Abstract
IgA nephropathy (IgAN), an immune-mediated chronic inflammatory kidney disease, is the most common primary glomerular disease in Asia, especially in China and Japan. The pathogenesis of IgAN is complex, and the main cause of IgAN is explained by the ‘multiple hit’ theory, which states that the deposition of immune complexes in renal mesangial cells induces chronic inflammation that leads to kidney damage. Chronic inflammation is associated with iron metabolism, which also plays an essential role in the pathogenesis, progression, diagnosis and prognosis of IgAN. Overall, this review aimed to explore the application of iron metabolism in IgAN by systematically elaborating the relationship between iron metabolism and chronic inflammation in IgAN to speculate on the possible diagnostic and therapeutic significance of iron metabolism indicators in IgAN.
Keywords: IgA nephropathy, iron metabolism, chronic inflammation, ferroptosis, treatment
1. Introduction
Iron is an indispensable element. Iron is regulated at the systemic level and the cellular level, mainly through the absorption, transport, distribution, storage, utilization and excretion of iron metabolic processes to maintain iron homeostasis in the human body [1]. Iron metabolism disorders are closely associated with a diversity of diseases, such as cancer, neurodegenerative diseases, ischemia–reperfusion injury, hematologic disorders, inflammatory bowel diseases, liver diseases, pulmonary fibrosis and chronic kidney disease (CKD) [2–9]. Additionally, the kidney influences the regulation of iron homeostasis [10–12], while imbalances in iron homeostasis can exacerbate kidney injury [11,13–15].
IgAN is the most common primary glomerulonephritis in the world, and approximately 30% of IgAN patients develop end-stage renal disease (ESRD) within 20 years [16,17]. IgAN accounts for approximately 40% of all natural kidney biopsies in Japan, 25% in Europe, and 12% in the United States but less than 5% in Central Africa [18]. IgAN is a chronic inflammatory disease, and its pathogenesis and progression are associated with many indicators of iron metabolism [19–21]. In addition, IgAN is characterized by hematuria and proteinuria as the main clinical manifestations [22]. Iron from hemoglobin metabolism in hematuria is toxic to proximal tubular cells [23], mainly because of the ability of renal tubular epithelial cells to phagocytose and degrade red blood cells [24–26], leading to an increase in Fe2+, which reacts with H2O2 to produce reactive oxygen species (ROS) to damage kidney cells [27]. Thus, IgAN, iron metabolism, and chronic inflammation are related and have a complex relationship. When elaborating this relationship, we need to focus on the importance of iron metabolism in IgAN and increase research on the mechanism of hematuria severity with IgAN.
2. Physiological iron metabolism
2.1. Body iron homeostasis
Iron metabolism is regulated at the systemic and cellular levels. Systemic iron homeostasis is mostly adjusted by the hepcidin-/ferroportin axis [28,29]. Because iron losses are relatively small, iron absorption and its regulation by hepcidin and ferroportin (FPN) determine systemic iron levels [30]. FPN exports iron from duodenal enterocytes that absorb dietary iron, from iron-recycling macrophages in the spleen and the liver, and from iron-storing hepatocytes [31]. Hemoglobin in mammals contains more than half of the total human iron content [32]. Hemo Oxygenase-1 (HO-1) degrades hemoglobin to bilirubin, Fe2+ and carbon monoxide, and the released iron can be exported from macrophages via FPN or stored intracellularly via ferritin [33,34]. Hepcidin is a negative regulator of iron metabolism that blocks iron export through FPN [30]. When iron deficiency or hemorrhage occurs, hepcidin decreases to allow iron delivery to plasma through FPN, promoting compensatory erythropoiesis. During infection or inflammation, hepcidin blocks iron delivery to plasma via FPN, limiting the supply of iron to invading microorganisms [29]. Cellular iron levels are largely modulated by the iron response element-iron regulatory protein (IRE-IRP) system, which adjusts the expression of transferrin (Tf), transferrin receptor 1 (TfR1), divalent metal transport protein 1 (DMT1), FPN, and ferritin [35]. When the cellular iron concentration is relatively low, IRP binding to IRE of TfR1 and DMT1 promotes the expression of both and augments iron uptake; conversely, IRE is occupied by Fe/S proteins, which prevents IRP from binding to IRE of TfR1 or DMT1, resulting in lower levels of TfR1 and DMT1 translation and less iron uptake when cellular iron levels are comparatively high [36]. Under iron loading conditions, this eventually leads to the over-saturation of the iron carrier protein transferrin and the generation of non-transferrin bound iron (NTBI)[37]. A growing body of literature indicates that NTBI uptake is mediated by non-transferrin-bound iron transporters such as ZIP14, L-type and T-type calcium channels, DMT1, ZIP8, and TRPC6 [38–42].
2.2. Renal iron homeostasis
Three regulatory mechanisms are relevant to iron metabolism in the kidney, including the IRE-IRP system, HIF regulatory system and renal reabsorption [11,12,43–48]. Many transport proteins and regulatory pathways involved in cellular iron handling have been identified in the kidney [10,45,47,48]. IRP1 and IRP2 are expressed in the kidney, with IRP1 being more distributed in the proximal tubule [45]. HIF1α is found in renal tubular cells, whereas HIF2α is restricted to renal endothelial cells and interstitial cells [47]. HIF2α promotes erythropoietin production by renal interstitial fibroblasts under hypoxic conditions, which can be inhibited by IRP1 [48]. Cultured human glomerular endothelial cells have been shown to express TfR1, FPN and DMT1 [49]. The basolateral membrane is the site of tubular iron export, and the only iron exporter that has been identified in the kidney tubules is FPN, which is present in the proximal tubules [50].
The renal reabsorption of Tf and iron plays an important role in iron regulation in the renal tubular epithelial system [11,12,51]. It has been shown that renal tubular dysfunction, as observed in patients with Fanconi syndrome, Dent disease and Lowe syndrome, results in reduced reabsorption [12,52,53]. Thus, renal tubular dysfunction can increase urinary Tf and iron excretion. In the glomeruli, transferrin-bound iron (TBI) can enter mesangial cells and endothelial cells via TfR1 and podocytes via an as yet unidentified transporter [11]. A limited iron-transferrin complex can be bound to TfR1 and megalin-cubilin complexes on the parietal membrane of proximal tubular epithelial cells by mediating the endocytosis of transferrin [54]. In addition, TfR1 and NGALR, which are correlated with apical membrane TBI uptake, are also expressed in the distal convoluted tubules and collecting ducts, respectively [55]. NTBI is currently considered as the main contributor to the pathology of iron-overload disorders [37,38]. Renal uptake of NTBI has been demonstrated in human and mouse proximal tubule cell lines and in distal tubule segments [51,56,57]. Therefore, the alteration of NTBI in kidney disease and the relationship with renal ferroptosis deserve to be noticed.
Erythropoietin (EPO) is largely produced by the kidney, and renal cell loss and inflammation-mediated inhibition of EPO synthesis in CKD lead to reduced erythropoiesis and contribute to anemia [58,59]. In glomerular disorders, glomerular leakage increases the renal filtration of plasma iron, leading to increased urinary excretion of iron and EPO, systemic iron deficiency and, ultimately, anemia [60–62]. In addition, chronic inflammation stimulates hepcidin synthesis, resulting in functional iron deficiency and anemia [63]. Therefore, the occurrence and progression of renal disease can be prevented or minimized by fully understanding the mechanisms regulating renal iron metabolism.
3. Inflammation and hematuria are key to iron metabolism in IgAN
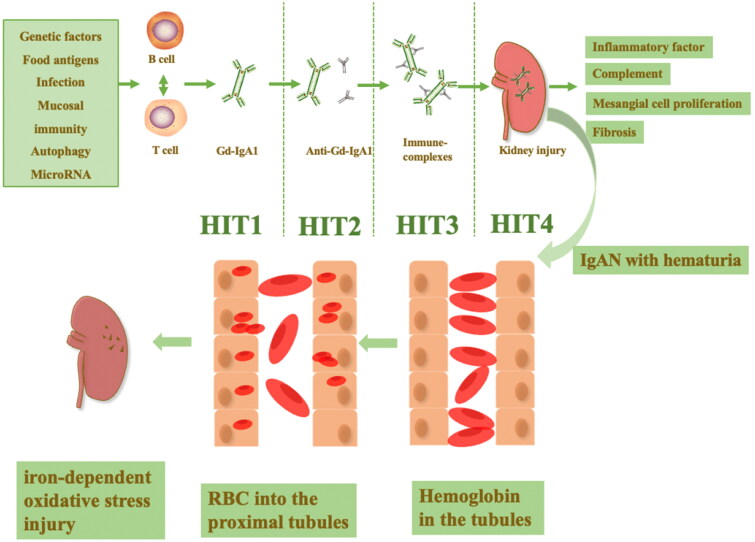
The pathogenesis of IgAN is complex and has been discovered to be associated with microbiota, genetic susceptibility, food antigens, infection, mucosal immunity, autophagy, and microRNAs [64–67]. The ‘multi-hit pathogenesis’ has been proposed to interpret the mechanism of renal damage by IgAN: the first hit is the generation of galactose-deficient IgA1 (Gd-IgA1); the second hit is the formation of IgA autoantibodies against (Gd-IgA1); the third hit autoantibodies bind to Gd-IgA1 and form a circulating immune complex that is not adequately cleared from the circulation; the fourth hit is this circulating complex depositing in the glomerular membranes, activating mesangial cells and complement and leading to kidney injury [68] (Figure 1). The ‘multi-hit pathogenesis’ is the key to the formation of inflammation in the glomerulus and cell–cell crosstalk pathways [69–72].
Figure 1.
The ‘multi-hit pathogenesis’ of IgAN and hematuria in IgAN. The microbiota, genetic susceptibility, food antigens, infection, mucosal immunity, autophagy and microRNA all contribute to the pathogenesis of IgAN. The ‘multi-hit pathogenesis’ explains the formation of circulating complexes deposited in the glomerular membranes, activating mesangial cells and complement and leading to kidney injury in IgAN. In IgAN patients with hematuria, the entry of red blood cells into the proximal tubule can contribute to red blood cell uptake by the renal tubules and the accumulation of intratubular hemoglobin, leading to iron-dependent oxidative stress renal interstitial injury.
IgAN is mainly characterized by hematuria and proteinuria as the main clinical symptoms [73]. For a long time, we have focused more on the damage caused by proteinuria in the kidneys and neglected the damage caused by hematuria. In 1992, Nath first suggested that hematuria can lead to progressive chronic kidney disease for the following reasons: (1) glomerular disease can cause the presence of red blood cells in the urinary tract, which are phagocytosed by the renal tubules and release hemoglobin in the tubules; (2) the injection of red blood cells into the proximal tubules of rodents can contribute to the renal tubular uptake of erythrocytes, intratubular hemoglobin accumulation and tubulointerstitial disease; and (3) hemoglobin facilitates iron-dependent oxidative stress injury [26] (Figure 1). These results demonstrate impairment of IgAN by hemoglobin, which may cause renal injury mainly by promoting iron-dependent oxidative stress; thus, exploring the relationship between IgAN and iron metabolism is of more interest.
4. Changes in iron metabolism indicators in IgAN
Iron metabolism is integrally related to the pathogenesis, pathological diagnosis, activity assessment and prognostic evaluation of IgAN. Among these processes, TfR might be involved in the pathogenesis and progression of IgAN. To some extent, sTfR is correlated with the assessment of the activity of IgAN. sTfR and Tf may indirectly reflect the degree of IgAN pathology. Hepcidin is expressed in the kidney, has a renoprotective effect in acute kidney injury (AKI) and is associated with renal anemia but is not currently studied in IgAN. In addition, the expression of GPX4, as the core factor of ferroptosis, is reduced in IgAN, and presumably ferroptosis also occurs in IgAN.
4.1. TfR and IgAN
The transferrin receptor (TfR, also known as CD71) in mammals has two types, TfR1 and TfR2. TfR1 is a 97-kDa type 2 membrane protein that is expressed as a homodimer in cell membranes [74]. TfR1 mainly forms the TfR1-Tf-Fe complex to mediate the entrance of iron into cells, and its expression is regulated by the cellular iron status [75]. When iron demand increases, TfR1 is upregulated in cells that require energy or are rapidly proliferating, such as erythrocytes, osteoblasts, cancer cells and activated lymphocytes [76]. TfR2 includes two types of receptors: TfR2-α and TfR2-β[77]. TfR2-α regulates iron by enhancing hepcidin expression in hepatocytes, regulating erythropoiesis and fostering the transportation of iron to mitochondria [78]. TfR2-β modulates iron metabolism by augmenting the expression of iron transport proteins in monocytes/macrophages [78]. In 2001, Ivan C. Moura et al. first identified TfR on renal mesangial cells as the major cell surface receptor for binding to IgA1 in IgAN and found that the proliferative state of mesangial cells was associated with TfR overexpression in IgAN [79,80]. A cohort study showed that Gd-IgA1 from IgAN patients combined more efficiently with TfR than healthy controls, suggesting that the formation of immune complexes favors mesangial TfR-IgA1 interactions and can cause a 3- to 4-fold increase in TfR expression via positive feedback [19,20]. In 2021, Jong Hyun Jhee et al. showed that mesangial TfR is significantly associated with disease progression and may play a biologic role in IgAN [81]. Additional studies have shown that IgA1 deposition leads to TfR1 upregulation because of the direct binding of sCD89 to TfR1 on mesangial cells and that the sCD89-TfR1 interaction induces the expression of transglutaminase 2 (TGase2) on the surface of mesangial cells, which in turn upregulates TfR1 [82]. However, it was found that in addition to TfR, other antibodies such as sCD89, β-1,4-GalT1 were also expressed on the mesangial cells of IgAN which bind to IgA1, because blocking TfR does not completely eliminate mesangial cell binding to IgA [82,83]. Marijn M et al. consequently identified a new IgAN antibody, β1,4-galactosyltransferase 1 (β-1,4-GalT1), which is a receptor that binds to the Fc portion of IgA, maintaining glomerular homeostasis and interacting with IgA-TfR to share intracellular signaling pathways [83]. Moreover, Feng et al. injected ferroptosis cell membranes into mice to generate an immune response and screened 3F3-FMA antibodies from antibodies whose antigen is transferrin [84], which indicated that TfR is a receptor in ferroptosis.
In conclusion, transferrin receptors are associated with the pathogenesis of IgAN. However, whether the high expression of TfR in the glomerular mesangial region causes iron overuptake in mesangial cells and further triggers cellular injury still needs to be further investigated.
4.2. sTfR and IgAN
Soluble transferrin receptor (sTfR) is a fragment of TfR on the cell membrane secreted into the circulation by protease hydrolysis, which can indirectly reflect the cytosolic expression of TfR [85]. sTfR can reflect iron deficiency in anemia and inflammatory status [86]. In addition, sTfR is also a strong predictor of heart failure. A study of 287 individuals with type 2 diabetes and coronary arteriosclerotic disease, with a mean follow-up of 45 months, showed that serum ferritin and sTfR independently of other risk factors strongly predict 5-year all-cause mortality [87]. Recent studies have shown that sTfR is also linked to rheumatoid arthritis, inflammatory bowel disease, obesity and postpartum depression [88–92]. A cohort study found that the concentration of sTfR was significantly higher in the blood and urine of IgAN patients than in controls [93]. Urinary sTfR concentrations were significantly reduced when active IgAN patients were in complete remission [21]. Hence, sTfR in blood and urine can be utilized as an indicator to facilitate early diagnosis and activity assessment of IgAN patients.
4.3. Tf and IgAN
Transferrin (Tf) is a 76-kDa glycoprotein generated by the liver with a half-life of approximately 8 days in serum. It is the principal iron transporter protein in the body and translocates circulating iron to cells [94]. Transferrin is very similar to albumin in molecular weight but has a higher isoelectric point, and urinary transferrin consequently precedes urine albumin in glomerular disease [95,96]. In addition, urinary transferrin can predict the severity of mesangial cell proliferation and glomerulosclerosis in the early stages of potentially progressive glomerular disease [97]. Urinary Tf can be evaluated to assess cardiovascular risk [98]. A cohort study showed significant differences in urinary Tf and the urinary/plasma Tf ratio in patients with type 2 diabetes compared to healthy controls, and urinary Tf was correlated with higher carotid intima-media thickness values [98]. In a retrospective study of 514 IgAN patients, urinary Tf was positively correlated with mesangial cell hyperplasia, endothelial cell hyperplasia, tubular atrophy or interstitial fibrosis in the Oxford classification of IgAN [99]. However, these studies [97–99] were only cross-sectional, and patient follow-up information needs to be added to explore the impact of urinary Tf on the prognosis of IgAN. In conclusion, Tf is relevant to the pathological manifestations of IgAN, and whether urinary Tf can be applied to assess cardiovascular risk in chronic kidney disease needs to be further investigated.
4.4. Hepcidin and IgAN
Hepcidin (also known as HAMP) is a 25-amino-acid peptide produced by hepatocytes that acts as a key negative regulator of small intestinal iron uptake and macrophage iron release [100,101]. Interestingly, a growing body of data suggests that hepcidin is expressed in the kidney and exerts a renoprotective effect [102,103]. Among these structures, hepcidin is preferentially expressed in the distal renal tubules, mainly in the thick segment of the ascending branches of the renal medulla but not in the proximal tubules [102]. The kidney was shown to defend against iron-mediated renal injury by reabsorbing hepcidin in the proximal tubule and synthesizing it in the distal tubule [104]. Mohammad et al. found that the hepcidin/FPN axis exerts is of crucial value in regulating renal and systemic iron homeostasis under iron overload in mouse kidneys [104]. In addition, hepcidin is closely related to renal anemia and shows a renoprotective effect in AKI [103,105,106]. Van Swelm et al. found that hepcidin reduces renal tubular damage from hemoglobin in mice with AKI [103,105] (Figure 2 and 3).
Figure 2.
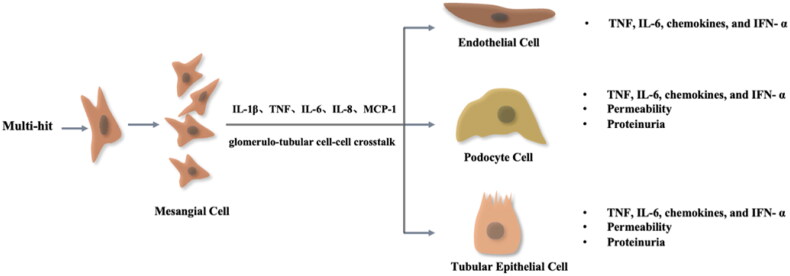
The activation of resident kidney cells contributes to chronic inflammation in IgAN. The ‘multi-hit’ hypothesis causes mesangial cells to proliferate and secrete inflammatory factors (IL-1β, TNF, IL-6, IL-8, and MCP-1). These inflammatory factors reactivate resident kidney cells by glomerulo-tubular cell–cell crosstalk pathways and produce proinflammatory chemokines responsible for perpetuating the cycle of chronic inflammation leading to kidney fibrosis.
Figure 3.
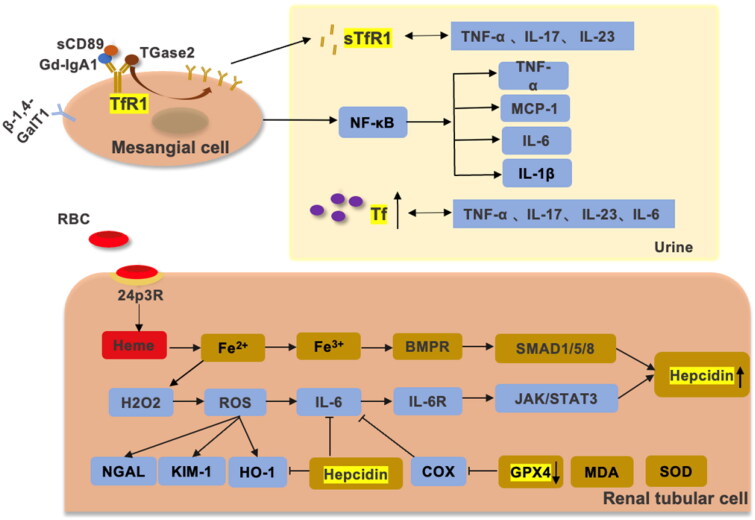
The relationship between iron metabolism, IgAN, and chronic inflammation. β-1,4-GalT, sTfR1, and CD89 are expressed by antibodies on mesangial cells in IgAN. Gd-IgA1 activates inflammation by promoting TfR1 overexpression through positive feedback to promote the secretion of IL-6, TNF-α, IL-1β and MCP-1 by the NF-κB pathway [107–109]. Overexpressed TfR1 can release sTfR1, which positively correlates with TNF-α, IL-17 and IL-23. When the glomerular filtration barrier is disrupted, Tf is increased in the urine. Tf is positively correlated with TNF-α, IL-17, IL-23, IL-6 and CRP. Ferroptosis is present in IgAN, and hemoglobin from hematuria is reabsorbed by 24p3R in renal tubular cells. Due to the tubular toxicity of hemoglobin, Fe3+ produced by the Fenton reaction promotes oxidative stress, causing increased expression of NGAL, KIM-1, HO1 and IL-6. The SMAD/BMP pathway and JAK/STAT3 pathway promote the expression of hepcidin [11,110]. GPX4 and SOD expression was decreased and MDA expression was increased in IgAN. GPX4 can suppress IL-6 by inhibiting LOX. In addition, hepcidin achieves renal protection by inhibiting IL-6 and HO-1 in hemoglobin-mediated renal tubular injury.
Persistent microscopic hematuria was shown to be a risk factor for IgAN progression, and in a study of up to 1 million Israeli middle-aged adults with primary glomerular disease, patients with primary glomerular disease exhibiting persistent microscopic hematuria had a markedly increased risk of ESRD compared to controls [111]. In a retrospective study of 1333 cases of IgAN in China, hematuria was found to still be an independent risk factor for renal composite endpoint events, and patients with hematuria remission occurring within 6 months of diagnosis had significantly lower renal composite endpoint events [111]. In a cohort study of 112 patients with IgAN, hematuria remission was identified as having a statistically significant favorable impact on IgAN outcomes, with a significantly higher proportion of patients with persistent hematuria reaching the renal composite endpoint event than patients with mild or negative hematuria [112]. Forty-six percent of patients with negative hematuria had a change in the rate of decline of renal function from −6.45 ± 14.66 to −0.18 ± 2.56 mL/min/1.73 m2/year [112].
In short, hepcidin in the kidney has a beneficial effect on hemoglobin-induced tubular injury, and hemoglobin in IgAN causes tubular injury by promoting iron-dependent oxidative stress. However, hepcidin has not been studied in IgAN at this time. Therefore, exploring the expression of hepcidin in IgAN patients and verifying the protective role of hepcidin in IgAN probably provides a basis for the future treatment of IgAN hematuria (Table 1).
Table 1.
The relationship between iron metabolism and IgAN.
| Iron index | Expression | Induction | Function | Association with IgAN | |
|---|---|---|---|---|---|
| TfR | TfR1 | Rapidly proliferating cells: erythrocytes, hepatocytes, osteoblasts, cancer cells and activated lymphocytes [113] | Cellular iron levels [114]; Up-regulated upon mitogenic stimulation [115] | Cellular uptake of iron by TfR1-Tf-Fe [75]; Endosomal acidification leads to iron release [116]; As a lipid sensor regulate mitochondrial fusion [117]; Microbial infection [118]; Ferroptosis marker [84] | As the major cell surface receptor for binding to IgA1 in IgAN [20,79]; Associated with the progression of IgAN [81] |
| TfR2 | Hepatocytes and erythroid cells [119] | Cellular iron levels [120] | Mediates cellular uptake of transferrin-bound iron in a non-iron dependent manner [120]; May be involved in iron metabolism, hepatocyte function and erythrocyte differentiation [119]; | No research | |
| sTfR | A fragment of TfR on the cell membrane [85] | Iron deficiency [86] | Reflect iron deficiency and inflammatory status [86] | A potential biomarker of IgAN [21]; Inhibited IgA1 binding (>50%) [19]; A marker of erythropoiesis in dialysis patients [121] | |
| Tf | liver [94] | Iron deficiency [122] | Translocate circulating iron to cells [94] | Associated with Oxford classification of IgAN; Predicts ESRD in T2DM patients [123] | |
| Hepcidin | Liver, heart, brain, lung, tonsils, salivary gland, trachea, prostate gland, adrenal gland, thyroid gland and kidney [104,124] | Erythropoiesis, anemia, and iron overload [100] | Main circulating regulator of iron [125,126]; Antimicrobial activity [127] | Regulated intrarenal iron handling at the distal nephron [128]; Defends against iron-mediated renal injury in AKI [104]; Renal anemia [129,130] | |
| GPX4 | Testis and platelets [131]; Cells undergoing lipid hydroperoxide toxicity: cancer [132], kidney [133], neuronal [134], gut [135] and liver [136] | Lipid oxidation [137] | Antioxidant peroxidase [132]; Against ferroptosis [131] | Related to the treatment of AKI [133,138], ADPKD [139] and DKD [140]. | |
| HO-1 | Macrophages in the spleen and liver [33]; Most cells in response to pro-oxidants [141] | Iron-recycling; Upregulated by pro-oxidants [142] | Decomposes heme to provide iron [33]; Antioxidants [141] | A risk factor of IgAN [143] | |
T2DM: type 2 diabetes mellitus; AKI: acute kidney injury; ADPKD: autosomal dominant polycystic kidney disease; DKD: diabetic kidney disease.
4.5. Ferroptosis and IgAN
Ferroptosis is an iron-dependent, novel form of programmed cell death that is distinct from apoptosis, cell necrosis and cell autophagy [144]. Ferroptosis is caused by unrestricted lipid peroxidation, eventually contributing to irreversible plasma membrane damage [145]. Ferroptosis includes three common metabolic pathways: iron ion metabolism, lipid peroxidation reactions and glutathione metabolism. When ferroptosis occurs, a large amount of free Fe2+ accumulates in the cell, which is highly oxidizing and easily reacts with H2O2 to produce hydroxyl radicals that can cause oxidative damage to DNA, proteins and membrane lipids, which promotes the occurrence of lipid peroxidation, damages the cell membrane and leads to cell death [146]. In ferroptosis, lipid peroxidation damage leads to the oxidative degradation of two important biofilm components, polyunsaturated fatty acids (PUFAs) and phosphatidylethanolamine (PE). The disruption of PUFA and PE structures in biofilms can result in cell rupture death and interference with cellular functions, such as oxidative phosphorylation, mitochondrial production and autophagy [147,148]. Currently, studies have confirmed that GPX4 is a central regulator of ferroptosis, and the depletion of glutathione (GSH) leads to GPX4 inactivation, which increases intracellular lipid peroxidation and ferroptosis [149].
The three metabolic pathways of ferroptosis can also occur in IgAN. In iron ion metabolism, the main manifestation is the abnormal deposition of iron ions in renal tissues. A retrospective study showed that in renal tissues, the amount of iron deposition directly correlated with the incidence of mean arterial pressure, serum creatinine level, urinary protein excretion and hematuria in patients with kidney disease [150]. A case report described an IgAN patient with acute kidney injury and massive hematuria, whose Prussian blue iron staining of renal biopsy tissue suggested acute tubular necrosis caused by massive deposits of iron-containing heme in the renal tubules; the authors speculated that iron-containing heme deposits may be a valid indicator of the pathophysiology of AKI associated with sarcoid hematuria [151]. Superoxide dismutase (SOD) and malondialdehyde (MDA) are involved in the lipid peroxidation process, where SOD and VitE attenuate the lipid peroxidation metabolic process and MDA is a toxic substance produced by the lipid peroxidation metabolic process [152]. In a cohort study, the activity of SOD and VitE in the serum was significantly lower and the level of MDA was significantly higher of IgAN patients than in healthy controls [153]. In patients with IgAN, MDA levels were significantly higher in the moderate pathology group than in the mild pathology group, and SOD activity was lower in the moderate pathology group than in the mild pathology group [153]. Compared to controls, the expression of GPX4 was significantly lower in the renal tissue of patients with IgAN [154]. Recent studies have shown that TfR is a specific receptor in ferroptosis [84], but the relationship between TfR and ferroptosis in IgAN still needs to be studied.
Based on the three metabolic pathways mentioned above, ferroptosis can be hypothesized to play an important role in the pathogenesis of IgAN and disease progression. Many drugs have been found to have a therapeutic effect in the clinic to reduce ferroptosis, including licorice, quercetin, apigenin and vitamin K [155–158]. In short, mitigating ferroptosis may have new value in the treatment of IgAN.
4.6. HO-1 and IgAN
Two functional isoforms of heme oxygenase exist in mammalian cells: HO-1 and HO-2. HO-2 is expressed in the brain, testis, cardiovascular and liver and can balance iron and redox metabolism as well as cellular messaging [159–161]. By contrast, HO-1 is a stress-inducible isozyme [162]. Under homeostatic conditions, HO-1 is constitutively expressed in iron-recycling macrophages in the spleen and liver and certain tolerogenic immune cells [163,164]. However, HO-1 is highly upregulated by most cells in response to free heme and many other pro-oxidants to provide protection against oxidative damage [141,142,165]. Micro and/or macroscopic hematuria is a typical symptom of IgAN that suggests potential induction of HO-1 in the glomeruli [166]. The HO-1 gene promoter length polymorphism was an important risk factor for mortality in IgAN [143].
5. The interconnection of iron metabolism, chronic inflammation and IgAN
Iron metabolism is connected with the occurrence of chronic inflammation in IgAN, and both promote each other. TfR enhances the proliferation of mesangial cells and inflammatory factor production in IgAN. Tf correlates with human immunity. When Tf decreases, bacterial, chlamydial, viral and other pathogenic microorganism infections can be increased. Tf, sTfR and inflammatory factors are positively correlated in IgAN. IL-6 is increased in IgAN tissue, blood, and urine, and IL-6 promotes hepcidin expression through the activation of the JAK/STAT signaling pathway. In addition, the reduction in GPX4 expression in IgAN may facilitate ferroptosis and interfere with inflammatory homeostasis.
5.1. Chronic inflammation is the essence of IgAN
Acute inflammation is marked by infiltrating leukocytes, but chronic inflammation is a prolonged, dysregulated and maladaptive response that involves active inflammation, tissue destruction and attempts at tissue repair [167,168]. In CKD, chronic inflammation is characterized by the recruitment of leukocytes and activation of resident kidney cells, including mesangial cells, endothelial cells, tubular epithelial cells, and podocytes, which exhibit a proinflammatory phenotype that eventually leads to kidney fibrosis and loss of kidney function [169].
In IgAN, the ''multi-hit’' hypothesis elaborates on the deposition of circulating immune complexes on mesangial cells that cause the secretion of extracellular matrix and release of proinflammatory and profibrotic cytokines [170–173], which leads to the stimulation of mesangial cell proliferation and recruitment of inflammatory cells into the glomerulus [174]. Inflammatory mediators also modify gene expression in podocytes, tubular epithelial cells, and endothelial cells, resulting in podocyte, tubulointerstitial and endothelial damage by glomerulo-tubular cell–cell crosstalk pathways in IgAN [170,175–178]. The above statements illustrate that IgAN is a typical chronic inflammatory disease.
5.2. TfR, chronic inflammation and IgAN
The overexpression of TfR on IgAN mesangial cells may promote the proliferation and inflammation of mesangial cells via positive feedback. Indirect immunofluorescence confirmed that TfR on IgAN mesangial cells preferentially binds to the poly IgA1 complex (pIgA1). The combination of pIgA1 and TfR enhanced the proliferation of mesangial cells and induced IL-6 and TGF production [19,20,179]. Additionally, a cohort study of 288 patients with IgAN found that TfR mRNA expression levels were higher in nonprogressive IgAN patients than in controls, and these differences were more pronounced in the progressive group. Furthermore, siRNA silencing of TfR significantly reduced IL-6, TNF-α, and MCP-1 expression [81] (Figure 3). Thus, TfR is closely correlated with mesangial cell activation and chronic inflammation in IgAN.
5.3. sTfR, chronic inflammation and IgAN
sTfR is mainly positively associated with the severity of iron deficiency. Under conditions of iron deficiency, a compensatory increment of TfR on the cell surface increases the cellular uptake of iron on the one hand and sTfR production on the other [180]. A prospective study showed that the levels of serum TNF-α, IL-17, and IL-23 in hemodialysis patients were positively correlated with Tf and sTfR [181] (Figure 3). This finding suggests a close relationship between abnormal iron metabolism and the microinflammatory response during hemodialysis, in which disturbances in the inflammatory response can affect iron metabolic processes and cause iron deficiency to varying degrees [181]. The current study confirms that sTfR levels in IgAN serum are increased and can be utilized for the assessment the activity of IgAN [21], but whether sTfR is involved in the inflammatory status of IgAN patients in vivo still needs to be further investigated.
5.4. Tf, chronic inflammation and IgAN
Tf is decreased when liver dysfunction and inflammation appear [78]. Almost all microbial pathogens need iron to successfully infect their mammalian hosts. Tf can provide a defense against systemic infections by inhibiting the binding of iron to potential pathogens, including coagulase-positive Staphylococcus aureus, Staphylococcus epidermidis, Pseudomonas aeruginosa, Bacillus anthracis, Chlamydia, SARS-CoV2 and other pathogenic microorganisms [182–184]. A retrospective study of 117 patients with primary glomerulonephritis showed a strong relationship between urinary Tf concentration and tubulointerstitial damage, where higher urinary Tf concentrations were found to be associated with more severe tubulointerstitial damage [185].
Studies on Tf and inflammatory factors in IgAN are currently lacking. Moreover, urinary Tf excretion is increased in patients with IgAN, and whether this increase further affects iron homeostasis in the circulatory system needs to be further investigated. In addition, Tf has anti-infective and immune defense effects, and whether IgAN patients are more susceptible to infection when they have less Tf in the circulation should be of interest.
5.5. Hepcidin, chronic inflammation and IgAN
Hepcidin is modulated by iron availability, erythropoiesis and inflammatory status [100]. Hepcidin expression increases under iron overload and inflammation and reduces under iron deficiency and hypoxia [186]. However, many of the current studies on hepcidin and inflammation are in the hepatocytes or AKI [187–189]. In IgAN, immune damage in the mesangial region promotes IL-6 secretion [171,190], and the tubular damage caused by hematuria also leads to an increase in IL-6 [26,191]. Thus, we speculate that IL-6 may promote hepcidin production by activating the JAK/STAT signaling pathway in IgAN (Figure 3).
5.6. Ferroptosis, chronic inflammation and IgAN
The relationship between ferroptosis and inflammation is complementary and mutually reinforcing. When ferroptosis occurs, a large amount of free Fe2+ accumulates in the cell, which is highly oxidizing and easily reacts with H2O2 to produce hydroxyl radicals (i.e., elevation of reactive oxygen species [ROS])[146]. Persistent low-grade inflammation and ROS are partners in crime, and the two are positive feedback loops in which one amplifies the other, ultimately leading to the progression of kidney disease [174]. Lipoxygenase (LOX) and cyclooxygenase (COX) are important factors in the metabolic process of inflammation, and GPX4 can suppress inflammation by inhibiting LOX and COX [192] (Figure 3). Therefore, the significantly reduced GPX4 expression in the renal tissues of IgAN patients may be relevant to the chronic inflammatory state of IgAN patients. Ferroptosis inhibitors have been shown to be effective in alleviating the inflammatory response, but the value of ferroptosis inhibitors in IgAN needs to be further explored.
5.7. HO-1, chronic inflammation and IgAN
HO-1 is upregulated during inflammation to protect against the potentially harmful effects of reactive oxygen species and pro-inflammatory cytokines [193]. Interestingly, HO-1 is also an important immune regulator in the macrophage population, associated with promoting anti-inflammatory M2 macrophage polarization and limiting the pro-inflammatory activity of M1 macrophages [194–196]. The HO-1 system allows macrophages to protect tissues from oxidative damage, a role that is associated with an important beneficial role in atherosclerosis, ischemic injury and kidney disease [197–199]. Although HO-1 is broadly anti-inflammatory in nature, it is not always beneficial and is associated with deleterious outcomes in diseases such as cancer, obesity, and chronic infections [200–202]. Thus, induction or inhibition of HO-1 in macrophages may have therapeutic effects, depending on the disease context. HO-1 expression was increased in IgAN because of its anti-inflammatory effects [143,166] and therefore may have a renoprotective role in IgAN. However, the specific application value of HO-1 in IgAN still needs to be further explored.
6. Therapeutic value of iron metabolism indicators in IgAN
The management of IgAN is focused on nonimmunosuppressive strategies. This strategy encompasses rigorous blood pressure control, optimal inhibition of the RAS, and lifestyle modification, including weight reduction, exercise, smoking cessation, and dietary sodium restriction [203].
TfR1 is required to regulate iron metabolism in high-metabolism cells in the human body, such as erythrocytes, hepatocytes, and lymphocytes [113], targeting TfR1 is highly likely to affect cellular iron metabolism. sTfR is the shedding product of TfR [85], and sTfR can essentially reflect the treatment effect of IgAN to some extent. Tf is associated with early kidney injury [96] and may reflect kidney recovery after treatment. Hepcidin has a central role in iron regulation, modulating the hepcidin-ferroportin axis by agonists/antagonists [100]. Hepcidin agonists may be able to prevent and treat iron overload. In mouse models, mini-hepcidin has been shown to control iron overload [204]. Hepcidin antagonists can inhibit hepcidin activity to form the basis of treatment of anemia characterized by iron restriction, such as anemia of inflammation and cancer [205]. Therefore, we speculate that the appropriate dose of hepcidin agonist that can be used in renal disease when excessive hematuria causes iron pigmentation still needs some exploration. When renal disease is complicated by anemia, the use of hepcidin antagonists is reasonable, but attention needs to be paid to the detection of iron concentrations to prevent iron overload. Iron storage, oxidative damage, and inflammation increase with kidney aging, iron dysregulation may be a causal factor in the renal dysfunction that accompanies aging [11,206]. Iron chelation improves renal function in animal models of renal lesions, as well as in humans with renal dysfunction [207,208]. Desferrioxamine (DFO) is an iron chelator approved for the treatment of iron overload in humans [209–211].
7. Conclusion
In summary, iron metabolism-related indicators are related to the pathogenesis, pathological diagnosis, activity and prognostic assessment of IgAN. However, there are still some iron metabolism pathways that have not been elucidated for different kidney cells. IgAN can cause disorders of iron metabolism, and disorders of iron metabolism can aggravate IgAN progression. Iron metabolism is closely related to chronic inflammation in IgAN. The hepcidin-ferroportin axis modulated by agonists/antagonists represents an attractive future target for novel preventive or therapeutic strategies for disorders of iron metabolism in IgAN.
Funding Statement
This study was funded by the National Natural Science Foundation of China [No. 81870498, No. 82000696], Natural Science Foundation of Hunan Province [No. 2021JJ40925] and Wisdom Accumulation and Talent Cultivation Project of the Third Xiangya Hospital of Central South University [YX202207].
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- 1.Anderson GJ, Frazer DM.. Current understanding of iron homeostasis. Am J Clin Nutr. 2017;106(Suppl 6):1559S–1566S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hu Y, Guo N, Yang T, et al. . The potential mechanisms by which artemisinin and its derivatives induce ferroptosis in the treatment of cancer. Oxid Med Cell Longev. 2022;2022:1458143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Qu X-X, He J-H, Cui Z-Q, et al. . PPAR-α agonist GW7647 protects against oxidative stress and iron deposit via GPx4 in a transgenic mouse model of alzheimer’s diseases. ACS Chem Neurosci. 2022;13(2):207–216. [DOI] [PubMed] [Google Scholar]
- 4.Li J-y, Liu S-Q, Yao R-Q, et al. . A novel insight into the fate of cardiomyocytes in Ischemia-Reperfusion injury: from iron metabolism to ferroptosis. Front Cell Dev Biol. 2021;9:799499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lanser L, Fuchs D, Kurz K, et al. . Physiology and inflammation driven pathophysiology of iron Homeostasis-Mechanistic insights into anemia of inflammation and its treatment. Nutrients. 2021;13(11):3732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mahadea D, Adamczewska E, Ratajczak AE, et al. . Iron deficiency anemia in inflammatory bowel Diseases-A narrative review. Nutrients. 2021;13(11):4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen S, Zhu J-y, Zang X, et al. . The emerging role of ferroptosis in liver diseases. Front Cell Dev Biol. 2021;9:801365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takahashi M, Mizumura K, Gon Y, et al. . Iron-Dependent mitochondrial dysfunction contributes to the pathogenesis of pulmonary fibrosis. Front Pharmacol. 2022;12:643980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang X, Li X.. Abnormal iron and lipid metabolism mediated ferroptosis in kidney diseases and its therapeutic potential. Metabolites. 2022;12(1):58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martines AMF, Masereeuw R, Tjalsma H, et al. . Iron metabolism in the pathogenesis of iron-induced kidney injury. Nat Rev Nephrol. 2013;9(7):385–398. [DOI] [PubMed] [Google Scholar]
- 11.van Swelm RPL, Wetzels JFM, Swinkels DW.. The multifaceted role of iron in renal health and disease. Nat Rev Nephrol. 2020;16(2):77–98. [DOI] [PubMed] [Google Scholar]
- 12.van Raaij SEG, Rennings AJ, Biemond BJ, et al. . Iron handling by the human kidney: glomerular filtration and tubular reabsorption both contribute to urinary iron excretion. American J Physiol Renal Physiol. 2019;316(3):F606–F614. [DOI] [PubMed] [Google Scholar]
- 13.Scindia Y, Dey P, Thirunagari A, et al. . Hepcidin mitigates renal Ischemia-Reperfusion injury by modulating systemic iron homeostasis. J Am Soc Nephrol JASN. 2015;26(11):2800–2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scindia Y, Leeds J, Swaminathan S.. Iron homeostasis in healthy kidney and its role in acute kidney injury. Semin Nephrol. 2019;39(1):76–84. [DOI] [PubMed] [Google Scholar]
- 15.Gao W, Li X, Gao Z, et al. . Iron increases diabetes-induced kidney injury and oxidative stress in rats. Biol Trace Elem Res. 2014;160(3):368–375. [DOI] [PubMed] [Google Scholar]
- 16.Hassler JR. IgA nephropathy: a brief review. Semin Diagnostic Pathol. 2020;37(3):143–147. [DOI] [PubMed] [Google Scholar]
- 17.Schena FP. A retrospective analysis of the natural history of primary IgA nephropathy worldwide. Am J Med. 1990;89(2):209–215. [DOI] [PubMed] [Google Scholar]
- 18.Schena FP, Nistor I.. Epidemiology of IgA nephropathy: a global perspective. Sem Nephrol. 2018;38(5):435–442. [DOI] [PubMed] [Google Scholar]
- 19.Moura IC, Arcos-Fajardo M, Sadaka C, et al. . Glycosylation and size of IgA1 are essential for interaction with mesangial transferrin receptor in IgA nephropathy. J Am Soc Nephrol JASN. 2004;15(3):622–634. [DOI] [PubMed] [Google Scholar]
- 20.Moura IC, Arcos-Fajardo M, Gdoura A, et al. . Engagement of transferrin receptor by polymeric IgA1: evidence for a positive feedback loop involving increased receptor expression and mesangial cell proliferation in IgA nephropathy. JASN. 2005;16(9):2667–2676. [DOI] [PubMed] [Google Scholar]
- 21.Delanghe SE, Speeckaert MM, Segers H, et al. . Soluble transferrin receptor in urine, a new biomarker for IgA nephropathy and Henoch-Schönlein purpura nephritis. Clin Biochem. 2013;46(7-8):591–597. [DOI] [PubMed] [Google Scholar]
- 22.Han X, Xiao Y, Tang Y, et al. . Clinical and pathological features of immunoglobulin a nephropathy patients with nephrotic syndrome. Clin Exp Med. 2019;19(4):479–486. [DOI] [PubMed] [Google Scholar]
- 23.Fan X, Zhang X, Liu LC, et al. . Hemopexin accumulates in kidneys and worsens acute kidney injury by causing hemoglobin deposition and exacerbation of iron toxicity in proximal tubules. Kid Inter. 2022;102(6):1320–1330. [DOI] [PubMed] [Google Scholar]
- 24.Sheerin NS, Sacks SH, Fogazzi GB.. In vitro erythrophagocytosis by renal tubular cells and tubular toxicity by haemoglobin and iron. Nephrol Dial Transplant. 1999;14(6):1391–1397. [DOI] [PubMed] [Google Scholar]
- 25.Gutiérrez E, Egido J, Rubio-Navarro A, et al. . Oxidative stress, macrophage infiltration and CD163 expression are determinants of long-term renal outcome in macrohematuria-induced acute kidney injury of IgA nephropathy. Nephron Clin Pract. 2012;121(1-2):c42–c53. [DOI] [PubMed] [Google Scholar]
- 26.Nath KA. Tubulointerstitial changes as a major determinant in the progression of renal damage. Am J Kidney Dis. 1992;20(1):1–17. [DOI] [PubMed] [Google Scholar]
- 27.Zager RA, Burkhart KM.. Differential effects of glutathione and cysteine on Fe2+, Fe3+, H2O2 and myoglobin-induced proximal tubular cell attack. Kidney Int. 1998;53(6):1661–1672. [DOI] [PubMed] [Google Scholar]
- 28.Doguer C, Ha J-H, Collins JF.. Intersection of iron and copper metabolism in the mammalian intestine and liver. Comprehen Physiol. 2018;8(4):1433–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nemeth E, Ganz T.. Hepcidin-Ferroportin interaction controls systemic iron homeostasis. IJMS. 2021;22(12):6493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ganz T. Systemic iron homeostasis. Physiol Rev. 2013;93(4):1721–1741. [DOI] [PubMed] [Google Scholar]
- 31.Thomas C, Oates PS.. Ferroportin/IREG-1/MTP-1/SLC40A1 modulates the uptake of iron at the apical membrane of enterocytes. Gut. 2004;53(1):44–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Andrews NC. Disorders of iron metabolism. N Engl J Med. 1999;341(26):1986–1995. [DOI] [PubMed] [Google Scholar]
- 33.Tenhunen R, Marver HS, Schmid R.. The enzymatic conversion of heme to bilirubin by microsomal heme oxygenase. Proc Natl Acad Sci U S A. 1968;61(2):748–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Soares MP, Hamza I.. Macrophages and iron metabolism. Immunity. 2016;44(3):492–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rouault TA. The role of iron regulatory proteins in mammalian iron homeostasis and disease. Nat Chem Biol. 2006;2(8):406–414. [DOI] [PubMed] [Google Scholar]
- 36.Leipuviene R, Theil EC.. The family of iron responsive RNA structures regulated by changes in cellular iron and oxygen. Cell Mol Life Sci. 2007;64(22):2945–2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brissot P, Ropert M, Le Lan C, et al. . Non-transferrin bound iron: a key role in iron overload and iron toxicity. Biochim Biophys Acta. 2012;1820(3):403–410. [DOI] [PubMed] [Google Scholar]
- 38.Knutson MD. Non-transferrin-bound iron transporters. Free Radical Biol Med. 2019;133:101–111. [DOI] [PubMed] [Google Scholar]
- 39.Liuzzi JP, Aydemir F, Nam H, et al. . Zip14 (Slc39a14) mediates non-transferrin-bound iron uptake into cells. Proc Natl Acad Sci USA. 2006;103(37):13612–13617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Trinder D. Localisation of divalent metal transporter 1 (DMT1) to the microvillus membrane of rat duodenal enterocytes in iron deficiency, but to hepatocytes in iron overload. Gut. 2000;46(2):270–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ji C, Kosman DJ.. Molecular mechanisms of non-transferrin-bound and transferring-bound iron uptake in primary hippocampal neurons. J Neurochem. 2015;133(5):668–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mwanjewe J, Grover AK.. Role of transient receptor potential canonical 6 (TRPC6) in non-transferrin-bound iron uptake in neuronal phenotype PC12 cells. Biochem J. 2004;378(Pt 3):975–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weiss A, Spektor L, A. Cohen L, et al. . Orchestrated regulation of iron trafficking proteins in the kidney during iron overload facilitates systemic iron retention. PLoS ONE. 2018;13(10):e0204471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Smith CP, Lee W-K, Haley M, et al. . Proximal tubule transferrin uptake is modulated by cellular iron and mediated by apical membrane megalin-cubilin complex and transferrin receptor 1. J Biol Chem. 2019;294(17):7025–7036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Meyron-Holtz EG, Ghosh MC, Iwai K, et al. . Genetic ablations of iron regulatory proteins 1 and 2 reveal why iron regulatory protein 2 dominates iron homeostasis. Embo J. 2004;23(2):386–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schödel J, Klanke B, Weidemann A, et al. . HIF-prolyl hydroxylases in the rat kidney: physiologic expression patterns and regulation in acute kidney injury. Am J Pathol. 2009;174(5):1663–1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shu S, Wang Y, Zheng M, et al. . Hypoxia and Hypoxia-Inducible factors in kidney injury and repair. Cells. 2019;8(3):207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Koury MJ, Haase VH.. Anaemia in kidney disease: harnessing hypoxia responses for therapy. Nat Rev Nephrol. 2015;11(7):394–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tajima S, Tsuchiya K, Horinouchi Y, et al. . Effect of angiotensin II on iron-transporting protein expression and subsequent intracellular labile iron concentration in human glomerular endothelial cells. Hypertens Res. 2010;33(7):713–721. [DOI] [PubMed] [Google Scholar]
- 50.van Raaij S, van Swelm R, Bouman K, et al. . Publisher correction: tubular iron deposition and iron handling proteins in human healthy kidney and chronic kidney disease. Sci Rep. 2018;8(1):13390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Thévenod F, Wolff NA.. Iron transport in the kidney: implications for physiology and cadmium nephrotoxicity. Metallomics Integrated Biometal Sci. 2016;8(1):17–42. [DOI] [PubMed] [Google Scholar]
- 52.Norden AG, Lapsley M, Lee PJ, et al. . Glomerular protein sieving and implications for renal failure in fanconi syndrome. Kidney Int. 2001;60(5):1885–1892. [DOI] [PubMed] [Google Scholar]
- 53.Vilasi A, Cutillas PR, Maher AD, et al. . Combined proteomic and metabonomic studies in three genetic forms of the renal fanconi syndrome. Am J Physiol Renal Physiol. 2007;293(2):F456–F467. [DOI] [PubMed] [Google Scholar]
- 54.Kozyraki R, Fyfe J, Verroust PJ, et al. . Megalin-dependent cubilin-mediated endocytosis is a major pathway for the apical uptake of transferrin in polarized epithelia. Proc Natl Acad Sci USA. 2001;98(22):12491–12496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Langelueddecke C, Roussa E, Fenton RA, et al. . Lipocalin-2 (24p3/neutrophil gelatinase-associated lipocalin (NGAL)) receptor is expressed in distal nephron and mediates protein endocytosis. J Biol Chem. 2012;287(1):159–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Haldar S, Tripathi A, Qian J, et al. . Prion protein promotes kidney iron uptake via its ferrireductase activity. J Biol Chem. 2015;290(9):5512–5522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.van Raaij SEG, Masereeuw R, Swinkels DW, et al. . Inhibition of Nrf2 alters cell stress induced by chronic iron exposure in human proximal tubular epithelial cells. Toxicol Lett. 2018;295:179–186. [DOI] [PubMed] [Google Scholar]
- 58.McGonigle RJ, Wallin JD, Shadduck RK, et al. . Erythropoietin deficiency and inhibition of erythropoiesis in renal insufficiency. Kidney Int. 1984;25(2):437–444. [DOI] [PubMed] [Google Scholar]
- 59.Weiss G, Goodnough LT.. Anemia of chronic disease. N Engl J Med. 2005;352(10):1011–1023. [DOI] [PubMed] [Google Scholar]
- 60.Inoue A, et al. . Albuminuria is an independent predictor of decreased serum erythropoietin levels in type 2 diabetic patients. Nephrol Dial Transplant. 2007;22(1):287–288. [DOI] [PubMed] [Google Scholar]
- 61.Yamaguchi-Yamada M, Manabe N, Uchio-Yamada K, et al. . Anemia with chronic renal disorder and disrupted metabolism of erythropoietin in ICR-derived glomerulonephritis (ICGN) mice. J Vet Med Sci. 2004;66(4):423–431. [DOI] [PubMed] [Google Scholar]
- 62.Harris ZL, Durley AP, Man TK, et al. . Targeted gene disruption reveals an essential role for ceruloplasmin in cellular iron efflux. Proc Natl Acad Sci USA. 1999;96(19):10812–10817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ganz T, Nemeth E.. Iron balance and the role of hepcidin in chronic kidney disease. Sem Nephrol. 2016;36(2):87–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li H, Chen Z, Chen W, et al. . MicroRNA-23b-3p deletion induces an IgA nephropathy-like disease associated with dysregulated mucosal IgA synthesis. JASN. 2021;32(10):2561–2578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Abbad L, Monteiro RC, Berthelot L.. Food antigens and transglutaminase 2 in IgA nephropathy: molecular links between gut and kidney. Mol Immunol. 2020;121:1–6. [DOI] [PubMed] [Google Scholar]
- 66.Gesualdo L, Di Leo V, Coppo R.. The mucosal immune system and IgA nephropathy. Semin Immunopathol. 2021;43(5):657–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wu C‐Y, Hua K‐F, Yang S‐R, et al. . Tris DBA ameliorates IgA nephropathy by blunting the activating signal of NLRP3 inflammasome through SIRT1- and SIRT3-mediated autophagy induction. J Cell Mol Med. 2020;24(23):13609–13622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Trimarchi H, Barratt J, Cattran DC, et al. . Oxford classification of IgA nephropathy 2016: an update from the IgA nephropathy classification working group. Kidney Inter. 2017;91(5):1014–1021. [DOI] [PubMed] [Google Scholar]
- 69.Wu MY, Chen CS, Yiang GT, et al. . The emerging role of pathogenesis of IgA nephropathy. J Clin Med. 2018;7(8):225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rauen T, Floege J.. Inflammation in IgA nephropathy. Pediatr Nephrol. 2017;32(12):2215–2224. [DOI] [PubMed] [Google Scholar]
- 71.Lai KN, Tang SCW, Guh J-Y, et al. . Polymeric IgA1 from patients with IgA nephropathy upregulates transforming growth factor-beta synthesis and signal transduction in human mesangial cells via the renin-angiotensin system. J Am Soc Nephrol. 2003;14(12):3127–3137. [DOI] [PubMed] [Google Scholar]
- 72.Lemley KV, Lafayette RA, Safai M, et al. . Podocytopenia and disease severity in IgA nephropathy. Kidney Int. 2002;61(4):1475–1485. [DOI] [PubMed] [Google Scholar]
- 73.Moriyama T. Clinical and histological features and therapeutic strategies for IgA nephropathy. Clin Exp Nephrol. 2019;23(9):1089–1099. [DOI] [PubMed] [Google Scholar]
- 74.Schneider C, Owen MJ, Banville D, et al. . Primary structure of human transferrin receptor deduced from the mRNA sequence. Nature. 1984;311(5987):675–678. [DOI] [PubMed] [Google Scholar]
- 75.Gao G, Li J, Zhang Y, et al. . Cellular iron metabolism and regulation. Adv Exp Med Biol. 2019;1173:21–32. [DOI] [PubMed] [Google Scholar]
- 76.Wieland E, Shipkova M.. Lymphocyte surface molecules as immune activation biomarkers. Clin Biochem. 2016;49(4-5):347–354. [DOI] [PubMed] [Google Scholar]
- 77.Kawabata H, Yang R, Hirama T, et al. . Molecular cloning of transferrin receptor 2. A new member of the transferrin receptor-like family. J Biol Chem. 1999;274(30):20826–20832. [DOI] [PubMed] [Google Scholar]
- 78.Kawabata H. Transferrin and transferrin receptors update. Free Rad Biol Med. 2019;133:46–54. [DOI] [PubMed] [Google Scholar]
- 79.Moura IC, Centelles MN, Arcos-Fajardo M, et al. . Identification of the transferrin receptor as a novel immunoglobulin (Ig)A1 receptor and its enhanced expression on mesangial cells in IgA nephropathy. J Exp Med. 2001;194(4):417–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Haddad E, Moura IC, Arcos-Fajardo M, et al. . Enhanced expression of the CD71 mesangial IgA1 receptor in berger disease and Henoch-Schönlein nephritis: association between CD71 expression and IgA deposits. J Am Soc Nephrol. 2003;14(2):327–337. [DOI] [PubMed] [Google Scholar]
- 81.Jhee JH, Nam BY, Park JT, et al. . CD71 mesangial IgA1 receptor and the progression of IgA nephropathy. Transl Res. 2021;230:34–43. [DOI] [PubMed] [Google Scholar]
- 82.Berthelot L, Papista C, Maciel TT, et al. . Transglutaminase is essential for IgA nephropathy development acting through IgA receptors. J Exp Med. 2012;209(4):793–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Molyneux K, Wimbury D, Pawluczyk I, et al. . β1,4-galactosyltransferase 1 is a novel receptor for IgA in human mesangial cells. Kidney Inter. 2017;92(6):1458–1468. [DOI] [PubMed] [Google Scholar]
- 84.Feng H, Schorpp K, Jin J, et al. . Transferrin receptor is a specific ferroptosis marker. Cell Reports. 2020;30(10):3411–3423.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Speeckaert MM, Speeckaert R, Delanghe JR.. Biological and clinical aspects of soluble transferrin receptor. Critic Rev Clin Laborat Sci. 2010;47(5-6):213–228. [DOI] [PubMed] [Google Scholar]
- 86.Neef V, Schmitt E, Bader P, et al. . The reticulocyte hemoglobin equivalent as a screening marker for iron deficiency and iron deficiency anemia in children. JCM. 2021;10(16):3506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ponikowska B, Suchocki T, Paleczny B, et al. . Iron status and survival in diabetic patients with coronary artery disease. Diabetes Care. 2013;36(12):4147–4156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sierpinski R, Josiak K, Suchocki T, et al. . High soluble transferrin receptor in patients with heart failure: a measure of iron deficiency and a strong predictor of mortality. Euro J Heart Fail. 2021;23(6):919–932. [DOI] [PubMed] [Google Scholar]
- 89.Tański W, et al. . Iron metabolism in patients with rheumatoid arthritis. Eur Rev Med Pharmacol Sci. 2021;25(12):4325–4335. [DOI] [PubMed] [Google Scholar]
- 90.Rodríguez-Mortera R, et al. . Higher hepcidin levels in adolescents with obesity are associated with metabolic syndrome dyslipidemia and visceral fat. Antioxidants. 2021;10(5):751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hameed S, et al. . Is iron deficiency a risk factor for postpartum depression? A Case-Control study in the Gaza Strip, palestine. Public Health Nutr. 2022;25(6):1631.–; . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Stojkovic Lalosevic M, Toncev L, Stankovic S, et al. . Hepcidin is a reliable marker of iron deficiency anemia in newly diagnosed patients with inflammatory bowel disease. Dis Markers. 2020;2020:8523205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.He L, Liu H, Peng Y. [Immune pathogenesis of IgA nephropathy and its drugable targets]. zhong nan da xue xue bao. Yi xue ban = journal of Central South university. Med Sci. 2014;39(1):96–101. [DOI] [PubMed] [Google Scholar]
- 94.Baker EN, Baker HM, Kidd RD.. Lactoferrin and transferrin: functional variations on a common structural framework. Biochem Cell Biol. 2002;80(1):27–34. [DOI] [PubMed] [Google Scholar]
- 95.Koshimura J, Narita T, Sasaki H, et al. . Urinary excretion of transferrin and orosomucoid are increased after acute protein loading in healthy subjects. Nephron Clin Pract. 2005;100(2):c33–c37. [DOI] [PubMed] [Google Scholar]
- 96.Bernard AM, Amor AA, Goemaere-Vanneste J, et al. . Microtransferrinuria is a more sensitive indicator of early glomerular damage in diabetes than microalbuminuria. Clin Chem. 1988;34(9):1920–1921. [PubMed] [Google Scholar]
- 97.Li Y, Wang J, Zhu X, et al. . Urinary protein markers predict the severity of renal histological lesions in children with mesangial proliferative glomerulonephritis. BMC Nephrol. 2012;13(1):29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sánchez-Hidalgo JJ, Suárez-Cuenca JA, Lozano-Nuevo JJ, et al. . Urine transferrin as an early endothelial dysfunction marker in type 2 diabetic patients without nephropathy: a case control study. Diabetol Metab Syndr. 2021;13(1):128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sa-Li LI, Qiu-Ling FAN, Jie ZHAO, et al. . Risk factors and correlation analysis between the oxford classification and clinical indicators of IgA nephropathy. J China Med Univ. 2017;46(1):1–6. [Google Scholar]
- 100.Agarwal AK, Yee J.. Hepcidin. Adv Chronic Kidney Dis. 2019;26(4):298–305. [DOI] [PubMed] [Google Scholar]
- 101.Nicolas G, Viatte L, Bennoun M, et al. . Hepcidin, a new iron regulatory peptide. Blood Cells Mol Dis. 2002;29(3):327–335. [DOI] [PubMed] [Google Scholar]
- 102.Houamel D, Ducrot N, Lefebvre T, et al. . Hepcidin as a major component of renal antibacterial defenses against uropathogenic Escherichia coli. J Am Soc Nephrol. 2016;27(3):835–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.van Swelm RP, Wetzels JF, Verweij VG, et al. . Renal handling of circulating and Renal-Synthesized hepcidin and its protective effects against Hemoglobin-Mediated kidney injury. JASN. 2016;27(9):2720–2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mohammad G, Matakidou A, Robbins PA, et al. . The kidney hepcidin/ferroportin axis controls iron reabsorption and determines the magnitude of kidney and systemic iron overload. Kidney Inter. 2021;100(3):559–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.van Swelm RPL, Vos M, Verhoeven F, et al. . Endogenous hepcidin synthesis protects the distal nephron against hemin and hemoglobin mediated necroptosis. Cell Death Dis. 2018;9(5):550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Agarwal AK. Iron metabolism and management: focus on chronic kidney disease. Kidney Inter Suppl. 2021;11(1):46–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kanamori Y, Murakami M, Sugiyama M, et al. . Interleukin-1β (IL-1β) transcriptionally activates hepcidin by inducing CCAAT enhancer-binding protein δ (C/EBPδ) expression in hepatocytes. J Biol Chem. 2017;292(24):10275–10287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chen S, Feng T, Vujić Spasić M, et al. . Transforming growth factor β1 (TGF-β1) activates hepcidin mRNA expression in hepatocytes. J Biol Chem. 2016;291(25):13160–13174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Canali S, Core AB, Zumbrennen-Bullough KB, et al. . Activin B induces noncanonical SMAD1/5/8 signaling via BMP type I receptors in hepatocytes: evidence for a role in hepcidin induction by inflammation in male mice. Endocrinology. 2016;157(3):1146–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wrighting DM, Andrews NC.. Interleukin-6 induces hepcidin expression through STAT3. Blood. 2006;108(9):3204–3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Yu G-Z, Guo L, Dong J-F, et al. . Persistent hematuria and kidney disease progression in IgA nephropathy: a cohort study. Am J Kidney Dis. 2020;76(1):90–99. [DOI] [PubMed] [Google Scholar]
- 112.Sevillano AM, Gutiérrez E, Yuste C, et al. . Remission of hematuria improves renal survival in IgA nephropathy. JASN. 2017;28(10):3089–3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gammella E, Buratti P, Cairo G, et al. . The transferrin receptor: the cellular iron gate. Metallomics Integrated Biometal Sci. 2017;9(10):1367–1375. [DOI] [PubMed] [Google Scholar]
- 114.Wang W, Di X, D'Agostino RB, et al. . Excess capacity of the iron regulatory protein system. J Biol Chem. 2007;282(34):24650–24659. [DOI] [PubMed] [Google Scholar]
- 115.Batista A, Millán J, Mittelbrunn M, et al. . Recruitment of transferrin receptor to immunological synapse in response to TCR engagement. J Immunol. 2004;172(11):6709–6714. [DOI] [PubMed] [Google Scholar]
- 116.Jabara HH, Boyden SE, Chou J, et al. . A missense mutation in TFRC, encoding transferrin receptor 1, causes combined immunodeficiency. Nat Genet. 2016;48(1):74–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Senyilmaz D, Virtue S, Xu X, et al. . Regulation of mitochondrial morphology and function by stearoylation of TFR1. Nature. 2015;525(7567):124–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zeltina A, Krumm SA, Sahin M, et al. . Convergent immunological solutions to argentine hemorrhagic fever virus neutralization. Proc Natl Acad Sci USA. 2017;114(27):7031–7036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Pagani A, Vieillevoye M, Nai A, et al. . Regulation of cell surface transferrin receptor-2 by iron-dependent cleavage and release of a soluble form. Haematologica. 2015;100(4):458–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Graham RM, Reutens GM, Herbison CE, et al. . Transferrin receptor 2 mediates uptake of transferrin-bound and non-transferrin-bound iron. J Hepatol. 2008;48(2):327–334. [DOI] [PubMed] [Google Scholar]
- 121.Yin P, Song Y, Li J.. Soluble transferrin receptor as a marker of erythropoiesis in patients undergoing high-flux hemodialysis. Bosn J of Basic Med Sci. 2017;17(4):333–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.McKnight GS, Lee DC, Hemmaplardh D, et al. . Transferrin gene expression. Effects of nutritional iron deficiency. J Biol Chem. 1980;255(1):144–147. [PubMed] [Google Scholar]
- 123.Zhao L, Zou Y, Zhang J, et al. . Serum transferrin predicts end-stage renal disease in type 2 diabetes mellitus patients. Int J Med Sci. 2020;17(14):2113–2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Krause A, Neitz S, Mägert HJ, et al. . LEAP-1, a novel highly disulfide-bonded human peptide, exhibits antimicrobial activity. FEBS Lett. 2000;480(2-3):147–150. [DOI] [PubMed] [Google Scholar]
- 125.Qiao B, Sugianto P, Fung E, et al. . Hepcidin-induced endocytosis of ferroportin is dependent on ferroportin ubiquitination. Cell Metab. 2012;15(6):918–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Aschemeyer S, Qiao B, Stefanova D, et al. . Structure-function analysis of ferroportin defines the binding site and an alternative mechanism of action of hepcidin. Blood. 2018;131(8):899–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Park CH, Valore EV, Waring AJ, et al. . Hepcidin, a urinary antimicrobial peptide synthesized in the liver. J Biol Chem. 2001;276(11):7806–7810. [DOI] [PubMed] [Google Scholar]
- 128.Moulouel B, Houamel D, Delaby C, et al. . Hepcidin regulates intrarenal iron handling at the distal nephron. Kidney Inter. 2013;84(4):756–766. [DOI] [PubMed] [Google Scholar]
- 129.Babitt JL, Lin HY.. Mechanisms of anemia in CKD. J Am Soc Nephrol. 2012;23(10):1631–1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ueda N, Takasawa K.. Impact of inflammation on ferritin, hepcidin and the management of iron deficiency anemia in chronic kidney disease. Nutrients. 2018;10(9):1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sutherland M, Shankaranarayanan P, Schewe T, et al. . Evidence for the presence of phospholipid hydroperoxide glutathione peroxidase in human platelets: implications for its involvement in the regulatory network of the 12-lipoxygenase pathway of arachidonic acid metabolism. Biochem J. 2001;353(1):91–100. [PMC free article] [PubMed] [Google Scholar]
- 132.Yang WS, SriRamaratnam R, Welsch ME, et al. . Regulation of ferroptotic cancer cell death by GPX4. Cell. 2014;156(1-2):317–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zhang J, Bi J, Ren Y, et al. . Involvement of GPX4 in irisin’s protection against ischemia reperfusion-induced acute kidney injury. J Cell Physiol. 2021;236(2):931–945. [DOI] [PubMed] [Google Scholar]
- 134.Yuan Y, Zhai Y, Chen J, et al. . Kaempferol ameliorates Oxygen-Glucose deprivation/Reoxygenation-Induced neuronal ferroptosis by activating Nrf2/SLC7A11/GPX4 axis. Biomolecules. 2021;11(7):923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Mayr L, Grabherr F, Schwärzler J, et al. . Dietary lipids fuel GPX4-restricted enteritis resembling crohn’s disease. Nat Commun. 2020;11(1):1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Capelletti MM, Manceau H, Puy H, et al. . Ferroptosis in liver diseases: an overview. IJMS. 2020;21(14):4908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Forcina GC, Dixon SJ.. GPX4 at the crossroads of lipid homeostasis and ferroptosis. Proteomics. 2019;19(18):e1800311. [DOI] [PubMed] [Google Scholar]
- 138.Hu Z, Zhang H, Yi B, et al. . VDR activation attenuate cisplatin induced AKI by inhibiting ferroptosis. Cell Death Dis. 2020;11(1):73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zhang X, Li LX, Ding H, et al. . Ferroptosis promotes cyst growth in autosomal dominant polycystic kidney disease mouse models. JASN. 2021;32(11):2759–2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kim S, Kang S-W, Joo J, et al. . Characterization of ferroptosis in kidney tubular cell death under diabetic conditions. Cell Death Dis. 2021;12(2):160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Gozzelino R, Jeney V, Soares MP.. Mechanisms of cell protection by heme oxygenase-1. Annu Rev Pharmacol Toxicol. 2010;50(1):323–354. [DOI] [PubMed] [Google Scholar]
- 142.Ferrándiz ML, Devesa I.. Inducers of heme oxygenase-1. CPD. 2008;14(5):473–486. [DOI] [PubMed] [Google Scholar]
- 143.Chin HJ, et al. . The heme oxygenase-1 genotype is a risk factor to renal impairment of IgA nephropathy at diagnosis, which is a strong predictor of mortality. J Korean Med Sci. 2009;24 Suppl(Suppl 1):S30–S37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Tang D, Kang R, Berghe TV, et al. . The molecular machinery of regulated cell death. Cell Res. 2019;29(5):347–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Dixon SJ, Lemberg KM, Lamprecht MR, et al. . Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149(5):1060–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Cao JY, Dixon SJ.. Mechanisms of ferroptosis. Cell Mol Life Sci. 2016;73(11-12):2195–2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Yang WS, et al. . Peroxidation of polyunsaturated fatty acids by lipoxygenases drives ferroptosis. Proc Natl Acad Sci USA. 2016;113(34):E4966–E4975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kagan VE, Mao G, Qu F, et al. . Oxidized arachidonic and adrenic PEs navigate cells to ferroptosis. Nat Chem Biol. 2017;13(1):81–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Ursini F, Maiorino M.. Lipid peroxidation and ferroptosis: the role of GSH and GPx4. Free Rad Biol Med. 2020;152:175–185. [DOI] [PubMed] [Google Scholar]
- 150.Wang H, Nishiya K, Ito H, et al. . Iron deposition in renal biopsy specimens from patients with kidney diseases. Am J Kidney Dis. 2001;38(5):1038–1044. [DOI] [PubMed] [Google Scholar]
- 151.Taguchi S, Hidaka S, Yanai M, et al. . Renal hemosiderosis presenting with acute kidney injury and macroscopic hematuria in immunoglobulin a nephropathy: a case report. BMC Nephrol. 2021;22(1):132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Tsikas D. Assessment of lipid peroxidation by measuring malondialdehyde (MDA) and relatives in biological samples: analytical and biological challenges. Anal Biochem. 2017;524:13–30. [DOI] [PubMed] [Google Scholar]
- 153.Tian J, et al. . Lipid peroxidation in IgA nephropathy and the effect of lipo-prostaglandin E1. J Nephrol. 2005;18(3):243–248. [PubMed] [Google Scholar]
- 154.Ya-Jun BAI, Yan-bin DU, Xin-Zhu YUAN, et al. . Regulation of chrysophanol-mediated TLR4/NF-κB pathway on renal injury and immune response in IgA nephropathy rats. Sichuan Da Xue Xue Bao Yi Xue Ban. 2019;50(6):840–846. [PubMed] [Google Scholar]
- 155.Zhang H-L, Hu B-X, Li Z-L, et al. . PKCβII phosphorylates ACSL4 to amplify lipid peroxidation to induce ferroptosis. Nat Cell Biol. 2022;24(1):88–98. [DOI] [PubMed] [Google Scholar]
- 156.Wang Y, Quan F, Cao Q, et al. . Quercetin alleviates acute kidney injury by inhibiting ferroptosis. J Adv Res. 2021;28:231–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Mishima E, Ito J, Wu Z, et al. . A non-canonical vitamin K cycle is a potent ferroptosis suppressor. Nature. 2022;608(7924):778–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Shao C, Yuan J, Liu Y, et al. . Epileptic brain fluorescent imaging reveals apigenin can relieve the myeloperoxidase-mediated oxidative stress and inhibit ferroptosis. Proc Natl Acad Sci USA. 2020;117(19):10155–10164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Trakshel GM, Kutty RK, Maines MD.. Purification and characterization of the major constitutive form of testicular heme oxygenase. The noninducible isoform. J Biol Chem. 1986;261(24):11131–11137. [PubMed] [Google Scholar]
- 160.Maines MD, Trakshel GM, Kutty RK.. Characterization of two constitutive forms of rat liver microsomal heme oxygenase. Only one molecular species of the enzyme is inducible. J Biol Chem. 1986;261(1):411–419. [PubMed] [Google Scholar]
- 161.Maines MD. The heme oxygenase system: a regulator of second messenger gases. Annu Rev Pharmacol Toxicol. 1997;37:517–554. [DOI] [PubMed] [Google Scholar]
- 162.Maines MD, Kappas A.. Cobalt induction of hepatic heme oxygenase; with evidence that cytochrome P-450 is not essential for this enzyme activity. Proc Natl Acad Sci USA. 1974;71(11):4293–4297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Korolnek T, Hamza I.. Macrophages and iron trafficking at the birth and death of red cells. Blood. 2015;125(19):2893–2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Theurl I, Hilgendorf I, Nairz M, et al. . On-demand erythrocyte disposal and iron recycling requires transient macrophages in the liver. Nat Med. 2016;22(8):945–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Applegate LA, Luscher P, Tyrrell RM.. Induction of heme oxygenase: a general response to oxidant stress in cultured mammalian cells. Cancer Res. 1991;51(3):974–978. [PubMed] [Google Scholar]
- 166.Shepard M, Dhulipala P, Kabaria S, et al. . Heme oxygenase-1 localization in the rat nephron. Nephron. 2002;92(3):660–664. [DOI] [PubMed] [Google Scholar]
- 167.Suzuki K. Chronic inflammation as an immunological abnormality and effectiveness of exercise. Biomolecules. 2019;9(6):223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Weiss U. Inflammation. Nature. 2008;454(7203):427. [DOI] [PubMed] [Google Scholar]
- 169.Kitching AR, Hutton HL.. The players: cells involved in glomerular disease. CJASN. 2016;11(9):1664–1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Lai KN, Leung JCK, Chan LYY, et al. . Activation of podocytes by mesangial-derived TNF-alpha: glomerulo-podocytic communication in IgA nephropathy. Am J Physiol Renal Physiol. 2008;294(4):F945–F955. [DOI] [PubMed] [Google Scholar]
- 171.Groza Y, Jemelkova J, Kafkova LR, et al. . IL-6 and its role in IgA nephropathy development. Cytokine Growth Factor Rev. 2022;66:1–14. [DOI] [PubMed] [Google Scholar]
- 172.Liang Y, Zhao G, Tang L, et al. . MiR-100-3p and miR-877-3p regulate overproduction of IL-8 and IL-1β in mesangial cells activated by secretory IgA from IgA nephropathy patients. Exp Cell Res. 2016;347(2):312–321. [DOI] [PubMed] [Google Scholar]
- 173.Zhang Y, Yan X, Zhao T, et al. . Targeting C3a/C5a receptors inhibits human mesangial cell proliferation and alleviates immunoglobulin a nephropathy in mice. Clin Experim Immunol. 2017;189(1):60–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Stenvinkel P, Chertow GM, Devarajan P, et al. . Chronic inflammation in chronic kidney disease progression: role of Nrf2. Kidney Inter Rep. 2021;6(7):1775–1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Lai KN, et al. . Podocyte injury induced by mesangial-derived cytokines in IgA nephropathy. Nephrology, dialysis, transplantation: official publication of the european dialysis and transplant association. Euro Renal Assoc. 2009;24(1):62–72. [DOI] [PubMed] [Google Scholar]
- 176.Chan LY, Leung JC, Tsang AW, et al. . Activation of tubular epithelial cells by mesangial-derived TNF-alpha: glomerulotubular communication in IgA nephropathy. Kidney Inter. 2005;67(2):602–612. [DOI] [PubMed] [Google Scholar]
- 177.Tang R, Meng T, Lin W, et al. . A partial picture of the Single-Cell transcriptomics of human IgA nephropathy. Front Immunol. 2021;12:645988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Zambrano S, He L, Kano T, et al. . Molecular insights into the early stage of glomerular injury in IgA nephropathy using single-cell RNA sequencing. Kidney Inter. 2022;101(4):752–765. [DOI] [PubMed] [Google Scholar]
- 179.Moura IC, Benhamou M, Launay P, et al. . The glomerular response to IgA deposition in IgA nephropathy. Sem Nephrol. 2008;28(1):88–95. [DOI] [PubMed] [Google Scholar]
- 180.Ricchi P, Meloni A, Costantini S, et al. . Soluble form of transferrin receptor-1 level is associated with the age at first diagnosis and the risk of therapeutic intervention and iron overloading in patients with non-transfusion-dependent thalassemia. Ann Hematol. 2017;96(9):1541–1546. [DOI] [PubMed] [Google Scholar]
- 181.Guang-Zhi Z, Peng-Fei , HU.. The correlation of serum hepcidin and transferrin receptor contents with iron deficiency and micro-inflammatory response in patients with hemodialysis. J Hainan Med Univ. 2018;24(1):30–33. in Chinese). [Google Scholar]
- 182.Brandsma ME, Jevnikar AM, Ma S.. Recombinant human transferrin: beyond iron binding and transport. Biotechnol Adv. 2011;29(2):230–238. [DOI] [PubMed] [Google Scholar]
- 183.De Puysseleyr L, De Puysseleyr K, Rybarczyk J, et al. . Transferrins reduce replication of in McCoy cells. Pathogens. 2021;10(7):858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Claise C, et al. . Low transferrin levels predict heightened inflammation in COVID-19 patients: new insights. Inter J Infect Dis. 2021;116:74–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Hui PENG, Xue-Qing YU, Tan-Qi LOU, et al. . Correlation between urinary protein components and renal pathology in primary glomerulonephritis patients of different pathological types. Chin J Nephrol. 2006;22(5):271–274. [Google Scholar]
- 186.Nicolas G, Chauvet C, Viatte L, et al. . The gene encoding the iron regulatory peptide hepcidin is regulated by anemia, hypoxia, and inflammation. J Clin Invest. 2002;110(7):1037–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Diepeveen LE, Stegemann G, Wiegerinck ET, et al. . Investigating the molecular mechanisms of renal hepcidin induction and protection upon Hemoglobin-Induced acute kidney injury. IJMS. 2022;23(3):1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Scindia Y, Wlazlo E, Leeds J, et al. . Protective role of hepcidin in polymicrobial sepsis and acute kidney injury. Front Pharmacol. 2019;10:615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Nemeth E, Tuttle MS, Powelson J, et al. . Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science. 2004;306(5704):2090–2093. [DOI] [PubMed] [Google Scholar]
- 190.Ranieri E, Gesualdo L, Petrarulo F, et al. . Urinary IL-6/EGF ratio: a useful prognostic marker for the progression of renal damage in IgA nephropathy. Kidney Int. 1996;50(6):1990–2001. [DOI] [PubMed] [Google Scholar]
- 191.Kayama F, Yoshida T, Elwell MR, et al. . Cadmium-induced renal damage and proinflammatory cytokines: possible role of IL-6 in tubular epithelial cell regeneration. Toxicol Appl Pharmacol. 1995;134(1):26–34. [DOI] [PubMed] [Google Scholar]
- 192.Sun Y, Chen P, Zhai B, et al. . The emerging role of ferroptosis in inflammation. Biomed Pharmacother Biomed Pharmacother. 2020;127:110108. [DOI] [PubMed] [Google Scholar]
- 193.Campbell NK, Fitzgerald HK, Dunne A.. Regulation of inflammation by the antioxidant haem oxygenase 1. Nat Rev Immunol. 2021;21(7):411–425. [DOI] [PubMed] [Google Scholar]
- 194.Naito Y, Takagi T, Higashimura Y.. Heme oxygenase-1 and anti-inflammatory M2 macrophages. Arch Biochem Biophys. 2014;564:83–88. [DOI] [PubMed] [Google Scholar]
- 195.Waltz P, Carchman EH, Young AC, et al. . Lipopolysaccaride induces autophagic signaling in macrophages via a TLR4, heme oxygenase-1 dependent pathway. Autophagy. 2011;7(3):315–320. [DOI] [PubMed] [Google Scholar]
- 196.Hull TD, Agarwal A, George JF.. The mononuclear phagocyte system in homeostasis and disease: a role for heme oxygenase-1. Antioxid Redox Signal. 2014;20(11):1770–1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Orozco LD, Kapturczak MH, Barajas B, et al. . Heme oxygenase-1 expression in macrophages plays a beneficial role in atherosclerosis. Circul Res. 2007;100(12):1703–1711. [DOI] [PubMed] [Google Scholar]
- 198.Zhang M, Nakamura K, Kageyama S, et al. . Myeloid HO-1 modulates macrophage polarization and protects against ischemia-reperfusion injury. JCI Insight. 2018;3(19):e120596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Morimoto K, Ohta K, Yachie A, et al. . Cytoprotective role of heme oxygenase (HO)-1 in human kidney with various renal diseases. Kidney Int. 2001;60(5):1858–1866. [DOI] [PubMed] [Google Scholar]
- 200.Kimura S, Aung NY, Ohe R, et al. . Increasing heme oxygenase-1-Expressing macrophages indicates a tendency of poor prognosis in advanced colorectal cancer. Digestion. 2020;101(4):401–410. [DOI] [PubMed] [Google Scholar]
- 201.Jais A, Einwallner E, Sharif O, et al. . Heme oxygenase-1 drives metaflammation and insulin resistance in mouse and man. Cell. 2014;158(1):25–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Carasi P, Rodríguez E, da Costa V, et al. . Heme-Oxygenase-1 expression contributes to the immunoregulation induced by Fasciola hepatica and promotes infection. Front Immunol. 2017;8:883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Kidney Disease: Improving Global Outcomes (KDIGO) Glomerular Diseases Work Group . KDIGO 2021 Clinical practice guideline for the management of glomerular diseases. Kidney Inter, 2021;100(4S):S1–S276. [DOI] [PubMed] [Google Scholar]
- 204.Preza GC, Ruchala P, Pinon R, et al. . Minihepcidins are rationally designed small peptides that mimic hepcidin activity in mice and may be useful for the treatment of iron overload. J Clin Invest. 2011;121(12):4880–4888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Sasu BJ, Cooke KS, Arvedson TL, et al. . Antihepcidin antibody treatment modulates iron metabolism and is effective in a mouse model of inflammation-induced anemia. Blood. 2010;115(17):3616–3624. [DOI] [PubMed] [Google Scholar]
- 206.Jing W, Nunes ACF, Farzaneh T, et al. . Phosphate binder, ferric citrate, attenuates anemia, renal dysfunction, oxidative stress, inflammation, and fibrosis in 5/6 nephrectomized CKD rats. J Pharmacol Exp Ther. 2018;367(1):129–137. [DOI] [PubMed] [Google Scholar]
- 207.Paller MS, Hedlund BE, Sikora JJ, et al. . Role of iron in postischemic renal injury in the rat. Kidney Inter. 1988;34(4):474–480. [DOI] [PubMed] [Google Scholar]
- 208.Rajapurkar MM, Hegde U, Bhattacharya A, et al. . Effect of deferiprone, an oral iron chelator, in diabetic and non-diabetic glomerular disease. Toxicol Mech Methods. 2013;23(1):5–10. [DOI] [PubMed] [Google Scholar]
- 209.Bloomer SA, Brown KE, Buettner GR, et al. . Dysregulation of hepatic iron with aging: implications for heat stress-induced oxidative liver injury. Am J Physiol Regulat Integr Compar Physiol. 2008;294(4):R1165–R1174. [DOI] [PubMed] [Google Scholar]
- 210.Bloomer SA, Brown KE, Kregel KC.. Renal iron accumulation and oxidative injury with aging: effects of treatment with an iron chelator. J Gerontol Series A Biol Sci Med Sci. 2020;75(4):680–684. [DOI] [PubMed] [Google Scholar]
- 211.Ramagiri S, Pan S, DeFreitas D, et al. . Deferoxamine prevents neonatal posthemorrhagic hydrocephalus through choroid Plexus-Mediated iron clearance. Transl Stroke Res. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]