Abstract
The small molecule characteristics and nutritional value of egg white hydrolysates have been widely used. In the present study, in vitro and in vivo models were used to investigate the hepatoprotective effect of egg protein hydrolysate (EWH) by regulating the expression of antioxidant enzymes. The in vitro experiment results showed that 0.1, 0.5, and 1 mg/mL of EWH enhanced antioxidant activity in HepG2 cells by increased glutathione peroxidase (GPx) activity and reduced glutathione (GSH) levels. The in vivo experiment results showed that EWH (L) (38.5 mg/kg BW) and EWH (H) (385 mg/kg BW) alleviated carbon tetrachloride (CCl4)-induced hepatotoxicity in SD rats through reduced levels of serum aspartate aminotransferase (AST) alanine aminotransferase (ALT), and lipid peroxidation products malondialdehyde (MDA). In addition, EWH also ameliorates CCl4-induced hepatotoxicity in SD rats by increasing the antioxidant activity of GSH levels with a decrease in oxidized glutathione (GSSG) levels. Besides, EWH ameliorates liver tissue injuries by CCl4-induction. EWH has the highest glutamic acid in free amino acid composition, the second highest was aspartic acid, and the third was cystine, 204, 141, and 125 mg/100 g, respectively. These results suggest EWH has hepatoprotective potential through reduced lipid peroxidation products and enhanced antioxidant activity.
Keywords: Egg white hydrolysates, Hepatoprotective, Carbon tetrachloride, Antioxidant activity
Introduction
Recently, many protein hydrolysates from food (animal and plant) obtained by enzymatic hydrolyses, such as walnut protein hydrolysates (Jahanbani et al. 2016), white shrimp protein hydrolysates (Latorres et al. 2018) and scallop protein hydrolysates (Wang et al. 2021) have been found to possess antioxidant activity. Protein hydrolysates have antioxidant properties attributed to their capacity to scavenge free radicals, quench oxygen, prevent lipid oxidation, and function as a metal-ion chelator (Moure et al. 2006).
Egg whites are the main component (60%) of eggs, frequently employed in food products as functional and nutritive components (Garcés-Rimón et al. 2016). Egg whites are currently utilized as texture improvers and stabilizers in foods due to their foaming, gelling, emulsifying, and heat-setting properties (Garcés-Rimón et al. 2016). In addition, egg whites are widely used in biomedicine, such as cell culture, tissue engineering, antimicrobial matrices, biosensors, and drugs by their high bioactivity, biocompatibility, ease of handling, and low cost (Jalili-Firoozinezhad et al. 2020). Recently, protein hydrolysates obtained by enzymatic hydrolysis can increase their value by changing food proteins' physical and nutritional properties (Garcés-Rimón et al. 2016). Studies have demonstrated that egg white protein hydrolysate obtained by trypsin or papain has antioxidant activity through reducing power, scavenging the 2,2 diphenyl-1-picrylhydrazyl (DPPH) radical and lipid peroxidation inhibitory activity (Chen et al. 2012).
Liver diseases are a common health problem worldwide because the liver is the primary site for detoxifying metabolism (Ganesan et al. 2018). When liver metabolism produces excess reactive oxygen species (ROS), homeostasis can be disturbed, resulting in oxidative stress (Almatroodi et al. 2020). In addition, ROS can cause liver damage by producing excess MDA, causing lipid peroxidation, and reducing antioxidant-related enzymes activity such as superoxide dismutase (SOD), glutathione peroxidase (GPx), catalase (CAT), and reduced glutathione (GSH) (Almatroodi et al. 2020). Therefore, these antioxidant enzymes are essential for repairing liver damage.
Research has demonstrated that egg white hydrolysates can ameliorate orotic acid-induced liver damage (Jiang et al. 2021). However, there are many different models of hepatoprotective experiments for hepatoprotection, such as CCl4, alcohol, diethylnitrosamine, and acetaminophen. CCl4 is a causative agent of various diseases due to its generation of oxidative stress (Ganesan et al. 2018). Therefore, this study used CCl4 as an inducer to evaluate the potential of EWH to be hepatoprotective.
To assess its hepatoprotective potential, this research examined the antioxidant effect of egg white hydrolysates (EWH) on the in vitro (HepG2 cells) and in vivo (carbon tetrachloride (CC14)-induced hepato-toxicity in Sprague–Dawley rats) experimental. The in vitro experimental of HepG2 cells (glutathione peroxidase (GPx) activity and reduced glutathione (GSH) levels) and in vivo experiments of CC14-induced hepatotoxicity in Sprague–Dawley rats (growth characteristics, serum biochemical parameters, malondialdehyde (MDA), oxidized glutathione (GSSG) and GSH levels) were evaluated to determine the potential of EWH for its hepatoprotective.
Materials and methods
Preparation of egg white hydrolysates
The egg white was purchased from Fu Che Frozen Food Co., Ltd. (Kaohsiung, Taiwan). Papain was purchased from Champion Co., Ltd. (Taipei, Taiwan). Protease A was purchased from Ho Jun Biotechnology Co., Ltd. (Taoyuan, Taiwan). The preparation of egg white hydrolysates (EWH) was based on the method developed by our previous study (Ho et al. 2021). Fresh egg whites were homogenized with water and heated to 50 °C. Then, 0.047% enzyme of papain and protease A (1:1 ratio) were added, and the mixture was hydrolyzed at 50 °C for 1 h, followed by 68 °C for 2 h. Subsequently, reacting at 95–98 °C for 10 min the enzyme was inactivated and centrifuged (at 4 °C for 5 min, 6000× g). Then the supernatant was taken and sterilized at 58 °C for 3.5 min, which is EWH. Our previous research has shown that egg whites hydrolyzed with enzymes (papain and protease A) can be obtained at a hydrolysis rate of 30.11% (Ho et al. 2021), which is higher than the previous reported 25% hydrolysis rate (Horimoto and Lim 2017).
Composition of free amino acid
High-performance liquid chromatography (HPLC) and reversed-phase high-performance liquid chromatography-mass spectrometry (RP-HPLC/MS/MS) were used to analyze the free amino acid compositions of EWH. Before injection, the EWH was filtered through a PVDF membrane (0.45 μm). Amino acids were chromatographically separated on the Capcell Pak C-18MGII 100A (250 nm × 4.6 mm) column. At a rate of 1 mL/min, a binary gradient of acetic acid (mobile phase A) and methanol (mobile phase B) containing acetonitrile (ratio 50: 50) was passed through the system. HPLC separated the product obtained after the EWH, 8 mL was taken and analyzed by RP-HPLC/MS/MS, and data processing was performed with the TurboSEQUEST (Therom Finning, San Jose, CA, USA).
The in vitro experimental
Cell culture
HepG2 cells obtained from the BCRC (Hsinchu, Taiwan) were cultured in Dulbecco’s Modified Eagle Medium (Gibco, Grand Island, NY, USA), supplemented with 1% penicillin/streptomycin (100 U/mL), and 10% FBS were purchased from Gibco (Grand Island, NY, USA) and 3.7 g/L sodium bicarbonate (Sigma-Aldrich, St. Louis, MO, USA) in humidified 5% CO2 at 37 °C.
Cell experimental design
HepG2 cells were seeded at a density of 3 × 106 cells/mL in a 3.5 cm dish overnight, followed by treatment with control (non-treated) and EWH (0.1, 0.5, 1, and 5 mg/mL) for 24 h to detect cell viability, GPx activity, and GSH levels.
Analysis of cell viability on HepG2 cells
HepG2 cells after EWH treatment, 1 mL of 0.1 mg/mL 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide solution (Sigma-Aldrich, St. Louis, MO, USA) solution was added, and cells were cultured for 3 h in a 37 °C, 5% CO2 humidified environment. The dish plates were shaken for 15 min to solubilize the formazan dye by adding 1 mL isopropanol (J.T Baker, Center Valley, Penn, USA). Subsequently, the mixture was centrifuged (9,560 × g for 10 min at 4 °C), and the absorbance of the supernatant was determined using ELISA (540 nm).
Analysis of GSH levels on HepG2 cells
After the EWH treatment, the cells were washed in 1 mL of phosphate-buffered saline (PBS; Unionward, Taipei, Taiwan), and then 1 mL of 1 M per-chloric acids (containing 2 mM 1,10-phenanthroline) (Sigma-Aldrich, St. Louis, MO, USA) was added for cell detachment. The supernatant was utilized as a sample for analysis after being centrifuged for 10,000 × g, 10 min. Iodoacetic acid (100 mM in PBS) was combined with the 400 mL supernatant for 15 min. After potassium hydrogen carbonate had been neutralized, HPLC was used to determine the amount of GSH in the supernatant (Wu et al. 2001).
The in vivo experimental
Experimental animals
Six-week-old male SD rats were purchased from the National Laboratory Animal Centre (Taipei, Taiwan). The SD rats were kept in polypropylene cages and kept in a controlled room environment at a temperature of 22 °C with a 12 h light/12 h dark cycle. All animal care and experimental methods used in this study were authorized by the National Kaohsiung University of Science and Technology's Institutional Animal Care and Use Committees (Kaohsiung, Taiwan).
Animal experimental design
The SD rats (n = 30; 6 per experimental group) were randomly divided into five groups. Group 1, the control group; group 2, the CCl4 group (20%, 0.5 mL/kg BW); group 3, silymarin (200 mg/kg BW) + CCl4 group; group 4, EWH (L) (38.5 mg/kg BW) + CCl4 group; group 5, EWH (H) (385 mg/kg BW) + CCl4 group for a period of 8 weeks. Groups 3 to group 5 SD rats were orally administered 20% CCl4 (0.5 mL/kg BW) of one hour after the administration of the experimental drugs twice a week. Silymarin was a positive control in this study (Madrigal-Santillán et al. 2014). The dose of EWH was converted based on the blood and body weight of the rats performed in the cellular model of this study.
Body weight and food intake were measured twice a week as part of the study. 12 h of fasting was followed by carbon dioxide euthanasia for SD rats. The chamber capacity was displaced at a rate of 20–30% per minute via CO2-based euthanasia. Blood samples were drawn via cardiac punctures to collect serum biochemical markers for further examination. The liver tissues detect MDA, GSH, and GSSG levels.
Analysis of serum biochemical parameters on CCl4-induced hepatotoxicity in SD rats
The blood was centrifuged (at 4 °C for 10 min, 1100× g), and the serum was used for biochemical parameters assays as a sample. Commercial kits (RANDOX, Ireland, UK) were used to determine the concentrations of total cholesterol (TC, CH202) and triglyceride (TG, TR212) decided according to the manufacturer’s description. Aspartate aminotransferase activity (AST, ab105135), blood urea nitrogen (BUN, ab83362), and alanine transaminase activity (ALT, ab105134) were purchased from Abcam (Cambridge, UK), and determined by manufacturer’s description.
Analysis of MDA levels on CCl4-induced hepatotoxicity in SD rats
The 400 μL of tissue homogenate sample was mixed in a test tube with 200 μL of 20 mM PBS (not including triton x-100), 250 μL of 3% SDS, 1 mL of 0.1 N HCl, 0.7% thiobarbituric acid (500 μL), and 10% phosphotungstic acid (150 μL) was heated for 30 min (boiling water bath), followed by rapid cooling. Subsequently, 2.5 mL of n-butyl alcohol was added and centrifuged at 4ºC for 15 min, 860 × g. With the use of an ELISA reader, the OD value of the supernatant was determined at 530 nm. MDA content was expressed as nmol/mg protein.
Analysis of GSH and GSSG levels on CCl4-induced hepatotoxicity in SD rats
1 M perchloric acid (1 mL) was mixed with tissue homogenate sample (400 μL) and centrifuged at 4 °C for 10,000 × g, 10 min). The 40 μL of iodoacetic acid (100 mM in PBS) was added to the sample for 15 min. HPLC was used to determine the GSH and GSSG concentration in the supernatants following neutralization with potassium hydrogen carbonate (Wu et al. 2001). GSH and GSSG level was expressed as nmol/mg protein.
Observation of histopathological condition on CCl4-induced hepatotoxicity in SD rats
The liver tissues were 10% formalin-treated before being embedded in paraffin. Hematoxylin and eosin staining was performed on the paraffin-embedded tissues by Harris' staining methodology. The microscope (Olympus, Tokyo, Japan) was used to observe the stained slides.
Statistical analysis
All data were expressed as mean ± SD. One-way ANOVA was used for statistical analysis (SPSS version 12.0, USA), followed by Duncan's multiple range tests. This research’s statistical significance was defined as a P value < 0.05.
Results
The free amino acids composition in EWH
The free amino acids are contained, as shown in Table 1. EWH contains 17 free amino acids, and the total amino acid content has 1443.7 mg/100 g. Among them, the content of glutamic acid was the highest, the second highest was aspartic acid, and the third was cystine, which was 204, 141, and 125 mg/100 g, respectively.
Table 1.
Free amino acid composition of EWH
| Sample | Parameter | |||
|---|---|---|---|---|
| Essential amino acid | Non-essential amino acid | |||
| EWH | Histidine | 45.7 ± 0.1 | Asparagine | 141.0 ± 0.1 |
| Threonine | 54.4 ± 0.1 | Glutamic acid | 204.0 ± 0.0 | |
| Valine | 85.3 ± 0.5 | Serine | 93.6 ± 0.2 | |
| Methionine | 19.9 ± 0.1 | Glycine | 45.0 ± 0.3 | |
| Phenylalanine | 94.0 ± 0.2 | Arginine | 85.0 ± 0.0 | |
| Isoleucine | 69.5 ± 0.1 | Alanine | 82.0 ± 0.1 | |
| Leucine | 113.0 ± 0.0 | Tyrosine | 48.8 ± 0.1 | |
| Lysine | 85.3 ± 0.1 | Cystine | 125.0 ± 0.3 | |
| Total | 567.1 ± 1.2 | Proline | 52.0 ± 0.2 | |
| Total | 876.4 ± 1.3 | |||
| Total free amino acids | 1443.7 ± 2.5 | |||
All values are presented as mean ± standard deviation (n = 3). EWH Egg white hydrolysates
The in vitro experiments
Effects of EWH on cell viability in HepG2 cells
The cell viability for HepG2 cells of the EWH at different concentrations was shown in Fig. 1. EWH at 0.1, 0.5, 1, and 5 mg/mL was treated to HepG2 cells for 24 h; the cell viability of the EWH group was not statistically different from that of control cells. According to the findings, EWH was not cytotoxic to HepG2 cells.
Fig. 1.
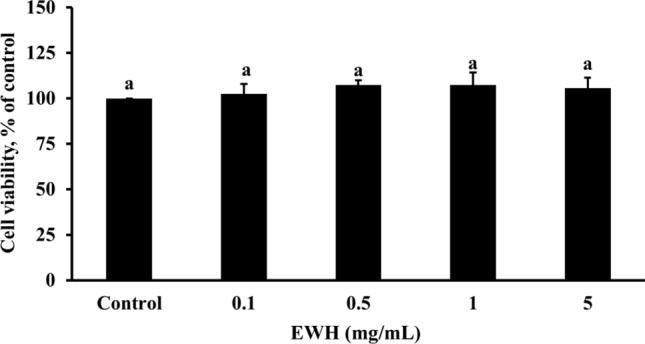
Effects of the EWH on cell viability in HepG2 cells. All values are presented as mean ± standard deviation (n = 4). According to Duncan's multiple range tests, an alphabet letter different from the others indicates a significant difference from the others at P < 0.05. EWH: Egg white hydrolysates
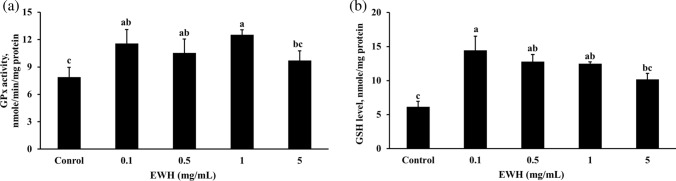
Effects of EWH on GPx activity and GSH levels in HepG2 cells
The GPx activity and GSH levels for HepG2 of the EWH at different concentrations were shown in Fig. 2. The GPx activity (Fig. 2a) and GSH levels (Fig. 2b) of cells that received 0.1, 0.5, and 1 mg/mL of EWH were significantly increased than that of the control group.
Fig. 2.
Effects of the EWH on GPx activity (a), and GSH levels (b) in HepG2 cells. All values are presented as mean ± standard deviation (n = 4). According to Duncan's multiple range tests, an alphabet letter different from the others indicates a significant difference from the others at P < 0.05. EWH: Egg white hydrolysates
The in vivo experiments
Effect of EWH on the growth performance and serum biochemical parameters of CCl4-induced hepatotoxicity in SD rats
The development traits and organ-related body weights for SD rats from the various groups are shown in Table 2. After 8 weeks of feeding, the groups had no remarkable change in weight gain and food intake. However, the CCl4 group caused a significantly increased in liver weight. Compared to the CCl4 group, SD rats treated with silymarin + CCl4, EWH (L) + CCl4, and EWH (H) + CCl4 showed a significant decrease in liver weight. The weights of the kidneys, spleen, and heart did not differ between the groups.
Table 2.
Effect of EWH on growth characteristics and serum biochemical parameters in CCl4-induced hepatotoxicity SD rats
| Sample | Control | CCl4 | CCl4 + Silymarin | CCl4 + EWH (L) | CCl4 + EWH (H) |
|---|---|---|---|---|---|
| Food intake (g) | 174.0 ± 11.3a | 174.5 ± 10.6a | 166.2 ± 5.80a | 177.3 ± 17.0a | 163.2 ± 10.1a |
| Weight gain (g) | 285.1 ± 0.65a | 285.3 ± 13.1a | 257.5 ± 12.0a | 270.6 ± 18.1a | 279.1 ± 10.5a |
| Heart (%) | 0.33 ± 0.01a | 0.34 ± 0.01a | 0.35 ± 0.02a | 0.33 ± 0.03a | 0.34 ± 0.01a |
| Liver (%) | 3.14 ± 0.32b | 4.28 ± 0.43a | 3.54 ± 0.06b | 3.65 ± 0.10b | 3.33 ± 0.07b |
| Spleen (%) | 0.17 ± 0.02a | 0.18 ± 0.02a | 0.21 ± 0.04a | 0.19 ± 0.02a | 0.21 ± 0.08a |
| Kidney (%) | 0.86 ± 0.02a | 0.90 ± 0.02a | 0.90 ± 0.03a | 0.94 ± 0.07a | 0.91 ± 0.05a |
| AST (U/L) | 151.8 ± 22.5b | 216.5 ± 4.9a | 162.5 ± 39.3b | 129.3 ± 9.8b | 158.0 ± 7.0b |
| ALT (U/L) | 46.0 ± 4.6c | 234.6 ± 26.4a | 136.5 ± 17.6b | 65.6 ± 29.4c | 80.0 ± 4.2c |
| BUN (mg/dL) | 17.9 ± 2.1a | 18.6 ± 1.1a | 17.2 ± 0.5a | 18.3 ± 1.4a | 17.6 ± 1.0a |
| TG (mg/dL) | 59.0 ± 17.3a | 68.4 ± 18.0a | 51.2 ± 8.8a | 57.8 ± 16.7a | 71.4 ± 27.7a |
| TC (mg/dL) | 64.5 ± 5.6a | 59.0 ± 1.0a | 63.0 ± 2.8a | 68.0 ± 15.3a | 68.6 ± 5.7a |
All values are presented as mean ± standard deviation (n = 6). According to Duncan's multiple range tests, an alphabet letter different from the others indicates a significant difference from the others at P < 0.05. The calculation for organ weight is (Organ weight/body weight) * 100. EWH Egg white hydrolysates, EWH (L) Egg white hydrolysates 38.5 mg/kg BW, EWH (H) Egg white hydrolysates 385 mg/kg BW
The changes in serum biochemical parameters for SD rats of the various groups are shown in Table 2. The AST level in the CCl4 group remarkably increased from 151.8 to 216.5. The ALT level in the CCl4 group remarkably increased from 46.0 to 234.6. Treatment with silymarin + CCl4 reduced AST and ALT levels to 24.9% and 41.8% (P < 0.05). In addition, treatment with EWH (L) + CCl4 and EWH (H) + CCl4 group reduced serum AST (40.3 and 27.0%) and ALT (72.0 and 65.9%) levels. The BUN, TG, and TC levels from various groups show no differences.
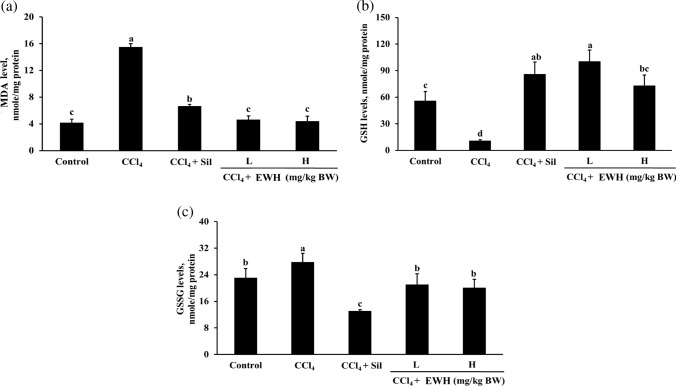
Effect of EWH on MDA, GSH and GSSG levels of CCl4-induced hepatotoxicity in SD rats
The MDA, GSH and GSSG levels in the liver for various groups of SD rats are shown in Fig. 3. The MDA levels of SD rats that received the CCl4 group were significantly increased than that of the control group (Fig. 3a). Silymarin + CCl4, EWH (L) + CCl4, and EWH (H) + CCl4 group significantly had reduced MDA levels compared with those obtained in the CCl4 group (P < 0.05). Notably, the effects of the EWH (L) + CCl4 and EWH (H) + CCl4 groups were better than that of silymarin + CCl4 in reducing the MDA levels. The GSH levels of SD rats that received the CCl4 group were significantly decreased than that of the control group (Fig. 3b). Silymarin + CCl4, EWH (L) + CCl4, and EWH (H) + CCl4 group significantly increased the GSH levels compared with those obtained in the CCl4 group (P < 0.05). The GSSG levels of the CCl4 group were considerably higher than the control group (Fig. 3c). Silymarin + CCl4, EWH (L) + CCl4, and EWH (H) + CCl4 group significantly reduced the GSSG levels compared with those obtained in the CCl4 group (P < 0.05).
Fig. 3.
Effects of the EWH on MDA (a), GSH (b), and GSSG (c) levels in HepG2 cells. All values are presented as mean ± standard deviation (n = 4). According to Duncan's multiple range tests, an alphabet letter different from the others indicates a significant difference from the others at P < 0.05. EWH: Egg white hydrolysates
Effect of EWH on histopathological examination of CCl4-induced hepatotoxicity in SD rats
A histological examination was performed to detect liver damage. The livers of mice from the control group exhibited normal hepatocyte structure (Fig. 4). In contrast, the CCl4 group treatment caused cell morphology disruption (cell structural damage) and hepatocyte vacuolation. However, CCl4-induced liver injuries were ameliorated by pre-administration with silymarin + CCl4, EWH (L) + CCl4, and EWH (H) + CCl4 group.
Fig. 4.
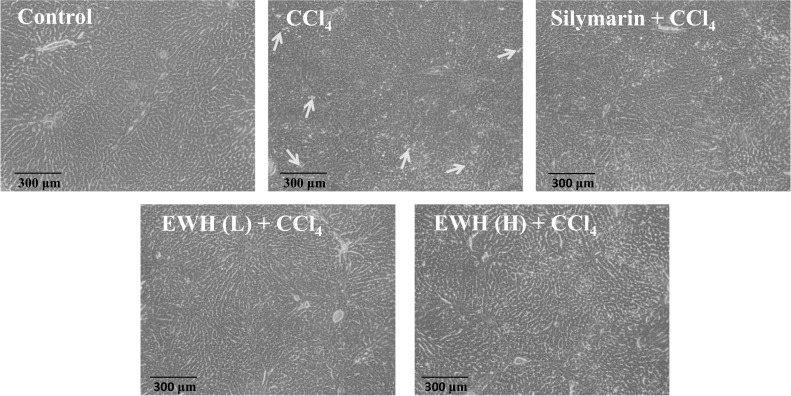
Effects of the EWH on liver photomicrographs in CCl4-induced hepatotoxicity SD rats (100 ×). The yellow arrowheads showed tissue damage. EWH: Egg white hydrolysates; EWH (L): Egg white hydrolysates 38.5 mg/kg BW; EWH (H): Egg white hydrolysates 385 mg/kg BW
Discussion
Research has demonstrated that the protein hydrolysate by enzymatic hydrolysis of food can enhance the antioxidant capability of the liver. Pine nut protein hydrolysate (Liu et al. 2021), and wild jujube seed protein hydrolysates (Han et al. 2022) can enhance GPx activity in HepG2 cells. In addition, silk protein hydrolysates (Kim et al. 2017) and Morchella esculenta protein hydrolysate (Zhang et al. 2019) increased GSH content in HepG2 cells. HepG2 cell line can be used as an alternative model for hepatocytes, which has the morphological and biochemical characteristics of normal hepatocytes and has been widely used to study the cytoprotective mechanisms of antioxidants (Gomez-Lechon et al. 2008). In addition, GSH is an important antioxidant molecule in organisms, mainly involved in detoxifying (Wahid et al. 2016). GPx is an important antioxidant enzyme in organisms, which can reduce harmful peroxides to non-toxic hydroxyl compounds and water with GSH (Liang et al. 2017). This study found that EWH can increasethe activity of GPx and GSH content in HepG2 cells. The study indicated that EWH could promote the activity of GPx and GSH, thereby enhancing the antioxidant capacity of the liver to maintain normal liver functioning.
CCl4 has been widely studied as causing liver damage in animal models (Ganesan et al. 2018). In addition, the degree of liver damage is assessed by the levels of AST and ALT in serum and histopathological examination (Recknagel et al. 1989). Elevated levels of AST and ALT in serum have been reported because liver damage releases these enzymes into the serum (Recknagel et al. 1989). Research has shown that food-derived protein hydrolysates can reduce the levels of ALT and AST in CCl4-induced rats, such as fish protein hydrolysate (Rabiei et al. 2022), and Klunzinger’s mullet protein hydrolysates (Rabiei et al. 2019). In this study, the CCl4 group elevated serum levels of ALT, AST and caused cell structural damage and hepatocyte vacuolation of liver tissues, which is due to liver damage, thereby allowing these enzymes to be released into the serum. EWH can restore the levels of AST and ALT in serum to near normal or be only slightly elevated and improve liver tissue damage, indicating protection against liver damage. On the other hand, oxidative stress contributes to the initiation and progression of liver injury (Ganesan et al. 2018). In addition, the hepatotoxic metabolites of CCl4 will bind to antioxidant enzymes and produce MDA that causes liver damage, reducing the level of the antioxidant molecule GSH, thereby causing oxidative stress (Ganesan et al. 2018). Therefore, natural substances with antioxidant capacity may regulate hepatotoxic metabolites produced by CCl4. Papain-hydrolyzed buffalo milk protein (Abdel-Hamid et al. 2020) and Pleurotus ostreatus protein hydrolysates (Zhang et al. 2020) can decrease MDA levels and increase GSH content in CCl4-induced hepatotoxicity in rats. In this study, EWH reduced levels of MDA and elevated the content of GSH in CCl4-induced hepatotoxicity in SD rats. These results indicated that EWH could reduce the levels of ALT, AST, and MDA and improve the damage to liver tissue, which may be attributed to the improvement of liver antioxidant capacity.
Amino acids are essential for metabolism and liver detoxification (Lee et al. 2019). Alanine, glutamic acid, aspartic acid, glycine, histidine, and arginine can prevent chemical-induced liver damage (including hepatocyte necrosis and hepatic fibrosis) (Lee et al. 2019). Serine is involved in glycogen storage in the liver and muscles and forms antibodies to increase immunity (Lee et al. 2019). In addition, some amino acids have been shown to have antioxidant activity, including phenylalanine, lysine, threonine, leucine, and isoleucine (Sarmadi et al. 2010). This study showed that EWH obtained by enzymatic hydrolysis of egg white contains amino acids (including serine, histidine, alanine, glycine, glutamic acid, arginine, and aspartic acid) that are advanced to liver detoxification. Moreover, EWH also contains amino acids with antioxidant activity (such as phenylalanine, lysine, threonine, leucine, and isoleucine). Therefore, EWH can enhance liver antioxidant activity and protect liver function, which may be attributed to the production of functional amino acids after enzymatic hydrolysis.
Egg protein has been widely used as a texture improver and stabilizer in food, due to foaming, emulsifying, and heat-setting properties (Garcés-Rimón et al. 2016). Enzyme hydrolysis improves the added value of food protein by modifying its physical and nutritional properties, such as improving the stabilization of emulsions and foams, as well as digestibility and nutrient bioavailability (Garcés-Rimón et al. 2016). Our previous research has shown that EWH can improve the viscosity, surface tension, and weight loss analysis of sterilized liquid egg white. In addition, the structure and appearance of the cakes were acceptable to consumers (Ho et al. 2021). Huang et al. (2022) indicated that the spoon cookies made by adding EWH to sterilized liquid egg white had good acceptability to consumers. Furthermore, López-Martínez et al. (2021) have shown that egg white hydrolysate can be used as a good raw material to replace dairy products in ice cream. Therefore, EWH has a high potential to be used in the food industry and can improve the sustainability of the egg industry.
Conclusion
This study suggested that EWH exhibited antioxidant activity of HepG2 cells and hepatoprotective activity against CCl4-induced hepatotoxicity in SD rats. EWH reduces MDA levels through the elevated antioxidant activity of livers such as GPx and GSH, thereby the significant decrease of serum ALT and AST levels. These results suggested that EWH may have a role in the prevention of associated liver disorders. In the future, EWH can be applied to the food industry, natural food supplements, and health products.
Acknowledgements
The authors are grateful to appreciate the invaluable support of the Ministry of Science and Technology of Taiwan in this study.
Abbreviations
- GPx
Glutathione peroxidase
- GSH
Reduced glutathione
- CCl4
Carbon tetrachloride
- AST
Serum aspartate aminotransferase
- ALT
Alanine aminotransferase
- MDA
Malondialdehyde
- GSSG
Oxidized glutathione
- ROS
Reactive oxygen species
- DMEM
Dulbecco’s modified eagle medium
- PBS
Phosphate-buffered saline
Author contributions
Conceptualization, Y-TC, and C-WT; methodology, Y-TC, and Y-AC; validation, C-YH, R-QX, and C-HK; formal analysis, C-WT, and C-YH; investigation, C-HK, and C-CW; resources, Y-AC, and R-QX; data curation, Y-TC; writing-original draft preparation, Y-TC; writing-review and editing, C-YH, C-CW and S-LH; supervision, S-LH; project administration, S-LH All authors read and agreed to the published version of the manuscript.
Funding
This study was funded with the support of the Ministry of Science and Technology of Taiwan (Grant No. MOST 103-2815-C-022-006-B).
Availability of data and materials
The datasets generated and/or analyzed during the current study are not publicly available due [REASON WHY DATA ARE NOT PUBLIC] but are available from the corresponding author at reasonable request.
Code availability
Not applicable.
Declarations
Conflict of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this manuscript.
Consent to participate
The MS has been approved by all authors.
Consent for publication
Not applicable.
Ethics approval
The work has not been published before and it is not under consideration for publication elsewhere.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Ya-Ting Chen, Email: melodyyu.chen@gmail.com.
Chao-Wen Tu, Email: e23magic@gmail.com.
Chih-Yao Hou, Email: chihyaohou@gmail.com.
Yu-An Chen, Email: michael81925@gmail.com.
Ruo-Qi Xu, Email: a1051234109@gmail.com.
Chia-Hung Kuo, Email: kuoch@nkust.edu.tw.
Chih-Chung Wu, Email: wuccmail@gmail.com.
Shu-Ling Hsieh, Email: slhsieh@nkust.edu.tw.
References
- Abdel-Hamid M, Osman A, El-Hadary A, Romeih E, Sitohy M, Li L. Hepatoprotective action of papain-hydrolyzed buffalo milk protein on carbon tetrachloride oxidative stressed albino rats. J Dairy Sci. 2020;103:1884–1893. doi: 10.3168/jds.2019-17355. [DOI] [PubMed] [Google Scholar]
- Almatroodi SA, Anwar S, Almatroudi A, Khan AA, Alrumaihi F, Alsahli MA, Rahmani AH. Hepatoprotective effects of garlic extract against carbon tetrachloride (CCl4)-induced liver injury via modulation of antioxidant, anti-inflammatory activities and hepatocyte architecture. Appl Sci. 2020;10(6200):6200. doi: 10.3390/app10186200. [DOI] [Google Scholar]
- Chen C, Chi YJ, Zhao MY, Lv L. Purification and identification of antioxidant peptides from egg white protein hydrolysate. Amino Acids. 2012;43:457–466. doi: 10.1007/s00726-011-1102-0. [DOI] [PubMed] [Google Scholar]
- Ganesan K, Jayachandran M, Xu B. A critical review on hepatoprotective effects of bioactive food components. Crit Rev Food Sci Nutr. 2018;58:1165–1229. doi: 10.1080/10408398.2016.1244154. [DOI] [PubMed] [Google Scholar]
- Garcés-Rimón M, Sandoval M, Molina E, López-Fandiño R, Miguel M. Egg protein hydrolysates: New culinary textures. Int J Gastron Food Sci. 2016;3:17–22. doi: 10.1016/j.ijgfs.2015.04.001. [DOI] [Google Scholar]
- Gomez-Lechon MJ, Donato MT, Lahoz A, Castell JV. Cell lines: a tool for in vitro drug metabolism studies. Curr Drug Metab. 2008;9:1–11. doi: 10.2174/138920008783331086. [DOI] [PubMed] [Google Scholar]
- Han R, Shao S, Zhang H, Qi H, Xiao F, Shen Y, Fan L, Wang H, Zhao D, Li G, Yan M. Physico-chemical properties, antioxidant activity, and ACE inhibitory activity of protein hydrolysates from wild jujube seed. J Food Sci. 2022;87:2484–2503. doi: 10.1111/1750-3841.16157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho HY, Ciou JY, Qiu YT, Hsieh SL, Shih MK, Chen MH, Tu CW, Hsieh CW, Hou CY. Improvement of foaming characteristics and stability of sterilized liquid egg with egg white hydrolysate (EWH) Foods. 2021;10:1326. doi: 10.3390/foods10061326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horimoto Y, Lim LT. Effects of different proteases on iron absorption property of egg white hydrolysates. Food Res Int. 2017;95:108–116. doi: 10.1016/j.foodres.2017.02.024. [DOI] [PubMed] [Google Scholar]
- Huang PH, Hazeena SH, Qiu YT, Ciou JY, Hsieh CW, Shih MK, Chen MH, Hou CY. Application of egg white hydrolysate (EWH) to improve frothing functionality of pasteurized liquid egg in large quantity production. Heliyon. 2022 doi: 10.1016/j.heliyon.2022.e12697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahanbani R, Ghaffari SM, Salami M, Vahdati K, Sepehri H, Sarvestani NN, Sheibani N, Moosavi-Movahedi AA. Antioxidant and anticancer activities of walnut (Juglans regia L.) protein hydrolysates using different proteases. Plant Foods Hum Nutr. 2016;71:402–409. doi: 10.1007/s11130-016-0576-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalili-Firoozinezhad S, Filippi M, Mohabatpour F, Letourneur D, Scherberich A. Chicken egg white: Hatching of a new old biomaterial. Mater Today. 2020;40:193–214. doi: 10.1016/j.mattod.2020.05.022. [DOI] [Google Scholar]
- Jiang Z, Kimura Y, Shirouchi B, Tanaka Y, Tsai WT, Yuan X, Sato M. Dietary egg white protein hydrolysate improves orotic acid-induced fatty liver in rats by promoting hepatic phospholipid synthesis and microsomal triglyceride transfer protein expression. J Nutr Biochem. 2021;98:108820. doi: 10.1016/j.jnutbio.2021.108820. [DOI] [PubMed] [Google Scholar]
- Kim JH, Suh HJ, Choi HS. Protective effect of silk protein hydrolysates against tert-BHP induced liver damage. Korean J Food Preserv. 2017;24:107–115. doi: 10.11002/kjfp.2017.24.1.107. [DOI] [Google Scholar]
- Latorres JM, Rios DG, Saggiomo G, Wasielesky W, Prentice-Hernandez C. Functional and antioxidant properties of protein hydrolysates obtained from white shrimp (Litopenaeus vannamei) J Food Sci Technol. 2018;55:721–729. doi: 10.1007/s13197-017-2983-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DY, Kim EH. Therapeutic effects of amino acids in liver diseases: current studies and future perspectives. Eur J Cancer Prev. 2019;24:72. doi: 10.15430/JCP.2019.24.2.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang R, Zhang Z, Lin S. Effects of pulsed electric field on intracellular antioxidant activity and antioxidant enzyme regulating capacities of pine nut (Pinus koraiensis) peptide QDHCH in HepG2 cells. Food Chem. 2017;237:793–802. doi: 10.1016/j.foodchem.2017.05.144. [DOI] [PubMed] [Google Scholar]
- Liu Z, Shi Y, Liu H, Jia Q, Liu Q, Tu J. Purification and identification of pine nut (Pinus yunnanensis Franch.) protein hydrolysate and its antioxidant activity in vitro and in vivo. Chem Biodivers. 2021;18:e2000710. doi: 10.1002/cbdv.202000710. [DOI] [PubMed] [Google Scholar]
- López-Martínez MI, Moreno-Fernández S, Miguel M. Development of functional ice cream with egg white hydrolysates. Int J Gastron Food Sci. 2021;25:100334. doi: 10.1016/j.ijgfs.2021.100334. [DOI] [Google Scholar]
- Madrigal-Santillán E, Madrigal-Bujaidar E, Álvarez-González I, Sumaya-Martínez MT, Gutiérrez-Salinas J, Bautista M, Morales-González A, González-Rubio MGL, Aguilar-Faisal JL, Morales-González JA. Review of natural products with hepatoprotective effects. World J Gastroenterol WJG. 2014;20:14787. doi: 10.3748/wjg.v20.i40.14787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moure A, Domínguez H, Parajó JC. Antioxidant properties of ultrafiltration-recovered soy protein fractions from industrial effluents and their hydrolysates. Process Biochem. 2006;41:447–456. doi: 10.1016/j.procbio.2005.07.014. [DOI] [Google Scholar]
- Rabiei S, Rezaei M, Abasian Z, Khezri M, Nikoo M, Rafieian-Kopaei M, Anjomshoaa M. The protective effect of Liza klunzingeri protein hydrolysate on carbon tetrachloride-induced oxidative stress and toxicity in male rats. Iran J Basic Med Sci. 2019;22:1203. doi: 10.22038/ijbms.2019.33201.7927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabiei S, Rezaei M, Nikoo M, Khezri M, Rafieian-Kopai M, Anjomshoaa M. Antioxidant properties of Klunzinger’s mullet (Liza klunzingeri) protein hydrolysates prepared with enzymatic hydrolysis using a commercial protease and microbial hydrolysis with Bacillus licheniformis. Food Sci Technol Int. 2022;28:233–246. doi: 10.1177/10820132211005297. [DOI] [PubMed] [Google Scholar]
- Recknagel RO, Glende EA, Jr, Dolak JA, Waller RL. Mechanisms of carbon tetrachloride toxicity. Pharmacol Ther. 1989;43:139–154. doi: 10.1016/0163-7258(89)90050-8. [DOI] [PubMed] [Google Scholar]
- Sarmadi BH, Ismail A. Antioxidative peptides from food proteins: a review. Peptides. 2010;31:1949–1956. doi: 10.1016/j.peptides.2010.06.020. [DOI] [PubMed] [Google Scholar]
- Wahid A, Hamed AN, Eltahir HM, Abouzied MM. Hepatoprotective activity of ethanolic extract of Salix subserrata against CCl 4-induced chronic hepatotoxicity in rats. BMC Complement Altern Med. 2016;16:1–10. doi: 10.1186/s12906-016-1238-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Liu X, Xie H, Liu Z, Rakariyatham K, Yu C, Shahidi Y, Zhou D. Antioxidant activity and functional properties of Alcalase-hydrolyzed scallop protein hydrolysate and its role in the inhibition of cytotoxicity in vitro. Food Chem. 2021;344:128566. doi: 10.1016/j.foodchem.2020.128566. [DOI] [PubMed] [Google Scholar]
- Wu CC, Sheen LY, Chen HW, Tsai SJ, Lii CK. Effects of organosulfur compounds from garlic oil on the antioxidation system in rat liver and red blood cells. Food Chem Toxicol. 2001;39:563–569. doi: 10.1016/S0278-6915(00)00171-X. [DOI] [PubMed] [Google Scholar]
- Zhang L, Lu Y, Feng X, Liu Q, Li Y, Hao J, Wang Y, Dong Y, Wang HD. Hepatoprotective effects of Pleurotus ostreatus protein hydrolysates yielded by pepsin hydrolysis. Catalysts. 2020;10:595. doi: 10.3390/catal10060595. [DOI] [Google Scholar]
- Zhang Q, Wu CE, Sun YJ, Li TT, Fan GJ. Cytoprotective effect of Morchella esculenta protein hydrolysate and its derivative against H2O2-induced oxidative stress. Pol J Food Nutr Sci. 2019 doi: 10.31883/pjfns/110134. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analyzed during the current study are not publicly available due [REASON WHY DATA ARE NOT PUBLIC] but are available from the corresponding author at reasonable request.
Not applicable.