Abstract
Coronavirus SARS-CoV-2, a causative agent for the global epidemic disease COVID-19, which has a highest modality rate. Several initiatives have been undertaken to repurpose current antiviral medications and tested the classic pyridine derivatives (PyDev), which have showed substantial therapeutic potential against a variety of illnesses and also have several biological functions such as, antibacterial, antiviral, and anti-inflammatory. However, limited reports are available for the treatment of Coronavirus SARS-CoV-2 using PyDev. Hence, the possibilities of the best-described PyDev molecules of powerful Coronavirus SARS-CoV-2 inhibitors have been attempted in this investigation. This study primarily focused on blocking four key targets of Coronavirus SARS-CoV-2 proteins. Terpyridine has shown the greatest inhibitory potential (with a binding energy of −8.8 kcal/mol) against all four coronavirus targets. This study results would pave the potential lead medication for Coronavirus SARS-CoV-2 therapeutic strategies.
Keywords: Coronavirus SARS-CoV-2, pyridine derivatives, molecular docking
Graphical Abstract
Introduction
The severe acute respiratory syndrome coronavirus 2 causes the COVID-19 coronavirus pandemic (SARS-CoV-2). In December 2019, coronavirus pathogen was reported at Wuhan, China, after the outbreak of acute pneumonia from an unknown source was spread all over the city. Because of its unusual genetic profile, it is resistant to current medical therapies, necessitating the hunt for new targets for vaccine development and medications to effectively prevent and cure nCOVID’19. This has resulted in a massive amount of nCOVID’19 genomic data being made public (https://www.ncbi.nlm.nih.gov/genbank/sars-cov-2-seqs/).1–8
Heterocycle molecules gained much importance in the medicinal chemistry; hence, they are widely investigated and used in many medications.9-11 Pyridine is a six-membered moiety among heterocyclic compounds which possess vast applicability in pharmaceuticals and agrochemicals.12,13 There is a clear medical need to create novel antiviral medicines, especially with the rise in medication resistance. Antimicrobial, antiviral, antioxidant, anti-diabetic, anti-cancer, anti-malarial, analgesic, and anti-inflammatory effects are all demonstrated by various derivatives of the pyridine scaffold. They also have psychopharmacological, antagonistic, anti-amoebic, and anti-thrombotic properties.14-17
These chemicals have shown good antiviral activity against a number of viruses, including HIV, hepatitis C, hepatitis B, respiratory syncytial virus (RSV), and cytomegalovirus, (CMV) according to current study. These derivatives hindered viral assessment by inhibiting RT, polymerase, suppression of viral thymidine kinase, RNase H activity, maturation, GAK (Cyclin G-associated kinase) inhibition, AAK1 (Adaptor-Associated Kinase 1) inhibition, inhibition of HDAC6, inhibition of post-integrational event, DNA and RNA replication, CCR5 antagonistic activity, cellulase inhibition, gene expression down regulation, cellular NF-jB signaling pathway and neuraminidase (NA) inhibition, protein synthesis inhibition, and generally inhibition of viral replication cycle.18,19
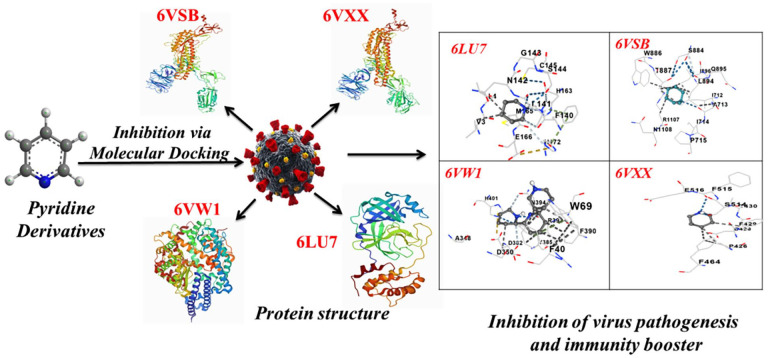
To find better therapeutic agents for the therapies of COVID-19 disease, we here include four different coronavirus targets. Even though various studies have been published that explain the inhibitory activity of natural products versus Coronavirus protein, this investigation is the first to determine the inhibitory potential of pyridine derivatives (PyDev) against these four critical coronavirus targets, which could pave the way for COVID-19 medication discovery.
Materials and Methods
The 3D structures of the molecular targets (Table 1), including 6VSB Prefusion 2019-nCoV spike glycoprotein,20,21 6LU7 COVID-19 3clpro/Mpro, 22 6VXX SARS-CoV-2 spike glycoprotein, 23 and 6VW1 SARS-CoV-2 chimeric receptor-binding domain, 24 was obtained from the RCSB protein data bank (https://www.rcsb.org), which are attributed for the COVID-19 disease progression. The ongoing research comprised of two medications (remdesivir and hydroxychloroquine) 25 as well as PyDev for molecular docking. For the Density Functional Theory (DFT) optimization of small molecules, software with the B3LYP/6-31G(d) basic functional set was employed. 26 The water molecules and native ligands bound to the target, as well as other heteroatoms that hampered the simulation, were eliminated to develop the ligands for the molecular docking investigation. The docking steps were then completed using the AutoDock Vina docking 27 and PatchDock 28 online docking software. AutoDock Vina software was utilized in all the docking experiments, with the optimized model as the docking target. Potential antiviral drugs were shown by the effective relationship between molecules and receptor targets. Grid box (126 Å × 126 Å × 126 Å) centered at (152.179, 167.664, 166.985) Å.
Table 1.
List of coronavirus disease targets, 3D structures, and its role in disease formation.
| Target | 6VSB Prefusion 2019-nCoV spike glycoprotein | 6VW1 SARS-CoV-2 chimeric receptor-binding domain complexed with its receptor human ACE2 | 6VXX SARS-CoV-2 spike glycoprotein | 6LU7 COVID-19 3clpro/Mpro |
| 3D structure |

|
|

|

|
| Role in Coronavirus | To achieve access to host cells, 2019-nCoV uses a highly glycosylated spike (S) protein. | SARS-CoV-2 receptor-binding domain (RBD) in combination with hACE2 (designed to enable crystallization). This research can help with intervention techniques that target SARS-CoV-2 receptor identification. | The receptor binding spheres for SARS-CoV S and that of SARS-CoV-2S are having similar nature as with human ACE2. Since, SARS-CoV-2S should enter ACE2, this indicates that SARS-CoV-2 would spreads progressively among humans. | COVID-19 virus Mpro’s substrate-binding pocket, which itself is functionally important across all CoVMpros. |
Drug-likeliness filters
We have selected the following specific filters which includes Lipinski (Pfizer) filter, Ghose filter, Veber filter, Egan (Pharmacia) filter, Muegge (Bayer) filter for the elucidation of drug-likeliness criteria of PyDev (http://www.swissadme.ch/).
ADMET properties
admetSAR was used to investigate the PyDev physicochemical and ADMET characteristics (http://lmmd.ecust.edu.cn/admetsar1). Using this, we have studied several factors such as Ames test, human intestinal absorption, blood-brain barrier, and Caco-2 cell permeability.
Results and discussion
Screening of PyDev for docking
The promising PyDev that have shown significant inhibitory potential against a variety of viral diseases such as hepatitis and HIV by inhibiting the functions of key enzymes includes DNA polymerase, reverse transcriptase, and protease inhibition, among others, to find a prevalent lead pyridine compound for COVID-19 rehabilitation. Figure 1 showed the 3D structures of 12 PyDev, which was used for the analysis of inhibitory activity against four different coronavirus targets. Figure 2 showed that the 3D structure of 2 positive drugs (recently identified for the treatment of COVID-19) remdesivir and hydroxychloroquine.
Figure 1.
Optimized structures of PyDev using DFT method. PyDev indicates pyridine derivatives.
Figure 2.

Structure of positive reference drugs: (A) remdesivir and (B) hydroxychloroquine.
Drug-likeliness criteria for PyDev
An additional pharmacokinetic analysis was performed over the 12 compounds: Pyridine, 4-Aminopyridine, Isonicotinic acid, Pyridinyl imidazole, 2,2′-Bipyridine, 4,4′-diamino-2,2′-bipyridine, 2 2′-bipyridine-4 4′-dicarboxylic acid, 4,4′-Bis(imidazolyl)-2,2′-bipyridine, 2,2′:6′,2″-terpyridine, 4′-Amino-2,2′:6′,2″-terpyridine, [2,2′:6′,2″-Terpyridine]-4′-carboxylic acid, and [2,2′:6′,2″-Terpyri-dine]-4′-imidazole (Tables 2 to 4). Among the 12 compounds, benzimidazole was showing excellent activity and determined to be in violation of five of the six criteria, indicating that the medicine would pave new strategy improvement in terms of drug delivery systems.
Table 2.
Analysis of Lipinski’s Ro5 of the PyDev.
| S. No. | Compound | Molecular weight (⩽500 g/mol) | Hydrogen bond acceptors (⩽10) | Hydrogen bond donors (⩽5) | Topological polar surface area (⩽140 Å) | Rotatable bonds (<10) | Log P (⩽5) |
|---|---|---|---|---|---|---|---|
| 1 | Pyridine | 79.04 | 1 | 0 | 12.89 | 0 | 0.658 |
| 2 | 4-Aminopyridine | 94.05 | 2 | 2 | 39.64 | 0 | –2.397 |
| 3 | Isonicotinic acid | 123.03 | 3 | 1 | 50.19 | 1 | 0.621 |
| 4 | Pyridinyl imidazole | 145.06 | 3 | 1 | 41.57 | 1 | 0.743 |
| 5 | 2,2′-Bipyridine | 156.07 | 2 | 0 | 25.78 | 1 | 1.521 |
| 6 | 4,4′-diamino-2,2′-bipyridine | 186.09 | 4 | 4 | 79.28 | 1 | –2.618 |
| 7 | 2 2′-bipyridine-4 4′-dicarboxylic acid | 244.05 | 6 | 2 | 100.38 | 3 | 1.697 |
| 8 | 4,4′-Bis(imidazolyl)-2,2′-bipyridine | 288.11 | 6 | 2 | 83.14 | 3 | 1.646 |
| 9 | 2,2′:6′,2″-terpyridine | 233.1 | 3 | 0 | 38.67 | 2 | 2.48 |
| 10 | 4′-Amino-2,2′:6′,2″-terpyridine | 248.11 | 4 | 2 | 65.42 | 2 | 1.392 |
| 11 | [2,2′:6′,2″-Terpyridine]-4′-carboxylic acid | 277.09 | 5 | 1 | 75.97 | 3 | 2.756 |
| 12 | [2,2′:6′,2″-Terpyridine]-4′-imidazole | 299.12 | 5 | 1 | 67.35 | 3 | 2.386 |
Abbreviation: PyDev, pyridine derivatives.
Table 4.
Selected pharmacokinetic parameters after ADME/T prediction.
| Compound | Human intestinal absorption | Caco-2 permeability | Blood-brain barrier penetration | Plasma protein binding | Volume distribution | CYP1A2-inhibitor | CYP1A2-substrate | CYP2C19-inhibitor | CYP2C19-substrate | CYP2 C9-inhibitor | CYP2 C9-substrate | CYP2D6-inhibitor | CYP2D6-substrate | CYP3A4-inhibitor | CYP3A4-substrate | Clearance | Human hepatotoxicity | Drug-induced liver injury | AMES toxicity |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 0.008 | –3.931 | 0.651 | 16.65% | 1.782 | 0.508 | 0.508 | 0.158 | 0.698 | 0.025 | 0.163 | 0.059 | 0.306 | 0.046 | 0.302 | 10.046 | 0.053 | 0.748 | 0.069 |
| 2 | 0.204 | –4.694 | 0.747 | 12.19% | 1.263 | 0.427 | 0.415 | 0.657 | 0.129 | 0.054 | 0.183 | 0.89 | 0.227 | 0.053 | 0.177 | 2.113 | 0.407 | 0.827 | 0.021 |
| 3 | 0.023 | –5.371 | 0.198 | 16.21% | 0.444 | 0.034 | 0.413 | 0.06 | 0.051 | 0.043 | 0.186 | 0.028 | 0.126 | 0.024 | 0.156 | 3.182 | 0.237 | 0.974 | 0.026 |
| 4 | 0.009 | –4.233 | 0.936 | 54.31% | 1.732 | 0.965 | 0.807 | 0.067 | 0.171 | 0.012 | 0.619 | 0.009 | 0.122 | 0.009 | 0.244 | 9.958 | 0.821 | 0.959 | 0.263 |
| 5 | 0.006 | –4.252 | 0.877 | 81.73% | 1.988 | 0.886 | 0.288 | 0.104 | 0.218 | 0.015 | 0.404 | 0.003 | 0.501 | 0.007 | 0.217 | 3.113 | 0.253 | 0.949 | 0.683 |
| 6 | 0.645 | –5.105 | 0.185 | 24.64% | 0.876 | 0.273 | 0.683 | 0.337 | 0.056 | 0.019 | 0.228 | 0.918 | 0.899 | 0.006 | 0.076 | 1.394 | 0.809 | 0.725 | 0.422 |
| 7 | 0.01 | –5.536 | 0.119 | 44.61% | 0.319 | 0.023 | 0.056 | 0.032 | 0.03 | 0.007 | 0.048 | 0.026 | 0.051 | 0.022 | 0.015 | 0.869 | 0.303 | 0.988 | 0.017 |
| 8 | 0.009 | –4.767 | 0.951 | 86.96% | 2.41 | 0.993 | 0.787 | 0.282 | 0.058 | 0.021 | 0.761 | 0.027 | 0.055 | 0.105 | 0.194 | 6.349 | 0.882 | 0.985 | 0.207 |
| 9 | 0.004 | –4.525 | 0.953 | 95.16% | 2.278 | 0.98 | 0.209 | 0.164 | 0.068 | 0.031 | 0.411 | 0.003 | 0.333 | 0.013 | 0.161 | 2.989 | 0.48 | 0.944 | 0.822 |
| 10 | 0.032 | –4.605 | 0.657 | 67.86% | 1.509 | 0.94 | 0.43 | 0.178 | 0.058 | 0.017 | 0.243 | 0.811 | 0.868 | 0.035 | 0.133 | 1.139 | 0.868 | 0.958 | 0.816 |
| 11 | 0.005 | –4.578 | 0.273 | 93.22% | 0.537 | 0.059 | 0.068 | 0.021 | 0.046 | 0.012 | 0.09 | 0.006 | 0.086 | 0.006 | 0.062 | 0.79 | 0.722 | 0.987 | 0.078 |
| 12 | 0.005 | –4.649 | 0.969 | 94.10% | 2.82 | 0.991 | 0.507 | 0.354 | 0.063 | 0.037 | 0.751 | 0.092 | 0.101 | 0.67 | 0.209 | 6.675 | 0.764 | 0.979 | 0.448 |
The drug-likeliness criteria of PyDev were assessed using five different filters (Lipinski, Ghose, Veber, Muegge, and Egan Filter). Apigenin has complied with all drug-likeliness criteria (Tables 2 and 3).
Table 3.
Screening of PyDev using several drug-likeliness filters.
| Compound | Canonical smile | Lipinski filter | Ghose filter | Veber filter | Egan filter | Muegge filter | Bio availability score |
|---|---|---|---|---|---|---|---|
| Pyridine | c1ccncc1 | Yes | No; 3 violations | Yes | Yes | No; 2 violations | 0.55 |
| 4-Aminopyridine | N=c1cc[nH]cc1 | Yes | No; 3 violations | Yes | Yes | No; 1 violations | 0.55 |
| Isonicotinic acid | O=C(O)c1ccncc1 | Yes | No; 3 violations | Yes | Yes | No; 1 violations | 0.85 |
| Pyridinyl imidazole | c1ccc(-c2ncc[nH]2)nc1 | Yes | No; 2 violations | Yes | Yes | No; 1 violations | 0.55 |
| 2,2′-Bipyridine | c1ccc(-c2ccccn2)nc1 | Yes | No; 1 violations | Yes | Yes | No; 1 violations | 0.55 |
| 4,4′-diamino-2,2′-bipyridine | N=c1cc[nH]c,-c2cc(=N,cc[nH]2)c1 | Yes | Yes | Yes | Yes | No;1 violations | 0.55 |
| 2 2′-bipyridine-4 4′-dicarboxylic acid | O=C,O)c1ccnc(-c2cc[(= O,O]ccn2)c1 | Yes | Yes | Yes | Yes | Yes | 0.56 |
| 4,4′-Bis(imidazolyl)-2,2′-bipyridine | c1cc(-c2ncc[nH]2)cc(-c2cc(-c3ncc[nH]3)ccn2)n1 | Yes | Yes | Yes | Yes | Yes | 0.55 |
| 2,2′:6′,2″-terpyridine | c1ccc(-c2cccc(-c3ccccn3)n2)nc1 | Yes | Yes | Yes | Yes | Yes | 0.55 |
| 4′-Amino-2,2′:6′,2″-terpyridine | N=c1cc(-c2ccccn2)[nH]c(-c2ccccn2)c1 | Yes | Yes | Yes | Yes | Yes | 0.55 |
| [2,2′:6′,2″-Terpyridine]-4′-carboxylic acid | O=C(O)c1cc(-c2ccccn2)nc(-c2ccccn2)c1 | Yes | Yes | Yes | Yes | Yes | 0.56 |
| [2,2′:6′,2′-Terpyridine]-4′-imidazole | c1ccc(-c2cc[c3cccnc3]n2)nc1 | Yes | Yes | Yes | Yes | Yes | 0.55 |
Abbreviation: PyDev, pyridine derivatives.
ADME/T prediction
Table 4 shows the ADMET profiles of PyDev bioactive chemicals. Due to their strong binding affinity with the target proteins, the putative immunomodulatory chemicals in PyDev were anticipated to have excellent in vitro activity. In vivo and in clinical settings, the binding free energy value, when paired with the ADMET profile, might to useful in predicting the safety and effectiveness of PyDev bioactive compounds.
All compounds have a high rate of intestinal absorption in humans, indicating that they are highly absorbed and may be ingested. The medications distribution was described using the permeability of the blood-brain barrier and their subcellular localization. The high permeability property of the compounds enables it to permeate and spread throughout the brain owing to the blood-brain barrier. The ability of the compounds to spread and permeate at the subcellular level was predicted by their subcellular location. Because none of the substances were CYP2D6 substrates, they were poorly metabolized in the body. On the other side, the human metabolism has the ability to vary a drug’s efficacy in the body. The toxicity parameters are hepatotoxicity and acute oral toxicity. Acute oral toxicity is a proxy for the maximal dosage of the researched substances that the body can tolerate, while hepatotoxicity is a proxy for organ toxicity.
Molecular docking by AutoDock for COVID-19 proteins with drugs PyDev
COVID-19 has posed a severe threat (to health and wealth) over the world, and no effective treatment has yet been discovered. Pyridine compounds have been demonstrated to be useful in the treatment of a wide range of ailments, such as with neurological disorders, cancer, gastrointestinal issues, and other inflammatory diseases. As a result, simulation studies have been performed to take advantage of the potential of pyridine compounds and in silico methods for obtaining a potent drug molecule that can help not only to prevent the virus pathogenesis (CoV 2019) but also boost the body’s immunity. For the identification of therapeutic targets and inhibitors, in silico approaches provide a safe and cost-effective solution.
As the chimeric receptor-binding sphere of SARS-CoV-2 is complexed with the receptor human ACE2 results in the stabilization of viral adherence toward the host cells. SARS-CoV-2 spike glycoprotein target has been linked to the current outbreak among humans to gain entry into the host tissues.
Using the molecular docking platform AutoDock, we investigated the ligand (PyDev) receptor (four different coronavirus targets) interaction. Our findings indicated that among the pyridine compounds tested, terpyridine showed the highest binding efficacy against all four Coronavirus targets (Table 5, Figure 3A to D). The keto form of [2,2′:6′,2"-Terpyridine]-4′-imidazole is represented by Ligand (12). It has a high binding affinity (−8.8 kcal/mol), and it binds to V209 via the imidazole (–NH) (distance 3.52), as shown in Figure 3D. In imidazole ring compounds, four distinct sites of coronavirus interaction have the greatest binding affinity.
Table 5.
Binding parameters of antiviral drugs against four different Coronavirus targets by molecular docking.
| S. No | Compound | Binding affinity (kcal/mol) 6VSB | Binding affinity (kcal/ mol) 6LU7 | Binding affinity (kcal/mol) 6VW1 | Binding affinity (kcal/ mol) 6VXX |
|---|---|---|---|---|---|
| 1 | Pyridine | –4.3 | –3.5 | –3.9 | –3.6 |
| 2 | 4-Aminopyridine | –4.7 | –3.8 | –4 | –4.1 |
| 3 | Isonicotinic acid | –5.2 | –4.9 | –5.2 | –5.1 |
| 4 | Pyridinyl imidazole | –5.2 | –4.7 | –5 | –5.9 |
| 5 | 2,2′-Bipyridine | –6.2 | –4.9 | –5.7 | –6.2 |
| 6 | 4,4′-diamino-2,2′-bipyridine | –6.3 | –5.3 | –6.2 | –6.3 |
| 7 | 2 2′-bipyridine-4 4′-dicarboxylic acid | –7.1 | –6.6 | –7.5 | –7.3 |
| 8 | 4,4′-Bis(imidazolyl)-2,2′-bipyridine | –7.9 | –6.7 | –7.8 | –7.6 |
| 9 | 2,2′:6′,2″-terpyridine | –7.2 | –6.2 | –7 | –7.2 |
| 10 | 4′-Amino-2,2′:6′,2″-terpyridine | –7.3 | –6 | –7.8 | –7.5 |
| 11 | [2,2′:6′,2″-Terpyridine]-4′-carboxylic acid | –8.0 | –7.1 | –8.2 | –7.8 |
| 12 | [2,2′:6′,2″-Terpyridine]-4′-imidazole | –8.7 | –7.3 | –8.8 | –8.5 |
| 13 | Remdesivir | –1.96 | –4.09 | 16.82 | –1.94 |
| 14 | Hydroxychloroquine | –4.13 | –3.61 | –4.83 | –3.95 |
Figure 3.
(A) Docking analysis (using AutoDock) of PyDev (12-ligands) and two standard drugs with (6LU7) targets of Coronavirus. (B) Docking Analysis (using AutoDock) of PyDev (12-ligands) and two standard drugs with (6VSB) targets of Coronavirus. (C) Docking Analysis (using AutoDock) of PyDev (12-ligands) and two standard drugs with (6VXX) targets of Coronavirus. (D) Docking Analysis (using AutoDock) of PyDev (12-ligands) and two standard drugs with (6VW1) targets of Coronavirus. PyDev indicates pyridine derivatives.
Evaluation of docking results by patch docking
PatchDock has been used to accomplish docking interpretation to confirm the AutoDock docking outcomes. The superior efficacy of terpyridine over the other 12 pyridine compounds was validated by the PatchDock. [2,2′:6′,2″-Terpyridine]-4′-imidazole showed that significant ligand-receptor interaction with the greatest possible ACE (atomic contact energy) value and patch dock score in correlation to other pyridine compounds (Table 6) and selected standard drugs (remdesivir and hydroxychloroquine).
Table 6.
Online docking software PatchDock was used to validate the manual docking results.
| S. No. | Compound | Targets in Coronavirus | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 6VSB | 6LU7 | 6VW1 | 6VXX | ||||||
| Score | ACE value | Score | ACE value | Score | ACE value | Score | ACE value | ||
| 1 | Pyridine | 2384 | –96.66 | 1820 | –61.17 | 2344 | –53 | 2406 | –99.79 |
| 2 | 4-Aminopyridine | 2626 | –115.27 | 2036 | –79.01 | 2594 | –58.96 | 2528 | –104.08 |
| 3 | Isonicotinic acid | 2878 | –130.42 | 2180 | –75.35 | 2776 | –42.58 | ||
| 4 | Pyridinyl imidazole | 3478 | –193.64 | 2562 | –78.63 | 3206 | –136.35 | 3646 | –190.84 |
| 5 | 2,2′-Bipyridine | 3524 | –73.72 | 2698 | –134.39 | 3222 | –64.66 | 3608 | –202.62 |
| 6 | 4,4′-diamino-2,2′-bipyridine | 3688 | –231.65 | 2842 | –108.66 | 3456 | –76.75 | 3716 | –244.65 |
| 7 | 2 2′-bipyridine-4 4′-dicarboxylic acid | 4168 | –256.09 | 3180 | –97.78 | 3908 | –92.27 | 3415 | –156.8 |
| 8 | 4,4′-Bis(imidazolyl)-2,2′-bipyridine | 5236 | –217.54 | 4052 | –246.62 | 5100 | –171.18 | 4912 | –216.38 |
| 9 | 2,2′:6′,2″-terpyridine | 4578 | –164.62 | 3624 | –158.53 | 4752 | –239.30 | 4420 | –203.5 |
| 10 | 4′-Amino-2,2′:6′,2″-terpyridine | 4742 | –216.30 | 3824 | –178.79 | 4802 | –231.30 | 4614 | –51.22 |
| 11 | [2,2′:6′,2″-Terpyridine]-4′-carboxylic acid | 5172 | –331.01 | 3824 | –178.75 | 4902 | –264.53 | 4766 | –213.05 |
| 12 | [2,2′:6′,2″-Terpyridine]-4′-imidazole | 5458 | –399.37 | 4224 | –217.40 | 5268 | –213.39 | 5118 | –150.37 |
| 13 | Remdesivir | 5624 | –197.02 | 7826 | –255.98 | 7040 | –159.45 | 7816 | –273.99 |
| 14 | Hydroxychloroquine | 4082 | –95.32 | 5456 | –219.80 | 4962 | –197.02 | 5550 | –180.21 |
Analysis of the inhibitory potential of PyDev with two standard drugs Remdesivir and hydroxychloroquine
Therapeutic repurposing has shown to be a new avenue toward the rapid understanding of inhibitory molecules for coronavirus drug discovery. Our research also looked into the potential of existing drugs to treat viral infections. In comparison with two conventional medications, remdesivir and hydroxychloroquine, in silico experimental findings in this work specifically stated that pyridines had strong binding capability with Coronavirus SARS-CoV-2 inhibitors (Tables 5 and 6). To confirm its inhibitory effect against coronavirus pathogenesis, more italic research is required.
Conclusion
This research could save time in the development of new drugs to treat COVID19 and other viral infections. The success of PyDev was compared with two well-known medications used in the treatment of COVID-19 using molecular docking (remdesivir and hydroxychloroquine). The compounds that were recommended and demonstrated great potential.
Footnotes
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: K.K. did the experimental part and designed the work and the Corresponding author, K.S. who is the research team supervisor compiled and corrected the full length article.
References
- 1.Xu J, Zhao S, Teng T, et al. Systematic comparison of two animal-to-human transmitted human coronaviruses: SARS-CoV-2 and SARS-CoV. Viruses. 2020;12:244. doi: 10.3390/v12020244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thakur N, Das S, Kumar S, et al. Tracing the origin of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2): a systematic review and narrative synthesis. J Med Virol. 2022;94:5766-5779. doi: 10.1002/jmv.28060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fengjiao S, Xiaodong L, Jian L, Hui L. Epidemiologic characteristics of SARS-CoV-2 in Wuhan, other regions of China, and globally based on data gathered from January 2020 to February 2021. Medicine. 2022;101:32. doi:10.1097%2FMD.0000000000030019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lai CC, Shih TP, Ko WC, Tang HJ, Hsueh PR. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): the epidemic and the challenges. Int J Antimicrob Agents. 2020;55:105924. doi: 10.1016/j.ijantimicag.2020.105924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Al-Awwal N, Dweik F, Mahdi S, El-Dweik M, Anderson SH. A review of SARS-CoV-2 disease (COVID-19): pandemic in our time. Pathogens. 2022;11:368. doi: 10.3390/pathogens11030368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shereen MA, Khan S, Kazmi A, Bashir N, Siddique R. COVID-19 infection: emergence, transmission, and characteristics of human coronaviruses. J Adv Res. 2020;24:91-98. doi: 10.1016/j.jare.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Park SE. Epidemiology, virology, and clinical features of severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2; coronavirus disease-19). Clin Exp Pediatr. 2020;63:119-124. doi:10.3345%2Fcep.2020.00493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schwartz DA, Graham AL. Potential maternal and infant outcomes from coronavirus 2019-nCoV (SARS-CoV-2) infecting pregnant women: lessons from SARS, MERS, and other human coronavirus infections. Viruses. 2020;12:194. doi: 10.3390/v12020194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pathania S, Narang RK, Rawal RK. Role of sulphur-heterocycles in medicinal chemistry: an update. Eur J Med Chem. 2019;180:486-508. doi: 10.1016/j.ejmech.2019.07.043. [DOI] [PubMed] [Google Scholar]
- 10.Keri RS, Patil MR, Patil SA, Budagumpi S. A comprehensive review in current developments of benzothiazole-based molecules in medicinal chemistry. Eur J Med Chem. 2015;89:207-251. doi: 10.1016/j.ejmech.2014.10.059. [DOI] [PubMed] [Google Scholar]
- 11.Kharb R, Sharma PC, Yar MS. Pharmacological significance of triazole scaffold. J Enzyme Inhib Med Chem. 2011;26:1-21. doi: 10.3109/14756360903524304. [DOI] [PubMed] [Google Scholar]
- 12.Eftekhari-Sis B, Zirak M, Akbari A. Arylglyoxals in synthesis of heterocyclic compounds. Chem Rev. 2013;113:2958-3043. doi: 10.1021/cr300176g. [DOI] [PubMed] [Google Scholar]
- 13.Suryanarayana K, Robert AR, Kerru N, et al. Design, synthesis, anticancer activity and molecular docking analysis of novel dinitrophenylpyrazole bearing 1, 2, 3-triazoles. J Mol Struct. 2021;1243:130865. doi: 10.1016/j.molstruc.2021.130865. [DOI] [Google Scholar]
- 14.Ling Y, Hao ZY, Liang D, Zhang CL, Liu YF, Wang Y. The expanding role of pyridine and dihydropyridine scaffolds in drug design. Drug Des Devel Ther. 2021;15:4289-4338. doi:10.2147%2FDDDT.S329547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Desai NC, Somani H, Trivedi A, et al. Synthesis, biological evaluation and molecular docking study of some novel indole and pyridine based 1, 3, 4-oxadiazole derivatives as potential antitubercular agents. Bioorg Med Chem Lett. 2016;26:1776-1783. doi: 10.1016/j.bmcl.2016.02.043. [DOI] [PubMed] [Google Scholar]
- 16.Altaf AA, Shahzad A, Gul Z, et al. A review on the medicinal importance of PyDev. J Drug Des Med Chem. 2015;1:1-1. doi: 10.11648/j.jddmc.20150101.11. [DOI] [Google Scholar]
- 17.Kishbaugh TL. Pyridines and imidazopyridines with medicinal significance. Curr Top Med Chem. 2016;16:3274-3302. [DOI] [PubMed] [Google Scholar]
- 18.Alizadeh SR, Ebrahimzadeh MA. Antiviral activities of pyridine fused and pyridine containing heterocycles, a review (from 2000 to 2020). Mini Rev Med Chem. 2021;21:2584-2611. doi: 10.2174/1389557521666210126143558. [DOI] [PubMed] [Google Scholar]
- 19.Wu YH, Zhang BY, Qiu LP, Guan RF, Ye ZH, Yu XP. Structure properties and mechanisms of action of naturally originated phenolic acids and their derivatives against human viral infections. Curr Med Chem. 2017;24:4279-4302. doi: 10.2174/0929867324666170815102917. [DOI] [PubMed] [Google Scholar]
- 20.Sinha SK, Shakya A, Prasad SK, et al. An in-silico evaluation of different Saikosaponins for their potency against SARS-CoV-2 using NSP15 and fusion spike glycoprotein as targets. J Biomol Struct Dyn. 2021;39:3244-3255. doi: 10.1080/07391102.2020.1762741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wrapp D, Wang N, Corbett KS, et al. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367:1260-1263. doi: 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khaerunnisa S, Kurniawan H, Awaluddin R, Suhartati S, Soetjipto S. Potential inhibitor of COVID-19 main protease (Mpro) from several medicinal plant compounds by molecular docking study. Preprints. 2020;2020:2020030226. doi: 10.20944/preprints202003.0226.v1. [DOI] [Google Scholar]
- 23.Henderson R, Edwards RJ, Mansouri K, et al. Controlling the SARS-CoV-2 spike glycoprotein conformation. Nat Struct Mol Biol. 2020;27:925-933. doi: 10.1038/s41594-020-0479-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang X, Pearce R, Zhang Y. De novo design of protein peptides to block association of the SARS-CoV-2 spike protein with human ACE2. Aging (Albany NY). 2020;12:11263. doi:10.18632%2Faging.103416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dabbous HM, El-Sayed MH, El Assal G, et al. RETRACTED ARTICLE: safety and efficacy of favipiravir versus hydroxychloroquine in management of COVID-19: a randomised controlled trial. Sci Rep. 2021;11:1-7. doi: 10.1038/s41598-021-85227-0. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 26.Yaqoob M, Gul S, Zubair NF, Iqbal J, Iqbal MA. Theoretical calculation of selenium N-heterocyclic carbene compounds through DFT studies: synthesis, characterization and biological potential. J Mol Struct. 2020;1204:127462. doi: 10.1016/j.molstruc.2019.127462. [DOI] [Google Scholar]
- 27.Scardino V, Bollini M, Cavasotto CN. Combination of pose and rank consensus in docking-based virtual screening: the best of both worlds. RSC Advances. 2021;11:35383-35391. doi: 10.1039/D1RA05785E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roy A, Menon T. Evaluation of bioactive compounds from Boswellia serrata against SARS-CoV-2. Vegetos. 2022;35:404-414. doi: 10.1007/s42535-021-00318-7. [DOI] [PMC free article] [PubMed] [Google Scholar]




