Medulloblastoma is the most common malignant brain tumor in pediatric patients and conventional treatment can result in devastating long-term sequalae.1–3 This highlights the essential need to develop more effective and less toxic therapies for children with this deadly cancer. While cancer immunotherapy has been revolutionary as a treatment modality in adult oncology and in pediatric acute lymphoblastic leukemia, the field has thus far been unsuccessful in harnessing the power of the immune system against the preponderance of pediatric solid tumors due to poor homogenously expressed antigenic targets, disease heterogeneity, ineffective immune trafficking/persistence within the tumor, and an immunosuppressive tumor microenvironment (TME).4,5
In the review by Schakelaar et al,6 the authors provide an excellent summary of the cellular immunotherapy landscape for medulloblastoma. Importantly, the authors pay attention not only to adaptive cell therapies (ie, T cells), but also innate therapies such as NK cells and dendritic cells. The authors consider agents which have entered early phase clinical trials, but also look toward the future and consider additional strategies which are in varying stages of preclinical development.
Any review of cellular cancer immunotherapy should begin with adoptive T cell therapy, as this approach has been most extensively studied. Several relevant targets are reviewed, including HER2, B7-H3, PRAME, and NKG2DLs. Of these, a number of clinical trials for HER2 CAR T cells are discussed demonstrating relative safety and feasibility. The authors also highlight 7 ongoing CAR T cell clinical trials for a range of targets including NKG2DL, GD2, HER2, EGFR, IL13-Rα2, and B7-H3.
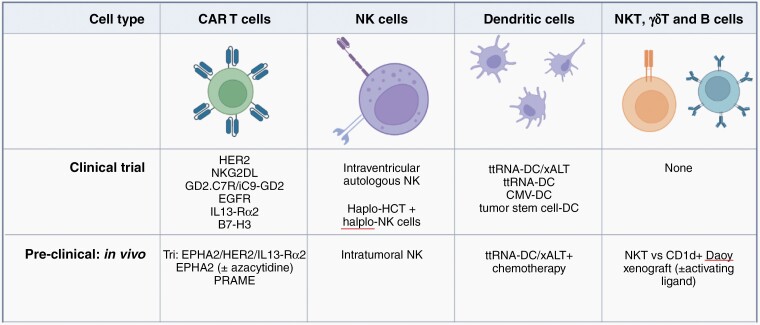
The authors then turn their attention to arbiters of the innate immune system, whose importance is increasingly becoming better understood. NK cells recognize non-self cells while dendritic cells serve as master antigen presenting cells; both have been extensively studied in preclinical testing and in early phase clinical trials. These approaches have proven safety, feasibility, and in a phase II study (NCT01326104), early signs of efficacy in medulloblastoma. These studies highlight how the field is moving beyond simply testing T cell-centric approaches as adoptive cellular therapies for medulloblastoma with additional approaches on the horizon including NKT cells, B cells, and γδ T cells (Figure 1). Some of these approaches alone or in combination may be better equipped to overcome medulloblastoma heterogeneity.
Figure 1.
Development status for medulloblastoma cellular immunotherapy, as discussed in Schakelaar et al. ALT, adoptive lymphocyte transfer; DC, dendritic cell; Haplo, haploidentical; HCT, hematopoietic cell transplant; ttRNA, total tumor RNA. Images created with Biorender.com.
It is now understood that medulloblastoma is not one disease entity and instead comprises several genetically distinct disease processes. Many of the early phase clinical trials discussed have not reported robustly annotated clinical data, including the medulloblastoma subtypes, and/or did not discriminate or stratify based on subtype. Incorporating subgroup stratification into clinical trial design will be important to ensure that we not only learn whether a novel therapy is safe/feasible, but also whether there is a specific subtype where cell therapy deserves particular attention in follow-up phase II studies.
As better targets are validated and new approaches tested with subgroup specific analyses, it will be essential that cell therapies culminate in immunologic memory, which is often stymied by inadequate cellular trafficking and persistence in the TME. To overcome these challenges, locoregional administration of adoptive cellular therapies, including CAR T-cells or NK cells, has been frequently employed in both clinical trials and in vivo studies. Evidence is emerging that route of administration is critical for success of cellular immunotherapy in other pediatric brain tumors such as diffuse midline glioma,7 and this line of investigation should be followed closely.
Tipping the balance in favor of effector immunity will be necessary to sustain cellular immunotherapy. One example includes targeting tumor metabolomics, which has shown promise in simultaneously activating tumor-infiltrating lymphocytes while also reprogramming immunosuppressive myeloid derived suppressor cells.8,9 Just as identifying novel cellular therapies with relevant targets in medulloblastoma is necessary, “arming” them to overcome a hostile TME will be equally essential. This necessitates a better understanding of the TME as well as the biology that may be unique to a given patient. We should commit to performing these arduous discovery studies and prospectively incorporate these questions into our ongoing clinical trials if we hope to uncover the specific combinations that better inform cellular immunotherapy in medulloblastoma.
Ultimately, combination therapies will be critical in overcoming cell therapy barriers while sustaining long-lived responses. These include combining cellular therapies with other potentially active agents such as immune checkpoint blockade. While checkpoint inhibition is frequently combined with other immunotherapies, and can overcome T cell exhaustion, alternative combinations may be necessary to maximize cellular therapeutic responses. Indeed, immunotherapy appears to be parroting the early days of chemotherapy when single agents were insufficient; however, through synergistic combinations that exploit oncologic vulnerabilities, combination chemotherapy rapidly emerged as a standard of care for medulloblastoma. Similarly, immunotherapeutic combinations must be leveraged to achieve the same synergy. While adoptive cellular therapy is ideally suited to elicit rapid de novo responses against immune “cold” tumors like childhood brain cancer, additional combinations to consolidate and intensify response will be necessary as will considerations for testing these in the upfront setting. Similar to how synergistic chemotherapy required a better understanding of the cell-cycle, synergistic immunotherapy will require a better understanding of the medulloblastoma ecosystem and immune TME. As new trials emerge, candidates for combinations may include small molecule inhibitors, vaccines, and immunomodulating agents. As responses are optimized, maintenance therapy with checkpoint inhibitors may be more ideal.
Immunotherapy represents a new frontier with significant unrealized potential in medulloblastoma. The early signs of safety and feasibility are encouraging as are the number of new approaches being developed. As we take the first steps to transform care and limit toxicity for medulloblastoma patients, there is hope that immunotherapy will fulfill its promise and engender long-lived memory that improves outcomes, limits relapse rates, and bypass late effects for the extraordinary patients battling this terrible disease.
Contributor Information
John A Ligon, Department of Pediatrics, Division of Hematology/Oncology, University of Florida, Gainesville, FL, USA.
Elias J Sayour, Department of Neurosurgery, University of Florida, Preston A. Wells, Jr. Center for Brain Tumor Therapy, Gainesville, FL, USA.
Disclosure
The text of this editorial is the sole product of the authors and no third party had input or gave support to its writing.
Conflict of interest
E.J.S. has pending patents related to vaccine technologies discussed in this editorial.
References
- 1. Juraschka K, Taylor MD. Medulloblastoma in the age of molecular subgroups: a review. J Neurosurg Pediatr. 2019;24(4):353–363. [DOI] [PubMed] [Google Scholar]
- 2. Thompson EM, Hielscher T, Bouffet E, et al. Prognostic value of medulloblastoma extent of resection after accounting for molecular subgroup: a retrospective integrated clinical and molecular analysis. Lancet Oncol. 2016;17(4):484–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Baroni LV, Sampor C, Gonzalez A, et al. Bridging the treatment gap in infant medulloblastoma: molecularly informed outcomes of a globally feasible regimen. Neuro Oncol. 2020;22(12):1873–1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ligon JA, Wessel KM, Shah NN, Glod J. Adoptive cell therapy in pediatric and young adult solid tumors: current status and future directions. Front Immunol. 2022;13:846346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hwang EI, Sayour EJ, Flores CT, et al. The current landscape of immunotherapy for pediatric brain tumors. Nat Cancer. 2022;3(1):11–24. [DOI] [PubMed] [Google Scholar]
- 6. Schakelaar MY, Monnikhof M, Crnko S, et al. Cellular immunotherapy for medulloblastoma. Neuro Oncol. 2023;25(4):617–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Majzner RG, Ramakrishna S, Yeom KW, et al. GD2-CAR T cell therapy for H3K27M-mutated diffuse midline gliomas. Nature. 2022;603(7903):934–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Leone RD, Zhao L, Englert JM, et al. Glutamine blockade induces divergent metabolic programs to overcome tumor immune evasion. Science. 2019;366(6468):1013–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Oh MH, Sun IH, Zhao L, et al. Targeting glutamine metabolism enhances tumor-specific immunity by modulating suppressive myeloid cells. J Clin Invest. 2020;130(7):3865–3884. [DOI] [PMC free article] [PubMed] [Google Scholar]