Abstract
Objective
The objective of this study is to establish which patient and lesion characteristics are related to the clinical outcome after microfracture of cartilage defects in the knee.
Study design
Systematic review.
Methods
After preregistration, PubMed, Embase, and Cochrane were searched for studies that analyzed prognostic factors for the outcome of microfracture treatment in the knee. The criteria for inclusion were outcome measured using Patient-Reported Outcome Measures (PROMs), a clinical study with ≥10 participants receiving microfracture, and a minimal follow-up period of 1 year.
Results
For none of the investigated prognostic factors, effect size reporting was sufficiently homogeneous to conduct a meta-analysis. However, a majority of the included studies identified higher age, larger lesion size, longer preoperative symptom duration, and previous surgery on the ipsilateral knee, especially meniscectomy and anterior cruciate ligament reconstruction, as factors that are reported to be correlated to a less favorable outcome. A lesion location that does not include the trochlea or the patellofemoral joint and is not weightbearing, a nondegenerative mechanism of injury, and a single lesion were reported as factors that predict a favorable outcome. As to gender, body mass index, preoperative activity level, smoking, and concomitant knee surgery, the included articles were inconclusive or no effect was reported.
Conclusions
Several factors correlated with the clinical result after microfracture treatment. However, the information on the effect sizes of the influence on clinical outcome is incomplete due to poor reporting. Large-scale registries or pooling of homogeneous, well-reported data is needed to work toward prognostic models. That would be an important step toward personalized treatment.
Keywords: bone marrow stimulation, osteochondral defect, risk factors
Introduction
Cartilage lesions in the knee can lead to pain and functional impairment, comparable to patients with end-stage osteoarthritis who are scheduled for total knee arthroplasty.1,2 Most cartilage lesions have a traumatic origin and are located on the medial femoral condyle and patellofemoral joint.3 The natural healing capacity of cartilage and regeneration after surgical repair are challenging, often only providing fibrocartilage defect filling rather than the native hyaline cartilage type. Untreated cartilage defects are a strong predictor for osteoarthritis development, which causes pain, a decrease in joint function, and a large demand for orthopedic care later in life.4 -6
First described by Steadman et al.,7 microfracture has been stated as the “golden standard” in the surgical treatment of full-thickness cartilage defects in the knee. Evidence is mostly derived from case series, cohort studies, and clinical trials. However, subjects in these studies often do not represent the typical patient in clinical practice.8 Namely, microfracture is mostly investigated and consequently advised for smaller primary defects in young individuals by international guidelines, but in clinical practice it is often applied in older individuals with more complex lesions and comorbidities.1,9 -12 Indeed, in clinical practice, microfracture is one of the most used cartilage repair techniques and mostly applied in middle-aged individuals.13 -16 Similarly, this is driven by its technical ease, low costs, and high availability.17 However, there is mounting evidence that the standard microfracture awls may cause subchondral bone sclerosis and cyst formation.18,19 Moreover, failed microfracture may also jeopardize the success of consecutive biological treatments such as autologous chondrocyte implantation (ACI).20 -23 The quality of the cartilage repair tissue after microfracture is also inconsistent and fluctuates, particularly with increasing age.12,24 -26 The exact reasons therefore remain unclear. Other techniques such as scaffold augmentations, osteochondral allografting (OCA) or autograft transplantation (OAT), and ACI challenge the position of microfracture as the first choice in operative management as there is increasing evidence that they are more durable than microfracture.12,27
There are particular concerns when microfracture is applied in larger defects and middle-aged patients.12 To optimize the proper use of microfracture treatment, it is important to gather the evidence on factors that should be taken into account during (shared) treatment decision-making. There are several prognostic factors that have been named for microfracture outcomes, such as age, lesion size, and location.28 Surprisingly and to the best of our knowledge, a study that scrutinizes the evidence for the influence of these factors has not been performed. Therefore, in this review, we aim to identify the prognostic factors that influence the clinical outcome of microfracture surgery of cartilage defects in the knee.
Methods
Literature Search and Selection of Studies
PubMed, Embase, and Cochrane were searched from inception until June 19, 2021 (registration in PROSPERO ID: CRD42020177512). The search was constructed using the following key words, including synonyms and closely related terms: “osteochondral lesion,” “osteochondritis dissecans,” “knee,” and “microfracture.” The full search strategies for all databases are listed in Supplemental Appendix I. Duplicates and manuscripts in any other language than English or German were excluded. Articles were included when (1) the study evaluated the outcome of microfracture treatment for cartilage defects based on Patient-Reported Outcome Measures (PROMs), (2) it was a clinical study with ≥10 participants in the microfracture-receiving group, (3) there was a minimal follow-up period of 1 year, and (4) the study addressed the preoperative or perioperative patient or lesion characteristics and their relation to the outcome. Concomitant procedures were allowed, but studies that only included patients with specific concomitant injuries were excluded. The primary outcomes were correlation coefficients, odds ratios, or differences between subgroups. Reviews, meta-analyses, cost-effectiveness analyses, congress abstracts, animal studies, and case series were excluded. Titles and abstracts were independently screened for relevance by 2 researchers (I.M.v.T. and K.S.E.) using Rayyan (www.rayyan.ai). If there was no consensus on the inclusion of a paper, a discussion with a third researcher (P.P.W.v.H.) was conducted until consensus or majority vote was reached.
Data Extraction
A data extraction form was created to obtain the following information: first author, journal, year of publication, number of patients receiving microfracture, lesion location, lesion size, age, etiology, history of knee trauma and/or surgery, follow-up, body mass index (BMI), smoking, concomitant injuries and/or procedures, and the International Cartilage Repair Society (ICRS) and/or Outerbridge Classification. When available, the PROMs, correlation (coefficient), uni- or multivariate analysis, subgroup analysis, and P values were collected for all identified following prognostic factors. If the primary outcomes were not completely reported, the corresponding authors of the included articles were contacted by e-mail, with a reminder e-mail after 8 to 10 weeks.
Quality Assessment
The included studies were subjected to a quality assessment based on the Methodological Index for Non-Randomized Studies (MINORS) by Slim et al.29 This instrument was designed to assess the methodological quality of nonrandomized surgical studies, whether comparative or noncomparative. The maximal score was 16 for noncomparative and 24 for comparative studies.
Data Analysis
A narrative synthesis of the evidence and the statistical significance of the findings are given.
Results
Study Identification
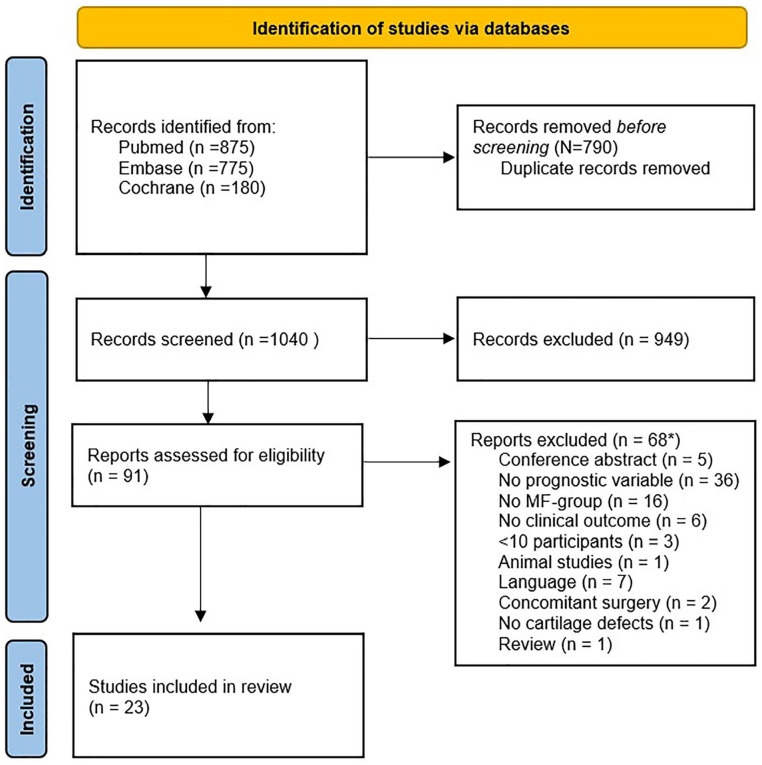
After the literature screening, 23 articles that met the inclusion criteria were identified (Fig. 1).30 -52 The most-studied prognostic factors were age, lesion size, gender, lesion location, and symptom duration. Other mentioned factors were defect count, BMI, smoking, concomitant knee surgery, mechanism of injury, previous knee surgery, activity level, and postoperative treatment, all of which will be addressed below.
Figure 1.
PRISMA flowchart of the selection process.53 PRISMA = Preferred Reporting Items for Systematic Reviews and Meta-Analyses; MF = microfracture.
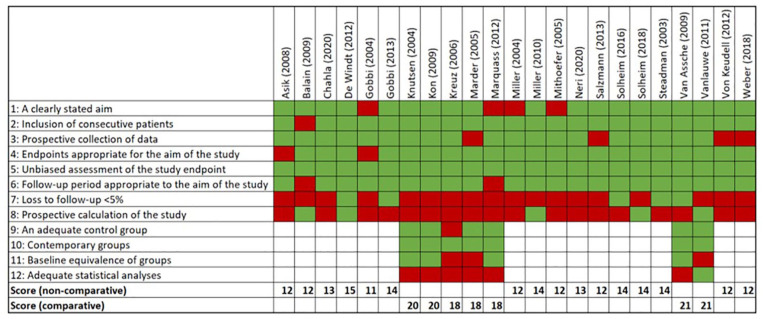
Quality Assessment
Overall, a reasonable study quality was found throughout the studies (Figure 2). The mean score for the included noncomparative studies was 13.9 (N = 16, range: 12-16), and for the comparative studies it was 20.4 (N = 7, range: 19-22). Most of the quality issues were due to loss to follow-up and the prospective calculation of the study. Due to prognostic factors being a secondary outcome measure in most studies, the prognostic calculation of the studies was usually not aimed at those.
Figure 2.
MINORS quality assessment. MINORS = Methodological Index for Non-Randomized Studies
Summary of Evidence
Due to poor reporting and heterogeneity of effect sizes, pooling of data was not possible. Therefore, a narrative synthesis of the evidence was given. Furthermore, the number of studies that studied a potential prognostic factor was counted and the ratio of studies that reported the factor to be significant to nonsignificant was reported.
Age
Age was the most frequently studied prognostic factor. Eighteen of the 23 included studies analyzed the relationship between the age of the patient and the clinical outcome of microfracture by means of either correlation (Table 1) or subgroup analysis (Table 2) with a cutoff age ranging between 25 and 45 years. Ten of the 18 papers reported a significant effect. A negative correlation with increasing age was reported in 4 of 10 studies (4/10). Lower scores in a subgroup analyses were observed in 7 of 10 studies (7/10, 1 study reported both). No significant effect of age was reported 8 times, either in correlation analysis (N = 6) or in subgroups (N = 2).
Table 1.
Correlations for Age.
| Author | N | Follow-Up (Months) | Variable 1 | Variable 2 | Correlation Coefficient | P Value |
|---|---|---|---|---|---|---|
| Asik et al.30 | 90 | 68.0 | Lower age | Higher Lysholm and lower Oxford | r = 0.623 r = 0.615 |
<0.001 |
| Balain et al.31 | 53 | 15-52 | Lower age | Higher Lysholm and IKDC | r = 0.24 | >0.001 |
| De Windt et al.33 | 56 | 34.0 | Age | Lysholm and KOOS | Not reported | n.s. |
| Kon et al.37 | 40 | 60.0 | Age | IKDC | Not reported | n.s. |
| Marquass et al.40 | 19 | 62.9 | Age | Lysholm and IKDC | Not reported | n.s. |
| Mithoefer et al.43 | 48 | 41.0 | Age | Decreasing IKDC scores | Not reported | n.s. |
| Neri et al.44 | 48 | 68.4 | Older age | Worse improvement in IKDC and VAS | Not reported | <0.05 |
| Salzmann et al.45 | 145 | 50.4 | Age | Lysholm and IKDC | Not reported | n.s. |
| Von Keudell et al.51 | 15 | 48.0 | Age | Lysholm and KOOS | Not reported | n.s. |
| Weber et al.52 | 10 | 67.9 | Older age | Lower symptom rate | Not reported | 0.0321 |
KOOS = Knee injury and Osteoarthritis Outcome Score; IKDC = International Knee Documentation Committee; VAS = Visual Analog Scale; n.s. = nonsignificant.
Table 2.
Subgroup Analyses for Age.
| Author | N | Follow-Up (Months) |
Outcome Variable | Subgroup 1 | Subgroup 2 | Mean Group 1 | Mean Group 2 | Results |
|---|---|---|---|---|---|---|---|---|
| Asik et al.30 | 90 | 68.0 | Lysholm Oxford |
<35 years | ≥35 years | 36.2 ± 5.8 21.7 ± 3.4 |
24.3 ± 6.1 16.5 ± 2.8 |
P < 0.001 |
| Chahla et al.32 | 206 | 92.1 | MCID PASS KOOS IKDC |
Lower age | Higher age | MCID: OR=0.61 PASS: OR=0.86 |
Not reported |
P = 0.036 P = 0.031 |
| Gobbi et al.35 | 61 | 181.2 | IKDC Lysholm KOOS |
<30 years | ≥30 years | Not reported | Not reported | n.s. |
| Knutsen et al.36 | 40 | 24.0 | KOOS Lysholm ICRS |
<30 years | ≥30 years | Not reported | Not reported | P ≤ 0.05 (all) |
| Kreuz et al.38 | 70 | 36.0 | ICRS Modified Cincinnati |
≤40 years | >40 years | Not reported | Not reported | P ≤ 0.01 |
| Miller et al.42 | 350 | 48.0 | Lysholm | ≤45 years | >45 years | Not reported | Not reported | P = 0.04 |
| Solheim et al.46 | 120 | 12-216 | Lysholm VAS | ≤25 years | >25 years | — | OR = 2.23 | P ≤ 0.05 |
| Steadman et al.47 | 72 | 135.6 | Lysholm WOMAC | <35 years | 35-45 years | Not reported | Not reported |
P ≤ 0.048 r = −0.28 |
| Vanlauwe et al.50 | 61 | 60.0 | KOOS VAS |
<35 years | ≥35 years | oKOOS: 16.59 ± 3.55 P = 0.262 |
oKOOS: 15.16 ± 4.02 P = 0.456 |
n.s. |
OR = odds ratio; ICRS = International Cartilage Repair Society; MCID = minimal clinically important difference; PASS = patient acceptable symptom state; KOOS = Knee injury and Osteoarthritis Outcome Score; oKOOS = Overall Knee injury and Osteoarthritis Outcome Score; IKDC = International Knee Documentation Committee; VAS = Visual Analog Scale; WOMAC = Western Ontario and McMaster Universities Arthritis Index; n.s. = nonsignificant.
Lesion size
Thirteen of the 23 studies reported the analysis of the influence of lesion size on patients’ clinical outcome via a correlation with PROMs or a subgroup analysis with a cutoff between 2 and 4 cm2. Eight of the 13 studies found a significant correlation (N = 2, Table 3) or a worse score in subgroup analysis (N = 6, Table 4) for larger lesions. The remaining 5 studies found no significant effect of lesion size.
Table 3.
Correlations for Lesion Size.
| Author | N | Follow-Up (Months) | Variable 1 | Variable 2 | Correlation Coefficient | P Value |
|---|---|---|---|---|---|---|
| De Windt et al.33 | 56 | 34.0 | Defect size | Lysholm and KOOS | Not reported | n.s. |
| Mithoefer et al.43 | 48 | 41.0 | Lesion size | Decreasing IKDC scores | Not reported | n.s. |
| Neri et al.44 | 48 | 68.4 | Larger cartilage defect | Poorer improvement in IKDC and VAS | Not reported | <0.05 |
| Salzmann et al.45 | 145 | 50.4 | Lesion size | Lysholm and IKDC | Not reported | n.s. |
| Weber et al.52 | 101 | 67.9 | Larger lesion size | Lower SF-12 physical component scores | Not reported | 0.0186 |
KOOS = Knee injury and Osteoarthritis Outcome Score; IKDC = International Knee Documentation Committee; VAS = Visual Analog Scale; SF-12 = 12-item Short Form Health Survey.
Table 4.
Subgroup Analyses for Lesion Size.
| Author | N | Follow-Up (Months) | Outcome Variable | Subgroup 1 | Subgroup 2 | Mean Group 1 | Mean Group 2 | Results | |
|---|---|---|---|---|---|---|---|---|---|
| Asik et al.30 | 90 | 68.0 | Lysholm Oxford | <2 cm2 | ≥2 cm2 | 37.4 ± 5.9 22.2 ± 3.6 |
26.9 ± 4.7 15.8 ± 2.8 |
P ≤ 0.001 | |
| Chahla et al.32 | 206 | 91.2 | KOOS IKDC | ≤2cm2 | 2 cm2 | Not reported | OR = 0.41 Lesions >2 cm2: OR = 0.89 |
P = 0.048 | |
| Gobbi et al.35 | 61 | 181.2 | IKDC Lysholm | 2-4 cm2 | 4.5-6 cm2 | 61.0 ± 2.9 67.2 ± 2.2 |
80.9 ± 2.6 86.3 ± 2.7 |
P ≤ 0.05 | |
| Knutsen et al.36 | 40 | 24.0 | KOOS Lysholm ICRS | <4 cm2 | ≥ 4cm2 | Not reported | Not reported | P ≤ 0.003 | |
| Kon et al.37 | 40 | 60.0 | IKDC | <2 cm2 | ≥2 to <3 cm2 | ≥3cm2 | Not reported | Not reported | n.s. |
| Miller et al.41 | 81 | 31.2 | Lysholm | <4 cm2 | >4 cm2 | Preoperative = 53.3 Postoperative = 83.8 |
Preoperative = 54.4 Postoperative = 81.0 |
n.s. | |
| Solheim et al.46 | 120 | 12-216 | Lysholm | ≤3 cm2
≤4 cm2 |
≥3 cm2
≥4 cm2 |
OR good Lysholm: 2.20 | OR poor Lysholm: 1.77 | P ≤ 0.05 | |
| Steadman et al.47 | 72 | 135.6 | Lysholm, WOMAC | <4 cm2 | >4 cm2 | Not reported | Not reported | n.s. | |
OR = odds ratio; ICRS = International Cartilage Repair Society; KOOS = Knee injury and Osteoarthritis Outcome Score; IKDC = International Knee Documentation Committee; VAS = Visual Analog Scale; n.s. = nonsignificant; WOMAC = Western Ontario and McMaster Universities Arthritis Index.
Lesion location
Seven studies conducted research on the effect of lesion location on postoperative scores and end results. The same PROMs as mentioned earlier were maintained. From the 3 studies mentioning a correlation for lesion location, 1 article found a significant correlation (Table 5). Five subgroup analyses were performed (Table 6). In 4 cases, a significant difference was found. Asik et al.30 found better scores at 68 months of follow-up comparing weightbearing to non-weightbearing zones. Gobbi et al.34 found better outcomes for lesions located at the medial compartment compared with the lateral compartment. Kreuz et al.38 reported worse outcome when the lesion was located at the patella or trochlea compared with the condyles or tibial plateau.
Table 5.
Correlations for Lesion Location.
| Author | N | Follow-Up (Months) | Variable 1 | Variable 2 | Correlation Coefficient | P Value |
|---|---|---|---|---|---|---|
| Miller et al.41 | 81 | 31.2 | Defect location | Clinical outcome | Not reported | n.s. |
| Steadman et al.47 | 72 | 135.6 | Defect location | Improvement in Lysholm score | Not reported | 0.104 |
n.s. = nonsignificant.
Table 6.
Subgroup Analyses for Lesion Location.
| Author | N | Follow-Up (Months) | Outcome Variable | Subgroup 1 | Subgroup 2 | Mean Subgroup 1 | Mean Subgroup 2 | Results |
|---|---|---|---|---|---|---|---|---|
| Asik et al.30 | 90 | 68.0 | Lysholm Oxford | Weightbearing surface | Non-weightbearing surface | 26.8 ± 5.3 16.2 ± 2.7 |
37.3 ± 6.4 23.2 ± 2.4 |
r = 0.658 r = 0.618 P ≤ 0.001 |
| De Windt et al.33 | 56 | 34.0 | VAS KOOS Lysholm |
Patellar or medial lesion | Other | Not reported | Not reported | n.s. |
| Gobbi et al.35 | 61 | 181.2 | IKDC Lysholm KOOS | Medial condyle | Lateral condyle | Not reported (better) | Not reported (worse) | P ≤ 0.05 |
| Kreuz et al.38 | 70 | 36.0 | ICRS Modified Cincinnati | Lesion on femoral condyles or tibia | Lesion on trochlea or patella | Not reported | Not reported | Defects on trochlea/patella in correlation with
deteriorated results: P ≤ 0.05 |
| Solheim et al.46 | 120 | 12-216 | Lysholm | Non-involvement of the patellofemoral joint | Involvement of the patellofemoral joint | Not reported | Not reported | OR = 2.27 P ≤ 0.05 |
ICRS = International Cartilage Repair Society; OR = odds ratio; KOOS = Knee injury and Osteoarthritis Outcome Score; IKDC = International Knee Documentation Committee; VAS = Visual Analog Scale.
Symptom duration
Seven of the 23 studies researched the influence of symptom duration prior to the surgical treatment, preoperative interval, delayed surgery, and chronicity of the complaints on the postoperative outcome. Four studies researched the correlation between these factors and the PROMs, and one of them found a significant correlation (Table 7). Three subgroup analyses were conducted in which a symptom duration ranging from ≤12 months to <36 months was compared with complaints that were already present for a longer period of time (Table 8). In 2 cases, a significant difference in the effect on PROMs was found. Overall, a shorter duration of symptoms was found to be more beneficial for the outcome.
Table 7.
Correlations for Symptom Duration.
| Author | N | Follow-Up (Months) | Variable 1 | Variable 2 | Correlation Coefficient | P value |
|---|---|---|---|---|---|---|
| Balain et al.31 | 53 | 15-52 | Symptom duration | Response shift | Not reported | n.s. |
| Mithoefer et al.43 | 48 | 41.0 | Preoperative interval | Decreasing IKDC scores | Not reported | n.s. |
| Neri et al.44 | 48 | 68.4 | Delayed surgery | Poorer improvement of IKDC and VAS | Not reported | <0.05 |
| Steadman et al.47 | 72 | 135.6 | Chronicity | Improvement in Lysholm score | r = −0.101 | <0.404 |
IKDC = International Knee Documentation Committee; VAS = Visual Analog Scale; n.s. = nonsignificant.
Table 8.
Subgroup Analyses for Symptom Duration.
| Author | N | Follow-Up (Months) | Outcome Variable | Subgroup 1 | Subgroup 2 | Mean Subgroup 1 | Mean Subgroup 2 | Results |
|---|---|---|---|---|---|---|---|---|
| Asik et al.30 | 90 | 68.0 | Lysholm Oxford | Symptom duration ≤12 months | Symptom duration >12 months | Not reported | Not reported | n.s. |
| Salzmann et al.45 | 145 | 50.4 | IKDC Lysholm NAS-P NAS-F |
Symptom duration <18 months | Symptom duration >18 months | 78.4 83.3 7.4 7.1 |
68.3 71.4 6.0, 5.9 |
0.001 0.001 0.003 0.003 |
| Solheim et al.46 | 120 | 12-216 | Lysholm VAS | Symptom duration ≥ 36 months | Symptom duration < 36 months | Not reported | Not reported | Shorter symptom duration: P = 0.005 OR: 2.45 |
OR = odds ratio; IKDC = International Knee Documentation Committee; NAS-P = Numeric Analogue Scale for Pain; NAS-F = Numeric Analogue Scale for Function; VAS = Visual Analogue Scale.
Gender
Seven studies investigated the influence of gender on the outcome of microfracture. All 7 articles performed subgroup analyses, out of which 2 showed significantly better PROMs at follow-up for male patients in comparison with females (Table 9). Chahla et al.32 found an odds ratio of 2.7 for males to reach the Patient Acceptable Symptom State (PASS) at 6 months after microfracture. Salzmann et al.45 found significantly better Lysholm, Knee injury and Osteoarthritis Outcome Score (KOOS), and International Knee Documentation Committee (IKDC) scores for males at an average of 50 months of follow-up. However, a majority of 5 papers did not report significant differences.
Table 9.
Subgroup Analyses for Gender.
| Author | N | Follow-Up (Months) | Outcome Variable | Subgroup 1 | Subgroup 2 | Mean Subgroup 1 | Mean Subgroup 2 | P Value |
|---|---|---|---|---|---|---|---|---|
| Balain et al.31 | 53 | 15-52 | Lysholm VAS IKDC |
Male | Female | Not reported | Not reported | n.s. |
| Chahla et al.32 | 206 | 91.2 | KOOS IKDC | Male | Female | Likelihood of achieving PASS at 6 months OR: 2.7 | 0.017 | |
| Marquass et al.40 | 19 | 62.9 | IKDC Lysholm |
Male | Female | Not reported | Not reported | n.s. |
| Miller et al.42 | 350 | 48.0 | Lysholm | Male | Female | Not reported | Not reported | n.s. |
| Salzmann et al.45 | 145 | 50.4 | IKDC Lysholm | Male | Female | Not reported (better) | Not reported (worse) | 0.032 0.026 |
| Solheim et al.46 | 120 | 12-216 | Lysholm | Male | Female | Not reported | Not reported | n.s. |
| Weber et al.52 | 101 | 67.9 | Lysholm IKDC KOOS |
Male | Female | Not reported | Not reported | n.s. |
PASS = patient acceptable symptom state; OR = odds ratio; KOOS = Knee injury and Osteoarthritis Outcome Score; IKDC = International Knee Documentation Committee; VAS = Visual Analog Scale.
Other factors and summary
Other prognostic factors that were addressed are defect count, BMI, smoking, concomitant knee surgery, mechanism of injury, previous knee surgery, activity level, and postoperative treatment. The tables displaying the extensive results of these factors can be found in Supplemental Appendix II, with a summary in Table 10. In short, the studies indicated that the presence of a single cartilage lesion compared with multiple lesions (significant in 3/4 studies) and a BMI <25-30 kg/m2 (3/6) have a beneficial effect on the clinical outcome after microfracture. Patients who have had previous, ipsilateral knee surgery were reported to have worse outcomes in 4 of 6 studies. Little or no evidence was found for the prognostic value of the variables smoking (0/2 significant), concomitant knee surgery (0/2), and the mechanism of injury (1/6).
Table 10.
Summary of Reported Effects.
| Significant Harm | No Significant Effect | Significant Benefit | |
|---|---|---|---|
| Higher age | 10 | 8 | 0 |
| Larger lesion size | 8 | 5 | 0 |
| Male gender | 0 | 5 | 2 |
| Lesion location: | |||
| Undefined | 0 | 2 | 0 |
| Non-weightbearing vs. weightbearing | 0 | 0 | 1 |
| Patellar/medial lesion vs. others | 0 | 1 | 0 |
| Medial vs. lateral condyle | 0 | 0 | 1 |
| Femoral condyles/tibia vs. trochlea/patella | 1 | 0 | 0 |
| Non-involvement of the PF joint vs. involvement of the PF joint | 0 | 0 | 1 |
| Shorter symptom duration | 0 | 3 | 4 |
| Higher BMI | 3 | 3 | 0 |
| Non-degenerative mechanism of injury | 0 | 5 | 1 |
| Previous knee surgery | 4 | 2 | 0 |
| Single lesions | 1 | 3 | |
| Smoking | 0 | 2 | 0 |
| Higher preoperative activity level | 0 | 1 | 1 |
| Concomitant knee surgery | 0 | 2 | 0 |
BMI = body mass index; PF = patellofemoral joint.
Discussion
In this systematic review, we identified the prognostic factors that influence the clinical outcome of microfracture surgery of cartilage defects in the knee. A lack of reporting of effect sizes made it impossible to pool the effect of the different variables. However, in a majority of the included studies, it was reported that patient age, lesion size, preoperative symptom duration, previous knee surgery, and lesion count were reported to have a significant effect on the outcome of microfracture. No study reported a significant effect of smoking (out of 2 studies). Conflicting evidence remains for BMI, with 3 studies reporting a significant negative effect and 3 studies stating no effect, and gender, with 3 studies describing a significant benefit for male patients and 4 studies stating no effect.
A harmful correlation between age and clinical outcome comes as no surprise, as with increasing patients’ age, their cell turnover and renewing capacities decline. In the case of articular chondrocytes, older cells exhibit a senescent phenotype.54 Moreover, advancing age may also impair the joint homeostasis. Taken together, chondrogenesis works insufficiently with increasing age, leading to inferior, fast deteriorating fibrous defect filling which is not able to withstand the so-called pothole effect.12 On the other hand, in the few studies on the natural history of cartilage defects—that is, untreated—a young age was often associated with stabilization of cartilage defects and high functional outcomes.55,56
A larger lesion size as risk factor is in accordance with biomechanical studies that have shown that defects larger than 2 cm2 increase the load on the base of the defect as well as on the rim.57 This will potentially negatively influence the development of the repair tissue in the defect and the progression of rim cartilage deformation, resulting in the pothole effect as mentioned before.58 The same biomechanics apply to lesion location as a prognostic factor. Lesion location showed an effect on the outcome in 4 of 7 mentioned studies, compared with 3 studies showing no significant effect. Particularly, patellofemoral defects have been well known to have inferior outcomes as this compartment endures high compression and shear forces.59
The effect of BMI on the outcome is inconclusive, with 3 studies showing significant harm (average N = 80) and 3 studies showing none (average N = 71). As BMI is a predictor for osteoarthritis disease development via both mechanical and inflammatory pathways, a negative effect on cartilage regeneration can be expected as well.60 From a pure mechanical point of view, this holds especially true for patients with a BMI >30 kg/m2, potentially amplifying the effect of concomitant mechanical-based prognostic factors such as defect size larger than 2 cm2.61
A shorter preoperative symptom duration showed a beneficial effect in 4 of 7 mentioned studies. As already stated, cartilage defects are a well-known risk factor for osteoarthritis development.4 As osteoarthritis is a whole-joint disease, this transition over time from local to generalized damage can only be explained by a biological process, that is, an impaired joint homeostasis.62 Indeed, in such impaired environment, cartilage repair is hampered.63 -65 The fact that we found 1 study with beneficial effects for nondegenerative cartilage defects over degenerative defects confirms this rationale. Namely, degenerative defects are probably either older traumatic defects or an early expression of osteoarthritis due to malalignment, for instance. Also, previous surgeries were shown to be harmful in 4 of the 6 studies, again highlighting the importance of a jeopardized/catabolic joint homeostasis.66
Gender did not show a significant effect on the outcome in 5 of the 7 studies. Two studies showed a better outcome for men, mostly based on subgroup analyses. The use of existing PROMs for comparing differences in outcome between various genders is debatable in general. Women and men may interpret their complaints differently, often resulting in worse outcome for women.67 Current PROMs do not correct this effect.
As for a higher preoperative activity level, the effect on the outcome is questionable as there was both a study showing an effect by preoperative activity and a study showing no effect.
For smoking, the mentioned studies (average group size N = 99) only specify the absence of a significant correlation between smoking and the response shift and clinical outcome. This is in contradiction to the study of Kraus et al.,68 which found significantly worse postoperative PROMs for smoking patients after arthroscopic partial meniscectomy. We postulate that this difference is perhaps due to more impairment of the micro-vascularization of the meniscus compared with the high blood flow in trabecular bone.
The results for concomitant knee surgery, in both cases reconstruction of the anterior cruciate ligament, found no significant difference. However, we postulate that the results could have been worse without ligament reconstruction due to increasing shear forces on the repair tissue.
Limitations
The limitations of this study lie predominantly in the poor quality and/or absence of the reporting of effect sizes, such as a correlation and an odds ratio. Furthermore, a wide variety of PROMs and outcome measures were used (Visual Analog Scale [VAS], KOOS, Lysholm, IKDC, Oxford, Western Ontario and McMaster Universities Arthritis Index [WOMAC], NAS-P, NAS-F). Combined, it was not possible to pool data and perform a classical meta-analysis. Being unable to pool the data was a limitation in itself. Therefore, the evidence was weighed based on the fraction of papers reporting a significant effect of the prognostic factor. With this approach, a higher risk of bias is unavoidable. Two factors should be considered: especially the smaller sized studies may have been underpowered for the detection of the prognostic factors, as the prognostic factors were rarely the main outcome variable in the studies, and therefore in no study a power analysis was performed for the prognostic factors. Second, for the same reason, a publication bias could be present. While the used PROMs slightly differ, they all aim to measure clinical satisfaction and function of the knee. Therefore, they are highly correlated and are therefore interchangeable in a linear regression analysis. This can also be found in the studies that reported correlations of one factor to multiple PROMs: all results were in agreement. For example, the relation between age and either Lysholm or Oxford score resulted in correlation coefficients of r = 0.623 and r = 0.615, respectively.
Recommendations
Most factors that are discussed above may already be in consideration in standard practice. Therefore, it is surprising that there is little research specifically aimed at testing these assumptions. That the results confirm the general assumptions could therefore also be enhanced by publication bias. This may be partly due to the large variations between patients, as cartilage defects often present with concomitant injuries. This makes it difficult to isolate prognostic factors. For the future, more research dedicated at investigating the impact of prognostic factors, optimally in larger cohorts, on clinical outcome is highly recommended. Furthermore, reportage of effect sizes should be standard practice to enable meta-analysis. Larger registries that combine data from various sources, such as the German Knorpel Register, could be used in future validation studies.69 The results from this review can be used to determine the variables of interest.
The results of this review indicate that the best prognosis for microfracture is for patients with cartilage defects smaller than 2 cm2, situated on the medial femoral condyle, that are of nondegenerative origin in healthy normal-weight patients younger than 40 years with less than 12 months of complaints. In clinical practice, this is a rare encounter. However, the alternatives (either conservative or with other surgical techniques) may share up until some extend the same risk factors. Comparative studies may therefore need to differentiate in population to find the best treatment for each subgroup and with increasing data availability work toward individual treatment advises. Ultimately, combining large-scale data collections with advanced data analysis holds the premise of fine-tuning the treatment algorithms based on patient factors, lesion characteristics, and clinical indications.
Finally, one of the key messages and recommendations which follows from this article is to emphasize the importance of the reporting of effect sizes, also for secondary outcome measure analysis.
Conclusion
In conclusion, the overall evidence regarding prognostic factors and their influence on clinical outcome is incomplete and possibly influenced by bias.
However, based on the reported evidence, the best prognosis after microfracture is for patients with lower age, smaller lesion size, a shorter preoperative symptom duration, a nondegenerative mechanism of injury, and a single lesion. Patients of previous surgery on the ipsilateral knee, especially meniscectomy and anterior cruciate ligament reconstruction, were indicated to be at risk. Taken together, the indication to perform microfracture is perhaps much smaller than reflected by current clinical practice. As to lesion location, gender, BMI, preoperative activity level, smoking, and concomitant knee surgery, the included articles are inconclusive or there is a lack of evidence. To determine effect sizes, better reporting is needed.
This study also highlights the need for collaboration on national and international level in the form of registries to generate larger data sets concerning cartilage repair.
Supplemental Material
Supplemental material, sj-docx-1-car-10.1177_19476035221147680 for Prognostic Factors for the Clinical Outcome after Microfracture Treatment of Chondral and Osteochondral Defects in the Knee Joint: A Systematic Review by Iris M. van Tuijn, Kaj S. Emanuel, Pieter P.W. van Hugten, Ralph Jeuken and Pieter J. Emans in CARTILAGE
Footnotes
Authors’ Note: This research was conducted at the Department of Orthopedic Surgery of Maastricht University Medical Center+.
Acknowledgments and Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: K.S.E. is supported by the Dutch Research Council (NWO) domain Applied and Engineering Sciences (P15-23).
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval: This systematic review was registered in PROSPERO on the 19th of June 2021 (id: CRD42020177512).
ORCID iDs: Iris M. van Tuijn  https://orcid.org/0000-0002-6201-1624
https://orcid.org/0000-0002-6201-1624
Ralph Jeuken  https://orcid.org/0000-0001-7729-8800
https://orcid.org/0000-0001-7729-8800
Supplementary material for this article is available on the Cartilage website at http://cart.sagepub.com/supplemental.
References
- 1. Makris EA, Gomoll AH, Malizos KN, Hu JC, Athanasiou KA. Repair and tissue engineering techniques for articular cartilage. Nat Rev Rheumatol. 2015;11:21-34. doi: 10.1038/nrrheum.2014.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Heir S, Nerhus TK, Røtterud JH, Løken S, Ekeland A, Engebretsen L, et al. Focal cartilage defects in the knee impair quality of life as much as severe osteoarthritis: a comparison of knee injury and osteoarthritis outcome score in 4 patient categories scheduled for knee surgery. Am J Sports Med. 2010;38(2):231-7. doi: 10.1177/0363546509352157. [DOI] [PubMed] [Google Scholar]
- 3. Widuchowski W, Widuchowski J, Trzaska T. Articular cartilage defects: study of 25,124 knee arthroscopies. Knee. 2007;14(3):177-82. doi: 10.1016/j.knee.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 4. Everhart JS, Abouljoud MM, Kirven JC, Flanigan DC. Full-thickness cartilage defects are important independent predictive factors for progression to total knee arthroplasty in older adults with minimal to moderate osteoarthritis: data from the Osteoarthritis Initiative. J Bone Joint Surg Am. 2019;101:56-63. doi: 10.2106/jbjs.17.01657. [DOI] [PubMed] [Google Scholar]
- 5. Houck DA, Kraeutler MJ, Belk JW, Frank RM, McCarty EC, Bravman JT. Do focal chondral defects of the knee increase the risk for progression to osteoarthritis? A review of the literature. Orthop J Sports Med. 2018;6(10):2325967118801931. doi: 10.1177/2325967118801931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Frappier J, Stanish W, Brittberg M, Steinwachs M, Crowe L, Castelo D, et al. Economic evaluation of BST-CarGel as an adjunct to microfracture vs microfracture alone in knee cartilage surgery. J Med Econ. 2014;17(4):266-78. doi: 10.3111/13696998.2014.897626. [DOI] [PubMed] [Google Scholar]
- 7. Steadman JR, Miller BS, Karas SG, Schlegel TF, Briggs KK, Hawkins RJ. The microfracture technique in the treatment of full-thickness chondral lesions of the knee in National Football League players. J Knee Surg. 2003;16:83-6. [PubMed] [Google Scholar]
- 8. Engen CN, Engebretsen L, Årøen A. Knee cartilage defect patients enrolled in randomized controlled trials are not representative of patients in orthopedic practice. Cartilage. 2010;1(4):312-9. doi: 10.1177/1947603510373917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cole BJ, Pascual-Garrido C, Grumet RC. Surgical management of articular cartilage defects in the knee. J Bone Joint Surg Am. 2009;91:1778-90. [PubMed] [Google Scholar]
- 10. Gomoll AH, Farr J, Gillogly SD, Kercher J, Minas T. Surgical management of articular cartilage defects of the knee. J Bone Joint Surg Am. 2010;92:2470-90. [PubMed] [Google Scholar]
- 11. Biant LC, McNicholas MJ, Sprowson AP, Spalding T. The surgical management of symptomatic articular cartilage defects of the knee: consensus statements from United Kingdom knee surgeons. Knee. 2015;22(5):446-9. doi: 10.1016/j.knee.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 12. Jeuken RM, van Hugten PPW, Roth AK, Timur UT, Boymans TAEJ, van Rhijn LW, et al. A systematic review of focal cartilage defect treatments in middle-aged versus younger patients. Orthop J Sports Med. 2021;9:23259671211031244. doi: 10.1177/23259671211031244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Montgomery SR, Foster BD, Ngo SS, Terrell RD, Wang JC, Petrigliano FA, et al. Trends in the surgical treatment of articular cartilage defects of the knee in the United States. Knee Surg Sports Traumatol Arthrosc. 2014;22(9):2070-5. doi: 10.1007/s00167-013-2614-9. [DOI] [PubMed] [Google Scholar]
- 14. McCormick F, Harris JD, Abrams GD, Frank R, Gupta A, Hussey K, et al. Trends in the surgical treatment of articular cartilage lesions in the United States: an analysis of a large private-payer database over a period of 8 years. Arthroscopy. 2014;30:222-6. doi: 10.1016/j.arthro.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 15. Gowd AK, Cvetanovich GL, Liu JN, Christian DR, Cabarcas BC, Redondo ML, et al. Management of chondral lesions of the knee: analysis of trends and short-term complications using the national surgical quality improvement program database. Arthroscopy. 2019;35(1):138-46. doi: 10.1016/j.arthro.2018.07.049. [DOI] [PubMed] [Google Scholar]
- 16. DeFroda SF, Bokshan SL, Yang DS, Daniels AH, Owens BD. Trends in the surgical treatment of articular cartilage lesions in the United States from 2007 to 2016. J Knee Surg. 2021;34(14):1609-16. doi: 10.1055/s-0040-1712946. [DOI] [PubMed] [Google Scholar]
- 17. Aae TF, Randsborg PH, Lurås H, Årøen A, Lian ØB. Microfracture is more cost-effective than autologous chondrocyte implantation: a review of level 1 and level 2 studies with 5 year follow-up. Knee Surg Sports Traumatol Arthrosc. 2018;26(4):1044-52. doi: 10.1007/s00167-017-4802-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Seow D, Yasui Y, Hutchinson ID, Hurley ET, Shimozono Y, Kennedy JG. The subchondral bone is affected by bone marrow stimulation: a systematic review of preclinical animal studies. Cartilage. 2019;10(1):70-81. doi: 10.1177/1947603517711220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shimozono Y, Coale M, Yasui Y, O’Halloran A, Deyer TW, Kennedy JG. Subchondral bone degradation after microfracture for osteochondral lesions of the talus: an MRI analysis. Am J Sports Med. 2018;46(3):642-8. doi: 10.1177/0363546517739606. [DOI] [PubMed] [Google Scholar]
- 20. Pestka JM, Bode G, Salzmann G, Südkamp NP, Niemeyer P. Clinical outcome of autologous chondrocyte implantation for failed microfracture treatment of full-thickness cartilage defects of the knee joint. Am J Sports Med. 2012;40(2):325-31. doi: 10.1177/0363546511425651. [DOI] [PubMed] [Google Scholar]
- 21. Minas T, Gomoll AH, Rosenberger R, Royce RO, Bryant T. Increased failure rate of autologous chondrocyte implantation after previous treatment with marrow stimulation techniques. Am J Sports Med. 2009;37(5):902-8. doi: 10.1177/0363546508330137. [DOI] [PubMed] [Google Scholar]
- 22. Levy YD, Görtz S, Pulido PA, McCauley JC, Bugbee WD. Do fresh osteochondral allografts successfully treat femoral condyle lesions? Clin Orthop Relat Res. 2013;471(1):231-7. doi: 10.1007/s11999-012-2556-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wang D, Kalia V, Eliasberg CD, Wang T, Coxe FR, Pais MD, et al. Osteochondral allograft transplantation of the knee in patients aged 40 years and older. Am J Sports Med. 2018;46(3):581-9. doi: 10.1177/0363546517741465. [DOI] [PubMed] [Google Scholar]
- 24. Erggelet C, Vavken P. Microfracture for the treatment of cartilage defects in the knee joint—a golden standard? J Clin Orthop Trauma. 2016;7(3):145-52. doi: 10.1016/j.jcot.2016.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Calcei JG, Ray T, Sherman SL, Farr J. Management of large focal chondral and osteochondral defects in the knee. J Knee Surg. 2020;33(12):1187-200. doi: 10.1055/s-0040-1721053. [DOI] [PubMed] [Google Scholar]
- 26. Smoak JB, Kluczynski MA, Bisson LJ, Marzo JM. Systematic review of patient outcomes and associated predictors after microfracture in the patellofemoral joint. J Am Acad Orthop Surg Glob Res Rev. 2019;3(11):e10.5435. doi: 10.5435/JAAOSGlobal-D-19-00151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Solheim E, Hegna J, Strand T, Harlem T, Inderhaug E. Randomized study of long-term (15-17 years) outcome after microfracture versus mosaicplasty in knee articular cartilage defects. Am J Sports Med. 2018;46(4):826-31. doi: 10.1177/0363546517745281. [DOI] [PubMed] [Google Scholar]
- 28. Mithoefer K, McAdams T, Williams RJ, Kreuz PC, Mandelbaum BR. Clinical efficacy of the microfracture technique for articular cartilage repair in the knee: an evidence-based systematic analysis. Am J Sports Med. 2009;37(10):2053-63. doi: 10.1177/0363546508328414. [DOI] [PubMed] [Google Scholar]
- 29. Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J. Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J Surg. 2003;73(9):712-6. doi: 10.1046/j.1445-2197.2003.02748.x. [DOI] [PubMed] [Google Scholar]
- 30. Asik M, Ciftci F, Sen C, Erdil M, Atalar A. The microfracture technique for the treatment of full-thickness articular cartilage lesions of the knee: midterm results. Arthroscopy. 2008;24(11):1214-20. doi: 10.1016/j.arthro.2008.06.015. [DOI] [PubMed] [Google Scholar]
- 31. Balain B, Ennis O, Kanes G, Singhal R, Roberts SN, Rees D, et al. Response shift in self-reported functional scores after knee microfracture for full thickness cartilage lesions. Osteoarthritis Cartilage. 2009;17(8):1009-13. doi: 10.1016/j.joca.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 32. Chahla J, Kunze KN, Tauro T, Wright-Chisem J, Williams BT, Beletsky A, et al. Defining the minimal clinically important difference and patient acceptable symptom state for microfracture of the knee: a psychometric analysis at short-term follow-up. Am J Sports Med. 2020;48(4):876-83. doi: 10.1177/0363546520903279. [DOI] [PubMed] [Google Scholar]
- 33. de Windt TS, Concaro S, Lindahl A, Saris DB, Brittberg M. Strategies for patient profiling in articular cartilage repair of the knee: a prospective cohort of patients treated by one experienced cartilage surgeon. Knee Surg Sports Traumatol Arthrosc. 2012;20(11):2225-32. doi: 10.1007/s00167-011-1855-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gobbi A, Nunag P, Malinowski K. Treatment of full thickness chondral lesions of the knee with microfracture in a group of athletes. Knee Surg Sports Traumatol Arthrosc. 2005;13(3):213-21. doi: 10.1007/s00167-004-0499-3. [DOI] [PubMed] [Google Scholar]
- 35. Gobbi A, Karnatzikos G, Kumar A. Long-term results after microfracture treatment for full-thickness knee chondral lesions in athletes. Knee Surg Sports Traumatol Arthrosc. 2014;22:1986-96. [DOI] [PubMed] [Google Scholar]
- 36. Knutsen G, Engebretsen L, Ludvigsen TC, Drogset JO, Grøntvedt T, Solheim E, et al. Autologous chondrocyte implantation compared with microfracture in the knee. A randomized trial. J Bone Joint Surg Am. 2004;86(3):455-64. doi: 10.2106/00004623-200403000-00001. [DOI] [PubMed] [Google Scholar]
- 37. Kon E, Gobbi A, Filardo G, Delcogliano M, Zaffagnini S, Marcacci M. Arthroscopic second-generation autologous chondrocyte implantation compared with microfracture for chondral lesions of the knee: prospective nonrandomized study at 5 years. Am J Sports Med. 2009;37(1):33-41. doi: 10.1177/0363546508323256. [DOI] [PubMed] [Google Scholar]
- 38. Kreuz PC, Erggelet C, Steinwachs MR, Krause SJ, Lahm A, Niemeyer P, et al. Is microfracture of chondral defects in the knee associated with different results in patients aged 40 years or younger? Arthroscopy. 2006;22:1180-6. doi: 10.1016/j.arthro.2006.06.020. [DOI] [PubMed] [Google Scholar]
- 39. Marder RA, Hopkins G, Jr, Timmerman LA. Arthroscopic microfracture of chondral defects of the knee: a comparison of two postoperative treatments. Arthroscopy. 2005;21(2):152-8. doi: 10.1016/j.arthro.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 40. Marquass B, Mahn T, Engel T, Gossner J, Theopold JD, von Dercks N, et al. [Clinical and radiological mid-term results after autologous osteochondral transplantation under consideration of quality of life]. Z Orthop Unfall. 2012;150(4):360-7. doi: 10.1055/s-0032-1314958. [DOI] [PubMed] [Google Scholar]
- 41. Miller BS, Steadman JR, Briggs KK, Rodrigo JJ, Rodkey WG. Patient satisfaction and outcome after microfracture of the degenerative knee. J Knee Surg. 2004;17(1):13-7. doi: 10.1055/s-0030-1247141. [DOI] [PubMed] [Google Scholar]
- 42. Miller BS, Briggs KK, Downie B, Steadman JR. Clinical outcomes following the microfracture procedure for chondral defects of the knee: a longitudinal data analysis. Cartilage. 2010;1(2):108-12. doi: 10.1177/1947603510366575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mithoefer K, Williams RJ, 3rd, Warren RF, Potter HG, Spock CR, Jones EC, et al. The microfracture technique for the treatment of articular cartilage lesions in the knee. A prospective cohort study. J Bone Joint Surg Am. 2005;87(9):1911-20. doi: 10.2106/jbjs.D.02846. [DOI] [PubMed] [Google Scholar]
- 44. Neri T, Dehon M, Klasan A, Putnis SE, Farizon F, Philippot R. Predictors of functional outcome after microfracture treatment of cartilage defects of the knee. Surg Technol Int. 2020;37:341-7. [PubMed] [Google Scholar]
- 45. Salzmann GM, Sah B, Südkamp NP, Niemeyer P. Clinical outcome following the first-line, single lesion microfracture at the knee joint. Arch Orthop Trauma Surg. 2013;133(3):303-10. doi: 10.1007/s00402-012-1660-y. [DOI] [PubMed] [Google Scholar]
- 46. Solheim E, Hegna J, Inderhaug E. Early determinants of long-term clinical outcome after cartilage repair surgery in the knee. J Orthop. 2018;15(1):222-5. doi: 10.1016/j.jor.2018.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Steadman JR, Briggs KK, Rodrigo JJ, Kocher MS, Gill TJ, Rodkey WG. Outcomes of microfracture for traumatic chondral defects of the knee: average 11-year follow-up. Arthroscopy. 2003;19(5):477-84. doi: 10.1053/jars.2003.50112. [DOI] [PubMed] [Google Scholar]
- 48. Solheim E, Hegna J, Inderhaug E, Øyen J, Harlem T, Strand T. Results at 10-14 years after microfracture treatment of articular cartilage defects in the knee. Knee Surg Sports Traumatol Arthrosc. 2016;24(5):1587-93. doi: 10.1007/s00167-014-3443-1. [DOI] [PubMed] [Google Scholar]
- 49. Van Assche D, Van Caspel D, Vanlauwe J, Bellemans J, Saris DB, Luyten FP, et al. Physical activity levels after characterized chondrocyte implantation versus microfracture in the knee and the relationship to objective functional outcome with 2-year follow-up. Am J Sports Med. 2009;37(Suppl 1):42S-49S. doi: 10.1177/0363546509350296. [DOI] [PubMed] [Google Scholar]
- 50. Vanlauwe J, Saris DB, Victor J, Almqvist KF, Bellemans J, Luyten FP, et al. Five-year outcome of characterized chondrocyte implantation versus microfracture for symptomatic cartilage defects of the knee: early treatment matters. Am J Sports Med. 2011;39(12):2566-74. doi: 10.1177/0363546511422220. [DOI] [PubMed] [Google Scholar]
- 51. Von Keudell A, Atzwanger J, Forstner R, Resch H, Hoffelner T, Mayer M. Radiological evaluation of cartilage after microfracture treatment: a long-term follow-up study. Eur J Radiol. 2012;81(7):1618-24. doi: 10.1016/j.ejrad.2011.04.071. [DOI] [PubMed] [Google Scholar]
- 52. Weber AE, Locker PH, Mayer EN, Cvetanovich GL, Tilton AK, Erickson BJ, et al. Clinical outcomes after microfracture of the knee: midterm follow-up. Orthop J Sports Med. 2018;6(2):2325967117753572. doi: 10.1177/2325967117753572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Leong DJ, Sun HB. Events in articular chondrocytes with aging. Curr Osteoporos Rep. 2011;9(4):196-201. doi: 10.1007/s11914-011-0070-3. [DOI] [PubMed] [Google Scholar]
- 55. Ding C, Cicuttini F, Scott F, Cooley H, Boon C, Jones G. Natural history of knee cartilage defects and factors affecting change. Arch Intern Med. 2006;166:651-8. doi: 10.1001/archinte.166.6.651. [DOI] [PubMed] [Google Scholar]
- 56. Shelbourne KD, Jari S, Gray T. Outcome of untreated traumatic articular cartilage defects of the knee: a natural history study. J Bone Joint Surg Am. 2003;85-A(Suppl 2):8-16. doi: 10.2106/00004623-200300002-00002. [DOI] [PubMed] [Google Scholar]
- 57. Lacy KW, Cracchiolo A, Yu S, Goitz H. Medial femoral condyle cartilage defect biomechanics: effect of obesity, defect size, and cartilage thickness. Am J Sports Med. 2016;44(2):409-16. doi: 10.1177/0363546515613517. [DOI] [PubMed] [Google Scholar]
- 58. Bergfeld JA. Articular cartilage injury: filling potholes. Orthopedics. 2004;27(9):973-4. doi: 10.3928/0147-7447-20040901-34. [DOI] [PubMed] [Google Scholar]
- 59. Brittberg M. Cartilage lesions in the patellofemoral joint. Aspetar Sports Med J. 2016;10:236-42. [Google Scholar]
- 60. Berenbaum F, Eymard F, Houard X. Osteoarthritis, inflammation and obesity. Curr Opin Rheumatol. 2013;25:114-8. doi: 10.1097/BOR.0b013e32835a9414. [DOI] [PubMed] [Google Scholar]
- 61. Chen L, Zheng JJY, Li G, Yuan J, Ebert JR, Li H, et al. Pathogenesis and clinical management of obesity-related knee osteoarthritis: impact of mechanical loading. J Orthop Translat. 2020;24:66-75. doi: 10.1016/j.jot.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Ryd L, Brittberg M, Eriksson K, Jurvelin JS, Lindahl A, Marlovits S, et al. Pre-osteoarthritis: definition and diagnosis of an elusive clinical entity. Cartilage. 2015;6(3):156-65. doi: 10.1177/1947603515586048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Saris DBF. Joint homeostasis in tissue engineering for cartilage repair. Utrecht: Universiteit Utrecht, Faculteit Geneeskunde; 2002. [Google Scholar]
- 64. Stolzing A, Jones E, McGonagle D, Scutt A. Age-related changes in human bone marrow-derived mesenchymal stem cells: consequences for cell therapies. Mech Ageing Dev. 2008;129(3):163-73. doi: 10.1016/j.mad.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 65. Toh WS, Brittberg M, Farr J, Foldager CB, Gomoll AH, Hui JHP, et al. Cellular senescence in aging and osteoarthritis. Acta Orthop. 2016;87:6-14. doi: 10.1080/17453674.2016.1235087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Mueller MB, Tuan RS. Anabolic/Catabolic balance in pathogenesis of osteoarthritis: identifying molecular targets. PM R. 2011;3(6, Suppl 1): S3-11. doi: 10.1016/j.pmrj.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 67. Hertler C, Seiler A, Gramatzki D, Schettle M, Blum D. Sex-specific and gender-specific aspects in patient-reported outcomes. ESMO Open. 2020;5(Suppl 4):e000837. doi: 10.1136/esmoopen-2020-000837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Kraus NR, Lowenstein NA, Garvey KD, Matzkin EG. Smoking negatively effects patient-reported outcomes following arthroscopic partial meniscectomy. Arthrosc Sports Med Rehabil. 2021;3(2):e323-8. doi: 10.1016/j.asmr.2020.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Niemeyer P. KnorpelRegister-Bilanz nach sieben Jahren. Z Orthop Unfall. 2021;11:63-4. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-car-10.1177_19476035221147680 for Prognostic Factors for the Clinical Outcome after Microfracture Treatment of Chondral and Osteochondral Defects in the Knee Joint: A Systematic Review by Iris M. van Tuijn, Kaj S. Emanuel, Pieter P.W. van Hugten, Ralph Jeuken and Pieter J. Emans in CARTILAGE