Abstract
Background:
It has been proposed that the immunomodulatory capacity of neuraltherapeutic medicine (NTM) functions by means of stimuli to the nervous system, which influences the self-regulatory and plastic capacity of the nervous system, especially through the autonomic balance between the sympathetic and parasympathetic nervous systems. Several studies report the usefulness of NTM in inflammatory pathologies.
Case presentation:
A case report through a retrospective review of the medical history of an 82-year-old male patient with a diagnosis of acute SARS-CoV-2 who received a therapeutic intervention of NTM at the beginning of his hospitalization and presented satisfactory clinical evolution, with a follow-up for 18 months without post-COVID sequelae. A patient diagnosed with acute pneumonia for SARS-CoV-2, and mild ARDS, with markers of severity given by the history of COPD, advanced age, and elevation of LDH, ferritin, and CRP. On the third day of hospitalization, he presented an episode of pulmonary thromboembolism. He presented significant clinical improvement with in-hospital management for 9 days and underwent out-patient control with no post-COVID sequelae.
Conclusions:
NTM could be useful for the management of acute inflammatory diseases, including viral diseases such as SARS-CoV-2, in a mild or severe state of inflammation, when added to allopathic medicine, and it can improve clinical evolution and long-term sequelae. More studies are needed to validate this information.
Keywords: Neural therapeutic medicine, neural therapy, COVID-19, SARS-CoV-2, inflammation, local anesthetics
Background
The world faces a major challenge that offers a chance for growth in the midst of adversity, with great changes in all societal systems. At the end of December, 2019, an infectious outbreak began in Wuhan, China, which spread rapidly to all of humanity, and on March 11, 2020, it was categorized as a pandemic. 1 As of May 1, 2022, there were 513 085 948 confirmed cases, including 6 234 079 deaths worldwide, 2 significantly affecting the global health system and socioeconomic dynamics.
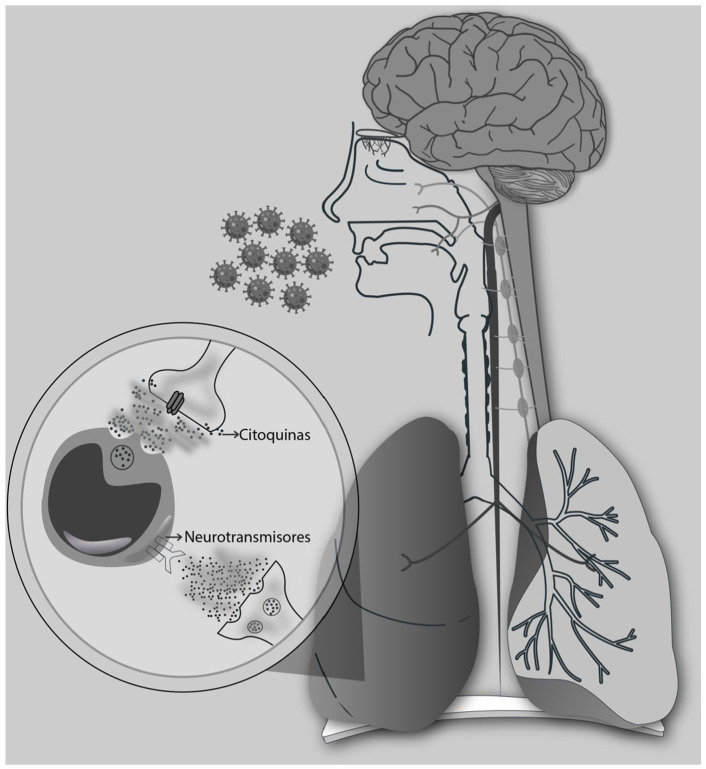
The physiopathology of SARS-CoV-2 infection has been described as cellular entry via endocytosis of angiotensin receptors, in a way similar to other virulent coronaviruses. 3 Initially, it infests nasal epithelial and bronchial cells, as well as pneumocytes. The mechanism of extrapulmonary dissemination is unclear. 4 The function of the immune system and inflammation in its 3 phases, cellular, humoral, 5 and the neuroimmune reflex, 6 is fundamental for controlling the infection (Figure 1). Nevertheless, when the inflammation presents a poor adaptive response in the cellular phase, there is a decrease in the circulation of T, regulatory T, B, and NK cells, 5 and in the humoral phase, it is manifested through a “cytokine storm,” characterized by excessive levels of pro-inflammatory cytokines and an increase in oxidative stress. 7 This process causes indirect damage, perpetuating the pro-inflammatory state, which causes platelet activation and coagulopathies, injury to the pulmonary tissues, and development of severe acute respiratory syndrome (SARS), and promotes multiple organ failure. 4 This mechanism is also seen in other viruses, such as SARS and MERS. 8
Figure 1.
Neuroimmune circuit, bidirectional communication between the nervous system and the immune system by means of neurotransmitters to membrane receptors in leukocytes and cytokines in the membrane receptors of nerve cells. (Source: author).
Recently, participation of the nervous system in the regulation of the immune response and inflammation by means of the “inflammatory reflex,” which monitors and modulates the inflammatory response as an arc reflex in a fast-tracked and unconscious manner in real-time, as occurs with the cardiac function and other vital functions, has been described. 6 The efferent arm of this reflex is called the “cholinergic anti-inflammatory pathway,” which inhibits macrophage activation and pro-inflammatory cytokine release. 9 The cytokines released by leukocytes and glial cells change the strength of the neuronal excitability, a link that directly contributes to the development of untreatable pain. 6 Furthermore, the cells of the neuroimmune system express ligands and receptors in common, forming a feedback loop. 6
The overload of the allostasis of the neural phase can be explained by an autonomic dysfunction called the “hyper-inflammatory reflex,” 10 which has been shown to be related to the pathophysiology of chronic inflammatory diseases such as diabetes, cardiovascular, respiratory, and mental illnesses, epilepsy, and obesity, among others, as well as rheumatoid arthritis and other autoimmune diseases. 6 In the case of acute illnesses, inflammation insufficiently modulated by the nervous system can lead to conditions such as shock. Currently, few known therapeutic developments take such a perspective into account. Several invasive and non-invasive implantable electrical devices that stimulate the vagus nerve have been patented, for the most part approved by the FDA and European agencies, 11 for the treatment of various inflammatory pathologies, but with very high costs and limited access for most of the population. 11 Other emerging approaches in medicine that include these innovative concepts of the role of the nervous system in modulating inflammation in their conceptual structure are becoming more prevalent.
In particular, a school of medicine that originally emerged in Germany under the name of “neural therapy according to Huneke” and later included input by different sources of scientific research in other latitudes has been proposed as a therapeutic possibility for modulating the reflexes of the nervous system.12,13 This school has experienced conceptual and scientific enrichment in Colombia from the sciences of complexity and the Pavlovian synthetic physiology school that had been developing since the 19th century, changing its name to neural therapeutic medicine (NTM).14,15
This school of synthetic physiology, also called the nervism school in Russia, after the Nobel Prize winner in physiology I. P. Pavlov, postulates the involvement of the nervous system in all physiopathological and trophic processes of organs and tissues, integrating the organism into a whole. This unity is generated through the reflexes of the nervous system called conditioned, which are those that allow the creation of an organism’s individuality in response to the external environment. 12 Other authors, such as Speransky, Bykov, Orbeli, and Vischnevsky, not only experimentally verified Pavlov’s findings but also developed therapeutic proposals to address nervous system dysfunctions using low doses of local anesthetics. 16 In this context, the use of anesthetics had purposes beyond a blockade; it sought to use the neural therapeutic effect to stimulate the natural reflexes that regulate inflammation, which for some reason were inhibited or diminished. This allowed them, in a surgical context, to manage critical acute conditions such as sepsis or shock in war victims (16), as well as to modulate inflammation in acute and chronic inflammatory and infectious pathologies of humans and animals. 17
The present article presents a report of a case of management with complementary NTM in a patient with acute SARS-CoV-2 and severe risk factors whose oxygenation disorder rapidly improved despite complications during hospitalization, requiring little time in hospital, and who did not present sequelae during the 18-month follow-up.
Case Presentation
Medical history
82-year-old male patient, known to the NMT service of the “El Tunal” Hospital in Bogotá, admitted for the management osteomuscular pain.
Consultation in January, 2021, for symptoms lasting 5 days suggestive of the evolution of SARS-CoV-2, given dyspnea, cough, anosmia, physical examination findings on admission of TA: 121/66 mmHg., HR: 75/min, FR: 19/min, Tº: 36º, SaO2: 70%, and bi-basal rhonchus, with a history of chronic obstructive pulmonary disease secondary to exposure to biomass smoke up to 60 years of age and low socioeconomic status.
Intervention
In the present case, a single NTM intervention is carried out according to the “visceral-cutaneous reflex” first proposed by Head based on the connection between the skin and the internal organs,18-20 with innervation at the same level as the metameric segment. The intervention with 0.5% procaine injections, consists of application to papules in the dermatomes that are correlated with the segmental level of the lung viscera. The patient was immediately referred to the emergency department.
However, it is important to note that each patient is unique, and an individualized approach based on patient life history and physical examination findings is needed.
Diagnosis, treatment, and follow-up
Admitted to the emergency room without clinical changes, receiving paraclinical service (Table 1), severity with Call score scale 21 class C [13 pts], high risk of progression of SARS-CoV-2 (>50%), hemogram with neutrophilia, renal and hepatic function preserved, with no hydro electrolytic disorder. Indications of severity given by elevation of PCR, ferritin, and lactate dehydrogenase (LDH). Chest x-ray showed an increase in bi-basal opacity with no signs of consolidation.
Table 1.
Paraclinical hospitalization.
| Paraclinical | Result |
|---|---|
| COVID PCR | Positive |
| C-reactive protein | 13.44 mg/L |
| Creatinine | 1.0 mg/L |
| LDH | 806 UI/L |
| D-dimer | 2.5 ug/mL |
| PaO2/FiO2 ratio | 118 |
| Na+ | 140.05 mEq/L |
| K+ | 4.37 mEq/L |
| Cl− | 106.89 mEq/L |
| GOT | 43.92 U/L |
| GPT | 23.46 U/L |
| BUN | 20.1 mg/dL |
| Hemogram | |
| Leukocytes | 8.210/mm |
| Neutrophils | 85.4% |
| Lymphocytes: | 10.6% |
| Platelets | 323.000/mm |
| Glycosylated hemoglobin | 5.3% |
Abbreviations: BUN, blood urea nitrogen; GOT, glutamic-oxaloacetic transaminase; GPT, glutamic pyruvic transaminase; LDH, lactate dehydrogenase.
In the emergency room, treatment was initiated with ampicillin-sulbactam, clarithromycin, dexamethasone, albendazole, and inhalations with ipratropium bromide and salbutamol. On the second day of hospitalization, the patient resolved the oxygenation disorder with a Pa/Fi: 357 and FiO2 32%.
On the third day, a complication occurred, with pulmonary thromboembolism and moderate oxygenation disorder; CT angiography showed pulmonary embolism of the segmental and subsegmental branches of both lower lobes of right predominance for which full anticoagulation was indicated with low molecular weight heparin at 100 mg.
On the seventh day of hospitalization, the oxygenation disorder improved without the requirement of oxygen, SaO2: 89% to the environment.
On the ninth day of hospitalization, the patient was discharged with supplemental oxygen.
1 month after discharge, a follow-up appointment reported the 12th day without supplemental oxygen requirement, with SaO2: >93% and no decrease in functional class, and the last follow-up appointment at 18 months showed no evidence of SARS-CoV-2 sequelae.
Discussion
SARS-CoV-2 infection can range from asymptomatic to mainly compromising the respiratory system, causing pneumonia and SARS, 4 compromising other systems such as the nervous, cardiovascular, gastrointestinal, skin, musculoskeletal, and endocrine ones, until becoming multisystemic. 22
Mortality is highly variable, associated with age, the severity of disease, and comorbidities; it has been described between 0.3% and 2.3% for all patients and 10% to 23% for hospitalized patients, 4 with higher incidence and mortality in populations with social vulnerability 23 and people with diets of low nutrient quality. 24 Furthermore, life expectancy also declined in 2020 in most upper- and upper-middle-income countries. 25
The physiopathology of SARS-CoV-2 is not fully understood. The severity of the disease and death have been related to a disproportionate inflammatory adaptive response known as a “cytokine storm,” 7 related to an autonomic imbalance of the nervous system. 10 Despite advances in prevention with immunization, there are still therapeutic limitations for acute and chronic management. 26 Therefore, it is necessary to generate novel approaches that may even be outside of the current biomedical paradigmatic conception.
Moreover, the systemic medical approach conceives of the human body as a complete biological system, complex systems within harmoniously related systems in homeodynamism, beyond the linearity in molecular biology,27-29 the relevance of the biological field, 30 the exposome, and the direct relationship with the internal and external environment 31 forming a symphony of multiple linearities functioning in unison, “because just as the body is one and has many members, all the members of the body, being many, are one body,” 32 also taking into consideration the complementarity of other complex medical systems. 33
NTM hierarchically conceives of the nervous system as the main regulator of all physiological and structural functions of the human biological system, maintaining a biological, functionally indivisible unit, with systemic responses as a whole, including the immune response, 14 and current evidence confirms the findings of the modulation of the nervous system in all cellular processes, such as cell division, cell differentiation, 34 regulation of gene expression, 35 tissue metabolism, and modulation of acute and chronic inflammation. 9
The stimuli of neural therapy through the regulatory and plastic functions of the nervous system, especially via the autonomic balance, modulates the immune system and the inflammatory reflex,10,13 which has been shown by several studies of the management of inflammatory pathologies and painegli.36-46 Additionally, immunomodulatory and anti-inflammatory properties of procaine, among other local anesthetics, have also been observed. 47 Finally, in the specific case of inflammatory syndrome secondary to SARS-CoV-2 with compromise of the respiratory system, Fischer et al 10 propose the use of neural therapy for the modulation of autonomic dysfunction through certain interventions such as, for example, on the cervical sympathetic chain.
References on the use of interventions as well as the therapeutic use of local anesthetics in NTM infections as well as SARS-CoV-2 are scarce in the medical literature; however, there are publications related to the physiopathogenesis of acute SARS-CoV-2.
Local anesthetic pharmacological properties.
Some studies have suggested that local anesthetics may have anti-inflammatory mechanisms of action, such as the release of inflammatory mediators, the reduction and inhibition of free radicals, as well as antioxidant properties 14 in the autonomic nervous system and nonneuronal tissues.48,49 Changes in the electrical potential of the cell membrane affect the penetration of viruses at the cellular level.50,51 In an in vitro model, an antiviral effect against SARS-CoV-2 was exhibited, 52 and Razavi and Fazly Bazzaz 53 described antimicrobial properties against bacteria and fungi in a review.
Intravenous use of local anesthetics
The intravenous use of local anesthetics has been studied as a potential way to reduce postoperative respiratory complications after extubation. Yang et al 54 performed a meta-analysis, and Aminnejad et al 55 pointed to its use in patients with SARS-CoV-2 undergoing intubation and extubation. Diaz-Vera et al 56 described the use of intravenous lidocaine in 28 SARS-CoV-2 patients, describing pain reduction, anti-stress properties, and improved mood and sleep patterns. Animal models suggest that lidocaine can have beneficial effects on lung function in the setting of lung injury and inflammation . 49
Use of inhaled local anesthetics
Malik et al 50 proposed the use of inhaled lidocaine in SARS-CoV-2 patients based on positive clinical trial results as a steroid-sparing agent in asthma and chronic cough. Ali and El-Mallakh 57 made the same recommendation. Both studies highlight the possibility of bronchospasm, especially in patients with a history of asthma or bronchial hyperreactivity.
Deep interventions with local anesthetics
On the other hand, deep interventions with local anesthetics at nerve centers can be performed. For example, Vishnevsky 58 and other Russian scientists describe a common denominator of neurodystrophy, regardless of the cause of the shock, and the development of other inflammatory processes always begins from a primary nervous system reaction, that is, one due to the overstimulation of the nervous system, which, according to Fischer et al 10 would be equivalent to the hyperinflammatory neuroimmune reflex. The severity of SARS-CoV-2 infection could be conditioned by the basal state of the nervous system, as comorbidities can induce a state of sympathetic arousal that makes the nervous system more prone to imbalance. 59
The use of “vagus-sympathetic” blocks with procaine in the lumbar sympathetic chain (LSC) or cervical sympathetic chain (CSC) or musculoskeletal blocks of the affected limb has beneficial effects on the control of various types of shock, 58 with similar findings to Ossipov. 60 In a randomized clinical study of 30 patients with severe trauma treated with ropivacaine versus saline solution in the CSC, Liu et al 61 recently reported the regulation of the early inflammatory response through the inhibition of the proinflammatory cytokines IL1, IL6, and TNFa (P < .01) and a decrease in the heart rate from 10 to 50 minutes after injection (P < .01) without other respiratory or hemodynamic changes.
In 1934, in a case series of 100 patients with different inflammatory processes of infectious origin, Pshenichnikov and Kharitonov reported the use of Novocaine (procaine) in the LSC, presacral region, and perilesional region, with findings of pain control and “sleeping peacefully.” It is noteworthy that the control of the inflammatory process and abscess formation and, in some patients the control of fever and a decrease in leukocytosis, were also observed 62 ; furthermore, it is important to remark that several of the interventions were unrelated to the metameric segment of tissue compromised by infection.
In a series of 2 cases of the post-acute syndrome of SARS-CoV-2 treated with intervention in the CSC, Liu et al focused on neuroimmune deregulation and related it to changes in cerebral blood flow that persisted after the anesthetic effect of local anesthetic. 63 In a patient who was refractory to conventional treatment, Vinyes et al reported improvement with 3 sessions of neural therapy interventions that were performed according to the patient’s life history and physical examination findings. 64
Conclusions
In this interesting case, the appropriate clinical evolution of a patient with complementary management with NTM was observed, represented by a shorter hospital stay and early rehabilitation. It can be difficult to distinguish the direct beneficial effect of NTM from the effect of conventional treatment with dexamethasone, as both can influence the inflammatory response.
Poor immunological-inflammatory adaptive response is the cornerstone of the severity of acute SARS-CoV-2 infection, a common cause of many acute and chronic diseases. A deep understanding of the physiopathology and modulation, integrating the functioning of the nervous system with the theory of the inflammatory reflex, is a promising field of research for the benefit of human beings and the growth of science in therapeutic conceptions of acute and chronic inflammatory conditions.
NMT could be useful in the treatment of some acute and chronic consequences associated with SARS-CoV-2 infection, and other kinds of inflammation-infection conditions, related to secondary autonomic dysfunction and inflammation by the stimulation of the inflammatory reflex.
Acknowledgments
To the living God for giving us the wisdom to understand the perfect creation of the human being a little and to my teachers for being a source of inspiration.
Footnotes
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by the authors.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: CB: conceptualization (coworking); writing – original draft (lead); formal analysis (lead); writing – review and editing (lead)
LP: Conceptualization (coworking); Writing – original draft (supporting); formal analysis (supporting); Writing – review and editing (supporting).
OM: Writing – review and editing (supporting).
Availability of Data and Materials: Data supporting the findings of this study are available from the corresponding author upon request.
Ethics Approval and Consent to Participate: This research was reviewed and approved by the institutional review board of the National University of Colombia and the Integrated Subnetwork of Southern Health Services, UHMES Tunal.
The participant’s informed consent was obtained.
Consent to Publish: The participant’s informed consent for publication was obtained.
ORCID iD: Carlos Bustamante  https://orcid.org/0000-0002-1366-5824
https://orcid.org/0000-0002-1366-5824
References
- 1.Cucinotta D, Vanelli M.WHO declares COVID-19 a pandemic. Acta Biomed. 2020;91:157-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ritchie H, Mathieu E, Rodés-Guirao L, et al. Coronavirus pandemic (COVID-19). 2022. Accessed April 30, 2022. https://ourworldindata.org/
- 3.Lukassen S, Chua RL, Trefzer T, et al. SARS-CoV-2 receptor ACE2 and TMPRSS2 are primarily expressed in bronchial transient secretory cells. EMBO J. 2020;39:e105114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC.Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;324:782-793. [DOI] [PubMed] [Google Scholar]
- 5.Liu K, Yang T, Peng X, et al. A systematic meta-analysis of immune signatures in patients with COVID-19. Rev Med Virol. 2021;31:e2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tracey KJ.The inflammatory reflex. Nature. 2002;420:853-859. [DOI] [PubMed] [Google Scholar]
- 7.Hu B, Huang S, Yin L.The cytokine storm and COVID-19. J Med Virol. 2021;93:250-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tay MZ, Poh CM, Rénia L, MacAry PA, Ng LFP. The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol. 2020;20:363-374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pavlov VA, Tracey KJ.The vagus nerve and the inflammatory reflex—linking immunity and metabolism. Nat Rev Endocrinol. 2012;8:743-754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fischer L, Barop H, Ludin SM, Schaible HG.Regulation of acute reflectory hyperinflammation in viral and other diseases by means of stellate ganglion block. A conceptual view with a focus on covid-19. Auton Neurosci. 2022;237:102903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnson RL, Wilson CG.A review of vagus nerve stimulation as a therapeutic intervention. J Inflamm Res. 2018;11:203-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dosch P, Dosch M.Theoretical principles. In: Dosch P, Dosch M, eds. Manual of Neural Therapy According to Huneke (Regulating Therapy with Local Anesthetics). Thieme; 2007;9-10, https://books.google.com/books?id=fdWZzgEACAAJ (accessed March 2022). [Google Scholar]
- 13.Engel R, Barop H, Giebel J, Ludin SM, Fischer L.The influence of modern neurophysiology on the previous definitions of “Segment” and “Interference Field” in neural therapy. Complement Med Res. 2022;29:257-267. doi: 10.1159/000522391 [DOI] [PubMed] [Google Scholar]
- 14.Tarazona J, Bonilla L, Adarme I, et al. La Terapia Neural/Medicina Neuralterapeútica(MNT) en contexto de pandemia. 2020. https://acolten.com.co/wp-content/uploads/2020/07/LA-TERAPIA-NEURAL-MEDICINA-NEURALTERAPEUTICA-MNT-EN-CONTEXTO-DE-PANDEMIA_compressed.pdf (accessed March 2022).
- 15.Dussán EHB, Oviedo JA v. Medicina Neuralterapéutica: Un Abordaje Desde Los Sistemas Médicos Complejos. Universidad Nacional de Colombia; 2013. https://books.google.nl/books?id=m7CftAEACAAJ (accessed March 2022). [Google Scholar]
- 16.Dosch P, Dosch M.Chronological survey. In: Dosch P, Dosch M, eds. Manual of Neural Therapy According to Huneke (Regulating Therapy With Local Anesthetics). Thieme; 2007;25-30, https://books.google.com.co/books?id=oiIl85eoBwoC (accessed March 2022). [Google Scholar]
- 17.Speransky A.A Basis for the Theory of Medicine. International Publishers; 2021. https://books.google.com/books?id=fdWZzgEACAAJ (accessed March 2022). [Google Scholar]
- 18.Beltrán Molano ML, Pinilla Bonilla LB, Beltrán Dussan EH, Vásquez Londoño CA.Anatomo-functional correlation between head zones and acupuncture channels and points: a comparative analysis from the perspective of neural therapy. Evid Based Complement Alternat Med. 2014;2014:836392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Head H.On disturbances of sensation with especial reference to the pain of visceral disease. Brain. 1893;16:1-133. [Google Scholar]
- 20.Navarro K, Pinilla L.The contributions of Henry Head to neuroanatomical and physiological bases of therapy segment. Rev Medicas UIS. 2013;26:33-44. http://www.scielo.org.co/scielo.php?script=sci_arttext&pid=S0121-03192013000300004 (accessed March 2022). [Google Scholar]
- 21.Ji D, Zhang D, Xu J, et al. Prediction for progression risk in patients with COVID-19 pneumonia: the CALL score. Clin Infect Dis. 2020;71:1393-1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Merad M, Blish CA, Sallusto F, Iwasaki A.The immunology and immunopathology of COVID-19. Science. 2022;375:1122-1127. [DOI] [PubMed] [Google Scholar]
- 23.Bilal U, Tabb LP, Barber S, Diez Roux AV.Spatial inequities in COVID-19 testing, positivity, confirmed cases, and mortality in 3 U.S. cities: an ecological study. Ann Intern Med. 2021;174:936-944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Merino J, Joshi AD, Nguyen LH, et al. Diet quality and risk and severity of COVID-19: a prospective cohort study. Gut. 2021;70:2096-2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Islam N, Jdanov DA, Shkolnikov VM, et al. Effects of covid-19 pandemic on life expectancy and premature mortality in 2020: time series analysis in 37 countries. BMJ. 2021;375:e066768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim JH, Marks F, Clemens JD.Looking beyond COVID-19 vaccine phase 3 trials. Nat Med. 2021;27:205-205-211. [DOI] [PubMed] [Google Scholar]
- 27.Kirschner MW.The meaning of systems biology. Cell. 2005;121:503-504. [DOI] [PubMed] [Google Scholar]
- 28.Goldstein DS.How does homeostasis happen? Integrative physiological, systems biological, and evolutionary perspectives. Am J Physiol Regul Integr Comp Physiol. 2019;316:R301-R317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rattan SI.Molecular gerontology: from homeodynamics to hormesis. Curr Pharm Des. 2014;20:3036-3039. [DOI] [PubMed] [Google Scholar]
- 30.Walma DAC, Yamada KM.The extracellular matrix in development. Development. 2020;147:dev175596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morales JS, Valenzuela PL, Castillo-García A, et al. The exposome and immune health in times of the COVID-19 Pandemic. Nutrients. 2021;14:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.De Tarso S. La Biblia. In: 1 Corintios. Reina Vale. 1960. [Google Scholar]
- 33.Badakhsh M, Dastras M, Sarchahi Z, Doostkami M, Mir A, Bouya S.Complementary and alternative medicine therapies and COVID-19: a systematic review. Rev Environ Health. 2021;36:443-450. [DOI] [PubMed] [Google Scholar]
- 34.Davis EA, Dailey MJ.A direct effect of the autonomic nervous system on somatic stem cell proliferation? Am J Physiol Regul Integr Comp Physiol. 2019; 316:R1-R5. [DOI] [PubMed] [Google Scholar]
- 35.Cole SW, Nagaraja AS, Lutgendorf SK, Green PA, Sood AK.Sympathetic nervous system regulation of the tumour microenvironment. Nat Rev Cancer. 2015;15:563-572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Garzón J.Modificaciones En La Calidad de Vida En Pacientes Con Dolor Osteomuscular Tratados Con Terapia Neural En La Consulta Externa. Universidad Nacional de Colombia Sede Bogotá; 2012. [Google Scholar]
- 37.Nazlıkul H, Ural FG, Öztürk GT, Öztürk ADT. Evaluation of neural therapy effect in patients with piriformis syndrome. J Back Musculoskelet Rehabil. 2018;31:1105-1110. [DOI] [PubMed] [Google Scholar]
- 38.Mermod J, Fischer L, Staub L, Busato A.Patient satisfaction of primary care for musculoskeletal diseases: a comparison between neural therapy and conventional medicine. BMC Complement Altern Med. 2008;8:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Egli S, Pfister M, Ludin SM, Puente de la Vega K, Busato A, Fischer L.Long-term results of therapeutic local anesthesia (neural therapy) in 280 referred refractory chronic pain patients. BMC Complement Altern Med. 2015;15:200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bölük Şenlikci H, Odabaşı ÖS, Ural Nazlıkul FG, Nazlıkul H.Effects of local anaesthetics (neural therapy) on pain and hand functions in patients with De Quervain tenosynovitis: a prospective randomised controlled study. Int J Clin Pract. 2021;75:e14581. [DOI] [PubMed] [Google Scholar]
- 41.Nieto F.Dolor Músculo Esquelético y Terapia Neural: Una Revisión Sistemática de La Literatura. Universidad Nacional de Colombia sede Bogotá; 2020. [Google Scholar]
- 42.Rey Novoa M, Muñoz-Sellart M, Catalán Soriano M, Vinyes D. Treatment of localized vulvar pain with neural therapy: a case series and literature review. Complement Med Res. 2021;28:571-577. [DOI] [PubMed] [Google Scholar]
- 43.Yılmaz E.The determination of the efficacy of neural therapy in conservative treatment-resistant patients with chronic low back pain. Spine. 2021;46:E752-E759. [DOI] [PubMed] [Google Scholar]
- 44.Ruiz JLEÓN. Evaluación de Pacientes Con Diagnostico de Dolor Pélvico Crónico Utilizando La Escala Visual Análoga, Luego de Ser Intervenidas Con Medicina Neuralterapéutica En La Consulta Externa de La Universidad Nacional y Hospital Meissen. Universidad Nacional de Colombia Sede Bogotá; 2016. [Google Scholar]
- 45.Ordoñez L.Evaluación Del Dolor y Calidad de Vida Relacionada Con La Salud En Pacientes Manejados Con Medicina Neuralterapéutica En La Consulta Externa. Universidad Nacional de Colombia Sede Bogotá; 2020. [Google Scholar]
- 46.Balevi Batur E, Atan T. Neural therapy for fibromyalgia: myth or improving quality of life? Int J Clin Pract. 2021;75:e13719. [DOI] [PubMed] [Google Scholar]
- 47.Gradinaru D, Ungurianu A, Margina D, Moreno-Villanueva M, Bürkle A.Procaine-the controversial Geroprotector candidate: new insights regarding its molecular and cellular effects. Oxid Med Cell Longev. 2021;2021:3617042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Altinbilek T, Terzi R, Kaya E, Murat S.Effects of local anesthetics on viruses from a neural therapy perspective. J Tradit Med Complement Ther. 2021;4:394-399. [Google Scholar]
- 49.Gs P.Local anesthetics and covid19 associated acute respiratory distress syndrome: a new therapeutic indication? Clin Res. 2020;6:1-5. [Google Scholar]
- 50.Malik NA, Hammodi A, Jaiswara DR.Lignocaine’s substantial role in COVID-19 management: potential remedial and therapeutic implications. Anaesth Pain Intens Care. 2020;24:59-63. [Google Scholar]
- 51.Dosch P, Dosch M.Infectious diseases. In: Dosch P, Dosch M, eds. Manual of Neural Therapy According to Huneke (Regulating Therapy With Local Anesthetics). Thieme; 2007;186. [Google Scholar]
- 52.Häring C, Schroeder J, Löffler B, Engert B, Ehrhardt C. The local anaesthetic procaine prodrugs ProcCluster® and Procaine-hydrochloride impair SARS-CoV-2 replication in vitro. Preprint. Published online June07, 2021. BioRxiv 447335. doi: 10.1101/2021.06.07.447335v1 [DOI] [Google Scholar]
- 53.Razavi BM, Fazly Bazzaz BS.A review and new insights to antimicrobial action of local anesthetics. Eur J Clin Microbiol Infect Dis. 2019;38:991-1002. [DOI] [PubMed] [Google Scholar]
- 54.Yang SS, Wang NN, Postonogova T, et al. Intravenous lidocaine to prevent postoperative airway complications in adults: a systematic review and meta-analysis. Br J Anaesth. 2020;124:314-323. [DOI] [PubMed] [Google Scholar]
- 55.Aminnejad R, Salimi A, Saeidi M.Lidocaine during intubation and extubation in patients with coronavirus disease (COVID-19). Can J Anaesth. 2020;67:759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Diaz-Vera MA, Santa Cruz J, Headrington A, et al. Lidocaine to reduce the severity of covid-19 cases. Ther Neural. 2020;2020:1-4. http://www.terapianeural.com/articulos/28-studies-estudios/495-lidocaine-to-reduce-the-severity-of-covid-19-cases (accessed March 2022). [Google Scholar]
- 57.Ali ZA, El-Mallakh RS.Nebulized lidocaine in COVID-19, an hypothesis. Med Hypotheses. 2020;144:109947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vishnevsky АА. Local infiltration anesthesia by the creeping infiltrate method. Kazan Med J. 1930;26:569-577. [Google Scholar]
- 59.Porzionato A, Emmi A, Barbon S, et al. Sympathetic activation: a potential link between comorbidities and COVID-19. FEBS J. 2020;287:3681-3688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ossipov BK.Local anesthesia in thoracic surgery: 20 years experience with 3265 cases. Anesth Analg. 1960;39:327-332. [PubMed] [Google Scholar]
- 61.Liu MH, Tian J, Su YP, Wang T, Xiang Q, Wen L.Cervical sympathetic block regulates early systemic inflammatory response in severe trauma patients. Med Sci Monit. 2013;19:194-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pshenichnikov VI, Kharitonov IF.Novocain block in acute inflammatory and purulent processes. Kazan Med J. 1934;30:564-572. [Google Scholar]
- 63.Liu LD, Duricka DL.Stellate Ganglion Block Successfully Treats Long COVID/PASC: A Case Series. 2021;1-8. 10.21203/rs.3.rs-873830/v1 [DOI]
- 64.Vinyes D, Muñoz-Sellart M, Caballero TG. Local anesthetics as a therapeutic tool for post COVID-19 patients: A case report. Medicine (Baltimore). 2022; 101(28):e29358. doi: 10.1097/MD.0000000000029358 [DOI] [PMC free article] [PubMed]