Abstract
Osteosarcoma (OS) is the most common malignant tumor of the bone tissue with the lowest survival rate among all pediatric cancers. OS cells grow vigorously under malnutrition; however, the mechanism by which they adapt to metabolic stress via metabolic reprogramming remains undefined. Here, we demonstrated that USP33, a member of the DUBs family, was significantly upregulated in the tissues of patients with OS compared to normal tissues. Moreover, high USP33 expression was significantly associated with poor survival. Functional assays suggested that USP33 promoted OS cell growth through the induction of aerobic glycolysis. Additionally, we confirmed that 6-phosphofructo-2-kinase/fructose-2, 6-biphosphatase 3 (PFKFB3) was critical for USP33-induced proliferation and aerobic glycolysis in OS cells, and the protein expression levels of PFKFB3 and USP33 were positively correlated in the OS tissues. Mechanistically, USP33 stabilized the expression of PFKFB3 by suppressing the ubiquitin mediated PFKFB3 degradation. Collectively, these findings reveal a mechanism by which OS cells survive in a dystrophic tumor microenvironment, with the USP33-PFKFB3 axis as a critical driver of aerobic glycolysis and OS proliferation. Furthermore, these findings reveal novel insights into the adaptation of cancer cells to metabolic stress in OS.
Keywords: Osteosarcoma, deubiquitinase, USP33, PFKFB3, ubiquitination
Introduction
Osteosarcoma (OS) is a malignant tumor derived from mesenchymal stem cells [1], most commonly found in children and adolescents [2,3]. Approximately 80% of all OS cases occur in the long bone of the extremities, especially in the epiphysis of the long shaft around the knee joint [4], with an annual incidence rate of 14/1 million. Currently, the standard treatment for OS is surgical resection followed by adjuvant chemotherapy [5,6]; however, the patient prognosis remains poor, with a five-year survival rate ranging from 37.5% to 77.6%. In addition, the recurrence rate remains high after treatment, with the 10-year survival rate of less than 50% [7,8]. Thus, investigating the molecular mechanism of OS and identifying key molecular targets of OS will unravel a novel direction for the effective treatment of patients with OS.
The abnormal glycolysis pathway is a vital feature of tumor cells, wherein it promotes tumor cell progression [9]. Notably, 6-phosphofructo-2-kinase/fructose-2, 6-biphosphatase 3 (PFKFB3), a key enzyme in the synthesis of fructose 2,6-diphosphate (F-2,6-DP), plays a vital role in cancer cell glycolysis and is widely expressed in numerous cells [10]. Moreover, F-2,6-DP is considered the most potent PFK-1 (6-phosphofructo-l-kinase) activator and glycolysis inducer [11,12]. Importantly, a significant upregulation of PFKFB3 has been reported in various cancers, including OS. Furthermore, PFKFB3 has been identified to be closely related to the poor prognosis and aggressiveness in cancers [13,14]. Similarly, high PFKFB3 expression is related to the progression and metastasis of OS [15,16]. Therefore, elucidating the regulatory mechanisms of PFKFB3 could shed light on the pathogenesis of OS and aid in developing novel therapeutic strategies for the treatment of this deadly disease.
Ubiquitination has been reported to play a critical role in inducing intracellular signal transduction and therefore may serve as new therapeutic target [17,18]. Deubiquitination, the reverse process of ubiquitination, is mediated by a group of proteins known as deubiquitinating enzymes [19,20]. To date, more than 100 types of deubiquitinase have been reported, among which deubiquitinase USP33 has recently been reported to be strongly correlated with tumor progression and invasion [21,22]. Mechanistically, USP33 can deubiquitinate substrates to antagonize the ubiquitination mediated by ubiquitin ligase [23-25]. USP33 is highly expressed in various tumor tissues, such as retinoblastoma, glioma, prostate cancer, and liver cancer [21,23,26,27]. Furthermore, USP33 can regulate DUSP1 and consequently alter the chemosensitivity of prostate cancer cells [27]. Moreover, Gan et al. has demonstrated that USP33 facilitates hepatocellular carcinoma metastasis via upregulating c-met expression [23]. However, studies have also reported that USP33 plays a tumor suppressor role in some tumors. For example, USP33 inhibits colorectal cancer cell migration via the slit Robo signalling pathway [25]. Importantly, we found that high USP33 protein expression was remarkably associated with poor OS of Sarcoma patient tissues by The Cancer Genome Atlas (TCGA) dataset, which was evaluated with the software of GEPIA (http://gepia.cancer-pku.cn/) (Supplementary Figure 1). These studies indicate that USP33 plays an essential role in sarcoma development and progression; nonetheless, the specific function of USP33 in OS progression remains to be determined.
In this study, we aimed to examine the biological function and prognostic value of USP33 as well as the underlying mechanisms in OS. Accordingly, we assessed the expression of USP33 in OS tissues and determined the association between the expression of USP33 and the patient survival as well as clinicopathological characteristics. We further investigated the effects of USP33 on the proliferation of OS in vitro and in vivo.
Materials and methods
Patient samples
A total of 86 OS tissues and their adjacent paracancerous tissues were obtained from the Second Affiliated Hospital of Nanchang University. The OS and adjacent tissue samples were stored at -80°C for further western blot and quantitative real-time polymerase chain reaction (qRT-PCR) analyses and fixed with 4% formaldehyde for immunohistochemistry (IHC) analysis. The clinical samples and clinical information used in this study were approved by the Clinical Research Ethics Committee of the Second Affiliated Hospital of Nanchang University, and informed consent was obtained from each patient.
Cell culture
Normal human osteoblasts cell line hfoBI-19 as well as the human OS cell lines, 143B, U2-OS, Saos-2 and MG-63, were obtained from the Shanghai Institute of Cell Biology, China. All cell lines were evaluated to confirm the authenticity by using short tandem repeat analysis in a cell bank. hfoBI-19 and all OS cell lines were cultured in DMEM (Gibco, Grand Island, NY, USA) supplemented with 10% foetal bovine serum.
qRT-PCR
Total RNA was extracted from OS tissues and OS cells using TRIzol (Invitrogen). PrimeScript RT kit (Invitrogen) was used to reverse transcribe RNA into cDNA. qRT-PCR was performed by using SYBR Green PCR Kit (TaKaRa, Dalian, China) on an ABI PRISM 7900 Sequence Detector (Applied Biosystems, Foster City, CA, USA). The primer sequences used were as follows: USP33 (Forward: 5’-ATCTGTTGTGCCTACTACTC-3’, Reverse: 5’-GCTCTTGTCTTCTTCCATTG-3’); GAPDH (Forward: 5’-AATCCCATCACCATCTTC-3’, Reverse: 5’-AGGCTGTTGTCATACTTC-3’); PFKFB3 (Forward: 5’-ATTGCGGTTTTCGATGCCAC-3’; Reverse: 5’-GCCACAACTGTAGGGTCGT-3’).
Western blot
Briefly, the total protein of OS tissues and OS cells was extracted using RIPA buffer (R0010; Solarbio) and quantified by BCA protein detection kit (Beyotime Biotechnology Co., Ltd, China). Next, the protein lysates (20 µg) were separated on SDS-PAGE and transferred onto a PVDF membrane. These membranes were then blocked with skimmed milk at room temperature for 2 h followed by incubation with a primary antibody overnight at 4°C. After extensive washing thrice with TBST (0.1% Tween-20), the membrane was incubated with HRP conjugated goat anti-rabbit IgG secondary antibody (1:5000; cat. No. ab6728; Abcam) overnight at 4°C. Finally, the ECL system and ImageJ software were used to detect and analyze the protein bands, respectively. The primary antibodies used were as follows: USP33 (1:1000, No. ab237510, Abcam), PFKFB3 (1:1000, No. ab181861, Abcam), and GADPH (1:5000, No. ab181602, Abcam).
shRNA constructs
USP33-overexpressing plasmid and the plasmids of shRNA for PFKFB3 and USP33 as well as the negative control were obtained from Gene Pharma (Shanghai, China). The previously validated shRNA sequences were as follows: shUSP33#1: 5’-GGACCAAAUCUUUGGGCAUUU-3’; shUSP33#2: 5’-GCCUACUACUCUGUUUCAAUU-3’; shNC: 5’-CAGUACUUUUGUGUAGUACAA-3’; shPFKFB3#1: 5’-AGUUGUAGGAGCUGUACUG-3’; shPFKFB3#2: 5’-CGGGTGCATGATTGTGCTTAA-3’. All plasmids were transfected using Lipofectamine 3000 transfection reagent (Invitrogen, USA) according to the manufacturer’s instruction.
Cell counting kit-8 (CCK-8) assay
The OS cells were seeded into 96 well plates at a density of 1×104 cells/well and cultured for 36 h. Cell proliferation was measured by CCK-8 assay. After incubation for 2 h at 37°C, the absorbance was determined using a microplate reader iMark (Bio-Rad) at 450 nm.
Statistical analysis
The data were described as the mean ± standard deviation of three individual tests. After analysis of variance, Tukey’s test and unpaired Student’s t-test were employed for evaluating the differences between multiple groups and two groups, respectively. Additionally, the association between the expression of USP33 and the clinicopathological characteristics of patients with OS was explored using Fisher’s exact test. Furthermore, GraphPad Prism 8.0 (GraphPad Software, Inc.) and SPSS 21.0 (IBM Corp.) were employed for all graphic construction and statistical calculations, respectively. P < 0.05 indicated statistical significance.
Results
Overexpression of USP33 was related to poor prognosis
We first determined the expression of USP33 in 26 OS tissues and adjacent tissues by qRT-PCR and western blot. qRT-PCR showed that USP33 mRNA level was upregulated in OS tissues compared with the adjacent tissues (Figure 1A, 1B). Consistently, western blot also revealed higher protein levels of USP33 in OS (Figure 1C). Furthermore, IHC analysis demonstrated that compared to adjacent tissues, USP33 protein level in OS tissues was upregulated (Figure 1D). Together, these data indicated that USP33 expression was increased in OS tissues compared to adjacent normal tissues.
Figure 1.
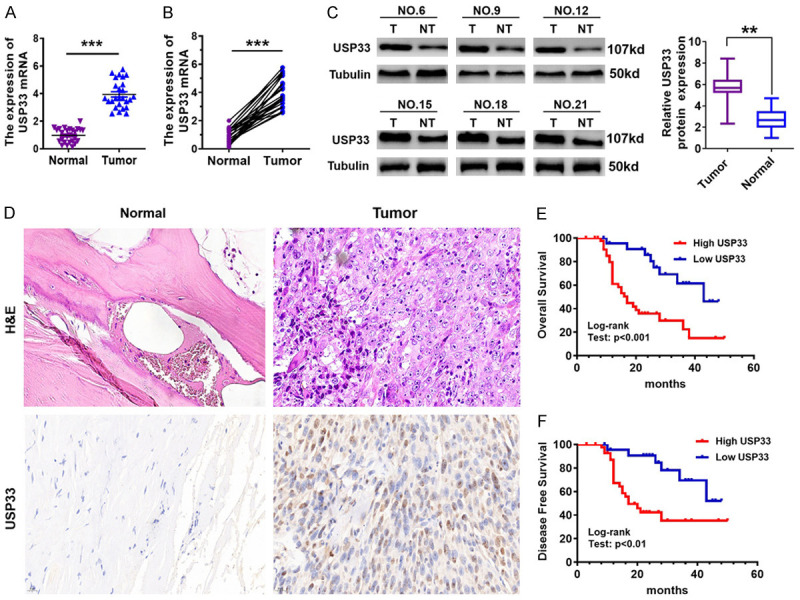
Overexpression of USP33 in osteosarcoma (OS) is strongly associated with the poor prognosis of patients. A, B. The USP33 mRNA levels in OS tissues and the adjacent normal tissues was analyzed by qRT-PCR. ***P < 0.001. C. The USP33 protein levels in OS tissues and adjacent normal tissues were determined by western blot. **P < 0.01. D. Representative images of IHC and H&E staining of USP33 in OS tissues and adjacent normal tissues. Scale bar, 20 μm. E, F. According to the expression level of USP33, Kaplan-Meier plots showed the probability of progression-free and overall survival of patients with OS.
Subsequently, we evaluated the association between the clinicopathological factors and USP33 expression in 86 patients with OS (Table 1). Although no clear correlation was observed between the expression of USP33 and age, sex and location, a significant association with Clinical stage (P < 0.001) and tumour size (P < 0.001) was observed. Furthermore, the role of USP33 expression as a predictor for patient survival and the correlation between USP33 expression level and patient survival were examined. As displayed in Figure 1E, 1F, Kaplan-Meier analysis revealed that OS specimens with high USP33 expression were significantly correlated with poorer overall survival and disease-free survival than those with low USP33 expression, suggesting that USP33 might be utilized as a potential prognostic factor for human OS.
Table 1.
Relationship between USP33 expression and clinicopathological features
| Parameters | n | USP33 expression | P value | |
|---|---|---|---|---|
|
| ||||
| Low (n = 30) | High (n = 56) | |||
| Age (years) | P = 0.808 | |||
| ≤ 18 | 70 | 24 | 46 | |
| > 18 | 16 | 6 | 10 | |
| Sex | P = 0.875 | |||
| Female | 44 | 15 | 29 | |
| Male | 42 | 15 | 27 | |
| Tumor size(cm) | P < 0.0001 | |||
| < 3 | 36 | 26 | 10 | |
| ≥ 3 | 50 | 4 | 46 | |
| Location | P = 0.119 | |||
| Upper limb bone | 58 | 17 | 41 | |
| Lower limb bone | 28 | 13 | 15 | |
| Clinical stage | P < 0.0001 | |||
| I/II | 31 | 20 | 11 | |
| III/IV | 55 | 10 | 45 | |
| Pathological differentiation | P = 0.353 | |||
| Well/Moderately | 40 | 16 | 24 | |
| Poor | 46 | 14 | 32 | |
| Recurrence | P = 0.094 | |||
| Absence | 41 | 18 | 23 | |
| Presence | 45 | 12 | 33 | |
USP33 facilitated the proliferation of OS cells in vitro
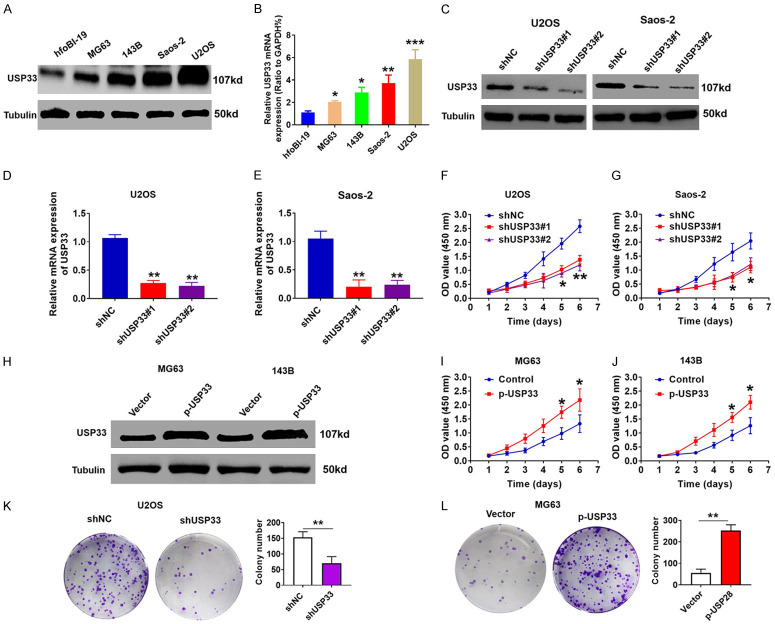
We also analyzed the expression of USP33 in OS cell lines as well as in normal hfoBI-19 osteoblasts by qRT-PCR and western blot and found a significantly higher USP33 expression in OS cell lines than in normal osteoblasts (Figure 2A, 2B). To assess the role of USP33 in cell proliferation, we transfected USP33-specific short hairpin RNA plasmids (shUSP33#1 and shUSP33#2) and control shNC plasmids into Saos-2 and U2OS cells, and the efficient knockdown of USP33 was confirmed by western blot and qRT-PCR (Figure 2C-E). Compared with the shNC group, the cell proliferation of shUSP33 group was significantly attenuated, as determined by CCK-8 assays (Figure 2F, 2G), while overexpressing USP33 in MG63 and 143B cells significantly enhanced cell proliferation (Figure 2H-J). Consistently, the colony formation assays also revealed that the knockdown of USP33 reduced the U2OS cell proliferation (Figure 2K), while USP33 overexpression increased the MG63 cell proliferation (Figure 2L). Together, these data revealed that USP33 exerts an oncogenic role in facilitating the growth of OS cells.
Figure 2.
USP33 promotes osteosarcoma (OS) cell proliferation. A, B. The USP33 expression levels in normal hfoBI-19 osteoblasts and four OS cell lines determined by qRT-PCR and western blot. C. The protein levels of USP33 in USP33 knockdown Saos-2 and U2OS stable cells determined by western blot. D, E. The mRNA levels of USP33 in USP33 knockdown Saos-2 and U2OS stable cells determined by qRT-PCR. F, G. The viability of USP33 knockdown Saos-2 and U2OS stable cells determined by CCK-8 assay. *P < 0.05, **P < 0.01. H. The protein levels of USP33 in p-USP33-overexpressing 143B and MG-63 stable cells determined by western blot. I, J. The viability of p-USP33-overexpressing 143B and MG-63 stable cells determined by CCK-8 assay. *P < 0.05, **P < 0.01. K. Colony formation analysis of USP33 knockdown U2OS stable cells. **P < 0.01. L. Colony formation analysis of USP33 knockdown MG-63 stable cells. **P < 0.01.
USP33 promoted aerobic glycolysis in OS cells
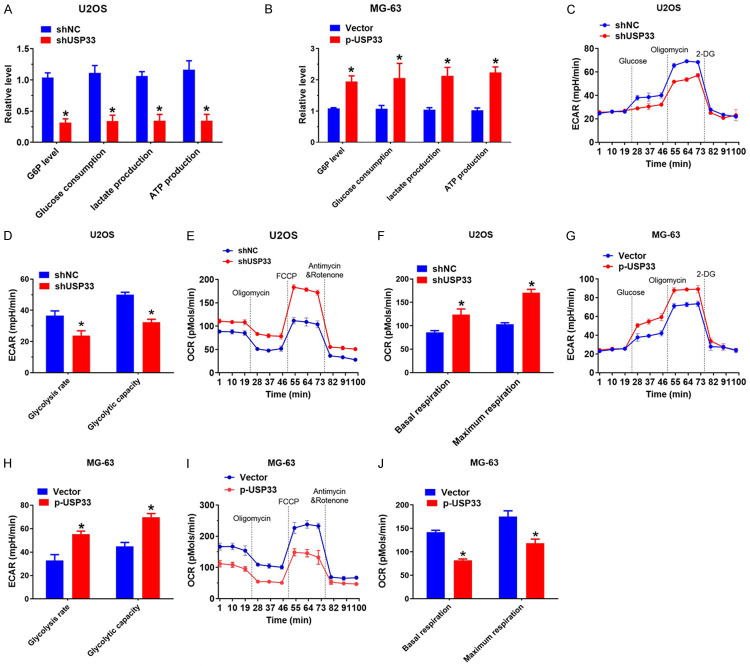
Given that aerobic glycolysis is a hall mark of cancer cells, we examined the role of USP33 in the glucose metabolism of OS cells. As shown in Figure 3A and 3B, Glucose-6-phosphate (G6P) production, lactate generation, glucose consumption and the cellular level of ATP were significantly reduced in U2OS cells with USP33 knockdown, whereas USP33 overexpression produced the opposite effect in MG-63 cells. To further validate the effect of USP33 on the glycolysis of OS, the extracellular acidification rate (ECAR) was determined, which revealed the overall glycolytic flux. As expected, the knockdown of USP33 attenuated the capacity and rate of glycolysis in U2OS cells (Figure 3C, 3D), while the overexpression of USP33 increased ECAR in MG-63 cells (Figure 3E, 3F). Furthermore, OCR, an indicator of mitochondrial respiration, was enhanced in U2OS cells with USP33 knockdown (Figure 3G, 3H), while USP33 overexpression decreased OCR in MG-63 cells (Figure 3I, 3J), indicating that USP33 facilitates aerobic glycolysis and suppresses mitochondrial respiration in OS cells.
Figure 3.
USP33 promotes osteosarcoma (OS) proliferation by enhancing the Warburg effect. A. Cellular glucose consumption, G6P levels, ATP levels and lactate generation in USP33 knockdown U2OS cells. *P < 0.05. B. Cellular glucose consumption, G6P levels, ATP levels and lactate generation in p-USP33-overexpressing MG-63 stable cells. *P < 0.05. C, D. ECAR data suggested the capacity and glycolytic rate in USP33 knockdown U2O cellss. *P < 0.05. E, F. OCR outcomes reveal maximum respiration and basal respiration in USP33 knockdown U2O cells. G, H. ECAR data exhibiting the capacity and glycolytic rate in p-USP33-expressing MG-63 cells. *P < 0.05. I, J. OCR outcomes reveal maximum respiration and basal respiration in p-USP33-expressing MG-63 cells.
USP33 positively modulated the protein level of PFKFB3
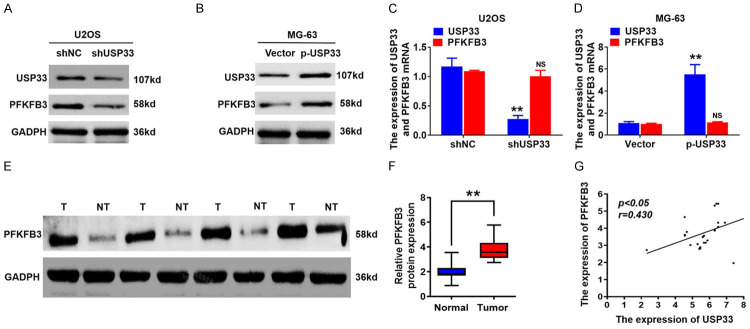
PFKFB3, a key enzyme in the synthesis of F-2, 6-DP, is involved in glycolysis and is highly expressed in numerous cancer cells. To determine if USP33 could modulate PFKFB3 expression, we evaluated PFKFB3 protein levels in USP33 knockdown or overexpressed OS cells. Western blot revealed that the knockdown of USP33 significantly reduced the expression of PFKFB3 in U2OS cells (Figure 4A), while USP33 overexpression increased PFKFB3 level in MG-63 cells (Figure 4B). In contrast, the PFKFB3 mRNA level was not affected by the altered expression of USP33 in OS cells (Figure 4C, 4D). Subsequently, we explored the association between the expression levels of PFKFB3 and USP33 in OS tissues. Western blot analysis reveals that, in the OS cancer tissues, PFKFB3 protein levels were significantly upregulated compared to those in the adjacent normal tissues (Figure 4E, 4F). Additionally, scatter plots showed that the protein levels of PFKFB3 and USP33 were positively associated with OS tissues (Figure 4G). Therefore, the knockdown of USP33 could decrease the PFKFB3 expression and hence suppress aerobic glycolysis in OS cells.
Figure 4.
USP33 positively modulates the protein levels of PFKFB3 in osteosarcoma (OS) cells. A. Western blot analysis of PFKFB3 and USP33 expression in USP33 knockdown U2OS cells. B. Western blot analysis of PFKFB3 and USP33 expression in p-USP33-expressing MG-63 cells. C. qRT-PCR analysis for USP33 and PFKFB3 mRNA expression in USP33 knockdown U2OS cells. D. qRT-PCR analysis for the mRNA expression of PFKFB3 and USP33 in p-USP33-expressing MG-63 cells. E, F. PFKFB3 protein expression in OS tissues and adjacent normal tissues determined by western blotting. G. Scatter plots exhibited a positive association between PFKFB3 and USP33 protein level in OS tissues. *P < 0.05.
USP33 stabilises PFKFB3 by regulating its ubiquitination in OS cells
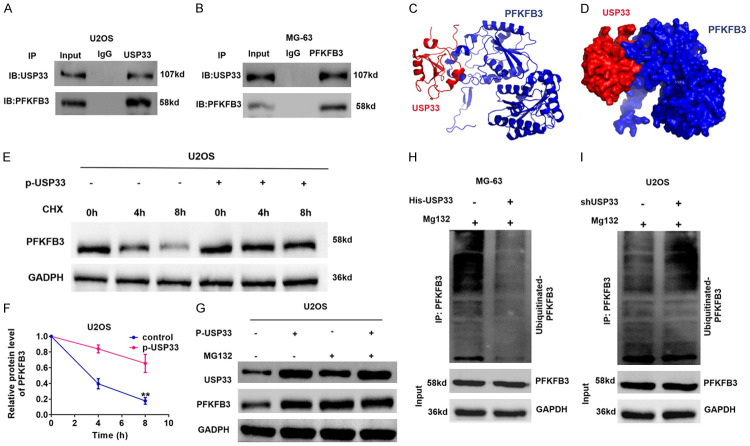
We further analyzed the mechanism of USP33 modulating the expression of PFKFB3 in OS. Since our data above showed that the PFKFB3 mRNA levels were not influenced by USP33 levels in OS cells, which suggested a post-transcriptional regulation, we hypothesised that USP33 could stabilize PFKFB3 by regulating its ubiquitination given that USP33 is a deubiquitinase. To test this hypothesis, we first determined the interaction between USP33 and PFKFB3 in OS cells. As shown in Figure 5A, 5B, Co-IP analysis validated the interaction between endogenous PFKFF3 and USP33 by utilizing PFKFB3 and USP33 antibodies in MG-63 and U2OS cells. Furthermore, docking analysis revealed the binding between PFKFB3 and USP33 (Figure 5C, 5D). Subsequently, we evaluated PFKFB3 protein degeneration in USP33-overexpressing MG-63 cells after exposure to cycloheximide. The overexpression of USP33 significantly inhibited the degradation of PFKFB3 protein in OS cells (Figure 5E, 5F). Additionally, after treatment with proteasome inhibitor MG132, upregulated USP33 had no significant effect on PFKFB3 protein expression in OS cells (Figure 5G). Importantly, ectopic expression of USP33 reduced the ubiquitination level of PFKFB3, whereas USP33 knockdown increased PFKFF3 polyubiquitination (Figure 5H, 5I). Therefore, these findings indicate that USP33 stabilizes PFKFB3 through the ubiquitin-proteasome pathway in OS cells.
Figure 5.
USP33 interacts with PFKFB3 and stabilises the expression of PFKFB2. A, B. Co-IP analyse indicated the interaction between PFKFB3 and USP33 in MG-63 and U2OS cells. C, D. 3D structure of PFKFB3 and USP33. USP33 and PFKFB3 were reflected in red and blue, respectively. E, F. The plasmid p-USP33 was transfected into MG-63 cells. Subsequently, MG-63 cells were exposed to CHX at a specific time, and the degradation of PFKFB3 was detected using Western blot analysis. *P < 0.05. G. MG132 was added to MG-63 cells, and the expression of USP33 was altered. The protein expression level of PFKFB3 was determined using Western blot. H. p-USP33 plasmid was transfected into MG-63 cells in the presence of MG132. Western blot analysis was performed to examine PFKFB3 ubiquitination. I. The shUSP33 plasmid was transfected into U2OS cells in the presence of MG132. Western blot analysis was performed to examine PFKFB3 ubiquitination.
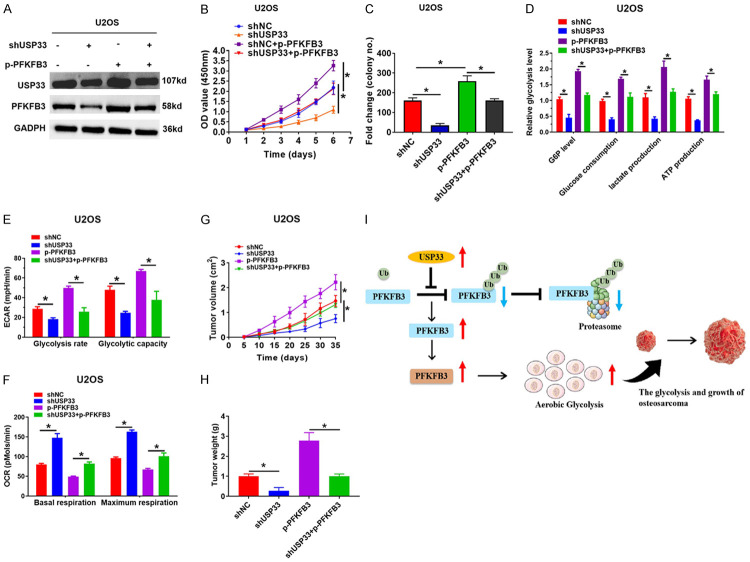
PFKFB3 mediated USP33-induced glycolysis and proliferation in OS cells
Finally, a rescue experiment was performed to determine the carcinogenic impact of USP33 in OS by assessing the stability of PFKFB3. U2OS cells with USP33 knockdown were transfected with HA-PFKFB3, and PFKFB3 overexpression was confirmed (Figure 6A). CCK-8 and colony formation assays demonstrated that USP33 knockdown or PFKFB3 overexpression significantly reduced the proliferation of OS cells (Figure 6B, 6C). However, increased PFKFB3 expression rescued the decrease in cellular G6P production, the consumption of glucose, lactate generation, and ATP levels in shUSP33-OS cells (Figure 6D). Additionally, the knockdown of USP33 reduced ECAR in OS cells, while the simultaneous overexpression of PFKFB3 decreased the glycolytic rate (Figure 6E). Furthermore, we also determined the physiological role of PFKFB3 in mitochondrial respiration mediated by USP33. The upregulation of PFKFB3 in USP33 knockdown OS cells offset the increase of OCR caused by USP33 knockdown (Figure 6F). Significantly, we subcutaneously transplanted the OS cells with USP33 knockdown only or with USP33 knockdown plus PFKFB3 overexpression into nude mice and monitored their tumour growth. As shown in Figure 6G, 6H, the volume and weight of the tumors in mice carrying USP33-silenced cells were reduced significantly; however, the simultaneous PFKFB3 overexpression eliminated the anti-tumor impact of USP33 knockdown. Thus, these findings suggested that PFKFB3 is critical for USP33-induced proliferation and aerobic glycolysis in the OS cells.
Figure 6.
PFKFB3 is critical for the USP33-induced increase of aerobic glycolysis and proliferation in osteosarcoma (OS) cells. A. The expression of PFKFB3 and USP331 in various groups was determined by western blot. B. CCK-8 analysis of U2OS cells transfected by a specific plasmid. *P < 0.05. C. Colony formation analysis of U2OS cells that were transfected by a specific plasmid. *P < 0.05. D. G6P levels, cellular glucose consumption, lactate formation and ATP levels in the indicated group. *P < 0.05. E. ECAR showed the capacity and glycolytic rate in the indicated group. *P < 0.05. F. OCR displayed the maximum respiration and the basal respiration in the indicated group. G, H. Tumor volume quantification or tumor weight determination was performed in the different groups. *P < 0.05. I. Diagram of the regulatory mechanism of USP33 in promoting the glycolysis and the growth of OS by modifying PFKFB3 ubiquitination and degradation.
Discussion
OS is the most common malignancy originating from human bone tissue, accounting for one-third of all bone malignant tumors and posing a great threat to patient health [28,29]. Importantly, approximately 20% of the patients with OS are in the middle and late stages at the time of diagnosis, with a high degree of malignancy [29]. Currently, the treatment for OS is primarily neoadjuvant chemotherapy and surgery. The patients’ 5-year survival rate is only approximately 50%, which is lower than other tumors [30]. Moreover, owing to its dynamic dependence on metabolism, the survival rate of patients is extremely low [31]. Thus, understanding the mechanism of OS from a metabolic perspective could provide novel insights into the disease. In this study, high USP33 expression suggested poor OS prognosis, with USP33 exerting an essential role in OS glucose metabolism reprogramming.
As a member of the ubiquitin-specific protease family, USP33 was initially identified as the substrate that binds to the VHL E3 ligase [32]. Recent studies also report that USP33 plays a critical role in modulating various physiological functions and the development of cancer. USP33 has been observed to be overexpressed in various primary tumors, including prostate cancer, retinoblastoma, hepatocellular carcinoma, gastric cancer, and lung cancer [23,26,27,33,34], suggesting USP33 is a oncogene playing a critical role in tumorigenesis. For example, Xia et al. discovered that USP33 expression was attenuated in gastric cancer cells, which inhibited the Slit2-Robo1 signal and thereby inhibited tumor cell invasion and EMT [33]. Similarly, Gan et al. demonstrated that USP33 increased c-Met expression via increasing SP1 levels, thus promoting the metastasis of hepatocellular carcinoma [23]. However, the detailed functional mechanisms and the role of USP33 in OS require exploration. In the current study, our data indicated that USP33 expression was increased in OS tissues compared to adjacent tissues, with high USP33 levels was associated with poor survival in patients with OS. Furthermore, USP33 suppresses oxidative phosphorylation while facilitating aerobic glycolysis in OS cells. Moreover, it also promoted the proliferation of OS cells through the enhancement of the Warburg effect. Hence, we speculate that USP33 could be a valuable novel prognosis predictor of human OS.
Numerous studies have reported that the metabolic changes and reprogramming in tumor cells could aid in tumor survival and growth in the harsh microenvironments [35-37]. Thus, a better understanding of the underlying regulatory mechanism of aerobic glycolysis in OS cells could help discover new therapeutic opportunities for patients with OS. As a key subtype of PFK1, PFKFB3 participates in cell glycolysis and has an essential role in tumor cell behavior [38-40]. Studies have found an abnormal upregulation of PFKFB3 in colorectal cancer, non-small cell lung cancer, gastric cancer, and other cancers [41,42]. In addition, the inhibition of PFKFB3 in endothelial cells has been reported to promote the normalization of blood vessels, to inhibit tumor metastasis, and to improve chemotherapy sensitivity [43]. In line with these findings, in hepatocellular carcinoma, high PFKFB3 expression confers drug resistance to sorafenib; accordingly, interfering with PFKFB3 expression can restore the sensitivity of HCC to sorafenib [44,45]. Nonetheless, the role of PFKFB3 expression in the development and progression of OS remains unclear. Here, we revealed a new mechanism to suppress aerobic glycolysis and tumor proliferation in OS, via USP33-regulated PFKFB3 activity. Furthermore, we also discovered that the protein levels of PFKFB3 and USP33 were significantly increased in OS tissues, suggesting a positive association between PFKFB3 and USP33 in the OS tissues. Consistently, the knockdown of USP33 decreased PFKFB3 expression and consequently suppressed aerobic glycolysis in the OS cells. Notably, PFKFB3 is critical for the USP33-induced increase of aerobic glycolysis and proliferation in OS cells. Therefore, these findings suggest that USP33 can modulate PFKFB3 expression to impact the malignant progression of OS.
Recent evidence reveals that post-translational modifications, especially ubiquitination, exert a critical role in regulating PFKFB3 [15,16]. Although PFKFB3 can undergo ubiquitination and degradation [16,46], how this process is regulated remains to be determined. In this study, we identified that USP33 as a deubiquitination enzyme of PFKFB3 in OS cells. Docking analysis and Co IP analysis demonstrated a direct interaction between PFKFB3 and USP33. Proteosome inhibitor MG132 and protein synthesis inhibitor CHX experiments indicated USP33 suppressed PFKFB polyubiquitination and degradation, thereby stabilizing PFKFB2 expression in OS cells. Moreover, direct ubiquitination assay demonstrated that the overexpression of USP33 decreased, whereas USP33 knockout increased, PFKFF3 polyubiquitination level.
In summary, this study is the first to report that USP33 is upregulated in OS tissues and is correlated with the progression of OS. Furthermore, USP33 facilitates the aerobic glycolysis and growth of OS cells and in vivo. Mechanistically, USP33 exerts its growth-promoting effects via PFKFB3 stabilization (Figure 6I). Thus, USP33 may serve as a candidate biomarker for the diagnosis and treatment of patients with OS.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 82060403 and 81860397). We thank Bullet Edits Limited and Veritas EdSci for the linguistic editing and proofreading of the manuscript.
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Zhang H, Wang J, Ren T, Huang Y, Liang X, Yu Y, Wang W, Niu J, Guo W. Bone marrow mesenchymal stem cell-derived exosomal miR-206 inhibits osteosarcoma progression by targeting TRA2B. Cancer Lett. 2020;490:54–65. doi: 10.1016/j.canlet.2020.07.008. [DOI] [PubMed] [Google Scholar]
- 2.Wang YM, Wang W, Qiu ED. Osteosarcoma cells induce differentiation of mesenchymal stem cells into cancer associated fibroblasts through Notch and Akt signaling pathway. Int J Clin Exp Pathol. 2017;10:8479–8486. [PMC free article] [PubMed] [Google Scholar]
- 3.Williams SA, Maecker HL, French DM, Liu J, Gregg A, Silverstein LB, Cao TC, Carano RA, Dixit VM. USP1 deubiquitinates ID proteins to preserve a mesenchymal stem cell program in osteosarcoma. Cell. 2011;146:918–930. doi: 10.1016/j.cell.2011.07.040. [DOI] [PubMed] [Google Scholar]
- 4.Pierce TT, Shailam R, Lozano-Calderon S, Sagar P. Inter-rater variability in the interpretation of pre and post contrast MRI for pre-surgical evaluation of osteosarcoma in long bones in pediatric patients and young adults. Surg Oncol. 2019;28:135–139. doi: 10.1016/j.suronc.2018.11.018. [DOI] [PubMed] [Google Scholar]
- 5.Baclesse F. Osteosarcoma of the long bones in young persons; radiodiagnosis and therapy. Brux Med. 1952;32:2599–2612. [PubMed] [Google Scholar]
- 6.Cates JM, Schoenecker JG. Proximal location in extremity long bones is a poor prognostic factor for osteosarcoma: a retrospective cohort study of 153 patients. Acta Oncol. 2016;55:1036–1039. doi: 10.3109/0284186X.2016.1156740. [DOI] [PubMed] [Google Scholar]
- 7.Kaneuchi Y, Yoshida S, Fujiwara T, Evans S, Abudu A. Limb salvage surgery has a higher complication rate than amputation but is still beneficial for patients younger than 10 years old with osteosarcoma of an extremity. J Pediatr Surg. 2022;57:702–709. doi: 10.1016/j.jpedsurg.2022.04.001. [DOI] [PubMed] [Google Scholar]
- 8.Gusho CA, Miller I, Clayton B, Colman MW, Gitelis S, Blank AT. The prognostic significance of lymphovascular tumor invasion in localized high-grade osteosarcoma: outcomes of a single institution over 10 years. J Surg Oncol. 2021;123:1624–1632. doi: 10.1002/jso.26445. [DOI] [PubMed] [Google Scholar]
- 9.Srivastava SP, Li J, Kitada M, Fujita H, Yamada Y, Goodwin JE, Kanasaki K, Koya D. SIRT3 deficiency leads to induction of abnormal glycolysis in diabetic kidney with fibrosis. Cell Death Dis. 2018;9:997. doi: 10.1038/s41419-018-1057-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hu M, Bao R, Lin M, Han XR, Ai YJ, Gao Y, Guan KL, Xiong Y, Yuan HX. ALK fusion promotes metabolic reprogramming of cancer cells by transcriptionally upregulating PFKFB3. Oncogene. 2022;41:4547–4559. doi: 10.1038/s41388-022-02453-0. [DOI] [PubMed] [Google Scholar]
- 11.Muroya S, Nomura R, Nagai H, Ojima K, Matsukawa K. Metabolomic profiling of postmortem aged muscle in Japanese brown beef cattle revealed an interbreed difference from Japanese black beef. Anim Biosci. 2022 doi: 10.5713/ab.22.0202. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yan F, Chen DQ, Tang J, Zhao YY, Guo Y. Serum metabolites associated with blood pressure in chronic kidney disease patients. Metabolites. 2022;12:281. doi: 10.3390/metabo12040281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kotowski K, Rosik J, Machaj F, Supplitt S, Wiczew D, Jablonska K, Wiechec E, Ghavami S, Dziegiel P. Role of PFKFB3 and PFKFB4 in cancer: genetic basis, impact on disease development/progression, and potential as therapeutic targets. Cancers (Basel) 2021;13:909. doi: 10.3390/cancers13040909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yu Y, Liang Y, Li D, Wang L, Liang Z, Chen Y, Ma G, Wu H, Jiao W, Niu H. Glucose metabolism involved in PD-L1-mediated immune escape in the malignant kidney tumour microenvironment. Cell Death Discov. 2021;7:15. doi: 10.1038/s41420-021-00401-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deng X, Deng J, Yi X, Zou Y, Liu H, Li C, Deng B, Fan H, Hao L. Ubiquitin-like protein FAT10 promotes osteosarcoma glycolysis and growth by upregulating PFKFB3 via stabilization of EGFR. Am J Cancer Res. 2020;10:2066–2082. [PMC free article] [PubMed] [Google Scholar]
- 16.Deng X, Yi X, Deng J, Zou Y, Wang S, Shan W, Liu P, Zhang Z, Chen L, Hao L. ROCK2 promotes osteosarcoma growth and metastasis by modifying PFKFB3 ubiquitination and degradation. Exp Cell Res. 2019;385:111689. doi: 10.1016/j.yexcr.2019.111689. [DOI] [PubMed] [Google Scholar]
- 17.Zadoroznyj A, Dubrez L. Cytoplasmic and nuclear functions of cIAP1. Biomolecules. 2022;12:322. doi: 10.3390/biom12020322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang Y, Ding H, Wang X, Wang X, Wan S, Xu A, Gan R, Ye SD. MK2 promotes Tfcp2l1 degradation via beta-TrCP ubiquitin ligase to regulate mouse embryonic stem cell self-renewal. Cell Rep. 2021;37:109949. doi: 10.1016/j.celrep.2021.109949. [DOI] [PubMed] [Google Scholar]
- 19.Zhang Z, Liu W, Bao X, Sun T, Wang J, Li M, Liu C. USP39 facilitates breast cancer cell proliferation through stabilization of FOXM1. Am J Cancer Res. 2022;12:3644–3661. [PMC free article] [PubMed] [Google Scholar]
- 20.Andreadis C, Li T, Liu JL. Ubiquitination regulates cytoophidium assembly in schizosaccharomyces pombe. Exp Cell Res. 2022;420:113337. doi: 10.1016/j.yexcr.2022.113337. [DOI] [PubMed] [Google Scholar]
- 21.Zhang A, Huang Z, Tao W, Zhai K, Wu Q, Rich JN, Zhou W, Bao S. USP33 deubiquitinates and stabilizes HIF-2alpha to promote hypoxia response in glioma stem cells. EMBO J. 2022;41:e109187. doi: 10.15252/embj.2021109187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Culver JA, Mariappan M. Deubiquitinases USP20/33 promote the biogenesis of tail-anchored membrane proteins. J Cell Biol. 2021;220:e202004086. doi: 10.1083/jcb.202004086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gan Q, Shao J, Cao Y, Lei J, Xie P, Ge J, Hu G. USP33 regulates c-Met expression by deubiquitinating SP1 to facilitate metastasis in hepatocellular carcinoma. Life Sci. 2020;261:118316. doi: 10.1016/j.lfs.2020.118316. [DOI] [PubMed] [Google Scholar]
- 24.Han PP, Zhang GQ, Li L, Yue L. Downregulation of USP33 inhibits Slit/Robo signaling pathway and is associated with poor patient survival of glioma. J Neurosurg Sci. 2023;67:113–120. doi: 10.23736/S0390-5616.20.04929-2. [DOI] [PubMed] [Google Scholar]
- 25.Huang Z, Wen P, Kong R, Cheng H, Zhang B, Quan C, Bian Z, Chen M, Zhang Z, Chen X, Du X, Liu J, Zhu L, Fushimi K, Hua D, Wu JY. USP33 mediates Slit-Robo signaling in inhibiting colorectal cancer cell migration. Int J Cancer. 2015;136:1792–1802. doi: 10.1002/ijc.29226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang H, Liu Z, Sun Z, Zhou D, Mao H, Deng G. Ubiquitin specific peptidase 33 promotes cell proliferation and reduces apoptosis through regulation of the SP1/PI3K/AKT pathway in retinoblastoma. Cell Cycle. 2021;20:2066–2076. doi: 10.1080/15384101.2021.1970305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guo F, Zhang C, Wang F, Zhang W, Shi X, Zhu Y, Fang Z, Yang B, Sun Y. Deubiquitinating enzyme USP33 restrains docetaxel-induced apoptosis via stabilising the phosphatase DUSP1 in prostate cancer. Cell Death Differ. 2020;27:1938–1951. doi: 10.1038/s41418-019-0473-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhong Z, Mao S, Lin H, Li H, Lin J, Lin JM. Alteration of intracellular metabolome in osteosarcoma stem cells revealed by liquid chromatography-tandem mass spectrometry. Talanta. 2019;204:6–12. doi: 10.1016/j.talanta.2019.05.088. [DOI] [PubMed] [Google Scholar]
- 29.Ross B, Helsper JT, Cox IJ, Young IR, Kempf R, Makepeace A, Pennock J. Osteosarcoma and other neoplasms of bone. Magnetic resonance spectroscopy to monitor therapy. Arch Surg. 1987;122:1464–1469. doi: 10.1001/archsurg.1987.01400240112021. [DOI] [PubMed] [Google Scholar]
- 30.Bielack SS, Blattmann C, Borkhardt A, Csoka M, Hassenpflug W, Kabickova E, Kager L, Kessler T, Kratz C, Kuhne T, Kevric M, Lehrnbecher T, Mayer-Steinacker R, Mettmann V, Metzler M, Reichardt P, Rossig C, Sorg B, von Luettichau I, Windhager R, Hecker-Nolting S. Osteosarcoma and causes of death: a report of 1520 deceased patients from the cooperative osteosarcoma study group (COSS) Eur J Cancer. 2022;176:50–57. doi: 10.1016/j.ejca.2022.09.007. [DOI] [PubMed] [Google Scholar]
- 31.Lee JS, Kelly CM, Bartlett EK. Management of pelvic sarcoma. Eur J Surg Oncol. 2022;48:2299–2307. doi: 10.1016/j.ejso.2022.09.011. [DOI] [PubMed] [Google Scholar]
- 32.Yuasa-Kawada J, Kinoshita-Kawada M, Rao Y, Wu JY. Deubiquitinating enzyme USP33/VDU1 is required for slit signaling in inhibiting breast cancer cell migration. Proc Natl Acad Sci U S A. 2009;106:14530–14535. doi: 10.1073/pnas.0801262106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xia Y, Wang L, Xu Z, Kong R, Wang F, Yin K, Xu J, Li B, He Z, Wang L, Xu H, Zhang D, Yang L, Wu JY, Xu Z. Reduced USP33 expression in gastric cancer decreases inhibitory effects of Slit2-Robo1 signalling on cell migration and EMT. Cell Prolif. 2019;52:e12606. doi: 10.1111/cpr.12606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang Y, Zhang S, Bao H, Mu S, Zhang B, Ma H, Ma S. MicroRNA-365 promotes lung carcinogenesis by downregulating the USP33/SLIT2/ROBO1 signalling pathway. Cancer Cell Int. 2018;18:64. doi: 10.1186/s12935-018-0563-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Su K, Peng Y, Yu H. Development of a prognostic model based on pyroptosis-related genes in pancreatic adenocarcinoma. Dis Markers. 2022;2022:9141117. doi: 10.1155/2022/9141117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xiao Y, Wang Z, Zhao M, Deng Y, Yang M, Su G, Yang K, Qian C, Hu X, Liu Y, Geng L, Xiao Y, Zou Y, Tang X, Liu H, Xiao H, Fan R. Single-cell transcriptomics revealed subtype-specific tumor immune microenvironments in human glioblastomas. Front Immunol. 2022;13:914236. doi: 10.3389/fimmu.2022.914236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Whitburn J, Rao SR, Morris EV, Tabata S, Hirayama A, Soga T, Edwards JR, Kaya Z, Palmer C, Hamdy FC, Edwards CM. Metabolic profiling of prostate cancer in skeletal microenvironments identifies G6PD as a key mediator of growth and survival. Sci Adv. 2022;8:eabf9096. doi: 10.1126/sciadv.abf9096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jones BC, Pohlmann PR, Clarke R, Sengupta S. Treatment against glucose-dependent cancers through metabolic PFKFB3 targeting of glycolytic flux. Cancer Metastasis Rev. 2022;41:447–458. doi: 10.1007/s10555-022-10027-5. [DOI] [PubMed] [Google Scholar]
- 39.Xing J, Jia Z, Xu Y, Chen M, Yang Z, Chen Y, Han Y. KLF9 (Kruppel Like Factor 9) induced PFKFB3 (6-Phosphofructo-2-Kinase/Fructose-2, 6-Biphosphatase 3) downregulation inhibits the proliferation, metastasis and aerobic glycolysis of cutaneous squamous cell carcinoma cells. Bioengineered. 2021;12:7563–7576. doi: 10.1080/21655979.2021.1980644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cargill KR, Stewart CA, Park EM, Ramkumar K, Gay CM, Cardnell RJ, Wang Q, Diao L, Shen L, Fan YH, Chan WK, Lorenzi PL, Oliver TG, Wang J, Byers LA. Targeting MYC-enhanced glycolysis for the treatment of small cell lung cancer. Cancer Metab. 2021;9:33. doi: 10.1186/s40170-021-00270-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yan S, Li Q, Li S, Ai Z, Yuan D. The role of PFKFB3 in maintaining colorectal cancer cell proliferation and stemness. Mol Biol Rep. 2022;49:9877–9891. doi: 10.1007/s11033-022-07513-y. [DOI] [PubMed] [Google Scholar]
- 42.Jin X, Qiao L, Fan H, Liao C, Zheng J, Wang W, Ma X, Yang M, Sun X, Zhao W. Long non-coding RNA MSC-AS1 facilitates the proliferation and glycolysis of gastric cancer cells by regulating PFKFB3 expression. Int J Med Sci. 2021;18:546–554. doi: 10.7150/ijms.51947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhu W, Ye L, Zhang J, Yu P, Wang H, Ye Z, Tian J. PFK15, a small molecule inhibitor of PFKFB3, induces cell cycle arrest, apoptosis and inhibits invasion in gastric cancer. PLoS One. 2016;11:e0163768. doi: 10.1371/journal.pone.0163768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Long Q, Zou X, Song Y, Duan Z, Liu L. PFKFB3/HIF-1alpha feedback loop modulates sorafenib resistance in hepatocellular carcinoma cells. Biochem Biophys Res Commun. 2019;513:642–650. doi: 10.1016/j.bbrc.2019.03.109. [DOI] [PubMed] [Google Scholar]
- 45.Li S, Dai W, Mo W, Li J, Feng J, Wu L, Liu T, Yu Q, Xu S, Wang W, Lu X, Zhang Q, Chen K, Xia Y, Lu J, Zhou Y, Fan X, Xu L, Guo C. By inhibiting PFKFB3, aspirin overcomes sorafenib resistance in hepatocellular carcinoma. Int J Cancer. 2017;141:2571–2584. doi: 10.1002/ijc.31022. [DOI] [PubMed] [Google Scholar]
- 46.Liu X, Li J, Wang Z, Meng J, Wang A, Zhao X, Xu Q, Cai Z, Hu Z. KDM2A targets PFKFB3 for ubiquitylation to inhibit the proliferation and angiogenesis of multiple myeloma cells. Front Oncol. 2021;11:653788. doi: 10.3389/fonc.2021.653788. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.