Abstract
Alteration of the outer retina leads to various diseases such as age-related macular degeneration or retinitis pigmentosa characterized by decreased visual acuity and ultimately blindness. Despite intensive research in the field of retinal disorders, there is currently no curative treatment. Several therapeutic approaches such as cell-based replacement and gene therapies are currently in development. In the context of cell-based therapies, different cell sources such as embryonic stem cells, induced pluripotent stem cells, or multipotent stem cells can be used for transplantation. In the vast majority of human clinical trials, retinal pigment epithelial cells and photoreceptors are the cell types considered for replacement cell therapies. In this review, we summarize the progress made in stem cell therapies ranging from the pre-clinical studies to clinical trials for retinal disease.
Key Words: age-related macular degeneration, cell transplantation, clinical trial, retinal disease, retinal dystrophy, stem cell
Introduction
The progressive degeneration of photoreceptors characterizes various retinal diseases such as age-related macular degeneration (AMD) and retinitis pigmentosa (RP), leading to progressive vision and visual field loss and ultimately to blindness (Verbakel et al., 2018; Voisin et al., 2022). In AMD, there is geographic atrophy (called atrophic AMD form) and abnormal and excessive growth of blood vessels in the eye called choroidal neovascularization (the exudative AMD form). Anti-vascular endothelial growth factor intravitreal therapy, which is the only effective treatment for exudative AMD, targets choroidal neovascularization but not the disease itself. To date, aside from some positive advances in gene therapy for very few particular forms of retinal dystrophy (i.e., voretigene neparvovec), there is no curative treatment for these retinal diseases (Stingl et al., 2022). In this context, over the past two decades, the development of cell-based therapy has been investigated as a means of replacing apoptotic or dystrophic cells and restoring visual function. Mammalian retinas have limited regenerative capacity, and stem cells in regenerative strategy seem to be a promoting approach (Salas et al., 2021). Despite encouraging results, stem cell therapy needs to be optimized to improve its effectiveness in the treatment of retinal diseases. This review focuses on the retinal dystrophies and the different stem cell populations and their use for transplantation in retinal diseases.
Search Strategy and Selection Criteria
Studies cited in this review were published between 2000 and 2022 and searched from PubMed or Google Scholar databases. The following keywords were used for this research: stem cell transplantation, retinal disease, retinal degeneration, retinal dystrophy, stem cell therapy, age-related macular degeneration, retina, retinitis pigmentosa, Stargardt disease, bone marrow-derived stem cells, retinal precursor cells, clinical trials, retinal pigment epithelial cells, mesenchymal stem cells, embryonic stem cells, and induced pluripotent stem cells.
Retinal Degeneration
The human adult retina is organized in ten histological layers with one glial cell type, the Müller glial cells, and six major types of neurons: two main cell types of photoreceptors (PRs; rods and cones), bipolar cells, amacrine cells, horizontal cells, and ganglion cells. Retinal cell bodies are organized into three layers from apical to basal; the outer nuclear layer, the inner nuclear layer, and the ganglion cell layer. Aside from these layers, the outer and inner plexiform layers contain axons and dendrites of neuronal cells. The outer part of the retina is limited by the retinal pigment epithelium (RPE) layer. These cells fulfill many functions such as epithelial transport, visual cycle, phagocytosis, secretion, and immune modulation (Voisin et al., 2019).
Recessive Stargardt macular degeneration
Recessive Stargardt macular degeneration (STGD1) is common retinal dystrophy of varying severity, the most common and severe form during childhood, and the less common form during adulthood (Tanna et al., 2017). STGD1 is an autosomal recessive disease with an incidence of 1:8000 to 1:10,000, caused by mutations in the ATP binding cassette subfamily A member 4 (ABCA4) gene (Spiteri Cornish et al., 2017; Cicinelli et al., 2019). The protein encoded by ABCA4 is involved in the visual cycle and localized in the photoreceptor outer segments. The disease is characterized by choriocapillaris atrophy and by accumulation of lipofuscin, an age-related pigment, in RPE, leading to PR degeneration (Figure 1A). Patients affected by STGD1 usually experience a rapid bilateral central visual loss with dyschomatopsia and central scotoma. Disease-causing variants of ABCA4 are also associated with cone, rod, and rod-cone dystrophies (Cremers et al., 2020).
Figure 1.
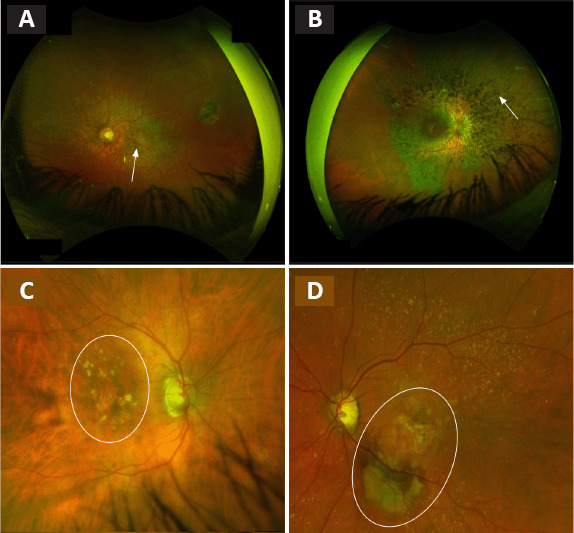
Fundus examination of patients affected by retinal diseases.
(A–D) STGD1 disease with the atrophy of the macular area (white arrow) (A), RP with the out bone spicule pigmentation (white arrow) (B), dry AMD with drusen and atrophy within the macular area (surrounded in white) (C) or wet AMD with fibrosis and retinal hemorrhage (surrounded in white) (D). AMD: Age-related macular degeneration; RP: retinit pigmentosa; STGD1: Stargardt macular degeneration. Unpublished data.
RP
RP is the most frequent hereditary (30–40% autosomal dominant, 40–60% autosomal recessive, and 5–15% X-linked) retinal disease with an incidence of approximately 1:4000 (Verbakel et al., 2018). Patients suffering from RP frequently report night blindness and progressive visual field loss beginning around the age of 20–30 years, and finally are complete blindness at the late stage. The disease is characterized by primary degeneration of PR rods (eventually secondary degeneration of cones in case of complete blindness) (Ali et al., 2017). At fundus examination, the disease is clinically characterized by dark bone-spicule pigmentation and attenuated blood vessels (Figure 1B; Verbakel et al., 2018).
AMD
AMD is a complex multifactorial disease characterized by degeneration of the macula usually preceded by drusen formation made up of the extracellular accumulation of proteins and lipids (Voisin et al., 2022). The disease usually affects older adult individuals and is the leading cause of blindness in industrialized countries. The pathogenesis of this retinal disease is associated with both genetic (CFH, ARMS2, etc.) and environmental factors (smoking and light exposure). AMD is classified in an atrophic form (Figure 1C) with the progressive development of macular atrophy and an exudative form (Figure 1D) with the development of choroidal neovascularization (Nashine, 2021). Both forms are clinically characterized by visual acuity decrease, central scotoma, and metamorphopsia.
Cell-Based Therapy
One approach for vision restoration in AMD, RP, or STGD1 is cell-based therapy, which has two objectives: (1) to replace dysfunctional cells with new retinal cells derived from stem cells that could integrate the host tissue and restore retina function, and (2) to release trophic factors that contribute to rescue effect (Figure 2). To be functional, transplanted cells must integrate, demonstrate long-term survival and form new synaptic connections with the host retina. Moreover, the secretion of neurotrophic factors by grafted cells can protect the retina from degeneration and contribute to vision restoration. For example, the brain-derived neurotrophic factor is expressed in Müller cells and retinal ganglion cells and is essential for the development of neurons, cell survival, and synaptic activity (Jindal et al., 2017). Mainly expressed in RPE end PR, ciliary neurotrophic factor and glial cell-derived neurotrophic factor also have neuroprotective effects. Ciliary neurotrophic factor enhances the survival of PR and promotes the axonal regeneration of retinal ganglion cells (Li et al., 2011). Glial cell-derived neurotrophic factor mediates retinal neuroprotection through Müller cell activation involved in PR survival, retinal structural stabilization, and inflammatory modulation (Tackenberg et al., 2009; Read et al., 2010). Moreover, it has been shown that intravitreal injection of ciliary neurotrophic factor inhibits the progression of retinal degeneration and preserves the retina function in animal models of retinal degeneration (Dulz et al., 2020). Based on these neuroprotective effects, ciliary neurotrophic factor-based therapy has been tested in several clinical trials, but therapeutic effects are not sufficient for AMD or RP (Zhang et al., 2011).
Figure 2.
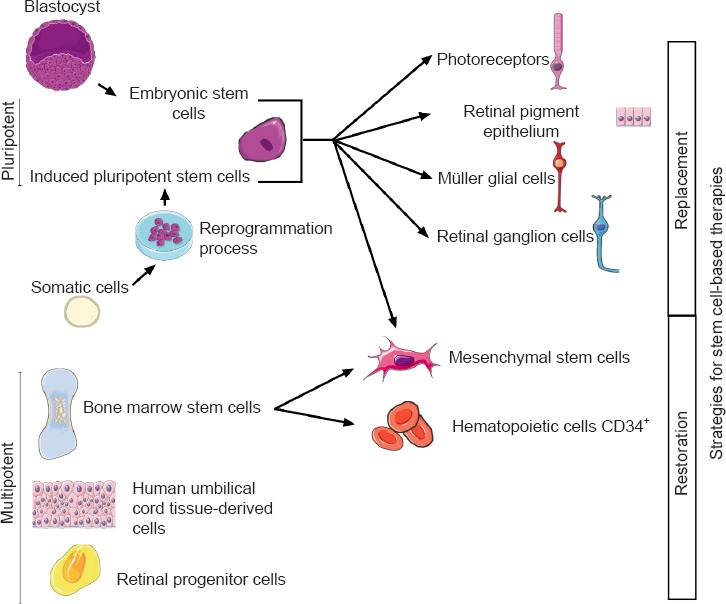
Two strategies for stem cell-based therapies: replacement or restoration of dysfunctional cells.
In cases of cell transplantation aimed at releasing trophic factors, different types of cells have been tested in clinical trials: bone marrow-derived mesenchymal stem cells (BMSCs), human umbilical tissue-derived cells (hUTCs), and retinal progenitor cells (RPCs) (Liu et al., 2017; Wiącek et al., 2021). For replacement of dysfunctional cells, candidate cells are PRs, RPEs, Müller glial cells, and retinal ganglion cell-derived from human embryonic stem cells (hESCs)/human induced pluripotent stem cells (hiPSCs), even if at present only hESC/hiPSC-RPE cells are used in clinical trials.
Cell Source
Stem cells have the ability to differentiate into other cell types from all three germ layers: ectoderm, mesoderm, and endoderm. Thanks to their potential, much research has been done to use stem cells to replace or repair damaged cells in many diseases.
Embryonic and pluripotent stem cells
Embryonic stem cells (ESCs) are pluripotent cells derived from the inner cell mass of blastocysts (Mackinlay et al., 2021). Since their derivation from human blastocysts, the potential application of ESCs was greatly studied in vitro, and in vivo for cell-based therapy. One major problem associated with human ESC-derived cell transplantation is an uncontrolled immune reaction that can lead to the rejection of grafted cells, requiring the use of immunosuppressors (Zhao et al., 2017). A potential alternative to avoid immunological rejection is the use of induced pluripotent stem cells (iPSCs), which could be derived from patient-specific somatic cells.
The first derivation of hiPSC occurred in 2007 (Takahashi et al., 2007). Like ESCs, iPSCs are pluripotent stem cells that can be differentiated into all lineages of the body. They are similar to ESC in terms of proliferation and differentiation capacities, and morphology (Logan et al., 2019). iPSCs are obtained by reprogramming various somatic cells, such as fibroblasts, blood cells, and urine cells, by different methods including episomal DNA plasmid, Sendai virus, adenovirus, mRNAs, and proteins (Gerami-Naini et al., 2016). At the beginning, the reprogramming method consisted in introduction into somatic cells of four transcription factors (c-Myc, Oct4, Sox2, and Flf4) by retroviral vectors (Takahashi et al., 2007). The use of c-Myc, a pro-oncogene, and retroviral vectors, which can cause genomic mutation, made this method inapplicable in human clinical trials (Nakagawa et al., 2010). Several studies have raised concerns over the genetic and epigenetic abnormalities of iPSC induced by the reprogramming process. At present, many protocols exist to obtain suitable iPSCs for clinical trials from somatic cells, with L-Myc instead of c-Myc, in non-integrative methods for reprogramming.
The generation of iPSCs from somatic cells enables us to obtain stem cells without using human embryos, which raises ethical issues (Takahashi et al., 2007). Cells for transplantation derived from iPSCs could overcome ethical concerns. In fact, the isolation and use of ESCs from preimplantation embryos produced by in vitro fertilization raise ethical issues. One approach to overcome this issue is the use of hiPSCs. One advantage of iPSC is the possibility to obtain cells from various human leucocyte antigen (HLA) types (Tu et al., 2019). HLA typing is used to match patients and donors for transplantation. In this context, the creation of HLA-iPSC obtained from donors of blood group O (compatible with all blood groups) bank is considered as a future clinical strategy for HLA-matched cell transplantation to minimize the risk of graft rejection (Taylor et al., 2005; Morizane et al., 2017).
However, the utilization of iPSCs for cell therapy also has some drawbacks such as epigenetic memory. Indeed, it has been shown that RPE cell derived from hiPSC (obtained after fibroblast reprogramming) retains a “memory” of gene expression patterns of the cell of origin (Hu et al., 2010). These lineage-specific imprints in hiPSC could affect certain cell properties such as proliferation and senescence. Moreover, due to their unlimited proliferative capacity, many questions have been raised about serious safety issues. Indeed, it is well-established that undifferentiated cells can generate teratomas or teratocarcinomas and induce potential immune reactions when transplanted (Schwartz et al., 2015). According to the literature, teratoma formation is expected to begin within the first few months after transplantation (Schwartz et al., 2015). Therefore, before clinical application of pluripotent stem cell materials, it is mandatory to develop robust differentiation protocols in order to eliminate pluripotent cells and minimize the risk of abnormal cell proliferation.
BMSCs and hUTCs
BMSCs are located in the bone marrow, which has the highest proportion of adult stem cells. Two types of BMSCs are defined: mesenchymal stem cells (MSCs) and hematopoietic stem cells, also called CD34+ cells (Enzmann et al., 2017; Aboutaleb Kadkhodaeian et al., 2019). These cells are multipotent, and they have a capacity for differentiation but it is more limited than pluripotent stem cells, and show paracrine trophic effects with secretion of neurotrophic factors or anti-inflammatory modulators. MSCs represent less than 0.1% of the cells in bone marrow but can be expanded easily in vitro. They are also found in many tissues such as teeth or liver.
BMSCs have many advantages: they are able to migrate toward lesion sites and they have the capacity for trans-differentiation (ability to differentiate into cells of other organs in specific environment) (Hong and Xu, 2011; Enzmann et al., 2017; Aboutaleb Kadkhodaeian et al., 2019). When RPE cells are damaged, they express specific chemoattractive cytokines/chemokines that induce migration of BMSCs to the injured site (Park et al., 2021). Once on the site, they can transdifferentiate into retinal cells [RPE cells (Enzmann et al., 2017) and PRs (Hong and Xu, 2011)] to repair the damaged tissue. BMSCs can also produce neurotrophic factors to promote cell survival and anti-inflammatory effects. Another advantage is that CD34+ cells are easily obtained from patients and require minimal manipulation before use during autologous procedures for transplantation.
hUTCs are derived from extra-embryonic mesoderm: cord tissue or cord blood component (Ho et al., 2017). The factors secreted by hUTCs include growth factors (hepatocyte growth factor, glial cell-derived neurotrophic factor, etc.), multiple receptor tyrosine kinase ligands, bridge molecules, and cytokines. These cells also secrete the thrombospondin family proteins involved in synaptic connectivity and neuronal growth (Koh et al., 2018).
RPCs
RPCs are the cells at the origin of retina formation during embryonic development and represent a highly interesting source of cells in retinal therapies. They can be obtained from the retina of human fetuses between 16 and 20 weeks of gestation (Wang et al., 2020). They are able to migrate and differentiate along the PR lineage and may have the potential to replace rods and cones in degenerative retinal disease. Transplantation of RPCs has two advantages: promoting neuroprotection by secretion of trophic factors that enhance retinal survival (insulin-like growth factor-1, hemodiafiltration, etc.), and photoreceptor replacement (Stern et al., 2018).
Which type of cells for transplantation?
Restoration of vision using a single type of retinal cell seems simplistic and optimistic especially in case of progressive retinal diseases. Indeed, the retina is composed of a highly complex layered structure where more than one type of cell is generally affected in retinal disease, due to the high degree of cellular interconnection. For example, during AMD, progressive RPE cell death is followed by underlying PR degeneration. Multiple studies have shown that hiPSCs/hESCs can evolve toward a three-dimensional (3D) retinal tissue with major retinal cell populations organized in different layers (Zhong et al., 2014; Hallam et al., 2018). The ability of ESCs/iPSCs to form retina in a dish is currently being developed to investigate retinal disease progression and as a tissue for cell transplantation.
Currently, clinical trials are focused on transplantation of RPE cells derived from hESCs or hiPSCs to cure retinal degeneration.
Preclinical Model
In retinal disease, despite robust genetic animal models, the lack of macula in rodents is a serious of limitations. The distribution of rods and cones differs among rodent, primate and human (Shirai et al., 2016). Despite these limitations, studies of integration, survival and visual improvement induced by cell-based transplantation in rodents have led to major advances in stem cell therapy.
hESC/hiPSC-derived cell transplantations
Since 2004, many reports have shown that mammalian retinas have a regenerative potency and can regain visual function after incorporating retinal cells derived from ESC/iPSC by transplantation (Table 1). In rho (rhodopsin) knockdown mice, embryonic retinal precursor cells differentiate into cells of retinal lineage after transplantation in eye (Klassen et al., 2004). Many studies have also shown that transplantation of mouse ESC/iPSC-derived cells can integrate and improve visual performance in a rat and mouse model of retinal degeneration (Sun et al., 2015; Tu et al., 2019; Salas et al., 2021). The adaptation of differentiation protocol to human stem cells has demonstrated the long-term safety of hESC-RPE cells grafted as a cell suspension into the retina in both rat and mice models with rescue PR and improvement of visual functions (Zhu et al., 2020; Salas et al., 2021). It has been shown that hESC/hiPSC-RPE cells grafted as monolayer can integrate the host retina and establish tight junctions (Riera et al., 2016). The Royal College Surgeons rat is an animal model with inherited retinal degeneration (mutation in the Mertk gene), widely used in preclinical studies for cell transplantations (Rajendran Nair et al., 2021). In this rat model, which is characterized by a phagocytosis defect in their RPE cells, hESC/hiPSC-RPE cell transplantation restored phagocytosis of photoreceptor, and improved retinal function up to 12 weeks post-transplantation (Riera et al., 2016; Salas et al., 2021). A neuroprotective effect of hESC/hiPSC-RPE cells has also been observed. Indeed, hESC/hiPSC-RPE cell transplantation protects retina from both cell degeneration and glial stress (Sun et al., 2015; Riera et al., 2016).
Table 1.
Summary of stem cell-based transplantation in the animal model for retinal disease therapy from 2004 to 2022
| Animal | Strain | Transplanted cells | Reference |
|---|---|---|---|
| Mouse | Rho –/– | RPCs | Klassen et al., 2004 |
| C57BL/6 | BMSCs | Li et al., 2007 | |
| ELOVL4 | hESC-RPE cells | Lu, 2009 | |
| Crx –/– | hESC-PR retinal cells | Lamba et al., 2009 | |
| Rpe65rd12/Rpe65rd12 | hESC-RPE cells | Wang, 2010 | |
| Crx –/– | hESC/hiPSC-PR precursors | Lamba et al., 2010 | |
| SCID | miPSC-PR precursors | Tucker, 2011 | |
| NOD-SCID | hCD34+ cells | Park et al., 2012 | |
| C57BL/6Jx129/SvJ | hES-RPCs | Hambright et al., 2012 | |
| Lrat –/– | hiPSC-RPE cells | Maeda, 2013 | |
| RPE65 –/– | hiPSC-RPE cells | Maeda, 2013 | |
| C57BL/6, Gnat1–/–, Prph2rd2/rd2, Nrlp.GFP+/+, Rho–/– | mESC-PR precursors | Gonzalez-Cordero et al., 2013 | |
| rd1 | mESC/miPSC-retina | Assawachanaont et al., 2014 | |
| rd1 | hiPSC-RPE cells | Sun et al., 2015 | |
| rd1 | human CD34+ cells | Moisseiev, 2016 | |
| rd1 | miPSC-retina | Mandai et al., 2017 | |
| Nr1–/–, Prph2rd2/rd2, RPE65R91W/R91W | mESC-retina | Waldron et al., 2018 | |
| NIH III | hESC-RPE cells | da Cruz et al., 2018 | |
| rd1 | hiPSC-RPE cells | Zhu et al., 2020 | |
| NaI03 | hMSCs | Pan, 2020 | |
| NOD-SCID rd1/RCS | hiPSC-RPE/hiPSC-PR precursors | Surendran, 2021 | |
| Rat | RCS | hESC-RPE cells | Lund, 2006 |
| RCS | hiPSC-RPE cells | Carr, 2009 | |
| RCS | hESC-RPE cells | Lu, 2009 | |
| rMSCs | Guan et al., 2013 | ||
| Nude | hESC-RPE cells | Diniz, 2013 | |
| RCS | hRPCs | Luo et al., 2014 | |
| RCS | rMSCs | Jian et al., 2015 | |
| RCS | hUTCs | Cao et al., 2016 | |
| RCS | hESC/hiPSC-RPE cells | Riera et al., 2016 | |
| RCS | hRPCs ± hMSCs | Qu, 2016 | |
| RCS | hESC-RPE cells 3D | Wu, 2016 | |
| RCS | CPCB-RPE1 | Thomas, 2016 | |
| SD-Foxn1 Tg(S334ter)3LavRrrc | hESC-retina | Iraha et al., 2018 | |
| S334ter-3 | hESC-retina | MacLelland, 2018 | |
| RCS | hUTCs | Koh et al., 2018 | |
| SD-Foxn1 Tg(S334ter)3LavRrrc | hiPSC-retina | Tu et al., 2019 | |
| RCS/Laser injury in pig | hiPSC-RPE patch | Sharma et al., 2019 | |
| RCS | hiPSC-RPE cells | Shrestha, 2020 | |
| RCS | hESC-retina | Lin et al., 2020 | |
| RCS | hiPSC-RPE patch | Nair, 2021 | |
| RCS | hiPSC-RPEs/hiPSC-RPCs | Salas et al., 2021 | |
| NOD-SCID rd1/RCS | hiPSC-RPE cells/hiPSC-PR precursors | Surendran, 2021 | |
| RCS | RPC-retina | He et al., 2021 | |
| Pig | Domestic | piPSC-PR precursors | Zhou et al., 2011 |
| Yùcatan | CPCB-RPE1 | Koss et al., 2016 | |
| Large white/Landrace hybrid | hESC-RPE cells | da Cruz et al., 2018 | |
| Yùcatan | CPCB-RPE1 | Fernandes et al., 2017 | |
| RCS/laser injury in pig | hiPSC-RPE patch | Sharma et al., 2019 | |
| Monkey | Macaca fasciularis/mulatta | hESC-retina | Shirai et al., 2016 |
| Macaca fasciularis/mulatta | hESC/hiPSC-retina | Tu et al., 2019 |
3D: Three-dimensional; BMSC: bone marrow stem cell; CPCB: mesh-supported submicron parylene C membrane; hESC: human embryonic stem cell; hiPSC: human induced pluripotent stem cell; hRPC: human retinal progenitor cell; hUTC: human umbilical tissuederived cell; miPSC: mouse induced pluripotent stem cell; MSC: mesenchymal stem cell; piPSC: porcine induced pluripotent stem cell; PR: photoreceptor; RCS: Royal College of Surgeons; rd1: retinal degeneration 1; rMSC: rat mesenchymal stem cell; RPE: retinal pigment epithelial cell.
hESC/hiPSC-PR precursors transplanted into the retina in animal models of retina degeneration have been robustly integrated into hosts and expressed typical markers of PR (Lamba et al., 2009, 2010; Zhou et al., 2011). Since 2011, numerous works have described protocols to obtain appropriate stages of development PR from 3D embryoid body (Eiraku et al., 2011; Gonzalez-Cordero et al., 2013). Rescue of visual function following transplantation of hESC/hiPSC-PR precursors is associated with cytoplasmic material transfer between transplanted cells and host cells (Waldron et al., 2018). This protein exchange is made by cytoplasmic fusion and is exclusively restricted to PR grafted-PR endogenous interaction and contributes to the rescue of dysfunctional PRs. For this phenomenon to take place, it is necessary that PR be present and functional in the host retina, highlighting the importance of the host environment for transplantation outcomes.
hESC/hiPSC-retinal sheet transplantations
Diniz et al. (2013) evaluated cell survival and tumorigenicity of suspension or patch hESC-RPE cell transplantation in rats. hESC-RPE patches were plated as a polarized monolayer on a parylene membrane. Parylene is an artificial Bruch membrane that allows adherence of epithelial monolayer RPE cells. This substrate is non-degradable, permeable, and stable in the subretinal space after transplantation. They showed that a hESC-RPE patch improved the survival of transplanted cells compared to suspension cells. In another study, the safety, survival, and functionality of the hiPSC-RPE patch transplantation were confirmed in rats (Rajendran Nair et al., 2021). In parallel, it has been shown that hESC-RPE patch transplantation in pig eyes could also survive (Fernandes et al., 2017; Sharma et al., 2019). The authors demonstrated the surgical feasibility and reliability of transplantation, and also a lack of systemic distribution of grafted cells in comparison with cell suspension transplantation (Koss et al., 2016; da Cruz et al., 2018; Sharma et al., 2019).
Development of 3D differentiation yields self-formation of the stratified retinal tissue at any development stage for transplantation (Eiraku et al., 2011). Numerous protocols have been established to obtain large populations of postnatal-stage hESCs/hiPSC-PRs from 3D culture that can be used in transplantation (Assawachananont et al., 2014; Reichman et al., 2014; Zhong et al., 2014). Assawachanaont et al. (2014) evaluated the ability of grafted 3D-differentiated mouse ESC- or mouse iPSC-derived retinal sheets to integrate the host retina. They generated efficient and reproducible mouse ESC/iPSC optic vesicles with retinal neuroepithelial-like layers, and showed that subretinal transplantation in a mouse model could lead to a well-developed stratified retinal layer. Moreover, transplanted mouse ESC- or iPSC-derived retinal sheets could form an integrated outer nuclear layer with mature PR able to respond to light (Shirai et al., 2016; Mandai et al., 2017; Iraha et al., 2018; McLelland et al., 2018; Lin et al., 2020). Shirai et al. (2016) showed that retinal sheets derived from hESCs could survive, mature (with the development of structured PR), and integrate after transplantation in monkey retina. Another study demonstrated that the transplantation of retinal cells derived from hiPSCs has the same potency as retinal cells derived from hESCs (Tu et al., 2019). They observed long-term survival of a transplanted retinal sheet derived from hiPSCs for more than 2 years in one monkey.
BMSC and hUTC transplantation
In rats, transplantation of hUTCs has been associated with the preservation of PR and visual function (Cao et al., 2016). In vitro analysis explained that hUTCs rescue the phagocytic defect function of RPE by secretion of neurotrophic factors such as brain-derived neurotrophic factor, hepatocyte growth factor, and glial cell-derived neurotrophic factor (Cao et al., 2016). Subretinal injection of hUTCs before PR loss in Royal College Surgeons rat could also preserve retinal synaptic connectivity and attenuates Müller glial reactivity by the secretion of thrombospondin family protein (Koh et al., 2018). Indeed, synaptic defects are associated with changes in Müller glial morphology and reactivity. By secretion of thrombospondin, hUTCs improve the retinal environment by decreasing the activation of Müller glial cells and protecting the synaptic connectivity.
Transplantation of BMSCs in a rat model of retinal degeneration can initiate regenerative mechanism (Guan et al., 2013; Jian et al., 2015). Moreover, transplanted BMSCs secreted factors such as nerve growth factor that could stimulate Müller cells to transdifferentiate into new retinal neurons and slow generation (Jian et al., 2015).
For intravitreal injection of CD34+ cells in NOD-SCID mice, no major safety concerns were observed in different studies (Park et al., 2012). Even if no recovery of retinal function was observed after transplantation of CD34+ cells, potential trophic regenerative effects have been observed with the change in gene expression involved in PR maintenance and apoptosis (Park et al., 2021).
RPC transplantation
Transplantation of RPCs has also been associated with the preservation of PRs and visual function in rats (Luo et al., 2014). Hambright et al. (2012) highlighted that transplantation of RPCs derived from hESCs can integrate the retina and differentiate into PRs in a mouse model. Moreover, a recent study showed that transplanted organoid-derived RPCs in rats are able to establish synaptic connections with the host retina (He et al., 2021). In 2016, co-transplantation of RPCs and MSCs in a rat model of degeneration showed better results than RPC or MSC alone (Qu et al., 2017). In this study, the Qu et al. (2017) showed that combined grafted cells migrated better and differentiated into PRs more often after transplantation compared to a single-cell strategy, thanks to the secretion of neurotrophic factors that improve the microenvironment. They also concluded that combined cell transplantation highlights a higher improvement of vision in this animal model.
Clinical Trials
Currently, the most important limitation in transplantation therapy is the lack of suitable donor organs, tissue or cells. In humans, only allogenic, or HLA-matched donor-derived stem cells can be used in cell-grafting therapy. Due to evidence that transplantation of cells in a preclinical model can rescue PR and prevent visual loss, in 2010 the US Food and Drug Administration approved the launch of phase I/II stem cell clinical trials for retinal diseases in humans (Schwartz et al., 2015) (Table 2). Currently, hESC/hiPSC-RPE is the only cell type suitable for the clinical grade.
Table 2.
Summary of hESC, hiPSC or multipotent cell transplantation trials in the world for retinal disease therapy from 2010 to 2022
| Disease | Phase | Identifier | Transplanted cells | Transplant | Location | Patient number | Immunosuppression | Sponsor | Study start |
|---|---|---|---|---|---|---|---|---|---|
| RP | I | NCT01068561* | BMSCs | Cell suspension (autologous) | IV | 5 | University of Sao Paulo | 2010 | |
| I | NCT01531348e | MSCs | Cell suspension (autologous) | IV | 10 | Mahidol University | 2012 | ||
| II | NCT01560715* | BMSCs | Cell suspension | IV | 50 | University of Sao Paulo | 2012 | ||
| I | NCT01736059r | CD34+ cells | Cell suspension (autologous) | IV | 15 | University of California Davis | 2012 | ||
| I | NCT02280135* | BMSCs | Cell suspension (autologous) | IV | 8 | Red de Terapia Celular | 2014 | ||
| I/II | NCT01914913u | BMSCs | Cell suspension (autologous) | 15 | Chaitanya Hospital, Pune | 2014 | |||
| I/II | NCT02464436r | hRPCs | Cell suspension | SR | 29 | Unknown | ReNeuron Limited | 2015 | |
| I/II | NCT02320812* | hRPCs | Cell suspension (allogenic) | IV | 28 | jCyte, Inc. | 2015 | ||
| II | NCT03073733* | RPCs | Cell suspension | IV | 84 | Unknown | jCyte, Inc. | 2017 | |
| I | NCT03772938u | BMSCs | Cell suspension (autologous) | IV | 30 | Pomeranian Medical University Szczecin | 2018 | ||
| I/II | NCT03963154r | hESC-RPE cells | – | SR | 12 | Unknown | Centre d’Etude des Cellules Souches | 2019 | |
| I | NCT04925687r | CD34+ cells | Cell suspension | IV | 4 | Unknown | University of California | 2021 | |
| AMD | I/II | NCT01344993* | hESC-RPE cells | Cell suspension | SR | 13 | Tacrolimus/mycophenolate mofetil | Astellas Institute for Regenerative Medicine | 2011 |
| I/II | NCT01518127* | BMSCs | Cell suspension (autologous) | IV | 20 | University of Sao Paulo | 2011 | ||
| I/II | NCT01674829u | hESC-RPE cells | Cell suspension | SR | 12 | Tacrolimus/mycophenolate mofetil | CHABiotech Co. | 2012 | |
| I | NCT01736059r | CD34+ celss | Cell suspension (autologous) | IV | 15 | University of California Davis | 2012 | ||
| I/II | NCT02463344og | hESC-RPE cells | Cell suspension | SR | 11 | Tacrolimus/mycophenolate mofetil | Astellas Institute for Regenerative Medicine | 2013 | |
| I/II | NCT02016508u | BMSCs | Cell suspension (autologous) | IV | 1 | Al-Azhar University | 2013 | ||
| UMIN000011929* | hiPSC-RPE cells | Cell suspension (autologous) | 2 | RIKEN | 2013 | ||||
| I/II | NCT02286089og | hESC-RPE cells | Cell suspension | SR | 24 | Unknown | Lineage Cell Therapeutics | 2015 | |
| I/II | NCT02749734u | hESC-RPE cells | Cell suspension | SR | 15 | Tacrolimus/mycophenolate mofetil/predisone | Southwest Hospital | 2015 | |
| II | NCT02563782w | hESC-RPE cells | Cell suspension | SR | 0 | Tacrolimus/mycophenolate mofetil | Astellas Institute for Regenerative Medicine | 2015 | |
| I/II | NCT02903576* | hESC-RPE cells | Patch/Cell suspension | SR | 15 | Unknown | Federal University of Sao Paulo | 2015 | |
| I | NCT01691261og | hESC-RPE cells | Patch | 2 | Fluocinolone/prednisolone | Pfizer/University College London | 2015 | ||
| – | ChiCTR-OCB-15007054* | hESC-RPE cells | SR | 10 | Unknown | Chinese Academy of Sciences | 2015 | ||
| I/II | NCT02590692og | hESC-RPE cells | Patch-CPCB | SR | 16 | Unknown | Regenerative Patch Technologies, Inc. | 2016 | |
| I | NCT03102138og | hESC-RPE cells | Patch | 2 | Unknown | Moorfields Eye Hospital NHS Foundation Trust | 2017 | ||
| I/II | NCT03178149og | hESC-ASP7317 | Cell suspension | 18 | Tacrolimus | Astellas Institute for Regenerative Medicine | 2017 | ||
| I/II | NCT03046407r | hESC-RPE cells | Cell suspension | SR | 10 | Unknown | Chinese Academy of Sciences | 2017 | |
| I/II | NCT02755428r | hESC-RPE cells | Cell suspension | SR | 10 | Unknown | Chinese Academy of Sciences | 2018 | |
| I/II | NCT03167203e | hESC-RPE cells | Cell suspension | SR | 36 | Unknown | Astellas Institute for Regenerative Medicine | 2018 | |
| I | NCT03772938u | BMSCs | Cell suspension (autologous) | IV | 30 | Pomeranian Medical University | 2018 | ||
| I/II | NCT04339764r | hiPSC-RPE cells | RPE-PLGA (autologous) | SR | 20 | National Eye Institute | 2020 | ||
| STGD1 | I/II | NCT01345006* | hESC-RPE cells | Cell suspension | SR | 13 | Tacrolimus/mycophenolate mofetil | Astellas Institute for Regenerative Medicine | 2011 |
| I/II | NCT01469832* | hESC-RPE cells | Cell suspension | SR | 12 | Tacrolimus/mycophenolate mofetil | Astellas Institute for Regenerative Medicine | 2011 | |
| I/II | NCT01518127* | BMSCs | Cell suspension (autologous) | IV | 20 | University of Sao Paulo | 2011 | ||
| I/II | NCT02445612og | hESC-RPE cells | Cell suspension | SR | 13 | Tacrolimus/mycophenolate mofetil | Astellas Institute for Regenerative Medicine | 2012 | |
| I | NCT01625559u | hESC-RPE cells | Cell suspension | SR | 3 | Tacrolimus/mycophenolate mofetil | CHABiotech Co. | 2012 | |
| I/II | NCT02941991* | hESC-RPE cells | Cell suspension | SR | 12 | Unknown | Astellas Institute for Regenerative Medicine | 2013 | |
| I/II | NCT02749734u | hESC-RPE cells | Cell suspension | SR | 15 | Tacrolimus/mycophenolate mofetil/predisone | Southwest Hospital | 2015 | |
| I/II | NCT02903576* | hESC-RPE cells | Patch/Cell suspension | SR | 15 | Unknown | Federal University of Sao Paulo | 2015 | |
| I | NCT03772938u | BMSCs | Cell suspension (autologous) | IV | 30 | Pomeranian Medical University | 2018 |
Clinical trials: Completed*, enrolling by invitatione, ongoingog, recruitingr, status unknownu, withdrawnw. AMD: Age-related macular degeneration; BMSC: bone marrow stem cell; CPCB: mesh-supported submicron parylene C membrane; hESC: human embryonic stem cell; hiPSC: human induced pluripotent stem cell; hRPC: human retinal progenitor cell; IV: intravitreal; MSC: mesenchymal stem cell; PR: photoreceptor; RP: retinitis pigmentosa; RPE: retinal pigment epithelial cell; SR: subretinal; STGD1: Stargardt macular degeneration.
RPE cell suspension or patch?
Up to now, clinical trials focused on the transplantation of RPE cells derived from hESCs or hiPSCs to prevent or slow down the retinal degeneration. Two different approaches to transplantation were evaluated: RPE cell suspension and RPE patch (Song et al., 2015; Kashani et al., 2018). Even if transplantation of cell suspension seems safe, without any serious adverse effect, it is not clear today whether RPE-transplanted cells can integrate or establish a monolayer organization after transplantation. For long-term efficacy, RPE cell-transplanted cells must be polarized into the host retina and establish connections with neighboring RPE cells to be able to play their role as phagocytosis of photoreceptor outer segment, maintenance of homeostasis and transport of nutriment into the new host retina. Moreover, RPE in suspension can be lost during the surgical procedure, due to reflux through the retinal hole created for the subretinal injection of donor cells (da Cruz et al., 2018).
Because they already form a monolayer epithelium, transplantation of a patch of RPE seems to be the ideal therapeutic substrate to treat retinal disease in stem cell therapy. Cells are fully differentiated, polarized with tight junctions, and a configuration close to their native ones (da Cruz et al., 2018). In addition, delivery of patches could decrease the risk of vitreoretinal proliferation (cellular migration and proliferation in vitreous cavity resulting in retinal detachment), which is the most severe clinical complication (da Cruz et al., 2018). Nevertheless, RPE sheet may also increase the risk of retinal detachment insofar as a larger retinotomy may be required to inject the RPE patch, compared to subretinal injection of RPE cell suspension (MacLaren et al., 2016). A recent study showed that absence of scaffold for the RPE patch led to aggregation of RPE in the region of atrophy rather than uniform distribution (Kashani et al., 2018). RPE with a supportive scaffold appears to show improved integration in the host retina. Bruch membrane (the basement membrane of RPE) changes are observed with aging and affect RPE metabolism and attachment. In this context, RPE monolayer cells adherent to a substrate as ultrathin parylene substrate (California Project to Cure Blindness Retinal Pigment Epithelium 1, CPCB-RPE1) that mimics properties of Bruch membrane (Kashani et al., 2018) or with a human vitronectin-coated polyester membrane (da Cruz et al., 2018) could be a good option for therapeutic purposes.
Transplantation approach
Successful treatment for retinal dystrophies also depends on effective methods of cell administration. Clinical trials have used two major approaches in stem cell-based therapy: subretinal injection of a cell suspension, and subretinal positioning of a patch after retinotomy. Intravitreal transplantation requires a vitrectomy followed by a limited retinotomy, usually in the upper part of the macula, and the introduction of the patch. For subretinal injection of cells, no retinotomy is required because the cell suspension can be delivered through a subretinal cannula (Wilson et al., 2017; da Cruz et al., 2018).
The main complications of these surgical procedures are retinal detachment, vitreoretinal proliferation, endophthalmitis, and graft rejection (Hartman and Kompella, 2018). Moreover, cells in suspension are not polarized and cells on patch can be lost or damaged in part, when delivered through the cannula (da Cruz et al., 2018).
Clinical trials using hESCs
Results of the first human trial using hESC-RPE cells were published by Schwartz et al. (2012) on 13 dry AMD patients and 13 STGD1 patients. Immunosuppressive systemic therapy was required for the first 3 months after cell transplantation to minimize cell rejection. However, immunosuppression was responsible for adverse effects in 28% of patients in this study (Schwartz et al., 2015). Indeed, the use of systemic immunosuppressants is related to complications such as urinary tract infection, gastrointestinal symptoms, or nonmelanoma skin cancers (Moutinho et al., 2020). In this first human trial with hESC-RPE cells, no signs of hyperproliferation could be observed after a 4-month follow-up. Furthermore, the safety and tolerability of the transplants in 18 patients with AMD (n = 9) or STGD1 (n = 9) were demonstrated during a longer follow-up between 12 and 36 months (Schwartz et al., 2015). In fact, the graft was present in 72% of patients after 1 year. They also observed an increase in best-corrected visual acuity in ten patients, while it remained stable in seven and deteriorated in one patient. No improvement was found in the patients’ untreated fellow eyes. Vision-related quality of life scoring increased by 25 points in cases of AMD and by 20 points in cases of Stargardt macular dystrophy. This study is the first to report the medium/long-term outcomes of stem cell application in AMD and STGD1 (Schwartz et al., 2015). However, there are still concerns considering the lack of a masked control group to control for the placebo effect and examiner bias in the subjective measure of visual acuity, the very limited sample size, and poor initial visual acuity. In another study, the long-term safety and tolerability of transplantation of hESC-RPE cells in seven patients with STGD1 were confirmed 5 years after transplantation (Li et al., 2021).
In parallel, Song et al. (2015) followed up four patients (two with dry AMD and two with STGD1) for 1 year after hESC-RPE transplantation and observed no evidence of adverse proliferation or tumorigenicity. Like Schwartz et al. (2012), hESC-RPE transplantation led to improvement in visual acuity in three patients (one remained stable). Subretinal injection of hESC-RPE cells has been shown to partially restore the structure and functionality of RPE cells (Kashani et al., 2018).
Since the initial report of hESC-RPE cell transplantation, many other stem cell treatment strategies have been proposed with the inclusion of artificial material with hiPSC-RPE sheets from HLA-matched with the host. The first transplantation of hESC-RPE cells plated on CPCB-RPE1 showed no evidence of safety concerns for patient and none of the implanted eyes showed progression of vision loss (Kashani et al., 2018). Moreover, one patient had improved vision, and two others improved fixation (ability to visually fixate in a specific location). hESC-RPE cells can also be plated onto a human vitronectin-coated polyester membrane to be transplanted into the subretinal space (da Cruz et al., 2018). In this first report, the authors suggested the efficacy and safety of RPE patch after 12 months of follow-up in two patients with severe wet AMD. In one patient, improvement of PR function was observed by electro-oculography. In parallel, 12 patients with severe STGD1 transplanted with hESC-RPE cells showed the safety and potential efficacy of the graft (Mehat et al., 2018).
Clinical trials using hiPSCs
The first human clinical trial using autologous hiPSC was investigated by RIKEN in 2014, a research institute in Japan, to treat one patient affected by AMD (Mandai et al., 2017). Because this cell transplantation is autologous, no systemic immunosuppression was needed. The trial was interrupted because of a new regulatory framework required for regenerative medicine adopted in 2014, even if the patient showed no serious adverse effects (Garber, 2015). There was no visual acuity gain either. The main concern was the formation of tumors but it was not observed during this trial. The second patient was not transplanted because three single nucleotide variations and three copy-variant numbers were observed in hiPSCs but not in the original patient’s somatic cells (Garber, 2015). Later, Mandai et al. (2017) demonstrated the feasibility of transplanting a sheet of autologous RPE cells derived from hiPSCs, obtained from skin fibroblasts, in one patient with wet AMD. No adverse events were observed within 25 months of follow-up and no improvement in visual acuity neither. As in RIKEN clinical trial, the second patient could not be transplanted due to the presence of mutations in his iPSCs.
One major issue with the utilization of hESC/hiPSC-derived cells for transplantation is the rejection by the host immune system. Autologous iPSC-based cell transplantation seems to be an appropriate alternative but has yet to become a standard treatment because of the costs and the amount of time required. In this context, RIKEN grafted five patients with HLA-matched allogenic hiPSC-RPE cells in 2017 (Sugita et al., 2020). During 1-year follow-up, no abnormal growth was observed but some adverse events occurred, such as corneal erosion (1/5), epiretinal membrane (1/5), elevated intralocular pressure (3/5), endophthalmitis (1/5), and mild immune rejection in eye (1/5). Despite these adverse events, this was the first proof of concept that it is possible to have the safety and survival of allogenic hiPSC-RPE cell grafts. A new clinical trial was initiated in 2020 by the National Eye Institute on 20 AMD patients to evaluate the safety of transplantation of hiPSC-RPE cells on poly(lactic-glycolic acid support. Poly(lactic-glycolic acid) is a biocompatible and biodegradable scaffold for RPE cell transplantation that has been shown to form a Bruch membrane-equivalent structure.
Clinical trials using BMSCs
The first trial preliminary reports after CD34+ cells intravitreal transplantation showed the safety and the feasibility of the procedure (Puertas-Neyra et al., 2020). Patients included in this trial presented AMD, retinal occlusion, STGD1, or RP. No intraocular inflammation or hyperproliferation was observed but there was no statistically significant visual acuity improvement after 6 months and the authors were not able to show the intraretinal incorporation of these CD34+ cells during this trial. In another clinical trial, they confirmed that BMSC transplantation is safe (no adverse event) and effective (improvement of visual acuity) for a long period of 12 months (Wiącek et al., 2021).
Clinical trials using RPCs
In 1999, when patients with RP received a suspension of hRPCs injected into the subretinal space (Das et al., 1999), no clinical appearance of adverse effects (inflammation, infection, or rejection) was observed. In another study, Liu et al. (2017) confirmed the safety and the feasibility of vision improvement through transplantation with RPCs in RP patients after a 24-month follow-up study. They observed a significant but transient improvement in visual acuity in five patients. However, this improvement was not maintained 12 months after transplantation. Another clinical trial using human RPCs began in 2015 for an enrollment of 28 patients with RPs (NCT02320812). These patients received a single intravitreal injection (0.5–1–2 or 3 million cells). Patients were followed for 2 years to evaluate the safety and tolerability of the transplantion. Preliminary results showed that intravitreal injection of RPCs was safe and seems to positively impact visual acuity at high dose cell level (Kuppermann et al., 2018).
Conclusion
Stem cell therapy is considered as a very promising therapeutic approach for many pathologies such as Parkinson’s disease, traumatic brain injury, and AMD. Recently, the US Food and Drug Administration provided a warning about stem cell therapies, recalling that any stem cell treatment must be Food and Drug Administration-approved in the US or must be under a clinical investigation plan submitted and allowed to proceed by the US Food and Drug Administration, because unscrupulous providers offered stem cell products that were both unapproved and unproven (US Food and Drug Administration, 2019).
In the case of retinal dystrophies, most clinical studies established the safety and tolerability of transplantation of RPE cells derived from pluripotent stem cells as a curative treatment (Song et al., 2015). Despite the major role of RPE cells for retina homeostasis and maintenance of the blood-retinal barrier, it could be better to co-transplant RPE cells with PRs to ensure the beneficial effect on vision. Indeed, in eye diseases such as AMD, multiple retinal cell subtypes are affected (RPE cells, PRs, choriocapillaris) and transplantation of one type of cells could not be sufficient (McLelland et al., 2018). In the same way, even if transplantation of other retinal cells would be needed in RPs, replacing only PRs without RPE cells seems not to be a good option for long-term efficacity and remains at a preclinical stage (Barnea-Cramer et al., 2016). In this context, the development of co-transplantation of organized layer cells for clinical trials is essential. In this way, optimization of protocols to obtain more complex multilayer retinal cells used for transplantation need to be developed to improve the integration and the survival of transplanted cells. Since the last decade, the emergence of retinal organoids, in vitro miniaturized and simplified model systems of organs, allow access to more physiologically relevant model systems for clinical transplantation (McLelland et al., 2018). Development of more complex systems could provide more functional cells, an essential criterion for graft cell survival (Qiu, 2019).
If it has been shown that RPE-grafted cells are able to establish tight junctions between each other and with host cells, transplantation of epithelium RPE cells could have a better benefit compared to suspension-grafted cells that do not form confluent monolayer RPE. This question is essential because (i) RPE epithelium organization is essential for retina morphological integrity and for maintenance of blood-retinal barrier (ii) proper connections among different retinal cell types are needed to transmit the electrical signal. In addition, in vitro studies have shown that embryonic RPE cells can adhere to normal but not aged Bruch membranes from post-mortem individuals and retinal detachment was the most severe clinical complication of AMD (da Cruz et al., 2018).
Another important question is which type of transplanted cells – allogenic or autologous – should be used in stem cell therapies. Autologous transplantation can be considered as optimal condition but requires time to prepare cell suspension or graft and are not available rapidly (da Cruz et al., 2018). In comparison, allogenic subretinal transplantation requires prolonged systemic immunosuppression. However, ocular side effects can occur using long-term systemic immunosuppressive drugs as higher risk of mortality and fatal cancer (Kempen et al., 2008). Indeed, it has been hypothesized that systemic immunosuppressive therapy, by impairing immunity itself, may increase the risk of malignancies. In order to avoid side effects of immune suppressive drugs, HLA-matched allogenic transplantation without immune suppression could be used but the cost of iPSC preparation is high.
In parallel, we have to determine at which disease stage, the cell transplantation will have the most beneficial effect. It could be better to perform stem cell therapy at the beginning of the retinal disease, when transplanted cells could be integrated into existing layers and help to ensure the survival of cell host, but at this stage of the disease, visual acuity is usually maintained.
In conclusion, developments of stem cell technologies have allowed multiple clinical trials to be currently ongoing. Despite encouraging results, novel therapies based on stem cell stills need to be optimized, including the surgical procedure, the conditioning and the subtypes of the cells, and the post-operative protocols.
Footnotes
Conflicts of interest: The authors declare no conflicts of interest.
C-Editors: Zhao M, Liu WJ, Yu J; T-Editor: Jia Y
References
- 1.Aboutaleb Kadkhodaeian H, Tiraihi T, Ahmadieh H, Ziaei H, Daftarian N, Taheri T. Generation of retinal pigmented epithelium-like cells from pigmented spheres differentiated from bone marrow stromal cell-derived neurospheres. Tissue Eng Regen Med. 2019;16:253–263. doi: 10.1007/s13770-019-00183-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ali MU, Rahman MSU, Cao J, Yuan PX. Genetic characterization and disease mechanism of retinitis pigmentosa;current scenario. 3 Biotech. 2017;7:251. doi: 10.1007/s13205-017-0878-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Assawachananont J, Mandai M, Okamoto S, Yamada C, Eiraku M, Yonemura S, Sasai Y, Takahashi M. Transplantation of embryonic and induced pluripotent stem cell-derived 3D retinal sheets into retinal degenerative mice. Stem Cell Reports. 2014;2:662–674. doi: 10.1016/j.stemcr.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barnea-Cramer AO, Wang W, Lu SJ, Singh MS, Luo C, Huo H, McClements ME, Barnard AR, MacLaren RE, Lanza R. Function of human pluripotent stem cell-derived photoreceptor progenitors in blind mice. Sci Rep. 2016;6:29784. doi: 10.1038/srep29784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cao J, Murat C, An W, Yao X, Lee J, Santulli-Marotto S, Harris IR, Inana G. Human umbilical tissue-derived cells rescue retinal pigment epithelium dysfunction in retinal degeneration. Stem Cells. 2016;34:367–379. doi: 10.1002/stem.2239. [DOI] [PubMed] [Google Scholar]
- 6.Carr AJ, Vugler AA, Hikita ST, Lawrence JM, Gias C, Chen LL, Buchholz DE, Ahmado A, Semo M, Smart MJ, Hasan S, da Cruz L, Johnson LV, Clegg DO, Coffey PJ. Protective effects of human iPS-derived retinal pigment epithelium cell transplantation in the retinal dystrophic rat. PLoS One. 2009;4:e8152. doi: 10.1371/journal.pone.0008152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cicinelli MV, Battista M, Starace V, Battaglia Parodi M, Bandello F. Monitoring and management of the patient with Stargardt disease. Clin Optom (Auckl) 2019;11:151–165. doi: 10.2147/OPTO.S226595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cremers FPM, Lee W, Collin RWJ, Allikmets R. Clinical spectrum, genetic complexity and therapeutic approaches for retinal disease caused by ABCA4 mutations. Prog Retin Eye Res. 2020;79:100861. doi: 10.1016/j.preteyeres.2020.100861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.da Cruz L, Fynes K, Georgiadis O, Kerby J, Luo YH, Ahmado A, Vernon A, Daniels JT, Nommiste B, Hasan SM, Gooljar SB, Carr AF, Vugler A, Ramsden CM, Bictash M, Fenster M, Steer J, Harbinson T, Wilbrey A, Tufail A, et al. Phase 1 clinical study of an embryonic stem cell-derived retinal pigment epithelium patch in age-related macular degeneration. Nat Biotechnol. 2018;36:328–337. doi: 10.1038/nbt.4114. [DOI] [PubMed] [Google Scholar]
- 10.Das T, del Cerro M, Jalali S, Rao VS, Gullapalli VK, Little C, Loreto DA, Sharma S, Sreedharan A, del Cerro C, Rao GN. The transplantation of human fetal neuroretinal cells in advanced retinitis pigmentosa patients:results of a long-term safety study. Exp Neurol. 1999;157:58–68. doi: 10.1006/exnr.1998.6992. [DOI] [PubMed] [Google Scholar]
- 11.Diniz B, Thomas P, Thomas B, Ribeiro R, Hu Y, Brant R, Ahuja A, Zhu D, Liu L, Koss M, Maia M, Chader G, Hinton DR, Humayun MS. Subretinal implantation of retinal pigment epithelial cells derived from human embryonic stem cells:improved survival when implanted as a monolayer. Invest Ophthalmol Vis Sci. 2013;54:5087–5096. doi: 10.1167/iovs.12-11239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dulz S, Bassal M, Flachsbarth K, Riecken K, Fehse B, Schlichting S, Bartsch S, Bartsch U. Intravitreal co-administration of GDNF and CNTF confers synergistic and long-lasting protection against injury-induced cell death of retinal ganglion cells in mice. Cells. 2020;9:2082. doi: 10.3390/cells9092082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eiraku M, Takata N, Ishibashi H, Kawada M, Sakakura E, Okuda S, Sekiguchi K, Adachi T, Sasai Y. Self-organizing optic-cup morphogenesis in three-dimensional culture. Nature. 2011;472:51–56. doi: 10.1038/nature09941. [DOI] [PubMed] [Google Scholar]
- 14.Enzmann V, Lecaudé S, Kruschinski A, Vater A. CXCL12/SDF-1-dependent retinal migration of endogenous bone marrow-derived stem cells improves visual function after pharmacologically induced retinal degeneration. Stem Cell Rev Rep. 2017;13:278–286. doi: 10.1007/s12015-016-9706-0. [DOI] [PubMed] [Google Scholar]
- 15.Fernandes RAB, Stefanini FR, Falabella P, Koss MJ, Wells T, Diniz B, Ribeiro R, Schor P, Maia M, Penha FM, Hinton DR, Tai YC, Humayun M. Development of a new tissue injector for subretinal transplantation of human embryonic stem cell derived retinal pigmented epithelium. Int J Retina Vitreous. 2017;3:41. doi: 10.1186/s40942-017-0095-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garber K. RIKEN suspends first clinical trial involving induced pluripotent stem cells. Nat Biotechnol. 2015;33:890–891. doi: 10.1038/nbt0915-890. [DOI] [PubMed] [Google Scholar]
- 17.Gerami-Naini B, Smith A, Maione AG, Kashpur O, Carpinito G, Veves A, Mooney DJ, Garlick JA. Generation of induced pluripotent stem cells from diabetic foot ulcer fibroblasts using a nonintegrative Sendai virus. Cell Reprogram. 2016;18:214–223. doi: 10.1089/cell.2015.0087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gonzalez-Cordero A, West EL, Pearson RA, Duran Y, Carvalho LS, Chu CJ, Naeem A, Blackford SJI, Georgiadis A, Lakowski J, Hubank M, Smith AJ, Bainbridge JWB, Sowden JC, Ali RR. Photoreceptor precursors derived from three-dimensional embryonic stem cell cultures integrate and mature within adult degenerate retina. Nat Biotechnol. 2013;31:741–747. doi: 10.1038/nbt.2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guan Y, Cui L, Qu Z, Lu L, Wang F, Wu Y, Zhang J, Gao F, Tian H, Xu L, Xu G, Li W, Jin Y, Xu GT. Subretinal transplantation of rat MSCs and erythropoietin gene modified rat MSCs for protecting and rescuing degenerative retina in rats. Curr Mol Med. 2013;13:1419–1431. doi: 10.2174/15665240113139990071. [DOI] [PubMed] [Google Scholar]
- 20.Hallam D, Hilgen G, Dorgau B, Zhu L, Yu M, Bojic S, Hewitt P, Schmitt M, Uteng M, Kustermann S, Steel D, Nicholds M, Thomas R, Treumann A, Porter A, Sernagor E, Armstrong L, Lako M. Human-induced pluripotent stem cells generate light responsive retinal organoids with variable and nutrient-dependent efficiency. Stem Cells. 2018;36:1535–1551. doi: 10.1002/stem.2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hambright D, Park KY, Brooks M, McKay R, Swaroop A, Nasonkin IO. Long-term survival and differentiation of retinal neurons derived from human embryonic stem cell lines in un-immunosuppressed mouse retina. Mol Vis. 2012;18:920–936. [PMC free article] [PubMed] [Google Scholar]
- 22.Hartman RR, Kompella UB. Intravitreal, subretinal , and suprachoroidal injections:evolution of microneedles for drug delivery. J Ocul Pharmacol Ther. 2018;34:141–153. doi: 10.1089/jop.2017.0121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.He XY, Zhao CJ, Xu H, Chen K, Bian BS, Gong Y, Weng CH, Zeng YX, Fu Y, Liu Y, Yin ZQ. Synaptic repair and vision restoration in advanced degenerating eyes by transplantation of retinal progenitor cells. Stem Cell Reports. 2021;16:1805–1817. doi: 10.1016/j.stemcr.2021.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ho AC, Chang TS, Samuel M, Williamson P, Willenbucher RF, Malone T. Experience with a subretinal cell-based therapy in patients with geographic atrophy secondary to age-related macular degeneration. Am J Ophthalmol. 2017;179:67–80. doi: 10.1016/j.ajo.2017.04.006. [DOI] [PubMed] [Google Scholar]
- 25.Hong Y, Xu GX. Proteome changes during bone mesenchymal stem cell differentiation into photoreceptor-like cells in vitro. Int J Ophthalmol. 2011;4:466–473. doi: 10.3980/j.issn.2222-3959.2011.05.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hu Q, Friedrich AM, Johnson LV, Clegg DO. Memory in induced pluripotent stem cells:reprogrammed human retinal-pigmented epithelial cells show tendency for spontaneous redifferentiation. Stem Cells. 2010;28:1981–1991. doi: 10.1002/stem.531. [DOI] [PubMed] [Google Scholar]
- 27.Iraha S, Tu HY, Yamasaki S, Kagawa T, Goto M, Takahashi R, Watanabe T, Sugita S, Yonemura S, Sunagawa GA, Matsuyama T, Fujii M, Kuwahara A, Kishino A, Koide N, Eiraku M, Tanihara H, Takahashi M, Mandai M. Establishment of immunodeficient retinal degeneration model mice and functional maturation of human ESC-derived retinal sheets after transplantation. Stem Cell Reports. 2018;10:1059–1074. doi: 10.1016/j.stemcr.2018.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jian Q, Li Y, Yin ZQ. Rat BMSCs initiate retinal endogenous repair through NGF/TrkA signaling. Exp Eye Res. 2015;132:34–47. doi: 10.1016/j.exer.2015.01.008. [DOI] [PubMed] [Google Scholar]
- 29.Jindal N, Banik A, Prabhakar S, Vaiphie K, Anand A. Alteration of neurotrophic factors after transplantation of bone marrow derived Lin-ve stem cell in NMDA-induced mouse model of retinal degeneration. J Cell Biochem. 2017;118:1699–1711. doi: 10.1002/jcb.25827. [DOI] [PubMed] [Google Scholar]
- 30.Kashani AH, Lebkowski JS, Rahhal FM, Avery RL, Salehi-Had H, Dang W, Lin CM, Mitra D, Zhu D, Thomas BB, Hikita ST, Pennington BO, Johnson LV, Clegg DO, Hinton DR, Humayun MS. A bioengineered retinal pigment epithelial monolayer for advanced, dry age-related macular degeneration. Sci Transl Med. 2018;10:eaao4097. doi: 10.1126/scitranslmed.aao4097. [DOI] [PubMed] [Google Scholar]
- 31.Kempen JH, Daniel E, Gangaputra S, Dreger K, Jabs DA, Kaçmaz RO, Pujari SS, Anzaar F, Foster CS, Helzlsouer KJ, Levy-Clarke GA, Nussenblatt RB, Liesegang T, Rosenbaum JT, Suhler EB. Methods for identifying long-term adverse effects of treatment in patients with eye diseases:the Systemic Immunosuppressive Therapy for Eye Diseases (SITE) cohort study. Ophthalmic Epidemiol. 2008;15:47–55. doi: 10.1080/09286580701585892. [DOI] [PubMed] [Google Scholar]
- 32.Klassen HJ, Ng TF, Kurimoto Y, Kirov I, Shatos M, Coffey P, Young MJ. Multipotent retinal progenitors express developmental markers, differentiate into retinal neurons, and preserve light-mediated behavior. Invest Ophthalmol Vis Sci. 2004;45:4167–4173. doi: 10.1167/iovs.04-0511. [DOI] [PubMed] [Google Scholar]
- 33.Koh S, Chen WJ, Dejneka NS, Harris IR, Lu B, Girman S, Saylor J, Wang S, Eroglu C. Subretinal human umbilical tissue-derived cell transplantation preserves retinal synaptic connectivity and attenuates Müller glial reactivity. J Neurosci. 2018;38:2923–2943. doi: 10.1523/JNEUROSCI.1532-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koss MJ, Falabella P, Stefanini FR, Pfister M, Thomas BB, Kashani AH, Brant R, Zhu D, Clegg DO, Hinton DR, Humayun MS. Subretinal implantation of a monolayer of human embryonic stem cell-derived retinal pigment epithelium:a feasibility and safety study in Yucatán minipigs. Graefes Arch Clin Exp Ophthalmol. 2016;254:1553–1565. doi: 10.1007/s00417-016-3386-y. [DOI] [PubMed] [Google Scholar]
- 35.Kuppermann BD, Boyer DS, Mills B, Yang J, Klassen HJ. Safety and activity of a single, intravitreal injection of human retinal progenitor cells (jCell) for treatment of retinitis pigmentosa (RP) Invest Ophthalmol Visual Sci. 2018;59:2987. [Google Scholar]
- 36.Lamba DA, Gust J, Reh TA. Transplantation of human embryonic stem cell-derived photoreceptors restores some visual function in Crx-deficient mice. Cell Stem Cell. 2009;4:73–79. doi: 10.1016/j.stem.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lamba DA, McUsic A, Hirata RK, Wang PR, Russell D, Reh TA. Generation, purification and transplantation of photoreceptors derived from human induced pluripotent stem cells. PLoS One. 2010;5:e8763. doi: 10.1371/journal.pone.0008763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li R, Wen R, Banzon T, Maminishkis A, Miller SS. CNTF mediates neurotrophic factor secretion and fluid absorption in human retinal pigment epithelium. PLoS One. 2011;6:e23148. doi: 10.1371/journal.pone.0023148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li SY, Liu Y, Wang L, Wang F, Zhao TT, Li QY, Xu HW, Meng XH, Hao J, Zhou Q, Wang L, Yin ZQ. A phase I clinical trial of human embryonic stem cell-derived retinal pigment epithelial cells for early-stage Stargardt macular degeneration:5-years’follow-up. Cell Prolif. 2021;54:e13100. doi: 10.1111/cpr.13100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lin B, McLelland BT, Aramant RB, Thomas BB, Nistor G, Keirstead HS, Seiler MJ. Retina organoid transplants develop photoreceptors and improve visual function in RCS rats with RPE dysfunction. Invest Ophthalmol Vis Sci. 2020;61:34. doi: 10.1167/iovs.61.11.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu Y, Chen SJ, Li SY, Qu LH, Meng XH, Wang Y, Xu HW, Liang ZQ, Yin ZQ. Long-term safety of human retinal progenitor cell transplantation in retinitis pigmentosa patients. Stem Cell Res Ther. 2017;8:209. doi: 10.1186/s13287-017-0661-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Logan S, Arzua T, Canfield SG, Seminary ER, Sison SL, Ebert AD, Bai X. Studying human neurological disorders using induced pluripotent stem cells:from 2D monolayer to 3D organoid and blood brain barrier models. Compr Physiol. 2019;9:565–611. doi: 10.1002/cphy.c180025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lu B, Malcuit C, Wang S, Girman S, Francis P, Lemieux L, Lanza R, Lund R. Long-term safety and function of RPE from human embryonic stem cells in preclinical models of macular degeneration. Stem Cells. 2009;27:2126–2135. doi: 10.1002/stem.149. [DOI] [PubMed] [Google Scholar]
- 44.Lund RD, Wang S, Klimanskaya I, Holmes T, Ramos-Kelsey R, Lu B, Girman S, Bischoff N, Sauvé Y, Lanza R. Human embryonic stem cell-derived cells rescue visual function in dystrophic RCS rats. Cloning Stem Cells. 2006;8:189–199. doi: 10.1089/clo.2006.8.189. [DOI] [PubMed] [Google Scholar]
- 45.Luo J, Baranov P, Patel S, Ouyang H, Quach J, Wu F, Qiu A, Luo H, Hicks C, Zeng J, Zhu J, Lu J, Sfeir N, Wen C, Zhang M, Reade V, Patel S, Sinden J, Sun X, Shaw P, et al. Human retinal progenitor cell transplantation preserves vision. J Biol Chem. 2014;289:6362–6371. doi: 10.1074/jbc.M113.513713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mackinlay KM, Weatherbee BA, Souza Rosa V, Handford CE, Hudson G, Coorens T, Pereira LV, Behjati S, Vallier L, Shahbazi MN, Zernicka-Goetz M. An in vitro stem cell model of human epiblast and yolk sac interaction. Elife. 2021;10:e63930. doi: 10.7554/eLife.63930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.MacLaren RE, Bennett J, Schwartz SD. Gene therapy and stem cell transplantation in retinal disease:the new frontier. Ophthalmology. 2016;123:S98–S106. doi: 10.1016/j.ophtha.2016.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Maeda T, Lee MJ, Palczewska G, Marsili S, Tesar PJ, Palczewski K, Takahashi M, Maeda A. Retinal pigmented epithelial cells obtained from human induced pluripotent stem cells possess functional visual cycle enzymes in vitro and in vivo. J Biol Chem. 2013;288:34484–34493. doi: 10.1074/jbc.M113.518571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mandai M, Watanabe A, Kurimoto Y, Hirami Y, Morinaga C, Daimon T, Fujihara M, Akimaru H, Sakai N, Shibata Y, Terada M, Nomiya Y, Tanishima S, Nakamura M, Kamao H, Sugita S, Onishi A, Ito T, Fujita K, Kawamata S, et al. Autologous induced stem-cell-derived retinal cells for macular degeneration. n engl j med. 2017;376:1038–1046. doi: 10.1056/NEJMoa1608368. [DOI] [PubMed] [Google Scholar]
- 50.McLelland BT, Lin B, Mathur A, Aramant RB, Thomas BB, Nistor G, Keirstead HS, Seiler MJ. Transplanted hESC-derived retina organoid sheets differentiate, integrate , and improve visual function in retinal degenerate rats. Invest Ophthalmol Vis Sci. 2018;59:2586–2603. doi: 10.1167/iovs.17-23646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mehat MS, Sundaram V, Ripamonti C, Robson AG, Smith AJ, Borooah S, Robinson M, Rosenthal AN, Innes W, Weleber RG, Lee RWJ, Crossland M, Rubin GS, Dhillon B, Steel DHW, Anglade E, Lanza RP, Ali RR, Michaelides M, Bainbridge JWB. Transplantation of human embryonic stem cell-derived retinal pigment epithelial cells in macular degeneration. Ophthalmology. 2018;125:1765–1775. doi: 10.1016/j.ophtha.2018.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Moisseiev E, Smit-McBride Z, Oltjen S, Zhang P, Zawadzki RJ, Motta M, Murphy CJ, Cary W, Annett G, Nolta JA, Park SS. Intravitreal administration of human bone marrow CD34+ stem cells in a murine model of retinal degeneration. Invest Ophthalmol Vis Sci. 2016;57:4125–4135. doi: 10.1167/iovs.16-19252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Morizane A, Kikuchi T, Hayashi T, Mizuma H, Takara S, Doi H, Mawatari A, Glasser MF, Shiina T, Ishigaki H, Itoh Y, Okita K, Yamasaki E, Doi D, Onoe H, Ogasawara K, Yamanaka S, Takahashi J. MHC matching improves engraftment of iPSC-derived neurons in non-human primates. Nat Commun. 2017;8:385. doi: 10.1038/s41467-017-00926-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Moutinho BD, de Barros JR, Baima JP, Saad-Hossne R, Sassaki LY. Immunosuppression and malignant neoplasms:risk-benefit assessment in patients with inflammatory bowel disease. Am J Case Rep. 2020;21:e920949. doi: 10.12659/AJCR.920949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nakagawa M, Takizawa N, Narita M, Ichisaka T, Yamanaka S. Promotion of direct reprogramming by transformation-deficient Myc. Proc Natl Acad Sci U S A. 2010;107:14152–14157. doi: 10.1073/pnas.1009374107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nashine S. Potential therapeutic candidates for age-related macular degeneration (AMD) Cells. 2021;10:2483. doi: 10.3390/cells10092483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pan T, Shen H, Yuan S, Lu G, Zhang Y, Wang H, Zhao Y, Sun X, Liu Q. Combined transplantation with human mesenchymal stem cells improves retinal rescue effect of human fetal rpe cells in retinal degeneration mouse model. Invest Ophthalmol Vis Sci. 2020;61:9. doi: 10.1167/iovs.61.8.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Park SS, Caballero S, Bauer G, Shibata B, Roth A, Fitzgerald PG, Forward KI, Zhou P, McGee J, Telander DG, Grant MB, Nolta JA. Long-term effects of intravitreal injection of GMP-grade bone-marrow-derived CD34+cells in NOD-SCID mice with acute ischemia-reperfusion injury. Invest Ophthalmol Vis Sci. 2012;53:986–994. doi: 10.1167/iovs.11-8833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Park UC, Park SS, Kim BH, Park SW, Kim YJ, Cary W, Anderson JD, Nolta JA, Yu HG. Subretinal versus intravitreal administration of human CD34+bone marrow-derived stem cells in a rat model of inherited retinal degeneration. Ann Transl Med. 2021;9:1275. doi: 10.21037/atm-20-4662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Puertas-Neyra K, Usategui-Martín R, Coco RM, Fernandez-Bueno I. Intravitreal stem cell paracrine properties as a potential neuroprotective therapy for retinal photoreceptor neurodegenerative diseases. Neural Regen Res. 2020;15:1631–1638. doi: 10.4103/1673-5374.276324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Qiu TG. Transplantation of human embryonic stem cell-derived retinal pigment epithelial cells (MA09-hRPE) in macular degeneration. NPJ Regen Med. 2019;4:19. doi: 10.1038/s41536-019-0081-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Qu L, Gao L, Xu H, Duan P, Zeng Y, Liu Y, Yin ZQ. Combined transplantation of human mesenchymal stem cells and human retinal progenitor cells into the subretinal space of RCS rats. Sci Rep. 2017;7:199. doi: 10.1038/s41598-017-00241-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Qu Z, Guan Y, Cui L, Song J, Gu J, Zhao H, Xu L, Lu L, Jin Y, Xu GT. Transplantation of rat embryonic stem cell-derived retinal progenitor cells preserves the retinal structure and function in rat retinal degeneration. Stem Cell Res Ther. 2015;6:219. doi: 10.1186/s13287-015-0207-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rajendran Nair DS, Zhu D, Sharma R, Martinez Camarillo JC, Bharti K, Hinton DR, Humayun MS, Thomas BB. Long-term transplant effects of iPSC-RPE monolayer in immunodeficient RCS rats. Cells. 2021;10:2951. doi: 10.3390/cells10112951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Read SP, Cashman SM, Kumar-Singh R. POD nanoparticles expressing GDNF provide structural and functional rescue of light-induced retinal degeneration in an adult mouse. Mol Ther. 2010;18:1917–1926. doi: 10.1038/mt.2010.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Reichman S, Terray A, Slembrouck A, Nanteau C, Orieux G, Habeler W, Nandrot EF, Sahel JA, Monville C, Goureau O. From confluent human iPS cells to self-forming neural retina and retinal pigmented epithelium. Proc Natl Acad Sci U S A. 2014;111:8518–8523. doi: 10.1073/pnas.1324212111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Riera M, Fontrodona L, Albert S, Ramirez DM, Seriola A, Salas A, Muñoz Y, Ramos D, Villegas-Perez MP, Zapata MA, Raya A, Ruberte J, Veiga A, Garcia-Arumi J. Comparative study of human embryonic stem cells (hESC) and human induced pluripotent stem cells (hiPSC) as a treatment for retinal dystrophies. Mol Ther Methods Clin Dev. 2016;3:16010. doi: 10.1038/mtm.2016.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Salas A, Duarri A, Fontrodona L, Ramírez DM, Badia A, Isla-Magrané H, Ferreira-de-Souza B, Zapata M, Raya Á, Veiga A, García-Arumí J. Cell therapy with hiPSC-derived RPE cells and RPCs prevents visual function loss in a rat model of retinal degeneration. Mol Ther Methods Clin Dev. 2021;20:688–702. doi: 10.1016/j.omtm.2021.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schwartz SD, Hubschman JP, Heilwell G, Franco-Cardenas V, Pan CK, Ostrick RM, Mickunas E, Gay R, Klimanskaya I, Lanza R. Embryonic stem cell trials for macular degeneration:a preliminary report. Lancet. 2012;379:713–720. doi: 10.1016/S0140-6736(12)60028-2. [DOI] [PubMed] [Google Scholar]
- 70.Schwartz SD, Regillo CD, Lam BL, Eliott D, Rosenfeld PJ, Gregori NZ, Hubschman JP, Davis JL, Heilwell G, Spirn M, Maguire J, Gay R, Bateman J, Ostrick RM, Morris D, Vincent M, Anglade E, Del Priore LV, Lanza R. Human embryonic stem cell-derived retinal pigment epithelium in patients with age-related macular degeneration and Stargardt’s macular dystrophy:follow-up of two open-label phase 1/2 studies. Lancet. 2015;385:509–516. doi: 10.1016/S0140-6736(14)61376-3. [DOI] [PubMed] [Google Scholar]
- 71.Sharma R, Khristov V, Rising A, Jha BS, Dejene R, Hotaling N, Li Y, Stoddard J, Stankewicz C, Wan Q, Zhang C, Campos MM, Miyagishima KJ, McGaughey D, Villasmil R, Mattapallil M, Stanzel B, Qian H, Wong W, Chase L, et al. Clinical-grade stem cell-derived retinal pigment epithelium patch rescues retinal degeneration in rodents and pigs. Sci Transl Med. 2019;11:eaat5580. doi: 10.1126/scitranslmed.aat5580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shirai H, Mandai M, Matsushita K, Kuwahara A, Yonemura S, Nakano T, Assawachananont J, Kimura T, Saito K, Terasaki H, Eiraku M, Sasai Y, Takahashi M. Transplantation of human embryonic stem cell-derived retinal tissue in two primate models of retinal degeneration. Proc Natl Acad Sci U S A. 2016;113:E81–90. doi: 10.1073/pnas.1512590113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shrestha R, Wen YT, Tsai RK. Induced pluripotent stem cells and derivative photoreceptor precursors as therapeutic cells for retinal degenerations. Ci Ji Yi Xue Za Zhi. 2019;32:101–112. doi: 10.4103/tcmj.tcmj_147_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Song WK, Park KM, Kim HJ, Lee JH, Choi J, Chong SY, Shim SH, Del Priore LV, Lanza R. Treatment of macular degeneration using embryonic stem cell-derived retinal pigment epithelium:preliminary results in Asian patients. Stem Cell Reports. 2015;4:860–872. doi: 10.1016/j.stemcr.2015.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Spiteri Cornish K, Ho J, Downes S, Scott NW, Bainbridge J, Lois N. The epidemiology of Stargardt disease in the United Kingdom. Ophthalmol Retina. 2017;1:508–513. doi: 10.1016/j.oret.2017.03.001. [DOI] [PubMed] [Google Scholar]
- 76.Stern JH, Tian Y, Funderburgh J, Pellegrini G, Zhang K, Goldberg JL, Ali RR, Young M, Xie Y, Temple S. Regenerating eye tissues to preserve and restore vision. Cell Stem Cell. 2018;22:834–849. doi: 10.1016/j.stem.2018.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Stingl K, Kempf M, Bartz-Schmidt KU, Dimopoulos S, Reichel F, Jung R, Kelbsch C, Kohl S, Kortüm FC, Nasser F, Peters T, Wilhelm B, Wissinger B, Wozar F, Zrenner E, Fischer MD, Stingl K. Spatial and temporal resolution of the photoreceptors rescue dynamics after treatment with voretigene neparvovec. Br J Ophthalmol. 2022;106:831–838. doi: 10.1136/bjophthalmol-2020-318286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sugita S, Mandai M, Hirami Y, Takagi S, Maeda T, Fujihara M, Matsuzaki M, Yamamoto M, Iseki K, Hayashi N, Hono A, Fujino S, Koide N, Sakai N, Shibata Y, Terada M, Nishida M, Dohi H, Nomura M, Amano N, et al. HLA-matched allogeneic iPS cells-derived RPE transplantation for macular degeneration. J Clin Med. 2020;9:2217. doi: 10.3390/jcm9072217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sun J, Mandai M, Kamao H, Hashiguchi T, Shikamura M, Kawamata S, Sugita S, Takahashi M. Protective effects of human iPS-derived retinal pigmented epithelial cells in comparison with human mesenchymal stromal cells and human neural stem cells on the degenerating retina in rd1 mice. Stem Cells. 2015;33:1543–1553. doi: 10.1002/stem.1960. [DOI] [PubMed] [Google Scholar]
- 80.Surendran H, Nandakumar S, Reddy K VB, Stoddard J, Mohan K V, Upadhyay PK, McGill TJ, Pal R. Transplantation of retinal pigment epithelium and photoreceptors generated concomitantly via small molecule-mediated differentiation rescues visual function in rodent models of retinal degeneration. Stem Cell Res Ther. 2021;12:70. doi: 10.1186/s13287-021-02134-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tackenberg MA, Tucker BA, Swift JS, Jiang C, Redenti S, Greenberg KP, Flannery JG, Reichenbach A, Young MJ. Müller cell activation, proliferation and migration following laser injury. Mol Vis. 2009;15:1886–1896. [PMC free article] [PubMed] [Google Scholar]
- 82.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 83.Tanna P, Strauss RW, Fujinami K, Michaelides M. Stargardt disease:clinical features, molecular genetics, animal models and therapeutic options. Br J Ophthalmol. 2017;101:25–30. doi: 10.1136/bjophthalmol-2016-308823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Taylor CJ, Bolton EM, Pocock S, Sharples LD, Pedersen RA, Bradley JA. Banking on human embryonic stem cells:estimating the number of donor cell lines needed for HLA matching. Lancet. 2005;366:2019–2025. doi: 10.1016/S0140-6736(05)67813-0. [DOI] [PubMed] [Google Scholar]
- 85.Thomas BB, Zhu D, Zhang L, Thomas PB, Hu Y, Nazari H, Stefanini F, Falabella P, Clegg DO, Hinton DR, Humayun MS. Survival and functionality of hESC-derived retinal pigment epithelium cells cultured as a monolayer on polymer substrates transplanted in RCS rats. Invest Ophthalmol Vis Sci. 2016;57:2877–2887. doi: 10.1167/iovs.16-19238. [DOI] [PubMed] [Google Scholar]
- 86.Tu HY, Watanabe T, Shirai H, Yamasaki S, Kinoshita M, Matsushita K, Hashiguchi T, Onoe H, Matsuyama T, Kuwahara A, Kishino A, Kimura T, Eiraku M, Suzuma K, Kitaoka T, Takahashi M, Mandai M. Medium- to long-term survival and functional examination of human iPSC-derived retinas in rat and primate models of retinal degeneration. EBioMedicine. 2019;39:562–574. doi: 10.1016/j.ebiom.2018.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tucker BA, Park IH, Qi SD, Klassen HJ, Jiang C, Yao J, Redenti S, Daley GQ, Young MJ. Transplantation of adult mouse iPS cell-derived photoreceptor precursors restores retinal structure and function in degenerative mice. PLoS One. 2011;6:e18992. doi: 10.1371/journal.pone.0018992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.US Food and Drug Administration (2019) FDA Warns About Stem Cell Therapies. [Accessed September 3, 2019]; https://www.fda.gov/consumers/consumer-updates/fda-warns-about-stem-cell-therapies. [Google Scholar]
- 89.Verbakel SK, van Huet RAC, Boon CJF, den Hollander AI, Collin RWJ, Klaver CCW, Hoyng CB, Roepman R, Klevering BJ. Non-syndromic retinitis pigmentosa. Prog Retin Eye Res. 2018;66:157–186. doi: 10.1016/j.preteyeres.2018.03.005. [DOI] [PubMed] [Google Scholar]
- 90.Voisin A, Monville C, Plancheron A, Béré E, Gaillard A, Leveziel N. Cathepsin B pH-dependent activity is involved in lysosomal dysregulation in atrophic age-related macular degeneration. Oxid Med Cell Longev 2019. 2019 doi: 10.1155/2019/5637075. 5637075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Voisin A, Gaillard A, Balbous A, Leveziel N. Proteins associated with phagocytosis alteration in retinal pigment epithelial cells derived from age-related macular degeneration patients. Antioxidants (Basel) 2022;11:713. doi: 10.3390/antiox11040713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Waldron PV, Di Marco F, Kruczek K, Ribeiro J, Graca AB, Hippert C, Aghaizu ND, Kalargyrou AA, Barber AC, Grimaldi G, Duran Y, Blackford SJI, Kloc M, Goh D, Zabala Aldunate E, Sampson RD, Bainbridge JWB, Smith AJ, Gonzalez-Cordero A, Sowden JC, et al. Transplanted donor- or stem cell-derived cone photoreceptors can both integrate and undergo material transfer in an environment-dependent manner. Stem Cell Reports. 2018;10:406–421. doi: 10.1016/j.stemcr.2017.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wang NK, Tosi J, Kasanuki JM, Chou CL, Kong J, Parmalee N, Wert KJ, Allikmets R, Lai CC, Chien CL, Nagasaki T, Lin CS, Tsang SH. Transplantation of reprogrammed embryonic stem cells improves visual function in a mouse model for retinitis pigmentosa. Transplantation. 2010;89:911–919. doi: 10.1097/TP.0b013e3181d45a61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wang Z, Gao F, Zhang M, Zheng Y, Zhang F, Xu L, Cao L, He W. Intravitreal injection of human retinal progenitor cells for treatment of retinal degeneration. Med Sci Monit. 2020;26:e921184. doi: 10.12659/MSM.921184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wiącek MP, Gosławski W, Grabowicz A, Sobuś A, Kawa MP, Baumert B, Paczkowska E, Milczarek S, Osękowska B, Safranow K, Zawiślak A, Lubiński W, Machaliński B, Machalińska A. Long-term effects of adjuvant intravitreal treatment with autologous bone marrow-derived lineage-negative cells in retinitis pigmentosa. Stem Cells Int 2021. 2021 doi: 10.1155/2021/6631921. 6631921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wilson DJ, Neuringer M, Stoddard J, Renner LM, Bailey S, Lauer A, McGill TJ. Subretinal cell-based therapy:an analysis of surgical variables to increase cell survival. Retina. 2017;37:2162–2166. doi: 10.1097/IAE.0000000000001462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wu W, Zeng Y, Li Z, Li Q, Xu H, Yin ZQ. Features specific to retinal pigment epithelium cells derived from three-dimensional human embryonic stem cell cultures - a new donor for cell therapy. Oncotarget. 2016;7:22819–22833. doi: 10.18632/oncotarget.8185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhang K, Hopkins JJ, Heier JS, Birch DG, Halperin LS, Albini TA, Brown DM, Jaffe GJ, Tao W, Williams GA. Ciliary neurotrophic factor delivered by encapsulated cell intraocular implants for treatment of geographic atrophy in age-related macular degeneration. Proc Natl Acad Sci U S A. 2011;108:6241–6245. doi: 10.1073/pnas.1018987108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhao HX, Jiang F, Zhu YJ, Wang L, Li K, Li Y, Wang XH, Li LS, Yao YQ. Enhanced immunological tolerance by HLA-G1 from neural progenitor cells (NPCs) derived from human embryonic stem cells (hESCs) Cell Physiol Biochem. 2017;44:1435–1444. doi: 10.1159/000485539. [DOI] [PubMed] [Google Scholar]
- 100.Zhong X, Gutierrez C, Xue T, Hampton C, Vergara MN, Cao LH, Peters A, Park TS, Zambidis ET, Meyer JS, Gamm DM, Yau KW, Canto-Soler MV. Generation of three-dimensional retinal tissue with functional photoreceptors from human iPSCs. Nat Commun. 2014;5 doi: 10.1038/ncomms5047. 4047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhou L, Wang W, Liu Y, Fernandez de Castro J, Ezashi T, Telugu BP, Roberts RM, Kaplan HJ, Dean DC. Differentiation of induced pluripotent stem cells of swine into rod photoreceptors and their integration into the retina. Stem Cells. 2011;29:972–980. doi: 10.1002/stem.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhu D, Xie M, Gademann F, Cao J, Wang P, Guo Y, Zhang L, Su T, Zhang J, Chen J. Protective effects of human iPS-derived retinal pigmented epithelial cells on retinal degenerative disease. Stem Cell Res Ther. 2020;11:98. doi: 10.1186/s13287-020-01608-8. [DOI] [PMC free article] [PubMed] [Google Scholar]


