Abstract
Background and objective
The fourth dose the COVID-19 vaccine was first proposed to immunocompromised patients. The aim of the article is to systematically review the literature and report the humoral response and outcomes after the fourth dose administration in people with impaired immune system.
Methods
Published studies on the humoral response, efficacy and safety of the fourth dose of the COVID-19 vaccine were analyzed in various settings of immunocompromised patients. We conducted systematic searches of PubMed, Cochrane Library and WHO COVID-19 Research Database for series published through January 31, 2023, using the search terms “fourth dose” or “second booster” or “4th dose” and “Coronavirus” or “COVID-19” or “SARS-CoV-2.” All articles were selected according to the PRISMA guidelines.
Results
A total of 24 articles including 2,838 patients were comprised in the systematic review. All the studies involved immunocompromised patients, including solid organ transplant recipients, patients with autoimmune rheumatic disease, patients with human immunodeficiency virus (HIV) and patients with blood cancers or diseases. Almost all patients received BNT162b2 or mRNA-1273 as fourth dose. All the studies demonstrated the increase of antibody titers after the fourth dose, both in patients who had a serological strong response and in those who had a weak response after the third dose. No serious adverse events after the 4th dose have been reported by 13 studies. COVID-19 infection after the fourth dose ranged from 0 to 21%.
Conclusion
The present review highlights the importance of the fourth dose of covid-19 vaccines for immunocompromised patients. Across the included studies, a fourth dose was associated with improved seroconversion and antibody titer levels. In particular, a fourth dose was associated with increasing immunogenicity in organ transplant recipients and patients with hematological cancers, with a very low rate of serious side effects.
Keywords: immunocompromised, fourth dose, immune system, second booster, COVID-19, SARS-CoV-2
Introduction
The infectious disease caused by the novel Coronavirus SARS-CoV-2 (COVID-19) has been deemed one of the most critical global health emergencies in recent years and vaccine development has become crucial for limiting disease transmission, especially in fragile people and patients with impaired immune system (1). Worldwide, more than 5 billion people have undergone at least one dose of the COVID-19 vaccine and ~ 4.9 billions were fully vaccinated according to World Health Organization (WHO) (2). In Europe, the percentage of people who received a booster dose is 30.9% (2). In the USA, a third dose of COVID-19 vaccine has been administered to ~ 33% of the population) (3).
The European Center for Disease Prevention and Control and European Medicine Agency recommend the administration of the fourth dose to people above 60 as well as vulnerable persons of any age, administered at least 4 months after the previous one, with a focus on people who have received a previous booster more than 6 months ago (4). In March 2022, the U.S. Food and Drug Administration allowed a fourth dose for immunocompromised people and anyone 50 years of age or older (5).
On the other hand, in Israel, administration of the fourth dose started from January 2022 for workers in health service and people over 60 years of age (6–8). Currently, a fourth dose has been granted for Israelis in immunocompromised groups.
Immunocompromised people represent ~3% of the overall population, and deserve particular attention because of possible suppression or over-activation of the immune system attributable to the primary disease or concurrent treatment (9). In this group, SARS-CoV-2 infection and viral shedding is more severe and persistent, and the risk of death is higher (10). Given the reduced immune responses, immunodeficient patients are less prone to develop serious complications of COVID-19 and cytokine storm. However, they are more likely to develop opportunistic infections that can mimic the symptoms of SARS-CoV-2 infection (11). Therefore, a fourth dose has been proposed for immunocompromised patients, including organ transplant recipients (12–14), people on active treatment for solid tumor, people with hematologic malignancies, patients treated with chimeric antigen receptor (CAR)-T-cell therapy or hematopoietic stem cell transplant, patients with moderate or severe primary immunodeficiency (e.g., common variable immunodeficiency disease, severe combined immunodeficiency, DiGeorge syndrome, Wiskott-Aldrich syndrome), with advanced or untreated human immunodeficiency virus (HIV) infection (people with HIV and CD4 cell counts < 200/mm3, history of an AIDS-defining illness without immune reconstitution, or clinical manifestations of symptomatic HIV), on active treatment with high-dose corticosteroids (i.e., 20 or more mg of prednisone or equivalent per day when administered for 2 or more weeks), alkylating agents, antimetabolites, transplant-related immunosuppressive drugs, cancer chemotherapeutic agents classified as severely immunosuppressive, tumor necrosis factor (TNF) blockers, and other biologic agents that are immunosuppressive or immunomodulatory (15).
To date, no systematic reviews have been performed on the immunogenicity of a fourth dose of COVID-19 vaccines in immunocompromised cohorts. The aim of the article is to systematically review the literature and report the current use of the fourth dose in immunocompromised people, the categories of involved patients, and the results obtained till now.
Methods
Study design
This is a systematic review of literature that was completed in accordance with Preferred Reporting Project for Systematic Evaluation and Meta-Analysis (PRISMA) guidelines (16, 17).
Literature search strategy
A literature search for the studies published up to January 31, 2023 was conducted. No restrictions on language or period of publications were applied. Three different electronic databases (Medline, Cochrane Library and WHO COVID-19 Research Database, which also includes Embase, medRxiv and Scopus articles about COVID-19) were searched employing the keywords “COVID-19” OR “coronavirus” OR “SARS-CoV-2” AND “fourth dose” OR “4th dose” OR “second booster”. Other relevant studies found in the references were also retrieved. The Boolean operator “AND” was used to combine parts of the subject terms and “OR” was used to expand the search. To increase the validity data, we removed non-peer-reviewed articles in the preprint database. Only the more informative publications would be chosen when there were similar studies carried out by the same authors and/or institutions.
Screening of articles for eligibility and data extraction
The articles identified from the databases and additional resources were screened for eligibility. First, the title and abstract were screened. The following inclusion criteria were used: (1) studies including men or non-pregnant women aged 18 and above, who had impaired immune system at the time of vaccination; (2) fourth dose of COVID-19 vaccination as the intervention measure; (3) randomized trials, observational studies, case series or retrospective studies including at least three patients. Studies were limited to human participants and of any follow-up duration and time points. The definition of immunocompromised patients was borrowed by the National Cancer Institute, identifying them as people with “reduced ability to fight infections and other diseases,… caused by certain diseases or conditions, such as AIDS, cancer, diabetes, malnutrition, and certain genetic disorders, … or by certain medicines or treatments, such as anticancer drugs, radiation therapy, and stem cell or organ transplant (18).”
Second, eligible studies that met the next circumstances were rejected: (1) medical news, popular science articles, non-medical papers, reviews, editorials, comments, basic research, conference abstracts; (2) in case of overlapping studies, the less informative was excluded.
Full articles were retrieved and read in the event of any doubt or uncertainty regarding the content relevance during the abstract screening. After a comprehensive list of abstracts was obtained, the articles were retrieved and reviewed in full text.
Two researchers (SM and DP) independently screened all studies and the results were collected and reviewed by a third researcher (PL). In the event of disagreement involving the study selection, the three reviewers collegially discussed to reach a consensus (PL). Two researchers (SM and DP) extracted data according to a predetermined proforma in Microsoft Excel Version 16.45. All key extracted data were reviewed and quality checked at the end of the data extraction phase by two researchers (SM and PL).
Data on study characteristics comprised setting, study design, sample size, dropout and non-response rates, and inclusion and exclusion criteria. Participant data comprised age, sex, and disease and treatment history, reason of impaired immune system or type of immunocompromising disease and immunosuppressive regimen. Intervention related data included vaccine type and brand, dosing schedule, number of participants receiving each type and brand of vaccine, and median or mean interval between doses. Outcome related data comprised assay type, antibody measured, method of measurement, intervals of sample collection, and number of measurements.
Data synthesis and quality assessment
Data retrieved was studied then synthesized using a descriptive method. The Risk of Bias In Non-randomized Studies of Interventions (ROBINS-I) tool was used to rate risk of bias for non-randomized included studies (19). This tool assesses seven domains: risk of bias from confounding, selection of participants, classification of interventions, deviations from intended interventions, missing data, measurement of outcomes, and selection of the reported results. The Cochrane Risk of Bias 2.0 tool was used for randomized trials (20). The tool is structured into five domains through which bias might be introduced into the result. These were identified based on both empirical evidence and theoretical considerations. Because the domains cover all types of bias that can affect results of randomized trials, each is mandatory, and no further domains should be added. The five domains for individually randomized trials (including cross-over trials) are: bias arising from the randomization process; bias due to deviations from intended interventions; bias due to missing outcome data; bias in measurement of the outcome; bias in selection of their ported result. A proposed judgment about the risk of bias arising from each domain is generated by an algorithm, based on answers to the signaling questions. Judgment can be “Low”, or “High” risk of bias, or can express “Some concerns”.
Two reviewers (SM and DP) independently judged these domains as having low, moderate, serious, or critical risk of bias, or no information. All discrepancies were resolved by the independent opinion of a third reviewer (PL). A study would be judged as having an overall low risk of bias if all the domains were judged as low risk. A study would be considered as having critical risk of bias if one domain was judged as high risk of bias.
The main results of this systematic review included the serological response after fourth dose vaccine in people with impaired immune system (primary endpoint). Furthermore, the safety and clinical effectiveness of the vaccine was evaluated as a secondary endpoint. The immunogenicity indicators included antibody titers, seroconversion rate, and the response of IgG or other specific antibodies to the receptor-binding domain. Indicators for evaluating safety included local adverse reactions and systemic adverse reactions. Data were reported as mean ± standard deviation or median (range), or number (%).
Results
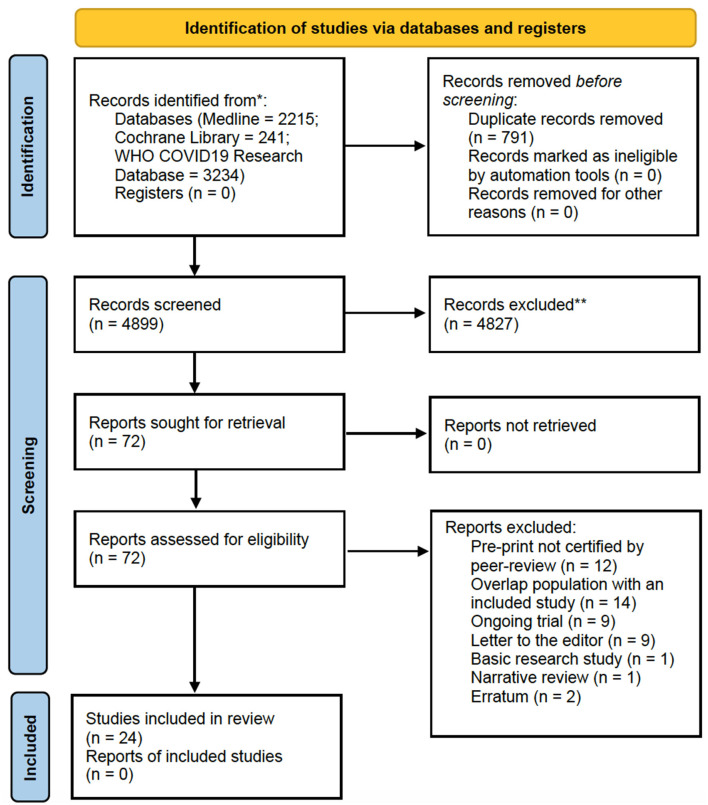
The selection process of articles and inclusion in the systematic review was summarized in Figure 1, showing the PRISMA flow diagram. The initial search included a total of 5,690 articles. After removing the duplicates, 4,899 articles were screened for keywords and relevance for the title and abstract. The full-text versions of the publications were reviewed in case of uncertainty. Only those that fulfilled the inclusion criteria were included for eligibility assessment. The full-text of these studies were fully examined. A total of 24 articles including 2,838 patients published since January 2023 were comprised in the systematic review (21–44), consisting mainly in retrospective cohort studies, followed by research letters, prospective cohort studies and case series. All the studies involved immunocompromised patients, including solid organ transplant recipients, patients with autoimmune rheumatic disease, patients with HIV and patients with blood cancers or diseases. The majority of studies were carried out in Europe, United States and Israel.
Figure 1.
PRISMA 2020 flow diagram for the systematic review.
Risk of bias
By using the Risk of Bias in Non-randomized Studies of Interventions (ROBINS-I), the risk of bias of the studies were summarized in Table 1 (19). In general, the individual studies had a low to moderate range of risk of bias due to adequate approach to the research question and findings, with presence of coherence among the sources of data collection and analysis.
Table 1.
Methodological quality evaluation of the included non-randomized studies according to ROBINS-1.
| References | Bias due to confounding domains relevant to the setting of the study | Bias in selection of participants into the study | Bias in classification of interventions | Bias due to deviations from intended interventions | Bias due to missing data | Bias in measurement of outcomes | Bias in selection of the reported results |
|---|---|---|---|---|---|---|---|
| Caillard et al. (21) | PY | PN | PN | PN | PN | N | N |
| Karaba et al. (22) | PN | N | N | N | PN | PN | N |
| Kamar et al. (23) | PN | N | N | N | PN | N | N |
| Teles et al. (24) | PN | PN | N | N | N | N | N |
| Mitchell et al. (25) | PN | N | PN | N | N | N | N |
| Osmanodja et al. (26) | PN | N | N | N | PN | PN | N |
| Aikawa et al. (27) | PN | N | N | N | PN | N | N |
| Mrake et al. (28) | PN | N | Y | PN | Y | PN | PN |
| Ntanasis-Stathopoulos et al. (29) | PN | N | PY | PN | PY | PN | PN |
| Perrier et al. (30) | PN | N | Y | PN | Y | PN | PN |
| Assawasaksakul et al. (31) | PN | N | Y | PN | Y | PN | PN |
| Gössi et al. (32) | PN | N | Y | PN | PY | PN | PN |
| Harberts et al. (33) | PN | N | PY | PN | Y | PN | PN |
| Benjamini et al. (34) | PN | N | Y | PN | PY | PN | PN |
| Midtvedt et al. (35) | PN | PN | PY | PN | PY | PN | PN |
| Peled et al. (36) | PN | N | Y | PN | PY | PN | PN |
| Thomson et al. (37) | PN | PN | PY | PN | PY | PN | PN |
| Busà et al. (38) | PN | PN | PY | PN | PY | N | PN |
| Brandstetter et al. (39) | PN | N | Y | PN | Y | PN | PN |
| Hod et al. (40) | PN | N | Y | PN | Y | PN | PN |
| Bjorlykke et al. (41) | PN | N | Y | PN | Y | PN | PN |
| Lamacchia et al. (42) | PN | PN | PY | PN | PY | N | PN |
| Affeldt et al. (43) | PN | PN | PY | PN | PY | N | PN |
| Davidov et al. (44) | PN | N | Y | PN | Y | PN | PN |
N, no; PN, probably no; PY, probably yes; Y, yes.
Main findings
This systematic review reports the use of a fourth dose of vaccine against COVID-19 worldwide in patients with impaired immune system. The characteristics of the included studies are summarized in Table 2, where details of vaccine characteristics and developer information are reported. A total of 2,838 patients were included. Characteristics of included patients are reported in Table 3. The majority of included patients were >50 years old. In twenty studies, 100% of patients were receiving immunosuppressive or immunomodulatory treatment following solid organ transplantation or as a treatment for autoimmune disease or cancer. All studies reported the type of vaccine used. Almost all patients received BNT162b2 or mRNA-1273 as fourth dose (Table 2). The time frame between the third and fourth dose was reported by 19 studies (21–23, 26–31, 33–42), and ranged from 22 to 201 days. All studies but two reported the antibody IgG titer before the 4th dose, using different units of measurement, as reported in Table 4. Table 5 reports the type of antibodies measured, the units of measure and the used assays, which were heterogeneous among the studies. The timing of antibody measurement after the 4th dose was reported by 21 studies and ranged from 14 to 65 days. The values of antibody titers after the 4th dose were reported with different units of measurement by all studies except two. All these studies demonstrated the increase of antibody titers after the fourth dose, both in patients who had a serological strong response and in those who had a weak response after the third dose. One study demonstrated different serological responses according to the evidence of a prior infection with SARS-CoV-2 before the fourth dose (37), reporting higher antibodies levels in patients with history of coronavirus infection. Another study pointed out a weaker serological response in patients who remained seronegative after the third dose (30).
Table 2.
Design and characteristics of the included studies.
| Author | Research type | Type of vaccine | Country | Number of included patients |
|---|---|---|---|---|
| Caillard | Retrospective study | BNT162b2 (34 patients)mRNA-1273 (58 patients) | France | 92 |
| Karaba | Prospective study | BNT162b2 (10 patients)mRNA-1273 (15 patients) | United States | 25 |
| Kamar | Retrospective study | BNT162b2 | France | 37 |
| Teles | Prospective study | BNT162b2 (11 patients)mRNA-1273 (6 patients)Ad.26.CoV2.S (1 patient) | United States | 18 |
| Mitchell | Prospective study | BNT162b2 (46 patients)mRNA-1273 (74 patients)Ad.26.CoV2.S (8 patients) | United States | 128 |
| Osmanodja | Retrospective study | mRNA vaccines (217 patients)Others (33 patients) | Germany | 250 |
| Aikawa | Prospective study | BNT162b2 (164 patients) | Brazil | 164 |
| Mrak | Prospective study | BNT162b2 (29 patients), mRNA-1273 (8 patients) | Austria | 37 |
| Ntanasis-Stathopoulos | Prospective study | BNT162b2 (201 patients) | Greece | 201 |
| Perrier | Retrospective study | BNT162b2 (507 patients), not specified (16 patients) | France | 523 |
| Assawasaksakul | Prospective study | BNT162b2 (28 patients) | Thailand | 28 |
| Gössi | Retrospective study | BNT162b2, mRNA-1273 | Switzerland | 7 |
| Harberts | Prospective study | BNT162b2, mRNA-1273 | Germany | 36 |
| Benjamini | Prospective study | BNT162b2 | Israel | 67 |
| Midtvedt | Prospective study | mRNA-1273 | Norway | 188 |
| Peled | Prospective study | BNT162b2 | Israel | 90 |
| Thomson | Prospective study | BNT162b2 (239 patients) | United Kingdom | 239 |
| Busà | Prospective study | BNT162b2, mRNA-1273 | Italy | 15 |
| Brandstetter | Retrospective study | BNT162b2 (3 patients), mRNA-1273 (38 patients) | Austria | 41 |
| Hod | Prospective study | BNT162b2 (29 patients) | Israel | 29 |
| Bjorlykke | Prospective study | BNT162b2, mRNA-1273 | Norway | 536 |
| Lamacchia | Prospective study | BNT162b2 (8 patients) | Italy | 8 |
| Affeldt | Prospective study | BNT162b2, mRNA-1273 | Germany | 29 |
| Davidov | Retrospective study | BNT162b2 (50 patients) | Israel | 50 |
| Total | 2,838 |
Table 3.
Characteristics of the included patients.
| Author | Age, years Median (IQR) | Male sex | Disease causing impair immune response | On medication with immunosuppressive or immunomodulatory therapy (%) |
|---|---|---|---|---|
| Caillard | 55.9 (47.1–64.2) | 64 (69.5%) | Kidney transplant recipients | 100% |
| Karaba | 59 (45–66) | 11 (44%) | Solid organ transplant recipients | 100% |
| Kamar | NR | NR | Solid organ transplant recipients | NR |
| Teles | 56 (52–66)** | 5 (27.8%) | Autoimmune rheumatic disease | 100% |
| Mitchell | ## 63.5 (54.2–71.6) 62.3 (49.6–69.5) 58.4 (48.4–68) | 58 (45.3%) | Solid organ transplant recipients | NR |
| Osmanodja | 61 (51–70) | 168 (67%) | Kidney transplant recipients | 100% |
| Aikawa | 55.7 (47.3–70.7) | 25 (20%) | Autoimmune rheumatic disease | 100% |
| Mrak | 62.1 (14.0)* | 11 (30.6%) | Autoimmune rheumatic disease | 100% |
| Ntanasis-Stathopoulos | 67 (15) | 114 (56.7%) | Multiple myeloma | 100% |
| Perrier | 61.2 (50.9–69.3)# | 550(66.7%) | Solid organ transplant recipients | 100% |
| Assawasaksakul | ## 39 (11.9)* 53.8 (9.3)* | 7 (7%) # | Autoimmune rheumatic disease | 97% |
| Gössi | 58.5 (17–78)** # | 28 (61%) | CAR-T-cell treated patients | 100% |
| Harberts | 61.0 (52.5–67.0) | 23 (63.9%) | Liver transplant recipients | 100% |
| Benjamini | 71.46 (64.90–75.82) | 47 (70.1%) | Chronic lymphocytic leukemia | 47.8% |
| Midtvedt | 60 ± 12* | 109 (58%) | Kidney transplant recipients | 100% |
| Peled | 57.2 ± 13.8* | 62 (68.9%) | Heart transplant recipients | 100% |
| Thomson | ## 61 (51–68)** 60 (49–67)** | 149 (62.3%) | Kidney transplant recipients | 100% |
| Busà | 58 ± 13* | 9 (60%) | Solid organ transplant recipients | 100% |
| Brandstetter | ## 66.8 (57.45–73.6) 67.75 (56.28–70.83) | 26 (63%) | Kidney transplant recipients | 100% |
| Hod | 64.2 (54.3–70.4) | 16 (55.2%) | Kidney transplant recipients | 100% |
| Bjorlykke | 59 (49–67) | 229 (43%) | Immune-mediated inflammatory diseases | 100% |
| Lamacchia | 58.5 ± 8.9* | NR | HIV | 100% |
| Affeldt | 55 (20) | 22 (61.1%) | Kidney transplant recipients | 100% |
| Davidov | 62.7 (53.1–70.6) | 26 (52%) | Liver transplant recipient | 100% |
mean (standard deviation).
median (range).
referred to the entire population of the study.
data referring to different groups according to the sierological response before the 4th vaccine dose.
NR, not reported; HIV, human immunodeficiency virus.
Table 4.
Immunological outcomes after 4th dose of vaccination.
| Author | Median (IQR) delay between 3rd and 4th dose, days | Median (IQR) antibody IgG titer before the 4rd dose, BAU/mL | Median (IQR) antibody IgG titer after the 4th dose, BAU/mL | Ratio of the median antibody titer after/before the 4th dose | Median (IQR) timing of antibody IgG titer exam after the 4th dose, days |
|---|---|---|---|---|---|
| Caillard | 68 (61–74.7) | 16.4 (5.9–62.3) | 145 (27.6–243) | 8.8 | 29 (26–34) |
| Karaba | 93 (28–134) | 42.3 (4.9–134.2) | 228.9 (115.4–655.8) | 5.4 | 29 (17–38) |
| Kamar | 65 (9)* | 4 (1–9)**α | 9.5 (1.7–658)**β | 2.4 | 28 |
| Teles | NR | < 0.4–>2,500 γ | 1,750 (26–2,500) γ | – | 32 (28–34) |
| Mitchell | NR | 207 (11.6–1,500) γ | 2,132.5 (96.9–>2,500) γ | 10.3 | 14–28** |
| Osmanodja | 64 (55–84) | 42% δ | 74.2% δ | – | NR |
| Aikawa | 90 | 29.5 (23.3–37.4) ε | 215.8 (180.5–257.9) ε | 7.3 | 30 |
| Mrak | 84 | 0.4 (0.4–8.1) | 12.4 (0.4–197.3) | 31 | 30 |
| Ntanasis–Stathopoulos | 180 (150–210)** | 80 ± 3.5% ζ | 96.1 ± 3.7% ζ | – | 30 |
| Perrier | 201 (173–221) | η no = 174 (32.3%); weak = 103 (19.1%); strong = 261 (48.5%) | θ no = 56 (23.8%); weak = 26 (11.1%); strong = 153 (65.1%) | – | 31.5** |
| Assawasaksakul | 22 | 88 (49–155) | 644 (398–1,041) | 7 | 15 |
| Gössi | NR | < 12 (< 12–>400)**ε | 30.4 (< 12–400)**ε | – | 48–59** |
| Harberts | 126.0(93.0–148.0) | 134.6 ε | 1,196.0 ε | 9 | NR |
| Benjamini | 175 (174–175) | 4.3 (0.1–117.65) | 41.3 (0.4–1,185) | 10 | 14 |
| Midtvedt | 18.0 (9.7–18.3) weeks | 4.6 (2.5–32) | 1,553 (356–3,703) in 79 patients with >200 BAU/ ml 53 (12–407) in 96 patients sero–negative before dose 4 | – | 3–4 weeks |
| Peled | 173.4 ± 4.2* | 12.5 ε | 96.9 ε | 7.8 | 16.1 ± 4* |
| Thomson | 92–130** | 3,791 (1,142–5,680) in patients with history of SARS-CoV-2 infection 295 (9.1–1,611) in patients without previous SARS-CoV-2 infection |
3,993 (835–5,680) in patients with history of SARS-CoV-2 infection 437 (26–2,211) in patients without previous SARS-CoV-2 infection | – | 23–66** |
| Busà | 168.3 (116–246)** | 330.2 (59.02–1,001) | 1,020 (366.6–5,486) | 65.33 (26–127**) | |
| Brandstetter | 26 (26–27) | NR | 44.7 (17.9–111.6) | 26 (26–27) | |
| Hod | 175 (164–176) | 345 (124–956) 699 (244–2,008) η, |
2,118 (761–5,900) 2,489 (1,098–5,640) η | 29 (25–33) | |
| Bjorlykke | 84 | 5,087 (1,250–9,081) | 6,192 (2,878–11,243) | 2–4 weeks | |
| Lamacchia | 119 ± 2* | NR | NR | 7, 30, 60 days | |
| Affeldt | NR | 134.4 | NR | NR | |
| Davidov | 175 (164–176) | 345 (124–955) | 2,118 (761–5,900) | 29 (25–33) |
Continuous data are reported as median (IQR range).
BAU, basal antibody units; AU, antibody units; ELU, Elisa laboratory units.
mean (standard deviation);
median (range);
range.
αdata referring to 5 patients seropositive before the 4th dose.
βdata referring to 31 patients seronegative before the 4th dose.
γreported in U/mL.
δserological response rate.
εreported in AU/mL.
ζmedian neutrilizing antibodies levels.
ηdata referring to different groups according to the sierological response before the 4th vaccine dose.
θ235 patients had serological tests after the fourth dose.
NR, not reported.
Table 5.
Type of antibody measured, units of measure and assays.
| Author | Antibodies measured | Unit of measure | Assay |
|---|---|---|---|
| Caillard | Anti-spike IgG | WHO BAU/mL | NR |
| Karaba | Antinucleocapsid antibody (anti-N), anti- RBD Protein, anti-S immunoglobulin IgG ACE2 neutralizing antibodies | WHO BAU/mL | Multiplex chemiluminescent Meso Scale Diagnostics (MSD, Rockville, MD) V-PLEX COVID-19 Respiratory Panel 3 Kit and ACE2 MSD V-PLEX SARS-CoV-2 ACE2 Panel 23 Kit |
| Kamar | Anti-spike total antibody concentration | WHO BAU/mL | Wantai enzyme-linked immunosorbent assay test |
| Teles | Anti-RBD Protein Ig | U/mL | Roche Elecsys immunoassay |
| Mitchell | Anti-RBD Protein Ig Anti-S1 domain of the spike protein | AU/mL | Roche Elecsys immunoassay and EUROIMMUN Anti-SARS-CoV-2 enzyme immunoassay |
| Osmanodja | Anti-spike protein S1 IgG Anti-RBD Protein Ig | Serological response rate | EUROIMMUN Medizinische Labordiagnostika AG and Roche Elecsys immunoassay |
| Aikawa | Total IgG against the SARS-CoV-2 S1 and S2 protein Circulating NAb | AU/mL | Chemiluminescent immunoassay on the ETI-MAX-3000, LIAISON SARS-CoV-2 S1/S2 IgG kit, DiaSorin SARS-CoV-2 sVNT kit GenScript |
| Mrak | Anti-RBD Protein Ig Anti-S1 domain of the spike protein | BAU/ml | Roche Elecsys immunoassay |
| Ntanasis-Stathopoulos | antibody-mediated reduction of SARS-CoV-2 RBD binding to the human host receptor angiotensin converting enzyme type 2 | Serological response rate | cPass SARS-CoV-2 Nabs Detection Kit (GenScript, Piscataway, NJ) |
| Perrier | Anti-RBD Protein Ig | BAU/ml | Wantai SARS-CoV-2 Ab ELISA (Beijing Wantaï Biological PharmacyEnterprise), VIDAS SARS CoV-2 IgG II ELF Aassay (Biomérieux), Alinityi SARS-CoV-2 IgG II Quantassay (Abbott), Elecsysanti-SARS CoV-2 S assay (Roche Diagnostics) Atellica sCOVG IgG assay (Siemens Healthineers). |
| Assawasaksakul | Anti-RBD of the SARS-CoV-2 spike protein | AU/ml | SARS-CoV-2IgG II Quant assay (Abbott Diagnostics) |
| Gössi | Anti-SARS-CoV-2 IgG antibodies binding to S1 and S2 antigens | AU/ml | automated immunoassay analyzer Liaison® XL by DiaSorin, Saluggia, Italy |
| Harberts | Anti-RBD Protein Ig | AU/ml | Roche Elecsys immunoassay |
| Benjamini | (IgG), aimed at the SARS-CoV-2 S protein receptor–binding domain (RBD) | BAU/mL | SARS-CoV-2 IgG II Quant (AbbottLaboratories, Abbott Park, Illinois), |
| Midtvedt | Anti-SARS-CoV-2 (Wuhan) receptor-binding domain (RBD) binding-(x-axis) and neutralizing-antibodies | BAU/mL | NR |
| Peled | SARS-CoV-2 anti-RBD IgGantibodies; IgG against sublineage B.1 of the wild-type virus, the B.1.617.2(delta) variant and the B.1.1.529(omicron) variant | AU/ml | SARS-CoV-2 IgGIIQuant assay, Abbott, USA and live virus microneutralization assays |
| Thomson | Antibodies to nucleocapsid protein (anti-NP) | BAU/mL | Abbott Architect SARS-CoV-2 IgG 2 step chemiluminescent immunoassay (CMIA) |
| Busà | IgG antibodies against S1 and S2 fragments of the Spike protein | BAU/mL | Chemiluminescent immunoassay (CLIA) LIAISON® Trimeric SARS-CoV-2 S1/S2 IgG (DiaSorin S.p.A., Saluggia, VC, Italy) |
| Brandstetter | Anti-SARS-CoV-2 antibodies directed against the receptor binding domain of the S1 subunit of the spike (S) protein | BAU/mL | SARS-CoV-2 IgG II Quant assay (Abbott Ireland Diagnostics Division, Sligo, Ireland) |
| Hod | IgG antibodies to the RBD of the SARS-CoV-2 spike protein | AU/ml | SARS-CoV-2 IgGIIQuantassay, Abbott, USA and live virus microneutralization assays |
| Bjorlykke | IgG antibodies to the RBD of the SARS-CoV-2 spike protein | BAU/mL | In-house bead-based method validated against a micro-neutralization assay at the Department of Immunology at Oslo University Hospital |
| Lamacchia | Evaluation of the SARS-CoV-2 spike protein antibodies, including the anti-spike-specific (in trimeric form) IgGs, anti-spike RBD-specific IgGs, anti-spike-specific IgMs, anti-nucleoprotein-specific IgGs, and neutralizing antibodies that block the binding of spike protein with the ACE2 receptor | BAU/ml | chemiluminescent immunoassay (CLIA) LIAISON® Trimeric SARS-CoV-2 S1/S2 IgG (DiaSorin S.p.A., Saluggia, VC, Italy), SARS-CoV-2 IgG II Quant (Abbott Rome, Italy), test for neutralizing antibodies that block the binding of spike protein with the ACE2 receptor (Dia.Pro Diagnostic Bioprobes, Milan, Italy) |
| Affeldt | IgG antibodies to the RBD of the SARS-CoV-2 spike protein, antibodies targeting additional regions of the spike protein, IgG against the S1 region | BAU/ml | Chemiluminescent microparticle immunoassay (CMIA) SARS-CoV-2 IgG II Quant by Abbott on the automated system Alinity I (Abbott, Abbott Park, IL, USA), (CLIA) LIAISON® SARS-CoV-2 TrimericS IgG assay by DiaSorin, Euroimmun anti-SARS-CoV-2-QuantiVac-ELISA (Enzyme-linked Immunosorbent Assay) |
| Davidov | Serum titers of IgG antibodies against the SARS-CoV-2 spike RBD | BAU/mL | SARS-CoV-2 IgGIIQuant assay, Abbott, USA and live virus microneutralization assays |
BAU, basal antibody units; NR, not reported; NA, not assessed; WHO, World Health Organization; ACE, angiotensin-converting enzyme; Ab, antibody; RBD, Receptor Binding Domain; U, unit; AU, antibody units, ELU, Elisa laboratory units; Nab, neutralizing antibodies.
No serious adverse events after the 4th dose have been reported by 13 studies (21, 23, 26–28, 31, 33–35, 39–41, 44) (Table 6). COVID-19 infection after the fourth dose was reported by 10 authors (21, 23, 28, 29, 32, 34, 40–42, 44) and ranged from 0 to 21%. Overall, all the authors recommended the 4th dose of vaccine against COVID-19 in immunocompromised patients, except for Karaba and Thomson et al. (22, 37).
Table 6.
Clinical outcomes after 4th dose of vaccination.
| Author | Side effects | COVID-19 infection | 4th dose recommended (yes/no) |
|---|---|---|---|
| Caillard | 0 (0%) | 1 (1%) | Yes |
| Karaba | NR | NR | No |
| Kamar | 0 (0%) | 0 (0%) | Yes |
| Teles | NR | NR | Yes |
| Mitchell | NR | NR | Yes |
| Osmanodja | 0 (0%) | NR | Yes |
| Aikawa | No serious adverse events | NR | Yes |
| Mrak | No serious adverse events | 1 (2.7%) | Yes |
| Ntanasis-stathopoulos | NR | 0 (0%) | Yes |
| Perrier | NR | NR | Yes |
| Assawasaksakul | No serious adverse events | NR | Yes |
| Gössi | NR | 1 (14.2%) | Yes |
| Harberts | No serious adverse events | NR | Yes |
| Benjamini | No serious adverse events | 14 (21%) |
Yes |
| Midtvedt | No serious adverse events | NR | Yes |
| Peled | NR | NR | Yes |
| Thomson | NR | NR | No |
| Busà | NR | NR | Yes |
| Brandstetter | No serious adverse events | NR | Yes |
| Hod | No serious adverse events | 9 | Yes |
| Bjorlykke | No serious adverse events | 35 (7% of 491) | Yes |
| Lamacchia | NR | 2 | Yes |
| Affeldt | NR | NR | Yes/No |
| Davidov | No serious adverse events | 9 (18%) | Yes |
NR, not reported.
Discussion
Given the continuing COVID-19 emergency associated with the risk of the virus undergoing new mutations and in the view of the fact that a clear reduction in vaccine coverage is evident 4 months after the third dose, it is hypothesized that administration of a fourth dose of vaccine may protect against the risk of severe illness and mortality from coronavirus infection. However, specific considerations must be made for immunocompromised patients. At first, efficacy and safety data on booster doses are less because large trials have often excluded patients with cancer, organ transplant recipients, and those with rheumatological disorders although they constitute 3% of the population (45). On the other side, these patients experience more severe and persistent infection and viral shedding (46) and are at increased risk of death (47).
The present systematic review provides relevant evidence about the current role of the fourth dose of vaccine against COVID-19 in immunocompromised people. At first, several considerations emerge on the population of immunocompromised patients who received the fourth dose. The majority of them had history of solid organ transplant, necessitating long-term immunosuppressive therapy to prevent rejection. Only a few data concern patients with hematological cancers or autoimmune rheumatological disease. Few data on HIV/AIDS patients and on patients with primary immunodeficiencies have been published till now.
It is clear that the availability of vaccines and the vaccination guidelines released from the single countries strongly influenced the use of the fourth dose. The inclusion of different ethnics groups better representing the global population may be limited according to the collected data. These findings generate ethical reflections in addition to scientific considerations.
Second, the types of vaccines used for the fourth dose were almost exclusively mRNA vaccines, produced by the two main companies, despite the large number of different types of vaccines available in the market (48). This observation may be explained because they have generally produced better antibody responses and are to some degree better available at least in the developed countries. Data about the other vaccine platforms should be accrued in the future.
The present review showed that the fourth dose was effective in increasing the antibody titer in immunocompromised patients. How the increase of the antibody titers impacted the rate and severity of COVID-19 infections was less clear, as the majority of studies did not follow the patients after the fourth dose to detect clinical infections, and follow-up data are scarcely reported. The COVID-19 infection rate after the fourth dose was reported only by 10 studies, with different follow-up times, and varied from 0% to 21%. Furthermore, the duration of further protection could not be assessed by the review due to the lack of long-term follow-up after the fourth dose at the time that the articles were published.
It should be emphasized that the circulating viral strain(s) are very different from the Wuhan strain, on the basis of which current vaccines were synthesized, and that antibodies elicited by vaccination are unlikely to provide good protection from mutated strains. However, the protection that vaccines provide is not completely dependent on antibodies, but T-cells play a role, particularly in protecting against severe forms of the disease. In addition, T-cells are likely to be less affected by spike protein mutations, given the promiscuity of antigen recognition by the T-cell receptor. The effect of repeated vaccination on the receptors of the adaptive immune system (whether antibody, T-cell receptor, or B-cell receptor) has not yet been extensively evaluated.
However, overall the systematic review demonstrated a very low rate of early major side effects after the fourth dose in immunocompromised patients. No severe adverse events occurred in 13 studies.
Most of the authors of the included studies recommended the use of the fourth dose, while only Karaba et al. and Thomson et al. dissented (22, 37). Karaba et al. argued that additional dosing of the original vaccines in solid organ transplant recipients might not produce valid defense against infection in the form of neutralizing antibodies against the Omicron variant or new variants generated by Omicron (22). These authors recommend additive approaches, such as modulation of immunosuppressive regimens before booster doses of vaccine, vaccines with alternative antigenic sequences, or extensively neutralizing passive immunity products. Thomson and colleagues (37) asserted that repeated vaccinations do not adequately protect all transplant recipients, as there is a spectrum of immune responses in patients in relation to vaccination and infection and it will disadvantage many immunocompromised people if they are managed as a uniform cohort irrespective of underlying disease, treatment or infection status. They recommend developing a more personalized approach, starting with antibody screening to identify the vaccine non-responders who are likely to be the most immune suppressed and at risk of an adverse outcome with infection.
We also highlight a lack of homogeneity of fourth dose use in the included studies. Particularly, the delay between the third and the fourth dose varied from a minimum median value of 22 days to a maximum of 201 days (Table 4). Also timing of antibody dosage after the fourth dose to assess its efficacy on seroconversion varied, as the type of assays (Tables 4, 5).
Globally, even if the data of the literature should be implemented in the future, our results demonstrate that a fourth dose of mRNA vaccine is effective in increasing the antibody titer and associated with a very low rate of side effects in immunocompromised patients, and therefore in our opinion should be proposed to fragile patients (immunocompromised, elderly) (experts' recommendation). Among the international societies, the World Health Organization recommended a fourth dose for immunocompromised patients (49).
Limitations
This study has several limitations. Firstly, the included studies are mostly observational and small case series. Secondly, the definition of immunocompromised is not universally shared and varied between studies. We therefore specified in the Methods the definition of immunocompromised that we choose to select the articles. Third, we mainly analyzed the seroconversion rate, which is an indication of an immune response to a vaccine, but is not necessarily related to clinical effectiveness. Data are still few on clinical efficacy endpoints such as COVID-19 infection rates in vaccinated immunocompromised populations. It was not possible to perform a meta-analysis for the heterogeneity of the studies and data and because most of the studies lack a comparator. Finally, the definition of seroconversion and the type of immunoassay used were not standardized across the included studies. Even if vaccine type might influence seroconversion rates after COVID-19 vaccination, the studies included in this review predominantly used mRNA vaccines, limiting potential bias.
Further issues
For long-term observations, there are relevant hesitations associated with the development of the virus and the specificities of new variants. The wide dissemination of new variants internationally suggests continued viral evolution with the emergence of future variants or sublines, as has been noted for some time. It has been shown in the literature that even a repetitive booster vaccination based on the Wuhan isolate has a limited ability to produce a durable humoral immune response toward a remote variant such as Omicron (50). This highlights the urgency of evaluating and adopting second-generation variant of SARS-CoV-2 vaccines. After all, a possible early availability of second-generation vaccines for the current SARS-CoV-2 variants might favor the administration of the aforementioned rather than the use of a fourth dose of first-generation vaccine. Therefore, further research is useful to study the long-term efficacy of vaccines and the influence of dose, seniority, and manufacturing process on protective efficacy (51).
Based on recent WHO guidance, we emphasize that future studies need to address other gaps in the evidence related to the need for additional booster doses, the duration of vaccine efficacy of inactivated, subunit, and viral vector vaccines over time, and according to disease outcome. Further data are required on the magnitude, extent, and duration of humoral and cell-mediated immune responses to variant (49).
Conclusion
Our findings highlight the importance of the fourth dose of COVID-19 vaccines for immunocompromised patients. Across the included studies, a fourth dose was associated with improved seroconversion and antibody titer levels. In particular, a fourth dose was associated with increasing immunogenicity in organ transplant recipients and patients with hematological cancers, with a very low rate of serious side effects.
Additional data are needed to define the long-term efficacy of the fourth dose and the influence of dose, age, immune disease and manufacturing process on the protective efficacy of different coronavirus variants.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
SM took care of the data synthesis, collection, and analysis. DP provided expert clinical advice on applied methodology, while PL provided expert clinical advice on community health. All authors critically corrected the manuscript for relevant scientific content and updated and approved the final version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1.Carneiro DC, Sousa JD, Monteiro-Cunha JP. The COVID-19 vaccine development: a pandemic paradigm. Virus Res. (2021) 301:198454. 10.1016/j.virusres.2021.198454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coronavirus (COVID-19) vaccinations . Geneva: World Health Organization. Available online at: https://covid19.who.int/data [Google Scholar]
- 3.COVID-19 Vaccinations in the United States . Center for Disease Control and Prevention. (2022). Available online at: https://covid.cdc.gov/covid-data-tracker/#vaccinations_vacc-total-admin-rate-total
- 4.ECDC and EMA update recommendations on additional booster doses of COVID-19 vaccines . Amsterdam: European Medicines Agency. Available online at: https://www.ecdc.europa.eu/en/news-events/ecdc-and-ema-update-recommendations-additional-booster-doses-covid-19-vaccines (accessed Oct 23, 2022). [Google Scholar]
- 5.Coronavirus (COVID-19) Update: FDA . Authorizes Second Booster Dose of Two COVID-19 Vaccines for Older and Immunocompromised Individuals. Silver Spring: U.S. Food and Drug Administration. Available online at: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-second-booster-dose-two-covid-19-vaccines-older-and
- 6.Burki TK. Fourth dose of COVID-19 vaccines in Israel. Lancet Respir Med. (2022) 10:e19. 10.1016/S2213-2600(22)00010-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Magen O, Waxman JG, Makov-Assif M, Vered R, Dicker D, Hernán MA, et al. Fourth dose of BNT162b2 mRNA COVID-19 vaccine in a nationwide setting. N Engl J Med. (2022) 386:1603–14. 10.1056/NEJMoa2201688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arbel R, Sergienko R, Friger M, Peretz A, Beckenstein T, Yaron S, et al. Effectiveness of a second BNT162b2 booster vaccine against hospitalization and death from COVID-19 in adults aged over 60 years. Nat Med. (2022) 28:1486–90. 10.1038/s41591-022-01832-0 [DOI] [PubMed] [Google Scholar]
- 9.Lee ARYB, Wong SY, Chai LYA, Lee SC, Lee MX, Muthiah MD, et al. Efficacy of covid-19 vaccines in immunocompromised patients: systematic review and meta-analysis. BMJ. (2022) 376:e068632. 10.1136/bmj-2021-068632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee LYW, Ionescu MC, Starkey T, Little M, Tilby M, Tripathy AR, et al. COVID-19: Third dose booster vaccine effectiveness against breakthrough coronavirus infection, hospitalisations and death in patients with cancer: a population-based study. Eur J Cancer. (2022) 175:1–10. 10.1016/j.ejca.2022.06.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tehrani S, Ziaie S, Kashefizadeh A, Fadaei M, Najafiarab H, Keyvanfar A. Case report: pneumonia in a patient with combined variable immunodeficiency: COVID-19 or pneumocystis pneumonia? Front Med. (2022) 9:814300. 10.3389/fmed.2022.814300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alejo JL, Mitchell J, Chiang TPY, Abedon AT, Boyarsky BJ, Avery RK, et al. Antibody response to a fourth dose of a SARS-CoV-2 vaccine in solid organ transplant recipients: a case series. Transplantation. (2021) 105:e280–1. 10.1097/TP.0000000000003934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Benotmane I, Bruel T, Planas D, Fafi-Kremer S, Schwartz O, Caillard S, et al. Fourth dose of the mRNA-1273 SARS-CoV-2 vaccine improves serum neutralization against the Delta variant in kidney transplant recipients. Kidney Int. (2022) 101:1073–6. 10.1016/j.kint.2022.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schrezenmeier E, Rincon-Arevalo H, Jens A, Stefanski AL, Hammett C, Osmanodja B, et al. Temporary antimetabolite treatment hold boosts SARS-CoV-2 vaccination-specific humoral and cellular immunity in kidney transplant recipients. JCI Insight. (2022) 7:e157836. 10.1172/jci.insight.157836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.People Who Are Immunocompromised . Centers for Disease Control and Prevention. Available online at: https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-who-are-immunocompromised.html (accessed Oct 23, 2022).
- 16.Page MJ, Moher D, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ. (2021) 372:n160. 10.1136/bmj.n160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moher D, Liberati A, Tetzlaff J, Altman DG. PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. (2009) 339:b2535. 10.1136/bmj.b2535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Immunocompromised . National Cancer Institute. Available online at: https://www.cancer.gov/publications/dictionaries/cancer-terms/def/immunocompromised (accessed Nov 13, 2022).
- 19.Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. (2016) 355:i4919. 10.1136/bmj.i4919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. (2019) 366:l4898. 10.1136/bmj.l4898 [DOI] [PubMed] [Google Scholar]
- 21.Caillard S, Thaunat O, Benotmane I, Masset C, Blancho G. Antibody response to a fourth messenger RNA COVID-19 vaccine dose in kidney transplant recipients: a case series. Ann Intern Med. (2022) 175:455–6. 10.7326/L21-0598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karaba AH, Johnston TS, Aytenfisu TY, Akinde O, Eby Y, Ruff JE, et al. A fourth dose of COVID-19 vaccine does not induce neutralization of the omicron variant among solid organ transplant recipients with suboptimal vaccine response. Transplantation. (2022) 106:1440. 10.1097/TP.0000000000004140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kamar N, Abravanel F, Marion O, Romieu-Mourez R, Couat C, Del Bello A, et al. Assessment of 4 doses of SARS-CoV-2 messenger RNA-based vaccine in recipients of a solid organ transplant. JAMA Netw Open. (2021) 4:e2136030. 10.1001/jamanetworkopen.2021.36030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Teles M, Connolly CM, Frey S, Chiang TPY, Alejo JL, Boyarsky BJ, et al. Attenuated response to fourth dose SARS-CoV-2 vaccination in patients with autoimmune disease: a case series. Ann Rheum Dis. (2022) 81:738–40. 10.1136/annrheumdis-2021-221641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mitchell J, Alejo JL, Chiang TPY, Kim J, Chang A, Abedon AT, et al. Antibody response to a fourth dose of SARS-CoV-2 vaccine in solid organ transplant recipients: an update. Transplantation. (2022) 106:e338. 10.1097/TP.0000000000004137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Osmanodja B, Ronicke S, Budde K, Jens A, Hammett C, Koch N, et al. Serological response to three, four and five doses of SARS-CoV-2 vaccine in kidney transplant recipients. Nephrology. (2022) 11:2565. 10.3390/jcm11092565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aikawa NE, Kupa LVK, Silva CA, Saad CGS, Pasoto SG, Yuki EFN, et al. Strong response after 4th dose of mRNA COVID-19 vaccine in autoimmune rheumatic diseases patients with poor response to inactivated vaccine. Rheumatol Oxf Engl. (2022) 62:keac301. 10.1093/rheumatology/keac301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mrak D, Simader E, Sieghart D, Mandl P, Radner H, Perkmann T, et al. Immunogenicity and safety of a fourth COVID-19 vaccination in rituximab-treated patients: an open-label extension study. Ann Rheum Dis. (2022) 81:1750–6. 10.1136/ard-2022-222579 [DOI] [PubMed] [Google Scholar]
- 29.Ntanasis-Stathopoulos I, Karalis V, Gavriatopoulou M, Malandrakis P, Sklirou AD, Eleutherakis-Papaiakovou E, et al. Second booster BNT162b2 restores SARS-CoV-2 humoral response in patients with multiple myeloma, excluding those under anti-BCMA therapy. HemaSphere. (2022) 6:e764. 10.1097/HS9.0000000000000764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perrier Q, Lupo J, Gerster T, Augier C, Falque L, Rostaing L, et al. SARS-CoV-2 anti-spike antibodies after a fourth dose of COVID-19 vaccine in adult solid-organ transplant recipients. Vaccine. (2022) 40:6404–11. 10.1016/j.vaccine.2022.08.065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Assawasaksakul T, Sathitratanacheewin S, Vichaiwattana P, Wanlapakorn N, Poovorawan Y, Avihingsanon Y, et al. Immunogenicity of the third and fourth BNT162b2 mRNA COVID-19 boosters and factors associated with immune response in patients with SLE and rheumatoid arthritis. Lupus Sci Med. (2022) 9:e000726. 10.1136/lupus-2022-000726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gössi S, Bacher U, Haslebacher C, Nagler M, Suter F, Staehelin C, et al. Humoral responses to repetitive doses of COVID-19 mRNA vaccines in patients with CAR-T-cell therapy. Cancers. (2022) 14:3527. 10.3390/cancers14143527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harberts A, Schaub GM, Ruether DF, Duengelhoef PM, Brehm TT, Karsten H, et al. Humoral and cellular immune response after third and fourth SARS-CoV-2 mRNA vaccination in liver transplant recipients. Clin Gastroenterol Hepatol. (2022) 20:2558–66.e5. 10.1016/j.cgh.2022.06.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Benjamini O, Gershon R, Haim EB, Lustig Y, Cohen H, Doolman R, et al. Cellular and humoral response to the fourth BNT162b2 mRNA COVID-19 vaccine dose in patients with CLL. Eur J Haematol. (2022) 81:1750–6. 10.1111/ejh.13878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Midtvedt K, Vaage JT, Heldal K, Munthe LA, Lund-Johansen F, Åsberg A. Fourth dose of the SARS-CoV-2 vaccine in kidney transplant recipients with previously impaired humoral antibody response. Am J Transplant. (2022). 10.1111/ajt.17091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peled Y, Afek A, Nemet I, Rahav G, Raanani E, Patel JK, et al. Fourth BNT162b2 vaccination neutralization of omicron infection after heart transplantation. J Heart Lung Transplant.. (2022) 41:1210–3. 10.1016/j.healun.2022.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thomson T, Prendecki M, Gleeson S, Martin P, Spensley K, De Aguiar RC, et al. Immune responses following 3rd and 4th doses of heterologous and homologous COVID-19 vaccines in kidney transplant recipients. EClinicalMedicine. (2022) 53:101642. 10.1016/j.eclinm.2022.101642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Busà R, Russelli G, Miele M, Sorrentino MC, Di Bella M, Timoneri F, et al. Immune response after the fourth dose of SARS-CoV-2 mRNA vaccine compared to natural infection in three doses' vaccinated solid organ transplant recipients. Viruses. (2022) 14:2299. 10.3390/v14102299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brandstetter C, Haller MC, Berger JM, Kerschner H, Apfalter P, Cejka D. Humoral response after a third and fourth dose of mRNA-based SARS-CoV-2 vaccine in previously seronegative kidney transplant recipients. Wien Klin Wochenschr. (2022) 134:815–21. 10.1007/s00508-022-02103-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hod T, Ben-David A, Mor E, Olmer L, Halperin R, Indenbaum V, et al. Humoral response to the fourth BNT162b2 vaccination and link between the fourth dose, omicron infection, and disease severity in renal transplant recipients. Transplantation. (2023) 107:192–203. 10.1097/TP.0000000000004383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bjørlykke KH, Ørbo HS, Tveter AT, Jyssum I, Sexton J, Tran TT, et al. Four SARS-CoV-2 vaccine doses or hybrid immunity in patients on immunosuppressive therapies: a Norwegian cohort study. Lancet Rheumatol. (2023) 5:e36–46. 10.1016/S2665-9913(22)00330-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lamacchia G, Salvati L, Kiros ST, Mazzoni A, Vanni A, Capone M, et al. Fourth dose of mRNA COVID-19 vaccine transiently reactivates spike-specific immunological memory in people living with HIV (PLWH). Biomedicines. (2022) 10:3261. 10.3390/biomedicines10123261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Affeldt P, Koehler FC, Brensing KA, Gies M, Platen E, Adam V, et al. Immune response to third and fourth COVID-19 vaccination in hemodialysis patients and kidney transplant recipients. Viruses. (2022) 14:2646. 10.3390/v14122646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Davidov Y, Indenbaum V, Atari N, Kliker L, Tsaraf K, Asraf K, et al. High immune response rate to the fourth boost of the BNT162b2 vaccine against the omicron variants of concern among liver transplant recipients. Viruses. (2022) 14:2769. 10.3390/v14122769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.COVID-19 vaccines for moderately to severely immunocompromised people . Centers for Disease Control and Prevention. Available Online at: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/recommendations/immuno.html (Accessed Nov 13, 2022).
- 46.Manuel O, Estabrook M. American society of transplantation infectious diseases community of practice. RNA respiratory viral infections in solid organ transplant recipients: guidelines from the American society of transplantation infectious diseases community of practice. Clin Transplant. (2019) 33:e13511. 10.1111/ctr.13511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mohammed AH, Blebil A, Dujaili J, Rasool-Hassan BA. The risk and impact of COVID-19 pandemic on immunosuppressed patients: cancer, HIV, and solid organ transplant recipients. AIDS Rev. (2020) 22:151–7. 10.24875/AIDSRev.20000052 [DOI] [PubMed] [Google Scholar]
- 48.Stasi C, Meoni B, Voller F, Silvestri C. SARS-CoV-2 Vaccination and the bridge between first and fourth dose: where are we? Vaccines. (2022) 10:444. 10.3390/vaccines10030444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.World Health Organization . Interim statement on the use of additional booster doses of Emergency Use Listed mRNA vaccines against COVID-19. Geneva: World Health Organization; (2022). [Google Scholar]
- 50.Grewal R, Kitchen SA, Nguyen L, Buchan SA, Wilson SE, Costa AP. Effectiveness of a Fourth Dose of COVID-19 Vaccine among Long-Term Care Residents in Ontario, Canada: Test-Negative Design Study. Public Global Health (2022). Available online at: http://medrxiv.org/lookup/doi/10.1101/2022.04.15.22273846 (accessed Jun 9, 2022). 10.1101/2022.04.15.22273846 [DOI] [PMC free article] [PubMed]
- 51.Napuri NI, Curcio D, Swerdlow DL, Srivastava A. Immune response to COVID-19 and mRNA vaccination in immunocompromised individuals: a narrative review. Infect Dis Ther. (2022) 11:1391–414. 10.1007/s40121-022-00648-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.