Abstract
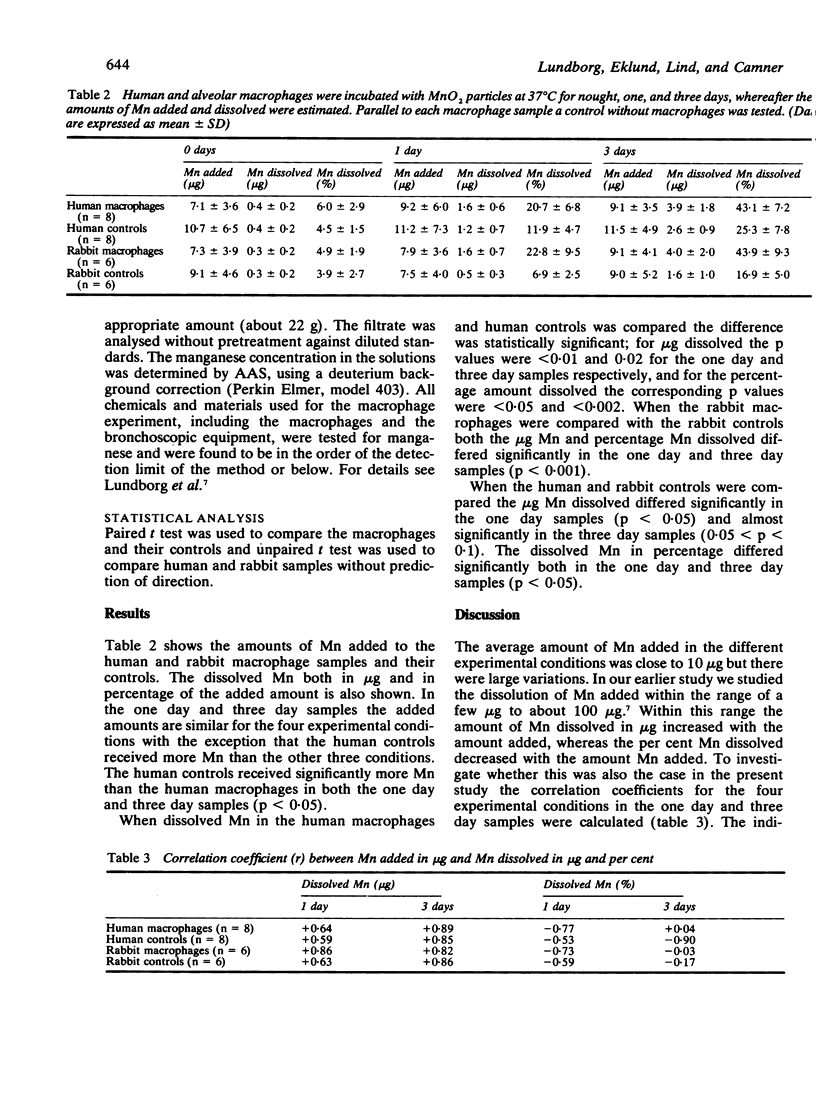
The ability of human and rabbit alveolar macrophages to dissolve 0.1-0.5 micron MnO2 particles in vitro was compared. The amount of Mn added and dissolved from the particles over periods of nought, one, and three days was determined by flame atomic absorption spectrophotometry. The amount dissolved by human and rabbit macrophages was similar; on average 43.1% and 43.9%, respectively, were dissolved within three days. But rabbit and human macrophages dissolved significantly more Mn than was dissolved in the respective culture medium without macrophages after one and three days. It is suggested that the dissolution of particles by alveolar macrophages should be one basic component in any model of alveolar clearance of inorganic particles.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bailey M. R., Fry F. A., James A. C. The long-term clearance kinetics of insoluble particles from the human lung. Ann Occup Hyg. 1982;26(1-4):273–290. [PubMed] [Google Scholar]
- Bohning D. E., Atkins H. L., Cohn S. H. Long-term particle clearance in man: normal and impaired. Ann Occup Hyg. 1982;26(1-4):259–271. [PubMed] [Google Scholar]
- Haslam P. L., Turton C. W., Heard B., Lukoszek A., Collins J. V., Salsbury A. J., Turner-Warwick M. Bronchoalveolar lavage in pulmonary fibrosis: comparison of cells obtained with lung biopsy and clinical features. Thorax. 1980 Jan;35(1):9–18. doi: 10.1136/thx.35.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen M. S., Bainton D. F. Temporal changes in pH within the phagocytic vacuole of the polymorphonuclear neutrophilic leukocyte. J Cell Biol. 1973 Feb;56(2):379–388. doi: 10.1083/jcb.56.2.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundborg M., Holma B. In vitro phagocytosis of fungal spores by rabbit lung macrophages. Sabouraudia. 1972 Jul;10(2):152–156. doi: 10.1080/00362177285190301. [DOI] [PubMed] [Google Scholar]
- Lundborg M., Lind B., Camner P. Ability of rabbit alveolar macrophages to dissolve metals. Exp Lung Res. 1984;7(1):11–22. doi: 10.3109/01902148409087905. [DOI] [PubMed] [Google Scholar]
- MORROW P. E., GIBB F. R., JOHNSON L. CLEARANCE OF INSOLUBLE DUST FROM THE LOWER RESPIRATORY TRACT. Health Phys. 1964 Aug;10:543–555. doi: 10.1097/00004032-196408000-00003. [DOI] [PubMed] [Google Scholar]
- MYRVIK Q., LEAKE E. S., FARISS B. Studies on pulmonary alveolar macrophages from the normal rabbit: a technique to procure them in a high state of purity. J Immunol. 1961 Feb;86:128–132. [PubMed] [Google Scholar]
- Mandell G. L. Intraphagosomal pH of human polymorphonuclear neutrophils. Proc Soc Exp Biol Med. 1970 Jun;134(2):447–449. doi: 10.3181/00379727-134-34810. [DOI] [PubMed] [Google Scholar]
- Mercer T. T. On the role of particle size in the dissolution of lung burdens. Health Phys. 1967 Nov;13(11):1211–1221. doi: 10.1097/00004032-196711000-00005. [DOI] [PubMed] [Google Scholar]
- Morrow P. E., Gibb F. R., Gazioglu K. M. A study of particulate clearance from the human lungs. Am Rev Respir Dis. 1967 Dec;96(6):1209–1221. doi: 10.1164/arrd.1967.96.6.1209. [DOI] [PubMed] [Google Scholar]
- Ohkuma S., Poole B. Fluorescence probe measurement of the intralysosomal pH in living cells and the perturbation of pH by various agents. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3327–3331. doi: 10.1073/pnas.75.7.3327. [DOI] [PMC free article] [PubMed] [Google Scholar]


