Abstract
Background
Molecularly-defined diffuse glioma types—including IDH-wildtype glioblastoma, IDH-mutant astrocytoma, IDH-mutant 1p/19q-codeleted oligodendroglioma, and H3 K27M-mutant diffuse midline glioma—were incorporated into U.S. cancer registry reporting for individuals with brain tumors beginning in 2018. We leveraged these new data to estimate the national-level overall survival (OS) patterns associated with glioma integrated diagnoses.
Methods
Individuals diagnosed with diffuse gliomas in 2018 and had brain molecular marker data were identified within the U.S. National Cancer Database. OS was estimated using Kaplan–Meier methods and stratified by WHO CNS grade, age, sex, tumor size, treatment, extent of resection, and MGMT promoter methylation. Additionally, the effects of WHO CNS grade were examined among individuals with IDH-wildtype astrocytic gliomas.
Results
8651 individuals were identified. One-year OS was 53.7% for WHO grade 4 IDH-wildtype glioblastomas; 98.0%, 92.4%, and 76.3% for WHO grade 2, 3, and 4 IDH-mutant astrocytomas, respectively; 97.9% and 94.4% for WHO grade 2 and 3 IDH-mutant 1p/19q-codeleted oligodendrogliomas, respectively; and 55.9% for H3 K27M-mutant diffuse midline gliomas. Among IDH-wildtype glioblastomas, median OS was 17.1 months and 12.4 months for methylated and unmethylated MGMT promoters. Additionally, IDH-wildtype diffuse astrocytic gliomas reported as WHO grade 2 or 3 demonstrated longer OS compared to grade 4 tumors (both P < .001).
Conclusions
Our findings provide the initial national OS estimates for molecularly-defined diffuse gliomas in the United States and illustrate the importance of incorporating such data into cancer registry reporting.
Keywords: diffuse glioma, glioblastoma, IDH mutation, MGMT promoter methylation, survival
Key Points.
Select glioma molecular data used for diagnosis are now available from U.S. cancer registries.
We report the first U.S. overall survival estimates for molecularly-defined glioma types.
Overall survival varied by glioma molecular type and WHO CNS grade.
Importance of the Study.
Diffuse glioma types are now defined by molecular markers (including IDH1/2 mutation and 1p/19q codeletion) that more accurately predict patients’ outcomes than histology alone. Despite this, existing U.S. survival statistics for diffuse gliomas have depended on histopathology-based classifications. In 2018, cancer registries in the United States implemented new integrated diagnosis ICD-O-3 codes and molecular site-specific data items. Using these novel data elements from a national dataset that represents >80% of new brain tumor diagnoses in the United States, we present the first overall survival estimates for patients with IDH-mutant astrocytomas, IDH-mutant and 1p/19q-codeleted oligodendrogliomas, IDH-wildtype glioblastomas, and H3 K27M-mutant diffuse midline gliomas—as stratified by patient and treatment characteristics, WHO CNS grade, and MGMT promoter methylation status. The inclusion of these data in national cancer registration is a critical step towards improving the clinical relevance of national datasets, and our results demonstrate that the prognostic value of these markers is broadly generalizable.
Isocitrate dehydrogenase 1/2 (IDH) mutation and codeletion of chromosomal arms 1p and 19q have long been shown to have a significant effect on survival in adult-type diffuse gliomas.1,2 With the 2016 revision to the 4th edition of the World Health Organization (WHO) classification of central nervous system (CNS) tumors (WHO-CNS4) and further codification of integrated diagnoses into the 5th edition (WHO-CNS5), these molecular markers now form the foundation of the classification of adult-type diffuse gliomas into IDH-mutant astrocytomas, IDH-mutant and 1p/19q-codeleted oligodendrogliomas, and IDH-wildtype glioblastomas.3 In parallel, molecular biomarkers have refined the classification of pediatric-type diffuse gliomas. The survival associated with these integrated diagnosis tumor types has been repeatedly shown in clinical cohorts but has not been previously estimated at the population level. Although national efforts such as the Central Brain Tumor Registry of the United States (CBTRUS) have provided indispensable population-level survival information regarding individuals with brain tumors, they were determined using older histology-based classification schema.
In accordance with the contemporary WHO-CNS4/5 schema, the North American Association of Central Cancer Registries (NAACCR) now requires registry collection of a newly developed site-specific data item (SSDI) to facilitate systematic reporting of Brain Molecular Markers (NAACCR data item #3816; Supplemental Table 1). Additionally, the NAACCR implemented a new International Classification of Disease, Oncology 3rd edition (ICD-O-3) code specifically for H3 K27M-mutant diffuse midline glioma—a diagnosis that is now included among H3 K27-altered diffuse midline gliomas. Collection of these data was required for all newly diagnosed gliomas beginning in 2018. While not mandatory for all central cancer registries, MGMT promoter methylation—which predicts response to alkylating chemotherapy in high-grade glioma—is also reported via an SSDI for primary brain tumors (NAACCR data item #3889). Standardized collection of these data now provides a unique opportunity to characterize the national survival patterns associated with these integrated histomolecular diagnoses that have not previously been possible.
We recently reported on the completeness and validity of the new molecular biomarker SSDIs for brain tumors in U.S. cancer registry data, and provided the first population-level description of the epidemiology of these molecular-defined brain tumor types in the United States – although their associated survival data were not yet available from the registries.4 Here, we expand upon those findings by describing the national survival patterns of molecularly-defined adult-type diffuse glioma tumor types and the pediatric-type H3 K27M-mutant diffuse midline glioma in a related database that includes more than 85% of newly diagnosed primary brain tumors in the United States.5 Additionally, among the subset of IDH-wildtype glioblastomas for which MGMT promoter methylation testing is particularly relevant, we further assessed the OS associated with MGMT promoter methylation status and therapeutic strategy. Finally, because the existence of lower grade (2 or 3) adult-type IDH-wildtype diffuse astrocytic gliomas remains unclear, we examined whether OS varied by WHO CNS grade when adjusting for key patient, tumor, and management characteristics among individuals diagnosed with adult-type IDH-wildtype diffuse astrocytic gliomas.
Methods
This analysis was approved by the institutional review boards of Mass General Brigham and Duke University Health System, and was conducted in accordance with the Declaration of Helsinki. The NCDB Participant User Files comprise deidentified national data for which consenting was not applicable.
Data Sources and Variable Design
Data were retrieved from the National Cancer Database (NCDB), which is a joint project of the Commission on Cancer of the American College of Surgeons and the American Cancer Society.5 Cancer histology and site were classified using ICD-O-3 morphology and site codes. Individuals presenting with histologically-confirmed adult-type diffuse gliomas of the brain (ICD-O-3 site code C71.0-C71.9) – including glioblastoma (ICD-O-3 morphology code 9440/3), astrocytoma (ICD-O-3 morphology codes 9400/3, 9401/3, 9445/3), and oligodendrogliomas (ICD-O-3 morphology codes 9450/3 and 9451/3) – or H3 K27M-mutant diffuse midline glioma (ICD-O-3 morphology code 9385/3) were identified in the NCDB for diagnoses made between January 1, 2018 and December 31, 2018. The study was restricted to 2018 because it was the only available year for which the NCDB reported (1) overall survival (OS) outcomes, (2) the new Brain Molecular Marker and MGMT promoter methylation data items, and (3) the new 9440/3 and 9385/3 ICD-O-3 entities. Individuals were excluded if all of their management subsequently occurred at an institution other than the reporting facility or if they lacked survival data.
Diffuse infiltrative glioma types are categorized in the WHO-CNS5 into “adult-type” and “pediatric-type high-grade” diffuse gliomas. Using the Brain Molecular Marker data item and ICD-O-3 codes, adult-diffuse gliomas were categorized into IDH-mutant and 1p/19q oligodendroglioma (ICD-O-3 code 9450/3 or 9451/3, with Brain Molecular Markers code 06 or 07), IDH-mutant astrocytoma (ICD-O-3 code 9400/3 or 9401/3, with Brain Molecular Markers code 01 or 03; or ICD-O-3 code 9445/3), or IDH-wildtype glioblastoma (ICD-O-3 code 9400/3, 9401/3, or 9440/3, with Brain Molecular Markers code 02, 04, or 05) (Please see Supplemental Table 1 for description of the included patient population). WHO CNS grade was reported by the NCDB using the histology-based revised 2016 WHO criteria, and assigned from either the pathological grade data item (determined from surgical resection) if available, or otherwise from the clinical grade data item (determined from biopsy). For IDH-mutant astrocytomas, IDH-wildtype glioblastomas, and IDH-mutant 1p/19q-codeleted oligodendrogliomas, reported as grade 1, low-grade, high-grade, or grade unknown were excluded. IDH-mutant 1p/19q-codeleted oligodendrogliomas reported as grade 4 were additionally excluded. Extent of resection was categorized as biopsy-only (either excisional biopsy or diagnostic biopsy), subtotal resection, or gross total resection. Additional variables included patient age at diagnosis, sex, maximal tumor dimension, first-line radiotherapy, and first-line chemotherapy. The MGMT promoter status from the MGMT SSDI was categorized into either unmethylated (code 0) or methylated (code 1, 2, or 3).
Although new Brain Molecular Marker and/or ICD-O-3 codes were also introduced for molecular subgroups of medulloblastomas, C19MC-altered embryonal tumor with multilayered rosettes, and ZFTA (ie, RELA) fusion-positive ependymoma, there were too few reported in 2018 to permit analysis of their survival.
Statistical Analysis
OS was estimated using Kaplan–Meier methods, measured from the date of initial diagnosis to the date of death (or censored at last follow-up), and reported with 95% confidence intervals (95CI). Patient follow-up was reported through December 31, 2019. Because median OS was not reached for several of the tumor types, one-year OS rates were reported. OS was reported by tumor type, WHO grade, patient characteristics, management, and MGMT promoter methylation status. As a secondary analysis, the OS associated with WHO CNS grades 2 and 3 (compared to grade 4) among individuals with IDH-wildtype glioblastomas was assessed using multivariable Cox regression. For the secondary analysis, an overall two-sided alpha level of .05 was used, corrected for the two hypotheses that were tested (P < .025 designated as significant for each). In accordance with the NCDB data use agreement, case counts <10 were suppressed. Statistics were conducted using Stata (v17.0, StataCorp).
Results
After all exclusions, 8651 individuals were identified with complete data for the primary analysis, including 6732 IDH-wildtype glioblastomas (77.8%; WHO grade 4), 1126 IDH-mutant astrocytomas (13.0%; WHO grades 2–4), 703 IDH-mutant 1p/19q-codeleted oligodendrogliomas (8.1%; WHO grades 2–3), and 90 H3 K27M-mutant diffuse midline gliomas (1.0%) (Supplemental Table 2). Overall, individuals were followed for a median of 14.8 months (interquartile range: 6.2–24.7 months). MGMT promoter methylation data were available for 72.3% of IDH-wildtype glioblastomas (n = 4864), 48.8% of IDH-mutant astrocytomas (n = 549), and 33.6% of IDH-mutant 1p/19q-codeleted oligodendrogliomas (n = 236). For exploratory analyses of OS by WHO CNS grade among IDH-wildtype astrocytic gliomas, there additionally were 213 grade 2 and 413 grade 3 tumors identified.
IDH-wildtype Glioblastoma
Among the 6732 individuals with WHO grade 4 IDH-wildtype glioblastoma, the one-year OS was 53.7% (95CI: 52.5–54.9) (Table 1, Figure 1). In terms of patient features, one-year OS was similar by sex (53.3% in females [95CI: 51.4%–55.2%] and 54% in males [95CI: 52.4–55.6]) and was higher in individuals < 50 at diagnosis (76%, 95CI: 72.9%–78.7%) as compared to those ≥ 50 (50.1%, 95CI: 48.7%–51.4%). Increasing tumor dimension was associated with reduced one-year OS. By extent of resection, the one-year OS was 37.1% in individuals who received biopsy only (95CI: 34.8–39.4), compared to 52.6% for STR (95CI: 50.5%–54.8%), and 65.5% for GTR (95CI: 63.6%–67.2%).
Table 1.
Kaplan–Meier Estimates of 1-Year Overall Survival Rate by Tumor Type and WHO CNS Grade
| Tumor Type | Glioblastoma, IDH-wildtype | Astrocytoma, IDH-mutant | Oligodendroglioma, IDH-mutant 1p/19q Codeleted | H3 K27M Diffuse Midline Glioma | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WHO CNS Grade | 4 | 2 | 3 | 4 | 2 | 3 | n/a | |||||||
| % | (95CI) | % | (95CI) | % | (95CI) | % | (95CI) | % | (95CI) | % | (95CI) | % | (95CI) | |
| Overall | 53.7 | (52.5–54.9) | 98.0 | (96.1–99.0) | 92.4 | (89.6–94.5) | 76.3 | (70.1–81.3) | 97.9 | (95.9–98.9) | 94.4 | (90.9–96.6) | 55.9 | (44.5–65.8) |
| Sex | ||||||||||||||
| Female | 53.3 | (51.4–55.2) | 98.9 | (95.5–99.7) | 92.3 | (87.5–95.3) | 79.1 | (69.4–86.0) | 99.0 | (96.0–99.8) | 94.5 | (88.9–97.4) | 53.7 | (38.2–66.9) |
| Male | 54.0 | (52.4–55.6) | 97.4 | (94.3–98.8) | 92.5 | (88.6–95.1) | 74.2 | (65.7–80.9) | 96.8 | (93.5–98.5) | 94.2 | (88.8–97.1) | 58.4 | (41.1–72.2) |
| Age at diagnosis, years | ||||||||||||||
| <50 | 76.0 | (72.9–78.7) | 99.4 | (97.6–99.9) | 96.5 | (94.0–98.0) | 84.6 | (76.9–89.9) | 99.0 | (96.8–99.7) | 98.6 | (94.4–99.6) | 56.7 | (44.3–67.3) |
| ≥50 | 50.1 | (48.7–51.4) | 91.8 | (82.6–96.2) | 78.4 | (69.3–85.1) | 66.2 | (55.8–74.6) | 95.3 | (89.8–97.9) | 89.1 | (81.9–93.5) | 50.0 | (18.4–75.3) |
| Maximal tumor dimension, cm | ||||||||||||||
| ≤3 | 57.4 | (54.6–60.1) | 98.7 | (91.0–99.8) | 89.9 | (79.9–95.1) | 74.9 | (56.0–86.6) | 98.3 | (88.6–99.8) | 93.2 | (75.5–98.3) | 86.7 | (56.4–96.5) |
| 3–6 | 53.7 | (51.9–55.4) | 97.4 | (93.3–99.0) | 93.6 | (88.7–96.4) | 76.3 | (67.0–83.3) | 98.1 | (94.2–99.4) | 94.9 | (88.1–97.8) | 52.8 | (36.8–66.5) |
| >6 | 49.5 | (46.1–52.8) | 100.0 | (100–100) | 95.7 | (88.8–98.4) | 85.5 | (70.6–93.2) | 97.4 | (89.9–99.3) | 93.5 | (85.1–97.3) | x | x |
| Therapy | ||||||||||||||
| No Chemo, No RT | 19.2 | (16.8–21.6) | 98.8 | (95.2–99.7) | 69.5 | (53.9–80.7) | 32.3 | (16.8–48.8) | 96.9 | (92.7–98.7) | 68.3 | (44.7–83.5) | x | x |
| Chemo & RT | 63.6 | (62.2–65.0) | 98.0 | (94.9–99.3) | 95.7 | (93.1–97.4) | 87.5 | (81.5–91.7) | 99.5 | (96.5–99.9) | 96.8 | (93.4–98.5) | 59.4 | (44.4–71.6) |
| Extent of resection | ||||||||||||||
| Biopsy-only | 37.1 | (34.8–39.4) | 94.2 | (86.7–97.6) | 78.8 | (68.8–85.9) | 52.9 | (35.0–68.0) | 97.8 | (91.3–99.4) | 85.9 | (69.5–93.9) | 55.1 | (40.4–67.6) |
| STR | 52.6 | (50.5–54.8) | 99.3 | (94.8–99.9) | 95.1 | (90.4–97.5) | 75.2 | (63.9–83.4) | 97.9 | (93.6–99.3) | 92.1 | (84.2–96.2) | 52.6 | (28.7–71.9) |
| GTR | 65.5 | (63.6–67.2) | 98.9 | (95.6–99.7) | 96.1 | (92.3–98.0) | 84.5 | (76.3–90.1) | 97.8 | (94.2–99.2) | 97.8 | (93.4–99.3) | x | x |
| MGMT promoter | ||||||||||||||
| Unmethylated | 51.7 | (49.9–53.6) | 96.3 | (89–98.8) | 90.8 | (83–95.1) | 80.5 | (67.6–88.7) | n/a | n/a | n/a | |||
| Methylated | 62.1 | (59.9–64.2) | 100 | (n/a) | 94.1 | (89–96.9) | 83.7 | (74.4–89.8) | n/a | n/a | n/a |
Abbreviations: Chemo, chemotherapy; CNS, Central nervous system; GTR, gross total resection; n/a, not applicable, too few events; RT, radiotherapy; STR, subtotal resection; WHO, World Health Organization; x, Estimates based on <10 cases were suppressed; 95CI, 95% confidence interval.
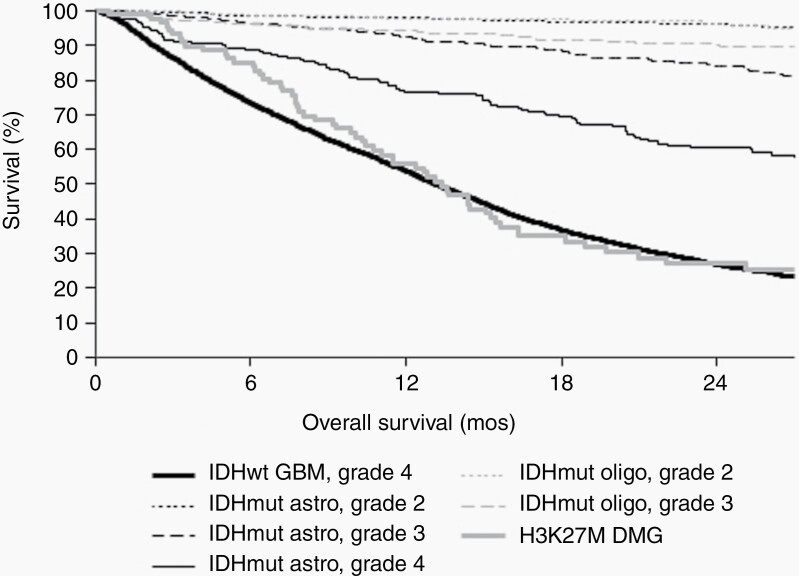
Figure 1.
Kaplan–Meier overall survival estimates by tumor integrated diagnosis and WHO CNS grade, including IDH-wildtype glioblastoma (WHO grade 4; ie, IDHwt GBM), IDH-mutant astrocytoma (WHO grades 2–4; ie, IDHmut astro), IDH-mutant and 1p/19q-codeleted oligodendroglioma (WHO grades 2–3; ie, IDHmut oligo), and H3 K27M-mutant diffuse midline glioma (DMG).
The effect of MGMT promoter methylation was further evaluated in IDH-wildtype glioblastoma, in which—overall—methylated tumors (n = 2015) displayed a median OS of 17.1 months (95CI: 16.1–18.6) and unmethylated tumors (n = 2849) a median of 12.4 months (95CI: 12.0–12.9). For individuals who either did not receive chemoradiotherapy or only received radiotherapy without chemotherapy, the median OS was similar between MGMT promoter methylated and unmethylated tumors (Table 2). However, among individuals who received both chemotherapy and radiotherapy, the median OS was 21.8 months (95CI: 20.3–23.2) for MGMT promoter methylated individuals and 14.1 months (95CI: 13.7–14.7) for MGMT promoter unmethylated individuals.
Table 2.
Median Overall Survival in Months Among WHO Grade 4 IDH-wildtype Glioblastomas by MGMT Promoter Status and Treatment
| MGMT Promoter Unmethylated (n = 2849) | MGMT Promoter Methylated (n = 2015) | |||
|---|---|---|---|---|
| Median OS | (95CI) | Median OS | (95CI) | |
| Overall | 12.4 | 12.0–12.9 | 17.1 | 16.1–18.6 |
| Therapy | ||||
| No chemo, no RT | 2.8 | 2.4–3.3 | 2.6 | 2.1–3.2 |
| RT, no chemo | 7.3 | 6.0–7.9 | 7.5 | 5.5–9.5 |
| Chemo, no RT | 7.9 | 6.6–12.3 | 10.0 | 7.5–13.0 |
| Chemo & RT | 14.1 | 13.7–14.7 | 21.8 | 20.3–23.2 |
Abbreviations: Chemo, chemotherapy; OS, overall survival; RT, radiotherapy; 95CI, 95% confidence interval.
IDH-mutant Astrocytoma
There was an inverse relationship between WHO CNS grade and one-year OS for individuals with IDH-mutant astrocytoma: 98.0% for grade 2 (n = 418; 95CI: 96.1%–99.0%), 92.4% for grade 3 (n = 474; 95CI: 89.6%–94.5%), and 76.3% for grade 4 (n = 234; 95CI: 70.1%–81.3%) (Table 1, Figure 1). In terms of patient features, there were not substantial differences in one-year OS by sex for any grade, while diagnosis at age <50 was associated with higher survival in all grades. Additionally, across all grades, there was substantial overlap in one-year OS by tumor size. In grade 2 tumors, the one-year OS was comparable for individuals receiving combination chemoradiotherapy as compared to no radiation or chemotherapy. However, in individuals with grade 3 and grade 4 tumors, chemoradiotherapy was associated with 95.7% (95CI: 93.1%–97.4%) and 87.5% (95CI: 81.5%–91.7%) one-year OS, respectively, as compared to individuals that received neither chemotherapy nor radiotherapy (69.5% [95CI: 53.9%–80.7%] and 32.3% [95CI: 16.8%–48.8%], respectively). Median age at presentation with interquartile range [IQR] was 35 (28–45.3), 37 (29–50), and 46.5 (35–60) years for WHO grade 2, 3, and 4 individuals, respectively.
IDH-mutant 1p/19q-codeleted Oligodendroglioma
The one-year OS in IDH-mutant 1p/19q-codeleted oligodendroglioma was high overall, but decreased slightly with increasing grade: 97.9% in grade 2 (n = 430; 95CI: 95.9%–98.9%) and 94.4% in grade 3 (n = 273; 95CI: 90.9%–96.6%) (Table 1, Figure 1). The one-year OS was similar by sex in tumors of both grades, as well as by age in grade 2 tumors. However, among individuals with grade 3 tumors, those diagnosed at <50 years old exhibited increased one-year OS (98.6% [95CI: 94.4%–99.6%]) compared to individuals ≥50 (89.1%% [95CI: 81.9%–93.5%]). One-year OS was also comparable across tumor dimensions. Similar to WHO grade 2 IDH-mutant astrocytomas, there was little difference in one-year OS among individuals with WHO grade 2 IDH-mutant 1p/19q-codeleted oligodendrogliomas who either received chemoradiotherapy or neither chemotherapy nor radiotherapy. However, among individuals with WHO grade 3 tumors, chemoradiotherapy was associated with a one-year OS of 96.8% (95CI: 93.4%–98.5%), whereas neither chemotherapy nor radiotherapy was associated with a one-year OS of 68.3% (95CI: 44.7%–83.5%). The one-year OS additionally was comparable across extent of resection for individuals with WHO grade 2 tumors; although in individuals with grade 3 tumors, greater extent of resection was associated with small increases in one-year OS. Among individuals with MGMT promoter status reported, 81.7% of grade 2 (n = 103) and 90.0% of grade 3 (n = 99) tumors were methylated. Median age at presentation with IQR was 43 (34–54) and 49 (37–57) years for WHO grade 2 and 3 individuals, respectively.
H3 K27M-mutant Diffuse Midline Glioma
Individuals with H3 K27M-mutant diffuse midline glioma had a one-year OS of 55.9% (95CI: 44.5%–65.8%) and a median OS of 13.4 months (95CI: 10.5–15.5; Table 1, Figure 1). There were no substantial differences in one-year OS by sex or age at diagnosis. The small size of the cohort complicated one-year OS estimates by different management approaches. The median age at presentation was 13.5 years (IQR 7–29).
Survival by WHO CNS Grade for Adult-type IDH-wildtype Diffuse Astrocytic Glioma
Following the observation that most adult-type IDH-wildtype diffuse astrocytic gliomas contain molecular alterations that portend high-grade biology (eg, TERT promoter mutations, EGFR amplification, and/or polysomy 7 with monosomy 10) even in the absence of the histological hallmarks of glioblastoma, the existence of lower grade adult-type IDH-wildtype diffuse astrocytic glioma (ie, grades 2 and 3) as a distinct entity has been unclear. Therefore as an exploratory analysis, we further evaluated the effect of WHO CNS grade on survival for IDH-wildtype diffuse astrocytomas. Irrespective of MGMT promoter methylation status, WHO grade 2–3 tumors demonstrated longer unadjusted OS compared to those reported as WHO grade 4 (Supplemental Figure 1A-B). After adjustment for age, tumor size, extent of resection, treatment, and MGMT status, WHO grade 2 (n = 49; HR: 0.35, 95CI: 0.21–0.57, P < .001) and grade 3 (n = 153; HR: 0.56, 95CI: 0.45–0.7, P < .001) IDH-wildtype diffuse astrocytic gliomas were associated with significantly improved OS as compared to grade 4 (n = 3889; referent; Supplemental Table 3). Median age at presentation with IQR was 57 (37.5–68), 60 (47–70), and 65 (56–72) years for WHO grade 2, 3, and 4 individuals, respectively.
Post hoc analysis of the CATNON clinical trial did not find an added survival benefit associated with temozolomide among radiotherapy-treated patients with histologically grade 3 IDH-wildtype diffuse astrocytic gliomas with molecular features of glioblastoma. Similarly, we did not observe a significant association between chemotherapy (overwhelmingly single-agent) and OS (HR 0.95, 95%CI: .41–2.22, P = .91) in the radiotherapy-treated patients with grade 3 IDH-wildtype diffuse astrocytic gliomas, including after accounting for patient and tumor features (n = 137; Supplemental Table 4).
Discussion
We have previously quantified the misclassification of diffuse gliomas in cancer registries, chronicled the design of novel brain molecular marker variables for cancer registries, determined the validity of those variables, and leveraged the variables to describe the epidemiology of molecularly-defined glioma types in the United States.4,6,7 Building on these efforts, our present findings provide the first U.S. OS estimates for molecularly-defined glioma types from a database that represents ~85% of newly-diagnosed primary brain tumors in the United States. Overall, IDH mutation and 1p/19q codeletion among adult-type diffuse gliomas were associated with improved OS that varied by WHO CNS grade, clinical characteristics, and treatment strategies—as has been persistently shown in prior clinical studies and codified in the revised WHO-CNS4.
For IDH-mutant astrocytomas, it has been suggested that grade 2 and grade 3 tumors occur in patients of the same age range and have a similar prognosis due to the inability in distinguishing these tumors in a clinically meaningful manner. Similarly, there has been substantial debate whether histopathologic grading criteria are capable of stratifying risk among grade 2/3 IDH-mutant astrocytomas.8 Our data indicate that grade 2 IDH-mutant astrocytomas occurred in patients that were younger. Moreover, patients with grade 2 IDH-mutant astrocytomas had higher one-year survival rates than grade 3, indicating that morphologic grading criteria from the CNS WHO 2016 are able to stratify risk at least modestly at the population level. Among individuals with WHO grade 3 or 4 tumors, treatment with combined chemoradiotherapy and increased extent of resection were associated with improved one-year OS. However, in WHO grade 2 IDH-mutant astrocytomas, an association with one-year OS was not observed with any specific treatment intervention, likely due to the prolonged OS and delayed effect of therapy in this subgroup. Standard practice recommendations including gross total resection and chemoradiotherapy, especially for high-risk low-grade gliomas, have been corroborated for such IDH-mutant astrocytomas in prior studies.9–11 Additionally, the recently released WHO-CNS5 eliminated “IDH-mutant glioblastoma” as a distinct entity, instead incorporating WHO grade 4 IDH-mutant tumors into the category of IDH-mutant astrocytomas based on the prognostic impact of IDH status despite shared histological features. Consistent with this notion that WHO grade 4 IDH-mutant astrocytoma and IDH-wildtype glioblastoma are distinct entities, a sizeable difference in one-year OS was observed between these two tumor types: approximately 76% and 54%, respectively. Unfortunately, we previously demonstrated that the incidence of IDH-wildtype glioblastoma in the United States was almost 25 times higher than that of WHO grade 4 IDH-mutant astrocytoma (age-adjusted annual incidence of 1.74 vs. 0.07 per 100 000 population).4
Individuals presenting with IDH-mutant 1p/19q-codeleted oligodendrogliomas, whether WHO grade 2 or 3, experienced high one-year OS (approximately 98% and 94%, respectively). The favorable one-year OS rate among WHO grade 2 tumors was similar across patient, tumor, and treatment characteristics. Individuals with WHO grade 3 tumors who were younger, treated with combined chemoradiotherapy, and underwent resection were associated with improved one-year OS—suggesting a benefit to upfront treatment for patients with grade 3 IDH-mutant 1p/19q-codeleted oligodendrogliomas, as opposed to watchful waiting. However, given that these data only include a median of 15 months of follow up, it is likely that the impact of these factors will become more pronounced with longer periods of follow up—for instance, the benefits chemoradiotherapy, as investigated by the EORTC 26951, RTOG 9402, and CODEL clinical trials.12–14 Increasingly, clinical trials are enrolling both grade 2 and 3 IDH-mutant 1p/19q-codeleted oligodendroglioma patients, which in part reflects the similarity in one-year OS between observed here for grade 2 and 3 tumors.
Unsurprisingly, the one-year OS for individuals with WHO CNS grade 4 IDH-wildtype glioblastoma was considerably lower as compared to WHO grade 4 astrocytic gliomas harboring an IDH mutation, underscoring the prognostic importance of IDH status. Attributes that were positively associated with one-year OS in IDH-wildtype glioblastoma included younger age, combined chemoradiotherapy in the first-line setting, and maximal extent of resection—all of which are supported by extensive literature and drive the current standard-of-care treatment paradigm.15,16 Alongside the Brain Molecular Marker SSDI, an updated MGMT promoter methylation variable was also introduced in 2018. MGMT promoter methylation has been long identified as an important predictive marker for response to temozolomide, although MGMT testing utilization has lagged in the United States.17–20 Herein, WHO grade 4 IDH-wildtype glioblastoma individuals were associated with similar OS irrespective of MGMT promoter methylation status, with a median of 17.1 months in methylated tumors and 12.4 months in unmethylated tumors – although these analyses did not account for important confounders such as patient performance status or age. Consistent with the pivotal trial by Stupp et al.,15,21 treatment with combined chemoradiotherapy was—overall—associated with improved OS regardless of MGMT promoter methylation status, with a substantially greater OS observed in MGMT promoter methylated tumors: a median of 21.8 months, in contrast to 14.1 months in MGMT promoter unmethylated tumors.
When considering adult-type IDH-wildtype diffuse gliomas with astrocytic histology, lower gradations (ie, WHO grade 2 and 3) are notably absent from the WHO-CNS5 classification. There has been debate regarding the existence of such lower-grade tumors given that numerous studies have suggested that the majority of IDH-wildtype astrocytomas with WHO grade 2/3 histology have clinical outcomes equivalent to IDH-wildtype glioblastoma and are often thought to represent under-sampled glioblastomas.22–27 Cancer registry reporting in 2018 included gliomas classified and graded according to WHO CNS 2016, which recognized grades 2, 3, and 4 IDH-wildtype astrocytic gliomas that were graded based on morphologic criteria. This allowed the analysis of one-year survival rates of these gliomas in the current analysis. It should be noted that a substantial percentage of histologically defined grade 2 and 3 IDH-wildtype astrocytic gliomas would be considered IDH-wildtype glioblastoma, WHO grade 4 if they met molecular criteria that include TERT promoter mutation, EGFR amplification, or polysomy 7/monosomy 10. Since we do not have this molecular data in the current analysis, it is not clear how many histologic grade 2/3 would be considered grade 4 by CNS WHO 2021 criteria. Nonetheless, the current data provide the opportunity to analyze the one-year survival data for IDH-wildtype astrocytic gliomas based on histologic grading. To help inform this discussion, we explored the association of WHO CNS grade with OS in the national cohort reported as adult-type IDH-wildtype diffuse astrocytic gliomas. Importantly, we adjusted our analysis for key confounders of this association: individuals’ age at diagnosis, tumor size, extent of resection (which may indirectly represent extent of tumor sampling), chemotherapy, radiotherapy, and MGMT promoter methylation status. We found that WHO grade 2 and 3 tumors were associated with significantly improved OS as compared to WHO grade 4 tumors, potentially supporting the notion that there may be “true” lower-grade adult-type IDH-wildtype astrocytomas.28 In tumors with WHO grade 2/3 histology lacking definitive molecular features of glioblastoma, recent clinical and molecular investigation suggests that this subset tends to have distinct features: occurring in younger individuals, having WHO grade 2 histology, and molecularly exhibiting less frequent CDKN2A homozygous deletions, less frequent PTEN and PIK3CA alterations, and more frequent NF1 alterations.29 However, DNA methylation profiling often grouped these tumors into other categories such as pilocytic astrocytomas, glioneuronal tumors, dysembryoplastic neuroepithelial tumors, and pediatric-type diffuse gliomas, rather than with adult-type diffuse gliomas.25,30 Although it is possible that some WHO grade 2/3 tumors were pediatric-type low-grade diffuse gliomas (for which distinct ICD-O-3 codes were not yet implemented) or erroneously coded pilocytic astrocytomas (for which unique ICD-O-3 codes did exist), the median age at diagnosis for WHO grade 2, 3, and 4 IDH-wildtype astrocytic gliomas was 57, 61, and 64 years, respectively; suggesting that such contamination would likely be limited. We also explored whether chemotherapy was associated with improved OS among morphologically grade 3 IDH-wildtype diffuse astrocytic gliomas that received radiotherapy, given that retrospective analysis of the CATNON clinical trial did not find an added survival benefit associated with temozolomide among such tumors with molecular features of glioblastoma.31 Our analyses also failed to find a significant association between chemotherapy use and OS in such individuals, including after accounting for patient and tumor features.
Although the Brain Molecular Marker SSDI was focused on reporting adult-type diffuse gliomas, in 2018, registries also implemented reporting of a novel ICD-O-3 specific for the pediatric-type high-grade diffuse glioma type of H3 K27M-mutant diffuse midline glioma (included among the broader H3 K27M-altered diffuse midline gliomas classification in WHO-CNS5). Using these data, we found that the OS for H3 K27M-mutant diffuse midline gliomas was similar to that of IDH-wildtype glioblastoma, with a median of 13.4 months and a one-year OS of 56% – which is comparable to what has been previously reported for midline gliomas by the much larger international meta-analysis of pediatric high-grade gliomas.32 Unfortunately, distinguishing brainstem from pontine or thalamic locations was not possible from NCDB data as there is currently no ICD-O-3 topography code for these sites in U.S. cancer registry, neither was differentiating between H3F3A (H3.3) and HIST1H3B (H3.1) alterations, which do not have distinct ICD-O-3 morphology codes.
Limitations
While notable as the first national-level analysis of survival patterns for molecularly-defined diffuse glioma types in the United States, there are several limitations germane to this study. First, the NCDB does not report details regarding the molecular testing methodology or assay (eg, whether IDH status was assessed by immunohistochemistry or sequencing). Second, the NCDB only reports overall survival for outcomes, not cancer-specific survival or progression-free survival. For adult-type diffuse glioma types with prolonged overall survival (ie, WHO grades 2–3 IDH-mutant astrocytomas and IDH-mutant 1p/19p-codeleted oligodendrogliomas) the presence or absence of one-year OS associations must therefore be interpreted with caution: whether these findings similarly correlate with progression-free survival and overall survival endpoints over longer periods of observation–which are ultimately more clinically relevant–has yet to be determined. The Brain Molecular Marker data item was not reported for all diffuse glioma histology codes, including “glioma, malignant” (ICD-O-3 9380/3), “gliosarcoma” (ICD-O-3 9442/3), “giant cell glioblastoma” (ICD-O-3 9441/3), or gemistocytic astrocytoma” (ICD-O-3 9411/3); so our survival results may not generalize to the minority of tumors reported to have these histological findings. The WHO CNS grade reported by NCDB reflected WHO-CNS4 histology-based criteria and so would not account for tumors that were higher grade by virtue of CDKN2A/B homozygous deletion. Consequently, some IDH-mutant astrocytoma or IDH-mutant and 1p/19q-codeleted oligodendrogliomas that were reported to be low grade based on histological criteria, may have actually been higher grade based on molecular criteria. Additionally, the NCDB lacks granularity regarding specific therapeutic agents or dosing, treatments beyond the first-line setting, MGMT promoter methylation testing methodology, symptomatology, or other molecular alterations with important diagnostic or prognostic utility. As understanding of these molecular patterns evolves, updated and integrated diagnosis variables will be necessary to capture such data in registry databases.
Conclusions
The standardized implementation of Brain Molecular Marker data collection by cancer registries has, for the first time, made possible the epidemiological analysis of histomolecularly-defined adult-type diffuse gliomas in the United States. Using this variable, we have defined the survival patterns associated with adult-type diffuse glioma types (as well as the pediatric-type H3 K27M-mutant diffuse midline glioma) across WHO CNS grade, patient and tumor characteristics, and treatment approaches for individuals in the United States. Our results build upon the many important historical epidemiological efforts—which often relied on now-outdated histological classifications—to provide updated prognostic information based on the contemporary, clinically-relevant integrated classification of diffuse gliomas. Taken together, these findings highlight the value of incorporating molecular biomarkers for patients with brain tumors into national cancer registry data.
Supplementary Material
Acknowledgments
J.B.S. is a full-time paid employee of the NIH/NCI. G.C. and K.A.W. are full-time contractors for the NIH/NCI. Contents are solely the responsibility of the authors and do not necessarily represent the official views of either the CDC or of the NIH/NCI. The data used in the study are derived from a de-identified NCDB file. The American College of Surgeons and the Commission on Cancer have not verified and are not responsible for the analytic or statistical methodology employed, or the conclusions drawn from these data by the investigator.
Contributor Information
Quinn T Ostrom, Central Brain Tumor Registry of the United States, Hinsdale, IL, USA; Department of Neurosurgery, Duke University School of Medicine, Durham, NC, USA; The Preston Robert Tisch Brain Tumor Center, Duke University School of Medicine, Durham, NC, USA; Duke Cancer Institute, Duke University Medical Center, Durham, NC, USA.
Madison L Shoaf, Department of Neurosurgery, Duke University School of Medicine, Durham, NC, USA.
Gino Cioffi, Central Brain Tumor Registry of the United States, Hinsdale, IL, USA; Trans-Divisional Research Program, Division of Cancer Epidemiology and Genetics, National Cancer Institute, Bethesda, MD, USA.
Kristin Waite, Central Brain Tumor Registry of the United States, Hinsdale, IL, USA; Trans-Divisional Research Program, Division of Cancer Epidemiology and Genetics, National Cancer Institute, Bethesda, MD, USA.
Carol Kruchko, Central Brain Tumor Registry of the United States, Hinsdale, IL, USA.
Patrick Y Wen, Division of Neuro-Oncology, Department of Medical Oncology, Dana-Farber Cancer Institute, Boston, MA, USA.
Daniel J Brat, Department of Pathology, Northwestern University Feinberg School of Medicine, Chicago, IL, USA.
Jill S Barnholtz-Sloan, Central Brain Tumor Registry of the United States, Hinsdale, IL, USA; Trans-Divisional Research Program, Division of Cancer Epidemiology and Genetics, National Cancer Institute, Bethesda, MD, USA; Center for Biomedical Informatics & Information Technology, National Cancer Institute, Bethesda, MD, USA.
J Bryan Iorgulescu, Division of Pathology and Laboratory Medicine, MD Anderson Cancer Center, Houston, TX, USA.
Conflict of interest statement
Jill S. Barnholtz-Sloan, Ph.D. is a full-time paid employee of the NIH/NCI. Gino Cioffi M.P.H. and Kristin A. Waite, Ph.D. are full-time contractors of the NIH/NCI.
Authorship statement. Conceptualization & supervision: Q.O., J.B.I.; methodology: Q.O., J.B.I.; formal analysis and investigation: J.B.I.; critical interpretation of results & writing: Q.O., M.S., J.B.I.; read and approved final version: all authors.
Funding
Funding for CBTRUS was provided by the Centers for Disease Control and Prevention (CDC) under Contract No. 75D30119C06056 Amendment/Modification No: 0002, the American Brain Tumor Association, Novocure, the Musella Foundation, National Brain Tumor Society, the Pediatric Brain Tumor Foundation, The Sontag Foundation, the Uncle Kory Foundation, the Zelda Dorin Tetenbaum Memorial Fund, as well as private and in-kind donations. JBI gratefully acknowledges funding support from the National Cancer Institute (K12CA090354) and Conquer Cancer Foundation/Sontag Foundation.
References
- 1. Jenkins RB, Blair H, Ballman KV, et al. A t(1;19)(q10;p10) mediates the combined deletions of 1p and 19q and predicts a better prognosis of patients with oligodendroglioma. Cancer Res. 2006; 66(20):9852–9861. [DOI] [PubMed] [Google Scholar]
- 2. Yan H, Parsons DW, Jin G, et al. IDH1 and IDH2 mutations in gliomas. N Engl J Med. 2009;360(8):765–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016; 131(6):803–820. [DOI] [PubMed] [Google Scholar]
- 4. Iorgulescu JB, Sun C, Neff C, et al. Molecular biomarker-defined brain tumors: epidemiology, validity, and completeness in the United States [published online ahead of print April 23, 2022]. Neuro Oncol. 2022;24(11):1989–2000.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mallin K, Browner A, Palis B, et al. Incident cases captured in the National Cancer Database compared with those in U.S. Population Based Central Cancer Registries in 2012-2014. Ann Surg Oncol. 2019; 26(6):1604–1612. [DOI] [PubMed] [Google Scholar]
- 6. Iorgulescu JB, Torre M, Harary M, et al. The misclassification of diffuse gliomas: rates and outcomes. Clin Cancer Res. 2019; 25(8):2656–2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kruchko C, Gittleman H, Ruhl J, et al. Cancer collection efforts in the United States provide clinically relevant data on all primary brain and other CNS tumors. Neurooncol Pract. 2019; 6(5):330–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brat DJ, Aldape K, Colman H, et al. cIMPACT-NOW update 5: recommended grading criteria and terminologies for IDH-mutant astrocytomas. Acta Neuropathol. 2020; 139(3):603–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bell EH, Zhang P, Shaw EG, et al. Comprehensive genomic analysis in NRG oncology/RTOG 9802: a phase III trial of radiation versus radiation plus Procarbazine, Lomustine (CCNU), and vincristine in high-risk low-grade glioma. J Clin Oncol. 2020; 38(29):3407–3417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kavouridis VK, Boaro A, Dorr J, et al. Contemporary assessment of extent of resection in molecularly defined categories of diffuse low-grade glioma: a volumetric analysis [published online ahead of print October 25, 2019]. J Neurosurg. 1–11. doi: 10.3171/2019.6.JNS19972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Patel SH, Bansal AG, Young EB, et al. Extent of surgical resection in lower-grade gliomas: differential impact based on molecular subtype. AJNR Am J Neuroradiol. 2019; 40(7):1149–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. van den Bent MJ, Brandes AA, Taphoorn MJ, et al. Adjuvant procarbazine, lomustine, and vincristine chemotherapy in newly diagnosed anaplastic oligodendroglioma: long-term follow-up of EORTC brain tumor group study 26951. J Clin Oncol. 2013; 31(3):344–350. [DOI] [PubMed] [Google Scholar]
- 13. Cairncross G, Wang M, Shaw E, et al. Phase III trial of chemoradiotherapy for anaplastic oligodendroglioma: long-term results of RTOG 9402. J Clin Oncol. 2013; 31(3):337–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jaeckle KA, Ballman KV, van den Bent M, et al. CODEL: phase III study of RT, RT + TMZ, or TMZ for newly diagnosed 1p/19q codeleted oligodendroglioma. Analysis from the initial study design. Neuro Oncol. 2021; 23(3):457–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Stupp R, Hegi ME, Mason WP, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009; 10(5):459–466. [DOI] [PubMed] [Google Scholar]
- 16. Brown TJ, Brennan MC, Li M, et al. Association of the extent of resection with survival in glioblastoma: a systematic review and meta-analysis. JAMA Oncol. 2016; 2(11):1460–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hegi ME, Diserens AC, Gorlia T, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005; 352(10):997–1003. [DOI] [PubMed] [Google Scholar]
- 18. Rivera AL, Pelloski CE, Gilbert MR, et al. MGMT promoter methylation is predictive of response to radiotherapy and prognostic in the absence of adjuvant alkylating chemotherapy for glioblastoma. Neuro Oncol. 2010; 12(2):116–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Olson RA, Brastianos PK, Palma DA. Prognostic and predictive value of epigenetic silencing of MGMT in patients with high grade gliomas: a systematic review and meta-analysis. J Neurooncol. 2011; 105(2):325–335. [DOI] [PubMed] [Google Scholar]
- 20. Lamba N, Chukwueke UN, Smith TR, et al. Socioeconomic disparities associated with MGMT promoter methylation testing for patients with glioblastoma. JAMA Oncol. 2020; 6(12):1972–1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005; 352(10):987–996. [DOI] [PubMed] [Google Scholar]
- 22. Tesileanu CMS, Dirven L, Wijnenga MMJ, et al. Survival of diffuse astrocytic glioma, IDH1/2 wildtype, with molecular features of glioblastoma, WHO grade IV: a confirmation of the cIMPACT-NOW criteria. Neuro Oncol. 2020; 22(4):515–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Reuss DE, Kratz A, Sahm F, et al. Adult IDH wild type astrocytomas biologically and clinically resolve into other tumor entities. Acta Neuropathol. 2015; 130(3):407–417. [DOI] [PubMed] [Google Scholar]
- 24. Weller M, Weber RG, Willscher E, et al. Molecular classification of diffuse cerebral WHO grade II/III gliomas using genome- and transcriptome-wide profiling improves stratification of prognostically distinct patient groups. Acta Neuropathol. 2015; 129(5):679–693. [DOI] [PubMed] [Google Scholar]
- 25. Fujimoto K, Arita H, Satomi K, et al. TERT promoter mutation status is necessary and sufficient to diagnose IDH-wildtype diffuse astrocytic glioma with molecular features of glioblastoma. Acta Neuropathol. 2021; 142(2):323–338. [DOI] [PubMed] [Google Scholar]
- 26. Suzuki H, Aoki K, Chiba K, et al. Mutational landscape and clonal architecture in grade II and III gliomas. Nat Genet. 2015; 47(5):458–468. [DOI] [PubMed] [Google Scholar]
- 27. Hasselblatt M, Jaber M, Reuss D, et al. Diffuse astrocytoma, IDH-wildtype: a dissolving diagnosis. J Neuropathol Exp Neurol. 2018; 77(6):422–425. [DOI] [PubMed] [Google Scholar]
- 28. Berzero G, Di Stefano AL, Ronchi S, et al. IDH-wildtype lower-grade diffuse gliomas: the importance of histological grade and molecular assessment for prognostic stratification. Neuro Oncol. 2021; 23(6):955–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Richardson TE, Hatanpaa KJ, Walker JM. Molecular characterization of “true” low-grade IDH-wildtype astrocytomas. J Neuropathol Exp Neurol. 2021; 80(5):431–435. [DOI] [PubMed] [Google Scholar]
- 30. Capper D, Stichel D, Sahm F, et al. Practical implementation of DNA methylation and copy-number-based CNS tumor diagnostics: the Heidelberg experience. Acta Neuropathol. 2018; 136(2):181–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tesileanu CMS, Sanson M, Wick W, et al. Temozolomide and radiotherapy versus radiotherapy alone in patients with glioblastoma, IDH-wildtype: post-hoc analysis of the EORTC randomized phase 3 CATNON trial. Clin Cancer Res. Published online March 11, 2022; clincanres.4283.2021. doi: 10.1158/1078-0432.CCR-21-4283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mackay A, Burford A, Carvalho D, et al. Integrated molecular meta-analysis of 1,000 pediatric high-grade and diffuse intrinsic pontine glioma. Cancer Cell. 2017; 32(4):520–537.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.