Abstract
National estimates suggest that kidney failure incidence is declining in the US. However, whether this trend is evident in areas with socioeconomic disadvantage is unknown. We examined trends in kidney failure incidence by county-level poverty between 2000 and 2017 and divided the study period into period 1 (2000–05), period 2 (2006–11), and period 3 (2012–17). The magnitude of disparity in kidney failure incidence between high- and low-poverty counties increased from 42.8 more incident cases per million in high-poverty counties in period 1 to 100.1 more in period 3. Despite a national decline, kidney failure incidence increased in high-poverty counties, and disparities between high- and low-poverty counties widened from 2000 to 2017. Achieving the Department of Health and Human Services objective of reducing incident kidney failure cases by 25 percent by 2030 will require focused attention on preventing kidney failure in counties with higher poverty.
Kidney failure, also referred to as end-stage renal disease (ESRD), is a costly, highly morbid medical condition affecting approximately 750,000 Americans.1 Inequities in kidney failure by socioeconomic status have been well documented.2,3 People from low-income or socioeconomically disadvantaged communities have disproportionately higher incidence rates of kidney failure.4–6 An estimated 34 percent of patients with newly diagnosed kidney failure are from neighborhoods in which more than one in five households live in poverty.6 Residing in high-poverty areas (such as counties or neighborhoods) is associated with a lower likelihood of receiving nephrology care before kidney failure,7,8 lower rates of transplantation,9,10 and worse health outcomes after the onset of kidney failure.8 Poverty is also associated with diabetes and hypertension—the two most common causes of kidney failure in the US.11 Effective treatment of diabetes and hypertension can prevent or delay kidney failure, but people with lower incomes are more likely to forgo necessary care because of financial barriers.12 Importantly, within high-poverty areas, racial and ethnic minority patients—particularly Black patients—have markedly higher kidney failure incidence and worse access to nephrology care than White patients.5,13
National trends suggest that the age- and sex-standardized incidence rate of treated kidney failure has declined since its peak in 2006 and in 2017 was its lowest since 1998.1 However, considering that there is wide geographic variation in kidney failure incidence,8 access to nephrology care before kidney failure,14 and transplantation rates,10 national estimates may mask disparities faced by socioeconomically disadvantaged communities. County-level analyses may identify populations with increasing kidney failure incidence, thereby informing more targeted policies, resource allocation, and public health interventions. We examined trends in the incidence of kidney failure by county-level poverty among US adults between 2000 and 2017.
Study Data And Methods
STUDY DESIGN
We examined changes in annual kidney failure incidence based on county-level poverty, separating our study period into three six-year periods: period 1 (2000–05), period 2 (2006–11), and period 3 (2012–17).
DATA SOURCES AND STUDY POPULATION
The study population included all adults (ages eighteen and older) who developed and were treated for incident kidney failure between January 1, 2000, and December 31, 2017, in all fifty states and Washington, D.C. To identify incident patients, we used data from the Renal Management Information System Medical Evidence Report (form CMS 2728), which is completed for all patients initiating dialysis or preemptive transplant, irrespective of insurance, citizenship, or treatment modality. The form includes sociodemographic and clinical information at treatment initiation, including the patient’s primary mailing address. Using previously described methods, we geolocated incident patients using ArcGIS spatial mapping software, version 10.5.1.15 More information about assigning patients to counties is described in the online appendix, and the construction of our study sample is described in appendix exhibit 1A.16
We used Census Bureau annual county population estimates to calculate annual adult population counts by age group and sex for each county. These data include estimates of the July 1 resident population by year, county, age, and sex. Consistent with US Renal Data System reports, age groups were defined as 18–44, 45–64, 65–74, and 75 or older.1 County-level population characteristics (racial and ethnic composition, rurality, educational attainment, and diagnosed diabetes prevalence) and supply-side characteristics (number of active nonfederal physicians per 1,000 residents and number of dialysis facilities per capita) were incorporated in the analysis (sources are in appendix exhibit 2A).16 Twelve counties containing a total of 355 incident kidney failure patients (0.02 percent of the sample) were excluded because of missing covariates (appendix exhibit 3A).16
MEASURES
We calculated annual county-level kidney failure incidence in the US adult population. Our main exposure of interest was county-level poverty, defined as the annual proportion of the population living below the federal poverty level. We assigned each county to a poverty quintile each year. Changes in quintile thresholds for high- and low-poverty counties are in “Detailed Description of Study Methods” in the appendix.16 As a secondary outcome, we examined the incidence of kidney failure caused by diabetes or hypertension.
STATISTICAL ANALYSIS
We used Pearson’s chi-square tests to examine changes in patient- and county-level characteristics by period. Building on previous county-level time trend analyses,6,17 we used linear regression models to calculate kidney failure incidence in each period. We constructed six models: Model 1 was unadjusted; model 2 was age and sex adjusted; model 3 added county-level racial and ethnic composition (county-level proportions of the population that were Black, Hispanic or Latino, American Indian or Native American, or Asian or Pacific Islander); model 4 added urban or rural designation, uninsurance rate, unemployment rate, and educational attainment; model 5 added prevalence of diagnosed diabetes among all adults in each county; and model 6 added supply-side characteristics (number of dialysis facilities per capita and number of active nonfederal physicians per 1,000 population). Models were weighted by the county-level adult population, and standard errors were clustered at the county level.
To assess whether the magnitude of disparity in kidney failure incidence between counties in the highest and lowest poverty quintiles changed between periods, we tested the significance of a poverty-by-period interaction term. We also calculated differences in age- and sex-adjusted county-level kidney failure incidence between periods 1 and 3.
STRATIFIED AND SENSITIVITY ANALYSES
To assess whether there were differential incidence trends for subpopulations, we stratified our analysis by age group, sex, and race and ethnicity (appendix exhibit 4A).16
To examine the robustness of our results, we used alternative approaches to assign a county to a poverty quintile (for instance, in an era rather than annually or fixed over time to its first poverty quintile) or to define the exposure as the proportion of the county population living below the federal poverty level. We also used alternative modeling and outcome specifications, such as adding median county-level household income as a covariate, using different referent periods, adding county and year fixed effects, and excluding preemptive kidney transplant patients.
To test whether there were differential trends over time by age and poverty, particularly among elderly patients in Medicare, we respecified our model to include a three-way interaction between age (younger than age sixty-five versus ages sixty-five and older), poverty quintile, and period. All analyses used Stata, version 15.
LIMITATIONS
Our study had several limitations. First, our data only included patients with incident kidney failure who initiated maintenance dialysis or received a preemptive transplant, and it may have excluded patients who received compassionate or emergency dialysis, forwent treatment, or were unable to access kidney replacement therapy. However, our methods to identify incident patients align with the US Renal Data System approach. Second, individual and household income data were not available. Although area-level poverty measures are associated with kidney failure incidence, they may mask more granular, person- or household-level heterogeneity; some evidence suggests that individual-level poverty measures (such as annual family income) are more strongly associated with incidence.11 Relatedly, county-level rates may inaccurately estimate the heterogeneity of risks for some groups within the same county (such as groups that face structural stigma or discrimination).18 We focused on county-level disparities because they can inform more targeted public health policies, programs, and interventions.
Third, we were unable to calculate age-, sex-, and race and ethnicity–adjusted incidence rates because some counties had little racial and ethnic variation; thus, we calculated age- and sex-adjusted incidence rates. Fourth, although we adjusted for several county-level sociodemographic, clinical, and supply-side characteristics, it is likely that we did not include all relevant factors that may have contributed to the differences observed, including the longer incubation period for development of kidney failure from certain conditions (such as diabetes or hypertension), the potential for population migration between counties, and the effect of spatial clustering in counties. Nevertheless, unadjusted differences are still meaningful regardless of unmeasured factors. Fifth, it is plausible that increases in incidence reflect population-level improvements in survival to kidney failure, but other studies have not provided evidence of larger reductions in all-cause or cause-specific mortality in high-poverty counties.17,19
Study Results
PATIENT CHARACTERISTICS
During the study period, 1,944,535 adults developed incident kidney failure (mean age: 62.9 years [standard deviation: 15.1]; 43.8 percent female; 27.6 percent Black, 12.9 percent Hispanic or Latino) (exhibit 1). Between period 1 (2000–05) and period 3 (2012–17) there were significant decreases in the proportion of patients with incident kidney failure who were uninsured (7.8 percent in period 1 versus 5.5 percent in period 3). There were significant increases in the proportion of patients with incident kidney failure who had a primary cause of kidney failure that was diabetes (45.0 percent in period 1 versus 47.1 percent in period 3) or hypertension (25.1 percent in period 1 versus 28.2 percent in period 3) and who had Medicaid insurance (11.7 percent in period 1 versus 12.8 percent in period 3).
EXHIBIT 1.
Characteristics of US adult patients with incident kidney failure at treatment initiation, 2000–17
| Characteristics | Overall (2000–17) | Period 1 (2000–05) | Period 2 (2006–11) | Period 3 (2012–17) |
|---|---|---|---|---|
| No. of incident patients | 1,944,535 | 585,322 | 653,136 | 706,077 |
| Age, mean years Age group (years), % | 62.9 | 62.7 | 63.0 | 62.9 |
| 18–44 | 12.5 | 13.4 | 12.4 | 11.9 |
| 45–64 | 37.9 | 36.2 | 38.5 | 38.9 |
| 65–74 | 24.7 | 24.6 | 23.5 | 25.9 |
| 75 or older | 24.9 | 25.8 | 25.7 | 23.4 |
| Female, % | 43.8 | 45.7 | 43.6 | 42.3 |
| Race and ethnicity, % | ||||
| White, non-Hispanic | 53.9 | 55.0 | 53.5 | 53.3 |
| Black, non-Hispanic | 27.6 | 28.2 | 28.1 | 26.5 |
| Hispanic or Latino | 12.9 | 11.2 | 13.0 | 14.2 |
| Other, non-Hispanic | 5.6 | 5.6 | 5.3 | 6.1 |
| Insurance type, % | ||||
| Medicare | 45.4 | 43.5 | 43.5 | 48.7 |
| Private | 14.8 | 15.1 | 16.4 | 13.1 |
| Dual eligible | 13.3 | 12.9 | 13.1 | 13.7 |
| Medicaid | 12.3 | 11.7 | 12.3 | 12.8 |
| VA or other | 7.3 | 9.0 | 7.1 | 6.2 |
| Uninsured | 6.9 | 7.8 | 7.6 | 5.5 |
| Primary cause of kidney failure, % | ||||
| Diabetes | 45.7 | 45.0 | 44.8 | 47.1 |
| Hypertension | 26.9 | 25.1 | 27.1 | 28.2 |
| Other | 27.4 | 29.9 | 28.1 | 24.7 |
| eGFR, mean | 9.8 | 9.0 | 10.3 | 10.0 |
| Preemptive transplant, % | 2.0 | 1.8 | 2.3 | 2.0 |
SOURCE Authors’ analyses of Medical Evidence Report data (form CMS 2728), 2000–17. NOTES Some group percentages may exceed 100 percent because of rounding. Authors used Pearson’s chi-square tests to compare changes in each characteristic by period; overall estimates are presented for reference. p < 0.001 for all comparisons. Dual eligible is eligible for both Medicare and Medicaid. VA is Veterans Affairs. eGFR is estimated glomerular filtration rate, a measure of normal kidney function; numbers below 15 indicate kidney failure.
COUNTY CHARACTERISTICS
The number of counties in our study ranged between 3,134 (period 1) and 3,141 (period 3); counties had mean annual adult populations of 68,855 in period 1 and 78,289 in period 3 (appendix exhibit 5A).16 There were significant increases in the county-level proportion of the population living below the federal poverty level and having diagnosed diabetes. The county-level uninsurance rate decreased significantly from 18.0 percent in period 1 to 14.0 percent in period 3.
CHANGES IN COUNTY-LEVEL KIDNEY FAILURE INCIDENCE
In counties in the lowest quintile of poverty (mean proportion of the population living below the federal poverty level: 8.4 percent), kidney failure incidence declined from 451.2 per million in period 1 to 432.5 per million in period 3 (change: −18.7 per million; relative change: −4.1 percent). In the highest poverty quintile (mean proportion of the population living below the federal poverty level: 24.9 percent), kidney failure incidence increased from 494.0 per million in period 1 to 532.6 per million in period 3 (change: 38.6 per million; relative change: 7.8 percent) (see “Detailed Description of Study Methods” in the appendix and appendix exhibit 6A).16 Annual trends in all-cause and cause-specific kidney failure incidence by quintile of county-level poverty are presented in in exhibits 2 and 3, respectively. Across the entire US adult population, the age- and sex-adjusted incidence of kidney failure declined from 471.7 per million in period 1 to 458.5 per million in period 3 (change: −13.2 per million [95% confidence interval: −16.0, −10.4]) (exhibit 4).
EXHIBIT 2. Trends in county-level all-cause kidney failure incidence per million US adults, by poverty quintile, 2000–17.
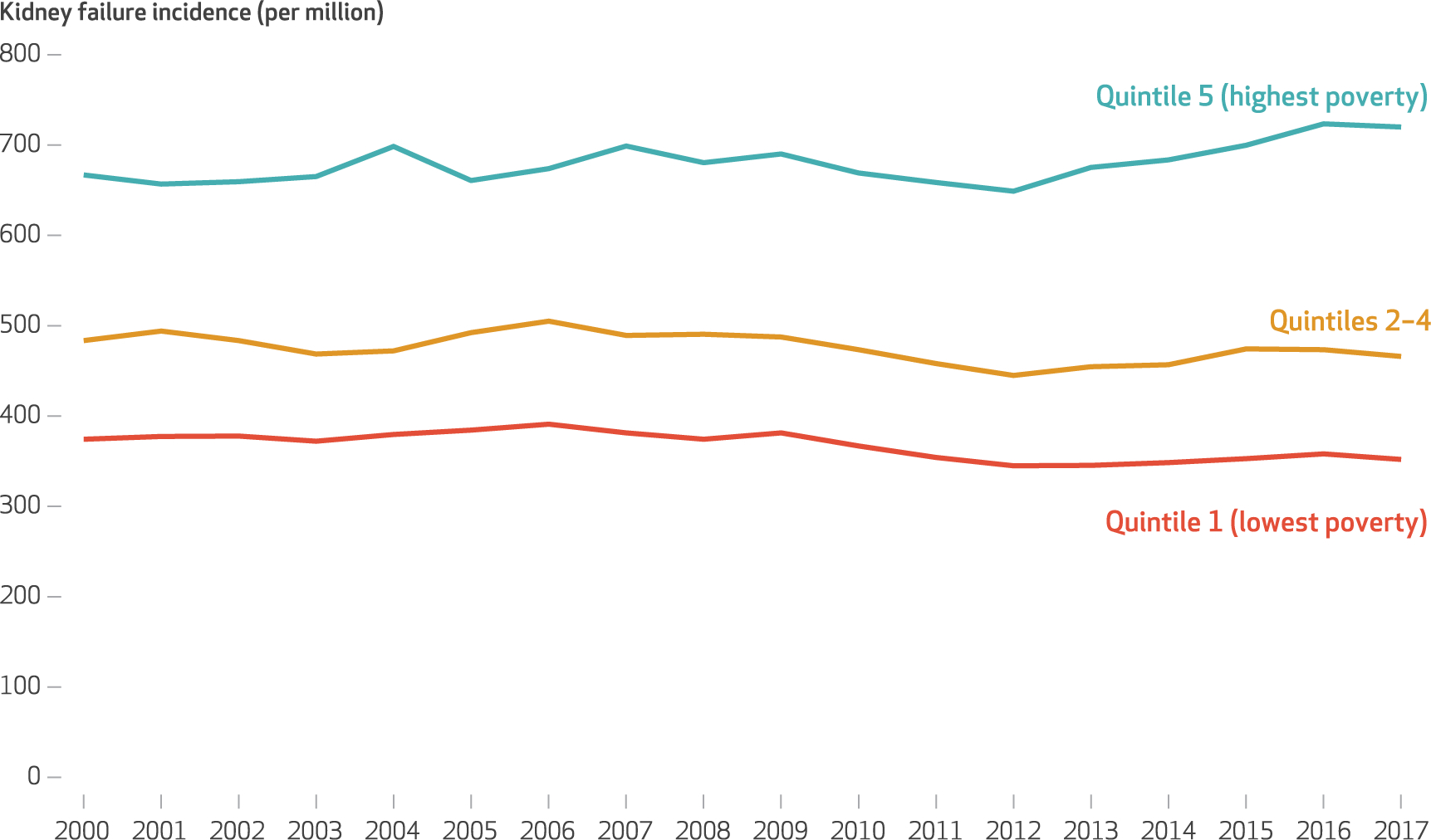
SOURCE Authors’ analyses of Medical Evidence Report data (form CMS 2728) and Census Bureau annual county population estimates, 2000–17. NOTE County-level kidney failure incidence rates are age and sex adjusted.
EXHIBIT 3. Trends in county-level cause-specific kidney failure incidence per million US adults, by poverty quintile, 2000–17.
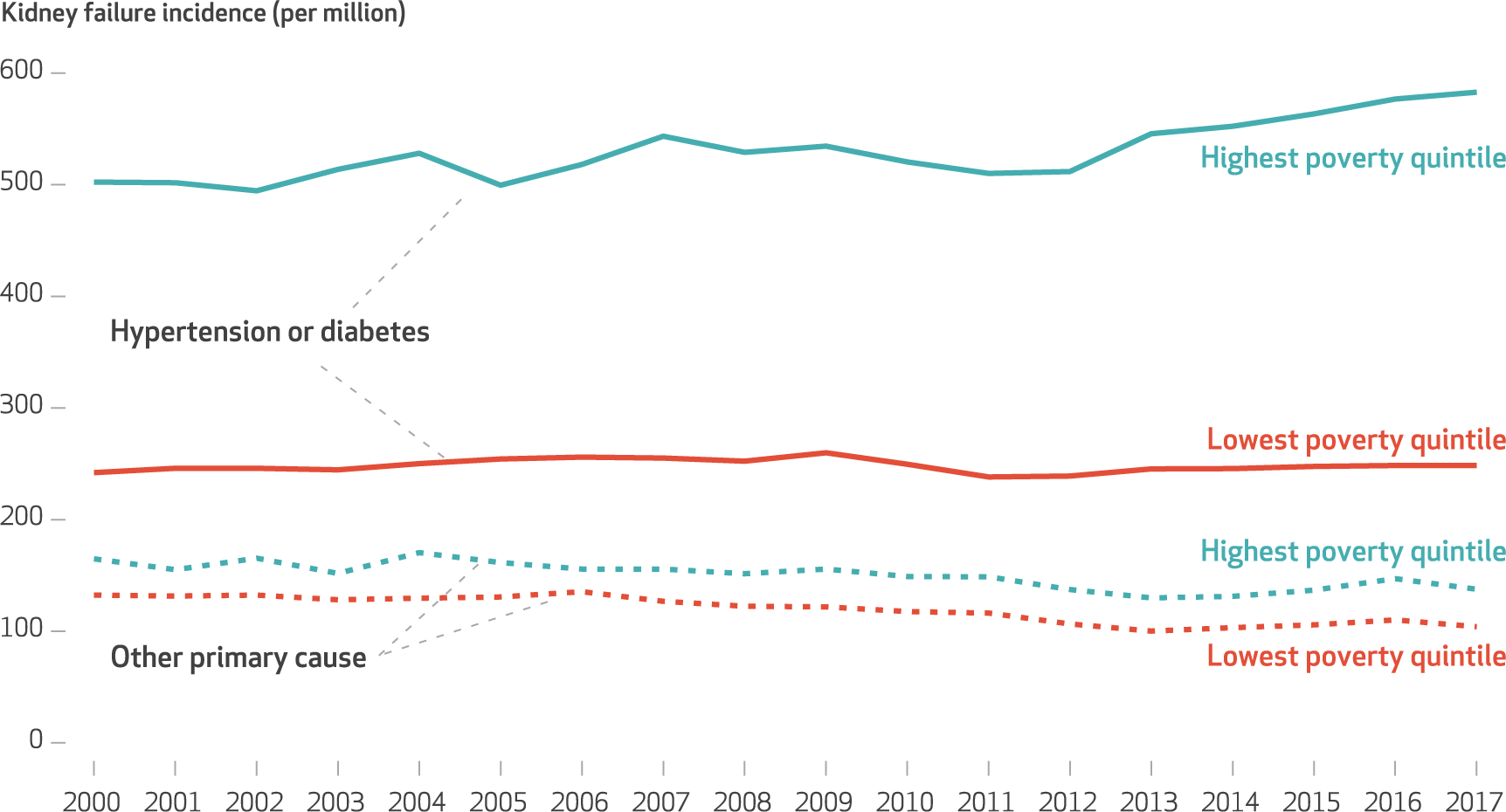
SOURCE Authors’ analyses of Medical Evidence Report data (form CMS 2728) and Census Bureau annual county population estimates between 2000–17. NOTE County-level kidney failure incidence rates are age and sex adjusted.
EXHIBIT 4.
Change in US county-level kidney failure incidence per million, by poverty quintile, 2000–17
| Kidney failure incidence (per million US adults) |
||||
|---|---|---|---|---|
| Period 1 (2000–05) | Period 2 (2006–11) | Period 3 (2012–17) | Adjusted change from period 1 to period 3 | |
| National estimates | 471.7 | 474.3 | 458.5 | −13.2*** |
| Model 1 (unadjusted) | ||||
| Highest poverty quintile | 641.0 | 662.8 | 691.2 | 50.3*** |
| Lowest poverty quintile | 356.0 | 378.1 | 377.1 | 21.2*** |
| Differencea | 285.0 | 284.7 | 314.1 | 29.1*** |
| Model 2 | ||||
| Highest poverty quintile | 664.0 | 678.2 | 692.5 | 28.5** |
| Lowest poverty quintile | 375.0 | 378.2 | 354.2 | −20.8*** |
| Differencea | 289.0 | 300.0 | 338.3 | 49.3*** |
| Model 3 | ||||
| Highest poverty quintile | 540.6 | 561.2 | 570.9 | 30.4** |
| Lowest poverty quintile | 435.5 | 427.4 | 398.2 | −37.3**** |
| Differencea | 105.1 | 133.8 | 172.7 | 67.7**** |
| Model 4 | ||||
| Highest poverty quintile | 522.2 | 551.4 | 556.4 | 34.2** |
| Lowest poverty quintile | 430.5 | 429.6 | 416.8 | −13.7** |
| Differencea | 91.7 | 121.8 | 139.6 | 47.9**** |
| Model 5 | ||||
| Highest poverty quintile | 489.9 | 502.5 | 536.3 | 37.4*** |
| Lowest poverty quintile | 450.7 | 433.0 | 430.5 | −20.3**** |
| Differencea | 48.2 | 69.5 | 105.8 | 57.7**** |
| Model 6 | ||||
| Highest poverty quintile | 494.0 | 501.9 | 532.6 | 38.6*** |
| Lowest poverty quintile | 451.2 | 435.2 | 432.5 | −18.7*** |
| Differencea | 42.8 | 66.6 | 100.1 | 57.3**** |
SOURCE Authors’analyses of Medical Evidence Report data (form CMS 2728) and Census Bureau annual county population estimates, 2000–17. NOTES National estimates are age and sex adjusted. Model 2 includes the indicators for poverty quintile, period, and their interaction (poverty quintile × period), as well as age, sex, and a linear time trend. Model 3 adds county-level proportions of the population that were Black, Hispanic or Latino, American Indian or Native American, or Asian or Pacific Islander. Model 4 adds county-level sociodemographic characteristics (urban or rural designation, uninsurance rate, unemployment rate, and educational attainment). Model 5 adds county-level prevalence of diagnosed diabetes among all adults. Model 6 adds county-level number of dialysis facilities per capita and number of active nonfederal physicians per 1,000 population. All models are weighted by the county’s adult population. Standard errors are clustered at the county level. Poverty quintiles are defined in the text.
Difference between highest and lowest poverty quintiles.
p < 0.05
p < 0.01
p < 0.001
CHANGES IN DISPARITIES IN KIDNEY FAILURE INCIDENCE
In unadjusted models (model 1), the magnitude of disparity in kidney failure incidence between high- and low-poverty counties increased from period 1 to period 3 (change: 29.1 per million) (exhibit 4). The magnitude of disparity between periods 1 and 3 increased by 49.3 per million after we adjusted for age and sex (model 2), by 67.7 per million with additional adjustment for county racial and ethnic composition (model 3), and by 47.9 per million when we added other county-level sociodemographic characteristics (model 4). The magnitude of disparity between periods 1 and 3 increased by 57.7 per million when we included the county-level prevalence of diagnosed diabetes (model 5). In a fully adjusted model that added supply-side characteristics (model 6), the magnitude of disparity in kidney failure incidence between high- and low-poverty counties increased from 42.8 per million in period 1 to 100.1 per million in period 3 (adjusted change in disparity: 57.3 per million).
The magnitude of disparity between high- and low-poverty counties between period 1 and period 3 was significantly higher for kidney failure due to hypertension or diabetes (52.3 per million), hypertension only (22.2 per million), and diabetes only (30.1 per million), but not for other causes (−0.9 per million) (appendix exhibit 6A).16
STRATIFIED AND SENSITIVITY ANALYSES
Changes in the magnitude of disparity in kidney failure incidence between high- and low-poverty counties were 76.0 per million for men and 41.1 per million for women (appendix exhibit 7A).16 In age-stratified analyses, changes in the magnitude of disparity between high- and low-poverty counties for patients ages seventy-five and older was 106.1 per million (appendix exhibit 8A).16 Changes in magnitude of disparity between high- and low-poverty counties were 63.3 per million for White, non-Hispanic patients; 111.6 per million for Hispanic or Latino patients; and 38.2 per million for Black, non-Hispanic patients (appendix exhibits 9A–12A).16
Estimates were robust to exclusion of preemptive kidney transplants, alternative definitions of county-level poverty, and alternative model specifications of referent periods (appendix exhibits 13A–15A).16 The three-way interaction term between poverty, age group, and period was statistically significant, suggesting that trends in kidney failure incidence between high- and low-poverty counties was statistically differential by age group (appendix exhibit 16A).16
Discussion
We examined trends in the county-level incidence of kidney failure between 2000 and 2017 in the US, using a national registry of all incident patients. Although kidney failure incidence rates declined nationwide,1 we found that incidence increased by 7.8 percent in high-poverty counties and that disparities between low-poverty and high-poverty counties widened from 2000 to 2017. Notably, this increasing disparity was largely observed for kidney failure due to diabetes or hypertension—causes amenable to health care intervention.
Our study built on prior studies of kidney failure incidence in four ways. First, our unit of analysis was the county instead of the entire US, which can inform local- and community-level public health solutions.20 Second, we stratified our estimates by county-level poverty rate, as disadvantaged communities have a higher kidney failure incidence.11,21 Stratification by annual quintiles, rather than a specific cutoff, is novel and addresses the dynamic nature of area-level poverty during our study period. Third, the analyses included a broad set of county-level factors related to sociodemographic composition, socioeconomic status, insurance coverage, diabetes prevalence, and provider supply. Our models suggest that county-level sociodemographic characteristics account for some of the observed poverty-based inequities in kidney failure incidence, whereas differences in the supply of physicians and dialysis facilities do not appear to explain observed differences in incidence between high- and low-poverty counties. Fourth, our stratified analyses identified that changes in poverty-related gaps were prominent among White, non-Hispanic patients and patients who were ages seventy-five and older. The changes in disparity for the latter group were largely driven by declines in treated kidney failure among those living in low-poverty counties.
Our age- and sex-adjusted rates were higher than US Renal Data System national estimates because our denominator included only adults (ages eighteen and older), rather than the entire US population, and kidney failure incidence is higher among adults than among children.1 Our results are consistent with previous analyses suggesting that there are strong associations between area-level poverty and kidney failure incidence and that the association between ZIP code–level poverty and incidence increased from 1995–2005 to 2005–10.2,6 Further, in stratified analyses, increases in disparity were concentrated among kidney failure cases where the primary cause was hypertension or diabetes—two conditions that may be prevented or delayed with effective medications for some patients. Although some forms of kidney failure can be delayed with preventive care, disparities are exacerbated because low-income people are at higher risk for antecedents (such as albuminuria) and clinical risk factors (such as diabetes and hypertension) for kidney failure, have lower access to care, and have unmet social needs including food and housing insecurity that are upstream causes of kidney failure.5,21,22 Additional attention to other barriers to managing and preventing chronic conditions, such as social risk factors, may be warranted.
Our study has several important implications for policy and practice. First, poverty is largely the product of social inequities and systemic barriers and also affects disease progression and access to necessary care. County-level poverty, for example, is likely associated with how geographic boundaries are drawn around populations of interest.18 There is increasing evidence that suggests a bidirectional relationship between poverty and kidney disease outcomes, where poverty can affect kidney disease through several pathways, including delayed access to health care and health-promoting goods, neighborhood stressors, environmental toxins, discrimination, and psychological stress.3,18,21 Kidney failure can also influence poverty through loss of wages or employment, catastrophic health care expenditures, and disability.21,23 Considering that these exposures occur over a lifetime, they cannot be addressed with a single intervention. Efforts must move beyond individual-level education or personal reflection and expand to changes in policies and social norms.
Second, our race-stratified analyses indicate that the largest increase in kidney failure incidence from 2000 to 2017 occurred among White patients living in high-poverty areas. This finding extends those of Anne Case and Angus Deaton, who reported that after decades of improvement, mortality rates for middle-aged White, non-Hispanic people have increased since 2000, particularly among people with a high school education or less, who are more likely to live in high-poverty areas.24–26 The increase in mortality rates was not observed among middle-aged Black, non-Hispanic people and Hispanic or Latino people or among adults in other high-income countries. The reasons for increased morbidity and mortality among middle-aged White populations in the US during the past two decades are unclear and deserve further investigation.
Importantly, despite increases in kidney failure incidence among White patients over time, kidney failure incidence was consistently higher among Black and Hispanic or Latino patients than among White patients throughout our study period, and substantial poverty-related gaps in incidence persisted over time. Dismantling systems and policies that perpetuate inequities in kidney failure incidence, such as residential segregation and structural racism, will be crucial to reduce the incidence of kidney failure.3,4,27,28 Because these systems are mutually reinforcing, it is critical to design comprehensive and cross-sectoral interventions that address the interrelated mechanisms through which racism and poverty operate and to measure whether such interventions attenuate inequitable gaps in health.29
Third, through the Advancing American Kidney Health initiative, the Department of Health and Human Services (HHS) has prioritized reducing the number of incident kidney failure cases by 25 percent by 2030. HHS aims to increase the detection of people in early stages of kidney disease through advanced public health surveillance and aims to delay progression to kidney failure through adoption of evidence-based interventions and increased use of effective medication therapy for diabetes and hypertension—the two leading causes of kidney failure in the US.30 Achieving the initiative’s goals and, more broadly, addressing poverty-based inequities in kidney failure incidence may require more targeted, multilevel interventions for patients residing in counties with higher poverty, including increasing health insurance coverage,15,30–32 improving receipt of nephrology care before kidney failure, and increasing surveillance and management of chronic kidney disease. Emerging evidence suggests that unmet social needs, such as food insecurity or housing insecurity, are associated with progression from chronic kidney disease to adverse kidney outcomes (such as rapid kidney function decline) and kidney failure.21,22,33 Thus, policy reforms to expand eligibility and access to social safety-net programs such as the Supplemental Nutrition Assistance Program may mitigate inequities in disease progression from chronic kidney disease to kidney failure among low-income patients.
Last, the COVID-19 pandemic threatens to exacerbate existing inequities in kidney disease: People experiencing unstable housing and members of racial and ethnic minority groups have higher infection rates, whereas increases in unemployment may lead to loss of insurance coverage, the forgoing of necessary care, food scarcity, an inability to manage kidney care, and kidney disease progression.34,35 In the first three months of the COVID-19 pandemic there were substantial racial and ethnic disparities in excess deaths among Black and Hispanic and Latino patients with kidney failure.36 It remains critical to identify approaches that maintain continuity of care for patients with chronic kidney disease. Federal and state policies that reduce financial instability (such as extending federal unemployment compensation and expanding Medicaid) may mitigate cost-related forgone care and prevent disease progression, particularly for communities that have been disproportionately affected by COVID-19.
Conclusion
Although the incidence of kidney failure decreased nationally from 2000 to 2017, disparities between high- and low-poverty counties began widening in 2013, and kidney failure incidence has increased in the highest-poverty counties. Addressing this inequity and achieving the national goal of a lower kidney failure incidence rate by 2030 will require changes in policy and care delivery, with a specific focus on low-income areas and communities.
Supplementary Material
Acknowledgments
A preliminary version of this study was presented at the AcademyHealth Annual Research Meeting (virtual), July 28, 2020. Kevin Nguyen completed this work while supported by an Agency for Healthcare Research and Quality National Research Service Award (Grant No. T32-HS 000011-32) and the Robert Wood Johnson Foundation Health Policy Research Scholars. Research reported in this publication was supported by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under award number R01DK113398-01. Rajnish Mehrotra has received personal honoraria from Baxter Healthcare for serving as an ad hoc consultant outside of the submitted work. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the US government.
Contributor Information
Kevin H. Nguyen, Department of Health Services, Policy, and Practice, Brown University School of Public Health, in Providence, Rhode Island.
Rebecca Thorsness, Department of Health Services, Policy, and Practice, Brown University School of Public Health, and a fellow in the Veterans Affairs New England Healthcare System, in Bedford, Massachusetts.
Shailender Swaminathan, professor of economics and the dean of the Division of Social Sciences at Sai University, in Chennai, India.
Rajnish Mehrotra, David S. and Nayda Utterberg Endowed Professor and interim head of the Division of Nephrology, University of Washington School of Medicine, in Seattle, Washington.
Rachel E. Patzer, Department of Surgery and the Department of Epidemiology at the Emory University Rollins School of Public Health and director of the Health Services Research Center at the Emory University School of Medicine, in Atlanta, Georgia.
Yoojin Lee, Department of Health Services, Policy, and Practice, Brown University School of Public Health.
Daeho Kim, Department of Health Services, Policy, and Practice, Brown University School of Public Health.
Maricruz Rivera-Hernandez, Department of Health Services, Policy, and Practice, Brown University School of Public Health.
Amal N. Trivedi, Department of Health Services, Policy, and Practice, Brown University School of Public Health, and a research health scientist at the Providence Veterans Affairs Medical Center, in Providence, Rhode Island.
NOTES
- 1.US Renal Data System. US Renal Data System 2019 annual data report: epidemiology of kidney disease in the United States [Internet]. Bethesda (MD): USRDS; 2019. [cited 2021 Oct 1]. Available from: https://usrds.org/media/2371/2019-executive-summary.pdf [Google Scholar]
- 2.Vart P, Gansevoort RT, Joosten MM, BÜltmann U, Reijneveld SA. Socioeconomic disparities in chronic kidney disease: a systematic review and meta-analysis. Am J Prev Med. 2015;48(5):580–92. [DOI] [PubMed] [Google Scholar]
- 3.Nicholas SB, Kalantar-Zadeh K, Norris KC. Socioeconomic disparities in chronic kidney disease. Adv Chronic Kidney Dis. 2015;22(1):6–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Norris K, Nissenson AR. Race, gender, and socioeconomic disparities in CKD in the United States. J Am Soc Nephrol. 2008;19(7):1261–70. [DOI] [PubMed] [Google Scholar]
- 5.Patzer RE, McClellan WM. Influence of race, ethnicity, and socioeconomic status on kidney disease. Nat Rev Nephrol 2012;8(9):533–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garrity BH, Kramer H, Vellanki K, Leehey D, Brown J, Shoham DA. Time trends in the association of ESRD incidence with area-level poverty in the US population. Hemodial Int. 2016;20(1):78–83. [DOI] [PubMed] [Google Scholar]
- 7.Nee R, Yuan CM, Hurst FP, Jindal RM, Agodoa LY, Abbott KC. Impact of poverty and race on pre–end-stage renal disease care among dialysis patients in the United States. Clin Kidney J. 2017;10(1):55–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schold JD, Flechner SM, Poggio ED, Augustine JJ, Goldfarb DA, Sedor JR, et al. Residential area life expectancy: association with outcomes and processes of care for patients with ESRD in the United States. Am J Kidney Dis. 2018;72(1):19–29. [DOI] [PubMed] [Google Scholar]
- 9.Patzer RE, Amaral S, Wasse H, Volkova N, Kleinbaum D, McClellan WM. Neighborhood poverty and racial disparities in kidney transplant waitlisting. J Am Soc Nephrol. 2009;20(6):1333–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mohan S, Mutell R, Patzer RE, Holt J, Cohen D, McClellan W. Kidney transplantation and the intensity of poverty in the contiguous United States. Transplantation. 2014;98(6):640–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crews DC, Gutiérrez OM, Fedewa SA, Luthi JC, Shoham D, Judd SE, et al. Low income, community poverty, and risk of end stage renal disease. BMC Nephrol. 2014;15:192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weissman JS, Stern R, Fielding SL, Epstein AM. Delayed access to health care: risk factors, reasons, and consequences. Ann Intern Med. 1991;114(4):325–31. [DOI] [PubMed] [Google Scholar]
- 13.Nzerue CM, Demissochew H, Tucker JK. Race and kidney disease: role of social and environmental factors. J Natl Med Assoc. 2002;94(8, Suppl):28S–38S. [PMC free article] [PubMed] [Google Scholar]
- 14.Hao H, Lovasik BP, Pastan SO, Chang HH, Chowdhury R, Patzer RE. Geographic variation and neighborhood factors are associated with low rates of pre–end-stage renal disease nephrology care. Kidney Int. 2015;88(3):614–21. [DOI] [PubMed] [Google Scholar]
- 15.Swaminathan S, Sommers BD, Thorsness R, Mehrotra R, Lee Y, Trivedi AN. Association of Medicaid expansion with 1-year mortality among patients with end-stage renal disease. JAMA. 2018;320(21):2242–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.To access the appendix, click on the Details tab of the article online.
- 17.Dwyer-Lindgren L, Bertozzi-Villa A, Stubbs RW, Morozoff C, Mackenbach JP, van Lenthe FJ, et al. Inequalities in life expectancy among US counties, 1980 to 2014: temporal trends and key drivers. JAMA Intern Med. 2017;177(7):1003–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clark CR, Williams DR. Understanding county-level, cause-specific mortality: the great value—and limitations—of small area data. JAMA. 2016;316(22):2363–5. [DOI] [PubMed] [Google Scholar]
- 19.Cosby AG, McDoom-Echebiri MM, James W, Khandekar H, Brown W, Hanna HL. Growth and persistence of place-based mortality in the United States: the rural mortality penalty. Am J Public Health. 2019;109(1):155–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thomas-Hawkins C, Flynn L, Zha P, Savage B. Associations among race, residential segregation, community income, and emergency department use by adults with end-stage renal disease. Public Health Nurs. 2019;36(5):645–52. [DOI] [PubMed] [Google Scholar]
- 21.Crews DC, Novick TK. Social determinants of CKD hotspots. Semin Nephrol. 2019;39(3):256–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crews DC, Kuczmarski MF, Grubbs V, Hedgeman E, Shahinian VB, Evans MK, et al. Effect of food insecurity on chronic kidney disease in lower-income Americans. Am J Nephrol. 2014;39(1):27–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morton RL, Schlackow I, Gray A, Emberson J, Herrington W, Staplin N, et al. Impact of CKD on household income. Kidney Int Rep. 2017;3(3):610–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khullar D, Chokshi DA. Health Policy Brief: Health, income, and poverty: where we are and what could help. Health Affairs [serial on the Internet]. 2018. Oct 4 [cited 2021 Oct 1]. Available from: 10.1377/hpb20180817.901935/full/ [DOI]
- 25.Case A, Deaton A. Mortality and morbidity in the 21st century [Internet]. Washington (DC): Brookings Institution; 2017. Mar 23 [cited 2021 Oct 1]. Available from: https://www.brookings.edu/bpeaarticles/mortality-and-morbidity-in-the-21st-century/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bosworth B. Increasing disparities in mortality by socioeconomic status. Annu Rev Public Health. 2018;39:237–51. [DOI] [PubMed] [Google Scholar]
- 27.Rodriguez RA, Sen S, Mehta K, Moody-Ayers S, Bacchetti P, O’Hare AM. Geography matters: relationships among urban residential segregation, dialysis facilities, and patient outcomes. Ann Intern Med. 2007;146(7):493–501. [DOI] [PubMed] [Google Scholar]
- 28.Kimmel PL, Fwu CW, Eggers PW. Segregation, income disparities, and survival in hemodialysis patients. J Am Soc Nephrol. 2013;24(2):293–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bailey ZD, Feldman JM, Bassett MT. How structural racism works—racist policies as a root cause of U.S. racial health inequities. N Engl J Med. 2021;384(8):768–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Crews DC, Novick TK. Achieving equity in dialysis care and outcomes: the role of policies. Semin Dial. 2020;33(1):43–51. [DOI] [PubMed] [Google Scholar]
- 31.Trivedi AN, Sommers BD. The Affordable Care Act, Medicaid expansion, and disparities in kidney disease. Clin J Am Soc Nephrol. 2018;13(3):480–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thorsness R, Swaminathan S, Lee Y, Sommers BD, Mehrotra R, Nguyen KH, et al. Medicaid expansion and incidence of kidney failure among nonelderly adults. J Am Soc Nephrol. 2021;32(6):1425–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Banerjee T, Crews DC, Wesson DE, Dharmarajan S, Saran R, Ríos Burrows N, et al. Food insecurity, CKD, and subsequent ESRD in US adults. Am J Kidney Dis. 2017;70(1):38–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Novick TK, Rizzolo K, Cervantes L. COVID-19 and kidney disease disparities in the United States. Adv Chronic Kidney Dis. 2020;27(5):427–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Crews DC, Purnell TS. COVID-19, racism, and racial disparities in kidney disease: galvanizing the kidney community response. J Am Soc Nephrol. 2020;31(8):1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim D, Lee Y, Thorsness R, Nguyen KH, Swaminathan S, Rivera-Hernandez M, et al. Racial and ethnic disparities in excess deaths among persons with kidney failure during the COVID-19 pandemic, March–July 2020. Am J Kidney Dis. 2021;77(5):827–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


