Abstract
Introduction
Autoimmune/inflammatory rheumatic diseases (AIRDs) patients might be at-risk of severe COVID-19. However, whether this is linked to the disease or to its treatment is difficult to determine. This study aimed to identify factors associated with occurrence of severe COVID-19 in AIRD patients and to evaluate whether having an AIRD was associated with increased risk of severe COVID-19 or death.
Materials and methods
Two databases were analyzed: the EDS (Entrepôt des Données de Santé, Clinical Data Warehouse), including all patients followed in Paris university hospitals and the French multi-center COVID-19 cohort [French rheumatic and musculoskeletal diseases (RMD)]. First, in a combined analysis we compared patients with severe and non-severe COVID-19 to identify factors associated with severity. Then, we performed a propensity matched score case–control study within the EDS database to compare AIRD cases and non-AIRD controls.
Results
Among 1,213 patients, 195 (16.1%) experienced severe COVID-19. In multivariate analysis, older age, interstitial lung disease (ILD), arterial hypertension, obesity, sarcoidosis, vasculitis, auto-inflammatory diseases, and treatment with corticosteroids or rituximab were associated with increased risk of severe COVID-19. Among 35,741 COVID-19 patients in EDS, 316 having AIRDs were compared to 1,264 Propensity score-matched controls. AIRD patients had a higher risk of severe COVID-19 [aOR = 1.43 (1.08–1.87), p = 0.01] but analysis restricted to rheumatoid arthritis and spondyloarthritis found no increased risk of severe COVID-19 [aOR = 1.11 (0.68–1.81)].
Conclusion
In this multicenter study, we confirmed that AIRD patients treated with rituximab or corticosteroids and/or having vasculitis, auto-inflammatory disease, and sarcoidosis had increased risk of severe COVID-19. Also, AIRD patients had, overall, an increased risk of severe COVID-19 compares general population.
Keywords: COVID-19, auto-immune diseases, inflammatory rheumatic diseases, rituximab, lupus, vasculitis, rheumatic and musculoskeletal diseases
Introduction
The COVID-19 pandemic has put the medical world in a new situation and has changed how we treat patients. In this context, access to the health database from all university hospitals of the Paris region (APHP, Assistance Publique–Hôpitaux de Paris), named Entrepôt des Données de Santé (EDS, Clinical Data Warehouse), has been set up for COVID-19-related research projects (EDS-COVID). This database captures all hospitalized cases of COVID-19 in these 39 university hospitals and allows for different kinds of studies in several populations.
One of risk population identified during the pandemic was patients with autoimmune/inflammatory rheumatic diseases (AIRDs). However, whether this increased risk was linked to the disease or with specific treatment was difficult to determine, and only anti-CD20 therapeutics, such as rituximab, have been associated with extensive risk of more severe COVID-19 disease (1). Moreover, despite the unprecedented speed of vaccine development against COVID-19, the emergence of new variants is very probable, with a “sword of Damocles” hanging over us (2). These variants threaten to overturn the significant progress made so far increasing resistance to vaccines or monoclonal antibody therapeutics (3, 4) which is very important in patients whom vaccines are not effective because of immunosuppressive treatments (such as anti-CD20 therapies).
Because most cases are identified by diagnostic testing and hospitalization, estimates of the incidence of this disease are greatly underestimated and biased toward moderate to severe disease forms. Mild forms treated in outpatient clinics, or even not treated, were rarely identified by health databases. For AIRDs, in France, a national cohort was set up at the beginning of the epidemic [French rheumatic and musculoskeletal diseases (RMD) COVID-19 cohort] (5). This cohort has collected more than 1,200 cases of COVID in AIRD patients. In this cohort, approximately 50% of cases are the benign forms, which are difficult to capture from other sources.
The accessibility of theses two databases for scientific projects is a unique opportunity to provide answers to these questions. The objectives of this study were to identify the factors associated with the occurrence of severe or moderate-to-severe COVID-19 in patients with AIRDs, by using a combination of these 2 databases, and to evaluate whether having an AIRD is associated with an increased risk of severe COVID-19, in a case–control study within the EDS.
Materials and methods
Methodology overview
This study had two parts:
-
1.
The first part aimed to identify factors associated with COVID-19 severity. For that purpose, we performed a comparative observational study of patients with COVID-19 and an AIRD by combining data from the EDS-COVID and RMD cohorts.
-
2.
The second part aimed to compare COVID-19 severity (risk of death) in patients with an AIRD and controls not having these underlying diseases. For that purpose, we performed a case–control study within the EDS-COVID database.
Data source
The data were provided by two registers: the EDS-COVID database based on the EDS (6) and the French RMD COVID-19 cohort (5). Both cohorts are described in Supplementary material.
Ethics
This present study was approved by the institutional review board (APHP Scientific and Ethical Committee, authorization no. CSE 20-60_CovAID) from the Scientific and Ethical Committee of the AP-HP and by the CNIL. All participants included were informed about the use of their data for research. Patients who expressed an objection to the use of their data were excluded from this study.
Patients
For the EDS-COVID, we performed a systematic electronic search based on International Classification of Disease, 10th revision (ICD-10) codes to identify patients with an AIRD of interest (list provide in Supplementary Table 1). Patients were included if they had confirmed COVID-19 [i.e., positive COVID-19 PCR or serology result or a chest CT-scan interpreted as possible or certain COVID-19 (7)] and a confirmed diagnosis of one of the AIRDs of interest. Patients were excluded if no information was found about the COVID-19 status (i.e., no proof of infection) or any underlying immune disease. All medical files were reviewed by one of the authors (KC) to validate the diagnosis. Data were retrospectively analyzed.
For the French RMD COVID-19 cohort, patients were enrolled if they had one of the AIRDs of interest and a highly suspected or confirmed diagnosis of COVID-19 (i.e., positive PCR or serology result or a chest CT-scan interpreted as possible or certain COVID-19, or anosmia or sudden ageusia in the absence of rhinitis or nasal obstruction, or typical clinical signs of COVID-19: cough, fever, nose/throat symptoms, digestive symptoms without any other diagnosis, flu-like syndrome in a patient with recent close contact with a known COVID-19-positive patient) (5).
For both cohorts, patients were included if COVID-19 was diagnosed between the start of the pandemic until 1 September 2020. We excluded patients under 18 years old. AIRDs were classified into groups of diseases (details in Supplementary Table 1).
Outcomes
Endpoints rely on COVID-19 severity according to WHO criteria (Supplementary Table 2). COVID-19 was defined as ambulatory mild disease if the WHO score was 1–3, moderate if the score was 4 or 5, and severe if the WHO score was 6–10 [hospitalization in an intensive care unit (ICU) and/or death]. We considered death from any cause within 90 days after COVID-19 diagnosis.
Statistical analyses
Identification of factors associated with COVID-19 severity among RMD patients: Combined analysis of EDS-COVID and RMD cohorts
To eliminate duplicates, data from EDS-COVID and RMD COVID-19 cohorts were pooled and then cross-referenced according to the birthdate, sex, date of first symptoms, date of diagnosis, date of hospitalization, and underlying disease. For this part, all medical files were reviewed to collect and confirm the following data: demographics data, comorbidities [interstitial pneumonia, chronic obstructive pulmonary disease (COPD), cardiovascular disease, stroke, diabetes, obesity, hypertension, smoking status, and cancer], underlying disease and its activity, AIRD treatments, dates of first symptoms, evolution, and outcome of COVID-19 (hospitalization, admission to an ICU, date of hospital discharge, and death; Supplementary Table 2).
For this part, the primary outcome was defined as severe COVID-19. Secondary outcomes were moderate-to-severe COVID-19. For these analyses, patients with severe (or moderate-to-severe) COVID-19 were compared to those with non-severe (or ambulatory mild) disease. Also, among patients with moderate-to-severe disease, patients with severe COVID-19 were compared to those with moderate disease to identify risk factors associated with ICU admission and/or death. Categorical variables are expressed as number (percentage) and quantitative variables as mean (SD). Comparisons of patients with severe versus non-severe disease and survivors versus non-survivors were assessed with logistic regression models. In case of cell frequency<5, a penalized logistic regression (Firth’s method) was used. Odds ratios (ORs) and their 95% confidence intervals (CIs) were calculated as the effect size. Factors associated with severity and death status on univariate analyses (p < 0.05) were introduced into multivariable penalized logistic regression models with a forward stepwise selection procedure (entrance criterion=0.05) to limit overfitting.
COVID-19 severity in AIRD patients compared to the general population: A propensity score-matched case–control study with the EDS-COVID database
For the second part of the study, we performed a case–control study within the EDS-COVID database. Cases were AIRD patients identified and manually confirmed in the EDS-COVID database. Non-RMD controls were identified in the EDS-COVID database, after excluding all patients with an ICD-10 code for an AIRD (Supplementary Table 1).
Because the EDS-COVID cohort included>35,000 patients, COVID-19 and comorbidity diagnoses could not be reviewed manually for all patients; comorbidity diagnoses were based on structured (ICD-10 codes) and/or unstructured data (rule-based phenotyping on clinical notes). Regarding the structured data approach, a set of ICD-10 codes was used for each comorbidity (Supplementary Table 3) and allowed for tagging each patient for this specific comorbidity. To extract the comorbidity status from clinical notes, the PyMedExt Python library (8) was used to extract both comorbidities and associated modifiers, namely, negation, family history context and hypothesis (9). The library leverages a set of regular expression to extract comorbidity mentions, along with a rule-based algorithm to qualify each mention. We considered only positive and certain occurrence concerning the patient. Finally, we considered that a given patient had a given comorbidity if we found at least one associated ICD-10 code or at least 2 occurrences of the comorbidity in the patient’s clinical notes. For each patient, we considered only ICD-10 codes and clinical notes edited before the COVID-19 diagnosis. For the RMD case cohort, those comorbidities were also extracted manually by KC who had access to each patient’s records. Thus, for this cohort, performance of this method (sensitivity, specificity, precision and F1 score; definitions available in Supplementary material 2 and Supplementary Table 4) were assessed by using manual identification as a reference (Supplementary Figure 1).
For cases and controls, the COVID-19 diagnosis was based on one of the following: positive PCR or serology result, chest-imaging report confirming a diagnosis of COVID-19, or COVID-19 ICD-10 code: U071 (excluding U0712 and U0713 which is the code for “SARS-CoV-2 carrier, asymptomatic” and “Other examinations and observations related to the outbreak COVID-19,” respectively). We then extracted dates of COVID-19 diagnosis. If multiple dates were available, the first was used.
Autoimmune/inflammatory rheumatic diseases patients (cases) and non-AIRD patients (controls) were matched on a propensity score. The propensity score included age, sex, and comorbidities and was estimated by means of multivariable logistic regression. These comorbidities were chosen because they were identified as COVID-19 severity risk factors at the time of the analysis (5, 10, 11). The two groups were matched (1:4) by using an optimal algorithm with caliper width 0.2 SD of logit for the propensity score (12, 13). To assess the bias reduction, absolute standardized differences (ASDs) were computed before and after matching. An ASD > 10% was considered a significant difference (14).
For this case–control study, the primary outcome was severe COVID-19 and secondary outcomes were ICU admission and death from any cause within 90 days.
ORs for severe COVID-19, death, and ICU admission were estimated by conditional logistic regression. If a confounding factor still had an ASD > 10% after matching, it was introduced in this previous model as an adjustment factor (15).
Sensitivity analyses
Because each AIRD might not have the same risk of severity or death, we performed a separate analysis of only patients with chronic inflammatory arthritis (CIA) including spondyloarthritis (SA), psoriasis arthritis (PsA) and rheumatoid arthritis (RA) to analyze whether these diseases were associated with increased risk of COVID-19 severity. Thus, we used the same analysis (identification of factors associated with COVID-19 severity and comparison with the general population) for the CIA population alone.
Results
Identification of factors associated with COVID-19 severity among RMD patients: Combined analysis of EDS-COVID and RMD cohorts
Patients
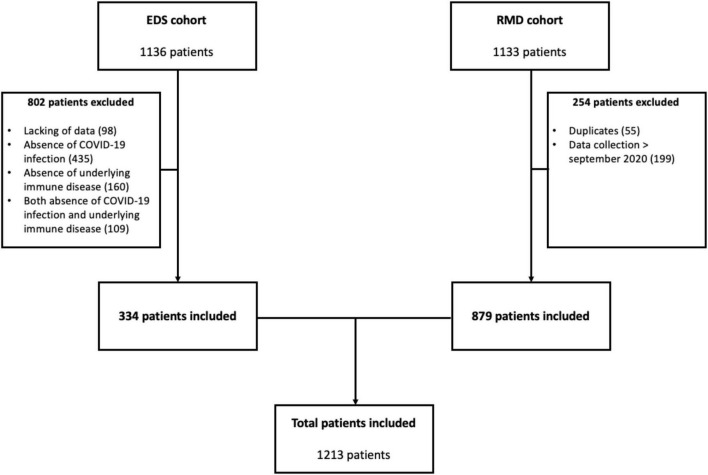
By 1 September 2020, 1,136 potential AIRD COVID-19 patients were identified within the EDS database, and after medical chart review 334 patients were included in the study (Figure 1). Also, 1,133 adults were identified from the French RMD COVID-19 cohort and among them 879 patients were included in the final analysis. Thus, 1,213 patients were included in this part of the study (Table 1). Eight hundred and twelve patients (66.9%) were diagnosed thanks to PCR or serology and 95 (0.8%) were diagnosed thanks to the typical CT scan pattern. The other patients were diagnosed thanks to a typical clinical symptomatology (i.e., fever, flue-like symptoms, anosmia, ageusia).
FIGURE 1.
Flow chart of patient inclusion. EDS, Entrepôt des Données de Santé; RMD, rheumatic and musculoskeletal diseases.
TABLE 1.
Characteristics of RMD patients in the pooled analysis of EDS and RMD COVID-19 French cohorts (N = 1213).
| Patient characteristics | RMD patients in EDS and RMD COVID-19 cohorts N = 1213 |
| Age | 58.2 (17.2) |
| Sex: male | 412 (34.0%) |
| Comorbidities | |
| Interstitial lung disease | 58 (4.78%) |
| COPD | 58 (4.78%) |
| Asthma | 86 (7.09%) |
| Coronary heart diseases | 172 (14.2%) |
| Stroke | 56 (4.62%) |
| Diabetes | 143 (11.8%) |
| Obesity | 181 (19.2%) |
| Hypertension | 382 (31.5%) |
| Smoking | 106 (8.74%) |
| Cancer | 81 (6.68%) |
| Number of patients with at least 1 comorbidity | 726 (65.1%) |
| Underlying disease | |
| Chronic inflammatory arthritis | 690 (56.9%) |
| Auto-inflammatory disease | 34 (2.81%) |
| Connective tissue diseases | 224 (18.5%) |
| Sarcoidosis | 64 (5.28%) |
| Vasculitis | 167 (13.8%) |
| Other | 33 (2.72%) |
| Ongoing rheumatic diseases or AID treatments | |
| Corticosteroids | 435 (35.9%) |
| Dose | 6.00 [5.00;10.0] |
| NSAIDs | 102 (8.41%) |
| Colchicine | 36 (2.97%) |
| Hydroxychloroquine | 116 (9.56%) |
| Methotrexate | 397 (32.7%) |
| Leflunomide | 46 (3.79%) |
| Salazopyrine | 13 (1.07%) |
| Mycophenolate mofetil/mycophenolic acid | 35 (2.89%) |
| Azathioprine | 20 (1.65%) |
| IVIg | 7 (0.58%) |
| Biologics | 474 (39.1%) |
| Anti-TNF alpha | 281 (23.4%) |
| Anti IL-6 | 33 (2.75%) |
| Anti IL-1 | 9 (0.75%) |
| Anti-IL-17 | 36 (3.00%) |
| Abatacept | 22 (1.83%) |
| Rituximab | 59 (4.92%) |
| JAK inhibitor | 31 (2.56%) |
| Other | 20 (1.67%) |
COPD, chronic obstructive pulmonary disease; IVIg, intravenous immunoglobulins; JAK, Janus kinase; NSAIDs, non-steroidal anti-inflammatory drugs; TNF, tumor necrosis factor; IL, interleukin.
The mean (SD) age of patients was 58.2 (17.2) years and many were women (n = 800, 66.0%). The most frequent underlying diseases were CIA (56.9%), connective tissue disease (56.9%) and vasculitis (13.8%). At least one comorbidity was recorded in 726 (65.1%) patients. Among the 1,213 patients, 195 (16.1%), 595 (49.1%), and 422 (34.8%) experienced severe, moderate and mild COVID-19, respectively (Table 2).
TABLE 2.
Factors associated with odds of severe COVID-19 in autoimmune/inflammatory rheumatic disease (AIRD) patients: Pooled analysis of EDS-COVID and the French RMD cohort patients.
| Overall | Patients with mild or moderate COVID-19 | Patients with severe COVID-19 | Univariate analysis |
N | Multivariate analysis |
|||
| N = 1213 | N = 1018 | N = 195 | OR (95% CI) | p -Value | aOR 95% CI | p -Value | ||
| Patient characteristics | ||||||||
| Age | 58.2 (17.2) | 55.9 (16.7) | 70.3 (14.3) | 1.06 [1.05–1.07] | <0.001 | 1,213 | 1.05 [1.03–1.07] | <0.001 |
| Sex: male | 412 (34.0%) | 323 (31.7%) | 89 (45.9%) | 1.82 [1.33–2.49] | <0.001 | 1,212 | 1.69 [1.08–2.64] | 0.0206 |
| Comorbidities | ||||||||
| Interstitial lung disease | 58 (4.78%) | 38 (3.73%) | 20 (10.3%) | 2.97 [1.67–5.15] | <0.001 | 1,213 | 2.94 [1.34–6.34] | 0.0077 |
| COPD | 58 (4.78%) | 38 (3.73%) | 20 (10.3%) | 2.97 [1.67–5.15] | <0.001 | 1,213 | ||
| Asthma | 86 (7.09%) | 68 (6.68%) | 18 (9.23%) | 1.45 [0.82–2.43] | 0.1922 | 1,213 | ||
| Coronary heart disease | 172 (14.2%) | 114 (11.2%) | 58 (29.7%) | 3.36 [2.33–4.82] | <0.001 | 1,213 | ||
| Stroke | 56 (4.62%) | 38 (3.73%) | 18 (9.23%) | 2.65 [1.46–4.67] | 0.0018 | 1,213 | ||
| Diabetes | 143 (11.8%) | 96 (9.43%) | 47 (24.1%) | 3.06 [2.06–4.49] | <0.001 | 1,213 | ||
| Obesity | 181 (19.2%) | 143 (17.8%) | 38 (27.7%) | 1.79 [1.17–2.68] | 0.0076 | 942 | 2.09 [1.26–3.43] | 0.0044 |
| Hypertension | 382 (31.5%) | 268 (26.3%) | 114 (58.5%) | 3.93 [2.87–5.40] | <0.001 | 1,213 | 1.81 [1.13–2.89] | 0.0128 |
| Smoking | 106 (8.74%) | 90 (8.84%) | 16 (8.21%) | 0.94 [0.53–1.59] | 0.8332 | 1,213 | ||
| Cancer | 81 (6.68%) | 60 (5.89%) | 21 (10.8%) | 1.95 [1.14–3.23] | 0.0157 | 1,213 | ||
| No. of patients with at least 1 comorbidity | 726 (65.1%) | 555 (59.9%) | 171 (90.5%) | 6.20 [3.87–10.52] | <0.001 | 1,115 | ||
| Underlying disease | <0.001 | 1212 | ||||||
| Chronic inflammatory arthritis | 690 (56.9%) | 618 (60.8%) | 72 (36.9%) | Ref. | Ref. | |||
| Auto-inflammatory disease | 34 (2.81%) | 29 (2.85%) | 5 (2.56%) | 1.59 [0.56–3.80] | 0.3571 | 3.91 [1.2–11.32] | 0.0251 | |
| Connective tissue diseases | 224 (18.5%) | 190 (18.7%) | 34 (17.4%) | 1.54 [0.99–2.37] | 0.0558 | 1.13 [0.62–2.01] | 0.6919 | |
| Sarcoidosis | 64 (5.28%) | 40 (3.93%) | 24 (12.3%) | 5.16 [2.93–8.97] | <0.001 | 5.19 [2.15–12.3] | 0.0003 | |
| Vasculitis | 167 (13.8%) | 111 (10.9%) | 56 (28.7%) | 4.32 [2.89–6.46] | <0.001 | 1.8 [1.02–3.16] | 0.0435 | |
| Other | 33 (2.72%) | 29 (2.85%) | 4 (2.05%) | 1.30 [0.41–3.29] | 0.6234 | 0.35 [0.06–1.41] | 0.1503 | |
| Ongoing rheumatic diseases or AIRD treatments | ||||||||
| Corticosteroids | 435 (35.9%) | 318 (31.2%) | 117 (60.0%) | 3.29 [2.41–4.52] | <0.001 | 1,213 | 2.47 [1.58–3.87] | 0.0001 |
| Dose | 6.0 [5.0–10.0] | 5.0 [5.0–10.] | 7.3 [5.0–13.1] | 1.01 [1.00–1.03] | 0.0229 | 431 | ||
| NSAIDs | 102 (8.41%) | 98 (9.63%) | 4 (2.05%) | 0.22 [0.07–0.51] | <0.001 | 1,213 | ||
| Colchicine | 36 (2.97%) | 31 (3.05%) | 5 (2.56%) | 0.91 [0.32–2.10] | 0.8298 | 1,213 | ||
| Hydroxychloroquine | 116 (9.56%) | 101 (9.92%) | 15 (7.69%) | 0.78 [0.43–1.32] | 0.3612 | 1,213 | ||
| Methotrexate | 397 (32.7%) | 349 (34.3%) | 48 (24.6%) | 0.63 [0.44–0.89] | 0.0077 | 1,213 | ||
| Leflunomide | 46 (3.79%) | 44 (4.32%) | 2 (1.03%) | 0.28 [0.06–0.84] | 0.0201 | 1,213 | 0.13 [0–0.97] | 0.0453 |
| Salazopyrine | 13 (1.07%) | 11 (1.08%) | 2 (1.03%) | 1.13 [0.22–3.87] | 0.8618 | 1,213 | ||
| Mycophenolate Mofetil/mycophenolic acid | 35 (2.89%) | 31 (3.05%) | 4 (2.05%) | 0.74 [0.23–1.82] | 0.5345 | 1,213 | ||
| Azathioprine | 20 (1.65%) | 16 (1.57%) | 4 (2.05%) | 1.43 [0.44–3.79] | 0.5219 | 1,213 | ||
| IVIg | 7 (0.58%) | 5 (0.49%) | 2 (1.03%) | 2.38 [0.43–9.95] | 0.2881 | 1,213 | ||
| Biologics | 474 (39.1%) | 430 (42.2%) | 44 (22.6%) | 0.40 [0.28–0.57] | <0.001 | 1,213 | ||
| Anti-TNF alpha | 281 (23.4%) | 271 (26.9%) | 10 (5.21%) | 0.16 [0.08–0.28] | <0.001 | 1,199 | ||
| Anti IL-6 | 33 (2.75%) | 30 (2.98%) | 3 (1.56%) | 0.59 [0.16–1.59] | 0.3256 | 1,199 | ||
| Anti IL-1 | 9 (0.75%) | 7 (0.70%) | 2 (1.04%) | 1.75 [0.33–6.61] | 0.4678 | 1,199 | ||
| Anti-IL17 | 36 (3.00%) | 34 (3.38%) | 2 (1.04%) | 0.37 [0.08–1.12] | 0.0831 | 1,199 | ||
| Abatacept | 22 (1.83%) | 21 (2.09%) | 1 (0.52%) | 0.36 [0.04–1.41] | 0.1617 | 1,199 | ||
| Rituximab | 59 (4.92%) | 37 (3.67%) | 22 (11.5%) | 3.42 [1.95–5.86] | <0.001 | 1,199 | 4.05 [1.96–8.27] | 0.0002 |
| JAK inhibitor | 31 (2.56%) | 25 (2.46%) | 6 (3.08%) | 1.34 [0.51–3.03] | 0.5275 | 1,213 | ||
| Other | 20 (1.67%) | 19 (1.89%) | 1 (0.52%) | 0.40 [0.04–1.57] | 0.2159 | 1,199 | ||
COPD, chronic obstructive pulmonary disease; IVIg, intravenous immunoglobulins; JAK, Janus kinase; NSAIDs, non-steroidal anti-inflammatory drugs; OR, odds ratio-aOR: adjusted odds ratio; 95% CI, 95% confidence interval. Bold values represent the significantive value.
Factors associated with COVID-19 severity
On univariate analyses (Table 2), the probability of severe versus mild or moderate COVID-19 was associated with mean age [70.3 (14.3) vs. 55.9 (16.7) years, p < 0.001], male sex (45.9% vs. 31.7%, p < 0.001), at least one comorbidity, history of interstitial lung disease (ILD), COPD, coronary heart disease. stroke, diabetes, obesity, arterial hypertension, and cancer. Regarding underlying diseases, sarcoidosis and vasculitis were associated with severe COVID-19. The use of corticosteroids (yes vs. no or ≥10 mg vs. <10 mg) was associated with poor outcomes, as was rituximab. All variables were entered in a multivariate model, for corticosteroids to avoid collinearity only corticosteroids use (but not the dose) was retained. On multivariate analysis, severe COVID-19 remained associated with age [adjusted OR (aOR) = 1.05 (95% CI 1.03–1.07), for each additional year], male sex [aOR = 1.69 (1.08–2.64)], history of ILD [aOR = 2.94 (1.34–6.34)], arterial hypertension [aOR = 1.81 (1.13–2.89)], obesity [aOR = 2.09 (1.26–3.43)], sarcoidosis [aOR = 5.19 (2.15–12.3)], vasculitis [aOR = 1.8 (1.02–3.16)], auto-inflammatory disease [OR = 3.91 (1.2–11.32)], corticosteroids treatment [aOR = 2.47 (1.58–3.87)] and rituximab treatment [aOR = 4.05 (1.96–8.27)]. By contrast, treatment with leflunomide was associated with better outcome [aOR = 0.13 (0–0.97)].
In patients with CIA (Supplementary Table 5), multivariate analyses revealed the same factors as those associated with severe COVID-19: older age [aOR = 1.05 (95% CI 1.03–1.07)], male sex [aOR = 2.19 (1.25–3.87)], history of ILD [aOR = 4.26 (1.22–14.39)] or arterial hypertension [aOR = 2.68 (1.48–4.94)], and use of corticosteroids [aOR = 2.44 (1.38–4.30)] or rituximab [aOR = 5.20 (1.83–14.01)].
Factors associated with death
Among the 790 patients admitted for moderate to severe COVID-19, survival status at 90 days was available for 590 (Table 3): 116 (19.7%) died within the 90 days after COVID-19 diagnosis. On univariate analyses, among hospitalized patients, probability of death was associated with older age; a history of COPD, coronary heart disease or arterial hypertension; a diagnosis of vasculitis; and corticosteroids or rituximab treatment. However, probability of survival was also associated with methotrexate or anti-tumor necrosis factor α treatment. On multivariable analysis, probability of death remained associated with older age [aOR = 1.06 (1.04–1.08)], a history of ILD [aOR = 2.34 (1.10–4.84)] or arterial hypertension [aOR = 1.64 (1.00–2.70)] and use of corticosteroids [aOR = 1.67 (1.05–2.66)] and rituximab [aOR = 3.32 (1.45–7.49)]. Also, methotrexate treatment remained significantly associated with better outcomes [aOR of death = 0.43 (0.25–0.73)]. Analyses restricted to CIA led to the identification of the same risk factors, except for methotrexate, which was longer significantly associated with better outcomes (Supplementary Table 6).
TABLE 3.
Factors associated with odds of death from COVID-19 in AIRD patients: Pooled analysis of EDS-COVID and French RMD cohorts.
| Univariate analyses | Multivariate analyses | |||||||
| Overall | Survivor | Non-survivor | OR (95% CI) | p -Value | N | aOR 95% CI | p -Value | |
| N = 590 | N = 474 | N = 116 | ||||||
| Patients characteristics | ||||||||
| Age | 66.3 (16.0) | 63.9 (16.0) | 76.3 (11.9) | 1.06 [1.04–1.08] | <0.001 | 590 | 1.06 [1.04–1.08] | <0.001 |
| Sex: male | 221 (37.5%) | 173 (36.5%) | 48 (41.7%) | 1.25 [0.82–1.88] | 0.2945 | 589 | ||
| Comorbidities | ||||||||
| Interstitial lung disease | 46 (7.80%) | 32 (6.75%) | 14 (12.1%) | 1.93 [0.97–3.65] | 0.059 | 590 | 2.34 [1.10–4.84] | 0.0283 |
| COPD | 43 (7.29%) | 28 (5.91%) | 15 (12.9%) | 2.39 [1.22–4.55] | 0.0124 | 590 | ||
| Asthma | 44 (7.46%) | 35 (7.38%) | 9 (7.76%) | 1.09 [0.49–2.22] | 0.8147 | 590 | ||
| Coronary heart disease | 151 (25.6%) | 107 (22.6%) | 44 (37.9%) | 2.10 [1.36–3.22] | <0.001 | 590 | ||
| Stroke | 48 (8.14%) | 34 (7.17%) | 14 (12.1%) | 1.81 [0.92–3.40] | 0.0852 | 590 | ||
| Diabetes | 120 (20.3%) | 89 (18.8%) | 31 (26.7%) | 1.59 [0.98–2.52] | 0.0584 | 590 | ||
| Obesity | 100 (24.5%) | 76 (23.2%) | 24 (29.6%) | 1.40 [0.81–2.38] | 0.2253 | 408 | ||
| Hypertension | 284 (48.1%) | 206 (43.5%) | 78 (67.2%) | 2.65 [1.74–4.09] | <0.001 | 590 | 1.64[1.00–2.70] | 0.048 |
| Smoking | 40 (6.78%) | 32 (6.75%) | 8 (6.90%) | 1.07 [0.46–2.24] | 0.8725 | 590 | ||
| Cancer | 65 (11.0%) | 49 (10.3%) | 16 (13.8%) | 1.41 [0.76–2.52] | 0.2695 | 590 | ||
| No. of patients with at least 1 comorbidity | 461 (83.5%) | 354 (80.6%) | 107 (94.7%) | 3.99 [1.88–10.06] | <0.001 | 552 | ||
| Underlying disease | 0.008 | 590 | ||||||
| Chronic inflammatory arthritis | 253 (42.9%) | 216 (45.6%) | 37 (31.9%) | Ref. | ||||
| Auto-inflammatory disease | 15 (2.54%) | 11 (2.32%) | 4 (3.45%) | 2.26 [0.65–6.72] | 0.1863 | |||
| Connective tissue diseases | 128 (21.7%) | 104 (21.9%) | 24 (20.7%) | 1.35 [0.77–2.36] | 0.2932 | |||
| Sarcoidosis | 49 (8.31%) | 38 (8.02%) | 11 (9.48%) | 1.72 [0.79–3.55] | 0.1638 | |||
| Vasculitis | 128 (21.7%) | 89 (18.8%) | 39 (33.6%) | 2.55 [1.53–4.26] | <0.001 | |||
| Other | 17 (2.88%) | 16 (3.38%) | 1 (0.86%) | 0.52 [0.06–2.20] | 0.4219 | |||
| Ongoing rheumatic diseases or AIRD treatments | ||||||||
| Corticosteroids | 295 (50.0%) | 222 (46.8%) | 73 (62.9%) | 1.92 [1.27–2.92] | 0.0019 | 590 | 1.67 [1.05–2.66] | 0.0302 |
| Dose | 7.00 [5.00–10.0] | 5.00 [5.00–10.0] | 7.50 [5.00–15.0] | 1.02 [1.00–1.03] | 0.043 | 291 | ||
| NSAIDs | 23 (3.90%) | 22 (4.64%) | 1 (0.86%) | 0.26 [0.03–1.03] | 0.0555 | 590 | ||
| Colchicine | 21 (3.56%) | 16 (3.38%) | 5 (4.31%) | 1.37 [0.46–3.48] | 0.5414 | 590 | ||
| Hydroxychloroquine | 55 (9.32%) | 48 (10.1%) | 7 (6.03%) | 0.60 [0.25–1.26] | 0.1893 | 590 | ||
| Methotrexate | 178 (30.2%) | 158 (33.3%) | 20 (17.2%) | 0.42 [0.25–0.70] | <0.001 | 590 | 0.43 [0.25–0.73] | 0.0015 |
| Leflunomide | 18 (3.05%) | 17 (3.59%) | 1 (0.86%) | 0.34 [0.04–1.37] | 0.1443 | 590 | 0.08 [0.00–0.67] | 0.0127 |
| Salazopyrine | 6 (1.02%) | 5 (1.05%) | 1 (0.86%) | NA | NA | 590 | ||
| Mycophenolate mofetil/mycophenolic acid | 20 (3.39%) | 17 (3.59%) | 3 (2.59%) | 0.81 [0.21–2.32] | 0.7111 | 590 | ||
| Azathioprine | 14 (2.37%) | 13 (2.74%) | 1 (0.86%) | 0.44 [0.05–1.84] | 0.2987 | 590 | ||
| IVIg | 7 (1.19%) | 5 (1.05%) | 2 (1.72%) | NA | NA | 590 | ||
| Biologics | 143 (24.2%) | 119 (25.1%) | 24 (20.7%) | 0.79 [0.47–1.27] | 0.3347 | 590 | ||
| Anti-TNF alpha | 53 (9.14%) | 49 (10.5%) | 4 (3.51%) | 0.34 [0.11–0.83] | 0.0155 | 580 | ||
| Anti IL-6 | 9 (1.55%) | 7 (1.50%) | 2 (1.75%) | NA | NA | 580 | ||
| Anti IL-1 | 6 (1.03%) | 4 (0.86%) | 2 (1.75%) | NA | NA | 580 | ||
| Anti-IL17 | 8 (1.38%) | 8 (1.72%) | 0 (0.00%) | NA | NA | 580 | ||
| Abatacept | 10 (1.72%) | 9 (1.93%) | 1 (0.88%) | 0.64 [0.07–2.80] | 0.5905 | 580 | ||
| Rituximab | 40 (6.90%) | 27 (5.79%) | 13 (11.4%) | 2.13 [1.04–4.15] | 0.0387 | 580 | 3.32 [1.45–7.49] | 0.0051 |
| JAK inhibitor | 10 (1.69%) | 7 (1.48%) | 3 (2.59%) | 1.92 [0.46–6.58] | 0.34 | 590 | ||
| Other | 7 (1.21%) | 7 (1.50%) | 0 (0.00%) | NA | NA | 580 | ||
COPD, chronic obstructive pulmonary disease; IVIg, intravenous immunoglobulins; JAK, Janus kinase; NA, not available; NSAIDs, non-steroidal anti-inflammatory drugs; OR, odds ratio; aOR, adjusted odds ratio; 95% CI, 95% confidence interval; TNF, tumor necrosis factor; IL, interleukin. Bold values represent the significantive value.
COVID-19 severity in AIRD patients compared to the general population: Propensity score-matched case–control study
Within the EDS-COVID cohort, we identified 35,741 adults with a diagnosis of COVID-19 according to our 4 pre-specified criteria. We identified 316 of the 334 (94.6%) previously described AIRD patients and compared them to 1,264 propensity score-matched controls (Table 4). Before matching, the controls were younger, more often male and had fewer comorbidities then AIRD patients. After the matching, the mean (SD) age was 66.3 (16.8) years for AIRDs patients and 68.7 (18.9) years for controls. The comorbidities were well-balanced between the cases and controls except for ILD (ASD = 0.14), arterial hypertension (ASD = 0.11), and cancer (ASD = 0.15). After conditional logistic regression (adjusted for age at COVID-19, arterial hypertension, ILD, and cancer), severe COVID-19 occurred in 118 (37.3%) AIRD patients and 384 (30.4%) controls [aOR for severe COVID-19 = 1.43 (1.08–1.87), p = 0.01] and death in 56 (19.6%) AIRD patients and 221 (17.5%) controls [aOR = 1.33 (0.95–1.86), p = 0.095]. Admission to an ICU was also numerically but not significantly more frequent in AIRD patients (n = 56, 17.7%) than controls (163, 12.9%) [aOR = 1.36 (0.96–1.93), p = 0.085].
TABLE 4.
Odds of severe COVID-19: Comparison between patients with or without AIRDs: case–control study within EDS database.
| Before PS matching | After PS matching | |||||||
| AIRD patients (N = 316) | Controls (N = 35425) | AIRD patients (N = 316) | Controls (N = 1264) | ASD | aOR | 95% CI | p -Value | |
| Severe COVID-19 infection | 118 (37.3%) | 6439 (18.2%) | 118 (37.3%) | 384 (30.4%) | 1.43 | [1.08–1.87] | 0.01 | |
| Death | 62 (19.6%) | 3235 (9.1%) | 62 (19.6%) | 221 (17.5%) | 1.33 | [0.95–1.86] | 0.10 | |
| ICU admission | 56 (17.7%) | 3204 (9.0%) | 56 (17.7%) | 163 (12.9%) | 1.36 | [0.96–1.93] | 0.09 | |
| Patients characteristics | ||||||||
| Age | 66.3 (16.8) | 55.4 (21.1) | 66.3 (16.8) | 68.7 (18.9) | 0.26 | |||
| Sex: male | 121 (38.3%) | 16676 (47.1%) | 121 (38.3%) | 519 (41.1%) | 0.03 | |||
| Comorbidities | ||||||||
| Interstitial lung disease | 42 (13.3%) | 376 (1.1%) | 42 (13.3%) | 140 (11.1%) | 0.14 | |||
| COPD | 29 (9.2%) | 1543 (4.4%) | 29 (9.2%) | 119 (9.4%) | 0.03 | |||
| Cardiovascular diseases | 119 (37.7%) | 5751 (16.2%) | 119 (37.7%) | 503 (39.8%) | 0.05 | |||
| Stroke | 53 (16.8%) | 2887 (8.1%) | 53 (16.8%) | 220 (17.4%) | 0.07 | |||
| Diabetes | 74 (23.4%) | 5123 (14.5%) | 74 (23.4%) | 324 (25.6%) | 0.03 | |||
| Obesity | 84 (26.6%) | 3730 (10.5%) | 84 (26.6%) | 349 (27.6%) | 0.06 | |||
| Hypertension | 180 (57.0%) | 10069 (28.4%) | 180 (57.0%) | 767 (60.7%) | 0.11 | |||
| Smoking | 37 (11.7%) | 1525 (4.3%) | 37 (11.7%) | 157 (12.4%) | 0.01 | |||
| Cancer | 66 (20.9%) | 3690 (10.4%) | 66 (20.9%) | 275 (21.8%) | 0.15 | |||
PS, propensity score; ASD, absolute standardized difference; COPD, chronic obstructive pulmonary disease, ICU, intensive care unit; aOR, adjusted odds ratio; 95% CI, 95% confidence interval. Conditional logistic regression was adjusted for age at COVID-19 diagnosis, hypertension, interstitial pneumonia and cancer.
We performed analyses restricted to patients with CIA; 102 patients with CIA were compared to 408 propensity score-matched controls (Table 5), but we found no increased probability of severe COVID-19 in CIA patients as compared with non-AIRD matched patients [aOR = 1.11 (0.68–1.81)].
TABLE 5.
Odds of severe COVID-19: Comparison between patients with or without chronic inflammatory arthritis–case control study within EDS database.
| CIA patients (N = 102) | Controls (N = 408) | ASD | aOR | 95% CI | p | |
| Severe COVID-19 | 38 (37.3%) | 141 (34.6%) | 1.111 | [0.68–1.81] | 0.67 | |
| Death | 18 (17.6%) | 83 (20.3%) | 1.001 | [0.55–1.81] | 1 | |
| ICU admission | 20 (19.6%) | 58 (14.2%) | 1.2153 | [0.65–2.26] | 0.54 | |
| Patient characteristics | ||||||
| Age | 66.4 (16.1) | 70.7 (17.3) | 0.2573 | |||
| Sex: male | 36 (35.3%) | 138 (33.8%) | 0.0309 | |||
| Comorbidities | ||||||
| Interstitial lung disease | 12 (11.8%) | 31 (7.6%) | 0.1413 | |||
| COPD | 9 (8.8%) | 39 (9.6%) | 0.0255 | |||
| Cardiovascular diseases | 40 (39.2%) | 171 (41.9%) | 0.0549 | |||
| Stroke | 12 (11.8%) | 57 (14.0%) | 0.0659 | |||
| Diabetes | 21 (20.6%) | 89 (21.8%) | 0.0300 | |||
| Obesity | 26 (25.5%) | 94 (23.0%) | 0.0572 | |||
| Hypertension | 62 (60.8%) | 269 (65.9%) | 0.1070 | |||
| Smoking | 14 (13.7%) | 55 (13.5%) | 0.0071 | |||
| Cancer | 20 (19.6%) | 106 (26.0%) | 0.1523 | |||
ASD, absolute standardized difference; CIA, chronic inflammatory arthritis; COPD, chronic obstructive pulmonary disease; aOR, adjusted odds ratio; 95% CI, 95% confidence interval. Bold values represent the significantive value.
Discussion
This observational, multicentric, French cohort study examined the frequency and predictors of severe COVID-19 in adults with AIRD. It included ambulatory and hospitalized patients covering the entire spectrum of the disease in this population. We confirmed that in AIRD patients, age, male sex, ILD, hypertension, obesity, and corticosteroids or rituximab use were associated with COVID-19 severity. Also, we identified age, ILD, hypertension, and corticosteroids or rituximab use as predictors of death in patients hospitalized with COVID-19. Finally, in our propensity score-matched case–control study, as compared with the general population, AIRD patients had higher risk of severe COVID-19 independent of their comorbidities. However, analyses restricted to CIA patients showed that these patients were not at increased risk of severe COVID-19. To our knowledge, few studies have compared patients with AIRD and matched controls (16–19), especially with a large number of patients.
Our study confirmed that older age, male sex, hypertension, obesity and ILD were associated with COVID-19 severity, in line with previous cohorts of COVID-19 in AIRD patients (11, 20–22) and in the general population (23–31). In the same way, predictors associated with death at 90 days (older age, hypertension or ILD) were concordant with the existing literature for the general population (32, 33), and with recent reports of the Global Rheumatology Alliance (10), and Japanese experience in 1,915 AIRD patients (34).
The association between ILD and severity of COVID-19 pneumonia might be explained by patients with previous injured pulmonary parenchyma being more susceptible to progression to severe COVID pneumonia because of impaired baseline pulmonary dysfunction and limited reserves. Moreover, COVID-19 could lead to an exacerbation of the ILD (27, 35–38). In the literature, the risk of death range from 30 to 60% for COVID-19 patients with pre-existing ILD, with ORs from 3.2 to 5.5 (32, 39, 40).
In our study, sarcoidosis, vasculitis and auto-inflammatory diseases were associated with severe COVID-19. These results confirmed those from the French RMD COVID-19 cohort study (5), that included some of our patients. However, here we had a much larger number of cases to confirm these findings: sarcoidosis (64 vs. 15), vasculitis (167 vs. 65) and auto-inflammatory disease (34 vs. 27). In sarcoidosis, two studies suggested that this association could be explained by ILD due to the disease, (41), with a specific increased risk of hospitalization in patients with stage III sarcoidosis (5, 42). Other underpowered cohorts did not identify sarcoidosis as a risk factor of severe COVID-19 (42, 43). Regarding vasculitis, in the absence of control populations, only few studies analyzed the risk of COVID severity associated with the vasculitis itself. The French RMD COVID-19 (5) and the Global Rheumatology Alliance (11) reported increased risk of severe or moderate COVID-19 requiring hospitalization. Nevertheless, these studies relied on a very low number of patients: 65 and 44, respectively. A few other cohorts have shown increased risk of death for patients with vasculitis, but the data are scarce and lack power (44). Additionally, in another cohort, the risk of severe COVID-19 was not higher in auto-inflammatory patients than in patients with other rheumatic conditions (45) or in the general population (46).
Use of corticosteroids and rituximab was associated with increased probability of severe COVID-19 or death. Overall, immunosuppressed patients are at increased risk of COVID-19 and severe COVID-19, even fully vaccinated individuals (47). However, this risk seems restricted to only some treatments, such as rituximab (10, 17, 34, 48–53), corticosteroids (5, 11, 19, 20, 22, 33, 54, 55), mycophenolate acid (5, 10), or cyclophosphamide (10), whereas other treatments, such as anti-tumor necrosis factor α, seem to be safe (11, 56). The negative impact of oral corticosteroids whatever the indication is well described (5, 11, 19, 20, 22, 33, 54, 55). In AIRD patients, the odds of hospitalization for COVID-19 were increased for those taking≥10 mg prednisone equivalent [aOR = 2.05 (1.06–3.96)] (11). This dose of corticosteroids was also found as an independent factor for COVID-19–related death (10). In CIA, this risk was not constantly observed (10, 57). Our result concerning the odds of corticosteroids with COVID-19 severity in our CIA patients, higher than in all AIRD patients, is puzzling. The difference could be explained by the other diseases included in our CIA category such as PsA and SA compared to RA patients alone. In contrast, the efficacy of high dose glucocorticoids to treat COVID-19 was demonstrated in the RECOVERY trial (58). The cause of this dichotomy may be related to the timing of use in relation to COVID-19 diagnosis (59).
The description of the high prevalence of COVID-19 and poorest outcome with rituximab is well described (17, 48–53). With COVID-19, the use of rituximab could lead to a persisting viremia without low viral clearance (60–64). In addition, even if our study was performed before the era of COVID-19 vaccination, anti-CD20 are well known to be associated with poor vaccinal response (65–67), so this treatment is still potentially risky in the era of vaccination. The impact of rituximab on COVID-19 prognosis might also explain, partly, the increased risk of severe COVID-19 in patients with vasculitis, who often receive rituximab. Nevertheless, even on multivariate analyses adjusted for underlying disease and in the analysis restricted to CIA, rituximab was still associated with increased probability of severity, with an even higher OR [5.20 (1.83;14.01)].
Role of AIRDs in COVID-19 severity
One of the strengths of our study was the propensity score-matched case–control study that allowed for controlling for confounding factors such as age, sex, and comorbidities and analyzing specifically the risk of COVID-19 severity associated with the underlying AIRD. Indeed, patients without AIRD were younger, often male and had fewer comorbidities than those with AIRDs, so a crude comparison would have led to highly biased results. Overall, patients with AIRD were at increased probability of severe COVID-19 and borderline increased risk of death.
In a previous study, D’Silva et al. found that patients with AIRD had the same odds of hospitalization and mortality but 3-fold higher odds of ICU admission as compared with those without rheumatic disease [aOR = 3.11 (1.07–9.05)] (17). However, this study relied on a small number of patients (n = 52), and AIRD patients were analyzed together, without an analysis restricted to CIA patients. In another study, Pablo et al. did not find any overall increased risk of severe COVID-19 in 228 AIRD patients analyzed altogether [risk ratio = 1.13 (0.84–1.49)] but found an increased risk of severe COVID-19 in patients with other AIRDs (connective tissue diseases, vasculitis, etc.) as compared with CIA patients (16). However, in these cohorts, non-AIRD controls were matched on only age and sex but not comorbidities, which are much more frequent in AIRD patients, as demonstrated by our results. Regarding specifically patients with RA of SpA, as in our study, in the Mena-Vasquez et al. study, 78 patients with CIA did not have increased risk of severe COVID-19 as compared with age-, sex-, hypertension- and diabetes-matched controls (18). By contrast, one study found that patients with RA were at higher risk of severe COVID-19 (hospitalization or death) compared with non-RA controls matched on sex and age (57). But, again controls were not matched on comorbidities and it is possible that, during the first months of COVID-19 breakout, physicians were more prone to hospitalize RA patients on immunosuppressants, even though not having severe COVID-19, since they thought that they were at risk of severe forms. Although we had a smaller sample, our methodology was less subject to bias, and we did not find any increased risk of severe COVID-19 [aOR = 1.11 (0.68–1.81)] or death [aOR = 1 (0.55–1.8)] in CIA patients. Finally, none of these studies found increased risk for COVID-19-related death, but they could lack power (68).
Limitations and strengths
Our study has some limitations. First, because of its retrospective nature, data collection might be subject to potential biases. However, this might have affected all groups of patients and both cases and controls in the same way. In addition, we had access to medical files of all patients, which allowed us to confirm all diagnoses of underlying AIRDs, comorbidities, treatments, and outcome in the first part of the study and to analyze the accuracy of our automated algorithms we developed in the second part. The use of an exhaustive database with an automatic search and a review by an investigator limits the risk of missing data and ensure its accuracy. Secondly, the utilization of two different sources can lead to a reporting bias. However, in both cases information was obtained from clinicians in charge of the patients, which is likely to minimize this bias. Finally, the data were collected before the development of COVID-19 vaccines and the emergence of virus variants and may not reflect the current situation, but studies of patients included after the vaccination period found the same factors as our study (34). Nevertheless, our study has several strengths. We were able to recruit a large patient population assessed within multiple centers in a single country. Moreover, we were able to collect patients with ambulatory and hospitalized forms of COVID-19, covering the entire clinical spectrum of the disease. Ambulatory patients are rarely considered in COVID-19 studies. Second, the use of the EDS-COVID database allowed us to run a propensity score-matched case–control study, including cases and controls from the same population and controlling for age and sex but also all comorbidities known to be associated with COVID-19 severity, which has never been done properly before.
Conclusion
In this multicenter study performed before the era of COVID-19 vaccines, we confirmed that AIRD patients receiving rituximab or corticosteroids were at increased risk of severe COVID-19, as were those with vasculitis, auto-inflammatory disease, and sarcoidosis. Also, as compared with non-AIRD controls from the same cohort of hospitalized patients, AIRD patients had an overall increased odds of severe COVID-19 and a borderline increased odd of death. However, a reassuring point is that these increased odds were not observed in an analysis restricted to patients with RA or SpA.
Data availability statement
The original contributions presented in this study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
This present study was approved by the institutional review board (APHP Scientific and Ethical Committee, authorization no. CSE 20-60_CovAID) from the Scientific and Ethical Committee of the AP-HP and by the CNIL. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
KC collected the data, analyzed them, and wrote the manuscript. RS, XM, and EH designed the study. MG and TJ performed the statistics and help for the data collection. JA, R-MF, SG-L, SE, EP, TP, AS, HM, FD, PC, MD, AM, JS, BF, DR, EE, NC-C, and CR collected data from the patients. All authors reviewed and corrected the manuscript.
Acknowledgments
We thank FAI2R/SFR/SNFMI/SOFREMIP/CRI/IMIDIATE consortium and contributors for having collecting the data of the French RMD COVID-19 Cohort.
Contributors
Florence Aeschlimann; Christian Agard; Nassim Ait-Abdallah; Jean-David Albert; Didier Alcais; Jean-Sébastien Allain; Yannick Allanore; Zahir Amoura; Adamah Amouzougan; Emma Andre; Anaïs Arbault; Jean-Benoît Arlet; Laurent Arnaud; Denis Arniaud; Herliette Arty-Hue; Lucie Atlan; François Aubin; Alexandra Audemard-Verger; Christine Audoin-Pajot; Victor Audren; Gilles Avenel; Jérôme Avouac; Maxime Bach-Bunner; Hélène Bacquet-Deschryver; Brigitte Bader-Meunier; Nathalie Balandraud; Jean-Charles Balblanc; Claire Ballot-Schmit; Stéphane Bally; Frédéric Banal; Béatrice Banneville; Pierre Barbery; Géraldine Bart; André Basch; Vincent Baumier; Guillaume Bayer; Sophie Bayle; Catherine Beauvais; Rudie Beinat; Véronique Belin; Rakiba Belkhir; Alexandre Belot; Auriélie Beltai; Ruben Benainous; Mohammed Benammar; Chahinez Bendahmane; Mathilde Benhamou; Ygal Benhamou; Ahmed Benmansour; Pascal Bennet; Brigitte Bernoux-Manat; Elise Berthet; Sabine Berthier; Olivia Berthoud; Emilie Berthoux; Ewa Bertolini; Adrien Bigot; Aurélia Bisson-Vaivre; Gilles Blaison; Gilles Bolla; Olivier Bonidan; Christine Bonnet; Raphaël Borie; Charlotte Borocco; Marie Bossert; Laurence Boudou; Françoise Bouhour; Kévin Bouiller; Laurence Bouillet; Bastien Bouldoires; Thomas Bourree; Ines Boussen; Karima Boussoualim; Eric Bouvard; Régine Brondino; Pierre Buchlin; Laurence Cabantous; Patrice Cacoub; Simon Cadiou; Alaine Cantagrel; Didier Caplanne; Aurélia Carbasse; Maurizio Carteni; Brice Castel; Pascal Cathebras; Hervé Caumont; Audrey Cayot-Bouillet; Annalisa Celant; Isabelle Cerf-Payrastre; Aurélie Chaffin; Benjamin Chaigne; Benoît Chaillous; Romuald Champy; Agnès Charcot; Pierre Charles; Caroline Charpin; Emmanuel Chatelus; Bernard Chaudier; Pascal Chazerain; Pascale Chertok; Xavier Chevalier; Maxime Chevreau; Emilie Chotard; Delphine Chu Miow Lin; Pascal Claudepierre; Gaëlle Clavel; Cyril Clavel-Osorio; Marine Clay; Johanna Clet; Astrid Coassy; Fleur Cohen; Gregory Cohen; Marie-Eve Colette-Cedoz; Nived Collercandy; Antoine Colombey; Chloé Comarmond; Bernard Combe; Céline Comparon; Elodie Constant; Arnaud Constantin; Pascal Coquerelle; Justine Corli; Clémence Corre; Nathalie Costedoat-Chalumeau; Marion Couderc; Marie Couret; Natacha Courvoisier; Fabienne Coury-Lucas; Cécile Coutarel; Fabrice Coutier; Richard Damade; Laurence Daver-Malaterre; Claire De Moreuil; Marijke Decrock; Michel Delahousse; Emilie Delattre Barrois; Christophe Deligny; Delphine Denarie; Amélie Denis; Camille Deprouw; Emanuelle Dernis; Alban Deroux; Renaud Desbarbieux; Elise Descamps; Alexandra Desdoits; Chantal Deslandre; Marie Desmurs; Jacques Despaux; Marie Desplats; Frédérick Detree; Valérie Devauchelle-Pensec; Mathilde Devaux; Robin Dhote; Harmonie Diaz; Philippe Dieude; Yannick Dieudonne; Elisabeth Diot; Guillaume Direz; Djamal-Dine Djeddi; Fanny Domont; Sarah Douvier; Béatrice Drouet; Jean-Jacques Dubost; Catherine Duc; Angélique Ducornet; Carine Dufauret-Lombard; Cécile Dumaine; Anne-Elisabeth Dumel; Chantal Dumoulin-Richez; Agnès Duquesne; Géraldine Durand; Mariane Durandin-Truffinet; Pierre-Marie Duret; Stéphanie Durieux-Mehlman; Perrine Dusser-Benesty; Maïka Duval; Mikaël Ebbo; Esther Ebstein; Andra Economu-Dubosc; Soumaya El Mahou; Stéphanie Emilie; Romain Euvrard; Philippe Evon; Claire Eymard-Gibert; Sylvie Fabre; Dorothée Fagedet; Dominique Farge-Bancel; Meryem Farhat; Marion Fauconier; Bruno Fautrel; Jacques Fechtenbaum; Renaud Felten; Nicole Ferreira-Maldent; Elodie Feurer; Amandine Fichet; Françoise Flaisler; René-Marc Flipo; Nans Florens; Violaine Foltz; Elisabeth Fontanges; Jennifer Foret; Anne-Claire Fougerousse; Anne Fouque-Aubert; Catherine Foutrier-Morello; Hélène Francois-Pradier; Léa Frantzen; Marie-Louise Fremond; Pierre Fritz; Antoine Froissart; Jean Fulpin; Piera Fuzibet; Francis Gaches; Laurence Gagneux-Lemoussu; Mélanie Gahier Penhoat; Joris Galland; Frédérique Gandjbakhch; Anaïs Gardette; Nicole Garnier; Thomas Garraud; Jean-François Garrot; Romain Gastaldi; Philippe Gaudin; Véronique Gaud-Listrat; Maud Gauthier-Prieur; Loraine Gauzere; Marion Geoffroy; Dana Georgescu; Sophie Georgin-Lavialle; Nathalie Gerard; Anne Gerber; Elisabeth Gervais; Christelle Gibert; Eric Gibert; Ghislaine Gill; Jérôme Gillard; Mélanie Gilson; Pauline Gimonnet; Jeanine-Sophie Giraudet-Le Quintrec; Aude Giraud-Morelet; Baptiste Glace; Camille Glanowski; Bertrand Godeau; Bruno Gombert; Camille Gonnet-Gracia; Tiphaine Goulenok; Philippe Goupille; Olivier Gourmelen; Sophie Govindaraju-Audouard; Franck Grados; Martine Grall-Lerosey; Bruno Grardel; Anne Grasland; Gilles Grateau; Monica Groza; Pascal Guggenbuhl; Isabelle Guichard; Constance Guillaud; Sévenrine Guillaume-Czitrom; Caroline Guillibert; Xavier Guillot; Philippe Guilpain; Aline Gury; Pauline Guyader; Marie-Hélène Guyot; Eric Hachulla; Cécile Hacquard-Bouder; Marie-Noelle Havard; Jean-Pierre Hellier; Pascal Hennequin; Julien Henry; Véronique Hentgen; Marion Hermet; Julie Hernandez; Miguel Hie; Pascal Hilliquin; Olivier Hinschberger; Ambre Hittinger-Roux; Jan Holubar; Elsa How Shing Koy; Charlotte Hua; Christophe Hudry; Serge Huguenel; Clara Jaccard; Jean-Michel Jacquemier; Bénédicte Jamard; Catherine Jan; Sylvie Jean; Laurie Joffres; Sandrine Jousse-Joulin; Mathieu Jouvray; Pierre-Antoine Juge; Laurent Juillard; Denis Jullien; Abdelkrim Kabchou; Ludovic Karkowski; Françoise Karman; Farid Kemiche; Jérémy Keraen; Pierre Kieffer; Isabelle Kone-Paut; Abdeldajallil Koreichi; Marie Kostine; Stéphanie Krebs; Sylvain La Batide Alanore; Valentin Lacombe; Pierre Lafforgue; Sophie Lahalle; Marc Lambert; Isabelle Lambrecht; François Lamer; Vincent Langlois; Sylvain Lanot; Aurélia Lanteri; Jean-Paul Larbre; Augustin Latourte; Christian Lavigne; Noémie Le Gouellec; Sophie Le Guen Guegan; Guillaume Le Guenno; Agnès Lebrun; Emmanuel Ledoult; Nathalie Legoupil; Erick Legrand; Diane Leguy; Vanessa Leguy-Seguin; Olivier Leloire; Christophe Leroux; Rémi Leroy; Marie Leroy-Gouix; Charles Leske; Tifenn Leturcq; Amélie Leurs; Céline Leveque-Michaud; François-Xavier Limbach; Frédéric Liote; Anne Lohse; Pierre Lozac’h; Charlotte Lucas; Etienne Mabrut; Aurélie Madelon; Marion Magnol; Nadine Magy-Bertrand; Matthieu Mahevas; Hélène Maillard; Thibault Maillet; François Maillot; Sandrine Malochet-Guinamand; Quentin Mangon; Julie Mankikian; Sylvie Marchou-Lopez; Nathalie Margarit; Thierry Marhadour; Alexandre Maria; Xavier Mariette; Hubert Marotte; Claire Martin; Thierry Martin; Alexis Mathian; Sylvain Mathieu; François Maurier; Frédéric Maury; Betty Mazet-Guillaume; Arnaud Mazouyez; Hassan Mazyad; Nadia Mehsen-Cetre; Arsène Mekinian; Isabelle Melki; Jean-Camille Meric; Laurent Messer; Martin Michaud; Catherine Michel; Matthias Michel; Mathilde Michon; Anne-Marie Milesi-Lecat; Anna Molto; Marie Moly; Olivier Moranne; Gautier Morel; Hugo Morel; Jacques Morel; Franck Morin; Laurence Moulinier; Guillaume Moulis; Bertrand Moura; Claudine Naude; Minh Nguyen; Sabine Nicolas-Vullierme; Hubert Nielly; Gaétane Nocturne; Aurore Nottez; Anne Olivier; Henri-Olivier Ollagnon; Eric Oziol; Isabelle Pacaud-Vitoux; Guillaume Padern; Anne Pagnier; Caroline Paris; Antoine Parrot; Tristan Pascart; Yasmina Pascaud-Mansour; Laetitia Paulin; Stephan Pavy; Judith Payet; Laurent Perard; Céline Pereira-Gillion; Yves-Marie Pers; Edouard Pertuiset; Juliette-Louise Petit; Micheline Pha; Thao Pham; Maud Pichon; Audrey Pierreisnard; Gabrielle Pizana; Sylvaine Poignant; Elsa Poix; Agnès Portier; Jacques Pouchot; Antoine Poulet; Nicolas Poursac; Grégory Pugnet; Déborah Puyraimond-Zemmour; Pierre Quartier-Dit-Maire; Marion Quenet; Viviane Queyrel; Loïc Raffray; Jérôme Razanamahery; Philippe Remy; Myriam Renard; Jessica Rene; Sabine Revuz; Bénédicte Rey; Gaëlle Richard-Colmant; Olivier Richer; Christophe Richez; Elodie Riviere; Etienne Riviere; Sébastien Riviere; Sophie Robin; Julien Rohmer; Isabelle Roitg; Michel Rolland; Mélanie Roriz; Carole Rosenberg; Linda Rossi; Olivier Roth; Sid-Ahmed Rouidi; Mathilde Roumier; Mickaël Rousiere; Clémentine Rousselin; Bénédicte Rouviere; Christian Roux; Fabienne Roux; Marielle Roux; Nicolas Roux; Diane Rouzaud; Sylvie Rozenberg; Adeline Ruyssen-Witrand; Isabelle Sacco; Fatiha Sadji; Laurent Sailler; Carine Salliot; Jean-Hugues Salmon; Charlotte Samaille; Isabelle Samjee; Maxime Samson; Alain Saraux; Jordane Saunier; Léa Savey; Jean Schmidt; Julie Seguier; Jérémie Sellam; Eric Senbel; Thomas Sene; Patricia Senet; Raphaële Seror; Amélie Servettaz; Pascal Seve; Aurélie Sicaud; Nadia Sivova; Perrine Smets; Vincent Sobanski; Christelle Sordet; Elisabeth Sornay-Rendu; Martin Soubrier; Odile Souchaud-Debouverie; Gaëlle Sourisseau-Diverres; Laetitia Sparsa; Lionel Spielmann; Chloé Stavris; Sarah Steib; Catherine Straus; Victor Strotz; Paulina Szafors; Séverine Taffignon-Clave; Déborah Talmud; Chloé Taraud; Nora Tenenbaum; Claire Theillac; Benoît Thomachot; Thierry Thomas; Nathalie Tieulie; Soizic Tiriau; Alice Tison; Anne Tournadre; Eric Toussirot; Ludovic Trefond; Sophie Trijau; Sébastien Trouillier; Anne-Priscille Trouvin; Marie-Elise Truchetet; Florence Uettwiller; Marc Ulrich; Yurdagul Uzunhan; Isabelle Valls-Bellec; Jacques Vaquier; Eric Veillard; Laurent Veillon; Guillaume Vial; Jean-François Viallard; Judith Victor; Claire Vidon; Mathias Vidon; Camille Vigne; Alexandre Virone; Ursula Warzocha; Daniel Wendling; Claude Werle; Cécile Wibaux; Michel Wisniewski; Juliette Woessner; Bernadette Xerri-Campano.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2023.1152587/full#supplementary-material
References
- 1.Avouac J, Drumez E, Hachulla E, Seror R, Georgin-Lavialle S, El Mahou S, et al. COVID-19 outcomes in patients with inflammatory rheumatic and musculoskeletal diseases treated with rituximab: a cohort study. Lancet Rheumatol. (2021). 3:e419–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cascella M, Rajnik M, Aleem A, Dulebohn S, Di Napoli R. Features, Evaluation, and Treatment of Coronavirus (COVID-19). StatPearls. Treasure Island, FL: StatPearls Publishing; (2022). [PubMed] [Google Scholar]
- 3.Ren S, Wang W, Gao R, Zhou A. Omicron variant (B.1.1.529) of SARS-CoV-2: mutation, infectivity, transmission, and vaccine resistance. WJCC. (2022) 10:1–11. 10.12998/wjcc.v10.i1.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen J, Wang R, Gilby N, Wei G. Omicron Variant (B.1.1.529): infectivity, vaccine breakthrough, and antibody resistance. J Chem Inf Model. (2022) 62:412–22. 10.1021/acs.jcim.1c01451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.FAI2R/SFR/SNFMI/SOFREMIP/CRI/IMIDIATE consortium and contributors. Severity of COVID-19 and survival in patients with rheumatic and inflammatory diseases: data from the French RMD COVID-19 cohort of 694 patients. Ann Rheum Dis. (2020) 80:527–38. 10.1136/annrheumdis-2020-218310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Czernichow S, Beeker N, Rives-Lange C, Guerot E, Diehl J, Katsahian S. Obesity doubles mortality in patients hospitalized for severe acute respiratory syndrome coronavirus 2 in Paris hospitals, France: a cohort study on 5,795 patients. Obesity. (2020) 28:2282–9. 10.1002/oby.23014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zarei F, Jalli R, Iranpour P, Sefidbakht S, Soltanabadi S, Rezaee M, et al. Differentiation of chest CT findings between influenza pneumonia and COVID-19: interobserver agreement between radiologists. Acad Radiol. (2021) 28:1331–8. 10.1016/j.acra.2021.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Digan W, Neuraz A, Rogier A, Baudoin D, Garcelon N, Rance B. PyMedExt, un couteau suisse pour le traitement des textes médicaux. Paris: AFIA-TLH/ATALA; (2021). [Google Scholar]
- 9.Garcelon N, Neuraz A, Benoit V, Salomon R, Burgun A. Improving a full-text search engine: the importance of negation detection and family history context to identify cases in a biomedical data warehouse. J Am Med Inform Assoc. (2017) 24:607–13. 10.1093/jamia/ocw144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Strangfeld A, Schäfer M, Gianfrancesco M, Lawson-Tovey S, Liew J, Ljung L, et al. Factors associated with COVID-19-related death in people with rheumatic diseases: results from the COVID-19 Global Rheumatology Alliance physician-reported registry. Ann Rheum Dis. (2021) 80:930–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gianfrancesco M, Hyrich K, Gossec L, Strangfeld A, Carmona L, Mateus E, et al. Rheumatic disease and COVID-19: initial data from the COVID-19 Global Rheumatology Alliance provider registries. Lancet Rheumatol. (2020) 2:e250–3. 10.1016/S2665-9913(20)30095-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Austin P. A comparison of 12 algorithms for matching on the propensity score. Statist Med. (2014) 33:1057–69. 10.1002/sim.6004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Austin P. Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharmaceut Statist. (2011) 10:150–61. 10.1002/pst.433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Austin P. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Statist Med. (2009) 28:3083–107. 10.1002/sim.3697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Austin P. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivar Behav Res. (2011) 46:399–424. 10.1080/00273171.2011.568786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pablos J, Galindo M, Carmona L, Lledó A, Retuerto M, Blanco R, et al. Clinical outcomes of hospitalised patients with COVID-19 and chronic inflammatory and autoimmune rheumatic diseases: a multicentric matched cohort study. Ann Rheum Dis. (2020) 79:1544–9. 10.1136/annrheumdis-2020-218296 [DOI] [PubMed] [Google Scholar]
- 17.D’Silva K, Serling-Boyd N, Wallwork R, Hsu T, Fu X, Gravallese E, et al. Clinical characteristics and outcomes of patients with coronavirus disease 2019 (COVID-19) and rheumatic disease: a comparative cohort study from a US ‘hot spot’. Ann Rheum Dis. (2020) 79:1156–62. 10.1136/annrheumdis-2020-217888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mena-Vázquez N, Manrique Arija S, Rojas-Giménez M, Raya-Álvarez E, Velloso-Feijoó M, López-Medina C, et al. Hospitalizaciones y mortalidad por COVID-19 en pacientes con enfermedades inflamatorias reumáticas en Andalucía. Reumatol Clín. (2021) 18:422–8. 10.1016/j.reuma.2021.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McKeigue P, Porter D, Hollick R, Ralston S, McAllister D, Colhoun H. Risk of severe COVID-19 in patients with inflammatory rheumatic diseases treated with immunosuppressive therapy in Scotland. Scand J Rheumatol. (2022) [Epub ahead of print]. 10.1080/03009742.2022.2063376 [DOI] [PubMed] [Google Scholar]
- 20.Montero F, Martínez-Barrio J, Serrano-Benavente B, González T, Rivera J, Molina Collada J, et al. Coronavirus disease 2019 (COVID-19) in autoimmune and inflammatory conditions: clinical characteristics of poor outcomes. Rheumatol Int. (2020) 40:1593–8. 10.1007/s00296-020-04676-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hasseli R, Mueller-Ladner U, Schmeiser T, Hoyer B, Krause A, Lorenz H, et al. National registry for patients with inflammatory rheumatic diseases (IRD) infected with SARS-CoV-2 in Germany (ReCoVery): a valuable mean to gain rapid and reliable knowledge of the clinical course of SARS-CoV-2 infections in patients with IRD. RMD Open. (2020) 6:e001332. 10.1136/rmdopen-2020-001332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haberman R, Castillo R, Chen A, Yan D, Ramirez D, Sekar V, et al. COVID-19 in patients with inflammatory arthritis: a prospective study on the effects of comorbidities and disease-modifying antirheumatic drugs on clinical outcomes. Arthritis Rheumatol. (2020) 72:1981–9. 10.1002/art.41456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guo L, Shi Z, Zhang Y, Wang C, Do Vale Moreira N, Zuo H. Comorbid diabetes and the risk of disease severity or death among 8807 COVID-19 patients in China: a meta-analysis. Diabetes Res Clin Pract. (2020) 166:108346. 10.1016/j.diabres.2020.108346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shi Q, Zhang X, Jiang F, Zhang X, Hu N, Bimu C, et al. Clinical characteristics and risk factors for mortality of COVID-19 patients with diabetes in Wuhan, China: a two-center, retrospective study. Dia Care. (2020) 43:1382–91. 10.2337/dc20-0598 [DOI] [PubMed] [Google Scholar]
- 25.Guan W, Ni Z, Hu Y, Liang W, Ou C, He J, et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. (2020) 382:1708–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. (2020) 395:1054–62. 10.1016/S0140-6736(20)30566-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wolff D, Nee S, Hickey N, Marschollek M. Risk factors for Covid-19 severity and fatality: a structured literature review. Infection. (2021) 49:15–28. 10.1007/s15010-020-01509-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ebinger J, Achamallah N, Ji H, Claggett B, Sun N, Botting P, et al. Pre-existing traits associated with Covid-19 illness severity. PLoS One. (2020) 15:e0236240. 10.1371/journal.pone.0236240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yildiz H, Castanares-Zapatero D, Pierman G, Pothen L, De Greef J, Aboubakar Nana F, et al. Validation of Neutrophil-to-Lymphocyte Ratio Cut-off Value Associated with High In-Hospital Mortality in COVID-19 Patients. IJGM. (2021) 14:5111–7. 10.2147/IJGM.S326666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ramos-Rincon J, Buonaiuto V, Ricci M, Martín-Carmona J, Paredes-Ruíz D, Calderón-Moreno M, et al. Clinical Characteristics and Risk Factors for Mortality in Very Old Patients Hospitalized With COVID-19 in Spain. J Gerontol A Biol Sci Med Sci. (2021) 76:e28–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Giorgi Rossi P, Marino M, Formisano D, Venturelli F, Vicentini M, Grilli R, et al. Characteristics and outcomes of a cohort of COVID-19 patients in the Province of Reggio Emilia, Italy. PLoS One. (2020) 15:e0238281. 10.1371/journal.pone.0238281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang H, Zhang M, Chen C, Zhang H, Wei Y, Tian J, et al. Clinical characteristics of COVID-19 in patients with preexisting ILD: A retrospective study in a single center in Wuhan, China. J Med Virol. (2020) 92:2742–50. 10.1002/jmv.26174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goldman J, Robinson P, Uldrick T, Ljungman P. COVID-19 in immunocompromised populations: implications for prognosis and repurposing of immunotherapies. J Immunother Cancer. (2021) 9:e002630. 10.1136/jitc-2021-002630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Isnardi C, Roberts K, Saurit V, Petkovic I, Báez R, Quintana R, et al. Sociodemographic and clinical factors associated with poor COVID-19 outcomes in patients with rheumatic diseases: data from the SAR-COVID Registry. Clin Rheumatol. (2022) 42:563–78. 10.1007/s10067-022-06393-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cao M, Zhang D, Wang Y, Lu Y, Zhu X, Li Y, et al. Clinical Features of Patients Infected with the 2019 Novel Coronavirus (COVID-19) in Shanghai, China. medRxiv [Preprint]. (2020) 10.1101/2020.03.04.20030395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Feng Z, Li J, Yao S, Yu Q, Zhou W, Mao X, et al. The use of adjuvant therapy in preventing progression to severe pneumonia in patients with coronavirus disease 2019: a multicenter data analysis. medRxiv [Preprint]. (2020) 10.1101/2020.04.08.20057539 [DOI] [Google Scholar]
- 37.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. (2020) 323:1061–9. 10.1001/jama.2020.1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jordan R, Adab P, Cheng K. Covid-19: risk factors for severe disease and death. BMJ. (2020) 368:m1198. [DOI] [PubMed] [Google Scholar]
- 39.Esposito A, Menon A, Ghosh A, Putman R, Fredenburgh L, El-Chemaly S, et al. Increased odds of death for patients with interstitial lung disease and COVID-19: a case–control study. Am J Respir Crit Care Med. (2020) 202:1710–3. 10.1164/rccm.202006-2441LE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Santos C, Morales C, Álvarez E, Castro C, Robles A, Sandoval T. Determinants of COVID-19 disease severity in patients with underlying rheumatic disease. Clin Rheumatol. (2020) 39:2789–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Southern B. Patients with interstitial lung disease and pulmonary sarcoidosis are at high risk for severe illness related to COVID-19. Cleve Clin J Med. (2020) 10.3949/ccjm.87a.ccc026 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 42.Brito-Zerón P, Gracia-Tello B, Robles A, Alguacil A, Bonet M, De-Escalante B, et al. Characterization and Outcomes of SARS-CoV-2 Infection in Patients with Sarcoidosis. Viruses. (2021) 13:1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Baughman R, Lower EE. COVID-19 infections in sarcoidosis: a prospective single center study of 886 sarcoidosis patients. Sarcoid Vasc Diffuse Lung Dis. (2021) 38:e2021029. 10.36141/svdld.v38i2.11646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kronbichler A, Geetha D, Smith R, Egan A, Bajema I, Schönermarck U, et al. The COVID-19 pandemic and ANCA-associated vasculitis – reports from the EUVAS meeting and EUVAS education forum. Autoimmun Rev. (2021) 20:102986. 10.1016/j.autrev.2021.102986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bourguiba R, Kyheng M, Koné-Paut I, Rouzaud D, Avouac J, Devaux M, et al. COVID-19 infection among patients with autoinflammatory diseases: a study on 117 French patients compared with 1545 from the French RMD COVID-19 cohort: COVIMAI – the French cohort study of SARS-CoV-2 infection in patient with systemic autoinflammatory diseases. RMD Open. (2022) 8:e002063. 10.1136/rmdopen-2021-002063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bourguiba R, Delplanque M, Vinit C, Ackermann F, Savey L, Grateau G, et al. Clinical course of COVID-19 in a cohort of 342 familial Mediterranean fever patients with a long-term treatment by colchicine in a French endemic area. Ann Rheum Dis. (2021) 80:539–40. 10.1136/annrheumdis-2020-218707 [DOI] [PubMed] [Google Scholar]
- 47.Shen C, Risk M, Schiopu E, Hayek S, Xie T, Holevinski L, et al. Efficacy of COVID-19 vaccines in patients taking immunosuppressants. Ann Rheum Dis. (2022) 81:875–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kow C, Hasan S. Use of rituximab and the risk of adverse clinical outcomes in COVID-19 patients with systemic rheumatic disease. Rheumatol Int. (2020) 40:2117–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Galimberti F, McBride J, Cronin M, Li Y, Fox J, Abrouk M, et al. Evidence-based best practice advice for patients treated with systemic immunosuppressants in relation to COVID-19. Clin Dermatol. (2020) 38:775–80. 10.1016/j.clindermatol.2020.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Guilpain P, Le Bihan C, Foulongne V, Taourel P, Pansu N, Maria A, et al. Rituximab for granulomatosis with polyangiitis in the pandemic of covid-19: lessons from a case with severe pneumonia. Ann Rheum Dis. (2021) 80:e10–10. [DOI] [PubMed] [Google Scholar]
- 51.Avouac J, Airó P, Carlier N, Matucci-Cerinic M, Allanore Y. Severe COVID-19-associated pneumonia in 3 patients with systemic sclerosis treated with rituximab. Ann Rheum Dis. (2021) 80:e37–37. [DOI] [PubMed] [Google Scholar]
- 52.Sharmeen S, Elghawy A, Zarlasht F, Yao Q. COVID-19 in rheumatic disease patients on immunosuppressive agents. Semin Arthr Rheum. (2020) 50:680–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schulze-Koops H, Krueger K, Vallbracht I, Hasseli R, Skapenko A. Increased risk for severe COVID-19 in patients with inflammatory rheumatic diseases treated with rituximab. Ann Rheum Dis. (2021) 80:e67–67. [DOI] [PubMed] [Google Scholar]
- 54.Gao Y, Ding M, Dong X, Zhang J, Kursat Azkur A, Azkur D, et al. Risk factors for severe and critically ill COVID-19 patients: A review. Allergy. (2021) 76:428–55. [DOI] [PubMed] [Google Scholar]
- 55.Shin Y, Shin J, Moon S, Jin H, Kim S, Yang J, et al. Autoimmune inflammatory rheumatic diseases and COVID-19 outcomes in South Korea: a nationwide cohort study. Lancet Rheumatol. (2021) 3:e698–706. 10.1016/S2665-9913(21)00151-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Baslılar S, Pehlivan O. Evaluation of factors affecting the frequency and clinical course of COVID-19 in patients using anti-TNF-alpha agents. Rev Assoc Med Bras. (2021) 67:1286–92. 10.1590/1806-9282.20210568 [DOI] [PubMed] [Google Scholar]
- 57.England B, Roul P, Yang Y, Kalil A, Michaud K, Thiele G, et al. Risk of COVID-19 in rheumatoid arthritis: a national veterans affairs matched cohort study in at-risk individuals. Arthritis Rheumatol. (2021) 73:2179–88. 10.1002/art.41800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Horby P, Lim W, Emberson J, Mafham M, Bell J, Linsell L, et al. Dexamethasone in hospitalized patients with COVID-19. N Engl J Med. (2021) 384:693–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Robinson P, Morand E. Divergent effects of acute versus chronic glucocorticoids in COVID-19. Lancet Rheumatol. (2021) 3:e168–70. 10.1016/S2665-9913(21)00005-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tepasse P, Hafezi W, Lutz M, Kühn J, Wilms C, Wiewrodt R, et al. Persisting SARS-CoV-2 viraemia after rituximab therapy: two cases with fatal outcome and a review of the literature. Br J Haematol. (2020) 190:185–8. 10.1111/bjh.16896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yasuda H, Tsukune Y, Watanabe N, Sugimoto K, Uchimura A, Tateyama M, et al. Persistent COVID-19 pneumonia and failure to develop Anti-SARS-CoV-2 antibodies during rituximab maintenance therapy for follicular lymphoma. Clin Lymph Myeloma Leukem. (2020) 20:774–6. 10.1016/j.clml.2020.08.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Leipe J, Wilke E, Ebert M, Teufel A, Reindl W. Long, relapsing, and atypical symptomatic course of COVID-19 in a B-cell-depleted patient after rituximab. Semin Arthr Rheum. (2020) 50:1087–8. 10.1016/j.semarthrit.2020.06.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kos I, Balensiefer B, Roth S, Ahlgrimm M, Sester M, Schmidt T, et al. Prolonged course of COVID-19-Associated pneumonia in a B-Cell depleted patient after rituximab. Front Oncol. (2020) 10:1578. 10.3389/fonc.2020.01578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Betrains A, Godinas L, Woei-A-Jin F, Rosseels W, Van Herck Y, Lorent N. Convalescent plasma treatment of persistent severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection in patients with lymphoma with impaired humoral immunity and lack of neutralising antibodies. Br J Haematol. (2021) 192:1100–5. 10.1111/bjh.17266 [DOI] [PubMed] [Google Scholar]
- 65.Vijenthira A, Gong I, Betschel S, Cheung M, Hicks L. Vaccine response following anti-CD20 therapy: a systematic review and meta-analysis of 905 patients. Blood Adv. (2021) 5:2624–43. 10.1182/bloodadvances.2021004629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Spiera R, Jinich S, Jannat-Khah D. Rituximab, but not other antirheumatic therapies, is associated with impaired serological response to SARS-CoV-2 vaccination in patients with rheumatic diseases. Ann Rheum Dis. (2021) 80:1357–9. [DOI] [PubMed] [Google Scholar]
- 67.Bitoun S, Henry J, Desjardins D, Vauloup-Fellous C, Dib N, Belkhir R. Rituximab impairs B cell response but not T Cell response to COVID-19 vaccine in autoimmune diseases. Arthr Rheumatol. (2022) 74:927–33. 10.1002/art.42058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Roseti L, Grigolo B. COVID-19 and rheumatic diseases: a mini-review. Front Med. (2022) 9:997876. 10.3389/fmed.2022.997876 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.