Abstract
The lithium (Li) metal anode (LMA) is susceptible to failure due to the growth of Li dendrites caused by an unsatisfied solid electrolyte interface (SEI). With this regard, the design of artificial SEIs with improved physicochemical and mechanical properties has been demonstrated to be important to stabilize the LMAs. This review comprehensively summarizes current efficient strategies and key progresses in surface engineering for constructing protective layers to serve as the artificial SEIs, including pretreating the LMAs with the reagents situated in different primary states of matter (solid, liquid, and gas) or using some peculiar pathways (plasma, for example). The fundamental characterization tools for studying the protective layers on the LMAs are also briefly introduced. Last, strategic guidance for the deliberate design of surface engineering is provided, and the current challenges, opportunities, and possible future directions of these strategies for the development of LMAs in practical applications are discussed.
Current surface engineering strategies to construct desirable protective layers for stable Li metal anodes are summarized.
INTRODUCTION
Modern society depends highly on high-performance electrochemical energy storage systems, battery for example, for portable electronic devices and the successful transition to renewable energy sources and electrified transportation (1, 2). Li metal has been regarded as the most promising battery anode material owing to its ultrahigh theoretical specific capacity (3860 mAh g−1), light weight (6.94 g mol−1), and the lowest redox potential (−3.04 V versus standard hydrogen electrode) (3). Despite these unique advantages, the practical application of lithium metal batteries (LMBs) is still severely hindered by Li dendrite growth, resulting in poor cycling performance and safety concerns (4).
Although various theories have been developed to unravel the mechanism of Li dendrite growth, including the nucleation and diffusion model, space-charge model, and stress-driven model (1, 5), it is recognized that Li dendrites are generally induced by an unsatisfied solid electrolyte interface (SEI) (6, 7). Because of the intrinsic ultrahigh chemical reactivity and thermodynamic instability of metallic Li, a passivation SEI layer is produced between Li metal and the electrolyte via spontaneous irreversible reactions (8). Nevertheless, the electrolyte-derived SEI is nonuniform and fragile, which induces inhomogeneous ion diffusion and the Li dendrite growth. The proliferating dendrite growth would result in continuous SEI breakage/reformation under the tremendous volume change of the Li metal anodes (LMAs) during the cycling, leading to the increase of interfacial Li+ ion diffusion resistance and higher voltage polarization (9, 10). In addition, because of the preferential dissolution at the dendrite base and the potential separation of Li dendrites from the current collector, the generation of residual inactive Li (“dead Li”) markedly decreases the coulombic efficiency (CE), resulting in unsatisfactory cycling performance and eventual cell failure (11, 12). Therefore, a stable interface between the Li metal and the electrolyte is perceived as the primary premise for a working LMB.
Precise control over the composition and properties of the SEI is desired to overcome the chemical and mechanical instabilities at the electrode/electrolyte interface, thereby achieving stable and high-performance LMAs (13, 14). A rationally designed artificial SEI positioned at the Li-electrolyte interface is thought to be a promising and efficient approach due to its high feasibility and simplicity in processing. Ideally, artificial SEIs should have the following desirable characteristics (15, 16): (i) superior chemical and electrochemical stability with electronic insulation to impede continuous electrolyte decomposition and Li consumption; (ii) high mechanical modulus and flexibility to suppress the dendrite growth and restrain the volume change during the repeated cycles; and (iii) high ionic conductivity and ability to allow homogeneous Li+ ion transport across the layer. In regard to the application of solid-state electrolyte (SSE) for LMAs, the design of an artificial SEI with more softness and stickiness to achieve intimate contact between the electrode and the SSE is an imperative criterion. Therefore, actively influencing and managing these features are critical for the development of advanced LMAs (17). Investigating the formation and manipulation of so-called artificial SEIs has been recognized as a rising research trend (18, 19). The development of such a functional layer requires an in-depth understanding of the relationship between its structure (morphology and composition) and its basic function in a battery cell (20). Rationally building an artificial SEI, including organic, inorganic, or hybrid one, is attracting more attention, giving rise to an opportunity to avoid continuous electrolyte decomposition and Li consumption to obtain a safe and highly stable LMA.
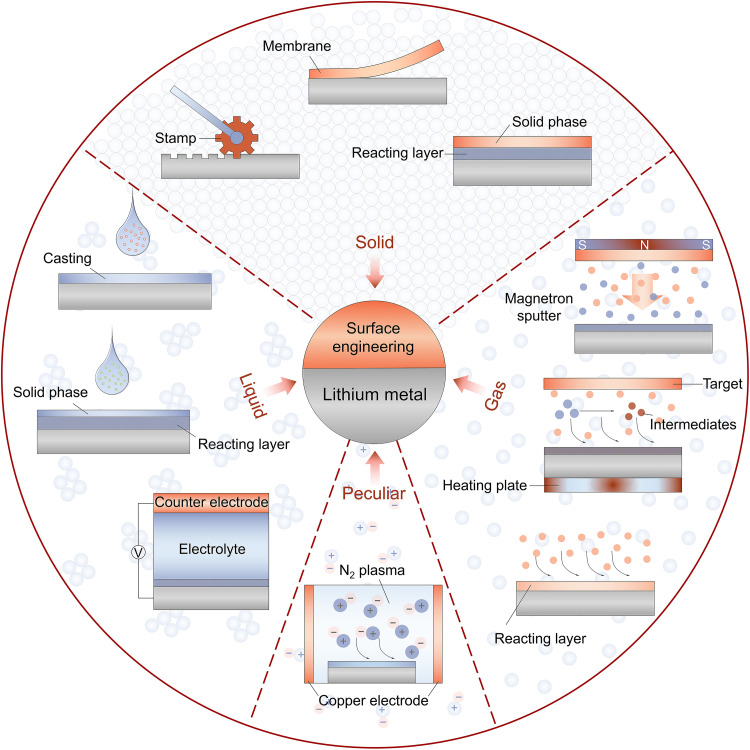
Surface and interface engineering has been found to play a key role in improving the electrochemical performance of LMAs by its strong capability of constructing various functional artificial SEIs (16, 21–26). As the physical/chemical properties of these artificial SEIs are essentially determined by the process of surface engineering, the states of matter (solid, liquid, and gas) of the reagents used for surface engineering deserve to be carefully considered. This is because different reagents will inevitably lead to variations in the structure and physicochemical properties of the formed artificial SEIs. For example, a solid reagent has a rigid structure and a stable, distinct shape that can only be altered through external force. These properties lead to poor interfacial contact between the solid reagents and the Li metal unless a spontaneous chemical reaction could occur on their interface. As compared to the solid, the molecules in the liquid reagents have a certain capacity to move around, which allows them a more intimate contact with the Li metal and diverse options for the SEI’s structures. As opposed to the solids and liquids, the atoms/molecules in the gas reagents have much higher kinetic energy due to their higher dispersity and smaller intermolecular force, endowing them with an improved chemical reaction kinetic with the Li metal.
This review summarizes recent efforts aimed at implementing surface engineering that involves pretreatment protocols before the stage of battery assembly on the LMAs to address the structural and electrochemical requirements for high-performance artificial SEIs. The surface engineering techniques are mainly categorized by the states of matter (solid, liquid, and gas) of the reagents used to pretreat the LMAs (Fig. 1). The category of using some peculiar pathways (plasma for example) is also included. Moreover, we will discuss the fundamental characterization techniques for elucidating the formation mechanism and physicochemical properties of these as-formed artificial SEIs. Last, potential future research directions regarding the rational design of surface engineering and advanced characterization tools for LMAs will be outlooked.
Fig. 1. Schematic illustration of the current surface engineering strategies for stabilizing LMAs.
SURFACE PRETEATMENT STRATEGIES
Solid-phase pathways
Since the SEI is made up of solids, the solid-phase approach is the simplest and most direct strategy to pretreat the surface of the LMAs, where researchers commonly just replace or enhance the naturally formed SEI by another predesigned solid. In this regard, three representative surface pretreatment processes are introduced as follows.
Mechanical processing
Mechanical processing is a simple, processable, and cost-effective process to realize dendrite-free LMAs (27, 28). The highly ductile Li metal can be easily molded or deformed to form surface patterns by large mechanical strains. The patterned surface could decrease the current density over the Li metal surface and diminish Li dendrite formation during cell operation by expanding the surface area of Li metal.
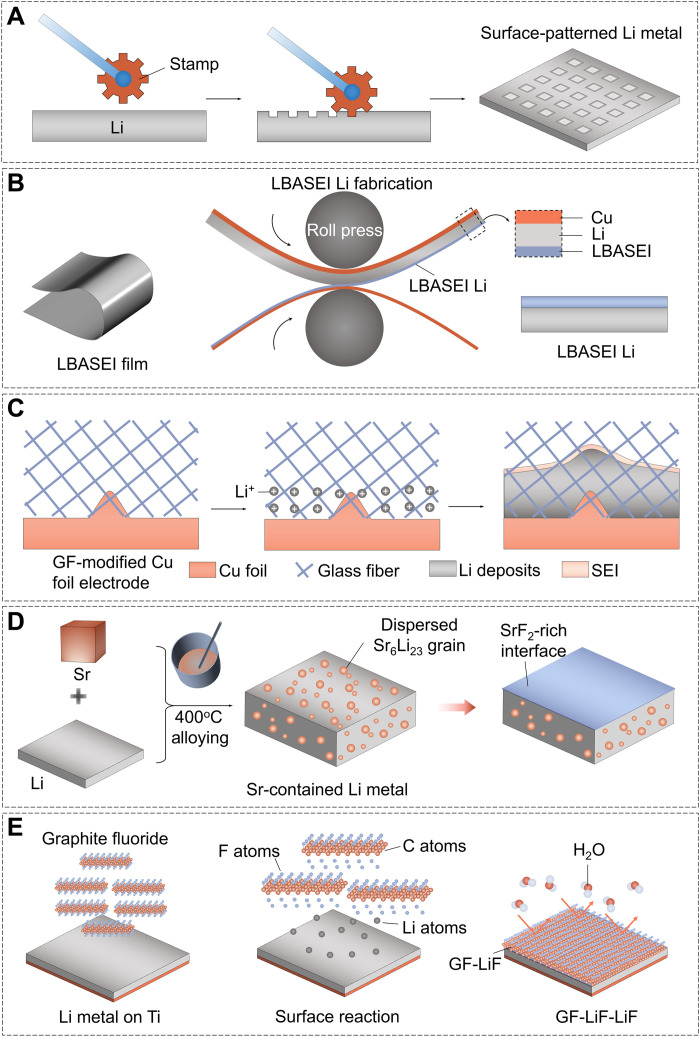
Stamp is an effective mechanical processing technique to construct surface patterns. In 2014, Ryou et al. (29) pioneered the use of skin needling for surface roughening to construct a large–surface area LMA with an abundance of Li plating sites. The microneedled Li metal with lower charge transfer resistance provided better electrochemical performance than bare Li. Motivated by the simplicity and effectiveness of this technique, Park et al. (30) demonstrated the possibility of employing a surface-patterned Li metal (spLi) pretreated with a commercially available microneedle roller in secondary batteries (Fig. 2A). They systematically verified the relationship between the pattern dimension and Li deposition behavior with the help of finite element analysis and postmortem analysis. After the stamping process, the spLi could be reversibly plated and stripped without Li dendrite growth in the surface-patterned holes. Creating a microsized array for guiding the Li plating/stripping behavior by micropattern stamping technique is another approach for stabilizing LMAs (31). In addition, a three-dimensional (3D) stainless-steel mesh and copper net–modified Li metal interlayer was used to stabilize LMAs (32–34).
Fig. 2. Schematic illustration of surface pretreatment using solid-phase pathways.
(A) The stamp modified process on Li metal. (B) Fabrication of the LBASEI Li via a roll-press process. (C) The glass fiber cloth is directly coated on copper foil to render the dendrite-free Li deposits. (D) The schematic diagram for the fabrication of the Li-Sr anode via a high-temperature alloying process. (E) Schematic illustration of GF-LiF-Li preparation and its protective effect for LMAs.
The mechanical processing approach is viable because it is economical, reproducible, and applicable to mass production lines. Nonetheless, there are two limitations: (i) The capacity for Li plating without the formation of Li dendrites is highly depend on the size of the holes. When the deposited Li exceeds the amount that can accommodated in the holes, Li metal will grow out of the holes and become dendrites. (ii) Without a preset protecting layer on Li metal, the microneedled Li will convert into 2D Li foil and fail to suppress dendritic Li growth during a long cycling test.
Membrane modification
Apart from the direct physical treatment of the Li metal, applying as-prepared free-standing films as the artificial SEIs would be an interesting approach to enhance the electrochemical performance of LMAs. The as-prepared film composed of inorganic components exhibits excellent chemical stability against Li metal and physically suppresses dendrite formation. Carbon-based materials are frequently adopted as such inorganic materials. For example, Zheng et al. (18) first reported a monolayer of interconnected amorphous hollow carbon nanospheres as an artificial SEI to isolate Li metal from the electrolyte. In addition, Kim et al. (35) used phosphate-functionalized reduced graphene oxides (rGO) to create Langmuir-Blodgett artificial SEIs (LBASEIs) on the surface of LMAs (Fig. 2B). The effectiveness of the LBASEI is primarily from its ability to regulate the electromigration. As a result, a LBASEI-modified 20-μm LMA paired with the Li[Ni0.8Co0.1Mn0.1]O2 (NCM811) cathode (negative/positive capacity ratio of around 1) was used to construct a full cell with superior cycling performance of over 200 cycles. Moreover, Li et al. (36) proposed a self-assembled rGO layer on LMA to achieve planar Li via in-plane lattice matching between the Li metal and the rGO.
3D structured SEIs have been used to alleviate the volume change and minimize the damage to the SEI during cycling. For instance, Cheng et al. (37) demonstrated an effective strategy to realize a dendrite-free LMA by using 3D glass fibers with large quantities of polar functional groups as the artificial SEIs (Fig. 2C). The polar functional groups of the glass fibers can offer strong interaction with Li+ ions, which induced a homogeneous Li+ ion flux and prevent the accumulation of Li+ ions around protuberances of Cu foil. Moreover, Zhai et al. (38) presented the design of a 3D architecture constructed by g-C3N4/graphene/g-C3N4 sandwiched nanosheets to guide homogeneous Li deposition. Its uniform lithiophilic sites and nanopore channels enable uniform Li plating between the graphene and g-C3N4, prohibiting direct contact of the electrolyte with the Li metal. In addition, diverse inorganic protective layers, such as Cu3P (39), carbon nanotube (CNT) film (40), graphene (41), Li3OCl (42), aluminum silicate (43), Mo6S8/C@Li (44), and α-Si3N4 (45), have been proposed to directly coated on the LMAs to solve the notorious surface problem.
Organic coating layers with superior mechanical deformability and low density are promising candidates to stabilize LMAs. For example, a modified polydimethylsiloxane (PDMS) film with nanopores has been used to improve the cycling stability of LMAs (46). The nanopores of the PDMS film show efficient Li+ ion transport, mechanical and chemical stability, and compatibility with different electrolytes. Moreover, a functional soft polymer with highly viscoelastic properties in the coating layer imparts a homogenizing effect on the Li+ ion flux to prevent rapid dendrite formation at hotspots in the SEI during cycling (47). Because of the trade-off between the Young’s modulus and toughness of a traditional polymer, the fixed cross-linking networks may break during stretching. To solve this problem, a tough polymer with a slide-ring structure as a self-adaptive layer was introduced on the LMAs (48). The slide-ring polymer with a dynamically cross-linked network moves freely while maintaining its toughness and fracture resistance, which allows it to dissipate the tension induced by the growth of the Li dendrites. Other reported organic protective layers, such as N-doped polyacrylonitrile fiber (49), nanoporous polyether sulfone (50), and clay/cross-linked network polymer (51), all have high ionic conductivity, resulting in a dendrite-free and stable LMA.
Mimicking the “early warning” defense responses in biological immunization mechanisms, Wang et al. (52) proposed that the fibroin molecules can work as an artificial SEI to prevent Li dendrite growth. The protein molecules are preferably adsorbed on the tips of Li buds through spatial conformation and secondary structure transformation from α-helix to β-sheets. This affects the local electric field intensity around the tips of Li buds and results in uniform Li plating and stripping during cycling. Moreover, biomineralization is a widespread phenomenon in nature leading to the formation of hierarchically structured minerals by living organisms (53). Inspired by the calcified crystallization on the eggshell membrane, Ju et al. (54) introduced a biomacromolecule matrix obtained from the natural membrane of eggshell to realize a dendrite-free LMA, and the regulatory mechanism was revealed by atomic-resolution cryo–transmission electron microscopy (cryo-TEM). The naturally soluble chemical species in the biomacromolecules was found to be able to participate in the formation of the SEI upon cycling, thus effectively enhancing the electrochemical performance.
Organic-inorganic composite layers with associated strengths from the organic and inorganic components have been presented to simultaneously provide fast Li+ ion diffusion, high modulus, and good shape conformability (55). For example, a poly(vinylidene-co-hexafluoropropylene) (PVDF-HFP) organic matrix with rigid LiF particles has been hybridized into a film, which serves as an artificial SEI on LMAs (56). To construct a protective layer with an ionic shielding property, Kim et al. (57) fabricated Li-terminated sulfonated titania–coated Li (LTST-Li) via direct transfer to Li using a roll-press machine. As a result, the LTST-Li anodes have high conductivity at the interface and high physical and chemical stability to shield the Li metal, which can stabilize electrochemical processes at both the anode and cathode of Li-S cells.
Note that the thickness of the as-prepared membrane is normally greater than a few micrometers, implying that the total volume energy density of the LMA would be sacrificed. In addition, the as-prepared membrane added onto the surface of Li metal can hardly manage the homogeneity of the SEI during the cycling, which may result in high electrochemical polarization.
Chemical reaction with solids
Designing a chemical reaction between metallic Li and solids has been reported to be an effective method to build an artificial SEI with high ionic conductivity and high mechanical property. Li-rich alloys have high Li+ ion diffusion coefficients and have proven to be beneficial in improving Li+ ion diffusion at the electrode/electrolyte interface (58, 59). For instance, a Li and Li-Sn alloy (Li22Sn5) integrated 3D networks have been in situ formed through the spontaneous reaction between periodically stacked nanolayers of Li and Sn (60). Such a 3D nanostructured Li22Sn5 network enables ultrafast charger diffusion across the entire electrode and inhibits the volume expansion during Li stripping/plating processes. A Li-Sr alloy electrode with a designed composition of 11 weight % (wt %) Sr was synthesized by heating and stirring Li and Sr metals at 400°C in a glovebox (Fig. 2D) (61). When the battery is cycling, a SrF2-rich SEI can be generated in fluoride-rich electrolytes. The SrF2-rich SEI enables outstanding cycling performance and improved CE of the Li-Sr anode in the half cell at an ultrahigh current density of 30 mA cm−2.
In addition to this alloy-derived SEI, some researchers have focused on constructing LiF-based protective layers on LMAs via a direct reaction between inorganic nonmetallic materials and Li metal, which enables a high mechanical modulus and high ionic conductivity (62). For example, a modified Li metal is fabricated by incorporating graphite fluoride (GF) in molten Li at 250°C (Fig. 2E) (63). The Li bonds strongly with the GF to form a LiF layer. The obtained GF-LiF-Li composite can sustain its initial structure in a humid air. In addition, this composite can prevent the parasitic reaction between Li metal and organic solvents owing to its hydrophobicity. As a result, the GF-LiF-Li can exhibit comparable electrochemical performance to bare LMA even after exposure to a moist atmosphere with a relative humidity of 20 to 35% for over 24 hours. In addition, a multifunctional complementary LiF-rich protective layer was formed on an LMA through in situ reaction of metallic Li with poly(tetrafluoroethylene) (64, 65). This gradient protective layer integrates the homogenizing function of the F-doped carbon for Li+ ion flux and the fast Li+ ion diffusion ability of LiF. Furthermore, a fluorinated protective layer with high ionic conductivity has been constructed in situ through a solid-state anodic oxidation method. The as-prepared F-rich protective film efficiently suppresses dendrite growth and promotes the electrochemical performance of the LMB in a commercial ester electrolyte (66).
In addition to LiF, Li3N and Li3P components have been demonstrated to suppress the formation of Li dendrites due to their high ionic conductivity and high mechanical modulus. For example, Ye et al. (67) reported that in a simple hyperthermal reduction process, g-C3N4 powder can react with molten Li to form a composite N-organic/Li3N artificial SEI on the surface of Li metal. Lee et al. (68) reported that Cu3N nanowires (NWs) was conformally printed onto the surface of Li metal through a one-step roll pressing to form a Li3N@Cu NW layer. The Li3N@Cu NW layer can assist homogeneous Li+ ion flux with the 3D channel structure and a high Li+ ion conductivity from the Li3N, which enhances the reversible Li plating/stripping behavior. In addition, an interconnected Li3P@Cu layer activated by the interfacial reaction between Cu3P arrays and Li metal has been efficiently constructed on the surface of Li foil through a room-temperature mechanical rolling process (69). It is demonstrated that Li3P could reduce the diffusion barrier of Li+ ions to ensure a well-dispersed current density.
Liquid-phase pathways
Taking advantage of the fluidity of liquids and its ability to dissolve chemicals or disperse the colloid materials, a uniform and component-tunable artificial SEI could be constructed on the surface of the LMAs via the liquid-phase strategy (22, 70). Therefore, direct contact between the LMA and electrolyte after the battery assembly could be restricted by this preformed artificial SEI, leading to a decreased consumption of the electrolyte and Li metal, preventing heterogeneous Li deposition, and suppressing the dendrite formation (24).
Solution casting
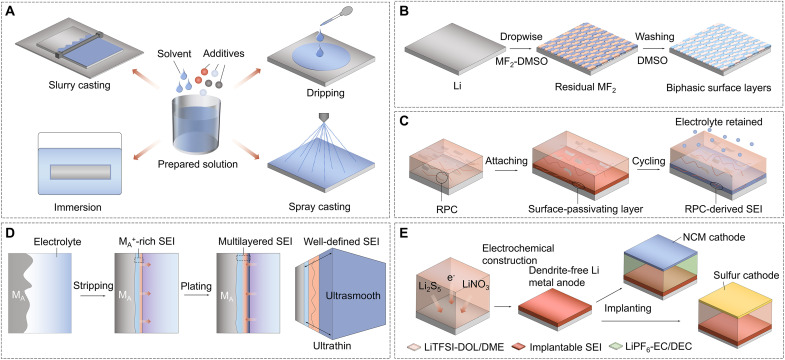
The solution-casting method, including immersion, drop coating, doctor blading, and spin coating, has been developed as a facile and reproducible way to create functional barrier layers to avoid the drawbacks of electrolyte-derived SEIs (Fig. 3A). Many of the presynthesized materials, including organic, inorganic, and the organic-inorganic composite, could be easily coated on the LMAs without any chemical reaction, efficiently enhancing the mechanical strength of the SEI to block Li dendrites and enabling homogeneous Li+ ion flux across the coating layer with the controllable interfacial resistance.
Fig. 3. Schematic illustration of surface pretreatment using liquid-phase pathways.
(A) Surface processing strategies via various solution casting methods, including slurry casting, immersion, dripping, and spray casting. (B) High-polarity DMSO was selected to dissolve sufficient metal fluorides (e.g., SnF2, InF3, and ZnF2), which is crucial to forming the uniform and robust BSLs on Li metal. (C) Design of a polymer-inorganic SEI using the RPC precursor rather than the electrolyte to trigger a chemical reaction with Li. (D) Potentiostatic stripping and galvanostatic plating for polishing of and formation of SEI on metal anode (MA) surface. (E) Ex situ SEI construction on the Li plate by electrochemical methods in 1.0 M LiTFSI-DOL/DME electrolyte with 0.02 M Li2S5–5.0 wt % LiNO3 hybrid additives.
Organic coating layers with superior mechanical deformability and low density are promising candidates to stabilize LMAs (71). In 1993, Takehara (72) pioneered the construction of a stable thin polymer film on the surface of Li metal by plasma-triggered polymerization of a liquid mixture. Thereafter, many researchers developed the polymer protective film on the Li surface via in situ polymerization, triggered polymerization, or direct coating. Recently, Zhao et al. (73) have spin-coated a mechanically interlocked network (MIN) on the surface of LMA under the argon atmosphere, followed by an ultraviolet-assisted polymerization to fabricate a MIN interfacial layer. The unique energy dissipation capability of the MIN layer could help the LMA survive from repeated volume variation during long-term cycling. In addition, Gao et al. (74) proposed a skin-grafting strategy that stabilizes the SEI and regulates the Li deposition/dissolution behavior by direct coating a chemically and electrochemically active polymer layer on the surface of Li metal. This layer contains cyclic ether groups with a stiff polycyclic main chain, incorporating ether-based polymeric components into the durable SEI. Furthermore, a polycationic and hydrophobic polymer protective layer built by a scalable tape-casting method has been developed to enable dendrite-free LMAs (75). The polymeric cations of poly(diallyl dimethyl ammonium) and bis(trifluoromethanesulfonyl)imide (TFSI) anions could provide a uniform Li+ ion flux at the coated LMA and a hydrophobic property, which improves homogeneous Li plating/stripping and moisture stability under air condition. In addition, Yang et al. (76) proposed a fluid fluorinated coating layer to stabilize LMAs and regulate Li plating by dripping a perfluoropolyether oil drop onto the surface of Li metal. The obtained SEI is reinforced with rich C-F and Li-F components, leading to homogeneous Li deposition and compact interconnected network morphology. Moreover, several stable PVDF and cross-linked PVDF-HFP films have also been formed on the Li metal surface via a simple casting method for prominent cycling performance (77, 78).
Carbonaceous materials including CNTs, graphene, and its derivatives are promising inorganic materials that can meet the requirements for artificial SEI due to their superior mechanical properties (79, 80). In addition, the mechanical and electronic properties of graphene can be selectively tuned or modified via proper functionalization and introduction of structural defects. For instance, GO layers have been coated onto LMAs to suppress dendrite formation and enhance cycling stability through a drop-casting process (81). Furthermore, 2D rGO layers have been coated on Li metal by a solvent evaporation-assisted self-assembly method (79). “Defect-free” graphene has also been synthesized via a special flow-aided sonication exfoliation method, allowing direct comparison of Li deposition behavior and electrochemical performance with those of common rGO (80). Moreover, a promising gradient layer with a lithiophobic CNT layer at the top and a lithiophilic ZnO-coated CNT layer at the bottom has been constructed on the surface of Li metal (82). The lithiophilic-lithiophobic gradient layer can stabilize the SEI and successfully suppress dendrite growth during Li plating/stripping.
Many researchers have developed special strategies to protect LMAs by a composite protective layer, which is composed of inorganic nanoparticles and a plastic polymer, effectively improving the SEI stability and inhibiting Li dendrite growth. An Al2O3/PVDF-HFP composite layer has been used as an artificial SEI because of its high mechanical properties and fast Li+ ion transport, resulting in a notable enhancement in the electrochemical performance of LMAs (83). A graphene-polydopamine composite has been introduced as a stable protective layer onto LMAs to inhibit Li dendrite growth and prevent parasitic reactions between Li metal and electrolyte (84). Inspired by the phenomenon that membrane proteins can selectively transport alkali ions across cell membranes, the building of biomimetic protective membranes with glutamate-like ionic channels for LMAs is expected to deliver an improved performance. A stable artificial SEI has been rationally designed and coated on the surface of Li metal by applying the ClO4−-decorated metal-organic framework (MOF) via drop-casting (85). The UIO-66-ClO4/Li-Nafion composite layer exhibits excellent mechanical strength, high Li+ ion mobility, and uniform Li+ ion flux, which can successfully restrain unnecessary reactions of the Li metal with the electrolyte and formation of fragile SEIs. Fan et al. (86) reported a similar work on polyvinyl alcohol cementing a Zn-MOF as a stable protective layer for homogeneous Li+ ion transport, suppressing Li dendrite growth, and reducing the volume change. Furthermore, a robust SEI with high Li+ ion transport efficiency and high stability has been rationally constructed by drop casting of 2D anionic covalent organic frameworks (ACOFs) (87). The ACOF layer served as an SEI offers strong affinity and fast translocation pathways for Li+ ions, thereby supporting ionically selective penetration into the liquid electrolyte and giving high conductivity of at least 3.7 mS cm−1.
Chemical reaction with liquids
Owing to the high reactivity of metallic Li, redox reactions can occur between Li and the liquid reagents, in situ generating an artificial SEI with much more intimate contact to the LMAs. Therefore, the method of chemical reaction with liquids offers the possibility of fine control of the composition and realization of gradient structure of the artificial SEIs according to the requirements.
The organic layers have a flexible nature, so they can mechanically accommodate the volume changes of LMA to a large extent (88). Some researchers reported an extremely simple method of constructing a protective layer on a Li metal surface via chemical reactions between Li metal and 1,4-dioxacyclohexane or 1,3-dioxolane (DOL) (89, 90). Moreover, to build a stable protective layer, researchers have immersed Li metal in fluoroethylene carbonate solvent to generate a passivation layer via spontaneous decomposition of the solvent (91, 92). The obtained artificial SEIs can protect the LMA from the corrosion of electrolytes and regulate the homogeneous plating of Li to achieve a dendrite-free LMA. Many other polymer layers can be formed on LMAs via in situ chemical reactions with metallic Li using polymers such as polyphosphoric ester, polyacrylic acid, polylactic acid and poly(ethylene oxide) (PEO) (93–95). To achieve long-term Li plating/stripping cycling at high current densities and high areal capacity, Wang et al. (95) designed a self-healable supramolecular copolymer (termed PEO-UPy) coating layer for providing diffusion pathways for Li+ ion transport, enabling uniform Li deposition during cycling. As a result, the LiPEO-UPy–coated LMA endows dendrite-free Li deposition at an ultrahigh current density of 20 mA cm−2 over 4000 cycles in symmetrical cells.
Commonly, inorganic protective layers have relatively high Li+ ions conductivity and Young’s modulus, which could physically suppress dendrite growth (96). For instance, Bai et al. (97) reported a simple and universal process to develop spontaneously reduced graphene (SR-G) directly coated on alkali metals (e.g., Li, Na, and K) under moderate conditions. The symmetric cell of SR-G–coated Li displays an ultralong life span of over 1000 cycles at a high current density of 5 mA cm−2 in carbonate electrolyte. Apart from the carbonaceous materials, lithium-based inorganic compounds with high ionic conductivity have been proven to be promising protective layers to suppress the growth of dendritic Li. For instance, Li2S has outstanding chemical stability against Li and the lowest formation energy among lithium polysulfides. Therefore, a high-quality SEI layer containing inorganic Li2S nanoparticles was constructed on LMAs via a simple reaction between Li and CS2 solution with 5 wt % S dissolved (98). Moreover, a thin Li3PS4 layer was fabricated by an in situ reaction between P4S16 and Li in “N-methyl-2-pyrrolidone solvent (99). Recently, a Li3PO4-Li3N hybrid layer was also constructed on LMAs via a LiNO3/H3PO4 pretreatment process (100).
In 2017, Liang et al. (101) reported that a series of Li-rich composite alloy films (such as Li13In3, LiZn, Li3Bi, and Li3As) can be synthesized by reacting Li with lithium-based compounds. These alloys can potently prevent the Li dendrite growth, which is attributed to the synergy of fast Li+ ion migration through Li-rich ion conductive alloys coupled with an electronically insulating surface component. Furthermore, more Li-rich alloy layers have been conducted on the surface of Li metal (102–104). For example, a polymer/alloy hybrid protective layer, consisting of polymer and LiF-rich Li-Sb alloy, was formed by an in situ chemical reaction process (103). The Li3Sb-LiF hybrid layer displays a superionic conductivity and electron-blocking capability to reduce the electron tunneling from the LMA into the SEI. A stable biphasic surface layer (BSL) was developed by a simple pretreatment reaction between Li metal and metal fluoride dissolved in the dimethylsulfoxide (DMSO) solution (Fig. 3B) (104). The obtained BSL is composed of lithiophilic alloy and LiF phase on Li metal, which prohibits the shuttle effect and improves the cycling stability of Li-S batteries, enabling fast Li+ ion transport and suppressing dendrite growth. Recently, gallium-based liquid metal (LM) alloys (such as Ga and GaInSn) have attracted some attention for the similar functionality as the alloys mentioned above. Moreover, these Ga-based LM alloys have unique properties beyond the solid ones, including good fluidity, low melting point, nontoxicity, high surface tension, and high electrical/thermal conductivities (105, 106).
To overcome some disadvantages arising from the single organic or inorganic layer, the strategy of building organic-inorganic composite layers has been proposed to take advantage of the merits of both components via spontaneous chemical reactions between the LMAs and liquids (107). A composite containing multiple components of polymer-tethered organo(poly)sulfide, inorganic Li3PSx, Li sulfides, and Li salts was designed as a multifunctional artificial SEI on the surface of Li metal (108). A stable hybrid SEI, composed of Si-interlinked OOCOR molecules and LiCl salt, was conducted on the LMA by in situ synthesis that uses readily accessible SiCl4 cross-linking chemistry (109). In particular, Gao et al. (110) described a molecule-level SEI design based on a reactive polymer composite (RPC), which can effectively reduce the electrolyte decomposition during SEI creation and maintenance (Fig. 3C). Combined cryo-TEM, atomic force microscopy (AFM), and surface-sensitive spectroscopy investigations revealed that the SEI layer is composed of polymeric Li salt, LiF nanoparticles, and GO sheets. The compact RPC-derived SEI exhibits excellent passivation, uniformity, and mechanical strength. Under lean electrolyte, limited Li excess, and high-capacity conditions, the polymer-inorganic SEI permits dendrite-free Li deposition with excellent efficiency and stable cycling of 4-V Li/NCM523 cells.
Electrochemical treatment
The electrochemical treatment process has been proposed to fabricate ideal SEIs under a dedicatedly designed electrochemical environment with specific parameters (including electrolyte formulation, voltage, operating temperature, etc.) (16, 111). As a result, these electrochemically constructed SEIs have more sophisticated components and structures as compared to single- (or dual-)component protective coatings synthesized by direct coating or regular chemical pretreatment.
Some recent work by Mao’s group (112–114) have shown that unique SEIs with multilayered structure could be developed and executed on the alkali metal surface by using electrochemical approaches. To be specific, ultrasmooth and ultrathin (USUT) SEI layers for Li and other alkali metals were designed on the basis of the manipulation of electrochemical polishing and follow-up electrolyte reduction (Fig. 3D) (112). The resulting SEI layer could be carefully regulated to manifest alternating inorganic-rich and organic-rich/mixed multilayered structures. The USUT SEI layer on polished LMA, which offers mechanical properties of linked stiffness and elasticity, improved the cycling stability to over 200 cycles at a current density of 2 mA cm−2. Furthermore, a variety of single-layered and multilayered SEIs with known compositions and structures can also be fabricated by using the similar electrochemical method (113). The applicability of the method was further demonstrated by using Cu foil as the current collector, and the mechanical properties of these SEIs can be evaluated using AFM nanoindentation. Recently, this research group developed a potentiostatic stripping-galvanostatic plating method to create stable LiNO3-derived multilayer-structured SEI film that successfully suppresses NO2 dissolution, Li dendrite formation, and O2 corrosion by encapsulating soluble NO2 species in the inner area of the SEI (114).
In addition to the electrochemical polishing procedure, in situ electrochemical precharging and electrodeposition techniques have been conducted to build a robust interlayer to safeguard LMAs. For example, an electrochemically activated Li surface with a homogeneous distribution of small pits, which act as preferential nucleation sites during Li plating, can be obtained by including a short oxidative potentiostatic pulse before the first galvanostatic stripping step in the first cycle (115). Under an inert atmosphere, the simple one-step in situ electrochemical precharging technique has been proven to create thin protective films on LMAs and other-type of electrodes (116). Moreover, Hou et al. (117) have proposed an effective strategy to stabilize Li metal by constructing an ex situ SEI film via an electroplating method involving precycling the LMA in an advanced electrolyte. The LiF-Li3N–enriched SEI is expected to be an electronically insulating film with satisfactory ionic conductivity, which improves the interfacial compatibility and suppresses Li dendrite formation during cell cycling. Gao et al. (118) described a nanocomposite layer composed of organic elastomeric salts [LiO-(CH2O)n-Li] and inorganic nanoparticle salts (LiF, -NSO2-Li, and Li2O) that protects the Li10GeP2S12 (LGPS) SSE and Li metal. Electrochemical decomposition of the liquid electrolyte produces the as-formed nanocomposite layer on Li, which has excellent chemical and electrochemical stability, an affinity for Li and LGPS, and low interfacial resistance.
A special implantable SEI was created using a general electroplating procedure incorporating Li metal precycling in a LiTFSI (1.0 M)–LiNO3 (5.0 wt %)–Li2S5 (0.02 M)–DOL/1,2-dimethoxyethane (DME) ternary salt electrolyte (Fig. 3E) (119). The LMA with the protection of the as-obtained SEI can efficiently match both sulfur and NCM523 cathodes and enhance the electrochemical performance. The excellent high-rate performance of the advanced full cells without any activation confirms the highly conductive properties of electroplated SEIs. In a latest study, Lu et al. (120) in situ constructed a stable electrolytic carbon-based hybrid (ECH) artificial SEI on the LMA via electrodeposition of the DME at a high voltage of 700 V. This ECH layer strongly increased the ionic conductivity and mechanical strength, allowing uniform Li+ ion diffusion, stabilizing the electrolyte-Li metal contact, and preventing Li dendritic growth and pulverization.
Considering the prospects for mass manufacturing, using as-prepared materials that immediately coat on or interact with Li metal to form an artificial SEI is usually more convenient than electrochemical pretreatment (15). Nevertheless, electrochemical pretreatment methods play a vital role in fundamental investigations, which enable deeper insights into the effects of Li salts, solvents, additives, decomposition products, and electrochemical parameters. This is crucial for a thorough understanding of the composition and structure of the SEI, as well as further electrolyte optimization and interfacial protection.
Gas-phase pathways
Apart from liquid pretreatment, surface modification with gaseous reagents to create a stable protective layer on LMAs is a desirable choice that provides high accessibility of the reagents to the Li surface and enhances the film uniformity. Therefore, the gas-phase pathway is regarded as an appropriate way to tackle the problems of interfacial issues and volume changes of the LMAs (121, 122).
Physical vapor deposition
Physical vapor deposition (PVD) is a technique that uses primarily physical means to deposit a thin layer of material. The PVD technique demonstrates special superiority in precisely controlling the components and thickness of the protective layer on LMAs based on the physical evaporation-deposition principle, when adopted as a primary or secondary manufacturing process.
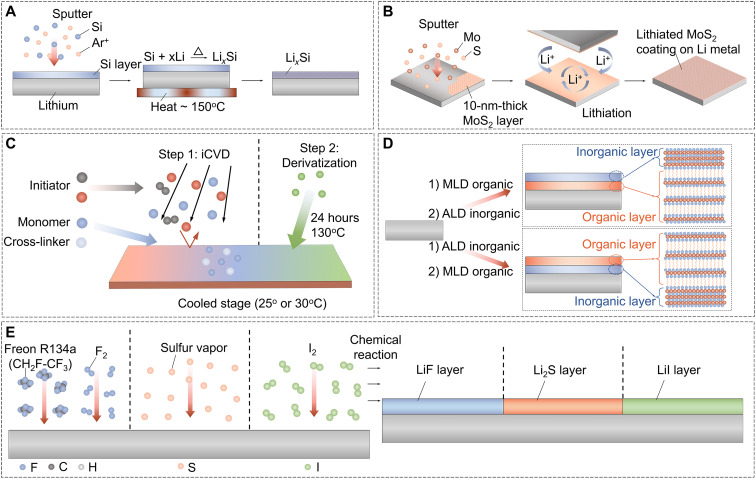
As a representative PVD method, magnetron sputtering (MS) allows the deposition of metals, alloys, ceramics, and polymer thin films onto Li metal in a vacuum environment to achieve homogeneous protective films with a controllable thickness (121). To build an ion-conducting layer on the Li surface, a silicon layer was fabricated in a radio frequency (RF)–MS system by Tang et al. (123). The Si-coated Li foil was heated to 250°C in a glovebox to speed up the alloying between the Li metal and deposited Si to form a LixSi layer (Fig. 4A). The LixSi-modified LMA shows much more uniform Li dissolution/deposition than the bare LMA. The S and LFP cathodes paired with the LixSi-modified LMA showed excellent electrochemical performance in terms of rate capability and cycling stability. A functional ultrathin graphite-SiO2 bilayered SEI on Li metal fabricated via RF sputtering was demonstrated to facilitate Li+ ion diffusion and had higher chemical stability, higher mechanical properties, and lower impedance, which guided a dendrite-free Li deposition (124). A uniform distribution of LiF film on LMAs was realized via the RF-MS process to avoid the side reactions between the LMA and electrolytes (125). As a result, the LiF-coated LMA efficaciously stabilized the interface that enables a uniform Li deposition and longer cycle life.
Fig. 4. Schematic illustration of surface pretreatment using gas-phase pathways.
(A) The preparation process of LixSi-modified lithium foil. (B) The fabrication method for a MoS2-coated LMA via sputtering and subsequent lithiation. (C) The fabrication of zwitterionic polymeric interphases. Step 1: iCVD precursor polymer film. Step 2: derivatization. (D) The fabrication process of the dual protective layer by ALD and MLD. (E) The surface processing strategies via chemical reactions with various gases.
A scalable sputter process proposed by Cha et al. (126) was shown to be able to passivate Li metal from the electrolyte with a 2D MoS2 film with the thickness of 10 nm (Fig. 4B). With a large amount of Li atoms intercalating into the unique atomically layered structure of the MoS2, consistent flow of Li+ ions in/out of the LMA is facilitated and the preferential sites for Li dendrite nucleation are eliminated. The symmetric cell of MoS2-coated LMA exhibits low-voltage hysteresis and threefold improvement in cycling performance even at 10 mA cm−2. Furthermore, in the Li-S full-cell configuration, the use of MoS2-coated LMA results in a specific energy density of ~589 Wh kg−1 and CE of ~98% for over 1200 cycles. A similar strategy via the MS deposition of MgF2 was also adopted by Li et al. (127). The MgF2 layer was served as a lithiophilic substrate to form a uniform LiF-Mg dual-layered SEI through its irreversible conversion reaction with Li metal. As a result, the LiF-Mg–coated LMA regulates dendrite-free deposition and provides long-cycle life and high CE in a half-cell. Moreover, Li et al. (128) have created a stable and thin Cu3N layer via the MS technique. The as-prepared Cu3N can be converted to Li3N/Cu composite film after Li deposition, working as a highly stable and homogeneous conductive SEI to reduce the formation of Li dendrites in working cells. In addition, Li3PO4 (129), Sb-doped Li3PO4 (130), Mg (131), ZnO (132), and amorphous carbon (133) were constructed as protective layers through the MS technique by other research groups, whereby considerable stability improvements in the LMA were also achieved.
Molecular beam epitaxy technique was used to deposit an ultrathin and uniform bismuth (Bi) film on the Li metal surface by thermally evaporating Bi precursor under an ultrahigh vacuum (134). The Bi film is prone to in situ alloying with Li to form a chemically stable alloy interface, which can reduce the harmful side reactions between Li and electrolyte. Moreover, the LixBi alloy–protected LMA offers rapid Li+ ion transport and lithiophilic nucleation sites to suppress Li dendrite formation and guide homogeneous Li plating.
Chemical vapor deposition (CVD)
The CVD method is an advanced synthetic approach for directly growing ultrathin films on electrodes through chemical interactions of precursors at high temperatures (135, 136). Highly uniform and stable protective films on Li metal with atomic-layer thickness can be achieved by this approach.
To rationally design an SEI layer with superior chemical, mechanical, and ion transport properties, Stalin et al. (137) used the initiated CVD (iCVD) technique to deposit ultrathin conformal zwitterionic polymeric films as a protective coating on Li metal to stabilize Li electrochemical deposition (Fig. 4C). The molecular structure of zwitterionic moieties at the polymer interface can adjust the solvation environment of the Li+ cation, which enables uniform, dendrite-free deposition of Li. As a result, the full cell by pairing a NCM622 cathode with the zwitterionic polymer–coated LMA (negative/positive capacity ratio of 1) exhibits superior capacity retention and stable CE compared to the uncoated LMA. An electron cyclotron resonance CVD apparatus was applied to passivate an LMA with Li4SiO4-based thin film (138). The in situ–converted Li4SiO4 layer efficiently improves Li+ ion conductivity and allows dendrite-free Li deposition. Moreover, 2D atomic crystal layers, including hexagonal boron nitride (h-BN) and graphene, can be directly grown on the surface of electrodes via the CVD process (135). The 2D layers afford excellent surface protection of Li metal due to their remarkable mechanical strength, flexibility, and stability. The point and line defects of the 2D layers allow Li+ ion infiltration into the electrode, resulting in smooth Li deposition without dendritic and mossy Li formation.
Although CVD is a well-established technique for depositing thin-film coatings, its relatively poor control over the thickness and composition makes it less ideal when applied for the LMA. Atomic layer deposition (ALD) is a self-controlled chemical reaction between gaseous precursors and a solid surface that enables excellent coverage and conformal deposition with rationally designed and precisely controlled composition and thickness (139). For instance, Xie et al. (140) demonstrated that LiF can be deposited on the line and point defects of h-BN by ALD. The chemically and mechanically stable LiF/h-BN film efficaciously suppresses Li dendrite formation and improves the CE during extended cycling. With an optimized ALD process, LMAs coated with other ultrathin metal oxide (Al2O3, TiO2, and ZrO2) layers achieve dendrite-free Li deposition and improved lifetime (141–143). Beyond ALD, molecular layer deposition (MLD) for the LMAs has been further developed by substituting the oxidizing precursor with organic linkers or molecular fragments (144). Pure polymer thin films and organic-inorganic hybrid protective layers can be obtained through the MLD technique. Sun’s group (145–148) has demonstrated that various functional protective films can be built on the Li metal by MLD to produce safe high-performance LMBs. For instance, they created an inorganic-organic interlayer (alucone) by MLD at the junction of Li metal and solid-state sulfide electrolytes (145). The interfacial side reactions between Li metal and solid-state sulfide electrolytes are greatly reduced with the aid of the alucone covering layer. In addition, a hybrid polyurea film with controllable components and improved strength was introduced as a protective layer on Li metal via the MLD process by introducing trimethylaluminum as a cross-linker into the polymer chains (147). A further study by Sun et al. (148) reported that a gradient hybrid inorganic-organic polyurea protection film can efficiently enhance the chemical and mechanical properties of the LMAs. The electrically insulating polymer on the coating surface can restrain the volume changes due to its good elasticity, while the inner inorganic lithiophilic sites can helpfully facilitate and regulate uniform Li nucleation and deposition. By combining the ALD and MLD techniques, a hybrid protective film on LMA with precisely controllable structures and robust mechanical properties was constructed (Fig. 4D) (149). The alucone-Al2O3 dual-layer shielded LMA exhibited high stability during the plating/stripping process thus greatly improved cycling performance.
Chemical reaction with gases
The chemical reaction with a gas to form an artificial SEI on LMAs is an alternate choice that provides high accessibility of reagents to the Li surface and improved film homogeneity. Multitudinous gases (e.g., CH2F-CH3, F2, S, SeS2 N2, I2, and CS2) have been conducted to construct a functional protective layer via a spontaneous chemical reaction with metallic Li (122, 150–155). For instance, a conformal and dense LiF layer with controllable thickness coated onto Li metal was developed by exposing Li foil to nonhazardous Freon R134a gas (CH2F-CH3) (Fig. 4E) (122). The symmetric cells of the layered Li-rGO anodes with the applied LiF layer demonstrated reduced side reactions and highly improved cycling stability without potential polarization augmentation. Furthermore, a homogeneous and compact LiF protective layer on LMAs was developed by a surface fluorination process, which is formed with in situ generated fluorine gas using a fluoropolymer as the precursor (Fig. 4E) (150). The LiF layer shows strong mechanical strength, chemical stability, and low solubility in electrolytes, which can minimize the corrosion reaction between Li metal and organic solvents and suppress Li dendrite formation. As a result, the LiF-coated LMA in symmetric cells enabled a dendrite-free Li deposition and stable cycling over 300 cycles in carbonate electrolytes. Lin et al. (152) reported a solid-gas reaction method to fabricate a dense LiI protective layer on the LMA surface by using a Li metal reaction with iodine vapor (Fig. 4E). In addition, a special “sauna” reaction between CS2-I2 mixed steam and Li metal was conducted to form a SEI layer with high mechanical property and high ionic conductivity, which consists of amorphous carbon and Li compounds (Li2S and LiI) (153). The obtained C-Li2S-LiI@Li anode exhibits outstanding electrochemical performance with low-voltage polarization and long-cycle life.
Apart from the lithium halides, lithium chalcogenides with high ionic conductivity are also attractive. Chen et al. (154) reported that a homogenous Li2S protective layer can be fabricated by reacting Li with sulfur vapor at an elevated temperature (Fig. 4E). Specifically, the uniform Li2S-based protective layer turned into a layered SEI that preserves protective function with high ionic conductivity, rather than into a disordered, broken SEI mainly composed of parasitic reaction products. Similarly, Liu et al. (155) proposed a gas-solid reaction to fabricate a stable Li2S/Li2Se mixed SEI via the reaction between SeS2 gas and Li metal at a low temperature. The migration barrier energy of Li2Se is lower than that of Li2S at different lattice planes, thus confirming the positive function of Li2Se in improving the integral ionic conductivity.
Li3N shows high ionic conductivity (up to 10−3 S cm−1) and low electronic conductivity (<10−12 S cm−1), which is suitable for Li metal protection. A pinhole-free and ionically conductive α-Li3N layer was prepared on the surface of Li metal by directly reacting clean molten Li foil with pure nitrogen gas at 450°C in the argon atmosphere (151). This Li3N layer is chemically stable, which can isolate the reactive Li metal from the organic solvent, prevent continuous electrolyte consumption during cycling, and suppress Li dendrite growth in the Li plating/stripping process.
Other peculiar pathways
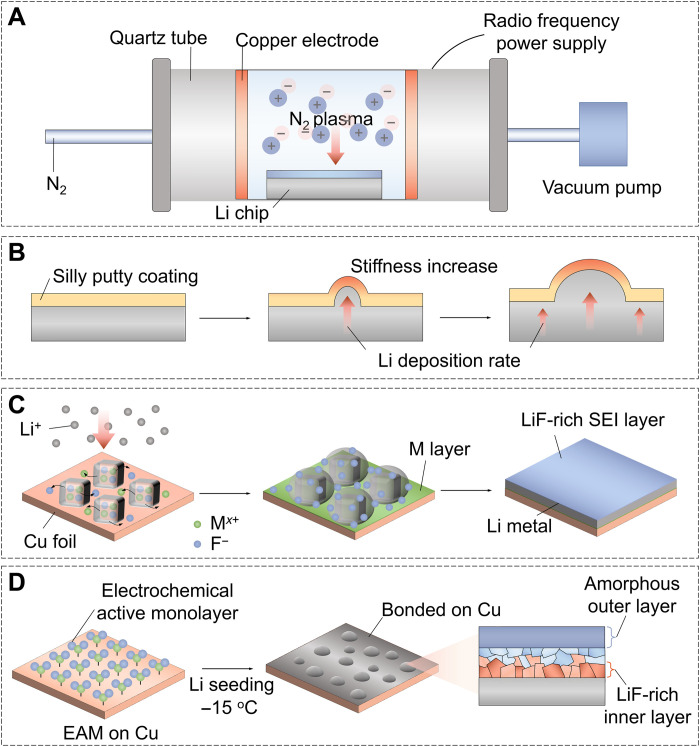
The plasma state is frequently referred to the fourth state of matter in the sequence: solid, liquid, gas, and plasma (156). In general, plasma is made up of extremely reactive electrons, ions, and neutral species that are created by high-voltage ionization (157). Through energy exchange, plasma reagents may quickly produce a large number of active sites on the material surface, allowing reactions with high barriers to proceed under mild circumstances in a matter of minutes (158). Therefore, plasma treatment appears to be a promising method for high-quality surface engineering of LMAs. For example, Chen et al. (159) obtained a Li3N protective layer with high modulus and high ionic conductivity on LMAs by a plasma activation of Li metal in a N2 environment (Fig. 5A). This desired Li3N protective layer can efficiently prevent spontaneous reactions between the highly reactive Li metal and the organic electrolyte and suppress dendritic Li generation during the Li plating/stripping process. Recently, Cao et al. (160) used a rapid CF4 plasma treatment process to construct an artificial SEI consisting of LiF and Li2C2 on LMAs via ion bombardment. Benefiting from the high adsorption energy, low diffusion barrier, and high mechanical strength of the LiF and Li2C2 components, the obtained composite layer guaranteed a stable interface and dendrite-free Li deposition after long cycling. Moreover, the room-temperature plasma treatment described in these works is highly tunable and environmentally friendly and has potential in large-scale energy storage applications.
Fig. 5. Schematic illustration of surface pretreatment using some peculiar pathways.
(A) A desired Li3N film can be formed on the Li metal as the protective layer by a plasma activation in a short time. (B) The design of silly putty modified LMA. (C) A designed “spansule” can sustainably supply functional ingredients that effectively guide dendrite-free Li deposition. (D) A self-assembled monolayer of electrochemically active 1,3-benzenedisulfonyl fluoride on Cu can lead to uniform seeding of Li with a stable LiF-rich SEI layer.
Some interesting and uncommon strategies beyond the above pathways, such as combining the different methods to construct multiple protective layers (161), the concepts of “solid-liquid membrane” (162), “spansule” (163), and “self-assembled monolayer” (164), have been proposed for creating various artificial SEIs on the LMAs. For example, Liu et al. proposed that a dynamically cross-linked polymer (silly putty) manifested a “solid-liquid” hybrid behavior, which enabled it to act as an adaptive interlayer for LMAs (Fig. 5B) (162). In response to the Li growth rate, the dynamic polymer can reversibly switch between “liquid” and “solid” qualities to support uniform coverage and prohibit Li dendrite formation, allowing reliable operation of LMAs. In addition, Yuan et al. (163) designed a smart “spansule” made of carbon-coated mixed metal fluoride (NMMF@C) core@shell microstructures to provide a long-lasting supply of functional ingredients for stabilizing LMAs (Fig. 5C). With the assistance of cryo-TEM, they found that the NMMF core would gradually dissolve and release functional metal and fluoride ions into the electrolyte during cycling, while the in situ–formed metal layer and LiF-involved bilayer interface would be beneficial for guiding uniform Li deposition. Consequently, a high CE of approximately 98% is achieved for over 1000 cycles at a current density of 2 mA cm−2 with a capacity of 1 mAh cm−2. To precisely construct a passive SEI to enable stable LMAs at low temperatures, Gao et al. (164) fabricated a self-assembled electrochemically active monolayer on the Cu current collector, which can regulate the nanostructure and component of the SEI and deposition behavior of Li metal (Fig. 5D). The as-formed SEI contains a LiF-rich inner phase and an amorphous outer layer that effectively sealed the Li surface. As a result, galvanic Li corrosion and self-discharge were suppressed, dendrite-free Li deposition was achieved from −60° to 45°C, and the LMB (Li/LiCoO2 cells) exhibits 200-cycle life span at −15°C with a recharge time of 45 min.
CHARACTERIZATION TECHNIQUES
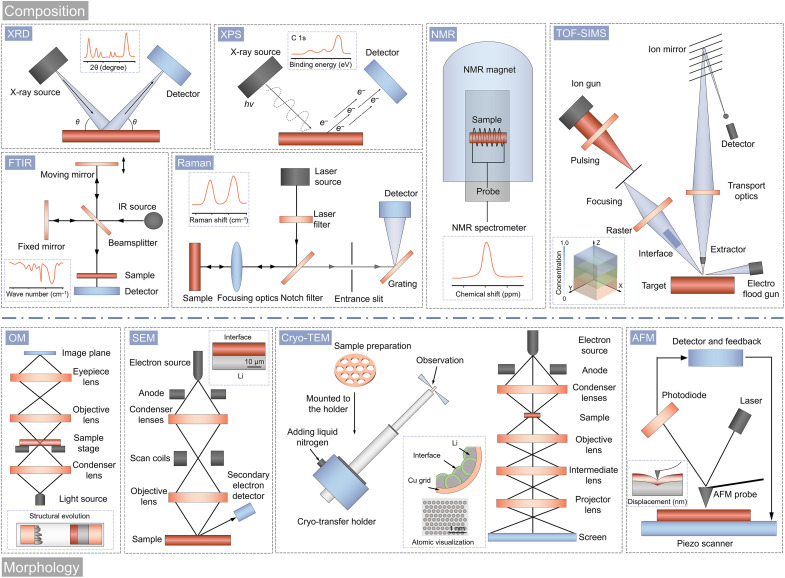
Since the surface of LMAs has been precisely modified using various surface engineering strategies, direct investigation of the physical and chemical properties of these protective layers is vitally important to understand the electrochemical behavior of LMAs. The following scientific issues are the major concerns during the surface investigation of LMAs: (i) the morphology, particle size, and thickness of the layers; (ii) the chemical composition, elemental content, and chemical state; (iii) the stiffness and elastic modulus; and (iv) the structural evolution at multiple spatial scales. Characterization tools play a key role in understanding these scientific issues, include x-ray diffraction (XRD), x-ray photoelectron spectroscopy (XPS), Fourier transform infrared (FTIR) spectroscopy, Raman spectroscopy, solid-state nuclear magnetic resonance (NMR), time-of-flight secondary ion mass spectrometry (TOF-SIMS), optical microscopy (OM), scanning electron microscopy (SEM), AFM, TEM, cryo-TEM, and several other advanced methods. All these techniques can be categorized in terms of the study of composition or morphology (Fig. 6).
Fig. 6. Schematic illustration of the current characterization techniques for the artificial SEIs on Li metal.
Composition
XRD is one of the well-established techniques to study the crystal structure and structural changes of surficial materials before and after reactions on LMAs. According to the peak positions and relative intensities, information about the crystal structure, phase purity, and structural evolution of various inorganic components within the protective layers can be obtained, such as Li-based alloys (LixM) and compounds (i.e., LiF, Li3N, LiCl, Li2S, LixPO4, etc.) (165).
XPS is the most extensively used analytical technique for surface analysis, providing element-specific composition and electronic state information (166). As a powerful quantitative technique, XPS has several merits as follows: (i) sensitivity to all the elements with the same order of magnitude; (ii) easy observation of the chemical shift information; (iii) ability to identify elements half-quantitatively; (iv) sensitivity for surface analysis with an ultrathin depth (2 to 5 nm). Theoretically, the electronic states of cations and anions in the entire artificial SEI can be expediently obtained by XPS analysis. Moreover, XPS can probe the change in the chemical state of elements in the protective layer after Li plating or cycling.
FTIR spectroscopy is not only a surface sensitive but also a nondestructive technique for acquiring accurate information about characteristic functional groups within the protective layers on the LMAs. It is especially suitable for investigating the organic species, which can complement the composition information from XRD and XPS. For qualitative analysis, FTIR spectroscopy has the advantages of short data collection time, small sample size requirement, and simple data collection procedure (167). Although the FTIR helps detect surface components, it only identifies dipole moment changes. Raman spectroscopy measures the inelastic scattering induced by polarizability changes, and it is especially helpful for analyzing carbon-based materials, such as graphene, GO, rGO, and their derivatives (168). Therefore, Raman and FTIR spectroscopies are complementary techniques in most circumstances of surface investigation.
Solid-state NMR spectroscopy is another important analytical technique to probe the local structural environments and electronic structure of surface materials for LMAs. A variety of precise structural details about the materials can be revealed based on the peak chemical shift, peak shape, and coupling constant of the nucleus (167). Therefore, many researchers have used the solid-state NMR to identify the organic components in the SEI of LMAs, which could assist to reveal their molecular structures and disclose the characteristics of Li-ion transport between SEI and the deposited Li (169).
TOF-SIMS is a material characterization technique with strong chemical sensitivity, high surface sensitivity (upper 2 to 3 nm probed), and molecular specificity (170). The depth profiling can identify species of the SEI and their 3D distribution on the micro- to the nanometer scale, which could be combined with atom probe tomography to show the chemistry and distribution of the SEI on the atomic scale. For example, Sun’s group (146–149) performed TOF-SIMS to identify the distribution and depth profiles of various molecular moieties within the artificial SEIs coated on LMAs, such as polyurea, zircone film, Al2O3, and alucone dual layer.
Morphology
OM observations can provide a direct imaging of structural evolution of the protective layers during the vertical growth and dissolution of Li metals in electrolytes but are limited at micron-level magnification (171). SEM has been mainly used for the observation of microstructures and thicknesses of the protective layers. However, traditional SEM has been confined to 2D information and relatively low resolutions. Advanced imaging techniques, including TEM and scanning TEM (STEM), operate at a high acceleration voltage, enabling highly enlarged orthographic images to be achieved with exceptional spatial resolution down to the atomic scale (25). In this regard, TEM and STEM are critical techniques for examining the morphological structure and thin layer on the Li surface. Combination of microscopic and spectroscopic techniques, such as SEM/TEM–energy-dispersive spectroscopy and STEM–electron energy loss spectroscopy, enables the multiangle investigation of not only the morphology but also the structure, local elemental distribution, chemical information, and bonding environment correlated to the local electrode materials simultaneously.
Nevertheless, the high-energy electron beams can permanently destroy the SEI layer and Li metal due to their weak atomic bonding and relatively low melting point. Therefore, observing the original morphology and chemical components of the SEI layer seems difficult. To discover more reliable characterization methods for SEI layers, scientists paid particular attention to cryo-TEM, which is mainly used for the characterization of biological materials. Cryo-TEM is thought to be capable of preserving the initial structure and morphology of materials at extremely low temperatures (172). Specifically, Cui’s group (173) has pioneered to use the cryo-TEM to investigate the atomic structure of SEI films formed on the surface of Li metal in different electrolyte. Motivated from the excellent effectiveness of the cryo-TEM for LMAs, more researchers have revealed the structure of the SEIs and their evolution behaviors (42, 110, 174–176). By using the cryo-TEM technique, a relationship between the SEI nanostructures and electrochemical properties may be established.
AFM is a type of microscopy that scans the surface of the sample and records the van der Waals force that exists between the probe and sample. It can measure the overall surface structure, height distribution, and roughness of the Li surface. Therefore, primary quality assessments of SEIs can be performed using the AFM technique to examine their morphological characteristics and mechanical properties (169). In particular, the nanoindentation characteristics, which are revealed by force probing in areas where surface deformation occurs, reveal the fine mechanical properties of SEIs that are governed by the chemical compositions and spatial arrangements, and they may be used to assess the SEI quality. For example, Mao’s group (113) used a set of unique nanoindentation features of mechanical properties as standards for evaluating the quality of unknown SEIs, including Young’s modulus, thickness, and smoothness.
CONCLUSION AND PERSPECTIVE
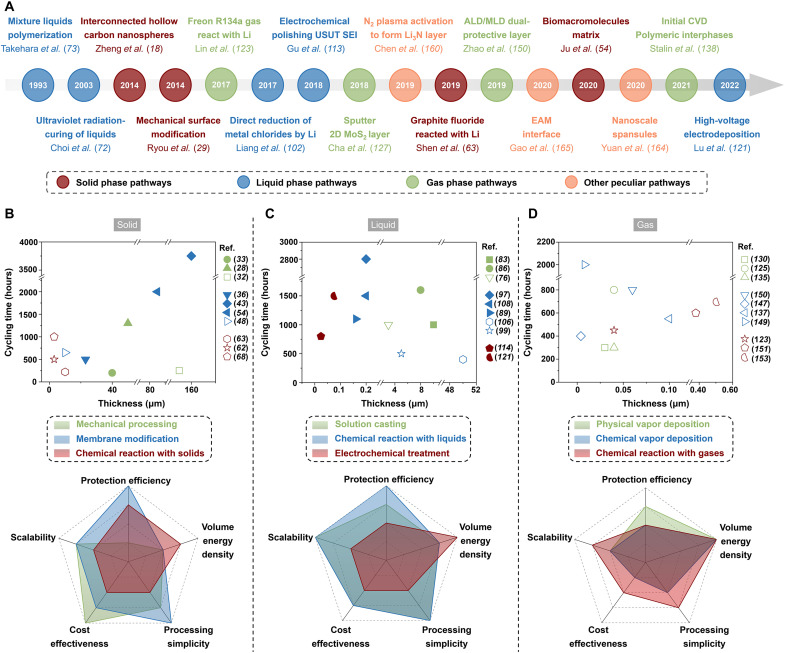
Surface engineering has been developed to address the critical application issues of LMAs for nearly three decades since its first demonstration in 1993 reported by Takehara et al. (72). However, this realm was developed slowly in the following two decades, which focused mainly on the liquid phase–dominated pathways. In 2014, Zheng et al. (18) first reported a solid phase–involved surface engineering approach, in which a protective layer assembled by free-standing interconnected hollow carbon nanospheres was built on the LMAs. Since then, research on the development of various surface engineering strategies is booming, including treating the Li metal toward the reagents under the states of matter beyond the solid or liquid (gas and plasma for instance) and using some unique pathways. The roadmap of this realm is illustrated in Fig. 7A. The cycling performance of the modified LMAs fabricated via different surface engineering strategies and the evaluation of these strategies from five practical application metrics are summarized in Fig. 7 (B to D). The LMAs pretreated by the solid-phase strategy have better cycling performance in ether-based electrolytes than those in carbonate-based ones, among which the method of membrane modification is the best (Fig. 7B). However, the disadvantage of using the solid-phase strategy is that the artificial SEI is usually thick (larger than 10 μm and up to 160 μm in thickness), resulting in a relatively small-volume energy density for practical application. Still, the solid-phase strategy scores well on the metrics of protection efficiency, cost effectiveness, and processing simplicity. Similar to the solid-phase strategy, the LMAs pretreated by the liquid-phase one also show better stability in the ether-based electrolyte, and the three representative methods are rivals in the cycling performance (Fig. 7C). In terms of the practical application, the “solution casting” and “chemical reaction with liquids” score well in all the five metrics, while the “electrochemical treatment” has only one comparative advantage in constructing thin artificial SEIs, which make it leading in the metrics of volume energy density. Compared with the previous two strategies, the cycling performance of the LMAs pretreated by the gas-phase one is generally mediocre, which might be due to that the electrolytes used are all carbonates (Fig. 7D). The cycling performance of these LMAs in ether-based electrolytes needs to be investigated. It is worth mentioning that the gas-phase strategy has a prominent merit for constructing ultrathin artificial SEIs, whose thickness can be as small as less than 10 nm, so its score for the “volume energy density” is the highest. However, as the equipment used in the gas-phase strategy is usually expensive, it is less cost-effective, while the other three metrics are moderate.
Fig. 7. Summary and comparison of various surface pretreatment strategies for LMAs.
(A) Roadmap of major achievements in the field of surface engineering. (B to D) The panels on the upper row are the cycling performance of Li symmetric cell (current density and capacity is 1 mA cm−2 and 1 mAh cm−2, respectively) using the modified LMAs pretreated by different methods. The solid symbols represent using the ether-based electrolytes, while the hollow ones mean using the carbonate-based electrolytes. The panels on the nether row are the evaluation of these methods from five practical application metrics: protective efficiency, volume energy density, processing simplicity, cost effectiveness, and scalability.
Although fruitful achievements have been achieved in the surface engineering for stabilizing the LMAs, many challenges still need to be considered: (i) The artificial SEIs constructed by current surface pretreatment techniques are still far from satisfactory for the LMAs. For example, the majority of the documented artificial SEIs are restricted to a single ingredient, which is insufficient to meet the longevity and safety requirements of LMAs. To create SEIs with multi-ingredient to realize the maximum of their functionality, combining multiple strategies is therefore worthwhile (161). Besides, potential new methods are always welcome to be explored, such as liquid phase–based SEI production assisted by speedy and accurate laser-mediated technology or solid phase–based patterned SEI constructed by 3D printing. (ii) The relationship between the structure of the artificial SEIs and battery performance is still not clear. Thus, more precise construction of the SEI’s components and thicknesses by advanced surface engineering protocols, for example, an affordable CVD-based technology, is needed. Accordingly, a selection rule for a specific component and its proper spatial and temporal distribution in the artificial SEIs can be established for an oriented preparation by surface engineering. (iii) Implementing surface engineering for practical applications in LMAs is still challenging. For instance, it is necessary to make the transition of the surface engineering from coin cells to pouch cells feasible for mass manufacturing. Furthermore, exploiting novel surface engineering strategies to build stable artificial SEIs that can work under certain challenging circumstances, such as low negative/positive capacity ratio, lean electrolytes, high current densities, and extreme temperature, should be put on the schedule. Last but not the least, more metrics must be considered for the evaluation of the practicality of the surface engineering processes (such as toxicity and environmental compatibility).
This review has also provided a concise summary of the current developments in the key characterization techniques used to examine the composition and structure of the artificial SEIs on the LMAs. However, these characterization techniques still have some limitations: (i) XRD has difficulty in obtaining detailed information on amorphous materials and organic components. (ii) It is challenging for XPS to provide deep-layer information. Also, the XPS etching procedure carries the risk of radiation damage, chemical-state changes brought by low-energy electrons, or sample contamination. (iii) Normal FTIR and Raman spectra have a relatively low spatial resolution due to the Abbe diffraction limit. (iv) The relatively low sensitivity and resolution of solid-state NMR poses challenges for studying the artificial SEIs. (v) The TOF-SIMS equipment is expensive and not readily available, while the sample testing process is also inconvenient. (vi) The SEI and Li metal will inevitably be irreversibly damaged by high-energy electron beams using SEM and TEM techniques. (vii) The AFM has strict criteria for the sample surface flatness. Therefore, a combination of diverse microscopic and spectroscopic techniques would be desirable, which enables simultaneous multi-angle analysis of the morphology, structure, elemental composition, and coordination chemistry of the electrode interface. For example, recent developments in combining light-induced molecular excitations with mechanical force detection are particularly interesting since they aim to improve the chemical selectivity of AFM (177). Nano-IR, which has been used effectively to visualize molecular layers on surfaces with chemical selectivity and with a spatial resolution as low as 25 nm (178), is a potentially powerful tool for this field. It should also be emphasized that the inherent sensitivity of Li metal and its SEI to air makes it difficult to transfer the samples to the instrument. Hence, how to achieve damage-free transfer and characterization is a challenge. On the one hand, researchers could interconnect these instruments with the glovebox via ducted vacuum for simultaneous characterization of the sample at the same location, as well as avoid potential contamination during the sample transfer. In addition, some nondestructive imaging techniques could be used, such as low-dose TEM imaging and x-ray computed tomography. On the other hand, more efforts need to be devoted to advance in situ/operando characterization tools, which can deliver more trustworthy and precise information by continually monitoring the evolution of the target materials and the intricate physical and chemical processes during the cycling, for example, the degradation mechanism of the artificial SEIs (169, 170).
In conjunction with interdisciplinary research (including chemical engineering, materials, nanotechnology, physics, chemistry, electrochemistry, etc.), more reliable artificial SEIs on LMAs can be rationally designed by advanced surface engineering. After more researchers joining in this fast-growing field with persistent cooperation and dedication, it is believed that many exciting discoveries on surface engineering can be expected in the years ahead, which will drive a greater breakthrough of the LMBs and other energy storage systems.
Acknowledgments
Funding: This work is supported by the funding of the National Key R&D Program of China (2022YFB2502000), the “Leading Innovative and Entrepreneur Team Introduction Program of Zhejiang” (2020R01002), and the National Natural Science Foundation of China (grant nos. 52225208, 51972285, and U21A20174).
Author contributions: G.L., J.N., D.L., X.T., and X.W.L. conceived the topic of this review. G.L. and J.N. cowrote the initial draft. X.T. and X.W.L. supervised the writing of the manuscript and made revisions.
Competing interests: The authors declare that they have no competing interests.
Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper.
REFERENCES AND NOTES
- 1.X. B. Cheng, R. Zhang, C. Z. Zhao, Q. Zhang, Toward safe lithium metal anode in rechargeable batteries: A review. Chem. Rev. 117, 10403–10473 (2017). [DOI] [PubMed] [Google Scholar]
- 2.X. Zhang, Y. Yang, Z. Zhou, Towards practical lithium-metal anodes. Chem. Soc. Rev. 49, 3040–3071 (2020). [DOI] [PubMed] [Google Scholar]
- 3.D. Lin, Y. Liu, Y. Cui, Reviving the lithium metal anode for high-energy batteries. Nat. Nanotechnol. 12, 194–206 (2017). [DOI] [PubMed] [Google Scholar]
- 4.W. Xu, J. Wang, F. Ding, X. Chen, E. Nasybulin, Y. Zhang, J.-G. Zhang, Lithium metal anodes for rechargeable batteries. Energ. Environ. Sci. 7, 513–537 (2014). [Google Scholar]
- 5.X. Zhang, A. Wang, X. Liu, J. Luo, Dendrites in lithium metal anodes: Suppression, regulation, and elimination. Acc. Chem. Res. 52, 3223–3232 (2019). [DOI] [PubMed] [Google Scholar]
- 6.T. Li, X.-Q. Zhang, P. Shi, Q. Zhang, Fluorinated solid-electrolyte interphase in high-voltage lithium metal batteries. Joule 3, 2647–2661 (2019). [Google Scholar]
- 7.W. Liu, P. Liu, D. Mitlin, Review of emerging concepts in SEI analysis and artificial SEI membranes for lithium, sodium, and potassium metal battery anodes. Adv. Energy Mater. 10, 2002297 (2020). [Google Scholar]
- 8.H. Wang, D. Yu, C. Kuang, L. Cheng, W. Li, X. Feng, Z. Zhang, X. Zhang, Y. Zhang, Alkali metal anodes for rechargeable batteries. Chem 5, 313–338 (2019). [Google Scholar]
- 9.J. W. Choi, D. Aurbach, Promise and reality of post-lithium-ion batteries with high energy densities. Nat. Rev. Mater. 1, 16013 (2016). [Google Scholar]
- 10.S. Wei, S. Choudhury, Z. Tu, K. Zhang, L. A. Archer, Electrochemical interphases for high-energy storage using reactive metal anodes. Acc. Chem. Res. 51, 80–88 (2018). [DOI] [PubMed] [Google Scholar]
- 11.C. B. Jin, T. F. Liu, O. W. Sheng, M. Li, W. K. Zhang, J. W. Nai, Z. J. Ju, Y. J. Liu, T. C. Liu, Y. Wang, Y. F. Yuan, Z. Lin, J. Lu, X. Y. Tao, Rejuvenating dead lithium supply in lithium metal anodes by iodine redox. Nat. Energy 6, 378–387 (2021). [Google Scholar]
- 12.F. Liu, R. Xu, Y. Wu, D. T. Boyle, A. Yang, J. Xu, Y. Zhu, Y. Ye, Z. Yu, Z. Zhang, X. Xiao, W. Huang, H. Wang, H. Chen, Y. Cui, Dynamic spatial progression of isolated lithium during battery operations. Nature 600, 659–663 (2021). [DOI] [PubMed] [Google Scholar]
- 13.P. C. Zou, Y. Sui, H. Zhan, C. Wang, H. L. Xin, H. M. Cheng, F. Kang, C. Yang, Polymorph evolution mechanisms and regulation strategies of lithium metal anode under multiphysical fields. Chem. Rev. 121, 5986–6056 (2021). [DOI] [PubMed] [Google Scholar]
- 14.Y. J. Liu, X. Y. Tao, Y. Wang, C. Jiang, C. Ma, O. W. Sheng, G. X. Lu, X. W. Lou, Self-assembled monolayers direct a LiF-rich interphase toward long-life lithium metal batteries. Science 375, 739–745 (2022). [DOI] [PubMed] [Google Scholar]
- 15.X.-Q. Zhang, X.-B. Cheng, Q. Zhang, Advances in interfaces between Li metal anode and electrolyte. Adv. Mater. Inter. 5, 1701097 (2018). [Google Scholar]
- 16.R. Xu, X.-B. Cheng, C. Yan, X.-Q. Zhang, Y. Xiao, C.-Z. Zhao, J.-Q. Huang, Q. Zhang, Artificial interphases for highly stable lithium metal anode. Matter 1, 317–344 (2019). [Google Scholar]
- 17.M. D. Tikekar, S. Choudhury, Z. Tu, L. A. Archer, Design principles for electrolytes and interfaces for stable lithium-metal batteries. Nat. Energy 1, 16114 (2016). [Google Scholar]
- 18.G. Y. Zheng, S. W. Lee, Z. Liang, H. W. Lee, K. Yan, H. Yao, H. Wang, W. Li, S. Chu, Y. Cui, Interconnected hollow carbon nanospheres for stable lithium metal anodes. Nat. Nanotechnol. 9, 618–623 (2014). [DOI] [PubMed] [Google Scholar]
- 19.Y. C. Yin, Q. Wang, J. T. Yang, F. Li, G. Zhang, C. H. Jiang, H. S. Mo, J. S. Yao, K. H. Wang, F. Zhou, H. X. Ju, H. B. Yao, Metal chloride perovskite thin film based interfacial layer for shielding lithium metal from liquid electrolyte. Nat. Commun. 11, 1761 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.H. Wu, H. Jia, C. Wang, J.-G. Zhang, W. Xu, Recent progress in understanding solid electrolyte interphase on lithium metal anodes. Adv. Energy Mater. 11, 2003092 (2021). [Google Scholar]
- 21.S. Gao, F. Sun, N. Liu, H. Yang, P.-F. Cao, Ionic conductive polymers as artificial solid electrolyte interphase films in Li metal batteries - A review. Mater. Today 40, 140–159 (2020). [Google Scholar]
- 22.Q. Zhang, S. Liu, Y. Lu, L. Xing, W. Li, Artificial interphases enable dendrite-free Li-metal anodes. J. Energy Chem. 58, 198–206 (2021). [Google Scholar]
- 23.M. Du, K. Liao, Q. Lu, Z. Shao, Recent advances in the interface engineering of solid-state Li-ion batteries with artificial buffer layers: Challenges, materials, construction, and characterization. Energ. Environ. Sci. 12, 1780–1804 (2019). [Google Scholar]
- 24.Z. Wang, Y. Wang, C. Wu, W. K. Pang, J. Mao, Z. Guo, Constructing nitrided interfaces for stabilizing Li metal electrodes in liquid electrolytes. Chem. Sci. 12, 8945–8966 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.J. Tan, J. Matz, P. Dong, J. Shen, M. Ye, A growing appreciation for the role of LiF in the solid electrolyte interphase. Adv. Energy Mater. 11, 2100046 (2021). [Google Scholar]
- 26.R. G. Fedorov, S. Maletti, C. Heubner, A. Michaelis, Y. Ein-Eli, Molecular engineering approaches to fabricate artificial solid-electrolyte interphases on anodes for Li-ion batteries: A critical review. Adv. Energy Mater. 11, 2101173 (2021). [Google Scholar]
- 27.Y.-J. Kim, H. S. Jin, D.-H. Lee, J. Choi, W. Jo, H. Noh, J. Lee, H. Chu, H. Kwack, F. Ye, H. Lee, M.-H. Ryou, H.-T. Kim, Guided lithium deposition by surface micro-patterning of lithium-metal electrodes. ChemElectroChem 5, 3169–3175 (2018). [Google Scholar]
- 28.H. Wang, P. Hu, X. Liu, Y. Shen, L. Yuan, Z. Li, Y. Huang, Sowing silver seeds within patterned ditches for dendrite-free lithium metal batteries. Adv. Sci. 8, 2100684 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.M.-H. Ryou, Y. M. Lee, Y. Lee, M. Winter, P. Bieker, Mechanical surface modification of lithium metal: Towards improved Li metal anode performance by directed Li plating. Adv. Funct. Mater. 25, 834–841 (2014). [Google Scholar]
- 30.J. Park, J. Jeong, Y. Lee, M. Oh, M.-H. Ryou, Y. M. Lee, Micro-patterned lithium metal anodes with suppressed dendrite formation for post lithium-ion batteries. Adv. Mater. Inter. 3, 1600140 (2016). [Google Scholar]
- 31.Q. Li, B. Quan, W. Li, J. Lu, J. Zheng, X. Yu, J. Li, H. Li, Electro-plating and stripping behavior on lithium metal electrode with ordered three-dimensional structure. Nano Energy 45, 463–470 (2018). [Google Scholar]
- 32.H. Kim, Y. J. Gong, J. Yoo, Y. S. Kim, Highly stable lithium metal battery with an applied three-dimensional mesh structure interlayer. J. Mater. Chem. A 6, 15540–15545 (2018). [Google Scholar]
- 33.D. Wang, C. Luan, W. Zhang, X. Liu, L. Sun, Q. Liang, T. Qin, Z. Zhao, Y. Zhou, P. Wang, W. Zheng, Zipper-inspired SEI film for remarkably enhancing the stability of Li metal anode via nucleation barriers controlled weaving of lithium pits. Adv. Energy Mater. 8, 1800650 (2018). [Google Scholar]
- 34.D. Liebenau, K. Jalkanen, S. Schmohl, M. C. Stan, P. Bieker, H.-D. Wiemhöfer, M. Winter, M. Kolek, Improved interfaces of mechanically modified lithium electrodes with solid polymer electrolytes. Adv. Mater. Inter. 6, 1900518 (2019). [Google Scholar]
- 35.M. S. Kim, J.-H. Ryu, Deepika, Y. R. Lim, I. W. Nah, K.-R. Lee, L. A. Archer, W. I. Cho, Langmuir-blodgett artificial solid-electrolyte interphases for practical lithium metal batteries. Nat. Energy 3, 889–898 (2018). [Google Scholar]
- 36.N. Li, K. Zhang, K. Xie, W. Wei, Y. Gao, M. Bai, Y. Gao, Q. Hou, C. Shen, Z. Xia, B. Wei, Reduced-graphene-oxide-guided directional growth of planar lithium layers. Adv. Mater. 32, 1907079 (2020). [DOI] [PubMed] [Google Scholar]
- 37.X. B. Cheng, T. Z. Hou, R. Zhang, H. J. Peng, C. Z. Zhao, J. Q. Huang, Q. Zhang, Dendrite-free lithium deposition induced by uniformly distributed lithium ions for efficient lithium metal batteries. Adv. Mater. 28, 2888, 2895 (2016). [DOI] [PubMed] [Google Scholar]
- 38.P. Zhai, T. Wang, H. Jiang, J. Wan, Y. Wei, L. Wang, W. Liu, Q. Chen, W. Yang, Y. Cui, Y. Gong, 3D artificial solid-electrolyte interphase for lithium metal anodes enabled by insulator-metal-insulator layered heterostructures. Adv. Mater. 33, 2006247 (2021). [DOI] [PubMed] [Google Scholar]
- 39.C. Sun, A. Lin, W. Li, J. Jin, Y. Sun, J. Yang, Z. Wen, In situ conversion of Cu3P nanowires to mixed ion/electron-conducting skeleton for homogeneous lithium deposition. Adv. Energy Mater. 10, 1902989 (2019). [Google Scholar]
- 40.K. Xie, K. Yuan, K. Zhang, C. Shen, W. Lv, X. Liu, J. G. Wang, B. Wei, Dual functionalities of carbon nanotube films for dendrite-free and high energy-high power lithium-sulfur batteries. ACS Appl. Mater. Inter. 9, 4605–4613 (2017). [DOI] [PubMed] [Google Scholar]
- 41.Y. Ma, P. Qi, J. Ma, L. Wei, L. Zhao, J. Cheng, Y. Su, Y. Gu, Y. Lian, Y. Peng, Y. Shen, L. Chen, Z. Deng, Z. Liu, Wax-transferred hydrophobic CVD graphene enables water-resistant and dendrite-free lithium anode toward long cycle Li-air battery. Adv. Sci. 8, 2100488 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.B. Han, D. Feng, S. Li, Z. Zhang, Y. Zou, M. Gu, H. Meng, C. Wang, K. Xu, Y. Zhao, H. Zeng, C. Wang, Y. Deng, Self-regulated phenomenon of inorganic artificial solid electrolyte interphase for lithium metal batteries. Nano Lett. 20, 4029–4037 (2020). [DOI] [PubMed] [Google Scholar]
- 43.Z. J. Ju, C. B. Jin, H. D. Yuan, T. Yang, O. W. Sheng, T. F. Liu, Y. J. Liu, Y. Wang, F. Y. Ma, W. K. Zhang, J. W. Nai, X. Y. Tao, A fast-ion conducting interface enabled by aluminum silicate fibers for stable Li metal batteries. Chem. Eng. J. 408, 128016 (2021). [Google Scholar]
- 44.K. Lu, S. Gao, R. J. Dick, Z. Sattar, Y. Cheng, A fast and stable Li metal anode incorporating an Mo6S8 artificial interphase with super Li-ion conductivity. J. Mater. Chem. A 7, 6038–6044 (2019). [Google Scholar]
- 45.N. Li, W. Wei, K. Xie, J. Tan, L. Zhang, X. Luo, K. Yuan, Q. Song, H. Li, C. Shen, E. M. Ryan, L. Liu, B. Wei, Suppressing dendritic lithium formation using porous media in lithium metal-based batteries. Nano Lett. 18, 2067–2073 (2018). [DOI] [PubMed] [Google Scholar]
- 46.B. Zhu, Y. Jin, X. Hu, Q. Zheng, S. Zhang, Q. Wang, J. Zhu, Poly(dimethylsiloxane) thin film as a stable interfacial layer for high-performance lithium-metal battery anodes. Adv. Mater. 29, 1603755 (2017). [DOI] [PubMed] [Google Scholar]
- 47.G. Y. Zheng, C. Wang, A. Pei, J. Lopez, F. Shi, Z. Chen, A. D. Sendek, H.-W. Lee, Z. Lu, H. Schneider, M. M. Safont-Sempere, S. Chu, Z. Bao, Y. Cui, High-performance lithium metal negative electrode with a soft and flowable polymer coating. ACS Energy Lett. 1, 1247–1255 (2016). [Google Scholar]
- 48.R. M. Gao, H. Yang, C. Y. Wang, H. Ye, F. F. Cao, Z. P. Guo, Fatigue-resistant interfacial layer for safe lithium metal batteries. Angew. Chem. Int. Ed. 60, 25508 (2021). [DOI] [PubMed] [Google Scholar]
- 49.Z. Zhang, Z. Peng, J. Zheng, S. Wang, Z. Liu, Y. Bi, Y. Chen, G. Wu, H. Li, P. Cui, Z. Wen, D. Wang, The long life-span of a Li-metal anode enabled by a protective layer based on the pyrolyzed N-doped binder network. J. Mater. Chem. A 5, 9339–9349 (2017). [Google Scholar]
- 50.W. Fan, R. Zhang, Z. Wang, X. Lei, C. Qin, X. Liu, Facile fabrication of polyether sulfone (PES) protecting layer on Cu foil for stable Li metal anode. Electrochim. Acta 260, 407–412 (2018). [Google Scholar]
- 51.H. Liu, R. Tao, C. Guo, W. Zhang, X. Liu, P. Guo, T. Zhang, J. Liang, Lithiated halloysite nanotube/cross-linked network polymer composite artificial solid electrolyte interface layer for high-performance lithium metal batteries. Chem. Eng. J. 429, 132239 (2022). [Google Scholar]
- 52.T. Wang, Y. Li, J. Zhang, K. Yan, P. Jaumaux, J. Yang, C. Wang, D. Shanmukaraj, B. Sun, M. Armand, Y. Cui, G. Wang, Immunizing lithium metal anodes against dendrite growth using protein molecules to achieve high energy batteries. Nat. Commun. 11, 5429 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.L. B. Mao, H. L. Gao, H. B. Yao, L. Liu, H. Colfen, G. Liu, S. M. Chen, S. K. Li, Y. X. Yan, Y. Y. Liu, S. H. Yu, Synthetic nacre by predesigned matrix-directed mineralization. Science 354, 107–110 (2016). [DOI] [PubMed] [Google Scholar]
- 54.Z. J. Ju, J. W. Nai, Y. Wang, T. F. Liu, J. H. Zheng, H. D. Yuan, O. W. Sheng, C. B. Jin, W. K. Zhang, Z. Jin, H. Tian, Y. J. Liu, X. Y. Tao, Biomacromolecules enabled dendrite-free lithium metal battery and its origin revealed by cryo-electron microscopy. Nat. Commun. 11, 488 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.D. Zhang, Y. Yin, C. Liu, S. Fan, Modified secondary lithium metal batteries with the polyaniline-carbon nanotube composite buffer layer. Chem. Commun. 51, 322–325 (2015). [DOI] [PubMed] [Google Scholar]
- 56.R. Xu, X.-Q. Zhang, X.-B. Cheng, H.-J. Peng, C.-Z. Zhao, C. Yan, J.-Q. Huang, Artificial soft-rigid protective layer for dendrite-free lithium metal anode. Adv. Funct. Mater. 28, 1705838 (2018). [Google Scholar]
- 57.M. S. Kim, M.-S. Kim, V. Do, Y. R. Lim, I. W. Nah, L. A. Archer, W. I. Cho, Designing solid-electrolyte interphases for lithium sulfur electrodes using ionic shields. Nano Energy 41, 573–582 (2017). [Google Scholar]
- 58.H. Xu, S. Li, C. Zhang, X. Chen, W. Liu, Y. Zheng, Y. Xie, Y. Huang, J. Li, Roll-to-roll prelithiation of Sn foil anode suppresses gassing and enables stable full-cell cycling of lithium ion batteries. Energ. Environ. Sci. 12, 2991–3000 (2019). [Google Scholar]
- 59.H. Wang, D. Lin, Y. Y. Liu, Y. Li, Y. Cui, Ultrahigh-current density anodes with interconnected Li metal reservoir through overlithiation of mesoporous AlF3 framework. Sci. Adv. 3, e1701301 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.M. Wan, S. Kang, L. Wang, H. W. Lee, G. W. Zheng, Y. Cui, Y. Sun, Mechanical rolling formation of interpenetrated lithium metal/lithium tin alloy foil for ultrahigh-rate battery anode. Nat. Commun. 11, 829 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.S. Liu, X. Ji, J. Yue, S. Hou, P. Wang, C. Cui, J. Chen, B. Shao, J. Li, F. Han, J. Tu, C. Wang, High interfacial-energy interphase promoting safe lithium metal batteries. J. Am. Chem. Soc. 142, 2438–2447 (2020). [DOI] [PubMed] [Google Scholar]
- 62.Z. Peng, N. Zhao, Z. Zhang, H. Wan, H. Lin, M. Liu, C. Shen, H. He, X. Guo, J.-G. Zhang, D. Wang, Stabilizing Li/electrolyte interface with a transplantable protective layer based on nanoscale LiF domains. Nano Energy 39, 662–672 (2017). [Google Scholar]
- 63.X. Shen, Y. Li, T. Qian, J. Liu, J. Zhou, C. Yan, J. B. Goodenough, Lithium anode stable in air for low-cost fabrication of a dendrite-free lithium battery. Nat. Commun. 10, 900 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Y. Yu, G. Huang, J. Z. Wang, K. Li, J. L. Ma, X. B. Zhang, In situ designing a gradient Li+ capture and quasi-spontaneous diffusion anode protection layer toward long-life Li-O2 batteries. Adv. Mater. 32, 2004157 (2020). [DOI] [PubMed] [Google Scholar]
- 65.S. Sun, S. Myung, G. Kim, D. Lee, H. Son, M. Jang, E. Park, B. Son, Y. Jung, U. Paik, T. Song, Facile ex situ formation of a LiF-polymer composite layer as an artificial SEI layer on Li metal by simple roll-press processing for carbonate electrolyte-based Li metal batteries. J. Mater. Chem. A 8, 17229–17237 (2020). [Google Scholar]
- 66.Y. He, Y. Zhang, Z. Wang, X. Li, Z. Lü, X. Huang, Z. Liu, In situ surface film formed by solid-state anodic oxidation for stable lithium metal anodes. Adv. Funct. Mater. 31, 2101737 (2021). [Google Scholar]
- 67.S. Ye, L. Wang, F. Liu, P. Shi, H. Wang, X. Wu, Y. Yu, g-C3N4 derivative artificial organic/inorganic composite solid electrolyte interphase layer for stable lithium metal anode. Adv. Energy Mater. 10, 2002647 (2020). [Google Scholar]
- 68.D. Lee, S. Sun, J. Kwon, H. Park, M. Jang, E. Park, B. Son, Y. Jung, T. Song, U. Paik, Copper nitride nanowires printed Li with stable cycling for Li metal batteries in carbonate electrolytes. Adv. Mater. 32, 1905573 (2020). [DOI] [PubMed] [Google Scholar]
- 69.Z. Luo, S. Li, L. Yang, Y. Tian, L. Xu, G. Zou, H. Hou, W. Wei, L. Chen, X. Ji, Interfacially redistributed charge for robust lithium metal anode. Nano Energy 87, 106212 (2021). [Google Scholar]
- 70.C. Yan, R. Xu, Y. Xiao, J.-F. Ding, L. Xu, B.-Q. Li, J.-Q. Huang, Toward critical electrode/electrolyte interfaces in rechargeable batteries. Adv. Funct. Mater. 30, 1909887 (2020). [Google Scholar]
- 71.Q. Zhang, P. Y. Chen, C. Yan, P. Chen, R. Zhang, Y. X. Yao, H. J. Peng, L. T. Yan, S. Kaskel, Selective permeable lithium-ion channels on lithium metal for practical lithium-sulfur pouch cells. Angew. Chem. Int. Ed. 133, 18179–18184 (2021). [DOI] [PubMed] [Google Scholar]
- 72.Z. Takehara, Z. Ogumi, Y. Uchimoto, K. Yasuda, H. Yoshida, Modification of lithium/electrolyte interface by plasma polymerization of 1,1-difluoroethene. J. Power Sources 43-44, 377–383 (1993). [Google Scholar]
- 73.J. Zhao, M. Hong, Z. Ju, X. Yan, Y. Gai, Z. Liang, Durable lithium metal anodes enabled by interfacial layers based on mechanically interlocked networks capable of energy dissipation. Angew. Chem. Int. Ed. 61, e202214386 (2022). [DOI] [PubMed] [Google Scholar]
- 74.Y. Gao, Y. Zhao, Y. C. Li, Q. Huang, T. E. Mallouk, D. Wang, Interfacial chemistry regulation via a skin-grafting strategy enables high-performance lithium-metal batteries. J. Am. Chem. Soc. 139, 15288–15291 (2017). [DOI] [PubMed] [Google Scholar]
- 75.J. Wu, Z. Rao, X. Liu, Y. Shen, C. Fang, L. Yuan, Z. Li, W. Zhang, X. Xie, Y. Huang, Polycationic polymer layer for air-stable and dendrite-free Li metal anodes in carbonate electrolytes. Adv. Mater. 33, 2007428 (2021). [DOI] [PubMed] [Google Scholar]
- 76.Q. Yang, J. Hu, J. Meng, C. Li, C–F-rich oil drop as a non-expendable fluid interface modifier with low surface energy to stabilize a Li metal anode. Energ. Environ. Sci. 14, 3621–3631 (2021). [Google Scholar]
- 77.Y. Xiao, R. Xu, C. Yan, Y. Liang, J.-F. Ding, J.-Q. Huang, Waterproof lithium metal anode enabled by cross-linking encapsulation. Sci. Bull. 65, 909 (2020). [DOI] [PubMed] [Google Scholar]
- 78.Z. Zhou, Y. Feng, J. Wang, B. Liang, Y. Li, Z. Song, D. M. Itkis, J. Song, A robust, highly stretchable ion-conducive skin for stable lithium metal batteries. Chem. Eng. J. 396, 125254 (2020). [Google Scholar]
- 79.Y. Yao, X. Zhao, A. A. Razzaq, Y. Gu, X. Yuan, R. Shah, Y. Lian, J. Lei, Q. Mu, Y. Ma, Y. Peng, Z. Deng, Z. Liu, Mosaic rGO layers on lithium metal anodes for the effective mediation of lithium plating and stripping. J. Mater. Chem. A 7, 12214–12224 (2019). [Google Scholar]
- 80.W. Liu, Y. Xia, W. Wang, Y. Wang, J. Jin, Y. Chen, E. Paek, D. Mitlin, Pristine or highly defective? Understanding the role of graphene structure for stable lithium metal plating. Adv. Energy Mater. 9, 1802918 (2018). [Google Scholar]
- 81.Y. T. Chen, S. A. Abbas, N. Kaisar, S. H. Wu, H. A. Chen, K. M. Boopathi, M. Singh, J. Fang, C. W. Pao, C. W. Chu, Mitigating metal dendrite formation in lithium-sulfur batteries via morphology-tunable graphene oxide interfaces. ACS Appl. Mater. Inter. 11, 2060–2070 (2019). [DOI] [PubMed] [Google Scholar]
- 82.H. Zhang, X. Liao, Y. Guan, Y. Xiang, M. Li, W. Zhang, X. Zhu, H. Ming, L. Lu, J. Qiu, Y. Huang, G. Cao, Y. Yang, L. Mai, Y. Zhao, H. Zhang, Lithiophilic-lithiophobic gradient interfacial layer for a highly stable lithium metal anode. Nat. Commun. 9, 3729 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.D. J. Lee, H. Lee, Y. J. Kim, J. K. Park, H. T. Kim, Sustainable redox mediation for lithium-oxygen batteries by a composite protective layer on the lithium-metal anode. Adv. Mater. 28, 857–863 (2016). [DOI] [PubMed] [Google Scholar]
- 84.W.-J. Kwak, S.-J. Park, H.-G. Jung, Y.-K. Sun, Optimized concentration of redox mediator and surface protection of Li metal for maintenance of high energy efficiency in Li-O2 batteries. Adv. Energy Mater. 8, 1702258 (2018). [Google Scholar]
- 85.G. Jiang, K. Li, F. Yu, X. Li, J. Mao, W. Jiang, F. Sun, B. Dai, Y. Li, Robust artificial solid-electrolyte interfaces with biomimetic ionic channels for dendrite-free Li metal anodes. Adv. Energy Mater. 11, 2003496 (2021). [Google Scholar]
- 86.L. Fan, Z. Guo, Y. Zhang, X. Wu, C. Zhao, X. Sun, G. Yang, Y. Feng, N. Zhang, Stable artificial solid electrolyte interphase films for lithium metal anode via metal-organic frameworks cemented by polyvinyl alcohol. J. Mater. Chem. A 8, 251–258 (2020). [Google Scholar]
- 87.X. Li, Y. Tian, L. Shen, Z. Qu, T. Ma, F. Sun, X. Liu, C. Zhang, J. Shen, X. Li, L. Gao, S. Xiao, T. Liu, Y. Liu, Y. F. Lu, Electrolyte interphase built from anionic covalent organic frameworks for lithium dendrite suppression. Adv. Funct. Mater. 31, 2009718 (2021). [Google Scholar]
- 88.Z. Wang, Y. Wang, Z. Zhang, X. Chen, W. Lie, Y.-B. He, Z. Zhou, G. Xia, Z. Guo, Building artificial solid-electrolyte interphase with uniform intermolecular ionic bonds toward dendrite-free lithium metal anodes. Adv. Funct. Mater. 30, 2002414 (2020). [Google Scholar]
- 89.X. Zhang, Q. Zhang, X. G. Wang, C. Wang, Y. N. Chen, Z. Xie, Z. Zhou, An extremely simple method for protecting lithium anodes in Li-O2 batteries. Angew. Chem. Int. Ed. 57, 12814–12818 (2018). [DOI] [PubMed] [Google Scholar]
- 90.P. Gao, C. Zhang, G. Wen, Equivalent circuit model analysis on electrochemical impedance spectroscopy of lithium metal batteries. J. Power Sources 294, 67–74 (2015). [Google Scholar]
- 91.N. D. Trinh, D. Lepage, D. Ayme-Perrot, A. Badia, M. Dolle, D. Rochefort, An artificial lithium protective layer that enables the use of acetonitrile-based electrolytes in lithium metal batteries. Angew. Chem. Int. Ed. 57, 5072–5075 (2018). [DOI] [PubMed] [Google Scholar]
- 92.C. Yan, X. B. Cheng, Y. Tian, X. Chen, X. Q. Zhang, W. J. Li, J. Q. Huang, Q. Zhang, Dual-layered film protected lithium metal anode to enable dendrite-free lithium deposition. Adv. Mater. 30, 1707629 (2018). [DOI] [PubMed] [Google Scholar]
- 93.X. Liu, J. Liu, T. Qian, H. Chen, C. Yan, Novel organophosphate-derived dual-layered interface enabling air-stable and dendrite-free lithium metal anode. Adv. Mater. 32, 1902724 (2020). [DOI] [PubMed] [Google Scholar]
- 94.N. W. Li, Y. Shi, Y. X. Yin, X. X. Zeng, J. Y. Li, C. J. Li, L. J. Wan, R. Wen, Y. G. Guo, A flexible solid electrolyte interphase layer for long-life lithium metal anodes. Angew. Chem. Int. Ed. 57, 1505–1509 (2018). [DOI] [PubMed] [Google Scholar]
- 95.G. Wang, C. Chen, Y. Chen, X. Kang, C. Yang, F. Wang, Y. Liu, X. Xiong, Self-stabilized and strongly adhesive supramolecular polymer protective layer enables ultrahigh-rate and large-capacity lithium-metal anode. Angew. Chem. Int. Ed. 59, 2055–2060 (2020). [DOI] [PubMed] [Google Scholar]
- 96.C. Chen, Q. Liang, G. Wang, D. Liu, X. Xiong, Grain-boundary-rich artificial SEI layer for high-rate lithium metal anodes. Adv. Funct. Mater. 32, 2107249 (2021). [Google Scholar]
- 97.M. Bai, K. Xie, K. Yuan, K. Zhang, N. Li, C. Shen, Y. Lai, R. Vajtai, P. Ajayan, B. Wei, A scalable approach to dendrite-free lithium anodes via spontaneous reduction of spray-coated graphene oxide layers. Adv. Mater. 30, 1801213 (2018). [DOI] [PubMed] [Google Scholar]
- 98.Y. L. Cui, S. F. Liu, D. H. Wang, X. L. Wang, X. H. Xia, C. D. Gu, J. P. Tu, A facile way to construct stable and ionic conductive lithium sulfide nanoparticles composed solid electrolyte interphase on Li metal anode. Adv. Funct. Mater. 31, 2006380 (2020). [Google Scholar]
- 99.J. Liang, X. Li, Y. Zhao, L. V. Goncharova, G. Wang, K. R. Adair, C. Wang, R. Li, Y. Zhu, Y. Qian, L. Zhang, R. Yang, S. Lu, X. Sun, In situ Li3PS4 solid-state electrolyte protection layers for superior long-life and high-rate lithium-metal anodes. Adv. Mater. 30, 1804684 (2018). [DOI] [PubMed] [Google Scholar]
- 100.H. Su, Y. Liu, Y. Zhong, J. Li, X. Wang, X. Xia, C. Gu, J. Tu, Stabilizing the interphase between Li and argyrodite electrolyte through synergistic phosphating process for all-solid-state lithium batteries. Nano Energy 96, 107104 (2022). [Google Scholar]
- 101.X. Liang, Q. Pang, I. R. Kochetkov, M. S. Sempere, H. Huang, X. Sun, L. F. Nazar, A facile surface chemistry route to a stabilized lithium metal anode. Nat. Energy 2, 17119 (2017). [Google Scholar]
- 102.T. Chen, W. Kong, P. Zhao, H. Lin, Y. Hu, R. Chen, W. Yan, Z. Jin, Dendrite-free and stable lithium metal anodes enabled by an antimony-based lithiophilic interphase. Chem. Mater. 31, 7565–7573 (2019). [Google Scholar]
- 103.A. Hu, W. Chen, X. Du, Y. Hu, T. Lei, H. Wang, L. Xue, Y. Li, H. Sun, Y. Yan, J. Long, C. Shu, J. Zhu, B. Li, X. Wang, J. Xiong, An artificial hybrid interphase for an ultrahigh-rate and practical lithium metal anode. Energ. Environ. Sci. 14, 4115–4124 (2021). [Google Scholar]
- 104.W. Guo, Q. Han, J. Jiao, W. Wu, X. Zhu, Z. Chen, Y. Zhao, In situ construction of robust biphasic surface layers on lithium metal for lithium-sulfide batteries with long cycle life. Angew. Chem. Int. Ed. 60, 7267–7274 (2021). [DOI] [PubMed] [Google Scholar]
- 105.C. Wei, L. Tan, Y. Tao, Y. An, Y. Tian, H. Jiang, J. Feng, Y. Qian, Interfacial passivation by room-temperature liquid metal enabling stable 5 V-class lithium-metal batteries in commercial carbonate-based electrolyte. Energy Storage Mater. 34, 12–21 (2021). [Google Scholar]
- 106.L. Lin, L. Suo, Y. Hu, H. Li, X. Huang, L. Chen, Epitaxial induced plating current-collector lasting lifespan of anode-free lithium metal battery. Adv. Energy Mater. 11, 2003709 (2021). [Google Scholar]
- 107.Q. Jin, X. Zhang, H. Gao, L. Li, Z. Zhang, Novel LixSiSy/nafion as an artificial SEI film to enable dendrite-free Li metal anodes and high stability Li-S batteries. J. Mater. Chem. A 8, 8979–8988 (2020). [Google Scholar]
- 108.Y. Zhao, G. Li, Y. Gao, D. Wang, Q. Huang, D. Wang, Stable Li metal anode by a hybrid lithium polysulfidophosphate/polymer cross-linking film. ACS Energy Lett. 4, 1271–1278 (2019). [Google Scholar]
- 109.Q. Zhao, Z. Y. Tu, S. Wei, K. H. Zhang, S. Choudhury, X. Liu, L. A. Archer, Building organic/inorganic hybrid interphases for fast interfacial transport in rechargeable metal batteries. Angew. Chem. Int. Ed. 130, 1004–1008 (2018). [DOI] [PubMed] [Google Scholar]
- 110.Y. Gao, Z. Yan, J. L. Gray, X. He, D. Wang, T. Chen, Q. Huang, Y. C. Li, H. Wang, S. H. Kim, T. E. Mallouk, D. Wang, Polymer-inorganic solid-electrolyte interphase for stable lithium metal batteries under lean electrolyte conditions. Nat. Mater. 18, 384–389 (2019). [DOI] [PubMed] [Google Scholar]
- 111.K. Yan, J. Wang, S. Zhao, D. Zhou, B. Sun, Y. Cui, G. Wang, Temperature-dependent nucleation and growth of dendrite-free lithium metal anodes. Angew. Chem. Int. Ed. 58, 11364–11368 (2019). [DOI] [PubMed] [Google Scholar]
- 112.Y. Gu, W. W. Wang, Y. J. Li, Q. H. Wu, S. Tang, J. W. Yan, M. S. Zheng, D. Y. Wu, C. H. Fan, W. Q. Hu, Z. B. Chen, Y. Fang, Q. H. Zhang, Q. F. Dong, B. W. Mao, Designable ultra-smooth ultra-thin solid-electrolyte interphases of three alkali metal anodes. Nat. Commun. 9, 1339 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.W. W. Wang, Y. Gu, H. Yan, S. Li, J.-W. He, H.-Y. Xu, Q.-H. Wu, J.-W. Yan, B.-W. Mao, Evaluating solid-electrolyte interphases for lithium and lithium-free anodes from nanoindentation features. Chem 6, 2728–2745 (2020). [Google Scholar]
- 114.X.-D. Lin, Y. Gu, X.-R. Shen, W.-W. Wang, Y.-H. Hong, Q.-H. Wu, Z.-Y. Zhou, D.-Y. Wu, J.-K. Chang, M.-S. Zheng, B.-W. Mao, Q.-F. Dong, An oxygen-blocking oriented multifunctional solid-electrolyte interphase as a protective layer for a lithium metal anode in lithium-oxygen batteries. Energ. Environ. Sci. 14, 1439–1448 (2021). [Google Scholar]
- 115.Y.-K. Huang, R. Pan, D. Rehnlund, Z. Wang, L. Nyholm, First-cycle oxidative generation of lithium nucleation sites stabilizes lithium-metal electrodes. Adv. Energy Mater. 11, 2003674 (2021). [Google Scholar]
- 116.B. Liu, W. Xu, J. Tao, P. Yan, J. Zheng, M. H. Engelhard, D. Lu, C. Wang, J.-G. Zhang, Enhanced cyclability of lithium-oxygen batteries with electrodes protected by surface films induced via in situ electrochemical process. Adv. Energy Mater. 8, 1702340 (2018). [Google Scholar]
- 117.G. Hou, X. Ma, Q. Sun, Q. Ai, X. Xu, L. Chen, D. Li, J. Chen, H. Zhong, Y. Li, Z. Xu, P. Si, J. Feng, L. Zhang, F. Ding, L. Ci, Lithium dendrite suppression and enhanced interfacial compatibility enabled by an ex situ SEI on Li anode for LAGP-based all-solid-state batteries. ACS Appl. Mater. Inter. 10, 18610–18618 (2018). [DOI] [PubMed] [Google Scholar]
- 118.Y. Gao, D. Wang, Y. C. Li, Z. Yu, T. E. Mallouk, D. Wang, Salt-based organic-inorganic nanocomposites: Towards a stable lithium metal/Li10GeP2S12 solid electrolyte interface. Angew. Chem. Int. Ed. 57, 13608–13612 (2018). [DOI] [PubMed] [Google Scholar]
- 119.X.-B. Cheng, C. Yan, X. Chen, C. Guan, J.-Q. Huang, H.-J. Peng, R. Zhang, S.-T. Yang, Q. Zhang, Implantable solid electrolyte interphase in lithium-metal batteries. Chem 2, 258–270 (2017). [Google Scholar]
- 120.G. Lu, J. Nai, H. Yuan, J. Wang, J. Zheng, Z. Ju, C. Jin, Y. Wang, T. Liu, Y. Liu, X. Tao, In-situ electrodeposition of nanostructured carbon strengthened interface for stabilizing lithium metal anode. ACS Nano 16, 9883–9893 (2022). [DOI] [PubMed] [Google Scholar]
- 121.Y. Ma, L. Li, J. Qian, W. Qu, R. Luo, F. Wu, R. Chen, Materials and structure engineering by magnetron sputtering for advanced lithium batteries. Energy Storage Mater. 39, 203–224 (2021). [Google Scholar]
- 122.D. C. Lin, Y. Y. Liu, W. Chen, G. M. Zhou, K. Liu, B. Dunn, Y. Cui, Conformal lithium fluoride protection layer on three-dimensional lithium by nonhazardous gaseous reagent freon. Nano Lett. 17, 3731–3737 (2017). [DOI] [PubMed] [Google Scholar]
- 123.W. Tang, X. Yin, S. Kang, Z. Chen, B. Tian, S. L. Teo, X. Wang, X. Chi, K. P. Loh, H. W. Lee, G. W. Zheng, Lithium silicide surface enrichment: A solution to lithium metal battery. Adv. Mater. 30, 1801745 (2018). [DOI] [PubMed] [Google Scholar]
- 124.R. Pathak, K. Chen, A. Gurung, K. M. Reza, B. Bahrami, F. Wu, A. Chaudhary, N. Ghimire, B. Zhou, W.-H. Zhang, Y. Zhou, Q. Qiao, Ultrathin bilayer of graphite/SiO2 as solid interface for reviving Li metal anode. Adv. Energy Mater. 9, 1901486 (2019). [Google Scholar]
- 125.L. Fan, H. L. Zhuang, L. Gao, Y. Y. Lu, L. A. Archer, Regulating Li deposition at artificial solid electrolyte interphases. J. Mater. Chem. A 5, 3483–3492 (2017). [Google Scholar]
- 126.E. Cha, M. D. Patel, J. Park, J. Hwang, V. Prasad, K. Cho, W. Choi, 2D MoS2 as an efficient protective layer for lithium metal anodes in high-performance Li-S batteries. Nat. Nanotechnol. 13, 337–344 (2018). [DOI] [PubMed] [Google Scholar]
- 127.P. Li, W. Feng, X. Dong, Y. Wang, Y. Xia, A new strategy of constructing a highly fluorinated solid-electrolyte interface towards high-performance lithium anode. Adv. Mater. Inter. 7, 2000154 (2020). [Google Scholar]
- 128.Q. Li, H. Pan, W. Li, Y. Wang, J. Wang, J. Zheng, X. Yu, H. Li, L. Chen, Homogeneous interface conductivity for lithium dendrite-free anode. ACS Energy Lett. 3, 2259–2266 (2018). [Google Scholar]
- 129.L. Wang, Q. Wang, W. Jia, S. Chen, P. Gao, J. Li, Li metal coated with amorphous Li3PO4 via magnetron sputtering for stable and long-cycle life lithium metal batteries. J. Power Sources 342, 175–182 (2017). [Google Scholar]
- 130.C. Gao, Q. Dong, G. Zhang, H. Fan, H. Li, B. Hong, Y. Lai, Antimony-doped lithium phosphate artificial solid electrolyte interphase for dendrite-free lithium-metal batteries. ChemElectroChem 6, 1134–1138 (2019). [Google Scholar]
- 131.K. K. Fu, Y. Gong, Z. Fu, H. Xie, Y. Yao, B. Liu, M. Carter, E. Wachsman, L. Hu, Transient behavior of the metal interface in lithium metal-garnet batteries. Angew. Chem. Int. Ed. 56, 14942–14947 (2017). [DOI] [PubMed] [Google Scholar]
- 132.X. Hao, Q. Zhao, S. Su, S. Zhang, J. Ma, L. Shen, Q. Yu, L. Zhao, Y. Liu, F. Kang, Y.-B. He, Constructing multifunctional interphase between Li1.4Al0.4Ti1.6(PO4)3 and Li metal by magnetron sputtering for highly stable solid-state lithium metal batteries. Adv. Energy Mater. 9, 1901604 (2019). [Google Scholar]
- 133.Y. J. Zhang, X. Y. Liu, W. Q. Bai, H. Tang, S. J. Shi, X. L. Wang, C. D. Gu, J. P. Tu, Magnetron sputtering amorphous carbon coatings on metallic lithium: Towards promising anodes for lithium secondary batteries. J. Power Sources 266, 43–50 (2014). [Google Scholar]
- 134.T. Chen, F. Meng, Z. Zhang, J. Liang, Y. Hu, W. Kong, X. L. Zhang, Z. Jin, Stabilizing lithium metal anode by molecular beam epitaxy grown uniform and ultrathin bismuth film. Nano Energy 76, 105068 (2020). [Google Scholar]
- 135.K. Yan, H. W. Lee, T. Gao, G. Zheng, H. Yao, H. Wang, Z. Lu, Y. Zhou, Z. Liang, Z. Liu, S. Chu, Y. Cui, Ultrathin two-dimensional atomic crystals as stable interfacial layer for improvement of lithium metal anode. Nano Lett. 14, 6016–6022 (2014). [DOI] [PubMed] [Google Scholar]
- 136.Q. Xu, J. Lin, C. Ye, X. Jin, D. Ye, Y. Lu, G. Zhou, Y. Qiu, W. Li, Air-stable and dendrite-free lithium metal anodes enabled by a hybrid interphase of C60 and Mg. Adv. Energy Mater. 10, 1903292 (2019). [Google Scholar]
- 137.S. Stalin, P. Chen, G. Li, Y. Deng, Z. Rouse, Y. Cheng, Z. Zhang, P. Biswal, S. Jin, S. P. Baker, R. Yang, L. A. Archer, Ultrathin zwitterionic polymeric interphases for stable lithium metal anodes. Matter 4, 3753–3773 (2021). [Google Scholar]
- 138.J. Y. Kim, A. Y. Kim, G. Liu, J. Y. Woo, H. Kim, J. K. Lee, Li4SiO4-based artificial passivation thin film for improving interfacial stability of Li metal anodes. ACS Appl. Mater. Inter. 10, 8692–8701 (2018). [DOI] [PubMed] [Google Scholar]
- 139.Y. Zhao, K. Zheng, X. L. Sun, Addressing interfacial issues in liquid-based and solid-state batteries by atomic and molecular layer deposition. Joule 2, 2583–2604 (2018). [Google Scholar]
- 140.J. Xie, L. Liao, Y. Gong, Y. Li, F. Shi, A. Pei, J. Sun, R. Zhang, B. Kong, R. Subbaraman, J. Christensen, Y. Cui, Stitching h-BN by atomic layer deposition of LiF as a stable interface for lithium metal anode. Sci. Adv. 3, eaao3170 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.M. Wang, X. Cheng, T. Cao, J. Niu, R. Wu, X. Liu, Y. Zhang, Constructing ultrathin TiO2 protection layers via atomic layer deposition for stable lithium metal anode cycling. J. Alloy. Compd. 865, 158748 (2021). [Google Scholar]
- 142.P. K. Alaboina, S. Rodrigues, M. Rottmayer, S. J. Cho, In situ dendrite suppression study of nanolayer encapsulated Li metal enabled by zirconia atomic layer deposition. ACS Appl. Mater. Inter. 10, 32801–32808 (2018). [DOI] [PubMed] [Google Scholar]
- 143.A. C. Kozen, C. F. Lin, A. J. Pearse, M. A. Schroeder, X. Han, L. Hu, S. B. Lee, G. W. Rubloff, M. Noked, Next-generation lithium metal anode engineering via atomic layer deposition. ACS Nano 9, 5884–5892 (2015). [DOI] [PubMed] [Google Scholar]
- 144.Y. Zhao, X. L. Sun, Molecular layer deposition for energy conversion and storage. ACS Energy Lett. 3, 899–914 (2018). [Google Scholar]
- 145.C. Wang, Y. Zhao, Q. Sun, X. Li, Y. Liu, J. Liang, X. Li, X. Lin, R. Li, K. R. Adair, L. Zhang, R. Yang, S. Lu, X. Sun, Stabilizing interface between Li10SnP2S12 and Li metal by molecular layer deposition. Nano Energy 53, 168–174 (2018). [Google Scholar]
- 146.Y. P. Sun, Y. Zhao, J. Wang, J. Liang, C. Wang, Q. Sun, X. Lin, K. R. Adai, J. Luo, D. Wang, R. Li, M. Cai, T. K. Sham, X. L. Sun, A novel organic "polyurea" thin film for ultralong-life lithium-metal anodes via molecular-layer deposition. Adv. Mater. 31, 1806541 (2019). [DOI] [PubMed] [Google Scholar]
- 147.Y. Sun, M. Amirmaleki, Y. Zhao, C. Zhao, J. Liang, C. Wang, K. R. Adair, J. Li, T. Cui, G. Wang, R. Li, T. Filleter, M. Cai, T.-K. Sham, X. L. Sun, Tailoring the mechanical and electrochemical properties of an artificial interphase for high-performance metallic lithium anode. Adv. Energy Mater. 10, 2001139 (2020). [Google Scholar]
- 148.Y. Sun, C. Zhao, K. R. Adair, Y. Zhao, L. V. Goncharova, J. Liang, C. Wang, J. Li, R. Li, M. Cai, T.-K. Sham, X. Sun, Regulated lithium plating and stripping by a nano-scale gradient inorganic-organic coating for stable lithium metal anodes. Energ. Environ. Sci. 14, 4085–4094 (2021). [Google Scholar]
- 149.Y. Zhao, M. Amirmaleki, Q. Sun, C. Zhao, A. Codirenzi, L. V. Goncharova, C. Wang, K. Adair, X. Li, X. Yang, F. Zhao, R. Li, T. Filleter, M. Cai, X. Sun, Natural SEI-inspired dual-protective layers via atomic/molecular layer deposition for long-life metallic lithium anode. Matter 1, 1215–1231 (2019). [Google Scholar]
- 150.J. Zhao, L. Liao, F. Shi, T. Lei, G. Chen, A. Pei, J. Sun, K. Yan, G. Zhou, J. Xie, C. Liu, Y. Li, Z. Liang, Z. Bao, Y. Cui, Surface fluorination of reactive battery anode materials for enhanced stability. J. Am. Chem. Soc. 139, 11550–11558 (2017). [DOI] [PubMed] [Google Scholar]
- 151.Y. Li, Y. Sun, A. Pei, K. Chen, A. Vailionis, Y. Li, G. Zheng, J. Sun, Y. Cui, Robust pinhole-free Li3N solid electrolyte grown from molten lithium. ACS Cent. Sci. 4, 97–104 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Y. Lin, Z. Wen, J. Liu, D. Wu, P. Zhang, J. Zhao, Constructing a uniform lithium iodide layer for stabilizing lithium metal anode. J. Energy Chem. 55, 129–135 (2021). [Google Scholar]
- 153.Y. Nan, S. Li, M. Zhu, B. Li, S. Yang, Endowing lithium metal surface with self-healing property via an in situ gas-solid reaction for high-performance lithium metal batteries. ACS Appl. Mater. Inter. 11, 28878–28884 (2019). [DOI] [PubMed] [Google Scholar]
- 154.H. Chen, A. Pei, D. Lin, J. Xie, A. Yang, J. Xu, K. Lin, J. Wang, H. Wang, F. Shi, D. Boyle, Y. Cui, Uniform high ionic conducting lithium sulfide protection layer for stable lithium metal anode. Adv. Energy Mater. 9, 1900858 (2019). [Google Scholar]
- 155.F. Liu, L. Wang, Z. Zhang, P. Shi, Y. Feng, Y. Yao, S. Ye, H. Wang, X. Wu, Y. Yu, A mixed lithium-ion conductive Li2S/Li2Se protection layer for stable lithium metal anode. Adv. Funct. Mater. 30, 2001607 (2020). [Google Scholar]
- 156.H. F. Zandy, Plasma: The fourth state of matter. The Physics Teacher 8, 27–31 (1970). [Google Scholar]
- 157.J. Zheng, R. Yang, L. Xie, J. Qu, Y. Liu, X. Li, Plasma-assisted approaches in inorganic nanostructure fabrication. Adv. Mater. 22, 1451–1473 (2010). [DOI] [PubMed] [Google Scholar]
- 158.H. F. Liang, F. W. Ming, H. N. Alshareef, Applications of plasma in energy conversion and storage materials. Adv. Energy Mater. 8, 1801804 (2018). [Google Scholar]
- 159.K. Chen, R. Pathak, A. Gurung, E. A. Adhamash, B. Bahrami, Q. He, H. Qiao, A. L. Smirnova, J. J. Wu, Q. Qiao, Y. Zhou, Flower-shaped lithium nitride as a protective layer via facile plasma activation for stable lithium metal anodes. Energy Storage Mater. 18, 389–396 (2019). [Google Scholar]
- 160.S. Cao, X. He, L. Nie, J. Hu, M. Chen, Y. Han, K. Wang, K. Jiang, M. Zhou, CF4 plasma-generated LiF-Li2C2 artificial layers for dendrite-free lithium-metal anodes. Adv. Sci. 9, 2201147 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.X. Jin, Z. Cai, X. Zhang, J. Yu, Q. He, Z. Lu, M. Dahbi, J. Alami, J. Lu, K. Amine, H. Zhang, Transferring liquid metal to form a hybrid solid electrolyte via a wettability-tuning technology for lithium-metal anodes. Adv. Mater. 34, e2200181 (2022). [DOI] [PubMed] [Google Scholar]
- 162.K. Liu, A. Pei, H. R. Lee, B. Kong, N. Liu, D. Lin, Y. Liu, C. Liu, P. C. Hsu, Z. Bao, Y. Cui, Lithium metal anodes with an adaptive "solid-liquid" interfacial protective layer. J. Am. Chem. Soc. 139, 4815–4820 (2017). [DOI] [PubMed] [Google Scholar]
- 163.H. D. Yuan, J. W. Nai, H. Tian, Z. J. Ju, W. K. Zhang, Y. J. Liu, X. Y. Tao, X. W. Lou, An ultrastable lithium metal anode enabled by designed metal fluoride spansules. Sci. Adv. 6, eaaz3112 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Y. Gao, T. Rojas, K. Wang, S. Liu, D. Wang, T. Chen, H. Wang, A. T. Ngo, D. Wang, Low-temperature and high-rate-charging lithium metal batteries enabled by an electrochemically active monolayer-regulated interface. Nat. Energy 5, 534–542 (2020). [Google Scholar]
- 165.X. Wei, X. Wang, Q. An, C. Han, L. Q. Mai, Operando X-ray diffraction characterization for understanding the intrinsic electrochemical mechanism in rechargeable battery materials. Small Methods 1, 1700083 (2017). [Google Scholar]
- 166.S. Mourdikoudis, R. M. Pallares, N. T. K. Thanh, Characterization techniques for nanoparticles: Comparison and complementarity upon studying nanoparticle properties. Nanoscale 10, 12871–12934 (2018). [DOI] [PubMed] [Google Scholar]
- 167.Z. Shadike, E. Zhao, Y.-N. Zhou, X. Yu, Y. Yang, E. Hu, S. Bak, L. Gu, X.-Q. Yang, Advanced characterization techniques for sodium-ion battery studies. Adv. Energy Mater. 8, 1702588 (2018). [Google Scholar]
- 168.R. Xu, J. Lu, K. Amine, Progress in mechanistic understanding and characterization techniques of Li-S batteries. Adv. Energy Mater. 5, 1500408 (2015). [Google Scholar]
- 169.H. Park, O. Tamwattana, J. Kim, S. Buakeaw, R. Hongtong, B. Kim, P. Khomein, G. Liu, N. Meethong, K. Kang, Probing lithium metals in batteries by advanced characterization and analysis tools. Adv. Energy Mater. 11, 2003039 (2020). [Google Scholar]
- 170.Y. Xu, K. Dong, Y. Jie, P. Adelhelm, Y. Chen, L. Xu, P. Yu, J. Kim, Z. Kochovski, Z. Yu, W. Li, J. LeBeau, Y. Shao-Horn, R. Cao, S. Jiao, T. Cheng, I. Manke, Y. Lu, Promoting mechanistic understanding of lithium deposition and solid-electrolyte interphase (SEI) formation using advanced characterization and simulation methods: Recent progress, limitations, and future perspectives. Adv. Energy Mater. 12, 2200398 (2022). [Google Scholar]
- 171.X. Q. Zhang, X. Chen, X. B. Cheng, B. Q. Li, X. Shen, C. Yan, J. Q. Huang, Q. Zhang, Highly stable lithium metal batteries enabled by regulating the solvation of lithium ions in nonaqueous electrolytes. Angew. Chem. Int. Ed. 57, 5301–5305 (2018). [DOI] [PubMed] [Google Scholar]
- 172.Y. Li, K. Wang, W. Zhou, Y. Li, R. Vila, W. Huang, H. Wang, G. Chen, G.-H. Wu, Y. Tsao, H. Wang, R. Sinclair, W. Chiu, Y. Cui, Cryo-EM structures of atomic surfaces and host-guest chemistry in metal-organic frameworks. Matter 1, 428–438 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Y. Li, Y. Li, A. Pei, K. Yan, Y. Sun, C.-L. Wu, L.-M. Joubert, R. Chin, A. L. Koh, Y. Yu, J. Perrino, B. Butz, S. Chu, Y. Cui, Atomic structure of sensitive battery materials and interfaces revealed by cryo-electron microscopy. Science 358, 506–510 (2017). [DOI] [PubMed] [Google Scholar]
- 174.H. Yuan, J. Nai, Y. Fang, G. Lu, X. Tao, X. W. D. Lou, Double-shelled C@MoS2 structures preloaded with sulfur: An additive reservoir for stable lithium metal anodes. Angew. Chem. Int. Ed. 59, 15839–15843 (2020). [DOI] [PubMed] [Google Scholar]
- 175.O. W. Sheng, J. H. Zheng, Z. J. Ju, C. B. Jin, Y. Wang, M. Chen, J. W. Nai, T. F. Liu, W. K. Zhang, Y. J. Liu, X. Y. Tao, In situ construction of a LiF-enriched interface for stable all-solid-state batteries and its origin revealed by cryo-TEM. Adv. Mater. 32, 2000223 (2020). [DOI] [PubMed] [Google Scholar]
- 176.O. W. Sheng, C. B. Jin, Z. J. Ju, J. H. Zheng, T. F. Liu, Y. J. Liu, Y. Wang, J. M. Luo, X. Y. Tao, J. W. Nai, Stabilizing Li4SnS4 electrolyte from interface to bulk phase with a gradient lithium iodide/polymer layer in lithium metal batteries. Nano Lett. 22, 8346–8354 (2022). [DOI] [PubMed] [Google Scholar]
- 177.A. Dazzi, F. Glotin, R. Carminati, Theory of infrared nanospectroscopy by photothermal induced resonance. J. Appl. Phy. 107, 124519 (2010). [Google Scholar]
- 178.F. Lu, M. Jin, M. A. Belkin, Tip-enhanced infrared nanospectroscopy via molecular expansion force detection. Nat. Photonics 8, 307–312 (2014). [Google Scholar]