Abstract
The school-age years is a period of increasing social interaction with peers and development of emotion regulation in facilitating that interaction. This study was an investigation of the neural correlates of emotional reactivity and reappraisal in typically developing school-age children elicited by threatening facial expressions of same-aged peers. This experimental paradigm is novel in eliciting event-related brain potentials (ERPs) to social stimuli that are ecologically valid to the everyday life of children. ERPs of 5- to 8-year-old children (N = 41, 18 females) were elicited by threatening (i.e., angry and fearful) and neutral child facial expressions, which were preceded by audio contextual cues. Three conditions differed in audio-image pairing: neutral context-neutral expression (neutral condition), negative context-threatening expression (threat condition), and reappraisal context-threatening expression (reappraisal condition). In addition, parental reporting of childhood temperament was collected to determine if elicited ERP morphologies were associated with temperamental dimensions of negative affect, extraversion, and effortful control. Elicitation of the P100 and N170 did not largely differ between conditions; however, amplitude of the late positive potential (LPP), a marker of heightened emotional reactivity and attention, was greater for threatening faces relative to neutral faces. During the reappraisal condition, no differences in ERP activity was observed compared to the threat condition. Neural substrates of emotional reactivity to social threat from peers were evident; however, the lack of ERP modulation facilitating reappraisal and the lack of strong associations between ERP morphology and temperamental dimensions is indicative of heterogeneity in LPP elicitation underlying emotion regulation in children.
Keywords: EEG, event-related potentials, late positive potential, emotional reactivity, emotion regulation, cognitive reappraisal
1. Introduction
Appropriate interpretation of another’s emotional state and the ability to regulate emotional arousal, is critical for effective social functioning and interpersonal communication (Garner & Waajid, 2012; Izard, Fine, Schultz, Mostow, Ackerman, & Youngstrom, 2001). Emotional reactivity refers to the arousal of cognitive, autonomic, and endocrine processes as attention is oriented towards a stimulus and appraised of its motivational significance (Rothbart & Derryberry, 1982). Social stimuli of varying valence and intensity elicit temperamental proclivities of negative affect and extraversion that shape a child’s experience with their social environment. Such reactivity can be regulated by developing executive function mechanisms, such as cognitive reappraisal – the reinterpretation of the salience of a stimulus given situational context (Goldin, McRae, Ramel, & Gross, 2008). The ability to regulate that reactivity through to suit environmental context has been defined as effortful control (Gross & John, 2003). Effective regulation of emotional reactivity to threatening information, such as fearful or sad facial expressions, is a necessary skill for contextually appropriate emotions and social relations that facilitate overall wellbeing (John & Gross, 2004).
The presentation of negatively valanced and arousing facial expressions capture attentional and visual processes associated with electrocortical potentiation across occipital and parietal brain regions (Öhman, Flykt, & Esteves, 2001; Vuilleumier, Richardson, Armony, Driver, & Dolan, 2004). Although peer interactions increase into the school-age years and neural activation associated with the processing of child facial expressions differs from commonly-used adult facial stimuli (Hoehl, Brauer, Brasse, Striano, & Friederici, 2010; Marusak, Carré, & Thomason, 2013), little is known regarding the neural correlates of emotional reactivity in children elicited by the facial expressions of own-age peers. In the current study, event-related brain potentials (ERPs) associated with emotional reactivity and regulation were recorded from school-age children in response to seeing facial expressions of own-age peers. Understanding these neural correlates of emotion to social threat in typically developing children, and potential associations with individual differences in childhood temperament, is a necessary step in identifying atypical emotional processes in children at risk for maladaptive social functioning.
1.1. Event-related potentials elicited by threatening facial expressions
The time course of an electrocortical response to an emotional stimulus can be measured in milliseconds with ERPs — averaged voltage fluctuations in electroencephalography (EEG) that are time-locked to the onset of a stimulus and associated with sensory, linguistic, and cognitive processes (Luck, 2014). ERP components reflecting visual/attentional processes and later indices of arousal have been observed to be sensitive to emotion, although this sensitivity is dependent on experimental parameters and methodologies. One such early component is the P100, an ERP with a positive polarity that typically peaks over occipital-parietal electrode sites approximately 100ms after stimulus onset and is sensitive to parameters of visual stimuli (Clark & Hillyard, 1996), including facial expressions (Batty & Taylor, 2003). For example, the amplitude of the P100 elicited over the visual cortex has been shown to increase in response to emotional compared to neutral stimuli (Eger, Jedynak, Iwaki, & Skrandies, 2003; Luo, Feng, He, Wang, & Luo, 2010). Clinically, increased P100 amplitude to emotional faces likely serves as a marker of increased attention allocation in anxious compared to typical populations (Bar-Haim, Lamy, & Glickman, 2005; Mueller, Hofmann, Santesso, Meuret, Bitran, & Pizzagalli, 2008). The N170 is a negative deflection that often follows the P100 and has been widely associated with face processing (Hinojosa, Mercado, & Carretié, 2015). According to a metaanalysis of over 100 studies, N170 amplitude is modulated by the emotional salience of facial expressions (Cuthbert, Schupp, Bradley, Birbaumer, & Lang, 2000).
The late positive potential (LPP) is a positive waveform that emerges approximately 300ms after stimulus onset in posterior electrode sites – the amalgamation of positive deflections underlying different cognitive processes (Foti, Hajcak, & Dien, 2009; Olofsson, Nordin, Sequeira, & Polich, 2008). As an index of emotional reactivity and motivated attention facilitated by increased concurrent activation of the amygdala and visual cortices, LPP amplitude is sensitive to the salience of emotional visual stimuli (Duval, Moser, Huppert, & Simons, 2013; Sabatinelli, Keil, Frank, & Lang, 2013). More specifically, LPP amplitude in adults is enhanced by arousing facial affect, such as angry and fearful expressions (Eimer & Holmes, 2007; Hajcak, & Dennis, 2009). Increased LPP amplitude in school-age children has been elicited over occipital–parietal electrode sites by negatively emotional stimuli, such as unpleasant scenes and threatening adult faces, compared to positive or neutral stimuli (Hajcak & Dennis, 2009; Hua, Han, Chen, Yang, Zhou, & Hu, 2014; Kujawa, Klein, & Hajcak, 2012; Kujawa, Klein, & Proudfit, 2013; Kujawa, MacNamara, Fitzgerald, Monk, & Phan, 2015; MacNamara et al., 2016; Solomon, DeCicco, & Dennis, 2012). An increase in LPP amplitude to emotional stimuli has been associated with heightened anxiety and fear in children (Kujawa et al., 2015), suggesting that electrocortical activity evoked by social threat may also be related to individual differences in the childhood temperament. Overall, elicitation of P100, N170, and LPP components likely develops into adulthood, including sensitivity to emotional stimuli, and thus may serve as markers of social cognitive development.
1.2. Late positive potential as an index of emotion regulation
Modulation of the LPP also reflects processes of emotion regulation, such as cognitive reappraisal (Campos, Frankel, & Camras, 2004). A similar regulatory process is cognitive preappraisal – the modification of emotional reactivity to a stimulus by manipulation of its context, prior to first exposure (Gross, 2013). Although preappraisal tasks involve the presentation of contextual information before target stimulus onset, the term “reappraisal” has been used (and will be used in the current study) to remain consistent with the broader research literature of emotion regulation (Gross, 2013). Previous ERP studies of cognitive reappraisal have reported reduced LPP amplitudes compared to non-reappraised stimuli, indicating a down-regulation of emotional reactivity in adults (Foti & Hajcak, 2008; Hajcak, Moser, & Simons, 2006; MacNamara, Foti, & Hajcak, 2009). For example, neutral auditory descriptions reduce the negative salience of subsequent emotional stimuli as evidenced by a reduction in LPP amplitude compared to stimuli preceded by negative descriptions.
ERP studies of cognitive reappraisal with children have produced mixed results (Babkirk, Rios, & Dennis, 2014; DeCicco, O’Toole, & Dennis, 2014; Dennis, & Hajcak, 2009; Hua, Han, & Zhou, 2015; Van Cauwenberge, Van Leeuwen, Hoppenbrouwers, & Wiersema, 2017). DeCicco and colleagues (2014) found that the provision of a positive contextual narrative did not influence the amplitude of LPP elicitation to negative images in school-age children, leading to the conclusion that the neural correlates for reappraisal may not develop until later childhood. To the contrary, an increase of LPP amplitude to unpleasant pictures preceded by negative descriptions compared to similar pictures preceded by neutral descriptions has been observed in preschool and school-age children (Dennis, & Hajcak, 2009; Hua, Han, & Zhou, 2015; Van Cauwenberge, Van Leeuwen, Hoppenbrouwers, & Wiersema, 2017). These findings are suggestive that LPP modulation as a marker of reappraisal may be contingent on specifics of the task and the social development of the child.
1.3. Purpose of the study
The aims of the current study were to identify ERP correlates of emotional reactivity and regulation in school-age (i.e., 5- to 8-year-old) typically developing children to threatening facial expressions (e.g., angry and fearful) of own-age peers. Given previous findings mentioned above, it was hypothesized that participants would exhibit greater emotional reactivity to threatening compared to neutral facial expressions, evidenced by potentiation of P100, N170, and LPP amplitudes over parietal-occipital electrode sites post stimulus onset. We also expected participants to down-regulate their reactivity to threatening faces during a reappraisal condition, as demonstrated by reduced LPP elicitation amplitude compared to the threat condition. In a secondary analysis, we hypothesized that LPP amplitude across conditions, as well as effects of emotional reactivity and regulation on the LPP (i.e., ΔLPP amplitude to threat and reappraisal condition relative to neutral condition), would be associated with parent-reported dimensions of childhood temperament (i.e., negative affect, extraversion, and effortful control) [42]. More specifically, it was expected that LPP enhancement to social threat would be positively correlated with higher negative affect and lower extraversion, while LPP reduction during reappraisal would be positively correlated with effortful control.
2. Materials and Methods
2.1. Participants
This study was approved by the Institutional Review Board at Purdue University. Participants were recruited from the local community, located within a rural region in the Midwest United States. Before data were collected, written informed consent from parents and assent from children were obtained. Participating in this study were typically developing children (N = 41, 18 females) between the ages of 5;1 and 8;11 (age in months: M = 84.65, SE = 2.39, range = 61–107). An additional five children were recruited; however, these children either did not complete the ERP paradigm or the resulting data was unusable due to noise artifact (and thus were not included in the sample size of 41 participants). All participants were reported by a parent to exhibit normal or corrected-to-normal vision and normal hearing, which was confirmed by a hearing screening at 20 dB HL for 500, 1000, 2000, 4000, and 6000 Hz and were native English speakers. No participants had significant medical conditions or developmental disabilities.
2.2. Stimuli
The ERP paradigm involved the audiovisual presentation of 150 trials divided into three conditions (neutral, threat, and reappraisal) that differed in audio-image pairing: neutral context-neutral expression (neutral condition), negative context-threatening expression (threat condition), and reappraisal context-threatening expression (reappraisal condition). For each condition, audio sentences provided a context in which to interpret the subsequent target facial expression. In the threat condition, fearful and angry facial expressions were preceded by a sentence of congruent negative context. In the reappraisal condition, the threatening facial expressions were preceded by an incongruent reappraisal context. Neutral facial expressions were preceded by a congruent neutral context. Table 1 provides the auditory cue stimuli presented in this study.
Table 1.
Auditory sentence stimuli for each condition.
| Condition | Auditory Sentence | Facial Expression |
|---|---|---|
| Neutral | Joe is seven. Joe looks outside. Joe sits in the chair. |
Neutral |
| Threat | Joe is mad. Joe lost his candy. Joe broke his toy. |
Angry |
| Joe is scared. Joe saw a ghost. Joe heard a scream. |
Fearful | |
| Reappraisal | Joe is acting. Joe is playing. Joe is pretending. |
Angry |
| Joe is acting. Joe is playing. Joe is pretending. |
Fearful |
The experimental design consisted of two blocks (A and B). Block A consisted of 60 trials of the threat condition (30 angry / 30 fearful) and 15 trials of the neutral condition. Block B consisted of 60 trials of the reappraisal condition (30 angry / 30 fearful) and 15 trials of the neutral condition. The presentation order of blocks A and B for each participant was randomly determined. Trials were sequenced quasi-randomly within each block to reduce redundancy in the presentation of condition or facial type. Each block was presented once and in alternating order between subjects to prevent an order effect. It should be noted that, although the order of block presentation was counterbalanced between participants, the possibility of an effect of block order on ERP elicitation was recognized. Thus, a preliminary analysis of ERP elicitation comparing the waveforms of participants exposed to A→B versus B→A ordering was performed. No differences in P100, N170 and LPP amplitude were found during visual inspection of overlaying waveforms, suggesting no overt order effects in ERP elicitation. The neutral, fearful, and angry facial expressions included those of ten1 (5 male, 5 female) child actors from the Child Affective Facial Expression (CAFE) set (LoBue & Thrasher, 2015). This set included photographs of 4- to 7-year-old racially and ethnically diverse children with stereotypical exemplars of angry, fearful, and neutral facial expressions. The ten actors were selected to correspond to the age-range of our participants.
Using Presentation software (Version 16.3), the visual stimuli was presented on an 18.5-inch computer monitor with a visual angle of 5 degrees horizontally and 4 degrees vertically and presented 60 inches in front of the seated participant. Before the onset of each facial expression, a contextual audio sentence was presented via a speaker placed directly above the monitor at an average intensity of 70–75 dB SPL. These audio cues were spoken half by a male and half by a female speaker at a natural rate and prosody and comprised of age-appropriate vocabulary. Trials for all three conditions began with a “ready?” screen and commenced with a button press. The appearance of a circular fixation point (duration of 2500ms and of alternating size and color) was followed by the presentation of a facial expression (2000ms). During the appearance of the circular fixation point, the auditory contextual sentence was presented. Given differences in the duration of the different sentences (1230 to 2021ms) during the presentation of the circular fixation point (2500ms), the interstimulus interval between the offset of the sentence and the onset of the face image was randomized (479 to 1270ms). Brief breaks (of approximately one minute) occurred at regular intervals (every 15 trials), during which time the child played with toy building set or fishing game. The start and end of each break was verbally acknowledged to the participant by an accompanying experimenter to facilitate their attention to the stimuli.
2.3. Measures
Parental reporting of temperamental tendencies related to emotional reactivity and regulation was undertaken through parental completion of the Children’s Behavior Questionnaire – Short Form Version I (CBQ), a standardized assessment of childhood temperament (Putnam & Rothbart, 2006). Parents rated their child using a seven-point Likert scale on 94 items. Three composite dimensions of temperament were analyzed and Cronbach’s alpha (in parentheses) indicated parental input was reliable for the majority of dimension subfactors: Extraversion (.70) [6 subfactors: approach/positive anticipation (.56), impulsivity (.64), high intensity pleasure (.83), activity level (.71), (reversed) shyness (.91), and smiling/laughter (.52)]; Negative Affect (.73) [5 subfactors: anger/frustration (.87), fear (.69), sadness (.66), discomfort (.76), (reversed) falling reactivity/soothability (.69)]; and Effortful Control (.60) [4 subfactors: attentional focusing (.69), inhibitory control (.33), low intensity pleasure (.67), and perceptional sensitivity (.71)].
2.4. Procedures
Before beginning the ERP paradigm, participants were shown a set of sample facial expressions (that were also from the CAFE set, but not used in the paradigm) to confirm that the participant could identify the emotional valence of each corresponding facial expression. For the ERP task, participants sat in a sound-attenuating booth with an accompanying experimenter. The experimenter sat next to the child throughout the stimuli presentation to remind the child to sit still if needed, to manually begin each trial, and to ensure the child was on task. The ERP task is illustrated in Fig 1. The following instructions were given to the child by the accompanying experimenter before commencing the task: “While you sit in this chair, you will see the faces of children your age on the screen. It is important to keep your arms, legs, and head as still as you can while you are watching the screen. When you are finished, you will get to pick out a prize!” At the beginning of each block, the experimenter presented the participant with a specific context for that block. Before Block A (which included the threat condition), the following sentence were added: “Some of these kids are having a bad day. They may look mad, afraid, or just fine.” Before Block B (which included the reappraisal condition), the participants were instead told, “Some of these kids are pretending. They may look mad or afraid, but they are just playing a game, they are really just fine.” Before the start of the paradigm, all participants confirmed that they understood the verbal directions following an example trial before the start of the task. Throughout the experiment, the accompanying experimenter monitored the participant to ensure that eye contact remained on the screen during stimuli presentation.
Fig 1.
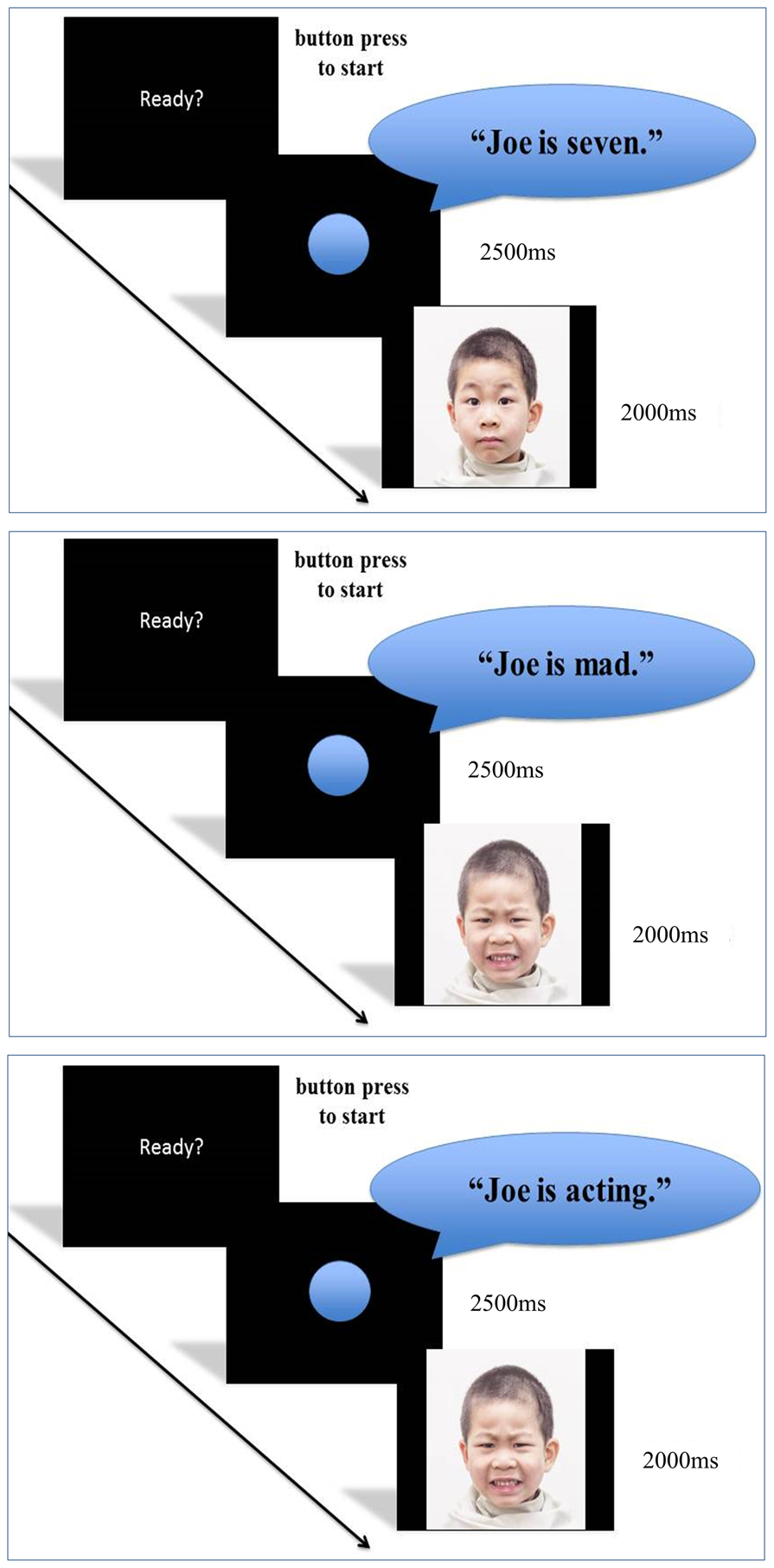
Experimental paradigm with neutral, threat (angry), and reappraisal (angry) conditions (fearful expression not shown).
2.5. Electroencephalographic Recording
Using the Biosemi ActiveTwo system, EEG signals time-locked to the onset of the visual facial expression were recorded from the scalp with a 32 Ag-Cl electrode elastic cap (Electro-Cap International, Inc.). Electrodes were positioned in homologous locations (FP1/2, AF3/4, F3/4, F7/8, FC1/2, FC5/6, C3/4, T7/8, CP1/2, CP5/6, P3/4, P7/8, PO3/4, O1/2), that are consistent with the international 10–10 system (Acharya, Hani, Cheek, Thirumala, & Tsuchida, 2016). Eye blinks were monitored with bipolar recordings from electrodes placed on the left superior and inferior orbital ridges. Bipolar recordings were taken from electrodes placed on the left and right outer canthi to monitor horizontal eye movements. Electrodes were also placed on the left and right mastoids. Recordings were then referenced offline to an average of the electrode recordings from the left and right mastoid placements. The EEG was digitized online at a rate of 512 Hz and band-pass filtered between 0.1 and 100 Hz.
2.6. ERP Analyses
ERP analyses were completed using EEGLAB (Delorme & Makeig, 2004) and ERPLAB (Lopez-Calderon & Luck, 2014) in Matlab (Mathworks). EEG data were downsampled to 256 Hz and low pass filtered at 30 Hz. Independent component analysis (ICA) was performed to detect and remove eye artifact. EEG waveforms were then isolated into time-locked epochs, between −200 and 2000ms, relative to the onset of the facial stimulus. An automatic detection algorithm removed trials with movement or other extraneous artifact. Artifact-free trials were averaged by condition (neutral, threat, reappraisal) for each participant and grand averages were created. The mean number of accepted trials is detailed in Table 2.
Table 2.
Mean (and standard error) for the number of trials accepted for each condition.
| Condition | # of accepted trials |
|---|---|
| Neutral | 27.10 (.53) |
| Threat (angry) | 27.02 (.44) |
| Threat (fearful) | 27.34 (.46) |
| Reappraisal (angry) | 27.39 (.52) |
| Reappraisal (fearful) | 27.63 (.63) |
ERP elicitation was analyzed in left and right hemisphere posterior regions of interest (P7, PO3, O1, P8, PO4, O2). This region was chosen to capture differences in ERP elicitation distribution previously reported by facial expressions over parietal-occipital sites in children (Hajcak & Dennis, 2009; Hua et al., 2014; Kujawa et al., 2012; Kujawa et al., 2013; Kujawa et al., 2015; MacNamara et al., 2016; Solomon et al., 2012). Time windows for the statistical analyses of ERPs were selected using common practice (Kappenman & Luck, 2016): windows were initially selected a priori based on time windows used in previous similar ERP studies with children (e.g., Kujawa, Klein, & Hajcak, 2012; Thom, Knight, Dishman, Sabatinelli, Johnson, & Clementz, 2013), and amended using visual inspection of ERP grand averages to ensure that the ERPs were captured. For example, the well-defined peaks of the P100 and N170 are the approximate center of their representative time windows. Time windows were as follows: P100 (130–170ms), N170 (170–270ms), and LPP (300–1000ms). Mean amplitudes of these ERP components were calculated as the amplitude (in μV) relative to baseline (correction set −200 to 0ms) within the designated temporal windows. P7 and P8 electrodes were not used for analysis of P100 amplitude, as no peak was observed at these sites.
Statistical analyses of the ERPs were performed, using SPSS Statistics for Windows (IBM Corp., Version 25.0), to evaluate overall differences in mean amplitude measures using a repeated-measures ANOVA. Significant statistical differences were considered using an alpha level of p < .05. If a significant Condition effect was observed, further analysis was conducted with a Bonferroni-corrected post-hoc test. Violations in sphericity were corrected by Huynh-Feldt-adjusted p-values when the degrees of freedom of the numerator were greater than 1 (Hays, 1994). Effect sizes, indexed by partial eta squared (ƞp2), were also reported. To test internal consistency of ERPs, split-half reliability of ERP amplitudes was determined by comparing two halves of trials within the threat and reappraisal conditions. ERPs to the threat condition revealed a split-half reliability of .86 (p < .001) for P100 amplitude, .79 (p < .001) for N170 amplitude, and .70 (p < .001) for LPP amplitude. To the reappraisal condition, split-half reliability for the P100 was .48 (p < .001), the N170 was .73 (p < .001), and the LPP was .61 (p < .001).
An additional analysis of the effects of emotional reactivity and regulation on LPP elicitation was conducted by measuring amplitude differences between conditions. To quantify the range of electrocortical modulation underlying emotional reactivity and reappraisal in each participant, a threat effect (ΔLPP amplitude to the threat condition relative to the neutral condition) and a reappraisal effect (ΔLPP amplitude to the reappraisal condition relative to the threat condition) were calculated using subtraction-based and reappraisal-based difference scores. Given that subtraction-based difference scores may not completely isolate the effects of threat and reappraisal (Meyer et al., 2017), residual-based difference scores (based on measuring the variance in regression in which the neutral condition predicts the threat condition and the threat condition predicts the reappraisal condition) were calculated from LPP amplitudes at electrode O2. This electrode was selected due to the prominence of LPP amplitude at this right occipital location (see Fig 2 and Table 3). Lastly, in an exploratory analysis, Pearson correlations (two-tailed) were calculated to determine possible relationships between LPP elicitation across conditions, effects of emotional reactivity and reappraisal on LPP amplitude, and dimensions of childhood temperament based on the CBQ: negative affect, extraversion, and effortful control. An alpha level of p < .005 was used to correct for multiple correlations.
Fig 2.

Grand averaged event-related potentials (ERPs) and topographic maps for each experimental condition.
Table 3.
Mean ERP amplitudes (in μV) and 95% Confidence Interval across conditions and electrodes
| ERP | Condition | Electrode | Mean | 95% CI |
|---|---|---|---|---|
| P100 | Neutral | P7 | −.87 | [−2.66, .92] |
| PO3 | 1.74 | [−.53, 4.01] | ||
| O1 | 11.46 | [7.97, 14.94] | ||
| P8 | −.39 | [−2.13, 1.35] | ||
| PO4 | 1.88 | [−.22, 3.98] | ||
| O2 | 11.59 | [8.20, 14.98] | ||
| Threat | P7 | −.33 | [−2.60, 1.95] | |
| PO3 | 1.90 | [−.42, 4.22] | ||
| O1 | 11.85 | [8.42, 15.28] | ||
| P8 | .62 | [−1.35, 2.59] | ||
| PO4 | 3.02 | [.72, 5.32] | ||
| O2 | 13.66 | [10.20, 17.11] | ||
| Reappraisal | P7 | −.23 | [−2.07, 1.60] | |
| PO3 | 2.37 | [.15, 4.59] | ||
| O1 | 10.71 | [6.90, 14.52] | ||
| P8 | .81 | [−.56, 2.17] | ||
| PO4 | 3.64 | [1.39, 5.89] | ||
| O2 | 12.70 | [9.58, 15.81] | ||
| N170 | Neutral | P7 | −2.14 | [−3.55, −.74] |
| PO3 | −2.08 | [−3.97, −.20] | ||
| O1 | 5.39 | [2.57, 8.22] | ||
| P8 | −1.82 | [−3.52, −.12] | ||
| PO4 | −1.52 | [−3.58, .54] | ||
| O2 | 5.97 | [2.97, 8.97] | ||
| Threat | P7 | −1.80 | [−3.18, −.42] | |
| PO3 | −1.31 | [−3.30, .68] | ||
| O1 | 6.31 | [3.59, 9.03] | ||
| P8 | −1.10 | [−2.76, .56] | ||
| PO4 | −.87 | [−2.95, 1.22] | ||
| O2 | 7.21 | [4.30, 10.11] | ||
| Reappraisal | P7 | −1.58 | [−2.86, −.30] | |
| PO3 | −.82 | [−2.88, 1.24] | ||
| O1 | 6.44 | [3.66, 9.22] | ||
| P8 | −.47 | [−2.02, 1.07] | ||
| PO4 | .35 | [−1.71, 2.42] | ||
| O2 | 7.75 | [4.97, 10.52] | ||
| LPP | Neutral | P7 | 1.98 | [.61, 3.34] |
| PO3 | 2.28 | [.68, 3.87] | ||
| O1 | 7.82 | [6.16, 9.48] | ||
| P8 | 4.44 | [2.89, 5.99] | ||
| PO4 | 3.40 | [1.35, 5.45] | ||
| O2 | 7.63 | [5.48, 9.78] | ||
| Threat | P7 | 2.09 | [.92, 3.27] | |
| PO3 | 4.08 | [2.17, 6.00] | ||
| O1 | 8.19 | [6.63, 9.74] | ||
| P8 | 6.16 | [4.59, 7.73] | ||
| PO4 | 5.61 | [3.83, 7.38] | ||
| O2 | 9.34 | [7.21, 11.47] | ||
| Reappraisal | P7 | 2.33 | [1.38, 3.28] | |
| PO3 | 3.52 | [1.86, 5.18] | ||
| O1 | 7.88 | [5.98, 9.77] | ||
| P8 | 4.98 | [3.62, 6.34] | ||
| PO4 | 4.63 | [2.88, 6.38] | ||
| O2 | 8.40 | [6.46, 10.35] |
3. Results
3.1. P100, N170, and LPP Elicitation
Grand averaged ERP waveforms elicited by neutral, threat, and reappraisal conditions are illustrated in Fig 2 and mean amplitudes are provided in Table 3. P100 amplitude did not significantly differ between conditions, F(2,80) = 1.80, p = .17, ƞp2 = .04, or hemispheres, F(1,40) = 1.81, p = .19, ƞp2 = .04. The interaction between Condition and Hemisphere was significant, F(2,80) = 3.92, p = .02, ƞp2 = .09, with P100 higher over the right hemisphere to the threat condition compared to the neutral condition. Not surprisingly, P100 amplitude differed across electrodes, F(2,80) = 66.03, p < .001, ƞp2 = .62, with P100 amplitude highest in bilateral occipital electrode sites. N170 amplitude also did not significantly differ between conditions, F(2,80) = 3.06, p = .05, ƞp2 = .07, or hemispheres, F(1,40) = 1.46, p = .24, ƞp2 = .04. The interaction between Condition and Hemisphere was not observed, F(2,80) = 1.16, p = .32, ƞp2 = .03. Similar to the P100, N170 amplitude was highest in bilateral occipital electrode sites, F(2,80) = 42.68, p < .001, ƞp2 = .52.
Modulation of the LPP was observed across conditions: A Condition effect was significant for LPP mean amplitude, F(2,80) = 4.19, p = .02, ƞp2 = .10, with a Bonferroni pairwise comparison revealing an increased LPP to the threat condition (M = 5.91, SE = .65 μV) compared to the neutral condition (M = 4.59, SE = .634, μV; p = .03). However, LPP amplitude for the reappraisal condition (M = 5.29, SE = .60 μV) did not differ significantly from that elicited by the neutral (p = .31) or the threat condition (p = .56). LPP mean amplitude was also higher over the right hemisphere overall (M = 6.07, SE = .67 μV) compared to the left hemisphere (M = 4.46, SE = .55 μV) overall, F(1,40) = 13.23, p < .001, ƞp2 = .25; but a Condition x Hemisphere interaction did not reach significance, F(2,80) = 2.99, p = .06, ƞp2 = .07. LPP amplitude was more prominent in the bilateral occipital region compared to other electrode sites, F(2,80) = 31.58, p < .001, ƞp2 = .44.
3.2. Difference Scores: Threat and Reappraisal Effects
The effects of emotional reactivity and reappraisal can be calculated by analyzing differences in LPP amplitudes between the threat and neutral conditions, and reappraisal and threat conditions, respectively. Given strong correlations in LPP elicitation were observed between conditions, rs ≥ .72, ps < .001 (see Fig 3 and Table 4), a hierarchical multiple regression analysis was used to investigate the association between LPP elicitation to threat and reappraisal conditions, while controlling for elictation to the neutral condition. A linear combination of LPP elicitation to the neutral (β = .51, t = 3.74, p = .001) and threat condition (β = .37, t = 2.69, p = .01) predicted LPP elicitation by the reappraisal condition, R2 = .66, F(2,38) = 36.57, p < .001). Addition of LPP to the threat condition significantly improved prediction beyond that of the neutral condition (R2 change = .07, F = 7.25, p = .01). LPP elicitation to threat and reappraisal conditions were associated across participants, evidenced by the strong correlation between LPP amplitude to threat and reappraisal conditions (see Table 4), after controlling for individual differences in the LPP to the neutral condition.
Fig 3.
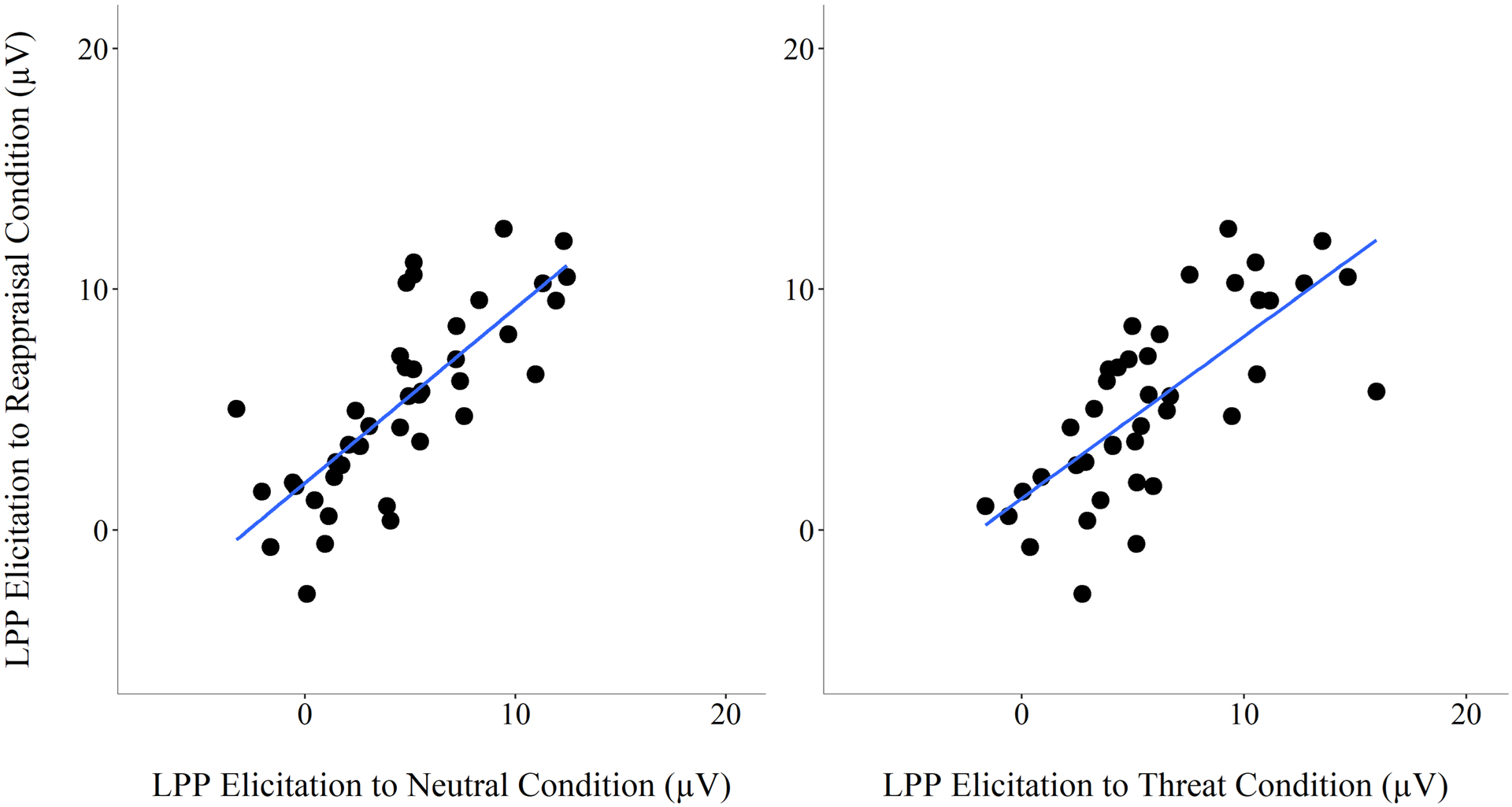
Scatterplots of late positive potential (LPP) elicitation show the relationship between neutral and reappraisal conditions (left), and between threat and reappraisal conditions (right). LPP amplitudes were averaged across the six posterior electrode sites.
Table 4.
Pearson correlations between LPP amplitudes across conditions, subtraction-based difference scores, and residual-based difference scores
| LPP (Neutral) | LPP (Threat) | LPP (Reapp) | ΔLPP (T-N) | ΔLPP (R-T) | LPPresid (Threat) | |
|---|---|---|---|---|---|---|
| LPP (Neutral) | — | — | — | — | — | — |
| LPP (Threat) | .72* | — | — | — | — | — |
| LPP (Reapp) | .77* | .73* | — | — | — | |
| ΔLPP (T-N) | −.38* | .35* | .001 | — | — | — |
| ΔLPP (R-T) | −.02 | −.41* | .27* | −.53* | — | — |
| LPPresid (Threat) | .02 | .39* | .23* | .51* | −.26* | — |
| LPPresid (Reapp) | .20 | .002 | .36* | −.29* | .51* | −.58* |
Note:
p < .005.
ΔLPP (T-N) = LPP amplitude to threat condition minus neutral condition; ΔLPP (R-T) = LPP amplitude to reappraisal condition minus threat condition; reapp = reappraisal.
Pearson correlations were computed between LPP amplitudes across conditions, subtraction-based difference scores and residual-based difference scores. LPP amplitude to both the threat and reappraisal conditions were strongly correlated with LPP amplitude to the neutral condition. Subtraction-based difference scores underlying a ΔLPP threat effect (i.e., threat minus neutral condition) and ΔLPP reappraisal effect (i.e., reappraisal minus threat condition) were negatively correlated. Residual-based difference scores (LPPresid) to the threat condition was significantly correlated with LPP amplitude to the threat and reappraisal conditions. LPPresid to the reappraisal condition was significantly correlated with LPP to the reappraisal condition. LPPresid to the threat and reappraisal conditions was significantly negatively correlated. This negative correlation between the residual scores reveal that participants with a larger LPP to the threat condition (i.e., threat effect) had a smaller LPP during reappraisal (i.e., reappraisal effect).
3.3. Correlations Between Difference Scores, Age, and Temperament
Pearson correlations between the residual-based difference scores for threat and reappraisal conditions and temperamental dimensions of negative affect, extraversion, and effortful control were largely not significant (Table 5). An exception was a negative correlation between increased LPP amplitude to the threat condition and decreased extraversion. Although not statistically significant (p = .02), extraversion was also negatively correlated with LPP to the reappraisal condition. The considerable individual differences across participants in threat and reappraisal effects suggests an influence by age; however, age was not strongly correlated with these LPP effects (−.01 < rs < .07, ps > .65) or temperamental dimensions (−.29 < rs < .02, ps > .07).
Table 5.
Pearson correlations (and associated p-values) between LPP amplitude across conditions, residual-based difference scores, and temperamental dimensions
| Negative Affect | Extraversion | Effortful Control | |
|---|---|---|---|
| LPP (neutral) | .08(.63) | −.22(.16) | .03(.87) |
| LPP (threat) | .09(.59) | −.46(.002)* | .04(.83) |
| LPP (reappraisal) | .02(.90) | −.37(.02) | .10(.39) |
| LPPresid (Threat) | −.10(.53) | .04(.79) | −.15(.35) |
| LPPresid (Reapp) | −.35(.03) | −.19(.24) | .16(.31) |
Note:
p < .005.
Residual scores taken from O2 electrode.
4. Discussion
The school-age years is a period of increasing interpersonal skills, including the ability to regulate emotions to threatening social stimuli. These stimuli include emotional peer facial expressions that can be reappraised with additional situational context. Emotion regulation abilities, which are still developing during the school-age years, have been associated with positive social functioning and wellbeing (Garner & Waajid, 2012). The purpose of this study was to observe the neural correlates of emotional reactivity and regulation in 5- to 8-year-old children elicited by the facial expressions of own-age peers within a social context.
Participants watched a sequence of threatening and neutral facial expressions, with each face preceded by a contextual auditory cue. Visual attention was automatically captured in our participants: P100 and N170 components were elicited over parietal and occipital electrode sites within the first 1000ms post stimulus onset. The morphology of the P100 and N170 were similar across neutral, threat, and reappraisal conditions. Putative enhancement of P100 amplitude to the threat versus neutral condition was most significant over the right occipital region (Fig 2). The overall lack of sensitivity of these early components (i.e., lack of condition effects) to the threat condition in the current study may be due to the lack of emotional salience that is characteristic of facial expressions, compared to scene stimuli (Thom et al., 2013).
Consistent with our hypothesis, increased LPP amplitude indicated greater levels of sustained attention and emotional reactivity to the threat relative to the neutral condition. This finding expands on previous studies of emotion processing in children that found similar LPP enhancement to negatively salient scenes and adult facial expressions (Hajcak & Dennis, 2009; Hua et al., 2014; Kujawa et al., 2012; Kujawa et al., 2013; Kujawa et al., 2015; MacNamara et al., 2016; Solomon et al., 2012). The morphology and duration of the LPP was comparably less sustained compared to adults (Hajcak, Dunning, & Foti, 2009), which is consistent with previous studies of children (e.g., Kujawa, Klein, & Hajcak, 2012). The increased LPP elicitation over the right compared to left hemisphere is also consistent with previous evidence of a right hemispheric bias in the processing of emotional stimuli (Cacioppo, Crites, & Gardner, 1996; Keil, Bradley, Hauk, Rockstroh, Elbert, & Lang, 2002). In addition, the neural processing of facial expressions has been found to be right lateralized compared to non-facial visual stimuli (Gauthier, Tarr, Anderson, Skudlarski, & Gore, 1999; LeGrand, Mondloch, Maurer, & Brent, 2003). Although speculative, the right-lateralization of LPP elicitation in our study may reflect the recruitment of lateral right cortical regions facilitating the evaluative processing of facial expressions in the context of differing precedent audio cues.
Although visual inspection of the LPP suggested down-regulation for the reappraisal compared to the threat condition, this decrease in LPP amplitude was not statistically significant. Similar to previous studies of cognitive reappraisal in young children (e.g., DeCicco et al., 2014), as a group, participants in this current study did not exhibit a significant reduction in LPP during reappraisal relative to the threat condition. The lack of an observed modulation of LPP amplitude during the reappraisal condition is likely because 1) the relatively weak emotional salience of child facial expressions did not require considerable emotion regulation demands (Thom et al., 2013), and 2) the neurodevelopment of emotion regulation for subtle social stimuli may take a protracted course beyond the school-age years for many children. Still, threat and reappraisal effects (calculated using subtraction- and residual-based difference scores) did reveal that participants exhibiting increased emotional reactivity to the threat condition also increased regulation of that activity during the reappraisal condition.
Overall, our findings suggest that LPP indices of emotional processes in 5- to 8-year-old children are modulated by threatening facial expressions of own-age peers. However, this finding is hampered by the large degree of heterogeneity across participants in LPP amplitude across threat, reappraisal, and neutral conditions. Threat and reappraisal effects on the LPP were only observed in approximately half of the participants. As a result, there was considerable individual differences in the appearance of threat and reappraisal effects. Strong correlations in LPP elicitation between conditions is suggestive that intra-individual differences in emotional reactivity was quite small compared to the indivdual differences across participants. This heterogeneity was not likely due to age differences across participants (e.g., ages 5–8) because threat or reappraisal effects were not significantly correlated with age. In regards to parental-reported measures of participant temperament, it was expected that LPP enhancement to the threat condition would be associated with higher negative affect and lower extraversion. This hypothesis was partly confirmed– children with less surgency may have exhibited greater emotionality in response to the threatening child emotional facial expressions. An association between extraversion and LPP elicitation to emotional stimuli has been observed in adolescents (Speed, Nelson, Perlman, Klein, Kotov, & Hajcak, 2015). However, Speed et al. found extraversion to be associated with increased LPP to pleasant and unpleasant pictures in adolescents, which is contrary to the relationship found in the current study. This difference may be due to the contribution of positive emotionality to the LPP in the study by Speed and colleagues. To the contrary, the current study did not evoke positive, but negative emotion. The current study is congruent with this previous work by revealing that LPP amplitude was not associated with other aspects of temperament such as negative affect. This is evidence that ERP correlates of emotional processes elicited during an experimental setting may not strongly correspond with differences in parential-reported childhood temperament. Further research with larger sample sizes and the use of behavioral measures in addition to parental reporting may be necessary to tease apart possible associations between ERP indices of emotional processes and childhood temperament.
5. Limitations
This study includes several limitations that should be considered for future research. First, the region of interest approach facilitated analysis of the distribution of ERP elicitation (including hemispheric differences); however, the shape of the ERP waveforms differed across the region of interest and likely contributed to the finding of considerable individual differences across participants. Recording from more electrode sites within the region of interest would have advantageous, but the need to minimize EEG preparation time for the young participants restricted the number of electrode sites. A second limitation was the lack of additional indices of emotional reactivity and regulation, such as autonomic nervous system measures (e.g., skin conductance), to support the interpretation of the ERPs. In addition to ERPs, time-frequency analysis of EEG, participant ratings of subjective arousal, and physiological measures would contribute to better understanding of individual differences in emotional reactivity and regulation that inform ERP measures.
Lastly, having the threat condition consist of both fearful and angry faces does not confound the interpretation of our research questions; however, future studies should consist of more trials for separate analysis of angry and fearful faces. Even if angry faces fearful faces differ in emotional salience (Bunford et al., 2017), the purpose of the current project was not to investigate potential effects of stimulus salience. Given the young age of our participants, the number of trials in the current study was restricted to prevent participant boredom and movement artifact. As a result, analysis of only fearful or angry faces would greatly reduce the number of trials used to estimate the LPP across our conditions (see Table 2).
6. Conclusions
The current study identified ERP activation underlying emotional reactivity and regulation by 5- to 8-year-old typically-developing children to the threatening facial expressions (e.g., angry and fearful) of own-age peers – a novel feature of this current study. The presented conditions reflected social stimuli that are ecologically-valid to everyday life of children. The presentation of threatening child facial expressions elicited an increase in LPP amplitude in school-age children, which is evidence of an increase in emotional reactivity to threatening social information from peers. However, this neural activity did not modulate significantly with reappraisal, which is suggestive that the neurodevelopment of emotion regulation for social stimuli may take a protracted course beyond the school-age years.
Highlights.
Neural correlates of emotional reactivity and regulation in typically developing school-age children were elicited by facial expressions of same-aged peers.
Event-related brain potentials (ERPs), including the P100, N170, and LPP, were elicited in a paradigm ecologically valid to the everyday life of children.
Elicitation of the P100 and N170 did not largely differ between conditions; however, amplitude of the late positive potential (LPP), a marker of heightened emotional reactivity and attention, was greater for threatening faces relative to neutral faces.
LPP amplitude elicited during a cognitive reappraisal condition did not differ from LPP elicitation in a threat condition; however, participants who exhibited more emotional reactivity also exhibited more cognitive reappraisal.
A lack of strong associations between ERP morphology and temperamental dimensions is indicative of heterogeneity in LPP elicitation underlying emotion regulation in children.
Acknowledgements
Data for this study was collected and analyzed as part of the first author’s dissertation work at Purdue University. We would also like to thank Eileen Haebig for her assistance with stimuli creation and Jennifer Schumaker for her input regarding an earlier draft of this manuscript. This work was funded by grants from the National Institute on Deafness and Other Communication Disorders, DC00559 and DC013017.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CAFE photographs used: 10025-angry_F-EA-32, 10405-fearful_F-EA-32, 10769-neutral_F-EA-32, 10062-angry_M-AS-06, 10432-fearful_M-AS-06, 10814-neutral_M-AS-06, 10068-angry_M-EA-06b-2, 10436-fearful_M-EA-06b, 10820-neutral_M-EA-06b, 10079-angry_M-EA-20, 10441-fearful_M-EA-20, 10832-neutral_M-EA-20, 10096-angry_M-LA-09, 10450-fearful_M-LA-09, 10855-neutral_M-LA-09, 9988-angry_F-AA-13, 10381-fearful_F-AA-13, 10737-neutral_F-AA-13, 9990-angry_F-AA-15, 10383-fearful_F-AA-15, 10739-neutral_F-AA-15, 10018angry_F-EA-25, 10400-fearful_F-EA-25, 10765-neutral_F-EA-25, 10036-angry_F-LA-05, 10411-fearful_F-LA-05–2, 10781-neutral_F-LA-05, 10037-angry_F-LA-12, 10415-fearful_F-LA-12, 10788-neutral_F-LA-12
References
- Acharya J, Hani A, Cheek J, Thirumala P, & Tsuchida T (2016). American clinical neurophysiology society guideline 2: Guidelines for standard electrode position nomenclature. Journal of Clinical Neurophysiology, 33(4), 308–311. [DOI] [PubMed] [Google Scholar]
- Babkirk S, Rios V, & Dennis TA (2014). The late positive potential predicts emotion regulation strategy use in school-aged children concurrently and two years later. Developmental Science, 18(5), 832–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Haim Y, Lamy D, & Glickman S (2005). Attentional bias in anxiety: A behavioral and ERP study. Brain and Cognition, 59(1), 11–22. [DOI] [PubMed] [Google Scholar]
- Batty M, & Taylor MJ (2003). Early processing of the six basic facial emotional expressions. Cognitive Brain Research, 17(3), 613–620. [DOI] [PubMed] [Google Scholar]
- Bunford N, Kujawa A, Fitzgerald KD, Swain JE, Hanna GL, Koschmann E, Simpson D, Connolly S, Monk CS, & Phan KL (2017). Neural reactivity to angry faces predicts treatment response in pediatric anxiety. Journal of Abnormal Child Psychology, 45(2), 385–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacioppo JT, Crites SL, & Gardner WL (1996). Attitudes to the right: Evaluative processing is associated with lateralized late positive event-related brain potentials. Personality and Social Psychology Bulletin, 22(12), 1205–1219. [Google Scholar]
- Campos JJ, Frankel CB, & Camras L (2004). On the nature of emotion regulation. Child Development, 75(2), 377–394. [DOI] [PubMed] [Google Scholar]
- Clark VP, & Hillyard SA (1996). Spatial selective attention affects early extrastriate but not striate components of the visual evoked potential. Journal of Cognitive Neuroscience, 8(5), 387–402. [DOI] [PubMed] [Google Scholar]
- Cuthbert BN, Schupp HT, Bradley MM, Birbaumer N, & Lang PJ (2000). Brain potentials in affective picture processing: Covariation with autonomic arousal and affective report. Biological Psychology, 52(2), 95–111. [DOI] [PubMed] [Google Scholar]
- DeCicco JM, O’Toole LJ, & Dennis TA (2014). The late positive potential as a neural signature for cognitive reappraisal in children. Developmental Neuropsychology, 39(7), 497–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delorme A, & Makeig S (2004). EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. Journal of Neuroscience Methods, 134(1), 9–21. [DOI] [PubMed] [Google Scholar]
- Dennis TA, & Hajcak G (2009). The late positive potential: A neurophysiological marker for emotion regulation in children. Journal of Child Psychology and Psychiatry, 50(11), 1373–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duval ER, Moser JS, Huppert JD, & Simons RF (2013). What’s in a face?: The late positive potential reflects the level of facial affect expression. Journal of Psychophysiology, 27, 1, 27–38. [Google Scholar]
- Eger E, Jedynak A, Iwaki T, & Skrandies W (2003). Rapid extraction of emotional expression: Evidence from evoked potential fields during brief presentation of face stimuli. Neuropsychologia, 41, 808–817. [DOI] [PubMed] [Google Scholar]
- Eimer M, & Holmes A (2007). Event-related brain potential correlates of emotional face processing. Neuropsychologia, 45(1), 15–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foti D, & Hajcak G (2008). Deconstructing reappraisal: Descriptions preceding arousing pictures modulate the subsequent neural response. Journal of Cognitive Neuroscience, 20(6), 977–988. [DOI] [PubMed] [Google Scholar]
- Foti D, Hajcak G, & Dien J (2009). Differentiating neural responses to emotional pictures: Evidence from temporal-spatial PCA. Psychophysiology, 46(3), 521–530. [DOI] [PubMed] [Google Scholar]
- Garner PW, & Waajid B (2012). Emotion knowledge and self-regulation as predictors of preschoolers’ cognitive ability, classroom behavior, and social competence. Journal of Psychoeducational Assessment, 30(4), 330–343. [Google Scholar]
- Gauthier I, Tarr MJ, Anderson AW, Skudlarski P, & Gore JC (1999). Activation of the middle fusiform ‘face area’ increases with expertise in recognizing novel objects. Nature Neuroscience, 2(6), 568–73. [DOI] [PubMed] [Google Scholar]
- Goldin PR, McRae K, Ramel W, & Gross JJ (2008). The neural bases of emotion regulation: Reappraisal and suppression of negative emotion. Biological Psychiatry, 63(6), 577–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross JJ (2013). Emotion regulation: Taking stock and moving forward. Emotion, 13(3), 359–365. [DOI] [PubMed] [Google Scholar]
- Gross JJ, & John OP (2003). Individual differences in two emotion regulation processes: Implications for affect, relationships, and well-being. Journal of Personality & Social Psychology, 85, 348–362. [DOI] [PubMed] [Google Scholar]
- Hajcak G, & Dennis TA (2009). Brain potentials during affective picture processing in children. Biological Psychology, 80(3), 333–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajcak G, Dunning JP, & Foti D (2009). Motivated and controlled attention to emotion: Time-course of the late positive potential. Clinical Neurophysiology, 120(3), 505–510. [DOI] [PubMed] [Google Scholar]
- Hajcak G, Moser JS, & Simons RF (2006). Attending to affect: Appraisal strategies modulate the electrocortical response to arousing pictures. Emotion, 6(3), 517–522. [DOI] [PubMed] [Google Scholar]
- Hays WL (1994). Statistics (5th edn.). Fort Worth, TX: Harcourt Brace College Publishers. [Google Scholar]
- Hinojosa JA, Mercado F, & Carretié L (2015). N170 sensitivity to facial expression: A meta-analysis. Neuroscience & Biobehavioral Reviews, 55, 498–509. [DOI] [PubMed] [Google Scholar]
- Hoehl S, Brauer J, Brasse G, Striano T, & Friederici AD (2010). Children’s processing of emotions expressed by peers and adults: An fMRI study. Social Neuroscience, 5, 543–559. [DOI] [PubMed] [Google Scholar]
- Hua M, Han ZR, Chen S, Yang M, Zhou R, & Hu S (2014). Late positive potential (LPP) modulation during affective picture processing in preschoolers. Biological Psychology, 101, 77–81. [DOI] [PubMed] [Google Scholar]
- Hua M, Han ZR, & Zhou R (2015). Cognitive reappraisal in preschoolers: Neuropsychological evidence of emotion regulation from an ERP study. Developmental Neuropsychology, 40(5), 279–290. [DOI] [PubMed] [Google Scholar]
- Izard CE, Fine S, Schultz D, Mostow AJ, Ackerman B, & Youngstrom E (2001). Emotion knowledge as a predictor of social behavior and academic competence in children at risk. Psychological Science, 12(1), 18–23. [DOI] [PubMed] [Google Scholar]
- John OP, & Gross JJ (2004). Healthy and unhealthy emotion regulation: Personality processes, individual differences, and life span development. Journal of Personality, 72(6), 1301–1334. [DOI] [PubMed] [Google Scholar]
- Kappenman ES, & Luck SJ (2016). Best practices for event-related potential research in clinical populations. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, 1, 2, 110–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keil A, Bradley MM, Hauk O, Rockstroh B, Elbert T, & Lang PJ (2002). Large-scale neural correlates of affective picture processing. Psychophysiology, 39(5), 641–649. [DOI] [PubMed] [Google Scholar]
- Kujawa A, Klein DN, & Hajcak G (2012). Electrocortical reactivity to emotional images and faces in middle childhood to early adolescence. Developmental Cognitive Neuroscience, 2, 458–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa A, Klein DN, & Proudfit GH (2013). Two-year stability of the late positive potential across middle childhood and adolescence. Biological Psychology, 94(2), 290–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa A, MacNamara A, Fitzgerald KD, Monk CS, & Phan KL (2015). Enhanced neural reactivity to emotional faces in anxious youth: Evidence from event-related potentials. Journal of Abnormal Child Psychology, 43(8), 1493–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeGrand R, Mondloch CJ, Maurer D, & Brent HP (2003). Expert face processing requires visual input to the right hemisphere during infancy. Nature Neuroscience, 6(10), 1108–12. [DOI] [PubMed] [Google Scholar]
- LoBue V, & Thrasher C (2015). The Child Affective Facial Expression (CAFE) set: Validity and reliability from untrained adults. Frontiers in Psychology, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Calderon J, & Luck SJ (2014). ERPLAB: an open-source toolbox for the analysis of event-related potentials. Frontiers in Human Neuroscience, 8, 213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luck SJ (2014). An introduction to the event-related potential technique. Cambridge, Massachusetts: MIT Press. [Google Scholar]
- Luo W, Feng W, He W, Wang N-Y, & Luo Y-J (2010). Three stages of facial expression processing: ERP study with rapid serial visual presentation. NeuroImage, 49(2), 1857–1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacNamara A, Foti D, & Hajcak G (2009). Tell me about it: Neural activity elicited by emotional pictures and preceding descriptions. Emotion, 9(4), 531–543. [DOI] [PubMed] [Google Scholar]
- Marusak HA, Carré JM, & Thomason ME (2013). The stimuli drive the response: An fMRI study of youth processing adult or child emotional face stimuli. NeuroImage, 83, 679–689. [DOI] [PubMed] [Google Scholar]
- Meyer A, Lerner MD, De Los Reyes A, Laird RD, & Hajcak G (2017). Considering ERP difference scores as individual difference measures: Issues with subtraction and alternative approaches. Psychophysiology, 54(1), 114–122. [DOI] [PubMed] [Google Scholar]
- Mueller EM, Hofmann SG, Santesso DL, Meuret AE, Bitran S, & Pizzagalli DA (2008). Electrophysiological evidence of attentional biases in social anxiety disorder. Psychological Medicine, 39(07), 1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Öhman A, Flykt A, & Esteves F (2001). Emotion drives attention: Detecting the snake in the grass. Journal of Experimental Psychology: General, 130(3), 466–478. [DOI] [PubMed] [Google Scholar]
- Olofsson JK, Nordin S, Sequeira H, & Polich J (2008). Affective picture processing: An integrative review of ERP findings. Biological Psychology, 77(3), 247–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacNamara A, Vergés A, Kujawa A, Fitzgerald KD, Monk CS, Phan KL, … Phan KL (2016). Age-related changes in emotional face processing across childhood and into young adulthood: Evidence from event-related potentials. Developmental Psychobiology, 58(1), 27–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putnam SP, & Rothbart MK (2006). Development of short and very short forms of the Children’s Behavior Questionnaire. Journal of Personality Assessment, 87(1), 102–12. [DOI] [PubMed] [Google Scholar]
- Rothbart MK, & Derryberry D (1982). Development of individual differences in temperament. Advances in Developmental Psychology, 37–86. [Google Scholar]
- Sabatinelli D, Keil A, Frank DW, & Lang PJ (2013). Emotional perception: Correspondence of early and late event-related potentials with cortical and subcortical functional MRI. Biological Psychology, 92(3), 513–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon B, DeCicco JM, & Dennis TA (2012). Emotional picture processing in children: An ERP study. Developmental Cognitive Neuroscience, 2(1), 110–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speed BC, Nelson BD, Perlman G, Klein DN, Kotov R, & Hajcak G (2015). Personality and emotional processing: A relationship between extraversion and the late positive potential in adolescence. Psychophysiology, 52(8), 1039–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thom N, Knight J, Dishman R, Sabatinelli D, Johnson DC, & Clementz B (2013). Emotional scenes elicit more pronounced self-reported emotional experience and greater EPN and LPP modulation when compared to emotional faces. Cognitive, Affective, & Behavioral Neuroscience, 14(2), 849–860. [DOI] [PubMed] [Google Scholar]
- Van Cauwenberge V, Van Leeuwen K, Hoppenbrouwers K, & Wiersema JR (2017). Developmental changes in neural correlates of cognitive reappraisal: An ERP study using the late positive potential. Neuropsychologia, 95, 94–100. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P, Richardson MP, Armony JL, Driver J, & Dolan RJ (2004). Distant influences of amygdala lesion on visual cortical activation during emotional face processing. Nature Neuroscience, 7(11), 1271–1278. [DOI] [PubMed] [Google Scholar]


