Abstract
Background:
Corticosteroids and tocilizumab have been shown to improve survival in patients who require supplemental oxygen from coronavirus disease 2019 (COVID-19) pneumonia. The optimal dose of immunosuppression for the treatment of COVID-19 acute respiratory distress syndrome (ARDS) is still unknown.
Objective:
The objective of this study was to evaluate the effectiveness and safety of high- versus low-dose corticosteroids with or without tocilizumab for the treatment of COVID-19 ARDS.
Methods:
This was a retrospective study of patients admitted to the intensive care unit (ICU) requiring mechanical ventilation who received high- versus low-dose corticosteroids with or without tocilizumab. The primary outcome was survival to discharge. Safety outcomes included infections and incidence of hyperglycemia.
Results:
In this cohort, 110 (54%) and 95 (46%) patients received high-dose (≥10 mg dexamethasone equivalent) and low-dose (<10 mg dexamethasone equivalent) corticosteroids for more than 3 consecutive days, respectively. Thirty-five patients (32%) in the high-dose group and 33 patients (35%) in the low-dose group survived to hospital discharge (P = 0.85). There was no difference in 28-day mortality in patients who received high-dose corticosteroids without tocilizumab compared with those who received low-dose corticosteroids with tocilizumab (n = 38/82, 46% vs n = 19/40, 48% P = 0.99); however, there was a higher mortality if patients received low-dose corticosteroids without tocilizumab (n = 39/55, 71%, P = 0.01). The highest rate of a bacterial pneumonia was in patients who received high-dose corticosteroids with tocilizumab.
Conclusions:
In critically ill patients with COVID-19 ARDS requiring mechanical ventilation, we found no difference in high- versus low-dose corticosteroids with regard to survival to hospital discharge. However, patients receiving only low-dose corticosteroids without tocilizumab did worse than the other groups. Larger prospective studies are needed to determine the optimal immunosuppression dosing strategy in this patient population.
Keywords: COVID-19, acute respiratory distress syndrome, critical care, corticosteroids, mechanical ventilation, pharmacology
Introduction
Acute respiratory distress syndrome (ARDS) due to severe acute respiratory syndrome-coronavirus 2 (SARS-CoV-2; coronavirus disease 2019 [COVID-19]) is associated with significant morbidity and mortality. 1 Infection with COVID-19 can lead to an intense inflammatory syndrome as evidenced by profound elevations in C-reactive protein (CRP), Interleukin (IL), and hyperthermia, which can progress to systemic inflammatory response syndrome (SIRS), septic shock, and refractory ARDS. 2 Despite initial concerns that systemic corticosteroids may increase viral replication, the Randomized Evaluation of COVID-19 (RECOVERY) trial demonstrated a mortality benefit for patients with COVID-19 ARDS requiring supplemental oxygen or mechanical ventilation when treated with dexamethasone 6 mg per day for 10 days in efforts to quell the hyperinflammatory syndrome. 3 Based on these findings, various society guidelines, including the World Health Organization, National Institutes of Health, and Infectious Diseases Society of America recommend corticosteroids for the treatment of acute COVID-19 respiratory disease. However, the RECOVERY group included patients who required significantly lower supplemental oxygen (with or without noninvasive ventilation) and did not increase the dose of corticosteroids for those who required mechanical ventilation. 3 Therefore, the recommended dosage is the same regardless of patients’ clinical status, severity of hyperinflammatory syndrome, or sequela of hypoxia.
Contemporary to the RECOVERY trial, studies have examined various corticosteroid dosing strategies to mitigate the inflammatory syndrome. Randomized, embedded, multifactorial, adaptive platform trial for community-acquired pneumonia (REMAP-CAP) examined hydrocortisone 50 mg every 6 hours (dexamethasone equivalent to 7.5 mg per day) for 7 days in patients on invasive, noninvasive ventilation or high-flow oxygen with a fraction of inspired oxygen (FiO2) of 40% or greater. 4 Another study by Dequin et al 5 had sought to elucidate the effects of a hydrocortisone continuous intravenous (IV) infusion of 200 mg per day (dexamethasone equivalent to 7.5 mg per day) for 7 days followed by a 14-day taper in patients who met specific severity criteria. Patients were included if they were receiving mechanical ventilation, or if they were receiving oxygen through high-flow nasal cannula or a reservoir mask with a PaO2:FiO2 ratio less than 300 or a pulmonary severity index greater than 130. 5 The COVID-19-associated ARDS treated with Dexamethasone (CODEX) study examined the use of dexamethasone 20 mg for 5 days followed by 10 mg for 5 days compared with standard of care (which did not include corticosteroids) in patients who were receiving mechanical ventilation within 48 hours of meeting criteria for moderate to severe ARDS with a PaO2:FiO2 ratio of 200 or less. 6 All 3 studies were halted early, given the rapidly shifting standard of care early in the pandemic.4-6 Since the time of this research, 2 trials have sought to investigate high-dose versus low-dose corticosteroids with different dosing strategies. The COVID STEROID 2 trial assessed the effects of dexamethasone 12 mg per day versus 6 mg per day in patients with COVID-19 requiring at least 10 L per minute of oxygen or mechanical ventilation and found no difference in days alive without life support. 7 The HIGHLOWDEXA-COVID trial compared high-dose dexamethasone (20 mg daily for 5 days followed by 10 mg daily for 5 days) with low-dose dexamethasone (6 mg daily for 10 days) in a less ill cohort of patients with COVID-19 pneumonia needing oxygen therapy. 8 The authors concluded that the higher dose of dexamethasone reduced clinical worsening within 11 days. 8 Finally, 3 main studies suggest benefit with the coadministration of corticosteroids and tocilizumab.9-11
Corticosteroids are immunomodulators with antiinflammatory effects. They primarily mediate their effects by activating the ubiquitously expressed glucocorticoid receptor (GR). 12 Upon ligand activation from corticosteroid binding (ie dexamethasone), the GR translocates to the cell nucleus and reversibly binds to specific DNA sites resulting in transactivation and transrepression, thereby producing genomic actions that alter protein expression. 13 Through these mechanisms, dexamethasone can inhibit the production of proinflammatory cytokines, including IL-1, IL-2, IL-6, IL-8, tumor necrosis factor, interferon-gamma, vascular endothelial growth factor, and prostaglandins, all of which have been linked to COVID-19 disease severity.13,14 The genomic process typically takes hours to days before changes at the cellular, tissue, or organism level become evident. 12 Corticosteroids also manifest almost immediate nongenomic actions on many signaling processes. 12 Through nonspecific interactions with the cell membrane, or interactions with cytosolic or membrane-bound GR, dexamethasone can rapidly modulate both intracellular calcium levels and a variety of cellular pathways, depending on the cell type. 12 Evidence also shows a rapid nontranscriptional action of corticosteroids on inflammation in immune cells, such as neutrophils, and has demonstrated a role in T-cell receptor signaling. 12 Higher doses of corticosteroids can fully saturate all GRs and induce the full range of genomic effects. 15 Although the genomic effects are responsible for increased expression of regulatory and antiinflammatory proteins (transactivation), their effects of transrepression are thought to be responsible for the numerous adverse effects of corticosteroids. 15 Questions still linger regarding the most appropriate dose of corticosteroids (along with other immunosuppressants) for the treatment of mechanically ventilated patients with COVID-19 ARDS, while balancing the known adverse effects associated with their use.
Materials and Methods
Study Design and Participants
This was a retrospective cohort study of patients admitted between July 2020 and March 2021 to NYU Langone Health, an ethnically and socioeconomically diverse health system with 3 campuses located in Manhattan, Long Island, and Brooklyn. The purpose of this study was to evaluate the effectiveness and safety of high-dose versus low-dose corticosteroids for the treatment of COVID-19-related ARDS. Based on local COVID-19 variants at the time of data collection, most cases were caused by the Delta strain. 16 All campuses used the same hospital guidelines for COVID-19 management. Each location houses a closed intensive care unit (ICU), with a team run by a critical care physician attending. Corticosteroids were indicated for hospitalized patients receiving supplemental oxygen, high flow nasal cannula, and noninvasive or invasive ventilator support. High-dose corticosteroids were defined as the equivalent of a dexamethasone dose >10 mg for more than 3 days consecutively. An equivalent dose of dexamethasone <10 mg for more than 3 consecutive days was considered low-dose treatment. The recommended treatment duration of corticosteroids was 10 days. Tocilizumab, at a dose of 8 mg/kg, was administered off-label under emergency use authorization. Patients were eligible to receive tocilizumab if they had a significant oxygen requirement (6 L nasal cannula, high-flow nasal cannula, or mechanical ventilation), had elevated biomarkers (ferritin >600 ng/mL, CRP>100 mg/L or lactate dehydrogenase >220 U/L), and had large consolidations or ground glass opacities on chest imaging. As per hospital guidance, it was recommended to administer tocilizumab within 24 hours of mechanical ventilation, if not already administered prior to intubation. The treating physician may have opted to administer the dose of tocilizumab beyond the recommended 24 hours of initiation of mechanical ventilation, based on clinical status and medication availability. The study was approved by the New York University (NYU) Grossman School of Medicine Institutional Review Board. Waiver of authorization requirement for informed consent was obtained due to the retrospective nature of the study.
Patients were included if they were aged >18 years, had a positive SARS-CoV-2 polymerase chain reaction (PCR) test by nasopharyngeal swab upon hospital admission, received corticosteroid therapy, and were admitted to the Medical Intensive Care Unit requiring mechanical ventilation for more than 24 hours. All patients had a diagnosis of ARDS in their electronic medical record, captured by International Classification of Diseases, Ninth Revision (ICD-9) billing code. Patients were excluded if they received corticosteroids for less than 72 hours during hospitalization or received extracorporeal membrane oxygenation support.
Study Variables
Data were collected through an automated extraction system from our electronic health record. We collected patient demographics that included age, body mass index, sex, and race. Pertinent past medical history and comorbidities were captured from physician documentation. Time to initiation, initial and cumulative doses, as well as duration of corticosteroid regimens were captured in dexamethasone equivalents (hydrocortisone 20 mg = prednisone 5 mg = methylprednisolone 4 mg = dexamethasone = 0.75 mg). There were patients who were transitioned between corticosteroid type or who were only on nondexamethasone regimens based on prescriber preference. We have converted all medications to dexamethasone as it was the most frequently prescribed corticosteroid and is considered standard of care. The index date was captured as the first day of corticosteroid therapy. Baseline inflammatory markers (including CRP, IL-6, and ferritin) and changes in biomarkers were collected when available. Relevant concomitant medications, including tocilizumab, remdesivir, anticoagulants, and antimicrobials were captured. Hospital length of stay, ICU length of stay, duration of mechanical ventilation and tracheostomy incidence were collected for each patient. All testing for bacterial and fungal infections ordered as part of routine care were collected, including cultures, T2Candida panel (manufacturer: T2 Biosystems), beta-d glucan, and white blood cell count. A superimposed bacterial pneumonia was diagnosed based on a positive tracheal aspirate in conjunction with clinical signs of ventilator-associated pneumonia (ie infiltrate on chest radiography, leukocytosis, purulent tracheobronchial secretions, or fever >38.3°C).
Outcomes
The primary outcome was survival to hospital discharge. Secondary outcomes included ventilator-free days at day 28 in the ICU, hospital length of stay, ICU length of stay, duration of mechanical ventilation, tracheostomy incidence, changes in biomarkers and 28-day mortality. Ventilator-free days at day 28 in the ICU was defined as number of days following liberation of mechanical ventilation, either from an endotracheal tube or tracheostomy tube. Safety outcomes measured bacterial and fungal infections, which were captured through positive cultures or positive fungal markers. Average glucose per day while receiving corticosteroid treatment was collected as well.
Statistics
Continuous variables were expressed as median values and as interquartile range (IQR), unless otherwise noted. Continuous variables were compared using a Mann-Whitney U test. Categorical data were analyzed using a χ2 or Fisher’s exact test, as appropriate. A P value of <0.05 was considered statistically significant. Data analysis was performed using IBM SPSS Statistic Software (Chicago, IL, Version 25). A 3-way analysis of variance (ANOVA) and a post hoc Tukey test were conducted to determine whether there were any significant differences between patients who received tocilizumab.
Results
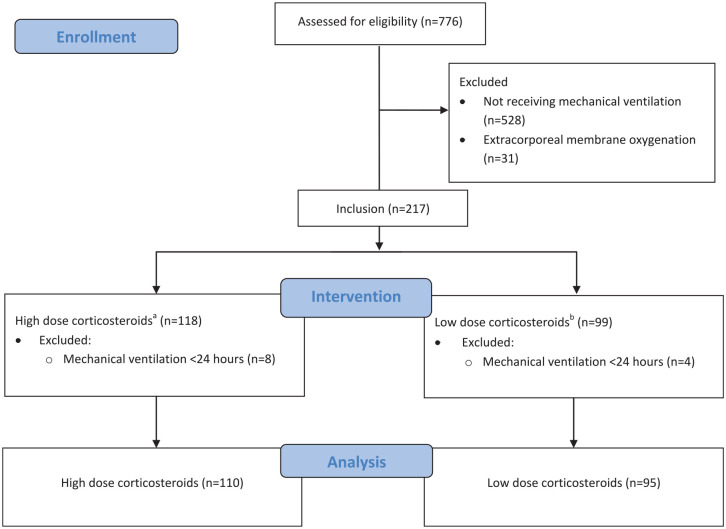
There were 776 patients assessed for eligibility. Upon initial screening, 559 met exclusion criteria (Figure 1). Of the 205 patients who were admitted to the ICU for active COVID-19 ARDS, 110 patients received high-dose and 95 patients received low-dose dexamethasone. Forty patients (42%) receiving low-dose dexamethasone also received tocilizumab. In the high-dose group, 28 patients (25%) received tocilizumab. No patients who met the inclusion criteria failed to receive corticosteroids. The median age was 69 years in both groups. Females represented 67% of the high-dose group and 70% of the low-dose group. The most common comorbidity was hypertension, occurring in roughly 35% of both groups. Other frequently observed comorbidities were distributed similarly in both groups and included pulmonary disease, chronic heart failure, and diabetes (Table 1). There were some statistical differences between corticosteroid distribution and racial groups. There was no difference in time from hospitalization to mechanical ventilation between the 2 groups (P = 0.47). The median sequential organ failure assessment (SOFA) score at the time of intubation was 7 in both groups. All other pertinent past medical history is listed in Table 1.
Figure 1.
Flow diagram for study inclusion.
aHigh-dose corticosteroids: >10 mg dexamethasone equivalent for more than 3 consecutive days.
bLow-dose corticosteroid: <10 mg dexamethasone equivalent for more than 3 consecutive days.
Table 1.
Baseline Characteristics.
| High dose (n = 110) | Low dose (n = 95) | P value | |
|---|---|---|---|
| Age, years | 69 (63-78) | 69 (59-76) | 0.69 |
| Male sex, n (%) | 43 (39) | 30 (32) | 0.26 |
| Body mass index, kg/m2 | 29.1 (25.1-33.3) | 28.6 (25.2-32.6) | 0.76 |
| Race, n (%) | |||
| Caucasian | 45 (41) | 45 (47) | 0.35 |
| African American | 11 (10) | 23 (24) | <0.01 |
| Asian | 24 (22) | 18 (19) | 0.61 |
| Hispanic | 14 (13) | 5 (5) | 0.07 |
| Other | 16 (15) | 4 (4) | 0.01 |
| Time from hospital to ICU admission (days) | 2 (0, 7) | 1 (0, 4) | 0.14 |
| Time from hospital admission to mechanical ventilation (days) | 6 (2-12) | 4 (0.8-8) | 0.47 |
| SOFA score at time of intubation | 7 (5-8) | 7 (5-9) | 0.24 |
| Past medical history, n (%) | |||
| Pulmonary disease | 17 (15.4) | 11 (11.5) | 0.42 |
| Chronic heart failure | 8 (7.2) | 9 (9.5) | 0.57 |
| Psychiatric disorder | 11 (10) | 5 (5.2) | 0.21 |
| Diabetes | 18 (16.3) | 19 (20) | 0.50 |
| Hypertension | 37 (33.6) | 34 (36) | 0.74 |
| Liver disease | 6 (5.4) | 6 (6.3) | 0.79 |
| Malignancy | 12 (11) | 4 (4.2) | 0.08 |
| Obesity | 15 (13.6) | 6 (6.2) | 0.09 |
| Renal failure | 11 (10) | 9 (9.5) | 0.90 |
| Valvular disease | 8 (7.2) | 3 (3.2) | 0.19 |
| Coagulation disorder | 5 (4.5) | 5 (5.3) | 1.00 |
| Vascular disease | 12 (11) | 8 (8.4) | 0.55 |
| Baseline laboratory markers ±24 hours of index corticosteroids a | |||
| C-reactive protein, mg/L | 123.3 (69.3-187.4) | 139.9 (69.9-212.45) | 0.29 |
| Ferritin, ng/mL | 969.0 (477.1-2386.3) | 782.7 (366.4-1689.9) | 0.17 |
| Lactate dehydrogenase, u/L | 476.5 (343.5-630.5) | 455 (357.5-666.5) | 0.87 |
| d-dimer, ng/mL | 434.0 (271-800.5) | 449.3 (284.8-903.8) | 0.60 |
| IL-6, pg/mL | 13.2 (3.9-37.9) | 12.6 (3.2-64) | 0.75 |
| Absolute lymphocyte count, 103/uL | 0.7 (0.6-1.1) | 0.8 (0.5-1) | 0.45 |
| White blood cell count, 103/uL | 7.8 (5.6-11.5) | 9.3 (6.2-13.5) | 0.04 |
All values listed in median (interquartile range = 25%-75%), unless otherwise noted.
Abbreviations: ICU, intensive care unit; SOFA, sequential organ failure assessment.
The index date was captured as the first day of corticosteroids.
A P value <0.05 indicates statistical significance.
Patients were admitted to the ICU at a median of 2 days (IQR = 0-7) and 1 day (IQR = 0-4) from hospital admis-sion in the high-dose and low-dose groups, respectively (P = 0.14). All patients had been receiving corticosteroids at the time of intubation. The median maximal daily dose was 12 mg (IQR = 10-17) in the high-dose group and 6 mg (IQR = 6-10) in the low-dose group (P < 0.01; Table 2). The total corticosteroid duration was 11 days (IQR = 6-15) and 10 days (IQR = 6-12) in the high-dose and low-dose groups, respectively (P = 0.09). Patients in the high-dose group had a lower baseline white blood cell count compared with the low-dose group (P = 0.04). There was no difference in any of the other baseline markers including IL-6, CRP, absolute lymphocyte count, d-dimer, ferritin, and lactate dehydrogenase. There were 94 patients (85%) and 75 patients (79%) who were treated with remdesivir in the high-dose and low-dose corticosteroid groups, respectively (P = 0.22). Tocilizumab was administered at a median of 2 days into hospitalization in both groups (P = 0.57).
Table 2.
Medications.
| High dose (n = 110) | Low dose (n = 95) | P value | |
|---|---|---|---|
| Time to corticosteroid initiation from hospital admission, (days) | 0 (0-1) | 0 (0-2) | 0.31 |
| Initial corticosteroid dose, average ± SD (mg) | 8 (±3.8) | 6 (±3.9) | <0.01 |
| Total corticosteroid dose, cumulative (mg) | 96 (58-142) | 60 (36-73) | <0.01 |
| Total corticosteroid duration, (days) | 11 (6-15) | 10 (6-12) | 0.09 |
| Maximal daily dose, (mg) | 12 (10-17) | 6 (6-10) | <0.01 |
| Concomitant COVID-19 directed and antiinfective medications | |||
| Remdesivir, n (%) | 94 (85) | 75 (79) | 0.22 |
| Duration of therapy, days | 10 (5-10) | 8 (5-10) | 0.78 |
| Tocilizumab, n (%) | 28 (25) | 40 (42) | 0.01 |
| Total dose (mg) | 400 (range = 400-1000) | 400 (range = 400-800) | 0.43 |
| Therapeutic anticoagulation, n (%) | 92 (83) | 78 (82) | 0.77 |
| Antibiotics, n (%) | 108 (98) | 92 (97) | 0.65 |
| Antifungals, n (%) | 61 (55) | 46 (48) | 0.28 |
All values listed in median (interquartile range = 25%-75%), unless otherwise noted. Therapeutic anticoagulation: Intravenous heparin or low molecular weight heparin 1 mg/kg q12 or 1.5 mg/kg q24, direct thrombin inhibitors, direct oral anticoagulants, and warfarin. Antibiotics: All antimicrobial agents used for empiric or confirmed treatment of a bacterial infection (does not include prophylactic agents). Antifungals: All antifungal agents used for empiric or confirmed treatment of a bacterial infection (does not include prophylactic agents).
Abbreviation: COVID-19, coronavirus disease 2019.
A P value <0.05 indicates statistical significance.
Primary and Secondary Outcomes
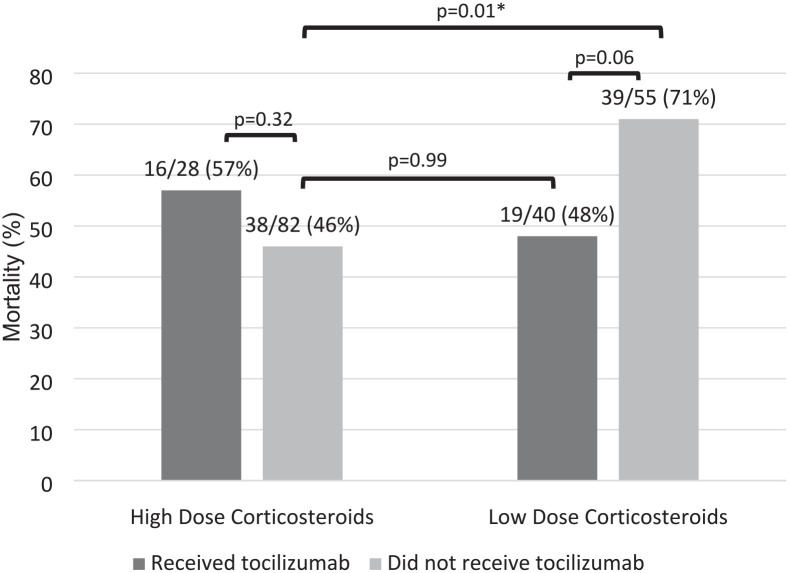
There was no significant difference in survival to discharge between the high- and low-dose corticosteroid groups (high-dose 32% vs low-dose 35%, P = 0.85). For those alive, there was no significant difference in ventilator-free days at day 28 in the ICU, high-dose (n = 56): 3 days (IQR = 0-9.5) vs low-dose (n = 37): 2 days (IQR = 0.5-19.5), P = 0.46. The median duration of mechanical ventilation was 14 days (IQR = 8-30) in the high-dose corticosteroid group and 10 days (IQR = 4-29) in the low-dose corticosteroid group. Patients who received low-dose corticosteroids had a shorter ICU length of stay compared with those who received high-dose corticosteroids, 12 days (IQR = 6-27) vs 18 days (IQR = 10-33), P = 0.05. There were 44 (40%) and 31 (33%) patients who required tracheostomy in the high- and low-dose groups, respectively. There was also no difference in 28-day mortality between the patients who received high-dose and low-dose corticosteroids (Table 3). There was a significant difference in 28-day mortality between patients who received high-dose corticosteroids without tocilizumab, low-dose corticosteroids without tocilizumab, and those who received low-dose corticosteroids with tocilizumab (P = 0.01). In the post hoc Tukey test, there was a significant difference in 28-day mortality in patients who received high-dose corticosteroids compared with low-dose corticosteroids without tocilizumab in either arm (46% vs 71%, P = 0.01). There was no significant difference in 28-day mortality between the high-dose corticosteroid group without tocilizumab compared with the patients who received low-dose corticosteroids with tocilizumab (P = 0.99). There was also no difference in 28-day mortality in patients who received high-dose corticosteroids with or without tocilizumab (57% vs 46%, P = 0.32). There was a numerical difference and a trend toward a higher 28-day mortality in patients who received low-dose corticosteroids without tocilizumab compared with the patients who received low-dose corticosteroids with tocilizumab (71% vs 48%, P = 0.06; Table 4, Figure 2).
Table 3.
Primary and Secondary Outcomes.
| High dose (n = 110) | Low dose (n = 95) | P value | |
|---|---|---|---|
| Survival to discharge, n (%) | 35 (32) | 33 (35) | 0.85 |
| Ventilator-free days at day 28 in the ICU, (days) | 3 (0-9.5) n = 56 |
2 (0.5-19.5) n = 37 |
0.46 |
| Hospital LOS, (days) | 25 (17-44) | 20 (12-41) | 0.06 |
| ICU LOS, (days) | 18 (10-33) | 12 (6-27) | 0.05 |
| Duration of mechanical ventilation, (days) | 14 (8-30) | 10 (4-29) | 0.08 |
| Tracheostomy, n (%) | 44 (40) | 31 (33) | 0.27 |
| Mortality at 28 days, n (%) | 54 (49) | 58 (61) | 0.09 |
All values listed in median (interquartile range = 25%-75%), unless otherwise noted.
Abbreviations: LOS, length of stay; ICU, intensive care unit.
A P value <0.05 indicates statistical significance.
Table 4.
The 28-Day Mortality Post Hoc Tukey test.
| High-dose corticosteroids | Low-dose corticosteroids | |
|---|---|---|
| Received tocilizumab | 16/28 (57%) a | 19/40 (48%) b |
| Did not receive tocilizumab | 38/82 (46%) c | 39/55 (71%) d |
All values listed as n (%), unless otherwise noted; a vs c: P = 0.32; c vs d: P = 0.01; c vs b: P = 0.99; b vs d: P = 0.06.
Figure 2.
The 28-Day mortality post hoc Tukey test.
*A P value <0.05 indicates statistical significance.
Biomarkers
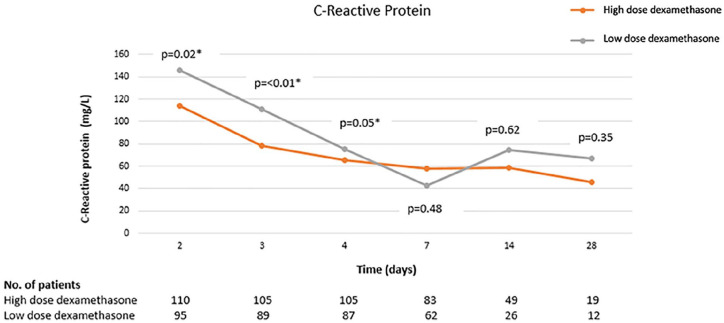
Biomarkers were followed at several time points following administration of corticosteroids. The CRP was significantly lower in the high-dose corticosteroid group through the first 96 hours (Figure 3). There was no difference in other sequentially monitored biomarkers between the 2 groups (Table 5).
Figure 3.
C-reactive protein trend over time.
*A P value <0.05 indicates statistical significance.
Table 5.
Biomarkers.
| High dose (n = 110) | Low dose (n = 95) | P value | |
|---|---|---|---|
| C-reactive protein, mg/L | |||
| 48 hours | 114.3 (56.1-151.8) | 145.6 (81.6-216.4) | 0.02 |
| 72 hours | 78.3 (37.5-131.4) | 110.5 (55.9-196.2) | <0.01 |
| 96 hours | 65.7 (37.2-127.2) | 75.3 (48.4-170) | 0.05 |
| 7 days | 57.6 (29-138.2) | 42.2 (17.0-168.5) | 0.48 |
| 14 days | 58.6 (8.9-178.5) | 74.2 (7.8-96.6) | 0.62 |
| 28 days | 45.5 (12.4-64) | 67.1 (15.6-107.0) | 0.35 |
| Ferritin, ng/mL | |||
| 48 hours | 1016.2 (535.9-2494) | 1208 (554-2443) | 0.92 |
| 72 hours | 1153.3 (553.7-2579.5) | 1420.5 (612.3-2694.5) | 0.63 |
| 96 hours | 1085.9 (518.4-2345.5) | 1429 (528.8-2513.3) | 0.57 |
| 7 days | 1127.9 (671.5-2059.8) | 1302 (685.4-1999.5) | 0.47 |
| 14 days | 882.5 (559.3-1857) | 1270 (734-2702.5) | 0.15 |
| 28 days | 737 (367-1362) | 920 (513.3-2330.5) | 0.48 |
| Lactate dehydrogenase, U/L | |||
| 48 hours | 547.5 (390.5-686) | 547 (412-757) | 0.44 |
| 72 hours | 591 (448.5-805.5) | 575.5 (415-773.8) | 0.66 |
| 96 hours | 618 (467.5-795) | 560 (458.5-741) | 0.21 |
| 7 days | 543.5 (425.5-668.7) | 542 (458-768.5) | 0.41 |
| 14 days | 437.5 (334.3-556) | 429.5 (336.3-643.3) | 0.79 |
| 28 days | 488 (436.8-678) | 369.5 (316.5-485) | 0.04 |
| d-dimer, ng/mL | |||
| 48 hours | 547 (294-1708) | 494 (332-1625) | 0.74 |
| 72 hours | 953 (339-2931) | 725 (406.5-2278) | 0.89 |
| 96 hours | 1303.5 (428-2511.3) | 1091 (486-3178) | 0.34 |
| 7 days | 1439-538.5 (2914) | 1851.5 (855.5-3830.8) | 0.06 |
| 14 days | 1260.3 (694-2555) | 1107.5 (536-3237) | 0.77 |
| 28 days | 938 (657-2117.5) | 723.5 (483.5-1825) | 0.29 |
| Absolute lymphocyte count, 103/uL | |||
| 48 hours | 0.7 (0.5-1) | 0.7 (0.5-1.1) | 0.97 |
| 72 hours | 0.8 (0.5-1.1) | 0.7 (0.5-1) | 0.19 |
| 96 hours | 0.7 (0.5-1.1) | 0.7 (0.4-1) | 0.31 |
| 7 days | 0.65 (0.4-1) | 0.7 (0.4-1) | 0.15 |
| 14 days | 0.8 (0.5-1.2) | 0.7 (0.5-1.1) | 0.05 |
| 28 days | 1.1 (0.8-1.7) | 1.5 (0.6-2.1) | 0.76 |
| White blood cell count, 103/uL | |||
| 48 hours | 7.9 (5.3-12.2) | 9.2 (6-14) | 0.04 |
| 72 hours | 9.8 (7.1-14.4) | 11.1 (7.9-13.9) | 0.19 |
| 96 hours | 11.1 (7.6-15.3) | 10.7 (8.2-15.7) | 0.63 |
| 7 days | 13.7 (10.6-18.2) | 13.3 (9.4-19.4) | 0.59 |
| 14 days | 15.1 (11.7-21.2) | 15.2 (11.6-22.3) | 0.94 |
| 28 days | 10 (7.9-13.2) | 11.7 (8.1-13.6) | 0.46 |
All values listed in median (interquartile range = 25%-75%), unless otherwise noted.
A P value <0.05 indicates statistical significance.
Adverse Events
Adverse events are listed in Table 6. Patients who received high-dose corticosteroids while on mechanical ventilation were more likely develop a concomitant bacterial pneumonia with a positive bacterial tracheal aspirate (65% vs 53%, P = 0.04). Furthermore, patients who received high-dose corticosteroids with tocilizumab had the highest rates of a positive tracheal aspirate culture (71%) compared with those who received high-dose corticosteroids without tocilizumab (63%), low-dose corticosteroids with tocilizumab (58%) and low-dose corticosteroids without tocilizumab (49%). There were no significant differences in fungal infections within 28 days and no differences in the incidence of hyperglycemia.
Table 6.
Adverse Events.
| High dose (n = 110) | Low dose (n = 95) | P value | |
|---|---|---|---|
| Fungal infections within 28 days, n (%) | |||
| 1,3-beta-d glucan>200 | 18 (16) | 11 (12) | 0.33 |
| Positive T2 candida panel | 15 (14) | 14 (15) | 0.66 |
| Antifungal use, n (%) | 61 (55) | 46 (48) | 0.28 |
| Duration of therapy, days | 5 (1-12) | 4 (2-13) | 0.98 |
| Bacterial infections within 28 days, n (%) | |||
| Tracheal aspirate culture | 72 (65) | 50 (53) | 0.04 |
| With tocilizumab | 20/28 (71) | 23/40 (58) | 0.24 |
| Without tocilizumab | 52/82 (63) | 27/55 (49) | 0.09 |
| Blood culture | 32 (29) | 24 (25) | 0.50 |
| Antibiotic use, n (%) | 108 (98) | 92 (97) | 0.65 |
| Duration of therapy, days | 15 (8-24) | 12 (6-27) | 0.48 |
| Average glucose/day, mean (SD) | 169 (±35) | 171 (±42) | 0.89 |
All values listed in median (interquartile range = 25%-75%), unless otherwise noted.
A P value <0.05 indicates statistical significance.
Discussion
Despite the RECOVERY trial, clinical equipoise exists in the dosage of corticosteroids for the treatment of COVID-19 ARDS. It is clear that immunosuppression improves ARDS but the optimal therapeutic regimen is not well established. This study suggests that, in patients with COVID-19 ARDS requiring mechanical ventilation, high-dose corticosteroids without tocilizumab did not show benefit over low-dose corticosteroids with tocilizumab but there is signal that both can improve outcomes over low-dose corticosteroids alone.
Severe inflammation secondary to SARS-CoV-2 infection can lead to prolonged lung injury. Mitigation of the inflammatory storm with corticosteroids have become the mainstay of therapy for the treatment of COVID-19 hypoxia.17,18 A meta-analysis of 7 randomized controlled trials demonstrated that, compared with placebo, corticosteroid therapy reduced the risk of all-cause mortality (relative risk [RR] 0.75; 95% confidence interval [CI] = [0.59-0.95]) and duration of mechanical ventilation (mean difference −4.93 days; 95% CI = [−7.81 to −2.06] days). 19 There is still a need for evidence for specific subgroups of disease severity, as well as for the different types and doses of corticosteroids. A subgroup analysis in the RECOVERY trial found a significantly lower mortality in patients requiring mechanical ventilation who received 6 mg of dexamethasone per day compared with those who received usual care. 3 When comparing this subgroup of mechanically ventilated patients with a similar patient population in the CODEX trial, with using higher doses of corticosteroids (20 mg per day for 5 days followed by 10 mg per day for 5 days), the CODEX trial was unable to replicate the mortality benefit that was demonstrated by the RECOVERY trial.3,6
The dose-dependent effects of corticosteroids may have an implication in viral replication. Earlier studies have shown that high doses of corticosteroids suppress the antiviral innate immune system by impairing T-lymphocyte-mediated responses, thus making it difficult to eradicate pathogens as efficiently.13,20 Slower clearance and prolonged shedding of viral RNA has been observed in patients with severe acute respiratory syndrome (SARS), Middle East respiratory syndrome (MERS), and influenza for those who were treated with high doses of systemic corticosteroid.3,21-23 It is likely that the beneficial effect of glucocorticoids in severe viral respiratory infections is dependent on the selection of the right dose, in the right patient. Early in the pandemic, Li et al20,24 demonstrated in a Cox regression analysis, that high-dose corticosteroids (dexamethasone equivalent 15 mg per day) but not low-dose corticosteroids (dexamethasone equivalent 7.5 mg per day) may potentially prolong viral shedding in patients with COVID-19. In addition, high-dose but not low-dose corticosteroids were potentially found to increase mortality of patients with severe COVID-19.20,24 Based on the analysis, Li et al 20 suggest that the effect of corticosteroids on viral shedding may occur in a dose-response manner.
The HIGHLOWDEXA-COVID trial evaluated the effect of high-dose (20 mg once daily for 5 days, followed by 10 mg once daily for 5 days) versus low-dose (6 mg once daily for 10 days) dexamethasone in a less severe cohort of patients with confirmed COVID-19 pneumonia needing oxygen therapy using nasal cannula or simple face mask. 8 Our patient population only comprises those receiving invasive ventilation and thus more severe and in line with the CODEX trial. 6 Patients in the high-dose group of the HIGHLOWDEXA-COVID trial were less likely to experience clinical worsening (defined as a need to increase FiO2>20%, need for FiO2> 50%, respiratory rate >25, or score higher than 4 on the 7-point ordinal scale WHO-CIS) within 11 days compared with the low-dose group (16.3% vs 31.4%, P = 0.01). 8 Those endpoints would not be meaningful in our patients as they were already more advanced.
The COVID STEROID 2 trial was the first study to evaluate the efficacy and safety of higher dose dexamethasone in hospitalized adult patients with COVID-19 and severe hypoxia. 7 Treatment with dexamethasone 12 mg per day did not result in statistically significantly more days alive without life support at 28 days compared with dexamethasone 6 mg per day. 7 Similarly, our study found no difference in survival at 28 days between the 2 groups. Our study included patients who were critically ill requiring mechanical ventilation, which was only 20% of the population in the COVID STEROID 2 trial. We report a higher mortality rate compared with both the COVID STEROID 2 trial and the HIGHLOWDEXA-COVID trial, which is likely because our patients were sicker to begin with, as reflected by the high SOFA score at the time of intubation in the ICU.
In addition to corticosteroids, the REMAP-CAP and RECOVERY trials have both reported a mortality benefit with tocilizumab among patients with rapid respiratory decompensation who require oxygen delivery through noninvasive ventilation.9,10 In both of these trials, the majority of patients received dexamethasone 6 mg per day.9,10 In our post hoc analysis, there was no difference in 28-day mortality for patients who received high-dose corticosteroids with or without tocilizumab. We saw that there was no difference in 28-day mortality in patients who received high-dose corticosteroids alone compared with those who received low-dose corticosteroids with tocilizumab. However, we did find a significant difference in 28-day mortality between patients who received high-dose corticosteroids compared with low-dose corticosteroids without tocilizumab in either arm. These data may support the idea that higher levels of immunosuppression may be beneficial in patients with severe ARDS from COVID-19, and that this may be achieved by the addition of tocilizumab, rather than with a higher dose of corticosteroids.
The immunosuppressive nature of corticosteroids leaves patients prone to secondary infections. The RECOVERY trial, HIGHLOWDEXA-COVID, and the COVID STEROID 2 trial reported no differences in adverse effects.3,7,8 In both the HIGHLOWDEXA-COVID trial and the STEROID 2 trial, only a very small number of patients received additional immunosuppression with tocilizumab. In our study, patients who received higher doses of dexamethasone were significantly more likely to have developed a superimposed bacterial pneumonia with a positive tracheal aspirate culture. Furthermore, patients who received high-dose corticosteroids with tocilizumab had the highest rates of a positive tracheal aspirate culture compared with patients in any of the other groups. Patients in the high-dose corticosteroid group also had a higher ICU length of stay. However, we are unable to conclude whether these patients developed a secondary infection due to high doses of corticosteroids, tocilizumab, or if they were already at an increased risk of developing a bacterial pneumonia due to longer durations of mechanical ventilation. Despite having a higher number of positive bacterial tracheal aspirate cultures in the high-dose corticosteroid group, both groups had high rates of antimicrobial prescriptions. COVID-19 pneumonia causes a clinical syndrome that is often difficult to distinguish from bacterial pneumonia, leading to high empiric antibiotic use despite negative cultures. 25
Finally, we recognize that racial disparities exist within health care. Throughout the pandemic, the NYC department of Health and Mental Hygiene has focused on understanding and addressing racial inequities that have led to higher rates of suffering in certain groups. 26 African American and Hispanic New Yorkers, and those in high poverty neighborhoods, have had less access to safe community and work environments, leading to increased rates of COVID-19 infection. 26 Due to financial hardships, these populations have had less access to COVID-19 testing, COVID-19 treatments, and COVID-19 vaccines. 26 We report an imbalance in population relative to race in our study. Most patients were white, with lower rates of both African American and Hispanic groups. Although we report these imbalances, we saw no significant differences between treatment sites that could have contributed to a difference between corticosteroid distributions.
Our analysis has substantial limitations. This was a retrospective analysis with a small sample size of patients. There was no standard protocol to determine whether the patient was to receive high-versus low-doses of corticosteroids, which exposes the analysis to treatment assignment bias. We address this concern by comparing baseline characteristics within the 2 groups and found that they were well matched. Tocilizumab was administered to patients at the discretion of the treating physicians, and patients in the low-dose group were more likely to receive tocilizumab. Many of the patients were treated before society guidelines included tocilizumab in their treatment algorithm. Furthermore, tocilizumab has a higher acquisition cost than dexamethasone and its availability may have been affected by drug shortages during this time. All these factors could have impacted the outcomes that we analyzed in this study. Despite this, we think that the results are notable in the absence of appropriately randomized dose–finding trials that examine the use of corticosteroids in COVID-19 ARDS.
Conclusion and Relevance
In critically ill patients with COVID-19 ARDS requiring mechanical ventilation, our study found no differences in survival to discharge between patients who received high- versus low-dose corticosteroids. Our study suggests that there was no benefit of high-dose corticosteroids without tocilizumab compared with low-dose corticosteroids with tocilizumab but there is signal that both can improve outcomes over low-dose corticosteroids alone. Our findings are compatible with current NIH treatment guidelines for patients with COVID-19 ARDS requiring mechanical ventilation, which recommend dexamethasone 6 mg per day (low dose) for 10 days plus tocilizumab. Future prospective studies are needed to determine the optimal dose of corticosteroids in this patient population.
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Alyson Katz  https://orcid.org/0000-0002-7177-4631
https://orcid.org/0000-0002-7177-4631
Xian Jie Cindy Chen  https://orcid.org/0000-0002-2258-9704
https://orcid.org/0000-0002-2258-9704
Shari B. Brosnahan  https://orcid.org/0000-0002-2092-3633
https://orcid.org/0000-0002-2092-3633
References
- 1.Xu B, Kraemer MUG; Open COVID-19 Data Curation Group. Open access epidemiological data from the COVID-19 outbreak. Lancet Infect Dis. 2020;20(5):534. doi: 10.1016/S1473-3099(20)30119-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Polidoro RB, Hagan RS, de Santis Santiago R, Schmidt NW. Overview: systemic inflammatory response derived from lung injury caused by SARS-CoV-2 infection explains severe outcomes in COVID-19. Front Immunol. 2020;11:1626. doi: 10.3389/fimmu.2020.01626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.RECOVERY Collaborative Group,Horby P, Lim WS, et al. Dexamethasone in hospitalized patients with COVID-19. N Engl J Med. 2021;384(8):693-704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Angus DC, Derde L, Al-Beidh F, et al. Effect of hydrocortisone on mortality and organ support in patients with severe COVID-19: the REMAP-CAP COVID-19 corticosteroid domain randomized clinical trial. JAMA. 2020;324(13):1317-1329. doi: 10.1001/jama.2020.17022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dequin PF, Heming N, Meziani F, et al. Effect of hydrocortisone on 21-day mortality or respiratory support among critically ill patients with COVID-19 a randomized clinical trial. JAMA. 2020;324(13):1298-1306. doi: 10.1001/jama.2020.16761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tomazini BM, Maia IS, Cavalcanti AB, et al. Effect of dexamethasone on days alive and ventilator-free in patients with moderate or severe acute respiratory distress syndrome and COVID-19: the CoDEX randomized clinical trial. JAMA. 2020;324(13):1307-1316. doi: 10.1001/jama.2020.17021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.COVID STEROID 2 Trial Group,Munch MW, Myatra SN, et al. Effect of 12 mg vs 6 mg of dexamethasone on the number of days alive without life support in adults with COVID-19 and severe hypoxemia: the COVID STEROID 2 randomized trial. JAMA. 2021;326(18):1807-1817. doi: 10.1001/jama.2021.18295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taboada M, Rodriguez N, Varela PM, et al. Effect of high versus low dose of dexamethasone on clinical worsening in patients hospitalised with moderate or severe COVID-19 pneumonia: an open-label, randomised clinical trial. Eur Respir J. 2021;59(5). doi: 10.1183/13993003.02518-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.RECOVERY Collaborative Group. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2021;397(10285):1637-1645. doi: 10.1016/S0140-6736(21)00676-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rosas IO, Brau N, Waters M, et al. Tocilizumab in hospitalized patients with severe COVID-19 pneumonia. N Engl J Med. 2021;384(16):1503-1516. doi: 10.1056/NEJMoa2028700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Salama C, Han J, Yau L, et al. Tocilizumab in patients hospitalized with COVID-19 pneumonia. N Engl J Med. 2021;384(1):20-30. doi: 10.1056/NEJMoa2030340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Panettieri RA, Schaafsma D, Amrani Y, Koziol-White C, Ostrom R, Tliba O. Non-genomic effects of glucocorticoids: an updated view. Trends Pharmacol Sci. 2019;40(1):38-49. doi: 10.1016/j.tips.2018.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ahmed MH, Hassan A. Dexamethasone for the treatment of coronavirus disease (COVID-19): a review. SN Compr Clin Med. 2020;2:1-10. doi: 10.1007/s42399-020-00610-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu QQ, Cheng A, Wang Y, et al. Cytokines and their relationship with the severity and prognosis of coronavirus disease 2019 (COVID-19): a retrospective cohort study. BMJ Open. 2020;10(11):e041471. doi: 10.1136/bmjopen-2020-041471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stahn C, Buttgereit F. Genomic and nongenomic effects of glucocorticoids. Nat Clin Pract Rheumatol. 2008;4(10):525-533. doi: 10.1038/ncprheum0898. [DOI] [PubMed] [Google Scholar]
- 16.World Health Organization. Tracking SARS-CoV-2 variants. Accessed March 18, 2022. https://www.who.int/en/activities/tracking-SARS-CoV-2-variants/.
- 17.Polak SB, Van Gool IC, Cohen D, von der Thusen JH, van Paassen J. A systematic review of pathological findings in COVID-19: a pathophysiological timeline and possible mechanisms of disease progression. Mod Pathol. 2020;33(11):2128-2138. doi: 10.1038/s41379-020-0603-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Borczuk AC, Salvatore SP, Seshan SV, et al. COVID-19 pulmonary pathology: a multi-institutional autopsy cohort from Italy and New York City. Mod Pathol. 2020;33(11):2156-2168. doi: 10.1038/s41379-020-00661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sterne JAC, Murthy S, Diaz JV, et al. Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19 a meta-analysis. JAMA. 2020; 324(13):1330-1341. doi: 10.1001/jama.2020.17023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li S, Hu Z, Song X. High-dose but not low-dose corticosteroids potentially delay viral shedding of patients with COVID-19. Clin Infect Dis. 2021;72(7):1297-1298. doi: 10.1093/cid/ciaa829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arabi YM, Mandourah Y, Al-Hameed F, et al. Corticosteroid therapy for critically ill patients with Middle East Respiratory Syndrome. Am J Respir Crit Care Med. 2018;197(6):757-767. doi: 10.1164/rccm.201706-1172OC. [DOI] [PubMed] [Google Scholar]
- 22.Lee N, Allen Chan KC, Hui DS, et al. Effects of early corticosteroid treatment on plasma SARS-associated Coronavirus RNA concentrations in adult patients. J Clin Virol. 2004;31(4):304-309. doi: 10.1016/j.jcv.2004.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee N, Chan PK, Hui DS, et al. Viral loads and duration of viral shedding in adult patients hospitalized with influenza. J Infect Dis. 2009;200(4):492-500. doi: 10.1086/600383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li X, Xu S, Yu M, et al. Risk factors for severity and mortality in adult COVID-19 inpatients in Wuhan. J Allergy Clin Immunol. 2020;146(1):110-118. doi: 10.1016/j.jaci.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Risa E, Roach D, Budak JZ, et al. Characterization of secondary bacterial infections and antibiotic use in mechanically ventilated patients with COVID-19 induced acute respiratory distress syndrome. J Intensive Care Med. 2021;36(10):1167-1175. doi: 10.1177/08850666211021745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.NYC Health. Racial Inequities in COVID-19 Hospitalizations During the Omicron Wave in NYC. 2022; Accessed March 20, 2022. https://www1.nyc.gov/assets/doh/downloads/pdf/covid/black-hospitalizations-omicron-wave.pdf.