ABSTRACT.
Approximately one-third of people with chronic Trypanosoma cruzi infection develop Chagas cardiomyopathy, which carries a poor prognosis. Accurate prediction of which individuals will go on to develop Chagas cardiomyopathy remains elusive. We performed a systematic review of literature comparing characteristics of individuals with chronic Chagas disease with or without evidence of cardiomyopathy. Studies were not excluded on the basis of language or publication date. Our review yielded a total of 311 relevant publications. We further examined the subset of 170 studies with data regarding individual age, sex, or parasite load. A meta-analysis of 106 eligible studies indicated that male sex was associated with having Chagas cardiomyopathy (Hedge’s g: 1.56, 95% CI: 1.07–2.04), and a meta-analysis of 91 eligible studies indicated that older age was associated with having Chagas cardiomyopathy (Hedge’s g: 0.66, 95% CI: 0.41–0.91). A meta-analysis of four eligible studies did not find an association between parasite load and disease state. This study provides the first systematic review to assess whether age, sex, and parasite load are associated with Chagas cardiomyopathy. Our findings suggest that older and male patients with Chagas disease are more likely to have cardiomyopathy, although we are unable to identify causal relationships due to the high heterogeneity and predominantly retrospective study designs in the current literature. Prospective, multidecade studies are needed to better characterize the clinical course of Chagas disease and identify risk factors for progression to Chagas cardiomyopathy.
INTRODUCTION
Almost 6 million people worldwide are estimated to have Chagas disease, caused by the protozoan parasite Trypanosoma cruzi.1 Considered a neglected tropical disease, Chagas disease is endemic to 21 countries in Latin America and is a major source of morbidity and mortality in this region.2 Globally, Chagas disease is associated with an annual burden of more than 800,000 disability-adjusted life years and $600 million (USD) in healthcare costs.3 Converted into lost productivity, the annual burden of Chagas disease is estimated at $7.2 billion, higher than that of other costly diseases, such as cervical cancer ($4.7 billion) or cholera ($5.4 billion).
The natural history of chronic Chagas disease comprises two stages: the indeterminate stage, characterized by positive serology but no clinical signs or symptoms, and a symptomatic stage, characterized by well-defined clinical syndromes. Although the majority of individuals remain in the indeterminate stage throughout their lifetime, approximately 30% of individuals develop clinical disease, characterized by cardiac, gastrointestinal, or mixed sequelae.4 The most common and severe complication is chronic Chagas cardiomyopathy, which results in the majority of mortality and morbidity from Chagas disease.5 Among individuals with indeterminate Chagas disease, the annual risk of developing cardiomyopathy is approximately 1.9%.6 Often initially asymptomatic, patients with Chagas cardiomyopathy may develop dilated cardiomyopathy, congestive heart failure, arrhythmias, thromboembolism, or sudden death.7 Although antitrypanosomal therapy can be used to successfully treat acute or indeterminate Chagas disease, these medications do not provide a mortality benefit or slow disease progression once cardiomyopathy has developed.8
Currently, no methodology exists to predict which patients with indeterminate Chagas disease will develop cardiomyopathy. Antitrypanosomal therapy frequently causes moderate to severe adverse reactions, including dermatologic manifestations, gastrointestinal upset, and neuropathy. Therefore, better prediction of high-risk patients could improve targeted efforts to provide antitrypanosomal therapy to patients who would benefit the most and to reduce the morbidity and mortality of Chagas disease. In this systematic review, we aim to identify risk factors for progression from the indeterminate form of Chagas disease to Chagas cardiomyopathy.
METHODS
Search strategy.
Our review followed Preferred Reporting Items for Systematic review and Meta-analyses (PRISMA) guidelines and was not limited by publication date, country of origin, or language.9 Searches were performed for relevant literature in PubMed, LILACS, and Embase electronic databases (Supplemental Table 1). An electronic search of gray literature and clinicaltrials.gov was also performed to identify ongoing or unpublished studies. The last search was performed on March 20, 2020. There was not a registered protocol.
Study selection.
Studies from the aforementioned searches were compiled for further review in Covidence, which removed duplicate studies. Two reviewers independently screened studies by title and abstract and removed irrelevant studies; conflicts were resolved through discussion. Next, full-text articles were screened for inclusion using eligibility criteria determined a priori (Table 1). Studies of adults aged 18 and older with chronic T. cruzi infection were included. Acceptable exposures included a broad array of potential risk factors for progression to Chagas cardiomyopathy, including patient, parasite, and environmental characteristics. We included studies that compared these risk factors between chronically infected patients with Chagas cardiomyopathy to those with the indeterminate form. Individuals were considered to have the indeterminate form of Chagas disease if they had positive serology confirmed by at least two serologic assays using different methods, with no signs or symptoms of cardiomyopathy or gastrointestinal disease. Individuals with mixed disease (combined cardiac and digestive forms) were excluded from the cardiomyopathy group. Studies in which participants had received antitrypanosomal treatment were excluded to avoid confounding because treatment may mask associations between a risk factor and the development of cardiomyopathy. In addition, the association between antitrypanosomal treatment and disease progression has been reviewed elsewhere.10 We also excluded studies that only examined known sequelae of Chagas cardiomyopathy or direct measures of cardiac function (such as cardiac imaging or electrocardiogram [ECG] findings). We included empirical interventional or observational study designs including randomized controlled trials, cohort studies, case–control studies, or cross-sectional studies. Review articles, case reports, abstracts, posters, theses or dissertations, animal-only studies, letters, comments, and editorials were excluded. No studies were excluded based on language, date of publication, publication status, country of origin, or clinical setting.
Table 1.
Inclusion and exclusion criteria
| PICOTSS | Inclusion criteria | Exclusion criteria |
|---|---|---|
| Population | Adults with Trypanosoma cruzi infection | Children under age 18 |
| Exposure | Risk factors such as age, sex, or parasite load | Known sequelae of Chagas cardiomyopathy or direct measures of cardiac function (e.g., cardiac imaging or electrocardiogram findings); treatment with antitrypanosomal therapy; accuracy of diagnostic testing |
| Comparison | Patients in the indeterminate stage of disease | Nonchagasic heart disease; nonchagasic healthy controls; chagasic patients with gastrointestinal or mixed manifestations of Chagas disease |
| Outcomes | Presence of or progression to Chagas cardiomyopathy | Acute myocarditis after acute infection with T. cruzi |
| Timing | Diagnosis at any point as an adult after chronic infection with T. cruzi | |
| Setting | Any country, medical setting, publication date, or language | |
| Study type | Empirical interventional or observational study designs including randomized controlled trials, cohort studies, case–control studies, or cross-sectional studies | Reviews, case reports, abstracts, posters, theses/dissertations, animal-only studies, letters, comments, or editorials |
PICOTSS = patients, interventions, comparators, outcomes, timing, setting, and study design.
An initial survey of the included studies revealed a heterogeneous array of risk factors. We identified age, sex, and parasite load among the most frequently examined risk factors. These factors could also be more effectively compared between studies due to relatively consistent measurement. Studies that included any of these risk factors subsequently underwent data extraction and study assessment.
Data extraction.
Data extracted for each study included population; study design; definitions of Chagas cardiomyopathy and indeterminate disease categories; and age, sex, and parasite load stratified by clinical form, if applicable (Supplemental Table 2). Study data were independently extracted manually by two reviewers; conflicts were resolved through discussion.
Study assessment.
Risk of bias in individual studies was assessed using the Joanna Briggs Institute Checklist for Analytical Cross Sectional Studies (Supplemental Table 3). Studies were independently evaluated by two reviewers; conflicts were resolved through discussion.
Statistics.
Data analysis was performed in Stata 16 (StataCorp LLC, College Station, TX). Forest plots were generated using random-effects meta-analysis among studies with nonoverlapping data to examine potential risk factors for cardiomyopathy development, including age, sex, and parasite load. If means and standard deviations were not available, these were calculated from the medians and interquartile ranges using established methodology.11 Hedge’s g was interpreted to signify a small (0.2 < g < 0.5), medium (0.5 ≤ g < 0.8), or high (g ≥ 0.8) effect size, respectively.
RESULTS
Search strategy.
The search identified a total of 7,917 publications (Figure 1). The publications underwent screening by title and abstract, and 7,377 were determined to be irrelevant. The remaining 540 were screened with full text review, and 229 met exclusion criteria. The final analysis included 311 publications; 170 publications were subsequently identified that included data on individual age (n = 162), sex (n = 133), and/or parasite load (n = 8), stratified by disease. Of these, six publications were available exclusively in Spanish12–17 and one exclusively in Portuguese.18 For studies available in multiple languages, the English text was used.
Figure 1.
Preferred Reporting Items for Systematic review and Meta-Analyses flow diagram.
Study characteristics and findings.
One hundred and seventy articles were identified that included information about patient age, sex, or parasite load (Supplemental Table 2). These articles were extracted for study population, study design, clinical form (indeterminate or cardiomyopathy) definitions, age by clinical form, and sex by clinical form.12–183 Patients described as having indeterminate or asymptomatic disease or those without suggestive ECG changes were considered to have indeterminate Chagas disease. Patients described as having cardiac symptoms or characteristic changes on ECG or echocardiogram were considered to have cardiomyopathy. Studies included participants in Argentina, Bolivia, Brazil, Chile, Colombia, Mexico, Spain, and Venezuela.
Risk of Chagas cardiomyopathy by age.
A total of 162 publications included age stratified by disease status. A meta-analysis was performed using 91 studies with sufficient, nonoverlapping data on patient age divided by disease stage (Supplemental Figure 1).12,16,20,23,25,27,29–32,34–38,41,44–47,49,50,52–54,57,58,60,62,64–68,70–75,77–79,81,90,93–97,99,100,106,107,109,110,112,113,115,117,118,122,124,126–128,133–135,137,139,141,143,145,147–149,151,153–155,157,158,161,165,168–170 In this analysis, older patient age was significantly associated with risk of Chagas cardiomyopathy, with an overall effect size (Hedge’s g) of 0.66 (95% CI: 0.41–0.91). Heterogeneity in this analysis was high (97%). A sensitivity analysis including 88 studies in which none of the patients had received antitrypanosomal therapy had similar results, with an overall effect size of 0.67 (95% CI: 0.41–0.93).12,16,23,25,27,30–32,34–38,41,44–47,49,50,52–54, 57,58,60,62,64–68,70–75,77–79,81,90,93–97,99,100,106,107,109,110,112,113,115,117,118,122,124,126–128,133–135,137,139,141,143,145,147–149,151, 153,155,157,158,161,165,168–170
Risk of Chagas cardiomyopathy by sex.
A total of 133 publications included sex stratified by disease status. A meta-analysis was performed using 106 studies with sufficient, nonoverlapping data on patient sex divided by disease stage (Supplemental Figure 2).11,12,16,18,19,21,22,24,28,30,31,34–37,40–47,53, 56,57,61–71,73,75–80,87,89,90,92,93,96,98,99,101–106,108,109,112,113,116,117, 121–128,130–136,140–144,146,148–150,152–158,160,164–169,173,175,177,179, 181 In this analysis, male sex was significantly associated with risk of Chagas cardiomyopathy, with an overall effect size (Hedge’s g) of 1.56 (95% CI: 1.07–2.04). Heterogeneity in this analysis was high (99%). A sensitivity analysis including 103 studies in which none of the patients had received antitrypanosomal therapy had similar results, with an overall effect size of 1.57 (95% CI: 1.07–2.07).12,13,17,19,22,23,25,29,31, 32,35–38,41–48,54,57,58,62–72,74,76–81,88,90,91,93,94,97,99,100,102–107, 109,110,113,114,117,118,122–129,131–137,141–145,147,149–151,153,154, 157–159,161,165–170,174,176,178,180,181
Risk of Chagas cardiomyopathy by parasite load.
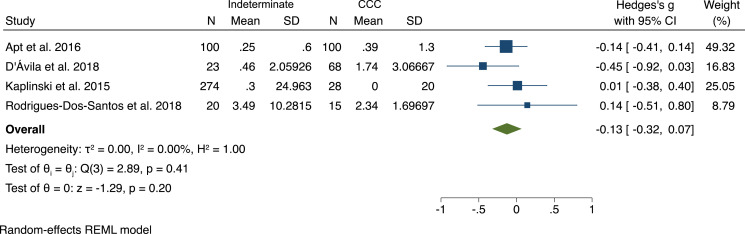
Among the eight studies that compared parasite load between individuals with indeterminate disease and cardiomyopathy, seven found no significant difference (Figure 2).24–26,41,75,115,189,190 One study found a higher parasite load among an established cohort of Chagas cardiomyopathy patients compared with seropositive blood donors without cardiomyopathy, although no significant difference was found between seropositive blood donors with and without cardiomyopathy.190
Figure 2.
Forest plot of parasite load in patients with Chagas cardiomyopathy vs. indeterminate disease. CCC = Chagas cardiomyopathy.
A meta-analysis was performed using four studies with sufficient nonoverlapping data and comparable units.25,41,75,189 A significant association between parasite load and disease status was not identified, with an overall effect size (Hedge’s g) of 0.13 (95% CI: −0.07 to 0.32).
Risk of bias.
The risk of bias in the included publications ranged from moderate to high, as assessed by the Joanna Briggs Institute Checklist for Analytical Cross Sectional Studies (Supplemental Table 3). The most common limitation was lack of identification of confounding factors or lack of strategies to deal with confounding factors. In addition, many studies did not provide statistical analysis comparing age and sex between clinical groups. We also noted that several studies excluded individuals of particular age ranges, such as those over age 65, which could bias results towards the null.
DISCUSSION
In this systematic review, we analyzed 311 publications that examined the role of age, sex, and parasite load in the progression from indeterminate Chagas disease to Chagas cardiomyopathy. Our findings suggest that male sex and older age are associated with Chagas cardiomyopathy.
Older age is one of the most well-established risk factors for progression to Chagas cardiomyopathy. This association is consistent with the clinical course of Chagas cardiomyopathy, which typically develops 10 to 30 years after acute infection.183 Older individuals are also more likely to have other forms of cardiac disease, including coronary artery disease (CAD), which is likely underdiagnosed in resource-poor areas and difficult to differentiate from Chagas cardiomyopathy.
The association with male sex has been previously reported by individual studies, but our study is the first meta-analysis to assess the association between sex and Chagas cardiomyopathy. Notably, male individuals have a higher incidence of CAD, particularly at younger ages.184 It is unknown whether preexisting cardiovascular disease synergistically increases the risk of developing Chagas cardiomyopathy. In addition, because CAD may be difficult to disentangle from Chagas cardiomyopathy in low-resource settings, this could lead to an overestimation of Chagas cardiomyopathy prevalence among males.
Our study did not identify a significant association between parasite load and clinical stage. Of the eight studies we identified that compared parasite load between individuals with indeterminate disease and cardiomyopathy, seven found no significant difference between groups. One large study found higher rates of polymerase chain reaction detection among an established cohort of Chagas cardiomyopathy individuals and seropositive blood donors diagnosed with Chagas cardiomyopathy compared with seropositive blood donors without cardiomyopathy. However, when the separate Chagas cardiomyopathy cohort was excluded, no significant difference was found in parasite load between individuals with and without cardiomyopathy. Our analysis was limited to a small number of studies that did not examine parasite load before evaluation of cardiac function; thus, we are unable to assess any causal effects between parasite load in early disease and development of cardiomyopathy. Additionally, peripheral parasitemia may not reflect parasite accumulation in cardiac tissue. Animal studies have demonstrated that T. cruzi tropism is influenced by a wide array of factors, including both host and parasite genetics.185 The pathogenesis of Chagas disease progression is a complex process thought to involve both parasite persistence and immunological mechanisms, which may not be mediated by parasite load.186
Our study has several limitations. Importantly, the existing literature that met our inclusion criteria was composed of retrospective case–control and cross-sectional studies. In addition, studies used a wide variety of definitions for Chagas cardiomyopathy and frequently did not control for confounding factors, such as other cardiac morbidities. Given the retrospective study designs and high heterogeneity among the existing literature, we are unable to identify causal relationships in our analysis. Finally, our inclusion criteria only considered studies that directly compared characteristics between individuals with cardiomyopathy and indeterminate disease. Additional literature exists that compares individuals with different stages of cardiomyopathy, compares individuals with Chagas cardiomyopathy to those with different types of cardiomyopathy, or include animal studies. A comprehensive understanding of risk factors for Chagas cardiomyopathy should consider this supportive evidence.
It is also worth noting that with the exception of one study in Mexico, almost all participants were from South America, predominantly in the Southern Cone and Bolivia. Given the geographic variation of T. cruzi genetic groups (discrete typing units [DTUs]), this potentially limits whether our results are applicable to Chagas infections in Central and North America.187,188 Past literature showed mixed results of associations between DTUs and clinical forms, including cardiomyopathy.188,189 The role of DTUs in clinical form, particularly in Central and North America where T. cruzi genetics and pathogenesis are less well studied, deserves future studies.
This study provided the first systematic review to assess whether age, sex, and parasite load are associated with Chagas cardiomyopathy. Our findings suggest that older and male patients with Chagas disease are more likely to have cardiomyopathy, but we cannot determine causal relationships because of the retrospective study designs and high heterogeneity in the current literature. Chagas cardiomyopathy typically develops 10 to 30 years after initial infection, and therefore prospective multidecade studies are needed to better evaluate risk factors for disease progression. In addition, given the limited benefits of antitrypanosomal therapy in patients with Chagas disease after cardiomyopathy has developed, preventive efforts are essential to reduce the morbidity and mortality associated with Chagas cardiomyopathy.
Supplemental files
ACKNOWLEDGMENTS
We thank Sarah Towner Wright and Dr. Daniel Jonas for their assistance in the development of this systematic review strategy.
Note: Supplemental Figures 1 and 2, and Tables 1–3 appear at www.ajtmh.org.
REFERENCES
- 1. 2015. Chagas disease in Latin America: an epidemiological update based on 2010 estimates. Wkly Epidemiol Rec 90: 33–43. [PubMed] [Google Scholar]
- 2. Pérez-Molina JA, Molina I, 2018. Chagas disease. Lancet 391: 82–94. [DOI] [PubMed] [Google Scholar]
- 3. Lee BY, Bacon KM, Bottazzi ME, Hotez PJ, 2013. Global economic burden of Chagas disease: a computational simulation model. Lancet Infect Dis 13: 342–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bern C, Martin DL, Gilman RH, 2011. Acute and congenital Chagas disease. Adv Parasitol 75: 19–47. [DOI] [PubMed] [Google Scholar]
- 5. Nunes MCP. et al. , 2018. Chagas cardiomyopathy: an update of current clinical knowledge and management: a scientific statement from the American Heart Association. Circulation 138: e169–e209. [DOI] [PubMed] [Google Scholar]
- 6. Chadalawada S. et al. , 2020. Risk of chronic cardiomyopathy among patients with the acute phase or indeterminate form of Chagas disease: a systematic review and meta-analysis. JAMA Netw Open 3: e2015072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Machado FS. et al. , 2012. Chagas heart disease: report on recent developments. Cardiology 20: 53–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Morillo CA. et al. , 2015. Randomized trial of benznidazole for chronic Chagas’ cardiomyopathy. N Engl J Med 373: 1295–1306. [DOI] [PubMed] [Google Scholar]
- 9. Page MJ. et al. , 2021. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Villar JC, Marin-Neto JA, Ebrahim S, Yusuf S, 2014. Trypanocidal drugs for chronic asymptomatic Trypanosoma cruzi infection. Cochrane Database Syst Rev 5: CD003463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wan X, Wang W, Liu J, Tong T, 2014. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol 14: 135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Giménez L, Mitelman J, González C, Borda E, Sterin Borda L, 2003. Anticuerpos antirreceptores autonómicos, alteraciones de la variabilidad de la frecuencia cardíaca y arritmias en sujetos con enfermedad de Chagas. Rev Argent Cardiol 71: 109–113. [Google Scholar]
- 13. González B. et al. , 2014. Factores de riesgo asociados con el diagnóstico de miocardiopatía chagásica crónica en individuos seropositivos del estado Barinas, Venezuela. Invest Clin 55: 119–132. [PubMed] [Google Scholar]
- 14. Martin UO, Afchain D, Marteleur de A, Ledesma O, Capron A, 1987. Complejos inmunes circulantes en los distintos estados evolutivos de la enfermedad de Chagas. Medicina (Buenos Aires) 47: 159–162. [PubMed] [Google Scholar]
- 15. Peverengo L, Rodeles LM, Prochetto E, Bertona D, Poato A, Cabrera G, Bontempi I, Vicco MH, Marcipar I, 2016. Presencia de anticuerpos inducidos por T. cruzi en pacientes con enfermedad de Chagas crónica y su relación con el perfil clínico. Rev Fed Arg Cardiol 45: 135–139. [Google Scholar]
- 16. Pozo-Pérez A, Jorquera-Fernández A, Rodríguez-Urbaneja F, Romero-Peña L, Geraldino-Carvajal O, Cáceres-Cauro A, Rosas-Martínez M, 2014. Péptido natriurético tipo B en pacientes con enfermedad de Chagas: utilidad diagnóstica en la insuficiencia cardíaca. Invest Clin 55: 321–331. [PubMed] [Google Scholar]
- 17. Storino R, Auger S, Caravello O, Urrutia MI, Sanmartino M, Jörg M, 2002. Cardiopatía chagásica en pacientes de área endémica versus contagiados en forma ocasional. Rev Saude Publica 36: 755–758. [DOI] [PubMed] [Google Scholar]
- 18. Peralta JM, Manigot DA, Muscelli EO, Magalhäes TC, de Almeida EA, Bastos A, 1982. Anticorpos EVI e NP na infeccao chagasica cronica: Estudo em pacientes com diferentes formas clinicas. Rev Inst Med Trop São Paulo 24: 6–10. [PubMed] [Google Scholar]
- 19. Abel LC. et al. , 2001. Chronic Chagas’ disease cardiomyopathy patients display an increased IFN-γ response to Trypanosoma cruzi infection. J Autoimmun 17: 99–107. [DOI] [PubMed] [Google Scholar]
- 20. Albareda MC, Laucella SA, Alvarez MG, Armenti AH, Bertochi G, Tarleton RL, Postan M, 2006. Trypanosoma cruzi modulates the profile of memory CD8+ T cells in chronic Chagas’ disease patients. Int Immunol 18: 465–471. [DOI] [PubMed] [Google Scholar]
- 21. Albareda MC, Perez-Mazliah D, Natale MA, Castro-Eiro M, Alvarez MG, Viotti R, Bertocchi G, Lococo B, Tarleton RL, Laucella SA, 2015. Perturbed T cell IL7 receptor-signaling in chronic Chagas disease. J Immunol 194: 3883–3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. de Angelis Alves RM, Thomaz RP, De Almeida EA, da Silva Wanderley J, Guariento ME, 2009. Chagas’ disease and ageing: the coexistence of other chronic diseases with Chagas’ disease in elderly patients. Rev Soc Bras Med Trop 42: 622–628. [DOI] [PubMed] [Google Scholar]
- 23. Aparecida da Silva C, Fattori A, Sousa AL, Mazon SB, Monte Alegre S, Almeida EA, Guariento ME, 2010. Determining the C-reactive protein level in patients with different clinical forms of Chagas disease. Rev Esp Cardiol 63: 1096–1099. [DOI] [PubMed] [Google Scholar]
- 24. Apt W, Arribada A, Zulantay I, Saavedra M, Araya E, Solari A, Ortiz S, Arriagada K, Rodríguez J, 2015. Trypanosoma cruzi burden, genotypes, and clinical evaluation of Chilean patients with chronic Chagas cardiopathy. Parasitol Res 114: 3007–3018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Apt W, Arribada A, Zulantay I, Saavedra M, Muñoz C, Toro B, Vega B, Rodríguez J, 2016. Chronic Chagas cardiopathy in Chile. Importance of Trypanosoma cruzi burden and clinical evaluation. Acta Trop 162: 155–166. [DOI] [PubMed] [Google Scholar]
- 26. Apt W, Carrasco D, Fuentealba C, Canals M, Muñoz G, Saavedra M, Castillo JP, Zulantay I, 2019. Chronic Chagas disease: quantification of Trypanosoma cruzi in peripheral blood and dejections of Triatoma infestans fed by xenodiagnosis in patients with and without cardiopathy. Acta Trop 200: 105167. [DOI] [PubMed] [Google Scholar]
- 27. Araújo-Jorge TC, Waghabi MC, Hasslocher-Moreno AM, Xavier SS, Higuchi MdL, Keramidas M, Bailly S, Feige JJ, 2002. Implication of transforming growth factor-b1 in Chagas disease myocardiopathy. J Infect Dis 186: 1823–1828. [DOI] [PubMed] [Google Scholar]
- 28. Argüello R, Albareda MC, Alvarez MG, Bertocchi G, Armenti AH, Vigliano C, Meckert PC, Tarleton RL, Laucella SA, 2012. Inhibitory receptors are expressed by Trypanosoma cruzi-specific effector T cells and in hearts of subjects with chronic Chagas disease. PLoS One 7: e35966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ayo CM, Reis PG, Dalalio MM, Visentainer JE, Oliveira CdF, de Araújo SM, de Oliveira Marques DS, Sell AM, 2015. Killer cell immunoglobulin-like receptors and their HLA ligands are related with the immunopathology of Chagas disease. PLoS Negl Trop Dis 9: e0003753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Batista AM. et al. , 2018. Genetic polymorphism at CCL5 is associated with protection in Chagas’ heart disease: antagonistic participation of CCR1+ and CCR5+ cells in chronic chagasic cardiomyopathy. Front Immunol 9: 615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bautista-López NL, Morillo CA, López-Jaramillo P, Quiroz R, Luengas C, Silva SY, Galipeau J, Lalu MM, Schulz R, 2013. Matrix metalloproteinases 2 and 9 as diagnostic markers in the progression to Chagas cardiomyopathy. Am Heart J 165: 558–566. [DOI] [PubMed] [Google Scholar]
- 32. Bravo-Tobar ID, Nello-Pérez C, Fernández A, Mogollón N, Pérez MC, Verde J, Concepción JL, Rodriguez-Bonfante C, Bonfante-Cabarcas R, 2015. Adenosine deaminase activity and serum C-reactive protein as prognostic markers of Chagas disease severity. Rev Inst Med Trop São Paulo 57: 385–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cetron MS, Basilio FP, Moraes AP, Sousa AQ, Paes JN, Kahn SJ, Wener MH, Van Voorhis WC, 1993. Humoral and cellular immune response of adults from northeastern Brazil with chronic Trypanosoma cruzi infection: depressed cellular immune response to T. cruzi antigen among Chagas’ disease patients with symptomatic versus indeterminate infection. Am J Trop Med Hyg 49: 370–382. [DOI] [PubMed] [Google Scholar]
- 34. Chaves AT. et al. , 2016. Immunoregulatory mechanisms in chagas disease: modulation of apoptosis in T-cell mediated immune responses. BMC Infect Dis 16: 191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Clark EH. et al. , 2015. Circulating serum markers and QRS scar score in Chagas cardiomyopathy. Am J Trop Med Hyg 92: 39–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Costa GC, da Costa Rocha MO, Moreira PR, Menezes CA, Silva MR, Gollob KJ, Dutra WO, 2009. Functional IL-10 gene polymorphism is associated with Chagas disease cardiomyopathy. J Infect Dis 199: 451–454. [DOI] [PubMed] [Google Scholar]
- 37. Curvo EO, Ferreira RR, Madeira FS, Alves GF, Chambela MC, Mendes VG, Sangenis LHC, Waghabi MC, Saraiva RM, 2018. Correlation of transforming growth factor-β1 and tumour necrosis factor levels with left ventricular function in Chagas disease. Mem Inst Oswaldo Cruz 113: e170440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cutrullis RA, Petray PB, Schapachnik E, Sánchez R, Postan M, González MN, Martín V, Corral RS, 2013. Elevated serum levels of macrophage migration inhibitory factor are associated with progressive chronic cardiomyopathy in patients with Chagas disease. PLoS One 8: e57181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. D’Ávila DA, Guedes PM, Castro AM, Gontijo ED, Chiari E, Galvão LM, 2009. Immunological imbalance between IFN-γ and IL-10 levels in the sera of patients with the cardiac form of Chagas disease. Mem Inst Oswaldo Cruz 104: 100–105. [DOI] [PubMed] [Google Scholar]
- 40. D’Ávila DA, Macedo AM, Valadares HM, Gontijo ED, de Castro AM, Machado CR, Chiari E, Galvão LM, 2009. Probing population dynamics of Trypanosoma cruzi during progression of the chronic phase in chagasic patients. J Clin Microbiol 47: 1718–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. D’Ávila DA, Galvão LMC, Sousa GR, Britto C, Moreira OC, Chiari E, 2018. Monitoring the parasite load in chronic Chagas disease patients: comparison between blood culture and quantitative real time PCR. PLoS One 13: e0208133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. De Melo AS, de Lorena VMB, de Moura Braz SC, Docena C, de Miranda Gomes Y, 2012. IL-10 and IFN-γ gene expression in chronic Chagas disease patients after in vitro stimulation with recombinant antigens of Trypanosoma cruzi. Cytokine 58: 207–212. [DOI] [PubMed] [Google Scholar]
- 43. De Moura Braz SC, de Melo AS, da Glória Aureliano de Melo Cavalcanti M, Martins SM, de Oliveira W, Jr., da Silva ED, Ferreira AG, de Lorena VM, de Miranda Gomes Y, 2014. Increase in the expression of CD4 + CD25+ lymphocytic T cells in the indeterminate clinical form of human Chagas disease after stimulation with recombinant antigens of Trypanosoma cruzi. J Clin Immunol 34: 991–998. [DOI] [PubMed] [Google Scholar]
- 44. Del Puerto R. et al. , 2010. Lineage analysis of circulating Trypanosoma cruzi parasites and their association with clinical forms of Chagas disease in Bolivia, 2010. PLoS Negl Trop Dis 4: e687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Dias FC. et al. , 2013. Polymorphic sites at the immunoregulatory CTLA-4 gene are associated with chronic Chagas disease and its clinical manifestations. PLoS One 8: e78367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Echeverría LE. et al. , 2020. Echocardiographic parameters, speckle tracking, and brain natriuretic peptide levels as indicators of progression of indeterminate stage to Chagas cardiomyopathy. Echocardiography 37: 429–438. [DOI] [PubMed] [Google Scholar]
- 47. Fabbro DL. et al. , 2011. Humoral immune response against P2β from Trypanosoma cruzi in persons with chronic Chagas disease: its relationship with treatment against parasites and myocardial damage. Am J Trop Med Hyg 84: 575–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Faé KC, Drigo SA, Cunha-Neto E, Ianni B, Mady C, Kalil J, Goldberg AC, 2000. HLA and beta-myosin heavy chain do not influence susceptibility to Chagas disease cardiomyopathy. Microbes Infect 2: 745–751. [DOI] [PubMed] [Google Scholar]
- 49. Fares RCG. et al. , 2013. Matrix metalloproteinases 2 and 9 are differentially expressed in patients with indeterminate and cardiac clinical forms of Chagas disease. Infect Immun 81: 3600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Fernandes F, Dantas S, Ianni BM, Ramires FJ, Buck P, Salemi VM, Lopes HF, Mady C, 2007. Leptin levels in different forms of Chagas’ disease. Braz J Med Biol Res 40: 1631–1636. [DOI] [PubMed] [Google Scholar]
- 51. Fernández-Mestre M, Jaraquemada D, Bruno R, Caro J, Layrisse Z, 2002. Analysis of the T-cell receptor β-chain variable-region (Vβ) repertoire in chronic human Chagas’ disease. Tissue Antigens 60: 10–15. [DOI] [PubMed] [Google Scholar]
- 52. Ferreira LRP. et al. , 2017. Blood gene signatures of Chagas cardiomyopathy with or without ventricular dysfunction. J Infect Dis 215: 387–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ferreira RC, Ianni MB, Abel LCJ, Buck P, Mady C, Kalil J, Cunha-Neto E, 2003. Increased plasma levels of tumor necrosis factor-alpha in asymptomatic/“indeterminate” and Chagas disease cardiomyopathy patients. Mem Inst Oswaldo Cruz 98: 407–411. [DOI] [PubMed] [Google Scholar]
- 54. Ferreira RR. et al. , 2018. TGF-β polymorphisms are a risk factor for Chagas disease. Dis Markers 2018: 4579198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Flórez O, Martín J, González C, 2011. Interleukin 4, interleukin 4 receptor-α and interleukin 10 gene polymorphisms in Chagas disease. Parasite Immunol 33: 506–511. [DOI] [PubMed] [Google Scholar]
- 56. Flórez O, Zafra G, Morillo C, Martín J, González CI, 2006. Interleukin-1 gene cluster polymorphism in Chagas disease in a Colombian case–control study. Hum Immunol 67: 741–748. [DOI] [PubMed] [Google Scholar]
- 57. Garcia-Alvarez A. et al. , 2010. Chagas cardiomyopathy: the potential of diastolic dysfunction and brain natriuretic peptide in the early identification of cardiac damage. PLoS Negl Trop Dis 4: e826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Garg NJ. et al. , 2016. Changes in proteome profile of peripheral blood mononuclear cells in chronic Chagas disease. PLoS Negl Trop Dis 10: e0004490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Gasparim AZ, Fontes CER, Rossoni DF, Toledo MJdO, 2018. Epidemiological and clinical profile of patients with Chagas disease in the central-north area of Paraná, southern Brazil. Rev Soc Bras Med Trop 51: 225–230. [DOI] [PubMed] [Google Scholar]
- 60. Gazzinelli RT, Leme VMC, Cancado JR, Gazzinelli G, Scharfstein J, 1990. Identification and partial characterization of Trypanosoma cruzi antigens recognized by T cells and immune sera from patients with Chagas’ disease. Infect Immun 58: 1437–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Gazzinelli R, Morato M, Nunes R, Cançado J, Brener Z, Gazzinelli G, 1988. Idiotype stimulation of T lymphocytes from Trypanosoma cruzi–infected patients. J Immunol 140: 3167–3172. [PubMed] [Google Scholar]
- 62. Georg I, Hasslocher-Moreno AM, Xavier SS, de Holanda MT, Roma EH, Bonecini-Almeida MdG, 2017. Evolution of anti-Trypanosoma cruzi antibody production in patients with chronic Chagas disease: correlation between antibody titers and development of cardiac disease severity. PLoS Negl Trop Dis 11: e0005796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Giraldo NA, Bolaños NI, Cuellar A, Roa N, Cucunubá Z, Rosas F, Velasco V, Puerta CJ, González JM, 2013. T lymphocytes from chagasic patients are activated but lack proliferative capacity and down-regulate CD28 and CD3ζ. PLoS Negl Trop Dis 7: e2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Gomes JAS. et al. , 2012. Impaired phagocytic capacity driven by downregulation of major phagocytosis-related cell surface molecules elicits an overall modulatory cytokine profile in neutrophils and monocytes from the indeterminate clinical form of Chagas disease. Immunobiology 217: 1005–1016. [DOI] [PubMed] [Google Scholar]
- 65. Gomes VAM. et al. , 2016. Analysis of regional left ventricular strain in patients with Chagas disease and normal left ventricular systolic function. J Am Soc Echocardiogr 29: 679–688. [DOI] [PubMed] [Google Scholar]
- 66. Gómez-Olarte S, Bolaños NI, Echeverry M, Rodríguez AN, Cuéllar A, Puerta CJ, Mariño A, González JM, 2019. Intermediate monocytes and cytokine production associated with severe forms of Chagas disease. Front Immunol 10: 1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. González F, Villar S, D'Attilio L, Leiva R, Marquez J, Lioi S, Beloscar J, Bottasso O, Perez AR, 2018. Dysregulated network of immune, endocrine and metabolic markers is associated to more severe human chronic Chagas cardiomyopathy. Neuroimmunomodulation 25: 119–128. [DOI] [PubMed] [Google Scholar]
- 68. Guedes PMM. et al. , 2016. Inflammation enhances the risks of stroke and death in chronic Chagas disease patients. PLoS Negl Trop Dis 10: e0004669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Gusmão RD, Rezende JM, Rassi A, Gam AA, Neva FA, 1982. Antibody levels to Trypanosoma cruzi in infected patients with and without evidence of chronic Chagas’ disease. Am J Trop Med Hyg 31: 452–458. [DOI] [PubMed] [Google Scholar]
- 70. Heringer-Walther S, Moreira MdC, Wessel N, Wang Y, Ventura TM, Schultheiss HP, Walther T, 2006. Does the C-type natriuretic peptide have prognostic value in Chagas disease and other dilated cardiomyopathies? J Cardiovasc Pharmacol 48: 293–298. [DOI] [PubMed] [Google Scholar]
- 71. De Lourdes Higuchi M, Kawakami J, Ikegami R, Clementino MB, Kawamoto FM, Reis MM, Bocchi E, 2009. Do Archaea and bacteria co-infection have a role in the pathogenesis of chronic chagasic cardiopathy? Mem Inst Oswaldo Cruz 104 (Suppl. 1): 199–207. [DOI] [PubMed] [Google Scholar]
- 72. De Lourdes Higuchi M. et al. , 2018. Archaea symbiont of T. cruzi infection may explain heart failure in Chagas disease. Front Cell Infect Microbiol 8: 412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Iosa D, DeQuattro V, Lee DDP, Elkayam U, Palmero H, 1989. Plasma norepinephrine in Chagas’ cardioneuromyopathy: a marker of progressive dysautonomia. Am Heart J 117: 882–887. [DOI] [PubMed] [Google Scholar]
- 74. Juiz NA, Estupiñán E, Hernández D, Garcilazo A, Chadi R, Morales Sanfurgo G, Schijman AG, Longhi SA, González CI, 2019. Association study between CCR2-CCR5 genes polymorphisms and chronic Chagas heart disease in Wichi and in admixed populations from Argentina. PLoS Negl Trop Dis 13: e30007033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Kaplinski M. et al. , 2015. Sustained domestic vector exposure is associated with increased Chagas cardiomyopathy risk but decreased parasitemia and congenital transmission risk among young women in Bolivia. Clin Infect Dis 61: 918–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Keating SM. et al. , 2015. Inflammatory and cardiac biomarkers are differentially expressed in clinical stages of Chagas disease. Int J Cardiol 199: 451–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Khan A, Wang Y, Schultheiss HP, Moreira MDCV, Walther T, 2016. Role of monokine induced by interferon gamma in discrimination and prognosis of patients with Chagas’ disease and idiopathic dilated cardiomyopathy. J Cardiovasc Pharmacol 67: 427–432. [DOI] [PubMed] [Google Scholar]
- 78. Larocca TF. et al. , 2017. Lack of association between serum syndecan-4, myocardial fibrosis and ventricular dysfunction in subjects with chronic Chagas disease. PLoS One 12: e0189408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Lassen O, Tabares S, Ojeda S, Dotto G, Bertolotto P, Sembaj A, 2018. Genetic polymorphisms of manganese-dependent superoxide dismutase in Chagas disease. Infect Dis Clin Pract 26: 159–164. [Google Scholar]
- 80. Lasso P, Mateus J, Pavía P, Rosas F, Roa N, Thomas MC, López MC, González JM, Puerta CJ, Cuéllar A, 2015. Inhibitory receptor expression on CD8+ T cells is linked to functional responses against Trypanosoma cruzi antigens in chronic chagasic patients. J Immunol 195: 3748–3758. [DOI] [PubMed] [Google Scholar]
- 81. Laucella SA, De Titto EH, Segura EL, 1996. Epitopes common to Trypanosoma cruzi and mammalian tissues are recognized by sera from Chagas’ disease patients: prognosis value in Chagas disease. Acta Trop 62: 151–162. [DOI] [PubMed] [Google Scholar]
- 82. Laucella S, Riarte A, Prado N, Zapata J, Segura E, 2001. α4 integrins and Sialyl Lewis × modulation in chronic Chagas disease: further evidence of persistent immune activation. Scand J Immunol 53: 514–519. [DOI] [PubMed] [Google Scholar]
- 83. Laucella S, de Titto EH, Segura EL, Orn A, Rottenberg ME, 1996. Soluble cell adhesion molecules in human Chagas’ disease: association with disease severity and stage of infection. Am J Trop Med Hyg 55: 629–634. [DOI] [PubMed] [Google Scholar]
- 84. Leon Rodriguez D, González C, Martin J, 2016. Analysis of association of FOXO3 gene with Trypanosoma cruzi infection and chronic Chagasic cardiomyopathy. HLA 87: 449–452. [DOI] [PubMed] [Google Scholar]
- 85. Leon Rodriguez DA, Acosta-Herrera M, Carmona FD, Dolade N, Vargas S, Echeverría LE, González CI, Martin J, 2018. Comprehensive analysis of three TYK2 gene variants in the susceptibility to Chagas disease infection and cardiomyopathy. PLoS One 13: e0190591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Leon Rodriguez DA, Carmona FD, Echeverría LE, González CI, Martin J, 2016. IL18 gene variants influence the susceptibility to Chagas disease. PLoS Negl Trop Dis 10: e0004583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Leon Rodriguez DA, Carmona FD, González CI, Martin J, 2016. Evaluation of VDR gene polymorphisms in Trypanosoma cruzi infection and chronic chagasic cardiomyopathy. Sci Rep 6: 31263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Lidani K, Sandri T, Andrade F, Bavia L, Nisihara R, Messias-Reason I, 2018. Complement Factor H as a potential atherogenic marker in chronic Chagas’ disease. Parasite Immunol 40: e12537. [DOI] [PubMed] [Google Scholar]
- 89. Llop E, Rothhammer F, Acuña M, Apt W, 1988. HLA sntigens in cardiomyopathic Chilean chagasics. Am J Hum Genet 43: 770–773. [PMC free article] [PubMed] [Google Scholar]
- 90. López L, Arai K, Giménez E, Jiménez M, Pascuzo C, Rodríguez-Bonfante C, Bonfante-Cabarcas R, 2006. C-reactive protein and interleukin-6 serum levels increase as Chagas disease progresses towards cardiac failure. Rev Esp Cardiol 59: 50–56. [PubMed] [Google Scholar]
- 91. Lorena VMB. et al. , 2010. Cytokine levels in serious cardiopathy of Chagas disease after in vitro stimulation with recombinant antigens from Trypanosoma cruzi. Scand J Immunol 72: 529–539. [DOI] [PubMed] [Google Scholar]
- 92. Luz PR, Boldt ABW, Grisbach C, Kun JFJ, Velavan TP, Messias-Reason IJT, 2013. Association of L-ficolin levels and FCN2 genotypes with chronic Chagas disease. PLoS One 8: e60237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Luz PR, Miyazaki MI, Chiminacio Neto N, Padeski MC, Barros AC, Boldt AB, Messias-Reason IJ, 2016. Genetically determined MBL deficiency is associated with protection against chronic cardiomyopathy in Chagas disease. PLoS Negl Trop Dis 10: e0004257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Marques DSDO, Canesin MF, Barutta F, Fuganti CJ, Barretto ACP, 2006. Evaluation of asymptomatic patients with chronic Chagas disease through ambulatory electrocardiogram, echocardiogram and B-Type natriuretic peptide analyses. Arq Bras Cardiol 87: 336–343. [DOI] [PubMed] [Google Scholar]
- 95. Medeiros NI. et al. , 2017. Differential expression of matrix metalloproteinases 2, 9 and cytokines by neutrophils and monocytes in the clinical forms of Chagas disease. PLoS Negl Trop Dis 11: e0005284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Medeiros NI, Pinto BF, Elói-Santos SM, Teixeira-Carvalho A, Magalhães LMD, Dutra WO, Correa-Oliveira R, Gomes JAS, 2019. Evidence of different IL-1β activation pathways in innate immune cells from indeterminate and cardiac patients with chronic Chagas disease. Front Immunol 10: 800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. de Melo RB, Parente GBdO, Victor EG, 2005. Measurement of human brain natriuretic peptide in patients with Chagas’ disease. Arq Bras Cardiol 84: 137–140. [DOI] [PubMed] [Google Scholar]
- 98. Messias-Reason I, Urbanetz L, Pereira da Cunha C, 2003. Complement C3 F and BF S allotypes are risk factors for Chagas disease cardiomyopathy. Tissue Antigens 62: 308–312. [DOI] [PubMed] [Google Scholar]
- 99. Miranda CP, Botoni FA, Nunes MDCP, Rocha MOdC, 2019. Analysis of iron metabolism in chronic chagasic cardiomyopathy. Arq Bras Cardiol 112: 189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Moreira MdC, Heringer-Walther S, Wessel N, Moreira Ventura T, Wang Y, Schultheiss HP, Walther T, 2008. Prognostic value of natriuretic peptides in Chagas’ disease: a 3-year follow-up investigation. Cardiology 110: 217–225. [DOI] [PubMed] [Google Scholar]
- 101. Moreira MdCV, Wang Y, Heringer-Walther S, Wessel N, Walther T, 2009. Prognostic value of natriuretic peptides in Chagas’ disease: a head-to-head comparison of the 3 natriuretic peptides. Congest Heart Fail 15: 75–81. [DOI] [PubMed] [Google Scholar]
- 102. Mundaray Fernández N, Fernández-Mestre M, 2014. The role of haptoglobin genotypes in Chagas disease. Dis Markers 2014: 793646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Muñoz-San Martín C, Zulantay I, Saavedra M, Fuentealba C, Muñoz G, Apt W, 2018. Discrete typing units of Trypanosoma cruzi detected by real-time PCR in Chilean patients with chronic Chagas cardiomyopathy. Acta Trop 185: 280–284. [DOI] [PubMed] [Google Scholar]
- 104. Munoz Saravia SG. et al. , 2013. Combined measurement of N-terminal pro-B-type natriuretic peptide and highly sensitive cardiac troponin T for diagnosis and monitoring of heart injury in chronic Chagas’ disease. Clin Biochem 46: 1615–1618. [DOI] [PubMed] [Google Scholar]
- 105. Negrão CE. et al. , 2009. Muscle sympathetic nerve activity in patients with Chagas’ disease. Int J Cardiol 137: 252–259. [DOI] [PubMed] [Google Scholar]
- 106. Nonaka CKV. et al. , 2019. Circulating miRNAs as potential biomarkers associated with cardiac remodeling and fibrosis in Chagas disease cardiomyopathy. Int J Mol Sci 20: 4064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Noya-Rabelo MM, Macedo CT, Larocca T, Machado A, Pacheco T, Torreão J, Souza BSF, Soares MBP, Ribeiro-Dos-Santos R, Correia LCL, 2018. The presence and extension of myocardial fibrosis in the undetermined form of Chagas’ disease: a study using magnetic resonance. Arq Bras Cardiol 110: 124–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Noya-Rabelo MM. et al. , 2017. Evaluation of galectin-3 as a novel biomarker for Chagas cardiomyopathy. Cardiology 136: 33–39. [DOI] [PubMed] [Google Scholar]
- 109. Nunes DF, da Matta Guedes PM, de Mesquita Andrade C, Jácome da Câmara AC, Chiari E, da Cunha Galvão LM, 2013. Troponin T autoantibodies correlate with chronic cardiomyopathy in human Chagas disease. Trop Med Int Health 18: 1180–1192. [DOI] [PubMed] [Google Scholar]
- 110. Okamoto EE. et al. , 2014. Biomarkers in Trypanosoma cruzi–infected and uninfected individuals with varying severity of cardiomyopathy in Santa Cruz, Bolivia. PLoS Negl Trop Dis 8: e3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Peralta J, Ginefra P, Dias J, Magalhães J, Szarfman A, 1981. Autoantibodies and chronic Chagas’s heart disease. Trans R Soc Trop Med Hyg 75: 568–569. [DOI] [PubMed] [Google Scholar]
- 112. Pérez AR, Silva-Barbosa SD, Berbert LR, Revelli S, Beloscar J, Savino W, Bottasso O, 2011. Immunoneuroendocrine alterations in patients with progressive forms of chronic Chagas disease. J Neuroimmunol 235: 84–90. [DOI] [PubMed] [Google Scholar]
- 113. Pérez-Fuentes R, López-Colombo A, Ordóñez-Toquero G, Gomez-Albino I, Ramos J, Torres-Rasgado E, Salgado-Rosas H, Romero-Díaz M, Pulido-Pérez P, Sánchez-Guillén MC, 2007. Correlation of the serum concentrations of tumour necrosis factor and nitric oxide with disease severity in chronic Chagas disease (American trypanosomiasis). Ann Trop Med Parasitol 101: 123–132. [DOI] [PubMed] [Google Scholar]
- 114. Pérez-Mazliah DE, Castro Eiro MD, Álvarez MG, Lococo B, Bertocchi G, César G, Natale MA, Albareda MC, Viotti R, Laucella SA, 2018. Distinct monocyte subset phenotypes in patients with different clinical forms of chronic Chagas disease and seronegative dilated cardiomyopathy. PLoS Negl Trop Dis 12: e0006887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Perez-Ramirez L. et al. , 1999. Clinical analysis and parasite genetic diversity in human immunodeficiency virus/Chagas’ disease coinfections in Brazil. Am J Trop Med Hyg 61: 198–206. [DOI] [PubMed] [Google Scholar]
- 116. Pissetti CW, De Oliveira RF, Correira D, Nascentes GAN, Llaguno MM, Rodrigues V, 2013. Association between the lymphotoxin-alpha gene polymorphism and chagasic cardiopathy. J Interferon Cytokine Res 33: 130–135. [DOI] [PubMed] [Google Scholar]
- 117. Puyó AM, Scaglione J, Auger S, Cavallero S, Donoso AS, Dupuy HA, Fernández BE, 2002. Atrial natriuretic factor as marker of myocardial compromise in Chagas’ disease. Regul Pept 105: 139–143. [DOI] [PubMed] [Google Scholar]
- 118. Ramasawmy R, Cunha-Neto E, Fae KC, Borba SC, Teixeira PC, Ferreira SC, Goldberg AC, Ianni B, Mady C, Kalil J, 2009. Heterozygosity for the S180L variant of MAL/TIRAP, a gene expressing an adaptor protein in the toll‐like receptor pathway, is associated with lower risk of developing chronic Chagas cardiomyopathy. J Infect Dis 199: 1838–1845. [DOI] [PubMed] [Google Scholar]
- 119. Ramasawmy R, Cunha-Neto E, Faé KC, Müller NG, Cavalcanti VL, Drigo SA, Ianni B, Mady C, Kalil J, Goldberg AC, 2006. BAT1, a putative anti-inflammatory gene, is associated with chronic Chagas cardiomyopathy. J Infect Dis 193: 1394–1399. [DOI] [PubMed] [Google Scholar]
- 120. Ramasawmy R, Cunha-Neto E, Fae KC, Martello FG, Müller NG, Cavalcanti VL, Ianni B, Mady C, Kalil J, Goldberg AC, 2006. The monocyte chemoattractant protein–1 gene polymorphism is associated with cardiomyopathy in human Chagas disease. Clin Infect Dis 43: 305–311. [DOI] [PubMed] [Google Scholar]
- 121. Ramasawmy R. et al. , 2007. Polymorphisms in the gene for lymphotoxin-α predispose to chronic Chagas cardiomyopathy. J Infect Dis 196: 1836–1843. [DOI] [PubMed] [Google Scholar]
- 122. Reis PG. et al. , 2017. Genetic polymorphisms of IL17 and Chagas disease in the south and southeast of Brazil. J Immunol Res 2017: 1017621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Ripoll JG, Giraldo NA, Bolaños NI, Roa N, Rosas F, Cuéllar A, Puerta CJ, González JM, 2018. T cells responding to Trypanosoma cruzi detected by membrane TNF-α and CD154 in chagasic patients. Immun Inflamm Dis 6: 47–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Rocha AL, Lombardi F, da Costa Rocha MO, Barros MV, Val Barros VdC, Reis AM, Ribeiro AL, 2006. Chronotropic incompetence and abnormal autonomic modulation in ambulatory Chagas disease patients. Ann Noninvasive Electrocardiol 11: 3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Rocha SC, Pérez AR, Beloscar J, Bottasso O, Silber AM, 2019. Diminished prolinemia in chronic Chagasic patients: a new clue for disease pathology? Mol 24: 3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Rodeles LM, Vicco MH, Bontempi IA, Siano A, Tonarelli G, Bottasso OA, Arias P, Marcipar IS, 2016. Combined analysis of cross-reacting antibodies anti-β1AR and anti-B13 in advanced stages of Chagas heart disease. Trop Med Int Health 21: 1545–1551. [DOI] [PubMed] [Google Scholar]
- 127. Salomone OA, Caeiro TF, Madoery RJ, Amuchástegui M, Omelinauk M, Juri D, Kaski JC, 2001. High plasma immunoreactive endothelin levels in patients with Chagas’ cardiomyopathy. Am J Cardiol 87: 1217–1220. [DOI] [PubMed] [Google Scholar]
- 128. Sánchez-Montalvá A, Salvador F, Rodríguez-Palomares J, Sulleiro E, Sao-Avilés A, Roure S, Valerio L, Evangelista A, Molina I, 2016. Chagas cardiomyopathy: usefulness of EKG and echocardiogram in a non-endemic country. PLoS One 11: e0157597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Sandri TL, Andrade FA, Lidani KCF, Einig E, Boldt ABW, Mordmüller B, Esen M, Messias-Reason IJ, 2019. Human collectin-11 (COLEC11) and its synergic genetic interaction with MASP2 are associated with the pathophysiology of Chagas disease. PLoS Negl Trop Dis 13: e0007324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Santos LdS, Torres RM, Machado-de-Assis GF, Bahia MT, Martins HR, Teixeira-Carvalho A, Coelho-Dos-Reis JG, Albajar-Viñas P, Martins-Filho OA, Lana Md, 2012. In-house ELISA method to analyze anti-Trypanosoma cruzi IgG reactivity for differential diagnosis and evaluation of Chagas disease morbidity. Rev Soc Bras Med Trop 45: 35–44. [DOI] [PubMed] [Google Scholar]
- 131. Saravia SGM, Haberland A, Bartel S, Araujo R, Valda G, Reynaga DD, Ramirez ID, Borges AC, Wallukat G, Schimke I, 2011. Cardiac troponin T measured with a highly sensitive assay for diagnosis and monitoring of heart injury in chronic Chagas disease. Arch Pathol Lab Med 135: 243–248. [DOI] [PubMed] [Google Scholar]
- 132. Schapachnik ES, Ramos AO, Reitburd CR, Maceri C, 1980. Enfermedad de Chagas crónica: correlación radiológica y electrocardiográfica. Rev Argent Cardiol 48: 256–263. [Google Scholar]
- 133. Silva SJ, Rassi S, Pereira AdC, 2017. Angiotensin-converting enzyme ID polymorphism in patients with heart failure secondary to Chagas disease. Arq Bras Cardiol 109: 307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Silva SDA, Gontijo ED, Amaral CFS, 2007. Case–control study of factors associated with chronic Chagas heart disease in patients over 50 years of age. Mem Inst Oswaldo Cruz 102: 845–851. [DOI] [PubMed] [Google Scholar]
- 135. Simões MV, Pintya AO, Bromberg-Marin G, Sarabanda AV, Antloga CM, Pazin-Filho A, Maciel BC, Marin-Neto JA, 2000. Relation of regional sympathetic denervation and myocardial perfusion disturbance to wall motion impairment in Chagas’ cardiomyopathy. Am J Cardiol 86: 975–981. [DOI] [PubMed] [Google Scholar]
- 136. de Araújo Soares AK, Neves PA, Cavalcanti MD, Marinho SM, Oliveira W, Jr., Souza JR, Lorena VM, Gomes YM, 2016. Expression of co-stimulatory molecules CD80 and CD86 is altered in CD14 + HLA-DR + monocytes from patients with Chagas disease following induction by Trypanosoma cruzi recombinant antigens. Rev Soc Bras Med Trop 49: 632–636. [DOI] [PubMed] [Google Scholar]
- 137. Sousa GR, Gomes JA, Damasio MP, Nunes MC, Costa HS, Medeiros NI, Fares RC, Chaves AT, Corrêa-Oliveira R, Rocha MO, 2017. The role of interleukin 17-mediated immune response in Chagas disease: high level is correlated with better left ventricular function. PLoS One 12: e0172833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Sousa GR. et al. , 2014. Plasma cytokine expression is associated with cardiac morbidity in Chagas disease. PLoS One 9: e87082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Strauss M, Acosta-Herrera M, Alcaraz A, Casares-Marfil D, Bosch-Nicolau P, Lo Presti MS, Molina I, González CI, Martín J , 2019. Association of IL18 genetic polymorphisms with Chagas disease in Latin American populations. PLoS Negl Trop Dis 13: e0007859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Szarfman A, Luquetti A, Rassi A, Rezende JM, Schmuñis GA, 1981. Tissue-reacting immunoglobulins in patients with different clinical forms of Chagas’ disease. Am J Trop Med Hyg 30: 43–46. [DOI] [PubMed] [Google Scholar]
- 141. Talvani A, Rocha MOC, Ribeiro AL, Borda E, Sterin-Borda L, Teixeira MM, 2006. Levels of anti-M2 and anti-beta1 autoantibodies do not correlate with the degree of heart dysfunction in Chagas’ heart disease. Microbes Infect 8: 2459–2464. [DOI] [PubMed] [Google Scholar]
- 142. Thomas MC, Fernández-Villegas A, Carrilero B, Marañón C, Saura D, Noya O, Segovia M, Alarcón de Noya B, Alonso C, López MC, 2012. Characterization of an immunodominant antigenic epitope from Trypanosoma cruzi as a biomarker of chronic Chagas’ disease pathology. Clin Vaccine Immunol 19: 167–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Torreão JA. et al. , 2015. Myocardial tissue characterization in Chagas’ heart disease by cardiovascular magnetic resonance. J Cardiovasc Magn Reson 17: 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Torres OA, Calzada JE, Beraún Y, Morillo CA, González A, González CI, Martín J, 2010. Role of the IFNG +874T/A polymorphism in Chagas disease in a Colombian population. Infect Genet Evol 10: 682–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Uellendahl M, Siqueira ME, Calado EB, Kalil-Filho R, Sobral D, Ribeiro C, Oliveira W, Martins S, Narula J, Rochitte CE, 2016. Cardiac magnetic resonance-verified myocardial fibrosis in Chagas disease: clinical correlates and risk stratification. Arq Bras Cardiol 107: 460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Valerio L, Roure S, Sabrià M, Balanzó X, Vallès X, Serés L, 2011. Clinical, electrocardiographic and echocardiographic abnormalities in Latin American migrants with newly diagnosed Chagas disease 2005–2009, Barcelona, Spain. Euro Surveill 16: 19971. [DOI] [PubMed] [Google Scholar]
- 147. Vasconcelos DF, Junqueira LF, 2009. Distinctive impaired cardiac autonomic modulation of heart rate variability in chronic Chagas’ indeterminate and heart diseases. J Electrocardiol 42: 281–289. [DOI] [PubMed] [Google Scholar]
- 148. Vasconcelos RHT, Azevedo EdA, Diniz GT, Cavalcanti MdG, de Oliveira W, Jr., de Morais CN, Gomes Yd M, 2015. Interleukin-10 and tumour necrosis factor-alpha serum levels in chronic Chagas disease patients. Parasite Immunol 37: 376–379. [DOI] [PubMed] [Google Scholar]
- 149. Venegas J. et al. , 2009. Differential distribution of Trypanosoma cruzi clones in human chronic chagasic cardiopathic and non-cardiopathic individuals. Acta Trop 109: 187–193. [DOI] [PubMed] [Google Scholar]
- 150. VerçoSsa AFA, Lorena VMB, Carvalho CL, Melo MFAD, Cavalcanti MGA, Silva ED, Ferreira AGP, Pereira VRA, Souza WV, Gomes YM, 2007. Chagas’ disease: IgG isotypes against cytoplasmic (CRA) and flagellar (FRA) recombinant repetitive antigens of Trypanosoma cruzi in chronic chagasic patients. J Clin Lab Anal 21: 271–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Vicco MH, Bontempi IA, Rodeles L, Yodice A, Marcipar IS, Bottasso O, 2014. Decreased level of antibodies and cardiac involvement in patients with chronic Chagas heart disease vaccinated with BCG. Med Microbiol Immunol (Berl) 203: 133–139. [DOI] [PubMed] [Google Scholar]
- 152. Vicco MH. et al. , 2013. Assessment of cross-reactive host-pathogen antibodies in patients with different stages of chronic Chagas disease. Rev Esp Cardiol 66: 791–796. [DOI] [PubMed] [Google Scholar]
- 153. Villacorta H, Bortolotto LA, Arteaga E, Mady C, 2006. Aortic distensibility measured by pulse-wave velocity is not modified in patients with Chagas’ disease. J Negat Results Biomed 5: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Villar JC, León H, Morillo CA, 2004. Cardiovascular autonomic function testing in asymptomatic T. cruzi carriers: a sensitive method to identify subclinical Chagas’ disease. Int J Cardiol 93: 189–195. [DOI] [PubMed] [Google Scholar]
- 155. Viotti R. et al. , 2009. The impact of socioeconomic conditions on chronic Chagas disease progression. Rev Esp Cardiol 62: 1224–1232. [DOI] [PubMed] [Google Scholar]
- 156. Vitelli-Avelar DM. et al. , 2008. Strategy to assess the overall cytokine profile of circulating leukocytes and its association with distinct clinical forms of human Chagas disease. Scand J Immunol 68: 516–525. [DOI] [PubMed] [Google Scholar]
- 157. Vizzoni AG, Varela MC, Sangenis LHC, Hasslocher-Moreno AM, Do Brasil PEAA, Saraiva RM, 2018. Ageing with Chagas disease: an overview of an urban Brazilian cohort in Rio de Janeiro. Parasit Vectors 11: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Volpato FCZ, Sousa GR, D’Ávila DA, Galvão LMdC, Chiari E, 2017. Combined parasitological and molecular-based diagnostic tools improve the detection of Trypanosoma cruzi in single peripheral blood samples from patients with Chagas disease. Rev Soc Bras Med Trop 50: 506–515. [DOI] [PubMed] [Google Scholar]
- 159. Wallukat G. et al. , 2010. Distinct patterns of autoantibodies against G-protein-coupled receptors in Chagas’ cardiomyopathy and megacolon. Their potential impact for early risk assessment in asymptomatic Chagas’ patients. J Am Coll Cardiol 55: 463–468. [DOI] [PubMed] [Google Scholar]
- 160. Wang Y, Khan A, Heringer-Walther S, Schultheiss H-P, Moreira MdCV, Walther T, 2013. Prognostic value of circulating levels of stem cell growth factor beta (SCGF beta) in patients with Chagas’ disease and idiopathic dilated cardiomyopathy. Cytokine 61: 728–731. [DOI] [PubMed] [Google Scholar]
- 161. Wang Y, Moreira MdC, Heringer-Walther S, Ebermann L, Schultheiss HP, Wessel N, Siems WE, Walther T, 2010. Plasma ACE2 activity is an independent prognostic marker in Chagas’ disease and equally potent as BNP. J Card Fail 16: 157–163. [DOI] [PubMed] [Google Scholar]
- 162. Wang Y, Moreira MdC, Heringer-Walther S, Khan A, Schultheiss HP, Wessel N, Siems WE, Walther T, 2011. Does the aminopeptidase a have prognostic and diagnostic value in Chagas disease and other dilated cardiomyopathies? J Cardiovasc Pharmacol 58: 374–379. [DOI] [PubMed] [Google Scholar]
- 163. Wang Y, Moreira MdC, Heringer-Walther S, Schultheiss HP, Siems WE, Wessel N, Walther T, 2010. Amino-terminal fragment of C-type natriuretic peptide precursor and C-type natriuretic peptide do not correlate in patients with Chagas disease: role for neutral endopeptidase. J Cardiovasc Pharmacol 55: 62–66. [DOI] [PubMed] [Google Scholar]
- 164. Wang Y, Moreira MdC, Khan A, Heringer-Walther S, Schultheiss HP, Wessel N, Siems WE, Walther T, 2012. Prognostic significance of circulating levels of hepatocyte growth factor in patients with Chagas’ disease and idiopathic dilated cardiomyopathy. Cardiology 121: 240–246. [DOI] [PubMed] [Google Scholar]
- 165. Ward LS, Guariento ME, Fernandes GA, Maciel RM, 1999. Serum cytokines in chronic Chagas’ disease. Rev Soc Bras Med Trop 32: 285–289. [DOI] [PubMed] [Google Scholar]
- 166. Zafra G, Flórez O, Morillo CA, Echeverría LE, Martín J, González CI, 2008. Polymorphisms of toll-like receptor 2 and 4 genes in Chagas disease. Mem Inst Oswaldo Cruz 103: 27–30. [DOI] [PubMed] [Google Scholar]
- 167. Zafra G, Morillo C, Martín J, González A, González CI, 2007. Polymorphism in the 30 UTR of the IL12B gene is associated with Chagas’ disease cardiomyopathy. Microbes Infect 9: 1049–1052. [DOI] [PubMed] [Google Scholar]
- 168. Zago MP. et al. , 2019. Potential utility of protein targets of cysteine-S-nitrosylation in identifying clinical disease status in human Chagas disease. Front Microbiol 9: 3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Grotto HZW, Costa FF, Carnéiro MV, Neto GCG, 1994. Serum neopterin in patients with Chagas disease. Trans R Soc Trop Med Hyg 88: 75. [DOI] [PubMed] [Google Scholar]
- 170. Zicker F, Netto JCDA, Zicker EMS, Oliveira RM, Smith PG, 1990. Trypanosoma cruzi infection and electrocardiographic findings among active manual workers. A population-based study in central Brazil. Int J Epidemiol 19: 182–186. [DOI] [PubMed] [Google Scholar]
- 171. Zicker F, Smith PG, Almeida Netto JC, Oliveira RM, Zicker EMS, 1990. Physical activity, opportunity for reinfection, and sibling history of heart disease as risk factors for Chagas’ cardiomyopathy. Am J Trop Med Hyg 43: 498–505. [DOI] [PubMed] [Google Scholar]
- 172. Mosca W, Castes M, Ojeda A, El Homsi A, 1986. Evaluation of the interaction of leucocytes from Chagas disease patients with trypomastigotes of Trypanosoma cruzi. Trans R Soc Trop Med Hyg 80: 975–977. [DOI] [PubMed] [Google Scholar]
- 173. Nunes MCP, Dones W, Morillo CA, Encina JJ, Ribeiro AL, 2013. Chagas disease: an overview of clinical and epidemiological aspects. J Am Coll Cardiol 62: 767–776. [DOI] [PubMed] [Google Scholar]
- 174. Pereira NdS, Queiroga TBD, Nunes DF, Andrade CM, Nascimento MSL, Do-Valle-Matta MA, da Câmara ACJ, Galvão LMDC, Guedes PMM, Chiari E, 2018. Innate immune receptors over expression correlate with chronic chagasic cardiomyopathy and digestive damage in patients. PLoS Negl Trop Dis 12: e0006589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Rocha ALL, Lombardi F, da Costa Rocha MO, Barros MV, Val Barros VdC, Reis AM, Ribeiro AL, 2006. Chronotropic incompetence and abnormal autonomic modulation in ambulatory Chagas disease patients. Ann Noninvasive Electrocardiol 11: 3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Almeida M, Lorena VMB, Medeiros CA, Junior WO, Cavalcanti MDGAM, Martins SM, de Morais CNL, 2018. Alternative Th17 and CD4+ CD25+ FoxP3+ cell frequencies increase and correlate with worse cardiac function in Chagas cardiomyopathy. Scand J Immunol 87: e12650. [DOI] [PubMed] [Google Scholar]
- 177. Faé KC, Drigo SA, Cunha-Neto E, Ianni B, Mady C, Kalil J, Goldberg AC, 2000. HLA and β-myosin heavy chain do not influence susceptibility to Chagas’ disease cardiomyopathy. Microbes Infect 2: 745–751. [DOI] [PubMed] [Google Scholar]
- 178. Frade AF. et al. , 2013. Genetic susceptibility to Chagas disease cardiomyopathy: involvement of several genes of the innate immunity and chemokine-dependent migration pathways. BMC Infect Dis 13: 587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Frade AF. et al. , 2013. Polymorphism in the alpha cardiac muscle actin 1 gene is associated to susceptibility to chronic inflammatory cardiomyopathy. PLoS One 8: e83446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Gomes JdAS. et al. , 2018. Systems biology reveals relevant gaps in Fc-γR expression, impaired regulatory cytokine microenvironment interfaced with anti–Trypanosoma cruzi IgG reactivity in cardiac Chagas disease patients. Front Microbiol 9: 1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Passos LSA, Magalhães LMD, Soares RP, Marques AF, Alves MLR, Giunchetti RC, Nunes MDCP, Gollob KJ, Dutra WO, 2019. Activation of human CD11b+ B1 B-cells by Trypanosoma cruzi-derived proteins is associated with protective immune response in human Chagas disease. Front Immunol 9: 3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Passos L, Magalhães LMD, Soares RP, Marques AF, Nunes MDCP, Gollob KJ, Dutra WO, 2017. Specific activation of CD4-CD8- double-negative T cells by Trypanosoma cruzi-derived glycolipids induces a proinflammatory profile associated with cardiomyopathy in Chagas patients. Clin Exp Immunol 190: 122–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Ribeiro AL, Nunes MP, Teixeira MM, Rocha MOC, 2012. Diagnosis and management of Chagas disease and cardiomyopathy. Nat Rev Cardiol 9: 576–589. [DOI] [PubMed] [Google Scholar]
- 184. Leening MJ. et al. , 2014. Sex differences in lifetime risk and first manifestation of cardiovascular disease: prospective population based cohort study. BMJ 349: g5992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Pereira SS, Trindade S, De Niz M, Figueiredo LM, 2019. Tissue tropism in parasitic diseases. Open Biol 9: 190036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Marin-Neto JA, Cunha-Neto E, Maciel BC, Simões MV, 2007. Pathogenesis of chronic Chagas heart disease. Circulation 115: 1109–1123. [DOI] [PubMed] [Google Scholar]
- 187. Brenière SF, Waleckx E, Barnabé C, 2016. Over six thousand Trypanosoma cruzi strains classified into discrete typing units (DTUs): attempt at an inventory. PLoS Negl Trop Dis 10: e0004792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Nielebock MAP, Moreira OC, Xavier SCDC, Miranda LFC, Lima ACB, Pereira TOJS, Hasslocher-Moreno AM, Britto C, Sangenis LHC, Saraiva RM, 2020. Association between Trypanosoma cruzi DTU TcII and chronic Chagas disease clinical presentation and outcome in an urban cohort in Brazil. PLoS One 15: e0243008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Rodrigues-dos-Santos Í, Melo MF, de Castro L, Hasslocher-Moreno AM, do Brasil PEAA, Silvestre de Sousa A, Britto C, Moreira OC, 2018. Exploring the parasite load and molecular diversity of Trypanosoma cruzi in patients with chronic Chagas disease from different regions of Brazil. PLoS Negl Trop Dis 12: e0006939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Sabino EC. et al. , 2015. Detection of Trypanosoma cruzi DNA in blood by PCR is associated with Chagas cardiomyopathy and disease severity. Eur J Heart Fail 17: 416–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.