Abstract
Bitter gourd extract (BGE) is rich in antioxidants and anti-diabetic components that promote good human health; however, its bitter taste makes it challenging to use in food. In this study, the effect of carboxymethyl cellulose and β-cyclodextrin (β-CD) on the bitterness and properties of BGE were investigated. The bitterness intensity was evaluated by the trained sensory panel, and the physicochemical properties were also determined, including viscosity, total saponin, polyphenol content, antioxidant capacity, and α-amylase inhibition activity. It was found that the bitterness of BGE with 0.75%, w/v β-cyclodextrin decreased significantly by more than 90%. Additionally, FTIR, 1 H-NMR, and thermogravimetric analysis of BGE supplemented with β-CD confirmed the formation of a complex between β-CD and components of BGE. The findings of the current study also reveal that debittering agents did not inhibit the bioactivities of BGE.
Keywords: Momordica charantia, Taste masking, Reducing bitterness, TGA, FTIR, NMR
Introduction
Diabetes is a severe health problem that has become alarmingly prevalent. In 2021, it was projected that there would be 537 million diabetic patients, and this figure is estimated to increase by more than 100 million by 2030 and reach 783 million by 2045. Moreover, over 6.7 million people between the age of 20–79 were reported to die due to diabetes related issues in 2021 (International Diabetes Federation 2021). Although there are a variety of synthetic drugs combating diabetes, almost all such drugs have multiple side effects, such as dyspepsia, diarrhea, and heart failure, etc. In addition, the access to modern drugs for all patients is limited because around 80% of diabetic people have low-medium income. Patients in developing countries usually prefer traditional medicines and complementary therapies due to better cultural acceptability and fewer adverse effects than modern remedies (Tran et al. 2020).
Bitter gourd (Momordica Charantia), also called bitter melon, is a traditional medicinal plant for dealing with diabetes. This tropical plant grows widely in India, China, Southeast Asia, Africa, and Latin America. This vegetable contains a variety of vitamins, carbohydrates, minerals, and dietary fibers, and is also rich in bioactive compounds such as phenolics, saponins, alkaloids, and flavonoids (Tan et al. 2014, 2016). Among these compounds, saponin is one of the critical components having physiological activities such as lowering blood glucose and improving insulin resistance, which are responsible for the antidiabetic effect of bitter gourd (Klomann et al. 2010). Bitter gourd extract (BGE) has also demonstrated hypoglycemic activity in both in vitro and in vivo studies using animal models. The antidiabetic ability of bitter gourd is comparable to synthetic drugs, namely metformin and glibenclamide (Deshaware et al. 2018). Despite having many health benefits, especially the hypoglycemic effect, bitter gourd is still underused due to its strong bitterness, which is usually associated with low acceptance by consumers. Bitter gourd’s bitter taste is linked to a presence of high concentration of triterpene glycosides known as saponins (Deng et al. 2022), especially momordicosides K and L (Deshaware et al. 2018). These compounds are also promising ingredients revealing beneficial effects on health in preventing diabetes (Deshaware et al. 2018; Tan et al. 2016; Tran et al. 2020). Therefore, it is essential to find a solution that can both reduce the bitterness of BGE and retain its bioactive compounds.
In the food industry, adding either carboxymethyl cellulose (CMC) or β-Cyclodextrin (β-CD) is an effective method for masking bitterness, based on their ability to prevent contact between the molecules causing bitterness and the receptors during the in-mouth processing. CMC, a cellulose derivative, is used as a thickening, stabilizing, and suspending agent in the food industry. It can also act as a physical barrier that restricts the interaction between bitterants and taste buds by increasing viscosity (Manninen et al. 2021), which can limit the spread of bitter compounds from the saliva to the taste receptors and reduce the perceived bitterness (Sun-Waterhouse and Wadhwa 2013). Numerous studies confirmed the effectiveness of CMC in masking the bitter taste. Manninen et al. (2021) also found that CMC can reduce quinine hydrochloride’s bitterness better than nanofibrillar cellulose. Compared to other food gums, including guar, arabic, and xanthan, CMC is the most effective agent in suppressing the astringency of polyphenol extracts obtained from walnut, black chokeberry, and green tea (Troszyńska et al. 2010). Besides CMC, application of β-CD to reduce bitterness has also attracted many researchers. β-CD is a cyclic oligomer formed from 7 α-D-glucopyranoside units. β-CD is an amphipathic molecule with a hydrophilic outer wall and hydrophobic inside cavity, which allows it to form inclusion complexes with the hydrophobic molecules called guests or the hydrophobic parts of some guests in the aqueous environment (Deshaware et al. 2018). Bitter compounds are usually hydrophobic, which can lead to the formation of the inclusion complex with β-CD. This complex can prevent the interaction between bitterants and receptors, decreasing perceived bitter intensity (Binello et al. 2004). In the food industry, β-CD was used to mask unfavorable tastes, such as bitterness. Some studies also used β-CD to lower the bitterness of products or extracts enriched with bio-compounds, involving bitter gourd juice (Deshaware et al. 2018), coffee, and catechin (Ho et al. 2019). Although many studies have evaluated the bitterness modifying ability of either CMC or β-CD for different extracts/substrates; however, no study has been carried out to compare their bitterness reducing ability for the BGE. Therefore, it is crucial to investigate and compare the impact of CMC and β-CD for reducing the bitterness of BGE as the ability of debittering agent to reduce bitterness depends on several factors, including type of bitter extract/substrate used and the type and concentration of bitterants present in it (Tamamoto et al. 2010; Gaudette and Pickering 2012).
Therefore, the objectives of the present study were to (1) identify the effective debittering agent and its concentration for reducing the bitterness of bitter gourd and (2) investigate the effects of treatments on the physicochemical properties of BGE. In this study, bitter gourd extract (BGE) separately supplemented with various concentrations of either CMC or β-CD were evaluated for bitterness intensity and alterations in physicochemical properties. The BGE supplemented with the debittering agent (CMC or β-CD) which was efficient in reducing bitterness and retaining the bio-activities, was assessed for physical characteristics. Since the extract from bitter gourd possesses health-promoting ability, especially the anti-diabetic effect, a step towards its debittering without affecting its bioactive properties may further contribute to the formation of functional food and beverages.
Materials and methods
Chemicals
α-Amylase from porcine pancreas, gallic acid, eacin, folin-ciocalteu’s phenol reagent, 2,2-diphenyl-1-picrylhydrazyl (DPPH), were procured from Sigma-Aldrich. β-CD and CMC were purchased from Tokyo Chemical Industry (Tokyo, Japan) and other chemicals were obtained from Showa (Japan). All the chemicals used in the study were of analytical grade.
Preparation of the bitter gourd extract
Fresh bitter gourd fruits used for the current study were collected from the local market in Dong Nai Province, Vietnam. These fruits were wild, dark green in color, and between 8 and 10 cm in length. The aqueous extract was prepared according to Tan et al. (2014) with modifications. Briefly, the fruits were washed with tap water and cut into circular shape of 1 to 2 mm thickness, and then dried using lyophilizer. The lyophilized slices were ground by blender and passed through a sieve (35 mesh), followed by sealing and storage at − 20 °C until use. Regarding extraction, the ground powder was extracted with water at a ratio of 20:1 (mL g− 1) at 40 °C and stirred for 15 min at 600 rpm (Thermo Scientific Cimarec, USA). The extract was filtered through cheesecloth and centrifuged at 4350×g for 10 min (H2100, Hanon, China) before passing through Whatman No.1 filter paper. The extract was used within 3 h for the sensory test or kept at − 20 °C until further analyses.
Addition of bitterness masking agents
Different concentrations of either CMC (0.2%, 0.4%, 0.6%, w/v) or β-CD (0.25%, 0.5%, 0.75%, 1%, w/v) were mixed with prepared extract. The resulting mixture was stirred at 600 rpm (Thermo Scientific Cimarec, USA) for 60 min at room temperature (25 ± 3 °C) to completely dissolve β-CD and CMC (Deshaware et al. 2018).
Sensory evaluation of bitterness intensity
A trained panel (n = 10, 6 males and 4 females) was used to evaluate the bitterness of samples. The panelists were students aged between 18 and 38 in Ho Chi Minh City, Vietnam, selected and trained according to ISO 8586 − 2014. In this study, the panel was only trained to evaluate bitter taste. Regarding the procedure for evaluating bitterness, each panelist was served 8 samples, including the extract without the bitter masking agent (the control sample) and the extract with the bitter masking agent (7 samples). 15 mL of each sample was contained in a 30 mL plastic cup and labeled with a 3-digit code number. Samples were served at room temperature at about 25 °C ± 3. On a 10 cm unstructured scale labeled “weak” and “strong” at the left and right extremities, the participants were asked to taste each sample and rate the strength of the bitterness. Distilled water and unsalted cracker were used for mouth rinsing between the samples. Sensory evaluation was conducted in duplicate on two different sessions and performed in isolated booths under red lighting. Informed consent was collected from each subject before their participation (Ares et al. 2009).
α-Amylase inhibition capacity (AIC)
-Amylase inhibition assay was conducted according to Lordan et al. (2013) with minor modifications. Concisely, 500 µL of test samples and 100 µL -amylase (7.5 U/mL) in 0.02 M phosphate buffer, (containing 6 mM NaCl, pH 6.9, PBS) were mixed well and incubated at 37 °C for 15 min. Next, 100 µL of starch solution (1%, w/v) was added to start the reaction and further incubated at 37 °C for 10 min. The reaction was stopped by adding 200 µL of DNS reagent, and these tubes were incubated at 100 °C for 5 min, followed by cooling the samples to room temperature. In the control, 500 µL of water was used instead of the sample. In the blank, the 100 µL -amylase was added after the addition of the DNS reagent. The resulting solution was diluted 5-fold by adding distilled water before absorbance was measured at 540 nm. The inhibitory activity (%) was estimated using Eq. 1:
| 1 |
Total saponin content (TSC)
TSC of extracts was determined according to Tan et al. (2014) with slight modifications. Extract (0.1 mL), 8% w/v vanillin solution (0.1 mL), and 72% v/v sulphuric acid (1 mL) were mixed well and incubated at 60 °C for 15 min. The mixture was then cooled using ice to rooms temperature, followed by measuring the absorption of the mixture at 560 nm using a spectrophotometer against blank (UV-1280, Shimadzu, Japan), and results are expressed as µg aescin equivalents (AE) per mL of extract.
Total phenolic content (TPC)
The TPC of the extract was measured using the method of Ainsworth and Gillespie (2007). Briefly, a mixture of 200 µL of folin-ciocalteu’s phenol reagent (10%, v/v) and 100 µL of the sample was thoroughly mixed. The solution was then incubated for 2 min at room temperature and subsequently mixed with 800 µL of a 7.5% (w/v) Na2CO3 solution. The tubes containing the above ingredients were covered with aluminum foil and incubated at room temperature (25 ± 2 °C) for 2 h. The absorbance of the solution was recorded at 765 nm. Results are reported as µg gallic acid equivalents (GAE) per mL of sample.
Total antioxidant capacity (TAC) s
In this study, the DPPH assay described by Deshaware et al. (2018) with some modifications was used to evaluate the TAC of the extract. Briefly, 30 µL of the extract was mixed with 470 µL of DPPH (120 µM) and incubated in the dark at room temperature (24 ± 2 °C) for 30 min. Following this, absorbance was recorded at 517 nm using a spectrophotometer (UV-1280, Shimadzu, Japan). Equation 2 was used to calculate the scavenging activity.
| 2 |
Viscosity measurement
The viscosity of the extracts was measured using a viscometer (VISCO™-895; ATAGO, Japan) equipped with an ultra-low adapter at a rotational speed of 200 rpm at 25 °C. Around 10 mL of sample was used for analysis.
Physical characterization of the complex between bitterness masking agent and bitter gourd extract
FTIR, 1 H-NMR, and TGA/DTG analysis were used to evaluate the change in physical properties of BGE after the addition of debittering agents. BGE mixed with the debittering agent which showed the maximum reduction in bitterness and preserved bio-activities was analyzed using these techniques. BGE and either of the debittering agents alone were used as control and also subjected to these analyses.
Regarding FTIR analysis, spectra of BGE, β-CD, and BGE with β-CD were obtained using a Thermo Scientific Nicole iS5 spectrometer with an iD5 ATR accessory over the range of 600–4000 cm− 1 with 64 scans and a resolution of 4 cm− 1. For thermogravimetric analysis (TGA), a thermogravimetric analyzer (Mettler-Toledo, 2-HT) was used to measure the thermal characteristics of the samples. The TGA measurements were carried out in a nitrogen environment. The samples were heated at a rate of 20 °C per min from 20 to 600 °C. Regarding 1 H-NMR analysis, NMR spectroscopy (Bruker, Germany) was used for the measurements, set to 600 MHz. The ppm units were used to measure the chemical shift of samples dissolved in D2O.
Statistical analyses
All the experiments in the current study were conducted in triplicates. A significant difference between results was determined from ANOVA analysis using R software, followed by Tukey test. A statistically significant difference was indicated as having a probability value of P < 0.05.
Results and discussion
Performance assessment of the trained sensory panel
Before the sensory data about the intensity of bitterness was used for comparing the bitterness of different extracts, the reliability of data was checked through the performance assessment of the panel. In the quantitative descriptive approach, reliable data were obtained from the panel satisfying three criteria, including sample discrimination ability, repeatability, and agreement of panelists (Lê and Worch 2018). The result of the performance assessment using ANOVA is shown in Table 1. As can be seen from the Table 1, the product effect was significant (P < 0.05), demonstrating that the panel has the ability to discriminate between samples. Additionally, insignificant interaction between the product and panelists was observed (P > 0.05), corresponding to achieving consensus among the panelists. Regarding repeatability, because the product-session interaction is insignificant (P > 0.05), it illustrates that the bitterness of samples was perceived similarly from one session to another, revealing the panel’s repeatability (Lê and Worch 2018). These results indicated that the panel in this study meets the identified requirements, and the reliable data obtained from this panel are suitable for further quantitative analysis.
Table 1.
Performance assessment of the trained sensory panel
| Source of variation | Df | F value | P value |
|---|---|---|---|
| Product | 7 | 324.355 | < 2e− 1* |
| Panelist | 9 | 0.669 | 0.734 |
| Session | 1 | 0.042 | 0.839 |
| Product* Panelist | 63 | 1.418 | 0.085 |
| Product* Session | 7 | 0.551 | 0.792 |
| Panelist* Session | 9 | 0.498 | 0.870 |
∗Statistical significance at P < 0.05
Panelist*Session = replication by panelist interaction; Product* Session = replication by sample interaction; Product* Panelist = panelist by sample interaction
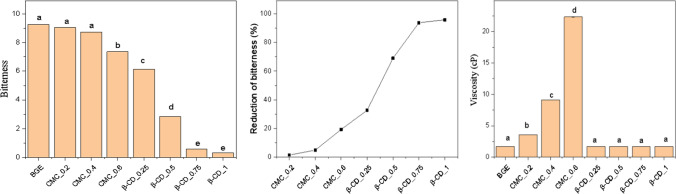
Effect of debittering agents on bitterness and viscosity of BGE
The impact of CMC and β-CD on the bitterness (Fig. 1A and B) and viscosity (Fig. 1C) of BGE was evaluated. The results reveal that the addition of CMC led to a considerable increase in the viscosity of samples, which was not observed in the case of β-CD. Moreover, both agents showed a remarkable ability to mask the bitterness, although this ability depended on the concentration of the bitterness inhibitor. At concentrations of 0.2% and 0.4% CMC could not mask the bitter taste, but adding 0.6% CMC resulted in lowering the bitterness significantly by 19%. CMC, a hydrocolloid compound, causes an increase in the viscosity of the extract and therefore, serves as a physical barrier that prevents the contact between the bitter compounds and taste receptors, and ultimately lowers the bitterness intensity (Manninen et al. 2021). Insignificant change in bitterness of samples mixed with either 0.2% or 0.4% CMC compared to the control (BGE) indicated that an appropriate amount of CMC is required to retard the release of bitterants during in-mouth processing. This is a considerable drawback of using CMC to inhibit bitter compounds because it cannot be applied to low-viscosity products.
Fig. 1.
Effect of addition of the bitterness masking agents on bitterness intensity (A), reduction of bitterness (B) and viscosity (C). With each graph, the same letters in columns were not significantly different (P ≥ 0.05)
Although the bitterness suppression capability of CMC was confirmed in this research, its power is negligible compared to the ability of β-CD. With the addition of only 0.25% β-CD, the bitterness was reduced by about 33%. The lowest bitter intensity was found in samples mixed with 0.75% or 1% β-CD as at these concentrations, the bitterness was reduced by more than 90%, indicating that almost all bitterants were enclosed by β-CD. Reddy et al. (2020) noted that the β-CD structure is shaped like a toroid with a hydrophilic outside wall and a hydrophobic interior component known as a “host” or cavity. This property makes β-CD receive hydrophobic molecules called “guests” to form a complex. In the bitter gourd extract, the bitter substances (mainly momordicoside K and L) are less polar glycosides (Okabe et al. 1982), especially all the carbons related to the C=C bond of momordicoside K and L are the hydrophobic triterpenoids (Deshaware et al. 2018). This hydrophobic region may allow momordicoside compounds to form complexes with β-CD and reduce their contact with taste receptors, thus lowering perceived bitterness. With the larger concentration of β-CD, the host molecules increased, and more guest compounds were enclosed, resulting in a higher ability to mask the bitterness. However, the results in this study revealed that the perceived bitterness of the samples containing 0.75% and 1% β-CDs was not different (P < 0.05). The reason may be that the number of β-CD molecules at 0.75% concentration was enough to trap the bitter molecules, and the addition of more amount of the bitterness inhibitor did not result in a higher debittering effect. The effect of β-CD in reducing bitter taste was also confirmed by some previous studies, such as using β-CD to mask the bitterness of catechins in different food matrices (Ho et al. 2019), to reduce the bitterness of ginseng solution, naringin, and limonin, and to modify the sensory properties of lingonberry juice (Kelanne et al. 2019).
Impact of bitterness inhibitors on total saponin content
Saponins are compounds containing one or more sugar chains (glycosides) and a non-sugar aglycone (steroid/triterpenoid). This ingredient can lower the glucose level in the blood by stimulating insulin secretion (Tran et al. 2020), enhancing glucose uptake, and inhibiting disaccharidase and pancreatic lipase activity (Oishi et al. 2007). In this study, the total saponin content of BGE was found to be 3181.9 µgAE mL− 1, as shown in Table 2. The presence of CMC and β-CD did not impact this content. Although the addition of CMC and β-CD reduced the bitter taste, which was caused mainly by the two kinds of saponin, including momordicosides K and L (Deshaware et al. 2018), the total saponin content was not affected significantly. This demonstrated that both β-CD and CMC masked bitter taste by limiting contact between bitter taste receptors and bitter compounds but did not destroy these bitter ingredients.
Table 2.
Effect of addition of bitterness masking agents on total phenolic content (TPC), saponin content (TSC), α-amylase inhibition activity (%) and total antioxidant capacity (TAC)
| Sample | TPC (µgGAE mL− 1) |
TSC (µgAE mL− 1) |
α-amylase inhibition (%) | TAC (%) | |
|---|---|---|---|---|---|
| Control (BGE) | 222.9a ± 4.8 | 3181.9a ± 154.5 | 73.5a ± 5.9 | 19.4a ± 0.6 | |
| BGE with CMC (w/v) | 0.2% | 219.0a ± 7.3 | 3266.3a ± 121.6 | 73.2a ± 5.9 | 19.2a ± 0.9 |
| 0.4% | 222.9a ± 4.3 | 3332.3a ± 64.4 | 72.6a ± 4.3 | 20.3a ± 0.4 | |
| 0.6% | 219.4a ± 3.1 | 3273.6a ± 143.8 | 75.5a ± 6.3 | 19.1a ± 1.4 | |
| BGE with β-CD (w/v) | 0.25% | 208.2a ± 2.8 | 3178.6a ± 159.8 | 72.5a ± 5.6 | 21a ± 1.2 |
| 0.5% | 221.0a ± 4.4 | 3272.9a ± 121 | 71.7a ± 6.3 | 20.9a ± 0.6 | |
| 0.75% | 216.6a ± 6.2 | 3362.3a ± 67.4 | 73.9a ± 5.4 | 20.9a ± 1.2 | |
| 1.0% | 220.4a ± 7.0 | 3280.3a ± 148.8 | 72.7a ± 5.3 | 20.3a ± 1.1 | |
The same letters in each column were not significantly different (p ≥ 0.05)
Impact of bitterness inhibitors on total polyphenol content and antioxidant capacity
Phenolics are also bioactive compounds responsible for the antidiabetic property of bitter gourd extract by delaying glucose absorption or stimulating insulin secretion (Poovitha and Parani 2016). The TPC analysis of the samples (Table 2) shows that the control sample contained 222.9 µg GAE mL− 1, and a significant difference in TPC was not found between the samples mixed with bitterness inhibitors and the control sample. Some previous studies reported that adding β-CD increased the TPC of the extract due to its ability to protect polyphenol from oxidation via formation of the complex (Deshaware et al. 2018). Moreover, CMC has also been reported to create a barrier against oxygen (Ćorković et al. 2021), hence protecting phenolic compounds. However, that property of β-CD and CMC is not observed in this study which may be due to the fact that oxidase activity, which is associated with phenol oxidation, only manifests after a certain interval of time (Rikhotso et al. 2019).
Concerning antioxidant capacity, the control sample’s DPPH free radical scavenging ability was 19.4%, and there was no difference in this property between samples. In general, antioxidant activity positively correlates with the content of polyphenol compounds owing to the hydrogen-donating capacity of these components (Lee et al. 2017). Therefore, insignificant changes in the polyphenol content might have retained the antioxidant property of samples.
Impact of bitterness inhibitors on inhibition of the α-amylase enzyme
The present treatment of diabetes mellitus is focused on controlling and lowering glucose levels in the blood to a standard rate. One of the strategies of both modern and traditional medicines for achieving the above aim is to inhibit the action of hormones which causes an increase in blood glucose concentration (Tran et al. 2020). This ability can be observed in bitter gourd extract, which can treat diabetes by inhibiting the enzyme α-amylase. Specifically, the α-D-(1,4) bonds in carbohydrates are broken by the enzyme α-amylase to produce oligosaccharides, which are then cleaved into glucose monosaccharides by the enzyme α-glucosidase. Therefore, if the extract can inhibit the activity of one of these enzymes, the extract is likely to prevent the increase in blood glucose levels for those consuming starchy foods (Poovitha and Parani 2016; Lordan et al. 2013). The analytical results show that (Table 2), the control sample could inhibit about 73.45% of the α-amylase’s ability. Moreover, the results also show that the addition of CMC and β-CD did not affect the enzyme-inhibitory ability of the extract. Because of the inclusion ability of β-CD, there is a probability of these enzyme inhibitors to interact with β-CD and affect the properties of antidiabetic compounds. However, this hypothesis is not confirmed in this study maybe because of the low and insufficient concentration of β-CD to form complexes with enzyme-inhibiting components. The result found in this study is also consistent with that of Deshaware et al. (2018) that adding 1.5% β-CD did not cause any change in the α-amylase inhibition activity; however, the addition of 2% β-CD limited this ability. Proteins, polysaccharides, and phenols are components in bitter gourd extract which are responsible for the inhibition of amylase enzymes (Gong et al. 2020; Poovitha and Parani 2016). α-Amylase inhibitors can act as carbohydrate blockers, restricting their ability to digest and absorb carbohydrates. Moreover, the inhibition of α-amylase by proteins usually occurs by directly blocking the active centers of several enzyme subsites. Gong et al. (2020) have reported that phenolic compounds bind at the active and allosteric positions, resulting in structural changes and enzyme inhibition. The key interactions which influence the bonding between polyphenols and amylase enzymes include hydrogen bonding, hydrophobic interactions, and van der Waals interactions (Aleixandre et al. 2022).
Physical characteristics of the BGE complex
Results obtained from evaluating bitterness intensity and physicochemical properties showed the addition of 0.75% and 1% β-CD were the best treatments, which lowered the bitterness and preserved bioactive compounds as well as biological activities of BGE. Therefore, the BGE mixed with 0.75% β-CD was freeze-dried for physical characteristics analysis using FTIR, 1 H-NMR, and TGA/DTG techniques with the aim of understanding the mechanism for debittering.
FTIR analysis
Based on changes in distinguished peaks pattern, FTIR analysis was used to reveal information regarding the establishment of an inclusion complex. Figure 2 shows the FTIR spectra of β-CD, BGE, β-CD/BGE. As per earlier reports, the spectrum of β-CD exhibited distinctive peaks (Reddy et al. 2020). In particular, its spectrum shows typical O–H stretching vibration bands at 3283 cm− 1, C-H stretching vibration bands at 2923 cm− 1, H–O–H bending vibration of water molecules sticking to β-CD at 1652 cm− 1, C–O–C stretching vibration at 1152 cm− 1, C–C stretching vibration at 1076 cm− 1, and C–O–C symmetric stretching vibration at 1019 cm− 1. There is also a peak at 937 cm− 1 for the vibration of the β-CD molecule’s α-1,4 bond.
Fig. 2.
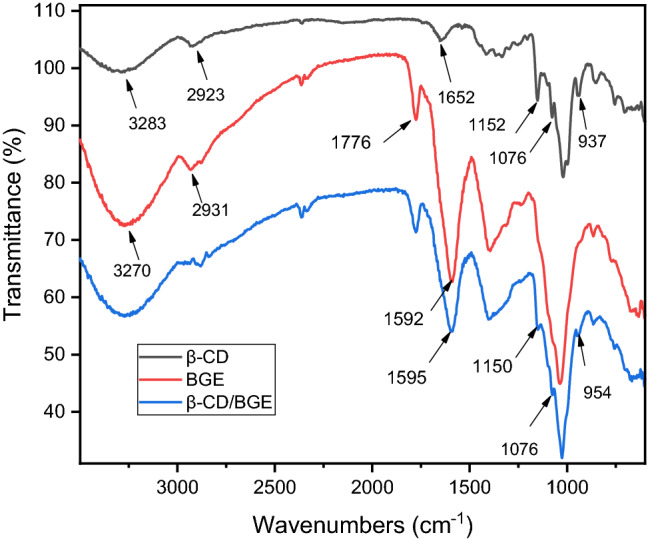
FTIR spectra of bitter gourd extract (BGE), free β-CD and inclusion complex (β-CD/BGE)
Regarding absorption peaks of BGE, there are peaks at 3270, 2931, 1776, and 1592 cm− 1, corresponding to stretching OH, stretching C-H, stretching C = O, and bending N–H vibrations, respectively. Compared to the spectra of BGE and free β-CD, the spectrum of β-CD/BGE changed significantly in absorption intensity and frequency. In particular, the peak of β-CD at 1652 cm− 1 belonging to water molecules sticking in the cavity of β-CD disappeared in the spectrum of β-CD/BGE. Pereva et al. (2019) found that the cavity of free β-CD has an affinity for accommodating water molecules at ambient conditions to form undefined hydrates in order to stabilize the crystal lattice. However, as the complex is formed, the entering guest compounds compete with the cavity-bound water molecules and may completely or partially replace their contents. The disappearance of 1652 cm− 1 peak demonstrated that compounds in BGE were incorporated into the hydrophobic cavity of the β-CD instead of former water molecules. Peaks’ intensity of β-CD at 1152 and 1076 cm− 1 in the spectrum of β-CD/BGE also decreased compared to that of the free β-CD spectrum. Along with these changes, upon complexation, the peak of BGE at 1592 cm− 1 shifted and experienced a decrease in intensity. These outcomes demonstrate that molecules in BGE and β-CDs have formed inclusion complexes (Reddy et al. 2020).
H-NMR analysis
The 1 H-NMR analysis is one of the most direct methods to confirm the occurrence of the inclusion complex based on differential changes in chemical shift between internal protons (H3 and H5) and external protons (H1, H2, H4, and H6). The former protons will undergo a significant shielding if guest molecules are inserted into the cavity of β-CD, whereas the latter protons will show little or minimal changes (Deshaware et al. 2018). The 1 H-NMR spectra of β-CD, BGE, and β-CD/BGE are shown in Table 3. Regarding the β-CD spectrum, it shows a set of significant peaks in the range of 3.556–5.042 ppm, including H1 (δ = 5.042 ppm), H2 (δ = 3.622 ppm), H3 (δ = 3.934 ppm), H4 (δ = 3.556 ppm) and H5 (δ = 3.833 ppm), H6 (δ = 3.842 ppm). When β-CD combined with BGE, the H3 and H5 protons of β-CD displayed more significant remarkable shifts (0.012 and 0.013 ppm, respectively) than shifts of H1, H2, H4, and H6 protons (0.003, 0.003, 0.001 and 0 ppm, respectively). These results are in agreement with the previous studies wherein β-cyclodextrin was mixed with rosa Damascena essential oil (Hadian et al. 2022).
Table 3.
1 H-NMR chemical shift of β-CD protons in the free status and in the inclusion complex (β-CD/BGE)
| Proton | Chemical shift (ppm) | ||
|---|---|---|---|
| β-CD | BGE- β-CD | ||
| H1 | 5.042 | 5.039 | 0.003 |
| H2 | 3.622 | 3.619 | 0.003 |
| H3 | 3.934 | 3.922 | 0.012 |
| H4 | 3.556 | 3.555 | 0.001 |
| H5 | 3.833 | 3.820 | 0.013 |
| H6 | 3.842 | 3.842 | 0 |
TGA analysis
TGA is an effective technique to authenticate complex formation based on assessing the thermal stabilities of materials. TGA thermograms and derivative thermogravimetry (DTG) for β-CD, BGE, and β-CD/BGE are shown in Fig. 3. The peak of DTG indicates the temperature at which the fastest rate of weight loss occurred. Two phases of mass loss are seen in the β-CD thermal profile. Water molecules that were adsorbed by the β-CD, and positioned in the cavity of β-CD, might have been evaporated during the initial phase, which occurred at roughly 88 °C. The later phase at 324 °C was related to β-CD degradation (Reddy et al. 2020). Concerning BGE, there are two stages of weight loss at temperatures around 125 and 194 °C, attributed to the thermal degradation of various ingredients of extract. In the case of β-CD/BGE, the profile shows four regions of mass loss at around 100, 149, 195, and 307 °C. The first could be associated with water evaporation, while the last perhaps corresponds to the decomposition of β-CD. The remaining peaks represent the temperature at which BGE’s ingredients in the complex were destroyed. The result reveals that the thermal profile of β-CD/BGE do not show a coincident thermogram which is made up of a mixture of peaks shown in the TGA profiles of each component. The degradation temperature of β-CD decreased from 321 to 307 °C when mixed with BGE. Additionally, the destruction of some components of BGE in the complex occurred at a higher temperature, at 149 °C instead of 125 °C as shown in the BGE curve. The results demonstrate that adding β-CD to BGE heightened the thermal stability of some components in the extract while lowering the thermal stability of β-CD, which confirmed that the complex formation was accomplished (Reddy et al. 2020; Hadian et al. 2022).
Fig. 3.
TGA/DTG thermograms of A β-CD, B BGE, C β-CD/BGE
Conclusion
This study compared the ability of CMC and β-CD to inhibit bitterness of BGE. The result shows that both CMC and β-CD were bitterness inhibitors and did not have any adverse impact on the biological activities of BGE. However, the effectiveness of β-CD surpassed that of CMC, especially the addition of 0.75% β-CD decreased the BGE bitterness almost completely. Results obtained from physical characteristics, including FTIR, 1 H-NMR, and TGA/DTG, confirmed β-CD successfully trapped molecules present in the extract. The results regarding the ability of β-CD to mask bitter taste and form complex lead to a conclusion that bitterants in the extract were enclosed in the cavity of β-CD. The findings of the present study suggest that supplementing BGE with β-CD is an effective approach to mask bitterness and preserve its biological properties. This finding may open up the opportunities to develop BGE containing functional foods or beverages with least or no bitterness.
Acknowledgements
The authors would like to acknowledge Ministry of Education (Taiwan) provided Taiwan Elite Scholarship, Van Thi Dung, Tran Nguyen Bao Chau, Do Thi Kim Anh, Faculty of Food Science and Technology, Ho Chi Minh City University of Food Industry (HUFI) for assistance in collecting sensory data in this study, Miss Ting-Yin Cheng at Instrumentation Centre of National Tsing Hua University for the Thermogravimetric analysis (TGA), and Miss Bi-Yin Lin at National Cheng Kung University for the NMR experiments.
Author contributions
CTHT: Investigation, Methodology, Formal analysis, Conceptualization, Writing-Original Draft. HTCP: Methodology. DML: Resources. PKH: Resources. PN: Validation, Revision. HMDW: Resources. CDD: Resources, Supervision. CHK: Resources, Supervision, Revision, Writing-Review & Editing.
Funding
This work was supported by research funding grants provided by the National Science and Technology Council of Taiwan (NSTC 111-2221-E-992-005-MY3).
Data Availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Code Availability
Not applicable.
Declarations
Conflict of interest
The author(s) declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Ainsworth EA, Gillespie KM. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin–Ciocalteu reagent. Nat Protoc. 2007;2:875–877. doi: 10.1038/nprot.2007.102. [DOI] [PubMed] [Google Scholar]
- Aleixandre A, Gil JV, Sineiro J, Rosell CM. Understanding phenolic acids inhibition of α-amylase and α-glucosidase and influence of reaction conditions. Food Chem. 2022;372:131231. doi: 10.1016/j.foodchem.2021.131231. [DOI] [PubMed] [Google Scholar]
- Ares G, Barreiro C, Deliza R, Gámbaro A. Alternatives to reduce the bitterness, astringency and characteristic flavour of antioxidant extracts. Food Res Int. 2009;42:871–878. doi: 10.1016/j.foodres.2009.03.006. [DOI] [Google Scholar]
- Binello A, Cravotto G, Nano GM, Spagliardi P. Synthesis of chitosan–cyclodextrin adducts and evaluation of their bitter-masking properties. Flavour Fragr J. 2004;19(5):394–400. doi: 10.1002/ffj.1434. [DOI] [Google Scholar]
- Ćorković I, Pichler A, Buljeta I, Šimunović J, Kopjar M. Carboxymethylcellulose hydrogels: effect of its different amount on preservation of tart cherry anthocyanins and polyphenols. Curr Plant Biol. 2021;28:100222. doi: 10.1016/j.cpb.2021.100222. [DOI] [Google Scholar]
- Deng Y, Ma Y, Liu H, Zhang Y, Wei Z, Liu G, Tang X, Jia X. Structure determination, bitterness evaluation and hepatic gluconeogenesis inhibitory activity of triterpenoids from the Momordica charantia fruit. Food Chem. 2022;372:131224. doi: 10.1016/j.foodchem.2021.131224. [DOI] [PubMed] [Google Scholar]
- Deshaware S, Gupta S, Singhal RS, Joshi M, Variyar PS. Debittering of bitter gourd juice using β-cyclodextrin: mechanism and effect on antidiabetic potential. Food Chem. 2018;262:78–85. doi: 10.1016/j.foodchem.2018.04.077. [DOI] [PubMed] [Google Scholar]
- Gaudette NJ, Pickering GJ. The efficacy of bitter blockers on health-relevant bitterants. J Funct Foods. 2012;4:177–184. doi: 10.1016/j.jff.2011.10.003. [DOI] [Google Scholar]
- Gong L, Feng D, Wang T, Ren Y, Liu Y, Wang J. Inhibitors of α-amylase and α‐glucosidase: potential linkage for whole cereal foods on prevention of hyperglycemia. Food Sci Nutr. 2020;8:6320–6337. doi: 10.1002/fsn3.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadian Z, Kamalabadi M, Phimolsiripol Y, Balasubramanian B, Rodriguez JML, Khaneghah AM. Preparation characterization and antioxidant activity of β-cyclodextrin nanoparticles loaded Rosa damascena essential oil for application in beverage. Food Chem. 2022 doi: 10.1016/j.foodchem.2022.134410. [DOI] [PubMed] [Google Scholar]
- Ho S, Thoo YY, Young DJ, Siow LF. Stability and recovery of cyclodextrin encapsulated catechin in various food matrices. Food Chem. 2019;275:594–599. doi: 10.1016/j.foodchem.2018.09.117. [DOI] [PubMed] [Google Scholar]
- International Diabetes Federation . IDF Diabetes Atlas. 10. Brussels: International Diabetes Federation; 2021. [PubMed] [Google Scholar]
- Kelanne N, Laaksonen O, Seppälä T, Yang W, Tuukkanen K, Loponen J, Yang B. Impact of cyclodextrin treatment on composition and sensory properties of lingonberry (Vaccinium vitis-idaea) juice. LWT. 2019;113:108295. doi: 10.1016/j.lwt.2019.108295. [DOI] [Google Scholar]
- Klomann SD, Mueller AS, Pallauf J, Krawinkel MB. Antidiabetic effects of bitter gourd extracts in insulin-resistant db/db mice. Br J Nutr. 2010;104:1613–1620. doi: 10.1017/S0007114510002680. [DOI] [PubMed] [Google Scholar]
- Lê S, Worch T. Analyzing sensory data with R. Boca Raton: Chapman and Hall/CRC; 2018. [Google Scholar]
- Lee SH, Jeong YS, Song J, Hwang K-A, Noh GM, Hwang IG. Phenolic acid, carotenoid composition, and antioxidant activity of bitter melon (Momordica charantia L.) at different maturation stages. Int J Food Prop. 2017;20:S3078–S3087. doi: 10.1080/10942912.2016.1237961. [DOI] [Google Scholar]
- Lordan S, Smyth TJ, Soler-Vila A, Stanton C, Ross RP. The α-amylase and α-glucosidase inhibitory effects of irish seaweed extracts. Food Chem. 2013;141:2170–2176. doi: 10.1016/j.foodchem.2013.04.123. [DOI] [PubMed] [Google Scholar]
- Manninen H, Sandell M, Mattila S, Hopia A, Laaksonen T. Comparing the taste-modifying properties of nanocellulose and carboxymethyl cellulose. J Food Sci. 2021;86:1928–1935. doi: 10.1111/1750-3841.15711. [DOI] [PubMed] [Google Scholar]
- Oishi Y, Sakamoto T, Udagawa H, Taniguchi H, Kobayashi-Hattori K, Ozawa Y, Takita T. Inhibition of increases in blood glucose and serum neutral fat by Momordica charantia saponin fraction. Biosci Biotechnol Biochem. 2007;71:735–740. doi: 10.1271/bbb.60570. [DOI] [PubMed] [Google Scholar]
- Okabe H, Miyahara Y, Yamauchi T. Studies on the constituents of Momordica charantia L. IV. Characterization of the new cucurbitacin glycosides of the immature fruits.(2) structures of the bitter glycosides, momordicosides K and L. Chem Pharm Bull. 1982;30:4334–4340. doi: 10.1248/cpb.30.4334. [DOI] [Google Scholar]
- Pereva S, Nikolova V, Angelova S, Spassov T, Dudev T. Water inside β-cyclodextrin cavity: amount, stability and mechanism of binding. Beilstein J Org Chem. 2019;15:1592–1600. doi: 10.3762/bjoc.15.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poovitha S, Parani M. In vitro and in vivo α-amylase and α-glucosidase inhibiting activities of the protein extracts from two varieties of bitter gourd (Momordica charantia L) BMC Complement Altern Med. 2016;16:1–8. doi: 10.1186/s12906-016-1085-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy CK, Jung ES, Son SY, Lee CH. Inclusion complexation of catechins-rich green tea extract by β-cyclodextrin: preparation, physicochemical, thermal, and antioxidant properties. LWT. 2020;131:109723. doi: 10.1016/j.lwt.2020.109723. [DOI] [Google Scholar]
- Rikhotso MM, Magwaza LS, Tesfay SZ, Mditshwa A. Evaluating the efficacy of chitosan and CMC incorporated with moringa leaf extracts on reducing peteca spot incidence on ‘Eureka’lemon. J Food Sci Technol. 2019;56:5074–5086. doi: 10.1007/s13197-019-03980-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun-Waterhouse D, Wadhwa SS. Industry-relevant approaches for minimising the bitterness of bioactive compounds in functional foods: a review. Food Bioprocess Technol. 2013;6(3):607–627. doi: 10.1007/s11947-012-0829-2. [DOI] [Google Scholar]
- Tamamoto LC, Schmidt SJ, Lee SY. Sensory properties of ginseng solutions modified by masking agents. J Food Sci. 2010;75:S341–S347. doi: 10.1111/j.1750-3841.2010.01749.x. [DOI] [PubMed] [Google Scholar]
- Tan SP, Kha TC, Parks SE, Roach PD. Bitter melon (Momordica charantia L.) bioactive composition and health benefits: a review. Food Rev Int. 2016;32(2):181–202. doi: 10.1080/87559129.2015.1057843. [DOI] [Google Scholar]
- Tan SP, Vuong QV, Stathopoulos CE, Parks SE, Roach PD. Optimized aqueous extraction of saponins from bitter melon for production of a saponin-enriched bitter melon powder. J Food Sci. 2014;79:E1372–E1381. doi: 10.1111/1750-3841.12514. [DOI] [PubMed] [Google Scholar]
- Tran N, Pham B, Le L. Bioactive compounds in anti-diabetic plants: from herbal medicine to modern drug discovery. Biology. 2020;9:252. doi: 10.3390/biology9090252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troszyńska A, Narolewska O, Robredo S, Estrella I, Hernández T, Lamparski G, Amarowicz R. The effect of polysaccharides on the astringency induced by phenolic compounds. Food Qual Prefer. 2010;21:463–469. doi: 10.1016/j.foodqual.2009.12.005. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Not applicable.