Abstract
Immune response to vaccines and pathogens remains unclear in patients with systemic lupus erythematosus (SLE). To investigate this, a single-center retrospective study was conducted with 47 SLE patients vaccinated against COVID-19, including 13 who subsequently developed an asymptomatic/mild disease. As compared to controls, post-vaccine response against Spike was reduced in SLE patients when considering both memory T-cells in a whole blood interferon gamma release assay (IGRA-S) and IgG anti-Spike antibody (Ab) responses. The SLE-associated defective IGRA-S response was associated with a serum albumin level below 40 g/L and with the use of glucocorticoids, while a defective IgG anti-Spike Ab response was associated with lower levels of anti-dsDNA and anti-SSA/Ro 52 kDa Abs. IGRA-S and IgG anti-Spike responses were independent from SLE activity and clinical phenotype, low complement, hypergammaglobulinemia, and lymphopenia. As compared to controls, SLE patients showed a rapid decay of anti-Spike T-cell memory and stable IgG anti-Spike Ab responses. In conclusion, both T cell and humoral anti-Spike responses were independently affected in our SLE patients cohort, which supports the exploration of both responses in the follow-up of SLE patients and especially in those receiving glucocorticoids.
Keywords: Systemic lupus erythematosus, Spike, Nucleocapsid, COVID-19, Antibody, Interferon gamma release assay
Highlights
-
•
Cellular and humoral anti-Spike responses were independently altered in our SLE patients.
-
•
Cellular anti-Spike response was affected by glucocorticoids and this was independent from disease activity.
-
•
A lower albumin level (<40 g/L) was associated with a defective T cell response.
-
•
SLE patients showed a more durable humoral than T-cell response against Spike antigen.
1. Introduction
Patients with systemic lupus erythematosus (SLE) are characterized by an increased risk of infections as compared to the general population [1], and infections are a leading cause of mortality, morbidity, and hospitalization in this disease [2]. The high burden of infection in SLE is caused by the disease itself as well as by the use of immunosuppressive therapies resulting in altered B and T cell immune responses. The recent pandemic caused by SARS-Cov2 has further confirmed sensitivity of SLE patients to infections resulting in extended COVID-19 symptom duration, an increased risk of hospitalization and severe fatal outcome with an Odds ratio of 2.2 [3,4]. Part of the risk of severe or fatal covid-19 infection is related to the use of glucocorticoids, antimetabolite drugs (e.g. mycophenolate mofetil, methotrexate, azathioprine), and biotherapies (e.g. belimumab, rituximab) [5]. Consequently, vaccination represents a primary means of protection against severe COVID-19 in SLE patients to reduce SARS-Cov2 complications [6]. However, lessons from influenza, varicella-zoster and other vaccination campaigns have revealed a defect in both cellular and humoral immunity among SLE patients that is not solely related to therapeutics [[7], [8], [9]]. Accordingly, this study was designed to describe the T cell and humoral responses to COVID-19 vaccine and SARS-Cov2 infection in SLE patients, taking into account the clinico-biological characteristics of the disease and therapy.
2. Material and methods
2.1. Patients
Forty-seven unselected SLE patients immunized against COVID-19 in response to the vaccine were included in the study (Table 1). All SLE patients met the 2019 ACR/EULAR classification criteria [10]. Data regarding disease activity using a cut-off point ≥5 from the SLEDAI-2K score [11], clinical presentation, current treatment, number and time of covid-19 vaccine injections or infectious episodes were retrospectively collected from medical records. Laboratory data included lymphocyte count, albumin and gamma-globulin levels using serum protein electrophoresis, IgG anti-double stranded (ds)DNA and anti-chromatin Abs (Bioplex, Biorad, Hercules, CA), IgG anti-extractable nuclear Abs, antiphospholipid Abs such as anticardiolipin (aCL) and anti-beta2 glycoprotein I (aβ2-GPI) Abs, and the complement fractions C3, C4 and CH50 (The Binding Site, Birmingham, UK) [[12], [13], [14], [15]].
Table 1.
Clinical and biological features in SLE patients.
| Post-vaccine only immunization | Post vaccine and Post-infection immunization | |
|---|---|---|
| Female/male | 30/4 | 13/0 |
| Age (mean ± SD) | 47 ± 13y | 43 ± 13 |
| Disease duration | 9±6y | 13±9y |
| Active disease (SLEDAI-2K ≥ 5) | 9 (26.5%) | 3 (23.1%) |
| Anti-dsDNA/Chromatin pos | 24 (70.6%) | 9 (62.2%) |
| Anti-Sm/RNP pos | 14 (41.1%) | 9 (62.2%) |
| Anti-SSA/SSB pos | 12 (35.3%) | 5 (38.5%) |
| Low complement | 5 (14.7%) | 2 (15.4%) |
| HCQ/GC/aMet/biotherapy | 24/15/15/9 | 10/3/8/6 |
| GC mg/days (mean ± SD) | 13.1 ± 4.7 | 7.5 ± 2.5 |
| Vaccine doses: 2/3/4 | 9/22/5 | 4/6/2 |
Abbreviations: GC: glucocorticoids; HCQ: hydroxychloroquine; SLEDAI-2K: SLE diseases activity index 2000, SD: standard deviation, dsDNA: double stranded DNA; Pos: positivity; Sm/RNP: Smith/ribonucleoprotein autoantibodies; SSA/SSB: sicca syndrome A/B autoantibodies.
Vaccinated (n = 64) and infected (n = 51) control groups comprised staff members from the medical laboratory of the University Hospital of Toulouse (CHU de Toulouse, Occitania, France), blood bank donors (EFS Toulouse, Occitania, France), and non-autoimmune patients recruited for COVID-19 infection follow-up at the Internal Medicine Department of the University Hospital of Toulouse. Some of them were described previously [16].
Blood from SLE patients was collected during a routine care visit in the medical departments to control for immunization after vaccination or infection (see Table 1), to advise patients on the risk of severe infection and opportunity of a new booster vaccine. Participants were informed and gave their consent, the related cohort ESSAi obtained approval from the ethics committee (CPP) in Paris Ile de France I under reference 2021-A03236-35.
2.2. Whole blood interferon gamma release assay (IGRA-covid)
As previously described [17], 1 mL of whole blood was distributed in 4 heparinized tubes with: (i) 20 μL of SARS-Cov2 full-length Spike protein (2 μg/tube); (ii) 2 μL of SARS-Cov2 Nuc protein (2 μg/tube); (iii) 20 μL of RMPI (negative control); and (iv) 20 μL of phytohemagglutinin (PHA, 40 μg/mL). Recombinant and endotoxin free Spike and Nuc proteins were produced by INVIVOGEN® (Toulouse, France) based on the Wuhan's strain protein sequences [18]. After 18–24 h incubation at 37 °C, tubes were centrifuged, supernatant concentration of IFN-γ quantified (Qiagen, Hilden, Germany), and results were expressed as international units (IU) of IFN-γ/mL (1 IU IFN-γ/mL = 2 × 104 μg IFN-γ). For analysis, value from the negative control tube was subtracted from the signal obtained after stimulation with recombinant proteins. IGRA-S and IGRA-Nuc thresholds for positivity were fixed at 0.040 IU IFN-γ/mL. The test is recorded as indeterminate when the negative control is > 8 IU IFN-γ/mL or when the mitogen control <0.5 IU IFN-γ/mL, but such cases were not observed in this study.
2.3. Serological tests
The serological tests were carried out on serum and the level of IgG antibodies to SARS-Cov2 Spike mammalian cell-expressed recombinant protein was assessed by using the SARS-CoV-2 IgG II Quant assay (Abbott Laboratories, IL, USA). ELISA total values are expressed in BAU/mL, and with an assigned cutoff at 7.14 BAU/mL, as previously described [19,20]. The SARS-CoV-2 IgG assay (Abbott Nuc) was used to detect anti-Nuc antibodies using a threshold fixed at 1.4 [21].
2.4. Statistics
Quantitative data are presented as mean ± standard deviation (SD) or as median and interquartile (IQ) 25th-75th percentile when analyzed using non-parametric assays. Receiver operating characteristic (ROC) curves were generated to determine the area under the curve (AUC), the optimal cut-off values were chosen using Youden's index, and the Cohen's kappa was used to compare techniques. Categorical data were analyzed using Fisher's exact test. Statistics were conducted using GraphPad Prism 9.2 (La Jolla, CA) software, and p-values<0.05 were considered significant.
3. Results
3.1. SLE patients' description
All SLE patients (n = 47) were immunized against COVID-19 mRNA Spike vaccine from Pfizer-BioNTech (BNT162b2) except one from Moderna (mRNA-1273). Among them 13 subsequently developed mild COVID-19 with symptoms such as fever, myalgia, asthenia and headache, but did not require hospitalization (Table 1). As a whole, a marked female predominance was observed (91.5%) and SLE disease duration was 10 ± 7 years. Active disease was reported in 12/47 (25.5%) SLE patients and the predominant organ involvements were joints (87.2%), skin (61.7%), heart (34.0%), brain (27.7%), kidney (21.3%), and the hematologic system (14.9%). Hydroxychloroquine was the most commonly prescribed medication (70.2%), followed by anti-metabolites (48.9%; mycophenolate mofetil n = 7, methotrexate n = 6, and azathioprine n = 10), prednisone as glucocorticoids (38.3%), and biotherapy with belimumab (27.7%) or rituximab (4.2%). At the time of blood analysis, 13 SLE patients had received two doses of COVID-19 vaccine, 28 three doses, and 7 four doses.
3.2. Cellular and humoral responses to COVID-19 are altered in SLE
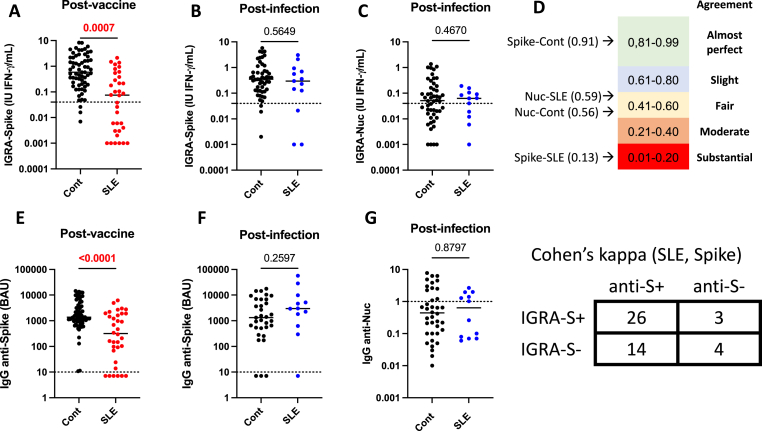
Memory T cell response against Spike (IGRA-S) was first evaluated in SLE patients and controls following COVID-19 vaccination and infection (Fig. 1A–C). Quantitative (p = 0.0007) and qualitative (p < 10−4) differences were retrieved following COVID-19 vaccine response for IGRA-S with a positive response in 18/34 (52.9%) SLE patients versus 61/64 (95.3%) in controls. In contrast, no difference was observed between 13 SLE patients and 51 controls infected with SARS-Cov2 when exploring IGRA-S and IGRA-Nuc levels and/or positivity. When considering the humoral response, IgG anti-Spike Ab level was reduced among the SLE COVID-19 vaccine sub-group (p < 10−4), and IgG anti-Spike Ab positivity was 28/34 (82.4%) in SLE patients versus 64/64 (100%) in controls (p = 0.001). Within the SLE COVID-19 infected sub-group, IgG anti-Spike Ab and IgG anti-Nuc Ab were similar to the control group.
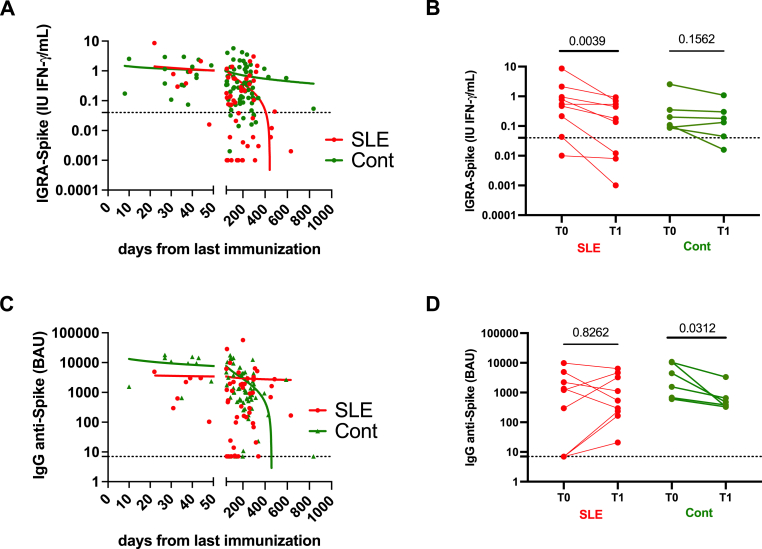
Fig. 1.
SARS-Cov2 memory T cell and humoral responses in patients with systemic lupus erythematosus (SLE) and controls (Cont) following post-COVID19 vaccine or infection. A/E: Anti-Spike cellular and humoral post-vaccine responses in Cont (n = 64) and SLE patients (n = 34). B/F: Anti-Spike memory T cell and humoral post-infection responses in Cont (n = 51) and SLE patients (n = 13). C/G: Anti-Nucleocapsid (Nuc) memory T cell and humoral post-infection responses in Cont and SLE patients. D: Concordance assessment using the Cohen's Kappa coefficient between cellular and humoral assays against Spike and Nuc in Cont and SLE patients. As an example, cross-tabulation between T cell and humoral anti-Spike responses in SLE patients. P values and assay thresholds (dot line) are indicated.
The level of agreement between T cell and humoral responses against Spike was further evaluated showing an almost perfect Cohens’ kappa (K) coefficient between IGRA-S and IgG anti-Spike Ab in controls (K = 0.91) as compared to a substantial agreement in the 47 SLE patients (K = 0.13) (Fig. 1D). Such agreement between T cell and humoral responses against Nuc was fair in both SLE patients (K = 0.59) and controls (K = 0.56), which reflects a higher sensitivity for the humoral assay.
Altogether this supports the idea that the cellular and humoral responses against SARS-Cov2 Spike are altered in SLE after covid-19 vaccination.
3.3. Serum albumin level can be used as a surrogate for IGRA-S reponse
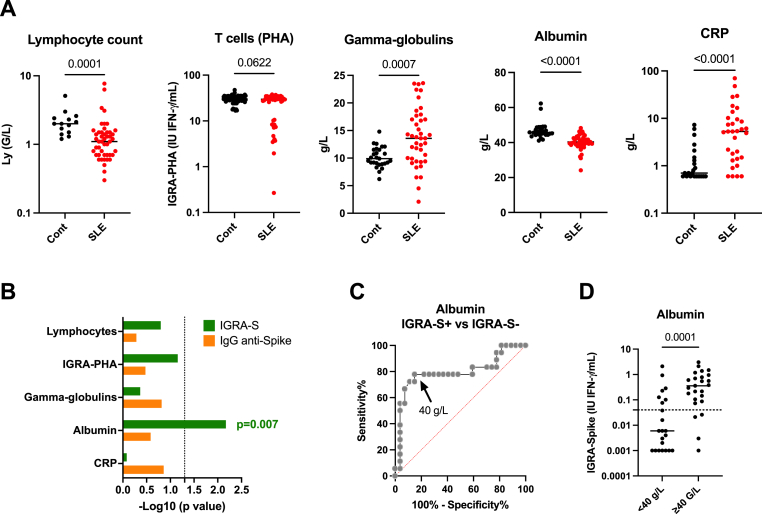
As presented in Fig. 2A, the 47 SLE patients were characterized as compared to controls by a lower lymphocyte count (p < 10−4), presence of hyper-gammaglobulinemia (p = 7 × 10−4), a lower serum albumin level (p < 10−4), and a higher CRP level (p < 10−4). Of note, T cell memory PHA response reflecting the functional integrity of T cells tended to be lower in SLE patients (p = 0,06). As these factors are known to influence IGRA and ELISA responses [[22], [23], [24]], their capacity to affect the IGRA-S and IgG anti-Spike vaccine response in SLE patients was evaluated (Fig. 2B). From such univariate analysis, only the serum albumin level was observed to be associated with IGRA-S response (p = 0.007), and the optimal threshold point using maximum Youden's index from the ROC curve analysis (AUC = 0.795) was fixed at 40 g/L with a sensitivity of 77.8% (95% CI: 58.8–91.0%) and a specificity of 85.2% (95% CI: 67.5–94.1%). This threshold may be used as a surrogate to predict a negative IGRA-S response (Fig. 2C).
Fig. 2.
Factors which interfere with the interferon gamma release assay (IGRA) and the enzyme linked immunosorbent assay (ELISA). A: Common immunoassay interfering factors to be considered in SLE: lymphocyte count, phytohemagglutinin (PHA) capacity to induce T cell memory and NK responses, gamma-globulinemia level, albuminemia level, and C-reactive protein (CRP) level. B: Comparison of these factors according to the IGRA-Spike and IgG anti-Spike antibody status in SLE patients. For statistical analysis a T-test was used and results are expressed as –log10 of the p value. C: Receiving operating characteristic (ROC) curve to establish at which serum albumin level can discriminate using Youden's index IGRA-S positive from IGRA-S negative SLE patients. D: IGRA-Spike results in SLE patients according to the serum albumin threshold fix at 40 g/L. P values and assay thresholds (dot line) are indicated.
3.4. Factors associated with a defective immune response in SLE
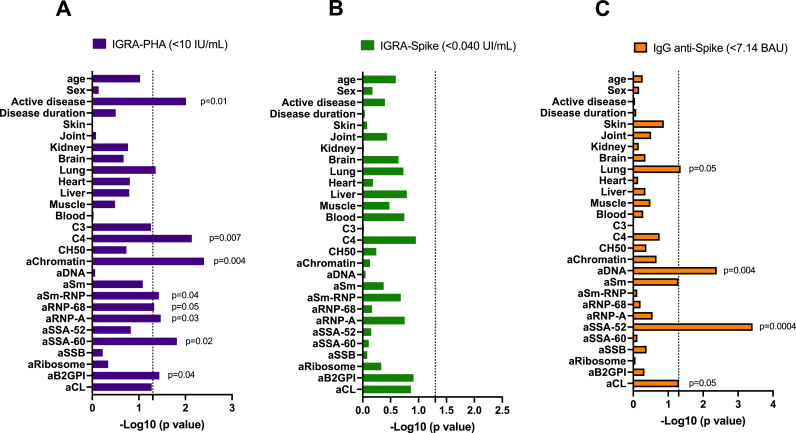
We further considered demographic parameters, clinical factors, complement fractions, and SLE-associated autoantibodies in order to test their associations with T cell activation in response to PHA used as mitogen (non specific) or Spike (specific) and to IgG anti-Spike positivity.
As previously described [24,25] and reported in Fig. 3A, a decreased production of IFNγ in T cells stimulated with PHA (<10 UI/mL) that reflects a defective T cell functional integrity was associated with disease activity (p = 0.01) and biological markers of disease activity such as C4 reduction (p = 0.007), anti-chromatin Abs (p = 0.004), anti-Sm-RNP/RNP-68kDa/RNP-A Abs (p = 0.03–0.05), anti-SSA/Ro-60kDa Abs (p = 0.02), and anti-β2GPI Abs (p = 0.04). While SLE disease activity affected T cell response to the mitogen, it did not influence the T-cell response to Spike Ag (Fig. 3B). Regarding IgG anti-Spike Ab production (Fig. 3C), positive results were associated with lung involvement (p = 0.05), elevated levels of anti-dsDNA Abs (p = 0.004), and anti-SSA-52kDa Abs (p = 0.0004) that reflects a polyclonal immunoglobulin production in SLE [26].
Fig. 3.
Comparison of demographic, clinical and biological characteristics in patients with SLE. A: according to phytohemagglutinin (PHA) mitogen-induced whole blood interferon gamma release assay (IGRA)-PHA status. B: according to the IGRA-Spike status. C: according to the IgG anti-Spike antibody status. The positive thresholds of the tests are indicated. For statistical analysis a T-test was used and results are expressed as –log10 of the p value, and significant p values < 0.05 are indicated (dot line).
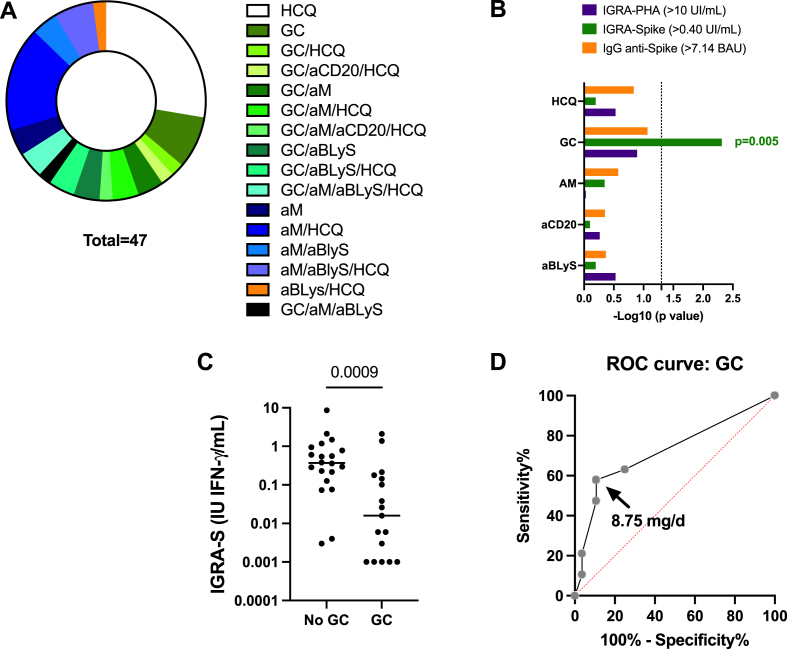
3.5. Drug exposures and immune response
As reported in Fig. 4, many drug combinations were used in SLE patients and among them glucocorticoid therapy was associated with IGRA-S negativity (p = 0.005). No association was retrieved with the other drug classes: hydroxychloroquine, antimetabolites, and biotherapies that included belimumab or rituximab. To determine the cut-off point of glucocorticoid dose that affects IGRA-S response, a ROC curve (AUC = 0.725, p = 0.01) was done and the greater sensitivity and specificity threshold was obtained at 8.75 mg/day of prednisone with a sensitivity of 47.4% (95% CI: 27–68%) and a specificity of 89% (95% CI: 73–96%). A defective IGRA-PHA response (<10 IFNγ IU/mL) and IgG anti-Spike Ab negativity were not associated with any drug exposure.
Fig. 4.
Glucocorticoids (GC) influence SARS-Cov2 memory T cell response in patients with SLE. A- Disease modifying therapies (DMT) repartition in the 47 SLE patients included in the study. B- Associations between DMT class usage and whole blood IGRA-S, IGRA-PHA, and IgG anti-Spike antibody (Ab) positivity. The positive thresholds of the tests are indicated. C- Whole blood IGRA-S results according to the usage of GC. D- Receiving operating characteristic (ROC) curve to establish the optimal level GC (Youden's index) to discriminate IGRA-S positive from IGRA-S negative SLE patients. Abbreviations: αBLys: anti-BLyS/BAFF monoclonal antibodies (mAb, belimumab); αCD20: anti-CD20 mAb (rituximab); αM: antimetabolite; HCQ: hydroxychloroquine; IGRA: interferon gamma release assay; PHA: phytohemagglutinin; S: spike; and p values < 0.05 are indicated when significant.
3.6. Accelerated T cell response decline, but not humoral response, following immunization
Finally and to complete the global analysis, the cellular and humoral responses against Spike were compared between SLE patients and controls according to time elapsed since last immunization in order to compare both the post-vaccine immune response (<50 days post-immunization), and the decline of the immune response (≥50 days) (Fig. 5). We further took advantage of data from 10 SLE patients and 6 controls that had been tested twice within a delay ranging from 6 to 14 months.
Fig. 5.
SARS-Cov2 anti-Spike cellular and humoral kinetics and responses are distinct between systemic lupus erythematosus (SLE) patients and Controls (Cont). A: Interferon gamma release assay (IGRA) response to the full length Spike recombinant protein according to the days from the last immunization. Short time post immune vaccine response (<50 days) and long-term decline of the immune response (≥50 days) are indicated. B: Follow-up levels of IGRA-Spike decline levels in 10 SLE patients and 6 Cont. C: Anti-Spike antibody response according to the days from the last immunization. D: Follow-up levels of anti-Spike antibody levels in SLE patients and Cont. P values and assay thresholds (dot line) are indicated.
Regarding memory T cell response against Spike, results revealed similar IGRA-S IFNγ levels between SLE and controls in response to the immunization (<50 days, p = 0.541), and, next, an accelerated decline was reported in SLE patients as compared to controls (>50 days, p = 0.001). The follow-up of SLE patients and controls further confirmed a significant decline of IGRA-S with time in SLE patients (p = 0.004), which was not the case in controls. Of note, such effect was conserved both when excluding from the analysis SLE patients receiving glucocorticoids and when excluding SLE patients with a serum albumin level <40 g/L (data not shown).
When considering IgG anti-Spike Ab responses (<50 days), IgG anti-Spike Ab levels were reduced in SLE patients as compared to controls (p = 0.05). After 50 days post-immunization, we observed a stable humoral response in SLE patients while there was a fast decline in controls (p = 0.02).
Altogether this supports that the reduction of the global anti-Spike immune response in SLE patients (Fig. 1) results both from an accelerated decline of the T cell immune response following a normal immunization, and from a weaker humoral response (<50 days) counterbalanced by a sustained humoral response with time.
4. Discussion
In this monocentric and retrospective study aimed at depicting the cellular and humoral response to SARS-Cov-2 in a cohort of SLE patients, we found strong evidence that the capacity to produce IgG anti-Spike Abs as well as maintaining a specific anti-Spike T-cell memory response are altered in SLE patients on various treatments. Our study further supports that COVID-19 mRNA does not protect from COVID-19 in 13/47 (27.7%) SLE patients but provides a protective cellular and/or humoral response after 2–4 vaccine injections against severe COVID-19. One should note, many of these lupus patients had a symptomatic COVID-19 infection during the era of the delta or omicron variant but none were severe enough to lead to hospitalization or death. Last but not least, better knowledge of the immune response following vaccination may help to better protect this population at high risk of infections.
In patients with SLE, several studies have established that mRNA COVID-19 vaccines are well tolerated, not associated with an increased risk of flares, but associated with a lower level of IgG anti-Spike Abs in association with a reduction of naïve B cell precursors at baseline [[27], [28], [29]]. The latter point is consistent with our report that the early stage of the humoral response is affected, which provides an argument to interrupt anti-metabolite drugs in the 2 weeks following COVID-19 vaccination as recently suggested [30]. Moreover, IgG anti-Spike Ab positivity with the presence of anti-dsDNA, anti-SSA/Ro 52 kDa, and anti-CL Abs together with sustained immune response after 50 days post immunization may reflect a post-vaccine expansion and survival of plasmablasts as previously reported in SLE [31,32]. In our study, immunosuppressive drugs did not significantly affect anti-Spike humoral response, which is in agreement with previous reports regarding glucocorticoids, hydroxychloroquine and belimumab [27,33]. Mycophenolate mofetil, methotrexate and rituximab are known to alter the post-vaccine humoral response but these drugs were infrequently used in our study [34]. Regarding glucocorticoids, a reduced vaccine humoral response is reported at high doses (>20 mg/day), which was prescribed in only 3 patients in our cohort [35].
Our results are consistent with previous reporting on T cell responses following influenza, varicella-zoster, and COVID-19 vaccine immunization in SLE [[7], [36], [37]]. These studies reported a protective but reduced memory T cell response in the majority of SLE patients, while the others remained unprotected. The diminished memory T cell vaccine response in SLE was associated in part with the use of glucocorticoids, but results were discordant between studies with regards to the effect of anti-metabolites, biotherapies, and hydroxychloroquine. The actual consensus is that disease flares, disease manifestations and changes in the level of SLE-autoantibodies and complement fractions are independent from the post-vaccine cellular immune response [7,36]. Our study further supports that the anti-Spike memory T cell reduction observed in SLE patients (Fig. 1, global analysis) results more from defective maintenance in the circulation rather from a defective expansion based on the report that IGRA-S levels are similar to controls in the first 50 days post-immunization. Such observation needs further prospective studies in a larger cohort [38]. Another point that deserves to be further explored is the difference observed between immunization in response to C0VID-19 vaccine and SARS-Cov2 infection in SLE patients, which supports a lower immunization of the vaccines as compared to the virus.
A decreased production of circulating IFNγ-producing T cells in response to mitogen stimulation is a hallmark of SLE patients and associated with active disease, autoantibody production, and complement activation as reported by others and us [25,[39], [40], [41], [42]]. Indeed it has been suggested that type II IFNγ dysregulation in T cells starts at the preclinical stage and precludes auto-Abs accrual (e.g. anti-chromatin, anti-Sm/RNP), and subsequently the type I IFNα signature occurs at SLE onset [43]. Type I IFNα is produced mainly by macrophages and dendritic cells and elicits a large spectrum of effects on the immune system including tissular T cell attraction and (autoreactive)-plasma cell differentiation, a process that can be reversed directly in the presence of anifrolumab and indirectly by belimumab [44,45]. Accordingly, it has been proposed that IGRA-PHA reduction reflects the IFN-dependent capacity of autoreactive memory T cells to migrate from peripheral blood to the inflamed tissues [44]. Another option is related to the polarization or exhaustion of memory T cells in SLE patients [[46], [47], [48]].
In conclusion, our study shows that the combined analysis of both humoral and T cell vaccine responses may help to depict vaccine protection against COVID-19 in SLE and other immunocompromised patients. In the previous years, protection against severe COVID-19 was provided by the infusion of monoclonal antibodies in immunosuppressed patients showing no humoral vaccine response. However, new variants are less sensitive to the more affordable monoclonal antibodies. Therefore, one could propose a new strategy of prevention of severe COVID-19 considering not only the humoral, but also the cellular response after vaccine or infection. It may be pertinent in high risk immunosuppressed patients to boost an altered cellular response, especially when no significant antibody response has been detected and when possible to promote quick tapering of glucocorticoids under 10 mg/day.
Author statement
Yves Renaudineau: Conceptualization, Data curation, Formal analysis, Investigation, Project administration, Methodology, Writing – original draft, review & editing, Laurent Sailler: Conceptualization, Formal analysis, Ressources, Writing – original draft, review & editing, Chloé Bost, Florence Abravanel, Jacques Izopet, Antoine Blancher, Nicolas Congy-Jolivet, Emmanuel Treiner: Investigation – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We are thankful to Dr. Wesley H. Brooks (University of South Florida, USA) and Gisèle Touzanne for editorial assistance, to Michèle Tiraby and Daniel Drocourt (INVIVOGEN®, Toulouse, France) for their precious advice, and to Elizabeth Argentin, Lorie Estrada, Fabienne Haudrechy and Elodie Martin for technical assistance.
Handling Editor: M.E. Gershwin
Contributor Information
Yves Renaudineau, Email: renaudineau.y@chu-toulouse.fr.
Chloé Bost, Email: bost.c@chu-toulouse.fr.
Florence Abravanel, Email: abravanel.f@chu-toulouse.fr.
Jacques Izopet, Email: izopet.j@chu-toulouse.fr.
Antoine Blancher, Email: blancher.antoine@neuf.fr.
Nicolas Congy, Email: congy.n@chu-toulouse.fr.
Emmanuel Treiner, Email: treiner.e@chu-toulouse.fr.
Laurent Sailler, Email: sailler.l@chu-toulouse.fr.
Data availability
Data will be made available on request.
References
- 1.Pego-Reigosa J.M., Nicholson L., Pooley N., Langham S., Embleton N., Marjenberg Z., Barut V., Desta B., Wang X., Langham J., Hammond E.R. The risk of infections in adult patients with systemic lupus erythematosus: systematic review and meta-analysis. Rheumatol. Oxf. Engl. 2021;60:60–72. doi: 10.1093/rheumatology/keaa478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yuan Q., Xing X., Lu Z., Li X. Clinical characteristics and risk factors of infection in patients with systemic lupus erythematosus: a systematic review and meta-analysis of observational studies. Semin. Arthritis Rheum. 2020;50:1022–1039. doi: 10.1016/j.semarthrit.2020.06.004. [DOI] [PubMed] [Google Scholar]
- 3.DiIorio M., Kennedy K., Liew J.W., Putman M.S., Sirotich E., Sattui S.E., Foster G., Harrison C., Larché M.J., Levine M., Moni T.T., Thabane L., Bhana S., Costello W., Grainger R., Machado P.M., Robinson P.C., Sufka P., Wallace Z.S., Yazdany J., Gore-Massy M., Howard R.A., Kodhek M.A., Lalonde N., Tomasella L.-A., Wallace J., Akpabio A., Alpízar-Rodríguez D., Beesley R.P., Berenbaum F., Bulina I., Chock E.Y., Conway R., Duarte-García A., Duff E., Gheita T.A., Graef E.R., Hsieh E., El Kibbi L., Liew D.F., Lo C., Nudel M., Singh A.D., Singh J.A., Singh N., Ugarte-Gil M.F., Hausmann J.S., Simard J.F., Sparks J.A. Prolonged COVID-19 symptom duration in people with systemic autoimmune rheumatic diseases: results from the COVID-19 Global Rheumatology Alliance Vaccine Survey. RMD Open. 2022;8 doi: 10.1136/rmdopen-2022-002587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Akiyama S., Hamdeh S., Micic D., Sakuraba A. Prevalence and clinical outcomes of COVID-19 in patients with autoimmune diseases: a systematic review and meta-analysis. Ann. Rheum. Dis. 2021;80:384–391. doi: 10.1136/annrheumdis-2020-218946. [DOI] [PubMed] [Google Scholar]
- 5.Bultink I.E.M., de Vries F., van Vollenhoven R.F., Lalmohamed A. Mortality, causes of death and influence of medication use in patients with systemic lupus erythematosus vs matched controls. Rheumatol. Oxf. Engl. 2021;60:207–216. doi: 10.1093/rheumatology/keaa267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mormile I., Della Casa F., Petraroli A., Furno A., Granata F., Portella G., Rossi F.W., de Paulis A. Immunogenicity and safety of mRNA anti-SARS-CoV-2 vaccines in patients with systemic lupus erythematosus. Vaccines. 2022;10:1221. doi: 10.3390/vaccines10081221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holvast A., van Assen S., de Haan A., Huckriede A., Benne C.A., Westra J., Palache A., Wilschut J., Kallenberg C.G.M., Bijl M. Studies of cell-mediated immune responses to influenza vaccination in systemic lupus erythematosus. Arthritis Rheum. 2009;60:2438–2447. doi: 10.1002/art.24679. [DOI] [PubMed] [Google Scholar]
- 8.Rondaan C., de Haan A., Horst G., Hempel J.C., van Leer C., Bos N.A., van Assen S., Bijl M., Westra J. Altered cellular and humoral immunity to varicella-zoster virus in patients with autoimmune diseases. Arthritis Rheumatol. Hoboken NJ. 2014;66:3122–3128. doi: 10.1002/art.38804. [DOI] [PubMed] [Google Scholar]
- 9.O'Neill S.G., Isenberg D.A. Immunizing patients with systemic lupus erythematosus: a review of effectiveness and safety. Lupus. 2006;15:778–783. doi: 10.1177/0961203306069355. [DOI] [PubMed] [Google Scholar]
- 10.Aringer M., Costenbader K., Daikh D., Brinks R., Mosca M., Ramsey-Goldman R., Smolen J.S., Wofsy D., Boumpas D.T., Kamen D.L., Jayne D., Cervera R., Costedoat-Chalumeau N., Diamond B., Gladman D.D., Hahn B., Hiepe F., Jacobsen S., Khanna D., Lerstrøm K., Massarotti E., McCune J., Ruiz-Irastorza G., Sanchez-Guerrero J., Schneider M., Urowitz M., Bertsias G., Hoyer B.F., Leuchten N., Tani C., Tedeschi S.K., Touma Z., Schmajuk G., Anic B., Assan F., Chan T.M., Clarke A.E., Crow M.K., Czirják L., Doria A., Graninger W., Halda-Kiss B., Hasni S., Izmirly P.M., Jung M., Kumánovics G., Mariette X., Padjen I., Pego-Reigosa J.M., Romero-Diaz J., Rúa-Figueroa Fernández Í., Seror R., Stummvoll G.H., Tanaka Y., Tektonidou M.G., Vasconcelos C., Vital E.M., Wallace D.J., Yavuz S., Meroni P.L., Fritzler M.J., Naden R., Dörner T., Johnson S.R. 2019 European league against rheumatism/American college of rheumatology classification criteria for systemic lupus erythematosus. Arthritis Rheumatol. Hoboken NJ. 2019;71:1400–1412. doi: 10.1002/art.40930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gladman D.D., Ibañez D., Urowitz M.B. Systemic lupus erythematosus disease activity index 2000. J. Rheumatol. 2002;29:288–291. [PubMed] [Google Scholar]
- 12.Carlé C., Fortenfant F., Tauber M., Tournier E., Paul C., Bost C., Renaudineau Y. Lupus band test can be used in combination with anti-chromatin antibodies and complement analysis to predict transition from cutaneous to systemic lupus. Clin. Immunol. Orlando Fla. 2022;234 doi: 10.1016/j.clim.2021.108908. [DOI] [PubMed] [Google Scholar]
- 13.Marziale A., Bettacchioli E., Picart G., Nafai S., Galinat H., Meroni P.L., Frostegard J., PRECISESADS Clinical Consortium. Alarcon-Riquelme M.E., Renaudineau Y. Antiphospholipid autoantibody detection is important in all patients with systemic autoimmune diseases. J. Autoimmun. 2020;115 doi: 10.1016/j.jaut.2020.102524. [DOI] [PubMed] [Google Scholar]
- 14.Bories E., Fortenfant F., Pugnet G., Renaudineau Y., Bost C. Myositis-specific autoantibodies in clinical practice: improving the performance of the immunodot. Semin. Arthritis Rheum. 2022;55 doi: 10.1016/j.semarthrit.2022.151998. [DOI] [PubMed] [Google Scholar]
- 15.Bost C., Fortenfant F., Blancher A., Pugnet G., Renaudineau Y. Combining multi-antigenic immunodot with indirect immunofluorescence on HEp-2 cells improves the diagnosis of systemic sclerosis. Clin. Immunol. Orlando Fla. 2021;229 doi: 10.1016/j.clim.2021.108774. [DOI] [PubMed] [Google Scholar]
- 16.Renaudineau Y., Sailler L., Abravanel F., Izopet J., Delourme A., Biotti D., Ciron J., Treiner E., Congy-Jolivet N., Bost C., Blancher A. Glucocorticoid use as a cause of non-cellular immune response to SARS-Cov2 Spike in patients with immune system diseases. J. Autoimmun. 2022;133 doi: 10.1016/j.jaut.2022.102912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Renaudineau Y., Abravanel F., Izopet J., Bost C., Treiner E., Congy N., Blancher A. Novel T cell interferon gamma release assay (IGRA) using spike recombinant protein for COVID19 vaccine response and Nucleocapsid for SARS-Cov2 response. Clin. Immunol. Orlando Fla. 2022;237 doi: 10.1016/j.clim.2022.108979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lu R., Zhao X., Li J., Niu P., Yang B., Wu H., Wang W., Song H., Huang B., Zhu N., Bi Y., Ma X., Zhan F., Wang L., Hu T., Zhou H., Hu Z., Zhou W., Zhao L., Chen J., Meng Y., Wang J., Lin Y., Yuan J., Xie Z., Ma J., Liu W.J., Wang D., Xu W., Holmes E.C., Gao G.F., Wu G., Chen W., Shi W., Tan W. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet Lond. Engl. 2020;395:565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abravanel F., Marion O., Del Bello A., Beunon T., Romieu-Mourez R., Couat C., Pucelle M., Staes L., Guitard J., Esposito L., Faguer S., Kamar N., Izopet J. Humoral and cellular immune responses of solid organ transplant patients on belatacept to three doses of mRNA-based anti-SARS-CoV-2 vaccine. Vaccines. 2022;10:354. doi: 10.3390/vaccines10030354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kamar N., Abravanel F., Marion O., Esposito L., Hebral A.L., Médrano C., Guitard J., Lavayssière L., Cointault O., Nogier M.B., Bellière J., Faguer S., Couat C., Del Bello A., Izopet J. Anti-SARS-CoV-2 spike protein and neutralizing antibodies at 1 and 3 months after three doses of SARS-CoV-2 vaccine in a large cohort of solid organ transplant patients. Am. J. Transplant. Off. J. Am. Soc. Transplant. Am. Soc. Transpl. Surg. 2022;22:1467–1474. doi: 10.1111/ajt.16950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Migueres M., Chapuy-Regaud S., Miédougé M., Jamme T., Lougarre C., Da Silva I., Pucelle M., Staes L., Porcheron M., Diméglio C., Izopet J. Current immunoassays and detection of antibodies elicited by Omicron SARS-CoV-2 infection. J. Med. Virol. 2023;95 doi: 10.1002/jmv.28200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Belliere J., Blancher A. QuantiFERON test interpretation in patients receiving immunosuppressive agents: an alert. Eur. Respir. J. 2017;49 doi: 10.1183/13993003.02102-2016. [DOI] [PubMed] [Google Scholar]
- 23.Kowdley K.V., Subler D.E., Scheffel J., Moore B., Smith H. Hepatitis C virus antibodies in systemic lupus erythematosus. J. Clin. Gastroenterol. 1997;25:437–439. doi: 10.1097/00004836-199709000-00008. [DOI] [PubMed] [Google Scholar]
- 24.Shin H.-J., Kim T.-O., Oh H.-J., Park H.-Y., Chang J.-S., Ahn S., Kim Y.-I., Lim S.-C., Kwon Y.-S. Impact of diabetes mellitus on indeterminate results of the QuantiFERON TB Gold In-Tube test: a propensity score matching analysis. PLoS One. 2017;12 doi: 10.1371/journal.pone.0181887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maharani W., Ratnaningsih D.F., Utami F., Yulianto F.A., Dewina A., Hamijoyo L., Atik N. Activity disease in SLE patients affected IFN-γ in the IGRA results. J. Inflamm. Res. 2020;13:433–439. doi: 10.2147/JIR.S258235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bettacchioli E., Le Gaffric C., Mazeas M., Borghi M.O., Frostegard J., Barturen G., Makowska Z., Babei S., Lesche R., PRECISESADS Clinical Consortium. Meroni P.L., Alarcon-Riquelme M.E., Renaudineau Y. An elevated polyclonal free light chain level reflects a strong interferon signature in patients with systemic autoimmune diseases. J. Transl. Autoimmun. 2021;4 doi: 10.1016/j.jtauto.2021.100090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moyon Q., Sterlin D., Miyara M., Anna F., Mathian A., Lhote R., Ghillani-Dalbin P., Breillat P., Mudumba S., de Alba S., Cohen-Aubart F., Haroche J., Pha M., Boutin T.H.D., Chaieb H., Flores P.M., Charneau P., Gorochov G., Amoura Z. BNT162b2 vaccine-induced humoral and cellular responses against SARS-CoV-2 variants in systemic lupus erythematosus. Ann. Rheum. Dis. 2022;81:575–583. doi: 10.1136/annrheumdis-2021-221097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Garcia-Cirera S., Calvet J., Berenguer-Llergo A., Pradenas E., Marfil S., Massanella M., Mateu L., Trinité B., Llop M., Arévalo M., Galisteo C., Orellana C., Gómez R., Gómez-Gerique M.N., Carmona I., Clotet B., Blanco J., Gratacós J. Glucocorticoids' treatment impairs the medium-term immunogenic response to SARS-CoV-2 mRNA vaccines in Systemic Lupus Erythematosus patients. Sci. Rep. 2022;12 doi: 10.1038/s41598-022-18996-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.So H., Li T., Chan V., Tam L.-S., Chan P.K. Immunogenicity and safety of inactivated and mRNA COVID-19 vaccines in patients with systemic lupus erythematosus. Ther. Adv. Musculoskelet. Dis. 2022;14 doi: 10.1177/1759720X221089586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abhishek A., Boyton R.J., Peckham N., McKnight Á., Coates L.C., Bluett J., Barber V., Cureton L., Francis A., Appelbe D., Eldridge L., Julier P., Valdes A.M., Brooks T., Rombach I., Altmann D.M., Nguyen-Van-Tam J.S., Williams H.C., Cook J.A., VROOM study investigators Effect of a 2-week interruption in methotrexate treatment versus continued treatment on COVID-19 booster vaccine immunity in adults with inflammatory conditions (VROOM study): a randomised, open label, superiority trial. Lancet Respir. Med. 2022;10:840–850. doi: 10.1016/S2213-2600(22)00186-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Szelinski F., Stefanski A.L., Schrezenmeier E., Rincon-Arevalo H., Wiedemann A., Reiter K., Ritter J., Lettau M., Dang V.D., Fuchs S., Frei A.P., Alexander T., Lino A.C., Dörner T. Plasmablast-like phenotype among antigen-experienced CXCR5-CD19low B cells in systemic lupus erythematosus. Arthritis Rheumatol. Hoboken NJ. 2022;74:1556–1568. doi: 10.1002/art.42157. [DOI] [PubMed] [Google Scholar]
- 32.Kotliarov Y., Sparks R., Martins A.J., Mulè M.P., Lu Y., Goswami M., Kardava L., Banchereau R., Pascual V., Biancotto A., Chen J., Schwartzberg P.L., Bansal N., Liu C.C., Cheung F., Moir S., Tsang J.S. Broad immune activation underlies shared set point signatures for vaccine responsiveness in healthy individuals and disease activity in patients with lupus. Nat. Med. 2020;26:618–629. doi: 10.1038/s41591-020-0769-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schiavoni I., Olivetta E., Natalucci F., Olivieri G., Lo Presti A., Fedele G., Stefanelli P., Ceccarelli F., Conti F. Evidence of immune response to BNT162b2 COVID-19 vaccine in systemic lupus erythematosus patients treated with Belimumab. Lupus. 2023 doi: 10.1177/09612033221151012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ammitzbøll C., Bartels L.E., Bøgh Andersen J., Risbøl Vils S., Elbaek Mistegård C., Dahl Johannsen A., From Hermansen M.-L., Kragh Thomsen M., Erikstrup C., Hauge E.-M., Troldborg A. Impaired antibody response to the BNT162b2 messenger RNA coronavirus disease 2019 vaccine in patients with systemic lupus erythematosus and rheumatoid arthritis. ACR Open Rheumatol. 2021;3:622–628. doi: 10.1002/acr2.11299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rondaan C., Furer V., Heijstek M.W., Agmon-Levin N., Bijl M., Breedveld F.C., D'Amelio R., Dougados M., Kapetanovic M.C., van Laar J.M., Ladefoged de Thurah A., Landewé R., Molto A., Müller-Ladner U., Schreiber K., Smolar L., Walker J., Warnatz K., Wulffraat N.M., van Assen S., Elkayam O. Efficacy, immunogenicity and safety of vaccination in adult patients with autoimmune inflammatory rheumatic diseases: a systematic literature review for the 2019 update of EULAR recommendations. RMD Open. 2019;5 doi: 10.1136/rmdopen-2019-001035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miossi R., Fuller R., Moraes J.C.B., Ribeiro A.C.M., Saad C.G.S., Aikawa N.E., Miraglia J.L., Ishida M.A., Bonfa E., Caleiro M.T.C. Immunogenicity of influenza H1N1 vaccination in mixed connective tissue disease: effect of disease and therapy. Clin. Sao Paulo Braz. 2013;68:129–134. doi: 10.6061/clinics/2013(02)oa02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yves Renaudineau, Sylviane Muller, Christian M. Hedrich, Dominique Chauveau, Julie Bellière, Sébastien De Almeida, Jan Damoiseaux, Marc Scherlinger, Jean Charles Guery, Laurent Sailler, Chloé Bost, Immunological and translational key challenges in systemic lupus erythematosus: A symposium update, Journal of Translational Autoimmunity, Volume 6, 2023, 100199, ISSN 2589-9090, 10.1016/j.jtauto.2023.100199. [DOI] [PMC free article] [PubMed]
- 38.Taves M.D., Ashwell J.D. Glucocorticoids in T cell development, differentiation and function. Nat. Rev. Immunol. 2021;21:233–243. doi: 10.1038/s41577-020-00464-0. [DOI] [PubMed] [Google Scholar]
- 39.Simon Q., Grasseau A., Boudigou M., Le Pottier L., Bettachioli E., Cornec D., Rouvière B., Jamin C., Le Lann L., PRECISESADS Clinical Consortium. PRECISESADS Flow Cytometry Study Group. Borghi M.O., Aguilar-Quesada R., Renaudineau Y., Alarcón-Riquelme M.E., Pers J.-O., Hillion S. A proinflammatory cytokine network profile in Th1/type 1 effector B cells delineates a Common group of patients in four systemic autoimmune diseases. Arthritis Rheumatol. Hoboken NJ. 2021;73:1550–1561. doi: 10.1002/art.41697. [DOI] [PubMed] [Google Scholar]
- 40.Barturen G., Babaei S., Català-Moll F., Martínez-Bueno M., Makowska Z., Martorell-Marugán J., Carmona-Sáez P., Toro-Domínguez D., Carnero-Montoro E., Teruel M., Kerick M., Acosta-Herrera M., Le Lann L., Jamin C., Rodríguez-Ubreva J., García-Gómez A., Kageyama J., Buttgereit A., Hayat S., Mueller J., Lesche R., Hernandez-Fuentes M., Juarez M., Rowley T., White I., Marañón C., Gomes Anjos T., Varela N., Aguilar-Quesada R., Garrancho F.J., López-Berrio A., Rodriguez Maresca M., Navarro-Linares H., Almeida I., Azevedo N., Brandão M., Campar A., Faria R., Farinha F., Marinho A., Neves E., Tavares A., Vasconcelos C., Trombetta E., Montanelli G., Vigone B., Alvarez-Errico D., Li T., Thiagaran D., Blanco Alonso R., Corrales Martínez A., Genre F., López Mejías R., Gonzalez-Gay M.A., Remuzgo S., Ubilla Garcia B., Cervera R., Espinosa G., Rodríguez-Pintó I., De Langhe E., Cremer J., Lories R., Belz D., Hunzelmann N., Baerlecken N., Kniesch K., Witte T., Lehner M., Stummvoll G., Zauner M., Aguirre-Zamorano M.A., Barbarroja N., Castro-Villegas M.C., Collantes-Estevez E., de Ramon E., Díaz Quintero I., Escudero-Contreras A., Fernández Roldán M.C., Jiménez Gómez Y., Jiménez Moleón I., Lopez-Pedrera R., Ortega-Castro R., Ortego N., Raya E., Artusi C., Gerosa M., Meroni P.L., Schioppo T., De Groof A., Ducreux J., Lauwerys B., Maudoux A.-L., Cornec D., Devauchelle-Pensec V., Jousse-Joulin S., Jouve P.-E., Rouvière B., Saraux A., Simon Q., Alvarez M., Chizzolini C., Dufour A., Wynar D., Balog A., Bocskai M., Deák M., Dulic S., Kádár G., Kovács L., Cheng Q., Gerl V., Hiepe F., Khodadadi L., Thiel S., de Rinaldis E., Rao S., Benschop R.J., Chamberlain C., Dow E.R., Ioannou Y., Laigle L., Marovac J., Wojcik J., Renaudineau Y., Borghi M.O., Frostegård J., Martín J., Beretta L., Ballestar E., McDonald F., Pers J.-O., Alarcón-Riquelme M.E. Integrative analysis reveals a molecular stratification of systemic autoimmune diseases. Arthritis Rheumatol. Hoboken NJ. 2021;73:1073–1085. doi: 10.1002/art.41610. [DOI] [PubMed] [Google Scholar]
- 41.Hagiwara E., Gourley M.F., Lee S., Klinman D.K. Disease severity in patients with systemic lupus erythematosus correlates with an increased ratio of interleukin-10:interferon-gamma-secreting cells in the peripheral blood. Arthritis Rheum. 1996;39:379–385. doi: 10.1002/art.1780390305. [DOI] [PubMed] [Google Scholar]
- 42.Chiche L., Jourde-Chiche N., Whalen E., Presnell S., Gersuk V., Dang K., Anguiano E., Quinn C., Burtey S., Berland Y., Kaplanski G., Harle J.-R., Pascual V., Chaussabel D. Modular transcriptional repertoire analyses of adults with systemic lupus erythematosus reveal distinct type I and type II interferon signatures. Arthritis Rheumatol. Hoboken NJ. 2014;66:1583–1595. doi: 10.1002/art.38628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Munroe M.E., Lu R., Zhao Y.D., Fife D.A., Robertson J.M., Guthridge J.M., Niewold T.B., Tsokos G.C., Keith M.P., Harley J.B., James J.A. Altered type II interferon precedes autoantibody accrual and elevated type I interferon activity prior to systemic lupus erythematosus classification. Ann. Rheum. Dis. 2016;75:2014–2021. doi: 10.1136/annrheumdis-2015-208140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Casey K.A., Guo X., Smith M.A., Wang S., Sinibaldi D., Sanjuan M.A., Wang L., Illei G.G., White W.I. Type I interferon receptor blockade with anifrolumab corrects innate and adaptive immune perturbations of SLE. Lupus Sci. Med. 2018;5 doi: 10.1136/lupus-2018-000286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wilkinson C., Henderson R.B., Jones-Leone A.R., Flint S.M., Lennon M., Levy R.A., Ji B., Bass D.L., Roth D. The role of baseline BLyS levels and type 1 interferon-inducible gene signature status in determining belimumab response in systemic lupus erythematosus: a post hoc meta-analysis. Arthritis Res. Ther. 2020;22:102. doi: 10.1186/s13075-020-02177-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhou H., Hu B., Huang N., Mo X., Li W., Zhang B., Wei B., Gao M., Wang Y., Liu X., Liao J. Aberrant T cell subsets and cytokines expression profile in systemic lupus erythematosus. Clin. Rheumatol. 2018;37:2405–2413. doi: 10.1007/s10067-018-4124-0. [DOI] [PubMed] [Google Scholar]
- 47.Choi J.-Y., Ho J.H., Pasoto S.G., Bunin V., Kim S.T., Carrasco S., Borba E.F., Gonçalves C.R., Costa P.R., Kallas E.G., Bonfa E., Craft J. Circulating follicular helper-like T cells in systemic lupus erythematosus: association with disease activity. Arthritis Rheumatol. Hoboken NJ. 2015;67:988–999. doi: 10.1002/art.39020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bost C., Arleevskaya M.I., Brooks W.H., Plaza S., Guery J.-C., Renaudineau Y. Long non-coding RNA Xist contribution in systemic lupus erythematosus and rheumatoid arthritis. Clin. Immunol. Orlando Fla. 2022;236 doi: 10.1016/j.clim.2022.108937. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.