Abstract
The aim of this study was to evaluate whether the addition of strawberry by-products (pulp and achene) and thermosonication offers a nectar with a potential contribution of health and safety benefits. Strawberry nectar with 0, 10 and 20% of strawberry by-products (SB) was subjected to thermosonication (24 kHz) at 70 and 80% for 8 min at 50 °C. Total soluble solids, pH, polyphenol oxidase (PO) and pectin methylesterase (PME) activities, total soluble phenols (TSP), ascorbic acid (AA), anthocyanins and antioxidant capacity (AOX) were evaluated. Microbiological reduction and inactivation of Escherichia coli was also determined. A limited activity was observed in PO and PME related to the SB percentage added. TSP, AA, anthocyanins, and AOX were increased due to the different percentages of SB added to the nectar. A reduction of aerobic mesophiles (1.28 Log CFU/mL), molds and yeast counts (1.23 Log CFU/mL) were achieved by thermosonication. E. coli inactivation was approximately 1 log CFU/mL in 20% SB nectar at 80% amplitude, 8 min at 50 °C, but increased during storage at 6 °C (0.915–5.86 Log CFU/mL). Thermosonication showed the possibility of employing strawberry by-products in nectars, improving the use of agro-industrial residues by non-thermal technologies.
Keywords: Thermosonication, Strawberry nectar, Bioactive compounds, E. coli inactivation
Introduction
The strawberry (Fragaria ananassa) is one of the most popular fruits around the world and strawberry beverages are becoming popular because of its appealing sensory properties, essential nutrients, and bioactive compounds (Cassani et al. 2020). Strawberry cloudy and clear beverages processing usually includes a step that removes pulp and seeds that can be considered as strawberry by-products. The removal of pulp and strawberry seeds called "achenes" causes flavor changes and a decrease in dietary fiber and phenolic compounds, especially those derived from ellagic acid (Navarro et al. 2018).
On the other hand, the main concern about raw fruit beverages is microbial contamination, especially by acid-tolerant microorganisms that include pathogenic bacteria such as E. coli (Alighourchi et al. 2014), that will eventually lead to Foodborne Illness (Ferrario et al. 2015). To ensure fruit juice safety, it is necessary to comply with the standard provided by the Food and Drug Administration (FDA) to reduce 5 logarithmic cycles in the pathogen population (FDA 2001; Demir and Kılınç 2019). Fruit beverage thermal processing before packaging is a very widespread practice employed to inactivate enzymes and microorganisms. However, high temperatures (60–121°C) can deteriorate the nutritional, physicochemical, rheological and sensory properties of beverages (Ferrario et al. 2015). Odriozola-Serrano et al. (2008) found that thermal processing has a significant reduction in antioxidant capacity (18%), total phenols (22%), flavonoids (25%), and ascorbic acid (36%) in strawberry juice.
Ultrasound processing is a technology that has been effective for microbial and enzyme inactivation on fruit beverages (Amador-Espejo et al. 2020). Ultrasound refers to pressure waves that spread within a medium at a frequency of 20 kHz or more (Sasikumar et al. 2019). This treatment results in the formation of microbubbles in the food system that eventually implodes in a process called cavitation. The application of ultrasound and mild heat treatment (< 100 °C, usually between 50 and 60 °C) is called thermosonication. In recent years, thermosonication process has been applied to fruit products in order to obtain the maximum yield of all the nutrients contained in raw fruits with minimal changes as a result of processing, thus achieving superior quality fruit products with maximum retention of phytonutrients, high consumer acceptance, and safety (Anaya-Esparza et al. 2017). Furthermore, different studies have found that ultrasound improves the content of bioactive compounds (such as total phenols and vitamin C) and antioxidant activity in fruit juice (Wang et al. 2019). Therefore, the aim of this study was to determine the effect of thermosonication on the microbiological (evaluating E. coli reduction in specific treatments) and enzymatic inactivation, and bioactive content compounds in strawberry nectar added with subproducts compared to heat-treated nectar.
Materials and methods
Strawberry nectar elaboration
Strawberry (Fragaria ananassa) fruits in commercial ripeness (was measured pH 3.2–3.6 and °Brix 7–9) were acquired from an orchard of Irapuato, Guanajuato, México (20°40′36.3′′N 101°21′22.6′′W), and transferred to the biotechnology laboratory (Instituto Tecnológico de Tepic, Nayarit, México). The fruits were cleaned and sanitized (sodium hypochlorite 5 ppm). First, strawberry puree was obtained using an extractor (Breville, BJE510XL/A, Australia) and filtered through a fine mesh stainless steel strainer (Double mesh, number 18 and 6). The pulp and retained achene (SB) were liquefied (Nutribullet, USA) and incorporated in different percentages (0, 10 and 20%, higher percentages was very viscous) to a 40% strawberry puree nectar. Sugar was added up to 14°Brix of total soluble solids according to the food codex (CODEX STAN 2005).
Thermosonication and pasteurization treatments
Nectar thermosonication treatment was carried out with an ultrasonic processor (Hielscher UP400S, Germany) using the 7 mm H7 sonotrode (400 W, 24 kHz). The experimental design was a 3 × 2 full factorial. Two factors X1, Amplitude with levels of 70 and 80%, and X2, Pulp/achene with levels of 0, 10 and 20% were considered for the ultrasound treatment (because higher percentages cause sedimentation and exceeded the soluble solids limit), the fixed temperature of 50 °C was maintained, as well as the time of 8 min. Experiment was made in triplicate and compared with pasteurized nectar (0, 10 and 20% pulp/achene) and control nectar (0, 10 and 20% pulp/achene). These treatments were applied using previous studies on fruit matrixes (juice and smoothie) with short time (less than 10 min), 70–85% of amplitude and moderate temperature (50–60 °C) (Amador-Espejo et al. 2019; Alves et al. 2020). Aliquots of 270 mL of nectar were sonicated at amplitudes of 70 and 80% for 8 min with a water bath coupled to the ultrasonic processor to reach at 50 °C outlet temperature. After thermosonication treatments, the nectar samples were stored at 4 °C or lyophilized until being analyzed. The effective power (EP) was calculated by the calorimetric method according to Eq. (1) as reported by Torkamani et al. (2014). The sample temperatures were recorded before (initial) and after (final) treatment. Thermal treatment (62.5 °C for 30 min) was performed in a water bath (Cat 13,100, USA), and untreated nectar (control sample) was also included for comparison, SB was added to both treatments at 10 and 20%.
| 1 |
where EP = Effective power determined for the treatment in W. mn = Mass used in treatment “n” in kg. Cp = Specific heat for sample “n” in J/ (kg °C).
Physicochemical parameters
The pH was measured using a potentiometer (Hach Sension, Spain) and total soluble solids (TSS) expressed in °Brix using a refractometer (HANNA HI 96,801, USA). Titratable acidity (TA) was measured according to the method suggested by the “Association of Official Analytical Chemists” (AOAC, 2005).
Enzyme (pectin methylesterase and polyphenol oxidase) activities
Pectin methylesterase (PME) enzymatic activity determination was carried out by titration of the carboxyl group, according to Raviyan et al. (2005). Nectar (20 mL) was added to 40 mL citrus pectin solution (1% diluted in 2N NaCl). The mixture was adjusted to pH 7.0 with 1 N NaOH. Once the pH of 7.0 was reached, 1 mL of 0.05 N NaOH was added, and the time required for the sample to return to pH 7.0 was measured. One unit of PME (UPME) is defined as the release of 1 μmol of the carboxyl group per minute at pH 7.0 at 30 °C. For the calculation of the enzymatic activity, the following formula was used:
Polyphenol oxidase (PPO) enzimatic activity was measured by spectrometry at 420 nm in a spectrophotometer (Genesys 10S, Uruguay) according to the methodology of Guerrero-Beltrán and Barbosa-Cánovas (2006). The enzyme extract was obtained by mixing 5 mL of the sample with 5 mL of Mcllevaine buffer (pH 6.6). This mixture was centrifuged (LabTech 1580R, Italy) for 40 min at 4 °C and 4000 rpm, and then filtered with Whatman paper no.1. Once the extract was obtained, 0.25 mL was taken and 1 mL of Mcllevaine buffer (pH 6.6) and 0.5 mL of catechol (0.175 M) were added. Absorbance was measured every 2 min for 20 min, and the linear portion of the curve was used to calculate units of enzyme activity (UAE). A UAE equals 0.001 A420/min/mL.
Microbiological analysis
Microbiological assays were performed according to the Official Mexican Norms. Pour plate technique was used for yeast and mold counts (NOM-111-SSA1-1994) on rose bengal agar base, and fluted quadrant technique was used for coliforms (NOM-113-SSA1-1994) on violet red bile glucose agar and Aerobic Plate Count (NOM-092-SSA1-1994) on plate count agar. The results were expressed as Log CFU/mL.
Ascorbic acid, total soluble phenolic and total monomeric anthocyanin content
Ascorbic acid (AA) determination was performed according to Dürüst et al. (1997) by diluting the strawberry nectar with 0.4% oxalic acid (1:10). Then, 100 µL were mixed with 100 µL of acetate buffer and 800 µL of 2,6-dichlorophenolindophenol sodium salt hydrate (DCPI). The absorbance was recorded at 525 nm every 15 s in a spectrophotometer (Genesys 10S, Uruguay). An ascorbic acid calibration curve of 0, 5, 10, 20, 30, and 40 mg/L was performed. The results were expressed as mg equivalents of ascorbic acid per gram of sample (mg EAA/g).
Total soluble phenols (TSP) content was determined using the Folin-Ciocalteu test in accordance with Alvarez-Parrilla et al. (2010). Sample (250 µL) Folin-Ciocalteu reagent (1250 µL) and sodium carbonate (7.5%) were incubated for 15 min at 50 °C. The reaction solution was measured at 750 nm in a microplate reader (Bio-Tek®, Synergy HT, Winooski, VT, USA) with the program Gen5. A calibration curve of gallic acid (GA) at different concentrations (0.0125 to 0.20 mg/mL) was performed. The results were expressed as mg equivalent of GA per gram of sample (mg EAG/g). For total anthocyanins determination, the method reported by Lee and Wrolstad (2004) was used. Sample (0.5 mL) was diluted using 4.5 mL potassium chloride buffer (0.025 M, pH 1). In another vial, the same amount of sample was diluted with 4.5 mL sodium acetate solution (0.4 M, pH 4.5 with 0.1 M HCl). The samples were placed 15 min in the dark to perform the reading at 510 and 700 nm on a microplate reader (Bio-Tek®, Synergy HT, Winooski, VT, USA) with the program Gen5. The anthocyanin content was calculated by the following equation:
where A = Subtraction of the absorbance of the values with potassium chloride (510–700 nm) minus the absorbance subtraction from the sodium acetate values (510–700 nm). MW = Molecular weight of cyanidin-3-glucoside (449.2 g/mol). FD = Dilution factor. E = Molar absorption (26900 L/mol cm)
Measurement of antioxidant capacity
In this study, antioxidant capacity (AOX) was evaluated by three methods: ABTS (2,2'-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid)) radical method, FRAP (Ferric reducing antioxidant power assay) and DPPH (free radical 2,2-diphenyl-1-picrylhydrazil scavening method). A microplate absorbance reader (Bio-Tek®, Synergy HT, Winooski, VT, USA) was used in the three methods. The determination of the antioxidant activity by means of the ABTS radical trapping was carried out according to Prior et al. (2005), absorbance was measured at 734 nm. FRAP, method described by Alvarez-Parrilla et al. (2011) was used to carry out the analysis, absorbance was measured at 595 nm at 37 °C. DPPH methodology was developed according to Alvarez-Parrilla et al. (2011), absorbance was measured at 517 nm. Trolox [((±)-6-hydroxy- 2,5,7,8-tetramethyl-chroman-2-carboxylic acid)] was used as a standard compound in the methods. AOX was expressed as µmol trolox equivalents per gram of sample (µmol ET/g).
Inactivation of Escherichia coli by thermosonication
Escherichia coli (ATCC 8739) was activated in tryptone soy broth (enriched with yeast extract 6 g/L) at 37 °C for 18–24 h. Subsequently, the broth was used to inoculate trypticaseine soy agar plates by surface inoculation and incubated at 37 °C for 24 h. Colonies were collected from plates to prepare a cell suspension in 11 mL of tryptone sodium chloride solution (1 g/L of tryptone pancreatic casein digestion) and 8.5 g/L of sodium chlroride. Optical density at 405 nm was measured using a spectrophotometer (Genesys 10S, Uruguay) to obtain 9–9.5 Log CFU/mL. Later, 10 mL of this cell suspension was inoculated in a liter of sterilized strawberry nectar to reach a load of 7 Log of CFU/mL of E. coli. Before nectar treatment, samples were refrigerated at 6 °C. After that, the pasteurization and thermosonication treatment were carried out, having as a control a sample of nectar inoculated without treatment. In order to compared the heat component from the thermosonication treatment, a thermal treatment (50 °C, 8 min) was applied in the nectar. The microbiological analyses were performed after 2 h, and 5 and 10 days of storage at 6 °C. To assess the treatment reduction, decimal dilutions were prepared and plated in trypticaseine soy agar with yeast extract and incubated at 37 °C for 48 h. Inactivation was determined as the Log (N/No) where No and N are colony counts in control nectar and after thermosonication treatment, respectively. To evaluate the effect of the food matrix in the inactivation achieved, E. coli suspensions the same procedure that was developed in nectar was applied in PBS (Phosphate buffered saline, 7.2 pH).
Finally, to determine conditions for achieving the 5 log CFU/mL required by FDA regulation, an inactivation kinetic was performed. Inoculated nectar with E. coli was treated at 80% of amplitude, 50 °C, and every 4 min to complete 20 min a 3 mL of sample was taken, placed in a 2 mL tube and each sample was refrigerated until analysis. Then, decimal dilutions were prepared and plated in trypticaseine soy agar with yeast extract and incubated at 37 °C for 48 h. The inactivation was calculated as the difference between the logarithms of the colony counts of the nectar at minute 0 and the nectar after every 4 min (Log No–Log N). A mathematical model was developed using a simple regression, taking treatment time as the independent variable and inactivation as the dependent variable. It was carried out with the statistical package Statistics 12 (TIBCO Software Inc. USA) for Windows.
Statistical analysis
Data from different strawberry nectars (thermosonicated, pasteurized, and untreated) were analyzed by ANOVA test and differences among means were compared using a Tukey test with a 95% confidence level using Statistica 12 (TIBCO Software Inc., USA) for Windows. All experiments were repeated three times and all tests were carried out in triplicate and reported as means with standard deviation (SD).
Results and discussion
Effect of thermosonication on TSS, pH and TA
Physicochemical results of the applied treatments are shown in Table 1. TSS (14.10–14.23°Brix) and pH (3.80–3.88) did not show significant differences between treatments or Strawberry by-products (SB), which complied with the quality parameters established for nectars, indicating that the pH should not be less than 2.5, and °Brix around 14 (CODEX STAN 2005). Other authors also have reported no changes in these parameters by thermosonication treatments applied in carambola juice (Nayak et al. 2018) and in camu-camu nectar (do Amaral Souza et al. 2019). However, A two-fold increase in TA was observed in thermosonicated samples compared to other treatments (p < 0.05). This increase in acidity may be related to the release of organic acids from the cell cytoplasm to the outside, caused by the rupture of the cell membrane due to the cavitation process (Anaya-Esparza et al. 2017; Oladunjoye et al. 2021).
Table 1.
Thermosonication effect on physicochemical, enzymatic and microbial parameters of strawberry nectar
| Treatments/Parameters | pH | Total soluble solids (°Brix) | Titratable acidity (%) | Polyphenol oxidase (UAE/min/mL) | Pectin metilesterase (UPME/mL) | Molds and yeast (Log CFU/mL) | Aerobic mesophilic bacteria (Log CFU/mL) |
|---|---|---|---|---|---|---|---|
| (0/70) | 3.82 ± 0.03a | 14.22 ± 0.05a | 0.250 ± 0.00a | 2.296 ± 0.387ab | 0.187 ± 0.030ac | 1.913 ± 0.104a | 2.166 ± 0.231a |
| (10/70) | 3.85 ± 0.01a | 14.23 ± 0.09a | 0.240 ± 0.00a | 2.706 ± 0.435a | 0.258 ± 0.008df | 2.156 ± 0.292a | 2.330 ± 0.173a |
| (20/70) | 3.84 ± 0.09a | 14.15 ± 0.16a | 0.240 ± 0.00a | 2.886 ± 0.566a | 0.287 ± 0.014f | 1.950 ± 0.177a | 2.073 ± 0.120a |
| (0/80) | 3.80 ± 0.04a | 14.10 ± 0.11a | 0.250 ± 0.00e | 2.396 ± 0.491ab | 0.148 ± 0.005bc | 2.083 ± 0.040a | 2.040 ± 0.115a |
| (10/80) | 3.87 ± 0.02a | 14.13 ± 0.26a | 0.240 ± 0.00a | 2.920 ± 0.590a | 0.224 ± 0.031ad | 2.003 ± 0.280a | 2.133 ± 0.100a |
| (20/80) | 3.88 ± 0.09a | 14.20 ± 0.23a | 0.240 ± 0.00a | 2.830 ± 0.287a | 0.218 ± 0.023ad | 1.966 ± 0.060a | 1.983 ± 0.215a |
| C (0/0) | 3.80 ± 0.04a | 14.11 ± 0.37a | 0.146 ± 0.01d | 2.400 ± 0.708a | 0.116 ± 0.007be | 2.780 ± 0.098b | 3.263 ± 0.177b |
| C (10/0) | 3.80 ± 0.04a | 14.16 ± 0.32a | 0.17 ± 0.00bc | 2.466 ± 0.448ab | 0.133 ± 0.009bce | 2.886 ± 0.380b | 3.096 ± 0.205b |
| C (20/0) | 3.85 ± 0.08a | 14.00 ± 0.08a | 0.176 ± 0.01c | 2.950 ± 1.213ab | 0.305 ± 0.041f | 3.143 ± 0.159b | 2.863 ± 0.184b |
| P (0/0) | 3.81 ± 0.04a | 14.42 ± 0.31a | 0.146 ± 0.01d | 0.683 ± 0.152b | 0.082 ± 0.004e | ND | ND |
| P (10/0) | 3.84 ± 0.05a | 14.33 ± 0.17a | 0.166 ± 0.01b | 1.550 ± 0.926ab | 0.173 ± 0.006abc | ND | ND |
| P (20/0) | 3.95 ± 0.03a | 14.33 ± 0.23a | 0.170 ± 0.00bc | 2.300 ± 0.563ab | 0.219 ± 0.024ad | ND | ND |
The results are expressed as the mean ± standard deviation (n = 3). Different letters in the same column are significantly different (p < 0.05). Equal letters do not show significant differences. (0/70): 0% SB (strawberry by-products) and 70% amplitude; (10/70): 10% SB and 70% amplitude; (20/70): 20% SB and 70% amplitude; (0/80): 0% SB and 80% amplitude; (10/80): 10% SB and 80% amplitude; (20/80): 20% SB and 80% amplitude; C (0/0): 0% SB; C (10/0): 10% SB; C (20/0): 20% SB; P (0/0): 0% SB; P (10/0): 10% SB; P (20/0): 20% SB. C CONTROL, P PASTEURIZED. ND not detected
Effect of thermosonication on enzymatic activities
In PPO activity a difference was observed between the pasteurized and the ultrasound treated samples, but with a low activity of the enzyme in the sonicated samples. It has been reported that activity of PPO is low in strawberries because of the slight content of copper, which is a cofactor of this enzyme. For example, avocado shows a value of 0.29 mg Cu/100 g in comparison with 0.09 mg Cu/100 g presented in strawberry (Jiménez-Vieyra and Zambrano-Zaragoza 2011). Besides, a fruit with polyphenol content and polyphenol oxidase is susceptible to darkening but a fruit that also has a high copper content announces an accelerated darkening (Es-Safi et al. 2003). Likewise, nectar has a low pH, which inhibits enzyme activity (Jiménez-Vieyra and Zambrano-Zaragoza 2011). Nonetheless, the pasteurized sample without the addition of strawberry subproduct addition showed the lowest activity of this enzyme, which can be explained because the pasteurization temperature applied (63 °C for 30 min). The optimal temperature for PPO is 25 °C, while a temperature of 65–90 °C is inactive (Vega Contreras et al. 2020).
Regarding PME, SB addition and thermosonication in nectars increases the enzymatic activity; however, thermal treatment tends to decrease it (Table 1). In this sense, the lowest value observed was in the pasteurized sample with 0% SB (0.082 UPME/mL) and the highest in the control sample with 20% SB (0.305 UPME/mL). Strawberry fruit has low pectin content and consequently low enzyme activity. Within this context, Draye and Van Cutsem (2008) mentions that most pectin in strawberry is soluble in oxalate (0.29 g galacturonic acid/100 g fresh basis) and this low-ester pectin fraction can favor calcium binding and form a crosslinking structure. Therefore, this enzyme does not represent an important activity in strawberry. That is a reason why choosing appropriate parameters can enhance enzyme inactivation. Amador-Espejo et al. (2020) observed that the amplitude and temperature were important parameters for the activity of PME, and when an amplitude of 80% was applied, the enzyme activity decreased.
Effect of thermosonication on microbial counts
Thermosonication treatments in nectar reduced about 1 Log CFU/mL of molds, yeasts, and aerobic mesophilic bacteria, with statistical differences in comparison to the control nectar (p > 0.05). These values reached the count limit allowed for fruit nectars by Mexican regulations (NOM-093-SSA1-1994) and codex alimentarius (CODEX STAN 2005). However, in the pasteurized nectar these microorganisms were not detected. For total coliforms, no counts were detected in any treated sample (data not shown). This confirms that the hygiene practices were followed and cross-contamination was avoided. Factors such as amplitude, temperature, and time treatment are key for microbial inactivation. Amador-Espejo et al. (2020) achieved a higher microbial reduction in fruit smoothies by increasing the amplitude to 70–85%, the temperature to 40–55 °C, and the time treatment to 15–25 min. Lethal effect of thermosonication on microorganisms is explain because the phenomenon of cavitation, which is capable of damaging the cell wall and membrane of microorganisms, forming pores (sonoporation) and causing the loss of intracellular content (Anaya-Esparza et al. 2017). Cavitation also increases the temperature and pressure of the medium, as well as the levels of free radicals with bactericidal properties, which can also contribute to microbial inactivation and the inhibition of microorganisms’ growth by inactivating the mitochondria enzymatic activity (Cassani et al. 2020).
Effect of thermosonication on ascorbic acid, total soluble phenolic and monomeric anthocyanin content
Table 2 shows the content of ascorbic acid, soluble phenols and monomeric anthocyanins in strawberry nectar. In TSP nectar samples presented a gradual increase related to the different percentages of SB addition from 9.061 to 20.017 mg GAE/g in thermosonicated nectar. This increase can be attributed to the achene which contains important concentrations of ellagic acid (Aaby et al. 2007). In the case of the anthocyanin content, nectar samples added with pulp/achene increased the anthocyanin content (p < 0.05), reaching the highest content at 20% SB and 80% amplitude treatment (111.32 mg Cy3gl/g). Ariza et al. (2016) reported a higher content of anthocyanin in the achene, being the main pelargonidin 3-glucoside and cyanidin 3-glucoside. In pasteurized nectar with 20% SB added, a reduction of half the value obtained with thermosonication was observed. In addition, in the treatment with thermosonication (20% pulp/achene and 80% amplitude), an increase of 20.97% in the content of anthocyanins was observed with respect to the control. This is due to the cavitation mechanism present in the thermosonication, which leads to a better extraction of the materials attached to the cells and to the fact of using a lower temperature than improves extraction (Sasikumar et al. 2019). However, in the pasteurized nectar a lower content was shown, unlike the thermosonicated ones, this may be directly related to anthocyanin degradation due to the heat treatment. In this sense, various studies have showed that anthocyanins present higher degradation rates at temperatures in the range of 65–90 °C (Wang and Xu 2007) for strawberry juice (Menelli et al. 2021) and khoonphal juice (Sasikumar et al. 2019).
Table 2.
Thermosonication effect on bioactive compounds and antioxidant capacity of strawberry nectar
| Treatment | Total phenolic content (mg GAE/g) | Anthocyanins (mg Cy3gl/g) | Ascorbic acid (mg EAA/g) | ABTS (mmol ET/g) | FRAP (mmol ET/g) | DPPH (mmol ET/g) |
|---|---|---|---|---|---|---|
| (0/70) | 9.777 ± 0.842abc | 54.920 ± 2.801bc | 154.571 ± 7.564 cd | 85.011 ± 7.590a | 35.869 ± 5.845ab | 7.604 ± 2.242ab |
| (10/70) | 15.318 ± 2.454de | 78.051 ± 6.682ef | 193.475 ± 10.332e | 116.660 ± 10.330b | 57.531 ± 1.980c | 18.726 ± 3.721c |
| (20/70) | 20.017 ± 2.824e | 70.135 ± 4.013de | 225.504 ± 10.209f | 194.387 ± 10.678c | 61.100 ± 5.643c | 44.449 ± 6.031f |
| (0/80) | 9.061 ± 0.383a | 41.932 ± 2.801ab | 165.819 ± 6.368d | 78.734 ± 1.143a | 35.026 ± 3.898ab | 8.434 ± 0.867ab |
| (10/80) | 13.700 ± 2.025abcd | 62.713 ± 5.491 cd | 182.362 ± 7.204e | 118.818 ± 12.680b | 49.518 ± 7.464bc | 22.689 ± 2.864 cd |
| (20/80) | 18.810 ± 0.742de | 111.325 ± 8.906 g | 249.854 ± 18.661 g | 191.010 ± 15.168c | 61.617 ± 6.606c | 38.034 ± 3.754ef |
| C (0/0) | 11.508 ± 1.870abc | 31.542 ± 3.578ª | 131.808 ± 2.186b | 78.328 ± 2.346a | 31.411 ± 3.599a | 2.873 ± 1.137a |
| C (10/0) | 15.042 ± 2.259bcde | 30.058 ± 2.945ª | 140.543 ± 11.570bc | 121.982 ± 8.654b | 55.932 ± 6.768c | 16.385 ± 3.135bc |
| C (20/0) | 20.981 ± 1.624e | 92.029 ± 2.801f | 190.566 ± 7.803e | 184.910 ± 13.613c | 58.998 ± 1.436c | 36.932 ± 4.284e |
| P (0/0) | 9.136 ± 0.915ab | 33.026 ± 2.570a | 32.793 ± 0.927a | 77.974 ± 7.577a | 33.599 ± 5.976a | 5.635 ± 1.282af |
| P (10/0) | 15.233 ± 2.653cde | 65.311 ± 1.700cde | 33.137 ± 1.564a | 142.776 ± 4.035b | 53.396 ± 1.286c | 29.129 ± 1.345de |
| P (20/0) | 19.068 ± 2.572cde | 55.662 ± 5.101bc | 29.193 ± 2.641a | 194.546 ± 12.640c | 58.022 ± 0.870c | 34.554 ± 3.896ef |
| SB | 51.668 ± 1.328f | 152.516 ± 5.101 h | 373.193 ± 11.705 h | 1147.332 ± 73.46d | 251.805 ± 30.84d | 125.312 ± 6.107 g |
| Achene (Ariza et al. 2016) | 382.87 ± 4.69 mg GAE/g | 6.97 ± 0.79 mg Pel-glc/g) | 34.7 mg/ 100 g de AA | 1236.015 ± 0.39 mmol ET/g | 642.627 ± 0.92 mmol ET/g | 595.575 ± 0.09 mmol ET/g |
The results are expressed as the mean ± standard deviation (n = 3). Different letters in the same column are significantly different (p < 0.05). Same letters do not show significant differences. (0/70): 0% SB (strawberry by-products) and 70% amplitude; (10/70): 10% SB and 70% amplitude; (20/70): 20% SB and 70% amplitude; (0/80): 0% SB and 80% amplitude; (10/80): 10% SB and 80% amplitude; (20/80): 20% SB and 80% amplitude; C (0/0): 0% SB; C (10/0): 10% SB; C (20/0): 20% SB; P (0/0): 0% SB; P (10/0): 10% SB; P (20/0): 20% SB. C CONTROL, P PASTEURIZED
Regarding Ascorbic acid, a 6.59-fold reduction was observed in pasteurized with 20% SB added compared to the control nectar (29.19 and 190.57 mg EAA/g). Ascorbic acid is known to be one of the least stable compounds with biological activity, because it is easily destroyed by heat, light, and oxygen exposure. High ascorbic acid contents were observed in nectar samples treated by thermosonication (80% Amplitude and 20% SB), presenting no statistical differences (p > 0.05) to the nectar without treatment. This could be attributed to the moderate temperature (50 °C) used and the removal of oxygen molecules trapped in the nectar by the thermosonication treatment (Anaya-Esparza et al. 2017). Besides, an increment in the ascorbic acid content was observed as the BP added percentage increased. Ariza et al. (2016) mention a higher content of ascorbic acid in strawberry pulp and achene (34.7 mg/100 g of AA) compared to the juice (6.7 mg/100 g of AA), which confirms the results obtained in Table 2, in which, the amount of ascorbic acid in the achene was higher (373.193 mg EAA/g). Further, it may be an effect of breaking structures within the achene containing ascorbic acid, which explains why at the same concentrations of pulp and achene, the concentration was higher in the nectars treated with thermosonication.
Effect of thermosonication on antioxidant capacity
In all three methodologies employed to measure the AOX, a gradual increment could be observed as the different percentages of SB increased, and the achene alone presented a higher content. Taking into account that 90% of the total weight of the fresh fruit is water, the achenes represent around 7.5% of the total dry weight of the fruit (Ariza et al. 2018). Ariza et al. (2016) used these percentages to calculate the relative contribution of achenes and pulp to the content and capacity of antioxidants in a whole fruit based on their estimation of the pulp finding 47, 55 and 19% for FRAP, DPPH and ABTS, respectively. Meanwhile, in the achene, the results were 53, 45 and 81% for FRAP, DPPH and ABTS, respectively. This confirms a higher content of these antioxidant compounds in the achene and was reflected in a gradual increase in the treatments as they were added in different percentages. An increase in ABTS, FRAP, and DPPH determinations could be observed in the applied thermosonication treatments with respect to the control, without significant differences (p > 0.05) (Table 2). In this sense, a similar increase has been reported in carrot juice (24 kHz, 120 µm, 50, 54, and 58 °C; 10 min) (Martínez-Flores et al. 2015). Ultrasound amplitude and temperature in the thermal processing did not affect the antioxidant capacity.
Effect of thermosonication on the inactivation of Escherichia coli
Table 3 shows the E. coli counts after thermosonication (80% of amplitude, 8 min, 50 °C outlet temperature), compared to thermal treatment and pasteurization in strawberry nectar with 20% of SB. Initial microbial load was 6.52 Log CFU/mL, after pasteurization treatment colonies were not detected in the nectar (aliquots of 270 mL). Besides, the thermal treatment (50 °C for 8 min) did not present significant differences (p > 0.05) compared to the control nectar, contrasting with thermosonication at 50 °C for 8 min, in which a 1 log CFU/mL of inactivation was achieved, pointing out the effect of applying a combination of ultrasound with temperature (50 °C). Nevertheless, a 1 log CFU/mL of reduction is not enough to fulfil the criteria imposed by the FDA. In this sense, Demir and Kılınç (2019) obtained a reduction of 2.98 log CFU/mL of E. coli K-12 (ATCC 25,253) in pumpkin juice treated at 60 °C for 9 min. It is important to stand out that after 5 days of storage at 6 °C the inactivationincreased in the thermosonicated nectar samples, reaching about 5.32 Log. To evaluate the effect of the food matrix, Inactivation was determined using PBS (Table 3), showing that reduction was 0.78 log CFU/mL. Comparing nectar and PBS results at day 5, it can be observed that E. coli inactivation was firstly influenced by the food matrix (pH, acidity, food components, among others), followed by termosonication effect, and finally by temperature. Figure 1 shows the inactivation kinetic of E. coli by thermosonication treatment (20/80) for 20 min at 50° C. The initial bacterial load was 6.69 log CFU/mL and after 20 min a inactivation of 4.15 log CFU/mL was obtained. To achieve a 5 log CFU/mL reduction as stated by the FDA, a longer treatment than 8 min is necessary. Martínez-Flores et al. (2015) stated that the objective set by the FDA can be achieved by extending the exposure time from 20 to 40 min. Another option to increase inactivation is applying higher temperatures as it provides a synergism between sonication by increasing microbial sensitivity to heat and low pH due to cavitation, and changes in the outer membrane of cell structures (Wordon et al. 2012). However, increasing the temperature can bring about a decrease in bioactive compounds since they are mostly thermolabile (Alves et al. 2020). Sasikumar et al. (2019) in khoonpahl juice achieved an inactivation of 5.18 Log CFU/mL in E. coli (MCC 452) and 5.93 in S. cerevisiae (MCC 1036) with a treatment of 50 °C for 21 min, 100% amplitude, and 700 W. Likewise, the authors mention in their study that thermosonication at 50 °C for 21 min did not affect the juice quality.
Table 3.
Escherichia coli counts in strawberry nectar and phosphate buffer after thermosonication, thermal and pasteurized treatments stored at 6 °C for 5 days
| Treatment | Counts (Log CFU/mL) | |||
|---|---|---|---|---|
| Nectar (20% SB) | PBS (pH 7.2) | |||
| 0 day | 5 day | 0 day | 5 day | |
| Control | 6.52 ± 0.007a | 3.13 ± 0.021a | 6.51 ± 0.016a | 5.95 ± 0.012a |
| Thermosonicated (80% amplitude, 50 °C, 8 min) | 5.63 ± 0.014b | 1.22 ± 0.042b | 5.85 ± 0.042b | 5.73 ± 0.053a |
| Thermal (50 °C, 8 min) | 5.91 ± 0.007a | 2.47 ± 0.014a | 5.77 ± 0.049c | 5.87 ± 0.084a |
| Pasteurized (63 °C, 30 min) | ND | ND | ND | ND |
Values are average of three determinations from two different experiments (n = 6) ± standard deviation (SD). Different letters in the same column indicate significant differences between treatments (p < 0.05). Same letters do not show significant differences. ND: not detected
Fig. 1.
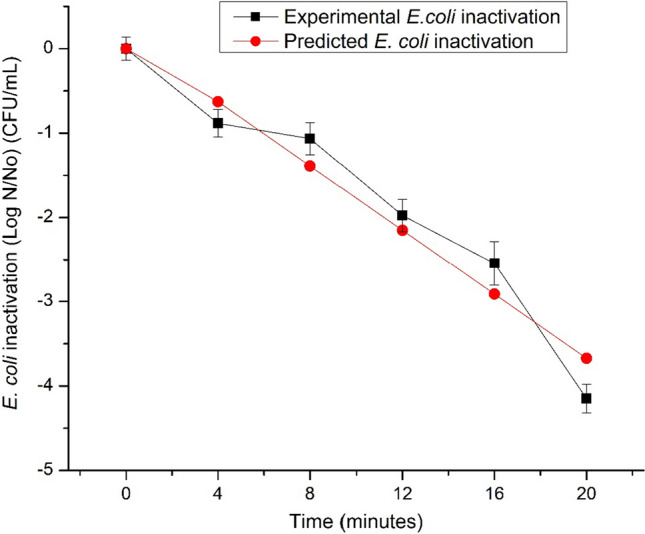
Experimental and predicted Escherichia coli inactivation by thermosonication at 80% amplitude and 50 °C. Values are the mean of three determinations from two different experiments (n = 6) ± standard deviation (SD)
Experimental data was used to fit a lineal model, in which, inactivation = − 0.133 + 0.190*Time (min). (R2 = 0.945). The experimental and predicted inactivation are showed in Fig. 1. According to the prediction model, up to minute 28, there would be a reduction of 5.1951 Log CFU/mL, while in the experimental curve, at minute 20, a 5.1951 Log CFU/mL reduction was achieved. However, these results may be influenced by the treated matrix since real food systems may influence the thermal resistance of microorganisms (Knorr et al. 2011), something that the mathematical model does not consider, as reported by Avila-Sosa et al. (2010).
Conclusions
The application of thermosonication (80% amplitude, 8 min) for a strawberry nectar added with SB (20%) leads to compliance with the regulation of physicochemical parameters, in addition to reducing enzymatic activity. On the other hand, the SB addition and the thermosonication treatment influenced the bioactive compound increment in the nectar. The 8-min thermosonication treatment was not enough to comply with the 5-log reduction required by the FDA. However, during storage nectar formulation helped to reach a significant inactivation increase. In this sense, the formulation effect shows the way for following studies to evaluate strawberry seed microbial effects. The proposed inactivation kinetics predict a 20-min treatment to reach the target of 5 log CFU/mL. For the above, it is important to consider a balance between treatment factors, the food matrix and the safety requirements. Higher temperatures or longer thermosonication treatments are recommended to achieve higher bacterial reduction, but the effect on the bioactive content must be evaluated.
Acknowledgements
Author Dalia M. Sotelo Lara was supported by a fellowship from the Consejo Nacional de Ciencia y Tecnología (CONACYT, Grant No. 760939). The authors have no conflict of interest to declare.
Abbreviations
- TSS
Total soluble solids
- PPO
Polyphenol oxidase
- PME
Pectin methylesterase
- TSP
Total soluble phenols
- AA
Ascorbic acid
- AOX
Antioxidant Capacity
- CFU
Colony forming unit
- FDA
Food and drug administration
- SB
Strawberry by-product
- SN
Strawberry nectar
- EP
Effective power
- UPME
Unit of pectin methylesterase
- DCPI
2,6-Dichlorophenolindophenol sodium salt hydrate
- EAA
Equivalents of ascorbic acid
- GA
Galic acid
- EGA
Equivalent of galic acid
- FRAP
Ferric reducing antioxidant power assay
- ABTS
2,2'-Azino-bis 3-ethylbenzothiazoline-6-sulfonic acid
- DPPH
Free radical 2,2-diphenyl-1-picrylhydrazil scavening method
- ATCC
American type culture collection
Author contributions
DMS-L: Conceptualization, Methodology, Writing-Original draft preparation. GGA-E: Conceptualization and Writing-Reviewing and Editing. VMZ-G: Supervision of the experiments. G-M: Supervision and investigation. V-E: Funding acquisition and Writing-Reviewing and Editing.
Funding
The author Dalia M. Sotelo-Lara thanks the Consejo Nacional de Ciencia y Tecnología Nacional (CONACYT, Mexico) for Grant No. 760939.
Availability of data and material
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Code availability
Not applicable.
Declarations
Conflict of interest
The authors have no conflicts of interest to declare.
Consent to participate
All authors have read and approved the MS. All co-authors are aware of its submission to JFST including the concerned authorities.
Consent for publication
Not applicable.
Ethics approval
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Aaby K, Wrolstad RE, Ekeberg D, Skrede G. Polyphenol composition and antioxidant activity in strawberry purees; impact of achene level and storage. J Agric Food Chem. 2007;55(13):5156–5166. doi: 10.1021/jf070467u. [DOI] [PubMed] [Google Scholar]
- Alighourchi H, Barzegar M, Sahari MA, Abbasi S. The effects of sonication and gamma irradiation on the inactivation of escherichia coli and saccharomyces cerevisiae in pomegranate juice. Iran J Microbiol. 2014;6(1):51–58. [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Parrilla E, De La Rosa LA, Legarreta P, Saenz L, Rodrigo-García J, González-Aguilar GA. Daily consumption of apple, pear and orange juice differently affects plasma lipids and antioxidant capacity of smoking and non-smoking adults. Int J Food Sci Nutr. 2010;61(4):369–380. doi: 10.3109/09637480903514041. [DOI] [PubMed] [Google Scholar]
- Alvarez-Parrilla E, De La Rosa LA, Amarowicz R, Shahidi F. Antioxidant activity of fresh and processed jalapeño and serrano peppers. J Agric Food Chem. 2011;59(1):163–173. doi: 10.1021/jf103434u. [DOI] [PubMed] [Google Scholar]
- Alves LDL, dos Santos RL, Bayer BL, Devens ALM, Cichoski AJ, Mendonça CRB. Thermosonication of tangerine juice: effects on quality characteristics, bioactive compounds, and antioxidant activity. J Food Process Preserv. 2020;44(12):1–9. doi: 10.1111/jfpp.14914. [DOI] [Google Scholar]
- Amador-Espejo GG, Chávez-Ocegueda J, Cruz-Cansino N, Suárez-Jacobo A, Gutiérrez-Martínez P, Valencia-Flores D, Velázquez Estrada R. Thermosonication parameter effects on physicochemical changes, microbial and enzymatic inactivation of fruit smoothie. J Food Sci Technol. 2020;57(5):1680–1688. doi: 10.1007/s13197-019-04201-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- do Amaral Souza FDC, Moura LGS, de Oliveira-Bezerra K, Aguiar JPL, Mar JM, Sanches EA, Campelo PH. Thermosonication applied on camu–camu nectars processing: effect on bioactive compounds and quality parameters. Food Bioprod Process. 2019;116:212–218. doi: 10.1016/j.fbp.2019.06.003. [DOI] [Google Scholar]
- Anaya-Esparza LM, Velázquez-Estrada RM, Roig AX, García-Galindo HS, Sayago-Ayerdi SG, Montalvo-González E. Thermosonication: an alternative processing for fruit and vegetable juices. Trends Food Sci Technol. 2017;61:26–37. doi: 10.1016/j.tifs.2016.11.020. [DOI] [Google Scholar]
- AOAC International (2005) Official methods of analysis of AOAC International. AOAC International
- Ariza MT, Reboredo-Rodríguez P, Mazzoni L, Forbes-Hernández TY, Giampieri F, Afrin S, Mezzetti B. Strawberry achenes are an important source of bioactive compounds for human health. Int J Mol Sci. 2016;17(7):1–14. doi: 10.3390/ijms17071103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariza MT, Reboredo-Rodríguez P, Cervantes L, Soria C, Martínez-Ferri E, González-Barreiro C, Simal-Gándara J. Bioaccessibility and potential bioavailability of phenolic compounds from achenes as a new target for strawberry breeding programs. Food Chem. 2018;248:155–165. doi: 10.1016/j.foodchem.2017.11.105. [DOI] [PubMed] [Google Scholar]
- Avila-Sosa R, Gastélum GG, López-Malo A, Palou E. Modelización de la inactivación termosónica de staphylococcus aureus, un enfoque multifactorial. CYTA J Food. 2010;8(3):177–183. doi: 10.1080/19476330903335251. [DOI] [Google Scholar]
- Cassani L, Tomadoni B, del Rosario Moreira M. Green ultrasound-assisted processing for extending the shelf-life of prebiotic-rich strawberry juices. J Sci Food Agric. 2020;100(15):5518–5526. doi: 10.1002/jsfa.10604. [DOI] [PubMed] [Google Scholar]
- CODEX STAN (2005) “Normas Generales Del Codex Para Zumos (Jugos) y Nectares de Frutas.” pp 1–17
- Demir H, Kılınç A. Effect of batch and continuous thermosonication on the microbial and physicochemical quality of pumpkin juice. J Food Sci Technol. 2019;56(11):5036–5045. doi: 10.1007/s13197-019-03976-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draye M, Van Cutsem P. Pectin methylesterases induce an abrupt increase of acidic pectin during strawberry fruit ripening. J Plant Physiol. 2008;165(11):1152–1160. doi: 10.1016/j.jplph.2007.10.006. [DOI] [PubMed] [Google Scholar]
- Dürüst N, Sümengen D, Dürüst Y. Ascorbic acid and element contents of foods of Trabzon (Turkey) J Agric Food Chem. 1997;45(6):2085–2087. doi: 10.1021/jf9606159. [DOI] [Google Scholar]
- Es-Safi NE, Cheynier V, Moutounet M. Effect of copper on oxidation of (+)-catechin in a model solution system. Int J Food Sci Technol. 2003;38(2):153–163. doi: 10.1046/j.1365-2621.2003.00656.x. [DOI] [Google Scholar]
- Ferrario M, Alzamora SM, Guerrero S. Study of the inactivation of spoilage microorganisms in apple juice by pulsed light and ultrasound. Food Microbiol. 2015;46:635–642. doi: 10.1016/j.fm.2014.06.017. [DOI] [PubMed] [Google Scholar]
- Food and Drug Administration (FDA) (2001) Hazard analysis and critical control point (HACCP); procedures for the safe and sanitary processing and importing of juices: final rule, Fed. Regist., vol 66, pp 6137–6202, Washington, D.C., USA
- Guerrero-Beltrán JA, Barbosa-Cánovas GV. Inactivation of saccharomyces cerevisiae and polyphenoloxidase in mango nectar treated with UV light. J Food Prot. 2006;69(2):362–368. doi: 10.4315/0362-028X-69.2.362. [DOI] [PubMed] [Google Scholar]
- Jiménez-Vieyra ME, Zambrano-Zaragoza ML. Cuantificación de cobre en polifenoloxidasa de frutas tropicales por espectrofotometría de absorción atómica. Inf Tecnol. 2011;22(2):15–22. doi: 10.4067/S0718-07642011000200003. [DOI] [Google Scholar]
- Knorr D, Froehling A, Jaeger H, Reineke K, Schlueter O, Schoessler K. Emerging technologies in food processing. Annu Rev Food Sci Technol. 2011;2:203–235. doi: 10.1146/annurev.food.102308.124129. [DOI] [PubMed] [Google Scholar]
- Lee J, Wrolstad RE. Extraction of anthocyanins and polyphenolics from blueberry-processing waste. J Food Sci. 2004;69(7):564–573. doi: 10.1111/j.1365-2621.2004.tb13651.x. [DOI] [Google Scholar]
- Martínez-Flores HE, Garnica-Romo MG, Bermúdez-Aguirre D, Pokhrel PR, Barbosa-Cánovas GV. Physico-chemical parameters, bioactive compounds and microbial quality of thermo-sonicated carrot juice during storage. Food Chem. 2015;172:650–656. doi: 10.1111/j.1365-2621.2004.tb13651.x. [DOI] [PubMed] [Google Scholar]
- Menelli GS, Fracalossi KL, Lepaus BM, De São José JFB. Effects of high-intensity ultrasonic bath on the quality of strawberry juice. CYTA J Food. 2021;19(1):501–510. doi: 10.1080/19476337.2021.1918768. [DOI] [Google Scholar]
- Navarro C, Ariza L, Gómez J, Martínez E, Cervantes L, Medina J (2018) Evaluación de variedades de fresa : resultados obtenidos en cultivo convencional con suelo. Red Andaluza de Experimentación Agraria: pp 1–36
- Nayak PK, Chandrasekar CM, Kesavan RK. Effect of thermosonication on the quality attributes of star fruit juice. J Food Process Eng. 2018;41(7):1–10. doi: 10.1111/jfpe.12857. [DOI] [Google Scholar]
- Odriozola-Serrano I, Soliva-Fortuny R, Martín-Belloso O. Phenolic acids, flavonoids, Vitamin C and antioxidant capacity of strawberry juices processed by high-intensity pulsed electric fields or heat treatments. Eur Food Res Technol. 2008;228(2):239–248. doi: 10.1007/s00217-008-0928-5. [DOI] [Google Scholar]
- Oladunjoye AO, Adeboyejo FO, Okekunbi TA, Aderibigbe OR. Effect of thermosonication on quality attributes of hog plum (Spondias Mombin L.) Juice. Ultrason Sonochem. 2021;70:105316. doi: 10.1016/j.ultsonch.2020.105316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prior RL, Wu X, Schaich K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J Agric Food Chem. 2005;53(10):4290–4302. doi: 10.1021/jf0502698. [DOI] [PubMed] [Google Scholar]
- Raviyan P, Zhang Z, Feng H. Ultrasonication for tomato pectinmethylesterase inactivation: effect of cavitation intensity and temperature on inactivation. J Food Eng. 2005;70(2):189–196. doi: 10.1016/j.jfoodeng.2004.09.028. [DOI] [Google Scholar]
- Sasikumar R, Pradhan D, Deka SC. Effects of thermosonication process on inactivation of Escherichia Coli and Saccharomyces Cerevisiae and Its survival kinetics modeling in khoonphal (Haematocarpus Validus) juice to extend its shelf life. J Food Process Preserv. 2019;43(11):1–11. doi: 10.1111/jfpp.14220. [DOI] [Google Scholar]
- Torkamani AE, Juliano P, Ajlouni S, Singh TK. Impact of ultrasound treatment on lipid oxidation of cheddar cheese whey. Ultrason Sonochem. 2014;21(3):951–957. doi: 10.1016/j.ultsonch.2013.11.021. [DOI] [PubMed] [Google Scholar]
- Vega Contreras NA, Mercado SAS, Bautista LT, Muñoz G. Evaluación Del Efecto Inhibidor de La Enzima Polifenol Oxidasa En Una Salsa de Aguacate (Persea Americana) Entre Ciencia e Ingeniería. 2020;14(27):58–62. doi: 10.31908/19098367.0007. [DOI] [Google Scholar]
- Wang J, Wang J, Ye J, Vanga SK, Raghavan V. Influence of high-intensity ultrasound on bioactive compounds of strawberry juice: Profiles of ascorbic acid, phenolics, antioxidant activity and microstructure. Food Control. 2019;96:128–136. doi: 10.1016/j.foodcont.2018.09.007. [DOI] [Google Scholar]
- Wang WD, Xu SY. Degradation kinetics of anthocyanins in blackberry juice and concentrate. J Food Eng. 2007;82(3):271–275. doi: 10.1016/j.jfoodeng.2007.01.018. [DOI] [Google Scholar]
- Wordon BA, Mortimer B, McMaster LD. Comparative REAL-TIME ANALYSIs of Saccharomyces Cerevisiae cell viability, injury and death induced by ultrasound (20kHz) and heat for the application of hurdle technology. Food Res Int. 2012;47(2):134–139. doi: 10.1016/j.foodres.2011.04.038. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Not applicable.


