Abstract
In this study, it was aimed to evaluate the cytotoxic and apoptotic activities of ethanolic extracts prepared from the roots of 5 Ferulago species [F. humilis Boiss., F. macrosciadia Boiss. & Balansa, F. sandrasica Peşmen & Quézel, F. silaifolia (Boiss.) Boiss., F. trojana Akalın & Pimenov] on various human cancer cell lines. The cytotoxicity analyses against human lung (A549), breast (MCF-7), prostate (PC3) and colon (SW480) cancer cell lines were determined by MTT test; while the apoptotic effect was evaluated by Annexin V binding assay. All studied extracts showed concentration-dependent cytotoxic activity with an IC50 value ranging from 0.416 to 5.336 mg/mL. The studied Ferulago species significantly induced apoptosis of cancer cells, while F. macrosciadia had the highest apoptotic activity on MCF-7 cells with 21.79 ± 1.63% apoptotic cell population (p < 0.0001). In addition, felamedin and prantschimgin content of the extracts, which are common coumarins in Ferulago species, were evaluated by HPLC. According to HPLC analysis, the highest amount of felamedin content was found in F. trojana, while the highest content of prantschimgin was found in F. sandrasica among the studied Ferulago species. This preliminary research has revealed that the studied Ferulago species have promising effects on various cancer cell lines. Further studies are planned to determine the compounds responsible for the effect and underlying mechanism.
Keywords: Ferulago sp., Antiproliferative, Apoptosis, Coumarin, Medicinal plant
Introduction
Cancer is a disease with an increasing number of cases all over the world. According to New Global Cancer Data, GLOBOCAN 2020, number of cancer cases was 19.3 million (excluding nonmelanoma skin cancer) and number of cancer related deaths were 9.9 million (again not including nonmelanoma skin cancer) with breast, lung, colorectal, prostate and stomach having the highest incidence and lung, colorectal, liver, stomach and breast having the highest number of deaths (Sung et al. 2022). Conventional therapies such as radiotherapy and chemotherapy are beneficial since they suppress different types of tumors, however they are associated with side effects and thus, researchers around the world started to focus on plants and plant products for their cancer preventing activities (Mirzaghaei et al. 2014).
Plants are considered to be promising sources against cancer since they have ability to prevent due to various secondary metabolites that they contain (Mirzaghaei et al. 2014). The most significant feature of human cancers is the fact that they have resistance to apoptosis, and therefore induction of apoptosis has become a valuable strategy that can be used in cancer therapy and in recent years, more than 3000 plant species have been utilized in the search for new sources against cancer (Heidari et al. 2014). Plant derived compounds utilize different pathways and mechanisms in inducing cell death such as programmed cell death (PCD)-type I (apoptosis), PCD-type II autophagic cell death and necrosis and apoptosis is the most important among them (Karimian et al. 2014). Induction of apoptosis with plant derived natural products have vital importance in the plants and plant derived products being considered as potent anticancer agents (Rahman and Hussain 2015).
One of the important anticancer secondary metabolite group found in plants is coumarins. However, it is known that coumarins have various biological activities such as anti-inflammatory, anticoagulant, antibacterial, antifungal, antiviral, antihypertensive, antitubercular, anticonvulsant, antiadipogenic, antihyperglycemic, antioxidant, neuroprotective, as well. Coumarins have significant anticancer activities, and they also have low side effects due to the functional groups that they have (Shokoohinia et al. 2018). Structure-activity relationship studies with different coumarins and different cancer cell lines revealed that; δ-lactone ring is required for activity, substitution of any one of the hydroxyl group at coumarin nucleus with methoxy, chloro or acetyl group reduced the activity, antiproliferative activity is increased upon the addition of electron-donating groups such as ortho-hydroxy-methoxy and ortho-dihydroxy groups on the aromatic ring A and B. C-4 with phenyl ring, C-7 with diethylamino, and C-2 substitution with phenyl acetate on coumarin nucleus increase the cytotoxic activity. Increase in the number of methoxy group on benzene ring attached to coumarin nucleus and the presence of tertiary butyl group on the benzene ring attached to coumarin segment strengthens the activity (Vázquez et al. 2012; Jeon et al. 2011; Kumar et al. 2017; Musa et al. 2011; Sashidhara et al. 2013). In addition to possessing anticancer effect, coumarins also have the ability to reduce side effects that are associated with radiotherapy and thus, they are being investigated in the search for promising anticancer compounds (Sandhu et al. 2014). In addition to above mentioned activities, they also have MAO-B inhibitory, anti-allergic, hepatoprotective, antiviral, antileishmanial, antidiabetic, anticoagulant, antithrombotic, anti-influenza, anti-Alzheimer activities (Kostova 2005; Salem et al. 2012; Sandhu et al. 2014; Gaudino et al. 2016; Koziol and Slakicka-Wozniak 2016; Thomas et al. 2017; Tosun and Miski 2020; Badalamenti et al. 2021).
More than 1300 molecules in the structure of coumarin have been isolated from plants, bacteria and fungi. It has been determined that approximately 150 genera from 30 different families, including Apiaceae, Rutaceae, Guttiferae, Caprifoliaceae, Oleaceae, contain coumarins (Venugopala et al. 2013). Among these families, Apiaceae, which is very important in terms of different coumarin content and diversity. The Apiaceae family is represented in Turkey with 100 genera, including Ferulago W. Koch. (Guner et al. 2012). The Ferulago genera has 34 taxa growing naturally in Turkey, and 19 of these taxa are endemics for Turkey (Guner et al. 2012), therefore Turkey is considered to be the gene center, i.e. center of diversity for this genus (Akalin and Kocyigit 2010–2011) and this is a fact that increases the importance of this genus for Turkey.
There are several studies in the literature investigating the anticancer activity of Ferulago species on various tumor cell lines and find high activity (Amirghofran et al. 2006; Thomas et al. 2017; Badalamenti et al. 2021; Bakar-Ates et al. 2021). Studies were performed on different Ferulago species; their extracts, fractions or coumarin derivatives isolated from them were tested against different cell lines and promising results were obtained. The anti-proliferative effect of F. angulata (Schlecht) Boiss., which is one of them, has been studied in detail. For example, methanolic extract of F. angulata aerial parts demonstrated anticancer effect on K562 leukemia and Jurkat cell lines (Amirghofran et al. 2006). Its methanol extract inhibited the proliferation of three leukemia and lymphoma tumor cell lines without significant effect on peripheral mononuclear cells (Shahneh et al. 2013a). In addition to F. angulata, other some Ferulago species have also been investigated for anticancer activity. Hexane and ethyl acetate fractions of aerial parts of F. carduchorum Boiss and Haussk exerted cytotoxic activity on cell lines due to its coumarin and phytosteroid content (Golfakhrabadi et al. 2013). The aqueous extracts of F. mughlae Peşmen roots and aerial parts were shown to inhibit proliferation of SW480 colorectal carcinoma cells (Bakar et al. 2016).
Despite the phytochemical richness of Ferulago species, there are limited number of activity studies on this genus in the literature. This rich phytochemical content and the anticancer activity results obtained from the studied species showed that Ferulago species have a very important potential in terms of anticancer activity. For this reason, our research group planned to conduct studies on the anticancer activity of Ferulago species, of which 34 species are found in Turkey and 19 of them are endemic (Guner et al. 2012). In a previous pre-screening research, we studied the cytotoxic and apoptotic effects of ethanol extracts from 5 different Ferulago species (F. cassia Boiss., F. isaurica Peşmen, F. syriaca Boiss, F. longistylis Boiss. and F. setifolia K.Koch) and found that all of the studied extracts inhibited cell cycle at different stages and cause apoptosis by increasing the effectiveness of Annexin V binding (Bakar-Ates et al. 2021). In the light of these literature data and as a continuation of our previous study, we aimed to investigate the cytotoxic activity of the root extracts of some other Ferulago species growing wildly in Turkey against different cancer cell lines. For this reason, ethanol extracts of F. humilis Boiss. F. macrosciadia Boiss. & Balansa, F. sandrasica Peşmen & Quézel, F. silaifolia (Boiss.) Boiss., F. trojana Akalın & Pimenov roots were tested against A549, MCF-7, PC3 and SW480 cancer cell lines.
Materials and methods
Plant material
The localities, where the Ferulago species collected from, are given in Table 1. Collected Ferulago species were identified by Hayri Duman and prepared voucher specimens are kept in AEF Herbarium.
Table 1.
Collection addresses of plant materials
| Species | Collection locality |
|---|---|
| F. humilis (F.h) | Muğla, Milas, north of İkizköy, Pinus brutia openings, 250 m, 9/6/2016 (35S 577097/4114855), AEF 28773 |
| F. macrosciadia (F.m) | Balıkesir, Bigadiç, north of Düğüncüler Village, under Quercus trees and in Cistus laurifolius openings, 8/6/2016 (39° 16.333’K/28° 32.141’D), AEF 28776 |
| F. sandrasica (F.sa) | Denizli, Beyağaç, Beyağaç-Köyceğiz road, Kartal Lake intersection, 1390 m, 9/6/2016 (37° 7.674’K/38° 50.267’D), AEF 28772 |
| F. silaifolia (F.si) | Bursa, Uludağ road, 10 km to the National Park, under Chestnut and BlackPine forest, 837 m 7/6/2016 (40° 8.69’K/29° 1.019’D), AEF 28771 |
| F. trojana (F.t) | Çanakkale, Çan, Terzialan-Bayramiç road, 5. Km, under Quercus tree forest, schist soil, 365 m, 8/6/2016 (39° 55.928’K / 27° 0.544’D), AEF 28774 |
Extraction procedure
The coarsely powdered Ferulago roots (30 g) were macerated in 80% ethanol by shaking in an orbital shaker for 48 h. Then the extracts were filtered and the residues were subjected to the same maceration process 6 times. Extracts obtained from each plant separately were combined and dried under low pressure by evaporating at a temperature not exceeding 45 °C.
HPLC studies
HPLC analyses of ethanol extracts and the standard compounds (felamedin and prantschimgin) were performed with HP Agilent 1100 (Germany) by adopting the procedure with detail given in previous researches (Kilic and Coskun 2006; Bakar-Ates et al. 2021).
Cell culture studies
A549 human lung carcinoma, MCF-7 human breast carcinoma, PC3 human prostate carcinoma, and SW480 human colon carcinoma cell lines (American Type Cell Culture Collection, Germany) were cultured in DMEM (Dulbecco’s Modified Eagle Medium). The cells were treated with extracts, in different concentration ranging from 0.01 to 1 mg/mL for 24 h under the same culture conditions. The non-treated cells were used as control. The details of the method were given in the previous research (Bakar-Ates et al. 2021).
Cytotoxicity assay
The cytotoxic effects of extracts were evaluated by MTT [3-(4,5-dimethylthiazol-2yl)-2,4-diphenyltetrazolium bromide] assay (Mosmann 1983). The cells were plated in 96-well plates at density of 9 × 103 cells/well and incubated for 24 h at 37 °C, then treated with extracts in different concentration ranging from 0.01 to 1 mg/mL for 24 h. Subsequently, the cells were treated with MTT solution and incubated for 2 h. The absorbance of the formed formazan crystals (in DMSO) were measured at 540 nm. Results of three independent experiments are expressed as the mean ± standard deviation (SD). The IC50 value (the half maximal inhibitory concentration) was calculated by linear regression analysis through GraphPad Prism 6.0 version (GraphPad Software Inc.). The details of the method were given in the previous research (Bakar-Ates et al. 2021).
Cell cycle analysis
The effect of the Ferulago extracts on cell cycle arrest was determined using the Muse Cell Cycle Assay Kit (Millipore, Germany). The cells (at a density of 2 × 105 cells/well) were plated on 12-well plates. After incubation for 24 h at 37 °C, the cells were treated with extracts at IC50 determined in MTT assay, and incubated again for 24 h. Harvested and 70% ethanol-fixed cells were collected by centrifugation and incubated with assay solution for 30 min. Three phases of the cell cycle (G0/G1, S, and G2/M) were detected using The Muse Cell Analyzer (Millipore, Germany). The details of the method were given in the previous research (Bakar-Ates et al. 2021).
Annexin V binding assay
The cells (at a density of 1 × 106 cells per well) were treated with extracts at IC50 concentrations, determined in MTT assay, for 24 h. Subsequently, the harvested, centrifuged and resuspended (in DMEM medium) cells were applied to Annexin V assay kit (Millipore, Germany). Manufacturer’s instructions, briefly summarized below, were followed for this process. Cell suspension is incubated with dead cell marker reagent, Annexin V and 7-aminoactinomycin D (7-AAD), for 20 min at room temperature. Then, the apoptotic cell population (non-apoptotic cells, early apoptotic cells, late stage apoptotic and dead cells, mostly nuclear debris) was detected by Muse Cell Analyzer (Millipore). Nontreated cells were defined as control group. The details of the method were given in the previous research (Bakar-Ates et al. 2021).
Statistical analysis
GraphPad Prism 6.0 version (GraphPad Software Inc.) were used for statistical analyses. Data obtained from the cell culture experiments were expressed as mean ± SD, and differences between control and extracts were statistically evaluated using one-way ANOVA. The IC50 values were calculated by linear regression analysis using same software.
Results
As described in the “Materials and methods” section, 80% ethanolic root extracts of 5 different Ferulago species were prepared initially for the assessment. The yields of each extracts are given in Table 2.
Table 2.
Extraction amounts of studied Ferulago roots
| Plant species | Extract (g) | Yield (%) (g extract/g dry root) |
|---|---|---|
| F. humilis | 5.29 | 17.66 |
| F. macrosciadia | 6.71 | 22.35 |
| F. sandrasica | 5.84 | 19.48 |
| F. silaifolia | 4.81 | 16.02 |
| F. trojana | 5.65 | 18.84 |
HPLC analysis
Both standard substance peaks were determined by two different methods: the analysis was started with the external standard method. In this method, a certain amount of standards was added to the extract and the increase in the peak areas in the obtained chromatogram was determined. Subsequently, peaks matching the signals of standard substances were determined in the chromatogram of the extracts. As a result of the analyzes, both felamedin and prantschimgin was understood to be found in all of the studied extracts. The results of the calculations of the parameters for the calibration of the standards and the validation of the method are presented in Table 3.
Table 3.
Calculated parameters for calibration the of standards and validation of analysis method
| Slope | Intersection | Regression coefficient (r2) | LOD | LOQ | |
|---|---|---|---|---|---|
| Felamedin | 17.430 | 2248.9 | 0.9684 | 0.0022 | 0.0069 |
| Prantschimgin | 17.673 | 2949.9 | 0.966 | 0.0381 | 0.0177 |
Felamedin and prantschimgin contents detected in the studied extracts as a result of HPLC analysis are presented in the Table 4. According to results, among the studied Ferulago species, F. trojana was determined as the species richest in felamedin; while the highest prantschimgin concentration was found in the F. sandrasica extract.
Table 4.
Felamedin and prantschimgin content of the 80% ethanolic extracts of root
| Species | Measured concentration (mg/mL) | |
|---|---|---|
| Felamedin | Prantschimgin | |
| F. humilis | 0.2251 | 0.2662 |
| F. macrosciadia | 0.2238 | 0.3501 |
| F. sandrasica | 0.5614 | 1.1040* |
| F. silalifolia | 0.0260 | 0.2344 |
| F. trojana | 0.5949* | 0.1425 |
*Specifies highest quantities of corresponding coumarin derivatives
Cytotoxicity assay
The cytotoxic activity of Ferulago extracts in various cancer cells was analyzed by MTT assay. Although F. sandrasica was found to have the lowest IC50 value in A549 cells (0.642 mg/mL, Table 5), from the cell lines to which the extract was applied at a concentration varying between 0.01 and 1 mg/mL, F. macrosiada and F. trojana were also found to inhibit cell viability at concentrations of 0.25 mg/mL and above significantly (p < 0.05). It was observed that the cytotoxic effect of the extracts on MCF-7 human breast cancer cells was lower than that of the other studied cell lines. F. humilis had the lowest IC50 value of 1.755 mg/mL in the aforementioned cell line and reduced cell viability to 70.05 ± 2.10% at a concentration of 1 mg/mL (p < 0.0001). Although the cytotoxic activity of the studied extracts in PC3 cells was close to each other, it was observed that F. humilis had the lowest IC50 value of 0.519 mg/mL (Table 5). In PC3 cells treated with F. humilis extract, cell viability was determined as 26.64 ± 0.37% at a concentration of 1 mg/mL (p < 0.0001). In SW480 cells, F. macrosciadia was the most effective extract with an IC50 value of 0.416 mg/mL (Table 5), and cell viability was found to be 35.11 ± 0.90% in the 1 mg/mL extract group (p < 0.0001).
Table 5.
IC50 values (mg/mL) of Ferulago root extracts in different cancer cell lines
| Species | A549 | MCF-7 | PC3 | SW480 |
|---|---|---|---|---|
| F. humilis | 0.655 | 1.755 | 0.519 | 0.552 |
| F. macrosciadia | 0.671 | 2.471 | 0.647 | 0.416 |
| F. sandrasica | 0.642 | 3.639 | 0.684 | 0.967 |
| F. silaifolia | 0.690 | 5.336 | 0.741 | 0.975 |
| F. trojana | 0.699 | 3.894 | 0.669 | 0.447 |
Cell cycle assay
After the cytotoxic activities of the extracts on the cells were determined, the relationship between the decrease in cell viability and the cell cycle was determined by the cell cycle assay. The plots showing the cell cycle activities of the extracts with the lowest IC50 value in the studied cancer cells are presented in Fig. 1. Accordingly, it was determined that F. sandrasica inhibited the cell cycle in G0/G1 phase in A549 cells. While the cell population in the G0/G1 phase in the aforementioned cells was 46.20 ± 1.28% in the control group, it was determined as 79.50 ± 3.44% in the extract treated group (p < 0.0001). It was observed that F. humilis inhibited the cell cycle in G2/M phase in MCF-7 cells. While the cell population in the G2/M phase was 35.60 ± 1.66% in the control group, it was found to be 69.30 ± 3.91% in the cells to which the extract was applied (p < 0.0001). It was determined that the same extract inhibited the cell cycle in PC3 cells in G0/G1 phase. Accordingly, while the G0/G1 phase cell population was 26.20 ± 2.15% in the control group, it was 79.70 ± 4.33% in the F. humilis treated group (p < 0.0001). It was determined that F. macrosciadia significantly arrested the cell cycle in SW480 cells in the G2/M phase (p < 0.05).
Fig. 1.
The cell cycle plot graphs of Ferulago species which showed the lowest IC50 values in MTT assay for each studied cell line (F. sandrasica for A549 cells, F. humilis for MCF-7 and PC3 cells, and F. macrosciadia for SW480 cells)
Apoptosis assay
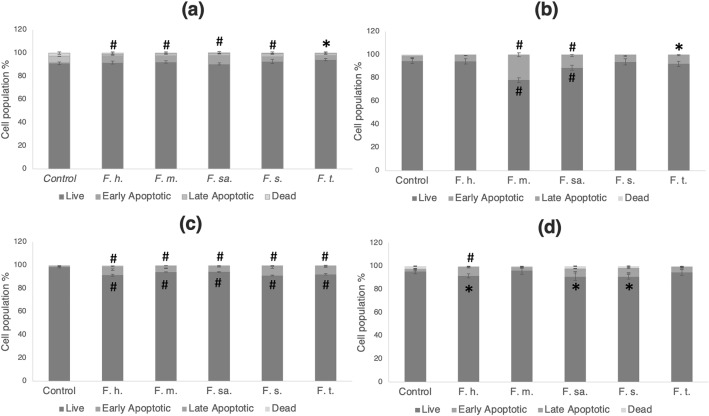
The apoptotic activities of Ferulago species, whose cytotoxic effects were evaluated within the scope of the study, on different cancer cells were analyzed with Annexin V binding assay. In this context, the effects of extracts on induction of apoptosis in different cell lines were evaluated as measuring the alteration on live, early apoptotic, late apoptotic and dead cell population % by cell analyzer. The cell population % were figured out in Fig. 2 and the plot graphics of the extracts led to the highest apoptotic cell population % in different cell lines are given in Fig. 3. Accordingly, by the treatment with F. sandrasica, the early apoptotic cell population % significantly increased to 7.05 ± 0.39% in A540 cells compared to the control group (p < 0.0001). While the early apoptotic cell population of control group was 2.52 ± 0.51% in MCF-7 cells, it was found to be significantly higher as 21.79 ± 1.63% in F. macrosciadia treated cells (p < 0.0001). In PC3 prostate cancer cells, the early apoptotic cell population, which was 0.53 ± 0.03% in the control group, was significantly higher as 6.98 ± 0.64% in the F. silaifolia extract group (p < 0.0001). In SW480 colon cancer cells, F. humilis increased the early apoptotic cell population by 7.35 ± 0.36% compared to the control group (p < 0.0001).
Fig. 2.
The results of the Annexin V binding assay for A549 (a), MCF-7 (b), PC3 (c) and SW480 (d) cells. Each value represents the mean ± standard deviation from triplicate experiments (*p < 0.05, #p < 0.0001, compared to control). F.h., F. hımilis; F.m., F. macrosciadia; F.sa., F. sandrasica; F.s., F. silaifolia; F.t., F. trojana
Fig. 3.
The annexin V binding assay plots graphs of Ferulago species which showed the highest increase in apoptotic cell population for each cell line (F. sandrasica for A549 cells; F. macrosciadia for MCF-7 cells; F. silaifolia for PC3 cells and F. humilis for SW480 cells)
Discussion
Natural products and the bioactive compounds isolated from these natural sources are the most important sources in the course of drug discovery, i.e. natural products provide prototypes for anticancer and antimicrobial agents (Amaral et al. 2019). Plant and animal originated natural products have been used in medicine for decades and recent investigations provide us important information related to the fact that active natural compounds could be effective against different cancer types (Emami and Dadashpour 2015; Harvey et al. 2015; Thakur et al. 2015; Salem et al. 2016; Sumorek-Wiadro et al. 2020). Considering all these studies and the results that they yielded, assessment of efficiency of crude extracts could be an important starting point, which would pave the way for more advanced studies directed to the determination of active compounds that are found in the extracts.
In this context, it is thought that Ferulago species due to the limited number of studies examining their anticancer activity could be a good starting point. This research is believed to make important contributions by filling an important gap in the literature in terms of examining the phytochemical compositions of 5 endemic Ferulago species (Guner et al. 2012) and determining their cytotoxic and apoptotic activity.
Various studies related to anticancer activities of Ferulago species are present. Three coumarins (aegelinol benzoate, decursin, samarcandin) were isolated from F. campestris and evaluated against HeLa, MCF-7, HT29, Jurkat, RS 4;11 and SEM cell lines. Aegelinol benzoate exhibited cytotoxic activity with IC50 value ranged from 29.2 to 61.7 µM against studied cell lines; while samarcandin determined to have cytotoxic activity only against SEM cell lines (IC50 value: 34.1 µM) (Dall’acqua et al. 2014). Shahneh et al (2013b) investigated the cytotoxic activity of aerial parts of F. angulata (Schlecht) Boiss methanolic extract against Raji U937, KG-1A, MCF-7, PC3, WEHI-164 and HUVEC cell lines by MTT test and as a result of the research, the extract was reported to show cytotoxic activity against studied cell lines, except HUVEC, with IC50 values ranging from 31.92 and 182.44 µg/mL (Shahneh et al. 2013a). Xanthotoxin, isoimperatorin, oxypeucedanin and oxypeucedannin hydrate were isolated from F. angulata roots and reported to have significant cytotoxic activities against HeLa cell line (Ameen 2014). In another research focused on F. angulata, its leaf hexane extract suppressed the expression of the tumor markers, induced apoptosis in breast tumor cells, and reduced the tumor size from 2031 to 432 mm3 in rats harboring LA7-induced breast tumors. Also, polycerasoidin (a prenylated benzopyran derivative) isolated from the plant showed cytotoxic activity against MCF7 cells with an IC50 value of 3.16 μg/mL by inducing mitochondrial-dependent apoptosis as a result of caspase activation and alteration in Bax and Bcl-2 expression (Karimian et al. 2015). Cytotoxic activity of alcoholic extracts of F. angulata flower and leaf were evaluated on AGS cell line. According to results, studied extracts displayed 100% cytotoxicity at a concentration of 140 μg/mL and above. In addition, leaf extract caused 49% late apoptosis in AGS cells at a concentration of 160 µg/mL; while the flower extract caused 45% late apoptosis at the same concentration (Heidari et al. 2014). Karimi et al. (2019) also investigated the anti-proliferative effect of F. angulata. The extract obtained from the leaves was found to have a cytotoxic effect with an IC50 value of 1000 µg/mL against HL-60 cell line with the MTS test, and it induced apoptosis by 37% at a concentration of 1000 µM. Golfakhrabadi et al. (2015) investigated the cytotoxic activity of F. carduchorum essential oil against T47D, HEP-G2 and HT-29 cell lines and reported that essential oil showed high cytotoxicity on studied cell lines (IC50 between 0.17 and 1.74 μg/mL). According to Zengin et al. (2020)’s investigation, F. setifolia aerial parts methanol extract has cytotoxic activity on HepG2 and S17 cell lines by reducing cellular viability to 49.35 and 17.36% at a concentration of 100 μg/mL, respectively. In our previous study (Bakar-Ates et al. 2021), we have determined that ethanol extracts of 5 Ferulago species growing in our country also had the ability to induce cell apoptosis in A549, MCF-7, PC3 and SW480 cancer cell lines. Furthermore, F. cassia ethanol extract was found to direct A549 and SW480 cancer cell lines to apoptosis more, compared to other Ferulago extracts, and F. syriaca was found to have the highest apoptotic activity against MCF-7 cell line. Then, bioactivity-guided fractionation studies were started from the F. syriaca.
Due to these encouraging results of our previous research and literature, we evaluated the cytotoxic and apoptotic activities of ethanolic extracts of other different Ferulago species on different cancer cells and in this context, it was observed that the extracts had significant cytotoxic and apoptosis-inducing activities in the studied cancer cells. Cell surface receptors play a role in various physiological and pathological processes (Mager et al. 2011; Ali et al. 2012), and it is known that a number of unique cell surface receptors exist in cancer cells (Shi et al. 2018). In addition, tumor cell proliferation rates vary depending on the type of tumor (Tubiana 1989). This may be the reason for the change in IC50 values determined for cytotoxic effect in different cancer cell lines in our study. Since the present research is a screening study, fractionation and purification processes have not yet been carried out for the determination of the compounds responsible for the activity. However, findings obtained with different Ferulago species show that coumarins have important contributions to cytotoxic activity (Rosselli et al. 2009; Ameen 2014; Sajjadi et al. 2015; Tavakoli et al. 2018). Therefore, it is possible that these two coumarins (felamedin and prantschimgin), which were determined and quantified in the five Ferulago species, will be encountered as responsible compound(s) for cytotoxic activity by further activity-directed fractionation studies. As far as we know, the cytotoxic activity of felamedin has not been evaluated before; while the effect of prantschimgin on some cancer cell lines has been investigated previously. However, there are findings in the literature showing the opposite for prantschimgin. For example, the antiproliferative effect of prantschimgin against MCF-7, HT-29 and H-1299 cell lines was previously investigated but not found to be effective (Sajjadi et al. 2015). In a study performed in 2009, synthetically produced prantschimgin was determined to show weak cytotoxicity in the MES-SA/DX5 cell line (Xia et al. 2009). In another study, it was determined that prantschimgin showed moderate growth inhibitory activity against HCT 116, MDA-MB-231, A-549, HT-29 and MRC-5 cell lines (Salem et al. 2012; Tavakoli et al. 2018). The cell lines dealed with in these studies are different from the present research (except MCF-7 and A549), so it is possible to obtain different results by future activity-directed fractionation studies. During our phytochemical studies, it was observed that the extracts, included in the present study, contained many other coumarins and compounds with different structures. In addition, not overlapping of felamedin and prantschimgin contents with the cytotoxic activities indicate that these two coumarins are not the mainly responsible compounds of the activity and other compounds in the extract have major role in the activity. Therefore, other compounds in the extracts may possibly be responsible for the activity. To determine this situation, bioactivity-guided fractionation studies should be carried out.
The chemical contents of plants vary according to environmental factors such as climate, soil composition, degree of exposure to sunlight, collection date and even collection time. Therefore, it is very important to study with standardized plants to give reproducible and comparable results in bioactivity researches. The use of more than one compounds in standardization is important in terms of obtaining more reliable results. In this study, the standardization of the extracts, prepared from 5 different Ferulago species and determined to have cytotoxic activity, was carried out using two marker compounds (felamedin and prantschimgin), previously found to be common in different Ferulago species (Doganca et al. 1991; Jimenez et al. 2000; Khalighi-Sigaroodi et al. 2006; Kılıç and Coşkun 2006; Basile et al. 2009; Rosselli et al. 2009; Venugopala et al. 2013; Razavi et al. 2015; Sajjadi et al. 2015; Valadbeigi et al. 2017; Malekshahi et al. 2018; Tavakoli et al. 2018; Karakaya et al. 2019). Thus, by conducting research with standardized extracts, bioactivity-directed fractionation studies that we planned as the next step, were tried to be built on more solid foundations.
On the other hand, one of the most important problems in evaluating the in vivo activities of plant extracts is their poor solubility in water. In this context, developments in nanotechnology in recent years have shown that by preparing various nanoformulations of plant extracts, the solubility problem can be reduced and the bioavailability of the extracts can be increased. For example, in a study, it was shown that conjugation of F. macrocarpa (Fenzl) Boiss. flower extract to silver nanoparticles increased cytotoxic activity in various gram negative and gram positive bacteria (Azarbani and Shiravand 2020). In another study, zinc oxide and copper oxide nanoparticles of F. angulata (Schlecht) Boiss. extract were produced and their photocatalytic degradation was compared (Shayegan Mehr et al. 2018). Studies show that developments in nanotechnology can also increase the activities of plant extracts, revealing the necessity of nanotechnology-based studies to increase the efficiency of Ferulago type extracts, whose cytotoxic and apototic activities were evaluated in this study. However, this is a preliminary screening study on the extracts. The active compounds in the extract are not yet clear. After the active compounds are isolated and structure determination studies are carried out, their solubility can be evaluated more clearly. The solubility of pure compounds in water may differ from that of the extract. If necessary, it may be possible to increase their solubility by preparing suitable derivatives from pure compounds. However, these are the subject of further studies.
Conclusions
In the present research, 80% ethanol extracts prepared from roots of 5 endemic Ferulago species (F. humilis, F. macrosciadia, F. sandrasica, F. silaifolia and F. trojana) were standardized over two coumarins, felamedin and prantschimgin, and their cytotoxic and apoptotic effects were investigated. According to obtained data, it was concluded that the all studied extracts showed concentration-dependent cytotoxic activity and induce apoptosis in human lung (A549), breast (MCF-7), prostate (PC3) and colon (SW480) cancer cell lines. The present research is a preliminary study and has revealed that aforementioned Ferulago species, whose cytotoxic activity has not been investigated before to the best of our knowledge, are promising on the investigated cancer types. Further studies are planned to determine the responsible compounds for the effect and underlying mechanism on these Ferulago species which were determined to have significant cytotoxic and apoptotic effects. With the results to be obtained, it will be possible to determine the drug candidate(s) that could be the subject of further studies.
Acknowledgements
This work was financially supported by The Scientific and Technological Research Council of Türkiye [grant number 115S364].
Data availability
The authors confirm that the data supporting the findings of this study are available within the article.
Declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Research involving human participants and/or animals
The authors declared that human participants or animals did not included in this study.
Informed consent
The authors declared that this research does not require informed consent since it is not a clinical trial.
References
- Akalin E, Kocyigit M (2010–2011) A chemotaxonomic study of Ferulago species in Turkey. J Fac Pharm Istanbul Univ 41:33–41
- Ali MM, Kang D, Tsang K, Fu M, Karp JM, Zhao W. Cell-surface sensors: lighting the cellular environment. Wiley Interdiscip Rev. 2012;4:547–561. doi: 10.1002/wnan.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaral RG, Santos SAD, Andrade LN, Severino P, Carvalho AA. Natural products as treatment against cancer: a historical and current vision. Clin Oncol. 2019;4:1562. [Google Scholar]
- Ameen BAH. Phytochemical study and cytotoxic activity of Ferulago angulata (Schlecht) Boiss, from Kurdistan-region of Iraq. Int J Innov Res Adv Eng. 2014;1:1–5. [Google Scholar]
- Amirghofran Z, Bahmani M, Azadmehr A, Javidnia K. Anticancer effects of various Iranian native medicinal plant on human cell lines. Neoplasma. 2006;53:418–433. [PubMed] [Google Scholar]
- Azarbani F, Shiravand S. Green synthesis of silver nanoparticles by Ferulago macrocarpa flowers extract and their antibacterial, antifungal and toxic effects. Green Chem Lett Rev. 2020;13:41–49. doi: 10.1080/17518253.2020.1726504. [DOI] [Google Scholar]
- Badalamenti N, Ilardi V, Rosseli S, Bruno M. The ethnobotany, phytochemistry and biological properties of genus Ferulago-a review. J Ethnopharmacol. 2021;274:114050. doi: 10.1016/j.jep.2021.114050. [DOI] [PubMed] [Google Scholar]
- Bakar F, Karakaya S, Bostanlık FGD, Kilic CS. Anticancer effect of Ferulago mughlae (Apiaceae) on cancer cell proliferation. Iranian J Pharm Res. 2016;15:501–504. [PMC free article] [PubMed] [Google Scholar]
- Bakar-Ates F, Hoti B, Gurbuz İ, Gunbatan T, Duman H, Kilic CS. The cytotoxic apoptotic effects of Ferulago W.Koch extracts on various cancer cell lines. Turk J Biochem. 2021;46:281–291. doi: 10.1515/tjb-2020-0225. [DOI] [Google Scholar]
- Basile A, Sorbo S, Spadaro V, Bruno M, Maggio A, Faraone N, Roselli S. Antimicrobial and antioxidant activities of coumarins from the roots of Ferulago campestris (Apiaceae) Molecules. 2009;14:939–952. doi: 10.3390/molecules14030939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dall’acqua S, Linardi MA, Bortolozzi R, Clauser M, Marzocchini S, Maggi F, Nicoletti M, Innocenti G, Basso G, Viola G, Natural daucane esters induces apoptosis in leukaemic cells through ROS production. Phytochemistry. 2014;108:147–156. doi: 10.1016/j.phytochem.2014.09.001. [DOI] [PubMed] [Google Scholar]
- Doganca S, Ulubelen A, Tuzlaci E. 1-Acetylhydroquinone 4-galactoside from Ferulago aucheri. Phytochem. 1991;30:2803–2805. doi: 10.1016/0031-9422(91)85152-P. [DOI] [Google Scholar]
- Emami S, Dadashpour S. Current developments of coumarin-based anti-cancer agents in medicinal chemistry. Eur J Med Chem. 2015;102:611–630. doi: 10.1016/j.ejmech.2015.08.033. [DOI] [PubMed] [Google Scholar]
- Gaudino EC, Tagliapietra S, Martina KI, Palmisano G, Cravotto G. Recent advances and perspectives in the synthesis of bioactive coumarins. RSC Adv. 2016;6:46394. doi: 10.1039/C6RA07071J. [DOI] [Google Scholar]
- Golfakhrabadi F, Ostad SN, Hafizi M, Ardekani MRS, Saeidnia S, Akbarzadeh T, Khanavi M. Phytochemical analysis and cytotoxic activity of Ferulago carduchorum. Res J Biol Sci. 2013;8:138–142. [Google Scholar]
- Golfakhrabadi F, Khanavi M, Ostad SN, Saeidnia S, Vatandoost H, Abai MR, Hafizi M, Yousefbeyk F, Rad YR, Baghenegadian A, Ardekani MRS. Biological activities and composition of Ferulago carduchorum essential oil. J Arthropod-Borne Dis. 2015;9:104–115. [PMC free article] [PubMed] [Google Scholar]
- Guner A, Aslan S, Ekim T, Vural M, Babac MT (2012) Turkiye Bitkileri Listesi (Damarli Bitkiler) [A Checklist of the Flora of Turkey (Vascular Plants)]. Nezahat Gokyigit Botanik Bahcesi ve Flora Arastırmaları Dernegi Yayini, Istanbul.
- Harvey AL, Edrada-Ebel R, Quinn RJ. The re-emergence of natural products for drug discovery in the genomics era. Nat Rev Drug Discov. 2015;14:111–129. doi: 10.1038/nrd4510. [DOI] [PubMed] [Google Scholar]
- Heidari S, Akrami H, Gharaei R, Jalili A, Mahdiuni H, Golezar E. Anti-tumor activity of Ferulago angulata Boiss. extract in gastric cancer cell line via induction of apoptosis. Iranian J Pharm Res. 2014;13:1335–1345. [PMC free article] [PubMed] [Google Scholar]
- Jeon J, Yang D, Jun J. Selective Synthesis of 3,4-dihydrocoumarins and chalcones from substituted aryl cinnamic esters. Bull Korean Chem Soc. 2011;32:65–70. doi: 10.5012/bkcs.2011.32.1.65. [DOI] [Google Scholar]
- Jimenez B, Grande MC, Anaya J, Torres P, Grande M. Coumarins from Ferulago capillaris and F. brachyloba. Phytochemistry. 2000;53:1025–1031. doi: 10.1016/S0031-9422(99)00524-5. [DOI] [PubMed] [Google Scholar]
- Karakaya S, Simsek D, Ozbek H, Guvenalp Z, Altanlar N, Kazaz C, Kiliç CS. Antimicrobial activities of extracts and isolated coumarins from the roots of four Ferulago species growing in Turkey. Iranian J Pharm Res. 2019;18:1516–1529. doi: 10.22037/ijpr.2019.1100718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karimi P, Pourgheysari B, Dezaki ZR, Soltani A, Zeilab F. The anti-proliferative effects of Ferulago angulata on human promyelocytic leukemia cell line (HL 60) Trends Pharm Sci. 2019;5:123–130. [Google Scholar]
- Karimian H, Oghadamtousi SZ, Golbabapour MFS, Razavi M, Hajrezaie M, Arya A, Abdulla MA, Mohan S, Ali HM, Noordin MI. Ferulago angulata activates intrinsic pathway of apoptosis in MCF-7 cells associated with G1 cell cycle arrest via involvement of p21/p27. Drug Des Dev Ther. 2014;8:1481–1497. doi: 10.2147/DDDT.S68818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karimian H, Fadaeinasab M, Moghadamtousi SZ, Hajrezaei M, Razavi M, Safi SZ, Abdulla MA, Ali HM, Noordin MI. Chemopreventive activity of Ferulago angulata against breast tumor in rats and the apoptotic effect of polycerasoidin in MCF-7 cells: a bioassay guided approach. PLoS ONE. 2015;10:e0127434. doi: 10.1371/journal.pone.0127434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalighi-Sigaroodi F, Hadjiakhoondi A, Shafiee A, Mozaffarian VA, Shahverdi AR, Alavi SHR. Phytochemical analysis of Ferulago bernardii Tomk & Pimen. DARU J Pharm Sci. 2006;14:214–221. [Google Scholar]
- Kilic CSE, Coskun M. Felamedin and prantschimgin content of chloroform fractions of Ferulago isaurica and F. syriaca growing in Turkey. Chem Nat Compd. 2006;42:351–352. doi: 10.1007/s10600-006-0119-1. [DOI] [Google Scholar]
- Kostova I. Synthetic and natural coumarins as cytotoxic agents. Curr Med Chem Anti Cancer Agents. 2005;5:29–46. doi: 10.2174/1568011053352550. [DOI] [PubMed] [Google Scholar]
- Koziol E, Slakicka-Wozniak K. Imperatorin-pharmacological meaning and analytical clues: profound investigation. Phytochem Rev. 2016;15:627–649. doi: 10.1007/s11101-016-9456-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar N, Bhatnagar A, Dudhe R. Synthesis of 3-(4, 5-dihydro-1-phenyl-5-substituted phenyl-1H-pyrazol-3-yl)-2H-chromen-2-one derivatives and evaluation of their anticancer activity. Arabian J Chem. 2017;10:S2443–S2452. doi: 10.1016/j.arabjc.2013.09.008. [DOI] [Google Scholar]
- Mager MD, LaPointe V, Stevens MM. Exploring and exploiting chemistry at the cell surface. Nat Chem. 2011;3:582–589. doi: 10.1038/nchem.1090. [DOI] [PubMed] [Google Scholar]
- Malekshahi Y, Gheibi S, Ghiasvand N, Jafari F, Mirabdali S, Kiani A, Yalda Shokoohinia Y. Effects of prantschimgin and grandivitin from Ferulago macrocarpa on VEGF, MMP9, MMP2 and research of binding modes using computational methods. Int Pharm Acta. 2018;1:92–93. [Google Scholar]
- Mirzaghaei S, Akrami H, Mansouri K. Ferulago angulata flower and leaf extracts inhibit angiogenesis in vitro through reducing VEGF-A and VEGFR-2 genes expression. Arch Iranian Med. 2014;17:278–285. [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Musa MA, Badisa VLD, Latinwo LM, Cooperwood J, Sinclair A, Abdullah A. Cytotoxic activity of new acetoxycoumarin derivatives in cancer cell lines. Anticancer Res. 2011;31:2017–2022. [PMC free article] [PubMed] [Google Scholar]
- Rahman A, Hussain A. Anticancer activity and apoptosis inducing effect of methanolic extract of Cordia dichotoma against human cancer cell line. Bangladesh J Pharmacol. 2015;10:27–34. doi: 10.3329/bjp.v10i1.20883. [DOI] [Google Scholar]
- Razavi SM, Ravansalar A, Mirinejad S. The investigation on phytochemicals from Ferulago angulata (Schlecht) Boiss, indigenous to central parts of Iran. Nat Prod Res. 2015;29:2037–2040. doi: 10.1080/14786419.2015.1017725. [DOI] [PubMed] [Google Scholar]
- Rosselli S, Maggio AM, Faraone N, Spadaro V, Morris-Natschke SL, Bastow KF, Lee K, Bruno M. The cytotoxic properties of natural coumarins isolated from roots of Ferulago campestris (Apiaceae) and of synthetic ester derivatives of aegelinol. Nat Prod Commun. 2009;4:1701–1706. [PubMed] [Google Scholar]
- Sajjadi SE, Jamali M, Shokoohinia Y, Abdi G, Shahbazi B, Fattahi A. Antiproliferative evaluation of terpenoids and terpenoid coumarins from Ferulago macrocarpa (Fenzl) Boiss. fruits. Pharmacogn Res. 2015;7:322–328. doi: 10.4103/0974-8490.158437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salem SB, Jabrane A, Harzallah-Skhiri F, Jannet HB. New bioactive dihydrofuranocoumarins from the roots of the Tunisian Ferula lutea (Poir.) Maire. Bioorg Med Chem Lett. 2012;23:4248–4252. doi: 10.1016/j.bmcl.2013.04.081. [DOI] [PubMed] [Google Scholar]
- Salem MA, Marzouk M, El-Kazak AM. Synthesis and characterization of some new coumarins with in vitro antitumor and antioxidant activity and high protective effects against DNA damage. Molecules. 2016;21:249. doi: 10.3390/molecules21020249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandhu S, Bansal Y, Silakari O, Bansal G. Coumarin hybrids as novel therapeutic agents. Bioorg Med Chem. 2014;22:3806–3814. doi: 10.1016/j.bmc.2014.05.032. [DOI] [PubMed] [Google Scholar]
- Sashidhara KV, Avula SR, Sharma K, Palnati GR, Bathula SR. Discovery of coumarin-monastrol hybrid as potential antibreast tumor-specific agent. Eur J Med Chem. 2013;60:120–127. doi: 10.1016/j.ejmech.2012.11.044. [DOI] [PubMed] [Google Scholar]
- Shahneh FZ, Valiyari S, Azadmehr A, Hajiahgaee R, Bandehagh A, Baradaran B. Cytotoxic activities of Ferulago angulata extract on human leukemia and lymphoma cells by induction of apoptosis. J Med Plants Res. 2013;7:677–682. [Google Scholar]
- Shahneh FZ, Baradan B, Orangi M, Zamani F. In vitro cytotoxic activity of four plants used in Persian Traditional Medicine. Adv Pharm Bull. 2013;3:453–455. doi: 10.5681/apb.2013.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shayegan Mehr E, Sorbiun M, Ramazani A, Fardood ST. Plant-mediated synthesis of zinc oxide and copper oxide nanoparticles by using Ferulago angulata (Schlecht) Boiss extract and comparison of their photocatalytic degradation of Rhodamine B (RhB) under visible light irradiation. J Mater Sci: Mater Electron. 2018;29:1333–1340. [Google Scholar]
- Shi T, Wang M, Li H, Wang M, Luo X, Huang Y, Wang H, Nie Z, Yao S. Simultaneous monitoring of cell-surface receptor and tumor-targeted photodynamic therapy via TdT-initiated poly-G-quadruplexes. Sci Rep. 2018;8:5551. doi: 10.1038/s41598-018-23902-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shokoohinia Y, Jafari F, Mohammadi Z, Bazvandi L, Hosseinzadeh L, Chow N, Bhattacharyya P, Farzaei MH, Farooqi AA, Nabavi SM, Yerer MB, Bishayee A. Potential anticancer properties of osthol: a comprehensive mechanistic review. Nutrients. 2018 doi: 10.3390/nu10010036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumorek-Wiadro J, Zajac A, Maciejczyk A, Jakubowicz-Gil J. Furanocoumarins in anticancer therapy-for and against. Fitoterapia. 2020;142:104492. doi: 10.1016/j.fitote.2020.104492. [DOI] [PubMed] [Google Scholar]
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J Clin. 2022;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- Tavakoli S, Delnavazi MR, Hadjiaghaee R, Jafari-Nodooshan S, Khalighi-Sigaroodi F. Bioactive coumarins from the roots and fruits of Ferulago trifida Boiss., an endemic species to Iran. Nat Prod Res. 2018;32:2724–2728. doi: 10.1080/14786419.2017.1375915. [DOI] [PubMed] [Google Scholar]
- Thakur A, Singla R, Jaitak V. Coumarins as anticancer agents: a review on synthetic strategies, mechanism of action and SAR studies. Eur J Med Chem. 2015;10:476–495. doi: 10.1016/j.ejmech.2015.07.010. [DOI] [PubMed] [Google Scholar]
- Thomas V, Giles D, Basavarajaswamy GPM, Das AK, Patel A. Coumarin derivatives as anti-inflammatory and anticancer agents. Anti Cancer Agents Med Chem. 2017;17:415–423. doi: 10.2174/1871520616666160902094739. [DOI] [PubMed] [Google Scholar]
- Tosun F, Miski F. Cytotoxic activity of the root and fruit extracts of Heptaptera anisoptera (DC) Tutin. ACTA Pharm Sci. 2020;58:21. [Google Scholar]
- Tubiana M. Tumor cell proliferation kinetics and tumor growth rate. Acta Oncol. 1989;28:113–121. doi: 10.3109/02841868909111193. [DOI] [PubMed] [Google Scholar]
- Val adbeigi S, Naderi-Moghadam M, Ghiasvand N, Jafari F, Jalilian F, Ahmadi F, Sajjadi S, Shokoohinia Y. In vitro study of the effects of dihydropyrano coumarins isolated from Ferulago macrocarpa on DNA by spectroscopic and molecular modeling methods. Res J Pharmacogn. 2017;4(Suppl):17. [Google Scholar]
- Vázquez R, Riveiro ME, Vermeulen M, Alonso E, Mondillo C, Facorro G, Piehl L, Gómez N, Moglioni A, Fernández N, Baldi A, Shayo C, Davio C. Structure-anti-leukemic activity relationship study of ortho-dihydroxycoumarins in U-937 cells: key role of the δ-lactone ring in determining differentiation-inducing potency and selective pro-apoptotic action. Bioorg Med Chem. 2012;20:5537–5549. doi: 10.1016/j.bmc.2012.07.043. [DOI] [PubMed] [Google Scholar]
- Venugopala KN, Rashmi V, Odhav B. Review on natural coumarin lead compounds for their pharmacological activity. BioMed Res Int. 2013 doi: 10.1155/2013/963248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Y, Min KH, Lee K. Synthesis and biologic evaluation of decursin, prantschimgin and their derivatives. Bull Korean Chem Soc. 2009;30:43–48. doi: 10.5012/bkcs.2009.30.1.043. [DOI] [Google Scholar]
- Zengin G, Sinan KI, Ak G, Mahomoodally MF, Paksoy MY, Picot-Allain C, Glamocilja J, Sokovic M, Jekő J, Cziáky Z, João RMJ, Pereira CG, Custodio L. Chemical profile, antioxidant, antimicrobial, enzyme inhibitory, and cytotoxicity of seven Apiaceae species from Turkey: a comparative study. Ind Crops Prod. 2020;153:112572. doi: 10.1016/j.indcrop.2020.112572. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article.