Abstract
This study measures patient’s concordance between clinical reference pathways with survival or cost among a population-based cohort of colon cancer patients applying a continuous measure of concordance. The primary hypothesis is that a higher concordance score with the clinical pathway is significantly associated with longer survival or lower cost. The study informs whether patient’s adherence to a defined clinical pathway is beneficial to patients’ outcomes or health system. An externally determined clinical pathway for colon cancer was used to identify treatment nodes in colon cancer care. Using observational data up to 2019, the study generated a continuous measure of pathway concordance. The study measured whether incremental improvements in pathway concordance were associated with survival and treatment costs. Concordance between patients’ reference pathways and their observed trajectories of care was highly statistically associated with survivorship [hazard ratio: 0.95 (95% confidence interval, CI, 0.95–0.96)], showing that adherence to the clinical pathway was associated with a lower mortality rate. An increase in concordance was statistically significantly associated with a decrease in health system cost. When patients’ care followed the clinical pathway, survival outcomes were better and total health system costs were lower in this cohort. This finding creates a compelling case for further research into understanding the barriers to pathway concordance and developing interventions to improve outcomes and help providers implement best practice care where appropriate.
Keywords: clinical pathway, colon cancer, concordance, cost, outcomes, survival
Introduction
Clinical pathways are an informational resource to support clinical judgment and present management options for standardized courses of care for a particular condition to improve treatment outcomes [1, 2]. Clinical pathways have been associated with reduced variability in clinical practice patterns, utilization rates, and patient length of stay; lower treatment, outpatient, and overall costs; and reduced inpatient mortality [3–7].
Implementation of clinical pathways has been slow. Barriers have included clinician reluctance due to a perceived loss of autonomy, outdated pathways, and inadequate resources for extracting clinical data from information systems [2, 4, 8]. Further contributing factors include the wide variation in the quality, content, and scope of clinical pathways and the limited evidence of their effectiveness [9, 10].
A systematic review of clinical pathway evaluation research identified cardiovascular, respiratory, gastrointestinal, and orthopaedic surgeries and oncology pathways as the most commonly studied clinical pathway diseases and interventions [10]. The impact of reducing variability in treatment may be relatively higher in cancer care than other clinical conditions, as cancer treatment often involves coordination between disciplines, costly interventions, and possibly multiple phases of treatment.
Clinical pathway concordance is an assessment of the similarity between the continuum of care presented by the pathway and a patient’s observed trajectory of care through care phases, settings, and treatment modalities. Simple measures of concordance indicate that a patient’s care was either ‘on’ or ‘off’ individual elements of the pathway, whereas more sophisticated measures of concordance indicate how closely a patient’s observed trajectory of care aligns with the pathway across multiple elements.
Previous studies have indicated that concordant care is associated with lower costs and similar or improved clinical outcomes [3, 5, 11]. Pathway concordance in bronchiolitis and syncope has been associated with lower lengths of stay and inpatient admissions [5, 6]. However, studies of pathway concordance among cancer populations have shown similar survival between ‘on’ and ‘off’ pathway subgroups [3, 11]. However, these studies are limited by their study population size, clinical setting (e.g. clinical pathways within the hospital only), and the definition of concordance measure (e.g. examining only parts of the full clinical pathway).
Ontario Health (previously named Cancer Care Ontario) is a provincial agency in Ontario, Canada, that provides oversight for the delivery of cancer services in the province. Ontario Health uses pathway maps as a quality improvement tool [12]. Pathway maps describe the recommended care for the disease/condition, encompassing prevention and screening to follow-up, surveillance, and palliative care.
This study employs Ontario Health’s Colon Cancer Pathway Map to measure the association between pathway concordance with survival and cost outcomes for colon cancer patients in Ontario. Colorectal cancer (including colon and rectal cancers) is the second most common cancer among Canadians with an estimated 26 800 new cases annually and is responsible for 11% of cancer deaths [13, 14].
This study measures whether incremental improvements in pathway concordance are associated with improved survival and lower health system spending using a population-level cohort. The results shed light on individual patients’ care continua relative to published pathways and identify opportunities for improving health and clinical outcomes among colon cancer patients in Ontario. This study provides novel information to influence policies regarding cancer treatment and the value of applying clinical pathways to population-level data to improve health and outcomes.
Methods
Study cohort
The study cohort included all Ontario residents with a valid Health Insurance Number who had an incident diagnosis of colon cancer between 28 September 2012 and 31 December 2016 and a malignant neoplasm of stage IIA, IIB, or IIC [15]. Patients were identified from the Ontario Cancer Registry, a population-based registry. Colon cancer was identified by International Classification of Diseases for Oncology codes (Supplementary Appendix A).
Patients with invalid or missing administrative data (e.g. neighbourhood income or immigration data) were excluded from the cohort. Patients who received surgery before diagnosis, patients receiving noncurative treatment, nonincident cases, cases identified at autopsy, cases with unknown histology, multiple primaries, or a nonrelevant diagnosis were also removed from the study.
The cohort was followed from 180 days prior to diagnosis to 4 years after diagnosis or 31 March 2019, whichever was earliest. 31 March 2019 corresponded with the end of the observational period, administrative censoring, and was the end of the setting’s fiscal year. The follow-up period ensured that the 4-year observation window was observable for all but those patients diagnosed in the last year of the cohort.
A number of patient characteristics and demographics were also included due to previous literature showing their association with cancer outcomes: age, sex, cancer substage, tumour grade, indicator of emergency admission for surgery, comorbidity measured by the Charlson comorbidity index, screening status, rurality (urban versus rural), percentage of neighbourhood population who were immigrants (reported in terciles), and neighbourhood income (reported in quintiles). Indicators of the number of outpatient, emergency department, and chemotherapy visits and surgical length of stay were also included in the survival analysis. Terciles and quintiles represent three and five equally sized subgroups to aid presentation of the data, respectively.
Pathway maps
This study was based on Ontario Health’s Colon Cancer Stage II Pathway Map for diagnosis and curative treatment [16]. Stage II colon cancer patients undergoing curative treatment were selected for this analysis of pathway concordance since the condition reflects a condition for which the mortality rate has a mix of survivors and decedents at the time of administrative censoring. The Stage II colon cancer pathway included endoscopy, computed tomography of the abdomen and pelvis, surgical resection, medical oncologist consultation, and chemotherapy (see Supplementary Appendix B for data sources). Simplified pathway maps have been evaluated elsewhere.
Concordance measure
One approach to measuring concordance between the reference pathway and patients’ observed trajectories of care converts observed healthcare utilization into character strings, where each unique character represents a different encounter with the healthcare system. For example, a unique character represents endoscopy (‘A’) and another represents surgical resection (‘C’). A distance measure is then calculated to represent the ‘difference’ between the character strings, a value representing how far patients’ observed healthcare utilization and the reference pathway diverge [17]. In this study, a modified Levenshtein distance measure looking only at insertions and deletions was used to calculate a concordance measure (Supplementary Appendix C), an integer count of the minimum number of character insertions and deletions required to transform the patients’ observed healthcare utilization to the reference pathway, normalized to range between 0 (complete discordance) and 100 (perfect concordance). An example of the application of the method for calculating the distance measure is shown in Fig. 1, showing that two deletions and one insertion were required to transform to the reference pathway. This measure has been piloted elsewhere [18] although other approaches to evaluating pathway concordance are also available including approaches that may provide different concordance statistics using substitutions or transpositions [19–21].
Figure 1.

Example of the Levenshtein distance algorithm; the algorithm counts the minimum number of insertions and deletions of letters required to make the observed pathway equal to a reference pathway; three changes were required to transform the patient pathway (A–F–B–F) into the reference pathway (A–B–C).
Survival
Patient survival was observable from Ontario’s Cancer Registry or Ontario’s Registered Persons Database, population-based registries that include the date of death. Survival was measured as the time between cancer surgery and the earliest of date of death, 4 years postdiagnosis or 31 March 2019.
Patient cost
Population-based clinical and administrative datasets were used to determine patients’ total publicly funded healthcare costs. The costs were determined by linking setting-specific utilization and cost over the observation period [22]. Patients’ costs were measured starting 180 days prior to their diagnosis date and ending one year after their diagnosis, and the duration of the observation period was longer than recent colon cancer treatment pathways or guidelines. Costs encompassed total identifiable healthcare costs for each patient, which included all public funding on healthcare whether related to their colon cancer diagnosis, although excluded patient or private insurance-borne costs (Supplementary Appendix D) [23]. All healthcare costs were adjusted for inflation to 2017 dollars [24].
Statistical analysis
The study cohort’s demographic characteristics were summarized, stratified by survivorship, concordance, and cost (Appendix Table S1). Patients’ survivorship over the observation period was reported by ‘yes’ or ‘no’. Summaries of patients’ concordance and cost were reported by tercile. Tercile (three equal-sized groups) was used to categorize concordance and cost to facilitate presentation, although continuously valued measures were used in the survival and cost regression analysis.
For presentation, health services utilization and patients’ cost were stratified by concordance tercile and healthcare sector; the healthcare sectors were presented in descending order with the sector contributing the highest proportion of total costs listed first. Multiple sectors were aggregated together for the ‘Other sectors’ section, as each sector individually made a small contribution to total cost and collectively, they contributed less than 20% of total costs across all terciles, with sectors shown in Supplementary Appendix E.
To measure factors associated with all-cause mortality, a time-dependent Cox proportional hazards model was used. The primary hypothesis was that a higher concordance score was significantly associated with longer survival. The time-dependent concordance metric was measured from 180 days prior to diagnosis to 1-year postdiagnosis.
The Cox regression was adjusted for age, sex, cancer substage, tumour grade, indicator of emergency surgery, comorbidity, screening status, the number of outpatient visits in the year prior to the observation period, an indicator of whether the inpatient length of stay was over 5 days following surgery, rurality, neighbourhood immigrant population tercile, and neighbourhood income quintiles. The concordance score and the number of ED visits and chemotherapy treatments after surgery and up to 1-year after diagnosis were included as time-dependent covariates in the model. The Martingale and Schoenfeld residuals were examined for violation of covariate linearity and proportionality of hazards.
For the Cox model, results were compared to a reference case: a male patient, 55 years old or younger, cancer substage A, low-grade tumour, planned surgery, no comorbidities, no screening history, no outpatient, chemotherapy or ED visits, surgical length of stay less than 5 days, living in a rural neighbourhood, lowest neighbourhood immigrant tercile, and lowest neighbourhood income quintile.
A generalized linear model with a Wald distribution and log-link function was used to model the relationship between patient cost and concordance, adjusting for patient and health system characteristics. The model was selected based on criteria outlined in the study by Manning [25], with the addition of a Vuong test for non-nested models. The regression model was adjusted for age, sex, cancer substage, tumour grade, indicator of emergency surgery, comorbidity, screening status, rurality, neighbourhood immigrant terciles, and neighbourhood income quintiles.
To assess the sensitivity of including cases with short follow-up due to death, a subanalysis was performed, which excluded cases where the patient died within 1 year of diagnosis. The differences between the cost analyses of the entire cohort and the cohort with first-year deaths removed were compared.
Simulations using the output of the Wald model were used to estimate the expected total costs and 95% confidence intervals (CIs) for a standard patient at each concordance value from 0 to 100. Simulations for the expected total cost and CIs were run using the algorithm outlined by King [26].
Results
Patient characteristics
The analyses included 4077 incident patients with Stage II colon cancer. Within the cohort, 83% survived the entire observation period. Those who survived tended to be younger and had fewer comorbidities. Those who did not survive were more likely to be over 75 years of age or male; have a history of higher healthcare utilization (e.g. outpatient and ED visits and longer length of stay), emergency surgery, and more comorbidities or high-grade tumours with a cancer substage of B or C; and were less likely to have undergone cancer screening or received chemotherapy treatment.
Patients in the lowest concordance tercile (low was less than 0.415 and high was greater than 0.552) were more likely to be over 75 years old; be male; have a history of more healthcare utilization, emergency surgery, more comorbidities, or tumours with unknown grade or a cancer substage of B or C; and have no history of screening.
This study found that the highest cost patients were: more likely to be over 75 years old, male, to have a history of more health care utilization, emergency surgery, more comorbidities, or tumours with unknown grade, a cancer substage of B or C, and no history of cancer screening. Neighbourhood characteristics (urban/rural, immigration tercile, and income quintile) were not significantly different between the cost terciles. Appendix Figure S2 illustrates the unadjusted relationship between the measure of concordance and health system costs. A negative relationship between concordance and cost was observed although there was variation in the data.
Health services utilization
As shown in Table 1, patient cost was highest among those with the lowest pathway concordance, while cost was lowest among those with the highest concordance. The average total healthcare cost over the observation period for a person in the lowest concordance tercile was twice as high as the average cost for a patient in the medium concordance group (∼$71 000 vs. $35 000) and nearly triple the cost in the highest concordance group (∼$24 000). Acute inpatient admissions represented the largest proportion of patients’ costs among all concordance categories, followed by specialist physician charges, collectively accounting for between 65% and 70% of costs across all categories. These results illustrated unadjusted associations between cost and concordance although factors such as age and clinical complexity were likely influencing the unadjusted results.
Table 1.
Description of health services utilization and corresponding costs by concordance tercile.
| Sector | Concordance tercile | N | Average visits | Average costs ($) | Total cost ($) | Percent of total cost |
|---|---|---|---|---|---|---|
| Total costs | High | 1373 | 24 261 | 33 309 827 | ||
| Total costs | Medium | 1346 | 34 918 | 46 999 619 | ||
| Total costs | Low | 1358 | 70 462 | 95 687 668 | ||
| Inpatient | High | 1373 | 1.2 | 12 054 | 16 550 112 | 50 |
| Inpatient | Medium | 1346 | 1.5 | 16 683 | 22 455 502 | 48 |
| Inpatient | Low | 1358 | 2.4 | 37 893 | 51 458 170 | 54 |
| Specialist physicans | High | 1373 | 21.8 | 4788 | 6 573 414 | 20 |
| Specialist physicans | Medium | 1346 | 28.3 | 6102 | 8 213 625 | 17 |
| Specialist physicans | Low | 1358 | 45.7 | 9736 | 13 221 981 | 14 |
| Home care | High | 1373 | 11.9 | 1138 | 1 562 727 | 5 |
| Home care | Medium | 1346 | 21.0 | 2119 | 2 852 536 | 6 |
| Home care | Low | 1358 | 50.5 | 5307 | 7 207 522 | 8 |
| Outpatient oncology | High | 1373 | 1.2 | 1314 | 1 804 059 | 5 |
| Outpatient oncology | Medium | 1346 | 1.6 | 1758 | 2 366 592 | 5 |
| Outpatient oncology | Low | 1358 | 3.9 | 3598 | 4 886 627 | 5 |
| Ontario drug benefit | High | 1373 | 17.9 | 1601 | 2 198 104 | 7 |
| Ontario drug benefit | Medium | 1346 | 21.9 | 1973 | 2 656 141 | 6 |
| Ontario drug benefit | Low | 1358 | 28.0 | 2448 | 3 323 853 | 3 |
| Other sectors | High | 1373 | 3366 | 4 621 411 | 14 | |
| Other sectors | Medium | 1346 | 6282 | 8 455 222 | 18 | |
| Other sectors | Low | 1358 | 11 480 | 15 589 515 | 16 |
Survival outcomes
Concordance between patients’ reference pathways and their observed trajectories of care was highly statistically associated with survivorship [hazard ratio: 0.95 (95% CI: 0.95–0.96)], adjusting for other patient characteristics and system-level variables (shown in Table 2). The model coefficients showed that a one-point increase in concordance was associated with a 5% lower death rate. Violation of the proportional hazards or linearity assumption was not detected.
Table 2.
Cox proportional hazards model output showing the relationship between 4-year survival and concordance for Stage II.
| 95% CI | ||||
|---|---|---|---|---|
| Regression variables | Hazard ratio | Lower | Upper | P-Value |
| Concordance (% similar) | 0.95 | 0.95 | 0.96 | 0.00 |
| Age (reference = ≤ 55 years) | ||||
| 56–64 years | 2.75 | 1.44 | 5.25 | 0.00 |
| 65–74 years | 4.45 | 2.44 | 8.12 | 0.00 |
| 75 years or greater | 11.51 | 6.41 | 20.68 | 0.00 |
| Sex (reference = male) | ||||
| Female | 0.76 | 0.66 | 0.89 | 0.00 |
| Cancer substage (reference = stage A) | ||||
| Stage B | 1.48 | 1.20 | 1.83 | 0.00 |
| Stage C | 1.31 | 0.98 | 1.76 | 0.07 |
| Tumour grade (reference = low grade) | ||||
| High grade | 1.33 | 1.08 | 1.64 | 0.01 |
| Unknown | 0.66 | 0.38 | 1.16 | 0.15 |
| Emergency surgery | 1.48 | 1.24 | 1.76 | 0.00 |
| Comorbidity score | 1.13 | 1.06 | 1.19 | 0.00 |
| Screening (reference = none) | ||||
| Diagnostic | 0.96 | 0.75 | 1.23 | 0.73 |
| Repeated | 0.84 | 0.50 | 1.41 | 0.51 |
| Sporadic | 0.90 | 0.75 | 1.08 | 0.26 |
| Outpatient visits (reference = none) | ||||
| 1 to 4 | 1.12 | 0.91 | 1.38 | 0.30 |
| 5 or more | 1.00 | 0.82 | 1.23 | 0.99 |
| Emergency Department visits (reference = none) | ||||
| 1 or 2 | 1.26 | 1.01 | 1.58 | 0.04 |
| 3 or more | 1.37 | 1.05 | 1.79 | 0.02 |
| Chemotherapy visits (reference = none) | ||||
| 1 to 4 | 1.02 | 0.61 | 1.70 | 0.94 |
| 5 to 8 | 0.79 | 0.46 | 1.37 | 0.41 |
| 8 or more | 0.68 | 0.38 | 1.24 | 0.21 |
| Inpatient surgery, Length of Stay > 5 days | 1.13 | 0.95 | 1.34 | 0.18 |
| Urban residency | 0.96 | 0.77 | 1.20 | 0.73 |
| Neighbourhood immigrant tercile (reference = lowest) | ||||
| Highest | 0.74 | 0.59 | 0.94 | 0.01 |
| Middle | 0.87 | 0.72 | 1.06 | 0.16 |
| Neighbourhood income quintile (reference = lowest) | ||||
| Highest | 0.70 | 0.55 | 0.90 | 0.01 |
| Medium | 0.80 | 0.63 | 1.01 | 0.06 |
| Middle | 0.71 | 0.56 | 0.89 | 0.00 |
| Medium to low | 0.88 | 0.71 | 1.09 | 0.26 |
Other covariates that were statistically significantly associated with shorter survival included age, being male, cancer Substage B relative to Substage A, high-grade versus low-grade tumour, comorbidity score, emergency surgery, and three or more emergency department visits. Covariates that were statistically significantly associated with longer survival included highest neighbourhood immigrant tercile relative to the lowest and neighbourhood income quintile of middle or higher relative to the lowest.
Cost outcomes
Table 3 presents the results of the cost model based on a Wald distribution and log-link function. The cost regression model coefficients showed that a one-point increase in pathway concordance was associated with a decrease in cost of approximately 2%, or $2450, adjusting for patient- and system-level factors. The coefficient of the concordance score was −0.022 (95% CI: −0.023, −0.021) and was statistically significant. Other covariates that were statistically significantly associated with higher total costs were age above 64 years, being male, cancer Substage of B or C relative to A, emergency surgery, comorbidity, and lowest neighbourhood immigrant tercile. Any cancer screening, such as repeated, diagnostic, or sporadic, was associated with a decrease in total costs.
Table 3.
Generalized linear model of total costs, measured with Wald distribution. Coefficients are presented on the model’s log-scale; to determine marginal monetary effects, exponentiate the coefficients as a proportion of the intercept.
| Regression variables | Coefficient | 95 % lower | 95 % upper | P-Value |
|---|---|---|---|---|
| Concordance (% similar) | −0.022 | −0.023 | −0.021 | 0.000 |
| Intercept | 11.44 | 11.33 | 11.54 | 0.00 |
| Age (reference = ≤ 55 years) | ||||
| 56–64 years | 0.06 | −0.01 | 0.13 | 0.07 |
| 65–74 years | 0.12 | 0.06 | 0.18 | 0.00 |
| 75 years or greater | 0.20 | 0.14 | 0.26 | 0.00 |
| Sex (reference = male) | ||||
| Female | −0.05 | −0.08 | −0.01 | 0.02 |
| Cancer substage (reference = stage A) | ||||
| Stage B | 0.23 | 0.16 | 0.30 | 0.00 |
| Stage C | 0.30 | 0.20 | 0.41 | 0.00 |
| Tumour grade (reference = low grade) | ||||
| High grade | 0.06 | 0.00 | 0.13 | 0.07 |
| Unknown | 0.08 | −0.03 | 0.21 | 0.18 |
| Emergency surgery | 0.22 | 0.16 | 0.27 | 0.00 |
| Comorbidity score (reference = none) | ||||
| 1 or 2 | 0.12 | 0.06 | 0.18 | 0.00 |
| 3 or more | 0.36 | 0.28 | 0.45 | 0.00 |
| Screening (reference = none) | ||||
| Diagnostic | −0.08 | −0.13 | −0.02 | 0.01 |
| Repeated | −0.13 | −0.23 | −0.03 | 0.01 |
| Sporadic | −0.09 | −0.13 | −0.05 | 0.00 |
| Urban residency | −0.01 | −0.06 | 0.05 | 0.82 |
| Neighbourhood immigrant tercile (reference = lowest) | ||||
| Highest | −0.08 | −0.14 | −0.03 | 0.00 |
| Middle | −0.05 | −0.10 | 0.00 | 0.03 |
| Neighbourhood income quintile (reference = lowest) | ||||
| Highest | −0.05 | −0.11 | 0.01 | 0.12 |
| Medium | −0.04 | −0.10 | 0.02 | 0.15 |
| Middle | −0.04 | −0.10 | 0.02 | 0.22 |
| Medium to low | −0.01 | −0.07 | 0.05 | 0.66 |
To assess the sensitivity of the cost analyses by including cases with short follow-up due to death, cases where the patient died within the year following diagnosis were excluded (a total of 307 cases). The effect of concordance on cost in the subanalysis was nearly identical to the main analysis, so these cases with short follow-up were not removed and were included in the presented analysis.
Figure 2 illustrates the expected cost, and 95% CIs, as a function of the concordance measure for a ‘standard patient’, describing a significant decrease in expected total cost from ∼$99 515 for a concordance value of 0 (discordant) to $10 800 for a concordance value of 100 (fully concordant). The covariate values were male, 65 to 74 years old, cancer Substage A, low-grade tumour, planned surgery, no comorbidities, no screening history, living in an urban neighbourhood with the lowest neighbourhood immigrant tercile, and medium to high neighbourhood income quintile.
Figure 2.
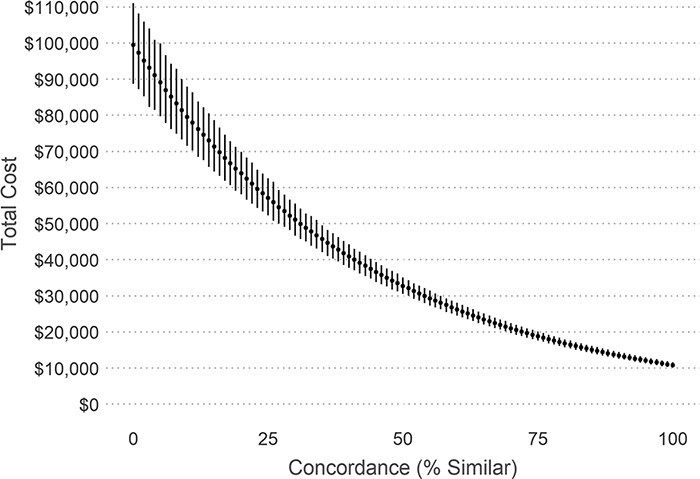
Association between pathway concordance and expected total costs for the standard patient; as pathway concordance increases, patients’ Stage II colon cancer costs tend to decrease.
Discussion
Statement of principal findings
Clinical pathways provide information to patients, providers, and healthcare administrators to understand treatment patterns over time, evaluate practice patterns, and facilitate system planning. This study found that higher concordance to the reference pathway had a statistically significant positive association with longer survival and negative association with health care costs, consistent with prior findings employing simpler concordance measures [11, 27].
Interpretation within the context of the wider literature
This study was novel in its application of clinical pathways to population-based clinical and cost datasets in the most populous province in Canada, where the majority of cancer-related healthcare costs were publicly funded. Moreover, the costs of care for colon cancer patients reported in this study were similar to results from other cancer cost studies conducted in Ontario [28–30], a finding that provides a higher degree of confidence in the directionality of the relationships between concordance to the reference pathway and costs.
Implications for policy, practice, and research
These findings lend evidence to support using Ontario Health’s clinical pathways for colon cancer to improve survival and reduce cost. For policy-makers and health system administrators, prospective measurement of pathway concordance may make a meaningful performance indicator that may change over time. This study should stimulate research in a number of directions. First, clinical pathways should be parsed into modifiable and nonmodifiable attributes so that modifiable factors can be targeted for review. Second, understanding the features of local delivery systems that inhibit or support high concordance is needed.
Strengths and limitations
While this study provides novel information regarding pathway concordance and survival or health system cost, there were limitations. The total costs included all sectors for which Ontario Health measured costs, not just costs related to their colon cancer diagnosis and treatment. It is possible that there was a different relationship between pathway concordance and colon cancer–specific costs. These data excluded non-fee-for-service physician payments, which could have underestimated total health system costs; this limitation is unlikely to affect the overall results of this study [31]. Although this study measured patients’ healthcare costs following 1 year from diagnosis of Stage II colon cancer, future research should link this study’s Stage II pathway with follow-up, Stage IV, or metastatic disease pathways, as appropriate for the patient, for a more comprehensive perspective of colon cancer treatment and pathway concordance.
This study also found that most deviations from concordant care were related to clinical activities beyond the reference pathway, including imaging not on the reference pathway and ED visits. Future applications of pathway concordance should ensure that concordance statistics reflect the desired health services interpretation; to do so, other studies may conduct further sensitivity analyses with different approaches to calculating concordance. Complementary studies may also evaluate whether the findings are robust to other settings’ pathways [32, 33]. Also, this study did not measure relationships between nonconcordant care and survival or cost, nor did it evaluate whether nonconcordant care was low-value; it is possible that patients may not have received pathway concordant care for a variety of reasons, including patient or family preferences or comorbidities.
Conclusion
Clinical pathways have the potential to provide significant value for improving outcomes for patients and the health system. This finding creates a compelling case for understanding the barriers to pathway concordance and developing interventions to help providers implement best practice care where appropriate.
Supplementary Material
Contributor Information
Shannon Milroy, Planning and Regional Programs, Ontario Health (Cancer Care Ontario), Toronto, Ontario, Canada.
Judith Wong, Planning and Regional Programs, Ontario Health (Cancer Care Ontario), Toronto, Ontario, Canada.
Maria Eberg, IQVIA, Kirkland, Quebec, Canada; Formerly of Ontario Health (Cancer Care Ontario), Toronto, Ontario, Canada.
Luciano Ieraci, Data and Decision Sciences, Systems and Infrastructure Planning, Ontario Health (Cancer Care Ontario), Toronto, Ontario, Canada.
Katharina Forster, Disease Pathway Management, Clinical Programs and Quality Initiatives, Ontario Health (Cancer Care Ontario), Toronto, Ontario, Canada.
Jason M Sutherland, Centre for Health Services and Policy Research, School of Population and Public Health, University of British Columbia, 201-2206 East Mall, Vancouver, British Columbia V6T 1Z3, Canada; Disease Pathway Management, Clinical Programs and Quality Initiatives, Ontario Health (Cancer Care Ontario), Toronto, Ontario, Canada; Department of Surgery, University of Toronto, Toronto, Ontario, Canada.
Author contributions
All authors meaningfully contributed to the conceptualization, data acquisition, analyses, drafting, and editing manuscript text. The opinions, results, and conclusions reported are those of the authors and may not reflect those of Ontario Health. No endorsement by Ontario Health should be inferred.
Supplementary data
Supplementary data are available at INTQHC Journal online.
Funding
This study was funded by a grant from Ontario Health (formerly known as Cancer Care Ontario).
Data availability
The population-based data held by Ontario Health and used in this study are not publicly available for reuse except through application to Ontario Health.
Ethics statement
Ontario Health is designated a ‘prescribed entity’ for the purposes of Section 45(1) of the Personal Health Information Protection Act of 2004. As a prescribed entity, Ontario Health (Cancer Care Ontario) is authorized to collect personal health information from health information custodians without the consent of the patient and to use such personal health information for the purpose of analysis or compiling statistical information with respect to the management, evaluation, or monitoring of the allocation of resources to or planning for all or part of the health system, including the delivery of services. Because our study was conducted in support of Ontario Health (formerly known as Cancer Care Ontario)’s mandate for health system monitoring and planning and is in compliance with privacy regulations, ethics review was not required.
References
- 1. Kinsman L, Rotter T, James E. et al. What is a clinical pathway? Development of a definition to inform the debate. BMC Med 2010;8:31. doi: 10.1186/1741-7015-8-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Noehammer E, Ponweiser M, Romeyke T. et al. Benefits, barriers and determinants of clinical pathway use in Germany, Austria and Switzerland. A pilot study. Health Serv Manage Res 2022;30. doi: 10.1177/09514848221107485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Neubauer MA, Hoverman JR, Kolodziej M. et al. Cost effectiveness of evidence-based treatment guidelines for the treatment of non-small-cell lung cancer in the community setting. J Oncol Pract 2010;6:12–8. doi: 10.1200/JOP.091058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zon RT, Frame JN, Neuss MN. et al. American society of clinical oncology policy statement on clinical pathways in oncology. J Oncol Pract 2016;12:261–6. doi: 10.1200/JOP.2015.009134. [DOI] [PubMed] [Google Scholar]
- 5. Bryan MA, Desai AD, Wilson L. et al. Association of bronchiolitis clinical pathway adherence with length of stay and costs. Pediatrics, 2017;139:e20163432. doi: 10.1542/peds.2016-3432. [DOI] [PubMed] [Google Scholar]
- 6. Brignole M, Ungar A, Bartoletti A. et al. Standardized-care pathway vs. usual management of syncope patients presenting as emergencies at general hospitals. Europace 2006;8:644–50. doi: 10.1093/europace/eul071. [DOI] [PubMed] [Google Scholar]
- 7. Panella M, Marchisio S, Di Stanislao F. Reducing clinical variations with clinical pathways: do pathways work? Int J Qual Health Care 2003;15:509–21. doi: 10.1093/intqhc/mzg057. [DOI] [PubMed] [Google Scholar]
- 8. American Society of Clinical Oncology . The State of Cancer Care in America, 2017: a report by the American Society of Clinical Oncology. J Oncol Pract 2017;13:e353–94. doi: 10.1200/JOP.2016.020743. [DOI] [PubMed] [Google Scholar]
- 9. Rotter T, Kinsman L, James E. et al. The quality of the evidence base for clinical pathway effectiveness: room for improvement in the design of evaluation trials. BMC Med Res Methodol 2012;12:80. doi: 10.1186/1471-2288-12-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. El Baz N, Middel B, Van Dijk JP. et al. Are the outcomes of clinical pathways evidence-based? A critical appraisal of clinical pathway evaluation research. J Eval Clin Pract 2007;13:920–9. doi: 10.1111/j.1365-2753.2006.00774.x. [DOI] [PubMed] [Google Scholar]
- 11. Hoverman JR, Cartwright TH, Patt DA. et al. Pathways, outcomes, and costs in colon cancer: retrospective evaluations in two distinct databases. J Oncol Pract 2011;7:52s–9s. doi: 10.1200/JOP.2011.000318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cancer Care Ontario . Pathway Map Development Methodology [Internet]. Toronto, Canada, 2017. https://www.cancercareontario.ca/sites/ccocancercare/files/assets/CCODPMDevelopmentMethodology.pdf (9 September 2020, date last accessed). [Google Scholar]
- 13. Smith L, Bryan S, De P. et al. Canadian Cancer Statistics 2018 [Internet]. Ottawa, Canada, 2018. https://cancer.ca/en/research/cancer-statistics/past-editions (9 September 2020, date last accessed). [Google Scholar]
- 14. Cancer Care Ontario . Ontario Cancer Statistics 2018 [Internet]. Toronto, Canada. https://www.cancercareontario.ca/en/statistical-reports/ontario-cancer-statistics-2018 (9 September 2020, date last accessed). [Google Scholar]
- 15. et al. (eds). American Joint Commission on Cancer Cancer Staging Manual [Internet]. 7th edn, London: Springer New York Dordrecht Heidelberg, 2010, 143–64, 7th Ed Cancer Staging Manual.pdf. https://cancerstaging.org/references-tools/deskreferences/Documents/AJCC (9 September 2020, date last accessed). [Google Scholar]
- 16. Cancer Care Ontario . Colorectal Cancer Pathway Map – Cancer Care Ontario [Internet]. Cancer Care Ontario, 2017. https://www.cancercareontario.ca/en/pathway-maps/colorectal-cancer. [Google Scholar]
- 17. Gao X, Xiao B, Tao D. et al. A survey of graph edit distance. Pattern Anal Appl 2010;13:113–29. doi: 10.1007/s10044-008-0141-y. [DOI] [Google Scholar]
- 18. Forster K, Tsang K, Li S. et al. Can concordance between actual care received and a pathway map be measured on a population based level? A pilot study. Curr Oncol 2020;27:e27–33. doi: 10.3747/co.27.5349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chan TCY, Eberg M, Forster K. et al. An inverse optimization approach to measuring clinical pathway concordance. Manage Sci 2022;68:1882–903. doi: 10.1287/mnsc.2021.4100. [DOI] [Google Scholar]
- 20. Lesnard L. Setting cost in optimal matching to uncover contemporaneous socio-temporal patterns. Sociol Methods Res 2010;38:389–419. doi: 10.1177/0049124110362526. [DOI] [Google Scholar]
- 21. Damerau FJ. A technique for computer detection and correction of spelling errors. Commun ACM 1964;7:171–6. doi: 10.1145/363958.363994. [DOI] [Google Scholar]
- 22. Wodchis WP, Bushmeneva K, Nikitovic M. et al. Guidelines on Person-Level Costing Using Administrative Databases in Ontario. Working Paper Series Volume 1 May 2013 [Internet]. Toronto, Canada, 2013. https://tspace.library.utoronto.ca/handle/1807/87373 (9 September 2020, date last accessed). [Google Scholar]
- 23. Canadian Institute for Health Information . National Health Expenditure Trends, 1975 to 2019. Ottawa, Canada: CIHI; 2019. https://www.cihi.ca/sites/default/files/document/nhex-trends-narrative-report-2019-en-web.pdf. [Google Scholar]
- 24. Statistics Canada . Consumer Price Index, Monthly, Not Seasonally Adjusted [Internet]. 2022. https://www150.statcan.gc.ca/t1/tbl1/en/tv.action?pid=1810000401 (9 September 2020, date last accessed).
- 25. Manning WG, and Mullahy J. Estimating log models: to transform or not to transform? J Health Econ 2001;20:461–94. doi: 10.1016/S0167-6296(01)00086-8. [DOI] [PubMed] [Google Scholar]
- 26. King G, Tomz M, Wittenberg J. Making the most of statistical analyses: improving interpretation and presentation. Am J Pol Sci 2000;44:347–61. doi: 10.2307/2669316. [DOI] [Google Scholar]
- 27. Ieraci I, Eberg M, Forster K. et al. Development of population-level colon cancer pathway concordance measures and association with survival. Int J Cancer 2022;15:2046–57. doi: 10.1002/ijc.33964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. de Oliveira C, Pataky R, Bremner KE. et al. Phase-specific and lifetime costs of cancer care in Ontario, Canada. BMC Cancer 2016;16:809. doi: 10.1186/s12885-016-2835-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. De Oliveira C, Pataky R, Bremner KE. et al. Estimating the cost of cancer care in British Columbia and Ontario: a canadian inter-provincial comparison. Healthc Policy 2017;12:95–108. [PMC free article] [PubMed] [Google Scholar]
- 30. de Oliveira C, Bremner KE, Pataky R. et al. Understanding the costs of cancer care before and after diagnosis for the 21 most common cancers in Ontario: a population-based descriptive study. C Open 2013;1:E1–8. doi: 10.9778/cmajo.20120013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wodchis WP, Arthurs E, Khan AI. et al. Cost trajectories for cancer patients. Curr Oncol 2016;23:S64–75. doi: 10.3747/co.23.2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. NHS . Implementing a Timed Colorectal Cancer Diagnostic Pathway. Guidance for Local Health and Care Systems [Internet]. London: England, 2022. https://www.england.nhs.uk/wp-content/uploads/2018/04/B1347_Colorectal-cancer-timed-diagnostic-pathway.pdf (9 September 2020, date last accessed). [Google Scholar]
- 33. Cancer Council Victoria and Department of Health Victoria . Optimal care pathway for people with colorectal cancer. Second Edition [Internet]. Melbourne, Australia, 2021. https://www.cancer.org.au/assets/pdf/colorectal-cancer-optimal-cancer-care-pathway (9 September 2020, date last accessed). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The population-based data held by Ontario Health and used in this study are not publicly available for reuse except through application to Ontario Health.


