Abstract
Medulloblastoma (MB) is the most common malignant brain tumor in children, making up ~20% of all primary pediatric brain tumors. Current therapies consist of maximal surgical resection and aggressive radio- and chemotherapy. A third of the treated patients cannot be cured and survivors are often left with devastating long-term side effects. Novel efficient and targeted treatment is desperately needed for this patient population. Cellular immunotherapy aims to enhance and utilize immune cells to target tumors, and has been proven successful in various cancers. However, for MB, the knowledge and possibilities of cellular immunotherapy are limited. In this review, we provide a comprehensive overview of the current status of cellular immunotherapy for MB, from fundamental in vitro research to in vivo models and (ongoing) clinical trials. In addition, we compare our findings to cellular immunotherapy in glioma, an MB-like intracranial tumor. Finally, future possibilities for MB are discussed to improve efficacy and safety.
Keywords: cellular immunotherapy, cytotoxic lymphocytes, medulloblastoma
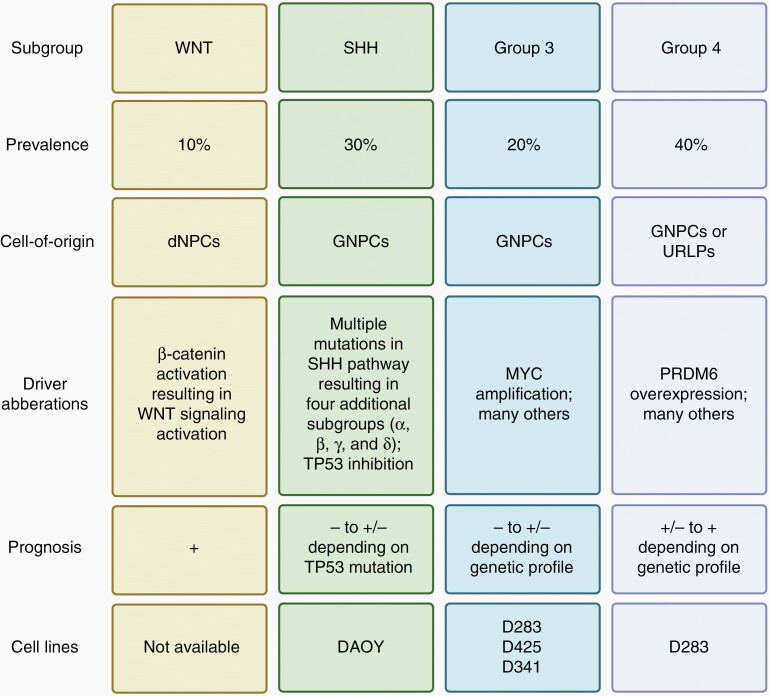
The incidence of central nervous system (CNS) tumors is most frequent in pediatric patients (0–21 years of age). Medulloblastoma (MB), a World Health Organization (WHO) grade IV classified malignancy, is the most prevalent1 and responsible for over 60% of all intracranial embryonal tumors in the USA diagnosed between 2011 and 2015.2 The peak incidence is between 6 and 8 years of age.1 In childhood, approximately 1 in 200 000 individuals is diagnosed with MB,2 whereas the incidence in adulthood is much lower (1 in 2 000 000).3 MB originates from the progenitor cell populations in the cerebellum during early life and has been linked to specific genetic aberrations. Based on these aberrations, four MB subgroups have been described; Wingless (WNT), Sonic Hedgehog (SHH), Group 3 (G3), and Group 4 (G4).4,5 Although morphologically all classified as MB, these subgroups are different in cell-of-origin, driver aberration, and prognosis thus should be treated as biologically distinct entities (Figure 1).
Figure 1.
Classification of medulloblastoma subgroups. dNPCs, differentiating neural stem and progenitor cells; GNPCs, granule neuron precursor cells; SHH, sonic hedgehog; URLPs, upper rhombic lip progenitors; WNT, wingless-related integration site. Figure was generated based on available literature.1,4,6–9.
In most WNT-MB patients, an activating mutation in the gene encoding β-catenin results in a constitutively active WNT signaling that contributes to enhanced cell growth and proliferation.6 Patients often have a favorable prognosis mainly attributed to an altered vasculature in the brain and higher permeability of blood brain barrier (BBB), making it more susceptible to systemic chemotherapies.1 SHH-MB is genetically best characterized with most mutations found within the SHH pathway. This subgroup is more heterogenetic compared to WNT-MB and is divided in four additional subgroups (SSHα, SSHβ, SSHγ, and SSHδ).7 Depending on the subtype of SSH-MB, adult patients can be treated by inhibiting proteins upstream of SSH. However, children cannot use these inhibitors because of potential skeletal abnormalities resulting in a far worse prognosis.1 Additionally, TP53-mutant tumors drastically decrease survival in both children and adults.8 For subgroups 3 and 4, less than a third of cases can be dedicated to one driver event and are therefore genetically not well defined.6 Genetic aberrations that are most prevalent in G3 and G4 are high-level MYC amplification and PRDM6 over-expression, respectively. G3-MB has the lowest survival rate and is considered the most aggressive and G4-MB has a moderate risk of progressive disease compared to other subtypes.9 However, precise prognoses of both subgroups depend on the exact genetic profile of the tumor.1
Conventional treatment options for MB patients include maximal surgical resection, aggressive chemotherapy, and radiation therapy.10 Surgery is often performed first and the prognosis strongly depends on the extent of tumor resection.11 This is followed by irradiation of the entire cranio-spinal axis and results in a multitude of severe adverse effects, such as neuroendocrine dysfunction, growth disturbances and deformities, neurocognitive disability, infertility, secondary malignancies, and a lowered quality of life.12 For this reason, infants are approached with a non-radiation strategy, being at highest risk for severe intellectual impairment. Studies that minimized or omitted radiation therapy were not effective to treat MB,13,14 emphasizing the trade-off between survival and neurocognitive disabilities.15 Adjuvant chemotherapy has greatly increased survival of MB patients but is still unable to cure over 30% of all patients (5-year survival).10,12
Cancer treatment was brought to a new era after the introduction of targeted immunotherapy. Immunotherapy shows a unique profile of toxicities and adverse events that require specific management16 but has been successfully implemented in treatment protocols for other cancers, for example, melanoma, urothelial cancer, non-small-cell lung cancer, and many others.17 Cellular immunotherapy, a subgroup in cancer immunotherapy, specifically focuses on infusing auto- or allogenic immune cells or modified immune cell lines to kill cancerous cells. This therapy exploits tumor-specific antigens and proteins to recognize and selectively target cancer cells, and has been proven to be successful in multiple hematologic tumors.18 In order to be effective as a therapy, immunotherapy requires a pro-inflammatory tumor microenvironment (TME), however MB tumors, due to a non-inflammatory microenvironment, have a generally low influx of immune cells, for example, B cells, T cells, γδ T cells, NK cells, and NKT cells.19–22 Immunosuppressive cells that are present in the TME include regulatory T cells, immunosuppressive M2 phenotype macrophages, and tumor-associated astrocytes.23,24 To overcome this suboptimal condition, cytotoxic cells can be engineered to be resistant to the inhibitory TME25,26 and perhaps combined with immune checkpoint blockade therapy.27 These findings suggest the potential benefit of (local) cellular immunotherapy in MB.
Recently, several chimeric antigen receptor (CAR) T cell therapies have been FDA (US Food and Drug Administration) approved as a novel cellular immunotherapy for refractory B cell malignancies.28,29 The first proof of principle30 for CAR T cells was reported in 1987 and, since then, several generations of CARs have been developed for efficient antitumor strategies. Basically, the constant region of the T cell receptor is fused to single-chain variable fragments of a monoclonal antibody through a flexible linker peptide. This enables CAR T cells to recognize antigens specific to the inserted monoclonal antibody without requiring major histocompatibility complex (MHC) presentation.31–33 Treatment of leukemia with CAR T cells targeting CD19, expressed on all B lineage cells, resulted in increased overall survival and complete remission.34 The most recent CAR is a fourth-generation, often referred to as an “armored” CAR because of the incorporation of additional stimulatory domains.31 By adding such domains, for example, to enhance IL-12 secretion, CAR T cells modulate the cytokine composition in the TME thereby stimulating innate immune cells and countering inhibitory elements of the original TME, the latter being especially important for solid tumors.35 However, long-term effects have not been studied well and CAR T cell therapies are technically complex, time-intensive, and expensive.36 Moreover, CAR T cell therapy does not yield a similar result in the treatment of solid tumors yet, especially in those with challenging microenvironments.36
Natural killer (NK) cells are innate lymphoid cells that are known to identify and target viral-infected cells and cancer cells. Their cytotoxic function has a lot of similarities to CD8+ T cells, as they both secrete cytotoxic granules and pro-inflammatory cytokines.37 Contradictory to T cells (and B cells), NK cells do not require somatic hypermutation of antigen receptors to target specific peptides. Rather, a combination of activating and inhibitory signals through non-specific receptors decide a potential response to a target cell.38 Effector functions of NK cells on cancerous cells can take one of three forms. First, NK cells can directly kill cancer cells using the “missing-self” mechanism, for example, the absence of MHC type I (MHC-I) expression. Many tumors, including MB,38 can downregulate MHC-I expression so that recognition by CD8+ T cells is limited. Since NK cells activation is inhibited by MHC-I molecules, its downregulation results in NK cell activation.38 Second, antibody-dependent cell-mediated cytotoxicity (ADCC) has shown to be successful in hematological as well as solid tumors.38 Third, NK cells secrete an abundance of pro-inflammatory cytokines, amongst which are tumor necrosis factor (TNF) and interferons (IFNs). These molecules can have anti-angiogenic, anti-proliferative, and pro-apoptotic effects on cancer cells and stimulate activation and recruitment of other immune cells.38 Despite this, NK cell therapy is not always beneficial for cancer patients. Well-known limitations include suppression of NK cells through hypoxia in the tumor microenvironment and anti-inflammatory molecules, such as TGF-β, IL-10, and prostaglandin E2, emphasizing the challenges of these tumor defense systems yet to be overcome.39–41
Natural killer T (NKT) cells, a subset of T cells with NK cell-associated markers, can recognize cells through lipid-based peptides presented on CD1d molecules.42 CD1d is an MHC-I-like molecule that presents hydrophobic structures. The recognition and effector functions of NKT cells are roughly similar to that of NK cells and T cells, and recently even CAR NKT cells have been synthesized to target B cell lymphoma in mice.43
Dendritic cells (DCs), although not cytotoxic cells by itself, are intensively studied for novel cancer treatments. DCs are professional antigen-presenting cells (APCs) that process and present antigens through MHC (I and II) as an integration of the innate and adaptive immune system for a specific and sustained immune response.44 DC therapy consists of monocyte or hematopoietic stem and progenitor cell (HSPC) extraction from the patient through leukapheresis, ex vivo “training” to present TAs through (1) loading of tumor antigenic peptides, (2) pulsing with whole tumor apoptotic bodies, (3) pulsing with tumor lysates, or (4) transfections with tumor-derived mRNA, and re-administration.45 Promising (pre)clinical data of DC therapy for several cancers have been reported together with satisfactory clinical safety studies.46
In this review, we provide an overview of current cellular immunotherapy in MB. Based on existing literature, we focus on cytotoxic T cell, NK cell, NKT cell, and DC therapy performed in vitro, in vivo, and in the clinic. We will compare these therapies with recent discoveries in glioma research, as it is the most studied brain cancer. Finally, future possibilities on cellular immunotherapy for MB will be discussed.
Cellular Immunotherapy
T Cells
CAR T cells have shown remarkable clinical results in hematological malignancies such as B cell leukemia or lymphoma.33 However, it has not been as successful in solid tumors because of the scarcity of tumor-specific antigens (TAs). To date, several molecules have been identified to be MB-specific. Rivero-Hinojosa et al. have screened 46 MB tumors and found a total of 362 peptides that were MB-specific, with one (NSSVSGIFTFQK) being present in more than 20% of all tested MB tumors.
Currently, also other tumor-specific proteins and peptides are tested for their potential role in (CAR) T cell therapy for MB. CAR T cell therapy targeting HER2 in MB has been studied most intensively in the past years. MB cell lines Daoy, D283, and D425,47 as well as 31.1% of primary MB tumors48 show HER2 overexpression. The first evidence was produced in 2007 when HER2 CAR T cells were found able to kill Daoy, D283, and primary tumor cells.49 Consequently, HER2 CAR T cell therapy was delivered intratumorally in Daoy xenogenic mice, showing significant tumor regression.49 Even though overall survival of the treated group was significantly higher than untreated control, all mice relapsed and died within 55 days post-treatment.49
The safety risk of an intravenous injection of HER2 CAR T cells was reported in a case report from 2010. A 39-year-old patient with colon cancer metastatic to the liver and lungs was treated with intravenous HER2 CAR T cell therapy (10 billion cells). Five days after treatment, the patient succumbed to a cytokine storm triggered by recognition of HER2 on lung epithelial cells.50 This on-tumor off-target effect underlines the importance of specific TAs for patient safety and effective treatment. Healthy brain tissue does not express HER2,51 suggesting minimal on-target off-tumor effects in the brain.
In 2018, Nellan et al.47 compared intratumorally delivered HER2 CAR T cells with intravenous therapy in Daoy xenograft-bearing mice. To accomplish complete tumor regression in both models, a five-fold higher cell dose was required intravenously compared to intratumorally. The same effect of complete tumor regression intratumorally versus intravenously was observed for mice with D283 xenografts.47 Overall survival of the treated groups was 100% and no mice relapsed. Notably, all mice were euthanized at 37 days post-treatment due to protocol restrictions. This short follow-up makes it impossible to compare the results with the 55 day follow-up of Ahmed et al.49 Furthermore, Nellan et al. also examined the safety of intra-ventricular therapy in non-human primates—rhesus macaques—which have a 98% homology to human HER2. CSF showed HER2 CAR T cells and increased IL-2 and IL-6 levels, whereas none were found in peripheral blood. Therefore the authors stated the superiority of intra-ventricular treatment compared to intravenous injection and concluded that it did not cause significant on-tumor off-target exposure or toxicity. These results therefore seem promising to start human HER2 CAR-T efficacy and safety studies.
In the most recent ongoing safety study, a phase I clinical trial (NCT03500991), an interim analysis of three MB patients was published.26 All three patients received HER2 CAR T cells intratumorally in week one, two, and three of each 4-week course. Patient one received two courses whereas patients two and three completed three courses of cell infusions. Of the patients, none experienced dose-limiting toxicities but adverse events consisted of headache, pain, and fever that were consistent with elevated systemic C-reactive protein levels.26 After treatment, patients were subjected to scheduled neuroimaging to examine disease progression. Patients one and three had progressive disease but patient two had stable disease.26 The authors conclude that locoregional administration of HER2 CAR T cells may be feasible and that assessing on-target CAR T cell activity in the CNS can be valuable.26 Nevertheless, the clinical benefit must be examined in a larger cohort of MB patients.
In a large group 3 MB study of 326 human samples from 2020, EPHA2, HER2, and IL13Rα2 were found to have a higher expression compared to normal brain tissue controls.52 CAR T cells mono-specific for EPHA2 and trivalent for co-targeting EPHA2, HER2, and IL13Rα2 were administered in the lateral ventricle of different group 3 MB xenograft mice models. A single dose of either CAR T cell increased survival and minimized tumor recurrence, whereas repeated CAR T cell therapy showed additional clinical benefits.52 Thereafter, in the same study, the authors compared intraventricular CAR T cell delivery with intravenous tail injections and concluded that while there is a clinical benefit for intravenous injections, intraventricular delivery has significant superior outcomes in group 3 MB in vivo models. Despite the success of the therapies performed in this study, all models eventually presented tumor recurrence. Further investigations where EPHA2 CAR T cell therapy was combined with the effective chemotherapeutic Azacytidine, showed a complete tumor clearance and relapse-free survival in 40% of the mice.52 Other mice experienced a significant reduction in tumor burden but eventually relapsed52
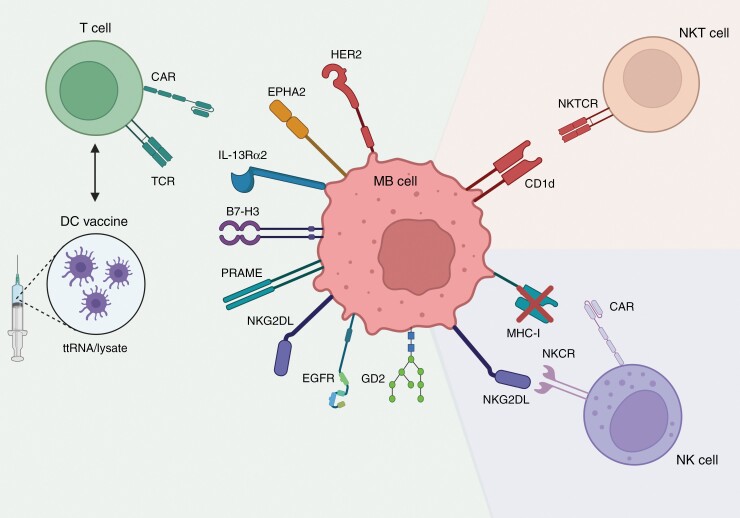
Besides HER2, other, less extensively studied proteins have been found on MB cells (Figure 2). Immune checkpoint B7-H3 (CD276) is expressed on 96% of examined MB tumors as was shown in two independent studies.61,62 A co-culture of B7-H3 CAR T cells with primary MB cells resulted in an upregulation of secreted IFN-γ, TNF-α, and IL-2 showing T cell activation.61 Subsequent in vivo studies in mice bearing Daoy and aggressive c-MYC-amplified MB xenografts showed total eradication of the tumors in 67% of the mice after intravenous injection of B7-H3 CAR T cells.61 Furthermore, a negative correlation was found between overall survival and the number of B7-H3 molecules expressed per cell of each tumor line after treatment.61 An antigen preferentially expressed in melanoma (PRAME) that is also expressed on MB63 can stimulate CD8+ T cells in melanoma patients.53 PRAME is evenly expressed by all four molecular subgroups of MB and high mRNA levels significantly correlate with worse overall survival.54 PRAME CAR T cells exerted antitumor activity towards Daoy and HLA-matched primary MB cells in co-cultures.54 In addition, Daoy xenograft-bearing mice showed an increased overall survival after intravenous infusion with PRAME CAR T cells.54 During the study, a total of 33 semiquantitative tests for health, behavior, and neurologic reflexes were performed and no signs of neurologic toxicity were discovered.54
Figure 2.
Schematic representation of the most important cellular immunotherapeutic strategies for medulloblastoma. B7-H3, B7 homolog 3; CAR, chimeric antigen receptor; CD1d, cluster of differentiation 1; DC, dendritic cell; EGFR, epidermal growth factor receptor; EPHA2, ephrin type-A receptor 2; HER2, human epidermal growth factor receptor 2; IL13-Rα2, interleukin-13 receptor subunit alpha-2; MHC-I, major histocompatibility complex type I; NK, natural killer; NKCR, NK cell receptor; NKG2DL, ligands of NKG2D; PRAME, antigen preferentially expressed in melanoma; TCR, T cell receptor, ttRNA; total tumor RNA. Figure was generated based on the available literature.22,26,47–49,52–63.
NK group 2 member D activatory receptor ligands (NKG2DLs) are expressed on MB cell lines (Daoy and D341) and most primary MB tumor samples.22,55,56 CAR T cells targeting these NKG2D ligands were used in an in vitro experiment with Daoy and resulted in killing of tumor cells through lysis and upregulation of cytokines TNF-α, IFN-γ, IL-10, and IL-2.56 As a result, the same CAR T cells were tested in mice with MB xenografts and eliminated tumors without exhibiting significant toxicity or pathological changes to organs.56 Nevertheless, due to selective pressure there is always a possibility of antigen escape. In this case, NKG2DL escape would mean CAR T resistance and partial NK cell evasion (since MHC-I downregulation could still be recognized). Therefore, CAR target ligands have to be chosen with extreme caution to limit potential tumor cell multi-resistance.
Because of the recent success of in vivo studies with CAR T cell therapy for MB models, several clinical trials have been started. In total, seven phase I clinical trials are ongoing and are testing the safety and dosing of CAR T cell therapy in MB patients (Table 1). However, to date, no phase II, III, or IV clinical trials have been registered yet to determine the clinical benefit of these treatments in MB patients.
Table 1.
List of ongoing CAR T cell and NK cell clinical trials for medulloblastoma patients
| Clinical trial | Phase | Cell therapy | Target | Delivery route | Estimated enrollment | Primary endpoint | Estimated primary completion date |
|---|---|---|---|---|---|---|---|
| NCT05131763 | I | CAR T | NKG2DL | i.v. | 3 | Treatment-related AEs | 12-2022 |
| NCT04099797 | I | CAR T | GD2.C7R | i.v. | 34 | Dose-limiting toxicity | 02-2023 |
| NCT03500991 | I | CAR T | HER2 | i.c. | 48 | Treatment-related AEs and establish feasibility | 07-2024 |
| NCT03638167 | I | CAR T | EGFR | i.c. | 36 | Treatment-related AEs and establish feasibility | 03-2025 |
| NCT04661384 | I | CAR T | IL13-Rα2 | i.c. | 30 | Overall survival and treatment-related AEs | 11-2025 |
| NCT04185038 | I | CAR T | B7-H3 | i.c. | 90 | Treatment-related AEs and establish feasibility | 05-2026 |
| NCT05298995 | I | CAR T | iC9-GD2 | i.v. | 54 | Treatment-related AEs and Dose-limiting toxicity | 05-2026 |
| NCT02100891 | II | NK | N.S. | i.v. | 15 | Disease control rate | 12-2022 |
| NCT01326104 | II | DC | ttRNA-DC/xALT | i.v. | 26 | 12 month PFS | 12-2022 |
Abbreviations: AE, adverse event; B7-H3, B7 homolog 3; C7R, interleukin-7 cytokine receptor; CAR, chimeric antigen receptor; EGFR, epidermal growth factor receptor; HER2, human epidermal growth factor receptor 2; iC9, inducible caspase-9; i.c., intracranial; i.v., intravenous; IL13-Rα2, interleukin-13 receptor subunit alpha-2; N.S., not specified; PFS, progression-free survival; ttRNA-DC/xALT, total tumor RNA-DC + expanded autologous lymphocyte transfer.
All data concerning the clinical trials were obtained from ClinicalTrials.gov (accessed on August 3rd, 2022).
In most studies with CAR T cell therapy in glioma patients, the initial patient response was beneficial and overall survival was increased, however, this was often followed by tumor recurrence.64 Therapy resistance can be explained by TA escape variants. Due to the heterogeneity of gliomas, antigens specific to the CAR may not be ubiquitously expressed or lose expression over time enabling subsequent survival of antigen negative clones and thus treatment resistance.64 To cope with this, combination treatment of multi-specific CAR T cells resulted in increased antitumor response and improved survival in glioblastoma mouse models.65,66 Due to the low mutational burden, antigen escape seems theoretical less likely in MB but no clinical data is available for MB. As of yet, no clinical study has started that examines bi- or multi-specific CAR T cell treatment in gliomas and MB but, based on mouse models, may provide an effective cellular immunotherapy with reduced risk of relapse in patients.
NK Cells
Compared to T cells, NK cells do not require specific TA recognition to kill tumor cells. Instead, they heavily rely on recognition of “induced self” and “missing self” antigen presentation to identify target cells,57 of which the most important host ligands include NKG2DLs (activatory) and MHC-I (inhibitory) (Figure 2). Therefore, a high NKG2DLs/MHC-I ratio results in high NK cell activation.22 In co-cultures between MB cell line Daoy and NK cells, NKG2DL/MHC-I ratio was shown to predict NK cell cytotoxicity better than NKG2DL expression alone.22
Furthermore, NK cells can efficiently kill several MB cell lines in in vitro co-cultures52,67 and NK cell tumor infiltration has been correlated with a better prognosis.68 Nevertheless, NK cell receptors that were involved in cytotoxicity differed between cell lines and correlated with expressed ligands on tumor cells.55 Cancer stem cell marker CD133 did not influence NK activation and cytotoxicity in similar experiments. Therefore, the authors concluded that in all MB cell lines tested, NK cell killing was present and independent of CD133 expression.55
The microenvironment of MB tumors is known to contain high levels of TGF-β which can inhibit NK cell function.25 Therefore, NK cells unsusceptible to TGF-β could prove to be extra efficient in MB treatment. To this end, an NK cell line that expresses a dominant-negative receptor (DNR) for TGF-β was produced and tested. DNR-transduced NK cells showed no impaired cytokine secretion, cytolytic activity, and cell expansion.25 However, no cytotoxic assays with MB cells have been reported with these NK cells yet.
To our knowledge, one study tested NK cell treatment for MB in mice. Two intratumoral infusions of NK cells were administered and Daoy xenograft-bearing mice showed a significant decrease in tumor size compared to the control.69 Worthwhile to mention is that additional labeling of NK cells with a fluorine-19 MRI probe did not change the cytotoxic outcome in vivo but does allow for the monitoring of intracranial delivery via MRI.69
In 2020, the previous group also completed the first phase I clinical trial (NCT02271711) of intracranial NK therapy for MB patients. Five MB patients enrolled and were treated with varying doses of NK cells. Delivery through either the fourth ventricle or the lateral ventricle of all cell doses did not lead to any dose-limiting toxicities and most patients only experienced grade one or two adverse effects.70 Two patients required brief hospitalization during treatment but were released after maximally three days. These data demonstrate the feasibility of intracranial NK cell treatment in MB patients.70 At the radiographic evaluation, one of five MB patients showed a nearly 30% size reduction of the tumor after five infusions. However, after two weeks, the patient experienced multiple seizures and had progressive disease.70 To examine clinical benefit correctly, longer treatment and follow-up are required. Only, one other clinical trial (NCT02100891) has been started in recent years and is summarized in Table 1.
When comparing T and NK cell therapies, it stands out that T cell treated patients more often develop severe toxic adverse effects in the form of cytokine release syndrome (CRS) and graft versus host disease (GvHD).71 However not yet fully understood, NK cell therapies show to be safer and patients have a reduced risk of CRS and GvHD, which explains the interest in NK cell immunotherapy candidates.72,73
For both MB and glioma patients, NK cell therapy lags behind when compared to T cell-based treatments. Most of the research is performed in pre-clinical models and only a handful of phase I clinical trials have been started. Moreover, NK cells are often, but not always, engineered as CAR NK. These cells can be engineered from primary NK cells or the NK-92 cell line of which both have been proven successful in glioma treatment.74 CAR NK cells could offer an advantage since they recognize TAs, but also have the intrinsic ability to kill tumor cells that do not present MHC-I. For MB, no studies for CAR NK cell therapy have been described yet. However, due to evidence of toxicity and adverse effects in T cell-based treatments in MB patients,26,50 CAR NK might offer a targeted and safer solution.
Dendritic Cells
DCs have limited cytotoxic properties and are best known as professional antigen-presenting cells. DC therapy strategies focus on ex vivo “training” of immune cells and can take one of two forms. Either DCs are introduced to TAs after which they are reinfused into the patient and activate the adoptive T cell response, or autologous T cells are expanded ex vivo with DC help and re-administered to the patient (Figure 2).75
Even though MB is known for its low mutational burden, Blaescke et al.76 showed that neoantigens can be used to stimulate a de novo T cell response after ex vivo co-culture with loaded DCs. An additional study showed that multiple MB cell lines were killed in vitro after a co-culture with similarly DC-activated T cells.77 However, another group showed no survival benefit after treatment with solely DC-activated T cells but required combination therapy with gemcitabine and rapamycin.60
Flores et al.74 used total tumor RNA (ttRNA)-pulsed DCs to generate a polyclonal T cell population for Group 3 MB treatment. In their mouse model, injection of this T cell population resulted in an increased survival compared to unreactive T cells. Furthermore, clonal expansion of the most efficient subpopulation of T cells was visible over the course of time, namely a 10% increase 120 days after initial infusion.74 A phase I/II clinical trial (Re-MATCH; FDA IND no. BB-14058) evaluated the feasibility and safety of this approach in recurrent MB patients.74 One patient demonstrated progression-free survival far exceeding that of other patients (13 months). It was found that clonal hyperexpansion of a single T cell clone could be attributed as a predictive biomarker for treatment response and thus survival. However, the authors did not elaborate on treatment efficacy and response.74
A clinical trial from 2009 examined injection of DCs loaded with lysate on pediatric patients with malignant CNS tumors, among which five MB.78 Although no clear clinical benefit was shown in any of the MB cases, median progression-free survival ranged from 3.0 to 17.7 months.78 As possible explanations of the treatment response, the authors mention that 60% of patients were aged <3 years and therefore have worse outcomes. Additionally, at the time of the experiment, no well-defined TAs for MB had been described.78
The most recent phase II clinical trial (NCT013261604) (Table 1) for DC therapy published ongoing study results of a significantly increased (P < .001) 12 months progression-free survival population as compared to historical control. However, raw data and elaborate patient data have not yet been published. Additional phase I clinical trials that have been completed (NCT03615404; NCT01171469; NCT00014573) showed safe application of DC therapy for brain tumors in general.
In the beginning of the 21st century, DC therapy was already tested on glioblastoma patients. But even after vaccinating over 1000 patients, results between studies differ drastically. Most find a stimulation of the T cell response and increased survival but two recent clinical trials failed to do so.79 In a glioblastoma review,79 authors elaborate on the hostile TME to DC therapies. Translating this to MB, with a similarly hostile TME, may mean that DC treatment also has to take immunosuppression into account to become more efficient. Additionally, inter-patient variability of the immunogenicity of tumors affect response to DC therapy.44 To overcome these hurdles, several strategies are being developed that exploit optimal DC subtypes, maturation cocktail, delivery, dosage, timing, and combination therapies to improve T cell-driven anticancer immunity.80
NKT Cells, B Cells, and γδ T Cells
NKT cells can be of particular interest in CNS malignancies as the brain is the second-most lipid enriched tissue in the body.81 Moreover, tumor lipid antigens that are presented via CD1d—an MHC-I-like molecule—can only be recognized by NKT cells (Figure 2). Although making up only a slight portion of all immune cells, NKT cells have been proven to kill tumor cells and reactivate exhausted CD8+ T cells and NK cells.82 In a panel of 38 primary MB tumors, 34.2% showed CD1d expression.58 Liu et al.59 found that NKT cells kill CD1d+ MB cells (Daoy) in in vitro cytotoxicity assays. Subsequently, intracranial treatment with ex vivo expanded NKT cells in mice with Daoy xenografts showed significant tumor growth delay. Co-administration of NKT cells with an activating ligand (7DW8-5) resulted in even more tumor regression.59 In contrast to some CAR T cell studies, intravenous injection of the same treatment did not affect tumor growth at all.59 Little research has been conducted on the role of NKT cells in MB, but glioma patient data shows inconclusive results.83 Promising results are seen in an in vivo model study with intracranial injection of NKT cells.84 However, in order to speculate about the implementation of NKT cell therapy in MB treatment plans, more (clinical) research is required. To date, no clinical studies regarding NKT cell treatment in MB patients have been started.
γδ T cells are a subgroup of T cells that produce large quantities of cytokines mostly present in peripheral tissues such as skin, lungs, and intestines.85 Vermeulen et al. (2018) showed low and heterogeneous influx of γδ T cells in MB. However, no other studies regarding the presence of γδ T cells in MB have been reported.
B cells can have multiple roles, both anti- and pro-tumorigenic, in the tumor microenvironment, for example, producing antibodies against TAs or inducing regulatory T cells.85 However, B cells have not received much attention compared to the other cell types described above and no studies have examined the therapeutic role of B cells in MB.85 In Table 2, the current advantages and disadvantages per treatment option have been summarized.
Table 2.
Advantages and disadvantages of cellular immunotherapy strategies for medulloblastoma
| Treatment | Advantages | Disadvantages |
|---|---|---|
| CAR T cell | • Best studied in MB • Specifically targeted • Designable affinity • Prolonged activity |
• TA escape • Off-tumor, on-target effects • Questionable high-dose safety • Expensive • Prone to hostile TME |
| NK cell | • Possible allogeneic therapy • Safe and affordable • CAR NK upgrade possible • Off-the-shelf application |
• Shorter activity • CAR is expensive • Clinical efficacy unclear • Prone to hostile TME |
| DC vaccine | • Polyantigenic therapy • Safe • Prolonged activity • Minimal TA escape |
• Expensive • Clinical efficacy unclear • Prone to hostile TME • Understudied in MB |
| NKT cell | • Promising mouse studies • Interesting because of lipid composition of CNS |
• Understudied in MB • No clinical data |
| γδ T cell | • None yet | • Understudied in MB • No clinical data |
| B cell | • None yet | • Understudied in MB • No clinical data |
Abbreviations: CAR, chimeric antigen receptor; CNS, central nervous system; DC, dendritic cell; MB, medulloblastoma; NK, natural killer; TA, tumor-specific antigen; TME, tumor microenvironment.
Discussion
In cellular immunotherapy for MB, researchers have come up with treatment options that are at the same time maximally efficient and minimally toxic to patients. For efficiency, immune cells need to be attracted to and activated by tumor cells, a process in which the hostile TME of MB plays a crucial—and understudied—role. Simultaneously, toxicity depends on specificity, location, and dosage of the given therapy.
In all MB subgroups, a hypoxic and anti-inflammatory state is induced by the tumor.86,87 However, differences in cytokine composition in the TME have been described depending on subgroups, genetic aberration within subgroups, and state of metastasis.88 Recently, Sreenivasan et al.89 found IL-6 to play an important role in acquiring drug resistance and metastatic potency in Group 3 MB and show a benefit of targeted blocking in vitro. However, in SHH MB, clinical benefit of IL-6 targeting was only found in male mice, suggesting a potential sexually dimorphic role,90 whereas in Group 4 MB, IL-6 did not correlate with drug resistance or metastasis.88 To date, many (clinical) studies are performed without specifying MB subgroup and, as a result, conflicting and non-comparable data could be acquired. Therefore, it will be increasingly important to start researching and treating MB subgroups as biologically distinct entities so that therapies can be tailored towards maximal efficiency.
The different efficacy in CAR T and NKT cell delivery – either intracranial or intravenous—has been noted by several groups in vivo.47,52,59 Intracranial injection was found to be significantly more efficient and presented less systemic side effects but also requires a more invasive approach. On the contrary, intravenous delivery of immune cells is minimally invasive but may lead to suboptimal BBB crossing and higher dosing thereby increasing risk of adverse effects. Whether this difference in delivery method is also visible in other cellular immunotherapies, for example, NK cell and DC is yet to be determined.
Besides a hostile TME and a delivery mechanism trade-off, targeted cellular immunotherapy poses another major challenge that needs to be overcome. TA escape leads to therapy resistance and tumor recurrence after initial response to treatment and is visible in most mouse and clinical studies described.26,49,52,64,70,74,78 A way to minimize TA escape could be combination therapy, combining multiple cellular and/or other immunotherapies. NK cells might be engineered as CAR NK cells to target cancer cells through neoantigens as well as the “induced-self” and “missing-self” mechanisms. Moreover, NK cells are known to promote DC attraction to the TME, possibly increasing the efficacy of DC vaccines.91 Alternatively, the MB field could learn from other combination therapy approaches that have been studied in other CNS tumors. In glioblastoma, a special subset of hematopoietic stem and progenitor cells was used to regain the tumors sensitivity to anti-PD-1 treatment.92
Conclusions
In conclusion, CAR-T cell, NK cell, NKT cell, and DC immunotherapy are effective and relatively safe in in vitro studies and MB mouse models. In (ongoing) MB phase I and II clinical studies, these cellular therapies are safe at low dosage with limited adverse events and show signs of clinical benefit. For MB, phase III clinical studies that specifically examine overall survival and other clinical benefits for these therapies have yet to be started. Intracranial cellular immunotherapy may provide better clinical results compared to intravenous administration and has a lower risk on adverse effects. However, the challenging and unique TME and high treatment resistance potential stand in the way of an efficient MB treatment. By combining individual therapies, such as DC vaccines, (engineered) cytotoxic cells, and immune checkpoint blockades, we believe these challenges can be overcome to produce treatment options for all MB subgroups.
Search Strategy and Selection Criteria
Relevant pre-clinical studies were identified in PubMed and Embase with the search terms “medulloblastoma” and specific terms for Mesh or title/abstract screening (eg, “medulloblast*”, “medulloblastoma[tiab]” in combination with “immunotherapy”, “immunotherapy[tiab]” “cellular*”, “cellular therapy’’ or specific cell therapies such as “CAR”, “NK”, “NKT”, “Dendritic*”, “Vaccin*” and “gamma delta T cells”. Relevant clinical studies were identified by searching using these same terms on ClinicalTrials.gov. No date restrictions were applied to either search; the last search of both databases was done on the 2nd of June, 2022. Only articles in English were reviewed. Publications corresponding to trials included in this Review were identified via search of National Clinical Trial numbers on PubMed. The final reference list for the pre-clinical studies was generated on the basis cellular injection therapy only, and relevance to medulloblastoma, mechanisms of targeting, and therapeutic avenues. The final reference list for the clinical studies was based on all current trials of cellular immunotherapy for medulloblastoma.
Contributor Information
Michael Y Schakelaar, Department of Pathology, University Medical Center Utrecht, 3584 CX Utrecht, The Netherlands.
Matthijs Monnikhof, Department of Pathology, University Medical Center Utrecht, 3584 CX Utrecht, The Netherlands.
Sandra Crnko, Department of Pathology, University Medical Center Utrecht, 3584 CX Utrecht, The Netherlands; Bachelor Research Hub, Education Center, University Medical Centre Utrecht, 3584 CX Utrecht, The Netherlands.
Emma W Pijnappel, Department of Pathology, University Medical Center Utrecht, 3584 CX Utrecht, The Netherlands; Bachelor Research Hub, Education Center, University Medical Centre Utrecht, 3584 CX Utrecht, The Netherlands.
Jan Meeldijk, Department of Pathology, University Medical Center Utrecht, 3584 CX Utrecht, The Netherlands; Center for Translational Immunology, University Medical Center Utrecht, 3584 CX Utrecht, The Netherlands; Bachelor Research Hub, Education Center, University Medical Centre Utrecht, 3584 CX Utrecht, The Netherlands.
Toine ten Broeke, Department of Pathology, University Medical Center Utrecht, 3584 CX Utrecht, The Netherlands; Bachelor Research Hub, Education Center, University Medical Centre Utrecht, 3584 CX Utrecht, The Netherlands.
Niels Bovenschen, Department of Pathology, University Medical Center Utrecht, 3584 CX Utrecht, The Netherlands; Center for Translational Immunology, University Medical Center Utrecht, 3584 CX Utrecht, The Netherlands; Bachelor Research Hub, Education Center, University Medical Centre Utrecht, 3584 CX Utrecht, The Netherlands.
Funding
This work was supported by grants from Cancer Foundation Koppie-Au and Foundation Team Doelbewust (grant no. 1522407). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Author Contributions
Conceptualization, M.Y.S., M.M., T.B., and N.B.; writing—original draft preparation, M.Y.S. and M.M.; writing—review and editing, M.Y.S., M.M., S.C., E.W.P., J.M., T.B., and N.B.; supervision, N.B. All authors have read and agreed to the published version of the manuscript.
References
- 1. Northcott PA, Robinson GW, Kratz CP, et al. Medulloblastoma. Nat Rev Dis Prim. 2019;5(1):1–20. [DOI] [PubMed] [Google Scholar]
- 2. Ostrom QT, Gittleman H, Truitt G, Boscia A, Kruchko C, Barnholtz-Sloan JS. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2011–2015. Neuro Oncol. 2018;20(suppl_4):iv1–iv86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Giordana MT, Schiffer P, Lanotte M, Girardi P, Chio A. Epidemiology of adult medulloblastoma. Int J Cancer. 1999;80(5):689–692. [DOI] [PubMed] [Google Scholar]
- 4. Voskamp MJ, Li S, van Daalen KR, et al. Immunotherapy in medulloblastoma: current state of research, challenges, and future perspectives. Cancers (Basel). 2021;13(21):1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ivanov DP, Coyle B, Walker DA, Grabowska AM. In vitro models of medulloblastoma: choosing the right tool for the job. J Biotechnol. 2016;236:10–25. [DOI] [PubMed] [Google Scholar]
- 6. Northcott PA, Buchhalter I, Morrissy AS, et al. The whole-genome landscape of medulloblastoma subtypes. Nature. 2017;547(7663):311–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cavalli FMG, Remke M, Rampasek L, et al. Intertumoral heterogeneity within medulloblastoma subgroups. Cancer Cell. 2017;31(6):737–754.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhukova N, Ramaswamy V, Remke M, et al. Subgroup-specific prognostic implications of TP53 mutation in medulloblastoma. J Clin Oncol. 2013;31(23):2927–2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Northcott PA, Dubuc AM, Pfister S, Taylor MD. Molecular subgroups of medulloblastoma. Expert Rev Neurother. 2012;12(7):871–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Juraschka K, Taylor MD. Medulloblastoma in the age of molecular subgroups: a review. J Neurosurg Pediatr. 2019;24(4):353–363. [DOI] [PubMed] [Google Scholar]
- 11. Thompson EM, Hielscher T, Bouffet E, et al. Prognostic value of medulloblastoma extent of resection after accounting for molecular subgroup: a retrospective integrated clinical and molecular analysis. Lancet Oncol. 2016;17(4):484–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gajjar A, Chintagumpala M, Ashley D, et al. Risk-adapted craniospinal radiotherapy followed by high-dose chemotherapy and stem-cell rescue in children with newly diagnosed medulloblastoma (St Jude Medulloblastoma-96): long-term results from a prospective, multicentre trial. Lancet Oncol. 2006;7(10):813–820. [DOI] [PubMed] [Google Scholar]
- 13. Michalski JM, Janss A, Vezina G, et al. Results of COG ACNS0331: a phase III trial of Involved-Field Radiotherapy (IFRT) and Low Dose Craniospinal Irradiation (LD-CSI) with chemotherapy in average-risk medulloblastoma: a report from the Children’s Oncology Group. Int J Radiat Oncol. 2016;96(5):937–938. [Google Scholar]
- 14. Gupta T, Pervez S, Dasgupta A, et al. Omission of upfront craniospinal irradiation in patients with low-risk WNT-pathway medulloblastoma is associated with unacceptably high risk of neuraxial failure. Clin Cancer Res. 2022;28(19):OF1–OF6. [DOI] [PubMed] [Google Scholar]
- 15. Baroni LV, Sampor C, Gonzalez A, et al. Bridging the treatment gap in infant medulloblastoma: molecularly informed outcomes of a globally feasible regimen. Neuro Oncol. 2020;22(12):1873–1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kennedy LB, Salama AKS. A review of cancer immunotherapy toxicity. CA Cancer J Clin. 2020;70(2):86–104. [DOI] [PubMed] [Google Scholar]
- 17. Riley RS, June CH, Langer R, Mitchell MJ. Delivery technologies for cancer immunotherapy. Nat Rev Drug Discov. 2019;18(3):175–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chow VA, Gopal AK, Maloney DG, et al. Outcomes of patients with large B-cell lymphomas and progressive disease following CD19-specific CAR T-cell therapy. Am J Hematol. 2019;94(8):e209–e213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vermeulen JF, Van Hecke W, Adriaansen EJM, et al. Prognostic relevance of tumor-infiltrating lymphocytes and immune checkpoints in pediatric medulloblastoma. Oncoimmunology. 2018;7(3):e1398877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Riemondy KA, Venkataraman S, Willard N, et al. Neoplastic and immune single-cell transcriptomics define subgroup-specific intra-tumoral heterogeneity of childhood medulloblastoma. Neuro Oncol. 2022;24(2):273–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bockmayr M, Mohme M, Klauschen F, et al. Subgroup-specific immune and stromal microenvironment in medulloblastoma. Oncoimmunology. 2018;7(9):e1462430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fernández L, Portugal R, Valentín J, et al. In vitro natural killer cell immunotherapy for medulloblastoma. Front Oncol. 2013;3(94):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tan IL, Arifa RDN, Rallapalli H, et al. CSF1R inhibition depletes tumor-associated macrophages and attenuates tumor progression in a mouse sonic Hedgehog-Medulloblastoma model. Oncogene. 2021;40(2):396–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Audi ZF, Saker Z, Rizk M, et al. Immunosuppression in medulloblastoma: insights into cancer immunity and immunotherapy. Curr Treat Options Oncol. 2021;22(9):1–28. [DOI] [PubMed] [Google Scholar]
- 25. Powell AB, Yadavilli S, Saunders D, et al. Medulloblastoma rendered susceptible to NK-cell attack by TGFβ neutralization. J Transl Med. 2019;17(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Vitanza NA, Johnson AJ, Wilson AL, et al. Locoregional infusion of HER2-specific CAR T cells in children and young adults with recurrent or refractory CNS tumors: an interim analysis. Nat Med. 2021;27(9):1544–1552. [DOI] [PubMed] [Google Scholar]
- 27. Robert C. A decade of immune-checkpoint inhibitors in cancer therapy. Nat Commun. 2020;11(1):1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bouchkouj N, Kasamon YL, de Claro RA, et al. FDA approval summary: axicabtagene ciloleucel for relapsed or refractory large B-cell lymphoma. Clin Cancer Res. 2019;25(6):1702–1708. [DOI] [PubMed] [Google Scholar]
- 29. O’Leary MC, Lu X, Huang Y, et al. FDA approval summary: tisagenlecleucel for treatment of patients with relapsed or refractory B-cell precursor acute lymphoblastic leukemia. Clin Cancer Res. 2019;25(4):1142–1146. [DOI] [PubMed] [Google Scholar]
- 30. Kuwana Y, Asakura Y, Utsunomiya N, et al. Expression of chimeric receptor composed of immunoglobulin-derived V resions and T-cell receptor-derived C regions. Biochem Biophys Res Commun. 1987;149(3):960–968. [DOI] [PubMed] [Google Scholar]
- 31. Hong M, Clubb JD, Chen YY. Engineering CAR-T cells for next-generation cancer therapy. Cancer Cell. 2020;38(4):473–488. [DOI] [PubMed] [Google Scholar]
- 32. Rafiq S, Hackett CS, Brentjens RJ. Engineering strategies to overcome the current roadblocks in CAR T cell therapy. Nat Rev Clin Oncol. 2020;17(3):147–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sterner RC, Sterner RM. CAR-T cell therapy: current limitations and potential strategies. Blood Cancer J. 2021;11(4):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Porter DL, Hwang WT, Frey NV, et al. Chimeric antigen receptor T cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukemia. Sci Transl Med. 2015;7(303):303ra139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hawkins ER, D’Souza RR, Klampatsa A. Armored CAR T-cells: the next chapter in T-cell cancer immunotherapy. Biol Targets Ther. 2021;15:95–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Levine BL, Miskin J, Wonnacott K, Keir C. Global manufacturing of CAR T cell therapy. Mol Ther Methods Clin Dev. 2017;4:92–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Myers JA, Miller JS. Exploring the NK cell platform for cancer immunotherapy. Nat Rev Clin Oncol. 2021;18(2):85–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bryceson YT, March ME, Ljunggren HG, Long EO. Activation, coactivation, and costimulation of resting human natural killer cells. Immunol Rev. 2006;214(1):73–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Delconte RB, Kolesnik TB, Dagley LF, et al. CIS is a potent checkpoint in NK cell-mediated tumor immunity. Nat Immunol. 2016;17(7):816–824. [DOI] [PubMed] [Google Scholar]
- 40. Molgora M, Bonavita E, Ponzetta A, et al. IL-1R8 is a checkpoint in NK cells regulating anti-tumour and anti-viral activity. Nature. 2017;551(7678):110–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sarhan D, Hippen KL, Lemire A, et al. Adaptive NK cells resist regulatory T-cell suppression driven by IL37. Cancer Immunol Res. 2018;6(7):766–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Godfrey DI, MacDonald HR, Kronenberg M, Smyth MJ, Kaer LV. NKT cells: what’s in a name? Nat Rev Immunol. 2004;4(3):231–237. [DOI] [PubMed] [Google Scholar]
- 43. Godfrey DI, Le Nours J, Andrews DM, Uldrich AP, Rossjohn J. Unconventional T cell targets for cancer immunotherapy. Immunity. 2018;48(3):453–473. [DOI] [PubMed] [Google Scholar]
- 44. Perez CR, De Palma M. Engineering dendritic cell vaccines to improve cancer immunotherapy. Nat Commun. 2019;10(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Yu J, Sun H, Cao W, Song Y, Jiang Z. Research progress on dendritic cell vaccines in cancer immunotherapy. Exp Hematol Oncol. 2022;11(1):1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Harari A, Graciotti M, Bassani-Sternberg M, Kandalaft LE. Antitumour dendritic cell vaccination in a priming and boosting approach. Nat Rev Drug Discov. 2020;19(9):635–652. [DOI] [PubMed] [Google Scholar]
- 47. Nellan A, Rota C, Majzner R, et al. Durable regression of Medulloblastoma after regional and intravenous delivery of anti-HER2 chimeric antigen receptor T cells. J ImmunoTher Cancer. 2018;6(1):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Varlet P, Bouffet E, Casanova M, et al. Comprehensive analysis of the ErbB receptor family in pediatric nervous system tumors and rhabdomyosarcoma. Pediatr Blood Cancer. 2022;69(1):1–16. [DOI] [PubMed] [Google Scholar]
- 49. Ahmed N, Ratnayake M, Savoldo B, et al. Regression of experimental medulloblastoma following transfer of HER2-Specific T cells. Cancer Res. 2007;67(12):5957–5964. [DOI] [PubMed] [Google Scholar]
- 50. Morgan RA, Yang JC, Kitano M, et al. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol Ther. 2010;18(4):843–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Uhlén M, Fagerberg L, Hallström BM, et al. Tissue-based map of the human proteome. Science. 2015;347(6220):1260419. [DOI] [PubMed] [Google Scholar]
- 52. Donovan LK, Delaidelli A, Joseph SK, et al. Locoregional delivery of CAR T cells to the cerebrospinal fluid for treatment of metastatic medulloblastoma and ependymoma. Nat Med. 2020;26(5):720–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Griffioen M, Kessler JH, Borghi M, et al. Detection and functional analysis of CD8 + T cells specific for PRAME: a target for T-cell therapy. Clin Cancer Res. 2006;12(10):3130–3136. [DOI] [PubMed] [Google Scholar]
- 54. Orlando D, Miele E, De Angelis B, et al. Adoptive immunotherapy using PRAME-specific T cells in medulloblastoma. Cancer Res. 2018;78(12):3337–3349. [DOI] [PubMed] [Google Scholar]
- 55. Castriconi R, Dondero A, Negri F, et al. Both CD133+ and CD133− medulloblastoma cell lines express ligands for triggering NK receptors and are susceptible to NK-mediated cytotoxicity. Eur J Immunol. 2007;37(11):3190–3196. [DOI] [PubMed] [Google Scholar]
- 56. Dai H, Sun B, Yang D, et al. Eradication of medulloblastoma by NKG2D-specific CAR T-cells. J Clin Oncol. 2020;38(15_suppl):2522–2522. [Google Scholar]
- 57. Wu SY, Fu T, Jiang YZ, Shao ZM. Natural killer cells in cancer biology and therapy. Mol Cancer. 2020;19(1):1–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Teo WY, Elghetany MT, Shen J, et al. Therapeutic implications of CD1d expression and tumor-infiltrating macrophages in pediatric medulloblastomas. J Neurooncol. 2014;120(2):293–301. [DOI] [PubMed] [Google Scholar]
- 59. Liu D, Song L, Brawley VS, et al. Medulloblastoma expresses CD1d and can be targeted for immunotherapy with NKT cells. Clin Immunol. 2013;149(1):55–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Lasky JL, Bradford KL, Wang Y, Pak Y, Panosyan EH. Chemotherapy can synergize with adoptive immunotherapy to inhibit medulloblastoma growth. Anticancer Res. 2022;42(4):1697–1706. [DOI] [PubMed] [Google Scholar]
- 61. Majzner RG, Theruvath JL, Nellan A, et al. CAR T cells targeting B7-H3, a pan-cancer antigen, demonstrate potent preclinical activity against pediatric solid tumors and brain tumors. Clin Cancer Res. 2019;25(8):2560–2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Li S, Poolen GC, van Vliet LC, et al. Pediatric medulloblastoma express immune checkpoint B7-H3. Clin Transl Oncol. 2022;24(6):1204–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Epping MT, Bernards R. A causal role for the human tumor antigen preferentially expressed antigen of melanoma in cancer. Cancer Res. 2006;66(22):10639–10642. [DOI] [PubMed] [Google Scholar]
- 64. Xu S, Tang L, Li X, Fan F, Liu Z. Immunotherapy for glioma: current management and future application. Cancer Lett. 2020;476:1–12. [DOI] [PubMed] [Google Scholar]
- 65. Bielamowicz K, Fousek K, Byrd TT, et al. Trivalent CAR T cells overcome interpatient antigenic variability in glioblastoma. Neuro Oncol. 2018;20(4):506–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Hegde M, Mukherjee M, Grada Z, et al. Tandem CAR T cells targeting HER2 and IL13Rα2 mitigate tumor antigen escape. J Clin Invest. 2016;126(8):3036–3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Pérez-Martínez A, Fernández L, Díaz MA. The therapeutic potential of natural killer cells to target medulloblastoma. Expert Rev Anticancer Ther. 2016;16(6):573–576. [DOI] [PubMed] [Google Scholar]
- 68. Liang KH, Chang CC, Wu KS, et al. Notch signaling and natural killer cell infiltration in tumor tissues underlie medulloblastoma prognosis. Sci Rep. 2021;11(1):23282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Kennis BA, Michel KA, Brugmann WB, et al. Monitoring of intracerebellarly-administered natural killer cells with fluorine-19 MRI. J Neurooncol. 2019;142(3):395–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Khatua S, Cooper LJN, Sandberg DI, et al. Phase I study of intraventricular infusions of autologous ex vivo expanded NK cells in children with recurrent medulloblastoma and ependymoma. Neuro Oncol. 2020;22(8):1214–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Singh AK, McGuirk JP. CAR T cells: continuation in a revolution of immunotherapy. Lancet Oncol. 2020;21(3):e168–e178. [DOI] [PubMed] [Google Scholar]
- 72. Olson JA, Leveson-Gower DB, Gill S, et al. NK cells mediate reduction of GVHD by inhibiting activated, alloreactive T cells while retaining GVT effects. Blood. 2010;115(21):4293–4301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Pan C, Zhai Y, Li G, Jiang T, Zhang W. NK cell-based immunotherapy and therapeutic perspective in gliomas. Front Oncol. 2021;11(751183):1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Flores C, Wildes T, Dean BD, et al. Massive clonal expansion of medulloblastoma-specific T cells during adoptive cellular therapy. Sci Adv. 2019;5(11):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Anguille S, Smits EL, Lion E, van Tendeloo VF, Berneman ZN. Clinical use of dendritic cells for cancer therapy. Lancet Oncol. 2014;15(7):e257–e267. [DOI] [PubMed] [Google Scholar]
- 76. Blaeschke F, Paul MC, Schuhmann MU, et al. Low mutational load in pediatric medulloblastoma still translates into neoantigens as targets for specific T-cell immunotherapy. Cytotherapy. 2019;21(9):973–986. [DOI] [PubMed] [Google Scholar]
- 77. Rivero-Hinojosa S, Grant M, Panigrahi A, et al. Proteogenomic discovery of neoantigens facilitates personalized multi-antigen targeted T cell immunotherapy for brain tumors. Nat Commun. 2021;12(1):6689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Ardon H, De Vleeschouwer S, Van Calenbergh F, et al. Adjuvant dendritic cell-based tumour vaccination for children with malignant brain tumours. Pediatr Blood Cancer. 2010;54(4):519–525. [DOI] [PubMed] [Google Scholar]
- 79. Datsi A, Sorg RV. Dendritic cell vaccination of glioblastoma: road to success or dead end. Front Immunol. 2021;12(770390):1–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Garg AD, Coulie PG, Van den Eynde BJ, Agostinis P. Integrating next-generation dendritic cell vaccines into the current cancer immunotherapy landscape. Trends Immunol. 2017;38(8):577–593. [DOI] [PubMed] [Google Scholar]
- 81. Hussain G, Wang J, Rasul A, et al. Role of cholesterol and sphingolipids in brain development and neurological diseases. Lipids Health Dis. 2019;18(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Bae EA, Seo H, Kim IK, Jeon I, Kang CY. Roles of NKT cells in cancer immunotherapy. Arch Pharm Res. 2019;42(7):543–548. [DOI] [PubMed] [Google Scholar]
- 83. Brettschneider EES, Terabe M. The role of NKT cells in glioblastoma. Cells. 2021;10(7):1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Hara A, Koyama-Nasu R, Takami M, et al. CD1d expression in glioblastoma is a promising target for NKT cell-based cancer immunotherapy. Cancer Immunol Immunother. 2021;70(5):1239–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Diao S, Gu C, Zhang H, Yu C. Immune cell infiltration and cytokine secretion analysis reveal a non-inflammatory microenvironment of medulloblastoma. Oncol Lett. 2020;20(6):1–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Reichl B, Niederstaetter L, Boegl T, et al. Determination of a tumor-promoting microenvironment in recurrent medulloblastoma: a multi-omics study of cerebrospinal fluid. Cancers (Basel). 2020;12(6):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Lee B, Mahmud I, Pokhrel R, et al. Medulloblastoma cerebrospinal fluid reveals metabolites and lipids indicative of hypoxia and cancer-specific RNAs. Acta Neuropathol Commun. 2022;10(1):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Low SYY, Bte Syed Sulaiman N, Tan EEK, et al. Cerebrospinal fluid cytokines in metastatic group 3 and 4 medulloblastoma. BMC Cancer. 2020;20(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Sreenivasan L, Wang H, Yap SQ, et al. Autocrine IL-6/STAT3 signaling aids development of acquired drug resistance in Group 3 medulloblastoma. Cell Death Dis. 2020;11(12):1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. White CL, Jayasekara WSN, Picard D, et al. A sexually dimorphic role for STAT3 in sonic hedgehog medulloblastoma. Cancers (Basel). 2019;11(11):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Böttcher JP, Bonavita E, Chakravarty P, et al. NK cells stimulate recruitment of cDC1 into the tumor microenvironment promoting cancer immune control. Cell. 2018;172(5):1022–1037.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Flores CT, Wildes TJ, Drake JA, et al. Lin−CCR2+ hematopoietic stem and progenitor cells overcome resistance to PD-1 blockade. Nat Commun. 2018;9(1):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]