Abstract
The larval form of the Phyllobothriidea cestode was found in the blubber of a Cape fur seal (Arctocephalus pusillus pusillus) from a zoo in Japan. Bladder-bearing larval cestodes with a scolex have been occasionally reported from blubbers of pinnipeds and morphologically identified as Clistobothrium delphini (formerly known as Phyllobothrium delphini) or rarely Clistobothrium grimaldii (Monorygma grimaldii). Although the larvae here morphologically resembled C. delphini, the 28S rDNA sequence was 100% (1,430/1,430 bp) homologous to the registered sequence of C. grimaldii (GenBank Accession No. KU724058). This discrepancy between morphological and molecular analyses confirms the difficulty of identifying C. delphini and C. grimaldii larvae based solely on morphology, and the need for molecular data to elucidate the morphological variations in Clistobothrium parasites.
Keywords: Cape fur seal, Clistobothrium, Phyllobothriidea
The order Phyllobothriidea currently includes 21 genera [5, 16], and is known to parasitize elasmobranch and holocephalan species as definitive hosts. The estimated diversity in this order is 669 species, but only approximately 20% of these have been discovered thus far [16]. Larval forms of Phyllobothriidea cestodes with a scolex and a bladder, generally referred to as merocercoids (terminology as in [7]), have been occasionally reported in the blubber of pinniped species and identified as Phyllobothrium delphini Bosc, 1802 or Monorygma grimaldii Moniez, 1889 [3, 9, 11, 13, 15, 17,18,19]. Recent phylogenetic analyses using 28S ribosomal RNA gene (rDNA) have invalidated the genus combination for both P. delphini and M. grimaldii, and both species have now been transferred to the genus Clistobothrium [1, 6, 14]. Although the great white shark (Carcharodon carcharias) is the most plausible definitive host for Clistobothrium delphini and C. grimaldii due to its regular consumption of large marine mammals, adult forms have not yet been found and its complete life cycle remains to be elucidated [6]. Here, we identified larval cestodes with an everted scolex and bladder from the subcutaneous adipose tissues of a >20-year-old captive Cape fur seal from a zoo in Japan. Morphological and molecular examinations of the larvae were conducted to provide additional information on the poorly understood Clistobothrium species.
A female Cape fur seal (Arctocephalus pusillus pusillus) kept at Tobu Zoo in Saitama, Japan was necropsied in January 2022. The seal was originally caught on the South African coast and brought to the Zoo in May 2000. Necropsy revealed that the cause of death was a cardiovascular complication possibly related to filarial infection. In addition to pathological findings directly related to death, large cavities containing larval cestodes within the subcutaneous adipose tissue were found and collected during necropsy. The collected larvae were washed with saline and preserved in 70% ethanol for morphological and molecular analyses. Morphological measurements were recorded for two larvae under a stereomicroscope using cellSens® Standard software (Olympus, Tokyo, Japan), according to the metrics defined by Agustí et al. [1].
DNA was extracted from one of the collected larva using a DNeasy Blood and Tissue Kit (Qiagen, Hilden, Germany) according to the manufacturer’s protocol. The D1-D3 region of the 28S rDNA gene was amplified using primer pairs LSU-5 (5′-TAGGTCGACCCGCTGAAYTTA-3′) [12] and LSU-1500R (5′-GCTATCCTGGAGGGAAACTTCG-3′) [20]. PCR was performed in a 50-μL reaction volume, including 1 μL of DNA template, 1.25 U of Takara Ex Taq®, 1× Ex Taq® Buffer, 0.2 mM dNTP mixture (Takara Bio Inc., Kusatsu, Japan), and 0.2 μM primers (FASMAC Co., Ltd., Atsugi, Japan). The reaction conditions included initial denaturation at 94°C for 3 min, followed by 40 cycles of denaturation at 94°C for 30 sec, annealing at 52°C for 30 sec, and extension at 72°C for 2 min, with a final extension at 72°C for 7 min [20]. The amplified products were visualized on 1.5% agarose gel. Positive PCR products were purified using the NucleoSpin Gel and PCR Clean-up kit (Macherey-Nagel, Düren, Germany) according to the manufacturer’s protocol and directly sequenced at a sequencing facility (FASMAC Co., Ltd.) using the same primers used for the PCR and additional primers LSU-55F (5′-AACCAGGATTCCCCTAGTAACGGC-3′) [4] and LSU-1200R (5′-GCATAGTTCACCATCTTTCGG-3′) [12]. The obtained sequences were compared with those available in GenBank (National Center for Biotechnology Information) using the nucleotide BLAST program (https://blast.ncbi.nlm.nih.gov/Blast.cgi). The sequence obtained in this study was deposited in the DNA Data Bank of Japan (DDBJ) under accession number LC718556.
The larval cestodes collected in this study were composed of a bladder connected to a filament ending with an everted scolex (Fig. 1). An anterior apical organ surrounded by four bothridia was observed on the scolex of one larva (Fig. 2A). The apical organ on the scolex of another larva was not visible. Each bothridium consisted of a foliate margin and an accessory sucker in the middle (Fig. 2B). Morphological measurements of the larvae are presented in Table 1 along with data from previous studies [1, 9, 13, 17]. According to a key to marine cestode larval types by Jensen and Bullard [8], the present larvae were identified as Type XV, which includes species historically referred to as Phyllobothrium delphini Bosc, 1802, and Monorygma grimaldii Moniez, 1889. These two species have been reported in cetaceans and pinnipeds and are distinguished by the morphology of the scolex and the length of the filament that connects the scolex and the bladder [1]. However, as described earlier, both P. delphini and M. grimaldii have recently been transferred to the genus Clistobothrium [1, 6, 14]. Morphological characteristics of the larval cestodes collected in this study were similar to those of C. delphini with shorter filaments and foliate bothrium (Figs. 1 and 2A, Table 1), although measurements of the bothridial accessory sucker length matched that of C. grimaldii (Table 1).
Fig. 1.
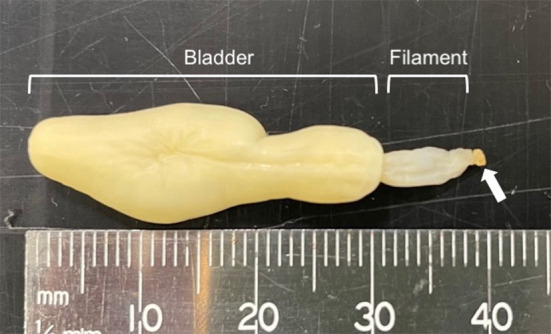
Larval cestodes collected in this study composed of a bladder connected to a filament ending with an everted scolex (arrow).
Fig. 2.
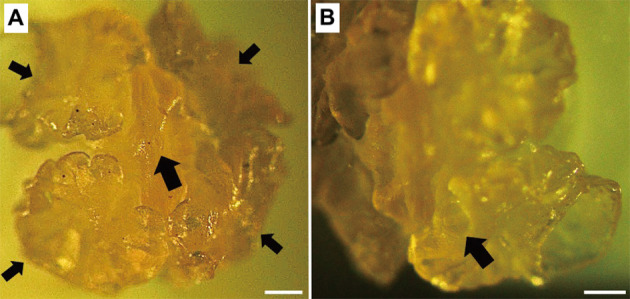
(A) Reduced anterior glandular apical sucker (large arrow) surrounded by four bothridia (small arrow) is observed on the everted scolex. (B) Each bothridium consists of a foliate margin and an accessory sucker in the middle (arrow). Scale bars=200 μm.
Table 1. Comparison of morphological measurements (range for more than two samples) of the Clistobothrium larvae from the present and previous studies.
| Host | Arctocephalus pusillus pusillus | Arctocephalus pusillus pusillus | Arctocephalus pusillus pusillus | Arctocephalus australis | Stenella coeruleoalbad | Stenella coeruleoalbad |
|---|---|---|---|---|---|---|
| Sample number | n=2 | n=2 | not reported | n=5 | n=20 | n=20 |
| Morphological identification | delphini | delphini | delphini | delphini | delphini | grimaldii |
| Molecular identification | grimaldii | grimaldii | ND | ND | delphini | grimaldii |
| Bladder length (mm) | 24.5, 31.0 | 35.0, 37.5 | 14.0–22.3 | 12.0–18.0 | 5.0–15.1 (10.3 ± 2.5) |
5.7–27.3 (13.7 ± 5.4) |
| Bladder width (mm) | 6.1, 8.9 | 7.0, 7.5 | 5.0–9.0 | 6.0–10.0 | 2.3–9.3 (5.9 ± 1.9) |
3.9–11.5 (7.7 ± 2.3) |
| Filament length (mm) | 6.9, 8.6 | 13.6, 18.2 | 5.0–12.0 | 12.0–14.0 | 1.5–12.9 (7.4 ± 2.7) |
3.0–415.7 (151.8 ± 122.9) |
| Filament width (mm) | 1.7, 3.3 | 1.5, 2.5 | 1.2–2.8 | 2.0–4.0 | 0.8–2.6 (1.6 ± 0.5) |
1.8–3.9 (2.7 ± 0.6) |
| Bothridium length (mm) | 0.6–0.7 (0.7), 0.8–1.2 (1.0)a |
1.5, ND | 0.9* | 1.6* | 1.2–2.1 (1.5 ± 0.2) |
3.8–6.0 (4.7 ± 0.6) |
| Bothridium width (mm) | 0.4–0.7 (0.6), 0.8–1.1 (0.9) a |
ND | 1.0* | 1.6* | 1.0–1.9 (1.3 ± 0.2) |
2.2–4.8 (3.1 ± 0.6) |
| Accessory sucker length (μm) | 79.1–84.2 (81.7), 122.5–137.2 (129.0) a |
400, 350 | 277* | 133–200* | 220–325 (274 ± 29) |
107–196 (148 ± 22) |
| Accessory sucker width (μm) | 85.2–95.7 (89.9), 134.4–148.7 (142.3) a |
500, 400 | 321* | 242–250* | 230–360 (288 ± 33) |
133–248 (172 ± 25) |
| Diameter of the apical organ (μm) | NDb, 87 | NDc | 240–400 | 120–150 | 50–170 (91 ± 30) |
53–131 (84 ± 20) |
| Reference | This study | [9] | [13] | [17] | [1] | |
a Range for four bothridia per scolex. Mean value in the parenthesis. b Apical organ not visible. c Although the study mentions the presence of an apical organ, description of the measurements were unavailable. d Mean ± Standard deviation described in the parenthesis. * Values measured by Agustí et al. [1] from published drawing by [13] and [17].
Larval cestodes found in the blubbers of pinniped species were identified solely based on morphology until the first molecular data were reported by Klotz et al. [9]. Although the morphology of the larvae in their study resembled that of C. delphini, DNA sequences (GenBank Accession No. KU724058) identified the larvae as C. grimaldii [6], displaying a lack of congruence between the results of molecular and morphological analyses. Such discrepancies between molecular and morphological analyses of larvae observed in pinnipeds have not been reported in cetacean host species [1]. Therefore, the larvae of Clistobothrium species have been hypothesized to develop different morphologies in different intermediate hosts [6, 9, 19], suggesting that the identification of C. delphini and C. grimaldii larvae solely based on morphology is unreliable. Larval cestodes collected in this study also resembled those of C. delphini, but the 28S rDNA sequence was 100% (1,430/1,430 bp) identical to that of C. grimaldii from Klotz et al. [9]. Considering the possibility that Clistobothrium larvae obtained from different host species exhibit morphological variation, molecular data were used to identify the larvae found in this study as C. grimaldii.
Several morphological characteristics of the larvae in this study, including filament length and bothridial accessory sucker length, did not match completely with those of the larvae described by Klotz et al. [9] (Table 1). Notably, the larvae in the present study had an everted scolex and filament, whereas the majority of the larval cestodes reported from pinnipeds, including those by Klotz et al. [9], had an invaginated scolex and a filament, generally referred to as a merocercoid [7, 9, 11, 19]. Mendonca [13] reported both everted and invaginated larval types from the same Cape fur seal and attributed these variations to different developmental stages. Such morphological variations, whether caused by different host species or developmental stages, re-affirm the difficulty of species identification based solely on morphology, highlighting the need for additional molecular information.
The life cycle of C. grimaldii is still unknown, although it is speculated to cycle between crustacean species as the first intermediate hosts, pinnipeds as the second intermediate hosts, and elasmobranch species as the definitive hosts [6, 9]. The coast of South Africa is a habitat of diverse elasmobranch species and a hot spot for the great white shark population, a possible candidate for the definitive host, as it regularly consumes large marine mammals [6, 10]. Indeed, 24.5% (13/53) of Cape fur seals on the eastern Cape coast of South Africa harbored Clistobothrium merocercoids [18], indicating that Clistobothrium parasites had been prevalent in the area where the seal was caught. The larvae collected in the present study seemed to be intact, considering the years of infection, although their infectivity to the final host is unknown. As suggested by Aznar et al. [2], it is reasonable that Clistobothrium parasites have adapted to and taken advantage of the longevity and body size of large marine mammals as intermediate hosts, instead of fish and cephalopods, to increase the chance of development and survival.
Here, we provide both morphological and molecular data for C. grimaldii from a Cape fur seal caught on the coast of South Africa. Molecular data for Clistobothrium species from pinnipeds are especially valuable, as the only DNA sequence data available to date are those by Klotz et al. [9]. Furthermore, the morphological features did not completely agree with those of C. grimaldii reported by Klotz et al. [9], although the 28S rDNA sequence was 100% identical. These results suggest that morphological variations exist, even among C. grimaldii larvae collected from the same host species, highlighting the importance of molecular data. Thus, to gain a better understanding of the development of Clistobothrium parasites, further molecular analyses in relation to morphological variations need to be conducted in future studies.
CONFLICT OF INTEREST
The authors declare that there are no known conflicts of interest associated with this publication.
Acknowledgments
The authors thank Dr. Hirotaka Kondo for his insightful comments.
REFERENCES
- 1.Agustí C, Aznar FJ, Olson PD, Littlewood DTJ, Kostadinova A, Raga JA. 2005. Morphological and molecular characterization of tetraphyllidean merocercoids (Platyhelminthes: Cestoda) of striped dolphins (Stenella coeruleoalba) from the Western Mediterranean. Parasitology 130: 461–474. doi: 10.1017/S0031182004006754 [DOI] [PubMed] [Google Scholar]
- 2.Aznar FJ, Agustí C, Littlewood DTJ, Raga JA, Olson PD. 2007. Insight into the role of cetaceans in the life cycle of the tetraphyllideans (Platyhelminthes: Cestoda). Int J Parasitol 37: 243–255. doi: 10.1016/j.ijpara.2006.10.010 [DOI] [PubMed] [Google Scholar]
- 3.Bester MN. 1989. Endoparasites of the subantarctic fur seal Arctocephalus tropicalis from Gough Island. S Afr J Zool 24: 363–365. [Google Scholar]
- 4.Bueno VM, Caira JN. 2017. Redescription and molecular assessment of relationships among three species of Echeneibothrium (Rhinebothriidea: Echeneibothriidae) parasitizing the yellownose skate, Dipturus chilensis, in Chile. J Parasitol 103: 268–284. doi: 10.1645/16-177 [DOI] [PubMed] [Google Scholar]
- 5.Caira JN, Bueno V, Jensen K. 2021. Emerging global novelty in phyllobothriidean tapeworms (Cestoda: Phyllobothriidea) from sharks and skates (Elasmobranchii). Zool J Linn Soc 193: 1336–1363. doi: 10.1093/zoolinnean/zlaa185 [DOI] [Google Scholar]
- 6.Caira JN, Jensen K, Pickering M, Ruhnke TR, Gallagher KA. 2020. Intrigue surrounding the life-cycles of species of Clistobothrium (Cestoda: Phyllobothriidea) parasitising large pelagic sharks. Int J Parasitol 50: 1043–1055. doi: 10.1016/j.ijpara.2020.08.002 [DOI] [PubMed] [Google Scholar]
- 7.Chervy L. 2002. The terminology of larval cestodes or metacestodes. Syst Parasitol 52: 1–33. doi: 10.1023/A:1015086301717 [DOI] [PubMed] [Google Scholar]
- 8.Jensen K, Bullard SA. 2010. Characterization of a diversity of tetraphyllidean and rhinebothriidean cestode larval types, with comments on host associations and life-cycles. Int J Parasitol 40: 889–910. doi: 10.1016/j.ijpara.2009.11.015 [DOI] [PubMed] [Google Scholar]
- 9.Klotz D, Hirzmann J, Bauer C, Schöne J, Iseringhausen M, Wohlsein P, Baumgärtner W, Herder V. 2018. Subcutaneous merocercoids of Clistobothrium sp. in two Cape fur seals (Arctocephalus pusillus pusillus). Int J Parasitol Parasites Wildl 7: 99–105. doi: 10.1016/j.ijppaw.2018.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kock A, O’Riain MJ, Mauff K, Meÿer M, Kotze D, Griffiths C. 2013. Residency, habitat use and sexual segregation of white sharks, Carcharodon carcharias in False Bay, South Africa. PLoS One 8: e55048. doi: 10.1371/journal.pone.0055048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lehnert K, Poulin R, Presswell B. 2019. Checklist of marine mammal parasites in New Zealand and Australian waters. J Helminthol 93: 649–676. doi: 10.1017/S0022149X19000361 [DOI] [PubMed] [Google Scholar]
- 12.Littlewood DTJ, Curini-Galletti M, Herniou EA. 2000. The interrelationships of proseriata (Platyhelminthes: seriata) tested with molecules and morphology. Mol Phylogenet Evol 16: 449–466. doi: 10.1006/mpev.2000.0802 [DOI] [PubMed] [Google Scholar]
- 13.Mendonca MM. 1984. Phyllobothrium delphini (Bosc, 1802)(Cestoda, Tetraphyllidea) from Arctocephalus pusillus (Schreber, 1778)(Carnivora, Otariidae) in captivity. Rev Iber Parasitol 44: 39–44. [Google Scholar]
- 14.Randhawa HS. 2011. Insights using a molecular approach into the life cycle of a tapeworm infecting great white sharks. J Parasitol 97: 275–280. doi: 10.1645/GE-2530.1 [DOI] [PubMed] [Google Scholar]
- 15.Rennie J, Reid A. 1912. XXII. The Cestoda of the Scottish National Antarctic Expedition. Earth Environ Sci Trans R Soc Edinb 48: 441–453. doi: 10.1017/S0080456800002945 [DOI] [Google Scholar]
- 16.Ruhnke TR, Caira JN, Pickering M. 2017. Phyllobothridea. pp. 305–326. In: Planetary Biodiversity Inventory (2008–2017): Tapeworms from Vertebrate Bowels of the Earth (Caira JN, Jensen K eds.), The University of Kansas Natural History Museum, Kansas. [Google Scholar]
- 17.Southwell T, Walker AJ. 1936. Notes on a larval Cestode from a Fur-Seal. Ann Trop Med Parasitol 30: 91–100. doi: 10.1080/00034983.1936.11684920 [DOI] [Google Scholar]
- 18.Stewardson CL, Fourie HJ. 1998. Endoparasites of the cape fur seal Arctocephalus pusillus pusillus from the eastern Cape Coast of South Africa. Trans R Soc S Afr 53: 33–51. doi: 10.1080/00359199809520372 [DOI] [Google Scholar]
- 19.Testa J, Dailey MD. 1977. Five new morphotypes of Phyllobothrium delphini (Cestoda: Tetraphyllidea), their relationship to existing morphotypes, and their zoogeography. Bull South Calif Acad Sci 76: 99–110. [Google Scholar]
- 20.Tkach VV, Littlewood DTJ, Olson PD, Kinsella JM, Swiderski Z. 2003. Molecular phylogenetic analysis of the Microphalloidea Ward, 1901 (Trematoda: Digenea). Syst Parasitol 56: 1–15. doi: 10.1023/A:1025546001611 [DOI] [PubMed] [Google Scholar]


