for pain relief during laser photocoagulation for retinopathy of prematurity (ROP) in preterm infants: a randomised trial. BMJ Open 2021;11(9):e046235. [DOI: 10.1136/bmjopen-2020-046235] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Manjunatha 2009 {published data only}
- Manjunatha CM, Ibhanesebhor SE, Rennix C, Fisher H, Abara R. Pain control during retinopathy of prematurity screening: double-blind, randomised, placebo-controlled study. Infant 2009;5(5):155-8. [DOI: 10.1345/aph.1E477] [PMID: ] [DOI] [PubMed] [Google Scholar]
Pokela 1994 {published data only}
- Pokela ML. Pain relief can reduce hypoxemia in distressed neonates during routine treatment procedures. Pediatrics 1994;93(3):379-83. [PMID: ] [PubMed] [Google Scholar]
Sethi 2020 {published data only}
- CTRI/2017/07/008977. A clinical study to compare the effect of fentanyl and oral sucrose in providing optimal pain relief in preterm babies during laser therapy for retinopathy of prematurity. trialsearch.who.int/Trial2.aspx?TrialID=CTRI/2017/07/008977 (first received 5 July 2017). [CENTRAL: CN-01887047]
- Sethi A, Sankar MJ, Kulkarni S, Thukral A, Chandra P, Agarwal R. Low dose fentanyl infusion versus 24% oral sucrose for pain management during laser treatment for retinopathy of prematurity-an open label randomized clinical trial. European Journal of Pediatrics 2020;179(2):285-92. [DOI: 10.1007/s00431-019-03514-5] [PMID: ] [DOI] [PubMed] [Google Scholar]
Sindhur 2020 {published data only}
- Sindhur M, Balasubramanian H, Srinivasan L, Kabra NS, Agashe P, Doshi A. Intranasal fentanyl for pain management during screening for retinopathy of prematurity in preterm infants: a randomized controlled trial. Journal of Perinatology 2020;40(6):881-7. [DOI: 10.1038/s41372-020-0608-2] [PMID: ] [DOI] [PubMed] [Google Scholar]
Taddio 2006 {published data only}
- NCT00213200. Intravenous and topical analgesics for procedural pain in neonates. clinicaltrials.gov/show/NCT00213200 (first received 21 September 2005). [CENTRAL: CN-02013389]
- Taddio A, Lee C, Yip A, Parvez B, McNamara PJ, Shah V. Intravenous morphine and topical tetracaine for treatment of pain in [corrected] neonates undergoing central line placement. JAMA 2006;295(7):793-800. [DOI: 10.1001/jama.295.7.793] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Taddio A, Lee C, Yip A, Parvez B, McNamara PJ, Shah V. Intravenous morphine and topical tetracaine for treatment of pain in preterm neonates undergoing central line placement. JAMA 2006;295(7):793‐800TN Erratum in JAMA. 2006 Apr 5;295(13):1518. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Axelin 2009 {published data only}
- Axelin A, Kirjavainen J, Salantera S, Lehtonen L. Effects of pain management on sleep in preterm infants. European Journal of Pain 2010;14(7):752-8. [DOI: 10.1016/j.ejpain.2009.11.007] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Axelin A, Salantera S, Kirjavainen J, Lehtonen L. Oral glucose and parental holding preferable to opioid in pain management in preterm infants. Clinical Journal of Pain 2009;25(2):138-45. [DOI: 10.1097/AJP.0b013e318181ad81] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Axelin A, Salantera S, Kirjavainen J, Lehtonen L. Oral glucose and parental holding superior to opioid in pain management in preterm infants. In: American Pediatric Society/Society for Pediatric Research Abstract. 2008.
Bell 2019 {published data only}
- Bell JL, Hardy P, Stallard N, Slater R, Hartley C, Adams E, et al. Setting up a stopping boundary for safety in a phase II trial: the Poppi trial. In: paediatrics.ox.ac.uk/publications/1070177. Vol. 20. 2019.
Campbell‐Yeo 2018 {published data only}
- Campbell-Yeo M, Benoit B. Use of morphine before retinopathy of prematurity examinations. Lancet 2018;392(10164):2521-3. [DOI: 10.1016/S0140-6736(18)32324-9] [PMID: ] [DOI] [PubMed] [Google Scholar]
Chambers 2002 {published data only}
- Chambers N, Lopez T, Thomas J, James MF. Remifentanil and the tunnelling phase of paediatric ventriculoperitoneal shunt insertion. A double-blind, randomised, prospective study. Anaesthesia 2002;57(2):133-9. [DOI: 10.1046/j.0003-2409.2001.02398.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
Moustogiannis 1996 {published data only}
- Moustogiannis AN, Raju TN, Roohey T, McCulloch KM. Intravenous morphine attenuates pain induced changes in skin blood flow in newborn infants. Neurological Research 1996;18(5):440-4. [DOI: 10.1080/01616412.1996.11740448] [PMID: ] [DOI] [PubMed] [Google Scholar]
NCT00571636 {published data only}
- NCT00571636. Continuous infusion of fentanyl in preterm on MV. clinicaltrials.gov/show/NCT00571636 (first received 12 December 2007). [CENTRAL: CN-02018526]
NCT03897452 {published data only}
- NCT03897452. NeoFent-I study; fentanyl treatment in newborn infants; a pharmacokinetic, pharmacodynamic and pharmacogenetic study. clinicaltrials.gov/ct2/show/NCT03897452 (first received 1 April 2019).
Rosen 2000 {published data only}
- Rosen DA, Morris JL, Rosen KR, Valenzuela RC, Vidulich MG, Steelman RJ, et al. Analgesia for pediatric thoracostomy tube removal. Anesthesia and Analgesia 2000;90(5):1025-8. [DOI: 10.1097/00000539-200005000-00005] [PMID: ] [DOI] [PubMed] [Google Scholar]
Shin 2013 {published data only}
Shin 2014 {published data only}
- Shin SH, Kim HS, Lee J, Choi KY, Lee JH, Kim EK, et al. A comparative study of two remifentanil doses for procedural pain in ventilated preterm infants: a randomized, controlled study. Pediatric Critical Care Medicine 2014;15(5):451-5. [DOI: 10.1097/PCC.0000000000000123] [PMID: ] [DOI] [PubMed] [Google Scholar]
Soffer 2019 {published data only}
- Soffer OD, Cornelissen L, Cummings C, Berde C. Morphine compared to placebo for procedural pain in preterm infants: safety, efficacy and equipoise. Journal of Perinatology 2019;39(10):1428-31. [DOI: 10.1038/s41372-019-0476-9] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Valkenburg 2015 {published data only}
- Valkenburg AJ, den Bosch GE, Graaf J, Lingen RA, Weisglas-Kuperus N, Rosmalen J, et al. Long-term effects of neonatal morphine infusion on pain sensitivity: follow-up of a randomized controlled trial. Journal of Pain 2015;16(9):926-33. [DOI: 10.1016/j.jpain.2015.06.007] [PMID: ] [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
ACTRN12612000385842 {published data only}
- ACTRN12612000385842. A randomised double-blind placebo-controlled trial of the efficacy of remifentanil for procedural pain in neonates. impact.psanz.com.au/clinical-trials/clinical-trials-2/?Sort=Topic&Dir=ASC&start=150&Filter=All (first received 4 April 2012). [CENTRAL: CN-01882516]
Gadzinowski 2000 {published data only}
- Gadzinowski J, Vidyasagar D, Bhat R, Rozycka J. Effect of short-term morphine infusion on premature infant pain profile (PIPP) and hemodynamics. In: The Abstracts of the XVII European Congress of Perinatal Medicine 2000. 2000.
Li 1997 {published data only}
- Li HK, Norcross-Nechay K, Marquez BA, Dejean BJ, Khorrami AM, Richardson J. Pain and stimulation in laser treatment for retinopathy of prematurity. In: IOVS 1997;38:ARVO Abstract 4515. 1997.
References to ongoing studies
CTRI/2017/12/011035 {published data only}
- CTRI/2017/12/011035. Single dose intranasal fentanyl for the reduction of pain associated with laser therapy for retinopathy of prematurity: a randomized controlled trial. trialsearch.who.int/Trial2.aspx?TrialID=CTRI/2017/12/011035 (first received 29 December 2017). [CENTRAL: CN-01900138]
CTRI/2018/04/012926 {published data only}
- CTRI/2018/04/012926. Oral morphine for analgesia in neonates undergoing ROP screening. trialsearch.who.int/?TrialID=CTRI/2018/04/012926 (first received 2 April 2018). [CENTRAL: CN-01904425]
CTRI/2020/08/027144 {published data only}
- CTRI/2020/08/027144. Analgesia during less invasive surfactant administration (LISA): a randomized controlled trial [Pain reducing drug administration during surfactant therapy by less invasive technique. Surfactant are alveolar surface reducing agents, used in baby having lower pool surfactant i.e. respiratory distress syndrome]. trialsearch.who.int/Trial2.aspx?TrialID=CTRI/2020/08/027144 (first received 14 August 2020). [CENTRAL: CN-02185616]
NCT02125201 {published data only}
- NCT02125201. Effectiveness of intranasal versus intravenous fentanyl in preterm and term newborns for pain prevention. clinicaltrials.gov/ct2/show/NCT02125201 (first received 29 April 2014). [CENTRAL: CN-01545341]
NCT03718507 {published data only}
- NCT03718507. Study on the effects of different premedication for LISA on stress and cerebral tissue oxygenation in preterm infants (SAFE LISA). clinicaltrials.gov/ct2/show/NCT03718507 (first received 24 October 2018). [CENTRAL: CN-01664417]
NCT03735563 {published data only}
- NCT03735563. Premedication for less invasive surfactant administration. clinicaltrials.gov/show/NCT03735563 (first received 8 November 2018). [CENTRAL: CN-01918347]
NCT04073173 {published data only}
- NCT04073173. Stress assessment with and without analgesia during surfactant therapy in preterm infants. clinicaltrials.gov/show/NCT04073173 (first received 29 August 2019). [CENTRAL: CN-01983867]
Additional references
AAP 2016
- American Academy of Pediatrics, Committee on Fetus and Newborn and Section on Anesthesiology and Pain Medicine. Prevention and management of procedural pain in the neonate: an update. Pediatrics 2016;137(2):e20154271. [DOI: 10.1542/peds.2015-4271] [PMID: ] [DOI] [PubMed] [Google Scholar]
Allegaert 2020
- Allegaert K. A critical review on the relevance of paracetamol for procedural pain management in neonates. Frontiers in Pediatrics 2020;8:89. [DOI: 10.3389/fped.2020.00089] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Anand 1999
- Anand KJ, Barton BA, McIntosh N, Lagercrantz H, Pelausa E, Young TE, et al. Analgesia and sedation in preterm neonates who require ventilatory support: results from the NOPAIN trial. Neonatal Outcome and Prolonged Analgesia in Neonates. Archives of Pediatrics and Adolescent Medicine 1999;153(4):331-8. [DOI: 10.1001/archpedi.153.4.331] [PMID: ] [DOI] [PubMed] [Google Scholar]
Anand 2005
- Anand KJ, Johnston CC, Oberlander TF, Taddio A, Lehr VT, Walco GA. Analgesia and local anesthesia during invasive procedures in the neonate. Clinical Therapeutics 2005;27(6):844-76. [DOI: 10.1016/j.clinthera.2005.06.018] [PMID: ] [DOI] [PubMed] [Google Scholar]
Anand 2007
- Anand KJ. Pharmacological approaches to the management of pain in the neonatal intensive care unit. Journal of Perinatology 2007;27(Suppl 1):S4-11. [DOI: 10.1038/sj.jp.7211712] [PMID: ] [DOI] [PubMed] [Google Scholar]
Andersen 2010
- Andersen RD, Meberg A, Wallin L, Jylli L. Clinical staff perceptions of the treatment of procedural pain in neonates: exploring a change process. International Journal of Medical and Biological Frontiers 2010;16(5/6):401-14. [ISSN: 1081-3829] [Google Scholar]
Antmen 2005
- Antmen B, Șașmaz I, Birbiçer H, Özbek H, Burgut R, Ișik G, et al. Safe and effective sedation and analgesia for bone marrow aspiration procedures in children with alfentanil, remifentanil and combinations with midazolam. Paediatric Anaesthesia 2005;15(3):214-9. [DOI: 10.1111/j.1460-9592.2004.01411.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
Ayed 2017
- Ayed M, Shah VS, Taddio A. Premedication for non-urgent endotracheal intubation for preventing pain in neonates. Cochrane Database of Systematic Reviews 2017, Issue 2. Art. No: CD012562. [DOI: 10.1002/14651858.CD012562] [DOI] [Google Scholar]
Balan 2018
- Balan S, Hassali MA, Mak VS. Two decades of off-label prescribing in children: a literature review. World Journal of Pediatrics 2018;14(6):528-40. [DOI: 10.1007/s12519-018-0186-y] [PMID: ] [DOI] [PubMed] [Google Scholar]
Bali 2015
- Bali A, Randhawa PK, Jaggi AS. Stress and opioids: role of opioids in modulating stress-related behavior and effect of stress on morphine conditioned place preference. Neuroscience and Biobehavioral Reviews 2015;51:138-50. [DOI: 10.1016/j.neubiorev.2014.12.018] [PMID: ] [DOI] [PubMed] [Google Scholar]
Bayley 1993
- Bayley N. Bayley Scales of Infant Development–II. San Antonio, Texas: Psychological Corporation, 1993. [Google Scholar]
Bayley 2005
- Bayley N. Bayley Scales of Infant and Toddler Development. 3rd edition. San Antonio, TX: Harcourt Assessment, 2005. [Google Scholar]
Bellieni 2018
- Bellieni CV, Tei M, Cornacchione S, Di Lucia S, Nardi V, Verrotti A, et al. Pain perception in NICU: a pilot questionnaire. Journal of Maternal-Fetal and Neonatal Medicine 2018;31(14):1921-3. [DOI: 10.1080/14767058.2017.1332038] [PMID: ] [DOI] [PubMed] [Google Scholar]
Bellieni 2005
- Bellieni CV, Bagnoli F, Sisto R, Neri L, Cordelli D, Buoocore G. Development and validation of the ABC pain scale for healthy full-term babies. Acta Paediatrica 2005;94(10):1432-6. [DOI: 10.1111/j.1651-2227.2005.tb01816.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
Bellù 2021
- Bellù R, Romantsik O, Nava C, Waal KA, Zanini R, Bruschettini M. Opioids for newborn infants receiving mechanical ventilation. Cochrane Database of Systematic Reviews 2021, Issue 3. Art. No: CD013732. [DOI: 10.1002/14651858.CD013732.pub2] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Borenstein 2013
- Borenstein M, Higgins JPT. Meta-analysis and subgroups. Prevention Science 2013;14:134-43. [DOI: 10.1007/s11121-013-0377-7] [PMID: ] [DOI] [PubMed] [Google Scholar]
Bruschettini 2022
- Bruschettini M, Olsson E, Persad E, Garratt A, Roger S. Clinical rating scales for assessing pain in newborn infants. Cochrane Database of Systematic Reviews 2022, Issue 2. Art. No: MR000064. [DOI: 10.1002/14651858.MR000064] [DOI] [Google Scholar]
Butt 2013
- Butt ML, McGrath JM, Samra HA, Gupta R. An integrative review of parent satisfaction with care provided in the neonatal intensive care unit. Journal of Obstetric, Gynecologic, and Neonatal Nursing 2013;42(1):105-20. [DOI: 10.1111/1552-6909.12002] [PMID: ] [DOI] [PubMed] [Google Scholar]
Bäcke 2022
- Bäcke P, Bruschettini M, Sibrecht G, Thernström Blomqvist Y, Olsson E. Pharmacological interventions for pain and sedation management in newborn infants undergoing therapeutic hypothermia. Cochrane Database of Systematic Reviews 2022, Issue 11. Art. No: CD015023. [DOI: 10.1002/14651858.CD015023] [DOI] [PMC free article] [PubMed] [Google Scholar]
CADTH 2016
- Canadian Agency for Drugs and Technologies in Health. Strings attached: CADTH database search filters. www.cadth.ca/strings-attached-cadths-database-search-filters (accessed 01 October 2021).
Carbajal 2005
- Carbajal R, Lenclen R, Jugie M, Paupe A, Barton BA, Anand KJ. Morphine does not provide adequate analgesia for acute procedural pain among preterm neonates. Pediatrics 2005;115(6):1494-500. [DOI: 10.1542/peds.2004-1425] [PMID: ] [DOI] [PubMed] [Google Scholar]
Carbajal 2008
- Carbajal R, Rousset A, Danan C, Coquery S, Nolent P, Ducrocq S, et al. Epidemiology and treatment of painful procedures in neonates in intensive care units. JAMA 2008;300(1):60-70. [DOI: 10.1001/jama.300.1.60] [PMID: ] [DOI] [PubMed] [Google Scholar]
Carbajal 2015
- Carbajal R, Eriksson M, Courtois E, Boyle E, Avila-Alvarez A, Andersen RD, et al, EUROPAIN Survey Working Group. Sedation and analgesia practices in neonatal intensive care units (EUROPAIN): results from a prospective cohort study. Lancet Respiratory Medicine 2015;3(10):796-812 [Erratum in: Lancet Respir Med. 2015 Dec;3(12):e44. doi: 10.1016/S2213-2600(15)00482-8. Epub 2015 Nov 30]. [DOI: 10.1016/S2213-2600(15)00331-8] [PMID: ] [DOI] [PubMed] [Google Scholar]
Carbajal 1997
- Carbajal R, Paupe A, Hoenn E, Lenclen R, Olivier-Martini M. APN: a behavioral acute pain rating scale for neonates [DAN: une échelle comportementale d'évaluation de la douleur aiguë du nouveau-né]. Archives de Pédiatrie 1997;4(7):623-8. [DOI: 10.1016/S0929-693X(97)83360-X] [PMID: ] [DOI] [PubMed] [Google Scholar]
Cerchione 2020
- Cerchione C, Martinelli G, Picardi M, Pugliese N, Nappi D, Casoria A, et al. Combined oral fentanyl citrate and midazolam as premedication for bone marrow aspiration and biopsy in patients with hematological malignancies: a randomized, controlled and patient-blinded clinical trial. Journal of Clinical Medicine 2020;9(2):395. [DOI: 10.3390/jcm9020395] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cignacco 2004
- Cignacco E, Mueller R, Hamers JP, Gessler P. Pain assessment in the neonate using the Bernese pain scale for neonates. Early Human Development 2004;78(2):125-31. [DOI: 10.1016/j.earlhumdev.2004.04.001] [PMID: ] [DOI] [PubMed] [Google Scholar]
Cochrane EPOC Group 2017
- Cochrane Effective Practice, Organisation of Care (EPOC). Data extraction and management. EPOC resources for review authors. Available from: epoc.cochrane.org/resources/epoc-resources-review-authors (accessed 01 October 2021).
Courtois 2016
- Courtois E, Droutman S, Magny JF, Merchaoui Z, Durrmeyer X, Roussel C, et al. Epidemiology and neonatal pain management of heelsticks in intensive care units: EPIPPAIN 2, a prospective observational study. International Journal of Nursing Studies 2016;59:79-88. [DOI: 10.1016/j.ijnurstu.2016.03.014] [PMID: ] [DOI] [PubMed] [Google Scholar]
Cruz 2016
- Cruz MD, Fernandes AM, Oliveira CR. Epidemiology of painful procedures performed in neonates: a systematic review of observational studies. European Journal of Pain 2016;20(4):489-98. [DOI: 10.1002/ejp.757] [PMID: ] [DOI] [PubMed] [Google Scholar]
Deeks 2020
- Deeks JJ, Higgins JP, Altman DG. Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.1 (updated September 2020). Cochrane, 2020. Available from training.cochrane.org/handbook/archive/v6.1.
Deeks 2022
- Deeks JJ, Higgins JPT, Altman DG (editors). Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane, 2022. Available from www.training.cochrane.org/handbook.
Dotta 2011
- Dotta A, Braguglia A, Salvatori G. Pharmacological research in neonatology. Journal of Maternal-Fetal and Neonatal Medicine 2011;24(Suppl 1):44-6. [DOI: 10.3109/14767058.2011.607580] [PMID: ] [DOI] [PubMed] [Google Scholar]
Duffett 2012
- Duffett M, Koop A, Menon K, Meade MO, Cook DJ. Clonidine for the sedation of critically ill children: a systematic review. Journal of Pediatric Intensive Care 2012;1(1):5-15. [DOI: 10.3233/PIC-2012-003] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gibbins 2014
- Gibbins S, Stevens B, Yamada J, Dionne K, Campbell-Yeo M, Lee G, et al. Validation of the premature infant pain profile-revised (PIPP-r). Early Human Development 2014;90(4):189-93. [DOI: 10.1016/j.earlhumdev.2014.01.005] [PMID: ] [DOI] [PubMed] [Google Scholar]
Giordano 2019
- Giordano V, Edobor J, Deindl P, Wildner B, Goeral K, Steinbauer P, et al. Pain and sedation scales for neonatal and pediatric patients in a preverbal stage of development: a systematic review. JAMA Pediatrics 2019;173(12):1186-97. [DOI: 10.1001/jamapediatrics.2019.3351] [PMID: ] [DOI] [PubMed] [Google Scholar]
Gitto 2012
- Gitto E, Pellegrino S, Manfrida M, Aversa S, Trimarchi G, Barberi I, et al. Stress response and procedural pain in the preterm newborn: the role of pharmacological and non-pharmacological treatments. European Journal of Pediatrics 2012;171(6):927-33. [DOI: 10.1007/s00431-011-1655-7] [PMID: ] [DOI] [PubMed] [Google Scholar]
Gomes Neto 2020
- Gomes Neto M, da Silva Lopes IA, Morais AC, Oliveira LS, Saquetto MB. The effect of facilitated tucking position during painful procedure in pain management of preterm infants in neonatal intensive care unit: a systematic review and meta-analysis. European Journal of Pediatrics 2020;179(5):699-709. [DOI: 10.1007/s00431-020-03640-5] [PMID: ] [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- GRADEpro GDT. Version accessed 11 January 2018. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015. Available at gradepro.org.
Griffiths 1954
- Griffiths R. The abilities of babies: a study of mental measurement. London: University of London Press, 1954. [Google Scholar]
Griffiths 1970
- Griffiths R. The abilities of young children: a comprehensive system of mental measurement for the first eight years. London: Child Development Research Center, 1970. [Google Scholar]
Guinsburg 1998
- Guinsburg R, Kopelman BI, Anand KJ, Almeida MF, Peres Cde A, Miyoshi MH. Physiological, hormonal, and behavioral responses to a single fentanyl dose in intubated and ventilated preterm neonates. Journal of Pediatrics 1998;132(6):954-9. [DOI: 10.1016/s0022-3476(98)70390-7] [PMID: ] [DOI] [PubMed] [Google Scholar]
Hall 2014
- Hall RW, Anand KJ. Pain management in newborns. Clinics in Perinatology 2014;41(4):895-924. [DOI: 10.1016/j.clp.2014.08.010] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hartley 2018
- Hartley C, Moultrie F, Hoskin A, Green G, Monk V, Bell JL, et al. Analgesic efficacy and safety of morphine in the Procedural Pain in Premature Infants (Poppi) study: randomised placebo-controlled trial. The Lancet 2018;392(10164):2595-605. [DOI: 10.1016/S0140-6736(18)31813-0] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Altman DG, Sterne JA: on behalf of the Cochrane Statistical Methods Group and the Cochrane Bias Methods Group. Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Higgins 2020
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). Cochrane, 2020. Available from training.cochrane.org/handbook/archive/v6.1.
Higgins 2021
- Higgins JP, Eldridge S, Li T (editors). Chapter 23: Including variants on randomized trials. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook/archive/v6.2.
Holsti 2008
- Holsti L, Grunau RE, Oberlander TF, Osiovich H. Is it painful or not? DIscriminant validity of the behavioral indicators of infant pain (BIIP) scale. Clinical Journal of Pain 2008;24(1):83-8. [DOI: 10.1097/AJP.0b013e318158c5e5] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hummel 2008
- Hummel P, Puchalski M, Creech SD, Weiss MG. Clinical reliability and validity of the N-PASS: neonatal pain, agitation and sedation scale with prolonged pain. Journal of Perinatology: Official Journal of the California Perinatal Association 2008;28(1):55-60. [DOI: 10.1038/sj.jp.7211861] [PMID: ] [DOI] [PubMed] [Google Scholar]
Hällström 2003
- Hällström M, Koivisto A, Janas M, Tammela O. Frequency of an risk factors for necrotizing enterocolitis in infants born before 33 weeks of gestation. Acta Paediatrica 2003;92(1):111-3. [DOI: 10.1111/j.1651-2227.2003.tb00479.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
Jacobs 2013
- Jacobs SE, Berg M, Hunt R, Tarnow-Mordi WO, Inder TE, Davis PG. Cooling for newborns with hypoxic ischaemic encephalopathy. Cochrane Database of Systematic Reviews 2013, Issue 1. Art. No: CD003311. [DOI: 10.1002/14651858.CD003311.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jiang 2012
- Jiang H, Cheng R, Kan Q, Shen X, Li F, Fu, C et al. Clinical evaluation of the effects of morphine in mechanical ventilation of neonates. Zhonghua Er Ke Za Zhi 2012;50:350-5. [PMID: ] [PubMed] [Google Scholar]
Johnston 2013
- Johnston C, Barrington K, Taddio A, Carbajal R, Filion F. Pain in Canadian NICUs: have we improved over the past 12 years? Clinical Journal of Pain 2013;27(3):225-32. [DOI: 10.1097/AJP.0b013e3181fe14cf] [PMID: ] [DOI] [PubMed] [Google Scholar]
Kassab 2019
- Kassab M, Alhassan A, Alzoubi K, Khader Y. Number and frequency of routinely applied painful procedures in university neonatal intensive care unit. Clinical Nursing Research 2019;28(4):488-501. [DOI: ] [PMID: ] [DOI] [PubMed] [Google Scholar]
Khanafer‐Larocque 2019
- Khanafer-Larocque I, Soraisham A, Stritzke A, Al Awad E, Thomas S, Murthy P, et al. Intraventricular hemorrhage: risk factors and association with patent ductus arteriosus treatment in extremely preterm neonates. Frontiers in Pediatrics 2019;22:408. [DOI: 10.3389/fped.2019.00408] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kinoshita 2020
- Kinoshita M, Stempel K, do Nascimento IJB, Vejayaram DN, Norman E, Bruschettini M. Opioids and alpha-2-agonists for analgesia and sedation in newborn infants: protocol of a systematic review. Systematic Reviews 2020;9(1):183. [DOI: 10.1186/s13643-020-01436-0] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kinoshita 2021
- Kinoshita M, Stempel KS, Borges do Nascimento IJ, Bruschettini M. Systemic opioids versus other analgesics and sedatives for postoperative pain in neonates. Cochrane Database of Systematic Reviews 2021, Issue 4. Art. No: CD014876. [DOI: 10.1002/14651858.CD014876] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kinoshita 2023
- Kinoshita M, Styrmisdóttir L, Borges do Nascimento IJ, Bruschettini M. Systemic opioid regimens for postoperative pain in neonates. Cochrane Database of Systematic Reviews 2023, Issue 1. Art. No: CD015016. [DOI: 10.1002/14651858.CD015016.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Krzyżaniak 2016
- Krzyżaniak N, Pawłowska I, Bajorek B. Review of drug utilization patterns in NICUs worldwide. Journal of Clinical Pharmacy and Therapeutics 2016;41(6):612-20. [DOI: 10.1111/jcpt.12440] [PMID: ] [DOI] [PubMed] [Google Scholar]
Ku 2019
- Ku LC, Simmons C, Smith PB, Greenberg RG, Fisher K, Hornik CD, et al. Intranasal midazolam and fentanyl for procedural sedation and analgesia in infants in the neonatal intensive care unit. Journal of Neonatal-Perinatal Medicine 2019;12(2):143-8. [DOI: 10.3233/NPM-17149] [PMID: ] [DOI] [PubMed] [Google Scholar]
Lawrence 1993
- Lawrence J, Alcock D, McGrath P, Kay J, MacMurray SB, Dulberg C. The development of a tool to assess neonatal pain. Neonatal Network 1993;12(6):59-66. [PMID: ] [PubMed] [Google Scholar]
Lefebvre 2021
- Lefebvre C, Glanville J, Briscoe S, Littlewood A, Marshall C, Metzendorf MI, et al. Chapter 4: Searching for and selecting studies. Section 4.S2 Supplementary material: Appendix of resources. In: Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). In: Higgins JP Thomas J Chandler J Cumpston M Li T Page MJ Welch VA, editors(s). Available from training.cochrane.org/handbook/archive/v6.2. Cochrane, 2021. [https://training.cochrane.org/handbook/current/chapter-04-appendix-resources] [Google Scholar]
Marshall 2018
- Marshall IJ, Noel-Storr AH, Kuiper J, Thomas J, Wallace BC. Machine learning for identifying randomized controlled trials: an evaluation and practitioner’s guide. Research Synthesis Methods 2018;9(4):602-14. [DOI: 10.1002/jrsm.1287] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Maxwell 2019
- Maxwell L, Fraga M, Malavolta C. Assessment of pain in the neonate: an update. Clinics in Perinatology 2019;46(4):693-707. [DOI: 10.1016/j.clp.2019.08.005] [PMID: ] [DOI] [PubMed] [Google Scholar]
McGowan 2016a
- McGowan J, Sampson M, Salzwedel D, Cogo E, Foerster V, Lefebvre C. PRESS Peer Review of Electronic Search Strategies: 2015 guideline Explanation and Elaboration (PRESS E&E). Ottawa: CADTH, 2016. [URL: https://www.cadth.ca/sites/default/files/pdf/CP0015_PRESS_Update_Report_2016.pdf] [DOI] [PubMed] [Google Scholar]
McGowan 2016b
- McGowan J, Sampson M, Salzwedel DM, Cogo E, Foerster V, Lefebvre C. PRESS Peer Review of Electronic Search Strategies: 2015 Guideline Statement. Journal of Clinical Epidemiology 2016;75:40-6. [DOI: 10.1016/j.jclinepi.2016.01.021] [PMID: ] [DOI] [PubMed] [Google Scholar]
McKechnie 2008
- McKechnie L, Levene M. Procedural pain guidelines for the newborn in the United Kingdom. Journal of Perinatology 2008;28(2):107-11. [DOI: 10.1038/sj.jp.7211822] [PMID: ] [DOI] [PubMed] [Google Scholar]
McPherson 2015
- McPherson C, Haslam M, Pineda R, Rogers C, Neil JJ, Inder TE. Brain injury and development in preterm infants exposed to fentanyl. Annals of Pharmacotherapy 2015;49(12):1291-7. [DOI: 10.1177/1060028015606732] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mehler 2013
- Mehler K, Oberthuer A, Haertel C, Herting E, Roth B, Goepel W. Use of analgesic and sedative drugs in VLBW infants in German NICUs from 2003-2010. European Journal of Pediatrics 2013;172(12):1633-9. [DOI: 10.1007/s00431-013-2095-3] [PMID: ] [DOI] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Journal of Clinical Epidemiology 2009;62(10):1006-12. [DOI: 10.1016/j.jclinepi.2009.06.005] [PMID: ] [DOI] [PubMed] [Google Scholar]
Monk 2019
- Monk V, Moultrie F, Hartley C, Hoskin A, Green G, Bell JL, et al. Oral morphine analgesia for preventing pain during invasive procedures in non-ventilated premature infants in hospital: the Poppi RCT. Efficacy and Mechanism Evaluation, No. 6.9. Southampton (UK): NIHR Journals Library 2019. [DOI: 10.3310/eme06090] [DOI] [PubMed]
Nafziger 2018
- Nafziger AN, Barkin RL. Opioid therapy in acute and chronic pain. Journal of Clinical Pharmacology 2018;58(9):1111-22. [DOI: 10.1002/jcph.1276] [PMID: ] [DOI] [PubMed] [Google Scholar]
Noel‐Storr 2020
- Noel-Storr AH, Dooley G, Wisniewski S, Glanville J, Thomas J, Cox S, et al. Cochrane Centralised Search Service showed high sensitivity identifying randomised controlled trials: a retrospective analysis. Journal of Clinical Epidemiology 2020;127:142-50. [DOI: 10.1016/j.jclinepi.2020.08.008] [PMID: ] [DOI] [PubMed] [Google Scholar]
Noel‐Storr 2021
- Noel-Storr AH, Dooley G, Elliott J, Steele E, Shemilt I, Mavergames C, et al. An evaluation of Cochrane Crowd found that crowdsourcing produced accurate results in identifying randomised trials. Journal of Clinical Epidemiology 2021;4356(21):00008-1. [DOI: 10.1016/j.jclinepi.2021.01.006] [PMID: ] [DOI] [PubMed] [Google Scholar]
Norman 2019
- Norman E, Kindblom JM, Rane A, Berg AC, Schubert U, Hallberg B, et al. Individual variations in fentanyl pharmacokinetics and pharmacodynamics in preterm infants. Acta Paediatrica 2019;108(8):1441-6. [DOI: 10.1111/apa.14744] [PMID: ] [DOI] [PubMed] [Google Scholar]
Ohlsson 2020
- Ohlsson A, Shah PS. Paracetamol (acetaminophen) for prevention or treatment of pain in newborns. Cochrane Database of Systematic Reviews 2020, Issue 1. Art. No: CD011219. [DOI: 10.1002/14651858.CD011219.pub4] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Olsson 2021
- Olsson E, Ahl H, Bengtsson K, Vejayaram DN, Norman E, Bruschettini M, et al. The use and reporting of neonatal pain scales: a systematic review of randomized trials. Pain 2021;162(2):353-60. [DOI: 10.1097/j.pain.0000000000002046] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Orsini 1996
- Orsini AJ, Leef KH, Costarino A, Dettorre MD, Stefano JL. Routine use of fentanyl infusions for pain and stress reduction in infants with respiratory distress syndrome. Journal of Pediatrics 1996;129(1):140-5. [DOI: 10.1016/s0022-3476(96)70201-9] [PMID: ] [DOI] [PubMed] [Google Scholar]
Osborn 2021
- Osborn DA, Jeffery HE, Cole MJ. Opiate treatment for opiate withdrawal in newborn infants. Cochrane Database of Systematic Reviews 2021, Issue 7. Art. No: CD002059. [DOI: 10.1002/14651858.CD002059.pub4] [DOI] [PubMed] [Google Scholar]
Papile 1978
- Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. Journal of Pediatrics 1978;92(4):529-34. [DOI: 10.1016/s0022-3476(78)80282-0] [PMID: ] [DOI] [PubMed] [Google Scholar]
Pirlotte 2019
- Pirlotte S, Beeckman K, Ooms I, Van Rompaey B, Cools F. Pharmacological interventions for the prevention of pain during endotracheal suctioning in ventilated neonates. Cochrane Database of Systematic Reviews 2019, Issue 6. Art. No: CD013355. [DOI: 10.1002/14651858.CD013355] [DOI] [PMC free article] [PubMed] [Google Scholar]
Prakeshkumar 2011
- Prakeshkumar S, Vibhuti S. Propofol for procedural sedation/anaesthesia in neonates. Cochrane Database of Systematic Reviews 2011, Issue 3. Art. No: CD007248. [DOI: 10.1002/14651858.CD007248.pub2] [PMID: ] [DOI] [PubMed] [Google Scholar]
Ranger 2013
- Ranger M, Chau C, Garg A, Woodward T, Beg M, Bjornson B, et al. Neonatal pain-related stress predicts cortical thickness at age 7 years in children born very preterm. PLoS One 2013;18(8):e76702. [DOI: 10.1371/journal.pone.0076702] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ranger 2014
- Ranger M, Grunau R. Early repetitive pain in preterm infants in relation to the developing brain. Pain Management 2014;4(1):57-67. [DOI: 10.2217/pmt.13.61] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
RCT‐Filter EMBASE
- Cochrane. How CENTRAL is created: Identifying RCTs for inclusion in CENTRAL. Available from: https://www.cochranelibrary.com/central/central-creation (accessed 01 October 2021). [cochranelibrary.com/central/central-creation]
Review Manager 2020 [Computer program]
- Review Manager 5 (RevMan 5). Version 5.4. Copenhagen: The Cochrane Collaboration, 2020.
Riskin 2015
- Riskin A, Shoris I, Kidalov G, Bader D, Borenstein-levin L, Kugelman A. Opiates use in very low birth weight infants—call for caution. Harefuah 2015;154:757-60. [PMID: ] [PubMed] [Google Scholar]
Romantsik 2020
- Romantsik O, Calevo MG, Norman E, Bruschettini M. Clonidine for pain in non-ventilated infants. Cochrane Database of Systematic Reviews 2020, Issue 4. Art. No: CD013104. [DOI: 10.1002/14651858.CD013104.pub2] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schünemann 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available from gdt.guidelinedevelopment.org/app/handbook/handbook.html.
Scott 1999
- Scott CS, Riggs KW, Ling EW, Fitzgerald CE, Hill ML, Grunau RV, et al. Morphine pharmacokinetics and pain assessment in premature newborns. Journal of Pediatrics 1999;135(4):423-9. [DOI: 10.1016/s0022-3476(99)70163-0] [PMID: ] [DOI] [PubMed] [Google Scholar]
Shah 2012
- Shah PS, Herbozo C, Aliwalas LL, Shah VS. Breastfeeding or breast milk for procedural pain in neonates. Cochrane Database of Systematic Reviews 2012, Issue 12. Art. No: CD004950. [DOI: 10.1002/14651858.CD004950.pub3] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Simons 2003
- Simons SH, Dijk M, Lingen RA, Roofthooft D, Duivenvoorden HJ, Jongeneel N, et al. Routine morphine infusion in preterm newborns who received ventilatory support: a randomized controlled trial. JAMA 2003;290(18):2419-27. [DOI: 10.1001/jama.290.18.2419] [PMID: ] [DOI] [PubMed] [Google Scholar]
Squillaro 2019
- Squillaro A, Mahdi EM, Tran N, Lakshmanan A, Kim E, Kelley-Quon LI. Managing procedural pain in the neonate using an opioid-sparing approach. Clinical Therapeutics 2019;41(9):1701-13. [DOI: 10.1016/j.clinthera.2019.07.014] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sterne 2017
- Sterne JA, Egger M, Moher D, Boutron I (editors). Chapter 10: Addressing reporting biases. In: Higgins JP, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), The Cochrane Collaboration, 2017. Available from training.cochrane.org/handbook/archive/v5.2.
Stevens 1996
- Stevens B, Johnstone C, Petryshen P, Taddio A. Premature infant pain profile: development and initial validation. Clinical Journal of Pain 1996;12(1):13-22. [DOI: 10.1097/00002508-199603000-00004] [PMID: ] [DOI] [PubMed] [Google Scholar]
Thigpen 2019
- Thigpen JC, Odle L, Harirforoosh S. Opioids: a review of pharmacokinetics and pharmacodynamics in neonates, infants, and children. European Journal of Drug Metabolism and Pharmacokinetics 2019;44(5):591-609. [DOI: 10.1007/s13318-019-00552-0] [PMID: ] [DOI] [PubMed] [Google Scholar]
Thomas 2020
- Thomas J, McDonald S, Noel-Storr AH, Shemilt I, Elliott J, Mavergames C, et al. Machine learning reduces workload with minimal risk of missing studies: development and evaluation of an RCT classifier for Cochrane Reviews. Journal of Clinical Epidemiology 2020;S0895-4356(20):31172-0. [DOI: 10.1016/j.jclinepi.2020.11.003vcvc] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tibboel 2005
- Tibboel D, Anand KJ, den Anker JN. The pharmacological treatment of neonatal pain. Seminars in Fetal & Neonatal Medicine 2005;10(2):195-205. [DOI: 10.1016/j.siny.2004.11.002] [PMID: ] [DOI] [PubMed] [Google Scholar]
van den 2016
- den Anker JN, Allegaert K. Treating pain in preterm infants: moving from opioids to acetaminophen. Journal of Pediatrics 2016;168:13-5. [DOI: 10.1016/j.jpeds.2015.09.061] [PMID: ] [DOI] [PubMed] [Google Scholar]
Walker 2019
- Walker SM. Long-term effects of neonatal pain. Seminars in Fetal & Neonatal Medicine 2019;24(4):101005. [DOI: 10.1016/j.siny.2019.04.005] [PMID: ] [DOI] [PubMed] [Google Scholar]
Walsh 1986
- Walsh MC, Kliegman RM. Necrotizing enterocolitis: treatment based on staging criteria. Pediatric Clinics of North America 1986;33(1):179-201. [DOI: 10.1016/s0031-3955(16)34975-6] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Williams 2020
- Williams M, Lascelles D. Early neonatal pain—a review of clinical and experimental implications on painful conditions later in life. Frontiers in Pediatrics 2020;7(8):30. [DOI: 10.3389/fped.2020.00030] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zvizdic 2019
- Zvizdic Z, Milisic E, Jonuzii A, Terzic S, Zvizdic D. The contribution of morphine sulfate to the development of necrotizing enterocolitis in preterm infants: a matched case control study. Turkish Journal of Pediatrics 2019;61(4):513-9. [DOI: 10.24953/turkjped.2019.04.007] [PMID: ] [DOI] [PubMed] [Google Scholar]
Zwicker 2016
- Zwicker JG, Miller SP, Grunau RE, Chau V, Brant R, Studholme C, et al. Smaller cerebellar growth and poorer neurodevelopmental outcomes in very preterm infants exposed to neonatal morphine. Journal of Pediatrics 2016;172:81-7.e2. [DOI: 10.1016/j.jpeds.2015.12.024] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Kinoshita 2021
- Kinoshita M, Olsson E, Borys F, Bruschettini M. Opioids for procedural pain in neonates. Cochrane Database of Systematic Reviews 2021, Issue 12. Art. No: CD015056. [DOI: 10.1002/14651858.CD015056] [DOI] [Google Scholar]


