Abstract
Background
Recurrent urinary tract infections (UTIs) are common, especially in women. When oral antimicrobial prophylaxis is ineffective or not possible due to allergies or antimicrobial resistance, intravesical aminoglycoside instillations (IAIs) are a non-systemic alternative.
Objectives
To assess treatment satisfaction, long-term safety and efficacy of IAIs for recurrent UTI.
Methods
We conducted a cohort study using data collected between January 2013 and June 2022 at the Leiden University Medical Center. Adult patients with recurrent UTI who received prophylactic IAI were eligible for inclusion. Treatment satisfaction was assessed through a survey. Data on serum aminoglycoside concentrations, cystoscopy results and number of recurrences were obtained through chart review. Number of recurrences and UTI characteristics were compared between patients on and off IAI using Poisson and logistic mixed effects models.
Results
Forty-four patients were included (median follow-up time 976 days) and 323 UTIs occurred during follow-up. Overall treatment satisfaction was high (median 79.2/100). All but one patient had undetectable serum aminoglycoside levels and no malignancies were found on follow-up cystoscopy. IAI increased the time to first recurrence (102 days versus 36 days, P = 0.02), reduced the number of recurrences (rate ratio 0.75, 95% CI 0.56–0.99, P = 0.04) and the necessity for systemic antibiotics (OR 0.33, 95% CI 0.13–0.86, P = 0.02).
Conclusions
In patients with recurrent UTI, IAI was associated with high treatment satisfaction, and was found to be a safe and effective alternative to oral antimicrobial prophylaxis.
Introduction
Recurrent urinary tract infection (UTI) refers to at least three episodes per year or two episodes per 6 months.1 Although the morbidity of a single UTI is low, the high incidence and recurrence risk lead to considerable healthcare costs and a reduced quality of life.2,3 In patients with high recurrence rates despite behavioural modifications and non-antimicrobial prophylaxis, oral antimicrobial prophylaxis is often initiated. Continuous antimicrobial prophylaxis reduces recurrence risk, including in patients who perform clean intermittent catheterization (CIC).4,5 However, an important disadvantage of continuous oral antimicrobial prophylaxis is the emergence of resistant pathogens, limiting treatment options.5,6 This is especially relevant for patients with an increased risk of infections with multidrug resistant organisms (MDROs), e.g. patients with neurogenic bladder and kidney transplant recipients.7,8 In addition to antimicrobial resistance (AMR), allergies and side effects may preclude oral antimicrobial prophylaxis as a viable treatment strategy for recurrent UTI.9
In an era of AMR being a rising threat to global health, direct instillation of antibiotics in the bladder may be an appealing alternative to systemic antimicrobial prophylaxis.10 With intravesical aminoglycoside instillation (IAI), high concentrations of aminoglycosides—which exhibit concentration-dependent killing—are achieved in the bladder. Consequently, uropathogens without high-level aminoglycoside resistance can still be treated with IAI because concentrations in the bladder exceed MIC breakpoints.11 Systemic uptake of aminoglycosides is rare, diminishing the concern for nephrotoxicity and ototoxicity.11 Because aminoglycosides stay in the bladder, it is hypothesized that the commensal flora of the gut, perineum and vagina may remain unaffected. In fact, Stalenhoef et al.11 showed a reduction in MDRO UTIs, possibly also explained by a decrease in overall systemic antibiotic use.12 Treatment satisfaction has not yet been assessed with validated tools. Evaluating treatment satisfaction is particularly relevant for patients receiving IAI, because it is more invasive than other prophylactic alternatives, and treatment satisfaction influences treatment-related behaviour (adherence and persistence), ultimately affecting treatment success.13 Since the study by Stalenhoef et al.11, the Leiden University Medical Center has implemented IAI in an increasing number of outpatients with recurrent UTI, most of them continuing IAI after 6 months. As a consequence, more long-term data have become available. The aim of this study was to assess treatment satisfaction, long-term safety and efficacy of IAI in patients with recurrent UTI.
Methods
We conducted a cohort study using data collected between January 2013 and June 2022 in our tertiary care hospital for assessment of long-term safety and efficacy. Treatment satisfaction was assessed through a cross-sectional survey (May 2022). This study was approved by the regional ethics committee (METC-LDD), and all patients provided written informed consent for the use of their data and survey participation. This study was registered at clinicaltrials.gov (NCT05376670).
Study population
Adult recurrent UTI patients who were on continuous or postcoital IAI were eligible for inclusion. Patients exclusively using IAI for on-demand treatment of recurrences (no prophylaxis) were excluded. Moreover, we excluded patients receiving IAI for chronic prostatitis and patients with an indwelling catheter. Patients with multiple treatment cycles (on and off IAI) acted as their own controls.
IAI treatment protocol
Patients received training for CIC and the preparation of the solution by a specialized nurse. They were instructed to mix 80 mg gentamicin with 20 mL 0.9% sodium chloride (tobramycin 80 mg or amikacin 250 mg were chosen in case of infections with a gentamicin-resistant pathogen within the preceding 6 months). To increase bladder time, patients were advised to administer the solution before bedtime. The standard treatment regimen consisted of daily instillations for 2 weeks, every other day for 10 weeks, and twice weekly for 12 weeks. In case of new-onset lower urinary tract symptoms (LUTS), daily instillations were reinitiated for 5–7 days if signs of systemic infection were absent. If LUTS persisted or systemic signs were present, oral or IV antibiotics were started. Patients were instructed to directly contact the outpatient clinic instead of their GP for all new-onset symptoms, regardless of whether they were on IAI at that time. After 6 months of IAI, discontinuation of treatment was discussed with all patients. If treatment was continued, IAI frequency was individualized and based on recurrence rate. Serum aminoglycoside levels were measured in the first month, after an overnight instillation. Cystoscopy was performed every 2 years.
Data collection
Clinical data were collected from electronic records and included baseline demographics, comorbidities, other prophylactic measures and previous MDRO UTIs. For safety endpoints we collected cystoscopy and serum aminoglycoside data. To establish efficacy, we recorded the number of recurrences during follow-up. For each UTI, additional information was collected on LUTS, fever (temperature ≥38.0°C), microbiological results, hospital admission and treatment.
We defined UTI as an episode with new-onset symptoms that was diagnosed as a UTI by a physician and was treated with an antimicrobial agent. Dysuria, frequency, urgency and suprapubic pain were classified as LUTS; other non-genitourinary symptoms were classified as ‘non-specific symptoms’. Both conversion to daily IAI and oral/IV antibiotics were considered treatment. We considered ESBL and carbapenemase-producing Enterobacterales, Enterobacterales with combined fluoroquinolone and aminoglycoside resistance, and vancomycin-resistant enterococci as MDROs. MIC breakpoints for resistance and intermediate sensitivity were based on EUCAST criteria.14
Treatment satisfaction
Treatment satisfaction was only assessed in patients who were on IAI at the time of data collection or had been using IAI no longer than 1 year before the start of data collection. Treatment satisfaction was assessed through a linguistically validated Dutch version of the Treatment Satisfaction Questionnaire for Medication-version II (TSQM-II) in a paper format.15 Permission was obtained from IQVIA Inc. (Plymouth Meeting, Pennsylvania). The TSQM-II consists of 11 questions, divided into four domains: effectiveness, side effects, convenience and global satisfaction. Scores are calculated by adding items in each domain and transforming the composite score into a value ranging from 0 to 100, where a score of 100 corresponds with the highest degree of satisfaction. For the side effects domain, a score of 100 indicates an absence of side effects.
Statistical analysis
Statistical analysis was performed using SPSS version 27.0 (IBM, Armonk, NY, USA) and R version 4.0.3 (R Foundation for Statistical Computing, Vienna, Austria). Data are presented as percentages, means with SDs, or medians with IQR based on the type and distribution of the data. To compare UTI characteristics between patients on and off IAI, a logistic mixed effects model with a varying intercept per patient was used, to take dependencies between observations (recurrences) per patient into account. The Kaplan–Meier method was used to estimate time to first UTI recurrence; results were graphically displayed and compared between patients on IAI and after cessation of IAI using a log-rank test. In case of multiple IAI cycles, only the first IAI cycle was included in the Kaplan–Meier analysis. To compare the incidence of UTI episodes between patients on and off IAI, a Poisson mixed effects model was used (with random intercept per patient). Because the duration of treatment cycles markedly varied, ‘duration’ was log-transformed and included as an offset in the model. For the Poisson model, we assumed that risk of recurrence was constant over time. Because this assumption may not hold true, we performed a sensitivity analysis in which these data were analysed using a Cox frailty model. Prior to data analysis, sample size was calculated for treatment satisfaction. To estimate the mean overall score on the TSQM-II questionnaire with a margin of error indicated by a 95% CI not wider than 20, a sample size of 25 patients was required, given the expected population SD of 25.4.5 Subgroup analyses were performed based on gender, menopausal status, history of kidney transplantation and history of CIC prior to IAI. To determine whether effects of IAI treatment differed between subgroups, Poisson mixed effects models with interaction terms were applied.
Results
Patient characteristics
In total, 44 patients were included (inclusion flowchart in Figure S1, available as Supplementary data at JAC-AMR Online). Patient characteristics are outlined in Table 1. Most patients in our cohort were postmenopausal women receiving IAI due to failure of oral antimicrobial prophylaxis (57%) or the lack of oral options due to AMR (36%). Twenty-eight patients (68%) were already performing CIC prior to the initiation of IAI, and 11 patients (25%) had a history of kidney transplantation. Median follow-up duration was 976 days (IQR 468–1637) and median number of IAI days was 602 (IQR 402–1212).
Table 1.
Baseline characteristics of patients with recurrent UTI starting IAI
| Baseline characteristics | n = 44 |
|---|---|
| Age in years | 61.9 (14) |
| Female | 31 (71) |
| Postmenopausal | 25/31 (81) |
| Sexually active | 13/21 (62) |
| Comorbidity | |
| Previous CIC | 28 (68) |
| Underactive/neurogenic bladder (including spina bifida) | 27 (61) |
| Kidney transplantation | 11 (25) |
| Urethral dilation/meatal dilation/urethrotomy | 10 (23) |
| Diabetes mellitus | 8 (18) |
| Cystocele/rectocele | 7 (16) |
| Nephrectomy | 5 (11) |
| TURP | 5 (11) |
| ADPKD | 3 (7) |
| Urolithiasis | 3 (7) |
| Urological malignancy | 0 |
| eGFR prior to start of IAI, mL/min/1.73 m2 | |
| ≥90 | 12 (27) |
| 60–89 | 21 (48) |
| 45–59 | 4 (9) |
| 30–44 | 3 (7) |
| 15–29 | 4 (9) |
| Non-antimicrobial prophylaxis | |
| Vaginal oestrogen | 22/31 (71) |
| d-Mannose | 13 (30) |
| Non-antibiotic irrigations | 11 (25) |
| UTI caused by MDRO in 6 months before IAI | 17 (39) |
| Indication for IAI | |
| Oral prophylaxis not efficacious | 25 (57) |
| No oral options due to resistance | 16 (36) |
| No oral options due to intolerance | 15 (34) |
| No oral options due to allergy | 4 (9) |
| Other reason | 6 (14) |
| Frequency of IAI at last follow-up | |
| Daily | 7 (16) |
| Every other day | 10 (23) |
| Twice weekly | 13 (30) |
| No IAI at last follow-up | 14 (32) |
Age is expressed as mean (SD); all other variables are expressed as n (%). Sexual activity was not reported for 10 women. Other reasons for initiation of IAI: patient preferred IAI over oral prophylaxis, patient already did CIC and had recurrent UTIs. ADPKD, autosomal dominant polycystic kidney disease; CIC, clean intermittent catheterization; eGFR, estimated glomerular filtration rate; IAI, intravesical aminoglycoside instillation; MDRO, multidrug-resistant organism; TURP, transurethral resection of the prostate; UTI, urinary tract infection.
Treatment satisfaction and (dis)continuation
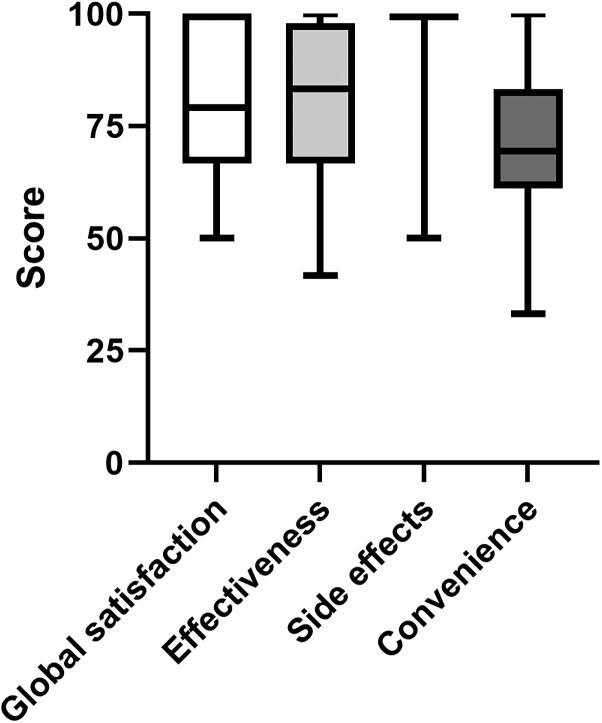
At 6 months, 80% of patients wished to continue IAI, because of fewer recurrences and an increased quality of life (self-reported). Two patients discontinued after 6 months due to insufficient efficacy, and one patient was switched to oral prophylaxis because resistance to oral antimicrobial therapy was lost. Of the 26 patients that discontinued IAI at some point during follow-up, 18 (69%) restarted IAI. The TSQM-II was filled out by 32 patients (73%), and results are summarized in Figure 1. Median scores of the four domains were: global satisfaction 79.2 (IQR 66.7–100.0), effectiveness 83.3 (IQR 66.7–97.9), side effects 100.0 (IQR 100.0–100.0) and convenience 69.4 (IQR 61.1–83.3). Two patients completing the questionnaire reported side effects of painful CIC. Global satisfaction was higher for patients who were already performing CIC before initiation of IAI compared with patients who did not have prior experience with CIC (median score 83.3 versus 58.3, P = 0.03). Discontinuation rates and TSQM-scores did not differ for the specified subgroups (data not shown).
Figure 1.
Box and whiskers plot of Treatment Satisfaction Questionnaire for Medication version II (TSQM-II) scores in patients with current or recent IAI treatment (n = 32). Median values are represented by the black line within the boxes; the median value of the side effects domain was 100.
Safety
Cystoscopy was performed in 29 patients (66%) after a median of 768 days (IQR 363–1327) since the start of IAI. No malignancies were found. Other cystoscopy findings included bladder trabeculation (n = 6), diverticula (n = 3) and cystitis cystica/glandularis (n = 2). Serum aminoglycoside levels were available for 40 patients (91%). All but one patient had undetectable serum aminoglycoside levels. The patient with a detectable aminoglycoside level (serum tobramycin 0.5 mg/L) had macroscopic haematuria (due to a recent bladder biopsy for a suspected fungal cystitis) at the time of measurement.
Efficacy
Recurrences and antimicrobial consumption
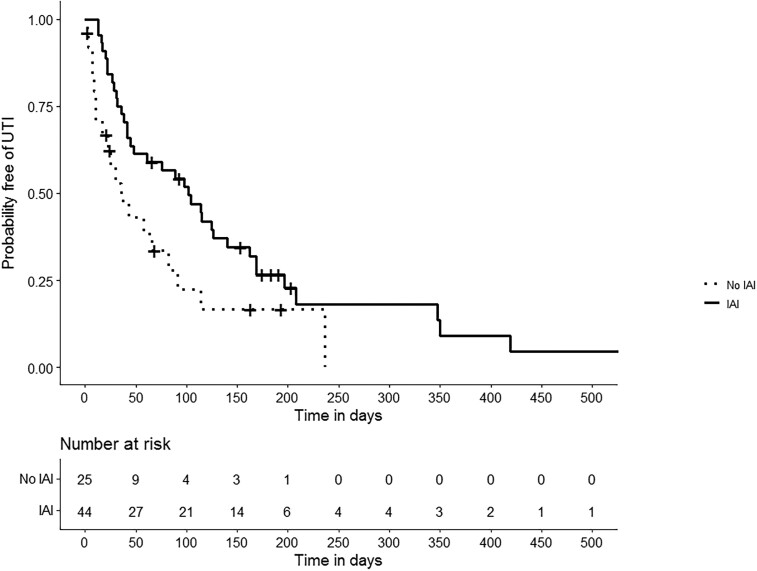
In total, 323 UTIs (207 during IAI prophylaxis, 116 after IAI prophylaxis) were reported in 44 patients. UTI characteristics are outlined in Table 2. LUTS were present in 209/268 (78.0%) episodes and fever in 44/323 (13.6%) episodes. Median time to first recurrence was longer for patients on IAI compared with after cessation of IAI (102 days versus 36 days, P = 0.02), as summarized in Figure 2. Moreover, IAI significantly decreased the number of recurrences (rate ratio 0.75, 95% CI 0.56–0.99, P = 0.04). A positive effect of IAI was also consistently seen in various Cox frailty models (Table S1). In patients on IAI, 75.2% of recurrences were treated with systemic (oral or IV) antibiotics, compared with 92.2% of recurrences after cessation of IAI (OR 0.33, 95% CI 0.13–0.86, P = 0.02).
Table 2.
Characteristics and treatment of UTIs in patients with IAI and after cessation of IAI.
| IAI, n (%) | No IAI, n (%) | OR (95% CI) | P value | |
|---|---|---|---|---|
| New-onset LUTS | 122/169 (72.2) | 87/99 (87.9) | 0.43 (0.16–1.18) | 0.10 |
| Fever | 30/207 (14.5) | 14/116 (12.1) | 1.23 (0.45–3.34) | 0.68 |
| UTI caused by classic GNR | 101/164 (61.6) | 75/102 (73.5) | 0.66 (0.31–1.43) | 0.29 |
| UTI caused by enterococci | 26/164 (15.9) | 5/102 (4.9) | 4.45 (1.40–12.88) | 0.01 |
| MDRO (including ESBL) | 22/155 (14.2) | 18/99 (18.2) | 0.78 (0.28–2.19) | 0.64 |
| Hospital admission | 30/206 (14.6) | 10/116 (8.6) | 1.09 (0.34–3.56) | 0.88 |
| Necessity for systemic (oral/IV) antibiotics | 155/206 (75.2) | 107/116 (92.2) | 0.33 (0.13–0.86) | 0.02 |
E. coli, Proteus mirabilis and Klebsiella pneumoniae were defined as classic Gram-negative rods. Missing data: new-onset LUTS (n = 55), hospital admission (n = 1), necessity for systemic antibiotics (n = 1). In 54 UTI episodes, no urine culture was performed. ORs were calculated using a logistic mixed effects model with a varying intercept per patient. GNR, Gram-negative rods; LUTS, lower urinary tract symptoms; MDRO, multidrug-resistant organism; UTI, urinary tract infection.
Figure 2.
Kaplan–Meier curve of time to first recurrence (UTI) in patients with intravesical aminoglycoside instillation (IAI) and after cessation of IAI. Patients on IAI treatment are indicated by the solid line, and patients that have stopped IAI by the dotted line.
The results of the subgroup analyses are provided in Table S2. In the subgroup of women, the time to first recurrence was 98 versus 23 days (P = 0.02), and the rate ratio of recurrences was 0.59 (95% CI 0.43–0.81, P = 0.001).
Microbiological characteristics
A urine culture was performed in 267 episodes (82.7%). In 216 cases (80.9%) a single uropathogen was found, whereas in 20 cases (7.5%) there were two uropathogens, in 21 cases (7.9%) there was a mixed flora, and in 10 cases (3.7%) no uropathogens were found. Recurrences that occurred during IAI were more often caused by enterococci than recurrences that occurred after cessation of IAI (OR 4.45, 95% CI 1.40–12.88, P = 0.01). No differences were found in the same comparison for classic Gram-negative rods (Escherichia coli, Klebsiella pneumoniae and Proteus mirabilis). In the 6 months before initiation of IAI, 17 patients had a UTI caused by an MDRO (5 were aminoglycoside resistant). Three of these 17 patients experienced a recurrence with the same MDRO in the 6 months after initiation of IAI.
Sensitivity analysis
Eight patients (18%) in our study had also participated in the study by Stalenhoef et al.11 Including only the remaining 36 patients (82%) in our Poisson model produced a rate ratio of 0.75 (95% CI 0.53–1.05). Furthermore, results of our logistic mixed effects model were not affected by missing clinical and microbiological data (Table S3).
Discussion
In patients with recurrent UTI, IAI is associated with high treatment satisfaction and continuation rates, and it appears to be a safe and effective alternative to oral antimicrobial prophylaxis.
Treatment satisfaction
Thus far, treatment satisfaction for IAI has not been assessed with a validated questionnaire. Stalenhoef et al.11 requested patients to grade their satisfaction by providing a score between 0 and 10 and found a mean score of 8 (SD 1.2) after 24 weeks of IAI. This score is similar to the overall satisfaction score that was found in our study (median 79.2 out of 100). However, an overall score does not give insight into the different domains of treatment satisfaction. The highest satisfaction scores were observed in the ‘effectiveness’ and ‘side effects’ domains. In fact, only two patients reported any side effects (painful catheterization). Contrary to previous studies, no gastrointestinal complaints or vaginal infections were reported.11,12 The validated questionnaire that we used in our study was also used in a randomized trial evaluating oral antimicrobial prophylaxis in patients with recurrent UTI and CIC use.5 Scores for effectiveness were comparable to our IAI cohort. However, convenience scores were lower in our patients with IAI (mean 71.2, SD 16.1) compared with patients in the oral prophylaxis study (mean 88.9, SD 13.9). Lower convenience scores for IAI are unsurprising because CIC is necessary for administration of the drug. In the oral prophylaxis study, all patients were already performing CIC and questions focused on convenience of oral therapy alone.
Safety
Serum aminoglycoside levels were undetectable in all but one patient, confirming results of previous studies that systemic uptake is very rare.11,16–18 In treatment of non-muscle-invasive bladder cancer, systemic uptake of intravesical agents occurs more frequently in case of mucosal damage, due to recent transurethral resection, traumatic catheterization or an active UTI.19 In an infected rat bladder model, systemic aminoglycoside absorption was observed in 3/7 rats, but serum aminoglycoside levels were all in the non-toxic range.20 The serum concentration that was found in one patient (0.5 mg/L) was likely related to disruption of the epithelial barrier due to recent bladder biopsies. This concentration is considered non-toxic because it falls below the usual trough levels for systemic aminoglycoside treatment.21 We propose that routine measurement of serum aminoglycoside concentration should no longer be performed in patients using IAI, except in patients with macroscopic haematuria.
Neither in our study, nor in the study by Stalenhoef et al.11 were malignancies found on follow-up cystoscopy. Our study had markedly longer follow-up times, with a quarter of patients having a follow-up cystoscopy more than 3.5 years after initiation of IAI. However, caution is warranted when interpreting these findings, because our sample size was relatively small, bladder cancer incidence is generally low, and the median age of our cohort was below the median age at bladder cancer diagnosis.
Efficacy
In our study, IAI significantly reduced the number of recurrences and necessity for systemic (oral/IV) antibiotics. These findings are consistent with previous studies, most of them including patients with neurogenic bladder.11,12,17,22,23 In subgroup analyses the effect of IAI seemed to be most pronounced in women, which contrasts with the results of two previous studies that also investigated the effect of gender.11,22 However, caution should be applied when interpreting results of subgroup analyses, because the subgroups were small and other determinants had a skewed distribution. For instance, 54% of men were kidney transplant recipients, compared with 13% of women.
The majority of studies compared the number of recurrences in the 6 months prior to IAI with the number of recurrences in the 6 months after initiation of IAI. However, Stalenhoef et al.11 showed that recurrence rates in the 6 months after cessation of IAI remained low. In this study, follow-up started at the initiation of IAI and recurrence rates were compared between on and off IAI cycles, meaning that patients off IAI had already used IAI in the past. It is possible that the reduction in recurrence rate would have been even more pronounced had we compared recurrence rates between patients on IAI and prior to initiation of IAI. A comparison between self-reported recurrence rate (before IAI) with physician-reported recurrence rate was not deemed ideal. In patients receiving IAI, we observed that fewer recurrences had to be treated with systemic antibiotics. This observation underestimates the reduction of the total antibiotic burden, because recurrence rates are also lower in patients with IAI use.
It is incompletely understood which mechanisms contribute to the efficacy of IAI. Worby et al.24 have shown that gut microbial richness is significantly lower in women with recurrent UTI. In this study, one in four recurrences were treated with daily IAI only. We hypothesize that a reduction in systemic antibiotic use (due to a decrease in recurrence rate as well as treating recurrences with IAI only) may promote a recovery of a dysbiotic gut microbiome, thereby potentially reducing recurrence risk. Another hypothesis is that IAI may eradicate intracellular bacterial reservoirs that can seed recurrent infection.25
Implications for clinical practice
Despite a lower recurrence rate on IAI, breakthrough infections do occur. If signs of systemic infection are absent, primary management with daily IAI is preferable, to avoid the drawbacks of systemic antimicrobials. If symptoms persist despite daily IAI, and systemic antimicrobial therapy is necessary, the different pathogen distribution among IAI users is relevant for empirical therapy. We observed that most patients who had had a UTI caused by an MDRO in the 6 months prior to IAI did not have a recurrence with that same pathogen. Moreover, recurrences that developed during IAI prophylaxis were more frequently caused by enterococci, which is likely explained by the fact that enterococci are frequently intrinsically resistant to high levels of aminoglycosides.
Strengths and limitations
Strengths of our study include the long follow-up time, the use of a validated questionnaire to assess treatment satisfaction, and the inclusion of subgroup analyses. Furthermore, the results regarding efficacy were consistent across different statistical approaches. Our study has several limitations. Firstly, the TSQM-II questionnaire was administered at the same time for all patients, which led to a variable timing of the questionnaire in relation to treatment duration. Most respondents were on IAI at the time of the survey, which might have led to an overestimation of treatment satisfaction. Secondly, due to the observational nature of this study we did not use an existing reference standard for UTI, which might have contributed to misclassification of UTI. However, this effect will have occurred in both ‘groups’ (on and off IAI) and biased results are therefore unlikely. Another limitation is the unblinded nature of this study. Finally, a limitation that is inherent to observational studies is unmeasured confounding.
Conclusion
In conclusion, IAI is a safe and effective non-systemic alternative for UTI prophylaxis with a high degree of treatment satisfaction. It should be considered in patients who fail oral antimicrobial prophylaxis or have allergies and resistance patterns that preclude oral prophylaxis as a viable strategy. Future studies should focus on elucidating the best regimen in terms of dosage and frequency.
Supplementary Material
Acknowledgements
We would like to thank P.H. Verbeek-Menken and I.G.A. Nellen for instructing patients on IAI use. Moreover, we thank B.E.L. Vrijsen for his valuable contribution to the statistical analysis of the data.
Contributor Information
Manu P Bilsen, Department of Infectious Diseases, Leiden University Medical Center, Leiden, The Netherlands.
Janneke I M van Uhm, Department of Urology, Leiden University Medical Center, Leiden, The Netherlands.
Janneke E Stalenhoef, Department of Internal Medicine, OLVG, Amsterdam, The Netherlands.
Cees van Nieuwkoop, Department of Internal Medicine, Haga Teaching Hospital, The Hague, The Netherlands.
Rolf H H Groenwold, Department of Clinical Epidemiology, Leiden University Medical Center, Leiden, The Netherlands.
Leo G Visser, Department of Infectious Diseases, Leiden University Medical Center, Leiden, The Netherlands.
Merel M C Lambregts, Department of Infectious Diseases, Leiden University Medical Center, Leiden, The Netherlands.
Funding
This study was carried out as part of routine work.
Transparency declarations
None of the authors have an association that might pose a conflict of interest.
Author contributions
Conceptualization and methodology, M.P.B., M.M.C.L., L.G.V. and R.H.H.G.; original draft preparation, M.P.B.; data interpretation, M.P.B., M.M.C.L., L.G.V. and R.H.H.G.; review and editing, M.P.B., M.M.C.L., J.I.M.V.U., J.E.S., C.V.N. and L.G.V.; supervision, M.M.C.L. and L.G.V. All authors have read and agreed to the final version of the manuscript.
Supplementary data
Figure S1 and Tables S1 to S3 are available as Supplementary data at JAC-AMR Online.
References
- 1. European Association of Urology . EAU guidelines on urological infections. 2021. https://uroweb.org/guidelines/urological-infections
- 2. Ennis SS, Guo H, Raman Let al. . Premenopausal women with recurrent urinary tract infections have lower quality of life. Int J Urol 2018; 25: 684–9. 10.1111/iju.13698 [DOI] [PubMed] [Google Scholar]
- 3. Foxman B. The epidemiology of urinary tract infection. Nat Rev Urol 2010; 7: 653–60. 10.1038/nrurol.2010.190 [DOI] [PubMed] [Google Scholar]
- 4. Beerepoot MA, ter Riet G, Nys Set al. . Cranberries vs antibiotics to prevent urinary tract infections: a randomized double-blind noninferiority trial in premenopausal women. Arch Intern Med 2011; 171: 1270–8. 10.1001/archinternmed.2011.306 [DOI] [PubMed] [Google Scholar]
- 5. Fisher H, Oluboyede Y, Chadwick Tet al. . Continuous low-dose antibiotic prophylaxis for adults with repeated urinary tract infections (AnTIC): a randomised, open-label trial. Lancet Infect Dis 2018; 18: 957–68. 10.1016/S1473-3099(18)30279-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Beerepoot MA, ter Riet G, Nys Set al. . Lactobacilli vs antibiotics to prevent urinary tract infections: a randomized, double-blind, noninferiority trial in postmenopausal women. Arch Intern Med 2012; 172: 704–12. 10.1001/archinternmed.2012.777 [DOI] [PubMed] [Google Scholar]
- 7. McKibben MJ, Seed P, Ross SSet al. . Urinary tract infection and neurogenic bladder. Urol Clin North Am 2015; 42: 527–36. 10.1016/j.ucl.2015.05.006 [DOI] [PubMed] [Google Scholar]
- 8. Korth J, Kukalla J, Rath PMet al. . Increased resistance of gram-negative urinary pathogens after kidney transplantation. BMC Nephrol 2017; 18: 164. 10.1186/s12882-017-0580-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Malik RD, Wu YR, Christie ALet al. . Impact of allergy and resistance on antibiotic selection for recurrent urinary tract infections in older women. Urology 2018; 113: 26–33. 10.1016/j.urology.2017.08.070 [DOI] [PubMed] [Google Scholar]
- 10. World Health Organization . Global antimicrobial resistance surveillance system (GLASS) report: early implementation. 2020. https://apps.who.int/iris/handle/10665/332081
- 11. Stalenhoef JE, van Nieuwkoop C, Menken PHet al. . Intravesical gentamicin treatment for recurrent urinary tract infections caused by multidrug resistant bacteria. J Urol 2019; 201: 549–55. 10.1016/j.juro.2018.10.004 [DOI] [PubMed] [Google Scholar]
- 12. Cox L, He C, Bevins Jet al. . Gentamicin bladder instillations decrease symptomatic urinary tract infections in neurogenic bladder patients on intermittent catheterization. Can Urol Assoc J 2017; 11: E350–4. 10.5489/cuaj.4434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jacobs JM, Pensak NA, Sporn NJet al. . Treatment satisfaction and adherence to oral chemotherapy in patients with cancer. J Oncol Pract 2017; 13: e474–85. 10.1200/JOP.2016.019729 [DOI] [PubMed] [Google Scholar]
- 14. The European Committee on Antimicrobial Susceptibility Testing . Breakpoint tables for interpretation of MICs and zone diameters. Version 10.0.2020.
- 15. Atkinson MJ, Kumar R, Cappelleri JCet al. . Hierarchical construct validity of the treatment satisfaction questionnaire for medication (TSQM version II) among outpatient pharmacy consumers. Value Health 2005; 8: S9–S24. 10.1111/j.1524-4733.2005.00066.x [DOI] [PubMed] [Google Scholar]
- 16. Marei MM, Jackson R, Keene DJB. Intravesical gentamicin instillation for the treatment and prevention of urinary tract infections in complex paediatric urology patients: evidence for safety and efficacy. J Pediatr Urol 2021; 17: 65.e1–65.e11. 10.1016/j.jpurol.2020.08.007 [DOI] [PubMed] [Google Scholar]
- 17. Abrams P, Hashim H, Tomson Cet al. . The use of intravesical gentamicin to treat recurrent urinary tract infections in lower urinary tract dysfunction. Neurourol Urodyn 2017; 36: 2109–16. 10.1002/nau.23250 [DOI] [PubMed] [Google Scholar]
- 18. Defoor W, Ferguson D, Mashni Set al. . Safety of gentamicin bladder irrigations in complex urological cases. J Urol 2006; 175: 1861–4. 10.1016/S0022-5347(05)00928-6 [DOI] [PubMed] [Google Scholar]
- 19. Koya MP, Simon MA, Soloway MS. Complications of intravesical therapy for urothelial cancer of the bladder. J Urol 2006; 175: 2004–10. 10.1016/S0022-5347(06)00264-3 [DOI] [PubMed] [Google Scholar]
- 20. Wan J, Kozminski M, Wang SCet al. . Intravesical instillation of gentamicin sulfate: in vitro, rat, canine, and human studies. Urology 1994; 43: 531–6. 10.1016/0090-4295(94)90249-6 [DOI] [PubMed] [Google Scholar]
- 21. Bertino JS Jr, Booker LA, Franck PAet al. . Incidence of and significant risk factors for aminoglycoside-associated nephrotoxicity in patients dosed by using individualized pharmacokinetic monitoring. J Infect Dis 1993; 167: 173–9. 10.1093/infdis/167.1.173 [DOI] [PubMed] [Google Scholar]
- 22. Huen KH, Nik-Ahd F, Chen Let al. . Neomycin-polymyxin or gentamicin bladder instillations decrease symptomatic urinary tract infections in neurogenic bladder patients on clean intermittent catheterization. J Pediatr Urol 2019; 15: 178.e1–e7. 10.1016/j.jpurol.2018.12.001 [DOI] [PubMed] [Google Scholar]
- 23. Chernyak S, Salamon C. Intravesical antibiotic administration in the treatment of recurrent urinary tract infections: promising results from a case series. Female Pelvic Med Reconstr Surg 2020; 26: 152–4. 10.1097/SPV.0000000000000810 [DOI] [PubMed] [Google Scholar]
- 24. Worby CJ, Schreiber HL 4th, Straub TJet al. . Longitudinal multi-omics analyses link gut microbiome dysbiosis with recurrent urinary tract infections in women. Nat Microbiol 2022; 7: 630–9. 10.1038/s41564-022-01107-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rosen DA, Hooton TM, Stamm WEet al. . Detection of intracellular bacterial communities in human urinary tract infection. PLoS Med 2007; 4: e329. 10.1371/journal.pmed.0040329 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.