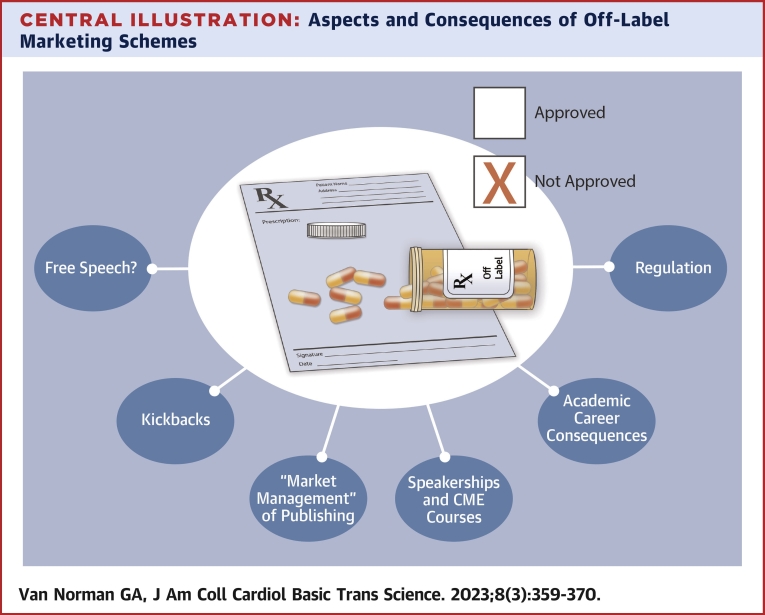
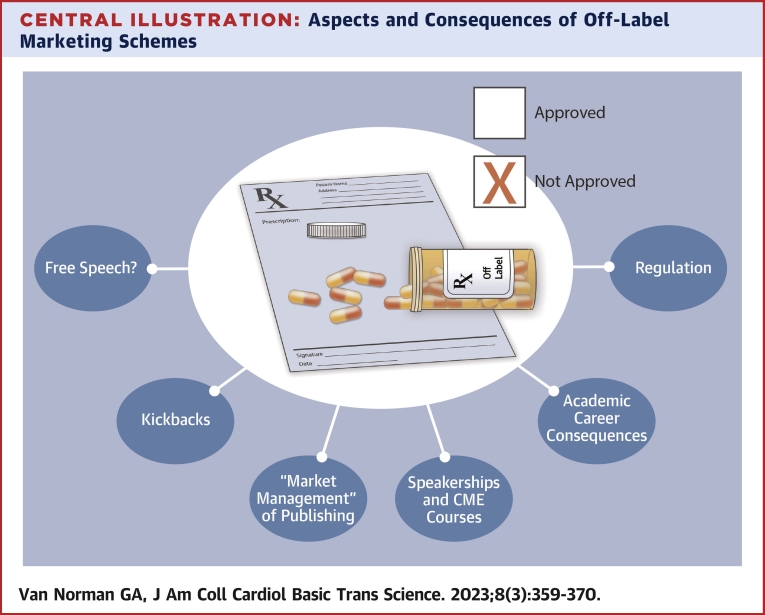
Central Illustration
Key Words: drugs and device marketing, ethics, off-label marketing, off-label use
Pharmaceutical and medical device companies are profit-driven, and drug and device pricing and sales are critical not only to revenue, but to company stock valuation.1,2 Off-label use is nearly impossible to track, and clinicians enjoy wide latitude in prescribing therapies for off-label use. Thus, physicians present obvious opportunities for off-label promotion. Part 1 of this 2-part review discussed off-label use of Food and Drug Administration (FDA)–approved drugs and devices. Part 2 addresses problems with off-label marketing and discusses ways in which manufacturers transform physicians and researchers through educational venues and manipulated publications into marketing opportunities for their products. It also discusses important steps that physicians and investigators can take to avoid the perils of participating in off-market labeling.
Off-Label Marketing
Advertising use of an FDA-approved drug or device for a purpose for which it is not approved is off-label marketing. Off-label use by a physician or other prescriber is legal,3 but off-label marketing of drugs or devices is not. It is illegal to promote or advertise use of a drug or device for anything other than its FDA-approved use, an act termed “misbranding.”4,5 To legally broaden the uses for which a drug or device can be advertised, the manufacturer must apply to the FDA and present evidence of safety and efficacy. Most companies do not seek expansion of FDA-approved indications after initial approval, allegedly due to the time and expense required. Yet clinical phase studies constitute on average <1% of a drug’s total development costs (even during initial approval),6,7 and the process of obtaining approval for additional use is generally shorter and less expensive than initial approval, because preclinical development is already complete and in the case of drug development early toxicity studies have already been performed.
Despite regulations prohibiting off-label marketing, a 2009 review found that off-label drug marketing occurred across “multiple manufacturers of various sizes and drugs in virtually every therapeutic class.”8,9 The prevalence of off-label promotion and use of medical devices and outcomes is less well studied and understood than off-label promotion and use of pharmaceuticals.10 However, off-label marketing also plagues the medical device industry, as evidenced in whistle-blower lawsuits (so-called Qui Tam law suits),11 and recent increased scrutiny by government prosecutors in the FDA, the Office of the Inspector General, and the Department of Justice on marketing practices in the medical device industry.12,13 Off-label device use is well known in cardiology: off-label use of cardiac and vascular stents,10,14 pulmonary vasodilators15 and percutaneous cardiac replacement valves16 are well known and useful in device-mediated treatment innovations. In 2007, the FDA found that 60% of drug-eluting stent placements were off-label, and that off-label use was associated with increased adverse events (although to be fair, off-label placements may well have generally occurred in more complex patients, who might already be at elevated risk for adverse events).10 Off-label marketing, on the other hand, can harm patients, third-party payors, competitor manufacturers, and researchers and clinicians in multiple ways (Box 1, Central Illustration).
Box 1. Patient Harms From Off-Label Promotion.
-
•
Prescription of more expensive but less effective or even nonsensical treatments
-
•
Exposure of patients to adverse side effects from drugs and devices that have not been adequately tested for safety and effectiveness in treatment of a particular condition
-
•
Increased harms to patients who receive treatments that are less effective in treating a particular condition
-
•
Increased out-of-pocket expenses for vulnerable patients who do not have insurance to help cover the costs
-
•
Misuse of funds from government programs such as Medicare and Medicaid to cover inadequately tested, more expensive, and less effective products, leading to funding shortfalls in programs established for patients in need
Central Illustration.
Aspects and Consequences of Off-Label Marketing Schemes
Off-label marketing promotes use associated with significantly heightened rates of adverse effects that have been demonstrated to be substantially increased in off-label use in general,17 and are particularly heightened in children.18 In 2009, for example, Eli Lilly pleaded guilty to illegally marketing Zyprexa as a treatment for dementia: in their own clinical trials twice as many people treated with Zyprexa died compared with controls.19 One hundred percent of deaths and 54.2% of the injuries associated with use of expandable biliary stents recently reported to the Medical Device Reporting data of the FDA involved the planned, off-label placement of the device in the vasculature instead of the biliary tree.14
Off-label marketing reduces innovation by allowing a manufacturer to achieve market expansion of a product without investing the time or money to either develop new drugs or devices with proven efficacy, or to demonstrate an existing drug or device will work for a new indication and still be safe—in effect stealing from other companies who have done the required homework.20,21 One example is the off-label marketing by Bard, Inc. of a coronary balloon angioplasty catheter that had an FDA label restriction permitting only limited manipulation (rotations) during use. Bard later pleaded guilty to both criminal and civil charges that it reconfigured the probe and marketed it off-label as not restricted, without FDA approval.22,23 The victims of this scheme were patients who underwent emergency surgery to remove catheter tips that fragmented, physicians and hospitals who purchased the catheter, and competing companies who had invested time and money to design probes that were safe to freely rotate in situ. To be clear, Bard did not intend to hurt patients. The promotion tapped a market demand without spending the time or money to get full safety clearance by the FDA.
Illegal marketing of all kinds harms third-party payers, including Medicare and Medicaid programs, by increasing the price of products, for unproven indications. Mallinckrodt, for example, paid kickbacks to physicians to prescribe repository corticotropin (RC) for transitory treatment of multiple sclerosis (MS).24 Originally costing $50/vial in 2001, the price of RC was raised to almost $40,000/vial in 2021.25 Yet there is little scientific evidence to support the use of RC for most indications,26 and a 2022 review concluded in fact that RC was not superior to other corticotropins in treatment of MS.27 RC appears more effective than placebo for treatment of MS, but no better than a much less expensive drug, methylprednisolone.28,29 In 2015-2016, annual cost of treatment with RC (including medication, hospitalization, and intravenous treatment) had risen to about $142,000.30 From 2011-2015 spending on RC in the United States increased 10-fold with aggressive marketing to more than $1 billion.31 In 2015, corticotropin was one of the most expensive drugs paid for by the Medicare program, which spent more than one-half billion dollars in that year for methylprednisolone alone.31
Kickbacks
It is illegal for pharmaceutical or medical device companies to offer money or “anything of value in any form whatsoever” to a provider in return for either on-label or off-label prescriptions under the Federal Anti-Kickback Statute—also known as the Medicare and Medicaid Fraud and Abuse Statute.32 Potentially illegal kickbacks to prescribers include cash payments, travel expenses to medical conferences, employment of the physician in some capacity by the company, and “honoraria” paid to physicians as “consultants” or “speakers” at medical conferences. Kickbacks and incentives need not be cash payments or gifts, however. Provision to a prescriber of anything that can be translated into monetary or professional value violates the law.
In the case of RC, about 88% of the most frequent physician prescribers were shown to have received corticotropin-related payments from Mallinckrodt (the maximum payment to an individual was $138,321),31 and the higher the payment, the more frequently they prescribed it. The manufacturer realized returns of approximately $53,000 for every $10,000 increase in prescriber payments—over quadruple the company’s “investment.”
Since the passage of the Federal Anti-Kickback Statute, companies have developed less obvious methods to influence prescribing that still can amount to illegal kickbacks, such as the provision of value services to physician offices and “patient assistance programs” or “prescription assistance programs” (both are referred to as PAPs). PAPs are funded by tax-exempt contributions from the pharmaceutical companies themselves. The funds are then supposed to support access to medications for patients who are uninsured or otherwise unable to pay for prescriptions. Although these programs provide significant tax breaks to companies, their benefits to patients in need have been questioned. Research shows that PAPs favor insured patients over the uninsured, and that programs maximize prescriptions for the more expensive drugs from the pharmaceutical companies that fund them, rather than less expensive alternatives—thus driving up health care costs.33
A 2019 study concluded that PAPs often represent a violation of the Antikickback Statute.34 In that same year, 3 pharmaceutical companies agreed to pay $122 million in penalties “to resolve allegations that they paid kickbacks through PAPs.” The Department of Justice settlement declared that PAP misconduct was “widespread” and called it a strategy by pharmaceutical companies to circumvent the antikickback laws “to artificially bolster high drug prices, all at the expense of American taxpayers.” They described the PAP payments as “illegal kickbacks.”35
Obtaining approval for insurance coverage for prescriptions for their patients is a significant expense for physician practices. The National Bureau of Economic Research found Medicaid claims are denied at least partial payment 25% of the time on first submission, that losses due to failure to pay and to office administrative costs averaged 17.5% of payment for a typical Medicaid visit, 5% for Medicare, and 2.8% for commercial insurers, and that these losses impact patient care.36 One study found costs to the physician’s office of PAP administration work was about $58 per patient, which translates into a significant annual expenditure; one of the study’s own authors found that his clinic spent $327,240 in submission costs alone over 4 years.34 Offering to provide a doctor’s office with patient assistance measures, prescription management, administrative assistance in applying for insurance approval or PAP money, or any other billing activities for the physician—or to subcontract with others to do so—can also constitute a “kickback” to the physician of similar monetary value in return for prescriptions of the company’s drug—an amount that these studies suggest can run up to hundreds of thousands of dollars in time and administrative costs to the physician’s office. Moreover, physicians who accept such services may be participating in a federal crime.
Market-Managing the Medical Literature
Reports of effective and safe off-label uses of drugs and devices is in the best interests of patients and restricting prescriber communication regarding off-label use is problematic. Thus, physicians and other prescribers have more regulatory freedom to discuss off-label uses than manufacturers. Physicians are allowed to discuss off-label uses with individual patients, and at medical conferences with other providers, but they are not allowed to promote off-label use to the general public, to a general practice, or to groups of physicians—this has been reinforced in warning letters from the FDA to physicians who violate the regulation.37 Commercial companies, on the other hand, are more restricted by federal regulations.
A manufacturer is allowed to distribute articles from peer-reviewed journals and reference books pertaining to off-label uses. The FDA cautions that publications should not be “articles in manufacturer-funded special supplements or publications”38 but does not specify that these need to be controlled studies,39,40 and manufacturers often include preliminary abstracts, case reports, case series, and partial data from clinical studies.39,41 Furthermore, many peer-reviewed studies of off-label use are actually written by the companies themselves, and many authors assert that such articles are merely marketing literature disguised as scientific evidence.40,42
Funding for drug research at academic centers shifted from primarily public to primarily commercial sponsorship beginning in the 1970s. By 2005, about 75% of clinical trials published in top medical journals were industry funded.43 Recently, only 25% of approved drugs had documentation of any significant contribution from the public sector.44,45 The influence of drug companies on the medical literature has grown as well, with companies routinely publishing “evidence” with selective data reporting and misleading conclusions.46
Industry-funded drug trials report positive results much more frequently than trials funded by the government and nonprofit organizations (85% vs 71.9%, respectively).47 Industry-funded trials have also sometimes selectively suppressed negative trial results,48 resulting in significant harm. JAMA published data in 2000 indicating that celecoxib was associated with lower gastrointestinal complications than other nonsteroidal anti-inflammatory agents.49 All 16 authors had financial ties to or were employed by the pharmaceutical company. The study ran for a year, but the last 6 months of study data were excluded from publication although available at the time. Nearly all serious complications with the drug (including heart attacks, strokes, and blood clots) occurred during that unreported period.50
In articles published in the New England Journal of Medicine, Merck was shown to have under-reported myocardial infarctions in their VIGOR (VIoxx Gastrointestinal Outcomes Research study) trial participants.51 In 2004, Forest Laboratories published a study as part of their “publication planning for off-label marketing”52 claiming that citalopram was safe and effective in off-label use in pediatric patients.53 Reanalysis demonstrated that there was no significant efficacy of citalopram over placebo, that negative adverse events had been unreported, and that adverse events were misleadingly analyzed.52
Manufacturers prepare a significant number of peer-reviewed articles that are written partially or entirely by a company-employed “ghost author”—someone who is not acknowledged as an author of the paper.54 To add legitimacy to these publications, companies frequently hire academic “opinion leaders” to lend their name as the primary or “guest author,”55 although they may have no direct involvement in the study design, data analysis, or writing process, and may not even have seen or analyzed the complete data set for the study.55,56 The 2004 study by Forest Laboratories, for example, was both ghost-written and guest authored.52 Ghost writers often have a scientific background or work for a university or drug company or medical communications firms, but are not necessarily involved directly with the study itself. Ghost authorship makes the authenticity of the research, the data, and the objectivity of the analysis difficult to trace. The World Association of Medical Editors describes ghost authorship as a threat to scientific integrity because its purpose may be “to persuade readers in favour of a special interest.”57 The International Committee of Medical Journal Editors has thus set forth specific criteria for authorship (Box 2).58
Box 2. International Committee of Medical Journal Editors’ Qualifications for Authorship58.
Authorship Criteria
-
•
Substantial contributions to the conception of the design of the work, or the acquisition, analysis, or interpretation of data for the work, AND
-
•
Drafting the work or revising it critically for important intellectual content, AND
-
•
Final approval of the version to be published, AND
-
•
Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved
Authorship Responsibilities
-
•
Should be able to identify which coauthors are responsible for specific other parts of the work
-
•
Should have confidence in the integrity of the contributions of their coauthors
All persons identified as authors should meet all 4 criteria and should be identified. Anyone not meeting all 4 criteria should be acknowledged (and should not be listed as authors).
A Pfizer company sales document disclosed in litigation states that “The purpose of data is to support, directly or indirectly the marketing of our product.”59 Another document cited in a 2010 Congressional inquiry into medical ghostwriting described the process one company follows in producing peer-reviewed manuscripts for a company product:
“The first step is to choose the target journal best suited to the manuscript’s content, thus avoiding the possibility of manuscript rejection. We will then analyze the data and write the manuscript, recruit a suitable well-recognized expert to lend his/her name as author of the document, and secure his/her approval of its content.”60
Ghost authorship aids concealment of conflicts of interest and research misconduct, and risks downstream detrimental impact on patient care and outcomes.61 Evidence of ghost authorship for studies initiated by commercial companies has been found to occur in 75%-91% of clinical trial protocols and their corresponding research publications.62
Merck & Co Inc., which was eventually fined $350 million in civil fines and nearly $1 billion in criminal penalties for illegal off-label promotion of rofecoxib, was shown to have “prewritten” a majority of journal articles regarding rofecoxib, through company employees or a medical publishing company—only later recruiting academically affiliated guest authors, presenting them with completed manuscripts, and paying them up to $2500 to participate.63 The first author of the published results of Merck’s “advantage” trial of rofecoxib admits the company designed, paid for, and ran the trial, and only afterward approached him with the paper they had already written.55
Ghost-written and guest-authored publications then become presentations at company-sponsored medical conferences, in “educational” webinars, and in presentations to physicians thorough other venues. Through “ghost management” the industry is able to publish selective, favorable data sets while suppressing negative data or even reforging negative data as positive data,55 raising the question of whether FDA allowance of the use of peer-review publications for “discussion” of off-label uses is specific enough and deserves reconsideration.
The full extent of ghost management by manufacturers is unknown because even the authors who lend their names to publications may be unaware of the extent to which the materials they are editing have already been manipulated in favor of the product. Such practices often come to public light through the media and/or litigation. One lawsuit involving the marketing of sertraline revealed a dossier of 85 manuscripts being prepared and/or coordinated for Pfizer by a medical education and communications company (MECC) called Current Medical Directions (CMD). The dossier included details of various vendors supplying the papers—with a number of authors listed as “to be determined,” indicating that the manuscripts had been written by hired writers with planned later recruitment of a guest author. Most were published during a 2-year period from 1998-2000, with academic authors added. These articles represented somewhere between 18% and 40% of all articles published on sertraline,55 and, with such a large proportion of the entire literature about sertraline being written by employees of Pfizer itself and its paid contractors, the company could exert substantial influence over the market. It is not surprising that the CMD articles were uniformly positive.64 CMD and Pfizer-prepared articles on sertraline were published in higher-profile journals, had more authors per article, included authors that were twice as prolific, and attained more than 2.6 times as many citations as articles not sponsored or produced by them.64
The services of “publication managers” such as CMD are in high demand; as early as 2001, there were 182 MECCs in the United States, many of them marketing through their websites directly to the pharmaceutical industry.55 And there is no room for doubt that these services are specifically geared toward product marketing and promotion.39 One MECC defines such publication planning on its website as “gaining product adoption and prescriptions through systematic, planned dissemination of key messages and data to appropriate target audiences at the optimum time using the most effective communication channels.” A video on the website then goes on to describe management of abstracts, posters, peer-reviewed papers in journals, reviews, journal supplements, and open access, and then strategic placement of them at “closed meetings paid for by the [pharmaceutical company] and open medical professional congresses.”65 As Sismondo states, “influencing scientific opinion in the service of marketing is the clearly stated goal here.”55
Academic meetings and continuing medical education (CME) courses have been incorporated in commercial influencing efforts, where company managed, peer-reviewed material is presented by company-hired speakers to promote drugs in the guise of scientific education. A congressional inquiry in 2007 revealed that the drug industry spent more than 1 billion dollars a year to fund CME programs.60 The marketing of Vyvanse (Shire Pharmaceuticals) is one case in point. Jung and Fugh-Berman identified 27 accredited CME courses posted online regarding binge eating disorder (BED) and discovered that all of the CME courses were funded by Shire Pharmaceuticals. Shire was at the time promoting Vyvanse, a drug that obtained FDA approval as a treatment for attention deficit disorder in adults, and then later in 2015 was approved for BED.66 Despite different presenters, the different CME courses shared common slides. Common educational themes included assertions that the mainstays of Bed therapy (i.e. cognitive behavioral therapy and other psychological therapies) negatively impact patients or even that they do not work—although psychotherapy is the first line of therapy in clinical guidelines and has been clearly shown to be highly effective in randomized controlled studies.67,68 The courses emphasized adverse effects of competitor drugs, while not a single Shire-sponsored course mentioned the serious adverse side effects of Vyvanse (eg, addiction, hypertension, tachycardiac, psychosis, manic symptoms, growth suppression, peripheral vascular disease, heart attack, stroke, and death). Based on analysis of the content of the presentations, the authors concluded that the Shire-funded courses were “being used to position Vyvanse as a diet pill,” an off-label use. They pointed out that 14 of these courses were published on-line before Vyvanse was approved for BED therapy and, therefore, constituted off-label marketing.66
Consequences to Physicians, Researchers, and Future Research Funding
There are few studies of financial, legal or academic consequences for researchers and clinicians who participate in off-label marketing and publishing misconduct, however, some reviews suggest that career consequences can be grim. Successful criminal prosecution of a physician speaker for conspiracy to illegally market drugs for off-label at CME conferences and other events use has occurred.69,70 Ghost authorship and guest authorship represent significant misconduct in research and publication, and, in 1 recent study, authorship issues accounted for 22.1% of article retractions.71 Publication misconduct can result in an author being banned from future submissions for a significant period of time (for 1 journal, 8 years72), retraction of the paper, and a report sent to the author’s institution for review and disciplinary action—there is no statute of limitations on academic misconduct. Murphy et al72 cite a case in which the head of a major hospital was forced to resign over publication misconduct that had occurred 20 years earlier.
Retractions result in a significant decrease in citations of an author’s prior work,73,74 and in future productivity, with a median decrease of 91.8% in new publications both 3 and 6 years after a single retraction.75 Publication and research misconduct can lead to loss of academic appointment and have devastating effects on career advancement. Article retraction due to misconduct also has serious negative impact on the availability of funds for research: the estimated mean cost to the National Institutes of Health for retraction of publications that were from publicly funded research is almost $400,000 per article.75 A finding of research misconduct leading to a retracted publication is associated with a sustained loss to the author of more than 70% in further research funding for the next 5 years.75 In at least 1 case, a finding of publication misconduct led to a physician resident being banned from any future research involving federal funds.75 Additional costs include loss of reputation to the author’s institution, legal fees, and salaries of individuals involved in the misconduct investigation, which in 1 cited case exceeded a half a million dollars.76
Legal Ramifications of Off-Label Marketing
Strong voices continue to seek ways to fight in the courts to curtail off-label marketing. In 2006, the Teamsters local union from New Jersey under the Racketeering Influenced and Corrupt Organizations Act sued Pfizer alleging that they and other third-party payors suffered financial harm (in the billions of dollars) in having to pay for prescriptions of atorvastatin that were written off-label as a result of an aggressive marketing campaign by Pfizer.21
Off-label marketing has also been successfully prosecuted using the Federal False Claims Act,77 under the theory that manufacturers cause pharmacies to claim payment for drugs used in ways that are not covered by Medicaid. Penalties accrued under the Federal False Claims Act can be substantial: up to 3 times the amount of damages plus an additional $11,000 per false claim (in the case of drugs, per prescription or each claim for payment) (Box 3).78 Whistleblowers can bring suit on behalf of the government (so-called Qui Tam lawsuits) and may collect up to 30% of the funds awarded to the government if the suit is successful (Box 4). Persons filing Qui Tam complaints are federally protected from retaliation, including reinstatement, twice the amount of back pay with interest, and attorney’s fees. Many states also have similar protection laws in additional to the federal protection.
Box 3. CMS List of Unlawful Means of Off-Label Promotion77.
-
•
Paying incentives to sales representatives based on sales for off-label use
-
•
Paying kickbacks to physicians to prescribe drugs for off-label use
-
•
Disseminating misleading posters promoting off-label use
-
•Paying physicians
-
○To pretend they are authors of articles about off-label uses when the articles were actually written by manufacturer’s agents
-
○To serve as members of “advisory boards” promoting off-label use
-
○To travel to resort locations to listen to promotions about off-label use
-
○To give promotional lectures in favor of off-label use to fellow practitioners
-
○
-
•
Providing advice to prescribers on how to code their claims and document their medical records to support payment for off-label uses not covered by Medicare or Medicaid
-
•
Publicizing studies showing efficacy of off-label uses while suppressing studies showing no efficacy
-
•
Making false representations directly to Medicare or Medicaid to influence decision about payment for drugs used off-label
Box 4. How to Report Unlawful Off-Label Marketing77.
-
•
Report concerns to the FDA via email at BadAD@fda.gov or 855-RX-BadAd (855-792-2323)
- •
-
•
The U.S. Department of Health and Human Services, Office of Inspector General at HHSTips@oig.hhs.gov or 1-800-447-8477 (1-800-HHS-TIPS)
The FDA offers an outreach program through the Agency’s Office of Prescription Drug Promotion to help providers identify potentially false or misleading prescription drug promotion, and to raise awareness among health care providers including physicians, physician assistants, nurse practitioners, nurses, pharmacists, pharmacy technicians, and trainees about potentially false or misleading prescription drug promotion and to provide a means of reporting suspected cases.79
Off-Label Promotion as Free Speech
In some cases, the courts have ruled that restricting a manufacturer’s “speech” in promoting a product for off-label use may run afoul of the First Amendment,80 a fact that would seem to make any regulation of the marketing of unapproved drugs and devices moot. Such claims appear to run against the very reason the regulatory powers of the FDA were originally established: to prevent manufacturers from making poorly or unsubstantiated claims of effectiveness and safety regarding their products because individual patients and doctors are unable to independently and individually undertake the scientific evaluation necessary to determine the veracity of the claims themselves. A 2015 pre-emptive lawsuit by Amarin Pharma asserting that FDA regulations preventing it from distributing information on the off-label use of its prescription fish oil violated its rights to free speech was partially successful. The court determined that Amarin “may engage in truthful and non-misleading speech promoting the off-label use of Vascepa,” leaving significant ambiguity about what would be considered “truthful and non-misleading.”81
Regulating Off-Label Marketing: a Daunting Challenge
Federal enforcement actions against off-label marketing do reduce prescriptions for off-label drug use, but only after they are settled.82 Off-label prescribing of Neurontin, for example, achieved annual sales of over $2.7 billion that only finally fell once a settlement in the case was reached.82 Such high penalties and settlement payments do little to deter the practice, largely because off-label marketing is so lucrative that they have come to be seen as the cost of doing business (Table 1).8 The $2.3 billion penalty extracted from Pfizer in 2009 for illegal marketing of Bextra and 3 other drugs represented a mere 14% of the $16.8 billion profit it earned from them.83 Pfizer continued to engage in off-label marketing, even as it was negotiating a settlement related to the marketing of Neurontin.82,84 Eli Lilly paid just 4% of the company’s earnings from Zyprexa as the 2009 penalty for illegal marketing of the drug; its off-label marketing continued thereafter unabated.8
Table 1.
Examples of Pharmaceutical Company Settlements for Off-Label Promotion Under the False Claims Act 2011-2017a
| Year | Company | Drug | Settlement Amount |
|---|---|---|---|
| 2011 | UCB | Keppra | $34 million combined civil and criminal penalties |
| 2011 | Novo Nordisk | NovoSeven | $25 million |
| 2011 | Pfizer | Detrol | $14.5 million |
| 2012 | Abbott Laboratories | Depakote | $800 million |
| 2012 | Glaxo Smith Kline | Paxil, Wellbutrin, Advair, Lamictal, Zofran | $1.043 billion |
| 2012 | Amgen | Aranesp, Enbrel, Neulasta | $612 million |
| 2013 | Par Pharmaceutical | Megace ES | $22.5 million |
| 2013 | Wyeth Pharmaceuticals | Rapamune | $257.4 million |
| 2013 | Johnson & Johnson | Risperdal, Invega, Natrecor | $1.391 billion |
| 2015 | Insys Therapeutics | Subsys (opioid) | $1.1 million in Oregon, $2.9 million in New Hampshire |
| 2016 | Bristol-Myers Squibb Co | Abilify | $19.5 million |
| 2017 | Celgene | Revlimid, Thalomid | $315 million |
| 2017 | Aegerion Pharmaceuticals | Juxtapid | $28.2 million civil damages, $7.2 million criminal fine |
| 2017 | Boehringer Ingelheim Pharmaceuticals | Aggrenox, Atrovent Combivent, Micardis | $13.5 million |
All settlements available at the U.S. Department of Justice Office of Public Affairs website at https://www.justice.gov/opa.
Some authors have proposed that sanctions against manufacturers should include assignment of personal criminal liability to company executives—similar to the requirements in the Sarbanes-Oxley Act of 200285 that required senior accounting executives to personally sign off on company financial statements, at the risk of criminal penalties including incarceration if they cleared statements that they knew to be inaccurate.84,86 Others have proposed that ghost writers and guest authors be named as defendants in litigation against manufacturers as a means of curtailing unethical research publication.59 And still others have proposed that state medical boards should take action against licensed health care providers who ghostwrite or guest write medical publications.87
Steps for Safeguarding Physicians and Investigators Against the Perils of Off-Label Marketing
Curtailing off-label marketing will require policies, regulation, and real penalties at all levels of involvement: clinicians and researchers, journal editors and reviewers, and commercial company executives. But off-label marketing methods also involve significant contributions from academic authors, clinicians, researchers, and medical education speakers, and exact a heavy toll. Solutions must also, therefore, include them. Most proposals are simple—but implementing them is not easy and has met significant resistance. The following are suggested steps to avoid engaging in off-market labeling:
-
1)
One should not agree to “author” a journal publication unless they meet the International Committee of Medical Journal Editors criteria for authorship, nor should anyone agree to “ghost author” a manuscript. All authors must have participated in the study, data analysis, and manuscript preparation to a very significant degree throughout the entire process. Anyone involved to lesser degrees in design, data analysis, and/or manuscript preparation for a study, but who do not meet qualifying criteria as an author must also be named in an acknowledgement that describes their involvement, however. All financial relationships between a commercial sponsor, authors, and acknowledged contributors must be explicitly disclosed.
-
2)
Before agreeing to participate in a commercially sponsored study, clinicians and researchers should secure an agreement that all data produced during a study will be available to all authors, journal reviewers, and editors. Ideally, data analysis for the study itself should be carried out by an entity that is completely independent of the commercial sponsor. Well-publicized instances of companies preventing publication of negative results by enforcing contracts with investigators that give the company veto rights to publication are a caution to researchers.88 Research/study contracts should not allow the commercial sponsor to veto or place restrictions on the rights of investigators to publish any results, even if they are negative.89
-
3)
Investigators should not work directly with MECCs in the drafting, editing, or critiquing of a manuscript in preparation, or any other activity that might affect nuances of the analysis and conclusions in the publication. If the manuscript was “managed” by either a commercial sponsor or a MECC, this fact should be clearly disclosed.
-
4)
Investigators should be especially wary of participating in so-called “seeding trials”—commercially sponsored postmarketing trials. In such trials, physicians are in effect paid to prescribe a sponsor’s drugs, circumventing rules against kickbacks—and physicians involved in seeding trials are known to increase their prescriptions of trial drugs.90 According to one contract research organization spokesperson, “such [seeding] studies are intended to increase the use of the manufacturer’s product …There is usually a marketing component to these studies, and by doing them the pharma companies get to speak with the doctors about a product and ultimately get them used to prescribing it. The intent is to influence physician and patient behavior.”90, 91, 92
-
5)
Researchers and clinicians should not join Speakers Bureaus for manufacturers. Speakerships are problematic even for “nonauthors” because they are proven to be biased toward the companies’ products, and to promote prescribing of more expensive drugs. For this reason, many universities no longer allow faculty members to join Speakers Bureaus. Clinicians and researchers should at the very least always disclose all such activities in full to audiences. The Accreditation Council for Continuing Medical Education (ACCME) has standards for integrity and independence in accredited CME, and it explicitly forbids pharmaceutical companies from “participating as planners, or faculty” and from influencing or controlling any aspect of the planning, delivery, or evaluation of accredited continuing education.93 If a participant feels that an accredited CME program violates these standards, it should be reported to the ACCME at https://accme.org/submit-complaint.
-
6)
Additionally, academic clinicians and researchers should work to promote academic ethical behavioral norms by actively participating on institutional committees to develop policies that do the following: 1) educate researchers and clinicians about off-label marketing; 2) establish policies regarding ethical research and author relationships with study sponsors; 3) prohibit study contracts that allow sponsors to draft, edit, or suppress articles or any part of the data contained therein, or to facilitate publication through publication planning; 4) set severe penalties for individuals that violate these norms; and 5) establish mechanisms for reporting suspected academic misconduct to the institution and to the journal involved. Policies should practice zero tolerance regarding ghost and guest authorships.
-
7)
Clinicians and researchers who serve as journal reviewers should always recuse themselves when asked to review manuscripts that originate with a commercial sponsor with whom they have any type of financial, contractual, or other relationship that might present a conflict of interest. It is not sufficient to simply disclose such relationships; because of reviewer anonymity, such disclosures will not be available to readers to include in assessment of the validity of the work.
-
8)
Researchers and clinicians who serve on the boards and committees of medical professional societies and on accrediting bodies for CME should work to establish policies prohibiting commercial sponsorship of CME activities and prohibiting manufacturer “participation” in CME courses through distribution of pamphlets or company-sponsored peer-reviewed publications, the provision of slides or talking points to speakers, and payment of speaker honoraria. This will undoubtedly increase registration costs for attendees, but in the words of Drummond Rennie, tireless advocate for steps to contain the influence of commercial sponsors on the quality of medical research: “That argument [that educational activities will not take place without drug company support] presupposes that some of the most well off in our society can’t afford to pay for their lunches, their education, or their conferences. But guess what. All sorts of poorer people pay every step of the way. No one is handing out money to them.”94
Summary
Off-label marketing presents harms to patients, third-party payors, and commercial competitors, and has had a corrupting influence on the integrity of the medical literature. Moreover, as discussed in this review, off-label marketing presents direct harms to physicians and investigators. Clinicians and researchers can be found legally liable for participating in activities that are determined to be off-label marketing schemes and may pay a grim price academically. Regulations regarding off-label marketing have thus far been unsuccessful in curbing the practice, in part because civil and criminal penalties and fines are insufficient to offset the profitability of the practice. Suggestions have included revisiting FDA guidance regarding off-label marketing and the institution of criminal penalties up to and including incarceration for company executives and others who participate in off-label marketing schemes. In the interest of preserving academic and professional integrity, clinicians and researchers need to become active participants in efforts to contain off-label marketing.
Funding Support and Author Disclosures
Dr Van Norman has received funding from the American College of Cardiology, and has provided expert witness testimony regarding off-label drug and device use and marketing.
Footnotes
The author attests they are in compliance with human studies committees and animal welfare regulations of the author’s institution and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
References
- 1.Grant W.C. Excess Medicaid payments and the stock prices of drug companies. J Manag Care Pharm. 2012;18:650. doi: 10.18553/jmcp.2012.18.8.650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bian B., Gorevski E., Kelton C.M.L., et al. Long-term Medicaid excess payments from alleged price manipulation of generic lorazepam. J Manage Care Pharm. 2012;18:506–515. doi: 10.18553/jmcp.2012.18.7.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.U.S. Food and Drug Administration Understanding unapproved use of approved drugs “off label. https://www.fda.gov/patients/learn-about-expanded-access-and-other-treatment-options/understanding-unapproved-use-approved-drugs-label
- 4.21 U.S. Code § 331—Prohibited acts. https://www.law.cornell.edu/uscode/text/21/331#
- 5.21 U.S. Code § 352—Misbranded drugs and devices. https://www.law.cornell.edu/uscode/text/21/352
- 6.Moore T.J., Zhang H., Anderson G., Alexander G.C. Estimated costs of pivotal trials for novel therapeutic agents approved by the US Food and Drug Administration. 2015-2016. JAMA Int Med. 2018;178:1451–1457. doi: 10.1001/jamainternmed.2018.3931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johns Hopkins Bloomberg School of Public Health. Cost of clinical trials for new drug FDA approval are fraction of the total tab. September 24, 2018. https://publichealth.jhu.edu/2018/cost-of-clinical-trials-for-new-drug-FDA-approval-are-fraction-of-total-tab#:∼:text=Clinical%20trials%20to%20obtain%20FDA%20approval%20typically%20account,from%20Johns%20Hopkins%20Bloomberg%20School%20of%20Public%20Health
- 8.Rodwin M.A. Rooting out institutional corruption to manage inappropriate off-label drug use. J Law Med Ethics. 2013;41:654–664. doi: 10.1111/jlme.12075. [DOI] [PubMed] [Google Scholar]
- 9.Kesselheim A.S., Mello M.M., Studder D.M. Strategies and practices in off-label marketing of pharmaceuticals: a retrospective analysis of whistleblower complaints. PLoS Med. 2011;8 doi: 10.1371/journal.pmed.1000431. 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grines C.L. Off-label use of drug eluting stents. Putting it in perspective. J Am Coll Cardiol. 2008;51:615–617. doi: 10.1016/j.jacc.2007.10.028. [DOI] [PubMed] [Google Scholar]
- 11.Phillips & Cohen. Medical devices—off-label marketing kickbacks and whistleblowers. https://www.phillipsandcohen.com/medical-devices-implants/
- 12.Colvin A., Ravits J.R. Off-label promotion of medical devices: seeking clues form the past to protect against increased enforcement in the future. Master Control. April 17, 2017 https://www.mastercontrol.com/gxp-lifeline/off_label_promotion_enforcement_0409/ [Google Scholar]
- 13.Dyson S. How to avoid off-label device promotion. Medical Device and Diagnostic Industry. February 17, 2010. https://www.mddionline.com/news/how-avoid-label-device-promotion
- 14.Marrone A.K., Gottschalk L., Chen A.L. US Food and Drug Administration and off-label use of metal expandable biliary stents within the peripheral vasculature—update. J Vasc Interv Radiol. 2020;31:622–628. doi: 10.1016/j.jvir.2019.10.016. [DOI] [PubMed] [Google Scholar]
- 15.Papthanasiou M., Rhparwar A., Kamler M., Rassaf T., Luedike P. Off-label use of pulmonary vasodilators after left ventricular assist device implantation: calling in the evidence. Pharm Ther. 2020;214:107619. doi: 10.1016/j.pharmthera.2020.107619. [DOI] [PubMed] [Google Scholar]
- 16.Werner N., Renker M., Dorr O., et al. Anatomical suitability and off-label use of contemporary transcatheter heart valves. Int J Cardiol. 2022;350:96–103. doi: 10.1016/j.ijcard.2021.12.044. [DOI] [PubMed] [Google Scholar]
- 17.Eguale T., Buckeridge D.L., Verma A., et al. Association of off-label drug use and adverse drug effects in an adult population. JAMA Int Med. 2016;176:55–63. doi: 10.1001/jamainternmed.2015.6058. [DOI] [PubMed] [Google Scholar]
- 18.European Medicines Agency Evidence of harm from off-label or unlicensed medicine in children. October 2004. https://www.ema.europa.eu/en/documents/other/evidence-harm-label-unlicensed-medicines-children_en.pdf
- 19.Ray W.A., Chung C.P., Murray K.T., et al. Atypical antipsychotic drugs and the risk of sudden cardiac death. N Engl J Med. 2009;360:225–235. doi: 10.1056/NEJMoa0806994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dresser R., Frader J. Off-label prescribing: a call for heightened professional and government oversight. J Law Med Ethics. 2009;37:476–496. doi: 10.1111/j.1748-720X.2009.00408.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Loucks M.K. Pros and cons of off-label promotion investigations and prosecutions. Food Drug Law. 2006;61:577–583. [PubMed] [Google Scholar]
- 22.Company news: C.R. Bard pleads guilty to criminal charges. New York Times. December 16, 1993 https://www.nytimes.com/1993/12/16/business/company-news-c-r-bard-pleads-guilty-to-criminal-charges.html [Google Scholar]
- 23.MedTech Insight. Bard to pay $61 mil. under plea agreement for criminal, civil charges. Oct 18, 1993. https://medtech.pharmaintelligence.informa.com/MT001319/BARD-TO-PAY-61-MIL-UNDER-PLEA-AGREEMENT-FOR-CRIMINAL-CIVIL-CHARGES
- 24.Department of Justice Office of Public Affairs. Drug maker Mallinckrodt agrees to pay over $15 million to resolve alleged false claims act liability for “wining and dining” doctors. September 4, 2019. https://www.justice.gov/opa/pr/drug-maker-mallinckrodt-agrees-pay-over-15-million-resolve-alleged-false-claims-act-liability#:∼:text=Pharmaceutical%20company%20Mallinckrodt%20ARD%20LLC%20%28formerly%20known%20as,H.P.%20Acthar%20Gel%20%28Acthar%29%20from%202009%20through%202013
- 25.United States Attorney’s Office, District of Massachusetts. Mallinkrodt agrees to pay nearly $234 million to resolve allegations related to price increases. March 7, 2022. https://www.justice.gov/usao-ma/pr/mallinckrodt-agrees-pay-nearly-234-million-resolve-allegations-related-price-increases#:∼:text=In%20March%202020%2C%20the%20government%20filed%20a%20complaint,in%202013%2C%20when%20in%20fact%2C%20it%20did%20not
- 26.Metersky M.L. Is there any reliable clinical evidence to suggest that Acthar is more effective than other forms of corticosteroids in treating sarcoidosis and other diseases it is being marketed to treat? Chest. 2016;149:886. doi: 10.1016/j.chest/2015.11.027. [DOI] [PubMed] [Google Scholar]
- 27.Tran K.A., Harrod C., Bourdette D.N. Characterization of the clinical evidence supporting repository corticotropin injection for FDA-approved indications. JAMA Int Med. 2022;182:206–217. doi: 10.1001/jamainternmed.2021.7171. [DOI] [PubMed] [Google Scholar]
- 28.Rose A.S., Kuzma J.W., Kurtzke J.F., et al. Cooperative study in the evaluation of therapy in multiple sclerosis. ACTH vs. placebo—final report. Neurology. 1970;20:1–59. doi: 10.1212/wnl.20.5_part_2.1. [DOI] [PubMed] [Google Scholar]
- 29.Thompson A.J., Kennard C., Swash M., et al. Relative efficacy of intravenous methylprednisolone and ACTH in the treatment of acute relapse in MS. Neurology. 1989;39:969–971. doi: 10.1212/wnl.39.7.969. [DOI] [PubMed] [Google Scholar]
- 30.Wan G.J., Chopra I., Niewoehner J., Hunter S.F. Cost per response analysis of repository corticotropin injection versus other alternative treatments for acute exacerbations of multiple sclerosis. Drugs Context. 2020;9 doi: 10.7573/dic.2020-9-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hartung D.M., Johnston K., Van Leuven S., et al. Trends and characteristics of US Medicare spending on repository corticotropin. JAMA Intern Med. 2017;177:1680–1682. doi: 10.1001/jamainternmed.2017.3631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.42 USC 1320a-7b Criminal penalties for acts involving Federal health care programs. https://uscode.house.gov/view.xhtml?edition=2016&req=granuleid%3AUSC-2017-title42-section1320a-7b&f=treesort&num=0
- 33.Kraschel K., Curfman G. Patient assistance programs and anti-kickback laws. JAMA. 2019;322:405–406. doi: 10.1001/jama.2019.9791. [DOI] [PubMed] [Google Scholar]
- 34.Kang S.Y., Sen A., Bai G., Anderson G.F. Financial eligibility criteria and medication coverage for independent charity patient assistance programs. JAMA. 2019;322:422–429. doi: 10.1001/jama.2019.9943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.U.S. Department of Justice Office of Public Affairs. Three pharmaceutical companies agree to pay a total of over $122 million to resolve allegations that they paid kickbacks through co-pay assistance foundations. April 4, 2019. https://www.justice.gov/opa/pr/three-pharmaceutical-companies-agree-pay-total-over-122-million-resolve-allegations-they-paid
- 36.Dunn A., Gottlieb J.D., Shapiro A., Sonnenstuhl D.J., Tebaldi P. A denial a day keeps the doctor away. National Bureau of Economic Research working paper 29010, July 2021. https://www.nber.org/system/files/working_papers/w29010/w29010.pdf
- 37.Sclafani A.P. Understanding physician responsibilities and limitations for drug and device off-label use. Facial Plast Surg Clin NA. 2007;15:251–254. doi: 10.1016/j.fsc.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 38.U.S. Food and Drug Administration Guidance for industry: good reprint practices for the distribution of medical journal articles and medical or scientific reference publications on unapproved new uses of approved drugs and approved or cleared medical devices: availability. January 13, 2009. https://www.federalregister.gov/documents/2009/01/13/E9-452/guidance-for-industry-on-good-reprint-practices-for-the-distribution-of-medical-journal-articles-and#:∼:text=In%20the%20Federal%20Register%20of%20February%2020%2C%202008,Approved%20Drugs%20and%20Approved%20or%20Cleared%20Medical%20Devices
- 39.Fugh-Berman A., Menick D. Off-label promotion, on-target sales. PLoS Med. 2008;5:1432–1435. doi: 10.1371/journal.pmed.0050210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.U.S. Food and Drug Administration Medical product communications that are consistent with the FDA-required labeling. Questions and answers. June 2018. https://www.fda.gov/media/133619/download
- 41.Mitka M. Critics say FDA/s off-label guidance allows marketing disguised as science. JAMA. 2008;299:1759–1761. doi: 10.1001/jama.299.15.1759. [DOI] [PubMed] [Google Scholar]
- 42.Smith R. Medical journals are an extension of the marketing arm of pharmaceutical companies. PLoS Med. 2005;2:e138. doi: 10.1371/journal.pmed.0020138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Abramson J., Starfield B. The effect of conflict of interest on biomedical research and clinical practice guidelines: can we trust the evidence in evidence-based medicine? J Amer Board Fam Prac. 2005;18:414–418. doi: 10.3122/jabfm.18.5.414. [DOI] [PubMed] [Google Scholar]
- 44.Conti R.M., David F.S. Public research funding and pharmaceutical prices: do Americans pay twice for drugs? F1000Research. 2020;9:707. doi: 10.12688/f1000research.24934.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nayak R.K., Avorn J., Kesselheim A.S. Public sector financial support for late stage discovery of new drugs in the United States: cohort study. BMJ. 2019;367:I5766. doi: 10.1136/bmj.l5766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Amsterdam J.D., McHenry L.B. The paroxetine 352 bipolar trial: a study in medical ghostwriting. Int J Risk Saf Med. 2012;24:221–231. doi: 10.3233/JRS-2012-0571. [DOI] [PubMed] [Google Scholar]
- 47.Bourgeois F.T., Murthy S., Mandl K. Outcome reporting among drug trials registered in ClinicalTrials.gov. Ann Int Med. 2010;153:158–166. doi: 10.1059/0003-4819-153-3-201008030-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lexchin J. Those who have the gold make the evidence: how the pharmaceutical industry biases the outcomes of clinical trials of medications. Sci Eng Ethics. 2012;18:247–261. doi: 10.1007/s11948-011-9265-3. [DOI] [PubMed] [Google Scholar]
- 49.Silverstein F., Faich G., Goldstein J.L., et al. Gastrointestinal toxicity with celecoxib vs nonsteroidal anti-inflammatory drugs for osteoarthritis and rheumatoid arthritis. The CLASS study: a randomized controlled trial. Celecoxib Long-term Arthritis Safety Study. JAMA. 2000;284:1247–1255. doi: 10.1001/jama.284.10.1247. [DOI] [PubMed] [Google Scholar]
- 50.Perry T. Selective reporting of pharmaceutical data leads major medical journals to change editorial policy. CJEM. 2001;3:321–322. doi: 10.1017/s1481803500005868. [DOI] [PubMed] [Google Scholar]
- 51.Kondro W. Dispute over Vioxx study plays out in New England journal. CMAJ. 2006;174:1397. doi: 10.1503/cmaj.060433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jureidini J.N., Amsterdam J.D., McHenry L.B. The citalopram CIT-MD-18 pediatric depression trial: deconstruction of medical ghostwriting, data mischaracterization and academic malfeasance. Int J Risk Saf Med. 2016;28:33–43. doi: 10.3233/JRS-160671. [DOI] [PubMed] [Google Scholar]
- 53.Wagner K.D., Robb A.S., Findling R.L., et al. A randomized, placebo-controlled trial of citalopram for the treatment of major depression in children and adolescents. Am J Psych. 2004;161:1079–1083. doi: 10.1176/appi.ajp.161.6.1079. [DOI] [PubMed] [Google Scholar]
- 54.Matthews A.W. At medical journals, writers paid by industry play a big role. Wall Street Journal. December 13, 2005 https://www.wsj.com/articles/SB113443606745420770 [PubMed] [Google Scholar]
- 55.Sismondo S. Ghost management: how much of the medical literature is shaped behind the scenes by the pharmaceutical industry? PLoS Med. 2007;4:1429–1433. doi: 10.1371/journal.pmed.0040286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Flaherty D.K. Ghost- and guest-authored pharmaceutical industry-sponsored studies: abuse of academic integrity, the peer review system, and public trust. Ann Phamacother. 2013;47:1081–1083. doi: 10.1345/aph.1R691. [DOI] [PubMed] [Google Scholar]
- 57.Aliukonis V., Poškute M., Gefenas E. Perish or publish dilemma: challenges to responsible authorship. Medicina. 2020;56:123. doi: 10.3390/medicina56030123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.International Committee of Medical Journal Editors Defining the role of authors and contributors. https://www.icmje.org/recommendations/browse/roles-and-responsibilities/defining-the-role-of-authors-and-contributors.html
- 59.Moffatt B., Elliott C. Ghost marketing: pharmaceutical companies and ghostwritten journal articles. Persp Biol Med. 2007;50:18–31. doi: 10.1353/pbm.2007.0009. [DOI] [PubMed] [Google Scholar]
- 60.Grassley C. Ghostwriting in medical literature. Minority staff report. Washington DC: US Senate. June 10, 2014. https://www.grassley.senate.gov/imo/media/doc/Senator-Grassley-Report.pdf
- 61.Ali M.J., Djalilian A. Readership awareness series—Paper 1: ghost authorship. Ocu Surf. 2022;26:209–210. doi: 10.1016/j.jtos.2022.07.011. [DOI] [PubMed] [Google Scholar]
- 62.Gotzsche P.C., Hrobjartsson A., Johansen H.K., et al. Ghost authorship in industry-initiated randomized trials. PLoS Med. 2007;4:e19. doi: 10.1371/journal.pmed.0040019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ross J.S., Hill K.P., Egilman D.S., Krumholz H.M. Guest authorship and ghostwriting in publications related to rofecoxib. JAMA. 2008;299:1800–1812. doi: 10.1001/jama.299.15.1800. [DOI] [PubMed] [Google Scholar]
- 64.Healy D., Cattrell D. Interface between authorship, industry and science in the domain of therapeutics. Br J Psychiatr. 2003;183:22–27. [PubMed] [Google Scholar]
- 65.NetworkPharma.tv. All about MedComms. Strategic communications planning and the role of the medcomms agency. April 10, 2017. https://networkpharma.tv/2017/04/10/strategic-communications-planning-and-the-role-of-the-medcomms-agency/#:∼:text=Strategic%20communications%20planning%20is%20about%20gaining%20product%20adoption,optimum%20times%2C%20using%20the%20most%20effective%20communication%20channels
- 66.Jung J., Fugh-Berman A. Marketing messages in continuing medical education (CME) modules on binge-eating disorder (BED) J Am Board Fam Med. 2020;33:240–251. doi: 10.3122/jabfm.2020.02.190129. [DOI] [PubMed] [Google Scholar]
- 67.American Psychiatric Association Steering Committee on Practice Guidelines Ptiracce guidelines for the treatment of patients with eating disorders, 3rd Edition. APA. 2006. https://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/eatingdisorders.pdf
- 68.Yager J., Devlin M.J., Halmi K.A., et al. Guideline watch (August 2012) Practice guideline for the treatment of patients with eating disorders, 3rd edition. https://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/eatingdisorders-watch.pdf
- 69.U.S. v. Caronia 576F. Supp. 2d 385 (E.D.N.Y. 2008) https://casetext.com/case/us-v-caronia Summary.
- 70.Syed S.A., Dixson B.A., Constantino E., Regan J. The law and practice of off-label prescribing and physician promotion. J Am Acad Psych Law. 2021;49:53–59. doi: 10.29158/JAAPL.200049-20. [DOI] [PubMed] [Google Scholar]
- 71.Bennett C., Chambers L.M., Al-Hafez L., et al. Retracted articles in the obstetrics literature: lessons from the past to change the future. Am J Obstet Gynecol MFM. 2020;2:100201. doi: 10.1016/j.ajogmf.2020.100201. [DOI] [PubMed] [Google Scholar]
- 72.Murphy S.P., Bulma C., Shariati B., Hausmann L. Submitting a manuscript for peer review—integrity, integrity, integrity. J Neurochem. 2014;128:341–343. doi: 10.1111/jnc.12644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lu S.F., Jin G.Z., Uzzi B., Jones B. The retraction penalty: evidence from the web of science. Scientif Rep. 2013;3 doi: 10.1038/srep03146. https://papers.ssrn.com/sol3/papers.cfm?abstract_id=2361984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Azoulay P., Bonatti A., Krieger J.L. The career effects of scandal: evidence from scientific retractions. Res Policy. 2017;46:1552–1569. [Google Scholar]
- 75.Stern A.M., Casadevall A., Steen R.G., Fang F.C. Financial cost and personal consequences of research misconduct resulting in retracted publications. eLife. 2014;3 doi: 10.7554/eLife.02956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Machalek A.M., Hutson A.D., Wicher C.P., Trump D.L. The costs and underappreciated consequences of research misconduct: a case study. PLoS Med. 2010;7 doi: 10.1371/journal.pmed.1000318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Off-label pharmaceutical marketing: how to recognize and report it. https://www.cms.gov/Medicare-Medicaid-Coordination/Fraud-Prevention/Medicaid-Integrity-Education/Downloads/off-label-marketing-factsheet.pdf
- 78.U.S. Department of Health and Human Services Office of Inspector General. False Claims Act. https://oig.hhs.gov/newsroom/oig-podcasts/false-claims-act/
- 79.U.S. Food and Drug Administration The bad ad program. 01/05/2021. https://www.fda.gov/drugs/office-prescription-drug-promotion/bad-ad-program
- 80.U.S. v. Caronia. 703 F.3d 149 (2012) https://www.leagle.com/decision/infco20121203037
- 81.Amarin Pharma, Inc et al v. United States Food & Drug Administration, et al, No. 1:2015cv03588-Document 73 (S.D.N.Y. 2015) https://law.justia.com/cases/federal/district-courts/new-york/nysdce/1:2015cv03588/441887/73/#:∼:text=Amarin%20Pharma%2C%20Inc.%20et%20al%20v.%20United%20States,Pharma%2C%20Inc.%2C%20Eric%20Rishe%2C%20Ralph%20Yung%2C%20Jonathan%20Herbst
- 82.Kesselheim A.S., Darby D., Studert D.M., et al. False claims act prosecution did not deter off-label drug use in the case of Neurontin. Health Aff. 2011;12:2318–2327. doi: 10.1377/hlthaff.2011.0370. [DOI] [PubMed] [Google Scholar]
- 83.Evans D. When drug makers’ profits outweigh penalties. Washington Post. March 21, 2010 https://www.washingtonpost.com/wp-dyn/content/article/2010/03/19/AR2010031905578.html [Google Scholar]
- 84.Ratner M. Pfizer settles largest every fraud suit for off-label promotion. Nat Biotech. 2009;27:961–962. doi: 10.1038/nbt1109-961. [DOI] [PubMed] [Google Scholar]
- 85.Sarbanes-Oxley Act of 2002. Public Law 107-204. 107th Congress of the United States. https://www.govinfo.gov/content/pkg/PLAW-107publ204/pdf/PLAW-107publ204.pdf
- 86.Kenton W. Sarbanes-Oxley Act: what it does to protect investors. May 8, 2022. https://www.investopedia.com/terms/s/sarbanesoxleyact.asp
- 87.Stern S., Lemmesn T. Legal remedies for medical ghostwriting: imposing fraud liability on gust authors of ghostwritten articles. PLoS Med. 2010;8 doi: 10.1371/journal.pmed.1001070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rennie D. Thyroid storm. JAMA. 1997;277:1238–1242. [PubMed] [Google Scholar]
- 89.Davidoff F., DeAngelis C.D., Drason J.M., et al. Sponsorship, authorship and accountability. Ann Intern Med. 2001;135:463–466. doi: 10.7326/0003-4819-135-6-200109180-00016. [DOI] [PubMed] [Google Scholar]
- 90.Anderson M., Kargstrup J., Sondergaard J. How conducting a clinical trial affects physician’s guideline adherence and drug preferences. JAMA. 2006;295:2759–2764. doi: 10.1001/jama.295.23.2759. [DOI] [PubMed] [Google Scholar]
- 91.Pena E. The value of phase IV. PharmaVOICE 2003. https://legacy.pharmavoice.com/article/220/
- 92.Psaty B.M., Rennie D. Clinical trial investigators and their prescribing patterns. Another dimension to the relationship between physician investigators and the pharmaceutical industry. JAMA. 2006;295:2787–2790. doi: 10.1001/jama.295.23.2787. [DOI] [PubMed] [Google Scholar]
- 93.Accreditation Council for Continuing Medical Education (ACCME). Standards for integrity and independence in accredited continuing education. ACCME, Chicago Il. December 2020. https://www.accme.org/accreditation-rules/standards-for-integrity-independence-accredited-ce
- 94.Moynihan R. Who pays for the pizza? Redefining the relationships between doctors and drug companies. 2: disentanglement. BMJ. 2003;326:1193–1196. doi: 10.1136/bmj.326.7400.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]