Abstract
Background
Bevacizumab is increasingly used in children with pediatric low-grade glioma (PLGG) despite limited evidence. A nationwide UK service evaluation was conducted to provide larger cohort “real life” safety and efficacy data including functional visual outcomes.
Methods
Children receiving bevacizumab-based treatments (BBT) for PLGG (2009–2020) from 11 centers were included. Standardized neuro-radiological (RANO-LGG) and visual (logMAR visual acuity) criteria were used to assess clinical–radiological correlation, survival outcomes and multivariate prognostic analysis.
Results
Eighty-eight children with PLGG received BBT either as 3rd line with irinotecan (85%) or alongside 1st/2nd line chemotherapies (15%). Toxicity was limited and minimal. Partial response (PR, 40%), stable disease (SD, 49%), and progressive disease (PD, 11%) were seen during BBT. However, 65% progressed at 8 months (median) from BBT cessation, leading to a radiology-based 3 yr-progression-free survival (PFS) of 29%. Diencephalic syndrome (P = .03) was associated with adverse PFS. Pre-existing visual morbidity included unilateral (25%) or bilateral (11%) blindness. Improvement (29%) or stabilization (49%) of visual acuity was achieved, more often in patients’ best eyes. Vision deteriorated during BBT in 14 (22%), with 3-year visual-PFS of 53%; more often in patients’ worst eyes. A superior visual outcome (P = .023) was seen in neurofibromatosis type 1-associated optic pathway glioma (OPG). Concordance between visual and radiological responses was 36%; optimized to 48% using only best eye responses.
Conclusions
BBTs provide effective short-term PLGG control and delay further progression, with a better sustained visual (best > worst eye) than radiological response. Further research could optimize the role of BBT toward a potentially sight-saving strategy in OPG.
Keywords: Bevacizumab, low-grade glioma, optic pathway glioma, pediatric, visual outcomes
Key Points.
Bevacizumab is a safe and effective treatment for pediatric low-grade glioma.
Benefits of BBT included speed to visual stability/improvement as sight-saving strategy.
Best eye visual responses benefit most from BBTs and correlate best with MRI responses.
Importance of the Study.
This study provides real-world clinical safety and efficacy data from the largest existing cohort of patients with pediatric low-grade glioma (PLGG) treated with Bevacizumab to date. This manuscript presents an unprecedented, in-depth visual outcome assessment of children with PLGGs receiving Bevacizumab as an important and meaningful functional outcome for patients; the majority of whom will survive into adulthood. This is in addition to a unique evaluation of visual and radiological outcome correlation, along with prognostic factor assessment for radiological and visual outcomes of Bevacizumab in PLGG to progress available published literature. Importantly, the novel and key assessments and conclusions within this manuscript are translatable to clinical practice to benefit patients; particularly the potential use of Bevacizumab with non-irinotecan chemotherapies at an earlier stage for visual preservation in optic pathway glioma. Overall, this work justifies further large cohort and prospective studies to refine the optimal use of Bevacizumab for PLGG.
Pediatric low-grade gliomas (PLGG) are the commonest central nervous system (CNS) tumors of childhood1; comprising a heterogeneous group with regards to age, tumor predisposition, location, and outcomes.2,3 For many patients with PLGG a complete surgical resection is curative and results in excellent survival rates.4 However, a significant proportion of children will have unresectable PLGG due to tumor location and/or risks of surgery. These are factors that are associated with inferior survival rates and a significantly increased risk of tumor progression requiring multiple treatments leading to a potential for suboptimal future function and quality of life.
Chemotherapy treatment is the mainstay in cases of unresectable PLGG, including optic pathway gliomas (OPG), which can delay or even omit the need for radiotherapy (RT) as a potentially more toxic treatment option, in a proportion of patients.5–7 However, with the current RT-sparing strategy, more than half of children suffer from progressive PLGG; often requiring multiple lines of treatments.8–10 In addition, whilst many available chemotherapy-based regimens might provide adequate or temporary tumor control, efficacy is diminished with subsequent chemotherapies at further progression.11,12
Importantly, patients with unresectable PLGG are vulnerable to chronic morbidities over prolonged periods due to both the tumor growth and the accumulation of toxicity from the various treatments required to limit it.11–15 In addition, a considerable proportion of unresectable OPGs with or without the involvement of the hypothalamus, can irreversibly affect and threaten visual and/or endocrine functions, thus contributing to the overall long-term morbidity and functional impairment for patients; the majority of who will survive long into adulthood.16–19 Continued efforts are therefore necessary to refine the optimal management of unresectable PLGGs as somewhat unpredictable and potentially progressive neoplasms, bearing in mind the long-term perspective of potentially life-long disabling morbidities and functional impairments.
Bevacizumab is a humanized monoclonal antibody against vascular endothelial growth factor (VEGF), which has been used in various pediatric tumors over the last 15 years with only limited benefit confined to selected groups such as PLGG.20–22 Bevacizumab was first demonstrated as effective in combination with irinotecan from a series of multiply relapsed PLGG (including a significant proportion of OPGs) published by Packer et al. in 2009, where radiological response alongside improvement in visual parameters such as visual fields (VF) or visual acuity (VA) were first documented.10 Since then, the increasing use of bevacizumab-based therapy (BBT) regimens has led to several small retrospective case series, with only one prospective phase II study confirming the activity of Bevacizumab for PLGG.10,20,23–28 Whilst the UK Cancer Drug Funding (CDF) has approved Bevacizumab use for PLGG as 3rd line in combination with Irinotecan, the limited data available has led to its increasingly varied, unlicensed, and off-label use across centers and countries.
We undertook a UK-nationwide service evaluation to obtain “real world data” on the outcomes, tolerability, and toxicities of BBT, to add data to the literature and to assist in refining and optimizing future management of PLGG.
Methods
We collected retrospective patient, tumor, and treatment data from children and young adults under 18 years of age diagnosed with progressive PLGG at UK tertiary pediatric oncology primary treatment centers (PTCs) who received a bevacizumab-based treatment (BBT) during their disease course.
Data including patient’s age at diagnosis and receipt of BBT, gender, presence of Neurofibromatosis type 1 (NF1), OPG staging according to the modified DODGE classification (PLAN),29,30 presence of metastatic disease, histopathological diagnosis (as per clinical diagnosis; not centrally reviewed), BRAF status (where available), and clinical presentation signs were retrospectively collected in all patients receiving BBT for PLGG since 2000. In addition, all surgical interventions (biopsy, CSF diversion, and tumor resections) and adjuvant therapies prior to Bevacizumab were recorded. BBT treatment data collected included indication for commencing BBT, time from diagnosis, and from last treatment to start of BBT, line of therapy BBT used, Bevacizumab partner drugs, number of Bevacizumab doses delivered, and overall duration of BBT. Bevacizumab-related toxicities were documented as per Common Terminology Criteria for Adverse Events (CTCAE version 4.0).
Primary outcomes for BBT efficacy were:
1) Radiological outcomes: assessed based on changes in tumor size on MRI scan according to the RANO-LGG assessment criteria,31 evaluated by the same neuro-radiologist or radiologist with expertise in pediatric neuro-oncology at each contributing center.
2) Visual outcomes: defined as better/stable/worse vision per eye and overall, per patient, based on monocular or, if not available, binocular best corrected visual acuity (VA) according to standardized criteria.32 Visual field data per patient was also collected where available and defined as better/stable/worse.
Radiological tumor measurements and VA data were gathered at BBT baseline, every 3 months until the completion of treatment, and at last available follow-up examination from the clinical standard of care MRI scans and visual assessments.
Visual outcomes were collated and reviewed by the same ophthalmologist or neuro-oncologist with expertise in ophthalmology and visual assessment at each contributing center. VA data obtained from the retrospective standard of care clinical ophthalmology assessments were translated to logMAR units, eg, 6/60 = 1.0 logMAR and quantified from 0 logMAR (normal vision) to 1.8 logMAR. In addition, the following logMAR units were assigned to low vision descriptive values: 2.0 logMAR for Counting Fingers (CF), 2.4 logMAR for Hand Movements (HM), 2.7 logMAR for Light Perception (LP), and 3.0 logMAR for No Perception of Light (NLP) based on the available references.33,34 Patients with NLP in both eyes were excluded from the visual changes analysis. Visual responses were assessed based on the change in monocular (if available) or binocular best corrected VA according to the REiNS recommendations.32Per patient changes in visual acuity were defined as “better” in case of improvement (reduction) of VA of ≥ 0.2 logMAR in one eye without deterioration in the contralateral eye, “worse” in case of deteriorating VA of 0.2 logMAR in at least one eye and regardless of the other eye’s changes, or “stable” otherwise. Per eye visual changes were also utilized with maximal–minimal and median logMAR changes from each eye reported. In addition, as best eyes more reliably reflect the overall residual and functional visual capacity in each patient, as an exploratory outcome we separately analyzed visual outcome from best (BE) and worst eyes (WE). As a secondary visual outcome measure, per patient visual field responses were included where performed clinically (using Goldmann and Humphrey Visual Field perimetry according to standardized clinical protocols at each contributing center), with response (from start to end of BBT) defined as “improved” (any visual field gain documented in at least one eye), “stable” (no change in documented visual fields), or “deteriorated” (any loss of visual field in at least one eye). VF responses were also compared to VA responses for assessment of concordance between the visual responses to BBT.
To evaluate clinical–radiological correlation, full/partial/no concordance between per patient visual outcome (VA) and radiological outcome from start to end of BBT were analyzed. Full Concordance (FC) was defined in cases with concordant outcomes, for example, radiological reduction in tumor size with associated improved vision, or radiologically progressive disease with concomitant visual deterioration. Full Discordance (FD) was characterized by conflicting outcomes, for example, radiological tumor shrinkage but with clinical visual deterioration. Partial concordance (PC) was defined by one parameter changing whilst the other remained stable, eg, visual deterioration with stable radiological findings. Visual field responses were also correlated with visual acuity responses and radiological responses as secondary correlation measures.
Overall survival (OS) and progression-free survival (PFS) curves (all causes, radiological responses only, and visual responses only) were calculated from the start of BBT to an event such as death, radiological and/or visual progression, or stable disease at most recent follow-up. Log-rank test (for univariate) and Cox proportional hazards models (for multivariate) analysis and hazard ratios (HR) with 95% CIs evaluated the effect of possible prognostic factors on time-dependent outcomes. Chi-square, T- or Fisher’s exact tests were used for comparison between groups and Odds Ratios (ORs) with 95% CIs obtained for each variable using logistic regression. Statistical analyses were performed using the IBM SPSS version 27 software for Windows (IBM Corp. Armonk, NY, USA) and R statistical packages; with P < .05 considered statistically significant.
This project was approved as a National Service Evaluation through Great Ormond Street Hospital (GOSH) London, with all contributing centres obtaining individual local service evaluation permissions. An appropriate Great Ormond Street Hospital committee approved the project as a national service evaluation. Data were anonymized, virtually stored, and analyzed at GOSH in a secure and data repository compliant with General Data Protection Regulation (GDPR).
Results
Baseline PLGG Patient and Tumor Characteristics
Eighty-eight children with progressive PLGG from 11 UK PTCs received BBT between November 2009 and December 2020. Baseline patient demographics, tumor characteristics and participating centers are detailed in Table 1 and Supplemental Table 1 respectively.
Table 1.
Patient, Disease and Treatment Characteristics
| Patient Characteristics | N = 88 | % | |
|---|---|---|---|
| Gender | Female: Male | 55:33 | 63%: 37 |
| Age at Diagnosis (months) | Median (range) | 26.6 (1.4–162.6) | |
| Associated NF1 | Presence of NF1 | 21 | 24 |
| Diencephalic Syndrome | Presence of diencephalic syndrome | 25 | 28 |
| Disease Characteristics | |||
| Tumor Site and Staging | Non-optic pathway glioma (OPG) | 11 | 12 |
| Optic pathway glioma (OPG) | 77 | 88 | |
| Optic Pathway Tumor Staging | Optic nerve/s +/- Chiasm (MDC 1/2) | 34 | 44 |
| Post-chiasmatic (MDC 3) | 31 | 40 | |
| Optic tracts (MDC 4) | 12 | 16 | |
| Hypothalamic involvement | 59 | 67 | |
| Metastatic Disease | Yes | 17 | 19 |
| No | 71 | 81 | |
| Histology (71 Patients) | Pilocytic astrocytoma (PA) | 58 | 82 |
| Non-pilocytic astrocytoma (Non-PA) | 13 | 18 | |
| BRAF Status (60 Patients) | BRAF-V600E mutation | 12 | 20 |
| BRAF:KIAA1549 fusion | 29 | 48 | |
| BRAF wild-type (WT) | 18 | 30 | |
| Other | 1 | 2 | |
| Pre-Bevacizumab Therapy Treatments | |||
| Surgical Intervention | Biopsy only | 36 | 41 |
| Resection/debulking | 33 | 38 | |
| No intervention | 19 | 21 | |
| Chemotherapy | Received chemotherapy | 81 | 92 |
| Bevacizumab Therapy Data | |||
| Indication for Bevacizumab Treatment | Clinical progression | 18 | 20 |
| Radiological progression | 38 | 44 | |
| Both | 24 | 27 | |
| Other | 8 | 9 | |
| Age at Starting Bevacizumab (months) | Median (range) | 73.5 (6.6–257) | |
| Number of Bevacizumab Doses | Median | 24 (2–49) | |
| Bevacizumab Partner Drug | Irinotecan | 76 | 86 |
| Vincristine-carboplatin | 7 | 7 | |
| Vinblastine | 2 | 2 | |
| Other (or monotherapy) | 3 | 3 | |
| Bevacizumab Line of Treatment | 1st-line (with VCR + carboplatin) | 7 | 8 |
| 2nd-line (with VBL or irinotecan) | 6 | 7 | |
| 3rd-line (with irinotecan) | 61 | 70 | |
| Further than 3rd-line | 14 | 16 |
The median age at PLGG diagnosis was 26 months. NF1 was present in 21 cases (24%). Optic pathway gliomas (OPG) were 77 (88%); either confined to optic chiasm +/− anterior visual pathway (PLAN 1-2) in 44%, or with post-chiasmatic involvement (PLAN 3-4) in 56%. Eleven (12%) tumors were non-OPGs located in the brainstem, posterior fossa, midbrain, spine, and temporal lobe locations. Histopathological findings from 68 patients (77%) biopsied either at diagnosis or progression were consistent with a WHO grade I Pilocytic Astrocytoma (PA) in 93% with an available tissue diagnosis, WHO grade I Ganglioglioma in 4 (6%) and a “LGG NOS” in 1 (1%). From the 60 PLGG in which molecular profiling was available, either a BRAF-fusion in 30 (50%) or BRAF-V600E mutation 12 (20%) was documented, while no known BRAF alterations (BRAF-WT) were found in 18 (30%). BRAF status was not available in the remaining 28 (32%) children. The diagnosis was based purely on pathognomonic MRI features without histological confirmation in 20 (23%).
Treatments before BBT included single (n = 27, 39%) or multiple surgical resections (n = 6, 9%) or biopsy only in 35 (52%) and was usually followed by chemotherapy (92%); either vincristine/carboplatin or vinblastine respectively as first- and second-line chemotherapies.
Bevacizumab-based Treatment (BBT) Data and Toxicity
As summarized in Table 1, Bevacizumab was administered at a dose of 10 mg/kg every 14 days, for a median of 24 doses (range 2–49) and for a median overall duration of 12 months (range 1–24). BBT was initiated after a median of 40 months (range 1–163) from PLGG diagnosis, at a median interval of 3.5 months (range 0–55) from the last treatment, and at a median age of 72 months (range 7–201). BBTs were used as 3rd or further-line therapy in 75 patients (85%) in combination with Irinotecan as per current Children’s Cancer and Leukemia group (CCLG) PLGG guidelines, as second-line treatment in combination with irinotecan in 6 (7%), or as an addition to upfront LGG regimens such as vincristine-carboplatin or vinblastine in 7 (8%). Altogether, 67% of patients received BBT for the licensed 12 months of therapy as per national CCLG guidelines.35 Twenty-six patients (30%) received shorter therapy durations (1–11 months) due to toxicities or patient/parent preference. Three patients with OPGs (3%) received prolonged BBT therapy (17 months, 18 months, and 72 months durations) with Irinotecan due to perceived exceptional clinical benefit on a case-by-case basis; with all 3 patients having perceived clinical benefit (stable or improved) in both radiological and visual responses justifying ongoing clinical benefit. All three patients remain alive and have relapsed at 5 months, 13 months, and 24 months (median 13 months) off BBT respectively. Bevacizumab was stopped outright (instead of a weaning dose) in the vast majority of patients.
Indications to start BBT were due to an isolated radiological progression (43%) or clinical (mainly visual) deterioration (20%), a combination of clinical and radiological deterioration (27%); or due to physician choice/allergy to previous chemotherapies in the remaining 10%. A significant correlation (Fisher’s exact test: P = .04) was found between isolated visual deterioration as BBT indication and BBT used earlier than 3rd line.
Most documented toxicities were limited to CTCAE grades I or II (76.1%); with 19.4% grade III toxicities including proteinuria in 14% or systemic hypertension in 6%, which were all transient and resolved after BBT interruption/completion. A total of 3 (5%) grade IV toxicities occurred which comprised an intracranial hemorrhage secondary to posterior reversible encephalopathy syndrome (PRES) soon after BBT commencement, and a gastrointestinal bleeding with subsequent sepsis within 6 months from start; both of which required immediate BBT cessation. Other potential toxicities which manifested within 12 months from BBT completion were a hemiplegic stroke with no alternative attributable causes and an asymptomatic occlusive vasculopathy in the absence of other known risk factors (no NF1 or radiotherapy). No significant differences were found between BBT toxicities with irinotecan or alternate chemotherapy regimens.
After a median follow-up of 43 months (range 4–143) from BBT start, 7 (8%) children died (none during or immediately after BBT) and 81 (92%) children remain alive, resulting in an estimated 3-year-overall survival (OS) of 92% and median OS time of 112 months (95% CI 81–143 months).
Radiological Response and Outcomes
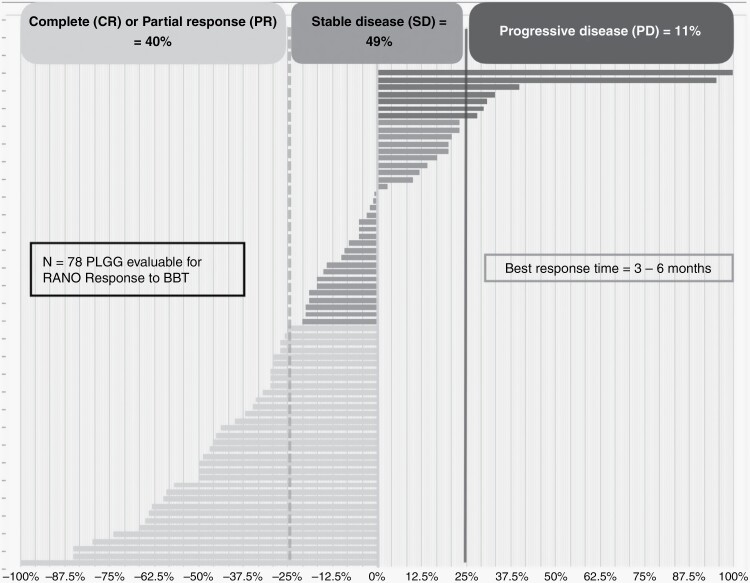
As determined by RANO criteria from 78 evaluable patients, the waterfall plot of radiological responses to BBT is illustrated in Figure 1. A partial response (PR) was seen in 31 (40%), stable disease (SD) in 38 (49%); and progressive disease (PD) in 9 (11%) children; resulting in an overall radiological disease control rate (PR + SD) of 88% during BBT. The median time to best response was 3 months from commencing treatment. No identified variables, such as indication to start BBT (radiological versus clinical progression), age at diagnosis (<1 year, 1-5, > 5), NF1-status, BRAF-status or timing of BBT (3rd line or further versus 1st/2nd line) influenced the likelihood of radiological outcomes.
Figure 1.
Waterfall plot of MRI (RANO) radiological responses to BBT. ).
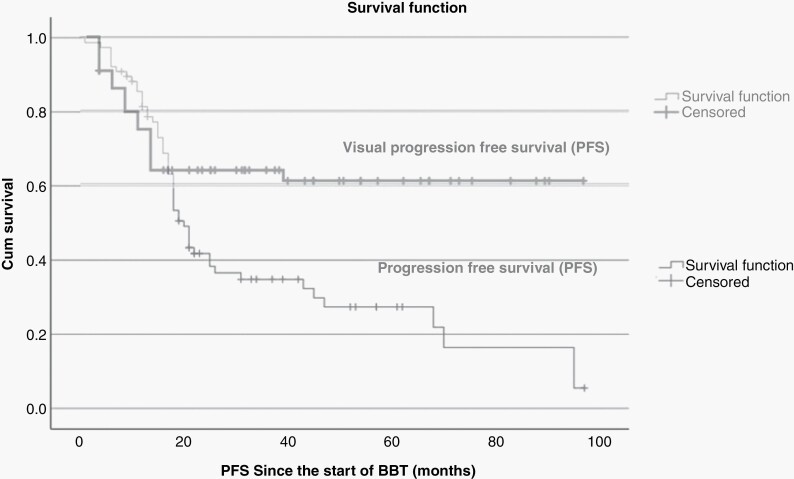
Of the 69 patients with on-treatment radiological response, 41 (59%) suffered from (radiological and/or clinical) progression at a median duration of 8 months after BBT cessation (range 4–23 months). The median PFS time from BBT start was 17 months (95% CI 14.54–19.46) with (all-cause) progression-free survival (PFS) rates at 1-year and 3-years of 67.5% and 21.7%, respectively (Figure 2). However, when radiological progression was analyzed independently from other events, the median radiological-PFS increased to 20 months (95% CI 17.65–22.34) with 1-year and 3-year radiological-PFS rates of 82.1% and 29.2%. Multivariate prognostic analysis (Cox-Hazard) revealed that the risk of radiological progression was increased in children presenting with Diencephalic Syndrome (DS) (P = .031) and those without NF1 (P = .021) (Supplemental Figures 1 and 2, respectively).
Figure 2.
Kaplan–Meier curves for PFS and visual-EFS with BBT.
Visual Morbidity, Outcomes, and Prognostic Factors
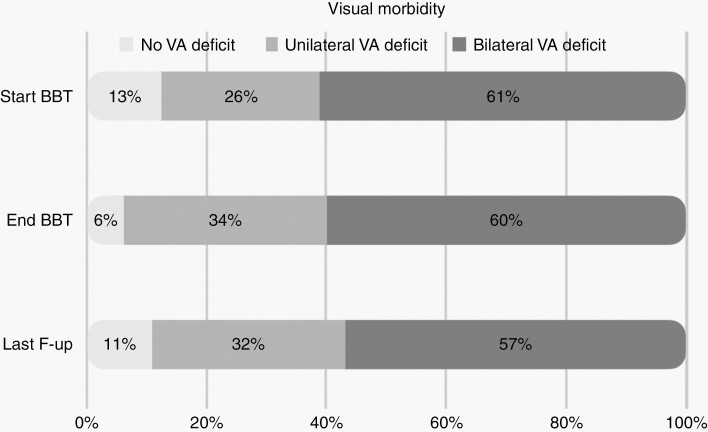
VA data of children with OPG with evaluable visual function at the start (n = 72) and end (n = 65) of BBT, and at last documented follow-up (n = 65) are summarized in Figure 3. Overall, the majority of children suffered VA deficits (VA logMAR > 0.2) in either one or both eyes, with the proportion of children having visual deficits similar between the start of BBT (87%) and at the last follow-up off BBT (89%). Prior to commencing BBT, 9 (13%) children had entirely normal vision, defined as VA logMAR ≤ 0.2 in both eyes, which reduced slightly to 4 (6%) at end of BBT and 7 (11%) at the last follow-up. The number of children with unilateral VA deficits increased from 19 (26%) pre-BBT to 22 (34%) at end of BBT and 21 (32%) at the last follow-up, whilst the number of children with bilateral VA deficits decreased from 44 (61%) pre-BBT to 39 (60%) at end of BBT and 37 (57%) at the last follow-up. Eighteen (25%) and 8 (11%) children were considered blind with LP/NLP in one or both eyes, respectively before BBT, which remained unchanged after BBT and at last follow-up.
Figure 3.
Visual morbidity per patient at start and end of BBT and at last follow-up (VA morbidity).
According to per patient VA outcomes using the REiNS criteria, vision improved in 19 (29%), remained stable in 32 (49%), and deteriorated in 14 (22%) patients; leading to an overall on-treatment visual stabilization rate (improved + stable vision) of 78.5%. The best visual response occurred at a median of 3 months from the start of BBT. Median time to visual progression after BBT was 73 months (95% CI 59.4–86.2), resulting in 1-year and 3-year visual-Event Free Survival (v-EFS) rates of 73% and 53%, respectively, and with an estimated 5-year v-EFS of 39% (see Figure 2).
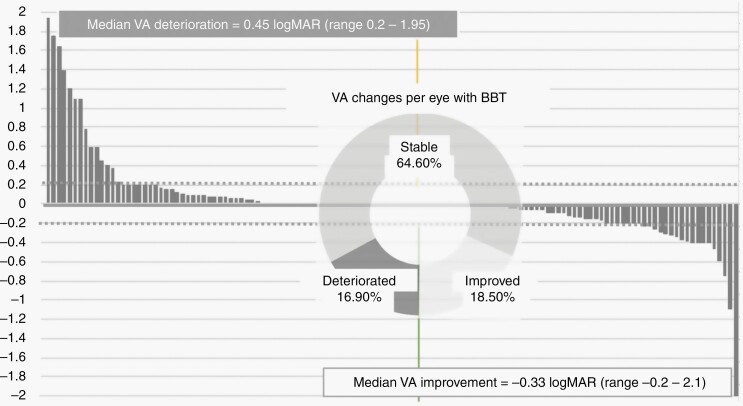
When each eye was analyzed separately for a visual outcome (per eye visual change) as illustrated in the waterfall plot in Figure 4, improved or stable vision (visual stabilization rate) was seen in 83% of eyes. Amongst the 24 (19%) eyes that improved on BBT, the median logMAR reduction was −0.33 (range 0.2–2.1). Best (BE) and worst eyes (WE) showed similar rates of VA improvement; 20% and 17%, respectively. The proportion of stable vision achieved between best and worst eyes was also similar (52% vs. 48%) out of the 84 (65%) stable eyes; 22 of which (18 WE and 4 BE) suffered from LP/NLP throughout BBT. Conversely, VA deteriorated in 22 (17%) eyes, with a median increase of +0.45 logMAR (range 0.2–1.95), which occurred more frequently in WE (22%) compared with BE (12%).
Figure 4.
Waterfall plot of VA changes per eye with BBT.
When the overall magnitude of VA changes per eye (BE/WE) was calculated, median VA was stable in the Best Eye (0.3–0.4 logMAR) throughout treatment and follow-up, whilst in the Worst Eye VA deteriorated (1.34–1.78 logMAR) at end of BBT and was stable thereafter (1.7 logMAR).
Visual field (VF) data was available in 24 (31%) patients with OPGs throughout BBTs; with only 16 (21%) having VF assessment from initial diagnosis. Of these 24, 18 (75%) had chiasmatic/hypothalamic tumors, and 6 (25%) post-chiasmatic tumors. Visual field abnormalities were documented in 10 patients (42%) at initial diagnosis, and 13 (54%) at the initiation of BBTs: 7 (54%) unilateral hemianopia, 3 (23%) unilateral quadrantanopia, 2 (15%) bitemporal hemianopia, and 1 (8%) unilateral blindness. Throughout BBT, VF abnormalities were recorded in 19 patients (79%), decreasing to 15 (63%) at the end of BBT. Overall VF responses (from start to end of BBT) were: stable in 20 (83%); with 7 (29%) having normal full VFs throughout, improved in 3 (13%), and deteriorated in 1 (4%). Of the 3 patients with VF improvement on BBT, 2 were sustained off BBT, with the third patient having deterioration in VF by the first follow-up 3 months after BBT cessation. Fourteen (58%) of 24 patients with VF data had VF responses concordant with VA responses; with 13 of these being stable responses and 1 being improvement.
The lack of NF1 was the only significant poor prognostic factor for a worse v-EFS on multivariate analysis (P = .023) (Supplemental Figure 2), with tumor location (PLAN staging I-II vs III-IV), age at PLGG diagnosis (<1 year, 1–5 years, and >5 years), BRAF status, and line of BBT not demonstrating independent prognostic influence for adverse visual outcome.
Clinical-Radiological Correlation
To determine the clinical–radiological outcome correlation throughout BBT treatments we analyzed the concordance between clinical/visual (VA) and MRI changes, as shown in Supplemental Table 2. A full concordance between both radiological and visual (VA) outcomes was present in 22 (36%) patients; whereas, a full discordance was found in 11 (18%) patients. In addition, a partial concordance—either improvement or deterioration of one parameter with the stability of the other parameter—was seen in 28 (46%) patients. When patients’ visual outcome based on best eye-only VA changes was considered, the concordance rate (full and partial) increased to 48% and 43%, respectively, with only 5 cases (8%) then fully discordant. A superior correlation between per patient visual field and radiological responses was demonstrated in 50%. Eight (33%) of 24 patients with VA, VF, and radiological response data available demonstrated a correlation of responses across the three outcome measures; all with stable disease (Supplemental Table 3).
Prognostic Factor Analysis
Cox regression analysis revealed children with NF1-related OPGs had more favorable outcomes after BBT in terms of both radiological-PFS (P = .021) and visual-EFS (P = .023) (Supplemental Figure 2a and 2b). DS was an independent poor prognostic factor for adverse radiological-PFS (P = .031) (Supplemental Figure 1). Age at PLGG diagnosis (<1 year, 1–5 years and >5 years) was not found to be an independent prognostic factor for either radiological or visual outcome, with similar outcomes between categorical age groups. However, children commencing BBT before 10 years old had a significantly higher chance of visual improvement compared with those commencing BBT over 10 years of age (Chi-squared P = .013).
No prognostic influence on radiological-PFS and/or visual EFS was seen for other parameters such as gender, tumor location (OPG/non-OPG), tumor stage (Modified Dodge classification), histopathology (pilocytic astrocytoma/non-pilocytic astrocytoma), BRAF status, presence of metastatic disease, partner drug, duration of BBT, or number of doses of Bevacizumab.
Due to the retrospective collection of clinically available data amongst a wide range of tumor locations in patients with non-OPG, functional outcome measures for patients with non-OPGs were not consistently available to allow accurate and objective description and comparison.
BBT Line of Therapy
Thirteen (15%) patients received BBT prior to third-line therapy and appear to have superior outcomes. Radiological outcomes were statistically superior in this group, with 62% PR, 38% SD, and 0 PD compared to 40% PR, 49% SD, and 11% PD in the whole cohort (P = .02, chi square 5.51). Similarly, BE visual outcomes were also significantly improved with earlier BBT when compared to the entire cohort (P = .03); with 54% of patients having improved VA, 31% stable vision (overall response of 85%), and 15% remaining blind throughout. The median time to both best radiological and visual responses in patients receiving BBT prior to third-line therapy was equal to the whole cohort at 3 months.
Discussion
This UK nationwide service evaluation provides the largest cohort of patients with PLGG treated with Bevacizumab to date; adding unprecedented real-world clinical data to the current existing limited knowledge confirming that BBTs represent a safe and effective treatment option for children with PLGG. Culminating our data with that of a recently published series from the French group36 as well as a recently published systematic review, this combined data of over 200 PLGG patients treated with BBTs unequivocally confirms the high response rate with BBT (including stable disease) between 80% and 96%; equal to response rates achieved with upfront carboplatin-based regimens (SIOP-LGG response rate at 24 weeks of 92%),37 and outweighing other chemotherapies used in PLGG such as Vinblastine (73%)38 and TPCV (68%).8 The responses from our data are particularly meaningful given that they are obtained in a heavily pre-treated PLGG population (85% 3rd line and beyond) in whom a decreasing efficacy of subsequent treatments is to be expected.12 Moreover, our data also supports that combining or adding Bevacizumab with agents other than irinotecan is tolerable without excessive or cumulative toxicities; further expanding the combinational options of BBT.
However, our data also supports limited existing evidence that the therapeutic effect of BBTs is not sustained after treatment, with tumor progressions amongst both this cohort and the recently published French cohort occurring soon after treatment cessation.36 The lack of a sustained therapeutic effect suggests that the particular role for BBT in PLGG is to provide short-term efficacy, in particular for visual preservation; which could delay the need for additional higher-morbidity treatments such as radiotherapy or TPCV chemotherapy whilst preventing further morbidity from tumor growth or progression.23,28,39 Possible hypotheses explaining the off-Bevacizumab rebound tumor growth may relate to the non-cytotoxic therapeutic effect of Bevacizumab as well as the limited evidence of any direct beneficial effect of irinotecan (as the commonest BBT partner) against PLGG. Furthermore, pre-clinical models of Bevacizumab indicate transient anti-angiogenesis followed by vasculature regrowth and remodeling which is seen at treatment interruption as an alternative hypothesis.40 This is in the context of the ongoing challenge of radiological PLGG response assessment owing to the lack of a consensus agreement as to the interpretation of cystic and contrast enhancement changes on MRI imaging.41 Similar concerns have been raised more recently with more novel targeted agents such as MAPK and BRAF inhibitors, emphasizing the need to better understand the dynamics of PLGG-related responses and tumor resistance mechanisms to optimize the efficacy and durability of new therapies for PLGG.
Overall, Bevacizumab was well-tolerated, with very few CTCAE grade 3 or 4 toxicities. However, albeit in very small numbers, serious adverse events (SAEs) did occur in this cohort and had the potential to cause significant and life-threatening complications. BBTs are therefore not suitable for all patients with PLGG and should be used with caution in patients with a high risk of bleeding; for example, in FGFR-altered PLGGs where an increased risk of bleeding has been suggested.42 In addition to published reports of Bevacizumab use with irinotecan, our data also supports that combining or adding Bevacizumab with alternative chemotherapy agents is tolerable without serious or significant toxicities.
The majority of patients (65%) received BBTs for the recommended licensed duration of 12 months which is largely determined by NHS funding in the UK. However, there is existing data suggesting that a more prolonged Bevacizumab duration may be confined by increased cumulative toxicities such as proteinuria and hypertension.26 Of the 3 patients with multiply relapsed OPG receiving BBTs for longer than 12 months, all had dual therapeutic effects with stable or improved visual acuity and radiological tumor responses. All three patients demonstrated tumor relapse after BBT but with a substantially increased PFS compared to that of the entire cohort, so it may be that prolonged BBT therapy increases the duration that tumor response is sustained. Prolongation of Bevacizumab therapy could be explored in further work using a reduced dose and/or less intensive maintenance schedule after an initial induction phase. From this retrospective study, an evidence-based suggestion for an alteration to the duration of Bevacizumab therapy cannot be made given that most patients received the standard licensed 1 year of treatment. However, the median time for to best radiological and best visual responses is 3 months, and therefore future work should evaluate the optimal duration of BBT to achieve the most favorable balance between short and longer-term efficacy with toxicity and treatment burden.
Whilst achieving tumor control is an important aim in PLGG, given that most patients will survive long into adulthood,43 the impact on patient function from both the tumor and its treatment is an equally important consideration in the management of these tumors. Owing to a specific role for the visual rescue of Bevacizumab being suggested from prior small case series, we have focused on visual morbidity and visual outcomes in response to BBTs as a key functional measure for patients; analyzing data from our large subgroup of 77 patients with OPG to provide more insight into this potential visual benefit. Based on standardized and quantifiable visual data, we demonstrate that visual morbidity in OPG was significantly before the use of BBT, with 61% of children suffering bilateral visual deficits before BBTs; including 25% and 11% of whom had LP/NLP in one or both eyes respectively. A stable VA was achieved in most cases, with a few patients benefiting from an improvement in VA offset by similar numbers of patients with visual deterioration. Notably, visual responses occurred quickly within the first 3–6 months of BBT treatment. In addition, VA in the BE showed very little variation over time across the cohort, whilst VA markedly deteriorated in the WE during BBT and remained stable thereafter. Although VF data is not widely available throughout our cohort of OPGs, the available VF responses demonstrate superior visual outcomes compared to VA responses with BBT, with almost all patients (96%) demonstrating the functional visual benefit of BBT with either stable (83%) or improved (13%) visual fields. Whilst the proportional gain in visual fields from this cohort is less than that previously published by Avery et al,44 the findings from Avery and this cohort justify routine visual field assessment in patients with OPGs to aid treatment response and functional outcome assessments; especially given that in both cohorts there are patients gaining VFs without change in VA which may confer important functional benefits for patients.
Our data suggest that the visual benefits achieved with BBTs, including halting further visual deterioration, are more often restricted to the best eye. Given that ongoing visual deterioration represents one of the main indications to start Bevacizumab, children with OPGs suffering from visual deterioration or threat to vision in the best eye may benefit most from this Bevacizumab-based visual rescue strategy. Moreover, the fact that significant visual morbidity was present prior but then persisted after BBT, raises concern that BBTs might currently be initiated too late once existent and established visual damage is irreversible. Patients’ VA improvement was not associated with an initial level of VA loss at the point of commencing BBT. Five (14%) of 35 patients with low vision values (initial VA >1.6) gained VA >0.2 or gained functional visual status. However, only one patient improved from LP/NLP to a functional level of vision (NLP and 1.55–0.8 bilaterally). As we strive to improve functional and quality of life outcomes for survivors of PLGG, this data provides justification for an earlier introduction of BBTs in the OPG treatment timeline to counteract and mitigate visual morbidity and to improve long-term visual outcomes to achieve a significant functional benefit for patients. Whilst the median time to best visual and radiological responses in patients treated with BBT before 3rd line therapy in this cohort was equal to the entire cohort of patients, the proportion of beneficial visual (VA) outcomes (improved and stable vision) along with the proportion of radiological responses (PR and SD) were significantly higher compared to the whole cohort. The concept of the addition of Bevacizumab to conventional first-line chemotherapy such as Vincristine/Carboplatin or Vinblastine in patients with a threat to vision at presentation or early during treatment is currently being evaluated prospectively in the Canadian Phase II study (NCT02840409) which has recently completed enrolment and may add further information as to the efficacy of earlier use of BBT for PLGG.
Despite the ongoing risk of visual deterioration after BBT in this uniquely large cohort of patients with OPG treated with BBTs, the visual-EFS showed superior survival rates at 3 years, with a less-steep decline over time and a longer v-EFS time, when compared to radiology- or all-causes-PFS; suggesting a more sustained effect on visual preservation compared to a more transient MRI response. Until now it has been unclear how to interpret the poor and discordant correlations between radiological and visual changes in children with OPG. The presence of a degenerative process underpinning the ongoing visual loss was demonstrated in NF1-OPG mouse models in which visual deficit caused by retinal ganglion cell (RGC) death significantly preceded radiological evidence of the progression.45,46 It is also postulated that the specific anti-angiogenic effect of Bevacizumab might reduce blood supply, thereby relieving the functional structures (e.g., optic nerves) in these highly vascularized tumors without significant effect on the tumor and its size.44,47 To obtain a deeper understanding of clinical-radiological changes, we were able to show that such correlation is maximized when BE response only is considered, which is also a more functionally relevant marker of residual vision.
On multivariate analysis, the presence of Diencephalic Syndrome (DS) and lack of NF1 were the only independent prognostic factors for adverse PFS outcomes for radiology-PFS and visual-EFS respectively. The role of age (<1 year) at diagnosis was not confirmed to be an independent poor prognostic factor and was therefore likely to have been associated with the presence of DS given the relatively young age of our cohort. Our prognostic findings are consistent with existing data from large cohorts of PLGG patients including the SIOP-LGG trial,9,43,48–50 although data from the recently published French cohort of PLGG patients treated with Bevacizumab did not report the absence of NF1 as an independent poor prognostic factor for radiological outcome.36 Possible explanations are that as our cohort contains the largest number of patients with OPGs treated with BBTs, we may have achieved a more accurate visual outcome assessment which facilitated the realization of the difference between radiology-based and visual outcomes which was not considered in the French prognostic analysis. Moreover, patients younger than 10 years of age at the start of BBT seemed to have an increased likelihood of visual response, which at least in part corresponds to the French data restricted to radiology-PFS highlighting a better radiological outcome if BBT is initiated in those aged between 5 and 10 years of age. Again, these findings seem to suggest that BBT exerts its optimal beneficial therapeutic effect in particular subsets of patients which justifies further research in large cohort series.
This service evaluation shares limitations with other retrospective studies which encumber multiple unintentional risks of bias. However, the multi-institutional cohort in this study and the standardized outcomes assessed by the same reviewer with relevant expertise at each contributing center (RANO-LGG for radiology and LogMAR visual acuity) mitigate these limitations as far as possible and provide additional value for this study. Visual field data were available in less than half of patients with OPGs which reflects the challenge of assessing a young patient population as well as the lack of standardized visual field assessment in clinical practice; an issue that current and future prospective trials will address moving forwards. Few differences in patient and disease characteristics emerged in comparison to other published series; including a younger patient age at PLGG diagnosis and a higher proportion of metastatic disease2 only minimally limiting the generalizability of our results. Furthermore, molecular profiling was only available in a limited proportion of cases at diagnosis or progression, emphasizing the need to promote diagnostic biopsy for molecular studies.
Conclusion
This nationally collaborative service evaluation provides the largest cohort of PLGG patients treated with BBT to date. We have confirmed that BBT is well-tolerated and efficacious; leading to at least comparable disease control (with potentially superior visual preservation) to existing chemotherapies, albeit the response is not sustained after Bevacizumab discontinuation in most. Further optimization of the use of Bevacizumab in PLGG is justified, either by introduction at an earlier stage to preserve optimal visual function or by combination with alternative agents including conventional chemotherapies or more novel targeted therapies to achieve the best possible balance between maximal clinical benefit and patient function with minimal toxicities for patients.
Supplementary Material
Acknowledgments
This work has only been possible due to the generous support and collaboration of the pediatric neuro-oncologists, pediatric neuro-radiologists, and pediatric ophthalmologists from each of the contributing Primary Treatment Centres in the UK.
The material included within this manuscript has been presented at the 20th International Symposium on pediatric Neuro-Oncology (ISPNO) conference (Hamburg) June 2022.
Contributor Information
Katherine Green, Great Ormond Street Hospital London, UK.
Paraskevi Panagopoulou, Great Ormond Street Hospital London, UK.
Felice D’Arco, Great Ormond Street Hospital London, UK.
Patricia O’Hare, Great Ormond Street Hospital London, UK.
Richard Bowman, Great Ormond Street Hospital London, UK.
Bronwen Walters, Great Ormond Street Hospital London, UK.
Christine Dahl, Great Ormond Street Hospital London, UK.
Mette Jorgensen, Great Ormond Street Hospital London, UK.
Pritesh Patel, Great Ormond Street Hospital London, UK.
Olga Slater, Great Ormond Street Hospital London, UK.
Rehana Ahmed, Nottingham Children’s Hospital, UK.
Simon Bailey, Great North Children’s Hospital Newcastle, UK.
Fernando Carceller, The Royal Marsden Hospital London, UK.
Rhiannon Collins, John Radcliffe Hospital Oxford, UK.
Elizabeth Corley, The Royal Marsden Hospital London, UK.
Martin English, Birmingham Children’s Hospital, UK.
Lisa Howells, Leeds Children’s Hospital, UK.
Ahmed Kamal, Birmingham Children’s Hospital, UK.
John-Paul (JP) Kilday, Royal Manchester Children’s Hospital, UK; The Centre for Paediatric, Teenage and Young Adult Cancer Sciences, The University of Manchester, UK.
Stephen Lowis, Bristol Royal Hospital for Children, UK.
Blanche Lumb, Noah’s Ark Children’s Hospital for Wales, UK.
Erika Pace, The Royal Marsden Hospital London, UK.
Susan Picton, Leeds Children’s Hospital, UK.
Barry Pizer, Alder Hey Children’s Hospital Liverpool, UK.
Ayad Shafiq, Great North Children’s Hospital Newcastle, UK.
Lena Uzunova, Noah’s Ark Children’s Hospital for Wales, UK.
Harriet Wayman, Royal Manchester Children’s Hospital, UK; The Centre for Paediatric, Teenage and Young Adult Cancer Sciences, The University of Manchester, UK.
Shaun Wilson, John Radcliffe Hospital Oxford, UK.
Darren Hargrave, Great Ormond Street Hospital London, UK.
Enrico Opocher, Great Ormond Street Hospital London, UK; Padua University Hospital, Padua, Italy.
Conflict of interest statement. None declared.
Author Contributions
All authors listed in this manuscript have contributed significantly to the design, implementation, analysis, or interpretation of the data. All authors have been involved in the writing of the manuscript at draft and revision stages, and have read and approved the final version.
Funding
No funding or sponsorship was received for this project.
References
- 1. Packer RJ. Brain tumors in children. Arch Neurol. 1999;56(4):421–425. [DOI] [PubMed] [Google Scholar]
- 2. Youland RS, Khwaja SS, Schomas DA, et al. Prognostic factors and survival patterns in pediatric low-grade gliomas over 4 decades. J Pediatr Hematol Oncol. 2013;35(3):197–205. [DOI] [PubMed] [Google Scholar]
- 3. Armstrong GT, Conklin HM, Huang S, et al. Survival and long-term health and cognitive outcomes after low-grade glioma. Neuro Oncol. 2011;13(2):223–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wisoff JH, Sanford RA, Heier LA, et al. Primary neurosurgery for pediatric low-grade gliomas: a prospective multi-institutional study from the Children’s Oncology Group. Neurosurgery 2011;68(6):1548–54; discussion 1554. discussion 15545. [DOI] [PubMed] [Google Scholar]
- 5. Ajithkumar T, Price S, Horan G, Burke A, Jefferies S. Prevention of radiotherapy-induced neurocognitive dysfunction in survivors of paediatric brain tumours: the potential role of modern imaging and radiotherapy techniques. Lancet Oncol. 2017;18(2):e91–e100. [DOI] [PubMed] [Google Scholar]
- 6. Grabenbauer GG, Schuchardt U, Buchfelder M, et al. Radiation therapy of optico-hypothalamic gliomas (OHG)--radiographic response, vision and late toxicity. Radiother Oncol. 2000;54(3):239–245. [DOI] [PubMed] [Google Scholar]
- 7. Williams NL, Rotondo RL, Bradley JA, et al. Late Effects After Radiotherapy for Childhood Low-grade Glioma. Am J Clin Oncol. 2018;41(6):307–312. [DOI] [PubMed] [Google Scholar]
- 8. Ater JL, Zhou T, Holmes E, et al. Randomized study of two chemotherapy regimens for treatment of low-grade glioma in young children: a report from the Children’s Oncology Group. J Clin Oncol. 2012;30(21):2641–2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gnekow AK, Falkenstein F, von Hornstein S, et al. Long-term follow-up of the multicenter, multidisciplinary treatment study HIT-LGG-1996 for low-grade glioma in children and adolescents of the German Speaking Society of Pediatric Oncology and Hematology. Neuro Oncol. 2012;14(10):1265–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Packer RJ, Jakacki R, Horn M, et al. Objective response of multiply recurrent low-grade gliomas to bevacizumab and irinotecan. Pediatr Blood Cancer 2009;52(7):791–795. [DOI] [PubMed] [Google Scholar]
- 11. Armstrong GT, Liu Q, Yasui Y, et al. Long-term outcomes among adult survivors of childhood central nervous system malignancies in the Childhood Cancer Survivor Study. J Natl Cancer Inst. 2009;101(13):946–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kandels D, Pietsch T, Bison B, et al. Loss of efficacy of subsequent nonsurgical therapy after primary treatment failure in pediatric low-grade glioma patients-Report from the German SIOP-LGG 2004 cohort. Int J Cancer 2020;147(12):3471–3489. [DOI] [PubMed] [Google Scholar]
- 13. Boele FW, Douw L, Reijneveld JC, et al. Health-related quality of life in stable, long-term survivors of low-grade glioma. J Clin Oncol. 2015;33(9):1023–1029. [DOI] [PubMed] [Google Scholar]
- 14. Mostow EN, Byrne J, Connelly RR, Mulvihill JJ. Quality of life in long-term survivors of CNS tumors of childhood and adolescence. J Clin Oncol. 1991;9(4):592–599. [DOI] [PubMed] [Google Scholar]
- 15. Shields LB, Choucair AK. Management of low-grade gliomas: a review of patient-perceived quality of life and neurocognitive outcome. World Neurosurg. 2014;82(1):e299–e309. [DOI] [PubMed] [Google Scholar]
- 16. Grill J, Laithier V, Rodriguez D, Raquin M A, Pierre-Kahn A, Kalifa C. When do children with optic pathway tumours need treatment? An oncological perspective in 106 patients treated in a single centre. Eur J Pediatr. 2000;159(9):692–6 [DOI] [PubMed] [Google Scholar]
- 17. Hill CS, Khan M, Phipps K, et al. Neurosurgical experience of managing optic pathway gliomas. Childs Nerv Syst. 2021;37(6):1917–1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Opocher E, Kremer LC, Da Dalt L, et al. Prognostic factors for progression of childhood optic pathway glioma: a systematic review. Eur J Cancer 2006;42(12):1807–1816. [DOI] [PubMed] [Google Scholar]
- 19. Falzon K, Drimtzias E, Picton S, Simmons I. Visual outcomes after chemotherapy for optic pathway glioma in children with and without neurofibromatosis type 1: results of the International Society of Paediatric Oncology (SIOP) Low-Grade Glioma 2004 trial UK cohort. Br J Ophthalmol. 2018;102(10):1367–1371. [DOI] [PubMed] [Google Scholar]
- 20. Couec ML, Andre N, Thebaud E, et al. Bevacizumab and irinotecan in children with recurrent or refractory brain tumors: toxicity and efficacy trends. Pediatr Blood Cancer 2012;59(1):34–38. [DOI] [PubMed] [Google Scholar]
- 21. Glade Bender JL, Adamson PC, Reid JM, et al. Phase I trial and pharmacokinetic study of bevacizumab in pediatric patients with refractory solid tumors: a Children’s Oncology Group Study. J Clin Oncol. 2008;26(3):399–405. [DOI] [PubMed] [Google Scholar]
- 22. Fangusaro J, Gururangan S, Poussaint TY, et al. Bevacizumab (BVZ)-associated toxicities in children with recurrent central nervous system tumors treated with BVZ and irinotecan (CPT-11): a Pediatric Brain Tumor Consortium Study (PBTC-022). Cancer 2013;119(23):4180–4187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gorsi HS, Khanna PC, Tumblin M, et al. Single-agent bevacizumab in the treatment of recurrent or refractory pediatric low-grade glioma: a single institutional experience. Pediatr Blood Cancer 2018;65(9):e27234. [DOI] [PubMed] [Google Scholar]
- 24. Gururangan S, Fangusaro J, Poussaint TY, et al. Efficacy of bevacizumab plus irinotecan in children with recurrent low-grade gliomas—a Pediatric Brain Tumor Consortium study. Neuro Oncol. 2014;16(2):310–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hsu CH, Lober RM, Li MD, et al. Decreased tumor apparent diffusion coefficient correlates with objective response of pediatric low-grade glioma to bevacizumab. J Neurooncol. 2015;122(3):491–496. [DOI] [PubMed] [Google Scholar]
- 26. Hwang EI, Jakacki RI, Fisher MJ, et al. Long-term efficacy and toxicity of bevacizumab-based therapy in children with recurrent low-grade gliomas. Pediatr Blood Cancer 2013;60(5):776–782. [DOI] [PubMed] [Google Scholar]
- 27. Kalra M, Heath JA, Kellie SJ, et al. Confirmation of bevacizumab activity, and maintenance of efficacy in retreatment after subsequent relapse, in pediatric low-grade glioma. J Pediatr Hematol Oncol. 2015;37(6):e341–e346. [DOI] [PubMed] [Google Scholar]
- 28. Zhukova N, Rajagopal R, Lam A, et al. Use of bevacizumab as a single agent or in adjunct with traditional chemotherapy regimens in children with unresectable or progressive low-grade glioma. Cancer Med. 2019;8(1):40–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lambron J, Rakotonjanahary J, Loisel D, et al. Can we improve accuracy and reliability of MRI interpretation in children with optic pathway glioma? Proposal for a reproducible imaging classification. Neuroradiology 2016;58(2):197–208. [DOI] [PubMed] [Google Scholar]
- 30. Taylor T, Jaspan T, Milano G, et al. Radiological classification of optic pathway gliomas: experience of a modified functional classification system. Br J Radiol. 2008;81(6):761–766. [DOI] [PubMed] [Google Scholar]
- 31. Chukwueke UN, Wen PY. Use of the Response Assessment in Neuro-Oncology (RANO) criteria in clinical trials and clinical practice. CNS Oncol. 2019;8(21):CNS28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fisher MJ, Avery RA, Allen JC, et al. Functional outcome measures for NF1-associated optic pathway glioma clinical trials. Neurology 2013;81(16):S15–S24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Huurneman B, Boonstra FN. Assessment of near visual acuity in 0-13 year olds with normal and low vision: a systematic review. BMC Ophthalmol. 2016;16(1):215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lange C, Feltgen N, Junker B, Schulze-Bonsel K, Bach M. Resolving the clinical acuity categories “hand motion” and “counting fingers” using the Freiburg Visual Acuity Test (FrACT). Graefes Arch Clin Exp Ophthalmol. 2009;247:137–142. [DOI] [PubMed] [Google Scholar]
- 35. Picton SK, Kilday JP, Hargrave D, et al. CCLG Guidelines for the diagnosis and management of paediatric and adolescent Low-Grade Glioma’ guidelines 2020. Children’s Cancer and Leukaemia Group (CCLG); 2020;1:6–35. [Google Scholar]
- 36. de Marcellus C, Tauziede-Espariat A, Cuinet A, et al. The role of irinotecan-bevacizumab as rescue regimen in children with low-grade gliomas: a retrospective nationwide study in 72 patients. J Neurooncol. 2022;2(81):355–364. [DOI] [PubMed] [Google Scholar]
- 37. Gnekow AK, Walker DA, Kandels D, et al. A European randomised controlled trial of the addition of etoposide to standard vincristine and carboplatin induction as part of an 18-month treatment programme for childhood (</=16 years) low grade glioma—a final report. Eur J Cancer 2017;81(12):206–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bouffet E, Jakacki R, Goldman S, et al. Phase II study of weekly vinblastine in recurrent or refractory pediatric low-grade glioma. J Clin Oncol. 2012;30(4):1358–1363. [DOI] [PubMed] [Google Scholar]
- 39. Lu VM, Welby JP, Nesvick CL, Daniels DJ. Efficacy and safety of bevacizumab in progressive pediatric low-grade glioma: a systematic review and meta-analysis of outcome rates. Neurooncol Pract. 2020;7(7):359–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yang Y, Zhang Y, Iwamoto H, et al. Discontinuation of anti-VEGF cancer therapy promotes metastasis through a liver revascularization mechanism. Nat Commun. 2016;7(6):12680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Fangusaro J, Witt O, Hernaiz Driever P, et al. Response assessment in paediatric low-grade glioma: recommendations from the Response Assessment in Pediatric Neuro-Oncology (RAPNO) working group. Lancet Oncol. 2020;21(3):e305–e316. [DOI] [PubMed] [Google Scholar]
- 42. Ishi Y, Yamaguchi S, Hatanaka KC, et al. Association of the FGFR1 mutation with spontaneous hemorrhage in low-grade gliomas in pediatric and young adult patients. J Neurosurg. 2020;134(3):733–741. [DOI] [PubMed] [Google Scholar]
- 43. Ryall S, Zapotocky M, Fukuoka K, et al. Integrated Molecular and Clinical Analysis of 1,000 Pediatric Low-Grade Gliomas. Cancer Cell 2020;37(1):569–583.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kalin-Hajdu E, Decarie JC, Marzouki M, Carret A-S, Ospina LH. Visual acuity of children treated with chemotherapy for optic pathway gliomas. Pediatr Blood Cancer 2014;61(1):223–227. [DOI] [PubMed] [Google Scholar]
- 45. Kim KY, Ju WK, Hegedus B, Gutmann DH, Ellisman MH. Ultrastructural characterization of the optic pathway in a mouse model of neurofibromatosis-1 optic glioma. Neuroscience 2010;170(2):178–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Gutmann DH, Avery R, Ferner RE, Listernick R. Visual function and optic pathway glioma: a critical response. JAMA Ophthalmol. 2013;131(1):120–121. [DOI] [PubMed] [Google Scholar]
- 47. Avery RA, Hwang EI, Jakacki RI, Packer Roger J.: Marked recovery of vision in children with optic pathway gliomas treated with bevacizumab. JAMA Ophthalmol. 2014;132(1):111–4 [DOI] [PubMed] [Google Scholar]
- 48. Dodgshun AJ, Hansford JR, Sullivan MJ. Risk assessment in paediatric glioma-Time to move on from the binary classification. Crit Rev Oncol Hematol. 2017;111(1):52–59. [DOI] [PubMed] [Google Scholar]
- 49. Mirow C, Pietsch T, Berkefeld S, et al. Children <1 year show an inferior outcome when treated according to the traditional LGG treatment strategy: a report from the German multicenter trial HIT-LGG 1996 for children with low grade glioma (LGG). Pediatr Blood Cancer 2014;61(1):457–463. [DOI] [PubMed] [Google Scholar]
- 50. El Beltagy MA, Reda M, Enayet A, et al. Treatment and outcome in 65 children with optic pathway gliomas. World Neurosurg. 2016;89(7):525–534. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.