Abstract
Objective:
To discuss the patient diagnosed with COVID-19 disease while receiving intravesical induction bacillus Calmette-Guérin (BCG) treatment for non-muscle-invasive bladder cancer, its management in the light of the literature.
Patient and methods:
A 52-year-old male patient, who received intravesical BCG treatment for high-grade pT1 papillary urothelial carcinoma, presented 12 h after taking the fourth dose of induction therapy 38.2° fever and chills. The patient’s reverse transcriptase-polymerase chain reaction test was positive, and Thorax CT imaging showed a few ground-glass pneumonic infiltrations in bilateral lung bases consistent with COVID-19 disease.
Results:
Although international urology associations have current recommendations regarding the pandemic process, only one study has made specific recommendations regarding the patient group diagnosed with COVID-19 while receiving intravesical BCG treatment. According to this recommendation, we interrupted our patient’s BCG treatment for 3 weeks and then completed the treatment for 6 weeks. A maintenance treatment not exceeding 1 year was planned.
Conclusion:
This group of patients’ recommendation is to delay BCG therapy for at least 3 weeks after initial symptoms to allow for complete recovery. Although the administration schedule varies, maintenance therapy is recommended for no more than 1 year.
Keywords: Bladder cancer, intravesical BCG, COVID-19, coronavirus, pandemic
Introduction
In the second half of 2019, new cases of pneumonia began to appear in Wuhan (Hubei province), China, and the World Health Organization named this coronavirus disease “COVID-19” and declared it a pandemic. 1 This current COVID-19 pandemic forces clinicians to adapt their clinical practice, especially for the management of life-threatening conditions such as urological malignancies. This adaptation is essential for patients with high-risk non-muscle-invasive bladder cancer (NMIBC) that requires repeat visits to the hospital for intravesical instillations of bacillus Calmette-Guérin (BCG) to reduce the risk of recurrence (60%–70%) and progression (26%) after transurethral bladder resection (TURB). 2 International urological societies, such as the European Association of Urology (EAU), 3 and the American Urological Association (AUA), 4 outline internationally recognized standards of care and publishes guidelines and protocols on how to triage patients during the COVID-19 outbreak. In this report, we aimed to present our patient diagnosed with COVID-19 disease during induction BCG treatment and the management of the disease in the light of literature and guidelines.
Case report
A 52-year-old male patient who underwent TURB for a bladder tumor about 5 cm size in an external center and whose pathology was reported as high-grade pT1 papillary urothelial carcinoma, but no muscle tissue was detected in the specimen, was admitted to our clinic for Re-TUR procedure. Thorax computerized tomography (CT) and CT-urography imaging was performed on the patient. CT-urography showed a 19 × 18 mm mass lesion with a filling defect in the bladder’s right lateral wall and thickening of the bladder wall (Figure 1). No pathology was found in thorax CT imaging. Cystoscopy revealed a papillary tumor with a solid component in the bladder’s right anterolateral wall with the previous resection area. Complete resection was performed separately for the tumor and tumor base. The bladder was mobile on bimanual examination. Pathology was reported as high-grade pT1 papillary urothelial carcinoma with no muscle invasion, and induction therapy with intravesical BCG was started.
Figure 1.
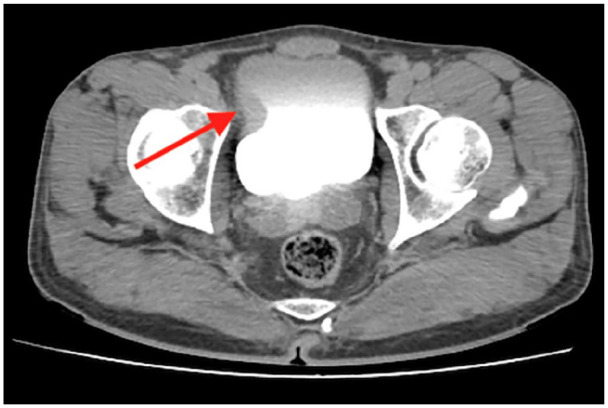
CT-urography showing a 19 × 18 mm mass lesion with a filling defect in the bladder’s right lateral wall and thickening of the bladder wall.
Twelve hours after the fourth session of induction therapy, the patient presented with a high fever (38.2°) and chills. There were 3+ blood and 3+ leukocytes in the urinary dipstick analysis and urinary infection was excluded by urine culture. While the white blood cell count was 4.21 (109/L), the c-reactive protein level was 24.6 mg/L. This situation was considered one of the systemic side effects of intravesical BCG (general malaise, fever), which is generally resolved within 48 h, with or without antipyretics. However, since the incidence of COVID-19 disease is high in our region, a reverse transcriptase-polymerase chain reaction (RT-PCR) test was performed. RT-PCR test was reported as positive. Thorax CT imaging showed a few ground-glass pneumonic infiltrations in bilateral lung bases consistent with COVID-19 disease (Figure 2). The patient had no symptoms associated with pneumonia, such as shortness of breath and cough, and oxygen saturation was above 93%. Since 5-day favipiravir treatment was recommended for asymptomatic COVID-19 outpatients in our country, this treatment was initiated. Ciprofloxacin was also added to the treatment as a prophylactic. When urinary tract infection was excluded as a result of urine culture, antibiotic treatment was stopped.
Figure 2.
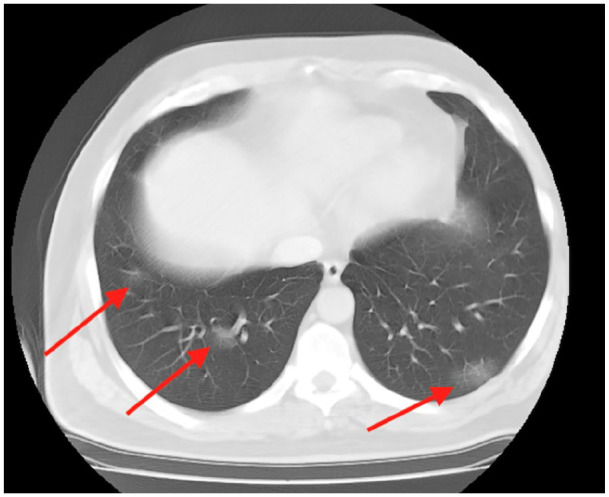
Thorax CT shows pneumonic infiltrates in bilateral lung basals with ground glass density compatible with COVID-19 disease.
The patient, who had a fever of >38° twice in the first 24 h after hospitalization, did not have fever in the following period, was discharged with the recommendations for isolation.
The induction BCG therapy was delayed for 3 weeks. Before continuing BCG treatment RT-PCR test was repeated and it was negative. Then, induction therapy was resumed and completed. His post-induction cystoscopy was negative. The patient was scheduled for a control cystoscopy 3 months later, and planned a maintenance BCG is not exceeding 1 year.
Discussion
The EAU recommends that all high-risk NMIBC patients should receive intravesical BCG therapy, which consists of induction and maintenance, and requires repeated hospital visits. 3 However, there is not enough data regarding the management of this patient group during the COVID-19 pandemic process. In this report, we evaluated our patient’s management with COVID-19 while receiving intravesical BCG treatment in the light of EAU, AUA, and current literature.
EAU made recommendations according to the priority level for situations affected by the pandemic crisis. High priority patients should be treated within 6 weeks. As cases of high-grade NMIBC most benefit from induction and initial maintenance BCG doses (so-called 6 + 3), this therapy should be recommended as a standard. The decision to start BCG therapy immediately or postpone (following a re-resection) depends on the risk of infection with SARSCoV-2 and the adverse course of COVID-19, the risk of bladder tumors, and current healthcare capacity. 3 BCG maintenance may not be performed after the first 3-month booster series until the risks of COVID-19 have been reduced. 5
Some theories suggest that BCG vaccination may protect against COVID-19 disease. BCG Vaccine has been shown to be protective against some DNA and RNA viruses by working on the innate immune system, producing a memory-like response. 6 Our patient’s overcoming the COVID-19 disease in mild form may be due to this effect of BCG. By the first half of 2020 it was thought that, the lack of a specific vaccine for COVID-19 could lead to a possible BCG shortage. AUA also made its recommendations for intravesical BCG application according to the possibility of this global BCG shortage that may occur. High-risk patients receiving induction therapy should be given priority for full BCG use. Where possible, if conditions are favorable, these high-risk patients should be reduced by 1/2–1/3 of the dose. If maintenance treatment is planned, 1/3 dose of BCG should be used, and an attempt should be made to limit the dose to 1 year. 4
Our case is the first reported in the literature to have COVID-19 disease while receiving induction BCG treatment. However, no specific direction has been reported for patients diagnosed with COVID-19 disease while receiving intravesical BCG therapy. Lenfant et al. 2 made some suggestions for this patient group. They stated that it is vital to complete the 6-week BCG induction dose for high-risk NMIBC cases without a diagnosis of COVID-19. They consider it acceptable to take at least two of the three doses of maintenance BCG therapy to minimize the number of hospital visits. They believe that patients who have been on intravesical BCG therapy for more than 1 year can safely terminate this therapy. Since there is not enough data on the reliability of BCG administration for patients with suspected or confirmed COVID-19. It is recommended to postpone BCG administration for at least 3 weeks after the first symptoms to allow full recovery. According to these recommendations, we interrupted our patient’s BCG treatment for 3 weeks and then completed the treatment for 6 weeks. The consensus on maintenance treatment is that it should not exceed 1 year.2–4 However, there is no consensus on the form of maintenance therapy. So that, we consider applying the first maintenance and arranging the next process according to the clinical response and pandemic intensity in our region.
Conclusion
This case report describes the management of a patient diagnosed with COVID-19 during induction BCG therapy. The recommendation is interrupt treatment for 3 weeks and then complete induction therapy to 6 weeks. Although the administration schedule varies, maintenance therapy is recommended no more than 1 year.
Footnotes
Authors’ contribution: F.O. and E.D., made substantial contributions to conception and design, or acquisition of data, or analysis and interpretation of data. F.O. and E.D. been involved in drafting the manuscript or revising it critically for important intellectual content. E.D. took care of the patient. E.D. took care of patient’s specimens. F.O. supervised the creation of the manuscript. All authors read and approved the final manuscript.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Consent for publication: Written informed consent for publication of clinical details and was obtained from the patient at the moment of the admission at our outpatient service.
ORCID iD: Fesih Ok  https://orcid.org/0000-0002-8785-9867
https://orcid.org/0000-0002-8785-9867
References
- 1.Ok F, Erdogan O, Durmus E, et al. Predictive values of blood urea nitrogen/creatinine ratio and other routine blood parameters on disease severity and survival of COVID-19 patients. J Med Virol 2021; 93(2): 786–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lenfant L, Seisen T, Loriot Y, et al. Adjustments in the use of intravesical instillations of Bacillus Calmette-Guérin for high-risk non-muscle-invasive bladder cancer during the COVID-19 pandemic. Eur Urol 2020; 78(1): 1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ribal MJ, Cornford P, Briganti A, et al. EAU Section Offices and the EAU Guidelines Panels. European Association of Urology Guidelines Office Rapid Reaction Group: an organisation-wide collaborative effort to adapt the European Association of Urology guidelines recommendations to the coronavirus disease 2019 era. Eur Urol 2020; 78(1): 21–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.American Urological Association. Important message about the BCG shortage, https://www.auanet.org/about-us/bcg-shortage-info (accessed 10 April 2020).
- 5.Wallis CJD, Novara G, Marandino L, et al. Risks from deferring treatment for genitourinary cancers: a collaborative review to aid triage and management during the COVID-19 pandemic. Eur Urol 2020; 78(1): 29–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hegarty PK, Kamat AM, Zafirakis H, et al. BCG vaccination may be protective against Covid-19. Preprint. 2020. [Google Scholar]


