Abstract
Patients with gastric cancer exhibit considerable genetic and phenotypic heterogeneity, necessitating the establishment of a model for personalized medicine screening. Here, we successfully established three-dimensional cell spheroids (3D cell spheroids) from fresh gastric cancer tissues (n = 30) as in vitro models for further therapeutic screening. Hematoxylin-eosin and immunohistochemical staining of whole spheroids and parental tumor tissues revealed that the 3D cell spheroids recapitulated the parental tissue structure and maintained the histological characteristics of the parental tumor tissue during long-term expansion in vitro. Further, transcriptome sequencing verified that the cell spheroids could recapitulate the gene expression profile characteristics of the parental tumor tissue. Drug susceptibility testing of the 3D cell spheroids demonstrated that these cell spheroids can be used as a reliable model for drug prediction.
Keywords: Gastric cancer, three-dimensional cell spheroids, drug screening
Introduction
Gastric cancer is histologically diverse; its treatment regimens and corresponding efficacy vary depending on clinical stage, pathological grade, and other clinical characteristics [1]. Most patients with gastric cancer who undergo surgical resection are administered chemotherapy; however, the recurrence and mortality rates of gastric cancer remain high. Owing to the poor response of patients with gastric cancer to various existing treatment methods, new methods are needed to predict the efficacy of individual treatment. Patient-derived 3D cell spheroids are cultures with morphological structure and genetic characteristics similar to those of the source organ; it is derived from stem cells via self-renewal and self-organization in vitro in three-dimensional (3D) cell cultures and can display some functions [2]. In the cancer research field, patient-derived 3D tumor cell spheroids exhibit a high degree of consistency with the original tumor tissue [3,4]. Studies have demonstrated the endless potential of 3D tumor cell spheroids in clinical settings and have summarized the characteristics of individual differences in patients [5,6]. Patient-derived tumor cell spheroids retain the histological complexity and genetic heterogeneity of parental tumors, even after several passages, providing a wide range of applications for cancer research. 3D cell spheroids have tremendous potential for identifying optimal treatment strategies for individual patients. Further, compared with the tumor tissues of patients, 3D cell spheroids have functions highly similar to spatial and corresponding tissues and can imitate pathophysiological processes such as tumor growth, metastasis, abscission, differentiation, and gene mutation in vivo.
For gastric cancer, specimens of radical surgical resection as well as gastroscopic biopsy have been successfully used to develop organoid cultures [7]. Studies have used gastric cancer organoids as a preclinical model to potentially evaluate the efficacy of cancer therapeutics [8]. In the present study, we aimed to generate and provide a detailed analysis of 30 gastric cancer patient-derived 3D cell spheroids. Further, we aimed to provide an in-depth phenotypic and molecular characterization of the developed 3D cell spheroid lines and their parental tumors, including histological architecture, clinical marker expression, and genomic landscape. We showed that these 3D cell spheroids frequently retain tumor heterogeneity. We also showed that these patient-derived 3D cell spheroids are a reliable model for studying tumor evolution and treatment response in precision cancer medicine.
Materials and methods
Sample collection and transfer
All the collected gastric cancer tissue samples received informed consent and signed informed consent forms from all patients, and the study has been approved by the Ethics Committee of the Affiliated Hospital of Jining Medical University. Fresh tumor samples (n=35) were collected from patients with gastric cancer undergoing subtotal gastrectomy (tumor tissue should avoid the necrotic area, with a volume of >1 cm3). The samples were immersed in RPMI 1640 medium supplemented with 2% penicillin-streptomycin solution (Biological Industries, Shanghai, China) and transported to the laboratory on ice.
Tissue dissociation and 3D cell spheroid culture
Fat, fiber, and necrotic tissues were removed from the tumor tissue sample, and the tumor tissue was cut into small sections 2-4 mm in size. Then, these sections were dissociated into a primary single-cell suspension using the gentle MACS™ Dissociator and Tumor Dissociation Kit, human (MiltenyiBiotec, Gergisch-Gladbach, Germany). Next, the cells were cultured with the 3D cell spheroid medium. The basal culture media contained advanced DMEM/F12 (Gibco, Carlsbad, CA, USA), 1× B27 (Absin, Shanghai, China), 1× HEPES (Thermo Fisher, Waltham, USA), and 1× GlutaMAX (Thermo Fisher) and was supplemented with various growth factors and small molecules to prepare the full culture media. The growth factors and small molecules included were Wnt3α (SGE BIOTECH, Suzhou, China) conditioned media, 500 ng/mL R-spondin-1 (SGE BIOTECH) conditioned media, 100 ng/mL noggin (SGE BIOTECH), 1 mM/mL acetylcysteine (GLPBIO, California, USA), 10 mM/mL nicotinamide (TargetMol, Massachusetts, USA), 50 ng/mL EGF (PeproTech Inc., PeproTech, USA), 500 nM/mL A83-01 (TargetMol), 1 nM/mL SB202190 (MCE, New Jersey, USA), 10 nM/mL prostaglandin E2 (MCE), and 1× penicillin-streptomycin solution (Biological Industries). The culture media was replaced every 2-3 days. 3D spheroids were passaged typically once every week.
Hematoxylin-eosin (HE) and immunohistochemical (IHC) staining
Tissues and 3D cell spheroids were paraffin-sectioned using standard protocols. The collected 3D cell spheroids were fixed with 10% neutral formalin (Sigma Aldrich, Shanghai, China) for 90 min and centrifuged at 1000 rpm for 60 s. The supernatant was discarded, and the 3D cell spheroids were mixed with liquid 5% agarose gel and then placed on ice to solidify, followed by routine dehydration and embedding. Paraffin sections were then prepared for subsequent staining. HE staining was automatically performed using a machine following standard procedures.
For IHC staining, the paraffin sections were dewaxed and subjected to antigen repair, followed by the blocking of endogenous peroxidase. After blocking with goat serum (ZSGB-BIO, Beijing, China), the sections were incubated with the following primary antibodies: Her-2, p53, and E-cadherin (all from MXB, Fuzhou, China). The primary antibodies were incubated at 4°C overnight. The next day, the slides were washed with PBS three times, 5 min each time. Then, the sample was incubated with the secondary antibody (MXB) for 60 min. Two pathologists blinded to the clinical and pathological parameters or patient outcome independently measured the positivity ratio. The positivity ratio was calculated as the average percentage of positive cells in five random fields under a 400-fold microscope (Olympus, Japan). Interobserver differences were resolved by consensus review using a double-headed microscope after independent reviews.
Periodic acid-Schiff (PAS) staining
Paraffin sections were stained with the Schiff periodate staining kit (Beyotime, Shanghai, China). After the sample was oxidized with periodic acid, it was dyed with Schiff reagent and placed in an oven at 37°C in the dark for 45 min. Finally, the sample was stained with hematoxylin.
Transcriptome sequencing and analysis
Parent tissue and 3D cell spheroids were subjected to total RNA extraction using the Trizol reagent (Invitrogen, 15596-026) according to the manufacturer’s instructions. The isolated RNA was treated with DNase I (Promega, M6101) to remove residual genomic DNA. RNA purity, concentration, and integrity were assessed using the Nano Photometer spectrophotometer (IMPLEN), Qubit RNA Assay Kit in Qubit 2.0 Fluorometer (Life Technologies), and Nano 6,000 Assay Kit of the Bioanalyzer 2,100 system (Agilent Technologies), respectively. Then, 1 μg of RNA per sample was used to prepare libraries, which were generated using the NEB Next Ultra RNA Library Prep Kit for Illumina (NEB) following the manufacturer’s recommendations; the average insert size for the paired-end libraries was 300 bp (± 50 bp). To ensure the quality of the libraries, the effective concentration of the libraries (>2 nM) was accurately quantified via real-time quantitative PCR. Paired-end sequencing (PE150) was performed using Illumina Novaseq 6000 at Berry Genomics Co., Ltd. (Beijing, China) following the vendor’s recommended protocol. After base composition and quality tests, the adaptor sequence and sequences with a high content of unknown bases (unknown bases of >10%) or low-quality reads were removed. The clean reads were used for bioinformatic analysis using DESeq2 version 1.16.1.
Statistical analyses
The significance of the results was tested via Student’s t-test using commercially available software (GraphPad Prism). A p-value of <0.05 was considered statistically significant.
Drug sensitivity test of the 3D cell spheroids
To analyze the drug reaction with 3D cell spheroids, 3D cell spheroids were collected and passed through a 100-µm cell filter (Corning, Corning, USA) to remove large 3D cell spheroids. The cell spheroids were treated with 10-1000 µM/ml 5-FU or 10-1000 nM/mL trastuzumab. After incubation for 3 days at 37°C under 5% carbon dioxide, 20 µL of the Cell Counting Kit-8 reagent (GLPBIO) was added to each well, followed by incubation for 1.5 h. Next, the absorbance was measured at 450 nm using a microplate reader (BioTek, USA). Data analyses and IC50 values were performed and determined using the GraphPad Prism software.
Measurement of serum tumor markers levels
We collected the data of serum tumor markers levels including CEA, CA72-4, CA199, CA15-3, and CA125 from GC patients (GC33, GC25, GC35) who tested serum tumor markers in the clinical laboratory of Affiliated Hospital of Jining Medical University.
Results of abdominal CT examination in patients with gastric cancer
CT images of gastric cancer patients (GC33, GC25, GC35) who underwent abdominal CT examination were collected.
Results
Establishment of 3D cell spheroids using human gastric cancer tissue
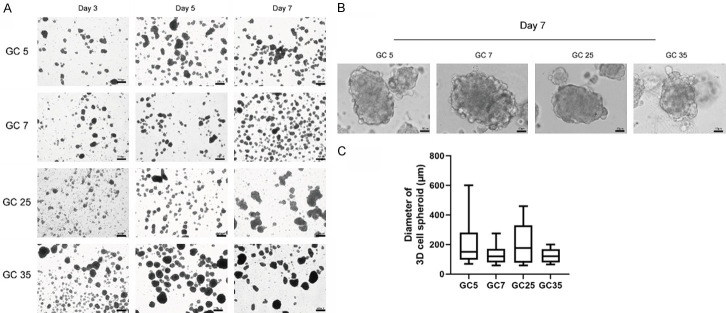
The tumor tissues of 35 patients with gastric cancer were collected. Finally, 30 tumor tissues were successfully cultured into 3D cell spheroids, with a success rate of 80% (consistent with the 70%-90% success rate reported in the literature [9]). The clinicopathologic features of all 35 patients with gastric cancer are shown in Table 1. We continuously observed the morphology of the 3D cell spheroids from 2-3 days of culture (Figure 1A). Gastric cancer 3D cell spheroids exhibited spheroid to asymmetric morphology. With time, the cell spheroids shifted from a relatively loose to a relatively aggregated pattern, and their size also increased (Figure 1A). On the seventh day of culture, the diameter of a single 3D spheroid reached approximately 100 µm (Figure 1B and 1C). The 3D cell spheroids were passaged every 1-2 weeks. These successfully developed spheroids were used for further experiments or were frozen and resuscitated.
Table 1.
Clinicopathological features of the 35 patients with gastric cancer
| Case | 3D cell spheroids | success | T stage | Sex | Age | Histopathological type | Degree of differentiation | Lymph node metastasis | MSS/MSI |
|---|---|---|---|---|---|---|---|---|---|
| 1 | GC1 | - | T3 | F | 65 | adenocarcinoma | Moderate | - | MSS |
| 2 | GC2 | + | T3 | M | 70 | adenocarcinoma | - | MSS | |
| 3 | GC3 | - | T4 | M | 69 | adenocarcinoma | Low | + | MSS |
| 4 | GC4 | + | T4 | M | 80 | adenocarcinoma | Moderate-Low | + | MSS |
| 5 | GC5 | + | T4 | M | 60 | Medullary carcinoma | + | ||
| 6 | GC6 | + | T3 | M | 65 | adenocarcinoma | Low | + | MSS |
| 7 | GC7 | + | T3 | M | 65 | adenocarcinoma | Moderate | - | MSS |
| 8 | GC8 | + | T3 | M | 52 | adenocarcinoma | Low | + | MSS |
| 9 | GC9 | + | T4 | M | 71 | adenocarcinoma | Low | + | |
| 10 | GC10 | - | T2 | M | 76 | adenocarcinoma | Well-Moderate | - | MSS |
| 11 | GC11 | + | T4 | M | 68 | adenocarcinoma | Moderate-Low | + | MSS |
| 12 | GC12 | + | T4 | M | 69 | adenocarcinoma | Moderate-Low | - | MSS |
| 13 | GC13 | + | T3 | M | 69 | adenocarcinoma | Low | + | MSS |
| 14 | GC14 | + | T3 | M | 49 | adenocarcinoma | Low | + | MSS |
| 15 | GC15 | + | T4 | M | 67 | adenocarcinoma | Low | + | MSS |
| 16 | GC16 | - | T3 | M | 56 | adenocarcinoma | Low | + | |
| 17 | GC17 | + | T3 | F | 74 | adenocarcinoma | Moderate-Low | + | MSS |
| 18 | GC18 | + | T4 | M | 52 | adenocarcinoma | + | MSS | |
| 19 | GC19 | + | T4 | M | 67 | adenocarcinoma | Moderate-Low | - | MSS |
| 20 | GC20 | + | T4 | M | 66 | adenocarcinoma | + | MSS | |
| 21 | GC21 | + | T4 | M | 57 | adenocarcinoma | Moderate | + | |
| 22 | GC22 | + | T3 | F | 33 | adenocarcinoma | Low | + | MSS |
| 23 | GC23 | + | T4 | M | 55 | adenocarcinoma | Well-Moderate | + | MSS |
| 24 | GC24 | + | T4 | M | 49 | adenocarcinoma | Moderate | + | MSI |
| 25 | GC25 | + | T3 | M | 51 | adenocarcinoma | Low | + | MSS |
| 26 | GC26 | + | T2 | M | 70 | adenocarcinoma | Well-Moderate | - | |
| 27 | GC27 | + | T4 | F | 64 | adenocarcinoma | Low | + | MSS |
| 28 | GC28 | + | T3 | M | 53 | adenocarcinoma | Low | - | MSS |
| 29 | GC29 | + | T3 | M | 64 | adenocarcinoma | Low | + | MSS |
| 30 | GC30 | + | T4 | M | 76 | adenocarcinoma | Moderate | + | MSS |
| 31 | GC31 | + | T3 | M | 66 | adenocarcinoma | Low | + | |
| 32 | GC32 | - | T3 | F | 65 | adenocarcinoma | Low | + | MSS |
| 33 | GC33 | + | T4 | M | 76 | adenocarcinoma | Well-Moderate | - | MSS |
| 34 | GC34 | + | T4 | F | 56 | adenocarcinoma | Moderate-Low | - | MSI |
| 35 | GC35 | + | T4 | M | 58 | adenocarcinoma | Moderate-Low | - | MSS |
Figure 1.
Microphotographs of the cultivated spheroids. A. Growth of the three-dimensional (3D) cell spheroids. B. Representative images of 3D cell spheroids cultured for 7 days. C. Diameters of the 3D spheroids.
Gastric cancer 3D cell spheroids maintain histological features of the parental tumor tissue
To compare the histological characteristics of 3D cell spheroids and parental tumor tissues, we collected 7-14-day-cultured 3D cell spheroids and their parental tumor tissues for HE staining. We observed that the histological features of parental tumors were highly recapitulated in the established 3D cell spheroids (Figure 2). For example, the 3D cell spheroids from poorly differentiated gastric cancer tissues (GC2) had similar morphology and exhibited low adhesion characteristics (Figure 2). The 3D cell spheroids derived from gastric cancer patients with mucinous adenocarcinoma (GC2) also produced a large amount of mucus (Figure 2).
Figure 2.
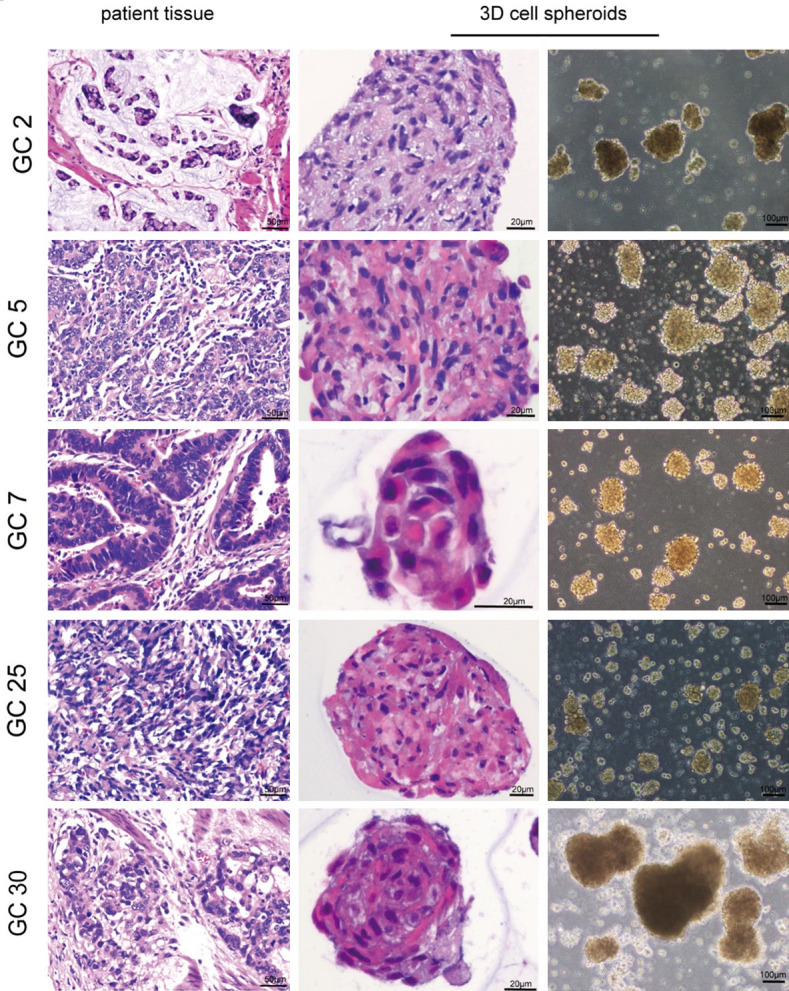
Histological characteristics of the three-dimensional (3D) cell spheroids derived from their parental tissue samples. Representative Hematoxylin-eosin (HE) staining images of the 3D cells spheroids and their parental tissues.
Gastric cancer patient-derived 3D cell spheroids are phenotypically similar to their parental tumor tissue
As human gastric cancer lesions exhibit histological heterogeneity, we further assessed whether the 3D cell spheroids originated from gastric cancer cells and whether they can recapitulate the parental tumor tissues. We selected whole spheroids, and parental tissue samples for HE staining and observed similar cell morphology between them (Figure 3A). As expected, cultured 3D cell spheroids could maintain the phenotype of parental tumors. IHC staining of Her-2 of whole spheroid and parental samples revealed the consistency of negative expression of Her-2 in tumor tissues and 3D cell spheroids (Figure 3B). Similarly, both the parental and spheroids sample’s expression of p53 was wild-type (Figure 3C). PAS staining of the 3D cell spheroids and their parental tissues revealed the presence of mucin in both samples, suggesting that the 3D cell spheroids fully display the characteristics of the parental tumor (Figure 3D, 3E). We selected the typical Epithelial marker E-cadherin and performed IHC staining on the 3D cell spheroids. We observed the consistency of positive expression of E-cadherin in tumor tissues and 3D cell spheroids (Figure 3E), further confirming similarities between the parental samples and 3D cell spheroids. Overall, by comparing the histological and IHC staining results of the 3D cell spheroids and their parental tissues, we successfully demonstrated that the 3D cell spheroids are derived from gastric cancer tissue and recapitulate the characteristics of the primary cancer tissue.
Figure 3.
Gastric cancer three-dimensional (3D) cell spheroids recapitulate the characteristics of the parental tissues. A. HE staining of the 3D cell spheroids and patient tumor tissue. B. Representative Her-2 staining of the 3D cell spheroids and patient tumor tissue. C. Representative images and quantification of p53 immunostaining of the 3D cell spheroids and patient tumor tissue. D. Representative images and quantification of PAS staining of the 3D cell spheroids and patient tumor tissue. E. Representative images of HE staining, E-cadherin immunostaining, and PAS staining of the 3D cell spheroids. Bar = 20 µm.
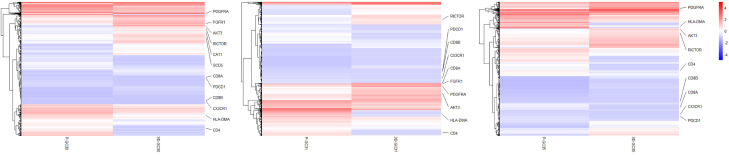
Gastric cancer 3D cell spheroids resemble the gene expression of the parental tumor tissue
To determine whether our 3D cell spheroids retained the gene expression profile of the parental tumor tissues, RNA sequencing of three pairs of 3D cell spheroids and the corresponding fresh-frozen gastric cancer tissues was performed. The result showed that the 3D cell spheroids derived from tumor tissues faithfully recapitulated the main gene expression signatures of cancer tissues in vivo (Figure 4). Tumor-derived 3D cell spheroids, as expected, expressed in vivo tumor-specific genes, including many that have been widely linked to gastric cancer progression and metastasis, such as PDGFRA, AKT3, SCD5, RICTOR and FGFR1. Besides, the downregulated genes in the 3D cell spheroids (such as CD4, CD8, PDCD1, HL-DMA, and CX3CR1) were mainly associated with immune and inflammatory responses. This is consistent with the lack of immune responses within 3D cell spheroids (Figure 4).
Figure 4.
Transcriptome analysis of gastric cancer tissues and the three-dimensional (3D) cell spheroids: heatmap analysis demonstrated that 3D cell spheroids derived from tumor tissues faithfully replicate the main gene expression signatures of cancer cells in vivo.
Drug susceptibility testing of gastric cancer patient-derived 3D cell spheroids
GC patients with microsatellite stability (MSS) were found to be more sensitive to 5-FU. Three cases of 3D cell spheroids with MSS status (GC25, GC33, and GC35) were tested for the 5-FU drug sensitivity. The results revealed that the above 3D cell spheroids were sensitive to 5-FU (IC50 = 5.74 ± 0.25, 11.16 ± 1.13, and 3.38 ± 0.13 µM, respectively) (Figure 5A). GC patients with positive Her-2 expression were found to be more sensitive to trastuzumab. Two cases of 3D cell spheroids with negative Her-2 expression (GC7 and GC30) were tested for the trastuzumab drug sensitivity. After 6 days of administration, it was found that the 3D cell spheroids were insensitive to trastuzumab (Figure 5B), confirming the efficacy of the 3D cell spheroid model in anti-cancer drug sensitivity testing.
Figure 5.
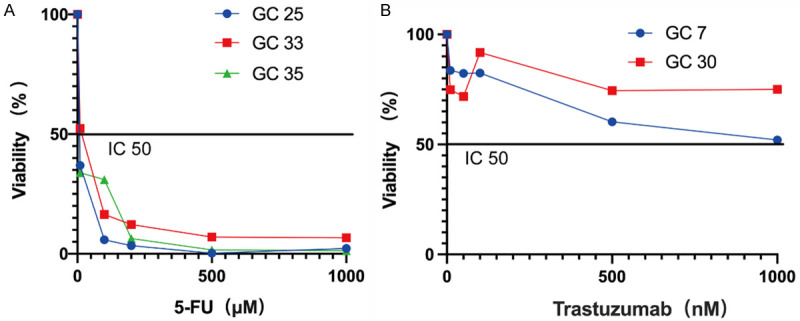
3D cell spheroids used for predicting therapeutic responses. A. Dose-response curves after 3 days of treatment with 5-FU. B. Dose-response curves after 3 days of treatment with trastuzumab.
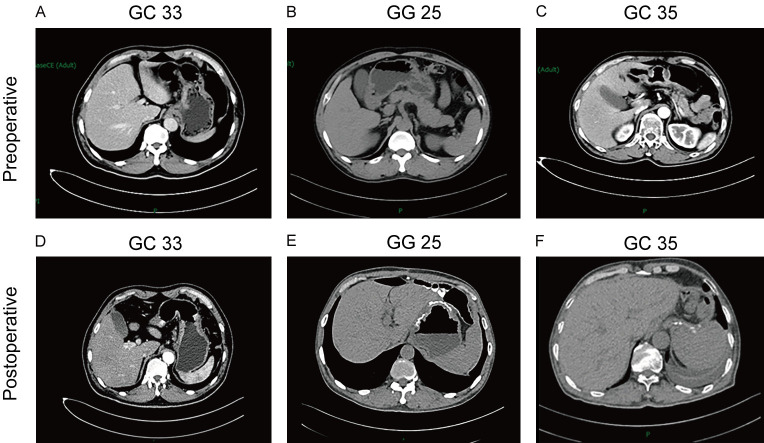
As mentioned above, the 3D cell spheroids with MSS status (GC25, GC33, and GC35) were tested to be sensitive to 5-FU. To further verify the reliability of the culture model, clinical observation of these three patients was performed. The three patients who were clinically treated with 5-FU clinically were postoperatively followed up on for 11, 18, 14 months, respectively. The patient’s blood CEA, CA72-4, CA199, CA15-3, and CA125 levels are presented in Table 2 which showed no progression in the three patients. Abdominal CT revealed the absence of abnormal lymph nodes around the stomach of these patients, with no metastases or implants in other abdominal organs (Figure 6). Physical examination revealed the absence of ascites. The above results indicated that clinical efficacy is consistent with drug sensitivity test in vitro.
Table 2.
Levels of serum tumor markers in postoperative patients with gastric cancer
| Case | Follow-up period (months) | CEA (U/mL) | CA72-4 (ng/mL) | CA199 (U/mL) | CA153 (U/mL) | CA125 (U/mL) |
|---|---|---|---|---|---|---|
| GC33 | 18 | 0.88 | 1.98 | 11.85 | 5.5 | 12.9 |
| GC25 | 11 | 1.35 | 0.17 | 3.01 | 6.6 | 5.1 |
| GC35 | 14 | 1.23 | 3.22 | 6.21 | - | - |
Reference value: CEA (0-5 U/mL), CA72-4 (0-6 ng/mL), CA199 (0-37 U/mL), CA153 (0-25 U/mL), CA125 (0-35 U/mL).
Figure 6.
Representative images of the abdomen CT in patients with gastric cancer. A-C. Preoperative abdominal CT images of gastric cancer patients. D-F. Postoperative abdominal CT images of GC patients (Follow-up time was 18, 11, and 14 months, respectively).
Discussion
Compared with the traditional two-dimensional culture and tumor-bearing animal experiments, the success rate of 3D cell spheroid culture is significantly higher, facilitating gene modification and drug screening. 3D cell spheroids retain the tissue characteristics of a part of the tumor microenvironment [5], thereby providing a more realistic environment for tumor drug development. In May 2018, the world’s first cancer organoid biological bank was published in Cell [10]. More importantly, tumor heterogeneity was preserved during in vitro culture [11,12].
In this study, we described the development of 3D cell spheroids using tumor tissues derived from patients with gastric cancer and collated the clinicopathological information of the selected patients, which summarized the characteristics of the parental tumors. In a 3D cell spheroid medium supplemented with growth factors, we successfully established a 3D cell spheroid biobank of 30 patients with gastric cancer with different differentiation degrees and pathological types (success rate = 85%). In our study, we not only successfully established gastric cancer 3D cell spheroids but also realized the cryopreservation and resuscitation of 3D cell spheroids. The cell spheroids retained the characteristic histological features of the parental tumor tissue, as assessed by light microscopy and HE staining. HE staining of whole spheroids revealed that the cells exhibited the typical morphology of gastric cancer cells, with large and hyperchromatic nuclei, and abundant cytoplasm in some cells.
Our study suggest that 3D cell spheroids derived from patients with gastric cancer can represent the parental tumor tissue. Gastric cancer 3D cell spheroids retained significant tumor heterogeneity, and their phenotypes and genes are consistent with the parent tissues during culture. The transcriptional sequencing analysis revealed the downregulated genes in the 3D cell spheroids (such as CD4, CD8, PDCD1, HL-DMA, and CX3CR1) were mainly associated with immune and inflammatory responses. This is consistent with the lack of an immune microenvironment in organoid culture which has been previously reported by researchers [13,14]. Recently, many studies on 3D culture have been performed with significant progress [15-17]. Compared with cell lines, 3D cell spheroids facilitate the generation of both tumor and normal 3D cell spheroid lines from one patient, making it possible to investigate the side effects of drugs on normal spheroids and help design targeted agents with higher selectivity. As an advanced cancer screening model, 3D cell spheroids are more reliable for predicting drug efficacy and toxicity. Yoshimasa et al. successfully screened 22 compounds from a group of 339 drugs that significantly inhibited cholangiocarcinoma organoids; two of these drugs were non-toxic or had low toxicity toward normal bile duct epithelial cell-derived organoids [18]. Several laboratories have established gastric cancer 3D cell cultures from different histological and clinical stages as preclinical models for therapeutic drug screening [19]. Therefore, 3D cell spheroid cultures for drug sensitivity screening can be used as a reliable tool to guide patient medication.
In the case of gastric cancer, patients are usually diagnosed in the early stages of disease progression [20,21]. Patients with non-advanced gastric cancer often undergo multiple excisions and treatments to avoid the impact of gastrectomy and its hazards on the quality of life [22]. The drug sensitivity data closest to the actual situation can be obtained by testing chemotherapeutic drugs on patient-derived 3D cell spheroids [8]. Through the drug sensitivity test for gastric cancer patients with MSS, follow-up observation of the patients, and determination of the effect of chemotherapy on patients, our study confirmed that the patient-derived 3D cell spheroids could be used as a reliable model to guide drug usage among patients. In the next study, we will continue conducting drug sensitivity tests on more samples to provide a more reliable basis for selecting chemotherapeutic drugs for patients with gastric cancer.
A notable feature of primary gastric cancer that is different from most solid tumors is the ability to obtain tumor samples from the same patient at multiple distinct disease states. In many cases, patients with recurrent or metastatic gastric cancer undergo surgical resection, and each resection provides an opportunity to generate corresponding 3D cell spheroids; this is feasible owing to the relatively high efficiency of the establishment of 3D cell spheroids. In future studies, these 3D cell spheroids from patients with recurrent or metastatic gastric cancer can be excellent model systems to investigate tumor evolution and drug response in primary solid tumors.
Acknowledgements
Supported by the National Natural Science Foundation of China (81972629), The Taishan Scholars Program of Shandong Province (tsqn201909193), Shandong Youth Innovation and Technology program (2020KJL003), Shandong Medical and Health Science and Technology Development Plan Project (202104080301) and Jining Research and Development Program (2021YXNS065, 2021YXNS075).
Disclosure of conflict of interest
None.
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Tuveson D, Clevers H. Cancer modeling meets human organoid technology. Science. 2019;364:952–955. doi: 10.1126/science.aaw6985. [DOI] [PubMed] [Google Scholar]
- 3.Lo YH, Karlsson K, Kuo CJ. Applications of organoids for cancer biology and precision medicine. Nat Cancer. 2020;1:761–773. doi: 10.1038/s43018-020-0102-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lancaster MA, Huch M. Disease modelling in human organoids. Dis Model Mech. 2019;12:dmm039347. doi: 10.1242/dmm.039347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li G, Ma S, Wu Q, Kong D, Yang Z, Gu Z, Feng L, Zhang K, Cheng S, Tian Y, Zhang W. Establishment of gastric signet ring cell carcinoma organoid for the therapeutic drug testing. Cell Death Discov. 2022;8:6. doi: 10.1038/s41420-021-00803-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yan HHN, Siu HC, Law S, Ho SL, Yue SSK, Tsui WY, Chan D, Chan AS, Ma S, Lam KO, Bartfeld S, Man AHY, Lee BCH, Chan ASY, Wong JWH, Cheng PSW, Chan AKW, Zhang J, Shi J, Fan X, Kwong DLW, Mak TW, Yuen ST, Clevers H, Leung SY. A comprehensive human gastric cancer organoid biobank captures tumor subtype heterogeneity and enables therapeutic screening. Cell Stem Cell. 2018;23:882–897. e811. doi: 10.1016/j.stem.2018.09.016. [DOI] [PubMed] [Google Scholar]
- 7.Song H, Park JY, Kim JH, Shin TS, Hong SA, Huda MN, Kim BJ, Kim JG. Establishment of patient-derived gastric cancer organoid model from tissue obtained by endoscopic biopsies. J Korean Med Sci. 2022;37:e220. doi: 10.3346/jkms.2022.37.e220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Steele NG, Chakrabarti J, Wang J, Biesiada J, Holokai L, Chang J, Nowacki LM, Hawkins J, Mahe M, Sundaram N, Shroyer N, Medvedovic M, Helmrath M, Ahmad S, Zavros Y. An organoid-based preclinical model of human gastric cancer. Cell Mol Gastroenterol Hepatol. 2019;7:161–184. doi: 10.1016/j.jcmgh.2018.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boehnke K, Iversen PW, Schumacher D, Lallena MJ, Haro R, Amat J, Haybaeck J, Liebs S, Lange M, Schäfer R, Regenbrecht CR, Reinhard C, Velasco JA. Assay establishment and validation of a high-throughput screening platform for three-dimensional patient-derived colon cancer organoid cultures. J Biomol Screen. 2016;21:931–941. doi: 10.1177/1087057116650965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sachs N, de Ligt J, Kopper O, Gogola E, Bounova G, Weeber F, Balgobind AV, Wind K, Gracanin A, Begthel H, Korving J, van Boxtel R, Duarte AA, Lelieveld D, van Hoeck A, Ernst RF, Blokzijl F, Nijman IJ, Hoogstraat M, van de Ven M, Egan DA, Zinzalla V, Moll J, Boj SF, Voest EE, Wessels L, van Diest PJ, Rottenberg S, Vries RGJ, Cuppen E, Clevers H. A living biobank of breast cancer organoids captures disease heterogeneity. Cell. 2018;172:373–386. e310. doi: 10.1016/j.cell.2017.11.010. [DOI] [PubMed] [Google Scholar]
- 11.Schütte M, Risch T, Abdavi-Azar N, Boehnke K, Schumacher D, Keil M, Yildiriman R, Jandrasits C, Borodina T, Amstislavskiy V, Worth CL, Schweiger C, Liebs S, Lange M, Warnatz HJ, Butcher LM, Barrett JE, Sultan M, Wierling C, Golob-Schwarzl N, Lax S, Uranitsch S, Becker M, Welte Y, Regan JL, Silvestrov M, Kehler I, Fusi A, Kessler T, Herwig R, Landegren U, Wienke D, Nilsson M, Velasco JA, Garin-Chesa P, Reinhard C, Beck S, Schäfer R, Regenbrecht CR, Henderson D, Lange B, Haybaeck J, Keilholz U, Hoffmann J, Lehrach H, Yaspo ML. Molecular dissection of colorectal cancer in pre-clinical models identifies biomarkers predicting sensitivity to EGFR inhibitors. Nat Commun. 2017;8:14262. doi: 10.1038/ncomms14262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pauli C, Hopkins BD, Prandi D, Shaw R, Fedrizzi T, Sboner A, Sailer V, Augello M, Puca L, Rosati R, McNary TJ, Churakova Y, Cheung C, Triscott J, Pisapia D, Rao R, Mosquera JM, Robinson B, Faltas BM, Emerling BE, Gadi VK, Bernard B, Elemento O, Beltran H, Demichelis F, Kemp CJ, Grandori C, Cantley LC, Rubin MA. Personalized in vitro and in vivo cancer models to guide precision medicine. Cancer Discov. 2017;7:462–477. doi: 10.1158/2159-8290.CD-16-1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ren X, Chen W, Yang Q, Li X, Xu L. Patient-derived cancer organoids for drug screening: basic technology and clinical application. J Gastroenterol Hepatol. 2022;37:1446–1454. doi: 10.1111/jgh.15930. [DOI] [PubMed] [Google Scholar]
- 14.Li Z, Xu H, Yu L, Wang J, Meng Q, Mei H, Cai Z, Chen W, Huang W. Patient-derived renal cell carcinoma organoids for personalized cancer therapy. Clin Transl Med. 2022;12:e970. doi: 10.1002/ctm2.970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yuki K, Cheng N, Nakano M, Kuo CJ. Organoid models of tumor immunology. Trends Immunol. 2020;41:652–664. doi: 10.1016/j.it.2020.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boucherit N, Gorvel L, Olive D. 3D tumor models and their use for the testing of immunotherapies. Front Immunol. 2020;11:603640. doi: 10.3389/fimmu.2020.603640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang J, Feng X, Li Z, Chen Y, Huang W. Patient-derived organoids as a model for tumor research. Prog Mol Biol Transl Sci. 2022;189:259–326. doi: 10.1016/bs.pmbts.2022.03.004. [DOI] [PubMed] [Google Scholar]
- 18.Saito Y, Muramatsu T, Kanai Y, Ojima H, Sukeda A, Hiraoka N, Arai E, Sugiyama Y, Matsuzaki J, Uchida R, Yoshikawa N, Furukawa R, Saito H. Establishment of patient-derived organoids and drug screening for biliary tract carcinoma. Cell Rep. 2019;27:1265–1276. e4. doi: 10.1016/j.celrep.2019.03.088. [DOI] [PubMed] [Google Scholar]
- 19.Fan H, Demirci U, Chen P. Emerging organoid models: leaping forward in cancer research. J Hematol Oncol. 2019;12:142. doi: 10.1186/s13045-019-0832-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nagtegaal ID, Odze RD, Klimstra D, Paradis V, Rugge M, Schirmacher P, Washington KM, Carneiro F, Cree IA WHO Classification of Tumours Editorial Board. The 2019 WHO classification of tumours of the digestive system. Histopathology. 2020;76:182–188. doi: 10.1111/his.13975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim J, Koo BK, Knoblich JA. Human organoids: model systems for human biology and medicine. Nat Rev Mol Cell Biol. 2020;21:571–584. doi: 10.1038/s41580-020-0259-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li S, Shan F, Zhang X, Li Y, Sun Y, Tang L, Wu Q, Yang W, Yang J, An Y, Deng M, Ji J. Chinese quality control indices for standardized diagnosis and treatment of gastric cancer (2022 edition) Chin J Cancer Res. 2022;34:623–632. doi: 10.21147/j.issn.1000-9604.2022.06.10. [DOI] [PMC free article] [PubMed] [Google Scholar]