Abstract
Background and Objectives
Spinal cord injury (SCI) disrupts the fine-balanced interaction between the CNS and immune system and can cause maladaptive aberrant immune responses. The study examines emerging autoantibody synthesis after SCI with binding to conformational spinal cord epitopes and surface peptides located on the intact neuronal membrane.
Methods
This is a prospective longitudinal cohort study conducted in acute care and inpatient rehabilitation centers in conjunction with a neuropathologic case-control study in archival tissue samples ranging from acute injury (baseline) to several months thereafter (follow-up). In the cohort study, serum autoantibody binding was examined in a blinded manner using tissue-based assays (TBAs) and dorsal root ganglia (DRG) neuronal cultures. Groups with traumatic motor complete SCI vs motor incomplete SCI vs isolated vertebral fracture without SCI (controls) were compared. In the neuropathologic study, B cell infiltration and antibody synthesis at the spinal lesion site were examined by comparing SCI with neuropathologically unaltered cord tissue. In addition, the CSF in an individual patient was explored.
Results
Emerging autoantibody binding in both TBA and DRG assessments was restricted to an SCI patient subpopulation only (16%, 9/55 sera) while being absent in vertebral fracture controls (0%, 0/19 sera). Autoantibody binding to the spinal cord characteristically detected the substantia gelatinosa, a less-myelinated region of high synaptic density involved in sensory-motor integration and pain processing. Autoantibody binding was most frequent after motor complete SCI (grade American Spinal Injury Association impairment scale A/B, 22%, 8/37 sera) and was associated with neuropathic pain medication. In conjunction, the neuropathologic study demonstrated lesional spinal infiltration of B cells (CD20, CD79a) in 27% (6/22) of patients with SCI, the presence of plasma cells (CD138) in 9% (2/22). IgG and IgM antibody syntheses colocalized to areas of activated complement (C9neo) deposition. Longitudinal CSF analysis of an additional single patient demonstrated de novo (IgM) intrathecal antibody synthesis emerging with late reopening of the blood-spinal cord barrier.
Discussion
This study provides immunologic, neurobiological, and neuropathologic proof-of-principle for an antibody-mediated autoimmunity response emerging approximately 3 weeks after SCI in a patient subpopulation with a high demand of neuropathic pain medication. Emerging autoimmunity directed against specific spinal cord and neuronal epitopes suggests the existence of paratraumatic CNS autoimmune syndromes.
Immune system function needs to be permanently orchestrated to deal with perturbations, complexities, and challenges.1 The coordination of appropriate immune system responses depends on timely and accurate information about the body's inflammatory state. After spinal cord injury (SCI), this feedback is blunted because of a disconnect from major adaptive sensor systems including the autonomic nervous system and stress axis (hypothalamic pituitary–adrenal axis).2,3 Uncoordinated immune responses lacking CNS supervision can trigger errors resulting in immunopathology. This may include adaptive immune responses able to propagate neuroaxonal pathology and demyelination.4,5 Despite sporadic reports of antimyelin antibodies in peripheral blood after SCI,6,7 it remains elusive whether these antibodies can access the CNS and cause immunopathology, particularly because immunoglobulin can cross the blood-brain barrier only to a very limited extent. Moreover, earlier neuropathologic studies did not detect B lymphocytes in the human spinal cord.8 These findings have been contrasted by experimental models reporting ectopic foci of infiltrating B lymphocytes/plasma cells, which synthesize neurotoxic antibodies associated with regional complement deposition after SCI.9 In this context, antibody synthesis evolves “behind” the blood-spinal cord barrier (BSB) at the lesion site and thereby does not require peripheral blood–derived antibodies to cross a BSB to reach the CNS epitopes. Autoimmune antibody synthesis can exert multiple effects, including antibody-mediated cellular toxicity, complement activation and associated neurodegeneration, and inhibition of saltatory nerve conduction and synaptic dysfunction.5,10,11 Hence, the emergence of autoantibody synthesis after human SCI might be a substantial contributor to (1) an impaired response to rehabilitation; (2) an overall failure of recovery; (3) the development of aberrant plasticity syndromes (including pain), (4) fatigue, or (5) if sustained delayed loss of recovered function (neuroworsening/degeneration).
In this study, we address the hypothesis that SCI triggers autoantibody synthesis causing humoral autoimmunity directed against spinal cord and neuronal epitopes. We apply tissue-based assays (TBA) and dorsal root ganglia (DRG) cell cultures to identify surface antibodies directed against spinal and neuronal epitopes.12-14 We interrogate whether the variable degree of disrupted interaction between the CNS and immune system caused by distinct SCI lesion level and severity (e.g., loss of autonomic control over lymphoid organ function) associates with the development of autoimmunity. Moreover, we examine human spinal cord specimens for cellular sources of autoimmunity including infiltration of B lymphocytes, plasma cells, antibody synthesis, and deposition of activated complement at the lesion site characterized by axonal pathology and myelin loss. Last, we assess the CSF in an additional independent patient to explore for dynamic changes in BSB dysfunction and for intrathecal antibody synthesis.
Methods
Multiple observational patient cohorts were interrogated to assess the hypothesis of an emerging autoantibody synthesis in patients after SCI including (1) a prospective, blinded, longitudinal observational study (n = 74), (2) a neuropathologic case-control study ranging from acute (baseline) to several months (follow-up) after SCI (n = 27), and (3) longitudinal CSF analysis of an additional single patient of a series of 10 cases.
Standard Protocol Approvals, Registrations, and Patient Consents
The cohort study was approved by the Research Ethics Board of Charite–Universitätsmedizin Berlin (EA1/001/09), University Health Network, Toronto (REB10-0384-AE), and Cantonal Ethics Commission, Zurich (KEK-ZH-Nr. 2011–0059). All participants were informed about the study and gave their written informed consent to participate. The neuropathologic case-control study was approved by the ethics committee of the Medical University of Vienna (EK. Nr.: 1454/2018 and 1636/2019). The study for the CSF analysis was approved by the Ethical Board Landesamt für Gesundheit und Soziales, Berlin, Germany (LaGeSo Nr. 0127-EK13). All participants were informed about the study and gave their written informed consent to participate.
Longitudinal Cohort Study
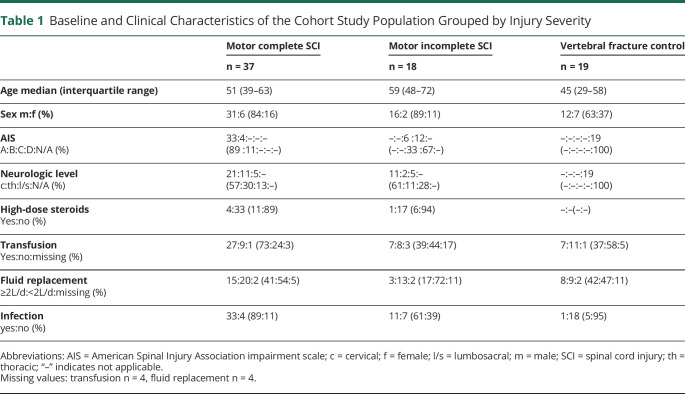
The prospective multicenter SCIentinel study (DRKS00000122) was designed to distinguish CNS-related effects on the immune system from systemic effects after trauma and therefore included both patients with acute traumatic SCI and patients with vertebral fracture (VF) without SCI.15 Patients were enrolled from 2011 to 2014 at 4 hospitals; BG Hospital Unfallkrankenhaus Berlin, Germany; Charité-Universitätsmedizin Berlin, Germany; University of Zurich, Switzerland; and University Health Network Toronto, Canada. Exclusion criteria were nontraumatic SCI, 2 or more distinct spinal cord lesions, life-threatening polytrauma, serious traumatic brain injury, preexisting health conditions, such as neoplasia, autoimmune diseases, chronic infections, systemic steroid treatment, severe alcohol or drug addiction, and pregnancy or lactation.15 All patients who completed the follow-up with available serum samples were included into the screening for autoantibodies that target conformational CNS epitopes (eFigure 1, links.lww.com/NXI/A814). Serum samples for the antibody detection and laboratory endpoints were collected at “baseline” (median [interquartile range] 8 [7–9] days after injury) antedating reactive de novo changes and at “follow-up” (median [interquartile range] 70 [66–74] days after injury). Lesion-dependent (neurogenic) aspects (SCI severity/completeness and neurologic level) were evaluated based on the International Standards for Neurological Classification of SCI at baseline.16 Demographic baseline data and clinical information on infections, medication, red blood transfusions, and fluid resuscitation were collected using paper case report forms.
Blinding of the Experiments, Assessment of Antibody Binding, and Exclusion of Heterophilic Binding
The samples for TBA and DRG assays were provided by Charité-Universitätsmedizin Berlin, Department of Neurology (J.M.S. and M.A.K.) in a blinded manner using a 6-digit pseudonym as identifier without any accompanying clinical information. The samples were evaluated microscopically by 2 independent observers at the Medical University of Vienna, Division of Neuropathology and Neurochemistry, Department of Neurology (C.H. and R.H.) including a board-certified neuropathologist. We performed indirect immunohistochemistry (IHC) (TBA) and immunofluorescence (DRG) assays with sera of patients with SCI and controls on frozen sections of healthy rat spinal cords or primary rat DRG cell cultures. The intensity of the immunoreactivity was evaluated and scored semiquantitatively (negative [−], marginal [±] slight staining [+], strong staining [++], and very strong staining [+++]). The samples were evaluated in a fully blinded setting in a randomly mixed pattern of patients with SCI at different time points after injury and controls. To reproduce the immunoreactivity, we performed TBA testing 3 times on different rat spinal cord sections from different animals and of various spinal cord levels. Moreover, we performed DRG assays 2 times for negative and 3–5 times for positive samples. For statistical analysis, semiquantitative TBA and DRG results were dichotomized into negative (− and +/−) and positive (+, ++, and +++) staining.
Because we used rat cells and tissues, putative binding of heterophilic antibodies was prevented using blocking strategies including 10% donkey serum (TBA) or fetal calf serum (DRG) incubation. The results of TBA and DRG assessments were sent to Charité-Universitätsmedizin Berlin, Department of Neurology (M.A.K and T.Lü.) for unblinding and statistical analysis.
Spinal Cord Tissue-Based Assay
Adult Sprague Dawley rats were euthanized and spinal cords removed and fixed in 4% paraformaldehyde for 4 hours at 4°C. The spinal cords were washed with 1× phosphate-buffered saline (PBS), cryoprotected with 20% sucrose for 48 hours at 4°C, embedded in freezing medium (optimal cutting temperature compound), snap frozen in isopentane chilled with liquid nitrogen, and then cut into 7-μm thick sections. For indirect IHC, the tissue sections were defrosted, washed in 1× PBS, and blocked with blocking solution (10% donkey serum diluted in 0.03% Triton X-100 [Sigma-Aldrich, St Louis, MO] in 1× PBS) for 30 minutes at room temperature (RT). Subsequently, sections were incubated with patients' sera (diluted 1:200 in blocking solution), according to previously published protocols,17 for 3 hours at 37°C, washed 3 times with 1× PBS, incubated with biotinylated goat antihuman IgG antibodies (Vector lab; 1:2,000 in 1× PBS) for 30 minutes at RT, and again washed with 1× PBS. Serial dilutions were performed in 5 representative serum samples. The last dilution before loss of the signal in the substantia gelatinosa was indicated as antibody titer. Antibody binding was visualized with 3,3′-diaminobenzidine (DAB). Omission of primary antisera served as controls.
Indirect Immunofluorescence of Living DRG Neurons Incubated With Patients' Sera
Live DRG cells (eMethods, links.lww.com/NXI/A814) were incubated with sera from patients with SCI or controls (1:200 diluted in cultivated Dulbecco's modified eagle medium) for 30 minutes at 37°C, washed 3 times with 1× PBS, fixed with 4% paraformaldehyde for 10 minutes, and then permeabilized with 0.3% Triton X-100 (Sigma-Aldrich, St. Louis, MO). Cells were then labeled with a goat antihuman Alexa Fluor 488–conjugated IgG antibody (AF488, 1:2,000; Jackson ImmunoResearch Laboratories, Cambridge, United Kingdom) and counterstained with 4′,6-Diamidin-2-phenylindol, 1 μg/mL in 1× PBS for 3 minutes, washed in distilled water, and mounted with Aqua-Poly/Mount (Polyscience, Warrington) on glass slides. Omission of primary antisera served as controls.
Cell-Based Assay to Detect α-Glial Fibrillary Acidic Protein Antibodies
To detect antibodies against the alpha subunit of glial fibrillary acidic protein (GFAP), we applied an indirect immunofluorescent cell-based assay, as previously described.18 For a brief description of the method, see eMethods, links.lww.com/NXI/A814.
Factor XII Assessment (ELISA)
Factor XII was measured in matched sera of patients with SCI demonstrating TBA+, DRG+ double reactivity compared to patients with SCI and VF control patients both without presence of TBA or DRG reactivity (TBA−, DRG−) (total n = 27). Matching was based on the best possible 1:1 allocation using the following variables in the order of descending priority. In the SCI group, matching was based on (1) the severity of SCI (American Spinal Injury Association impairment scale [AIS]), (2) neurologic level, (3) age, and (4) sex. Subsequently, patients with VF were matched to patients with SCI based on (1) age and (2) sex (eTable 1, links.lww.com/NXI/A814). Factor XII ELISA was performed according to the manufacturer's instructions (LSBio Inc. Seattle, WA; Human F12/Factor XII ELISA Kit; Catalog No. LS-F10418).
CD8/CD3 Ratio
In the similar matched sample analyzed for Factor XII, the CD8/CD3 ratio was assessed (n = 27). Blood samples collected in Cyto-Chex tubes (Streck, La Vista, NE) were used to quantify lymphocyte subpopulations within 36 hours after blood withdrawal. The mouse antihuman fluorescence-labeled monoclonal antibodies cluster of differentiation 3 Allophycocyanine-Alexa Fluor 750 (APC-A750, clone UCHT1; Beckman Coulter, Krefeld, Germany, catalog number A94680), CD8 APC (clone B9.11; Beckman Coulter IM2469), and CD45RA Pacific-Blue (clone J33; A74763)–stained samples were acquired on a ten-color Navios flow cytometer and analyzed using Navios software (Beckman Coulter). The gating strategy for the enumeration of CD3+ and CD8+ T cells in peripheral blood is described in eFigure 2, links.lww.com/NXI/A814. The CD8/CD3 ratio was calculated based on the absolute frequencies of CD3+ and CD8+ T cells that were estimated based on their relative frequency within the parent population.
Neuropathologic Case-Control Study
In an independent neuropathologic study, we examined archival human autopsy tissue from 22 patients with SCI and 5 nontraumatic control patients with clinical data, demographics, and histologic characterization, as previously described19 (eTable 2, links.lww.com/NXI/A814). An archival collection of spinal cord autopsy tissues (collected between 1960 and 2019) in the Division of Neuropathology and Neurochemistry, Department of Neurology, Medical University of Vienna, was screened. All available samples associated with traumatic SCI (n = 22) and a control group of 5 nontraumatic patient samples were included in analogy to a previous study.19 The controls were selected after exclusion of pathologic white and gray matter alterations, inflammatory infiltrates, or signs of tissue injury and under the condition that age (between 32 and 73 years) and sex (2 females and 3 males) of the control patients correspond to the composition of the SCI group. For clinical data collection, all available medical records, laboratory charts, and autopsy charts were screened.
Spinal Cord Injury Neuropathology
IHC was performed on formalin-fixed paraffin-embedded tissue with a biotin/avidin detection system.20 All primary antibodies and respective antigen retrieval methods are listed in eTable 3, links.lww.com/NXI/A814. Inflammatory infiltrates were characterized by IHC, using antibodies targeting CD3, CD4, CD8 (T cells), CD20 (B cells), CD79a (B cells and plasma cells), CD138 (plasma cells), and IgG or IgM antibodies and visualized with DAB as chromogen. C9neo detection was used to identify complement activation.21 Omission of primary antibodies served as controls. Quantitative assessment of inflammatory cells was performed in the lesion core and directly surrounding tissue margin (lesion rim). For quantitative evaluation of lymphocytes, sections were overlaid by a morphometric grid (0.2256 mm2) placed within the ocular lens and manually counted within the entire lesion (5–20 fields of 0.2256 mm2). Cells expressing the respective markers were counted separately for perivascular and parenchymal areas and pooled afterward. The values were expressed as cell counts per square millimeter, as previously described.19
Intrathecal Immunoglobulin Synthesis After Spinal Cord Injury
In an index patient derived from an extra case series of 10 patients with SCI, albumin, IgG, IgA, and IgM from the CSF and serum samples were quantified by routine nephelometry. BSB dysfunction was determined on the basis of age-related albumin quotients of the CSF/serum. For the detection of intrathecal antibody synthesis, the antibody index was calculated as the ratio between the CSF/serum quotient for IgG, IgM, and IgA antibodies and the CSF/serum albumin quotient using the Reiber calculation.22,23 Three weeks after SCI, almost no BSB disturbance was noted.24
Statistical Analysis
Continuous variables were reported as median and quartiles and categorical variables as absolute and relative frequencies. To examine the neurogenic, SCI-lesion associated aspects of autoantibody binding, the exposed groups were defined based on injury severity (AIS A or B vs AIS C or D vs VF without SCI), and neurologic level (cervical vs thoracic, lumbar, or sacral vs VF without SCI) and compared using the χ2 test. All tests were 2-sided, and the statistical significance level was set to <0.05. Explorative p values should be interpreted cautiously because no adjustment for multiple testing was performed. An analysis of baseline and clinical characteristics of the SCI population in association with TBA+, DRG+ double and single reactivity at follow-up was performed in a descriptive manner comparing TBA+, DRG+ reactivity status by age, sex, acquired infections (pulmonary, urinary, or other infections), red blood transfusion, fluid replacement, and the early application of high-dose steroids according to National Acute Spinal Cord Study (NASCIS) schemes. Continuous age was dichotomized by the median age of the SCI population (54 years). Fluid replacement was analyzed by dividing into >2 and ≥2 L/d at least once within the first week after SCI. The temporal changes in Factor XII levels in serum and CD8/CD3 ratio in peripheral blood were evaluated descriptively, and the analysis was stratified according to whether the TBA+, DRG+ double reactivity was de novo or had already existed at baseline. The medication administered for the treatment of strong pain in the cohort study and the neuropathologic study data were analyzed primarily in a casuistic manner. Statistical analysis was performed with SPSS 28.0.1 (IBM Corp. in Armonk, NY).
Data Availability
Individual-level data collected in the cohort study associated with the SCIentinel study cannot be fully shared for data protection reasons. However, dataset extracts of deidentified patient data from the analysis dataset in connection with a data dictionary can be made available on written request and signature of a data transfer agreement by the corresponding authors with the publication of the article. For the neuropathologic study, in addition to the clinical information already published, the individual IHC findings can be provided in a tabular format on request to the corresponding authors. The statistical syntax code (SPSS) used to analyze the study data can be made available on written request to the corresponding authors with publication of the article.
Results
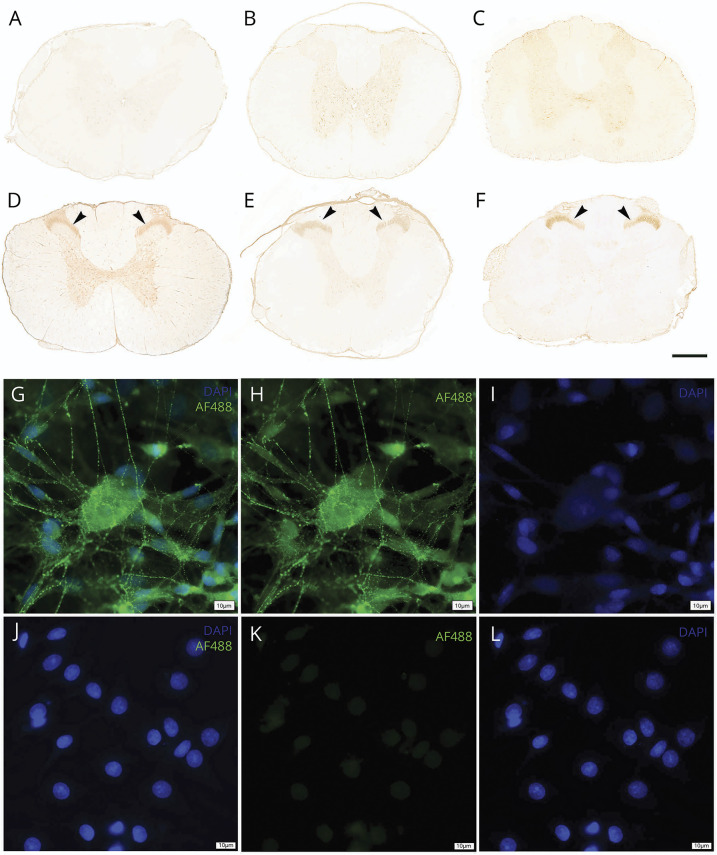
Sera of patients with SCI recognize spinal cord epitopes and form a characteristic binding pattern de novo in areas of high synaptic density. Sera of 55 patients with SCI were assessed for reactivity with spinal cord epitopes and were compared with those of 19 controls (Figure 1). For the analysis flowchart see eFigure 1, links.lww.com/NXI/A814. The patient characteristics are reported in Table 1. Dynamic changes in autoimmunity about to develop over time in the context of neoantigen exposition in an inflammatory lesion milieu25 were assessed by comparing the acute SCI stage (baseline; median 8, interquartile range 7–9, range 1–24 days after injury) with a follow-up time point (median 70, interquartile range 66–74, range 43–107 days), applying a longitudinal study design by blinded observers. To determine the reactivity of patient sera to spinal cord epitopes, a spinal cord TBA was developed in analogy to diagnostic brain tissue assays used for example to determine the presence of autoimmune encephalitis or paraneoplastic syndromes (Figure 1, A–F).12,14 These assays use postfixed rat tissue that maintain 3D epitope conformation to increase accuracy and biological relevance and reduce the likelihood of false-positive or false-negative binding associated with denatured proteins. In 11 of 55 (20%) patients with SCI, a TBA reactivity was apparent by 10 weeks after injury, whereas none of the VF control sera were TBA immunoreactive (Figure 2A). This profound SCI-dependent increase in labeling was restricted to the dorsal horn localized to Rexed Laminae II and III displaying the typical fine granular staining pattern. These laminae include the substantia gelatinosa, a less myelinated region characterized by high synaptic density, which receives input from A-delta (mechanoreceptor) or C-fibers (nociceptor). The dorsal horn is considered crucial for sensory-motor integration and serves as an entry gate for propriospinal information, mediating considerable effects of activity-dependent neurorehabilitation.26 One anatomical substrate of sensory-motor integration is descending corticospinal tract fibers synapsing on dorsal horn neurons including Laminae III.27 The titer of this reactivity pattern ranged from 1:800 to 1:3,200 and was not detected in sera of control patients (Figure 2A), neither at baseline nor at follow-up time points, and can be considered specific to develop with time after SCI (Figure 2B). The axons and myelin of the dorsal root fibers showed negative results. Moreover, selected positive sera reactive with spinal cord TBA were also probed for binding on well-established TBA on rat brain, as previously described, 10 and showed negative results for any signs of autoreactivity on rat brain tissues17 (data not shown).
Figure 1. Antibody Binding of Sera of Patients With Spinal Cord Injury (SCI) to Native Spinal Cord Epitopes as Detected by Tissue-Based Assays (TBA) and Binding to Living Dorsal Root Ganglia Cells (DRG).
Sera of patients with SCI are applied to native rat spinal cord sections and incubated for 3 hours at 37°C (1:200 in blocking solution). A representative SCI patient serum from the acute phase (1 week post-SCI) (A) and 2 representative vertebral fracture control sera (B, C) illustrate a negative staining pattern. By contrast, 10 weeks after SCI, de novo neuropil labeling of the Rexed Laminae II and III in the dorsal horn becomes apparent (arrowheads; D: follow-up serum of A; E, F: 2 representative sera of patients with SCI). It contains the substantia gelatinosa, a less myelinated region characterized by high synaptic density, which receives input from A-delta (mechanoreceptor) or C fibers (nociceptor). The dorsal horn is considered crucial for sensory-motor integration and serves as an entry gate for propriospinal information mediating considerable effects of neurorehabilitation.26,27 The role of the dorsal horn is further emphasized by evidence demonstrating even descending corticospinal tract (CST) fibers27 to synapse on dorsal horn neurons. Last, corresponding dorsal horn laminae contain Calcitonin Gene-Related Peptide positive fibers as candidates involved in neuropathic pain formation and autonomic control.35 This pattern is recapitulated by several SCI patient sera and demarcates a characteristic recognition pattern emerging after SCI. The targeted gray matter neuropil is composed of a dense unmyelinated fiber network of neuronal process, containing synapses, axons, and dendrites. The neuropil represents an established target for antibody-mediated autoimmune disease.12 Tissue-based assays are an established and validated diagnostic mean to determine autoantibodies in neurologic disease.10,13 Extending from autoantibodies recognizing spinal cord epitopes, the binding to living primary DRG cultured cells is considered relevant for functional relevance. The presence of autoantibodies binding to the membrane of DRG neurons and glia cells in patient sera is visualized by antihuman IgG antibodies (AF488, green) and counterstained with 4′,6-Diamidin-2-phenylindol (DAPI, blue). Sera from patients with SCI demonstrate binding of human IgG antibodies to the DRG cells (G–I). The surface staining pattern is absent in the serum of a control patient (J–L). Scale bar A–F = 500 μm, scale bar G–L = 10 μm.
Table 1.
Baseline and Clinical Characteristics of the Cohort Study Population Grouped by Injury Severity
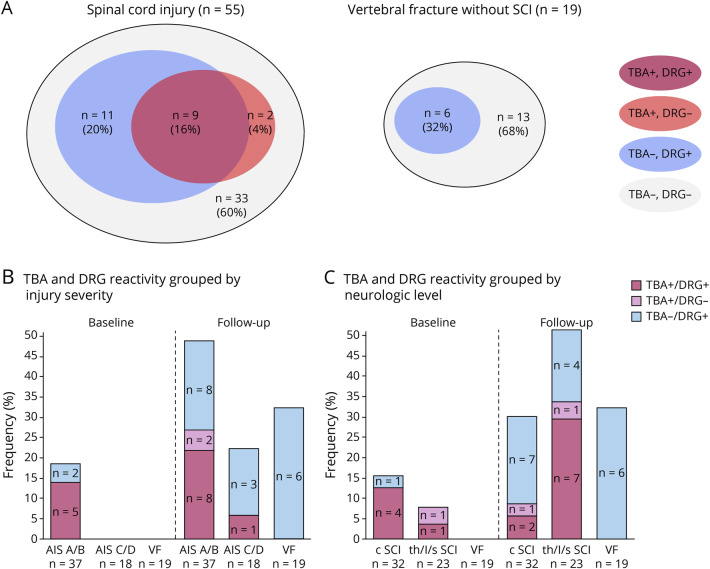
Figure 2. Dynamic Emergence of Autoantibodies Detecting Epitopes of the Spinal Cord and Living Dorsal Root Ganglia (DRG) Cells in Patients After Spinal Cord Injury (SCI) Compared With That in Controls.
Tissue-based assay (TBA) and DRG screening at baseline (median [interquartile range] 8 [7–9] days after injury) and follow-up (median [interquartile range] 70 [66–74] days after injury). Applying 2 diagnostic assessments testing for different aspects of autoantibody binding, patients with SCI develop in 16% of the total SCI sample autoantibodies as being double positive in TBA and DRG assays, as visualized in a Venn-like diagram depicting the overlap of TBA and DRG immune reactivity relative to the total SCI or vertebral fracture (VF) group (A) Patient sera that contain autoantibodies detecting spinal cord epitopes in situ (TBA) and having the ability to bind to living DRG cells constitute higher odds for functional relevance than those being immune positive in a singular assay alone (A, left).10,13 By contrast, in the vertebral fracture control cohort, no patient has been detected with double-positive serum (A, right). Neurogenic SCI-lesion-associated patterns of autoantibody binding were analyzed further by segregating SCI severity graded using the American Spinal Injury Association impairment scale (AIS) (“motor complete SCI” [AIS A/B]; “motor incomplete SCI” [AIS C/D]; “vertebral fracture without SCI” [VF]) (B) or for neurologic level of injury (“cervical SCI” [c SCI]; “thoracic, lumbar or sacral SCI” [th/l/s SCI]; “vertebral fracture without SCI” [VF]) (C). Applying 2 diagnostic assay patients testing for different aspects of autoantibody binding, patients with motor complete SCI develop in 22% autoantibodies as being double positive in TBA and DRG. By contrast, in the motor incomplete group, 1 patient (5%) and in the control cohort, no patient has been detected with double-positive serum (B). In the thoracic, lumbar, or sacral SCI group, 7 patients (30%) revealed double reactivity, whereas only 2 sera of patients with cervical SCI (5%) were double positive (C). Relative frequencies of TBA+, DRG+ double immune reactivity and single reactivity are expressed as percentages with absolute frequencies indicated in the bars. Comparison of TBA and DRG double-assay reactivity (TBA+, DRG+) vs single assay or no reactivity (TBA+, DRG− or TBA−, DRG+ or TBA−, DRG−) using the χ2 test: At baseline by injury severity, p = 0.070; at follow-up by injury severity, p = 0.031 (B); at baseline by neurologic level, p = 0.26; at follow-up by neurologic level, p = 0.003 (C).
Autoantibodies in Sera of Patients With SCI Bind to the Surface/Membrane of DRG Neurons
Besides antibody binding to spinal cord epitopes, the capacity to recognize and bind to epitopes of living DRG cells allows further predictions for putative functional relevance.10 Relevance to use living DRG neurons is further provided by the TBA results as the immunoreactive substantia gelatinosa is relaying DRG-derived fibers. The presence of surface (membrane) antibody binding to DRG cells is visualized by antihuman antibodies, as illustrated in Figure 1, G–I. The absence of surface antibodies in a control patient serum is illustrated in Figure 1, J–L. The presence of antibodies targeting cell surface epitopes indicates clinical relevance.10,13 Following-up on reports demonstrating GFAP antibodies after human SCI,28 we screened all sera for their capacity to the detect the alpha subunit of GFAP, applying specialized cellular assays.18 Only antibodies directed against the α-GFAP subunit can cause a specific autoimmune neurologic syndrome, while antibodies against the linear GFAP protein are not associated with a known clinical phenotype. All tested serum samples remained α-GFAP negative (data not shown).
Dynamic Emergence of Autoantibody Synthesis Detecting Neoepitopes of the Spinal Cord and Living DRG Neurons After SCI
Overall, the total number of patient samples displaying immunoreactivity was higher in the DRG neuron assay when compared with that in the TBA assessment. Venn-like plot analysis was used to visualize differences in antibody binding patterns in TBA and DRG neuron assays between SCI and control patients at follow-up (Figure 2). In contrast to the control group where no patient sera have been TBA+ and DRG+, 9 patients (16%) of the spinal cord group were double immunoreactive (Figure 2A). Therefore, in line with other neuropathologic conditions characterized by functional relevant antibody formation, we considered only the combination of TBA+, DRG+ (double-positive immune reactivity) as an indicator specific for autoantibody binding. Immunoreactivity restricted to the DRG assay only (TBA negative) can be classified of unknown significance. In 6 of 55 cases (13%), the reactivity was de novo (developing after the baseline).
Lesion-Dependent (“Neurogenic”) Patterns Associated With Developing Immunoreactivity
Combinatorial evidence from TBA and DRG binding assays indicate that immune reactivity of patient sera after motor complete SCI (AIS A or B), motor incomplete SCI (AIS C or D), and the control group differ substantially in their capacity to recognize spinal cord and DRG epitopes. At the acute phase (baseline), 5 of 37 sera (14%) from patients with motor complete SCI were TBA+, DRG+ double-immune reactive. By contrast, none of the 18 motor incomplete SCI and of the 19 control patient sera were double-assay or single-assay reactive (p = 0.070) (Figure 2B). At follow-up, 8 of 37 sera (22%) of patients with motor complete SCI were TBA+, DRG+ double immune reactive, whereas one of 18 (5%) motor incomplete SCI and 0 of 19 control patient sera were TBA+, DRG+ double positive (p = 0.031). Considering the neurologic level, TBA+, DRG+ double-immunoreactive results were obtained at baseline in 4 of 32 patients with cervical SCI (13%) and in 1 of 23 patients with lower neurologic level (4%). At follow-up, the proportion of double-positive findings in the cervical SCI group was diminished to 2 of 32 (6%), and in lower neurologic level group, it was increased to 7 of 23 patients (30%) (Figure 2C).
Putative Nonlesion-Dependent Denominators Associated With the Development of Autoantibodies Detecting Epitopes of the Spinal Cord and DRG Neurons
Patient and clinical characteristics associated with TBA and DRG immunoreactivity were explored in the SCI population (n = 55) (eFigure 3, links.lww.com/NXI/A814). The patients' age and sex were not associated with TBA+, DRG+ double immunoreactivity nor were infectious complications or early high-dose corticosteroid treatment of SCI. Fluid replacement and red blood cell transfusions revealed an association with both higher TBA+, DRG+ double and single-assay immunoreactivity. Coagulation is altered by CNS trauma itself and subsequently by blood volume or red blood substitution. Factor XII is a member of the coagulation cascade, which can propagate autoimmunity in animal models of MS.29 We evaluated whether the level of coagulation factors known to drive autoimmunity against CNS epitopes29 are present at higher levels in patients with SCI who developed de novo antibody binding (TBA+, DRG+) relative to SCI or VF controls without emerging autoimmune antibody synthesis (TBA−/DRG−). Individuals demonstrating de novo immunoreactivity also frequently displayed an increase in systemic Factor XII levels (eFigure 4, links.lww.com/NXI/A814) and CD8/CD3 lymphocyte ratios as another possible contributing element21 (eFigure 5, links.lww.com/NXI/A814).
Autoantibody Binding and Its Association With Neuropathic Pain Medication
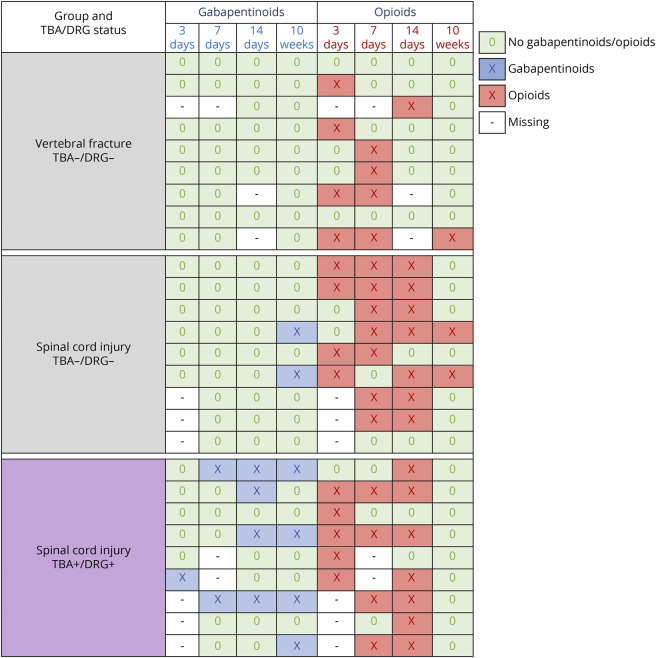
Whether TBA and DRG immunoreactivity were associated with pain was examined in the matched sample of patients with SCI and VF control patients (n = 27, eTable 1, links.lww.com/NXI/A814) using data on pain medication administered specifically for neuropathic pain (gabapentinoids) or strong nociceptive and neuropathic pain (opioids). Patients with SCI revealing TBA+, DRG+ double immunoreactivity displayed an earlier and more frequent use of gabapentinoids compared to patients with SCI or VF who were not demonstrating immunoreactivity (Figure 3). The pattern of opioid prescription was not markedly different between the groups (Figure 3).
Figure 3. Medication Administered for the Treatment of Strong Pain in the Cohort Study.
Patients with spinal cord injury and antibody binding detected in tissue-based assays (TBA) and dorsal root ganglia (DRG) cells (TBA+, DRG+ double positive) received earlier and more frequently gabapentinoid (pregabalin or gabapentin) medication for the specific treatment of neuropathic pain compared with TBA−, DRG− double-negative patients with SCI and VF. Regarding opioid medication used mainly for strong non-neuropathic but also for neuropathic pain, no clear differences between the groups were observed. Patients with SCI were matched for American Spinal Injury Association impairment scale, neurologic level, age, and sex. Patients with VF were matched for age and sex.
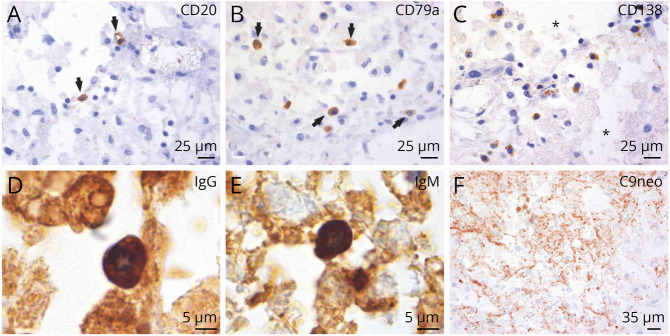
Neuropathologic Evidence of Infiltrating B Lymphocytes and Antibody-Synthesizing Plasma Cells in the Injured Human Spinal Cord
We examined archival human autopsy tissue from 22 patients with traumatic SCI and 5 nontraumatic control patients (eTable 2, links.lww.com/NXI/A814).19 In an extended analysis, we observed infiltrating B lymphocytes, CD20+ (Figure 4A), or CD79a+ (Figure 4B) at the spinal cord lesion site in 6 of 22 spinal cords (27%). CD138+ plasma cells were visible in 2 of the 6 cases in injured spinal cord parenchyma (Figure 4C). In these 2 cases, infiltration of CD79a+ B cells and CD138+ IgG+–producing plasma cells ranged from 4,95 to 5.875 CD79a+ cells/mm2 and 0.55–3.85 CD138+ cells/mm2. The other 4 cases showed a range of 0.55–1.825 CD20+ cells/mm2 and 0.285–1.375 CD79a+ cells/mm2. B cells and plasma cells became apparent starting 15 days after SCI and were detected up to 36 days after SCI. CD138+ plasma cell infiltration was detected at the lesion site by day 15–18 days after injury. CD138+ plasma cells were IgG+ (Figure 4D) and IgM+ (Figure 4E), indicating active antibody synthesis. Deposition of synthesized IgM and IgG antibodies was observed in lesion parenchyma adjacent to IgG+ and IgM+ plasma cells (Figure 4, D and E). Binding to the respective cognate antigen on glia and neurons can not only trigger functional changes but also induce complement-mediated cell cytotoxicity.5 In addition, antibody-dependent cell-mediated cytotoxicity is induced when antibody-coated cells are recognized and lysed by Fc receptor–bearing cells.5 We observed substantial deposition of activated complement (C9neo antigen) close to plasma cell–derived antibody synthesis and in the presence of antibody–coated cells (Figure 4F). Parenchymal plasma cells or B lymphocytes were absent in the control group.
Figure 4. B Cells Extravasate, Convert Into Plasma Cells, and Synthesize Antibodies in Subacute Spinal Cord Injury (SCI) Lesions After Human SCI.
B cells leave the vessel lumen (extravasate) and populate the perivascular space (2 weeks after SCI). These findings are corroborated by the detection of 2 different B cell antigens, CD20 (A) and CD79a (B) (black arrows). B cells can convert into plasma cells as the appearance of CD138+ plasma cells coincided with the presence of B cells. CD138+ plasma cells are not restricted to the perivascular space and are interspersed in the reorganizing lesion milieu characterized by spongiotic tissue changes (C) (asterisk). Plasma cells synthesize IgG (D) and IgM antibodies (E). IgG synthesis is localized in the deposition areas of activated complement (C9neo antigen) (F).
Reopening of the BSB Occurs Concomitant With Intrathecal IgM Antibody Synthesis After SCI
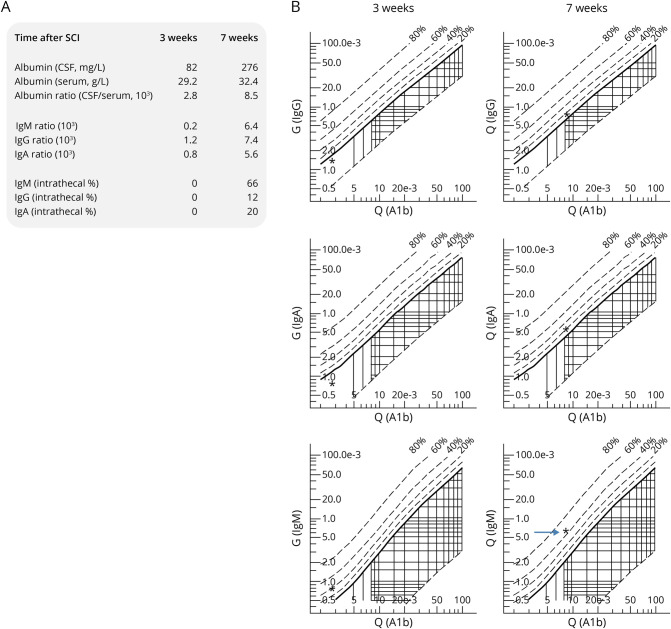
In a single patient of a series of 10 cases with radiologically confirmed motor-sensory complete SCI and without CNS infection, preinjury demyelinating disease, or intracerebral neoplasm, we evaluated BSB permeability and measured CSF antibody ratios. In this patient, the CSF albumin concentration increased from 82 to 276 mg/L between 3 and 7 weeks postinjury, indicating a delayed reopening of the BSB (Figure 5A). The CSF/serum albumin ratio confirmed a BSB breach, resulting in a 3-fold higher permeability at 7 weeks compared with 3 weeks after SCI, indicating BSB dysfunction. While IgG, IgM, and IgA antibody indices (CSF/serum difference of antibody amounts) were zero at 3 weeks post-SCI, they increased to 66 (IgM), 20 (IgA) and 12 (IgG), respectively, at 7 weeks. The corresponding Reiber diagrams (Figure 5B) illustrate a strong association between increasing IgG and IgA levels and reflect the breach of the BSB (as measured by higher albumin CSF levels). However, the most pronounced increase in IgM antibodies exceeded beyond what can be expected by BSB dysfunction (passive transport), indicating de novo intrathecal synthesis of IgM.
Figure 5. Intrathecal De Novo IgM Synthesis Emerging After Spinal Cord Injury (SCI) Coinciding a Late Reboosted Inflammatory Milieu.
Consecutive CSF diagnostics over time in a 55-year-old female patient with motor-sensory complete SCI of the neurologic level C4 illustrated in absolute measures (A) and plotted as Reiber diagram (B).22,23 Three weeks after SCI, despite a remaining mild disturbance of the blood-spinal cord barrier (BSB), no intrathecal antibody synthesis can be detected (IgG, IgA, and IgG CSF/serum ratios are within the normal range relative to the albumin CSF/serum ratio, as the asterisks are below the solid line in each graph on the left). Within the subsequent 4 weeks, a dynamic autoimmune process develops at the lesion site until week 7. Evolving post-traumatic CNS autoimmunity is characterized by a (1) reopening of the BSB (3-fold albumin CSF/serum ratio) indicative for a mild BBB disturbance and (2) remarkable de novo antibody synthesis detected in the CSF compartment only (IgG, IgA, and IgM CSF/serum ratios are substantially increased relative to the only slightly increased albumin CSF/serum ratio (asterisks are above the solid line in each graph on the right). A predominant IgM class response confirms its novel onset (de novo synthesis).
Discussion
We set out to investigate the hypothesis that SCI triggers autoantibody synthesis causing humoral autoimmunity. We addressed the void of missing systematic longitudinal studies, applying enabling methodology to detect conformational spinal cord and DRG surface epitope binding. This is required to gauge functional relevance of the autoantibody synthesis emerging with time compared with baseline assessments and controls.
First, we provided aggregate evidence for (1) the presence of de novo post-traumatic antibody synthesis at the lesion site and (2) an emerging antibody-mediated autoimmunity after human SCI directed against conformational spinal cord and DRG neuronal surface epitopes. Dissociated DRG cultures are well established to study cellular and molecular aspects relevant for neuroaxonal plasticity and repair including neurite outgrowth and belong to the diagnostic armamentarium to identify autoimmune syndromes associated with antineuronal or antiglial antibodies.14 Synthesis of autoantibodies detecting conformational spinal cord and DRG surface epitopes were observed in the SCI group only, compared with those in controls. Sera of patients with SCI bind to the substantia gelatinosa as a less myelinated region of high synaptic density. Besides relevance for neuropathic pain, this region hosts propriospinal tract fibers relaying considerable aspects of neurorehabilitation.26 So far, the target antigen is not known, and future studies with immunoprecipitation and mass spectrometry will be necessary for the identification.13 Nevertheless, evaluation of the medication data provided evidence of an increased need for gabapentinoids in patients with TBA+, DRG+ double immunoreactivity, which can be interpreted as a surrogate for possible enhancement of neuropathic pain. Increase of sera immunoreactivity already during the acute SCI phase is in line with a natural antibody response and consistent with low-affinity polyreactive antibodies driving clearance of cellular debris after massive cell injury.30
Second, we provide neuropathologic proof that after B cells infiltrate the lesioned spinal cord, it is followed by the emergence of plasma cells, which demonstrate IgM and IgG antibody syntheses. Regions of IgG and IgM syntheses colocalize with areas of complement activation (C9neo) as putative self-sufficient mechanism of neurodegeneration.5,21 Intrathecal immunoglobulin synthesis developing de novo after SCI has been determined by an elevated IgM antibody serum to CSF index coincident with a reopening of the BSB (Figure 5). B cell/plasma cell infiltration or intrathecal antibody synthesis becomes apparent in a delayed manner after SCI as putative underlying element of emerging secondary humoral autoimmunity. The time window of plasma cell infiltration into spinal cord lesion matches with the occurrence of intrathecal IgM synthesis in the index patient (onset approximately 3 weeks after SCI). The time window of an emerging antibody-mediated autoimmune response further corresponds with the rodent SCI model, where antibody-mediated autoimmunity emerges within 4 weeks post-SCI.9
Third, the development of autoimmunity depends on severity and lesion level and provides neurogenic considerations. Motor complete lesions (AIS A/B) cause in more than 90% at-level autonomic denervation (“sympathetic decentralization”)31 and are associated with elevated ratios of autoimmunity (Figure 2B). Sympathetic decentralization withdraws supraspinal autonomic control and disconnects a major adaptive integrative feedback system required to sense and control inflammation.27 By contrast, autoantibody binding occurs less frequent in patients with high lesion (cervical) SCI, in line with the limited ability to develop a humoral immune response to “de-novo” antigens after high-injury level SCI in rodents.32 Non-neurogenic candidate factors putatively associated with de novo autoimmunity are increased FXII levels and CD8/CD3 ratios (eFigure 3 and 4, links.lww.com/NXI/A814). While we did not observe antibodies directed against the α-GFAP subunit in serum (GFAP autoimmunity), this does not exclude the possibility of CSF antibodies.18
Our work has limitations. One is that serum samples are not accompanied by CSF samples; however, while desirable, also the detection of serum immunopositivity in TBA and DRG alone being considered sufficient and diagnostic for autoimmune diseases in humans.10,13 Moreover, the identification of plasma cells directly at the human spinal cord lesion site provides more direct and neuropathologic evidence of antibody synthesis behind the BSB compared with CSF analysis.
Applying several lines of evidence, we provide immunologic, neurobiological, and neuropathologic proof-of-principle for an antibody-mediated autoimmunity response emerging after SCI affecting an SCI patient subpopulation. The dimension ranges from 16% TBA+, DRG+ double positive immune reactivity to 27% with relevant plasma cell infiltration at the lesion site.
This dimension matches with the occurrence of oligoclonal bands in the CSF (B-cell clonal expansion) after acute ischemic brain injury providing cross-pathology verification in human.33 Emerging antibody-mediated reactivity after SCI, which detects epitopes present in the spinal cord and on the surface/membrane of living DRG cells, is in line with “epitope spreading” as an underlying mechanism contributing to antibody-mediated autoimmune diseases.25 Future studies are needed to identify specific cognate surface epitopes of neurons, synapses, and glia.34 In analogy to paraneoplastic syndromes,14 the emerging autoimmunity directed against spinal cord and DRG epitopes suggests the existence of functional relevant neuronal paratraumatic autoimmune syndromes.
Acknowledgment
The authors thank Gabriele Gaupmann for sharing her expertise in DRG cell cultures and Irene Erber and Susanne Schmid for excellent technical assistance. The authors thank Anastasia Zekeridou MD PhD (Mayo Clinic, Dept Neurology, Rochester, MN) for providing the alpha-GFAP plasmid to screen for anti-GFAP-antibodies. The work of J.M. Schwab is supported by the National Institutes of Neurologic Disorders-NIH (Grant R01 NS118200), the National Institute of Disability, Independent Living and Rehabilitation Research (NIDILRR Grant 90SI5020), the Craig H Neilsen Foundation (Grant 596,764), and the William E. Hunt and Charlotte M. Curtis endowment. J.M. Schwab is a Discovery Theme Initiative Scholar (Chronic Brain Injury) of the Ohio State University. P.G. Popovich is supported by the NIH (R01NS099532, R01NS083942, and R35NS111582 to P.G. Popovich) and the Ray W. Poppleton Endowment.
Glossary
- AIS
American Spinal Injury Association impairment scale
- BSB
blood-spinal cord barrier
- DAB
diaminobenzidine
- DRG
dorsal root ganglia
- GFAP
glial fibrillary acidic protein
- IgG/M/A
immunoglobulin G/M/A
- IHC
immunohistochemistry
- SCI
spinal cord injury
- TBA
tissue-based assay
- VF
vertebral fracture
Appendix. Authors
Study Funding
This work received funding by the European Union (EU Era Net–Neuron Program, SILENCE Grant 01 EW170A), the Austrian Science Funds (FWF, project I3334-B27, SILENCE; I4685-B, SYNABS; DOC 33-B27), and the Wings for Life Spinal Cord Research Foundation (Grants WfL-DE-006/12, WfL-DE-16/16, and WfL-DE-11/20) and the Else Kröner-Fresenius-Stiftung (EKFS 2012_A32).
Disclosure
The authors report no relevant disclosures. Go to Neurology.org/NN for full disclosures.
References
- 1.Cohen IR. Biomarkers, self-antigens and the immunological homunculus. J Autoimmun. 2007;29(4):246-249. [DOI] [PubMed] [Google Scholar]
- 2.Pruss H, Tedeschi A, Thiriot A, et al. Spinal cord injury-induced immunodeficiency is mediated by a sympathetic-neuroendocrine adrenal reflex. Nat Neurosci. 2017;20(11):1549-1559. [DOI] [PubMed] [Google Scholar]
- 3.Zhang Y, Guan Z, Reader B, et al. Autonomic dysreflexia causes chronic immune suppression after spinal cord injury. J Neurosci. 2013;33(32):12970-12981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sabatino JJ Jr, Probstel AK, Zamvil SS. B cells in autoimmune and neurodegenerative central nervous system diseases. Nat Rev Neurosci. 2019;20(12):728-745. [DOI] [PubMed] [Google Scholar]
- 5.Brimberg L, Mader S, Fujieda Y, et al. Antibodies as mediators of brain pathology. Trends Immunol. 2015;36(11):709-724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zajarias-Fainsod D, Carrillo-Ruiz J, Mestre H, Grijalva I, Madrazo I, Ibarra A. Autoreactivity against myelin basic protein in patients with chronic paraplegia. Eur Spine J. 2012;21(5):964-970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hayes KC, Hull TC, Delaney GA, et al. Elevated serum titers of proinflammatory cytokines and CNS autoantibodies in patients with chronic spinal cord injury. J Neurotrauma. 2002;19(6):753-761. [DOI] [PubMed] [Google Scholar]
- 8.Fleming JC, Norenberg MD, Ramsay DA, et al. The cellular inflammatory response in human spinal cords after injury. Brain. 2006;129(12):3249-3269. [DOI] [PubMed] [Google Scholar]
- 9.Ankeny DP, Guan Z, Popovich PG. B cells produce pathogenic antibodies and impair recovery after spinal cord injury in mice. J Clin Invest. 2009;119(10):2990-2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pruss H. Autoantibodies in neurological disease. Nat Rev Immunol. 2021;21(12):798-813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vincent A, Roberts M, Willison H, Lang B, Newsom-Davis J. Autoantibodies, neurotoxins and the nervous system. J Physiol Paris. 1995;89(3):129-136. [DOI] [PubMed] [Google Scholar]
- 12.Dalmau J, Geis C, Graus F. Autoantibodies to synaptic receptors and neuronal cell surface proteins in autoimmune diseases of the central nervous system. Physiol Rev. 2017;97(2):839-887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ricken G, Schwaiger C, De Simoni D, et al. Detection methods for autoantibodies in suspected autoimmune encephalitis. Front Neurol. 2018;9:841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoftberger R, Rosenfeld MR, Dalmau J. Update on neurological paraneoplastic syndromes. Curr Opin Oncol. 2015;27(6):489-495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kopp MA, Druschel C, Meisel C, et al. The SCIentinel study: prospective multicenter study to define the spinal cord injury-induced immune depression syndrome (SCI-IDS)--study protocol and interim feasibility data. BMC Neurol. 2013;13(1):168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kirshblum SC, Waring W, Biering-Sorensen F, et al. Reference for the 2011 revision of the international Standards for neurological classification of spinal cord injury. J Spinal Cord Med. 2011;34(6):547-554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dalmau J, Tuzun E, Wu HY, et al. Paraneoplastic anti-N-methyl-D-aspartate receptor encephalitis associated with ovarian teratoma. Ann Neurol. 2007;61(1):25-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Flanagan EP, Hinson SR, Lennon VA, et al. Glial fibrillary acidic protein immunoglobulin G as biomarker of autoimmune astrocytopathy: analysis of 102 patients. Ann Neurol. 2017;81(2):298-309. [DOI] [PubMed] [Google Scholar]
- 19.Zrzavy T, Schwaiger C, Wimmer I, et al. Acute and non-resolving inflammation associate with oxidative injury after human spinal cord injury. Brain. 2021;144(1):144-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bauer J, Lassmann H. Neuropathological techniques to investigate central nervous system sections in multiple sclerosis. Methods Mol Biol. 2016;1304:211-229. [DOI] [PubMed] [Google Scholar]
- 21.Bien CG, Vincent A, Barnett MH, et al. Immunopathology of autoantibody-associated encephalitides: clues for pathogenesis. Brain. 2012;135(5):1622-1638. [DOI] [PubMed] [Google Scholar]
- 22.Reiber H, Lange P. Quantification of virus-specific antibodies in cerebrospinal fluid and serum: sensitive and specific detection of antibody synthesis in brain. Clin Chem. 1991. Jul;37(7):1153-60. [PubMed] [Google Scholar]
- 23.Reiber H, Ungefehr S, Jacobi C. The intrathecal, polyspecific and oligoclonal immune response in multiple sclerosis. Mult Scler. 1998;4(3):111-117. [DOI] [PubMed] [Google Scholar]
- 24.Kleiser R, Rademacher GU, Niedeggen A, Muller HW, Seitz RJ. Assessment of gadolinium leakage into traumatic spinal cord lesion using magnet resonance imaging. Spine (Phila Pa 1976). 2010;35(26):E1604-E1609. [DOI] [PubMed] [Google Scholar]
- 25.Vanderlugt CJ, Miller SD. Epitope spreading. Curr Opin Immunol. 1996;8(6):831-836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Formento E, Minassian K, Wagner F, et al. Electrical spinal cord stimulation must preserve proprioception to enable locomotion in humans with spinal cord injury. Nat Neurosci. 2018;21(12):1728-1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu Y, Latremoliere A, Li X, et al. Touch and tactile neuropathic pain sensitivity are set by corticospinal projections. Nature. 2018;561(7724):547-550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hergenroeder GW, Moore AN, Schmitt KM, Redell JB, Dash PK. Identification of autoantibodies to glial fibrillary acidic protein in spinal cord injury patients. Neuroreport. 2016;27(2):90-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gobel K, Pankratz S, Asaridou CM, et al. Blood coagulation factor XII drives adaptive immunity during neuroinflammation via CD87-mediated modulation of dendritic cells. Nat Commun. 2016;7(1):11626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Needham EJ, Stoevesandt O, Thelin EP, et al. Complex autoantibody responses occur following moderate to severe traumatic brain injury. J Immunol. 2021;207(1):90-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Previnaire JG, Soler JM, El Masri W, Denys P. Assessment of the sympathetic level of lesion in patients with spinal cord injury. Spinal Cord. 2009;47(2):122-127. [DOI] [PubMed] [Google Scholar]
- 32.Oropallo MA, Held KS, Goenka R, et al. Chronic spinal cord injury impairs primary antibody responses but spares existing humoral immunity in mice. J Immunol. 2012;188(11):5257-5266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pruss H, Iggena D, Baldinger T, et al. Evidence of intrathecal immunoglobulin synthesis in stroke: a cohort study. Arch Neurol. 2012;69(6):714-717. [DOI] [PubMed] [Google Scholar]
- 34.Palmers I, Ydens E, Put E, et al. Antibody profiling identifies novel antigenic targets in spinal cord injury patients. J Neuroinflammation. 2016;13(1):243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ackery AD, Norenberg MD, Krassioukov A. Calcitonin gene-related peptide immunoreactivity in chronic human spinal cord injury. Spinal Cord. 2007;45(10):678-686. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Individual-level data collected in the cohort study associated with the SCIentinel study cannot be fully shared for data protection reasons. However, dataset extracts of deidentified patient data from the analysis dataset in connection with a data dictionary can be made available on written request and signature of a data transfer agreement by the corresponding authors with the publication of the article. For the neuropathologic study, in addition to the clinical information already published, the individual IHC findings can be provided in a tabular format on request to the corresponding authors. The statistical syntax code (SPSS) used to analyze the study data can be made available on written request to the corresponding authors with publication of the article.